As
filed with the Securities and Exchange Commission on July 2, 2019.
Registration
No. 333-
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
ADVAXIS,
INC.
(Exact
Name of Registrant as Specified in its Charter)
|
Delaware
|
|
2836
|
|
02-0563870
|
|
(State
or other jurisdiction of
incorporation
or organization)
|
|
(Primary
Standard Industrial
Classification
Code Number)
|
|
(I.R.S.
Employer
Identification
Number)
|
305
College Road East
Princeton,
New Jersey 08540
(609)
452-9813
(Address,
including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Kenneth
Berlin
Chief Executive Officer
305 College Road East
Princeton, New Jersey 08540
(609) 452-9813
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
|
Kristopher
D. Brown
Goodwin
Procter LLP
620 Eighth Avenue
New York, NY 02109
(212) 813-8800
|
|
Oded
Har-Even, Esq.
Robert V. Condon III, Esq.
Zysman, Aharoni, Gayer and
Sullivan & Worcester LLP
1633 Broadway
New York, NY 10019
(212) 660-3000
|
Approximate
date of commencement of proposed sale to public:
As soon as practicable after this Registration Statement is declared effective.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under
the Securities Act of 1933, check the following box. [X]
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the
same offering. [ ]
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list
the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list
the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”,
“smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large
accelerated filer
|
[ ]
|
|
Accelerated
filer
|
[X]
|
|
Non-accelerated
filer
|
[ ]
|
|
Smaller
reporting company
|
[X]
|
|
Emerging
Growth Company
|
[ ]
|
|
|
|
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for
complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. [ ]
CALCULATION
OF REGISTRATION FEE
|
Title of Each Class of Securities to be Registered
|
|
Proposed Maximum Aggregate Offering Price(1)(2)(3)
|
|
|
Amount of Registration Fee
|
|
|
Common Stock, par value $0.001 per share
|
|
$
|
|
|
|
$
|
|
|
|
Pre-funded warrants to purchase shares of common stock and shares of common stock issuable upon exercise thereof(4)
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
17,250,000
|
|
|
$
|
2,091
|
|
|
(1)
|
Includes
additional shares of common stock that the underwriters have the option to purchase solely to cover over-allotments, if any.
|
|
(2)
|
Estimated
solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended.
|
|
(3)
|
The
proposed maximum aggregate offering price of the common stock proposed to be sold in the offering will be reduced on a dollar-for-dollar
basis based on the aggregate offering price of the pre-funded warrants offered and sold in the offering (plus the aggregate
exercise price of the common stock issuable upon exercise of the pre-funded warrants), and as such the proposed aggregate
maximum offering price of the common stock and pre-funded warrants (including the common stock issuable upon exercise of the
pre-funded warrants), if any, is $ , or if the
underwriter’s option to purchase additional shares of common stock is exercised in full.
|
|
(4)
|
Pursuant
to Rule 416 under the Securities Act of 1933, as amended, the securities being registered hereunder include such indeterminate
number of additional securities as may be issuable to prevent dilution resulting from stock splits, dividends or similar transactions.
|
The
Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until
the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become
effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective
on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The
information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration
statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell
these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not
permitted.
PRELIMINARY
PROSPECTUS SUBJECT TO COMPLETION DATED JULY 2, 2019
Shares
of Common Stock
Pre-Funded
Warrants to Purchase Up to Shares
of Common Stock

This
is an offering of shares of our common stock,
$0.001 par value per share. We are also offering to certain purchasers whose purchase of shares of common stock in this
offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially
owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following
the consummation of this offering, the opportunity to purchase, if any such purchaser so chooses, pre-funded warrants, in
lieu of shares of common stock that would otherwise result in such purchaser’s beneficial ownership exceeding 4.99%
(or, at the election of the purchaser, 9.99%) of our outstanding common stock. Each pre-funded warrant is exercisable for one
share of our common stock.
The
purchase price of each pre-funded warrant is equal to the price at which a share of common stock is sold to the public in this
offering, minus $0.01, and the exercise price of each pre-funded warrant will be $0.01 per share. The pre-funded warrants are
immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full. For each
pre-funded warrant that we sell, the number of shares of common stock we are offering will be reduced on a one-for-one basis.
This offering also relates to the shares of common stock issuable upon exercise of the pre-funded warrants sold in this offering.
The shares of common stock and pre-funded warrants can only be purchased together in this offering but will be issued separately
and will be immediately separable upon issuance.
Our
common stock is listed on The Nasdaq Global Select Market under the symbol “ADXS.” The last reported sale price of
our common stock on The Nasdaq Global Select Market on July 1, 2019 was $2.25 per share. There is no established public trading
market for the pre-funded warrants, and we do not expect a market to develop. In addition, we do not intend to apply for a listing
of the pre-funded warrants on any national securities exchange or other nationally recognized trading system.
The
final public offering price will be determined through negotiation between us and the underwriters in the offering, and the recent
market price used throughout this prospectus may not be indicative of the final public offering price.
Investing
in our securities involves a high degree of risk. See “Risk Factors“ beginning on page 11 for a discussion of information
that should be considered in connection with an investment in our securities.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or
passed on the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
|
|
|
Per Share
|
|
|
Per Pre-Funded Warrant
|
|
|
Total
|
|
|
Public offering price
(1)
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
|
Underwriting discounts and commissions
(2)
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
|
Proceeds to us, before expenses
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
|
(1)
|
The
public offering price is $ per share of common
stock and $ per pre-funded warrant.
|
|
(2)
|
We
have agreed to reimburse the underwriters for certain expenses. See “Underwriting” beginning on page 32
of this prospectus for a description of the compensation payable to the underwriters.
|
We
have granted a 30-day option to the representative of the underwriters to purchase up to additional shares of common stock from
us solely to cover over-allotments, if any.
The
underwriters expect to deliver the shares to purchasers in the offering on , 2019.
A.G.P.
Prospectus
dated , 2019
TABLE
OF CONTENTS
We
are responsible for the information contained in this prospectus and in any free-writing prospectus we prepare or authorize. We
have not, and the underwriters have not, authorized anyone to provide you with different information, and we take no responsibility
for any other information others may give you. We are not, and the underwriters are not, making an offer to sell these securities
in any jurisdiction where the offer or sale is not permitted. You should not assume that the information contained in this prospectus
is accurate as of any date other than the date on the cover page of this prospectus. Our business, financial condition, results
of operations and prospects may have changed since that date.
Through
and including , 2019 (the 25th day after the date of this prospectus), all dealers effecting transactions in these
securities, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement
is in addition to the obligation of dealers to deliver a prospectus when acting as underwriters and with respect to their
unsold allotments or subscriptions.
For
investors outside the United States: We have not, and the underwriters have not, done anything that would permit this offering
or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in
the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about,
and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside
the United States.
This
prospectus and the documents incorporated by reference contain references to our trademarks and to trademarks belonging to other
entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other
visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that
we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks
and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship
with, or endorsement or sponsorship of us by, any other companies.
PROSPECTUS
SUMMARY
This
summary highlights information contained elsewhere or incorporated by reference in this prospectus and does not contain all of
the information that you should consider in making your investment decision. Before investing in our common stock or pre-funded
warrants, you should read the entire prospectus carefully, including the section entitled “Risk Factors” and the information
in our filings with the U.S. Securities and Exchange Commission, or the SEC, incorporated by reference in this prospectus. Unless
the context otherwise requires, we use the terms “Advaxis,” “the Company,” “we,” “us,”
“our” and similar designations in this prospectus to refer to Advaxis, Inc. and its wholly owned subsidiaries.
Overview
Advaxis,
Inc. (“Advaxis” or the “Company”) is a clinical-stage biotechnology company focused on the discovery,
development and commercialization of proprietary
Listeria monocytogenes
(“
Lm
”) based antigen delivery
products. We are using our
Lm
platform directed against tumor-specific targets in order to engage the patient’s immune
system to destroy tumor cells. Through a license from the University of Pennsylvania, we have exclusive access to this proprietary
formulation of attenuated
Lm
called
Lm
Technology
TM
. Our proprietary approach is designed to deploy a
unique mechanism of action that redirects the immune system to attack cancer in three distinct ways:
|
|
●
|
Alerting
and training the immune system by activating multiple pathways in Antigen-Presenting Cells (“APCs”) with the equivalent
of multiple adjuvants;
|
|
|
|
|
|
|
●
|
Attacking
the tumor by generating a strong, cancer-specific T cell response; and
|
|
|
|
|
|
|
●
|
Breaking
down tumor protection through suppression of the protective cells in the tumor microenvironment (“TME”) that shields
the tumor from the immune system. This enables the activated T cells to begin working to attack the tumor cells.
|
Our
proprietary
Lm
platform technology has demonstrated clinical activity in several of its programs and has been dosed in
over 470 patients across multiple clinical trials and in various tumor types. We believe that
Lm
Technology immunotherapies
can complement and address significant unmet needs in the current oncology treatment landscape. Specifically, our product candidates
have the potential to work synergistically with other immunotherapies, including checkpoint inhibitors, while having a generally
well-tolerated safety profile.
The
Advaxis Corporate Strategy
Our
strategy is to advance the
Lm
Technology platform and leverage its unique capabilities to design and develop an array of
cancer treatments. We are currently conducting or planning clinical studies of
Lm
Technology immunotherapies in, non-small
cell lung cancer, melanoma, bladder, head and neck, and prostate cancers. We are working with, or are in the process of identifying,
collaborators for many of these programs.
Moving
forward, we expect that we will continue to invest in our core clinical program areas and will also remain opportunistic in evaluating
Investigator Sponsored Trials (“ISTs”) as well as licensing opportunities. The
Lm
Technology platform is protected
by a range of patents, covering both product and process, some of which we believe can be maintained into 2039.
Clinical
Pipeline

Personalized
Neoantigen-Directed Therapies (ADXS-NEO)
ADXS-NEO
is an individualized
Lm
Technology antigen delivery product developed using whole-exome sequencing of a patient’s
tumor to identify mutation specific neoantigens. ADXS-NEO is designed to work by presenting a large payload of neoantigens directly
into dendritic cells within the patient’s immune system and stimulating a T cell response against cancerous cells.
The U.S.
Food and Drug Administration (“FDA”) allowed the Investigational New Drug (“IND”)
application of ADXS-NEO and in June 2018 we announced the commencement of a Phase 1 trial with the dosing of the first
patient with ADXS-NEO. ADXS-NEO is being evaluated in an open-label, dose-escalation, multicenter clinical trial in the
United States. The study is open to patients with metastatic non-small cell lung cancer (NSCLC), metastatic microsatellite
stable colon cancer and metastatic squamous head and neck cancer. The study had been in development in collaboration with
Amgen until December 2018, when Amgen provided us with a notice of termination of their existing collaboration. During 2019,
we presented early immune response and clinical data from this study, which showed that two microsatellite stable (MSS)
colorectal cancer patients dosed with ADXS-NEO at 1x10
8
colony forming units (cfu) demonstrated increased
CD8+ T cell infiltration in the tumor microenvironment after three doses of ADXS-NEO. Both patients had metastatic colorectal
cancer, which is considered to be a “cold” tumor and typically exhibits little CD8+ T cell infiltration and
resistance to immunotherapy, yet both successfully transitioned from “cold” tumors into “hot” tumors
with ADXS-NEO therapy. An estimated 80-85% of colorectal cancer patients are MSS. Dosing of ADXS-NEO at 1x10
8
cfu
has been well-tolerated in two patients. ADXS-NEO dosed at 1x10
9
cfu was beyond the maximum tolerated dose (MTD)
leading to reversible Grade 3 hypoxia (n=2) and Grade 3 hypotension (n=1) and as a result were dose-limiting toxicities
(DLTs).
We
plan to continue to provide updates on this study during 2019, as data becomes available.
Disease
Focused Hotspot/Off-the-Shelf Neoantigen Therapies (ADXS-HOT)
We
have created a new group of immunotherapy constructs for major cancers that is designed to combine our optimized
Lm
Technology
vector with promising targets to generate potent anti-cancer immunity. The ADXS-HOT program is a series of novel cancer immunotherapies
that target somatic mutations (“hotspots”), cancer testis antigens (“CTAs”) and oncofetal antigens (“OFAs”).
These three types of targets form the basis of the ADXS-HOT program because they are designed to be more capable of generating
potent, tumor specific, and high strength killer T cells, versus more traditional over-expressed native sequence TAAs. Most hotspot
mutations and OFA/CTA proteins play critical roles in oncogenesis; targeting both at once could significantly impair cancer proliferation.
The ADXS-HOT products combine many of the potential high avidity targets that are expressed in all patients with the target disease
into one “off-the-shelf,” ready to administer treatment. The ADXS-HOT technology has a strong Intellectual Property
(“IP”) position, with potential protection into 2038, and an IP filing strategy providing for broad coverage opportunities
across multiple disease platforms and combination therapies.
In
July 2018, we announced that the FDA allowed our IND application for our ADXS-HOT drug candidate for non-small cell
lung cancer (NSCLC). In February 2019, we announced that the first patient has been enrolled into the study. We anticipate a preliminary
readout of safety, tolerability and immune correlative data during 2019.
HPV-Related
Cancers
Cervical
Cancer: Axalimogene Filolisbac
In
June 2019, we announced the closing of our AIM2CERV Phase 3 clinical trial with axalimogene filolisbac (AXAL) in high-risk locally
advanced cervical cancer. We intend to continue to support the clinical development of AXAL, our single-antigen construct, in
other HPV-related cancers including an anticipated Investigator Sponsor Trial (IST) in head and neck cancer to be initiated near
the end of 2019. We estimated that the remaining cost to complete the AIM2CERV trial ranged from $80 million to $90 million, and
initial efficacy data was not anticipated for at least three years. Therefore, results from the clinical trial were not the basis
for the decision to close the study, nor was safety as the trial recently underwent its third Independent Data Monitoring Committee
(IDMC) review with no safety issues noted. We plan to unblind the AIM2CERV clinical data generated to date and anticipates submitting
these data for publication. In addition, we will continue to pursue monetization opportunities for AXAL.
Head
and Neck Cancer
Squamous
Cell Carcinoma of the Head and Neck (“SCCHN”) is the most frequently occurring malignant tumor of the head and neck
and is a major cause of morbidity and mortality worldwide. More than 90% of SCCHNs originate from the mucosal linings of the oral
cavity, pharynx, or larynx and 70% of these cancers are caused by HPV. According to the Surveillance, Epidemiology, and End Results
(“SEER”) database head and neck cancer accounts for about 3% of all cancers in the United States. But while the Pap
smear and other HPV tests have reduced rates of cervical cancer, rates of oral cavity and pharynx cancer are growing, with 53,000
new cases projected to be diagnosed in the United Stated in 2019 according to the SEER database.
A
study published in the Annals of Internal Medicine found that approximately 12% of U.S. men and 3% of women were actively infected
with oral HPV between 2011 and 2014. That totals 11 million men and 3 million women who are at risk for developing SCCHN. SCCHN
is typically asymptomatic until it has metastasized, and screening options do not exist. The only way to prevent infection is
the HPV vaccine, but compliance has been low to date. Another challenge is that preventative vaccines cannot protect those already
infected or older than age 26, leaving several generations of Americans vulnerable to SCCHN with no way of knowing if cancer is
silently growing.
We
conducted a clinical trial in collaboration with MedImmune on a Phase 1/2, open-label, multicenter, two part trial to evaluate
safety and efficacy of axalimogene filolisbac, in combination with durvalumab (MEDI4736), for patients with metastatic squamous
or non-squamous carcinoma of the cervix and metastatic HPV-associated SCCHN. Part 1 of this trial is complete and the Company
and MedImmune have decided to not continue further enrollment into the expansion phases of this study.
We
plan to initiate an investigator-sponsored trial with a major research center in head and neck cancer by the end of 2019 or in
the first half of 2020. Axalimogene filolisbac has received FDA orphan drug designation for HPV-associated head and neck cancer.
Prostate
Cancer (ADXS-PSA)
According
to the American Cancer Society, prostate cancer is the second most common type of cancer found in American men and is the second
leading cause of cancer death in men, behind only lung cancer. According to the SEER database, more than 174,000 men are estimated
to be diagnosed with prostate cancer in 2019, with approximately 32,000 deaths each year. Unfortunately, in about 10 – 20%
of cases, men with prostate cancer will go on to develop castration-resistant prostate cancer (“CRPC”), which refers
to prostate cancer that progresses despite androgen deprivation therapy. Metastatic CRPC (“mCRPC”) occurs when the
cancer spreads to other parts of the body and there is a rising prostate-specific antigen (“PSA”) level. This stage
of prostate cancer has an average survival of 9-13 months, is associated with deterioration in quality of life, and has few therapeutic
options available.
According
to a data review published by MD Anderson Cancer Center in 2016, checkpoint inhibitor monotherapy has not shown significant activity
in mCRPC to date. The authors hypothesize that may be due to the inability of the checkpoint inhibitor to infiltrate the tumor
microenvironment, and that combination therapy with agents that induce T cell infiltration within the tumor may improve performance
of checkpoints in prostate cancer. Data from the Keynote-199 trial in bone predominant-mCRCP patients treated with KEYTRUDA
®
(“pembrolizumab”) was presented at the 2018 ASCO Annual Meeting. In this trial, only 4 out of 60 patients (7%)
had decrease PSA post-baseline, with only one case that was ≥50%. The total SD/disease stabilization rate was 37%.
Lm
Technology constructs have been shown by multiple labs to reduce number and suppressive function of Tregs and MDSCs in the
tumor microenvironment and cause the destruction of Tregs in the TME as soon as five days after dosing in models. This reduction
of immune suppression in the tumors has been attributed to our proprietary
tLLO
-fusion peptides expressed by multiple
copies of the plasmids in each bacteria. We feel that the combination of ADXS-PSA, our immunotherapy designed to target the PSA
antigen, with a checkpoint inhibitor may provide an alternative treatment option for patients with mCRPC. Clinical benefit in
prostate cancer could be a significant value creator to expand the
Lm
Technology platform into the prostate cancer market.
