Use these links to rapidly review the document
TABLE OF CONTENTS Prospectus Supplement
TABLE OF CONTENTS
Table of Contents
Filed Pursuant to Rule 424(b)(5)
Registration Statement No. 333-227363
PROSPECTUS SUPPLEMENT
(To Prospectus dated October 1, 2018)

21,818,544 Shares of Common Stock
Pre-Funded Warrants to Purchase up to 1,360,265 shares of Common Stock
Warrants to Purchase up to 23,178,809 shares of Common Stock
(and the shares of Common Stock underlying such Pre-Funded
Warrants and Warrants)
We are offering an aggregate of 21,818,544 shares of our common stock, par value $0.0001 per share (the "Common Stock"), and warrants to purchase
up to 23,178,809 shares of our Common Stock (the "Warrants") at a combined purchase price equal to $1.51 per share to several institutional and accredited investors. Each Warrant is exercisable for
one share of our Common Stock at an exercise price of $1.80 per share. The Warrants are immediately exercisable and will expire 24 months from the issue date. We are also offering to certain
purchasers pre-funded warrants to purchase up to an aggregate of 1,360,265 shares of Common Stock (the "Pre-Funded Warrants"), in lieu of shares of Common Stock. Each Pre-Funded Warrant is exercisable
for one share of our Common Stock. The purchase price of each Pre-Funded Warrant is equal to the price at which a share of Common Stock is sold to the public in this offering, minus $0.001, and the
exercise price of each pre Pre-Funded Warrant is $0.001 per share. The Pre-Funded Warrants are immediately exercisable and may be exercised at any time until all of the Pre-Funded Warrants are
exercised in full. This offering also relates to the shares of Common Stock issuable upon exercise of the Warrants and Pre-Funded Warrants sold in this offering.
Our
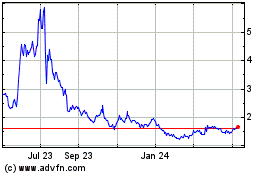
Common Stock is listed on The Nasdaq Global Market, or Nasdaq, under the symbol "RMTI." The last reported sale price of our Common Stock on September 22, 2020 was $1.68 per
share.
You
should read this prospectus supplement and the accompanying prospectus and the documents incorporated by reference in this prospectus supplement carefully before you invest.
See "Risk Factors" on page S-8 of this prospectus supplement to read about factors you should consider before buying shares of our Common
Stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these
securities or determined if this prospectus supplement is truthful or complete. Any representation to the contrary is a criminal offense.
We
have engaged H.C. Wainwright & Co., LLC, or the placement agent, as our exclusive placement agent in connection with this offering. The placement agent has no
obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of securities. We have agreed to pay the placement agent a total cash fee
equal to 6.0% of the aggregate gross proceeds of raised in the offering. Excluding any proceeds we may receive if the Warrants and Pre-Funded Warrants are exercised, the total proceeds to us, after
deducting placement agent fee but before offering expenses, will be approximately $32.9 million. See "Plan of Distribution" beginning on page S-15 of this prospectus supplement for more
information regarding these arrangements.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Per Share and
Accompanying
Common Stock
Warrant
|
|
Per Pre-Funded
Warrant and
Accompanying
Common Stock
Warrant
|
|
Total
|
|
|
|
Offering Price
|
|
$1.51
|
|
$1.50
|
|
$34,998,641.33
|
|
|
|
Placement Agent Fees(1)
|
|
$0.0906
|
|
$0.0906
|
|
$2,100,000.10
|
|
|
|
Proceeds, before expenses, to us(2)
|
|
$1.4194
|
|
$1.4094
|
|
$32,898,641.23
|
|
|
-
(1)
-
In
addition, we have agreed to reimbursements for certain out-of-pocket expenses. See "Plan of Distribution" beginning on page S-15 of this prospectus supplement.
-
(2)
-
The
amount of the offering proceeds to us presented in this table does not give effect to any exercise of the warrants being issued in this offering.
Delivery
of the shares of our Common Stock, Warrants and Pre-Funded Warrants being offered pursuant to this prospectus supplement and the accompanying prospectus is expected to be made
on or about September 25, 2020, subject to satisfaction of certain closing conditions.
H.C. Wainwright & Co.
The date of this prospectus supplement is September 23, 2020.
Table of Contents
TABLE OF CONTENTS
Prospectus Supplement
Prospectus
Table of Contents
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying prospectus relate to an offering of shares of our common stock. Before buying any of the
securities we are offering, we urge you to carefully read this prospectus supplement and the accompanying prospectus, together with the information incorporated by reference as described under the
headings "Where You Can Find Additional Information" and "Incorporation of Certain Information by Reference" in this prospectus supplement. These documents contain important information that you
should consider when making your investment decision. Unless the context otherwise requires, references in this prospectus supplement to "Rockwell," "we," "us," "our" and "ours" refer to Rockwell
Medical, Inc., and include its consolidated subsidiaries where the context so requires.
This
document consists of two parts. The first part is this prospectus supplement, which describes the specific terms of the offering and other matters relating to us. The second part is
the accompanying prospectus, which provides more general information about the securities we may offer from time to
time, some of which may not apply to this offering of Common Stock, Warrants and Pre-funded Warrants (and the shares of Common Stock underlying the Warrants and Pre-Funded Warrants). This prospectus
supplement and the accompanying prospectus are part of a registration statement that we filed with the Securities and Exchange Commission (the "SEC") using the SEC's shelf registration rules. You
should read both this prospectus supplement and the accompanying prospectus, together with the documents incorporated by reference and the additional information described under the heading "Where You
Can Find More Information" in this prospectus supplement and the accompanying prospectus before making an investment decision. Generally, when we refer to this prospectus, we are referring to both
parts of this document combined.
To
the extent there is a conflict between the information contained in this prospectus supplement, on the one hand, and the information contained in the accompanying prospectus, on the
other hand, the information contained in this prospectus supplement shall control. If any statement in this prospectus supplement conflicts with any statement in a document that has been incorporated
herein by reference, then you should consider only the statement in the more recent document. You should assume that the information contained in this prospectus supplement, the accompanying
prospectus and the documents incorporated by reference is accurate only as of their respective dates.
We
have not authorized, and the placement agent has not authorized, any person to provide you with any information or to make any representation other than as contained in this
prospectus supplement or in the accompanying prospectus and the information incorporated by reference herein and therein. We do not take any responsibility for, and can provide no assurance as to the
reliability of, any information that others may provide you. The information appearing or incorporated by reference in this prospectus supplement and the accompanying prospectus is accurate only as of
the date of this prospectus supplement or the date of the document in which incorporated information appears unless otherwise noted in such documents. Our business, financial condition, results of
operations and prospects may have changed since those dates.
We
further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference into this
prospectus supplement or the accompanying prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties
to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made.
Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
The
distribution of this prospectus supplement and the accompanying prospectus and the offering of the Common Stock, Warrants and Pre-funded Warrants (and the shares of Common Stock
S-1
Table of Contents
underlying
the Warrants and Pre-Funded Warrants) in certain jurisdictions may be restricted by law. We are not making an offer of the Common Stock, Warrants or Pre-funded Warrants (or the shares of
Common Stock underlying the Warrants or Pre-Funded Warrants) in any jurisdiction where the offer is not permitted. Persons who come into possession of this prospectus supplement and the accompanying
prospectus should inform themselves about and observe any such restrictions. This prospectus supplement and the accompanying prospectus do not constitute, and may not be used in connection with, an
offer or solicitation by anyone in any jurisdiction in which such offer or solicitation is not authorized or in which the person making such offer or solicitation is not qualified to do so or to any
person to whom it is unlawful to make such offer or solicitation.
This
prospectus supplement, the accompanying prospectus and the information incorporated herein and therein by reference include trademarks, servicemarks and tradenames owned by us or
other companies. All trademarks, servicemarks and tradenames included or incorporated by reference in this prospectus supplement or the accompanying prospectus are the property of their respective
owners.
S-2
Table of Contents
NOTE REGARDING FORWARD-LOOKING STATEMENTS
We make forward-looking statements in this report and may make such statements in future filings with the Securities and Exchange Commission, or
SEC. We may also make forward-looking statements in our press releases or other public or shareholder communications. Our forward-looking statements are subject to risks and uncertainties and include
information about our expectations and possible or assumed future results of our operations. When we use words such as "may," "might," "will," "should," "believe," "expect," "anticipate," "estimate,"
"continue," "could," "plan," "potential," "predict," "forecast," "project," "intend," or similar expressions, or make statements regarding our intent, belief, or current expectations, we are making
forward-looking statements. Our forward looking statements also include, without limitation, statements about our liquidity and capital resources; our plans and ability to successfully commercialize
our products; our timing and ability to obtain add-on reimbursement for our products; our ability to successfully launch FDA approved Triferic AVNU; whether we can successfully execute on our business
strategy and development of new indications; and statements regarding our anticipated future financial condition, operating results, cash flows and business plans.
While
we believe that our forward-looking statements are reasonable, you should not place undue reliance on any such forward-looking statements, which are based on information available
to us on the date of this report or, if made elsewhere, as of the date made. Because these forward-looking statements are based on estimates and assumptions that are subject to significant business,
economic and competitive uncertainties, many of which are beyond our control or are subject to change, actual results could be materially different. Factors that might cause such a difference include,
without limitation, the risks and uncertainties discussed in this prospectus, "Item 1A—Risk Factors" in our Form 10-K for the year ended December 31, 2019 and from
time to time in our other reports filed with the SEC.
Other
factors not currently anticipated may also materially and adversely affect our results of operations, cash flow and financial position. There can be no assurance that future
results will meet expectations. Forward-looking statements speak only as of the date of this report and we expressly disclaim any intent to update or alter any statements whether as a result of new
information, future events or otherwise, except as may be required by applicable law.
S-3
Table of Contents
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights selected information contained elsewhere in this prospectus supplement or incorporated by
reference in this prospectus supplement, and does not contain all of the information that you need to consider in making your investment decision. You should carefully read the entire prospectus
supplement, the accompanying prospectus and any related free writing prospectus that we authorize for use in connection with this offering, including the risks of investing in our securities discussed
under the heading "Risk Factors" beginning on page S-8 of this prospectus supplement and under similar headings in the accompanying prospectus and in the other documents that are incorporated
by reference herein or therein. You should also carefully read the information incorporated by reference into this prospectus supplement and the accompanying prospectus, including our financial
statements and the exhibits to the registration statement of which this prospectus supplement is a part.
Overview
We are a biopharmaceutical company dedicated to improving outcomes for patients with iron deficiency and iron-deficiency anemia, with an initial
focus on patients with end-stage kidney disease (ESKD) and on dialysis. The Company is focused on developing its proprietary ferric pyrophosphate citrate ("FPC") therapeutic platform. The first
product developed from this platform is Triferic, the first-FDA approved product for the replacement of iron and maintenance of hemoglobin in adult hemodialysis patients. We initiated commercial sales
of Triferic Dialysate, during the second quarter of 2019 and received approval by the U.S. Food and Drug Administration ("FDA") for the intravenous formulation of Triferic, Triferic AVNU, on
March 27, 2020. We plan to leverage our experience with Triferic to develop our FPC platform for iron deficiency and iron deficiency anemia in other disease states. We are also a manufacturer
of hemodialysis concentrates for dialysis providers and distributors in the United States and abroad. We supply the domestic market with dialysis concentrates and we also supply dialysis concentrates
to distributors serving a number of foreign countries, primarily in the Americas and the Pacific Rim.
Our
mission is to transform anemia management in a wide variety of disease states across the globe, while improving patients' lives. Accordingly, we are building the foundation to become
a leading medical and commercial organization in the field of iron deficiency.
Recent Developments
As of June 30, 2020, the Company had approximately $26.7 million of cash and cash equivalents, $13.3 million of investments
available-for-sale, working capital of $40.0 million and an accumulated deficit of $321.4 million. Net cash used in operating activities for the six months ended June 30, 2020 was
approximately $16.2 million. Management evaluated the Company's ability to continue as going concern for at least the next 12 months from the filing of this prospectus supplement and
accompanying prospectus. Based on the currently available working capital, capital raise, debt financing described below and the expected proceeds from this offering, management believes the Company
currently has sufficient funds to meet its operating requirements for at least the next twelve months from the date of the filing of this prospectus supplement and accompanying prospectus.
In
February 2020, the Company sold 3,670,212 shares of its common stock for proceeds of $8.0 million, net of issuance costs. On March 16, 2020, the Company closed a debt
financing transaction with net proceeds at closing of approximately $21.2 million, net of fees and expenses.
During
the six months ended June 30, 2020, the Company sold 987,716 shares of its common stock as part of its sales agreement with Cantor Fitzgerald & Co. for
proceeds of $2.0 million, net of issuance costs. Approximately $32.6 million remains available for sale under this facility.
