Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) Slide � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � $PHU���119&� 3UHVHQWHG�E�� $QLO�5��'LZDQ��3K'� 3UHVLGHQW� �([HF��&KDLUPDQ 0RELOH�� ��� � ��� � ���� DGLZDQ#QDQRYLULFLGHV�FRP “Bind - Encapsulate - Destroy” 1DQR9LULFLGHV¶�7RSLFDO�'UXJ�&DQGLGDWH�$JDLQVW�6KLQJOHV��9=9��� 3UHSDULQJ�IRU�5HJXODWRU�7ULDOV�� $Q�$UUD�RI�,QGLFDWLRQV� LQ�+HUSHFLGHŒ�3URJUDP�$ORQH &RUSRUDWH�3UHVHQWDWLRQ 'HFHPEHU� �� �� ���� 1 NNVC (NYSE American) Issuer Free - Writing Prospectus Filed Pursuant to Rule 433 Form S - 1 Registration Number: 333 - 235306, filed November 29, 2019.

Slide � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � $PHU���119&� 6OLGH © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) NanoViricides , Inc. (the “Company”) has filed a Registration Statement on Form S - 1 (including a preliminary prospectus) with the Securities and Exchange Commission (the “SEC”) for the offering to which this communication relates. This document does not provide full di sclosure of all material facts relating to the securities offered. Before you invest, you should read the prospectus and other documents the company has filed with the SEC incorporated therein for more complete information about us and the offering, including the risk factors r ela ting to us and the securities offered. You may obtain these documents for free on the SEC website www.sec.gov or by contacting Aegis Capital Co rp., 810 7th Avenue, 18th Floor, New York, New York 10019, Attn: Syndicate Department, Email: syndicate@aegiscap.com , (212) 813 - 1010 . This free writing prospectus relates to the offering of shares of our common stock, par value $0.001 per share (the “Common St ock”). This company presentation may include forward - looking statements. All forward - looking statements are subject to a number of ris ks, uncertainties and assumptions, and you should not rely upon forward - looking statements as predictions of future events. You can identify forward - looking statements by words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “will,” “pl an,” “potential,” “predict,” “project,” “should,” “would” or the negative of those terms, and similar expressions that convey uncertainty of fut ure events or outcomes. This presentation discusses drug candidates that are under pre - clinical study and anticipated to advance into human clinical tr ials and that have not yet been approved for marketing by the Food and Drug Administration. No representation is made as to the sa fet y or efficacy of these product candidates for the use for which they are being studied. All forward - looking statements are based upon current estimates and expectations about future events and financial and other trends. There is no guarantee that future results, per for mance or events reflected in the forward - looking statements will be achieved or occur. No person assumes responsibility for the accuracy and completeness of the forward - looking statements, and, except as required by law, no person undertakes any obligation to update an y forward - looking statements for any reason after the date of this company presentation. Certain data in this presentation was obtained fr om various external sources. Neither the Company nor its affiliates, advisers or representatives have verified such data with independen t s ources. Accordingly, neither the Company nor any of its affiliates, advisers or representatives make any representations as to the ac cur acy or completeness of that data or to update such data after the date of this presentation. Such data involves risks and uncertaint ies and is subject to change based on various factors. Actual results could differ materially from those in forward - looking statements because of, among other reasons, the factors des cribed in the prospectus and in the periodic reports that we file with the SEC from time to time, including Forms 10 - K, 10 - Q and 8 - K and any a mendments thereto. The forward - looking statements are not guarantees of future performance. They are based on numerous assumptions that we believe are reasonable, but they are open to a wide range of uncertainties and business risks. � ,VVXHU�)UHH�:ULWLQJ�3URVSHFWXV� GDWHG�'HFHPEHU� �� �� ���� )LOHG�3XUVXDQW�WR�5XOH� ��� 5HODWLQJ�WR�3UHOLPLQDU�3URVSHFWXV� GDWHG�1RYHPEHU �� �� ���� 5HJLVWUDWLRQ 1R� ��� � ������ “Bind - Encapsulate - Destroy” NNVC (NYSE American)

