SUPPLEMENT NO 2. TO
TO REGULATION A OFFERING CIRCULAR
WEED, INC.
WEED, Inc.
4920 N. Post Trail, Tucson, AZ 85750
(520) 818-8582
www.WEEDincUSA.com.
March 8, 2021
This Supplement amends and
supplements the Offering Circular of WEED, Inc. (the
“Company”)
dated “December __ 2020” which forms a part of the
offering statement on Form 1-A qualified on January 15, 2021, as
the same may be amended or supplemented from time to time (the
“Offering
Circular”) by (i) dating the
Offering Circular for March 8, 2021, (ii) announcing the
Company’s entry into an Investment Banking Agreement with
Great Point Capital, LLC (“GPC”), (iii) updating
“Risk Disclosures” to reflect the risks related to lack
of certain insurances, and (iv) revising the amount of commissions
and proceeds to the Company as a result of the Investment Banking
Agreement.
A complete Offering Circular, containing language
encompassing items (i)-(iv) above, is attached hereto as
Exhibit
A. Except as described above,
this Supplement to the Offering Circular does not modify or update
disclosure in, or exhibits to, the original Offering Circular. This
Supplement to the Offering Circular should be read in conjunction
with the Offering Circular and is qualified by reference to the
Offering Circular except to the extent that the information
contained herein supplements the information contained in the
Offering Circular.
Date of Offering Circular
The
Offering Circular is dated March 8, 2021.
Description of Investment Banking Agreement With GPC
The
following is a summary of the material terms of the Investment
Banking Agreement with GPC:
On
March 3, 2021, WEED, Inc. (the “Company”) and Great
Point Capital, LLC (“GPC”) entered into an Investment
Banking Agreement (the “Agreement”) whereby GPC shall
serve as a nonexclusive investment banker for the Company in
connection with the identification of investors who may be
interested in funding the Company’s capital needs via the
Company’s qualified Reg A+ offering (the
“Raise”). GPC is an independent contractor for the
purposes of the Agreement.
The
Term of the Agreement shall be for the duration of the Raise or
until 15 days after effective notice of termination has been
received by either party. Company may not terminate until 45 days
from the Effective Date, March 3, 2021.
In
consideration of GPC’s services, the Company will pay to GPC
2% of gross proceeds of funds raised by GPC during the term of the
Agreement and a one-time payment of 120,000 shares of the
Company’s common stock, which shares are Restricted
Securities under Rule 144. Company shall reimburse all expenses of
GPC up to an aggregate of $2,500, after which approval is required.
The Company has significant indemnification obligations pursuant to
the Agreement, whereby GPC is indemnified from most actions falling
short of intentional misconduct. Company’s recovery for any
liability is also strictly limited.
SEC Disclaimer
An
offering statement regarding the offering described in the Offering
Circular has been filed with the Commission. The Commission has
qualified that offering statement, which only means that the
Company may make sales of the securities described by that offering
statement. It does not mean that the Commission has approved,
passed upon the merits or passed upon the accuracy or completeness
of the information in the offering statement. You should read the
offering circular before making any investment.
SIGNATURES
Pursuant to the requirements of Regulation A, the Issuer certifies
that it has reasonable grounds to believe that it meets all of the
requirements for filing on Form 1-A and has duly caused this
Supplement No. 2 to Regulation A Offering Statement to be signed on
its behalf by the undersigned, thereunto duly authorized, in the
City of Tucson, Arizona, on March 8, 2021.
|
|
WEED, Inc.
|
|
|
|
|
|
By:
|
/s/
Glenn E. Martin
|
|
|
Name:
|
Glenn
E. Martin
|
|
|
Title:
|
Chief
Executive Officer and Chief Financial Officer
|
This offering statement has been signed by the following persons in
the capacities and on the dates indicated.
|
Signature
|
Title
|
Date
|
|
/s/
Glenn E. Martin
|
Director
|
March
8, 2021
|
|
Glenn
E. Martin
|
|
|
|
|
|
|
/s/
Nicole M. Breen
|
Director
|
|
|
Nicole
M. Breen
|
March
8, 2021
|
Exhibit A
Offering Circular
REGULATION
A OFFERING CIRCULAR
Dated
March 8, 2021
WEED,
INC.
4920
N. Post Trail
Tucson,
AZ 85750
(520)
818-8582
www.WEEDincUSA.com
Up
to $40,000,000
Units
Consisting of 1 Share of Common Stock and
A
Warrant to Purchase 1 Share of Common Stock
Price:
$1.00
per
Unit
Minimum
Investment Amount: $1,000
WEED, Inc., a Nevada corporation (the Company,
WEED, we, or our) is offering (the “Offering”) up to a
maximum of 40,000,000 units (“Units”), with each Unit
consisting of one (1) share of our common stock, par value $0.001
(“Common Stock”) and one (1) warrant to purchase (1)
share of our common stock, at a purchase price of $1.00 per Unit.
The exercise price on the warrant will be 150% of the price of the
Unit sold to the investor and
cannot be exercised by the holder until at least twelve months
after issuance.
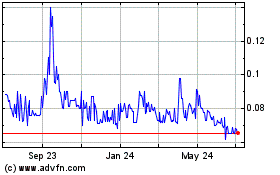
Our Common Stock currently trades on the
OTCQB-tier of OTC Markets under the symbol “BUDZ” and
the closing price of our Common Stock on November 30, 2020 was
$0.27. Our Common Stock currently trades on a sporadic and limited
basis. The Units and warrants
offered hereunder will not be quoted on any OTC
market.
No Escrow
The
proceeds of this offering will not be placed into an escrow
account. We will offer the Units on a best effort’s basis. As
there is no minimum offering, upon the approval of any subscription
to this Offering Circular, we will immediately deposit said
proceeds into our bank account and may dispose of the proceeds in
accordance with the Use of Proceeds.
Subscriptions are irrevocable and the purchase
price is non-refundable as expressly stated in this Offering
Circular. We, by determination
of our Board of Directors, in its sole discretion, may issue the
Securities under this Offering for cash, promissory notes,
services, and/or other consideration without notice to
subscribers. All proceeds
received by us from subscribers for this Offering will be available
for use by us upon acceptance of subscriptions for the Securities
by us.
Sale
of these shares will commence within two calendar days of the
qualification date (the “Qualification Date”) and it
will be a continuous Offering pursuant to Rule
251(d)(3)(i)(F).
We are
selling the Units on a “best efforts” basis through a
Tier 2 offering pursuant to Regulation A (Regulation A+) under the
Securities Act of 1933, as amended (the “Securities
Act”), and we intend to sell the Shares either directly to
investors or through registered broker-dealers who are paid
commissions. This Offering will terminate on the earlier of (i)
November 30, 2021, (ii) the date on which the Maximum Amount is
sold, or (iii) when the Board of Directors of the Company elects to
terminate the Offering (in each such case, the Termination Date).
There is no escrow established for this Offering. We will hold
closings upon the receipt of investors’ subscriptions and
acceptance of such subscriptions by the Company. If, on the initial
closing date, we have sold less than the Maximum Amount, then we
may hold one or more additional closings for additional sales,
until the earlier of: (i) the sale of the Maximum Amount or (ii)
the Termination Date. There is no aggregate minimum requirement for
the Offering to become effective, therefore, we reserve the right,
subject to applicable securities laws, to begin applying
“dollar one” of the proceeds from the Offering towards
our business strategy, including without limitation, research and
development expenses, offering expenses, acquisitions and joint
ventures, working capital and general corporate purposes and other
uses as more specifically set forth in the “Use of
Proceeds” section of this Offering Circular. The minimum
investment amount from an investor is $1,000; however, we expressly
reserve the right to waive this minimum in the sole discretion of
our management. See “Securities Being Offered”
beginning on page 26 for a discussion of certain items required by
Item 14 of Part II of Form 1-A.
Investing
in the Units involves a high degree of risk. These are speculative
securities. You should purchase these securities only if you can
afford a complete loss of your investment. See Risk Factors”
starting on page 7 for a discussion of certain risks that you
should consider in connection with an investment in the
Shares.
THE
SEC DOES NOT PASS UPON THE MERITS OF OR GIVE ITS APPROVAL TO ANY
SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS
UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER
SOLICITATION MATERIALS. THESE SECURITIES ARE OFFERED PURSUANT TO AN
EXEMPTION FROM REGISTRATION WITH THE COMMISSION; HOWEVER, THE
COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE
SECURITIES OFFERED ARE EXEMPT FROM REGISTRATION.
|
Title
of each
class
of
Securities
|
Maximum
Number of Units to be Offered
|
Proposed
offering
price
per
Unit (1)(2)
|
Proposed
aggregate
offering
proceeds (1)
|
Broker-Dealer
discount and commissions (2) (5)
|
Proceeds
to Company(3)(4)(5)
|
|
Units
consisting of shares of Common Stock, $0.001 par value, and a
Warrant to purchase Common Stock offered by the
Company
|
40,000,000
|
$1.00
|
$40,000,000
|
$1,200,000
|
$38,800,000
|
(1)
We
are offering on a continuous basis starting on the Qualification
Date.