S-4
Table of Contents
The
Company will require additional capital to sustain its operations and make the investments it needs to execute upon its longer-term business plan, including the continued
commercialization of Triferic Dialysate and Triferic AVNU, executing plans for enhancing its medical capabilities, generating additional data for Triferic and developing Triferic for new therapeutic
indications. If the Company is unable to generate sufficient revenue from its existing long-term business plan, the Company may not be able to satisfy certain covenants in its loan agreement with
Innovatus and will need to obtain
additional equity or debt financing. If the Company attempts to obtain additional debt or equity financing, the Company cannot assume that such financing will be available on favorable terms, if at
all.
We
are regulated by the FDA under the Federal Drug and Cosmetics Act, as well as by other federal, state and local agencies. We hold several FDA product approvals including for both
drugs and medical devices.
Coronavirus
The Coronavirus pandemic and resulting global disruptions have adversely affected our business and operations, including, but not limited to,
our sales and marketing efforts and our research and development activities, and the operations of third parties upon whom we rely. As noted above, we intend to initiate a sample evaluation program
for Triferic AVNU during the third quarter of 2020 in order to prepare for a commercial launch. Quarantines, shelter-in-place, executive and similar government orders may negatively impact our sales
and marketing activities, particularly if our sales representatives are unable to interact with current and potential customers to the same extent as before onset of the Coronavirus pandemic.
Depending on the severity of the impact on our sales and marketing efforts, the timing of our commercial launch of Triferic AVNU could be adjusted into the first quarter of 2021.
The
Coronavirus pandemic and resulting global disruptions have caused significant volatility in financial and credit markets. We have utilized a range of financing methods to fund our
operations in the past; however, current conditions in the financial and
Company Information
We were incorporated in the state of Michigan in 1996 and we reincorporated in the state of Delaware in August 2019. Our principal executive
offices are located at 411 Hackensack Avenue, Suite 501, Hackensack, New Jersey 07601. Our telephone number is (248) 960-9009 and our Internet website address
is www.rockwellmed.com. We do not incorporate the information on our website into this prospectus supplement, and you should not consider it part of
this prospectus supplement.
S-5
Table of Contents
SUMMARY OF THE OFFERING
|
|
|
|
|
Common Stock offered
|
|
21,818,544 shares.
|
|
Warrants offered
|
|
We are also offering Warrants to purchase up to 23,178,809 shares of Common Stock. Each Warrant is exercisable for one share
of our Common Stock at an exercise price of $1.80 per share. The Warrants are exercisable immediately and will expire 24 months from the issue date. This offering also relates to the shares of Common Stock issuable upon exercise of the Warrants
sold in this offering.
|
|
Pre-Funded Warrants offered
|
|
We are also offering Pre-Funded Warrants to purchase up to 1,360,265 shares of Common Stock to certain purchasers whose
purchase of shares of Common Stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 9.99% (or, at the election of the purchaser, 4.99%) of our
outstanding Common Stock immediately following the consummation of this offering, in lieu of shares of Common Stock that would otherwise result in each such purchaser's beneficial ownership exceeding 9.99% (or, at the election of the purchaser,
4.99%) of our outstanding Common Stock. Each Pre-Funded Warrant is exercisable for one share of our Common Stock. The purchase price of each Pre-Funded Warrant is equal to the price at which the share of Common Stock is being sold to the public in
this offering, minus $0.001, and the exercise price of each Pre-Funded Warrant is $0.001 per share. The Pre-Funded Warrants are exercisable immediately and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. This
offering also relates to the shares of Common Stock issuable upon exercise of the Pre-Funded Warrants sold in this offering.
|
|
Common stock to be outstanding after the offering
|
|
91,975,466 shares (assuming no exercise of the Warrants or the Pre-Funded Warrants). Assuming all of the Warrants and the
Pre-Funded Warrants were immediately exercised, there would be 116,655,432 shares of Common Stock outstanding after this offering.
|
|
Use of proceeds
|
|
We expect to receive net proceeds of approximately $32.9 million from this Offering, before expenses. The Company
intends to use the net proceeds to advance the development of ferric pyrophosphate citrate (FPC), currently indicated for the maintenance of hemoglobin in dialysis, in new indications, including for the treatment of anemia in the home infusion
setting, as well as for working capital and general corporate purposes. See "Use of Proceeds."
|
|
Nasdaq Global Market symbol
|
|
Our Common Stock is listed on Nasdaq under the symbol "RMTI". We do not intend to list the Warrants or the Pre-Funded
Warrants on any securities exchange or nationally recognized trading system.
|
S-6
Table of Contents
|
|
|
|
|
Risk factors
|
|
Investing in our securities involves a high degree of risk. See "Risk Factors" on page S-8 of this prospectus supplement to read about
factors you should consider carefully before buying shares of our Common Stock.
|
The
number of shares of Common Stock that will be outstanding after this offering is based on 70,156,922 shares of Common Stock outstanding as of June 30, 2020, and also
excludes:
-
•
-
5,625,562 shares of common stock issuable upon the exercise of outstanding stock service-based options as of June 30, 2020 at a
weighted-average exercise price of $4.90 per share;
-
•
-
600,000 shares of common stock issuable upon the exercise of outstanding stock performance-based options as of June 30, 2020 at a
weighted-average exercise price of $2.45 per share;
-
•
-
146,800 shares of common stock issuable upon the vesting of restricted stock awards as of June 30, 2020 at a weighted-average exercise
price of $5.70 per share;
-
•
-
420,062 shares of common stock issuable upon the vesting of service-based restricted stock units outstanding as of June 30, 2020 at a
weighted-average exercise price of $3.27 per share;
-
•
-
600,000 shares of common stock issuable upon the vesting of service-based restricted stock units outstanding as of June 30, 2020 at a
weighted-average exercise price of $2.45 per share;
-
•
-
3,248,054 shares of common stock issuable upon the exercise of outstanding warrants as of June 30, 2020 at a weighted-average exercise
price of $4.48 per share; and
-
•
-
3,178,082 shares of common stock reserved for issuance pursuant to future equity awards under our 2018 Long-Term Incentive Plan as of
June 30, 2020, as well as any future increases in the number of shares of our common stock reserved for future issuance under this plan.
Unless
otherwise indicated, all information in this prospectus supplement, including share and per share amounts assumes no exercise of the Warrants or Pre-Funded Warrants to purchase
shares of our Common Stock issued in this offering.
S-7
Table of Contents
RISK FACTORS
Investing in our securities involves a high degree of risk. You should carefully consider the risks and uncertainties
described below and discussed under the section entitled "Risk Factors" contained in our
Annual Report on Form 10-K for the year ended December 31,
2019, as amended, and our Quarterly Reports on Form 10-Q for the quarters ended
March 31, 2020 and
June 30, 2020, respectively, which are incorporated by
reference in this prospectus supplement, together with all of the other information contained in, or incorporated by reference, in this prospectus supplement and the accompanying prospectus, before
purchasing any of our securities. These risks and uncertainties are not the only ones facing us. Additional risks and uncertainties that we are unaware of, or that we currently deem immaterial, also
may become important factors that affect us. If any of these risks actually occur, our business, financial condition, results of operations and future prospects could be
materially and adversely affected. In that case, the trading price of our Common Stock could decline, and you may lose some or all of your investment.
You will experience immediate and substantial dilution in the net tangible book value per share of the Common
Stock you purchase.
Since the price per share of our Common Stock being offered is substantially higher than the net tangible book value per share of our Common
Stock, you will suffer immediate and substantial dilution in the net tangible book value of the Common Stock you purchase in this offering. As of June 30, 2020, our net tangible book value was
approximately $15.3 million, or $0.218 per share. As discussed in greater detail in the "Dilution" section of this prospectus supplement, based on the combined offering price of $1.51 per share
of Common Stock and Warrants and our as adjusted net tangible book value as of June 30, 2020, if you purchase securities in this offering, you will suffer immediate and substantial dilution of
$0.986 per share with respect to the pro forma net tangible book value of our Common Stock.
There is no public market for the Warrants or Pre-Funded Warrants being offered in this offering.
There is no established public trading market for the Warrants or Pre-Funded Warrants being offered in this offering, and we do not expect a
market to develop. In addition, we do not intend to apply to list the Warrants or the Pre-Funded Warrants on any securities exchange or nationally recognized trading system, including Nasdaq. Without
an active market, the liquidity of the Warrants and Pre-Funded Warrants will be limited.
Holders of Warrants or Pre-Funded Warrants purchased in this offering will have no rights as common
stockholders until such holders exercise such Warrants or Pre-Funded Warrants and acquire our Common Stock.
Until holders of Warrants or Pre-Funded Warrants acquire shares of our Common Stock upon exercise of such Warrants or Pre-Funded Warrants,
holders of Warrants or Pre-Funded Warrants will have no rights with respect to the shares of our Common Stock underlying such Warrants or Pre-Funded Warrants. Upon exercise of the Warrants or
Pre-Funded Warrants, the holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
If we sell shares of our Common Stock in future financings, stockholders may experience immediate dilution
and, as a result, our stock price may decline.
We may from time to time issue additional shares of Common Stock at a discount from the current market price of our Common Stock. As a result,
our stockholders would experience immediate dilution upon the purchase of any shares of our Common Stock sold at such discount. In addition, as opportunities present themselves, we may enter into
financings or similar arrangements in the future,
S-8
Table of Contents
including
the issuance of debt securities, preferred stock or Common Stock. If we issue Common Stock or securities convertible or exercisable into Common Stock, our common stockholders would
experience additional dilution and, as a result, our stock price may decline.
Our management team may invest or spend the proceeds of this offering in ways with which you may not agree or
in ways which may not yield a significant return.
The Company intends to use the net proceeds to advance the development of ferric pyrophosphate citrate (FPC), currently indicated for the
maintenance of hemoglobin in dialysis, in new indications, including for the treatment of anemia in the home infusion setting, as well as for working capital and general corporate purposes. Our
management will have considerable discretion in the application of the net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are
being used appropriately. The net proceeds may be used for purposes that do not increase our operating results or enhance the value of our common stock. Pending their use, we may invest the net
proceeds from this offering in short-term, investment-grade, interest-bearing securities. These investments may not yield a favorable return to our stockholders. If we do not invest or apply the net
proceeds from this offering in ways that enhance stockholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
S-9
Table of Contents
USE OF PROCEEDS
We expect to receive net proceeds of approximately $32.9 million from this offering, after deducting placement agent fees and estimated
offering expenses payable by us, and excluding the proceeds, if any, from the exercise of the Warrants or the Pre-Funded Warrants issued in this offering. We intend to use the net proceeds to advance
the development of ferric pyrophosphate citrate (FPC), currently indicated for the maintenance of hemoglobin in dialysis, in new indications, including for the treatment of anemia in the home infusion
setting, as well as for working capital and general corporate purposes.
The
amounts and timing of our actual expenditures will depend on numerous factors, including our development and commercialization efforts, as well as the amount of cash used in our
operations. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering, and investors will be relying on the judgment of our management
regarding the application of the net proceeds from this offering. We therefore cannot estimate with certainty the portion of the net proceeds to be used for each of the purposes described above. We
may also find it necessary or advisable to use the net proceeds for other purposes. Pending the uses described above, we plan to invest the net proceeds from this offering in short-term,
investment-grade, interest-bearing obligations, certificates of deposit or obligations of the United States.
S-10
Table of Contents
DILUTION
If you purchase securities in this offering, your ownership interest will be diluted immediately to the extent of the difference between the
offering price per share you will pay in this
offering and the pro forma as adjusted net tangible book value per share of our common stock after this offering.
Our
net tangible book value as of June 30, 2020 was approximately $15.3 million, or $0.218 per share. We calculate net tangible book value per share by dividing the net
tangible book value, which is tangible assets less total liabilities, by the number of outstanding shares of our common stock. Dilution represents the difference between the amount per share paid by
purchasers of shares in this offering and the as adjusted net tangible book value per common share immediately after giving effect to this offering.
After
giving effect to this offering (the issuance and sale of 21,818,544 shares of our Common Stock, Pre-Funded Warrants to purchase up to an aggregate of 1,360,265 shares of Common
Stock, and Warrants to purchase up to 23,178,809 shares of our Common Stock) and after deducting placement agent fees and estimated offering expenses payable by us, and assuming no exercise of the
Warrants or Pre-Funded Warrants, our as adjusted net tangible book value as of June 30, 2020 would have been approximately $48.0 million, or $0.524 per share. This represents an
immediate increase of $0.306 in as adjusted net tangible book value per share to existing stockholders and immediate dilution of $0.986 in as adjusted net tangible book value per share to investors
purchasing securities in this offering.