6OLGH © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) 6OLGH � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � $PHU���119&� Presentation Layout Industry - Leading Platform Technology Exclusively Licensed from TheraCour Pharma, Inc. Developing Drugs that Virus May Not Escape due to Mutations Our Own cGMP - Capable Manufacturing, R&D, and Nanomedicine Characterization Facility Enables Rapid Development and Potential for Early Commercialization Revenues On Our Own Broad Pipeline with Multi - Billion Dollar Markets - Current Focus on HerpeCide™ Program with a Franchise of Drugs Lead Drug Candidate - NV - HHV - 101 for Shingles Rash Indication Investment Thesis - Company Valuation, cGMP Manufacturing Capabilities, Platform Technology, Multiple Candidates and Programs, Poised for a Strong Future NV - HHV - 101 - Shingles Rash - First Drug Candidate Technology Strategy, Other Programs Team 3

Slide � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � PNW��119&� 6OLGH � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � PNW��119&� 1RYHO�3ODWIRUP�7HFKQRORJ��$�QDQRYLULFLGHŠ�LV�D�&HOO�0LPLF� 4 A nanoviricide “Looks Like” a Human Cell to the Virus HIV Particle binding to a Cell via CD4 and a Coreceptor gp120 on virus (binds CD4, CCR5 and CXCR4 on the Cell) &' �� RQ�&HOO 7KH�&HOO &&5 � &' �� ³.H´�WKDW� ELQGV�WR�+,9 CCR5 “Key” that binds to HIV Make Ligands to Mimic the “Keys” ® 9LUDO�5HVLVWDQFH�WR�WKH�1DQRYLULFLGH�'UXJ�LV�8QOLNHO� EHFDXVH�(YHQ�DV�WKH�9LUXV�0XWDWHV�� ,W�6WLOO�%LQGV�WR�WKH�6DPH�&HOO�6XUIDFH�5HFHSWRU�V�� � LQ�WKH�6DPH�)DVKLRQ� $�QDQRYLULFLGH�LV�ODUJH�HQRXJK�IRU�D�YLUXV�SDUWLFOH�WR�ODWFK�RQWR�LW� <HW�VPDOO�HQRXJK�WR�FLUFXODWH�UHDGLO�LQ�WKH�ERG��� 5DWKHU�WKDQ�WKH�YLUXV�SDUWLFOH�HQWHULQJ�LQWR�D�QDQRYLULFLGH�� D�QDQRYLULFLGH�ZUDSV�DURXQG�WKH�YLUXV�SDUWLFOH�DQG�HQFDSVXODWHV�LW�� E�XVLQJ�WKH�YLUXV�SDUWLFOH¶V�YHU�VDPH�DELOLW�WR�HQWHU�D�FHOO

6OLGH � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � PNW��119&� Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - mkt: NNVC) 5 A NanoViricide® Attacking a Virus Particle: Unique, Novel, Nanotech Design %,1' (1&$368/$7( '(6752<

Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - mkt: NNVC) Slide � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � PNW��119&� 1DQRYLULFLGHV�'LVPDQWOLQJ�0&09�9LUXV�3DUWLFOH &RQWURO Treated $ C Virus Dismantled; Capsids Spilling Out A: intermediate state; C: total dismantling MCMV Virus Particle Containing Multiple Capsids � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��119&� �

Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) 6OLGH © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) NanoViricides is a Unique Drug Developer Company with Its Own cGMP - Capable Manufacturing Capability Nanomedicines Characterization Facility Virology BSL - 2 Certified Lab Protect Proprietary Technology & Intellectual Property Rapid Transfer from Lab Bench to cGMP Manufacture Highly Customizable and Flexible Pharma Manufacturing Capability Skin Creams, Eye Drops, Gels, Injectables, Oral… � &OLQLFDO�3URGXFW�6XSSO�&DSDELOLW�IRU� 0RVWO�$OO�RI�2XU�1DQRYLULFLGHV 6LJQLILFDQW�7LPH�DQG�&RVW�6DYLQJV 3RWHQWLDO�IRU�0DQXIDFWXULQJ�&RPPHUFLDO� 3URGXFW� � � 0DUNHW�(QWU� �(DUO�5HYHQXHV

Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) 6OLGH © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) 8 FluCide™ Injectable FluCide™ Oral HIVCide™ DengueCide™ Other Programs: Platform Technology Potentially One Drug for All Influenzas Potentially One Drug for All Influenzas Potentially “Functional Cure” Avoid ADE Effect Select Ligand for Different Virus Injectable for High Potency Oral for Ease of Use by Out - Patients Possibly the Only Technology Platform that Can Enable
Total Cure of HIV by Hunting Out Latent Infection NanoViricides has Orphan Drug Benefits in US & EU Select Polymer Backbone for Desired Route of Administration Pre - Clinical Successes Achieved in Several Programs 1DQR9LULFLGHV�3ODWIRUP�7HFKQRORJ�+DV� (QDEOHG�6HYHUDO�'UXJ�3URJUDPV�IRU�D� %URDG�'UXJ�3LSHOLQH 7KH�RYHUDOO�DQWL � YLUDO�PDUNHW�DGGUHVVHG�E�RXU�SURJUDPV�ZDV�HVWLPDWHG� WR�EH�� ��� ELOOLRQ�LQ� ����� DQG�� ����� ELOOLRQ�LQ� ���� D a. Jain Pharma Biotech - Antivirals Report, 2014 )OX&LGHŒ D��,QMHFWDEOH For Hospitalized Patients DengueCide™ Avoid ADE West Nile Virus, Yellow Fever Virus, Jap. Encephalitis +LY&LGHŒ ³)XQFWLRQDO� &XUH"´ +,9 Rabies Ebola/M arburg )OX&LGHŒ E��2UDO Many More Possibilities for the Platform Other Programs For Out - Patients

Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) 6OLGH © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) 9 VZV
Lead Drug Candidate
NV - HHV - 101 HSV - 2 HSV - 1 Herpes Keratitis Acute Retinal Necrosis Shingles Rash Treatment Genital Ulcers Treatment Cold Sores
Treatment Herpes Keratitis Treatment Acute Retinal Necrosis Treatment IND - Enabling Studies;
Pre - Clinical Pre - Clinical Optimization Pre - Clinical Optimization Pre - Clinical Pre - Clinical Dermal Topical
Skin Cream Dermal Topical
Skin Cream Dermal Topical
Skin Cream Eye Drops Injectable Additional Future Indications in HerpeCide Program Chickenpox (Possibly Orphan Drugs) PHN (Post - Herpetic Neuralgia) Recurrent Herpes Labialis HSV - 1 Cold Sores Dermal Topical Cream +HUSHV� .HUDWLWLV VZV Shingles HSV - 2 Genital Lesions Genital Lesions Topical Cream ³$51´�$FXWH� 5HWLQDO� 1HFURVLV VZV, HSV - 1, HSV - 2 mainly Eye Drops Dermal Topical Cream 0DUNHW�6L]H�IRU�+HUSH&LGHŒ�3URJUDP�'UXJV�HVWLPDWHG�DW�2YHU�� � � � �� %LOOLRQ /HDG�,QGLFDWLRQ�6KLQJOHV�5DVK�0DUNHW�6L]H�HVWLPDWHG�DW�� �� %LOOLRQ�RU� 0RUH� �WDNHV�LQWR�DFFRXQW�LPSDFW�RI�6KLQJUL[�DQG�RWKHU�9DFFLQHV� � Our Current Focus is on the HerpeCide™ Program Regulatory Development NanoViricides Leveraging Herpecide Program Developments into Multiple Drugs Franchise

Slide � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � $PHU���119&� Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) NanoViricides’ Regulatory Strategy 'HUPDO�7RSLFDO�'UXJ�DV�)LUVW�&DQGLGDWH� � !�4XLFNHU�3DWK UDWKHU�WKDQ�VVWHPLF�WKHUDSHXWLFV� 0XOWLSOH�,QGLFDWLRQV�ZLWK�6DPH�'UXJ�&DQGLGDWH�RU�LWV�'HULYDWLYHV� WR�0D[LPL]H�6KDUHKROGHU�9DOXH�DQG�52, 9=9�6KLQJOHV +69 � �� *HQLWDO�+HUSHV +69 � �� +HUSHV�/DELDOLV�´&ROG�6RUHVµ� 10 Shingles Rash from img.webmd.com Herpes Keratitis Stromal vascularization, Lipid keratopathy +HUSHV�&ROG�6RUHV� IURP� ZZZ�UHPRYHFROGVRUHV�FRP Herpes Keratitis (HSV - 1 >95%) (External Eye) Also: Ocular Herpes; Acute Retinal Necrosis; Other Indications Distribute Development Costs over Multiple Indications

