Filed
pursuant to Rule 424(b)(5)
Registration
Statement No. 333-229584
PROSPECTUS
SUPPLEMENT
(To
Prospectus dated February 22, 2019)

PolarityTE,
Inc.
Up
to $50,000,000 of Shares of Common Stock
We
have entered into a Controlled Equity OfferingSM Sales Agreement (the “sales agreement”) with Cantor Fitzgerald
& Co. (“Cantor”) relating to shares of our common stock offered by this prospectus supplement and the accompanying
prospectus. In accordance with the terms of the sales agreement, we may offer and sell shares of our common stock, from time to
time, having an aggregate offering price of up to $50.0 million through Cantor, acting as sales agent, pursuant to this prospectus
supplement.
Our
common stock is listed on The Nasdaq Capital Market under the trading symbol “PTE.” The last reported sale price of
our common stock on The Nasdaq Capital Market on March 29, 2021 was $1.23 per share.
Sales
of shares of our common stock, if any, under this prospectus supplement and the accompanying prospectus will be made in sales
each deemed to be an “at the market offering” as defined in Rule 415(a)(4) under the Securities Act of 1933, as amended
(the “Securities Act”). Cantor is not required to sell any specific number or dollar amount of securities, but will
act as our sales agent using commercially reasonable efforts consistent with its normal trading and sales practices, on mutually
agreed terms between Cantor and us. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
Cantor
will be entitled to compensation at a fixed commission rate of 4% of the gross proceeds from the sale of shares of our common
stock. In connection with the sale of shares of our common stock on our behalf, Cantor will be deemed to be an “underwriter”
within the meaning of the Securities Act, and the compensation of Cantor will be deemed to be underwriting commissions or discounts.
See “Plan of Distribution” for more information regarding Cantor’s compensation.
Investing
in our common stock involves a high degree of risk. You should review carefully the risks and uncertainties referenced under the
heading “Risk Factors” beginning on page S-5 of this prospectus supplement, and under similar headings in the accompanying
prospectus and in the documents that are incorporated by reference herein and therein.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or
passed upon the adequacy or accuracy of this prospectus supplement or the accompanying prospectus. Any representation to the contrary
is a criminal offense.

The
date of this prospectus supplement is March 30, 2021
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS SUPPLEMENT
This
document consists of two parts. The first part is this prospectus supplement, which describes the terms of this offering and also
adds to and updates information contained in the accompanying prospectus and the documents incorporated by reference into this
prospectus supplement. The second part is the accompanying prospectus, which includes the documents incorporated by reference
therein and provides more general information. To the extent the information contained in this prospectus supplement differs or
varies from the information contained in the accompanying prospectus or the documents incorporated by reference herein or therein,
you should rely on the information in this prospectus supplement.
Generally,
when we refer to the prospectus, we are referring to this prospectus supplement and the accompanying prospectus combined. You
should read both this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and
therein, together with additional information described under the headings “Where You Can Find More Information” and
“Incorporation of Certain Information By Reference.”
You
should rely only on the information contained in or incorporated by reference in this prospectus supplement, the accompanying
prospectus and in any free writing prospectus that we have authorized for use in connection with this offering. Neither we nor
Cantor has authorized anyone to provide you with information that is different. We and Cantor are offering to sell the securities
offered hereby and seeking offers to buy such securities only in jurisdictions where offers and sales are permitted. The information
appearing in this prospectus supplement, the accompanying prospectus, the documents incorporated by reference in this prospectus
supplement and the accompanying prospectus, and in any free writing prospectus that we have authorized for use in connection with
this offering, is accurate only as of the date of those respective documents, regardless of the time of delivery of those respective
documents or sale of our common stock.
Neither
we nor Cantor has done anything that would permit this offering or possession or distribution of this prospectus supplement, the
accompanying prospectus and in any free writing prospectus that we have authorized for use in connection with this offering in
any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States
who come into possession of this prospectus supplement, the accompanying prospectus and any free writing prospectus that we have
authorized for use in connection with this offering must inform themselves about, and observe any restrictions relating to, the
offering of the securities offered hereby and the distribution of this prospectus supplement, the accompanying prospectus and
any free writing prospectus that we have authorized for use in connection with this offering outside the United States.
Unless
the context otherwise indicates, references in this prospectus supplement to “PolarityTE,” the “Company,”
“we,” “us,” and “our” refer, collectively, to PolarityTE, Inc., a Delaware corporation, and
where appropriate, its subsidiaries.
We
use various trademarks and trade names in our business, including without limitation our corporate name and logo. All other trademarks
or trade names referred to in this prospectus are the property of their respective owners. Solely for convenience, the trademarks
and trade names in this prospectus may be referred to without the ® and ™ symbols, but such references should not be
construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights
thereto.
PROSPECTUS
SUPPLEMENT SUMMARY
This
summary highlights certain information about us, this offering and selected information contained elsewhere in or incorporated
by reference into this prospectus supplement. This summary provides an overview of selected information and does not contain all
of the information you should consider before deciding whether to invest in our common stock. Therefore, you should read the entire
prospectus supplement and the accompanying prospectus carefully (including the documents incorporated by reference herein and
therein), especially the “Risk Factors” section beginning on page S-5 and in the documents incorporated by reference
and our consolidated financial statements and the related notes incorporated by reference in this prospectus supplement and the
accompanying prospectus, before deciding to invest in our common stock.
Overview
PolarityTE,
Inc., headquartered in Salt Lake City, Utah, is a biotechnology company developing regenerative tissue products and biomaterials.
We also operate a laboratory testing and clinical research business using equipment, personnel, and facilities we acquired to
advance our development of regenerative tissue products.
Regenerative
Tissue Product
Our
first regenerative tissue product is SkinTE, which is intended for the repair, reconstruction, replacement, and supplementation
of skin in patients who have a need for treatment of acute or chronic wounds, burns, surgical reconstruction events, scar revision,
or removal of dysfunctional skin grafts. SkinTE was registered and listed with the United States Food and Drug Administration
(“FDA”) in August 2017 based on our determination that SkinTE is appropriately regulated solely under Section 361
of the Public Health Service Act and Part 1271 of Title 21 of the Code of Federal Regulations (i.e., as a so-called 361 HCT/P)
and that, as a result, no premarket review or approval by the FDA was required. We proceeded to develop sales and manufacturing
capabilities for SkinTE and focused on advancing commercialization of SkinTE. We began a regional commercial rollout of SkinTE
in October 2018.
Following
informal, voluntary discussions between us and the FDA we were advised by the FDA in April 2020 that its preliminary assessment
is that SkinTE does not meet the requirements to be regulated solely as a 361 HCT/P. Rather, the FDA’s preliminary assessment
was that SkinTE is a biological product that should be regulated under Section 351 of the Public Health Service Act. We re-evaluated
our regulatory approach and determined it is prudent to submit an investigational new drug application (“IND”) for
SkinTE and an eventual biologics license application (“BLA”) because we believe it will create a more valuable asset
with a greater likelihood of achieving widespread commercial adoption, and to avoid the possibility of a protracted dispute with
the FDA. As a result of the change in the regulatory approach for SkinTE, we decided to adjust our SkinTE commercial operations
accordingly.
The
FDA developed and published in November 2017 a regenerative medicine policy framework to help facilitate regenerative medicine
therapies. Under the framework, the FDA stated its intent to exercise enforcement discretion until November 2020 with respect
to the FDA’s IND and premarket approval requirements, which was subsequently extended through May 2021. We continued to
sell SkinTE as a 361 HCT/P in 2020 and into 2021 in reliance on our view that there is a reasonable basis for regulating SkinTE
as a 361 HCT/P and also in reliance on the enforcement discretion position stated in the policy framework. In May 2020, we effectuated
a reduction in force within our regenerative medicine business segment to reduce historical monthly cash burn and preserve capital
for pursuing the filing of an IND. Since then we have focused our commercial effort for SkinTE on the territories where we have
current and repeat users of SkinTE.
We
have evaluated the question of whether the FDA may extend enforcement discretion on regenerative medical products, and as of the
date of this report the FDA has not taken any action on extending enforcement discretion. Following the end of the FDA’s
period of enforcement discretion, we may need to cease selling SkinTE until the FDA approves a BLA, and then we will only be able
to market the product for indications that have been approved in a BLA. We cannot predict at this time when we may decide on continuing
SkinTE sales because of the uncertainty around the decisions the FDA may make in this area.
We
plan to focus our SkinTE activity on the preparation and submission of an IND in the second half of 2021, and the commencement
of clinical trials under that IND once it is open. We believe that the network of physicians and other healthcare providers who
have treated more than 1,100 patients to date with SkinTE will provide valuable support for our clinical development program as
we work towards a BLA for SkinTE.
Testing
and Research Services
Beginning
in 2017 we developed internally a laboratory and research capability to advance the development of SkinTE and related technologies,
which we operate through our subsidiary, Arches Research, Inc. (“Arches”). At the beginning of May 2018, we acquired
a preclinical research and veterinary sciences business to be used, in part, for preclinical studies on our regenerative tissue
products, which we operate through our subsidiary IBEX Preclinical Research, Inc. (“IBEX”). Through Arches and IBEX,
we also offer research and laboratory testing services to unrelated third parties on a contract basis.
There
was a substantial surge in COVID-19 testing throughout the United States as a result of the COVID-19 pandemic, which began in
the spring of 2020. In the course of its operations, Arches maintains equipment and staff capable of performing molecular polymerase
chain reaction testing for COVID-19, which made it possible for Arches to begin providing COVID-19 testing services at the end
of May 2020. We believe that COVID-19 testing offers an opportunity to use existing resources to generate additional revenue in
the contract services segment and thereby help defray our operating expenses. We provided COVID-19 testing services through the
end of 2020, which we expect will continue in 2021.
Corporate
Information
We
were incorporated in 1998 in the state of Delaware. Effective January 11, 2017, we changed our name to PolarityTE, Inc. from “Majesco
Entertainment Company.”
Our
principal executive offices are located at 1960 S. 4250 West, Salt Lake City, Utah 84104 and our telephone number is (800) 560-3983.
Our web site address is www.PolarityTE.com. Information contained on, or that can be accessed through, our website does not constitute
a part of this prospectus supplement and is not incorporated by reference herein.
THE
OFFERING
|
Common
Stock Offered by Us
|
|
Shares
of our common stock, par value $0.001, having an aggregate offering price of up to $50,000,000.
|
|
|
|
|
|
Common
Stock to be Outstanding after this Offering
|
|
On
March 25, 2021, there were 80,319,378 shares of our common stock issued and outstanding. Assuming all $50,000,000 of our common
stock is sold in this offering at an assumed offering price of $1.23 per share, which was the last reported sale price
of our common stock on The Nasdaq Capital Market on March 29, 2021, we would have had 120,969,784 shares of common
stock outstanding as of March 25, 2021. The actual number of shares issued will vary based on the prices at which shares of
our common stock are sold in this offering.
|
|
|
|
|
|
Manner
of Offering
|
|
“At
the market offering” that may be made from time to time through our sales agent, Cantor. See “Plan of Distribution.”
|
|
|
|
|
|
Use
of Proceeds
|
|
We
expect to use the net proceeds of this offering for general corporate purposes and working capital, including among other
things, capital expenditures and research and development expenses. See “Use of Proceeds.”
|
|
|
|
|
|
Risk
Factors
|
|
Investing
in our securities involves a high degree of risk. See the “Risk Factors” section in this prospectus supplement
and other information included and incorporated by reference in this prospectus supplement and the accompanying prospectus
for a discussion of factors that you should carefully consider before deciding to invest in our securities.
|
|
|
|
|
|
Listing
|
|
Our
common stock is listed on The Nasdaq Capital Market under the trading symbol “PTE.”
|
The
number of shares of our common stock to be outstanding immediately after this offering, should it be completed in full using the
assumptions stated above, and, unless otherwise indicated, the information in this prospectus supplement, is based on 80,319,378
shares of our common stock outstanding as of March 25, 2021, and excludes as of that date:
|
|
●
|
539,500
shares of our common stock issuable upon the exercise of outstanding warrants with an exercise price of $0.10 per share;
|
|
|
|
|
|
|
●
|
641,283
shares of our common stock issuable upon the exercise of outstanding warrants with an exercise price of $0.9356 per share;
|
|
|
|
|
|
|
●
|
17,587,905
shares of our common stock issuable upon the exercise of
outstanding warrants with an exercise price of $1.20 per share;
|
|
|
|
|
|
|
●
|
545,455
shares of our common stock issuable upon the exercise of outstanding warrants with an exercise price of $1.375 per share;
|
|
|
|
|
|
|
●
|
6,079,210
shares of our common stock issuable upon the exercise of outstanding stock options having a weighted-average exercise price
of $8.13 per share;
|
|
|
|
|
|
|
●
|
2,652,498
shares of our common stock issuable upon the vesting of outstanding restricted stock units; and
|
|
|
|
|
|
|
●
|
4,271,350
shares of our common stock reserved for issuance in connection with future grants under our stock incentive plans.
|
RISK
FACTORS
An
investment in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider
carefully the risks described below and discussed under the section captioned “Risk Factors” beginning on page 4 of
the accompanying prospectus, together with other information in this prospectus supplement, the accompanying prospectus, and the
documents incorporated by reference in this prospectus supplement and the accompanying prospectus, including the information set
forth under the caption “Risk Factors” in our annual report on Form 10-K for the fiscal year ended December 31, 2020.
If any of these risks actually occurs, our business, financial condition or results of operations could be seriously harmed. This
could cause the trading price of our common stock to decline, resulting in a loss of all or part of your investment.