(2)
We are offering the
Units without an underwriter. However, we have engaged Dalmore Group, LLC, a registered
FINRA/SIPC broker-dealer (“Dalmore”), to perform
administrative and technology related functions in connection with
the Offering, but not for underwriting or placement agent services.
This includes a 1% commission, but it does not include the one-time
set-up fees payable to Dalmore of $31,500. We may
pay other broker-dealers
and finders as well, but
information as to the finder or brokers must be disclosed in an
amendment to this offering circular. See “Plan of Distribution” for more
detail.
(3)
This is a
“best efforts” offering. The proceeds of this offering
will not be placed into an escrow account. We will offer the Units
on a “best efforts” basis primarily through our
management and/or an online platform. Any subscription to this
Offering Circular meeting the Minimum Investment Amount ($1,000),
shall be immediately deposited into the bank account of the Company
and we may dispose of the proceeds in accordance with the Use of
Proceeds. See “Use of Proceeds.”
(4)
Excludes estimated
total offering expenses, not including underwriting discount and
commissions, will be approximately $75,000. See “Use of
Proceeds.”
(5)
We engaged Great Point Capital
LLC pursuant to an Investment Banking Agreement to assist in
identification and qualification of identification of investors who
may be interested in funding the Company’s capital needs via
the Company’s qualified Reg A+ offering. Their fee include a
2% commission of all funds raised through their introductions, as
well as a one-time payment of 120,000 shares of the
Company’s common stock which shares are Restricted Securities
under Rule 14. We may pay other
broker-dealers and finders as well, but information as to the finder
or brokers must be disclosed in an amendment to this offering
circular. See
“Plan of Distribution” for more
detail.”
This
Offering Circular relates to the sale of Units by WEED, Inc., a
Nevada corporation (“we” or the “Company”)
to certain investors at $1.00 per Unit, up to 40,000,000
Units.
Our
Common Shares are quoted on OTC Market’s “OTCQB”
tier under the ticker symbol “BUDZ.” The Units and
warrants offered hereunder will not be quoted on any OTC
market.
Investing
in the securities involves risks. WEED, Inc., currently has limited
operations, limited income, and limited assets, is in unsound
financial condition, and you should not invest unless you can
afford to lose your entire investment. See “Risk
Factors” beginning on page 7. Neither the Securities and
Exchange Commission nor any state securities commission has
approved or disapproved of these securities or determined if this
prospectus is truthful or complete. Any representation to the
contrary is a criminal offense.
OFFERING
CIRCULAR SUMMARY
You should read the following summary together with the more
detailed information and the financial statements appearing
elsewhere in this Offering Circular. This Offering Circular
contains forward-looking statements that involve risks and
uncertainties. Our actual results could differ materially from
those anticipated in these forward-looking statements as a result
of certain factors, including those set forth under “Risk
Factors” and elsewhere in this Offering Circular. Unless the
context indicates or suggests otherwise, references to
“we,” “our,” “us,” the
“Company,” or the “Registrant” refer to
WEED, Inc., a Nevada corporation.
WEED,
INC.
We are
a USA-based fully reporting public company currently specializing
in the medicinal cannabis, adult-use cannabis & hemp space. We
are a multi-national, multi-faceted, vertically-integrated
organization. We are structured as a holding company doing business
through our divisions, wholly-owned subsidiaries, and strategically
placed collaborative partners to achieve and promote a global
brand. We are dedicated to global goals and outreach across the
full spectrum of the cannabis and hemp industry to find treatments,
therapies and medical cures utilizing the Cannabaceae plant family.
We do not grow, harvest, produce, or sell any substance in
violation of US Federal law under The Federal Controlled Substances
Act, and we meet all standards of international law for WEED, Inc.
and its subsidiaries in foreign locations.
Currently, we are
either working or planning for several different business
opportunities in the cannabis and hemp space. Our first business
opportunity is through our wholly-owned subsidiary, Sangre AT, LLC
(“Sangre”), where we are focused on the development and
application of cannabis-derived compounds for the treatment of
human disease. To that end Sangre, is working on a planned
five-year Cannabis Genomic Study to complete a genetic blueprint of
the Cannabis plant genus, by creating a global genomic
classification of the entire plant. Second, we are under contract
to purchase a golf course property located in Westfield, New York.
We own the “unlimited water extraction rights” from
Lake Erie to the property already, but need to raise at least
$500,000 to acquire the property. If we are successful in acquiring
the property we plan to utilize the property to gain access to the
HEMP and infused beverage industry to establish a foothold for the
New York marketplace. Third, we have established WEED Australia
Ltd. and The Cannabis Institute of Australia (C.I.A.) in Australia
for the purpose of conducting cannabis and hemp research and
potentially developing products in Australia. C.I.A. is a
non-profit entity formed for the purpose of conducting cannabis and
hemp research and potentially developing products in Australia for
domestic research and development of products, services and
educational purposes to all 7 States and territories including
Tasmania. Fourth, we have an arrangement with Professor Elka
Touitou to assist us with cannabis research and studies in Israel.
Professor Touitou was the Head of the Innovative Dermal,
Transdermal and Transmucosal Delivery Lab at the Institute of Drug
Research, The School of Pharmacy, HUJ, now retired but still has
HUJ clinical trial & independent studies/lab privileges.
Professor Touitou is an internationally renowned authority in the
field of drug delivery and design of new technologies for efficient
administration of drugs and development of new products. Professor
Touitou has been involved in cannabinoid research since 1988 at The
Hebrew University of Jerusalem, (HUJ) Jerusalem,
Israel.
Through
these business divisions and subsidiaries, we plan to advance our
research and development of cannabis-related compounds for the
treatment of human and animal disease, as well as potentially
commercialize other cannabis-related products, both pharmaceutical
and non-pharmaceutical. The business segments are detailed
herein.
By targeting
cannabis-derived molecules that stimulate the endocannabinoid
system, Sangre’s research team plans to develop
scientifically-valid and evidence-based cannabis strains for the
production of disease-specific medicines. The goal of the research
is to identify, collect, patent, and archive a collection of
highly-active medicinal strains. We plan to conduct this study in
accordance with U.S. and state laws and regulations, as well as in
foreign locations where it is legal, such as Israel (including at
The Ministry of Health in Israel) and Australia (at the Therapeutic
Drug Administration (TGA) & Office of Drug Control (ODC).
Although we have had discussions with these organizations we have
not contracted with any of these organizations to conduct our study
and will not be able to do so until we raise sufficient funding.
Israel allows medical research on cannabis, and actually funds
certain research clinics and studies. The Federal Government of
Australia has recently amended the Narcotic Drugs Act of 1967 to
allow cultivation of cannabis for medicinal or scientific
purposes.
Using
annotated genomic data and newly generated phenotypic data, Sangre
plans to identify and isolate regions of the plant genome which are
related to growth, synthesis of desired molecules, and drought,
heavy metals and pest resistance. This complex data set would then
be utilized in a breeding program to generate and establish new
hybrid cultivars which exemplify the traits that are desired by the
medical and patient community. This breeding program would produce
new seed stocks, clones, and tissue cultures which we plan on
patenting. If successful this intellectual property should generate
immense value for the Company. After developing a comprehensive
understanding of the annotated genome of a variety of cannabis
strains and obtaining intellectual property protection over the
most promising strains, we plan to move forward either
independently or with strategic partners to develop medicinal
products for the treatment of a multitude of human and animal
diseases.
Currently, we do
not have the money or funding to achieve the above goals and we
will not be able to achieve our goals unless we are successful in
obtaining additional funding, likely through sales of our
securities, all which may serve to dilute the ownership position of
our current and future shareholders.
Corporate
Information
We were
originally incorporated under the name Plae, Inc., in the State of
Arizona on August 20, 1999. At the time we operated under the name
Plae, Inc., no business was conducted. No books or records were
maintained and no meetings were held. In essence, nothing was done
after incorporation until Glenn E. Martin took possession of Plae,
Inc. in January 2005. On February 18, 2005, the corporate name was
changed to King Mines, Inc. and then subsequently changed to its
current name, United Mines, Inc., on March 30, 2005. No shares were
issued until the Company became United Mines, Inc. From 2005 until
2015, we were an exploration stage mineral exploration company that
owned a number of unpatented BLM mining claims and Arizona State
Land Department exploration leases.