Slide � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � $PHU���119&� Slide Lead Drug Candidate NV - HHV - 101: Business Case Potentially Billion+ Dollar Market for Topical Shingles Rash Treatment alone, even after the new vaccine Shingrix® has been introduced 500,000 to 1,000,000 Cases Annually in the USA Alone Additionally, NV - HHV - 101 is Expected to Lead into Dermal Topical Treatments for: HSV - 1 “Cold Sores”, and HSV - 2 “Genital Ulcers” Open Multi - Billion Dollar Additional Markets Many Additional Indications Expanding Indications means Improving ROI and Maximizing Shareholder Returns ��

6OLGH © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) Slide Lead Drug Candidate NV - HHV - 101 Status - Getting Ready for IND Filing Indication: Shingles Rash Treatment. Form: Topical Skin Cream Anticipate Human Clinical Trials to Begin in CY 2020 Q1/Q2 FDA Response “Generally Adequate” on Pre - IND Filed with FDA Non - Clinical Safety/Toxicology Studies Complete - except certain analyses Awaiting GLP Safety/Toxicology Study Reports External Collaborations for IND Write - ups, Clinical Trial Design, eCTD Conversion, and FDA Submission in Progress Clinical Trial Site Selection in Progress Proposed Adaptive Protocol Phase I Plus Phase IIa Combined IND Filing - Major Milestone Program Expected to Provide Value - Driving Data Reads As the Trials Progress 12 Shingles Rash from img.webmd.com

Slide � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � $PHU���119&� 6OLGH © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) Investment Thesis: NanoViricides Platform Technology and Facility Platform Technology - A “Venus - Fly - Trap” for Viruses - “Bind - Encapsulate - Destroy” - Drugs that Viruses Cannot Escape by Mutations Multiple Drug Programs with Many Candidates having demonstrated Successful Animal Model Effectiveness and Safety Enable Continuing Future Growth Own cGMP Manufacturing Facility Supplying Clinical Product Needs Saves Money, Time, and Minimizes IP Exposure May Enable Production for Early Commercial Market Entry Early Successes - Strong Effectiveness in Influenza, HIV Now Focused on Regulatory Development of Drugs in HerpeCide™ Program IND - Enabling Safety and Effectiveness of First Drug Candidate Demonstrated Validate Platform Substantially as First Drug Goes Through Clinical Trials 13

6OLGH © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) Slide � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � $PHU���119&� Investment Thesis: NanoViricides Valuation Company Founded and taken public (reverse shell merger) in 2005 - Platform Technologies Licensed from TheraCour Pharma, Inc. Market Cap Historically Around $100~150M Uplisted to NYSE - American in September 2013 Moved to Integrated cGMP Manufacturing & R&D Facility in 2015 Fully Owned Facility Asset Value ~$10M Net - of - Depreciation Focused on Regulatory Development of HerpeCide Program “Valley of Death” Phenomenon Valuation May be Anticipated to Go Substantially Higher with First Drug Entering Clinical Trials Large Market Sizes Enable Strong Future Potential 14

Slide � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � $PHU���119&� Slide Shingles Rash breakouts - HHV - 3 aka VZV � 8QPHW�0HGLFDO�1HHG���$YDLODEOH�GUXJV�LQ�XVH�QRW�YHU� HIIHFWLYH��YDFFLQHV�H[LVW� 7KH�FKLFNHQSR[�YLUXV�VXUYLYLQJ�LQ�JDQJOLD�FDXVHV�6KLQJOHV�LQ�DGXOWV $ERXW� �������� WR� �� 0LOOLRQ�&DVHV�3HU�<HDU�LQ�WKH�86$�$ORQH 5LVN�,QFUHDVHV�ZLWK�$JH 7ULJJHUHG�E�5HGXFHG�,PPXQH�)XQFWLRQ 6WUHVV��$JH��,PPXQH�&RPSURPLVHG�6XSSUHVVHG 6HYHUH�6WLQJLQJ�3DLQ� �=RVWHULIRUP�5DVK� 3DLQ�0D�3HUVLVW�IRU�0RQWKV�RU�<HDUV� $IWHU�5HVROXWLRQ�RI�2XWEUHDN� � � 3+1 9LUXV�'DPDJHG�1HUYHV�&RQWLQXH�WR�6LJQDO�6KDUS��'HELOLWDWLQJ�3DLQ 3DLQ�&RXOG�EH�$YRLGHG�0LQLPL]HG�LI�9LUXV�LV�&RQWUROOHG %URDG � 6SHFWUXP�+HUSH&LGHŒ�&RXOG�EH�D�+LJKO�(IIHFWLYH�'UXJ 15 Shingles Rash from img.webmd.com Potential Billion+ Dollar Market Size projected even after the new vaccine introduction

