Current Report Filing (8-k)
March 13 2020 - 6:52AM
Edgar (US Regulatory)
U.S. SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported):
March 13, 2020 (March 11, 2020)
iBio, Inc.
(Exact name of registrant as specified in
its charter)
Delaware
(State or jurisdiction of incorporation
or organization)
001-35023
(Commission File Number)
26-2797813
(I.R.S. Employer Identification Number)
600 Madison Avenue, Suite 1601, New
York, NY 10022-1737
(Address of principal executive offices
(Zip Code)
Registrant's telephone number: (302) 355-0650
N/A
(Former name or former address, if changed
since last report)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction
A.2. below):
|
|
¨
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
|
|
|
¨
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
|
|
|
¨
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17CFR 240.14d-2(b))
|
|
|
¨
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17CFR 240.13e-4(c))
|
Indicate by check mark whether the registrant is an emerging
growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities
Exchange Act of 1934 (§240.12b-2 of this chapter).
If an emerging growth company, indicate by check mark if the
registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards
provided pursuant to Section 13(a) of the Exchange Act.
¨ Emerging growth company
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
|
Ticker symbol(s)
|
|
Name of each exchange on which registered
|
|
Common Stock
|
|
IBIO
|
|
NYSE American
|
Patents
On
March 11, 2020, iBio, Inc. (the “Company” or “iBio”) filed four provisional patent applications (the “Patent
Applications”) that apply its Virus Like Particle [VLP] platform technology, or its lichenase carrier immunostimulatory (“LickM”)
adjuvant technology, in conjunction with its FastPharming™ Manufacturing
System for treating or preventing infections with the SARS-CoV-2 virus, which is the agent that causes coronavirus disease 2019
(COVID-19).
Master
Joint Development Agreement with Beijing CC-Pharming Ltd.
On
August 8, 2018, the Company and Beijing CC-Pharming Ltd. (“BCCP”) entered into
a Master Joint Development Agreement (the “MJDA”) in which the Company and BCCP established
a strategic commercial relationship for the joint development of products and manufacturing facilities for the Chinese biopharmaceutical
market, utilizing iBio’s technology. The Company’s relationship with BCCP initially focused on the development
of an oncology biosimilar or bio-better drug and license and transfer of the Company’s proprietary technology for drug development
and manufacture to BCCP for use only in China.
When the coronavirus problems emerged in
China, BCCP sought to extend the collaboration with the Company to process development and testing of coronavirus vaccine candidates.
On February 6, 2020, the Company
and BCCP executed a Statement of Work 2 (“SOW2”) memorializing their collaborative efforts to develop and test a new
BCCP 2019-nCoV vaccine to be manufactured using iBio’s FastPharming System™.
The SOW2 is governed by the terms of the
MJDA, which provides BCCP with a nonexclusive, non- assignable, non-sublicensable, limited license to use iBio technology in order
to manufacture, process, prepare, and obtain regulatory approval for the development and production of biopharmaceuticals products
based on iBio’s proprietary and patented plant-based protein production technology and know-how. The non-exclusive license
granted under the MJDA extends only to China.
The contemplated collaborative effort with
BCCP is still in early stages and has not yet progressed in any material respect.
The Company’s separate activities
have resulted in the development of intellectual property in the field of vaccine candidate development for the SARS-CoV-2 virus.
Those separate activities are reflected in the filing of the Patent Applications described above.
The foregoing descriptions of the terms
of the SOW2 and MJDA do not purport to be complete and are subject to, and qualified in their entirety by reference to, such agreements,
which are filed herewith as Exhibit 10.2, and Exhibit 10.1, respectively, and are incorporated herein by reference.
* Portions of this exhibit, which are
not material and would likely cause competitive harm to the Company if publicly disclosed, have been omitted. Omitted information
is indicated by brackets in the exhibit.
Signatures
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf
by the undersigned hereunto duly authorized.
|
|
IBIO INC.
|
|
|
|
|
|
|
|
|
|
|
Date: March 13, 2020
|
By:
|
/s/ Thomas F. Isett
|
|
|
|
|
Name:
|
Thomas F. Isett
|
|
|
|
|
Title:
|
Chief Executive Officer and
|
|
|
|
|
|
Executive Co-Chairman
|
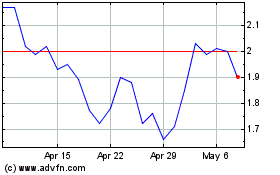
iBio (AMEX:IBIO)
Historical Stock Chart
From Mar 2024 to Apr 2024
iBio (AMEX:IBIO)
Historical Stock Chart
From Apr 2023 to Apr 2024