We
have entered into a clinical trial collaboration and supply agreement with Merck to evaluate the safety and efficacy of ADXS-PSA
as monotherapy and in combination with KEYTRUDA
®
(“pembrolizumab”), Merck’s anti PD-1 antibody,
in a Phase 1/2, open-label, multicenter, dose determination and expansion trial in patients with previously treated metastatic,
castration-resistant prostate cancer (Keynote-046). ADXS-PSA was tested alone or in combination with KEYTRUDA
®
in an advanced and heavily pretreated patient population who had progressed on androgen deprivation therapy. A total of 13 and
37 patients were evaluated on monotherapy and combination therapy, respectively. For the ADXS-PSA monotherapy dose escalation
and determination portion of the trial, cohorts were started at a dose of 1x10
9
cfu (n=7) and successfully escalated
to higher dose levels of 5x10
9
cfu (n=3) and 1x10
10
cfu (n=3) without achieving a maximum tolerated dose.
Treatment emergent adverse events noted at these higher dose levels were generally consistent with those observed at the lower
dose level (1x10
9
cfu) other than a higher occurrence rate of Grade 2/3 hypotension. The ADXS-PSA monotherapy dose-determination
phase of the trial has been completed. The Recommended Phase II Dose (RP2D) of ADXS-PSA monotherapy was determined to be 1x 10
9
cfu based on a review of the totality of the clinical data. This dose was used in combination with 200mg of pembrolizumab
in a cohort of six patients to evaluate the safety of the combination before moving into an expanded cohort of patients. The safety
of the combination was confirmed and enrollment in the expansion cohort phase was initiated. Enrollment in this phase of the trial
(n=37) was completed in January 2017.
Data
from this study in mCRPC patients treated with ADXS-PSA monotherapy (Part A) and in combination with pembrolizumab (Part
B) were presented at the American Society of Clinical Oncology (ASCO) Annual Meeting in June 2018 and updated survival data presented
at the American Association of Cancer Research (AACR) in March 2019. At entry, Part A and Part B patients were similar in age
(~70 yrs), Gleason score (~8.3), absence of visceral metastases (71% vs. 70%) and prior abiraterone use. Part B patients had higher
median baseline PSA values (40.6 vs. 20.8 ng/ml), and more prior enzalutamide (53% vs. 26%) and chemotherapy (49% vs. 36%) use
versus Part A patients. A total of 49 patients (98%) experienced treatment-related adverse events (TRAE), mainly chills, fever,
nausea and hypotension. Five Part A and 13 Part B patients had grade 3-4 events: fatigue, hypotension, hypertension, anemia. Treatment-related
adverse events (TRAEs) were mostly mild or moderate constitutional symptoms such as fever, chills, rigors, hypotension, nausea
and fatigue, consistent with immune activation and manageable with standard care. One patient in the monotherapy arm was discontinued
from the study due to a grade 4 TRAE related to cytokine release, which resolved within 24 hours using medical management. Overall,
two Part A (14%) v 16 Part B patients (43%) had a decreased PSA post-baseline. Of these, seven Part B (22%) versus 0 Part A patients
achieved a PSA reduction ≥50% from baseline. Part B patients had higher rates (56.8%) of stable disease/disease stabilization
than Part A patients (38.5%). Part B patients had higher rates (27%) of stable disease than monotherapy patients (7.7%). Median
overall survival (mOS) was 21.1 months at data cutoff in Part B patients and 36 of the 37 patients were microsatellite instability-high
(MSI-high) negative which often do not respond to checkpoint inhibitors. In this population of heavily pretreated mCRPC patients,
ADXS-PSA + pembrolizumab had a manageable safety profile (mostly grade 1-2 TRAEs) and showed a greater level of activity compared
to monotherapy.
Other
Lm Technology Products
HER2
Expressing Solid Tumors
HER2
is overexpressed in a percentage of solid tumors including osteosarcoma. According to published literature, up to 60% of osteosarcomas
are HER2 positive, and this overexpression is associated with poor outcomes for patients. ADXS-HER2 is an
Lm
Technology
antigen delivery product candidate designed to target HER2 expressing solid tumors including human and canine osteosarcoma. ADXS-HER2
has received FDA and EMA orphan drug designation for osteosarcoma and has received Fast Track designation from the FDA for patients
with newly-diagnosed, non-metastatic, surgically-resectable osteosarcoma.
In
September 2018, we announced that we had granted a license to OS Therapies, LLC (“OS Therapies”) for the use of ADXS31-164,
also known as ADXS-HER2, for evaluation in the treatment of osteosarcoma in humans. Under the terms of the license agreement,
OS Therapies, will be responsible for the conduct and funding of a clinical study evaluating ADXS-HER2 in recurrent, completely
resected osteosarcoma. Pursuant to the agreement, we are to receive an upfront payment, reimbursement for product supply and other
support, clinical, regulatory, and sales-based milestone payments, and royalties on future product sales. Additional details of
the financial terms have not been disclosed.
Canine
Osteosarcoma
On
March 19, 2014, we entered into a definitive Exclusive License Agreement (the “Aratana Agreement”) with Aratana Therapeutics,
Inc. (“Aratana”), where we granted Aratana an exclusive, worldwide, royalty-bearing license, with the right to sublicense,
certain of our proprietary technology that enables Aratana to develop and commercialize animal health products that will be targeted
for treatment of osteosarcoma and other cancer indications in animals. A product license request was filed by Aratana for ADXS-HER2
(also known as AT-014 by Aratana) for the treatment of canine osteosarcoma with the United States Department of Agriculture (“USDA”).
Aratana received communication in December 2017 that the USDA granted Aratana conditional licensure for AT-014 for the treatment
of dogs diagnosed with osteosarcoma, one year of age or older. Aratana is currently conducting an extended field study which is
a requirement for full USDA licensure.
Under
the terms of the Aratana Agreement, Aratana paid an upfront payment to us in the amount of $1,000,000 upon signing of the Aratana
Agreement. Aratana will also pay us: (a) up to $36.5 million based on the achievement of milestone relating to the advancement
of products through the approval process with the USDA in the United States and the relevant regulatory authorities in the European
Union (“E.U.”) in all four therapeutic areas and up to an additional $15 million in cumulative sales milestones based
on achievement of gross sales revenue targets for sales of any and all products for use in non-human animal health applications
(the “Aratana Field”) (regardless of therapeutic area), and (b) tiered royalties starting at 5% and going up to 10%,
which will be paid based on net sales of any and all products (regardless of therapeutic area) in the Aratana Field in the United
States. Royalties for sales of products outside of the United States will be paid at a rate equal to half of the royalty rate
payable by Aratana on net sales of products in the United States (starting at 2.5% and going up to 5%). Royalties will be payable
on a product-by-product and country-by-country basis from first commercial sale of a product in a country until the later of (a)
the 10th anniversary of first commercial sale of such product by Aratana, its affiliates or sub licensees in such country or (b)
the expiration of the last-to-expire valid claim of our patents or joint patents claiming or covering the composition of matter,
formulation or method of use of such product in such country. Aratana will also pay us 50% of all sublicense royalties received
by Aratana and its affiliates. In fiscal year 2018, we received approximately $3,000 in royalty revenue from Aratana.
Risks
Affecting Us
Our
business is subject to a number of risks and uncertainties, including those highlighted in the section titled “Risk Factors”
immediately following this prospectus summary and in our Annual Report on Form 10-K for the year ended October 31, 2018, which
is incorporated by reference in this prospectus. These risks and uncertainties include, but are not limited to, the following:
|
|
●
|
We
are a clinical-stage company.
|
|
|
●
|
The
successful development of immunotherapies is highly uncertain.
|
|
|
●
|
If
we are unable to establish, manage or maintain strategic collaborations in the future, our revenue and drug development may
be limited.
|
|
|
●
|
The
biotechnology and immunotherapy industries are characterized by rapid technological developments and a high degree of competition.
We may be unable to compete with more substantial enterprises.
|
|
|
●
|
Drug
discovery and development is a complex, time-consuming and expensive process that is fraught with risk and a high rate of
failure.
|
|
|
●
|
We
may be required to suspend or discontinue clinical trials for a number of reasons, which could delay or preclude approval
of any of our product candidates.
|
|
|
●
|
We
can provide no assurance that our clinical product candidates will obtain regulatory approval or that the results of clinical
studies will be favorable.
|
|
|
●
|
We
rely upon patents to protect our technology. We may be unable to protect our intellectual property rights and we may be liable
for infringing the intellectual property rights of others.
|
|
|
●
|
We
have incurred significant losses since our inception and anticipate that we will continue to incur losses for the foreseeable
future.
|
|
|
●
|
We
will require additional capital to fund our operations and if we fail to obtain necessary financing we will not be able to
complete the development and commercialization of our product candidates.
|
|
|
●
|
Our
auditor’s report includes a going concern paragraph.
|
|
|
●
|
The
price of our common stock and warrants may be volatile.
|
Recent
Developments
Resignation
of Robert Petit
On
May 13, 2019,
Robert Petit, Ph.D., our Executive Vice President and Chief Scientific Officer,
announced that he is stepping down as our Chief Scientific Officer effective as of the close of business on June 3, 2019. Dr.
Petit will continue to support us as an advisor and consultant as the new Chair of the Advaxis Scientific Advisory Board.
FDA
Clinical Hold Release
On
May 15, 2019, we announced that the United States Food and Drug Administration (“FDA”) has lifted the partial clinical
hold on AIM2CERV, (AXAL) for the treatment of patients with high-risk locally advanced cervical cancer. In its letter, the FDA
acknowledged that we satisfactorily addressed all hold questions.
Closing
of AIM2CERV
On
June 27, 2019, we announced the closing of our AIM2CERV Phase 3 clinical trial with axalimogene filolisbac (AXAL) in high-risk
locally advanced cervical cancer. We intend to continue to support the clinical development of AXAL, our single-antigen construct,
in other HPV-related cancers including an anticipated Investigator Sponsor Trial (IST) in head and neck cancer to be initiated
by the end of 2019 or in the first half of 2020. We plan to increase our focus on our neoantigen programs, specifically, ADXS-NEO
and ADXS-HOT.
Corporate
Information
We
were originally incorporated in the State of Colorado on June 5, 1987 under the name Great Expectations, Inc. We were a publicly-traded
“shell” company without any business until November 12, 2004 when we acquired Advaxis, Inc., a Delaware corporation,
through a Share Exchange and Reorganization Agreement, dated as of August 25, 2004, which we refer to as the Share Exchange, by
and among Advaxis, the stockholders of Advaxis and us. As a result of the Share Exchange, Advaxis became our wholly owned subsidiary
and our sole operating company. On December 23, 2004, we amended and restated our articles of incorporation and changed our name
to Advaxis, Inc. On June 6, 2006, our stockholders approved the reincorporation of our company from Colorado to Delaware by merging
the Colorado entity into our wholly owned Delaware subsidiary. Our date of inception, for financial statement purposes, is March
1, 2002 and we were uplisted to Nasdaq in 2013.
Our
principal executive offices are located at 305 College Road East, Princeton, New Jersey 08540 and our telephone number is (609)
452-9813. We maintain a corporate website at www.advaxis.com which contains descriptions of our technology, our /product candidates
and the development status of each drug. We are not including the information on our website as a part of, nor incorporating it
by reference into, this prospectus. For further information regarding us and our financial information, you should refer to our
recent filings with the SEC. See “Where You Can Find More Information” and “Incorporation of Certain Information
by Reference.”
THE
OFFERING
|
Common
stock offered by us
|
|
shares.
|
|
|
|
|
|
Pre-funded
warrants offered by us
|
|
We
are also offering to certain purchasers whose purchase of shares of common stock in this offering would otherwise result in
the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election
of the purchaser, 9.99%) of our outstanding common stock immediately following the closing of this offering, the opportunity
to purchase, if such purchasers so choose, pre-funded warrants to purchase shares of common stock, in lieu of shares of common
stock that would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election
of the purchaser, 9.99%) of our outstanding common stock. Each pre-funded warrant is exercisable for one share of our common
stock. The purchase price of each pre-funded warrant is equal to the price at which a share of common stock is being sold
to the public in this offering, minus $0.01, and the exercise price of each pre-funded warrant is $0.01 per share. The pre-funded
warrants are exercisable immediately and may be exercised at any time until all of the pre-funded warrants are exercised in
full. The pre-funded warrants also provide that in the event of a fundamental transaction we are required to cause any successor
entity to assume our obligations under the pre-funded warrants. In addition, the holder of the pre-funded warrant will be
entitled to receive upon exercise of the pre-funded warrant the kind and amount of securities, cash or property that the holder
would have received had the holder exercised the pre-funded warrant immediately prior to such fundamental transaction. This
offering also relates to the shares of common stock issuable upon exercise of any pre-funded warrants sold in this offering.
For each pre-funded warrant that we sell, the number of shares of common stock that we are offering will be reduced on a one-for-one
basis.
|
|
Common
stock to be outstanding immediately after this offering
|
|
shares (or shares if the underwriter exercises its option to purchase additional shares in full),
in each case assuming no exercise of the pre-funded warrants issued in this offering.
|
|
Option
to purchase additional shares
|
|
We
have granted a 30-day option to the representative of the underwriters to purchase up to an aggregate of additional shares
of common stock.
|
|
Use
of proceeds
|
|
We
estimate that our net proceeds from this offering will be approximately $ million (or $ million if the underwriters exercise
their option to purchase additional shares in full), based upon an assumed public offering price of $ per share, the last
reported sale price of our common stock on The Nasdaq Global Select Market on , 2019,
and
assuming no sale of the pre-funded warrants
, after deducting estimated underwriting
discounts and commissions and estimated offering expenses payable by us
.
|
|
|
|
We
intend to use the net proceeds from this offering, together with our existing cash, cash equivalents and investments, to fund
our continued research and development initiatives in connection with our product pipeline including, but not limited to (i)
investment in our ADXS- HOT program in both monotherapy and combination therapy and new cancer types; (ii) investment in ongoing
clinical research in ADXS-PSA and ADXS-NEO, in combination therapy; and (iii) general corporate purposes. See “Use of
Proceeds” for additional information.
|
|
Risk
factors
|
|
See
“Risk Factors” beginning on page 11 of this prospectus and the other information included in, or incorporated
by reference into, this prospectus for a discussion of factors you should carefully consider before deciding to invest in
our common stock and pre-funded warrants.
|
|
Nasdaq
Global Select Market symbol
|
|
“ADXS.”
There is no established trading market for the pre-funded warrants and we do not expect a market to develop. In addition,
we do not intend to apply for the listing of the pre-funded warrants on any national securities exchange or other trading
market. Without an active trading market, the liquidity of the pre-funded warrants will be limited.
|
The
number of shares of our common stock to be outstanding after this offering is based on 8,020,370 shares of our common stock outstanding
as of April 30, 2019, and excludes:
|
|
●
|
400,260
shares of common stock issuable upon the exercise of stock options outstanding as of April 30, 2019 at a weighted-average
exercise price of $101.82 per share;
|
|
|
|
|
|
|
●
|
17,063
shares of common stock reserved for issuance upon settlement of restricted stock units;
|
|
|
|
|
|
|
●
|
72,304
shares of common stock issuable upon the exercise of warrants outstanding as of April 30, 2019 at a weighted-average exercise
price of $3.82 per share; and
|
|
|
|
|
|
|
●
|
188,658
shares of common stock reserved for the future awards under our 2015 Incentive Plan.
|
Unless
otherwise indicated, all information in this prospectus reflects or assumes the following:
|
|
●
|
No
exercise or forfeiture of the outstanding options or remaining warrants or settlement of restricted stock units after April
30, 2019;
|
|
|
|
|
|
|
●
|
N
o
sale of any pre-funded warrants in this offering; and
|
|
|
|
|
|
|
●
|
No
exercise by the underwriters of their option to purchase up to an additional
shares of common stock in this
offering.
|
SUMMARY
CONSOLIDATED FINANCIAL DATA
You
should read the following summary consolidated financial data together with the section titled “Management’s Discussion
and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes
included in our Annual Report on Form 10-K for the year ended October 31, 2018, which is incorporated by reference in this prospectus.
We have derived the consolidated statement of operations data for the years ended October 31, 2018 and 2017 from our audited consolidated
financial statements included in our Annual Report on Form 10-K for the year ended October 31, 2018, which is incorporated by
reference in this prospectus. The consolidated statements of operations data for the six months ended April 30, 2018 and 2019
and the consolidated balance sheet data as of April 30, 2019 have been derived from our unaudited consolidated financial statements
included elsewhere in this prospectus and have been prepared on the same basis as the audited consolidated financial statements.
In the opinion of management, the unaudited data reflects all adjustments, consisting only of normal recurring adjustments, necessary
for a fair statement of the financial information contained in those statements. Our historical results are not necessarily indicative
of the results that may be expected in the future, and interim results are not necessarily indicative of results to be expected
for the full year or any other period.
ADVAXIS,
INC.