Risks
Related to Our Business
You
should read and consider risk factors specific to our business before making an investment decision. Those risks are described
in the sections entitled “Risk Factors” in our annual report on Form 10-K for the fiscal year ended December 31, 2020
and in other documents incorporated by reference into this prospectus supplement. Please be aware that additional risks and uncertainties
not currently known to us or that we currently deem to be immaterial could also materially and adversely affect our business,
results of operations, financial condition, cash flows or prospects.
Risks
Related to this Offering and Our Common Stock
The
trading price of the shares of our common stock has been and may continue to be volatile, and the market price of our common stock
may drop.
You
should consider an investment in our securities as risky and invest only if you can withstand a significant loss and wide fluctuations
in the market value of your investment. You may be unable to sell your shares of common stock at or above the price paid for the
shares due to fluctuations in the market price of our common stock arising from changes in our operating performance or prospects.
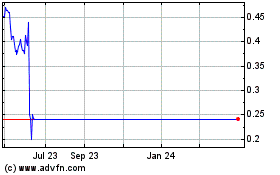
Our stock price has been highly volatile during the 12-month period ended February 28, 2021, with closing stock prices ranging
from a high of $1.85 per share to a low of $0.61 per share. The stock market in general, and the market for biotech companies
in particular, have experienced extreme volatility that, at times, has been unrelated to the operating performance of particular
companies. Some of the factors that may cause the market price of our common stock to fluctuate include:
|
|
●
|
the
timing or success of obtaining regulatory licenses or approvals for marketing our products;
|
|
|
●
|
the
initiation, timing, progress, and results of our pre-clinical or clinical trials;
|
|
|
●
|
sufficiency
of our working capital to fund our operations over the next 12 months and beyond;
|
|
|
●
|
infrastructure
required to support operations in future periods, including the expected costs thereof;
|
|
|
●
|
estimates
associated with revenue recognition, asset impairments, and cash flows;
|
|
|
●
|
variance
in our estimates of future operating costs;
|
|
|
●
|
the
impact of new accounting pronouncements;
|
|
|
●
|
size
and growth of our target markets;
|
|
|
●
|
the
initiation, timing, progress, and results of our research and development programs;
|
|
|
●
|
issues
in manufacturing our product candidates or future approved products;
|
|
|
●
|
regulatory
developments or enforcement in the United States and foreign countries with respect to our product candidates or our competitors’
products;
|
|
|
●
|
competition
from existing products or new products that may emerge;
|
|
|
●
|
developments
or disputes concerning patents, patent applications, or other proprietary rights;
|
|
|
●
|
introduction
of technological innovations or new commercial products by us or our competitors;
|
|
|
●
|
announcements
by us, our collaborators, or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations,
or capital commitments;
|
|
|
●
|
changes
in estimates or recommendations by securities analysts, if any, who cover our common stock;
|
|
|
●
|
fluctuations
in the valuation of companies perceived by investors to be comparable to us;
|
|
|
●
|
public
concern over our product candidates or any future approved products;
|
|
|
●
|
threatened
or actual litigation;
|
|
|
●
|
future
or anticipated sales of our common stock;
|
|
|
●
|
share
price and volume fluctuations attributable to inconsistent trading volume levels of our shares;
|
|
|
●
|
additions
or departures of key personnel;
|
|
|
●
|
changes
in the structure of health care payment systems in the United States or overseas;
|
|
|
●
|
failure
of any of our products or product candidates to perform safely or effectively or achieve commercial success;
|
|
|
●
|
economic
and other external factors or other disasters or crises;
|
|
|
●
|
period-to-period
fluctuations in our financial condition and results of operations;
|
|
|
●
|
general
market conditions and market conditions for biopharmaceutical stocks; and
|
|
|
●
|
overall
fluctuations in U.S. equity markets.
|
In
addition, in the past, when the market price of a stock has been volatile, holders of that stock have instituted securities class
action litigation against the company that issued the stock. Defending such litigation could result in substantial defense costs
and divert the time and attention of our management, which could seriously harm our business.
You
may experience immediate and substantial dilution in the net tangible book value per share of the common stock you purchase.
Since
the price per share of our common stock being offered is expected to be substantially higher than the net tangible book value
per share of our common stock, your interest will be diluted to the extent of the difference between the price per share you pay
and the net tangible book value per share of our common stock. Assuming that an aggregate of 40,650,406 shares of our common
stock are sold at an assumed offering price of $1.23 per share, which was the last reported sale price of our common stock
on The Nasdaq Capital Market on March 29, 2021, in the aggregate gross amount of $50,000,000, after deducting commissions and
estimated aggregate offering expenses payable by us, you would experience substantial and immediate dilution of $0.47 per
share with respect to the net tangible book value of the common stock. To the extent that outstanding options or warrants are
exercised, you will experience additional dilution. For a further description of the dilution that you may experience, see “Dilution.”
Our
management will have broad discretion as to the use of the proceeds of this offering.
Our
management will have broad discretion as to the application of the net proceeds from this offering. You will be relying on the
judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your
investment decision, to assess whether the proceeds are being used appropriately. It is possible that, pending their use, we may
invest the net proceeds in a way that does not yield a favorable, or any, return for the Company.
You
may experience future dilution as a result of future equity offerings or other equity issuances.
To
raise additional capital, we may in the future offer additional shares of our common stock, preferred stock or other securities
convertible into or exchangeable for our common stock. We cannot assure you that we will be able to sell shares or other securities
in any other offering at a price per share that is equal to or greater than the price per share paid by investors in this offering.
The price per share at which we sell additional shares of our common stock or other securities convertible into or exchangeable
for our common stock in future transactions may be higher or lower than the price per share in this offering. Investors purchasing
shares or other securities in the future could have rights superior to existing stockholders.
As
of the date of this prospectus supplement we had a significant number of securities convertible into, or allowing the purchase
of, our common stock, including 19,314,143 common stock purchase warrants, 6,079,210 options to purchase shares of our
common stock, 2,652,498 restricted stock units, and 4,271,350 shares of common stock reserved for future issuance under our stock
incentive plans.
Future
sales of our common stock in the public market could cause our stock price to fall.
Sales
of a substantial number of shares of our common stock in the public market, or the perception that these sales might occur, could
depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity
securities. As of March 25, 2021, we had 80,319,378 shares of common stock outstanding, all of which, other than shares held by
our directors and certain officers and affiliates, were eligible for sale in the public market, subject in some cases to compliance
with the requirements of Rule 144, including the volume limitations and manner of sale requirements.
If
there are more shares of our common stock offered for sale than buyers are willing to purchase, then the market price of our common
stock may decline to a market price at which buyers are willing to purchase the offered shares of our common stock and sellers
remain willing to sell the shares. All of the shares of our common stock sold in this offering will be freely tradable without
restriction or further registration under the Securities Act upon issuance.
Our
Restated Certificate of Incorporation, our Restated Bylaws and Delaware law could deter a change of our management, which could
discourage or delay offers to acquire us.
Certain
provisions of Delaware law and of our Restated Certificate of Incorporation, as amended, and by-laws, could discourage or make
it more difficult to accomplish a proxy contest or other change in our management or the acquisition of control by a holder of
a substantial amount of our voting stock. It is possible that these provisions could make it more difficult to accomplish, or
could deter, transactions that stockholders may otherwise consider to be in their best interests or in our best interests. These
provisions include:
|
|
●
|
we
have a classified Board of Directors (the “Board”) requiring that members of the Board be elected in different
years, which lengthens the time needed to elect a new majority of the Board;
|
|
|
●
|
our
Board is authorized to issue up to 25,000,000 shares of preferred stock without stockholder approval, which could be issued
by our Board to increase the number of outstanding shares or change the balance of voting control and thwart a takeover attempt;
|
|
|
●
|
stockholders
are not entitled to remove directors other than by a two-thirds vote and only for cause;
|
|
|
●
|
stockholders
cannot call a special meeting of stockholders;
|
|
|
●
|
we
require all stockholder actions be taken at a meeting of our stockholders, and not by written consent; and
|
|
|
●
|
stockholders
must give advance notice to nominate directors or submit proposals for consideration at stockholder meetings.
|
We
are subject to the anti-takeover provisions of Section 203 of the Delaware General Corporation Law, which regulates corporate
acquisitions by prohibiting Delaware corporations from engaging in specified business combinations with particular stockholders
of those companies. These provisions could discourage potential acquisition proposals and could delay or prevent a change in control
transaction. They could also have the effect of discouraging others from making tender offers for our common stock, including
transactions that may be in your best interests. These provisions may also prevent changes in our management or limit the price
that investors are willing to pay for our stock.
Because
we do not expect to declare cash dividends on our common stock in the foreseeable future, stockholders must rely on appreciation
of the value of our common stock for any return on their investment.
While
we have in the past declared and paid cash dividends on our capital stock, we currently anticipate that we will retain future
earnings for the development, operation and expansion of our business and do not expect to declare or pay any additional cash
dividends in the foreseeable future. As a result, only appreciation of the price of our common stock, if any, will provide a return
to investors in this offering.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein include “forward-looking
statements” within the meaning of Section 27A of the Securities Act, and Section 21E of the Exchange Act. All statements
other than statements of historical fact are “forward-looking statements” for purposes of this prospectus, the accompanying
prospectus and the documents incorporated by reference herein and therein. In some cases, you can identify forward-looking statements
by words such as “anticipate,” “believe,” “contemplate,” “continue,” “could,”
“estimate,” “expect,” “intend,” “may,” “plan,” “potential,”
“predict,” “project,” “seek,” “should,” “target,” “would,”
or the negative of these words or other comparable terminology. These forward-looking statements include, but are not limited
to, statements about:
|
|
●
|
the
timing or success of obtaining regulatory licenses or approvals for marketing our products;
|
|
|
●
|
the
initiation, timing, progress, and results of our pre-clinical tests or clinical trials;
|
|
|
●
|
sufficiency
of our working capital to fund our operations over the next 12 months;
|
|
|
●
|
infrastructure
required to support operations in future periods, including the expected costs thereof;
|
|
|
●
|
estimates
associated with revenue recognition, asset impairments, and cash flows;
|
|
|
●
|
variance
in our estimates of future operating costs;
|
|
|
●
|
future
vesting and forfeitures of compensatory equity awards;
|
|
|
●
|
the
effectiveness of our disclosure controls and our internal control over financial reporting;
|
|
|
●
|
the
impact of new accounting pronouncements;
|
|
|
●
|
size
and growth of our target markets; and
|
|
|
●
|
the
initiation, timing, progress, and results of our research and development programs.
|
Factors
that may cause actual results to differ materially from those contemplated by such forward-looking statements include, without
limitation:
|
|
●
|
the
ability to comply with regulations applicable to the manufacture and distribution of our products and delivery of our services;
|
|
|
●
|
the
ability to meet demand for our products and services;
|
|
|
●
|
the
ability to deliver our products and services if employees are quarantined due to the impact of COVID-19;
|
|
|
●
|
the
scope of protection we can establish and maintain for intellectual property rights covering our product candidates and technology;
|
|
|
●
|
developments
relating to our competitors and industry;
|
|
|
●
|
new
discoveries or the development of new therapies or technologies that render our products or services obsolete or unviable;
|
|
|
●
|
outbreaks
of disease, including the COVID-19 pandemic, and related stay-at-home orders, quarantine policies and restrictions on travel,
trade, and business operations;
|
|
|
●
|
political
and economic instability, whether resulting from natural disasters, wars, terrorism, pandemics, or other sources;
|
|
|
●
|
the
ability to gain adoption by healthcare providers of our products for patient care;
|
|
|
●
|
the
ability to find and retain skilled personnel;
|
|
|
●
|
the
need for, and ability to obtain, additional financing in the future;
|
|
|
●
|
general
economic conditions;
|
|
|
●
|
inaccuracies
in estimates of our expenses, future revenues, and capital requirements;
|
|
|
●
|
future
accounting pronouncements;
|
|
|
●
|
unauthorized
access to confidential information and data on our information technology systems and security and data breaches; and
|
|
|
●
|
the
other risks and uncertainties described under “Risk Factors” beginning on page S-5 of this prospectus supplement,
and in our annual report on Form 10-K for the fiscal year ended December 31, 2020.
|
Forward-looking
statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and
other factors that may cause our actual results, performance or achievements to be materially different from any future results,
performance or achievements expressed or implied by these forward-looking statements. Any forward-looking statement in this prospectus
supplement, the accompanying prospectus and the documents incorporated by reference herein and therein reflects our current view
with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our operations,
results of operations, industry and future growth. Given these uncertainties, you should not place undue reliance on these forward-looking
statements. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason,
even if new information becomes available in the future.
INDUSTRY
AND MARKET DATA
This
prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein contain estimates,
projections and other information concerning our industry, our business and the markets for certain products, including data regarding
the estimated size of those markets, their projected growth rates and the incidence and prevalence of certain medical conditions.
Information that is based on estimates, forecasts, projections, market research, or similar methodologies is inherently subject
to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information.
Unless otherwise expressly stated, we obtained these industry, business, market and other data from our own research as well as
from reports, research surveys, studies, and similar data prepared by market research firms and other third parties, industry,
medical and general publications, government data and similar sources. These data involve a number of assumptions and limitations,
and you are cautioned not to give undue weight to such estimates. Projections, assumptions and estimates of our future performance
and the future performance of the industry in which we operate is necessarily subject to a high degree of uncertainty and risk
due to a variety of factors, including those described in “Risk Factors” and elsewhere in this prospectus supplement.
These and other factors could cause results to differ materially from those expressed in the estimates made by independent third
parties and by us.
