Edwards Gets FDA OK for Konect Resilia Aortic Valved Conduit
July 15 2020 - 9:17AM
Dow Jones News
By Colin Kellaher
Edwards Lifesciences Corp. Wednesday said the U.S. Food and Drug
Administration approved its Konect Resilia aortic valved conduit
for complex aortic-valve surgeries.
The Irvine, Calif., company said the product is the first
ready-to-implant solution for bio-Bentall procedures, which involve
replacement of a patient's aortic valve, aortic root and the
ascending aorta.
Edwards said the device can streamline treatment for patients
requiring the complex and technical surgery, noting that up to 30%
of Bentall procedures are performed in an emergency setting.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
July 15, 2020 09:02 ET (13:02 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
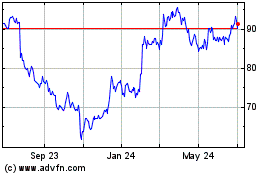
Edwards Lifesciences (NYSE:EW)
Historical Stock Chart
From Mar 2024 to Apr 2024
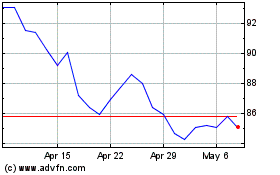
Edwards Lifesciences (NYSE:EW)
Historical Stock Chart
From Apr 2023 to Apr 2024