STATEMENTS
OF OPERATIONS
(In
thousands, except share and per share data)
|
|
|
Year Ended
October 31,
|
|
|
Six Months Ended
April 30,
|
|
|
|
|
2018
|
|
|
2017
|
|
|
2019
|
|
|
2018
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
$
|
6,063
|
|
|
$
|
12,031
|
|
|
$
|
20,877
|
|
|
$
|
3,803
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses
|
|
|
56,970
|
|
|
|
70,508
|
|
|
|
12,675
|
|
|
|
27,119
|
|
|
General and administrative expenses
|
|
|
19,472
|
|
|
|
39,969
|
|
|
|
5,759
|
|
|
|
10,785
|
|
|
Total operating expenses
|
|
|
76,442
|
|
|
|
110,477
|
|
|
|
18,434
|
|
|
|
37,904
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Loss) income from operations
|
|
|
(70,379
|
)
|
|
|
(98,446
|
)
|
|
|
2,443
|
|
|
|
(34,101
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income, net
|
|
|
577
|
|
|
|
670
|
|
|
|
259
|
|
|
|
291
|
|
|
Net changes in fair value of derivative liabilities
|
|
|
3,400
|
|
|
|
20
|
|
|
|
2,395
|
|
|
|
-
|
|
|
Loss on shares issued in settlement of warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,607
|
)
|
|
|
-
|
|
|
Other expense
|
|
|
(63
|
)
|
|
|
(82
|
)
|
|
|
(6
|
)
|
|
|
(40
|
)
|
|
Net (loss) income before benefit for income taxes
|
|
|
(66,465
|
)
|
|
|
(97,838
|
)
|
|
|
3,484
|
|
|
|
(33,850
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense (benefit)
|
|
|
50
|
|
|
|
(4,403
|
)
|
|
|
50
|
|
|
|
50
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income
|
|
$
|
(66,515
|
)
|
|
|
(93,435
|
)
|
|
$
|
3,434
|
|
|
$
|
(33,900
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income per common share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
(19.36
|
)
|
|
$
|
(34.58
|
)
|
|
$
|
0.65
|
|
|
$
|
(11.16
|
)
|
|
Diluted
|
|
$
|
(19.36
|
)
|
|
$
|
(34.58
|
)
|
|
$
|
0.20
|
|
|
$
|
(11.16
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of common shares outstanding
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
3,434,824
|
|
|
|
2,701,856
|
|
|
|
5,259,677
|
|
|
|
3,038,439
|
|
|
Diluted
|
|
|
3,434,824
|
|
|
|
2,701,856
|
|
|
|
5,282,772
|
|
|
|
3,038,439
|
|
The
accompanying notes should be read in conjunction with the financial statements.
|
|
|
As of April 30, 2019
|
|
|
|
|
Actual
|
|
|
As Adjusted
(2)(3)
|
|
|
|
|
(in thousands)
|
|
|
Consolidated Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
33,706
|
|
|
$
|
|
|
|
Working capital
(1)
|
|
|
32,965
|
|
|
|
|
|
|
Total assets
|
|
|
49,771
|
|
|
|
|
|
|
Loan payable, net of current portion and discount
|
|
|
-
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
43,170
|
|
|
|
|
|
|
(1)
|
We
define working capital as current assets less current liabilities. See our consolidated financial statements incorporated
by reference into this prospectus for further details regarding our current assets and current liabilities.
|
|
(2)
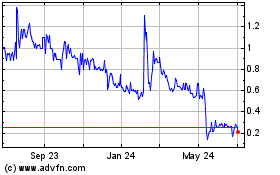
|
The
as adjusted data reflects the sale by us of shares of our
common stock in this offering at an assumed public offering price of $ per
share, the last reported sale price of our common stock on The Nasdaq Global Select Market on ,
2019, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
|
|
(3)
|
A
$0.50 increase (decrease) in the assumed public offering price of $
per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on ,
2019, would increase (decrease) the as adjusted amount of each of cash and cash equivalents, working capital, total assets
and total stockholders’ equity by $ million, assuming that
the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting
estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase (decrease) of
500,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease)
the as adjusted amount of each of cash and cash equivalents, working capital, total assets and total stockholders’ equity
by $ million, assuming no change in the assumed public
offering price per share and after deducting estimated underwriting discounts and commissions and estimated offering expenses
payable by us. The as adjusted information discussed above is illustrative only and will be adjusted based on the actual public
offering price and other terms of this offering determined at pricing.
|
RISK
FACTORS
Investing
in our common stock or pre-funded warrants involves a high degree of risk. You should carefully consider the risks and uncertainties
described below together with all of the other information contained in this prospectus, or incorporated by reference, including
our financial statements and the related notes and the risks and uncertainties discussed under “Risk Factors” in our
Annual Report on Form 10-K for the year ended October 31, 2018 and our Quarterly Report on Form 10-Q for the quarter ended April
30, 2019, which are incorporated by reference herein in their entirety, before deciding to invest in our common stock or pre-funded
warrants. If any of these risks actually occur, our business, prospects, operating results and financial condition could suffer
materially. In such event, the trading price of our common stock and value of the pre-funded warrants could decline and you might
lose all or part of your investment. The risks and uncertainties described below are not the only ones we face. Additional risks
and uncertainties not presently known to us or that we currently believe to be immaterial may also adversely affect our business.
Certain statements below are forward-looking statements. See “Cautionary Note Regarding Forward-Looking Statements”
in this prospectus.
Risks
Related to the Development and Regulatory Approval of Our Product Candidates
We
may elect or be required to suspend or discontinue clinical trials for a number of reasons, which could preclude approval of any
of our product candidates.
Our
clinical trials may be suspended at any time for a number of reasons. A clinical trial may be suspended or terminated by us,
an Institutional Review Board, the FDA or other regulatory authorities due to a failure to conduct the clinical trial
in accordance with regulatory requirements or our clinical protocols, presentation or identification of unforeseen safety
signals or issues, failure to demonstrate a benefit from using the investigational drug, changes in governmental regulations
or administrative actions, lack of adequate funding to continue the clinical trial, or for other business-related reasons,
including a decision by us to allocate our resources to our other existing or potential development programs. For example, in
June 2019, we announced that we are increasing our focus on neoantigen-directed immunotherapies and are terminating our
AIM2CERV Phase 3 clinical trial with AXAL in high-risk locally advanced cervical cancer. While we intend to continue to
support the clinical development of AXAL in HPV-related cancers and expect to continue to seek monetization opportunities for
AXAL, the full effects of our termination of the AIM2CERV trial cannot yet be predicted and our AXAL program as well as our
business in general may be materially impacted by the termination of AIM2CERV. As a result of the decision to terminate
our AIM2CERV Phase 3 clinical trial, we expect to record a writedown of property and equipment and intangible assets of
approximately $500,000 for the period ended July 31, 2019.
In
addition, clinical trials for our product candidates could be suspended due to adverse side effects. Drug-related side effects
could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability
claims. We may also voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable
risk to patients or do not demonstrate clinical benefit. If we elect or are forced to suspend or terminate any clinical trial
of any product candidates that we develop, the commercial prospects of such product candidates will be harmed and our ability
to generate product revenues from any of these product candidates will be delayed or eliminated. In addition, to the extent that
we suspend or discontinue a clinical trial for a program on which we are pursuing with one or more collaborators, such suspension
or discontinuation may damage our relationships with our collaborators or increase the challenges of entering into collaborations
in the future. Any of these occurrences may significantly harm our business, financial condition, results of operations and prospects.
Risks
Related to this Offering and Ownership of our Common Stock and Pre-Funded Warrants
Our
stock price can be volatile, which increases the risk of litigation, and may result in a significant decline in the value of your
investment.
The
trading price of our common stock is likely to be highly volatile and subject to wide fluctuations in price in response to various
factors, many of which are beyond our control and may not be related to our operating performance. These fluctuations could cause
you to lose part or all of your investment in our common stock. These factors include, but are not limited to, the following:
|
|
●
|
price
and volume fluctuations in the overall stock market from time to time;
|
|
|
●
|
changes
in the market valuations, stock market prices and trading volumes of similar companies;
|
|
|
●
|
actual
or anticipated changes in our net loss or fluctuations in our operating results or in the expectations of securities analysts;
|
|
|
●
|
the
issuance of new equity securities pursuant to a future offering, including potential issuances of preferred stock;
|
|
|
●
|
general
economic conditions and trends;
|
|
|
●
|
positive
and negative events relating to healthcare and the overall pharmaceutical and biotech sector;
|
|
|
●
|
major
catastrophic events;
|
|
|
●
|
sales
of large blocks of our stock;
|
|
|
●
|
additions
or departures of key personnel;
|
|
|
●
|
changes
in the regulatory status of our immunotherapies, including results of our pre-clinical and clinical trials;
|
|
|
●
|
positive
and negative changes in relationships with partners;
|
|
|
●
|
events
affecting the University of Pennsylvania or any of our other current or future collaborators;
|
|
|
●
|
announcements
of new products or technologies, commercial relationships or other events by us or our competitors;
|
|
|
●
|
regulatory
developments in the United States and other countries;
|
|
|
●
|
failure
of our common stock or warrants to be listed or quoted on the Nasdaq Stock Market, NYSE Amex Equities or other national market
system;
|
|
|
●
|
changes
in accounting principles; and
|
|
|
●
|
discussion
of us or our stock price by the financial and scientific press and in online investor communities.
|
In
addition, equity markets in general, and the market for biotechnology and life sciences companies in particular, have experienced
extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of companies
traded in those markets. These broad market and industry factors may materially affect the market price of our common stock, regardless
of our development and operating performance. In the past, following periods of volatility in the market price of a company’s
securities, securities class-action litigation has often been instituted against that company. Due to the volatility of our stock
price, we have been and may be the target of securities litigation in the future. Securities litigation could result in substantial
costs and divert management’s in the future attention and resources from our business.
Future
sales or other issuances of our common stock could depress the market for our common stock.
Sales
of a substantial number of shares of our common stock, or the perception by the market that those sales could occur, could cause
the market price of our common stock to decline or could make it more difficult for us to raise funds through the sale of equity
in the future.
In
connection with this offering, we and our directors and executive officers have entered into lock-up agreements for a period of
90 days, respectively, following this offering (which period may be extended under certain circumstances). We and our directors
and executive officers may be released from lock-up prior to the expiration of the lock-up period at the sole discretion of A.G.P./Alliance
Global Partners (See “Underwriting” beginning on page 32 of this prospectus). Upon expiration or earlier release
of the lock-up, we and our directors and executive officers may sell shares into the market, which could adversely affect the
market price of shares of our common stock.
Future
issuances of common stock or other equity securities could further depress the market for our common stock. We expect to continue
to incur drug development and selling, general and administrative costs, and to satisfy our funding requirements, we will need
to sell additional equity securities, which may be subject to registration rights and warrants with anti-dilutive protective provisions.
The sale or the proposed sale of substantial amounts of our common stock or other equity securities in the public markets may
adversely affect the market price of our common stock and our stock price may decline substantially. Our stockholders may experience
substantial dilution and a reduction in the price that they are able to obtain upon sale of their shares. New equity securities
issued may have greater rights, preferences or privileges than our existing common stock. In addition, we have a significant number
of shares of restricted stock, restricted stock units, stock options and warrants outstanding. To the extent that outstanding
stock options or warrants have been or may be exercised or other shares issued, investors purchasing our common stock in this
offering may experience further dilution.
If
we make one or more significant acquisitions in which the consideration includes stock or other securities, our stockholders’
holdings may be significantly diluted. In addition, stockholders’ holdings may also be diluted if we enter into arrangements
with third parties permitting us to issue shares of common stock in lieu of certain cash payments upon the achievement of milestones.
There
is no public market for the pre-funded warrants being offered in this offering.
There
is no established public trading market for the pre-funded warrants being offered in this offering, and we do not expect a market
to develop. In addition, we do not intend to apply to list the pre-funded warrants on any securities exchange or nationally recognized
trading system. Without an active market, the liquidity of the pre-funded warrants will be limited.
Holders
of our pre-funded warrants will have no rights as a common stockholder until they acquire our common stock.
Until
you acquire shares of our common stock upon exercise of your pre-funded warrants, you will have no rights with respect to shares
of our common stock issuable upon exercise of your pre-funded warrants. Upon exercise of your pre-funded warrants, you will be
entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise
date.
The
pre-funded warrants are speculative in nature.
The
pre-funded warrants offered hereby do not confer any rights of common stock ownership on their holders, such as voting rights
or the right to receive dividends, but rather merely represent the right to acquire shares of common stock at a fixed price. Specifically,
commencing on the date of issuance, holders of the pre-funded warrants may acquire the common stock issuable upon exercise of
such warrants at an exercise price of $0.01 per share of common stock. Moreover, following this offering, the market value
of the pre-funded warrants is uncertain and there can be no assurance that the market value of the pre-funded warrants will equal
or exceed their public offering price. There can be no assurance that the market price of the common stock will ever equal or
exceed the exercise price of the pre-funded warrants, and consequently, whether it will ever be profitable for holders of the
pre-funded warrants to exercise the pre-funded warrants.
We
have broad discretion to use the net proceeds from this offering and our investment of these proceeds pending any such use may
not yield a favorable return.
Our
management has broad discretion as to how to spend the proceeds from this offering and may spend these proceeds in ways with which
our stockholders may not agree. Pending any such uses, we plan to invest the net proceeds of this offering in short-term and long-term,
investment-grade, interest-bearing securities. These investments may not yield a favorable return to our stockholders. See “Use
of Proceeds.”
Even
if this offering is successful, we expect that we will need to raise additional funding to complete the development and commercialization
of our product candidates. This additional financing may not be available on acceptable terms, or at all. Failure to obtain this
necessary capital when needed may force us to delay, limit, or terminate our product development efforts or other operations.
We
estimate that our current cash, cash equivalents and investments, along with the net proceeds from this offering, will be sufficient
for us to fund our operating expenses and capital expenditure requirements through July 2020.
Without
giving effect to the anticipated net proceeds from this offering, we do not believe that our existing capital resources will be
sufficient to meet our projected operating requirements for at least 12 months from the date of issuance of our unaudited interim
condensed consolidated financial statements as of April 30, 2019 and for the six months then ended, and
our audit for the
year ended October 31, 2018,
our independent registered public accounting firm has concluded
that there is substantial doubt about our ability to continue as a going concern. Therefore, the net proceeds from this offering
would remove such doubt regarding our ability to continue as a going concern
. We have based this estimate on assumptions
that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect. In addition,
the expected net proceeds of this offering will not be sufficient for us to fund any of our product candidates through regulatory
approval, and we will need to raise substantial additional capital to complete the development and commercialization of our product
candidates. We will continue to seek funds through equity or debt financings, collaborative or other arrangements with corporate
sources, or through other sources of financing. Adequate additional funding may not be available to us on acceptable terms, or
at all. Any failure to raise capital as and when needed, as a result of insufficient authorized shares or otherwise, could have
a negative impact on our financial condition and on our ability to pursue our business plans and strategies.
If
you purchase shares of common stock and pre-funded warrants in this offering, you will suffer immediate dilution in the book value
of your shares and pre-funded warrants you purchase.
The
offering price of our common stock and each pre-funded warrant is substantially higher than the net tangible book value per share
of our common stock. Therefore, if you purchase shares of our common stock in this offering, you will pay a price per share that
substantially exceeds our net tangible book value per share after this offering. After giving effect to the sale of shares of
our common stock, at a public offering price of $ per share and the sale but not the exercise of pre-funded warrants to purchase
shares of our common stock at an offering price of $ per pre-funded warrant (which is $0.01 less than the public offering price
per share of common stock) and after deducting the estimated underwriting discount and estimated offering expenses payable by
us, purchasers of our common stock or pre-funded warrants in this offering will incur immediate dilution of $ per share in the
net tangible book value of the common stock they acquire, representing the difference between our as adjusted net tangible book
value per share after giving effect to this offering. For a further description of the dilution that you will experience immediately
after this offering, see “Dilution.”
In
addition, to the extent that outstanding stock options or warrants (including the exercise of any pre-funded warrants) have been
or may be exercised or other shares issued, you may experience further dilution.
We
do not intend to pay cash dividends.
We
have not declared or paid any cash dividends on our common stock, and we do not anticipate declaring or paying cash dividends
for the foreseeable future. Any future determination as to the payment of cash dividends on our common stock will be at our Board
of Directors’ discretion and will depend on our financial condition, operating results, capital requirements and other factors
that our Board of Directors considers to be relevant.
Certain
anti-takeover provisions in our charter documents and Delaware law could make a third-party acquisition of us difficult. This
could limit the price investors might be willing to pay in the future for our common stock.
Provisions
in our amended and restated certificate of incorporation and amended and restated bylaws could have the effect of making it more
difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, or control us. These factors
could limit the price that certain investors might be willing to pay in the future for shares of our common stock. Our amended
and restated certificate of incorporation allows us to issue preferred stock without the approval of our stockholders. The issuance
of preferred stock could decrease the amount of earnings and assets available for distribution to the holders of our common stock
or could adversely affect the rights and powers, including voting rights, of such holders. In certain circumstances, such issuance
could have the effect of decreasing the market price of our common stock. Our amended and restated bylaws also provide our board
of directors with the ability to alter such bylaws without stockholder approval. Any of these provisions could also have the effect
of delaying or preventing a change in control.
Provisions
of the pre-funded warrants offered by this prospectus could discourage an acquisition of us by a third party.
In
addition to the discussion of the provisions of our amended and restated certificate of incorporation and our amended and restated
bylaws, certain provisions of the pre-funded warrants offered by this prospectus could make it more difficult or expensive for
a third party to acquire us. The pre-funded warrants prohibit us from engaging in certain transactions constituting “fundamental
transactions” unless, among other things, the surviving entity assumes our obligations under the pre-funded warrants. Further,
the pre-funded warrants provide that, in the event of certain transactions constituting “fundamental transactions,”
with some exception, holders of such warrants will have the right, at their option, to require us to repurchase such pre-funded
warrants at a price described in such pre-funded warrants. These and other provisions of the pre-funded warrants offered by this
prospectus could prevent or deter a third party from acquiring us even where the acquisition could be beneficial to you.
The
exercise price of the pre-funded warrants offered by this prospectus will not be adjusted for certain dilutive events.
The
exercise price of the pre-funded warrants offered by this prospectus is subject to adjustment for certain events, including, but
not limited to, certain issuances of capital stock, options, convertible securities and other securities. However, the exercise
prices will not be adjusted for dilutive issuances of securities considered “excluded securities” and there may be
transactions or occurrences that may adversely affect the market price of our common stock or the market value of such pre-funded
warrants without resulting in an adjustment of the exercise prices of such pre-funded warrants.
The
tax reform bill passed in 2017 could adversely affect our business and financial condition.
The
“Tax Cuts and Jobs Act,” or the TCJA, was enacted in 2017 and significantly amends the Internal Revenue Code of 1986,
or the Code. The TCJA, among other things, reduces the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%,
limits the tax deduction for interest expense to 30% of adjusted earnings (except for certain small businesses), limits the deduction
for net operating losses to 80% of current year taxable income and eliminates net operating loss carrybacks, in each case, for
losses generated after December 31, 2017 (though any such net operating losses may be carried forward indefinitely), and modifies
or repeals many business deductions and credits (including reducing the business tax credit for certain clinical testing expenses
incurred in the testing of certain drugs for rare diseases or conditions generally referred to as “orphan drugs”).
We continue to examine the impact these changes may have on our business.
Our ability to use estimated net operating
losses and research and development credits to offset future taxable income may be subject to certain limitations.