USE
OF PROCEEDS
We
may issue and sell shares of our common stock having aggregate sales proceeds of up to $50,000,000 from time to time. Because
there is no minimum offering amount required as a condition to close this offering, the actual total public offering amount, commissions
and proceeds to us, if any, are not determinable at this time. There can be no assurance that we will sell any shares under or
fully utilize the sales agreement with Cantor as a source of financing.
We
intend to use the net proceeds from this offering for general corporate purposes and working capital, including among other things,
capital expenditures and research and development expenses. These expected uses represent our intentions based upon our current
plans and business conditions, which could change in the future as our plans and business conditions evolve. We have not determined
the amounts we plan to spend or the timing of expenditures. As a result, our management will have broad discretion to allocate
the net proceeds from the sale of the common stock that we may offer under this prospectus supplement and the accompanying prospectus.
Pending
their ultimate use, we intend to invest the net proceeds in a variety of securities, including commercial paper, government and
non-government debt securities and/or money market funds that invest in such securities.
Offering
expenses will be paid by us using cash-on-hand.
DILUTION
Dilution
is the amount by which the price paid by the purchaser of the securities sold in this offering exceeds the net tangible book value
per share of our common stock after the offering. Net tangible book value per share is determined by subtracting our total liabilities
from the total book value of our tangible assets and dividing the difference by the number of shares of our common stock deemed
to be outstanding at that date.
Our
historical net tangible book value as of December 31, 2020 was $26.5 million, or $0.48 per share. In January 2021, we sold 25,042,496
shares of our common stock in a registered direct offering and through the exercise of warrants resulting in estimated net proceeds
after offering costs of $16.7 million. After giving effect to these transactions and no other transactions subsequent to December
31, 2020, our pro forma net tangible book value as of December 31, 2020, would be $43.3 million, or $0.54 per share.
The
table below assumes for illustrative purposes that 40,650,406 shares of our common stock are sold at a price of $1.23,
which was the closing price of our common stock on The Nasdaq Capital Market on March 29, 2021, for aggregate gross proceeds
of $50.0 million. After giving effect to the offering, based on these assumptions and after deducting commissions and estimated
aggregate offering expenses payable by us, and after giving effect to the sales of stock and exercise of warrants described in
the preceding paragraph, our as adjusted net tangible book value as of December 31, 2020 would have been approximately $91.1
million, or $0.76 per share. This represents an immediate increase in as adjusted net tangible book value of $0.22
per share to our existing stockholders and immediate dilution of $0.47 per share to new investors participating in
this offering.
The
following table illustrates this dilution on a per share basis to new investors:
|
Assumed offering price per share
|
|
|
|
|
|
$
|
1.23
|
|
|
Historical net tangible book value per share as of December 31, 2020
|
|
$
|
0.48
|
|
|
|
|
|
|
Pro forma net tangible book value per share as of December 31, 2020
|
|
$
|
0.54
|
|
|
|
|
|
|
Increase in pro forma net tangible book value per share attributable to new investors
|
|
$
|
0.22
|
|
|
|
|
|
|
As adjusted pro forma net tangible book value per share after giving effect to this offering
|
|
|
|
|
|
$
|
0.76
|
|
|
Dilution per share to new investors
|
|
|
|
|
|
$
|
0.47
|
|
Notwithstanding
the assumptions reflected in this table, the shares of our common stock sold in this offering, if any, will be sold from time
to time at various prices. The dilution per share to new investors purchasing our common stock in this offering will depend
on the number and price of shares of our common stock that are sold in this offering. For example, an increase of $1.00 per
share in the price at which the shares are sold from the assumed public offering price of $1.23 per share shown in the
table above, assuming all of our common stock registered for sale pursuant to this prospectus supplement is sold at that
price would result in a pro forma as adjusted net tangible book value after the offering of $0.89 per share and would
increase the dilution in net tangible book value per share to new investors in this offering to $1.34 per share, after
deducting commissions and estimated aggregate offering expenses payable by us. A decrease of $1.00 per share in the price at
which the shares are sold from the assumed public offering price of $1.23 per share shown in the table above, assuming
all of our common stock registered for sale pursuant to this prospectus is sold at that price would result in a pro forma as
adjusted net tangible book value of $0.31 per share and would decrease the dilution in net tangible book value per
share to new investors in this offering to $0.08 per share, after deducting commissions and estimated offering
expenses payable by us.
To
the extent that outstanding options or warrants are exercised, investors purchasing securities in this offering will experience
further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations
even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is
raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution
to our stockholders.
The
table above is based on 79,900,200 shares of our common stock outstanding after giving effect to the sale of 25,042,476
shares of common stock in a registered direct offering and through the exercise of warrants in January 2021, and excludes
as of that date:
|
|
●
|
419,158
shares of our common stock issued pursuant to the exercise of outstanding warrants and stock awards under our stock incentive
plans since December 31, 2020;
|
|
|
|
|
|
|
●
|
539,500
shares of our common stock issuable upon the exercise of outstanding warrants with an exercise price of $0.10 per share;
|
|
|
|
|
|
|
●
|
641,283
shares of our common stock issuable upon the exercise of outstanding warrants with an exercise price of $0.9356 per share;
|
|
|
|
|
|
|
●
|
17,587,905
shares of our common stock issuable upon the exercise of
outstanding warrants with an exercise price of $1.20 per share;
|
|
|
|
|
|
|
●
|
545,455
shares of our common stock issuable upon the exercise of outstanding warrants with an exercise price of $1.375 per share;
|
|
|
|
|
|
|
●
|
6,079,210
shares of our common stock issuable upon the exercise of outstanding stock options having a weighted-average exercise price
of $8.13 per share;
|
|
|
|
|
|
|
●
|
2,652,498
shares of our common stock issuable upon the vesting of outstanding restricted stock units; and
|
|
|
|
|
|
|
●
|
4,271,350
shares of our common stock reserved for issuance in connection with future grants under our stock incentive plans.
|
PLAN
OF DISTRIBUTION
On
March 30, 2021, we entered into a sales agreement with Cantor Fitzgerald & Co. In accordance with the terms of the
sales agreement, we may offer and sell shares of our common stock, from time to time, having an aggregate offering price of up
to $50.0 million through Cantor acting as sales agent. A copy of the sales agreement is filed as an exhibit to our Annual Report
on Form 10-K for the year ended December 31, 2020 and is incorporated by reference in this prospectus supplement.
Upon
delivery of a placement notice and subject to the terms and conditions of the sales agreement, Cantor may sell our common stock
by any method permitted by law deemed to be an “at the market offering” as defined in Rule 415(a)(4) promulgated under
the Securities Act, including sales made directly or through the Nasdaq Capital Market or on any other existing trading market
for our common stock. We may instruct Cantor not to sell common stock if the sales cannot be effected at or above the price
designated by us from time to time. We or Cantor may suspend the offering of common stock upon notice and subject to other conditions.
We
will pay Cantor commissions, in cash, for their services in acting as agent in the sale of our common stock. Cantor will be entitled
to compensation at a fixed commission rate of up to 4% of the gross proceeds from each sale of our common stock. In the event
the total amount of commissions paid to Cantor is not at least $400,000 as of March 30, 2022, we will pay to Cantor the difference
between $400,000 and the total amount of commissions paid to Cantor as of that date, and the amount so paid will be credited against
any payments due to Cantor after March 30, 2022. We will reimburse Cantor up to $50,000 for its fees and expenses incurred in
connection with this offering. We also have paid Cantor a $100,000 fee pursuant to a financial advisory services agreement we
have with them. In accordance with FINRA Rule 5110 these fees and commissions are deemed sales compensation in connection with
this offering. Because there is no minimum offering amount required as a condition to close this offering, the actual total public
offering amount, commissions and proceeds to us, if any, are not determinable at this time. We estimate that the total expenses
for the offering excluding commissions will be approximately $180,000.
Settlement
for sales of common stock will occur on the second business day following the date on which any sales are made, or on some other
date that is agreed upon by us and Cantor in connection with a particular transaction, in return for payment of the net proceeds
to us. Sales of our common stock as contemplated in this prospectus supplement will be settled through the facilities of The Depository
Trust Company or by such other means as we and Cantor may agree upon. There is no arrangement for funds to be received in an escrow,
trust or similar arrangement.
Cantor
will use its commercially reasonable efforts, consistent with its sales and trading practices, to sell on our behalf shares of
our common stock as designated by us. In connection with the sale of the common stock on our behalf, Cantor will be deemed to
be an “underwriter” within the meaning of the Securities Act and the compensation of Cantor will be deemed to be underwriting
commissions or discounts. We have agreed to provide indemnification and contribution to Cantor against certain civil liabilities,
including liabilities under the Securities Act.
The
offering of our common stock pursuant to the sales agreement will terminate upon the termination of the sales agreement as permitted
therein. We and Cantor may each terminate the sales agreement at any time upon three days’ prior notice, and the sales agreement
may also be terminated by Cantor at any time in certain circumstances, including the occurrence of a material and adverse change
in our business or financial condition that makes it impractical or inadvisable to market our common stock or to enforce contracts
for the sale of our common stock.
Cantor
and its affiliates may in the future provide various investment banking, commercial banking and other financial services for us
and our affiliates, for which services they may in the future receive customary fees. To the extent required by Regulation M,
Cantor will not engage in any market making activities involving our common stock while the offering is ongoing under this prospectus.
This prospectus supplement and the accompanying prospectus in electronic format may be made available on a website maintained
by Cantor and Cantor may distribute this prospectus electronically.
LEGAL
MATTERS
King
& Spalding LLP will pass upon the validity of the securities offered hereby. Covington & Burling LLP is acting as counsel
to the sales agent in connection with the offering.
EXPERTS
The
consolidated balance sheets of PolarityTE, Inc. and subsidiaries as of December 31, 2020 and December 31, 2019, and the related
consolidated statements of operations, comprehensive loss, stockholders’ equity, and cash flows for each of the years then
ended, have been audited by EisnerAmper LLP, independent registered public accounting firm, as stated in their report, which is
incorporated herein by reference, which report expresses an unqualified opinion on the financial statements. Such financial statements
have been incorporated herein by reference in reliance on the report of such firm given upon their authority as experts in accounting
and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
This
prospectus supplement and the accompanying prospectus form part of a registration statement on Form S-3 that we filed with the
SEC. This prospectus supplement does not contain all of the information set forth in the registration statement and the exhibits
to the registration statement. For further information with respect to us and the securities we are offering under this prospectus
supplement, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement.
Neither we nor any agent, underwriter or dealer has authorized any person to provide you with different information. We are not
making an offer of these securities in any state where the offer is not permitted. You should not assume that the information
in this prospectus supplement is accurate as of any date other than the date on the front page of this prospectus supplement,
regardless of the time of delivery of this prospectus supplement or any sale of the securities offered by this prospectus supplement.
We
file annual, quarterly and current reports, proxy statements and other information with the SEC. These periodic reports, proxy
statements and other information are available on the SEC’s website at www.sec.gov. Our website is located at www.polarityte.com.
Information contained on our website is not incorporated by reference into this prospectus supplement and, therefore, is not part
of this prospectus supplement.
INCORPORATION
OF CERTAIN INFORMATION BY REFERENCE
The
SEC allows us to “incorporate by reference” into this prospectus supplement the information in documents we file with
it, which means that we can disclose important information to you by referring you to those documents. The information incorporated
by reference is considered to be a part of this prospectus, and information that we file later with the SEC will automatically
update and supersede this information. Any statement contained in any document incorporated or deemed to be incorporated by reference
herein shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in
or omitted from this prospectus, or in any other subsequently filed document that also is or is deemed to be incorporated by reference
herein, modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed, except as so
modified or superseded, to constitute a part of this prospectus.
We
incorporate by reference the documents listed below and any future documents that we file with the SEC under Sections 13(a), 13(c),
14 or 15(d) of the Exchange Act until the offering of the securities is terminated:
|
|
●
|
our
Annual Report on Form 10-K for the fiscal year ended December 31, 2020, filed with the SEC on March 30, 2021;
|
|
|
|
|
|
|
●
|
our
Current Reports on Form 8-K filed with the SEC on January 11, 2021, January 14, 2021, January 26, 2021, January 27, 2021 and
February 3, 2021; and
|
|
|
|
|
|
|
●
|
the
description of our common stock contained in Exhibit 4.13 to our Annual Report on Form 10-K for the fiscal year ended December
31, 2020, filed with the SEC on March 30, 2021.
|
We
will not, however, incorporate by reference in this prospectus supplement any documents or portions thereof that are not deemed
“filed” with the SEC, including any information furnished pursuant to Item 2.02 or Item 7.01 of our current reports
on Form 8-K unless, and except to the extent, specified in such current reports.
Upon
written or oral request, we will provide, without charge, to each person, including any beneficial owner, to whom a copy of this
prospectus supplement is delivered, a copy of the documents incorporated by reference into this prospectus supplement but not
delivered with the prospectus supplement. You may request a copy of these filings, and any exhibits we have specifically incorporated
by reference as an exhibit in this prospectus supplement, at no cost by writing or telephoning us at the following address:
PolarityTE,
Inc.
1960
S. 4250 West
Salt
Lake City, Utah 84104
(800)
560-3983
You
may also access these documents, free of charge on the SEC’s website at www.sec.gov or on our website at www.polarityte.com.
Information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information
on, or that can be accessed from, our website as part of this prospectus supplement.
This
prospectus supplement is part of a registration statement we filed with the SEC. We have incorporated exhibits into this registration
statement. You should read the exhibits carefully for provisions that may be important to you.
You
should rely only on the information incorporated by reference or provided in this prospectus supplement. We have not authorized
anyone to provide you with different information. We are not making an offer of these securities in any state where the offer
is not permitted. You should not assume that the information in this prospectus supplement or in the documents incorporated by
reference is accurate as of any date other than the date on the front of this prospectus supplement or those documents.