On
November 26, 2014, our Board of Directors approved the
redomestication of our company from Arizona to Nevada (the
“Articles of Domestication”), and approved Articles of
Incorporation in Nevada, which differed from then-Articles of
Incorporation in Arizona, primarily by (a) changing our name from
United Mines, Inc. to WEED, Inc., (b) authorizing Twenty Million
(20,000,000) shares of preferred stock, with blank check rights
granted to our Board of Directors, and (c) authorizing Two Hundred
Million (200,000,000) shares of common stock (the “Nevada
Articles of Incorporation”). On December 19, 2014, the
holders of a majority of our outstanding common stock approved the
Articles of Domestication and the Nevada Articles of Incorporation
at a Special Meeting of Shareholders. On January 16, 2015, the
Articles of Domestication and the Nevada Articles of Incorporation
went effective with the Secretary of State of the State of Nevada.
On February 2, 2015, our name change to WEED, Inc., and a
corresponding ticker symbol change to “BUDZ” went
effective with FINRA and was reflected on the quotation of our
common stock on OTC Markets.
These
changes were effected in order to make our corporate name and
ticker symbol better align with our short-term and long-term
business focus, which in the short-term is to conduct
Sangre’s Cannabis Genomic Study over the next 5 years,
process those results, and in the long-term to be an international
cannabis and hemp research and product development company, with a
globally-recognized brand focusing on building and purchasing labs,
land and building commercial grade “Cultivation
Centers” to consult, assist, manage & lease to
universities, state governments, licensed dispensary owners and
worldwide organic grow operators on a contract basis, with a
concentration on the legal and medical Cannabis sector. Our
long-term plan is to become a True “Seed-to-Sale”
global holding company providing infrastructure, financial
solutions, product development and real estate options in this new
emerging market. Our long term plans may also include acquisitions
of synergistic businesses, such as distilleries to make infused
beverages and/or super oxygenated water with CBD and THC. We have
also formed WEED Australia Ltd., registered as an unlisted public
company in Australia, to address future global demand, however the
entity has been essentially dormant other than building
relationships and speaking at The Pharmaceutical Guild of Australia
conference event in Sydney Australia, September 2019, since its
inception.
Our
corporate offices are located at 4920 N. Post Trail, Tucson, AZ
85750, telephone number (520) 818-8582.
SUMMARY
OF THE OFFERING
|
Units offered by Company
|
|
40,000,000 Units,
with each Unit consisting of one (1) share of our Common Stock and
one (1) warrant to purchase (1) share of our Common Stock, at a
purchase price of $1.00 per Unit. The exercise price on the warrant
included in each Unit will be $1.50 and the warrant cannot be
exercised by the holder for at least twelve months from the date of
issuance. The warrants will expire two years from the issuance
date.
|
|
|
|
|
|
Common Shares outstanding before the offering
|
|
112,972,685
Common Shares as of the date hereof.
|
|
|
|
|
|
Common Shares outstanding after the offering (not including the
exercise of any warrants that are part of the Units)
|
|
152,972,685 Common Shares.
|
|
|
|
|
|
Common Shares outstanding after the offering (including the
exercise of all warrants that are part of the Units)
|
|
192,972,685 Common Shares.
|
|
|
|
|
|
Price per Unit
|
|
$1.00 per Unit.
|
|
|
|
|
|
Use of proceeds
|
|
If
we sell all the Units for the Maximum Price Per Unit, our proceeds
will be $40,000,000 less fees and commissions. We intend to use
these proceeds primarily for:
- Acquisition
and Build-Out of New York Property
- Completion
of 5-Year Genomic Sequencing Study
- Research
and Development in United States, Australia and Israel
See
“Use of Proceeds” on page 12 of this Offering
Circular.
|
|
|
|
|
|
Offering Amount
|
|
$40,000,000
|
|
|
|
|
|
Quotation of Common Shares
|
|
Our
Common Shares are quoted on the OTCQB-tier of OTC Markets under the
ticker symbol “BUDZ”. The
Units and warrants offered hereunder will not be quoted on any OTC
market.
|
|
|
|
|
|
Risk Factors
|
|
The
Units offered hereby involves a high degree of risk and should not
be purchased by investors who cannot afford the loss of their
entire investment. See “Risk Factors”.
|
RISK
FACTORS
Investing in the Units involves a high degree of risk. In
evaluating WEED, Inc. and investing in the Units, careful
consideration should be given to the following risk factors, in
addition to the other information included in this Offering
Circular. Each of these risk factors could materially adversely
affect WEED, Inc.’s business, operating results or financial
condition, as well as adversely affect the value of an investment
in our Units. The following is a summary of the most significant
factors that make this Offering speculative or substantially risky.
We are still subject to all the same risks that all companies in
our industry, and all companies in the economy, are exposed to.
These include risks relating to economic downturns, political and
economic events and technological developments. You should consider
general risks as well as specific risks when deciding whether to
invest.
We have a limited operating history and historical financial
information upon which you may evaluate our
performance.
You
should consider, among other factors, our prospects for success in
light of the risks and uncertainties encountered by companies that,
like us, are in their early stages of development. We may not
successfully address these risks and uncertainties or successfully
complete our studies and/or implement our existing and new
products. If we fail to do so, it could materially harm our
business and impair the value of our common stock. Even if we
accomplish these objectives, we may not generate the positive cash
flows or profits we anticipate in the future. We were incorporated
in the State of Arizona on August 20, 1999. From 2005 until 2015,
we were an exploration stage mineral exploration company that owned
a number of unpatented mining claims and Arizona State Land
Department claims. On November 26, 2014, our Board of Directors
approved the redomestication of our company from Arizona to Nevada
and we shifted our business focus to a company concentrating on the
development and application of cannabis-derived compounds for the
treatment of human disease. Although our subsidiary, Sangre, has
begun its planned five-year Cannabis Genomic Study to complete a
global genomic classification of the Cannabis plant genus the
completion of the study is likely years away. Unanticipated
problems, expenses and delays are frequently encountered in
establishing a new business, conducting research, and developing
new products. These include, but are not limited to, inadequate
funding, unforeseen research issues, lack of consumer acceptance,
competition, product development, and inadequate sales and
marketing. The failure by us to meet any of these conditions would
have a materially adverse effect upon us and may force us to reduce
or curtail operations. No assurance can be given that we can or
will ever operate profitably.
We may not be able to meet our future capital needs.
To
date, we have not generated any revenue and we have limited cash
liquidity and capital resources. Our
future capital requirements will depend on many factors, including
the progress and results of our Cannabis Genomic Study, our ability
to develop products, cash flow from operations, and competing
market developments. We anticipate the Cannabis Genomic Study will
cost approximately $15,000,000 to complete. We will need additional
capital in the near future. Any equity financings will result in
dilution to our then-existing stockholders. Although we currently
do not have any debt financing, any sources of debt financing in
the future may result in a high interest expense. Any financing, if
available, may be on unfavorable terms. If adequate funds are not
obtained, we will be required to reduce or curtail
operations.
If we cannot obtain additional funding, our research and
development efforts may be reduced or discontinued and we may not
be able to continue operations.
We
have historically experienced negative cash flows from operations
since our inception and we expect the negative cash flows from
operations to continue for the foreseeable future. Unless and until
we are able to generate revenues, we expect such losses to continue
for the foreseeable future. As discussed in our financial
statements, there exists substantial doubt regarding our ability to
continue as a going concern.
Research
and development efforts are highly dependent on the amount of cash
and cash equivalents on hand combined with our ability to raise
additional capital to support our future operations through one or
more methods, including but not limited to, issuing additional
equity or debt.
In
addition, we may also raise additional capital through additional
equity offerings and licensing our
research and/or future products in development. While we will
continue to explore these potential opportunities, there can be no
assurances that we will be successful in raising sufficient capital
on terms acceptable to us, or at all, or that we will be successful
in licensing our future products. Based on our current projections,
we believe we have insufficient cash on hand to meet our
obligations as they become due based on current assumptions. The
uncertainties surrounding our future cash inflows have raised
substantial doubt regarding our ability to continue as a going
concern.
One of our current projects is our 5-year cannabis genomic study
being conducted by Sangre. In the event that we are unable to
complete that study for any reason, such as inability to complete
our human clinical trials, or if those trials are not successful,
then it could significantly impact our business.
Although
we have plans to be a company with a multitude of business
segments, one of our first foray into medical cannabis research is
the 5-year cannabis genomic study being conducted by Sangre. In the
event that we are unable to complete the 5-year study for any
reason, such as the inability to complete our planned human
clinical trials in phases 2 and 3 of the study, or if those trials
are not successful, then it could significantly impact our
business.