As of October 31, 2018, we had federal and
state net operating loss carryforwards of $265.8 million and $129.2 million, respectively, which begin to expire in various amounts
in 2023. As of October 31, 2018, we also had federal and state research and development tax credit carryforwards of $6.3 million
and $0.5 million, respectively, which begin to expire in 2025. These net operating loss and tax credit carryforwards could expire
unused and be unavailable to offset future income tax liabilities (except for federal net operating loss carryforwards generated
in taxable years ending after December 31, 2017, which are not subject to expiration). In addition, in general, under Sections
382 and 383 of the Code a corporation that undergoes an “ownership change” is subject to limitations on its ability
to utilize its pre-change net operating losses or tax credits, or NOLs or credits, to offset future taxable income or taxes. For
these purposes, an ownership change generally occurs where the aggregate stock ownership of one or more stockholders or groups
of stockholders who owns at least 5% of a corporation’s stock increases its ownership by more than 50 percentage points over
its lowest ownership percentage within a specified testing period. Our existing NOLs or credits are subject to limitations arising
from previous ownership changes, and if we undergo an ownership change in connection with or after this offering, our ability to
utilize NOLs or credits could be further limited by Sections 382 and 383 of the Code. In addition, future changes in our stock
ownership, many of which are outside of our control, could result in an ownership change under Sections 382 and 383 of the Code.
Our NOLs or credits may also be impaired under state law. Accordingly, we may not be able to utilize a material portion of our
NOLs or credits.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus and the documents incorporated by reference contain forward-looking statements, contains forward-looking statements.
These statements include all matters that are not related to present facts or current conditions or that are not historical facts,
including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects,
plans, objectives of management and expected market growth. The words “anticipate,” “believe,” “could,”
“continue,” “should,” “predict,” “estimate,” “expect,” “intend,”
“may,” “plan,” “potentially,” “will,” “may,” “would,”
or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not
all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations
disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements.
By
their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics,
and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the
future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each
forward-looking statement contained in this prospectus, we caution you that forward-looking statements are not guarantees of future
performance and that our actual results of operations, financial condition and liquidity, and the development of the industry
in which we operate may differ materially from the forward-looking statements contained in this prospectus. In addition, even
if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent
with the forward-looking statements contained in this prospectus, they may not be predictive of results or developments in future
periods.
Some
of the factors that we believe could cause actual results to differ from those anticipated or predicted include:
|
|
●
|
the
success and timing of our clinical trials, including patient accrual;
|
|
|
|
|
|
|
●
|
our
ability to obtain and maintain regulatory approval and/or reimbursement of our product candidates for marketing;
|
|
|
|
|
|
|
●
|
our
ability to obtain the appropriate labeling of our products under any regulatory approval;
|
|
|
|
|
|
|
●
|
our
plans to develop and commercialize our products;
|
|
|
|
|
|
|
●
|
the
successful development and implementation of our sales and marketing campaigns;
|
|
|
|
|
|
|
●
|
the
change of key scientific or management personnel;
|
|
|
|
|
|
|
●
|
the
size and growth of the potential markets for our product candidates and our ability to serve those markets;
|
|
|
|
|
|
|
●
|
our
ability to successfully compete in the potential markets for our product candidates, if commercialized;
|
|
|
|
|
|
|
●
|
regulatory
developments in the United States and other countries;
|
|
|
|
|
|
|
●
|
the
rate and degree of market acceptance of any of our product candidates;
|
|
|
|
|
|
|
●
|
new
products, product candidates or new uses for existing products or technologies introduced or announced by our competitors
and the timing of these introductions or announcements;
|
|
|
|
|
|
|
●
|
market
conditions in the pharmaceutical and biotechnology sectors;
|
|
|
|
|
|
|
●
|
our
available cash;
|
|
|
|
|
|
|
●
|
the
accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional financing;
|
|
|
|
|
|
|
●
|
our
ability to obtain additional funding;
|
|
|
|
|
|
|
●
|
our
ability to obtain and maintain intellectual property protection for our product candidates;
|
|
|
●
|
the
success and timing of our preclinical studies including IND enabling studies;
|
|
|
|
|
|
|
●
|
the
ability of our product candidates to successfully perform in clinical trials;
|
|
|
|
|
|
|
●
|
our
ability to obtain and maintain approval of our product candidates for trial initiation;
|
|
|
|
|
|
|
●
|
our
ability to manufacture and the performance of third-party manufacturers;
|
|
|
|
|
|
|
●
|
our
ability to identify license and collaboration partners and to maintain existing relationships;
|
|
|
|
|
|
|
●
|
the
performance of our clinical research organizations, clinical trial sponsors, clinical trial investigators and collaboration
partners for any clinical trials we conduct; and
|
|
|
|
|
|
|
●
|
our
ability to successfully implement our strategy.
|
These
factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are
included in this prospectus and the documents incorporated by reference. Other sections of this prospectus may include additional
factors that could adversely impact our business and financial performance. Moreover, we operate in a very competitive and rapidly
changing environment. New risk factors emerge from time to time, and it is not possible for our management to predict all risk
factors nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors,
may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements.
We
have included important factors in the cautionary statements included in this prospectus, particularly those described or incorporated
by reference in the “Risk Factors” section, that we believe could cause actual results or events to differ materially
from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future
acquisitions, mergers, dispositions, joint ventures or investments we may make. No forward-looking statement is a guarantee of
future performance.
You
should read this prospectus and the documents that we reference and incorporate by reference in this prospectus and have filed
as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our
actual future results may be materially different from what we expect. The forward-looking statements in this prospectus, and
the documents incorporated by reference, represent our views as of the date of this prospectus. We anticipate that subsequent
events and developments will cause our views to change. However, while we may elect to update these forward-looking statements
at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should,
therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this
prospectus.
INDUSTRY
AND MARKET DATA
This
prospectus and the documents incorporated by reference include
statistical and other industry
and market data that we obtained from our own internal estimates and research, as well as from industry publications and research,
surveys and studies conducted by us and third parties. Industry publications, studies, and surveys generally state that they have
been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information.
While we believe that each of these studies and publications is reliable, we have not independently verified market and industry
data from third-party sources. While we believe our internal company research is reliable and the market definitions are appropriate,
neither such research nor these definitions have been verified by any independent source. The industry in which we operate is
subject to a high degree of uncertainty and risks due to various factors, including those described in the section titled “Risk
Factors.”
USE
OF PROCEEDS
We
estimate that the net proceeds to us from the sale of the shares of our common stock offered by us in this offering will be approximately
$ million, based on an assumed public offering price of $
per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on ,
2019, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us and assuming
no sale of any pre-funded warrants in this offering and excluding the proceeds, if any, from the exercise of the pre-funded warrants.
If the underwriters’ option to purchase additional shares in this offering is exercised in full, we estimate that our net
proceeds from this offering will be approximately $ million,
after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
A
$0.50 increase (decrease) in the assumed public offering price of $
per share, the last reported sale price of the our common stock on The Nasdaq Global Select Market on ,
2019, would increase (decrease) the net proceeds to us from this offering by approximately $
million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and
after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, an
increase (decrease) of 500,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus,
would increase (decrease) the net proceeds to us from this offering by approximately $ million,
assuming no change in the assumed public offering price and after deducting estimated underwriting discounts and commissions and
estimated offering expenses payable by us.
We
expect to use the net proceeds from this offering to fund our continued research and development initiatives in connection with
expanding our product pipeline including, but not limited to:
|
|
●
|
investment
in our ADXS-HOT program in both monotherapy and combination therapy and new cancer types;
|
|
|
|
|
|
|
●
|
investment
in ongoing clinical research in ADXS-PSA and ADXS-NEO, in combination therapy; and
|
|
|
|
|
|
|
●
|
general
corporate purposes.
|
Our
expected use of net proceeds from this offering represents our current intentions based upon our present plans and business condition.
As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received
upon the closing of this offering or the amounts that we will actually spend on the uses set forth above. The amounts and timing
of our actual use of the net proceeds from this offering will vary depending on numerous factors, including the progress of our
clinical trials and other development efforts for our product candidates and other factors described in “Risk Factors”
beginning on page 11 or incorporated by reference herein, as well as the amount of cash we use in our operations. As a
result, our management will have broad discretion in the application of the net proceeds, and investors will be relying on our
judgment regarding the application of the net proceeds from this offering. In addition, we might decide to postpone or not pursue
clinical trials or preclinical activities if the net proceeds from this offering and the other sources of cash are less than expected.
Pending
application of the net proceeds, we intend to invest the net proceeds from this offering in short- and intermediate-term, interest-bearing
obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
DIVIDEND
POLICY
We
have never declared or paid any cash dividends on our common stock and do not anticipate paying any cash dividends in the foreseeable
future. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial
condition, operating results, capital requirements and other factors that our board of directors considers to be relevant.
CAPITALIZATION
The
following table sets forth our cash, cash equivalents and investments and capitalization as of April 30, 2019:
|
|
●
|
on
an actual basis;
|
|
|
|
|
|
|
●
|
on
a pro forma basis to reflect the shares of common stock offered by us in this offering at an assumed public offering price
of $ per share (which was the last reported sale price of our common stock on the Nasdaq Global Select Market on , 2019),
after deducting underwriting discounts and estimated offering expenses payable by us. The pro forma basis assumes no sale
of pre-funded warrants, which, if sold, would reduce the number of shares of common stock that we are offering on a one-for-one
basis, and excludes the proceeds, if any, from the exercise of any pre-funded warrants issued in this offering.
|
You
should read this information together with our financial statements and the notes to those statements incorporated by reference
into this prospectus.
|
April
30, 2019 (unaudited) (in thousands, except share and par value data)
|
|
Actual
|
|
|
Pro
Forma(1)
|
|
|
Cash,
cash equivalents and investments
|
|
$
|
33,706
|
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’
equity:
|
|
|
|
|
|
|
|
|
|
Preferred
stock, $0.001 par value per share, 5,000,000 shares authorized; 0 shares issued and outstanding actual, pro forma and pro
forma as adjusted
|
|
|
—
|
|
|
|
|
|
|
Common
stock, $0.001 par value per share, 170,000,000 shares authorized; 8,020,370 shares issued and outstanding, actual, shares
issued and outstanding, pro forma
|
|
|
8
|
|
|
|
|
|
|
Additional
paid-in capital
|
|
|
407,385
|
|
|
|
|
|
|
Accumulated
deficit
|
|
|
(364,223
|
)
|
|
|
|
|
|
Total
stockholders’ equity
|
|
|
43,170
|
|
|
|
|
|
|
Total
capitalization
|
|
$
|
43,170
|
|
|
$
|
|
|
(1)
A $0.50 increase (decrease) in the assumed public offering price of $
per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on
, 2019, would increase (decrease) the as adjusted amount of each of cash and cash equivalents, additional paid-in capital, total
assets and total stockholders’ equity by $ million,
assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after
deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase (decrease)
of 500,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease)
the as adjusted amount of each of cash and cash equivalents, working capital, total assets and total stockholders’ equity
by $ million, assuming no change in
the assumed public offering price per share and after deducting estimated underwriting discounts and commissions and estimated
offering expenses payable by us. The as adjusted information discussed above is illustrative only and will be adjusted based on
the actual public offering price and other terms of this offering determined at pricing.
The
number of shares of common stock to be outstanding immediately after the offering is based on 8,020,370 shares of common stock
outstanding as of April 30, 2019. The number of shares outstanding as of April 30, 2019 excludes:
|
|
●
|
400,260
shares of common stock issuable upon the exercise of stock options outstanding as of
April 30, 2019 at a weighted-average exercise price of $101.82 per share;
|
|
|
|
|
|
|
●
|
17,063
shares of common stock reserved for issuance upon settlement of restricted stock units;
|
|
|
|
|
|
|
●
|
72,304
shares of common stock issuable upon the exercise of warrants outstanding as of April 30, 2019 at a weighted-average exercise
price of $3.82 per share; and
|
|
|
|
|
|
|
●
|
188,658
shares of common stock reserved for the future awards under our 2015 Incentive Plan
.
|
DILUTION
If
you invest in our common stock in this offering, your ownership interest will be diluted immediately to the extent of the difference
between the public offering price per share of our common stock and pre-funded warrant and the as adjusted net tangible book value
per share of our common stock immediately after this offering.
As
of April 30, 2019, our historical net tangible book value was $38.2 million, or $4.77 per share of common stock. Our net tangible
book value represents total tangible assets less total liabilities and convertible preferred stock. Our net tangible book value
per share is our net tangible book value divided by the number of shares of our common stock outstanding as of April 30, 2019.
Dilution
in net tangible book value per share represents the difference between the amount per share paid by purchasers in this offering
and the net tangible book value per share of our common stock immediately after this offering.
After
giving further effect to the sale of shares of common stock in this offering at an assumed public offering price of $
per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on ,
2019, assuming no sale of any pre-funded warrants in this offering, and after deducting estimated underwriting discounts and commissions
and estimated offering expenses payable by us, our as adjusted net tangible book value as of April 30, 2019 would have been approximately
$ million, or approximately
$ per share of common stock, and after deducting the underwriting
discounts and commissions and the estimated offering expenses payable by us. This represents an immediate increase in net tangible
book value of $ per
share of common stock to our existing stockholders and an immediate dilution in net tangible book value of $
per share of common stock to purchasers in this offering.
The
following table illustrates this dilution on a per share basis:
|
Assumed
public offering price per share
|
|
|
|
|
|
$
|
|
|
|
Historical
net tangible book value (deficit) per share as of April 30, 2019
|
|
$
|
4.77
|
|
|
|
|
|
|
Increase
in as adjusted net tangible book value per share attributable to new investors purchasing common stock in this offering
|
|
|
|
|
|
|
|
|
|
As
adjusted net tangible book value per share, after giving effect to this offering
|
|
|
|
|
|
|
|
|
|
Dilution
per share to new investors purchasing common stock in this offering
|
|
|
|
|
|
$
|
|
|
A
$0.50 increase (decrease) in the assumed public offering price of $
per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on
, 2019, would increase (decrease) our as adjusted net tangible book value per share after this offering by $ and
dilution per share to new investors purchasing common stock in this offering by $ ,
assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after
deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase of 500,000
shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase our as adjusted
net tangible book value per share after this offering by $
and decrease the dilution per share to new investors purchasing common stock in this offering by $ ,
assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions
and estimated offering expenses payable by us. A decrease of 500,000 shares in the number of shares offered by us, as set forth
on the cover page of this prospectus, would decrease our as adjusted net tangible book value per share after this offering by
$ and increase the dilution per share to new
investors purchasing common stock in this offering by $
, assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions
and estimated offering expenses payable by us.
The
following table summarizes, as of April 30, 2019, on the as adjusted basis described above, the total number of shares of common
stock purchased from us, the total consideration paid or to be paid, and the average price per share paid or to be paid by existing
stockholders and by new investors in this offering at an assumed public offering price of $
per share, the last reported sale price of our common stock on The Nasdaq Global Select Market on ,
2019, before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. As the
table shows, new investors purchasing common stock in this offering will pay an average price per share substantially higher than
our existing stockholders paid.
|
|
|
Shares
Purchased
|
|
|
Total
Consideration
|
|
|
Average
Price
|
|
|
|
|
Number
|
|
|
Percent
|
|
|
Amount
|
|
|
Percent
|
|
|
Per
share
|
|
|
|
|
(in
thousands)
|
|
|
Existing
stockholders
|
|
|
|
|
|
|
|
%
|
|
$
|
|
|
|
|
|
%
|
|
$
|
|
|
|
New
investors
|
|
|
|
|
|
|
|
%
|
|
|
|
|
|
|
|
%
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
|
%
|
|
$
|
|
|
|
|
|
%
|
|
|
|
|
A
$0.50 increase (decrease) in the assumed public offering price of $ per
share, the last reported sale price of our common stock on The Nasdaq Global Select Market on ,
2019, would increase (decrease) the total consideration paid by new investors by $ million
and, in the case of an increase, would increase the percentage of total consideration paid by new investors by percentage
points and, in the case of a decrease, would decrease the percentage of total consideration paid by new investors by percentage
points, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same.
An increase (decrease) of 500,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus,
would increase (decrease) the total consideration paid by new investors by $ million
and, in the case of an increase, would increase the percentage of total consideration paid by new investors by percentage
points and, in the case of a decrease, would decrease the percentage of total consideration paid by new investors by percentage
points, assuming no change in the assumed public offering price per share.
The
table above assumes no exercise of the underwriters’ option to purchase additional shares in this offering and that no
pre-funded warrants are issued in this offering
. If the underwriters’ option to purchase additional shares is
fully exercised, the number of shares of our common stock held by existing stockholders would be reduced to
% of the total number of shares of our common stock outstanding after this
offering, and the number of shares of common stock held by new investors purchasing common stock in this offering would be
increased to % of the total number of shares of our common stock outstanding after this offering.
The
number of shares of our common stock to be outstanding after this offering is based on 8,020,370 shares of our common stock outstanding
as of April 30, 2019, and excludes:
|
|
●
|
400,260
shares of common stock issuable upon the exercise of stock options outstanding as of
April 30, 2019 at a weighted-average exercise price of $101.82 per share;
|
|
|
|
|
|
|
●
|
17,063
shares of common stock reserved for issuance upon settlement of restricted stock units;
|
|
|
|
|
|
|
●
|
72,304
shares of common stock issuable upon the exercise of warrants outstanding as of April 30, 2019 at a weighted-average exercise
price of $3.82 per share; and
|
|
|
|
|
|
|
●
|
188,658
shares of common stock reserved for the future awards under our 2015 Incentive Plan.
|
New
investors will experience further dilution if any of our outstanding options or warrants are exercised, new options are issued
and exercised under our equity incentive plans or we issue additional shares of common stock, other equity securities or convertible
debt securities in the future.