$200,000,000

Common
Stock
Preferred
Stock
Debt
Securities
Warrants
Rights
Units
We
may from time to time issue, in one or more series or classes, up to $200,000,000 in aggregate principal amount of our common
stock, preferred stock, debt securities, warrants or rights in one or more offerings. We may offer these securities separately
or together in units. We may also offer common stock or preferred stock upon conversion of or exchange for the debt securities;
common stock upon conversion of or exchange for preferred stock; or common stock, preferred stock or debt securities upon the
exercise of warrants or rights. We will specify in a supplement to this prospectus the terms of the securities being offered.
We may sell these securities to or through underwriters, to other purchasers or through agents. We will set forth the names of
any underwriters or agents, and any fees, conversions or discount arrangements, in the prospectus supplement. We may not sell
any securities under this prospectus without delivery of the applicable prospectus supplement.
You
should read this document and any prospectus supplement or amendment carefully before you invest in our securities.
Our
common stock is listed on The Nasdaq Capital Market under the symbol “PTE.” On February 7, 2019, the closing price
for our common stock, as reported on The Nasdaq Capital Market, was $16.54 per share. Our principal executive office is located
at 123 Wright Brothers Drive, Salt Lake City, Utah 84116. Our telephone number is (800) 560-3983.
Investing
in our securities involves a high degree of risk. You should review carefully the risks and uncertainties referenced under the
heading “Risk Factors” contained in this prospectus beginning on page 4 and any applicable prospectus supplement,
and under similar headings in the other documents that are incorporated by reference into this prospectus.
This
prospectus may not be used to offer or sell securities unless accompanied by a prospectus supplement.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or
determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The
date of this Prospectus is February 22, 2019.
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
This
prospectus is part of a registration statement that we filed with the United States Securities and Exchange Commission (the “SEC”),
using a “shelf” registration process. Under this shelf registration process, we may from time to time sell any combination
of the securities described in this prospectus in one or more offerings up to a total amount of $200,000,000.
This
prospectus provides you with a general description of the securities we may offer. Each time we sell securities, we will provide
one or more prospectus supplements that will contain specific information about the terms of the offering. The prospectus supplement
may also add, update or change information contained in this prospectus. You should read both this prospectus and the accompanying
prospectus supplement together with the additional information described under the heading “Where You Can Find More Information”
beginning on page 24 of this prospectus.
You
should rely only on the information contained in or incorporated by reference in this prospectus, any accompanying prospectus
supplement or in any related free writing prospectus filed by us with the SEC. We have not authorized anyone to provide you with
different information. This prospectus and the accompanying prospectus supplement do not constitute an offer to sell or the solicitation
of an offer to buy any securities other than the securities described in the accompanying prospectus supplement or an offer to
sell or the solicitation of an offer to buy such securities in any circumstances in which such offer or solicitation is unlawful.
You should assume that the information appearing in this prospectus, any prospectus supplement, the documents incorporated by
reference and any related free writing prospectus is accurate only as of their respective dates. Our business, financial condition,
results of operations and prospects may have changed materially since those dates.
Unless
the context otherwise indicates, references in this prospectus to “PolarityTE,” the “Company,” “we,”
“us,” and “our” refer, collectively, to PolarityTE, Inc., a Delaware corporation, and its subsidiaries.
We
use various trademarks and trade names in our business, including without limitation our corporate name and logo. All other trademarks
or trade names referred to in this prospectus are the property of their respective owners. Solely for convenience, the trademarks
and trade names in this prospectus may be referred to without the ® and ™ symbols, but such references should not be
construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights
thereto.
RISK
FACTORS
Investing
in our securities involves a high degree of risk. You should carefully consider the risks referenced below and described in the
documents incorporated by reference in this prospectus and any prospectus supplement, as well as other information we include
or incorporate by reference into this prospectus and any applicable prospectus supplement, before making an investment decision.
Our business, financial condition or results of operations could be materially adversely affected by the materialization of any
of these risks. The trading price of our securities could decline due to the materialization of any of these risks, and you may
lose all or part of your investment. This prospectus and the documents incorporated herein by reference also contain forward-looking
statements that involve risks and uncertainties. Actual results could differ materially from those anticipated in these forward-looking
statements because of certain factors, including the risks referenced below and described in the documents incorporated herein
by reference, including our annual report on Form 10-K for the fiscal year ended October 31, 2018, which is on file with the SEC,
and other documents we file with the SEC that are deemed incorporated by reference into this prospectus.
CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus, including the documents that we incorporate by reference, may contain forward-looking statements within the meaning
of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities
Exchange Act of 1934, as amended (the “Exchange Act”).
Forward-looking
statements in this prospectus and any accompanying prospectus supplement give our current expectations or forecasts of future
events. You can identify these statements by the fact that they do not relate strictly to historical or current facts. You can
find many (but not all) of these statements by looking for words such as “approximates,” “believes,” “hopes,”
“expects,” “anticipates,” “estimates,” “projects,” “intends,” “plans,”
“would,” “should,” “could,” “may” or other similar expressions in this prospectus
and any prospectus supplement. In particular, forward-looking statements include statements relating to future actions, prospective
products and applications, customers, technologies, future performance or future financial results. These forward-looking statements
are subject to certain risks and uncertainties that could cause actual results to differ materially from our historical experience
and our present expectations or projections. Factors that could cause actual results to differ from those discussed in the forward-looking
statements include, but are not limited to:
|
|
●
|
our
limited cash and our history of losses;
|
|
|
|
|
|
|
●
|
our
ability to achieve profitability;
|
|
|
|
|
|
|
●
|
our
limited operating history;
|
|
|
|
|
|
|
●
|
emerging
competition and rapidly advancing technology;
|
|
|
|
|
|
|
●
|
whether
the FDA will object to our registration of SkinTE solely under Section 361 of the Public Health Service Act, which permits
marketing of SkinTE without obtaining prior FDA marketing approval;
|
|
|
|
|
|
|
●
|
whether
future changes in regulation of biotechnology products or the interpretation and application of existing regulations could
adversely affect development or commercialization of our products;
|
|
|
|
|
|
|
●
|
whether
demand develops for our medical products;
|
|
|
|
|
|
|
●
|
the
impact of competitive or alternative products, technologies and pricing;
|
|
|
|
|
|
|
●
|
our
success in obtaining patents under the applications we have filed;
|
|
|
|
|
|
|
●
|
the
adequacy of protections afforded to us by any patents we may obtain, and the cost to us of maintaining, enforcing and defending
those patents;
|
|
|
|
|
|
|
●
|
our
ability to obtain, expand and maintain patent protection in the future, and to protect our non-patented intellectual property;
|
|
|
|
|
|
|
●
|
our
exposure to and ability to defend third-party claims and challenges to our intellectual property rights;
|
|
|
|
|
|
|
●
|
our
ability to obtain adequate financing in the future, as and when we need it;
|
|
|
|
|
|
|
●
|
our
ability to continue as a going concern;
|
|
|
|
|
|
|
●
|
our
success at managing the risks involved in the foregoing items; and
|
|
|
|
|
|
|
●
|
other
factors discussed in this prospectus.
|
Although
we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results,
levels of activity, performance or achievements. The forward-looking statements are based upon management’s beliefs and
assumptions and are made as of the date of this prospectus. We undertake no obligation to publicly update or revise any forward-looking
statements included in this prospectus to conform such statements to actual results or changes in our expectations. You should
not place undue reliance on these forward-looking statements.
THE
COMPANY
The
following summary highlights selected information contained in this prospectus. This summary does not contain all the information
you should consider before investing in the securities. Before making an investment decision, you should read the entire prospectus
carefully.
PolarityTE
- Welcome to the SHIFT
PolarityTE
Inc., headquartered in Salt Lake City, Utah, is a young and growing commercial-stage, biotechnology company founded in 2016 -
and we believe the first of its kind. We are focused on the design and development of novel technology platforms that promote
the regeneration of complex, cellular-derived tissue substrates and the propagation of self-organizing composite systems. We have
developed, and will continue to evolve these technologies and platforms through uniquely targeted and yet comprehensive approaches
to the interactome. The interactome is the complete set of physical interactions between molecules within a cell that underlies
most genotype-to-phenotype relationships and modulates nearly all complex biological pathways and cellular networks seen in living
systems. Understanding this, we believe that to effectively deliver our advanced technologies to patients we must not simply deliver
products, but rather robust platform systems and evolving technology foundations that are intelligent, multi-functional, and able
to adapt and evolve. Over the last year we have established and advanced three of our pipeline programs consisting of our core
“TE” program, (which includes our first commercial product, SkinTE), our Related Technology Derivative program (“RTD”),
and our Advanced Research Center program (“ARC”).
Vision
We
aspire to be a global biotechnology company that provides superior, tangible, and pragmatic platform technologies that provide
superior results to patients, while reducing costs and promoting improved health economics for patients, providers, and payors.
We believe this can be accomplished through our pursuit of complex simplicity, which embodies the development of robust cell/tissue-derived
therapies that can be efficiently produced and deployed. PolarityTE is committed to delivering transformative technology that
positively impacts humanity.
PolarityTE
was founded by a dedicated group of doctors and scientists from The Johns Hopkins University School of Medicine, who left to become
part of something bigger. Something that could transform the future of medicine. We believe that living systems require more than
a simple singular input (for example a growth factor, stem cell, or nano-particle), to produce a complex output. Therefore, we
took a different direction and developed multi-tiered platform technologies that propagate the necessary complex substrate required
for regenerating fully-functional tissue, such as skin, bone, cartilage, muscle, blood vessels, and neural elements, as well as
solid and hollow organ composite tissue systems. We have engineered and developed our regenerative materials and core tissue substrate
technology platforms to allow us to induce, maintain, and promote the integrated polarity, organized assembly, and interface development
of cells and tissues, so that they replicate regenerative healing in the body and are not seen as foreign by the immune system.
The
core technology of TE products is minimally polarized functional units (“MPFUs”) consisting of self-complexing intelligent
regenerative materials (“SCIRM”). SCIRM within an MPFU form polarizing, multi-cellular aggregates that act as an intrinsic,
regenerative bio-reactor capable of expanding, proliferating, and synthesizing cells, materials, factors, or systems necessary
for regenerating full-thickness, three-dimensional tissue. The TE products we develop begin with the patient’s own tissue
to produce SCIRM that address the specific tissue or system needed for the patient’s care. Our product pipeline focuses
on the development of regenerative products for a variety of tissue types and organ systems that are commonly altered, injured,
or destroyed by a variety of diseases, pathologies, traumatic events, and medical interventions.
SkinTE,
our first tissue product, was registered with the United States Food and Drug Administration (FDA) in August 2017, and is now
commercially available for the repair, reconstruction, replacement, and regeneration of skin in patients who have a need for treatment
of acute or chronic wounds, burns, surgical reconstruction events, scar revision, or removal of dysfunctional skin grafts. We
are pursuing a regional plan for commercial rollout that began in late October 2018, and at the beginning of January 2019 we had
24 sales representatives in the field marketing SkinTE.
OsteoTE
is designed to utilize the patient’s bone to repair, reconstruct, replace, supplement, or regenerate bone damage or defects.
We registered OsteoTE with the FDA in December 2018. We are preparing for the first application of the product in a clinical setting,
which we are endeavoring to achieve in the first half of 2019.
Human
cells, tissues and cellular and tissue-based products (“HCT/Ps”) are governed by specific FDA regulations that provide
for a registration pathway that is different than the pathway for traditional drug candidates. SkinTE and OsteoTE are both registered
as HCT/Ps under Section 361 of the Public Health Service Act.
We
have a number of additional TE products under development, including the following:
|
|
●
|
AdipoTE
to optimize the delivery of autologous fat beyond the capabilities of current fat transfer techniques utilized in procedures
on, among others, the breast, buttocks, and face;
|
|
|
|
|
|
|
●
|
AngioTE
to address vascular regeneration including microscopic capillary networks all the way up to great vessel replacement;
|
|
|
|
|
|
|
●
|
NeuralTE
for peripheral nerve injuries of the extremities, as well as for patients with neuromas or chronic compression due to joint
replacements, migraines, craniofacial injuries, carpal tunnel syndrome, and those who have undergone hernia or abdominal-based
procedures;
|
|
|
|
|
|
|
●
|
UroTE
targeting the delivery of autologous urogenital epithelium and submucosa across a spectrum of diseases and processes, including
urethral strictures, urethral creation, bladder reconstruction, and ureter reconstruction;
|
|
|
|
|
|
|
●
|
LiverTE
to address numerous causes of liver failure, including NASH, fibrosis/cirrhosis, surgical resection of the liver; and
|
|
|
|
|
|
|
●
|
BowelTE
to deliver an optimized autologous construct to aid in the regeneration of bowel tissue.
|
RTD
and ARC represent research and development of new science and product opportunities based on what we learned while developing
the TE platform. RTD is focused on altered state analytes for the generation of composite materials that can be utilized for the
augmentation, modulation, and regulation of cell and tissue-derived systems. ARC is focused on the design and development of gene
transfer, small molecule synthesis, composite therapeutics, and alteration of self-propagating cell/tissue-derived bioreactors.
We
have significant research facilities and a well-educated and skilled team of scientists and researchers. These resources are highly
beneficial to the work we are doing on our TE products and in RTD and ARC. We also offer research services to unrelated third
parties on a contract basis, which we offer under the trademark POLARITYRD. Contract research services help us defray the costs
of maintaining a first-rate research facility and allow us to meet companies pursuing new technologies that may be opportunities
for collaborative or strategic relationships going forward.