Our research plan, which is focused on the development and
application of cannabis-derived compounds for the treatment of
human disease, and includes our 5-year cannabis genomic study being
conducted by Sangre, is dependent upon our ability to complete the
necessary research and clinical human trials.
Our
research plan, which is focused on the development and application
of cannabis-derived compounds for the treatment of human disease,
and includes our 5-year cannabis genomic study being conducted by
Sangre, is dependent upon our ability to complete the necessary
research and clinical human trials. In the event that we are unable
to complete those research and/or human clinical trials, or if
those trials are not successful, then it could significantly,
negatively impact all phases of our research plan and significantly
impact our business.
Current economic conditions and capital markets are in a period of
disruption and instability which could adversely affect our ability
to access the capital markets, and thus adversely affect our
business and liquidity.
The
current economic conditions largely caused by the coronavirus
pandemic have had, and likely will continue to have for the
foreseeable future, a negative impact on our ability to access the
capital markets, and thus have a negative impact on our business
and liquidity. The recent, substantial losses in worldwide equity
markets could lead to an extended worldwide recession. We may face
significant challenges if conditions in the capital markets do not
improve. Our ability to access the capital markets has been and
continues to be severely restricted at a time when we need to
access such markets, which could have a negative impact on our
business plans. Even if we are able to raise capital, it may not be
at a price or on terms that are favorable to us. We cannot predict
the occurrence of future disruptions or how long the current
conditions may continue.
The coronavirus pandemic is causing disruptions in the workplace,
which will have negative repercussions on our business if they
continue for an extended period time.
We
are closely monitoring the coronavirus pandemic and the directives
from federal and local authorities regarding not only our
workforce, but how it impacts companies we work with for our
various projects. As more states and localities continue with
social distancing and “work from home” regulations more
and more companies will be forced to either shut down, slow down or
alter their work routines. This could have a negative impact our
business going forward if these conditions persist for an extended
period of time.
Our proposed business is dependent on laws pertaining to the
cannabis industry.
Continued
development of the cannabis industry is dependent upon continued
legislative authorization of marijuana at the state level. Any
number of factors could slow or halt progress in this area.
Further, progress for the industry, while encouraging, is not
assured. While there may be ample public support for legislative
action, numerous factors impact the legislative process. Any one of
these factors could slow or halt use of marijuana, which would
negatively impact our business.
As
of the end of February 2019, 33 states and the District of Columbia
allow its citizens to use medical marijuana. Voters in the states
of Colorado, Washington, Alaska, Oregon and the District of
Columbia have approved ballot measures to legalize cannabis for
adult use. Currently as of the date of this Offering, there are 11
states that have legalized adult recreational use. The state laws
are in conflict with the Federal Controlled Substances Act, which
makes cannabis use and possession illegal on a national level. The
prior administration (President Obama) effectively stated that it
is not an efficient use of resources to direct law federal law
enforcement agencies to prosecute those lawfully abiding by
state-designated laws allowing the use and distribution of medical
cannabis. However, the Trump administration has indicated the
potential for stricter enforcement of the cannabis industry at the
federal level, but to date there has been very little in terms of
action. There is no guarantee that the Trump administration or
future administrations will maintain the low-priority enforcement
of federal laws in the cannabis industry that was adopted by the
Obama administration. The Trump administration or any new
administration that follows could change this policy and decide to
enforce the federal laws strongly. Any such change in the federal
government’s enforcement of current federal laws could cause
significant financial damage to our business and our
shareholders.
Further,
and while we do not intend to harvest, distribute or sell cannabis,
if we conduct research with the cannabis plant or lease buildings
to growers of cannabis, etc., we could be deemed to be
participating in cannabis cultivation, which remains illegal under
federal law, and exposes us to potential criminal liability, with
the additional risk that our properties could be subject to civil
forfeiture proceedings.
The
cannabis industry faces strong opposition.
It
is believed by many that large well-funded businesses may have a
strong economic opposition to the cannabis industry. We believe
that the pharmaceutical industry clearly does not want to cede
control of any product that could generate significant revenue. For
example, medical cannabis will likely adversely impact the existing
market for the current “marijuana pill” sold by
mainstream pharmaceutical companies. Further, the medical cannabis
industry could face a material threat from the pharmaceutical
industry, should cannabis displace other drugs or encroach upon the
pharmaceutical industry’s products. The pharmaceutical
industry is well funded with a strong and experienced lobby that
eclipses the funding of the medical cannabis movement. Any inroads
the pharmaceutical industry could make in halting or impeding the
cannabis industry could have a detrimental impact on our proposed
business.
Cannabis
remains illegal under Federal law.
Cannabis
is a schedule-I controlled substance and is illegal under federal
law. Even in those states in which the use of cannabis has been
legalized, its production and use remains a violation of federal
law. Since federal law criminalizing the use of cannabis preempts
state laws that legalize its use, strict enforcement of federal law
regarding cannabis would likely result in our inability to proceed
with our business plan.
Laws and regulations affecting the medical cannabis industry are
constantly changing, which could detrimentally affect our proposed
operations.
Local,
state and federal medical cannabis laws and regulations are broad
in scope and subject to evolving interpretations, which could
require us to incur substantial costs associated with compliance or
alter our business plan. In addition, violations of these laws, or
allegations of such violations, could disrupt our business and
result in a material adverse effect on our operations. In addition,
it is possible that regulations may be enacted in the future that
will be directly applicable to our proposed business. We cannot
predict the nature of any future laws, regulations, interpretations
or applications, nor can we determine what effect additional
governmental regulations or administrative policies and procedures,
when and if promulgated, could have on our business.
If we are unable to recruit and retain qualified personnel, our
business could be harmed.
Our
growth and success highly depend on qualified personnel.
Competition in the industry could cause us difficulty in recruiting
or retaining a sufficient number of qualified technical personnel,
which could harm our ability to develop new products. Also, the
fact cannabis remains illegal at the federal level may dissuade
qualified personnel from working in the cannabis industry, thus
limiting the pool of qualified individuals to run our business. If
we are unable to attract and retain necessary key talents, it would
harm our ability to develop competitive product and retain good
customers and could adversely affect our business and operating
results.
We may be unable to adequately protect our proprietary
rights.
Our
ability to compete partly depends on the superiority, uniqueness
and value of our intellectual property. To protect our proprietary
rights, we will rely on a combination of patent, copyright and
trade secret laws, confidentiality agreements with our employees
and third parties, and protective contractual provisions. Despite
these efforts, any of the following occurrences may reduce the
value of our intellectual property:
●
Our applications for patents relating to our business may not be
granted and, if granted, may be challenged or
invalidated;
●
Issued patents may not provide us with any competitive
advantages;
●
Our efforts to protect our intellectual property rights may not be
effective in preventing misappropriation of our
technology;
●
Our efforts may not prevent the development and design by others of
products or technologies similar to or competitive with, or
superior to those we develop;
●
Another party may obtain a blocking patent and we would need to
either obtain a license or design around the patent in order to
continue to offer the contested feature or service in our products;
or
●
The fact cannabis is illegal at the federal level may impact our
ability to secure patents from the United States Patent and
Trademark Office, and other intellectual property protections may
not be available to us.
We may become involved in lawsuits to protect or enforce our
patents that would be expensive and time consuming.
In
order to protect or enforce our patent rights, we may initiate
patent or trademark litigation against third parties. In addition,
we may become subject to interference or opposition proceedings
conducted in patent and trademark offices to determine the priority
and patentability of inventions. The defense of intellectual
property rights, including patent rights through lawsuits,
interference or opposition proceedings, and other legal and
administrative proceedings, would be costly and divert our
technical and management personnel from their normal
responsibilities. An adverse determination of any litigation or
defense proceedings could put our pending patent applications at
risk of not being issued.
Furthermore,
because of the substantial amount of discovery required in
connection with intellectual property litigation, there is a risk
that some of our confidential information could be compromised by
disclosure during this type of litigation. For example, during the
course of this kind of litigation, confidential information may be
inadvertently disclosed in the form of documents or testimony in
connection with discovery requests, depositions or trial testimony.
This disclosure could have a material adverse effect on our
business and our financial results.
We may be involved in litigation at some in the
future.
In
the ordinary course of business, we are from time to time involved
in various pending or threatened legal actions. The litigation
process is inherently uncertain and it is possible that the
resolution of such matters might have a material adverse effect
upon our financial condition and/or results of operations.
Litigation is also expensive and could cause us to spend
substantial sums on legal fees even if we are eventually successful
in the litigation.