PRINCIPAL
STOCKHOLDERS
Except
as noted below, the following table sets forth certain information with respect to the beneficial ownership of our Common Stock
as of June 1, 2019:
|
|
●
|
each
person who is known by us to be the beneficial owner of more than 5% of our outstanding Common Stock;
|
|
|
|
|
|
|
●
|
each
of our directors;
|
|
|
|
|
|
|
●
|
each
of our named executive officers and current executive officers; and
|
|
|
|
|
|
|
●
|
all
of our current directors and executive officers as a group.
|
As
used in the table below, the term beneficial ownership with respect to our Common Stock consists of sole or shared voting power
(which includes the power to vote, or to direct the voting of shares of our Common Stock) or sole or shared investment power (which
includes the power to dispose, or direct the disposition of, shares of our Common Stock) through any contract, arrangement, understanding,
relationship or otherwise, including a right to acquire such power(s) during the 60 days following April 30, 2019.
Unless
otherwise indicated in the footnotes to this table, and subject to community property laws where applicable, we believe each of
the stockholders named in this table has sole voting and investment power with respect to the shares indicated as beneficially
owned. Applicable percentages are based on 8,020,370 shares of Common Stock outstanding as of April 30, 2019, adjusted as required
by the rules promulgated by the SEC. Unless otherwise indicated, the address for each of the individuals and entities listed in
this table is 305 College Road East, Princeton, New Jersey 08540.
|
Name
of Beneficial Owner
|
|
Total
# of
Shares
Beneficially
Owned
|
|
|
Percentage
of
Ownership
|
|
|
Kenneth
Berlin (1)
|
|
|
27,223
|
|
|
|
*
|
%
|
|
David
Sidransky (2)
|
|
|
15,357
|
|
|
|
*
|
%
|
|
Roni
Appel (3)
|
|
|
23,157
|
|
|
|
*
|
%
|
|
Richard
Berman (4)
|
|
|
11,770
|
|
|
|
*
|
%
|
|
Samir
Khleif (5)
|
|
|
14,751
|
|
|
|
*
|
%
|
|
James
Patton (6)
|
|
|
27,375
|
|
|
|
*
|
%
|
|
Andres
Gutierrez (7)
|
|
|
9,306
|
|
|
|
-
|
%
|
|
Molly
Henderson (8)
|
|
|
11,389
|
|
|
|
*
|
%
|
|
Robert
Petit (9)
|
|
|
34,647
|
|
|
|
*
|
%
|
|
All
Directors and Officers as a Group (10)
|
|
|
174,975
|
|
|
|
2.16
|
%
|
*Less
than 1%
(1)
Represents 10,556 issued shares of our Common Stock and options to purchase 16,667 shares of our Common Stock exercisable
within 60 days.
(2)
Represents 7,357 issued shares of our Common Stock and options to purchase 8,000 shares of our Common Stock exercisable
within 60 days.
(3)
Represents 13,476 issued shares of our Common Stock, options to purchase 7,792 shares of our Common Stock exercisable within
60 days and warrants to purchase 1,889 shares of our Common Stock exercisable within 60 days.
(4)
Represents 3,711 issued shares of our Common Stock and options to purchase 8,059 shares of our Common Stock exercisable
within 60 days.
(5)
Represents 4,639 issued shares of our Common Stock and options to purchase 10,112 shares of our Common Stock exercisable
within 60 days.
(6)
Represents 19,116 issued shares of our Common Stock and options to purchase 8,259 shares of our Common Stock exercisable
within 60 days.
(7)
Represents 3,750 issued shares of our Common Stock and options to purchase 5,556 shares of our Common Stock exercisable
within 60 days.
(8)
Represents 5,833 issued shares of our Common Stock and options to purchase 5,556 shares of our Common Stock exercisable
within 60 days.
(9)
Represents 12,856 issued shares of our Common Stock and options to purchase 21,791 shares of our Common Stock exercisable
within 60 days. On May 13, 2019,
Dr. Petit stepped down as our Chief Scientific Officer
effective as of the close of business on June 3, 2019.
(10)
Represents 81,294 issued shares of our Common Stock and options to purchase 91,792 shares of our Common Stock exercisable
within 60 days and warrants to purchase 1,889 shares of our Common Stock exercisable within 60 days.
DESCRIPTION
OF CAPITAL STOCK
The
following description of our common stock and preferred stock, together with the additional information we may incorporate by
reference herein, summarizes the material terms and provisions of our capital stock. The following description of our capital
stock does not purport to be complete and is subject to, and qualified in its entirety by, our Amended and Restated Certificate
of Incorporation and our Amended and Restated Bylaws, which are exhibits to the registration statement of which this prospectus
forms a part, and by applicable law. We refer in this section to our Amended and Restated Certificate of Incorporation as our
“certificate of incorporation”, and we refer to our Amended and Restated Bylaws as our “bylaws.” The terms
of our common stock and preferred stock may also be affected by Delaware law.
Authorized
Capital Stock
Under
our certificate of incorporation, we are authorized to issue a total of 170,000,000 shares of common stock, par value $0.001 per
share, and 5,000,000 shares of “blank check” preferred stock, par value $0.001 per share. As of June 1, 2019, we had
issued and outstanding 8,020,815 shares of our common stock and no shares of preferred stock outstanding. As of such date, there
were approximately 102 holders of record.
Common
Stock
Holders
of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of shareholders
and do not have any cumulative voting rights. Holders of our common stock are entitled to receive ratably any dividends declared
by the board of directors out of funds legally available for that purpose, subject to any preferential dividend rights of any
outstanding preferred stock. Our common stock has no preemptive rights, conversion rights or other subscription rights or redemption
or sinking fund provisions. In the event of our liquidation, dissolution or winding up, holders of our common stock will be entitled
to share ratably in all assets remaining after payment of all debts and other liabilities and any liquidation preference of any
outstanding preferred stock. All outstanding shares are fully-paid and non-assessable.
Listing
Our
common stock is listed on the Nasdaq Global Select Market under the symbol “ADXS.” On July 1, 2019, the closing
price for our common stock, as reported on the Nasdaq Global Select Market, was $2.25 per share.
Transfer
Agent
The
transfer agent and registrar for our common stock is Continental Stock Transfer and Trust Company, 17 Battery Place, 8th Floor,
New York, NY 10004.
Preferred
Stock
Our
board of directors is authorized, without action by the stockholders, to designate and issue up to an aggregate of 5,000,000 shares
of preferred stock in one or more series. Our board of directors can designate the rights, preferences and privileges of the shares
of each series and any of its qualifications, limitations or restrictions. Our board of directors may authorize the issuance of
preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of
common stock.
The
issuance of preferred stock, while providing flexibility in connection with possible future financings and acquisitions and other
corporate purposes could, under certain circumstances, have the effect of restricting dividends on our common stock, diluting
the voting power of our common stock, impairing the liquidation rights of our common stock, or delaying, deferring or preventing
a change in control of our company, which might harm the market price of our common stock. See also “Antitakeover Effects
of Delaware Law and Provisions of our Amended and Restated Certificate of Incorporation and Amended and Restated By-laws—Provisions
of our Amended and Restated Certificate of Incorporation and Amended and Restated By-laws—Undesignated preferred stock”
below.
Dividends
Subject
to the dividend rights of the holders of any outstanding series of preferred stock, holders of our common stock are entitled to
receive ratably such dividends and other distributions of cash or any other right or property as may be declared by our board
of directors out of our assets or funds legally available for such dividends or distributions.
Voting
Rights
The
holders of our common stock are entitled to one vote for each share held of record on each matter submitted to a vote of stockholders.
Holders of our common stock do not have a cumulative voting right, which means that the holders of more than one-half of the outstanding
shares of common stock, subject to the rights of the holders of the preferred stock, if any, can elect all of our directors, if
they choose to do so. In this event, the holders of the remaining shares of common stock would not be able to elect any directors.
Except as otherwise required by Delaware law, and subject to the rights of the holders of preferred stock, if any, all stockholder
action is taken by the vote of a majority of the outstanding shares of common stock voting as a single class present at a meeting
of stockholders at which a quorum consisting of one-third of the outstanding shares of common stock is present in person or proxy.
Liquidation
and Dissolution
In
the event of any voluntary or involuntary liquidation, dissolution or winding up of our affairs, holders of common stock would
be entitled to share ratably in our assets that are legally available for distribution to stockholders after payment of liabilities.
If we have any preferred stock outstanding at such time, holders of the preferred stock may be entitled to distributions and/or
liquidation preferences. In either such case, we must pay the applicable distribution to the holders of our preferred stock (if
any) before we may pay distributions to the holders of common stock.
Anti-Takeover
Provisions
Delaware
Law
We
are subject to Section 203 of the Delaware General Corporation Law, or Section 203. This provision generally prohibits a Delaware
corporation from engaging in any business combination with any interested stockholder for a period of three years following the
date the stockholder became an interested stockholder, unless:
|
|
●
|
prior
to such date, the board of directors approved either the business combination or the transaction that resulted in the stockholder
becoming an interested stockholder;
|
|
|
●
|
upon
consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder
owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for
purposes of determining the number of shares outstanding those shares owned by persons who are directors and also officers
and by employee stock plans in which employee participants do not have the right to determine confidentially whether shares
held subject to the plan will be tendered in a tender or exchange offer; or
|
|
|
●
|
on
or subsequent to such date, the business combination is approved by the board of directors and authorized at an annual meeting
or special meeting of stockholders and not by written consent, by the affirmative vote of at least 66-2/3% of the outstanding
voting stock that is not owned by the interested stockholder.
|
Section
203 defines a business combination to include:
|
|
●
|
any
merger or consolidation involving the corporation and the interested stockholder;
|
|
|
●
|
any
sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder;
|
|
|
●
|
subject
to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation
to the interested stockholder;
|
|
|
●
|
any
transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class
or series of the corporation beneficially owned by the interested stockholder; or
|
|
|
●
|
the
receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits
provided by or through the corporation.
|
In
general, Section 203 defines an “interested stockholder” as any entity or person beneficially owning 15% or more of
the outstanding voting stock of a corporation, or an affiliate or associate of the corporation and was the owner of 15% or more
of the outstanding voting stock of a corporation at any time within three years prior to the time of determination of interested
stockholder status; and any entity or person affiliated with or controlling or controlled by such entity or person.
These
statutory provisions could delay or frustrate the removal of incumbent directors or a change in control of our company. They could
also discourage, impede, or prevent a merger, tender offer, or proxy contest, even if such event would be favorable to the interests
of stockholders.
Amended
and Restated Certificate of Incorporation and Bylaw Provisions
Our
amended and restated certificate of incorporation and bylaws contain provisions that could have the effect of discouraging potential
acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a stockholder
might consider favorable. In particular, the certificate of incorporation and bylaws, as applicable, among other things:
|
|
●
|
provide
our board of directors with the ability to alter its bylaws without stockholder approval; and
|
|
|
●
|
provide
that vacancies on our board of directors may be filled by a majority of directors in office, although less than a quorum.
|
Such
provisions may have the effect of discouraging a third-party from acquiring us, even if doing so would be beneficial to our stockholders.
These provisions are intended to enhance the likelihood of continuity and stability in the composition of our board of directors
and in the policies formulated by them, and to discourage some types of transactions that may involve an actual or threatened
change in control of our company. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal
and to discourage some tactics that may be used in proxy fights. We believe that the benefits of increased protection of our potential
ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure our company outweigh
the disadvantages of discouraging such proposals because, among other things, negotiation of such proposals could result in an
improvement of their terms. However, these provisions could have the effect of discouraging others from making tender offers for
our shares that could result from actual or rumored takeover attempts. These provisions also may have the effect of preventing
changes in our management.
DESCRIPTION
OF SECURITIES WE ARE OFFERING
We
are offering shares of our common stock and/or
pre-funded warrants to purchase shares of common stock. The shares of common stock and pre-funded warrants will be issued separately.
We are also registering the shares of common stock issuable from time to time upon exercise of the pre-funded warrants offered
hereby.
Common
Stock
The
material terms and provisions of our common stock and each other class of our securities which qualifies or limits our common
stock are described under the caption “Description of Capital Stock” in this prospectus.
Pre-funded
Warrants
The
following summary of certain terms and provisions of pre-funded warrants that are being offered hereby is not complete and is
subject to, and qualified in its entirety by, the provisions of the pre-funded warrant, the form of which is filed as an exhibit
to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and
provisions of the form of pre-funded warrant for a complete description of the terms and conditions of the pre-funded warrants.
Duration
and Exercise Price.
Each pre-funded warrant offered hereby will have an initial exercise price per share equal to $0.01. The
pre-funded warrants will be immediately exercisable and may be exercised at any time until the pre-funded warrants are exercised
in full. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in
the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price.
Exercisability.
The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly
executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise
(except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion
of the pre-funded warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of
the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder
to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s pre-funded warrants
up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage
ownership is determined in accordance with the terms of the pre-funded warrants. Purchasers of pre-funded warrants in this offering
may also elect prior to the issuance of the pre-funded warrants to have the initial exercise limitation set at 9.99% of our outstanding
common stock. No fractional shares of common stock will be issued in connection with the exercise of a pre-funded warrant. In
lieu of fractional shares, we will either pay the holder an amount in cash equal to the fractional amount multiplied by the exercise
price or round up to the next whole share.
Cashless
Exercise.
If, at the time a holder exercises its pre-funded warrants, a registration statement registering the issuance of
the shares of common stock underlying the pre-funded warrants under the Securities Act is not then effective or available, then
in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise
price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common
stock determined according to a formula set forth in the pre-funded warrants.
Transferability.
Subject to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded
warrant to us together with the appropriate instruments of transfer.
Exchange
Listing.
There is no trading market available for the pre-funded warrants on any securities exchange or nationally recognized
trading system. We do not intend to list the pre-funded warrants on any securities exchange or nationally recognized trading system.
Right
as a Stockholder.
Except as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of
shares of our common stock, the holders of the pre-funded warrants do not have the rights or privileges of holders of our common
stock, including any voting rights, until they exercise their pre-funded warrants.
Fundamental
Transaction.
In the event of a fundamental transaction, as described in the pre-funded warrants and generally including any
reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially
all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of
our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our
outstanding common stock, the holders of the pre-funded warrants will be entitled to receive upon exercise of the pre-funded warrants
the kind and amount of securities, cash or other property that the holders would have received had they exercised the pre-funded
warrants immediately prior to such fundamental transaction.
SHARES
ELIGIBLE FOR FUTURE SALE
We
cannot predict the effect, if any, that future sales of shares of common stock, or the availability for future sale of shares
of common stock, will have on the market price of shares of our common stock prevailing from time to time. The sale of substantial
amounts of shares of our common stock in the public market, or the perception that such sales could occur, could harm the prevailing
market price of shares of our common stock.
Based
on the number of shares outstanding as of April 30, 2019, upon completion of this offering, we will have a total of shares
of our common stock outstanding, assuming no sale of pre-funded warrants, (or
shares of common stock if the underwriters exercise in full their option to purchase additional shares of common stock). Of the
outstanding shares, all of the shares sold in this offering will be freely tradable, except that any shares, including shares
sold to an entity affiliated with an existing shareholder that may purchase shares in this offering, held by our affiliates, as
that term is defined in Rule 144 under the Securities Act, may only be sold in compliance with the limitations described below.
Rule
144
In
general, a person who has beneficially owned restricted stock for at least six months would be entitled to sell their securities
provided that (i) such person is not deemed to have been one of our affiliates at the time of, or at any time during the 90 days
preceding, a sale and (ii) we are subject to the Securities Exchange Act of 1934, as amended, or the Exchange Act, periodic reporting
requirements for at least 90 days before the sale. Persons who have beneficially owned restricted shares for at least six months
but who are our affiliates at the time of, or any time during the 90 days preceding, a sale, would be subject to additional restrictions,
by which such person would be entitled to sell within any six-month period only a number of securities that does not exceed the
greater of either of the following:
|
|
●
|
1%
of the number of shares then outstanding, which will equal approximately shares
immediately after this offering assuming no exercise of the underwriters’ option
to purchase additional shares, and assuming no sale of pre-funded warrants, based on
the number of shares outstanding as of April 30, 2019; or
|
|
|
|
|
|
|
●
|
the
average weekly trading volume of our common stock on The Nasdaq Global Select Market
during the four calendar weeks preceding the filing of a notice on Form 144 with respect
to the sale.
|
Provided,
in each case, that we are subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale. Such
sales both by affiliates and by non-affiliates must also comply with the manner of sale, current public information and notice
provisions of Rule 144 of the Securities Act, or Rule 144.
Rule
701
Rule
701 under the Securities Act, or Rule 701, as in effect on the date of this prospectus, permits resales of shares in reliance
upon Rule 144 but without compliance with certain restrictions of Rule 144, including the holding period requirement. Most of
our employees, executive officers or directors who purchased shares under a written compensatory plan or contract may be entitled
to rely on the resale provisions of Rule 701, but all holders of Rule 701 shares are required to wait until 90 days after the
date of this prospectus before selling their shares. However, substantially all Rule 701 shares are subject to lock-up agreements
as described below in the section titled “Underwriting” included elsewhere in this prospectus and will become eligible
for sale upon the expiration of the restrictions set forth in those agreements.
Lock-up
Agreements
In
connection with this offering, we, along with our directors, executive officers and certain stockholders have agreed with the
underwriters that for a period of 90 days (the restricted period), after the date of this prospectus, subject to specified exceptions,
we or they will not offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract
to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any
shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock, or enter
into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership
of the common stock.
MATERIAL
UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
FOR
NON-U.S. HOLDERS
The
following discussion is a summary of the material U.S. federal income tax considerations applicable to non-U.S. holders (as defined
below) with respect to their ownership and disposition of shares of our common stock or pre-funded warrants issued pursuant to
this offering. For purposes of this discussion, a non-U.S. holder means a beneficial owner of our common stock and/or pre-funded
warrants that is for U.S. federal income tax purposes:
|
|
●
|
a
non-resident alien individual;
|
|
|
●
|
a
foreign corporation or any other foreign organization taxable as a corporation for U.S.
federal income tax purposes; or
|
|
|
●
|
a
foreign estate or trust, the income of which is not subject to U.S. federal income tax
on a net income basis.
|
This
discussion does not address the tax treatment of partnerships or other entities that are pass-through entities for U.S. federal
income tax purposes or persons that hold their common stock and/or pre-funded warrants through partnerships or other pass-through
entities. A partner in a partnership or other pass-through entity that will hold our common stock and/or pre-funded warrants should
consult his, her or its tax advisor regarding the tax consequences of acquiring, holding and disposing of our common stock and/or
pre-funded warrants through a partnership or other pass-through entity, as applicable.