Company
Background
Our
principal executive offices are located at 123 Wright Brothers Drive, Salt Lake City, UT 84116 and our telephone number is (800)
560-3983. Our website address is www.polarityte.com.
On
December 1, 2016, Majesco Acquisition Corp., a Nevada corporation and wholly-owned subsidiary of Majesco Entertainment Company,
a Delaware corporation (“Majesco DE”) entered into an Agreement and Plan of Reorganization with PolarityTE, Inc.,
a Nevada corporation (“PolarityTE NV”) and Dr. Denver Lough, the owner of 100% of the issued and outstanding shares
of capital stock of PolarityTE NV. The asset acquisition was subject to shareholder approval, which was received on March 10,
2017, and the transaction closed on April 7, 2017. In January 2017, Majesco DE changed its name to “PolarityTE, Inc.”
(“PolarityTE”). Majesco Acquisition Corp. was then merged with PolarityTE NV, which remains a subsidiary of PolarityTE.
Majesco Acquisition Corp. II, formed in November 2016 under Majesco Entertainment Company, changed its name to “PolarityTE
MD, Inc.,” and remains a wholly-owned subsidiary of PolarityTE.
USE
OF PROCEEDS
We
intend to use the net proceeds from the sale of any securities offered under this prospectus for general corporate purposes unless
otherwise indicated in the applicable prospectus supplement. General corporate purposes may include research and development and
clinical development costs to support the advancement of our product candidates and the expansion of our product candidate pipeline;
repayment and refinancing of debt; working capital; and capital expenditures. We may also use a portion of the net proceeds to
acquire or invest in businesses, products and technologies that are complementary to our own, although we have no commitments
or agreements with respect to any acquisitions as of the date of this prospectus. Pending these uses, we may invest the net proceeds
in a variety of capital preservation instruments, including short-term, investment-grade, interest-bearing instruments and U.S.
government securities, or may hold such proceeds as cash, until they are used for their stated purpose. We have not determined
the amount of net proceeds to be used specifically for such purposes. As a result, management will retain broad discretion over
the allocation of net proceeds.
SECURITIES
WE MAY OFFER
This
prospectus contains summary descriptions of the securities we may offer from time to time. These summary descriptions are not
meant to be complete descriptions of each security. The terms of any security will be described in the applicable prospectus supplement.
DESCRIPTION
OF CAPITAL STOCK
The
following description of our common stock and preferred stock, together with the additional information we include in any applicable
prospectus supplements, summarizes the material terms and provisions of the common stock and preferred stock that we may offer
under this prospectus. The following description of our capital stock does not purport to be complete and is subject to, and qualified
in its entirety by, our certificate of incorporation and bylaws, which are exhibits to the registration statement of which this
prospectus forms a part, and by applicable law. The terms of our common stock and preferred stock may also be affected by Delaware
law.
Authorized
Capital Stock
Our
authorized capital stock consists of 250,000,000 shares of common stock, par value $0.001 per share, and 25,000,000 shares of
preferred stock, par value $0.001 per share, all of which are undesignated preferred stock. As of February 5, 2019, we had 21,653,524
shares of common stock outstanding and no shares of preferred stock outstanding.
Common
Stock
The
holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of the stockholders.
The holders of our common stock do not have any cumulative voting rights. Holders of our common stock are entitled to receive
ratably any dividends declared by our board of directors out of funds legally available for that purpose, subject to any preferential
dividend rights of any outstanding preferred stock. Our common stock has no preemptive rights, conversion rights or other subscription
rights or redemption or sinking fund provisions.
In
the event of our liquidation, dissolution or winding up, holders of our common stock will be entitled to share ratably in all
assets remaining after payment of all debts and other liabilities and any liquidation preference of any outstanding preferred
stock. All outstanding shares are fully paid and non-assessable.
When
we issue shares of common stock under this prospectus, the shares will fully be paid and non-assessable and will not have, or
be subject to, any preemptive or similar rights.
Undesignated
Preferred Stock
Our
board of directors is authorized to issue up to 25,000,000 shares of undesignated preferred stock in one or more series without
stockholder approval. Our board of directors may determine the rights, preferences, privileges and restrictions, including voting
rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock.
The
purpose of authorizing our board of directors to issue preferred stock in one or more series and determine the number of shares
in the series and its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances.
Examples of rights and preferences that the Board may fix are:
|
|
●
|
dividend
rights;
|
|
|
|
|
|
|
●
|
conversion
rights;
|
|
|
●
|
voting
rights;
|
|
|
|
|
|
|
●
|
preemptive
rights;
|
|
|
|
|
|
|
●
|
terms
of redemption;
|
|
|
|
|
|
|
●
|
liquidation
preferences;
|
|
|
|
|
|
|
●
|
sinking
fund terms; and
|
|
|
|
|
|
|
●
|
the
number of shares constituting, or the designation of, such series, any or all of which may be greater than the rights of common
stock.
|
The
existence of authorized but unissued shares of undesignated preferred stock may enable our board of directors to render more difficult
or to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise. For example,
if in the due exercise of its fiduciary obligations, our board of directors were to determine that a takeover proposal is not
in the best interests of us or our stockholders, our board of directors could cause shares of preferred stock to be issued without
stockholder approval in one or more private offerings or other transactions that might dilute the voting or other rights of the
proposed acquirer, stockholder or stockholder group. The rights of holders of our common stock described above, will be subject
to, and may be adversely affected by, the rights of any preferred stock that we may designate and issue in the future. The issuance
of shares of undesignated preferred stock could decrease the amount of earnings and assets available for distribution to holders
of shares of common stock. The issuance may also adversely affect the rights and powers, including voting rights, of these holders
and may have the effect of delaying, deterring or preventing a change in control of us.
We
will incorporate by reference as an exhibit to the registration statement, which includes this prospectus, the form of any certificate
of designation that describes the terms of the series of preferred stock we are offering. This description and the applicable
prospectus supplement will include:
|
|
●
|
the
title and stated value;
|
|
|
|
|
|
|
●
|
the
number of shares authorized;
|
|
|
|
|
|
|
●
|
the
liquidation preference per share;
|
|
|
|
|
|
|
●
|
the
purchase price;
|
|
|
|
|
|
|
●
|
the
dividend rate, period and payment date, and method of calculation for dividends;
|
|
|
|
|
|
|
●
|
whether
dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate;
|
|
|
|
|
|
|
●
|
the
procedures for any auction and remarketing, if any;
|
|
|
|
|
|
|
●
|
the
provisions for a sinking fund, if any;
|
|
|
|
|
|
|
●
|
the
provisions for redemption or repurchase, if applicable, and any restrictions on our ability to exercise those redemption and
repurchase rights;
|
|
|
|
|
|
|
●
|
any
listing of the preferred stock on any securities exchange or market;
|
|
|
|
|
|
|
●
|
whether
the preferred stock will be convertible into our common stock, and, if applicable, the conversion price, or how it will be
calculated, and the conversion period;
|
|
|
|
|
|
|
●
|
whether
the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange price, or how it will be calculated,
and the exchange period;
|
|
|
|
|
|
|
●
|
voting
rights, if any, of the preferred stock;
|
|
|
|
|
|
|
●
|
preemptive
rights, if any;
|
|
|
|
|
|
|
●
|
restrictions
on transfer, sale or other assignment, if any;
|
|
|
|
|
|
|
●
|
whether
interests in the preferred stock will be represented by depositary shares;
|
|
|
|
|
|
|
●
|
a
discussion of any material United States federal income tax considerations applicable to the preferred stock;
|
|
|
|
|
|
|
●
|
the
relative ranking and preferences of the preferred stock as to dividend rights and rights if we liquidate, dissolve or wind
up our affairs;
|
|
|
|
|
|
|
●
|
any
limitations on issuance of any class or series of preferred stock ranking senior to or on a parity with the series of preferred
stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; and
|
|
|
|
|
|
|
●
|
any
other specific terms, preferences, rights or limitations of, or restrictions on, the preferred stock.
|
When
we issue shares of preferred stock under this prospectus, the shares will fully be paid and non-assessable and will not be subject
to any preemptive or similar rights.
Antitakeover
Effects of Delaware Law and Provisions of our Restated Certificate of Incorporation and Amended and Restated Bylaws
Certain
provisions of the Delaware General Corporation Law and of our restated certificate of incorporation and amended and restated bylaws
could have the effect of delaying, deferring or discouraging another party from acquiring control of us unless such takeover or
change of control is approved by the board of directors. These provisions, which are summarized below, are expected to discourage
certain types of coercive takeover practices and inadequate takeover bids and, therefore, they might also inhibit temporary fluctuations
in the market price of our common stock that often result from actual or rumored hostile takeover attempts. These provisions are
also designed in part to encourage anyone seeking to acquire control of us to first negotiate with our board of directors. These
provisions might also have the effect of preventing changes in our management. It is possible that these provisions could make
it more difficult to accomplish transactions that stockholders might otherwise deem to be in their best interests. However, we
believe that the advantages gained by protecting our ability to negotiate with any unsolicited and potentially unfriendly acquirer
outweigh the disadvantages of discouraging such proposals, including those priced above the then-current market value of our common
stock, because, among other reasons, the negotiation of such proposals could improve their terms.
Delaware
Takeover Statute. We are subject to the provisions of Section 203 of the Delaware General Corporation Law. In general, Section
203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested
stockholder” for a three-year period following the time that this stockholder becomes an interested stockholder, unless
the business combination is approved in a prescribed manner. Under Section 203, a business combination between a corporation and
an interested stockholder is prohibited unless it satisfies one of the following conditions:
|
|
●
|
before
the stockholder became interested, our board of directors approved either the business combination or the transaction which
resulted in the stockholder becoming an interested stockholder;
|
|
|
|
|
|
|
●
|
upon
consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder
owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for
purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee
stock plans, in some instances, but not the outstanding voting stock owned by the interested stockholder; or
|
|
|
|
|
|
|
●
|
at
or after the time the stockholder became interested, the business combination was approved by our board of directors and authorized
at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting
stock which is not owned by the interested stockholder.
|
|
|
|
|
|
|
●
|
Section
203 defines a business combination to include:
|
|
|
|
|
|
|
●
|
any
merger or consolidation involving the corporation and the interested stockholder;
|
|
|
|
|
|
|
●
|
any
sale, transfer, lease, pledge, exchange, mortgage or other disposition involving the interested stockholder of 10% or more
of the assets of the corporation;
|
|
|
|
|
|
|
●
|
subject
to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation
to the interested stockholder;
|
|
|
|
|
|
|
●
|
subject
to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the
stock of any class or series of the corporation beneficially owned by the interested stockholder; or
|
|
|
|
|
|
|
●
|
the
receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits
provided by or through the corporation.
|
In
general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding
voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Provisions
of our Restated Certificate of Incorporation and Amended and Restated Bylaws. Our restated certificate of incorporation and
amended and restated bylaws include several provisions that may have the effect of delaying, deferring or discouraging another
party from acquiring control of us and encouraging persons considering unsolicited tender offers or other unilateral takeover
proposals to negotiate with our board of directors rather than pursue non-negotiated takeover attempts. These provisions include
the items described below.
Board
composition and filling vacancies. In accordance with our restated certificate of incorporation, our board is divided into
three classes serving staggered three-year terms, with one class being elected each year. Our restated certificate of incorporation
also provides that directors may be removed only for cause and then only by the affirmative vote of the holders of two-thirds
or more of the shares then entitled to vote at an election of directors. Furthermore, any vacancy on our board of directors, however
occurring, including a vacancy resulting from an increase in the size of our board, may only be filled by the affirmative vote
of a majority of our directors then in office even if less than a quorum.
No
written consent of stockholders. Our restated certificate of incorporation provides that all stockholder actions are required
to be taken by a vote of the stockholders at an annual or special meeting, and that stockholders may not take any action by written
consent in lieu of a meeting. This limit may lengthen the amount of time required to take stockholder actions and would prevent
the amendment of our bylaws or removal of directors by our stockholder without holding a meeting of stockholders.
Meetings
of stockholders. Our bylaws provide that only a majority of the members of our board of directors then in office or stockholders
holding at least one-quarter of the voting power of all the then outstanding shares of our capital stock entitled to vote generally
in the election of directors may call special meetings of stockholders and only those matters set forth in the notice of the special
meeting may be considered or acted upon at a special meeting of stockholders. Our bylaws limit the business that may be conducted
at an annual meeting of stockholders to those matters properly brought before the meeting.
Advance
notice requirements. Our bylaws establish advance notice procedures regarding stockholder proposals pertaining to the nomination
of candidates for election as directors or new business to be brought before meetings of our stockholders. These procedures provide
that notice of stockholder proposals must be timely given in writing to our corporate secretary prior to the meeting at which
the action is to be taken. Generally, to be timely, notice must be received at our principal executive offices not less than 45
days or more than 75 days prior to the first anniversary date of the annual meeting for the preceding year. The notice must contain
certain information specified in our bylaws.
Amendment
to certificate of incorporation and bylaws. As required by the Delaware General Corporation Law, any amendment of our restated
certificate of incorporation must first be approved by a majority of our board of directors, and if required by law or our restated
certificate of incorporation, must thereafter be approved by a majority of the outstanding shares entitled to vote on the amendment,
and a majority of the outstanding shares of each class entitled to vote thereon as a class, except that the amendment of the provisions
relating to stockholder action, directors, amending our bylaws, limitation of liability and the amendment of our restated certificate
of incorporation must be approved by not less than two-thirds of the outstanding shares entitled to vote on the amendment, and
a majority of the outstanding shares of each class entitled to vote thereon as a class. Our bylaws may be amended by the affirmative
vote of a majority vote of the directors then in office, subject to any limitations set forth in the bylaws; and may also be amended
by the affirmative vote of at least two-thirds of the voting power of all the then outstanding shares of our capital stock entitled
to vote generally in the election of directors, voting together as a single class.