We have several opportunities that we may not be able to take
advantage of or close without substantial funding.
As
detailed elsewhere in this Offering Circular we have several
business opportunities that we either cannot continue or cannot
begin without raising substantial funds either in this Offering or
through other sources. Notably, we have an opportunity to close on
a golf course property in New York, with unlimited water extraction
rights from Lake Erie, but currently need approximately $500,000 to
pay the remainder of the purchase price and close on the
acquisition. We are not sure if we will be able to secure the
necessary funds to acquire the property within the current
timeframe. If we are unable to acquire the property we could miss a
significant opportunity and it could affect our future business
plans.
Our common stock has been thinly traded and we cannot predict the
extent to which a trading market will develop.
Our
common stock is traded on the OTC Markets’ “Pink
Current Information” tier. Our common stock is thinly traded
compared to larger more widely known companies. Thinly traded
common stock can be more volatile than common stock trading in an
active public market. We cannot predict the extent to which an
active public market for our common stock will develop or be
sustained after this Offering.
Because we are subject to the “penny stock” rules, the
level of trading activity in our stock may be reduced.
Our
common stock is traded on the OTC Markets’
“OTCQB” tier. Broker-dealer practices in connection
with transactions in “penny stocks” are regulated by
certain penny stock rules adopted by the Securities and Exchange
Commission. Penny stocks, like shares of our common stock,
generally are equity securities with a price of less than $5.00,
other than securities registered on certain national securities
exchanges or quoted on NASDAQ. The penny stock rules require a
broker-dealer, prior to a transaction in a penny stock not
otherwise exempt from the rules, to deliver a standardized risk
disclosure document that provides information about penny stocks
and the nature and level of risks in the penny stock market. The
broker-dealer also must provide the customer with current bid and
offer quotations for the penny stock, the compensation of the
broker-dealer and its salesperson in the transaction, and, if the
broker-dealer is the sole market maker, the broker-dealer must
disclose this fact and the broker-dealer's presumed control over
the market, and monthly account statements showing the market value
of each penny stock held in the customer's account. In addition,
broker-dealers who sell these securities to persons other than
established customers and “accredited investors” must
make a special written determination that the penny stock is a
suitable investment for the purchaser and receive the purchaser's
written agreement to the transaction. Consequently, these
requirements may have the effect of reducing the level of trading
activity, if any, in the secondary market for a security subject to
the penny stock rules, and investors in our common stock may find
it difficult to sell their shares.
The lack of available and cost-effective directors and
officer’s insurance coverage in our industry may cause us to
be unable to attract and retain qualified executives, and this may
result in our inability to further develop our
business.
Our
business depends on attracting independent directors, executives
and senior management to advance our business plans. We currently
do not have directors and officer’s insurance to protect our
directors, officers and the company against to possible third-party
claims. This is due to the significant lack availability of such
policies in the cannabis industry at reasonably competitive prices.
As a result, the Company and our executive directors and officers
are susceptible to liability claims arising by third parties, and
as a result, we may be unable to attract and retain qualified
independent directors and executive management causing the
development of our business plans to be impeded as a
result.
If product liability lawsuits are successfully brought against us,
we will incur substantial liabilities.
From
time to time, we may receive complaints from customers regarding
our goods and services. We may become subject to product liability
lawsuits from customers alleging injury because of a purported
defect in our products or services, claiming substantial damages
and demanding payments from us. Liability claims may include
allegations of defects in manufacturing, defects in design, a
failure to warn of dangers inherent in the product, negligence,
strict liability and a breach of warranties. Claims could also be
asserted under state consumer protection acts. We may be in the
chain of ownership when we supply or distributes products, and
therefore is subject to the risk of being held legally responsible
for such products. Given the nature of these products (including
their relation to cannabis or for other reasons), these claims may
not be subject to insurance coverage or covered by insurance
policies. Any resulting litigation, regardless of the merits or
eventual outcome, could decrease demand for our products, result in
product recalls or withdrawals, be costly, divert management
attention, result in increased costs of doing business, or
otherwise have a material adverse effect on our business, results
of operations, and financial condition. Any litigation or even
negative publicity generated as a result of customer frustration or
disagreement with the products or services could damage our
reputation and diminish the value of our brand name, which could
have a material adverse effect on our business, results of
operations, and financial condition.
Due to our involvement in the cannabis industry, we may have a
difficult time obtaining the various insurances that are desired to
operate our business, which may expose us to additional risk and
financial liability.
Insurance
that is otherwise readily available, such as general liability, and
directors and officer’s insurance, is more difficult for us
to find, and more expensive, because we are service providers to
companies in the cannabis industry. There are no guarantees that we
will be able to find such insurances in the future, or that the
cost will be affordable to us. If we are forced to go without such
insurances, it may prevent us from entering into certain business
sectors, may inhibit our growth, and may expose us to additional
risk and financial liabilities.
SPECIAL
NOTE ABOUT FORWARD-LOOKING STATEMENTS
We have
made forward-looking statements in this Offering Circular,
including the sections entitled “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations” and “Business,” that are based on our
management’s beliefs and assumptions and on information
currently available to our management. Forward-looking statements
include the information concerning our possible or assumed future
results of operations, business strategies, financing plans,
competitive position, industry environment, potential growth
opportunities, the effects of future regulation and the effects of
competition. Forward-looking statements include all statements that
are not historical facts and can be identified by the use of
forward-looking terminology such as the words
“believe,” “expect,”
“anticipate,” “intend,” “plan,”
“estimate” or similar expressions. These statements are
only predictions and involve known and unknown risks and
uncertainties, including the risks outlined under “Risk
Factors” and elsewhere in this prospectus.
Although we believe
that the expectations reflected in our forward-looking statements
are reasonable, we cannot guarantee future results, events, levels
of activity, performance or achievement. We are not under any duty
to update any of the forward-looking statements after the date of
this prospectus to conform these statements to actual results,
unless required by law.
USE
OF PROCEEDS
If we raise Maximum
Offering hereunder, our net proceeds (after our estimated offering
expenses of $75,000, plus payments to
Dalmore Group) will be $38,693,500.(5) We will use these net
proceeds for the following:
|
%
of Offering Amount Sold)(1)
|
100%
of Offering Amount Sold
|
75%
of Offering Amount Sold
|
50%
of Offering Amount Sold
|
25% of Offering Amount Sold
|
|
Gross
Offering Proceeds(3)(5)
|
$40,000,000
|
$30,000,000
|
$20,000,000
|
$10,000,000
|
|
Approximate Offering Expenses
|
|
|
|
|
|
Misc.
Expenses
|
35,000
|
35,000
|
35,000
|
35,000
|
|
Legal
and Accounting
|
40,000
|
40,000
|
40,000
|
40,000
|
|
Dalmore – One-Time Fees (4)
|
31,500
|
31,500
|
31,500
|
31,500
|
|
Dalmore – 1% Commission (4)
|
400,000
|
300,000
|
200,000
|
100,000
|
|
GPC – 2% Commission
(5)
|
800,000
|
600,000
|
400,000
|
200,000
|
|
Total
Offering Expenses
|
75,000
|
75,000
|
75,000
|
75,000
|
|
Total
Net Offering Proceeds(3)
|
38,693,500
|
29,593,500
|
19,293,500
|
9,593,500
|
|
Principal Uses of Net Proceeds (2)
|
|
|
|
|
|
Employee/Officers
& Directors / Independent Contractor Compensation
|
$4,000,000
|
$3,000,000
|
$1,500,000
|
$600,000
|
|
Marketing
|
$2,000,000
|
$1,000,000
|
$500,000
|
-0-
|
|
Public
Company Costs
|
$750,000
|
$500,000
|
$300,000
|
$200,000
|
|
Sangre
Genomic Study
|
$10,000,000
|
$7,500,000
|
$5,000,000
|
$3,000,000
|
|
Other
Research & Development
|
$3,200,000
|
$2,400,000
|
$600,000
|
$418,500
|
|
Insurance
(Directors, Officers, Product, General Liability)
|
$1,000,000
|
$500,000
|
$250,000
|
$100,000
|
|
WEED
Israel Cannabis, Ltd.
|
$4,000,000
|
$3,000,000
|
$2,800,000
|
$1,500,000
|
|
WEED
Australia Ltd.
|
$4,000,000
|
$3,000,000
|
$2,800,000
|
$1,500,000
|
|
Purchase and
Build-Out of NY Property
|
$3,000,000
|
$3,000,000
|
$3,000,000
|
$2,000,000
|
|
Acquisitions
|
$3,000,000
|
$1,500,000
|
$1,000,000
|
-0-
|
|
Travel
|
$1,000,000
|
$1,000,000
|
$500,000
|
$150,000
|
|
Misc.