The
following table illustrates this calculation:
|
|
|
|
|
|
|
|
|
|
Offering price per share of common stock and accompanying common stock warrant
|
|
|
|
|
$
|
1.51
|
|
|
Historical net tangible book value per share as of June 30, 2020
|
|
$
|
0.218
|
|
|
|
|
|
Increase per share attributable to the adjustments attributable to this offering
|
|
$
|
0.306
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As adjusted net tangible book value per share as of June 30, 2020
|
|
$
|
0.524
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dilution in as adjusted net tangible book value to new investors in this offering
|
|
|
|
|
$
|
0.986
|
|
The
number of shares of Common Stock that will be outstanding after this offering is based on 70,156,922 shares of Common Stock outstanding as of June 30, 2020, and also
excludes:
-
•
-
5,625,562 shares of common stock issuable upon the exercise of outstanding stock service-based options as of June 30, 2020 at a
weighted-average exercise price of $4.90 per share;
-
•
-
600,000 shares of common stock issuable upon the exercise of outstanding stock performance-based options as of June 30, 2020 at a
weighted-average exercise price of $2.45 per share;
-
•
-
146,800 shares of common stock issuable upon the vesting of restricted stock awards as of June 30, 2020 at a weighted-average exercise
price of $5.70 per share;
-
•
-
420,062 shares of common stock issuable upon the vesting of service-based restricted stock units outstanding as of June 30, 2020 at a
weighted-average exercise price of $3.27 per share;
-
•
-
600,000 shares of common stock issuable upon the vesting of service-based restricted stock units outstanding as of June 30, 2020 at a
weighted-average exercise price of $2.45 per share;
-
•
-
3,248,054 shares of common stock issuable upon the exercise of outstanding warrants as of June 30, 2020 at a weighted-average exercise
price of $4.48 per share; and
-
•
-
3,178,082 shares of common stock reserved for issuance pursuant to future equity awards under our 2018 Long-Term Incentive Plan as of
June 30, 2020, as well as any future increases in the number of shares of our common stock reserved for future issuance under this plan.
S-11
Table of Contents
DESCRIPTION OF THE SECURITIES WE ARE OFFERING
We are offering shares of our Common Stock, Warrants and Pre-Funded Warrants. The following description of our Common Stock, Warrants and
Pre-Funded Warrants summarizes the material terms and provisions thereof, including the material terms of the Common Stock, Warrants and Pre-Funded Warrants we are offering under this prospectus
supplement and the accompanying prospectus.
Common Stock
See "Description of Capital Stock" on page 20 of the accompanying prospectus for a description of the material terms of our Common Stock.
Warrants
The following summary of certain terms and provisions of the Warrants that are being offered hereby is not complete and
is subject to, and qualified in its entirety by, the provisions of the Warrant, the form of which will be filed as an exhibit to our Current Report on Form 8-K. Prospective investors should
carefully review the terms and provisions of the form of Warrant for a complete description of the terms and conditions of the Warrants.
Duration and Exercise Price. Each Warrant offered hereby has an initial exercise price per share equal to $1.80. The Warrants are
immediately
exercisable and will expire 24 months from the issue date. The exercise price and number of shares of Common Stock issuable upon exercise is subject to appropriate adjustment in the event of
stock dividends, stock splits, reorganizations or similar events affecting our Common Stock and the exercise price.
Exercisability. The Warrants are exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed
exercise notice
accompanied by payment in full for the number of shares of our Common Stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). Purchasers of the Warrants in
this offering may elect to deliver their exercise notice following the pricing of the offering and prior to the issuance of the Warrants at closing to have their Warrants exercised immediately upon
issuance and receive shares of Common Stock underlying the Warrants upon closing of this offering. A holder (together with its affiliates) may not exercise any portion of the Warrant to the extent
that the holder would own more than 9.99% of the outstanding Common Stock (or, at the election of the purchaser, 4.99%). No fractional shares of Common Stock will be issued in connection with the
exercise of a Warrant. In lieu of fractional shares, we will round down to the next whole share.
Cashless Exercise. In lieu of making the cash payment otherwise contemplated to be made to us upon exercise of a Warrant in payment of
the aggregate
exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of Common Stock determined according to a formula set forth in the
Warrants, provided that such cashless exercise shall only be permitted if the registration statement to which this prospectus is a part is not effective at the time of such exercise.
Transferability. Subject to applicable laws, a Warrant may be transferred at the option of the holder upon surrender of the Warrant to
us together
with the appropriate instruments of transfer.
Exchange Listing. There is no trading market available for the Warrants on any securities exchange or nationally recognized trading
system. We do not
intend to list the Warrants on any securities exchange or nationally recognized trading system.
Right as a Stockholder. Except as otherwise provided in the Warrants or by virtue of such holder's ownership of shares of our Common
Stock, the
holders of the Warrants do not have the rights or
S-12
Table of Contents
privileges
of holders of our Common Stock, including any voting rights, until they exercise their Warrants.
Fundamental Transaction. In the event of a fundamental transaction, as described in the Warrants and generally including any
reorganization,
recapitalization or reclassification of our Common Stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into
another person, the acquisition of more than 50% of our outstanding Common Stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding Common
Stock, the holders of the Warrants will be entitled to receive upon exercise of the Warrants the kind and amount of securities, cash or other property that the holders would have received had they
exercised the Warrants immediately prior to such fundamental transaction.
Pre-Funded Warrants
The following summary of certain terms and provisions of the Pre-Funded Warrants that are being offered hereby is not
complete and is subject to, and qualified in its entirety by, the provisions of the Pre-Funded Warrant, the form of which will be filed as an exhibit to our Current Report on Form 8-K.
Prospective investors should carefully review the terms and provisions of the form of Pre-Funded Warrant for a complete description of the terms and conditions of the Pre-Funded
Warrants.
Duration and Exercise Price. Each Pre-Funded Warrant offered hereby has an initial exercise price per share equal to $0.001. The
Pre-Funded Warrants
are immediately exercisable and may be exercised at any time until the Pre-Funded Warrants are exercised in full. The exercise price and number of shares of Common Stock issuable upon exercise is
subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our Common Stock and the exercise price.
Exercisability. The Pre-Funded Warrants are exercisable, at the option of each holder, in whole or in part, by delivering to us a duly
executed
exercise notice accompanied by payment in full for the number of shares of our Common Stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). Purchasers of
the Pre-Funded Warrants in this offering may elect to deliver their exercise notice following the pricing of the offering and prior to the issuance of the Pre-Funded Warrants at closing to have their
Pre-Funded Warrants exercised immediately upon issuance and receive shares of Common Stock underlying the Pre-Funded Warrants upon closing of this offering. A holder (together with its affiliates) may
not exercise any portion of the Pre-Funded Warrant to the extent that the holder would own more than 9.99% of the outstanding Common Stock (or, at the election of the purchaser, 4.99%). No fractional
shares of Common Stock will be issued in connection with the exercise of a Pre-Funded Warrant. In lieu of fractional shares, we will round down to the next whole share.
Cashless Exercise. In lieu of making the cash payment otherwise contemplated to be made to us upon exercise of a Pre-Funded Warrant in
payment of the
aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of Common Stock determined according to a formula set forth
in the Pre-Funded Warrants.
Transferability. Subject to applicable laws, a Pre-Funded Warrant may be transferred at the option of the holder upon surrender of the
Pre-Funded
Warrant to us together with the appropriate instruments of transfer.
Exchange Listing. There is no trading market available for the Pre-Funded Warrants on any securities exchange or nationally recognized
trading
system. We do not intend to list the Pre-Funded Warrants on any securities exchange or nationally recognized trading system.
S-13
Table of Contents
Right as a Stockholder. Except as otherwise provided in the Pre-Funded Warrants or by virtue of such holder's ownership of shares of
our Common
Stock, the holders of the Pre-Funded Warrants do not have the rights or privileges of holders of our Common Stock, including any voting rights, until they exercise their Pre-Funded Warrants.
Fundamental Transaction. In the event of a fundamental transaction, as described in the Pre-Funded Warrants and generally including any
reorganization, recapitalization or reclassification of our Common Stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger
with or into another person, the acquisition of more than 50% of our outstanding Common Stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our
outstanding Common Stock, the holders of the Pre-Funded Warrants will be entitled to receive upon exercise of the Pre-Funded Warrants the kind and amount of securities, cash or other property that the
holders would have received had they exercised the Pre-Funded Warrants immediately prior to such fundamental transaction.
S-14
Table of Contents
PLAN OF DISTRIBUTION
We have engaged H.C. Wainwright & Co., LLC (the "placement agent") to act as our exclusive placement agent in connection
with this offering. The placement agent proposes to arrange for the sale of the Common Stock, Pre-Funded Warrants and Warrants we are offering pursuant to this prospectus supplement and accompanying
prospectus. The placement agent has no commitment to buy any of the securities. We will make offers only to a limited number of institutional buyers and accredited investors. We will enter into a
securities purchase agreement directly with investors in connection with this offering and we will only sell to investors who have entered into securities purchase agreements with us. We may not sell
the entire amount of shares of our Common Stock, Warrants and Pre-Funded Warrants offered pursuant to this prospectus supplement. The placement will have no authority to bind us by virtue of its
engagement. Further, the placement agent does not guarantee that it will be able to raise new capital in any prospective offering. The placement agent may retain sub-agents and selected dealers in
connection with this offering. We may not sell the entire amount of the securities being offered pursuant to this prospectus supplement.
Delivery
of the shares of our Common Stock, Warrants and Pre-Funded Warrants being offered pursuant to this prospectus supplement and the accompanying prospectus is expected to be made
on or about September 25, 2020, subject to satisfaction of certain closing conditions.
We
have agreed to indemnify the placement agent against specified liabilities relating to or arising out of the agent's activities as placement agent.
Fees and Expenses
We have agreed to pay the placement agent a total cash fee equal to 6.0% of the aggregate gross proceeds of raised in the offering, minus
$420,000 payable by the Company to a financial advisory firm for services related to this offering.
In
addition, the Company shall pay the placement agent (i) 6.0% of the aggregate gross proceeds to be received, if any, from the cash exercise of any warrants issued to investors
in this offering from the exercise of warrants during the fifteen (15) months period commencing on the issuance date and (ii) 4.0% of the aggregate gross proceeds to be received, if any,
from the cash exercise of any warrants issued in the offering from the exercise of warrants during the remainder term of such warrants.
The
Company has also agreed to pay the placement agent non-accountable expenses of $50,000 as well as $12,900 for the clearing fees of the placement agent in connection with this
offering.
We
estimate the total expenses of this offering paid or payable by us will be approximately $2.3 million. After deducting the fees due to the placement agent and our estimated
expenses in connection with this offering, we expect the net proceeds from this offering will be approximately $32.7 million.
Tail Financing Payments
We have also agreed to pay the placement agent, subject to certain exceptions, a tail fee equal to the cash and warrant compensation in this
offering, if any investor, who was contacted or introduced to the Company by placement agent during the term of its engagement or introduced to us by placement agent during the term of its engagement,
provides us with capital in any public or private offering or other financing or capital raising transaction during the 6-month period following the termination or expiration of our engagement
agreement.
S-15
Table of Contents
Regulation M
The placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions
received by it and any profit realized on the resale of the securities sold by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. As an
underwriter, the placement agent would be required to comply with the requirements of the Securities Act and the Exchange Act, including, without limitation, Rule 415(a)(4) under the Securities Act
and Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of common shares by Wainwright acting as principal. Under these rules
and regulations, the placement agent:
-
•
-
may not engage in any stabilization activity in connection with our securities; and
-
•
-
may not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted
under the Exchange Act, until it has completed its participation in the distribution.
Other Relationships
From time to time, the placement agent may provide in the future various advisory, investment and commercial banking and other services to us in
the ordinary course of business, for which they have received and may continue to receive customary fees and commissions. However, except as disclosed in this prospectus supplement, we have no present
arrangements with the placement agent for any further services.
Listing of Common Stock
Our Common Stock is listed on The Nasdaq Global Market, or Nasdaq, under the symbol "RMTI." The last reported sale price of our Common Stock on
September 22, 2020 was $1.68 per share.
S-16
Table of Contents
LEGAL MATTERS
Certain legal matters relating to the issuance of the securities offered by this prospectus supplement will be passed upon for us by Gibson,
Dunn & Crutcher, LLP, San Francisco, California.
EXPERTS
The financial statements incorporated in this prospectus supplement by reference to our
Annual Reports on Form 10-K for the years ended December 21,
2019 and December 31, 2018, respectively, have been so
incorporated in reliance on the report of Marcum LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
S-17
Table of Contents
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. We also filed a registration statement on
Form S-3, including exhibits, under the Securities Act with respect to the securities offered by this prospectus supplement and the accompanying prospectus. This prospectus supplement and the
accompanying prospectus are a part of that registration statement, but do not contain all of the information included in the registration statement or the exhibits. You can find our public filings
with the SEC on the internet at a web site maintained by the SEC located at www.sec.gov.