Undesignated
preferred stock. Our restated certificate of incorporation provides for authorized shares of preferred stock. The existence
of authorized but unissued shares of preferred stock may enable our board of directors to render more difficult or to discourage
an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise. For example, if in the due
exercise of its fiduciary obligations, our board of directors were to determine that a takeover proposal is not in the best interests
of us or our stockholders, our board of directors could cause shares of preferred stock to be issued without stockholder approval
in one or more private offerings or other transactions that might dilute the voting or other rights of the proposed acquirer or
insurgent stockholder or stockholder group. In this regard, our restated certificate of incorporation grants our board of directors’
broad power to establish the rights and preferences of authorized and unissued shares of preferred stock. The issuance of shares
of preferred stock could decrease the amount of earnings and assets available for distribution to holders of shares of common
stock. The issuance may also adversely affect the rights and powers, including voting rights, of these holders and may have the
effect of delaying, deterring or preventing a change in control of us.
DESCRIPTION
OF DEBT SECURITIES
The
following description, together with the additional information we include in any applicable prospectus supplements, summarizes
the material terms and provisions of the debt securities that we may offer under this prospectus. While the terms we have summarized
below will apply generally to any future debt securities we may offer pursuant to this prospectus, we will describe the terms
of any debt securities that we may offer in more detail in the applicable prospectus supplement. If we so indicate in a prospectus
supplement, the terms of any debt securities offered under such prospectus supplement may differ from the terms we describe below,
and to the extent the terms set forth in a prospectus supplement differ from the terms described below, the terms set forth in
the prospectus supplement shall control.
We
may sell from time to time, in one or more offerings under this prospectus, debt securities, which may be senior or subordinated.
We will issue any such senior debt securities under a senior indenture that we will enter into with a trustee to be named in the
senior indenture. We will issue any such subordinated debt securities under a subordinated indenture, which we will enter into
with a trustee to be named in the subordinated indenture. We have filed forms of these documents as exhibits to the registration
statement, of which this prospectus is a part. We use the term “indentures” to refer to either the senior indenture
or the subordinated indenture, as applicable. The indentures will be qualified under the Trust Indenture Act of 1939, as in effect
on the date of the indenture. We use the term “debenture trustee” to refer to either the trustee under the senior
indenture or the trustee under the subordinated indenture, as applicable.
The
following summaries of material provisions of the senior debt securities, the subordinated debt securities and the indentures
are subject to, and qualified in their entirety by reference to, all the provisions of the indenture applicable to a particular
series of debt securities.
General
Each
indenture provides that debt securities may be issued from time to time in one or more series and may be denominated and payable
in U.S. or foreign currencies or units based on or relating to U.S or foreign currencies. Neither indenture limits the amount
of debt securities that may be issued thereunder, and each indenture provides that the specific terms of any series of debt securities
shall be set forth in, or determined pursuant to, an authorizing resolution and/or a supplemental indenture, if any, relating
to such series.
We
will describe in each prospectus supplement the following terms relating to a series of debt securities:
|
|
●
|
the
title or designation;
|
|
|
|
|
|
|
●
|
the
aggregate principal amount and any limit on the amount that may be issued;
|
|
|
|
|
|
|
●
|
the
currency or units based on or relating to currencies in which debt securities of such series are denominated and the currency
or units in which principal or interest or both will or may be payable;
|
|
|
|
|
|
|
●
|
whether
we will issue the series of debt securities in global form, the terms of any global securities and who the depositary will
be;
|
|
|
|
|
|
|
●
|
the
maturity date and the date or dates on which principal will be payable;
|
|
|
|
|
|
|
●
|
the
interest rate, which may be fixed or variable, or the method for determining the rate and the date interest will begin to
accrue, the date or dates interest will be payable and the record dates for interest payment dates or the method for determining
such dates;
|
|
|
|
|
|
|
●
|
if
the debt securities will be secured or unsecured, and the terms of any secured debt;
|
|
|
●
|
the
terms of the subordination of any series of subordinated debt;
|
|
|
|
|
|
|
●
|
the
place or places where payments will be payable;
|
|
|
|
|
|
|
●
|
our
right, if any, to defer payment of interest and the maximum length of any such deferral period;
|
|
|
|
|
|
|
●
|
the
date, if any, after which, and the price at which, we may, at our option, redeem the series of debt securities pursuant to
any optional redemption provisions;
|
|
|
|
|
|
|
●
|
the
date, if any, on which, and the price at which we are obligated, pursuant to any mandatory sinking fund provisions or otherwise,
to redeem, or at the holder’s option to purchase, the series of debt securities;
|
|
|
|
|
|
|
●
|
whether
the indenture will restrict our ability to pay dividends, or will require us to maintain any asset ratios or reserves;
|
|
|
|
|
|
|
●
|
whether
we will be restricted from incurring any additional indebtedness;
|
|
|
|
|
|
|
●
|
a
discussion of any material or special U.S. federal income tax considerations applicable to a series of debt securities;
|
|
|
|
|
|
|
●
|
the
denominations in which we will issue the series of debt securities, if other than denominations of $1,000 and any integral
multiple thereof; and
|
|
|
|
|
|
|
●
|
any
other specific terms, preferences, rights or limitations of, or restrictions on, the debt securities.
|
We
may issue debt securities that provide for an amount less than their stated principal amount to be due and payable upon declaration
of acceleration of their maturity pursuant to the terms of the indenture. We will provide you with information on the federal
income tax considerations and other special considerations applicable to any of these debt securities in the applicable prospectus
supplement.
Conversion
or Exchange Rights
We
will set forth in the prospectus supplement the terms, if any, on which a series of debt securities may be convertible into or
exchangeable for our common stock or our other securities. We will include provisions as to whether conversion or exchange is
mandatory, at the option of the holder or at our option. We may include provisions pursuant to which the number of shares of our
common stock or our other securities that the holders of the series of debt securities receive would be subject to adjustment.
Consolidation,
Merger or Sale; No Protection in Event of a Change of Control or Highly Leveraged Transaction
The
indentures do not contain any covenant that restricts our ability to merge or consolidate, or sell, convey, transfer or otherwise
dispose of all or substantially all our assets. However, any successor to or acquirer of such assets must assume all our obligations
under the indentures or the debt securities, as appropriate.
Unless
we state otherwise in the applicable prospectus supplement, the debt securities will not contain any provisions that may afford
holders of the debt securities protection in the event we have a change of control or in the event of a highly leveraged transaction
(whether or not such transaction results in a change of control), which could adversely affect holders of debt securities.
Events
of Default Under the Indenture
The
following are events of default under the indentures with respect to any series of debt securities that we may issue:
|
|
●
|
if
we fail to pay interest when due and our failure continues for 90 days and the time for payment has not been extended or deferred;
|
|
|
|
|
|
|
●
|
if
we fail to pay the principal, or premium, if any, when due and the time for payment has not been extended or delayed;
|
|
|
|
|
|
|
●
|
if
we fail to observe or perform any other covenant set forth in the debt securities of such series or the applicable indentures,
other than a covenant specifically relating to and for the benefit of holders of another series of debt securities, and our
failure continues for 90 days after we receive written notice from the debenture trustee or holders of not less than a majority
in aggregate principal amount of the outstanding debt securities of the applicable series; and
|
|
|
|
|
|
|
●
|
if
specified events of bankruptcy, insolvency or reorganization occur as to us.
|
No
event of default with respect to a series of debt securities (except as to certain events of bankruptcy, insolvency or reorganization)
necessarily constitutes an event of default with respect to any other series of debt securities. The occurrence of an event of
default may constitute an event of default under any bank credit agreements we may have in existence from time to time. In addition,
the occurrence of certain events of default or an acceleration under the indenture may constitute an event of default under certain
of our other indebtedness outstanding from time to time.
If
an event of default with respect to debt securities of any series at the time outstanding occurs and is continuing, then the trustee
or the holders of not less than a majority in principal amount of the outstanding debt securities of that series may, by a notice
in writing to us (and to the debenture trustee if given by the holders), declare to be due and payable immediately the principal
(or, if the debt securities of that series are discount securities, that portion of the principal amount as may be specified in
the terms of that series) of and premium and accrued and unpaid interest, if any, on all debt securities of that series. Before
a judgment or decree for payment of the money due has been obtained with respect to debt securities of any series, the holders
of a majority in principal amount of the outstanding debt securities of that series (or, at a meeting of holders of such series
at which a quorum is present, the holders of a majority in principal amount of the debt securities of such series represented
at such meeting) may rescind and annul the acceleration if all events of default, other than the non-payment of accelerated principal,
premium, if any, and interest, if any, with respect to debt securities of that series, have been cured or waived as provided in
the applicable indenture (including payments or deposits in respect of principal, premium or interest that had become due other
than as a result of such acceleration). We refer you to the prospectus supplement relating to any series of debt securities that
are discount securities for the provisions relating to acceleration of a portion of the principal amount of such discount securities
upon the occurrence of an event of default.
Subject
to the terms of the indentures, if an event of default under an indenture shall occur and be continuing, the debenture trustee
will be under no obligation to exercise any of its rights or powers under such indenture at the request or direction of any of
the holders of the applicable series of debt securities, unless such holders have offered the debenture trustee reasonable indemnity.
The holders of a majority in principal amount of the outstanding debt securities of any series will have the right to direct the
time, method and place of conducting any proceeding for any remedy available to the debenture trustee, or exercising any trust
or power conferred on the debenture trustee, with respect to the debt securities of that series, provided that:
|
|
●
|
the
direction so given by the holder is not in conflict with any law or the applicable indenture; and
|
|
|
|
|
|
|
●
|
subject
to its duties under the Trust Indenture Act, the debenture trustee need not take any action that might involve it in personal
liability or might be unduly prejudicial to the holders not involved in the proceeding.
|
A
holder of the debt securities of any series will only have the right to institute a proceeding under the indentures or to appoint
a receiver or trustee, or to seek other remedies if:
|
|
●
|
the
holder previously has given written notice to the debenture trustee of a continuing event of default with respect to that
series;
|
|
|
|
|
|
|
●
|
the
holders of at least a majority in aggregate principal amount of the outstanding debt securities of that series have made written
request, and such holders have offered reasonable indemnity to the debenture trustee to institute the proceeding as trustee;
and
|
|
|
|
|
|
|
●
|
the
debenture trustee does not institute the proceeding, and does not receive from the holders of a majority in aggregate principal
amount of the outstanding debt securities of that series (or at a meeting of holders of such series at which a quorum is present,
the holders of a majority in principal amount of the debt securities of such series represented at such meeting) other conflicting
directions within 60 days after the notice, request and offer.
|
These
limitations do not apply to a suit instituted by a holder of debt securities if we default in the payment of the principal, premium,
if any, or interest on, the debt securities.
We
will periodically file statements with the applicable debenture trustee regarding our compliance with specified covenants in the
applicable indenture.
Modification
of Indenture; Waiver
The
debenture trustee and we may change the applicable indenture without the consent of any holders with respect to specific matters,
including:
|
|
●
|
to
fix any ambiguity, defect or inconsistency in the indenture; and
|
|
|
|
|
|
|
●
|
to
change anything that does not materially adversely affect the interests of any holder of debt securities of any series issued
pursuant to such indenture.
|
In
addition, under the indentures, the rights of holders of a series of debt securities may be changed by us and the debenture trustee
with the written consent of the holders of at least a majority in aggregate principal amount of the outstanding debt securities
of each series (or, at a meeting of holders of such series at which a quorum is present, the holders of a majority in principal
amount of the debt securities of such series represented at such meeting) that is affected. However, the debenture trustee and
we may make the following changes only with the consent of each holder of any outstanding debt securities affected:
|
|
●
|
extending
the fixed maturity of the series of debt securities;
|
|
|
|
|
|
|
●
|
reducing
the principal amount, reducing the rate of or extending the time of payment of interest, or any premium payable upon the redemption
of any debt securities;
|
|
|
|
|
|
|
●
|
reducing
the principal amount of discount securities payable upon acceleration of maturity;
|
|
|
|
|
|
|
●
|
making
the principal of or premium or interest on any debt security payable in currency other than that stated in the debt security;
or
|
|
|
|
|
|
|
●
|
reducing
the percentage of debt securities, the holders of which are required to consent to any amendment or waiver.
|
Except
for certain specified provisions, the holders of at least a majority in principal amount of the outstanding debt securities of
any series (or, at a meeting of holders of such series at which a quorum is present, the holders of a majority in principal amount
of the debt securities of such series represented at such meeting) may on behalf of the holders of all debt securities of that
series waive our compliance with provisions of the indenture. The holders of a majority in principal amount of the outstanding
debt securities of any series may on behalf of the holders of all the debt securities of such series waive any past default under
the indenture with respect to that series and its consequences, except a default in the payment of the principal of, premium or
any interest on any debt security of that series or in respect of a covenant or provision, which cannot be modified or amended
without the consent of the holder of each outstanding debt security of the series affected; provided, however, that
the holders of a majority in principal amount of the outstanding debt securities of any series may rescind an acceleration and
its consequences, including any related payment default that resulted from the acceleration.