Costs and Expenses
|
$1,268,500
|
$1,643,500
|
$543,500
|
$25,000
|
|
Legal,
IP & Compliance
|
$1,475,000
|
$1,000,000
|
$500,000
|
$100,000
|
|
Total
Principal Uses of Net Proceeds(3)
|
39,493,500
|
29,593,500
|
19,693,500
|
9,793,500
|
|
Amount
Unallocated
|
-0-
|
-0-
|
-0-
|
-0-
|
(1)
We are offering the
Units at $1.00 per Unit.
(2)
These amounts are
estimated. The expected use of net proceeds from this Offering
represents our intentions based upon our current plans and business
conditions, which could change in the future as our plans and
business conditions evolve and change. The amounts and timing of
our actual expenditures, specifically with respect to working
capital, may vary significantly depending on numerous factors. The
precise amounts that we will devote to each of the foregoing items,
and the timing of expenditures, will vary depending on numerous
factors. As a result, our management will retain broad discretion
over the allocation of the net proceeds from this
offering.
(3)
For the Offering
Proceeds we did not account for any money received by us for the
exercise of the warrants that are a part of the Units since there
is no guarantee such warrants would ever be exercised.
(4)
We
have engaged Dalmore Group, LLC (“Dalmore”) a
broker-dealer registered with the SEC and a member of FINRA, to
perform the following administrative and technology related
functions in connection with this Offering, but not for
underwriting or placement agent services. The total fees that we
estimate that we will pay Dalmore Group, pursuant to a
fully-subscribed offering would be $431,500.
(5)
We engaged Great Point Capital
LLC (“GPC”), a broker-dealer registered with the SEC
and a member of FINRA, pursuant to an Investment Banking Agreement,
to assist in identification and qualification of identification of
investors who may be interested in funding the Company’s
capital needs via the Company’s qualified Reg A+ offering.
Their fee includes a 2% commission of all funds raised through
their introductions, as well as a one-time payment of
120,000 shares of the Company’s common stock which shares are
Restricted Securities under Rule 14. We may pay other
broker-dealers and finders as well, but information as to the finder
or brokers must be disclosed in an amendment to this offering
circular. The
maximum amount of fees we would pay to GPC pursuant to a
fully-subscribed offering would be $800,000, though it is unlikely
that all investors would come through GPC introductions and the
amount would likely be less than
$800,000.
In the
event we do not sell all of the Units being offered, we may seek
additional financing from other sources in order to support the
intended use of proceeds indicated above. If we secure additional
equity funding, investors in this offering would be diluted. In all
events, there can be no assurance that additional financing would
be available to us when wanted or needed and, if available, on
terms acceptable to us.
DILUTION
We
are offering for sale to new investors up to 40,000,000 Units at
$1.00 per Unit with one share per Unit. The following
table sets forth on a pro forma basis at September 30, 2020, the
differences between existing stockholders and new investors with
respect to the number of shares of common stock purchased from us,
the total consideration paid to us, and the price paid per Unit,
which is $1.00 per Unit.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Existing
Shareholders
|
112,972,685
|
74%
|
$80,200,784(2)
|
67%
|
$0.72
|
|
|
|
|
|
|
|
|
New
Investors
|
40,000,000(1)
|
26%
|
$40,000,000
|
33%
|
$1.00
|
|
|
|
|
|
|
|
|
Total
|
152,972,685
|
100%
|
$120,200,784
|
100%
|
$0.79
|
(1)
Does not include any shares from the exercise of warrants from the
Units.
(2)
Includes the value of shares issued for services.
If
you purchase Units in this offering, your ownership interest in our
Common Stock will be diluted immediately. The difference between
the public offering price per share of common stock and the net
tangible book value per share of common stock after this offering
constitutes the dilution to investors in this offering. Net
tangible book value per share is determined by dividing the net
tangible book value (total assets less intangible assets and total
liabilities) by the number of outstanding shares of common
stock. The dilution calculations we have set forth in
this section reflects an offering price of $1.00 per
share.
As
of September 30, 2020, we had a net tangible book value of
($959,221) or ($0.008) per share of issued and outstanding common
stock. After giving effect to the sale of the Units
proposed to be offered in the maximum offering of 40,000,000 Units
(40,000,000 shares), the net tangible book value at that date would
have been $40,959,221 or approximately $0.27 per
share. This represents an immediate increase in net
tangible book value of approximately $0.26 per share to existing
shareholders and an immediate dilution of approximately $0.74 per
share to new investors.
The
following table illustrates such per share dilution:
|
Proposed
public offering price (per share)
|
|
$1.00
|
|
Net
tangible book value per share (September 30, 2020)
|
$0.008
|
|
|
Increase in net
tangible book value per share attributable to proceeds from the
maximum offering
|
$0.26
|
|
|
Pro
forma net tangible book value per share after the
offering
|
|
$0.27
|
|
|
|
|
|
Dilution
to new investors
|
$0.74
|
|
PLAN
OF DISTRIBUTION
This
Offering Circular is part of an Offering Statement that we filed
with the SEC, using a continuous offering process. Periodically, as
we have material developments, we will provide an Offering Circular
supplement that may add, update or change information contained in
this Offering Circular. Any statement that we make in this Offering
Circular will be modified or superseded by any inconsistent
statement made by us in a subsequent Offering Circular supplement.
The Offering Statement we filed with the SEC includes exhibits that
provide more detailed descriptions of the matters discussed in this
Offering Circular. You should read this Offering Circular and the
related exhibits filed with the SEC and any Offering Circular
supplement, together with additional information contained in our
annual reports, semi-annual reports and other reports and
information statements that we will file periodically with the SEC.
See the section entitled “Additional Information” below
for more details.
Pricing of the Offering
Prior
to the Offering, our common stock trades on OTC Markets. However,
the public offering price herein ($1 per share) was determined by
our management. The principal factors considered in determining the
public offering price include:
-the
information set forth in this Offering Circular and otherwise
available;
-our
history and prospects and the history of and prospects for the
industry in which we compete;
-our
past and present financial performance;
-our
prospects for future earnings and the present state of our
development;
-the
general condition of the securities markets at the time of this
Offering;
-the
recent market prices of, and demand for, publicly traded common
stock of generally comparable companies; and
-other
factors deemed relevant by us.
Offering Period and Expiration Date
This
Offering will start on or after the Qualification Date and will
terminate at our discretion or, on the Termination
Date.
Dalmore Group
We have engaged Dalmore Group, LLC (“Dalmore”) a
broker-dealer registered with the SEC and a member of FINRA, to
perform the following administrative and technology related
functions in connection with this Offering, but not for
underwriting or placement agent services:
|
|
●
|
Review investor information, including KYC (“Know Your
Customer”) data,
AML (“Anti Money Laundering”) and other compliance
background checks, and provide a
recommendation to us whether or not to accept investor as a
customer.
|
|
|
●
|
Review each investors subscription agreement to confirm such
investors participation in the
Offering, and provide a determination to us whether or not to
accept the use of the
subscription agreement for the investor’s
participation.
|
|
|
●
|
Contact and/or notify us, if needed, to gather additional
information or clarification
on an investor.
|
|
|
●
|
Not provide any investment advice nor any investment
recommendations to any investor.
|
|
|
●
|
Keep investor details and data confidential and not disclose to any
third-party except as required
by regulators or pursuant to the terms of the agreement (e.g. as
needed for AML and background checks).
|
|
|
●
|
Coordinate with third party providers to ensure adequate review and
compliance.
|
As compensation for the services listed above, the company has
agreed to pay Dalmore $31,500 in one-time set up fees, consisting
of the following:
|
|
●
|
$5,000 advance payment for out of pocket expenses.
|
|
|
●
|
$20,000 consulting fee due and payable immediately after FINRA
issues a no objection letter.
|
|
|
●
|
$6,500 for fees to be paid to FINRA.
|
In addition, we will pay Dalmore Group a commission equal to 1% of
the amount raised in the Offering to support the Offering. Assuming
that the Offering is open for 12 months, we estimate that fees due
to pay Dalmore, pursuant to the 1% commission would be $400,500 for
a fully-subscribed Offering. Finally, the total fees that we
estimate that we will pay Dalmore Group, pursuant to a
fully-subscribed offering would be $431,500. These assumptions were
used in estimating the fees due in the “Use of
Proceeds.”