S-18
Table of Contents
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
We are "incorporating by reference" specific documents that we file with the SEC, which means that we can disclose important information to you
by referring you to those documents that are considered part of this prospectus supplement and the accompanying prospectus. Information that we file subsequently with the SEC will automatically update
and supersede this information. We incorporate by reference the documents listed below, and any documents that we file with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Exchange Act,
after the date of this prospectus supplement until the termination of the offering of all of the securities registered pursuant to the registration statement of which the accompanying prospectus is a
part (excluding any portions of such documents that have been "furnished" but not "filed" for purposes of the Exchange Act):
-
•
-
Our Annual report on
Form 10-K for the year ended December 31, 2019, filed with the SEC on March 17, 2020;
-
•
-
Our Current Reports on
Form 10-Q for the quarter ended March 31, 2020, filed with the SEC on May 11, 2020, and the
quarter ended on June 20, 2020, filed with the SEC on August 10,
2020;
-
•
-
Our Definitive Proxy Statement
filed with the SEC on April 20, 2020;
-
•
-
Our Current Reports on Form 8-K, filed with the SEC on
January 9, 2020,
January 15, 2020
(two reports),
February 6, 2020,
February 21, 2020,
March 2, 2020,
March 12, 2020,
March 20, 2020,
March 27, 2020,
April 1, 2020,
April 20, 2020,
May 11, 2020,
May 21, 2020,
June 10, 2020,
June 19, 2020,
June 29, 2020,
July 13, 2020,
July 22, 2020,
August 10, 2020,
September 9, 2020, and
September 17, 2020 (except for the information furnished under
Items 2.02 or 7.01 and the exhibits furnished thereto); and
-
•
-
the description of our common stock
contained in our registration statement on Form 8-A which was filed on January 23, 1998, including any amendments or reports filed for the purpose of updating such
description.
We
also incorporate by reference all documents (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that
are related to such items) that are filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act on or after the date of this prospectus supplement but prior to
the termination of this offering. These documents include periodic reports, such as Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as
well as proxy statements.
Any
statement contained herein or in a document incorporated or deemed to be incorporated by reference into this document will be deemed to be modified or superseded for purposes of the
document to the extent that a statement contained in this document or any other subsequently filed document that is deemed to be incorporated by reference into this document modifies or supersedes the
statement.
We
will provide, without charge to you, upon written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus supplement, other than exhibits
to those documents, unless the exhibits are specifically incorporated by reference in those documents. Requests should be directed to our principal executive offices at:
Rockwell Medical, Inc.
411 Hackensack Ave., Suite 501
Hackensack, NJ 07601
(248) 960-9009
Attention: David Kull, Secretary
You
can also find these filings on our website at www.rockwellmed.com. We are not incorporating the information on our website other than
these filings into this prospectus supplement.
S-19
Table of Contents
PROSPECTUS

ROCKWELL MEDICAL, INC.
DEBT SECURITIES
COMMON STOCK
PREFERRED STOCK
WARRANTS
SUBSCRIPTION RIGHTS
SECURITIES PURCHASE CONTRACTS
UNITS
We may offer and sell from time to time up to $200 million of any combination of the securities described in this prospectus, from time to
time, in one or more offerings, in amounts, at prices and on terms determined at the times of offerings.
This
prospectus describes the general manner in which our securities may be offered using this prospectus. We will provide specific terms of the securities, including the offering
prices, in one or more supplements to this prospectus. The supplements may also add, update or change information contained in this prospectus. You should read this prospectus and the prospectus
supplement relating to the specific issue of securities carefully before you invest.
We
may offer the securities for sale directly to the purchasers or through one or more underwriters, dealers and agents to be designated at a future date. The supplements to this
prospectus will provide the specific terms of the plan of distribution.
Our
common stock is listed on the Nasdaq Global Market and traded under the symbol "RMTI." The last reported sale price of the common stock on September 13, 2018 was $4.12 per
share. Each prospectus supplement will indicate if the securities offered thereby will be listed on any securities exchange.
Investing in our securities involves risk. Please read carefully the section entitled "Risk Factors" on Page 4 of this prospectus and any
similar section contained in the applicable prospectus supplement and/or other offering material concerning factors you should consider before investing in our securities which may be offered
hereby.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these
securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The
date of this prospectus is October 1, 2018
Table of Contents
TABLE OF CONTENTS
Table of Contents
ABOUT THIS PROSPECTUS
Unless the context otherwise requires, references in this prospectus to "Rockwell," "we," "us," "our" and "ours" refer to Rockwell
Medical, Inc., and include its consolidated subsidiaries where the context so requires.
This
prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or the SEC, using a "shelf" registration process. Under
this shelf registration process, we may, from time to time, sell the securities described in this prospectus, in one or more offerings, up to the maximum aggregate dollar amount $200,000,000. This
prospectus provides you with a general description of the securities that we may offer. Each time we offer securities, we will provide a prospectus supplement and/or other offering material that will
contain specific information about the terms of that offering. The prospectus supplement and/or other offering material may also add, update or change information contained in this prospectus. You
should read this prospectus and the applicable
prospectus supplement and any other offering material together with the additional information described under the heading "Where You Can Find More Information."
You
should rely only on the information contained or incorporated by reference in this prospectus and in any prospectus supplement or other offering material. We have not authorized any
other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not making offers to sell the securities in
any jurisdiction in which an offer is not authorized or in which the person making that offer is not qualified to do so or to anyone to whom it is unlawful to make an offer. You should not assume that
the information contained in this prospectus or any prospectus supplement or any other offering material, or the information we previously filed with the SEC that we incorporate by reference in this
prospectus or any prospectus supplement, is accurate as of any date other than its respective date. Our business, financial condition, results of operations and prospects may have changed since those
dates.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
We make forward-looking statements in this registration statement and may make such statements in future filings with the Securities and
Exchange Commission, or SEC. We may also make forward-looking statements in our press releases or other public or shareholder communications. Our forward-looking statements are subject to risks and
uncertainties and include information about our expectations and possible or assumed future results of our operations. When we use words such as "may," "might," "will," "should," "believe," "expect,"
"anticipate," "estimate," "continue," "could," "plan," "potential," "predict," "forecast," "project," "intend," or similar expressions, or make statements regarding our intent, belief, or current
expectations, we are making forward-looking statements.
These
forward-looking statements are neither promises nor guarantees of future performance due to a variety of risks and uncertainties, many of which are beyond our control, which could
cause actual results to differ materially from those indicated by these forward-looking statements, including, without limitation, risks relating to:
-
•
-
the timing and commercial success of the commercial launch of our proprietary products and new business strategy;
-
•
-
the timing and success of obtaining Medicare and other third-party reimbursement approval for our products, including Triferic;
-
•
-
the timing and success of filing of applications for new regulatory approvals in the United States and abroad, including for our planned
intravenous formulation of Triferic;
-
•
-
our liquidity and capital resources;
1
Table of Contents
-
•
-
future results of operations and the financial condition of the Company;
-
•
-
our dependence on key employees and our ability to integrate new members of our management team;
-
•
-
the timing and success of clinical studies of the Company's drug candidates, including planned studies of Triferic in China and a pediatric
study of Triferic;
-
•
-
the manufacture of our products in compliance with the FDA's current Good Manufacturing Practices;
-
•
-
the ability to maintain compliance with SEC and Nasdaq rules; and
-
•
-
other risks more fully discussed in the "Risk Factors" section in this prospectus, the section of any accompanying prospectus supplement
entitled "Risk Factors" and the risk factors and cautionary statements described in other documents that we file from time to time with the SEC, specifically under "Risk Factors" and elsewhere in our
most recent Annual Report on Form 10-K and subsequent Quarterly Reports on Form 10-Q.
We
claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995 for all of our forward-looking statements.
While we believe that our forward-looking statements are reasonable, you should not place undue reliance on any such forward-looking statements, which are based on information available to us on the
date of this report or, if made elsewhere, as of the date made. Because these forward-looking statements are based on estimates and assumptions that are subject to significant business, economic and
competitive uncertainties, many of which are beyond our control or are subject to change, actual results could be materially different. See "Risk Factors" in this prospectus for more information. You
should consider these factors and other cautionary statements made in this prospectus and in the documents we incorporate by reference as being applicable to all related forward-looking statements
wherever they appear in this prospectus and in the documents incorporated by reference.
Other
factors not currently anticipated may also materially and adversely affect our results of operations, cash flows and financial position. We do not undertake any obligation to
update or alter any statements whether as a result of new information, future events or otherwise, except as required by law.
2
Table of Contents
OUR COMPANY
We are a specialty pharmaceutical company targeting end-stage renal disease ("ESRD") and chronic kidney disease with products for the treatment
of iron deficiency, secondary hyperparathyroidism and hemodialysis (also referred to as "dialysis").
We
are currently marketing and developing unique, proprietary renal drug therapies. These renal drug therapies support disease management initiatives to improve the quality of life and
care of dialysis patients and are designed to deliver safe and effective therapy, while decreasing drug administration costs and improving patient convenience and outcome. We have also obtained
licenses for certain dialysis related drugs which we are developing and planning to market in major markets globally either directly or through license partners.
We
are also a manufacturer of hemodialysis concentrates/dialysates to dialysis providers and distributors in the United States and abroad. We manufacture, sell and distribute
hemodialysis concentrates and other ancillary medical products and supplies used in the treatment of patients with ESRD. We also supply dialysis concentrates to distributors serving a number of
foreign countries, primarily in the Americas and the Pacific Rim. The majority of our sales occur in the United States.
We
are regulated by the United States Food and Drug Administration ("FDA") under the Federal Drug and Cosmetics Act, as well as by other federal, state and local agencies. We hold
several FDA product approvals including both drugs and medical devices.
We
are a Michigan corporation and our corporate headquarters are located at 30142 Wixom Road, Wixom, Michigan 48393. Our telephone number is (248) 960-9009 and our Internet
website address is www.rockwellmed.com. We do not incorporate the information on our website into this prospectus, and you should not consider it part of this prospectus.
3
Table of Contents
RISK FACTORS
Investing in our securities involves risks. Before making an investment decision, you should carefully consider the risks and other information
we include or incorporate by reference in this prospectus and any prospectus supplement. In particular, you should consider the risk factors described under the heading "Risk Factors" in our most
recent Annual Report on Form 10-K and in Item 1A of our Quarterly Report on Form 10-Q for the period ended June 30, 2018 under the heading "Risk Factors," as may be revised
or supplemented by our subsequent Quarterly Reports on Form 10-Q or Current Reports of Form 8-K, each of which are on file with the SEC and are incorporated herein by reference, and
which may be amended, supplemented or superseded from time to time by other reports we file with the SEC in the future. In addition to those risk factors, there may be additional risks and
uncertainties of which are not currently known to us or that we currently deem immaterial. Our business, financial condition or results of operations could be materially adversely affected by any of
these risks. The occurrence of any of these risks might cause you to lose all or part of your investment in the offered securities. Additional risk factors may be included in a prospectus supplement
relating to a particular offering of securities.
4
Table of Contents
USE OF PROCEEDS
Except as may be otherwise set forth in the applicable prospectus supplement accompanying this prospectus, the net proceeds from the sale of the
securities will be used for general corporate purposes, including potentially expanding existing businesses, acquiring businesses and investing in other business opportunities. Pending such use, we
may temporarily invest the net proceeds in short-term investments.
5
Table of Contents
DILUTION
We will set forth in a prospectus supplement the following information regarding any material dilution of the equity interests of investors
purchasing securities in an offering under this prospectus:
-
•
-
the net tangible book value per share of our equity securities before and after the offering;
-
•
-
the amount of the increase in such net tangible book value per share attributable to the cash payments made by purchasers in the offering; and
-
•
-
the amount of the immediate dilution from the public offering price which will be absorbed by such purchasers.
6
Table of Contents
SECURITIES TO BE OFFERED
We may offer, from time to time and in one or more offerings, debt securities, shares of common stock, shares of preferred stock, warrants,
subscription rights, securities purchase contracts and units. Set forth herein and below is a general description of the securities that we may offer hereunder. We will set forth in the applicable
prospectus supplement a specific description of the securities that may be offered under this prospectus. The terms of the offering of securities, the initial offering price and the net proceeds will
be contained in the prospectus supplement and/or other offering material relating to such offering.