6OLGH © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) Slide Lead Drug Candidate NV - HHV - 101 Dermal Topical Skin Cream for Treating Shingles Rash 6WDQGDUG�RI�&DUH��9DOWUH[Š�2UDO��LV�1RW�9HU�(IIHFWLYH %HFDXVH�YLUDO�7.�HQ]PH�DFWLYLW�UHTXLUHG�IRU�FRQYHUWLQJ� $FFORYLU�FODVV�RI�GUXJV�WR�DFWLYH�IRUP�LV� YHU�SRRU�LQ�9=9�FRPSDUHG�WR�+69 � �� RU�+69 � � � [�0RUH�(IIHFWLYH�WKDQ�$FFORYLU�LQ�&HOO�&XOWXUH� +XPDQ�6NLQ�3DWFK�2UJDQ�&XOWXUH�6WXGLHV� � ! +LJKO�(IIHFWLYH� 9HU�6DIH� � � 1R�+LVWRORJLFDO�&KDQJHV 6WXGLHV�LQ�3URIHVVRU�-HQQLIHU�0RIIDW�/DE��8SVWDWH�0HGLFDO� &HQWHU��681<��6UDFXVH��1< 3URIHVVRU�0RIIDW�LV�D�/HDGLQJ�5HVHDUFKHU�LQ�9=9�,QIHFWLRQ 16 Risk is Minimized for Human Clinical Studies, Since Efficacy and Safety Data are Obtained in Human Skin Itself

Slide � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � $PHU���119&� Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) Optimization Studies VZV (Shingles) Topical Candidates Highly Effective - Five Times More Effective against VZV than Acyclovir in Cell Cultures (Pre - Clinical) �� Also, Against HSV - 1 and HSV - 2, Found Similar Strong Effectiveness in Cell Cultures using multiple virus strains and multiple cell lines Poster Presented by NanoViricides at the American Society of Virology Annual Meeting, 2018, Madison, WI.

6OLGH © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) VZV Infected Skin - Basal Layer Breaches seen, Also, Multinucleated Giant Cells seen Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) VZV (Shingles) Topical Drug Candidates Are Highly Effective Match Efficacy of Cidofovir in Human Skin Organ Culture Model of VZV Infection (Pre - Clinical) �� Note: Cidofovir is higly toxic ( https://reference.medscape.com/drug/vistide - cidofovir - 342606#5 ) Poster Presented by Moffat Group, SUNY Syracuse Upstate Medical Center, at the International Conference on Antiviral Research, June 11 - 15, 2018, Porto, Portugal 19 � ���� 7UHDWHG�9=9�,QIHFWHG�6NLQ 1RUPDO�'HUPLV�DQG�(SLGHUPLV�� 1R�YLUDO�LQILOWUDWLRQ�RU�OHVLRQV� VHHQ�

Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) Slide VZV (Shingles) Topical Candidate Moving Rapidly To Clinical Trials Preliminary Non - GLP Safety/Tolerability Studies in Rats Successful (2018) 19 1R�FOLQLFDOO�REVHUYDEOH�DGYHUVH�VDIHW�DQG�WR[LFRORJ�HIIHFWV�LQ� WRSLFDO�DGPLQLVWUDWLRQ 1R�FOLQLFDOO�REVHUYDEOH�DGYHUVH�VDIHW�DQG�WR[LFRORJ�HIIHFWV�LQ� VVWHPLF�DGPLQLVWUDWLRQ��,9�RU�,3� 1R�REVHUYDEOH�GLUHFW�HIIHFWV�RQ�WKH�SULPDU�RUJDQ�IXQFWLRQV� ZKHWKHU�WKH�GUXJ�ZDV�DGPLQLVWHUHG�WR�WKH�VNLQ�RU�VVWHPLFDOO OLYHU�DQG�NLGQH�IXQFWLRQ�LV�XQDIIHFWHG��DPRQJ�RWKHU�RUJDQV &RQVLVWHQW�ZLWK�6WURQJ�6DIHW�2EVHUYHG�LQ�+XPDQ�6NLQ�3DWFK� (IILFDF�0RGHO�6WXGLHV Thereupon Undertook Manufacturing Scale - up, Final Drug Candidate Optimization, Final Drug Product Formulation, and Entry into IND - enabling Safety/Toxicology Studies (“Tox Package”)

Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) 6OLGH 20 Non - GLP Portion of IND - Enabling Safety/Toxicology Studies Completed by BASi, IN ca. February, 2019 Excellent Safety Profile Observed cGMP Manufacture for GLP Safety/Toxicology Studies Completed GLP Studies began in May, 2019, at BASi, IN VZV (Shingles) Topical Drug Candidate : IND - Enabling Safety/Toxicology Package Studies Non - GLP Studies ,Q � OLIH�3RUWLRQ�RI�*/3�6WXGLHV�FRPSOHWHG�LQ�-XO� ���� ��DW�%$6L��,1� ([FHOOHQW�6DIHW�3URILOH�2EVHUYHG�LQ�&OLQLFDO�2EVHUYDWLRQV 7R[LFRNLQHWLFV�6WXGLHV�,Q�3URJUHVV� � � $OPRVW�&RPSOHWH +LVWRORJLFDO�6WXGLHV�&RPSOHWH $ZDLWLQJ�5HSRUWV� GLP Safety/Toxicology Studies

Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) 6OLGH HerpeCide™ Program Future Expansion Epstein - Barr Virus: Mononucleosis Monoclonal Gammopathy other B - cell diseases, etc. Cytomegalovirus: Retinitis, Organ Transplant etc. HHV - 6A, HHV - 6B, HHV - 7 several diseases are associated encephalopathy, epilepsy sialoadenopathy, Sicca syndrome T - cell diseases 21 'UXJ�&DQGLGDWHV�LQ�&XUUHQW�+HUSH&LGH�3URJUDP � 0D�+DYH�$SSOLFDWLRQV�$JDLQVW�2WKHU�+HUSHV9LUXVHV�

Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) Slide � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � $PHU���119&� Strong Executive Team Anil R. Diwan, PhD President & Exec. Chairman Co - Founder Led Uplisting to NYSE - American Exchange in 2013 Raised $65M Co - Inventor of Nanoviricides® & of TheraCour ® 25+ years Leadership & Entrepreneurial experience Key Patents, Several NIH SBIR Awards PhD (Biochem Eng - Rice), BTech (ChemEng - IITB) 22 5DQGDOO�:��%DUWRQ��3K' &62�DQG�$FWLQJ�&52 �� ��<HDUV�RI�3KDUPDFHXWLFDO�,QGXVWU�([SHULHQFH�� LQ��'UXJ�'LVFRYHU�DQG�3UH � FOLQLFDO�5HJXODWRU� 'HYHORSPHQW� )RUPHU�'LUHFWRU�RI�,Q � 9LWUR�&DUGLRYDVFXODU� 5HVHDUFK�DW�%RHKULQJHU�,QJHOKHLP 1HYLUDSLQH��9LUDPPXQHŒ��'HYHORSPHQW 9LVLWLQJ�)DFXOW�DW�WKH�8QLYHUVLW�RI� &RQQHFWLFXW�0HGLFDO�6FKRRO��)DUPLQJWRQ��&7 Meeta R. Vyas, MBA CFO 30+ years Experience in Corporate Performance Improvement, Finance, M&A, EBITDA Growth… Previously: Principal, The Gores Group; Director, Kamylon Capital; CEO, Signature Brands, Inc. (a public company, known for “Mr. Coffee”); Ran $1B GE Appliances Division; Consultant, McKinsey & Company MBA (Fin.) Columbia, BS (ChemEng) MIT Jayant Tatake, PhD VP, R&D 30+ Years of Pharmaceutical Industry Experience in Drug Discovery, Manufacturing, QA/QC, CRO Synthesis, Scale - up, Formulations, and Pharmaceutical cGMP Expertise Former Asst. Director, Pharma. Analytics, InterPharm, Inc. Co - Inventor of Nanoviricides® & TheraCour® PhD UICT, Bombay