This
discussion is based on current provisions of the U.S. Internal Revenue Code of 1986, as amended, which we refer to as the Code,
existing and proposed U.S. Treasury Regulations promulgated thereunder, current administrative rulings and judicial decisions,
all as in effect as of the date of this prospectus and, all of which are subject to change or to differing interpretation, possibly
with retroactive effect. Any such change or differing interpretation could alter the tax consequences to non-U.S. holders described
in this prospectus. There can be no assurance that the Internal Revenue Service, which we refer to as the IRS, will not challenge
one or more of the tax consequences described herein. We assume in this discussion that a non-U.S. holder holds shares of our
common stock and/or pre-funded warrant as a capital asset within the meaning of Section 1221 of the Code, generally property held
for investment.
This
discussion does not address all aspects of U.S. federal income taxation that may be relevant to a particular non-U.S. holder in
light of that non-U.S. holder’s individual circumstances nor does it address any U.S. state, local or non-U.S. taxes, the
alternative minimum tax, the Medicare tax on net investment income, the rules regarding qualified small business stock within
the meaning of Section 1202 of the Code, or any other aspect of any U.S. federal tax other than the income tax. This discussion
also does not consider any specific facts or circumstances that may apply to a non-U.S. holder and does not address the special
tax rules applicable to particular non-U.S. holders, such as:
|
|
●
|
insurance
companies;
|
|
|
●
|
tax-exempt
or governmental organizations;
|
|
|
●
|
financial
institutions;
|
|
|
●
|
brokers
or dealers in securities;
|
|
|
●
|
regulated
investment companies;
|
|
|
●
|
pension
plans;
|
|
|
●
|
“controlled
foreign corporations,” “passive foreign investment companies,” and
corporations that accumulate earnings to avoid U.S. federal income tax;
|
|
|
●
|
“qualified
foreign pension funds,” or entities wholly owned by a “qualified foreign
pension fund”;
|
|
|
●
|
persons
deemed to sell our common stock and/or pre-funded warrants under the constructive sale
provisions of the Code;
|
|
|
●
|
persons
that hold our common stock and/or pre-funded warrants as part of a straddle, hedge, conversion
transaction, synthetic security or other integrated investment; and
|
|
|
●
|
certain
U.S. expatriates.
|
This
discussion is for general information only and is not tax advice. Accordingly, all prospective non-U.S. holders of our common
stock and/or pre-funded warrants should consult their tax advisors with respect to the U.S. federal, state, local and non-U.S.
tax consequences of the purchase, ownership and disposition of our common stock and/or pre-funded warrants.
Treatment
of Pre-funded Warrants
Although
it is not entirely free from doubt, a pre-funded warrant should be treated as a share of our common stock for U.S. federal income
tax purposes and a holder of pre-funded warrants should generally be taxed in the same manner as a holder of common stock, as
described below. Accordingly, no gain or loss should be recognized upon the exercise of a pre-funded warrant and, upon exercise,
the holding period of a pre-funded warrant should carry over to the share of common stock received. Similarly, the tax basis of
the pre-funded warrant should carry over to the share of common stock received upon exercise, increased by the exercise price
of $0.01 per share. Each holder should consult his, her or its own tax advisor regarding the risks associated with the acquisition
of pre-funded warrants pursuant to this offering (including potential alternative characterizations). The balance of this discussion
generally assumes that the characterization described above is respected for U.S. federal income tax purposes.
Distributions
on Our Common Stock
Distributions,
if any, on our common stock will constitute dividends for U.S. federal income tax purposes to the extent paid from our current
or accumulated earnings and profits, as determined under U.S. federal income tax principles. If a distribution exceeds our current
and accumulated earnings and profits, the excess will be treated as a tax-free return of the non-U.S. holder’s investment,
up to such holder’s tax basis in the common stock. Any remaining excess will be treated as capital gain, subject to the
tax treatment described below in “Gain on Sale or Other Taxable Disposition of Our Common Stock and/or Pre-Funded Warrants.”
Any such distributions will also be subject to the discussions below under the sections titled “Backup Withholding and Information
Reporting” and “Withholding and Information Reporting Requirements—FATCA.”
Subject
to the discussion in the following two paragraphs in this section, dividends paid to a non-U.S. holder generally will be subject
to withholding of U.S. federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty
between the United States and such holder’s country of residence.
Dividends
that are treated as effectively connected with a trade or business conducted by a non-U.S. holder within the United States and,
if an applicable income tax treaty so provides, that are attributable to a permanent establishment or a fixed-base maintained
by the non-U.S. holder within the United States, are generally exempt from the 30% withholding tax if the non-U.S. holder satisfies
applicable certification and disclosure requirements. However, such U.S. effectively connected income, net of specified deductions
and credits, is taxed at the same graduated U.S. federal income tax rates applicable to United States persons (as defined in the
Code). Any U.S. effectively connected income received by a non-U.S. holder that is a corporation may also, under certain circumstances,
be subject to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable
income tax treaty between the United States and such holder’s country of residence.
A
non-U.S. holder of our common stock who claims the benefit of an applicable income tax treaty between the United States and such
holder’s country of residence generally will be required to provide a properly executed IRS Form W-8BEN or W-8BEN-E (or
successor form) to the applicable withholding agent and satisfy applicable certification and other requirements. Non-U.S. holders
are urged to consult their tax advisors regarding their entitlement to benefits under a relevant income tax treaty. A non-U.S.
holder that is eligible for a reduced rate of U.S. withholding tax under an income tax treaty may obtain a refund or credit of
any excess amounts withheld by timely filing a U.S. tax return with the IRS.
The
pre-funded warrants are not entitled to any distributions until such warrant is exercised.
Gain
on Sale or Other Taxable Disposition of Our Common Stock and/or Pre-Funded Warrants
Subject
to the discussions below under “Backup Withholding and Information Reporting” and “Withholding and Information
Reporting Requirements—FATCA,” a non-U.S. holder generally will not be subject to any U.S. federal income tax on any
gain realized upon such holder’s sale or other taxable disposition of shares of our common stock and/or Pre-Funded Warrants
unless:
|
|
●
|
the
gain is effectively connected with the non-U.S. holder’s conduct of a U.S. trade
or business and, if an applicable income tax treaty so provides, is attributable to a
permanent establishment or a fixed-base maintained by such non-U.S. holder in the United
States, in which case the non-U.S. holder generally will be taxed on a net income basis
at the graduated U.S. federal income tax rates applicable to United States persons (as
defined in the Code) and, if the non-U.S. holder is a foreign corporation, the branch
profits tax described above in “Distributions on Our Common Stock” also may
apply;
|
|
|
|
|
|
|
●
|
the
non-U.S. holder is a nonresident alien individual who is present in the United States
for 183 days or more in the taxable year of the disposition and certain other conditions
are met, in which case the non-U.S. holder will be subject to a 30% tax (or such lower
rate as may be specified by an applicable income tax treaty between the United States
and such holder’s country of residence) on the net gain derived from the disposition,
which may be offset by certain U.S. source capital losses of the non-U.S. holder, if
any (even though the individual is not considered a resident of the United States), provided
that the non-U.S. holder has timely filed U.S. federal income tax returns with respect
to such losses; or
|
|
|
●
|
we
are, or have been, at any time during the five-year period preceding such sale or other
taxable disposition (or the non-U.S. holder’s holding period, if shorter) a “U.S.
real property holding corporation,” unless any class of our stock is regularly
traded on an established securities market and the non-U.S. holder disposes of such class
of stock and holds no more than 5% of such class of stock, directly or indirectly, actually
or constructively, during the shorter of the 5-year period ending on the date of the
disposition or the period that the non-U.S. holder held such class of stock. Generally,
a corporation is a U.S. real property holding corporation only if the fair market value
of its U.S. real property interests equals or exceeds 50% of the sum of the fair market
value of its worldwide real property interests plus its other assets used or held for
use in a trade or business. Although there can be no assurance, we do not believe that
we are, or have been, a U.S. real property holding corporation, or that we are likely
to become one in the future. Our pre-funded warrants are expected to constitute a class
of stock for purposes of the rules described above. However, we do not expect our pre-funded
warrants to be regularly traded on an established securities market for purposes of such
rule. Further, there can be no assurance that our common stock will be regularly traded
on an established securities market for purposes of the rules described above.
|
Backup
Withholding and Information Reporting
We
must report annually to the IRS and to each non-U.S. holder the gross amount of the distributions on our common stock paid to
such holder and the tax withheld, if any, with respect to such distributions. Non-U.S. holders may have to comply with specific
certification procedures to establish that the holder is not a United States person (as defined in the Code) in order to avoid
backup withholding at the applicable rate with respect to dividends on our common stock. Dividends paid to non-U.S. holders subject
to withholding of U.S. federal income tax, as described above in “Distributions on Our Common Stock,” generally will
be exempt from U.S. backup withholding.
Information
reporting and backup withholding will generally apply to the proceeds of a disposition of our common stock and/or pre-funded warrants
by a non-U.S. holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status
as a non-U.S. holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting
and backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected
outside the United States through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected
through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar
to dispositions effected through a U.S. office of a broker.
Non-U.S.
holders should consult their tax advisors regarding the application of the information reporting and backup withholding rules
to them. Copies of information returns may be made available to the tax authorities of the country in which the non-U.S. holder
resides or is incorporated under the provisions of a specific treaty or agreement. Backup withholding is not an additional tax.
Any amounts withheld under the backup withholding rules from a payment to a non-U.S. holder can be refunded or credited against
the non-U.S. holder’s U.S. federal income tax liability, if any, provided that an appropriate claim is filed with the IRS
in a timely manner.
Withholding
and Information Reporting Requirements—FATCA
Provisions
of the Code commonly referred to as the Foreign Account Tax Compliance Act, or FATCA, generally impose a U.S. federal
withholding tax at a rate of 30% on payments of dividends on our common stock paid to a foreign entity unless (i) if the
foreign entity is a “foreign financial institution,” such foreign entity undertakes certain due diligence,
reporting, withholding, and certification obligations, (ii) if the foreign entity is not a “foreign financial
institution,” such foreign entity identifies certain of its U.S. investors, if any, or (iii) the foreign entity is
otherwise exempt under FATCA. Such withholding may also apply to gross proceeds from the sale or other disposition of our
common stock and/or pre-funded warrants, although under recently proposed U.S. Treasury Regulations, no withholding would
apply to such gross proceeds. The preamble to the proposed regulations specifies that taxpayers (including withholding
agents) are permitted to rely on the proposed regulations pending finalization. Under certain circumstances, a non-U.S.
holder may be eligible for refunds or credits of this withholding tax. An intergovernmental agreement between the United
States and an applicable foreign country may modify the requirements described in this paragraph. Non-U.S. holders should
consult their tax advisors regarding the possible implications of this legislation on their investment in our common stock
and/or pre-funded warrants and the entities through which they hold our common stock and/or pre-funded warrants, including,
without limitation, the process and deadlines for meeting the applicable requirements to prevent the imposition of the 30%
withholding tax under FATCA.
UNDERWRITING
We
have entered into an underwriting agreement, dated , 2019, with A.G.P./Alliance Global Partners, acting as the representative
of the several underwriters named below, with respect to the shares of common stock and pre-funded warrants (if any). Subject
to certain conditions, we have agreed to sell to the underwriters, and the underwriters have severally agreed to purchase, the
shares of common stock and pre-funded warrants provided below opposite their respective names.
|
Underwriters
|
|
Number
of Shares
|
|
|
Number
of
Pre-Funded
Warrants
|
|
|
Total
|
|
|
A.G.P./Alliance
Global Partners
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
The
underwriters are committed to purchase all the shares of common stock and the pre-funded warrants offered by us other than those
covered by the option to purchase additional shares and pre-funded warrants described below. The obligations of the underwriters
may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the
underwriting agreement, the underwriters’ obligations are subject to customary conditions, representations and warranties
contained in the underwriting agreement, such as receipt by the underwriters of officers’ certificates and legal opinions.
Discount,
Commissions and Expenses
The
underwriters have advised us that they propose to offer the shares of common stock and the pre-funded warrants at the public offering
price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of
$ per share of common stock and pre-funded warrant. The underwriters may allow, and certain dealers
may reallow, a discount from the concession not in excess of $ per share of common stock and
pre-funded warrant to certain brokers and dealers. After this offering, the public offering price, concession and reallowance
to dealers may be changed by the representative. No such change shall change the amount of proceeds to be received by us as set
forth on the cover page of this prospectus. The shares of common stock and the pre-funded warrants are offered by the underwriters
as stated herein, subject to receipt and acceptance by them and subject to their right to reject any order in whole or in part.
The underwriters have informed us that they do not intend to confirm sales to any accounts over which they exercise discretionary
authority.
The
following table shows the underwriting discount payable to the underwriters by us in connection with this offering.
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
Per
Share
|
|
|
Per
Pre-
Funded
Warrant
|
|
|
Without
Over-
Allotment
|
|
|
With
Over-
Allotment
|
|
|
Public
offering price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Underwriting
discounts and commissions (7%)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds,
before expenses, to us
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
We
have agreed to reimburse the underwriters for accountable legal expenses not to exceed $85,000 and non-accountable expenses not
to exceed $20,000 in the aggregate. We estimate that expenses payable by us in connection with this offering, including reimbursement
of the underwriters’ out-of-pocket expenses, but excluding the underwriting discount referred to above, will be approximately
$ .
The
Representatives have informed us that they do not expect sales to accounts over which the underwriters have discretionary authority
to exceed 5% of the shares of common stock and the pre-funded warrants being offered.
Over-allotment
Option
We
have granted to the underwriters an option exercisable not later than 30 days after the date of this prospectus to purchase
up to additional shares of common stock at the
public offering price per share of common stock set forth on the cover page hereto less the underwriting discounts and
commissions. The underwriters may exercise the option solely to cover overallotments, if any, made in connection with this
offering. If any additional shares of common stock are purchased pursuant to the over-allotment option, the underwriters will
offer these shares of common stock on the same terms as those on which the other securities are being offered.
Indemnification
We
have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act and liabilities
arising from breaches of representations and warranties contained in the underwriting agreement, or to contribute to payments
that the underwriters may be required to make in respect of those liabilities.
Lock-up
Agreements
Our
directors and executive officers have entered into lock-up agreements. Under these agreements, these individuals have agreed,
subject to specified exceptions, not to sell or transfer any shares of common stock or securities convertible into, or exchangeable
or exercisable for, our shares of common stock during a period ending 90 days after the date of this prospectus, without first
obtaining the written consent of A.G.P./Alliance Global Partners. Specifically, these individuals have agreed, in part, not to:
|
|
●
|
offer,
pledge, sell, contract to sell, grant, lend or otherwise transfer or dispose of, directly or indirectly, any shares of common
stock or any securities convertible into or exercisable or exchangeable for shares of common stock, whether now owned or hereafter
acquired or with respect to which such person has or later acquires the power of disposition, whether any such transaction
is to be settled by delivery of our securities, in cash, or otherwise;
|
|
|
|
|
|
|
●
|
enter
into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership
of our securities, whether any such transaction is to be settled by delivery of our shares of common stock, in cash or otherwise;
|
|
|
|
|
|
|
●
|
make
any demand for or exercise any right with respect to the registration of any of our securities; or
|
|
|
|
|
|
|
●
|
publicly
disclose the intention to make any offer, sale, pledge or disposition, or to enter into any transaction, swap, hedge or other
arrangement relating to any of our securities.
|
Notwithstanding
these limitations, these shares of common stock may be transferred under limited circumstances, including, without limitation,
by gift, will or intestate succession.
In
addition, we have agreed that, for a period of sixty (60) days from the date of this prospectus, we will not (i) offer, pledge,
sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right
or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any of our shares of common stock or
any securities convertible into or exercisable or exchangeable for our shares of common stock; (ii) file or caused to be filed
any registration statement with the SEC relating to the offering of any of our shares of common stock or any securities convertible
into or exercisable or exchangeable for our shares of common stock; (iii) enter into any swap or other arrangement that transfers
to another, in whole or in part, any of the economic consequences of ownership of capital stock of the Company, whether any such
transaction described in clause (i), (ii) or (iii) is to be settled by delivery of our shares of common stock or such other securities,
in cash or otherwise. However, we will not be precluded from selling over 25% of our outstanding common stock to a strategic investor,
so long as such investor agrees not to resell such shares into the public markets during such three month period.
Stabilization
In
connection with this offering, the underwriters may engage in over-allotment transactions, syndicate-covering transactions, stabilizing
transactions, penalty bids and purchases to cover positions created by short sales.
|
|
●
|
Stabilizing
transactions permit bids to purchase shares, so long as the stabilizing bids do not exceed a specified maximum and are engaged
in for the purpose of preventing or retarding a decline in the market price of the shares while the offering is in progress.
|
|
|
●
|
Over-allotment
transactions involve sales by the underwriters of shares of common stock in excess of the number of shares the underwriters
are obligated to purchase. This creates a syndicate short position, which may be either a covered short position or a naked
short position. In a covered short position, the number of shares of common stock over-allotted by the underwriters is not
greater than the number of shares of common stock that they may purchase in the over-allotment option. In a naked short position,
the number of securities involved is greater than the number of shares in the over-allotment option. The underwriters may
close out any short position by exercising their over-allotment option and/or purchasing common stock in the open market.
|
|
|
|
|
|
|
●
|
Syndicate
covering transactions involve purchases of common stock in the open market after the distribution has been completed in order
to cover syndicate short positions. In determining the source of common stock to close out the short position, the underwriters
will consider, among other things, the price of common stock available for purchase in the open market as compared to the
price at which they may purchase common stock through exercise of the over-allotment option. If the underwriters sell more
shares of common stock than could be covered by exercise of the over-allotment option, and, therefore, have a naked short
position, the position can be closed out only by buying common stock in the open market. A naked short position is more likely
to be created if the underwriters are concerned that after pricing there could be downward pressure on the price of the shares
in the open market that could adversely affect investors who purchase in the offering.
|
|
|
|
|
|
|
●
|
Penalty
bids permit the underwriters to reclaim a selling concession from a syndicate member when the common stock originally sold
by that syndicate member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions.
|
These
stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market
price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price
of our common stock in the open market may be higher than it would otherwise be in the absence of these transactions.