7
Table of Contents
RATIO OF EARNINGS TO FIXED CHARGES
As of the date of this prospectus and for the previous five fiscal years, we had no fixed charges and no shares of preferred stock for which we
are required to make dividend payments. Accordingly, we have no ratio of earnings to fixed charges and no ratio of earnings to combined fixed charges and preferred stock dividends, to illustrate for
these periods. To the extent applicable at the
time of filing, we will provide any ratios of earnings to fixed charges in the applicable prospectus supplement or in a document that we file with the SEC and incorporate by reference in the future.
8
Table of Contents
DESCRIPTION OF DEBT SECURITIES
The following description of the terms of the debt securities sets forth general terms that may apply to the debt securities and provisions of
the indenture that will govern the debt securities, and is not complete. We will describe the particular terms of any debt securities in the prospectus supplement relating to those debt securities.
The
debt securities will be our senior debt securities and will be issued under an indenture between us and a trustee, a form of which is incorporated by reference into this prospectus
and attached as an exhibit to the registration statement of which this prospectus is a part. See "Where You Can Find More Information." We refer to this indenture as the "indenture."
The
following is a summary of some provisions of the indenture. The following summary does not purport to be complete, and is subject to, and qualified in its entirety by reference to,
all of the provisions of the indenture, including the definitions of specified terms used in the indenture, and the debt securities. We encourage you to read the indenture and the debt securities
because they, and not this description, set forth your rights as a holder of our debt securities. We will describe the particular terms of any debt securities in the prospectus supplement relating to
those debt securities. Parenthetical section references under this heading are references to sections in the indenture unless we indicate otherwise.
General Terms
The indenture does not limit the amount of debt securities that we may issue. (Section 301). The indenture provides that debt securities
may be issued up to the principal amount authorized by us from time to time. The debt securities will be unsecured and will have the same rank as all of our other unsecured debt. None of our
subsidiaries, if any, will have any obligations with respect to the debt securities. Therefore, our rights and the rights of our creditors, including holders of senior debt securities and subordinated
debt securities, to participate in the assets of any subsidiary will be subject to the prior claims of the creditors of any such subsidiaries.
We
may issue the debt securities in one or more separate series of senior debt securities. (Section 301). The prospectus supplement relating to the particular series of debt
securities being offered will specify the particular amounts, prices and terms of those debt securities. These terms may include:
-
•
-
the title of the debt securities and the series in which the debt securities will be included;
-
•
-
the authorized denominations and aggregate principal amount of the debt securities;
-
•
-
the date or dates on which the principal and premium, if any, are payable;
-
•
-
the rate or rates per annum at which the debt securities will bear interest, if there is any interest, or the method or methods of calculating
interest and the date from which interest will accrue;
-
•
-
the place or places where the principal of and any premium and interest on the debt securities will be payable;
-
•
-
the dates on which the interest will be payable and the corresponding record dates;
-
•
-
the period or periods within which, the price or prices at which, and the terms and conditions on which, the debt securities may be redeemed,
in whole or in part, at our option;
-
•
-
whether the debt securities of the series will be issued in whole or in part;
-
•
-
whether the debt securities of the series will be issued in the form of a global security and, if so, the name of the applicable depositary and
global exchange agent;
9
Table of Contents
-
•
-
any obligation to redeem, repay or purchase debt securities pursuant to any sinking fund or analogous provisions or at the option of a holder;
-
•
-
the portion of the principal amount of the debt securities payable upon declaration of the acceleration of the maturity of the debt securities;
-
•
-
the person to whom any interest on any debt security will be payable if other than the person in whose name the debt security is registered on
the applicable record date;
-
•
-
any events of default, covenants or warranties applicable to the debt securities;
-
•
-
the currency, currencies or composite currency of denomination of the debt securities;
-
•
-
the currency, currencies or composite currencies in which payments on the debt securities will be payable and whether the holder may elect
payment to be made in a different currency;
-
•
-
whether and under what conditions we will pay additional amounts to holders of the debt securities;
-
•
-
the terms and conditions of any conversion or exchange provisions in respect of the debt securities;
-
•
-
the terms pursuant to which our obligation under the indenture may be terminated through the deposit of money or government obligations;
-
•
-
whether the debt securities of the series will be subordinated in right of payment to senior indebtedness; and
-
•
-
any other specific terms of the debt securities not inconsistent with the indenture. (Section 301).
Unless
otherwise specified in the applicable prospectus supplement, the debt securities will not be listed on any securities exchange.
Unless
the applicable prospectus supplement specifies otherwise, we will issue the debt securities in fully registered form without coupons. If we issue debt securities of any series in
bearer form, the applicable prospectus supplement will describe the special restrictions and considerations, including special offering restrictions and special federal income tax considerations,
applicable to those debt securities and to payment on and transfer and exchange of those debt securities.
U.S. Federal Income Tax Considerations
We may issue the debt securities as original issue discount securities, bearing no interest or bearing interest at a rate, which, at the time of
issuance, is below market rates, to be sold at a substantial discount below their principal amount. We will describe some special U.S. federal income tax and other considerations applicable to any
debt securities that are issued as original issue discount securities in the applicable prospectus supplement. We encourage you to consult with your own tax and financial advisors on these important
matters.
Payment, Registration, Transfer and Exchange
Subject to any applicable laws or regulations, we will make payments on the debt securities at a designated office or agency, unless the
applicable prospectus supplement otherwise sets forth. At our option, however, we may also make interest payments on the debt securities in registered form:
-
•
-
by checks mailed to the persons entitled to interest payments at their registered addresses; or
-
•
-
by wire transfer to an account maintained by the person entitled to interest payments as specified in the security register.
10
Table of Contents
Unless
the applicable prospectus supplement otherwise indicates, we will pay any installment of interest on debt securities in registered form to the person in whose name the debt security is
registered at the close of business on the regular record date for that installment of interest. (Section 307). If a holder wishes to receive payment by wire transfer, the holder should provide
the paying agent with written wire transfer instructions at least 15 days prior to the payment date.
Unless
the applicable prospectus supplement otherwise sets forth, debt securities issued in registered form will be transferable or exchangeable at the agency we may designate from time
to time. Debt securities may be transferred or exchanged without service charge, other than any tax or other governmental charge imposed in connection with the transfer or exchange.
(Section 305).
Book-Entry Procedures
The applicable prospectus supplement for each series of debt securities will state whether those debt securities will be subject to the
following provisions.
Unless
debt securities in physical form are issued, we will issue the debt securities in whole or in part in the form of one or more global certificates, which we refer to as global
securities, in denominations of $1,000 or any integral multiple of $1,000. We will deposit the global securities with or on behalf of The Depository Trust Company, which we refer to as DTC, and
registered in the name of Cede & Co., as nominee of DTC. Beneficial interests in the global securities may be held through the Euroclear System ("Euroclear") and Clearstream
Banking, S.A. ("Clearstream") (as indirect participants in DTC).
We
have provided the following descriptions of the operations and procedures of DTC, Euroclear and Clearstream solely as a matter of convenience. These operations and procedures are
solely within the control of DTC, Euroclear and Clearstream and are subject to change by them from time to time. Neither we, any underwriter nor the trustee take any responsibility for these
operations or procedures, and you are urged to contact DTC, Euroclear or Clearstream directly to discuss these matters.
DTC
has advised us that:
-
•
-
DTC is a limited-purpose trust company organized under the New York Banking Law, a "banking organization" within the meaning of the New York
Banking Law, a member of the Federal Reserve System, a "clearing corporation" within the meaning of the New York Uniform Commercial Code and a "clearing agency" registered under Section 17A of
the Exchange Act;
-
•
-
DTC holds securities that its direct participants deposit with DTC and facilitates the settlement among direct participants of securities
transactions, such as transfers and pledges, in deposited securities through electronic computerized book-entry changes in direct participants' accounts, thereby eliminating the need for physical
movement of securities certificates;
-
•
-
direct participants include securities brokers and dealers, trust companies, clearing corporations and other organizations;
-
•
-
DTC is a wholly-owned subsidiary of The Depository Trust & Clearing Corporation, which is owned by the users of its regulated
subsidiaries;
-
•
-
access to the DTC system is also available to indirect participants such as securities brokers and dealers, banks and trust companies that
clear through or maintain a custodial relationship with a direct participant, either directly or indirectly; and
-
•
-
the rules applicable to DTC and its direct and indirect participants are on file with the SEC.
11
Table of Contents
We
expect that under procedures established by DTC:
-
•
-
upon deposit of the global securities with DTC or its custodian, DTC will credit on its internal system the accounts of direct participants
designated by the underwriters with portions of the principal amounts of the global securities; and
-
•
-
ownership of the debt securities will be shown on, and the transfer of ownership of the debt securities will be effected only through, records
maintained by DTC or its nominee, with respect to interests of direct participants, and the records of direct and indirect participants, with respect to interests of persons other than participants.
Investors
in the global securities who are participants in DTC's system may hold their interests therein directly through DTC. Investors in the global notes who are not participants may
hold their interests therein indirectly through organizations (including Euroclear and Clearstream) which are participants in such system. Euroclear and Clearstream may hold interests in the global
securities on behalf of their participants through customers' securities accounts in their respective names on the books of their respective depositories, which are Euroclear Bank S.A./N.V., as
operator of Euroclear, and Citibank, N.A., as depository of Clearstream. All interests in a securities, including those held through Euroclear or Clearstream, may be subject to the procedures and
requirements of DTC. Those interests held through Euroclear or Clearstream may also be subject to the procedures and requirements of such systems.
The
laws of some jurisdictions require that purchasers of securities take physical delivery of those securities in the form of a certificate. For that reason, it may not be possible to
transfer interests in a global security to those persons. In addition, because DTC can act only on behalf of its participants, who in turn act on behalf of persons who hold interests through
participants, the ability of a person having an interest in a global security to pledge or transfer that interest to persons or entities that do not participate in DTC's system, or otherwise to take
actions in respect of that interest, may be affected by the lack of a physical definitive security in respect of that interest.
So
long as DTC or its nominee is the registered owner of a global security, DTC or that nominee will be considered the sole owner or holder of the debt securities represented by that
global security for all purposes under the indenture and under the debt securities. Except as described below, owners of beneficial interests in a global security will not be entitled to have debt
securities represented by that global security registered in their names, will not receive or be entitled to receive the debt securities in the form of a physical certificate and will not be
considered the owners or holders of the debt securities under the indenture or under the debt securities, and may not be entitled to give the trustee directions, instructions or approvals. For that
reason, each holder owning a beneficial interest in a global security must rely on DTC's procedures and, if that holder is not a direct or indirect participant in DTC, on the procedures of the DTC
participant through which that holder owns its interest, to exercise any rights of a holder of debt securities under the indenture or the global security.
Neither
we nor the trustee will have any responsibility or liability for any aspect of DTC's records relating to the debt securities or relating to payments made by DTC on account of the
debt securities, or any responsibility to maintain, supervise or review any of DTC's records relating to the debt securities.
We
will make payments on the debt securities represented by the global securities to DTC or its nominee, as the registered owner of the debt securities. We expect that when DTC or its
nominee receives any payment on the debt securities represented by a global security, DTC will credit participants' accounts with payments in amounts proportionate to their beneficial interests in the
global security as shown in DTC's records. We also expect that payments by DTC's participants to owners of beneficial interests in the global security held through those participants will be governed
by standing instructions and customary practice as is now the case with securities held for the accounts of
12
Table of Contents
customers
registered in the names of nominees for such customers. DTC's participants will be responsible for those payments.
Payments
on the debt securities represented by the global securities will be made in immediately available funds. Transfers between participants in DTC will be made in accordance with
DTC's rules and will be settled in immediately available funds.
Transfers
between participants in DTC will be effected in accordance with DTC's procedures, and will be settled in same-day funds, and transfers between participants in Euroclear and
Clearstream will be effected in accordance with their respective rules and operating procedures.
Cross-market
transfers between the participants in DTC, on the one hand, and Euroclear or Clearstream participants, on the other hand, will be effected through DTC in accordance with
DTC's rules on behalf of Euroclear or Clearstream, as the case may be, by its depository; however, such cross-market transactions will require delivery of instructions to Euroclear or Clearstream, as
the case may be, by the counterparty in such system in accordance with the rules and procedures and within the established deadlines (European time) of such system. Euroclear or Clearstream, as the
case may be, will, if the transaction meets its settlement requirements, deliver instructions to its respective depository to take action to effect final settlement on its behalf by delivering or
receiving interests in the relevant global security in DTC, and making or receiving payment in accordance with normal procedures for same-day funds settlement applicable to DTC. Euroclear participants
and Clearstream participants may not deliver instructions directly to the depositories for Euroclear or Clearstream.
DTC
has advised us that it will take any action permitted to be taken by a holder of notes only at the direction of one or more participants to whose account DTC has credited the
interests in the global securities and only in respect of such portion of the aggregate principal amount of the notes as to which such participant or participants has or have given such direction.