Great
Point Capital
On
March 3, 2021, WEED, Inc. (the “Company”) and Great
Point Capital, LLC (“GPC”) entered into an Investment
Banking Agreement (the “Agreement”) whereby GPC shall
serve as a nonexclusive investment banker for the Company in
connection with the identification of investors who may be
interested in funding the Company’s capital needs via the
Company’s qualified Reg A+ offering (the
“Raise”). GPC is an independent contractor for the
purposes of the Agreement.
The
Term of the Agreement shall be for the duration of the Raise or
until 15 days after effective notice of termination has been
received by either party. Company may not terminate until 45 days
from the Effective Date, March 3, 2021.
In
consideration of GPC’s services, the Company will pay to GPC
2% of gross proceeds of all money raised by GPC during the term of
the Agreement and a one-time payment of 120,000 shares of the
Company’s common stock, which shares are Restricted
Securities under Rule 144. Company shall reimburse all expenses of
GPC up to an aggregate of $2,500, after which approval is required.
The Company has significant indemnification obligations pursuant to
the Agreement, whereby GPC is indemnified from most actions falling
short of intentional misconduct. Company’s recovery for any
liability is also strictly limited.
Please
see Exhibit 10.1 to the
Company’s 8k filed on March 8, 2021for the full text of the
Agreement with GPC.
Procedures for Subscribing
When
you decide to subscribe for Offered Shares in this Offering, you
should:
Contact
us via phone or email.
1.
Electronically
receive, review, execute and deliver to us a subscription
agreement; and
2.
Deliver
funds directly by wire or electronic funds transfer via ACH to the
specified account maintained by us.
Any
potential investor will have ample time to review the subscription
agreement, along with their counsel, prior to making any final
investment decision. We shall only deliver such subscription
agreement upon request after a potential investor has had ample
opportunity to review this Offering Circular.
Right to Reject
Subscriptions. After we receive
your complete, executed subscription agreement and the funds
required under the subscription agreement have been transferred to
the escrow account, we have the right to review and accept or
reject your subscription in whole or in part, for any reason or for
no reason. We will return all monies from rejected subscriptions
immediately to you, without interest or
deduction.
Acceptance of
Subscriptions. Upon our
acceptance of a subscription agreement, we will countersign the
subscription agreement and issue the shares and warrants subscribed
at closing. Once you submit the subscription agreement and it is
accepted, you may not revoke or change your subscription or request
your subscription funds. All accepted subscription agreements are
irrevocable.
No Escrow
The
proceeds of this offering will not be placed into an escrow
account. We will offer the Units on a best effort’s basis. As
there is no minimum offering, upon the approval of any subscription
to this Offering Circular, we will immediately deposit said
proceeds into our bank account and may dispose of the proceeds in
accordance with the Use of Proceeds at Management’s
discretion.
MANAGEMENT'S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Disclaimer Regarding Forward Looking Statements
Our
Management’s Discussion and Analysis or Plan of Operations
contains not only statements that are historical facts, but also
statements that are forward-looking. Forward-looking statements
are, by their very nature, uncertain and risky. These risks and
uncertainties include international, national and local general
economic and market conditions; demographic changes; our ability to
sustain, manage, or forecast growth; our ability to successfully
make and integrate acquisitions; raw material costs and
availability; new product development and introduction; existing
government regulations and changes in, or the failure to comply
with, government regulations; adverse publicity; competition; the
loss of significant customers or suppliers; fluctuations and
difficulty in forecasting operating results; changes in business
strategy or development plans; business disruptions; the ability to
attract and retain qualified personnel; the ability to protect
technology; and other risks that might be detailed from time to
time in our filings with the Securities and Exchange
Commission.
Although
the forward-looking statements in this Registration Statement
reflect the good faith judgment of our management, such statements
can only be based on facts and factors currently known by them.
Consequently, and because forward-looking statements are inherently
subject to risks and uncertainties, the actual results and outcomes
may differ materially from the results and outcomes discussed in
the forward-looking statements. You are urged to carefully review
and consider the various disclosures made by us in this report and
in our other reports as we attempt to advise interested parties of
the risks and factors that may affect our business, financial
condition, and results of operations and prospects.
Overview
We
are an early stage holding company currently focused on the
development and application of cannabis-derived compounds for the
treatment of human disease. Our wholly-owned subsidiary, Sangre AT,
LLC (“Sangre”), has begun a planned five-year Cannabis
Genomic Study to complete a genetic blueprint of the Cannabis plant
genus, by creating a global genomic classification of the entire
plant. By targeting cannabis-derived molecules that stimulate the
endocannabinoid system, Sangre’s research team plans to
develop scientifically-valid and evidence-based cannabis strains
for the production of disease-specific medicines. The goal of the
research is to identify, collect, patent, and archive a collection
of highly-active medicinal strains. We plan to conduct this study
only in states where cannabis has been legalized for medicinal
purposes.
Using
annotated genomic data and newly generated phenotypic data, Sangre
plans to identify and isolate regions of the plant genome which are
related to growth, synthesis of desired molecules, and drought and
pest resistance. This complex data set would then be utilized in a
breeding program to generate and establish new hybrid cultivars
which exemplify the traits that are desired by the medical and
patient community. This breeding program would produce new seed
stocks and clones, which we plan on patenting. If successful this
intellectual property should generate immense value for the
Company. After developing a comprehensive understanding of the
annotated genome of a variety of cannabis strains, and obtaining
intellectual property protection over the most promising strains,
we plan move forward either independently or with strategic
partners to develop medicinal products for the treatment of a
multitude of human diseases.
Our
current, short-term goals relate to the Cannabis Genomic Study and
the resulting development of a variety of new cannabis strains,
and, over the next 5 years, we plan to process those results in
order to become an international cannabis research and product
development company, with a globally-recognized brand focusing on
building and purchasing labs, land and building commercial grade
“Cultivation Centers” to consult, assist, manage &
lease to universities, state governments, licensed dispensary
owners and organic grow operators on a contract basis with a
concentration on the legal and medical cannabis
sector.
Our
long-term plan is to become a true “Seed-to-Sale”
global holding company providing infrastructure, financial
solutions, product development, and real estate options in this new
emerging market. Our long term growth may also come from the
acquisition of synergistic businesses, such as distilleries, to
make anything from infused beverages to super oxygenated water with
CBD and THC. Currently, we have formed WEED Australia Ltd.,
registered as an unlisted public company in Australia to address
this Global demand. We have also formed WEED Australia Ltd.,
registered as an unlisted public company in Australia, to address
future global demand, however the entity has been dormant since its
inception. We will look to conduct future research, marketing,
import/exporting, and manufacturing of our proprietary products on
an international level.
In
furtherance of our current, short terms goals, Sangre initiated the
cannabis genome project in April 2017, by extracting DNA from seven
cannabis strains in Tucson, Arizona. Sangre followed the initial
extraction with a second round of extractions in July 2017. The
extracted DNA is currently being sequenced by the Sangre team using
a binary sequencing approach based on the use of two distinct
sequencing technologies and a proprietary bioinformatics database.
Following the generation of genomic data, the sequences will be
annotated (compared) against over 300,000 plant genes to elucidate
specific de novo pathways responsible for the synthesis of specific
compounds and classes of compounds.
Under
the genome project directives, additional strains are slated for
sequencing and annotation as part of the overall expansion of this
research project. An integral part of this expansion is the
acquisition of additional DNA extraction, amplification, and
sequencing technologies. The expansion also includes the
installation of high-level IT networks for data acquisition,
analysis, and storage.
On
July 26, 2017, we acquired a property located in La Veta, Colorado
in order for Sangre to complete its 5-Year, $15+ million Cannabis
Genomic Study. The site includes a 10,000+ sq. ft. building that
will house Sangre’s genomic research facility, a 4,000+
square foot building for plant product analytics and plant product
extraction, a 3,500 sq. ft. corporate office center, and 25 RV
slots with full water and electric, which we plan to convert into a
series of small research pods. Under the terms of the purchase
agreement, we paid $525,000 down, along with 25,000 shares of our
common stock, and Sangre took immediate possession of the property.
We were obligated to pay an additional $400,000 in cash and issue
an additional 75,000 shares of our common stock over the two next
years in order to pay the entire purchase price. To date we have
spent $354,000 renovating the property and an additional $400,000
on extraction and analytical lab equipment. We plan to complete the
property renovations by Q3 of 2019, at an estimated cost of
$300,000. We will need additional extraction equipment and
analytical lab equipment, totaling approximately $700,000. During
the year ended December 31, 2019, construction in progress in the
amount of $499,695 was fully impaired due to the Company may not
receive funds to complete the research facility center project.
There was no work performed in 2019. We will need to raise
additional funds in order to complete the planned renovations and
pay the purchase price for the equipment.