Discharge
Each
indenture provides that we can elect to be discharged from our obligations with respect to one or more series of debt securities,
except for obligations to:
|
|
●
|
the
transfer or exchange of debt securities of the series;
|
|
|
|
|
|
|
●
|
replace
stolen, lost or mutilated debt securities of the series;
|
|
|
|
|
|
|
●
|
maintain
paying agencies;
|
|
|
|
|
|
|
●
|
hold
monies for payment in trust;
|
|
|
|
|
|
|
●
|
compensate
and indemnify the trustee; and
|
|
|
|
|
|
|
●
|
appoint
any successor trustee.
|
To
exercise our rights to be discharged with respect to a series, we must deposit with the trustee money or government obligations
sufficient to pay all the principal of, the premium, if any, and interest on, the debt securities of the series on the dates payments
are due.
Form,
Exchange and Transfer
We
will issue the debt securities of each series only in fully registered form without coupons and, unless we otherwise specify in
the applicable prospectus supplement, in denominations of $1,000 and any integral multiple thereof. The indentures provide that
we may issue debt securities of a series in temporary or permanent global form and as book-entry securities that will be deposited
with, or on behalf of, The Depository Trust Company or another depositary named by us and identified in a prospectus supplement
with respect to that series.
At
the option of the holder, subject to the terms of the indentures and the limitations applicable to global securities described
in the applicable prospectus supplement, the holder of the debt securities of any series can exchange the debt securities for
other debt securities of the same series, in any authorized denomination and of like tenor and aggregate principal amount.
Subject
to the terms of the indentures and the limitations applicable to global securities set forth in the applicable prospectus supplement,
holders of the debt securities may present the debt securities for exchange or for registration of transfer, duly endorsed or
with the form of transfer endorsed thereon duly executed if so required by us or the security registrar, at the office of the
security registrar or at the office of any transfer agent designated by us for this purpose. Unless otherwise provided in the
debt securities that the holder presents for transfer or exchange or in the applicable indenture, we will make no service charge
for any registration of transfer or exchange, but we may require payment of any taxes or other governmental charges.
We
will name in the applicable prospectus supplement the security registrar, and any transfer agent in addition to the security registrar,
that we initially designate for any debt securities. We may at any time designate additional transfer agents or rescind the designation
of any transfer agent or approve a change in the office through which any transfer agent acts, except that we will be required
to maintain a transfer agent in each place of payment for the debt securities of each series.
If
we elect to redeem the debt securities of any series, we will not be required to:
|
|
●
|
issue,
register the transfer of, or exchange any debt securities of that series during a period beginning at the opening of business
15 days before the day of mailing of a notice of redemption of any debt securities that may be selected for redemption and
ending at the close of business on the day of the mailing; or
|
|
|
|
|
|
|
●
|
register
the transfer of or exchange any debt securities so selected for redemption, in whole or in part, except the unredeemed portion
of any debt securities we are redeeming in part.
|
Information
Concerning the Debenture Trustee
The
debenture trustee, other than during the occurrence and continuance of an event of default under the applicable indenture, undertakes
to perform only those duties as are specifically set forth in the applicable indenture. Upon an event of default under an indenture,
the debenture trustee under such indenture must use the same degree of care as a prudent person would exercise or use in the conduct
of his or her own affairs. Subject to this provision, the debenture trustee is under no obligation to exercise any of the powers
given it by the indentures at the request of any holder of debt securities unless it is offered reasonable security and indemnity
against the costs, expenses and liabilities that it might incur.
Payment
and Paying Agents
Unless
we otherwise indicate in the applicable prospectus supplement, we will make payment of the interest on any debt securities on
any interest payment date to the person in whose name the debt securities, or one or more predecessor securities, are registered
at the close of business on the regular record date for the interest.
We
will pay principal of and any premium and interest on the debt securities of a series at the office of the paying agents designated
by us, except that unless we otherwise indicate in the applicable prospectus supplement, will we make interest payments by check
which we will mail to the holder. Unless we otherwise indicate in a prospectus supplement, we will designate the corporate trust
office of the debenture trustee in the City of New York as our sole paying agent for payments with respect to debt securities
of each series. We will name in the applicable prospectus supplement any other paying agents that we initially designate for the
debt securities of a series. We will maintain a paying agent in each place of payment for the debt securities of a series.
All
money we pay to a paying agent or the debenture trustee for the payment of the principal of or any premium or interest on any
debt securities which remains unclaimed at the end of two years after such principal, premium or interest has become due and payable
will be repaid to us, and the holder of the security thereafter may look only to us for payment thereof.
Governing
Law
The
indentures and the debt securities will be governed by and construed in accordance with the laws of the State of New York, except
to the extent that the Trust Indenture Act is applicable.
Subordination
of Subordinated Debt Securities
Our
obligations pursuant to any subordinated debt securities will be unsecured and will be subordinate and junior in priority of payment
to certain of our other indebtedness to the extent described in a prospectus supplement. The subordinated indenture does not limit
the amount of senior indebtedness we may incur. It also does not limit us from issuing any other secured or unsecured debt.
DESCRIPTION
OF WARRANTS
General
We
may issue warrants to purchase shares of our common stock, preferred stock and/or debt securities in one or more series together
with other securities or separately, as described in the applicable prospectus supplement. Below is a description of certain general
terms and provisions of the warrants that we may offer. Terms of the warrants will be described in the warrant agreements and
the prospectus supplement relating to the warrants.
The
applicable prospectus supplement will contain, where applicable, the following terms of and other information relating to the
warrants:
|
|
●
|
the
specific designation and aggregate number of, and the price at which we will issue, the warrants;
|
|
|
|
|
|
|
●
|
the
currency or currency units in which the offering price, if any, and the exercise price are payable;
|
|
|
|
|
|
|
●
|
the
designation, amount and terms of the securities purchasable upon exercise of the warrants;
|
|
|
|
|
|
|
●
|
if
applicable, the exercise price for shares of our common stock and the number of shares of common stock to be received upon
exercise of the warrants;
|
|
|
|
|
|
|
●
|
if
applicable, the exercise price for shares of our preferred stock, the number of shares of preferred stock to be received upon
exercise, and a description of that series of our preferred stock;
|
|
|
|
|
|
|
●
|
if
applicable, the exercise price for our debt securities, the amount of debt securities to be received upon exercise, and a
description of that series of debt securities;
|
|
|
|
|
|
|
●
|
the
date on which the right to exercise the warrants will begin and the date on which that right will expire or, if you may not
continuously exercise the warrants throughout that period, the specific date or dates on which you may exercise the warrants;
|
|
|
|
|
|
|
●
|
whether
the warrants will be issued in fully registered form or bearer form, in definitive or global form or in any combination of
these forms, although, in any case, the form of a warrant included in a unit will correspond to the form of the unit and of
any security included in that unit;
|
|
|
|
|
|
|
●
|
any
applicable material U.S. federal income tax consequences;
|
|
|
|
|
|
|
●
|
the
identity of the warrant agent for the warrants and of any other depositaries, execution or paying agents, transfer agents,
registrars or other agents;
|
|
|
|
|
|
|
●
|
the
proposed listing, if any, of the warrants or any securities purchasable upon exercise of the warrants on any securities exchange;
|
|
|
|
|
|
|
●
|
if
applicable, the date from and after which the warrants and the common stock, preferred stock and/or debt securities will be
separately transferable;
|
|
|
|
|
|
|
●
|
if
applicable, the minimum or maximum amount of the warrants that may be exercised at any one time;
|
|
|
|
|
|
|
●
|
information
with respect to book-entry procedures, if any;
|
|
|
|
|
|
|
●
|
the
anti-dilution provisions of the warrants, if any;
|
|
|
|
|
|
|
●
|
any
redemption or call provisions;
|
|
|
|
|
|
|
●
|
whether
the warrants may be sold separately or with other securities as parts of units; and
|
|
|
|
|
|
|
●
|
any
additional terms of the warrants, including terms, procedures and limitations relating to the exchange and exercise of the
warrants.
|
Transfer
Agent and Registrar
The
transfer agent and registrar for any warrants will be set forth in the applicable prospectus supplement.
DESCRIPTION
OF RIGHTS
General
We
may issue rights to our stockholders to purchase shares of our common stock, preferred stock or the other securities described
in this prospectus. We may offer rights separately or together with one or more additional rights, debt securities, preferred
stock, common stock or warrants, or any combination of those securities in the form of units, as described in the applicable prospectus
supplement. Each series of rights will be issued under a separate rights agreement to be entered into between us and a bank or
trust company, as rights agent. The rights agent will act solely as our agent regarding the certificates relating to the rights
of the series of certificates and will not assume any obligation or relationship of agency or trust for or with any holders of
rights certificates or beneficial owners of rights. The following description sets forth certain general terms and provisions
of the rights to which any prospectus supplement may relate. The terms of the rights to which any prospectus supplement may relate
and the extent, if any, to which the general provisions may apply to the rights so offered will be described in the applicable
prospectus supplement. To the extent that any terms of the rights, rights agreement or rights certificates described in a prospectus
supplement differ from any of the terms described below, then the terms described below will be deemed to have been superseded
by that prospectus supplement. We encourage you to read the applicable rights agreement and rights certificate for additional
information before you decide whether to purchase any of our rights. We will provide in a prospectus supplement the following
terms of the rights being issued:
|
|
●
|
the
date of determining the stockholders entitled to the rights distribution;
|
|
|
|
|
|
|
●
|
the
aggregate number of shares of common stock, preferred stock or other securities purchasable upon exercise of the rights;
|
|
|
|
|
|
|
●
|
the
exercise price;
|
|
|
|
|
|
|
●
|
the
aggregate number of rights issued;
|
|
|
|
|
|
|
●
|
whether
the rights are transferrable and the date, if any, on and after which the rights may be separately transferred;
|
|
|
|
|
|
|
●
|
the
date on which the right to exercise the rights will commence, and the date on which the right to exercise the rights will
expire;
|
|
|
|
|
|
|
●
|
the
method by which holders of rights will be entitled to exercise;
|
|
|
|
|
|
|
●
|
the
conditions to the completion of the offering, if any;
|
|
|
|
|
|
|
●
|
the
withdrawal, termination and cancellation rights, if any;
|
|
|
|
|
|
|
●
|
whether
there are any backstop or standby purchaser or purchasers and the terms of their commitment, if any;
|
|
|
|
|
|
|
●
|
whether
stockholders are entitled to oversubscription rights, if any;
|
|
|
|
|
|
|
●
|
any
applicable material U.S. federal income tax considerations; and
|
|
|
|
|
|
|
●
|
any
other terms of the rights, including terms, procedures and limitations relating to the distribution, exchange and exercise
of the rights, as applicable, including any provisions for modifying any of the terms of the rights.
|
Each
right will entitle the holder of rights to purchase for cash the principal number of shares of common stock, preferred stock or
other securities at the exercise price provided in the applicable prospectus supplement. Rights may be exercised at any time up
to the close of business on the expiration date for the rights provided in the applicable prospectus supplement.
Holders
may exercise rights as described in the applicable prospectus supplement. Upon receipt of payment and the rights certificate properly
completed and duly executed at the corporate trust office of the rights agent or any other office indicated in the prospectus
supplement, we will, as soon as practicable, forward the shares of common stock, preferred stock or other securities, as applicable,
purchasable upon exercise of the rights. If less than all the rights issued in any rights offering are exercised, we may offer
any unsubscribed securities directly to persons other than stockholders, to or through agents, underwriters or dealers or through
a combination of such methods, including pursuant to standby arrangements, as described in the applicable prospectus supplement.
Rights
Agent
The
rights agent for any rights we offer will be set forth in the applicable prospectus supplement.
DESCRIPTION
OF UNITS
The
following description, together with the additional information that we include in any applicable prospectus supplements summarizes
the material terms and provisions of the units that we may offer under this prospectus. While the terms we have summarized below
will apply generally to any units that we may offer under this prospectus, we will describe the terms of any series of units in
more detail in the applicable prospectus supplement. The terms of any units offered under a prospectus supplement may differ from
the terms described below.
We
will incorporate by reference from reports that we file with the SEC, the form of unit agreement that describes the terms of the
series of units we are offering, and any supplemental agreements, before the issuance of the related series of units. The following
summaries of material terms and provisions of the units are subject to, and qualified in their entirety by reference to, all the
provisions of the unit agreement and any supplemental agreements applicable to a series of units. We urge you to read the applicable
prospectus supplements related to the series of units that we may offer under this prospectus, as well as any related free writing
prospectuses and the complete unit agreement and any supplemental agreements that contain the terms of the units.
General
We
may issue units consisting of common stock, preferred stock, one or more debt securities, warrants or rights for the purchase
of common stock, preferred stock and/or debt securities in one or more series, in any combination. Each unit will be issued so
that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the
rights and obligations of a holder of each security included in the unit. The unit agreement under which a unit is issued may
provide that the securities included in the unit may not be held or transferred separately, at any time or at any time before
a specified date.
We
will describe in the applicable prospectus supplement the terms of the series of units being offered, including:
|
|
●
|
the
designation and terms of the units and of the securities comprising the units, including whether and under what circumstances
those securities may be held or transferred separately;
|
|
|
|
|
|
|
●
|
any
provisions of the governing unit agreement that differ from those described below; and
|
|
|
|
|
|
|
●
|
any
provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units,
including any provisions for modifying any the terms of the units.
|
The
provisions described in this section, as well as those set forth in any prospectus supplement or as described under “Description
of Common Stock,” “Description of Preferred Stock,” “Description of Debt Securities,” “Description
of Warrants,” and “Description of Rights” will apply to each unit, as applicable, and to any common stock, preferred
stock, debt security, warrant or right included in each unit, as applicable.
Unit
Agent
The
name and address of the unit agent, if any, for any units we offer will be set forth in the applicable prospectus supplement.
Issuance
in Series
We
may issue units in such amounts and in such numerous distinct series as we determine.