Slide � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � $PHU���119&� Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) Board of Directors $QLO�5��'LZDQ��3K' 3UHVLGHQW� �([HF��&KDLUPDQ &R � )RXQGHU��/HG�8SOLVWLQJ�WR�1<6( � $PHU��LQ� ���� ��5DLVHG�� �� 0���&R � ,QYHQWRU�RI� 1DQRYLULFLGHVŠ� �RI�7KHUD&RXU�Š �� ��HDUV�/HDGHUVKLS� �(QWUHSUHQHXULDO� H[SHULHQFH 1RW�DQ�,QGHSHQGHQW�%RDUG�0HPEHU 'LUHFWRU�DQG�&KDLUPDQ�6LQFH�)RXQGLQJ�LQ� ���� 23 6WDQOH�*OLFN��&3$�� ط &KDLUPDQ��$XGLW�&RPPLWWHH $XGLWLQJ��$FFRXQWLQJ��7D[�� �0JPW��$GYLVRU� 6HUYLFHV )LQDQFLDO�0DQDJHPHQW�2YHUVLJKW��&LYLF�/HDGHU� ,QGHSHQGHQW�%RDUG�0HPEHU�DQG� &KDLU�RI�$XGLW�&RPPLWWHH�VLQFH�-XQH� ���� 0DUN�'D��3K'� ط �� ��<HDUV�RI�3KDUPDFHXWLFDO�,QGXVWU� ([SHULHQFH��$VVRFLDWH�3URIHVVRU��7UDQVODWLRQDO� 1HXURVFLHQFH��$GMXQFW��DW�<DOH�8QLYHUVLW��([ � &(2�RI�%LRDVLV��27&��%,2$)���3UHYLRXVO��([HF� 93�RI�([W��5HVHDUFK� �6FRXWLQJ�DW�$OH[LRQ�� *OREDO�/HDG�%XV��'HY��DQG�+HDG�RI�9LURORJ�DQG� &16�'LVHDVHV�DW�%06��+HDG��7UDQVODWLRQDO� ,PDJLQJ� �%LRPDUNHUV�DW�$EERWW�/DEV��'LVFRYHU� +HDG��&16�7UDQVO��0HGLFLQH�DW�:HWK�5HVHDUFK� ,QGHSHQGHQW�%RDUG�0HPEHU�VLQFH�-XQH�� ���� -DPHV�6DSLUVWHLQ��0%$� ط � �� ��<HDUV�RI�3KDUPDFHXWLFDO�,QGXVWU� ([SHULHQFH��ZLWK�RYHU� �� GR]HQ�3URGXFW�/DXQFKHV� DW�%LJ�3KDUPD�DQG�6PDOO�3KDUPD��H[ � &(2�RI� &RQWUD9LU��&759���%RDUG�PHPEHU�RI�(QRFKLDQ� %LRVFL���(12%���5HVSLUH5[�3KDUPD���563,���DQG� /HDGLQJ�%LRVFL���SYW����$OVR�D�%RDUG�PHPEHU�RI� %,21-�DQG�D�%RDUG�'LUHFWRU�IRU�%,2��RQ�ERWK�WKH� +HDOWK�6HFWLRQ�*RYHUQLQJ� �(PHUJLQJ�&RPSDQLHV� 6HFWLRQ� ,QGHSHQGHQW�%RDUG�0HPEHU�VLQFH�1RYHPEHU�� ���� ط � �,QGHSHQGHQW�%RDUG�0HPEHU

Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) 5HFDS “Resistance is Futile” - Antiviral Nanomachines Designed to Destroy Viruses Despite Viral Mutations Broad and Deep Pipeline based on Platform Technology In - house cGMP Manufacture Enabling Early Commercial Revenues On Its Own Major Regulatory Progress and Milestones to Occur Throughout Next Several Years Starting with Upcoming IND for NV - HHV - 101 Shingles Rash Human Skin Pre - clinical Studies De - risk Clinical Development Strong Asset Position Expert team Valuation

Slide © All Rights Reserved, NanoViricides, Inc., A Publicly Traded Company (NYSE - Amer.: NNVC) 6OLGH � ‹�$OO�5LJKWV�5HVHUYHG��1DQR9LULFLGHV��,QF���$�3XEOLFO�7UDGHG�&RPSDQ��1<6( � $PHU���119&� �� 7KH�(QG
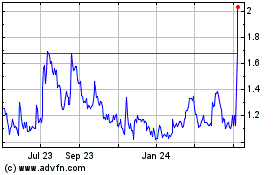
NanoViricides (AMEX:NNVC)
Historical Stock Chart
From Mar 2024 to Apr 2024
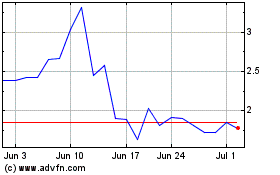
NanoViricides (AMEX:NNVC)
Historical Stock Chart
From Apr 2023 to Apr 2024