However, if there is an event of default under the notes, DTC reserves the right to exchange the global securities for certificated notes, and to distribute such notes to its participants.
Although
DTC, Euroclear and Clearstream have agreed to the foregoing procedures to facilitate transfers of interests in the global securities among participants in DTC, Euroclear and
Clearstream, they are under no obligation to perform or to continue to perform such procedures, and may discontinue such procedures at any time. None of the trustee, us or any of their or our
respective agents will have any responsibility for the performance by DTC, Euroclear or Clearstream or their respective direct or indirect participants of their respective obligations under the rules
and procedures governing their operations.
Physical
certificates will be issued to holders of a global security, or their nominees, if:
-
•
-
DTC advises the trustee in writing that DTC is no longer willing, able or eligible to discharge properly its responsibilities as depository and
we are unable to locate a qualified successor; or
-
•
-
we decide in our sole discretion to terminate the book-entry system through DTC. (Section 305).
In
such event, the trustee will notify all holders of debt securities through DTC participants of the availability of such physical debt securities. Upon surrender by DTC of a definitive global note
representing the debt securities and receipt of instructions for reregistration, the trustee will reissue the debt securities in physical form to holders or their nominees. (Section 305).
Debt
securities in physical form will be freely transferable and exchangeable at the office of the trustee upon compliance with the requirements set forth in the indenture.
No
service charge will be imposed for any registration of transfer or exchange, but payment of a sum sufficient to cover any tax or other governmental charge may be required.
(Section 305).
13
Table of Contents
Same Day Settlement and Payment
We will make payments in respect of the notes represented by the global securities (including principal, premium, if any, and interest) by wire
transfer of immediately available funds to the accounts specified by the global securities holder. We will make all payments of principal, interest and premium, if any, with respect to certificated
notes by wire transfer of immediately available funds to the accounts specified by the holders of the certificated notes or, if no such account is specified, by mailing a check to each such holder's
registered address. The notes represented by the global securities are expected to be eligible to trade in DTC's Same-Day Funds Settlement System, and any permitted secondary market trading activity
in such notes will, therefore, be required by DTC to be settled in immediately available funds. We expect that secondary trading in any certificated notes will also be settled in immediately available
funds.
Because
of time zone differences, the securities account of a Euroclear or Clearstream participant purchasing an interest in a global security from a participant in DTC will be credited,
and any such crediting will be reported to the relevant Euroclear or Clearstream participant, during the securities settlement processing day (which must be a business day for Euroclear and
Clearstream) immediately following the settlement date of DTC. DTC has advised us that cash received in Euroclear or Clearstream as a result of sales of interests in a global securities by or through
a Euroclear or Clearstream participant to a participant in DTC will be received with value on the settlement date of DTC but will be available in the relevant Euroclear or Clearstream cash account
only as of the business day for Euroclear or Clearstream following DTC's settlement date.
Consolidation, Merger or Sale by the Company
The indenture generally permits a consolidation or merger between us and another U.S. legal entity. It also permits the sale or transfer by us
of all or substantially all of our property and assets to another legal entity. These transactions are permitted if:
-
•
-
(A) we are the continuing or surviving legal entity, or (B) the resulting or acquiring legal entity, if other than us, assumes all of
our responsibilities and liabilities under the indenture, including the payment of all amounts due on the debt securities and performance of the covenants in the indenture;
-
•
-
immediately after the transaction, no event of default exists. (Section 801); and
-
•
-
the trustee shall have received an officer's certificate and an opinion stating such consolidation, merger, conveyance, transfer or lease and,
if applicable, the corresponding supplemental indenture, are in compliance with the base indenture.
Even
though the indenture contains the provisions described above, we are not required by the indenture to comply with those provisions if we sell all of our property and assets to
another U.S. legal entity if, immediately after the sale, that legal entity is one of our wholly-owned subsidiaries. (Section 801).
If
we consolidate or merge with or into any other legal entity or sell all or substantially all of our assets according to the terms and conditions of the indenture, the resulting or
acquiring legal entity will be substituted for us in the indenture with the same effect as if it had been an original party to the indenture. As a result, the successor legal entity may exercise our
rights and powers under the indenture, in our name or in its own name and we will be released from all our liabilities and obligations under the indenture and under the debt securities.
(Section 801).
14
Table of Contents
Events of Default, Notice and Certain Rights on Default
Unless otherwise stated in the applicable prospectus supplement, an "event of default," when used with respect to any series of debt securities,
means any of the following:
-
•
-
failure to pay interest on any debt security of that series for 30 days after the payment is due;
-
•
-
failure to pay the principal of or any premium on any debt security of that series when due;
-
•
-
failure to deposit any sinking fund payment on debt securities of that series when due;
-
•
-
failure to perform any other covenant in the indenture that applies to debt securities of that series for 90 days after we have received
written notice of the failure to perform in the manner specified in the indenture;
-
•
-
an event of default under any debt by the company or any significant subsidiary of the company (including a default with respect to any series
of debt securities) that results in debt of an outstanding principal amount greater than $75,000,000 becoming or being declared due and payable;
-
•
-
certain events in bankruptcy, insolvency or reorganization; or
-
•
-
any other event of default that may be specified for the debt securities of that series when that series is created. (Section 502).
If
an event of default for any series of debt securities occurs and continues, the trustee or the holders of at least 25% in aggregate principal amount of the outstanding debt securities
of the series may declare the entire principal of all the debt securities of that series to be due and payable immediately.
If a declaration occurs, the holders of a majority of the aggregate principal amount of the outstanding debt securities of that series can, subject to certain conditions, rescind the declaration.
(Section 502).
The
prospectus supplement relating to each series of debt securities which are original issue discount securities will describe the particular provisions that relate to the acceleration
of maturity of a portion of the principal amount of that series when an event of default occurs and continues.
An
event of default for a particular series of debt securities does not necessarily constitute an event of default for any other series of debt securities issued under the indenture.
The
indenture requires us to furnish an officer's certificate to the trustee each year as to the knowledge of our principal executive, financial or accounting officer of our compliance
with all conditions and covenants under the indenture. (Section 1008). The trustee will transmit by mail to the holders of debt securities of a series notice of any default.
Other
than its duties in the case of a default, the trustee will not be obligated to exercise any of its rights or powers under an indenture at the request, order or direction of any
holders, unless the holders offer the trustee indemnification satisfactory to the trustee. (Section 603). If indemnification satisfactory to the trustee is provided, then, subject to certain
other rights of the trustee, the holders of a majority in principal amount of the outstanding debt securities of any series may, with respect to the debt securities of that series, direct the time,
method and place of:
-
•
-
conducting any proceeding for any remedy available to the trustee; or
-
•
-
exercising any trust or power conferred upon the trustee. (Section 512).
15
Table of Contents
The
holder of a debt security of any series will have the right to begin any proceeding with respect to the indenture or for any remedy only
if:
-
•
-
the holder has previously given the trustee written notice of a continuing event of default with respect to that series;
-
•
-
the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series have made a written request of, and
offered reasonable indemnification to, the trustee to begin the proceeding;
-
•
-
the trustee has not started the proceeding within 60 days after receiving the request; and
-
•
-
the trustee has not received directions inconsistent with the request from the holders of a majority in aggregate principal amount of the
outstanding debt securities of that series during those 60 days. (Section 507).
The
holders of not less than a majority in aggregate principal amount of any series of debt securities, by notice to the trustee for that series, may waive, on behalf of the holders of
all debt securities of that series, any past default or event of default with respect to that series and its consequences. (Section 513). A default or event of default in the payment of the
principal of, or premium or interest on, any debt security and certain other defaults may not, however, be waived. (Sections 508 and 513).
Modification of the Indenture
We, as well as the trustee for a series of debt securities, may enter into one or more supplemental indentures, without the consent of, or
notice to, the holders of any of the debt securities, in order to:
-
•
-
evidence the succession of another corporation to us and the assumption of our covenants by a successor;
-
•
-
add to our covenants or surrender any of our rights or powers;
-
•
-
add additional events of default for any series;
-
•
-
change or eliminate any restrictions on the payment of principal of (or premium, if any, on) debt securities, provided such action will not
adversely affect the interest of holders of any series of debt securities in any material respect;
-
•
-
permit or facilitate the issuance of debt securities in uncertificated form, provided such action will not adversely affect the interests of
holders of any series of debt securities in any material respect;
-
•
-
secure the debt securities;
-
•
-
establish the form or terms of debt securities not yet issued;
-
•
-
evidence and provide for successor trustees;
-
•
-
add, change or eliminate any provision affecting registration as to principal of debt securities;
-
•
-
change or eliminate provisions or add any other provisions that are required or desirable in accordance with any amendments to the Trust
Indenture Act of 1939, which we refer to in this prospectus as the Trust Indenture Act, on the condition that this action does not adversely affect the interests of any holder of debt securities of
any series issued under the indenture in any material respect;
-
•
-
comply with requirements of the SEC in order to maintain the qualification of the indenture under the Trust Indenture Act;
16
Table of Contents
-
•
-
provide for uncertificated debt securities in addition to or in place of certificated debt securities;
-
•
-
make any change that would provide additional rights or benefits to holders of debt securities or any series, or that does not adversely affect
the legal rights of such holders under the indenture;
-
•
-
supplement any provisions of the indenture to facilitate defeasance and discharge of any series of debt securities, provided such action will
not adversely affect the interest of the holders of debt securities of such series or any other series;
-
•
-
conform text of the indenture or any debt securities to the description thereof in any prospectus supplement;
-
•
-
cure any ambiguity or correct any mistake; or
-
•
-
to make any other provision with respect to the indenture, provided that such actions will not adversely affect the interests of the holders,
as determined in good faith by the board of directors of the company. (Section 901).
In
addition, with the consent of the holders of not less than a majority in aggregate principal amount of the outstanding debt securities of all series affected by the supplemental
indenture, we and the trustee may execute supplemental indentures adding any provisions to or changing or eliminating any of the provisions of the indenture or any supplemental indenture or modifying
the rights of the holders of debt securities of that series. No such supplemental indenture may, however, without the consent of the holder of each debt security that is
affected:
-
•
-
change the time for payment of principal or interest on any debt security;
-
•
-
reduce the principal of, or any installment of principal of, or interest on, any debt security;
-
•
-
reduce the amount of premium, if any, payable upon the redemption of any debt security;
-
•
-
change any obligation of the company to pay additional amounts;
-
•
-
reduce the amount of principal payable upon acceleration of the maturity of an original issue discount debt security;
-
•
-
impair the right to institute suit for the enforcement of any payment on or for any debt security;
-
•
-
reduce the percentage in principal amount of the outstanding debt securities of any series the consent of whose holders is required for
modification or amendment of the indenture or for waiver of compliance with certain provisions of the indenture or for waiver of certain defaults;
-
•
-
modify the provisions relating to waiver of some defaults or any of the foregoing provisions;
-
•
-
change the currency of payment;
-
•
-
adversely affect the right to repayment of debt securities of any series at the option of the holders of those debt securities; or
-
•
-
change the place of payment. (Section 902).
Any
supplemental indenture will be filed with the SEC as an exhibit to:
-
•
-
a post-effective amendment to the registration statement of which this prospectus is a part;
-
•
-
an annual report on Form 10-K;
-
•
-
a quarterly report on Form 10-Q; or
-
•
-
a current report on Form 8-K.
17
Table of Contents
Defeasance and Covenant Defeasance
When we use the term defeasance, we mean discharge from some or all of our obligations under the indenture. If we deposit with the trustee
sufficient cash or government obligations to pay the principal, interest, any premium and any mandatory sinking fund or analogous payments due to the stated maturity or a redemption date of the debt
securities of a particular series, then at our option:
-
•
-
we will be discharged from our obligations for the debt securities of that series, the holders of the debt securities of the affected series
will no longer be entitled to the benefits of the indenture, except for registration of transfer and exchange of debt securities and replacement of lost, stolen or mutilated debt securities, and those
holders may look only to the deposited funds or obligations for payment, which is referred to as "defeasance"; or
-
•
-
we will no longer be under any obligation to comply with certain covenants under the indenture as it relates to that series, and some events of
default will no longer apply to us, which is referred to as "covenant defeasance." (Sections 403 and 1501).
Unless
the applicable prospectus supplement specifies otherwise and except as described below, the conditions to both defeasance and covenant defeasance are as
follows:
-
•
-
it must not result in a breach or violation of, or constitute a default or event of default under, the indenture, or result in a breach or
violation of, or constitute a default under, any other of our material agreements or instruments;
-
•
-
certain bankruptcy-related defaults or events of default with respect to us must not have occurred and be occurring during the period
commencing on the date of the deposit of the trust funds to defease the debt securities and ending on the 91st day after that date;
-
•
-
we must deliver to the trustee an officer's certificate and an opinion of counsel addressing compliance with the conditions of the defeasance
or covenant defeasance; and
-
•
-
we must comply with any additional conditions to the defeasance or covenant defeasance that the indenture may impose on us.