Neither
we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have
on the prices of our securities. These transactions may occur on the Nasdaq Global Select Market or on any other trading market.
If any of these transactions are commenced, they may be discontinued without notice at any time.
Passive
Market Making
In
connection with this offering, the underwriters and any selling group members may engage in passive market making transactions
in our common shares on Nasdaq in accordance with Rule 103 of Regulation M under the Exchange Act during a period before the commencement
of offers or sales of common shares and extending through the completion of the distribution. A passive market maker must display
its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered
below the passive market maker’s bid that bid must then be lowered when specified purchase limits are exceeded.
Electronic
Distribution
This
prospectus in electronic format may be made available on websites or through other online services maintained by one or more of
the underwriters, or by their affiliates. Other than this prospectus in electronic format, the information on any underwriter’s
website and any information contained in any other website maintained by an underwriter is not part of this prospectus or the
registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or any underwriter in
its capacity as underwriter, and should not be relied upon by investors.
Other
From
time to time, certain of the underwriters and/or their affiliates have provided, and may in the future provide, various investment
banking and other financial services for us for which services they have received and, may in the future receive, customary fees.
In the course of their businesses, the underwriters and their affiliates may actively trade our securities or loans for their
own account or for the accounts of customers, and, accordingly, the underwriters and their affiliates may at any time hold long
or short positions in such securities or loans.
Offer
restrictions outside the United States
Other
than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities
offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus
and the accompanying prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering
material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction,
except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons
into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to
the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of
an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This
prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian
Securities and Investments Commission and does not purport to include the information required of a disclosure document under
Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made
to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act
under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available
in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance
that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and,
unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities
sold to the offeree within twelve (12) months after its transfer to the offeree under this prospectus.
China
The
information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in
the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau
Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal
or natural persons other than directly to “qualified domestic institutional investors.”
European
Economic Area-Belgium, Germany, Luxembourg and Netherlands
The
information in this document has been prepared on the basis that all offers of common stock will be made pursuant to an exemption
under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic
Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An
offer to the public of the securities has not been made, and may not be made, in a Relevant Member State except pursuant
to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
(a)
to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated,
whose corporate purpose is solely to invest in securities;
(b)
to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total
balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements)
and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial
statements);
(c)
to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus
Directive) subject to obtaining the prior consent of the Company or any underwriter for any such offer; or
(d)
in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of common stock
shall result in a requirement for the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus Directive.
France
This
document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers)
in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier)
and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”).
The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This
document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval
in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such
offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés)
acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D. 744-1,
D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number
of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance
with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing
regulation.
Pursuant
to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the common stock cannot be distributed
(directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and
L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The
information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been
filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering
of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus
Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly
in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations
and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
The
securities offered by this prospectus has not been approved or disapproved by the Israeli Securities Authority, or “ISA,”
nor have such been registered for sale in Israel. The securities may not be offered or sold, directly or indirectly, to the public
in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the
offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness,
or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the
public of the offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance
with the Israeli securities laws and regulations.
Italy
The
offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission
(Commissione Nazionale per le Società e la Borsa, “CONSOB”) pursuant to the Italian securities legislation
and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered
or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree
No. 58”), other than:
|
|
●
|
to
Italian qualified investors, as defined in Article 100 of Decree no. 58 by reference to Article 34-ter of CONSOB Regulation
no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and
|
|
|
|
|
|
|
●
|
in
other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter
of Regulation No. 11971 as amended.
|
Any
offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding
placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
|
|
●
|
made
by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative
Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other
applicable laws; and
|
|
|
|
|
|
|
●
|
in
compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws.
|
Any
subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement
rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure
to comply with such rules may result in the sale of such shares being declared null and void and in the liability of the entity
transferring the shares for any damages suffered by the investors.
Japan
The
securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law
of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements
applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article
2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securitiess may not be offered or sold,
directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors.
Any Qualified Institutional Investor who acquires common stock may not resell them to any person in Japan that is not a Qualified
Institutional Investor, and acquisition by any such person of common stock is conditional upon the execution of an agreement to
that effect.
Portugal
This
document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários)
in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários).
The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese
Securities Market Commission (Comissão do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly,
may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances
that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of
shares in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code).
Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This
document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority).
Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances
that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980)
om handel med finansiella instrument)). Any offering of common stock in Sweden is limited to persons who are “qualified
investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they
may not distribute it or the information contained in it to any other person.
Switzerland
The
securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or
on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the
disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure
standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange
or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the shares may
be publicly distributed or otherwise made publicly available in Switzerland.
Neither
this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss
regulatory authority. In particular, this document will not be filed with, and the offer of common stock will not be supervised
by, the Swiss Financial Market Supervisory Authority.
This
document is personal to the recipient only and not for general circulation in Switzerland.
United
Arab Emirates
Neither
this document nor the securities has been approved, disapproved or passed on in any way by the Central Bank of the United Arab
Emirates or any other governmental authority in the United Arab Emirates, nor has the Company received authorization or licensing
from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or
sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of
an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or
redemption of such shares, may be rendered within the United Arab Emirates by us.
No
offer or invitation to subscribe for the securities is valid or permitted in the Dubai International Financial Centre.
United
Kingdom
Neither
the information in this document nor any other document relating to the offer has been delivered for approval to the Financial
Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets
Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the securities. This
document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA)
in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying
letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section
86(1) FSMA. This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed
by recipients to any other person in the United Kingdom.
Any
invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with
the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused
to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to the Company.
In
the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience
in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets
Act 2000 (Financial Promotions) Order 2005 (“FPO”); (ii) who fall within the categories of persons referred to in
Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO; or (iii) to whom it may otherwise
be lawfully communicated (together “relevant persons”). The investments to which this document relates are available
only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is
not a relevant person should not act or rely on this document or any of its contents.
LEGAL
MATTERS
The
validity of the common stock and pre-funded warrants offered in this prospectus will be passed upon for us by Goodwin Procter
LLP, New York, NY. Certain legal matters will be passed upon for the underwriters by Zysman, Aharoni, Gayer and Sullivan &
Worcester LLP, New York, NY.
EXPERTS
The
consolidated financial statements of Advaxis, Inc. appearing in the Annual Report on Form 10-K for the year ended October 31,
2018 and 2017 and the effectiveness of internal control over financial reporting as of October 31, 2018 and 2017 have been audited
by Marcum LLP, independent registered public accounting firm, as set forth in their reports thereon, included therein, and incorporated
herein by reference. Such consolidated financial statements are incorporated herein by reference in reliance upon such report
given on the authority of such firm as experts in accounting and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock
and pre-funded warrants offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain
all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information
with respect to us and the common stock and pre-funded warrants offered hereby, reference is made to the registration statement
and the exhibits and schedules filed therewith. Statements contained in this prospectus regarding the contents of any contract
or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement
is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration
statement. The SEC maintains a website that contains reports, proxy and information statements and other information regarding
registrants, including this registration statement that file electronically with the SEC. The address is
www.sec.gov
.
We
are subject to the information and periodic reporting requirements of the Exchange Act. Under the Exchange Act, we will file annual,
quarterly and current reports, as well as proxy statements and other information with the SEC. These periodic reports, proxy statements,
and other information will be available for inspection and copying at the SEC’s Public Reference Room and the website of
the SEC referred to above.
INCORPORATION
OF CERTAIN INFORMATION BY REFERENCE
The
SEC allows us to “incorporate by reference” information from other documents that we file, which means that we can
disclose important information to you by referring you to those documents. The information incorporated by reference is considered
to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with
the SEC prior to the date of this prospectus. We incorporate by reference into this prospectus and the registration statement
of which this prospectus is a part the information or documents listed below that we have filed with the SEC (SEC File No. 001-38268):
|
|
●
|
our
Annual Report on Form 10-K for the year ended October 31, 2018, filed with the SEC on January 11, 2019;
|
|
|
|
|
|
|
●
|
our
Quarterly Reports on Form 10-Q for the quarters ended January 31, 2019, filed with the SEC on March 12, 2019, and April 30,
2019, filed with the SEC on June 10, 2019;
|
|
|
|
|
|
|
●
|
our
Definitive Proxy Statement on Schedule 14A, filed with the SEC on January 11, 2019; and
|
|
|
|
|
|
|
●
|
our
Current Reports on Form 8-K filed with the SEC on December 13, 2018, January 23, 2019, February 7, 2019, February 22, 2019,
March 1, 2019, March 14, 2019, March 15, 2019, March 29, 2019, April 5, 2019, April 15, 2019, May 13, 2019, May 15, 2019 and
June 27, 2019.
|
We
will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference
in this prospectus, including exhibits to these documents. You should direct any requests for documents to our corporate headquarters
at the following address: 305 College Road East, Princeton, New Jersey 08540 Attn: Molly Henderson, or by calling (609) 452-9813.
You
also may access these filings on our website at
www.advaxis.com.
We do not incorporate the information contained on or
accessible through our website into this prospectus or any supplement to this prospectus and you should not consider any information
on, or that can be accessed through, our website as part of this prospectus or any supplement to this prospectus (other than those
filings with the SEC that we specifically incorporate by reference into this prospectus or any supplement to this prospectus).
Any
statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified,
superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes
or replaces such statement.
Shares
of Common Stock
Pre-Funded
Warrants to Purchase Up to Shares of Common Stock

PROSPECTUS
A.G.P.
Through
and including , 2019 (the 25th day after the date of this prospectus), all dealers that effect transactions in these securities,
whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’
obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
|
Item
13.
|
Other
Expenses of Issuance and Distribution
|
The
following table sets forth the costs and expenses, other than the underwriting discounts and commissions, payable by the registrant
in connection with the sale of common stock being registered. All amounts are estimates except for the SEC registration fee and
the Financial Industry Regulatory Authority, or FINRA, filing fee.
|
Item
|
|
Amount to be
paid
|
|
|
SEC registration fee
|
|
$
|
1,818
|
|
|
FINRA filing fee
|
|
|
2,750
|
|
|
Printing and engraving expenses
|
|
|
*
|
|
|
Legal fees and expenses
|
|
|
*
|
|
|
Accounting fees and expenses
|
|
|
*
|
|
|
Transfer Agent fees and expenses
|
|
|
*
|
|
|
Miscellaneous expenses
|
|
|
*
|
|
|
|
|
|
*
|
|
|
Total
|
|
$
|
*
|
|
|
*
|
To
be provided by amendment.
|
|
Item
14.
|
Indemnification
of Directors and Officers
|
Section
145 of the Delaware General Corporation Law, or the DGCL, authorizes a corporation to indemnify its directors and officers against
liabilities arising out of actions, suits and proceedings to which they are made or threatened to be made a party by reason of
the fact that they have served or are currently serving as a director or officer to a corporation. The indemnity may cover expenses
(including attorneys’ fees) judgments, fines and amounts paid in settlement actually and reasonably incurred by the director
or officer in connection with any such action, suit or proceeding. Section 145 permits corporations to pay expenses (including
attorneys’ fees) incurred by directors and officers in advance of the final disposition of such action, suit or proceeding.
In addition, Section 145 provides that a corporation has the power to purchase and maintain insurance on behalf of its directors
and officers against any liability asserted against them and incurred by them in their capacity as a director or officer, or arising
out of their status as such, whether or not the corporation would have the power to indemnify the director or officer against
such liability under Section 145.
We
have adopted provisions in our certificate of incorporation and bylaws to be in effect at the consummation of this offering that
limit or eliminate the personal liability of our directors to the fullest extent permitted by the DGCL, as it now exists or may
in the future be amended. Consequently, a director will not be personally liable to us or our stockholders for monetary damages
or breach of fiduciary duty as a director, except for liability for:
|
|
●
|
any breach of the director’s duty of loyalty to us or our stockholders;
|
|
|
|
|
|
|
●
|
any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
|
|
|
|
|
|
|
●
|
any unlawful payments related to dividends or unlawful stock purchases, redemptions or other distributions; or
|
|
|
|
|
|
|
●
|
any transaction from which the director derived an improper personal benefit.
|
These
limitations of liability do not alter director liability under the federal securities laws and do not affect the availability
of equitable remedies such as an injunction or rescission.
In
addition, our bylaws provide that:
|
|
●
|
we
will indemnify our directors, officers and, in the discretion of our board of directors, certain employees to the fullest
extent permitted by the DGCL, as it now exists or may in the future be amended; and
|
|
|
|
|
|
|
●
|
we
will advance reasonable expenses, including attorneys’ fees, to our directors and, in the discretion of our board of
directors, to our officers and certain employees, in connection with legal proceedings relating to their service for or on
behalf of us, subject to limited exceptions.
|
We
have entered into indemnification agreements with each of our directors and intend to enter into such agreements with certain
of our executive officers. These agreements provide that we will indemnify each of our directors, certain of our executive officers
and, at times, their affiliates to the fullest extent permitted by Delaware law. We will advance expenses, including attorneys’
fees (but excluding judgments, fines and settlement amounts), to each indemnified director, executive officer or affiliate in
connection with any proceeding in which indemnification is available and we will indemnify our directors and officers for any
action or proceeding arising out of that person’s services as a director or officer brought on behalf of the Company and/or
in furtherance of our rights. Additionally, each of our directors may have certain rights to indemnification, advancement of expenses
and/or insurance provided by their affiliates, which indemnification relates to and might apply to the same proceedings arising
out of such director’s services as a director referenced herein. Nonetheless, we have agreed in the indemnification agreements
that the Company’s obligations to those same directors are primary and any obligation of the affiliates of those directors
to advance expenses or to provide indemnification for the expenses or liabilities incurred by those directors are secondary.
We
also maintain general liability insurance which covers certain liabilities of our directors and officers arising out of claims
based on acts or omissions in their capacities as directors or officers, including liabilities under the Securities Act.
The
underwriting agreement filed as Exhibit 1.1 to this registration statement provides for indemnification of us and our directors
and officers by the underwriters against certain liabilities under the Securities Act and the Exchange Act.
|
Item
15.
|
Recent
Sales of Unregistered Securities
|
In
the three years preceding the filing of this registration statement, we have issued the following securities which were not registered
under the Securities Act:
|
|
1.
|
On
January 7, 2016, we issued 5,000 shares of Common Stock to an accredited investor as payment for consulting services.
|
|
|
|
|
|
|
2.
|
On
August 11, 2016, we issued 28,838 shares of Common Stock to accredited investors as payment for consulting services.
|
|
|
|
|
|
|
3.
|
On
August 31, 2016, we issued 974 shares of Common Stock to its Executive Officers, pursuant to their Employment Agreements.
|
|
|
|
|
|
|
4.
|
On
September 30, 2016, we issued 962 shares of Common Stock to its Executive Officers, pursuant to their Employment Agreements.
|
|
|
|
|
|
|
5.
|
On
October 19, 2016, we issued 20,000 shares of Common Stock to an accredited investor as payment for consulting services.
|
|
|
|
|
|
|
6.
|
On
October 31, 2016, we issued 972 shares of Common Stock to our Executive Officers, pursuant to their Employment Agreements.
|
|
|
|
|
|
|
7.
|
On
November 15, 2016, we issued 32,500 shares of Common Stock to accredited investors as payment for consulting services.
|
|
|
|
|
|
|
8.
|
On
November 30, 2016, we issued 1,205 shares of Common Stock to our Executive Officers, pursuant to their Employment Agreements.
|
|
|
|
|
|
|
9.
|
On
December 30, 2016, we issued 2,011 shares of Common Stock to its Executive Officers, pursuant to their Employment Agreements.
|
|
|
10.
|
On
October 24, 2017, we issued 10,000 shares of Common Stock to accredited investors as payment for consulting services.
|
|
|
|
|
|
|
11.
|
On
October 31, 2017 we issued 1,322 shares of Common Stock to our Executive Officers, pursuant to their Employment Agreements.
|
|
|
|
|
|
|
12.
|
On
November 30, 2017 we issued 2,919 shares of Common Stock to our Executive Officers, pursuant to their Employment Agreements.
|
|
|
|
|
|
|
13.
|
On
December 29, 2017 we issued 1,968 shares of Common Stock to its Executive Officers, pursuant to their Employment Agreements.
|
|
|
|
|
|
|
14.
|
On
January 31, 2018 we issued 1,839 shares of Common Stock to its Executive Officers, pursuant to their Employment Agreements.
|
|
|
|
|
|
|
15.
|
On
February 28, 2018 we issued 3,340 shares of Common Stock to its Executive Officers, pursuant to their Employment Agreements.
|
|
|
|
|
|
|
16.
|
On
March 7, 2018, we issued 8,648 shares of Common Stock to an employee.
|
|
|
|
|
|
|
17.
|
On
March 29, 2018, we issued 2,889 shares of common stock to its Executive Officers, pursuant to their Employment Agreements
|
|
|
|
|
|
|
18.
|
On
April 17, 2018, we issued 13,949 shares of Common Stock to an employee.
|
|
|
|
|
|
|
19.
|
On
April 30, 2018, we issued 2,902 shares of common stock to its Executive Officers, pursuant to their Employment Agreements.
|
|
|
|
|
|
|
20.
|
On
May 31, 2018, we issued 2,707 shares of common stock to its Executive Officers, pursuant to their Employment Agreements.
|
|
|
21.
|
On
May 31, 2018, we issued 26,112 shares of Common Stock to an employee.
|
|
|
|
|
|
|
22.
|
On
June 29, 2018, we issued 1,516 shares of common stock to its Executive Officers, pursuant to their Employment Agreements.
|
|
|
|
|
|
|
23.
|
On
July 31, 2018, we issued 1,505 shares of common stock to an Executive Officer, pursuant to his Employment Agreement.