Enforceability
of Rights by Holders of Units
Each
unit agent will act solely as our agent under the applicable unit agreement and will not assume any obligation or relationship
of agency or trust with any holder of any unit. A single bank or trust company may act as unit agent for more than one series
of units. A unit agent will have no duty or responsibility in case of any default by us under the applicable unit agreement or
unit, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any
holder of a unit may, without the consent of the related unit agent or the holder of any other unit, enforce by appropriate legal
action its rights as holder under any security included in the unit.
PLAN
OF DISTRIBUTION
We
may sell securities:
|
|
●
|
through
underwriters;
|
|
|
|
|
|
|
●
|
through
dealers;
|
|
|
|
|
|
|
●
|
through
agents;
|
|
|
|
|
|
|
●
|
directly
to purchasers;
|
|
|
|
|
|
|
●
|
in
“at the market offering”, within the meaning of Rule 415(a)(4) of the Securities Act; or
|
|
|
|
|
|
|
●
|
through
a combination of any of these methods or any other method permitted by law.
|
In
addition, we may issue the securities as a dividend or distribution or in a subscription rights offering to our existing security
holders.
We
may directly solicit offers to purchase securities, or agents may be designated to solicit such offers. In the prospectus supplement
relating to such offering, we will name any agent that could be viewed as an underwriter under the Securities Act and describe
any commissions that we must pay to any such agent. Any such agent will be acting on a best efforts basis for the period of its
appointment or, if indicated in the applicable prospectus supplement, on a firm commitment basis. This prospectus may be used
in connection with any offering of our securities through any of these methods or other methods described in the applicable prospectus
supplement.
The
distribution of the securities may be effectuated from time to time in one or more transactions:
|
|
●
|
at
a fixed price, or prices, which may be changed from time to time;
|
|
|
|
|
|
|
●
|
at
market prices prevailing at the time of sale;
|
|
|
|
|
|
|
●
|
at
prices related to such prevailing market prices; or
|
|
|
|
|
|
|
●
|
at
negotiated prices.
|
Each
prospectus supplement will describe the method of distribution of the securities and any applicable restrictions.
The
prospectus supplement with respect to the securities of a series will describe the terms of the offering of the securities, including
the following:
|
|
●
|
the
name of the agent or any underwriters;
|
|
|
|
|
|
|
●
|
the
public offering or purchase price;
|
|
|
|
|
|
|
●
|
any
discounts and commissions to be allowed or paid to the agent or underwriters;
|
|
|
|
|
|
|
●
|
all
other items constituting underwriting compensation;
|
|
|
|
|
|
|
●
|
any
discounts and commissions to be allowed or paid to dealers; and
|
|
|
|
|
|
|
●
|
any
exchanges on which the securities will be listed.
|
If
any underwriters or agents are used in the sale of the securities in respect of which this prospectus is delivered, we will enter
into an underwriting agreement, sales agreement or other agreement with them at the time of sale to them, and we will set forth
in the prospectus supplement relating to such offering the names of the underwriters or agents and the terms of the related agreement
with them.
In
connection with the offering of securities, we may grant to the underwriters an option to purchase additional securities with
an additional underwriting commission, as may be set forth in the accompanying prospectus supplement. If we grant any such option,
the terms of such option will be set forth in the prospectus supplement for such securities.
If
a dealer is used in the sale of the securities in respect of which the prospectus is delivered, we will sell such securities to
the dealer, as principal. The dealer, who may be deemed to be an “underwriter” as that term is defined in the Securities
Act, may then resell such securities to the public at varying prices to be determined by such dealer at the time of resale.
If
we offer securities in a subscription rights offering to our existing security holders, we may enter into a standby underwriting
agreement with dealers, acting as standby underwriters. We may pay the standby underwriters a commitment fee for the securities
they commit to purchase on a standby basis. If we do not enter into a standby underwriting arrangement, we may retain a dealer-
manager to manage a subscription rights offering for us.
Agents,
underwriters, dealers and other persons may be entitled under agreements which they may enter into with us to indemnification
by us against certain civil liabilities, including liabilities under the Securities Act, and may be customers of, engage in transactions
with or perform services for us in the ordinary course of business.
If
so indicated in the applicable prospectus supplement, we will authorize underwriters or other persons acting as our agents to
solicit offers by certain institutions to purchase securities from us pursuant to delayed delivery contracts providing for payment
and delivery on the date stated in the prospectus supplement. Each contract will be for an amount not less than, and the aggregate
amount of securities sold pursuant to such contracts shall not be less nor more than, the respective amounts stated in the prospectus
supplement. Institutions with whom the contracts, when authorized, may be made include commercial and savings banks, insurance
companies, pension funds, investment companies, educational and charitable institutions and other institutions, but shall in all
cases be subject to our approval. Delayed delivery contracts will not be subject to any conditions except that:
|
|
●
|
the
purchase by an institution of the securities covered under that contract shall not at the time of delivery be prohibited under
the laws of the jurisdiction to which that institution is subject; and
|
|
|
|
|
|
|
●
|
if
the securities are also being sold to underwriters acting as principals for their own account, the underwriters shall have
purchased such securities not sold for delayed delivery. The underwriters and other persons acting as our agents will not
have any responsibility in respect of the validity or performance of delayed delivery contracts.
|
Offered
securities may also be offered and sold, if so indicated in the prospectus supplement, in connection with a remarketing upon their
purchase, in accordance with a redemption or repayment pursuant to their terms, or otherwise, by one or more remarketing firms,
acting as principals for their own accounts or as agents for us. Any remarketing firm will be identified and the terms of its
agreement, if any, with us and its compensation will be described in the applicable prospectus supplement. Remarketing firms may
be deemed to be underwriters because of their remarketing of offered securities.
Certain
agents, underwriters and dealers, and their associates and affiliates, may be customers of, have borrowing relationships with,
engage in other transactions with, or perform services, including investment banking services, for us or one or more of our respective
affiliates in the ordinary course of business.
To
facilitate the offering of the securities, any underwriters may engage in transactions that stabilize, maintain or otherwise affect
the price of the securities or any other securities the prices of which may be used to determine payments on such securities.
Specifically, any underwriters may over-allot in connection with the offering, creating a short position for their own accounts.
In addition, to cover overallotments or to stabilize the price of the securities or of any such other securities, the underwriters
may bid for, and purchase, the securities or any such other securities in the open market. Finally, in any offering of the securities
through a syndicate of underwriters, the underwriting syndicate may reclaim selling concessions allowed to an underwriter or a
dealer for distributing the securities in the offering if the syndicate repurchases previously distributed securities in transactions
to cover syndicate short positions, in stabilization transactions or otherwise. Any of these activities may stabilize or maintain
the market price of the securities above independent market levels. Any such underwriters are not required to engage in these
activities and may end any of these activities at any time.
We
may engage in at the market offerings into an existing trading market in accordance with Rule 415(a)(4) under the Securities Act.
In addition, we may enter into derivative transactions with third parties, or sell securities not covered by this prospectus to
third parties in privately negotiated transactions. If the applicable prospectus supplement so indicates, in connection with those
derivatives, the third parties may sell securities covered by this prospectus and the applicable prospectus supplement, including
in short sale transactions. If so, the third party may use securities pledged by us or borrowed from us or others to settle those
sales or to close out any related open borrowings of stock, and may use securities received from us in settlement of those derivatives
to close out any related open borrowings of stock. The third party in such sale transactions will be an underwriter and, if not
identified in this prospectus, will be named in the applicable prospectus supplement (or a post-effective amendment). In addition,
we may otherwise loan or pledge securities to a financial institution or other third party that in turn may sell the securities
short using this prospectus and an applicable prospectus supplement. Such financial institution or other third party may transfer
its economic short position to investors in our securities or in connection with a concurrent offering of other securities.
Under
Rule 15c6-1 of the Exchange Act, trades in the secondary market generally are required to settle in three business days, unless
the parties to any such trade expressly agree otherwise. The applicable prospectus supplement may provide that the original issue
date for your securities may be more than three scheduled business days after the trade date for your securities. Accordingly,
in such a case, if you wish to trade securities on any date prior to the third business day before the original issue date for
your securities, you will be required, by virtue of the fact that your securities initially are expected to settle in more than
three scheduled business days after the trade date for your securities, to make alternative settlement arrangements to prevent
a failed settlement.
The
securities may be new issues of securities and may have no established trading market. The securities may or may not be listed
on a national securities exchange. We can make no assurance as to the liquidity of or the existence of trading markets for any
of the securities.
The
specific terms of any lock-up provisions in respect of any given offering will be described in the applicable prospectus supplement.
The
underwriters, dealers and agents may engage in transactions with us, or perform services for us, in the ordinary course of business
for which they receive compensation.
The
anticipated date of delivery of offered securities will be set forth in the applicable prospectus supplement relating to each
offer.
LEGAL
MATTERS
Certain
legal matters relating to this offering will be passed upon for us by Goodwin Procter LLP, San Francisco, California. Any underwriters
will also be advised about the validity of the securities and other legal matters by their own counsel, which will be named in
the prospectus supplement.
EXPERTS
The
consolidated balance sheets of PolarityTE, Inc. and Subsidiaries as of October 31, 2018 and 2017, and the related consolidated
statements of operations, stockholders’ equity, and cash flows for each of the years then ended, have been audited by EisnerAmper
LLP, independent registered public accounting firm, as stated in their reports that are incorporated herein by reference, which
reports (1) express an unqualified opinion on the financial statements, and (2) express an adverse opinion on the effectiveness
of internal control over financial reporting. Such financial statements have been incorporated herein by reference in reliance
on the reports of such firm given upon their authority as experts in accounting and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
This
prospectus is part of a registration statement that we have filed with the SEC. Certain information in the registration statement
has been omitted from this prospectus in accordance with the rules of the SEC. We are subject to the information requirements
of the Exchange Act and, in accordance therewith, file annual, quarterly and special reports, proxy statements and other information
with the SEC. You may read and copy any document we file at the SEC’s Public Reference Room at 100 F Street, N.E., Washington,
D.C. 20549. You may call the SEC at 1-800-SEC-0330 for further information on the operation of the Public Reference Room. These
documents also may be accessed through the SEC’s electronic data gathering, analysis and retrieval system, or EDGAR, via
electronic means, including the SEC’s home page on the Internet (www.sec.gov).
We
have the authority to designate and issue more than one class or series of stock having various preferences, conversion and other
rights, voting powers, restrictions, limitations as to dividends, qualifications, and terms and conditions of redemption. See
“Description of Capital Stock.” We will furnish a full statement of the relative rights and preferences of each class
or series of our stock which has been so designated and any restrictions on the ownership or transfer of our stock to any stockholder
upon request and without charge. Written requests for such copies should be directed to PolarityTE, Inc., 1960 South 4250 West,
Salt Lake City, Utah, 84104, Attention: Chief Legal Officer, by telephone request to (385) 237-2279, or by e-mail to marklehman@polarityte.com.
Our website is located at www.polarityte.com. Information contained on our website is not incorporated by reference into
this prospectus and, therefore, is not part of this prospectus or any accompanying prospectus supplement.
INCORPORATION
BY REFERENCE
The
SEC allows us to incorporate by reference the information and reports we file with it, which means that we can disclose important
information to you by referring you to these documents. The information incorporated by reference is an important part of this
prospectus, and information that we file later with the SEC will automatically update and supersede the information already incorporated
by reference. We are incorporating by reference the documents listed below, which we have already filed with the SEC, and any
future filings we make with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, including all filings made after
the date of the filing of this registration statement and prior to the effectiveness of this registration statement, except as
to any portion of any future report or document that is not deemed filed under such provisions, after the date of this prospectus
and prior to the termination of this offering:
|
|
●
|
Our
Annual Report on Form 10-K for the year ended October 31, 2018 filed with the SEC on January 14, 2019;
|
|
|
|
|
|
|
●
|
Our
Current Reports on Form 8-K filed with the SEC on December 11, 2018, and January 29, 2019; and
|
|
|
|
|
|
|
●
|
The
description of our common stock contained in our Registration Statement on Form 8-A filed with the SEC on January 21, 2005
(File No. 000-51128), including any amendment or report filed to update such description.
|
Upon
request, we will provide, without charge, to each person, including any beneficial owner, to whom a copy of this prospectus is
delivered, a copy of the documents incorporated by reference into this prospectus but not delivered with the prospectus. You may
request a copy of these filings, and any exhibits we have specifically incorporated by reference as an exhibit in this prospectus,
at no cost by writing or telephoning us at the following address:
PolarityTE,
Inc.
123
Wright Brothers Drive
Salt
Lake City, Utah 84116
(800)
560-3983
You
may also access these documents, free of charge on the SEC’s website at www.sec.gov or on our website at www.polarityte.com.
Information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information
on, or that can be accessed from, our website as part of this prospectus or any accompanying prospectus supplement.
This
prospectus is part of a registration statement we filed with the SEC. We have incorporated exhibits into this registration statement.
You should read the exhibits carefully for provisions that may be important to you.
You
should rely only on the information incorporated by reference or provided in this prospectus or any prospectus supplement. We
have not authorized anyone to provide you with different information. We are not making an offer of these securities in any state
where the offer is not permitted. You should not assume that the information in this prospectus or in the documents incorporated
by reference is accurate as of any date other than the date on the front of this prospectus or those documents.

PolarityTE,
Inc.
Up to $50,000,000 of Shares of Common Stock

March
30, 2021
PolarityTE (NASDAQ:PTE)
Historical Stock Chart
From Mar 2024 to Apr 2024
PolarityTE (NASDAQ:PTE)
Historical Stock Chart
From Apr 2023 to Apr 2024