(Sections 403 and 1501).
In
the event that government obligations deposited with the trustee for the defeasance of such debt securities decrease in value or default subsequent to their being deposited, we will
have no further obligation, and the holders of the debt securities will have no additional recourse against us, for any decrease in value or default. If indicated in the prospectus supplement, in
addition to obligations of the United States or an agency or instrumentality of the United States, government obligations may include obligations of the government or an agency or instrumentality of
the government issuing the currency in which debt securities of such series are payable.
We
may exercise our defeasance option for the debt securities even if we have already exercised our covenant defeasance option. If we exercise our defeasance option, payment of the debt
securities may not be accelerated because of default or an event of default. If we exercise our covenant defeasance option, payment of the debt securities may not be accelerated because of default or
an event of default with respect to the covenants to which the covenant defeasance is applicable. If, however, acceleration occurs, the realizable value at the acceleration date of the money and
government obligations in the defeasance trust could be less than the principal and interest then due on the debt securities, because the required deposit in the defeasance trust is based on scheduled
cash flow rather than market value, which will vary depending on interest rates and other factors.
18
Table of Contents
Conversion and Exchange Rights
The debt securities of any series may be convertible into or exchangeable for other securities of our company or another issuer or property or
cash on the terms and subject to the conditions set forth in the applicable prospectus supplement. (Section 301).
Governing Law
The indenture and the debt securities will be governed by, and construed under, the laws of the State of New York without regard to conflicts of
laws principles thereof.
Regarding the Trustee
We may from time to time maintain lines of credit, and have other customary banking relationships, with the trustee under the indenture.
The
indenture and provisions of the Trust Indenture Act that are incorporated by reference therein contain limitations on the rights of the trustee, should it become one of our
creditors, to obtain payment of claims in certain cases or to realize on certain property received by it in respect of any such claim as security or otherwise. The trustee is permitted to engage in
other transactions with us or any of our affiliates; provided, however, that if it acquires any conflicting interest (as defined under the Trust Indenture Act), it must eliminate such conflict or
resign.
19
Table of Contents
DESCRIPTION OF CAPITAL STOCK
The following description of our capital stock summarizes general terms and provisions that apply to our capital stock and provisions of our
restated articles of incorporation and amended and restated bylaws. This description is only a summary. For more detailed information, you should refer to our restated articles of incorporation and
amended and restated bylaws filed as exhibits to the registration statement, of which this prospectus is a part and incorporated by reference into this prospectus. See "Where You Can Find More
Information."
General
Our authorized capital stock consists of 120,000,000 shares of common stock, no par value per share, and 2,000,000 shares of preferred stock, no
par value per share. As of September 13, 2018, 51,435,224 shares of our common stock were outstanding. As of the date of this prospectus, no shares of our preferred stock were outstanding.
Common Stock
Holders of our common stock are entitled to one vote for each share held of record on all matters on which shareholders are generally entitled
to vote. The majority of votes cast by the holders of shares entitled to vote on an action at a meeting at which a quorum is present is generally required to take shareholder action, unless a greater
vote is required. Directors are elected by a plurality of the votes cast at any election, but the election of a director-nominee in an uncontested election requires a majority vote under our
Principles of Corporate Governance. There is no cumulative voting of shares.
Holders
of our common stock are entitled to receive dividends when, as and if declared by our board of directors out of funds legally available for the payment of dividends. Upon the
liquidation, dissolution or winding up of the Company, holders of common stock are entitled to share pro rata in any assets available for distribution to shareholders after payment of all obligations
of the Company and after provision has been made with respect to each class of stock, if any, having preference over the common stock. Holders of common stock do not have cumulative voting rights or
preemptive, subscription or conversion rights and shares of common stock are not redeemable. The shares of common stock presently outstanding are duly authorized, validly issued, fully paid and
non-assessable. There will be a prospectus supplement relating to any offering of common stock offered by this prospectus.
Preferred Stock
Our board of directors is authorized to issue from time to time up to 2 million shares of preferred stock without shareholder approval.
Our board of directors has the discretion to determine the rights, preferences and limitations, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation
preferences. It is not possible to state the actual effect of
the issuance of any shares of preferred stock on the rights of the holders of common stock until our board of directors determines the specific rights associated with the preferred stock. The effects
of issuing preferred stock could include one or more of the following:
-
•
-
decreasing the amount of earnings and assets available for distribution to holders of common stock;
-
•
-
restricting dividends on the common stock;
-
•
-
diluting the voting power of the common stock;
-
•
-
impairing the liquidation rights of the common stock; or
-
•
-
delaying, deferring or preventing changes in our control or management.
20
Table of Contents
As
of the date of this prospectus, there were no shares of preferred stock outstanding.
Anti-Takeover Effects of Michigan Law and Our Certificate of Incorporation and Bylaws
The directors of the Company serve staggered three-year terms. Directors may not be removed without cause. The restated articles of
incorporation also set the minimum and maximum number of directors constituting the entire board at three and fifteen, respectively, with the exact number to be determined by the board from time to
time.
Our
restated articles of incorporation and amended and restated bylaws contain provisions that could have the effect of delaying, deterring or preventing a merger, tender offer or other
takeover attempt. Our restated articles of incorporation authorize the board to issue up to 120 million shares of common stock (less shares already outstanding or reserved for issuance) and up
to two million shares of preferred stock without shareholder approval. In addition, the restated articles of incorporation provide that shareholder action without a meeting requires the unanimous
consent of the shareholders, unless the applicable action has been approved by the Board prior to execution of the shareholder consent. Our amended and restated bylaws permit incumbent directors to
fill any vacancies on the board of directors, however occurring, whether by an increase in the number of directors, death, resignation, retirement, disqualification, removal from office or otherwise,
unless filled by proper action of the shareholders. Furthermore, our amended and restated bylaws require shareholders to give advance notice of director nominations and proposals to be presented at
meetings of shareholders.
These
provisions may delay shareholder actions with respect to business combinations and the election of new members to our board of directors. As such, the provisions could discourage
open market purchases of our common stock because a shareholder who desires to participate in a business combination or elect a new director may consider them disadvantageous.
Subject
to certain exceptions, Chapter 7A of the Michigan Business Corporation Act prohibits a corporation from engaging in any business combination with an interested shareholder
(generally defined as a shareholder who beneficially owns 10% or more of the voting power of the Company) unless approved by (1) 90% of the votes of each class of stock entitled to vote and
(2) two-thirds of the votes of each class of stock entitled to be cast by the shareholders other than the interested shareholder. We are currently not subject to Chapter 7A but may opt
in at any time by resolution of our board of directors.
Nasdaq Global Market Listing
Our common stock is listed on the Nasdaq Global Market under the symbol "RMTI."
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company.
21
Table of Contents
DESCRIPTION OF WARRANTS
We may issue other warrants in the future for the purchase of debt securities, common stock, preferred stock, units or other securities.
Warrants may be issued independently or together with debt securities, common stock, preferred stock or units offered by any prospectus supplement and/or other offering material and may be attached to
or separate from any such offered securities. Each series of warrants will be issued under a separate warrant agreement to be entered into between us and a bank or trust company, as warrant agent,
provided that we may also act as warrant agent and enter into warrant agreements directly with the purchasers of securities offered pursuant to this prospectus. In each case, the terms of the warrants
will be set forth in the prospectus supplement and/or other offering material relating to the particular issue of warrants. The warrant agent, if any, will act solely as our agent in connection with
the warrants and will not assume any obligation or relationship of agency or trust for or with any holders of warrants or beneficial owners of warrants.
The
following summary of certain provisions of the warrants we may issue in the future does not purport to be complete and is subject to, and is qualified in its entirety by reference
to, all provisions of the warrant agreements.
Reference
is made to the prospectus supplement and/or other offering material relating to the particular issue of warrants offered pursuant to such prospectus supplement and/or other
offering material for the terms of and information relating to such warrants, including, where applicable:
-
•
-
the designation, aggregate principal amount, currencies, denominations and terms of the series of debt securities purchasable upon exercise of
warrants to purchase debt securities and the price at which such debt securities may be purchased upon such exercise;
-
•
-
the number of shares of common stock or preferred stock purchasable upon the exercise of warrants and the price at which such number of shares
of common stock or preferred stock may be purchased upon such exercise;
-
•
-
the designation and number of units of other securities purchasable upon the exercise of warrants to purchase other securities and the price at
which such number of units of such other securities may be purchased upon such exercise;
-
•
-
the date on which the right to exercise such warrants shall commence and the date on which such right shall expire;
-
•
-
U.S. federal income tax consequences applicable to such warrants;
-
•
-
the amount of warrants outstanding as of the most recent practicable date; and
-
•
-
any other terms of such warrants.
Warrants
will be issued in registered form only. The exercise price for warrants will be subject to adjustment in accordance with the applicable prospectus supplement and/or other
offering material.
Each
warrant will entitle the holder thereof to purchase such principal amount of debt securities or such number of shares of common stock, preferred stock, units or other securities at
such exercise price as shall in each case be set forth in, or calculable from, the prospectus supplement and/or other offering material relating to the warrants, which exercise price may be subject to
adjustment upon the occurrence of certain events as set forth in such prospectus supplement and/or other offering material. After the close of business on the expiration date, or such later date to
which such expiration date may be extended by us, unexercised warrants will become void. The place or places where, and the manner in which, warrants may be exercised shall be specified in the
prospectus supplement and/or other offering material relating to such warrants.
22
Table of Contents
Prior
to the exercise of any warrants to purchase debt securities, common stock, preferred stock, units or other securities, holders of such warrants will not have any of the rights of
holders of the underlying securities, as the case may be, purchasable upon such exercise, including the right to receive payments of principal of, premium, if any, or interest, if any, on the debt
securities purchasable upon such exercise or to enforce covenants in the applicable indenture, or to receive payments of dividends, if any, on the common stock purchasable upon such exercise, or to
exercise any applicable right to vote.
23
Table of Contents
DESCRIPTION OF SUBSCRIPTION RIGHTS
We may issue subscription rights to purchase debt securities, common stock, preferred stock, warrants, units other securities described in this
prospectus or any combination thereof. These subscription rights may be issued independently or together with any other security offered by us and may or may not be transferable by the shareholder
receiving the subscription rights in such offering. In connection with any offering of subscription rights, we may enter into a standby arrangement with one or more underwriters or other investors
pursuant to which the underwriters or other investors may be required to purchase any securities remaining unsubscribed for after such offering.
To
the extent appropriate, the applicable prospectus supplement will describe the specific terms of the subscription rights to purchase shares of our securities offered thereby,
including the following:
-
•
-
the date of determining the shareholders entitled to the rights distribution;
-
•
-
the price, if any, for the subscription rights;
-
•
-
the exercise price payable for the debt securities, common stock, preferred stock, warrants, units or other securities upon the exercise of the
subscription right;
-
•
-
the number of subscription rights issued to each shareholder;
-
•
-
the amount of debt securities, common stock, preferred stock, warrants, units or other securities that may be purchased per each subscription
right;
-
•
-
any provisions for adjustment of the amount of securities receivable upon exercise of the subscription rights or of the exercise price of the
subscription rights;
-
•
-
the extent to which the subscription rights are transferable;
-
•
-
the date on which the right to exercise the subscription rights shall commence, and the date on which the subscription rights shall expire;
-
•
-
the extent to which the subscription rights may include an over-subscription privilege with respect to unsubscribed securities;
-
•
-
the material terms of any standby underwriting or purchase arrangement entered into by us in connection with the offering of subscription
rights;
-
•
-
any applicable federal income tax considerations; and
-
•
-
any other terms of the subscription rights, including the terms, procedures and limitations relating to the transferability, exchange and
exercise of the subscription rights.
24
Table of Contents
DESCRIPTION OF SECURITIES PURCHASE CONTRACTS
We may issue securities purchase contracts, which consist of contracts obligating holders to purchase from us, and obligating us to sell to the
holders, a specified number of shares of common stock, preferred stock, warrants, units, debt securities or other securities at a future date or dates, which we refer to in this prospectus as
"securities purchase contracts." The terms and conditions for any purchase and sale rights or obligations, as well as the price per share of the underlying securities (if applicable) and the number or
value of the underlying securities, may be fixed at the time the securities purchase contracts are issued or may be determined by reference to a specific formula set forth in the securities purchase
contracts.
The
securities purchase contracts may be issued separately or as part of units, other securities or debt obligations of third parties, including U.S. treasury securities, securing the
holders' obligations to purchase the securities under the securities purchase contracts. The securities purchase contracts may require holders to secure their obligations under the securities purchase
contracts in a specified manner. The securities purchase contracts also may require us to make periodic payments to the holders thereof or vice versa, and those payments may be unsecured or refunded
on some basis.