|
No
underwriters were involved in the foregoing issuances of securities. The offers, sales and issuances of the securities described
above were deemed to be exempt from registration under the Securities Act in reliance upon Rule 701 or Section 4(a)(2) of the
Securities Act. The offers, sales and issuances of the securities that were deemed to be exempt in reliance on Rule 701 were transactions
under compensatory benefit plans and contracts relating to compensation as provided under Rule 701. The offers, sales and issuances
of the securities that were deemed to be exempt in reliance upon Section 4(a)(2) were each transactions not involving any public
offering, and all recipients of these securities were accredited investors within the meaning of Rule 501 of Regulation D of the
Securities Act who were acquiring the applicable securities for investment and not distribution and had represented that they
could bear the risks of the investment. Each of the recipients of securities in these transactions had adequate access, through
employment, business or other relationships, to information about us.
|
Item
16.
|
Exhibits
and financial statement schedules
|
(a)
Exhibits
See
the Exhibit Index List below, which is incorporated by reference herein.
|
|
Exhibit
Number
|
|
Description
of Exhibits
|
|
|
|
|
|
|
|
1.01*
|
|
Form
of Underwriting Agreement
|
|
|
|
|
|
|
|
3.1
|
|
Amended and Restated Certificate of Incorporation. Incorporated by reference to Annex C to DEF 14A Proxy Statement filed with the SEC on May 15, 2006.
|
|
|
|
|
|
|
|
3.2
|
|
Certificate of Designations of Preferences, Rights and Limitations of Series A Preferred Stock of the registrant, dated September 24, 2009. Incorporated by reference to Exhibit 4.1 to Current Report on Form 8-K filed with the SEC on September 25, 2009.
|
|
|
|
|
|
|
|
3.3
|
|
Certificate of Designations of Preferences, Rights and Limitations of Series B Preferred Stock of the registrant, dated July 19, 2010. Incorporated by reference to Exhibit 4.1 to Current Report on Form 8-K filed with the SEC on July 20, 2010.
|
|
|
|
|
|
|
|
3.4
|
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation filed with the Delaware Secretary of State on August 16, 2012. Incorporated by reference to Exhibit 3.1 to Current Report on Form 8-K filed with the SEC on August 17, 2012.
|
|
|
|
|
|
|
|
3.5
|
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation filed with the Delaware Secretary of State on July 11, 2013 (reverse stock split). Incorporated by reference to Exhibit 3.1 to Current Report on Form 8-K filed with the SEC on July 15, 2013.
|
|
|
|
|
|
|
|
3.6
|
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation filed with the Delaware Secretary of State on July 12, 2013 (reverse stock split). Incorporated by reference to Exhibit 3.2 to Current Report on Form 8-K filed with the SEC on July 15, 2013.
|
|
|
|
|
|
|
|
3.7
|
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation filed with the Delaware Secretary of State on July 9, 2014. Incorporated by reference to Exhibit 3.1 to Current Report on Form 8-K filed with the SEC on July 10, 2014.
|
|
|
|
|
|
|
|
3.8
|
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation filed with the Delaware Secretary of State on March 10, 2016. Incorporated by reference to Exhibit 3.1 to Current Report on Form 8-K filed with the SEC on March 11, 2016.
|
|
|
|
|
|
|
|
3.9
|
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation filed with the Delaware Secretary of State on March 21, 2018. Incorporated by reference to Exhibit 3.1 to Current Report on Form 8-K filed with the SEC on March 21, 2018.
|
|
|
|
|
|
|
|
3.10
|
|
Certificate of Amendment of the Amended and Restated Certificate of Incorporation of Advaxis, Inc. filed with the Delaware Secretary of State on February 28, 2019, Incorporated by reference to Exhibit 3.1 to Current Form on Form 8-K filed with the SEC on March 1, 2019.
|
|
|
|
|
|
|
|
3.11
|
|
Certificate of Amendment of the Amended and Restated Certificate of Incorporation of Advaxis, Inc. filed with the Delaware Secretary of State on March 29 28, 2019, Incorporated by reference to Exhibit 3.1 to Current Form on Form 8-K filed with the SEC on March 29, 2019
|
|
|
4.5
|
|
Form of Warrant Agency Agreement, dated as of September 11, 2018 between Advaxis, Inc. and Continental Stock Transfer and Trust Company (and Form of Warrant contained therein), Incorporated by reference to Exhibit 4.1 to Current Report on Form 8-K filed with the SEC on September 11, 2018.
|
|
|
|
|
|
|
|
4.6
|
|
Form of Common Stock Warrant dated September 11, 2018 (included in Exhibit 4.5)
|
|
|
|
|
|
|
|
4.7*
|
|
Form
of Pre-Funded Warrant
|
|
|
|
|
|
|
|
5.1*
|
|
Opinion
of Goodwin Procter LLP
|
|
|
|
|
|
|
|
10.1
|
|
2004 Stock Option Plan of the registrant. Incorporated by reference to Exhibit 4.1 to Report on Form S-8 filed with the SEC on December 1, 2005.
|
|
|
|
|
|
|
|
10.2
|
|
2005 Stock Option Plan of the registrant. Incorporated by reference to Annex A to DEF 14A Proxy Statement filed with the SEC on May 15, 2006.
|
|
|
|
|
|
|
|
10.3
|
|
License Agreement, between the Trustees of the University of Pennsylvania and the registrant dated as of June 17, 2002, as Amended and Restated on February 13, 2007. Incorporated by reference to Exhibit 10.11 to Annual Report on Form 10-KSB filed with the SEC on February 13, 2007.
|
|
|
10.4
|
|
Amended and Restated 2009 Stock Option Plan of the registrant. Incorporated by reference to Annex A to DEF 14A Proxy Statement filed with the SEC on April 30, 2010.
|
|
|
|
|
|
|
|
10.5
|
|
Second Amendment to the Amended and Restated Patent License Agreement between the registrant and the Trustees of the University of Pennsylvania dated as of May 10, 2010. Incorporated by reference to Exhibit 10.1 to Quarterly Report on Form 10-Q filed with the SEC on June 3, 2010.
|
|
|
|
|
|
|
|
10.6
|
|
2011 Omnibus Incentive Plan of registrant. Incorporated by reference to Annex A to DEF 14A Proxy Statement filed with the SEC on August 29, 2011.
|
|
|
|
|
|
|
|
10.7
|
|
Amendment No. 1 to the Advaxis, Inc. 2011 Employee Stock Purchase Plan. Incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the SEC on December 20, 2011.
|
|
|
|
|
|
|
|
10.8
|
|
Amendment No. 1, dated as of March 26, 2007, to the License Agreement, between the Trustees of the University of Pennsylvania and Advaxis, Inc. dated as of June 17, 2002, as amended and restated on February 13, 2007. Incorporated by reference to Exhibit 10.1 to Quarterly Report on Form 10-Q filed with the SEC on June 14, 2012.
|
|
|
|
|
|
|
|
10.9
|
|
Amendment No. 3, dated as of December 12, 2011, to the License Agreement, between the Trustees of the University of Pennsylvania and Advaxis, Inc. dated as of June 17, 2002, as amended and restated on February 13, 2007. Incorporated by reference to Exhibit 10.5 to Quarterly Report on Form 10-Q filed with the SEC on June 14, 2012.
|
|
|
|
|
|
|
|
10.10
|
|
Amendment No. 1 to 2011 Omnibus Incentive Plan of registrant. Incorporated by reference to Annex B to DEF 14A Proxy Statement filed with the SEC on July 19, 2012.
|
|
|
10.12
‡
|
|
Employment Agreement between Advaxis, Inc. and Robert Petit, dated September 26, 2013. Incorporated by reference to Exhibit 10.70 to Registration Statement on Form S-1/A (File No. 333-188637) filed with the SEC on September 27, 2013.
|
|
|
|
|
|
|
|
10.13
|
|
Exclusive License and Technology Transfer Agreement by and between Advaxis, Inc. and Global BioPharma, Inc., dated December 9, 2013. Incorporated by reference to Exhibit 10.79 to Annual Report on Form 10-K/A filed with the SEC on February 6, 2014.
|
|
|
|
|
|
|
|
10.14‡
|
|
Amendment No. 1, dated as of December 19, 2013, to the Employment Agreement by and between Advaxis, Inc. and Robert G. Petit. Incorporated by reference to Exhibit 10.82 to Annual Report on Form 10-K/A filed with the SEC on February 6, 2014.
|
|
|
|
|
|
|
|
10.15
|
|
Distribution and Supply Agreement, dated as of January 20, 2014, by and between Advaxis, Inc. and Biocon, Limited. Incorporated by reference to Exhibit 10.7 to Quarterly Report on Form 10-Q filed with the SEC on March 17, 2014.
|
|
|
|
|
|
|
|
10.16
|
|
Exclusive License Agreement, dated March 19, 2014, by and between Advaxis, Inc. and Aratana Therapeutics, Inc. Incorporated by reference to Exhibit 10.1 to Quarterly Report on Form 10-Q filed with the SEC on June 10, 2014.
|
|
|
|
|
|
|
|
10.17‡
|
|
Amendment No. 2, dated as of June 5, 2014, to the Employment Agreement by and between Advaxis, Inc. and Robert G. Petit. Incorporated by reference to Exhibit 10.6 to Quarterly Report on Form 10-Q filed with the SEC on June 10, 2014.
|
|
|
|
|
|
|
|
10.18
|
|
Clinical Trial Collaboration Agreement, dated July 21, 2014, by and between Advaxis, Inc. and MedImmune, LLC. Incorporated by reference to Exhibit 10.1 to Quarterly Report on Form 10-Q filed with the SEC on September 9, 2014.
|
|
|
|
|
|
|
|
10.19
|
|
5
th
Amendment to the Amended & Restated License Agreement, dated July 25, 2014, by and between Advaxis, Inc. and University of Pennsylvania. Incorporated by reference to Exhibit 10.2 to Quarterly Report on Form 10-Q filed with the SEC on September 9, 2014.
|
|
|
|
|
|
|
|
10.20
|
|
Amendment No. 2 to the Advaxis, Inc. 2011 Omnibus Incentive Plan, effective July 9, 2014. Incorporated by reference to Annex A to Current Report on Schedule 14A filed with the SEC on May 20, 2014.
|
|
|
|
|
|
|
|
10.21
|
|
Amended and Restated 2011 Omnibus Incentive Plan, dated September 8, 2014. Incorporated by reference to Exhibit 10.4 to Quarterly Report on Form 10-Q filed with the SEC on September 9, 2014.
|
|
|
|
|
|
|
|
10.22
|
|
Master Services Agreement for Technical Transfer and Clinical Supply, dated February 5, 2014, by and between Advaxis, Inc. and SynCo Bio Partners B.V. Incorporated by reference to Exhibit 10.1 to Current Report to Form 8-K filed with the SEC on February 11, 2014.
|
|
|
|
|
|
|
|
10.23
|
|
Clinical Trial Collaboration and Supply Agreement by and between Advaxis, Inc. and Merck & Co. dated August 22, 2014. Incorporated by reference to Exhibit 10.101 to Annual Report on Form 10-K filed with the SEC on January 6, 2015
|
|
|
|
|
|
|
|
10.24‡
|
|
Amendment No. 3, dated as of April 17, 2015, to the Employment Agreement by and between Advaxis, Inc. and Robert G. Petit. Incorporated by reference to Exhibit 10.6 to Quarterly Report on Form 10-Q filed with the SEC on June 15, 2015.
|
|
|
|
|
|
|
|
10.25
|
|
Exclusive License Agreement, dated August 25, 2015, by and between Advaxis, Inc. and Knight Therapeutics, Inc. Incorporated by reference to Exhibit 10.57 to Annual Report on Form 10-K filed with the SEC on January 8, 2016.
|
|
|
10.26‡
|
|
Amendment
No. 4, dated as of December 31, 2015, to the Employment Agreement by and between Advaxis, Inc. and Robert G. Petit. Incorporated
by reference to Exhibit 10.59 to Annual Report on Form 10-K filed with the SEC on January 8, 2016.
|
|
|
|
|
|
|
|
10.27
|
|
Co-Development and Commercialization Agreement between Advaxis, Inc. and Especificos Stendhal SA de CV dated February 3, 2016. Incorporated by reference to Exhibit 10.1 to Quarterly Report on Form 10-Q filed with the SEC on February 26, 2016.
|
|
|
|
|
|
|
|
10.28‡
|
|
Separation Agreement and General Release, dated July 6, 2017, between Advaxis, Inc. and Daniel J. O’Connor. Incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the SEC on July 7, 2017.
|
|
|
|
|
|
|
|
10.29
|
|
2015 Incentive Plan of registrant. Incorporated by reference to Annex A to DEF 14A Proxy Statement filed with the SEC on April 7, 2015.
|
|
|
|
|
|
|
|
10.30‡
|
|
Separation Agreement and General Release, dated April 23, 2018, between Advaxis, Inc. and Anthony Lombardo. Incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the SEC on April 23, 2018.
|
|
|
|
|
|
|
|
10.31‡
|
|
Employment Agreement between Advaxis, Inc. and Molly Henderson, dated June 6,2018. Incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the SEC on June 6, 2018.
|
|
|
|
|
|
|
|
14.1
|
|
Code of Business Conduct and Ethics dated July 9, 2014. Incorporated by reference to Exhibit 14.1 to Current Report on Form 8-K filed with the SEC on July 10, 2014.
|
|
|
|
|
|
|
|
23.1
|
|
Consent of Independent Registered Public Accounting Firm.
|
|
|
|
|
|
|
|
23.2*
|
|
Consent
of Goodwin Procter LLP (included in Exhibit 5.1).
|
|
|
|
|
|
|
|
24.1
|
|
Power of Attorney (included on signature page)
|
|
*
|
To
be filed by amendment.
|
|
|
|
|
‡
|
Denotes
management contract or compensatory plan or arrangement.
|
(b)
Financial statement schedules
Schedules
not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the
financial statements or notes thereto.
The
undersigned registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreement,
certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each
purchaser.
Insofar
as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons
of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the
Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred
or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding)
is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant
will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate
jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed
by the final adjudication of such issue.
The
undersigned registrant hereby undertakes:
|
|
(1)
|
To
file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
|
|
|
(i)
|
To
include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
|
|
|
|
|
|
|
(ii)
|
To
reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most
recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information
set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered
(if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low
or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant
to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum
aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration
statement;
|
|
|
|
|
|
|
(iii)
|
To
include any material information with respect to the plan of distribution not previously disclosed in the registration statement
or any material change to such information in the registration statement;
|
|
|
(2)
|
That,
for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be
deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities
at that time shall be deemed to be the initial bona fide offering thereof.
|
|
|
|
|
|
|
(3)
|
To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold
at the termination of the offering.
|
|
|
|
|
|
|
(4)
|
That,
for the purpose of determining liability under the Securities Act of 1933 to any purchaser in the initial distribution of
the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant
pursuant to this registration statement, regardless of the method used to sell the securities to the purchaser, if the securities
are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be
a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
|
|
|
(i)
|
Any
preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant
to Rule 424 (§230.424 of this chapter);
|
|
|
|
|
|
|
(ii)
|
Any
free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred
to by the undersigned registrant;
|
|
|
|
|
|
|
(iii)
|
The
portion of any other free writing prospectus relating to the offering containing material information about the undersigned
registrant or its securities provided by or on behalf of the undersigned registrant; and
|
|
|
|
|
|
|
(iv)
|
Any
other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
|
|
|
(5)
|
For
purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed
as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant
pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement
as of the time it was declared effective.
|
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Registration Statement on Form
S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Princeton, New Jersey on July 2,
2019.
|
|
ADVAXIS,
INC.
|
|
|
|
|
|
|
By:
|
/s/
Kenneth Berlin
|
|
|
Name:
|
Kenneth Berlin
|
|
|
Title:
|
President and
Chief Executive Officer
|
POWER
OF ATTORNEY
Each
person whose signature appears below hereby constitutes and appoints Kenneth Berlin and Molly Henderson, and each of them singly,
his true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him and in his name,
place and stead, in any and all capacities, to sign any or all amendments (including, without limitation, post-effective amendments)
to this Registration Statement and any subsequent registration statement filed by the Registrant pursuant to Rule 462(b) of the
Securities Act of 1933, which relates to this Registration Statement, and to file the same, with all exhibits thereto, and all
documents in connection herewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent,
full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises,
as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact
and agent, or his substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant
to the requirements of the Securities Act of 1933, as amended, this Registration Statement on Form S-1 has been signed by the
following persons in the capacities and on the dates indicated.
|
SIGNATURE
|
|
Title
|
|
DATE
|
|
|
|
|
|
|
|
/s/
Kenneth Berlin
|
|
|
|
|
|
Kenneth
Berlin
|
|
President,
Chief Executive Officer and Director
|
|
July
2, 2019
|
|
|
|
(Principal
Executive Officer)
|
|
|
|
|
|
|
|
|
|
/s/
Molly Henderson
|
|
|
|
|
|
Molly
Henderson
|
|
Chief
Financial Officer, Executive Vice President
|
|
July
2, 2019
|
|
|
|
(Principal
Financial and Accounting Officer)
|
|
|
|
|
|
|
|
|
|
/s/
David Sidransky
|
|
|
|
|
|
David
Sidransky
|
|
Chairman
of the Board
|
|
July
2, 2019
|
|
|
|
|
|
|
|
/s/
James Patton
|
|
|
|
|
|
James
Patton
|
|
Vice
Chairman of the Board
|
|
July
2, 2019
|
|
|
|
|
|
|
|
/s/
Richard Berman
|
|
|
|
|
|
Richard
Berman
|
|
Director
|
|
July
2, 2019
|
|
|
|
|
|
|
|
/s/
Samir Khleif
|
|
|
|
|
|
Samir
Khleif
|
|
Director
|
|
July
2, 2019
|
|
|
|
|
|
|
|
/s/
Roni Appel
|
|
|
|
|
|
Roni
Appel
|
|
Director
|
|
July
2, 2019
|
Ayala Pharmaceuticals (QX) (USOTC:ADXS)
Historical Stock Chart
From Mar 2024 to Apr 2024
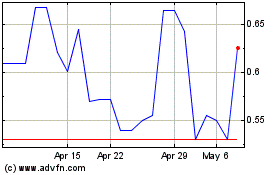
Ayala Pharmaceuticals (QX) (USOTC:ADXS)
Historical Stock Chart
From Apr 2023 to Apr 2024