The
securities purchase contracts, and, if applicable, collateral or depositary arrangements, relating to the securities purchase contracts, will be filed with the SEC in connection with
the offering of securities purchase contracts. The prospectus supplement and/or other offering material relating to a particular issue of securities purchase contracts will describe the terms of those
securities purchase contracts, including the following:
-
•
-
if applicable, a discussion of material U.S. federal income tax considerations; and
-
•
-
any other information we think is important about the securities purchase contracts.
25
Table of Contents
DESCRIPTION OF UNITS
As specified in the applicable prospectus supplement, we may issue units consisting of one or more shares of common stock, shares of preferred
stock, debt securities, warrants, subscription rights and securities purchase contracts, or any combination of the foregoing.
The
applicable prospectus supplement will specify the following terms of the units:
-
•
-
the terms of the underlying securities comprising the units, including whether and under what circumstances the underlying securities may be
traded separate of the units;
-
•
-
a description of the terms of any unit agreement governing the units (if any);
-
•
-
if appropriate, a discussion of material U.S. federal income tax considerations; and
-
•
-
a description of the provisions for the payment, settlement, transfer or exchange of the units.
26
Table of Contents
PLAN OF DISTRIBUTION
We may sell securities in any one or more of the following ways from time to time: (i) through agents; (ii) to or through
underwriters; (iii) through brokers or dealers; (iv) directly to purchasers, including through a specific bidding, auction or other process; (v) upon the exercise of subscription
rights that may be distributed to our shareholders; or (vi) through a combination of any of these methods of sale. The applicable prospectus supplement and/or other offering material will
contain the terms of the transaction, name or names of any underwriters, dealers, agents and the respective amounts of securities underwritten or purchased by them, the initial public offering price
of the securities, and the applicable agent's commission, dealer's purchase price or underwriter's discount. Any dealers and agents participating in the distribution of the securities may be deemed to
be underwriters, and compensation received by them on resale of the securities may be deemed to be underwriting discounts.
Any
initial offering price, dealer purchase price, discount or commission may be changed from time to time.
The
securities may be distributed from time to time in one or more transactions, at negotiated prices, at a fixed price or fixed prices (that may be subject to change), at market prices
prevailing at the time of sale, at various prices determined at the time of sale or at prices related to prevailing market prices.
Offers
to purchase securities may be solicited directly by us or by agents designated by us from time to time. Any such agent may be deemed to be an underwriter, as that term is defined
in the Securities Act, of the securities so offered and sold.
If
underwriters are utilized in the sale of any securities in respect of which this prospectus is being delivered, such securities will be acquired by the underwriters for their own
account and may be resold from time to time in one or more transactions, including negotiated transactions, at fixed public offering prices or at varying prices determined by the underwriters at the
time of sale. Securities may be offered to the public either through underwriting syndicates represented by managing underwriters or directly by one or more underwriters. If any underwriter or
underwriters are utilized in the sale of securities, unless otherwise indicated in the applicable prospectus supplement and/or other offering material, the obligations of the underwriters are subject
to certain conditions precedent, and that the underwriters will be obligated to purchase all such securities if any are purchased.
If
a dealer is utilized in the sale of the securities in respect of which this prospectus is delivered, we will sell such securities to the dealer, as principal. The dealer may then
resell such securities to the public at varying prices to be determined by such dealer at the time of resale. Transactions through brokers or dealers may include block trades in which brokers or
dealers will attempt to sell shares as agent but may position and resell as principal to facilitate the transaction or in crosses, in which the same broker or dealer acts as agent on both sides of the
trade. Any such dealer may be deemed to be an underwriter, as such term is defined in the Securities Act, of the securities so offered and sold.
Offers
to purchase securities may be solicited directly by us and the sale thereof may be made directly to institutional investors or others, who may be deemed to be underwriters within
the meaning of the Securities Act with respect to any resale thereof.
If
so indicated in the applicable prospectus supplement and/or other offering material, we may authorize agents and underwriters to solicit offers by certain institutions to purchase
securities at the public offering price set forth in the applicable prospectus supplement and/or other offering material pursuant to delayed delivery contracts providing for payment and delivery on
the date or dates stated in the applicable prospectus supplement and/or other offering material. Such delayed delivery contracts will be subject only to those conditions set forth in the applicable
prospectus supplement and/or other offering material.
27
Table of Contents
Agents,
underwriters and dealers may be entitled under relevant agreements to indemnification against certain liabilities, including liabilities under the Securities Act, or to
contribution with respect to payments which such agents, underwriters and dealers may be required to make in respect thereof. The terms and conditions of any indemnification or contribution will be
described in the applicable prospectus supplement and/or other offering material.
We
may also sell shares of our common stock through various arrangements involving mandatorily or optionally exchangeable securities, and this prospectus may be delivered in connection
with those sales.
We
may engage in at-the-market offerings into an existing trading market in accordance with Rule 415(a)(4) under the Securities Act. To the extent that we make sales through one
or more underwriters or agents in at-the-market offerings, we will do so pursuant to the terms of a sales agency financing agreement or other at-the-market offering arrangement between us and the
underwriters or agents. If we engage in at-the-market sales pursuant to any such agreement or arrangement, we will issue and sell our securities through one or more underwriters or agents, which may
act on an agency basis or a principal basis. During the term of any such agreement or arrangement, we may sell securities on a daily basis in exchange transactions or otherwise as we agreement with
the underwriters or agents. Any such agreement or arrangement will provide that any securities sold will be sold at prices related to the then-prevailing market prices for our securities. Therefore,
exact figures regarding proceeds that will be raised or commissions to be paid cannot be determined at this time. Pursuant to the terms of the agreement or arrangement, we may agree to sell, and the
relevant underwriters or agents may agree to solicit offers to purchase blocks of our common stock. The terms of any such agreement or arrangement will be set forth in more detail in the applicable
prospectus supplement.
We
may enter into derivative, sale or forward sale transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated
transactions. If the applicable prospectus supplement and/or other offering material indicates, in connection with those transactions, the third parties may sell securities covered by this prospectus
and the applicable prospectus supplement and/or other offering material, including in short sale transactions and by issuing securities not covered by this prospectus but convertible into, or
exchangeable for or representing beneficial interests in such securities covered by this prospectus, or the return of which is derived in whole or in part from the value of such securities. The third
parties may use securities received under derivative, sale or forward sale transactions, or securities pledged by us or borrowed from us or others to settle those sales or to close out any related
open borrowings of stock, and may use securities received from us in settlement of those transactions to close out any related open borrowings of stock. The third party in such sale transactions will
be an underwriter and will be identified in the applicable prospectus supplement (or a post-effective amendment) and/or other offering material.
Underwriters,
broker-dealers or agents may receive compensation in the form of commissions, discounts or concessions from us. Underwriters, broker-dealers or agents may also receive
compensation from the purchasers of shares for whom they act as agents or to whom they sell as principals, or both. Compensation as to a particular underwriter, broker-dealer or agent might be in
excess of customary commissions and will be in amounts to be negotiated in connection with transactions involving shares. In effecting sales, broker-dealers may arrange for other broker-dealers to
participate in the resales.
Each
series of securities will be a new issue and, other than the common stock, which is listed on the Nasdaq Global Market, will have no established trading market. We may elect to list
any series of securities on an exchange, and in the case of the common stock, on any additional exchange, but, unless otherwise specified in the applicable prospectus supplement and/or other offering
material, we shall not be obligated to do so. No assurance can be given as to the liquidity of the trading market for any of the securities.
28
Table of Contents
Agents,
underwriters and dealers may engage in transactions with, or perform services for us and our respective subsidiaries in the ordinary course of business.
Any
underwriter may engage in overallotment, stabilizing transactions, short covering transactions and penalty bids in accordance with Regulation M under the Exchange Act.
Overallotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do
not exceed a specified maximum. Short covering transactions involve purchases of the securities in the open market after the distribution is completed to cover short positions. Penalty bids permit the
underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer are purchased in a covering transaction to cover short positions. Those activities may
cause the price of the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time. An underwriter may carry out these
transactions on the Nasdaq Global Market, in the over-the-counter market or otherwise.
The
place and time of delivery for securities will be set forth in the accompanying prospectus supplement and/or other offering material for such securities.
29
Table of Contents
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. We also filed a registration statement on
Form S-3, including exhibits, under the Securities Act with respect to the securities offered by this prospectus. This prospectus is a part of that registration statement, but does not contain
all of the information included in the registration statement or the exhibits. You may read and copy the registration statement and any other document we file at the SEC's Public Reference Room at
100 F Street, N.E., Washington D.C. 20549. You can call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference room. You can also find our public
filings with the SEC on the internet at a web site maintained by the SEC located at www.sec.gov.
We
are "incorporating by reference" specified documents that we file with the SEC, which means:
-
•
-
incorporated documents are considered part of this prospectus;
-
•
-
we are disclosing important information to you by referring you to those documents; and
-
•
-
information we file with the SEC will automatically update and supersede information contained in this prospectus.
We
incorporate by reference the documents listed below and any future filings we make with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Exchange Act (i) after the
date of the registration statement on Form S-3 filed under the Securities Act with respect to securities offered by this prospectus and prior to the effectiveness of such registration statement
and (ii) after the date of this prospectus and before the end of the offering of the securities pursuant to this prospectus:
-
•
-
our Annual Report on
Form 10-K for the year ended December 31, 2017;
-
•
-
our Quarterly Report on
Form 10-Q (as amended) for the quarter ended March 31, 2018 and
our Quarterly Report on Form 10-Q for the quarter ended June 30,
2018;
-
•
-
our Definitive Proxy Statement on
Schedule 14A filed April 30, 2018 and the Supplement to the
Definitive Proxy Statement on Schedule 14A filed June 7, 2018;
-
•
-
our Current Reports on Form 8-K filed
March 21, 2018;
April 19, 2018;
May 23, 2018
(two filing);
May 25, 2018;
June 7, 2018;
June 19, 2018;
June 21, 2018;
June 21, 2018;
June 27, 2018;
July 13, 2018;
July 25, 2018;
July 30, 2018;
August 3, 2018;
August 7, 2018;
August 8, 2018;
August 14, 2018
(two filing); and
September 4, 2018; and
-
•
-
the description of our common stock contained in or incorporated into our Registration Statement on
Form 8-A filed with the SEC on January 23, 1998, including any
amendment or reports filed for the purpose of updating such description.
Information
in this prospectus supersedes related information in the documents listed above, and information in subsequently filed documents supersedes related information in both this
prospectus and the incorporated documents.
We
will provide, without charge to you, upon written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus, other than exhibits to those
documents, unless the exhibits are specifically incorporated by reference in those documents. Requests should be directed to our principal executive offices at:
Rockwell Medical, Inc.
30142 Wixom Road
Wixom, Michigan 48393
(248) 960-9009
Attention: David Kull, Secretary
You
can also find these filings on our website at www.rockwellmed.com. We are not incorporating the information on our website other than these filings into this prospectus.
30
Table of Contents
LEGAL MATTERS
The validity of the securities offered by this prospectus will be passed upon for us by Foley & Lardner LLP. The validity of the
securities offered by this prospectus will be passed upon for any underwriters or agents by counsel named in the applicable prospectus supplement. The opinions of Foley & Lardner LLP and
counsel for any underwriters or agents may be conditioned upon and may be subject to assumptions regarding future action required to be taken by us and any underwriters, dealers or agents in
connection with the issuance of any securities. The opinions of Foley & Lardner LLP and counsel for any underwriters or agents may be subject to other conditions and assumptions, as
indicated in the prospectus supplement.
EXPERTS
The consolidated financial statements incorporated in this prospectus by reference from the Company's
Annual Report on Form 10-K for the year ended December 31,
2017, and the effectiveness of the Company's internal control over financial reporting as of December 31, 2017, have been audited by Plante & Moran, PLLC, independent
auditors, as stated in their reports which are incorporated in this prospectus by reference, and have been so incorporated in reliance upon the reports of such firm given upon their authority as
experts in accounting and auditing.
31
Table of Contents

21,818,544 Shares of Common Stock
Pre-Funded Warrants to Purchase up to 1,360,265 shares of Common Stock
Warrants to Purchase up to 23,178,809 shares of Common Stock
(and the shares of Common Stock underlying such Pre-Funded
Warrants and Warrants)
PROSPECTUS SUPPLEMENT
H.C. Wainwright & Co.
September 23, 2020
Rockwell Medical (NASDAQ:RMTI)
Historical Stock Chart
From Mar 2024 to Apr 2024
Rockwell Medical (NASDAQ:RMTI)
Historical Stock Chart
From Apr 2023 to Apr 2024