WEED
Inc. acquired the property in La Veta, Colorado in order to
facilitate the expansion of the genomic studies and the development
of new hybrid strains. The facility is currently under re-design
and renovation to convert the existing structures into a
world-class genetics research center.
A
gene-based breeding program will allow us to root out inferior
cultivars and replace them with fully-validated and patentable
cultivars which produce consistent plant products for the medicinal
markets. The gene-based breeding program will improve cultivars and
introduce integrity, stability, and quality to the market in the
following ways:
|
●
|
accelerated
and optimized growth rates; modern genomic resources will enhance
traditional breeding methods
|
|
●
|
generate
new cultivars, accelerating and perfecting the art of selective
breeding
|
|
●
|
provide
the ability to assay for specific genes within the crop, establish
strain tracking, and promote market quality assurance
|
|
●
|
improved
disease, pest, and drought resistance of the Cannabis
plant
|
We
believe the gene-based breeding program will facilitate and
accelerate:
|
●
|
improved
therapeutic properties, i.e., increased THC/CBD concentration and
the production of specific classes of oils and
terpenses
|
|
●
|
enhanced
opportunities for new drug discovery
|
|
●
|
accelerated
breeding of super-cultivars: drought, pest, and mold resistant,
increased %THC
|
|
●
|
revenue
generation through our unique ability to breed and genetically
fingerprint new, super-cultivars: establish strong patent
protection; and provide these cultivars to the market on a
favorable cost and royalty basis.
|
Our
goal with this program is to develop a translational breeding
program to establish a new collection of Cannabis cultivars for the
Colorado, national, and international markets. Through the use of
genetic screening technology, cultivars can be up-selected for
specific traits and grown to address the needs of consumers in the
medicinal market.
Corporate Overview
We
were originally incorporated under the name Plae, Inc., in the
State of Arizona on August 20, 1999. At the time we operated under
the name Plae, Inc., no business was conducted. No books or records
were maintained and no meetings were held. In essence, nothing was
done after incorporation until Glenn E. Martin took possession of
Plae, Inc. in January 2005. On February 18, 2005, the corporate
name was changed to King Mines, Inc. and then subsequently changed
to its current name, United Mines, Inc., on March 30, 2005. No
shares were issued until the Company became United Mines, Inc. From
2005 until 2015, we were an exploration stage mineral exploration
company that owned a number of unpatented mining claims and Arizona
State Land Department claims.
On
November 26, 2014, our Board of Directors approved the
redomestication of our company from Arizona to Nevada (the
“Articles of Domestication”), and approved Articles of
Incorporation in Nevada, which differed from then-Articles of
Incorporation in Arizona, primarily by (a) changing our name from
United Mines, Inc. to WEED, Inc., (b) authorizing Twenty Million
(20,000,000) shares of preferred stock, with blank check rights
granted to our Board of Directors, and (c) authorizing Two Hundred
Million (200,000,000) shares of common stock (the “Nevada
Articles of Incorporation”). On December 19, 2014, the
holders of a majority of our outstanding common stock approved the
Articles of Domestication and the Nevada Articles of Incorporation
at a Special Meeting of Shareholders. On January 16, 2015, the
Articles of Domestication and the Nevada Articles of Incorporation
went effective with the Secretary of State of the State of Nevada.
On February 2, 2015, our name change to WEED, Inc., and a
corresponding ticker symbol change to “BUDZ” went
effective with FINRA and was reflected on the quotation of our
common stock on OTC Markets.
These
changes were affected in order to make our corporate name and
ticker symbol better align with our short-term and long-term
business focus. Our current, short-term goals relate to the
Cannabis Genomic Study and the resulting development of a variety
of new cannabis strains, and, over the next 5 years, we plan to
process those results in order to become an international cannabis
research and product development company, with a
globally-recognized brand focusing on building and purchasing labs,
land and building commercial grade “Cultivation
Centers” to consult, assist, manage & lease to
universities, state governments, licensed dispensary owners and
organic grow operators on a contract basis with a concentration on
the legal and medical cannabis sector.
Our
long-term plan is to become a true “Seed-to-Sale”
global holding company providing infrastructure, financial
solutions, product development, and real estate options in this new
emerging market. Our long term growth may also come from the
acquisition of synergistic businesses, such as distilleries, to
make anything from infused beverages to super oxygenated water with
CBD and THC. Currently, we have formed WEED Australia Ltd.,
registered as an unlisted public company in Australia to address
this Global demand. We have also formed WEED Israel Cannabis Ltd.,
an Israeli corporation, to address future global demand. We will
look to conduct future research, marketing, import/exporting, and
manufacturing of our proprietary products on an international
level.
On
April 20, 2017, we entered into a Share Exchange Agreement with
Sangre AT, LLC, a Wyoming limited liability company, under which we
acquired all of the issued and outstanding limited liability
company membership units of Sangre in exchange for Five Hundred
Thousand (500,000) shares of our common stock, restricted in
accordance with Rule 144. As a result of this agreement, Sangre is
a wholly-owned subsidiary of WEED, Inc.
This
discussion and analysis should be read in conjunction with our
financial statements included as part of our Annual Report for the
year ended December 31, 2019.
Results of Operations for the Years Ended December 31, 2019 and
2018
|
|
|
|
|
|
|
|
Revenue
|
$ -
|
$ -
|
|
|
|
|
|
Operating
expenses:
|
|
|
|
|
|
|
|
General and
administrative expenses
|
969,913
|
1,358,178
|
|
Professional
fees
|
26,287,730
|
26,866,800
|
|
Depreciation and
amortization
|
159,424
|
180,640
|
|
Total operating
expenses
|
27,417,067
|
28,405,618
|
|
|
|
|
|
Loss from
operations
|
(27,417,067)
|
(28,405,618)
|
|
|
|
|
|
Other
expense
|
|
|
|
Interest
income
|
-
|
9,338
|
|
Interest
expense
|
(11,672)
|
(12,179)
|
|
Other
income
|
1,016
|
155,701
|
|
Loss on
deposit
|
(100,000)
|
(110,000)
|
|
Loss on
extinguishment of debt
|
-
|
(1,064,720)
|
|
Gain on
extinguishment of debt
|
-
|
121,475
|
|
Other
expense
|
(1,956)
|
(9,004)
|
|
Total other
expense, net
|
(112,612)
|
(909,389)
|
|
|
|
|
|
Net income
(loss)
|
$ (27,529,679)
|
$ (29,315,007)
|
Operating Loss; Net Loss
Our
comprehensive net loss decreased by $1,784,719, from ($29,315,007)
to ($27,530,288), from the year ended 2018 compared to 2019. Our
operating loss decreased by $988,551, from ($28,405,618) to
($27,417,067) for the same period. The decrease in operating loss
is primarily a result of our decrease in our professional fees
partially offset by a decrease in our general and administrative
expenses. The increase in our net loss is also a result of our
operating loss, partially offset by decreases in impairment expense
and loss on extinguishment of debt. These changes are detailed
below.
Revenue
We
have not had any revenues since our inception. Prior to October 1,
2014, we were an exploration stage mineral exploration company that
owned a number of unpatented mining claims and Arizona State Land
Department claims. In late 2014, we changed our short-term and
long-term business focus to the medical cannabis sector. In the
short-term we plan to conduct Sangre’s Cannabis Genomic Study
over the next 5 years and process those result, and in the
long-term is to be a company focused on purchasing land and
building commercial grade “Cultivation Centers” to
consult, assist, manage & lease to licensed dispensary owners
and organic grow operators on a contract basis, with a
concentration on the legal and medical marijuana (Cannabis) sector.
Our long-term plan is to become a True “Seed-to-Sale”
company providing infrastructure, financial solutions and real
estate options in this new emerging market, worldwide. We plan to
make our brand global and therefore we will look for opportunities
to conduct future research, marketing, import and exporting, and
manufacturing of any proprietary products on an international
level.
General and Administrative Expenses
General and administrative expenses decreased by
$388,265, from $1,358,178 for the year ended December 31, 2018 to
$969,913 for the year ended December 31, 2019, primarily due
to decreases in our lab supplies and
construction labor for the research facilities.
Professional Fees
Our
professional fees decreased during the year ended December 31, 2019
compared to the year ended December 31, 2018. Our professional fees
were $26,287,730 for the year ended December 31, 2019 and
$26,866,800 for the year ended December 31, 2018. These fees are
largely related to fees paid for legal and accounting services,
along with compensation to independent contractors, and increased
primarily as a result of increased stock-based compensation awards
and the value attributed to those shares of stock. We expect the
amount of professional fees we pay in cash to grow steadily as our
business expands. However, the amount attributed to the stock-based
compensation could decrease in periods when our stock price is
lower, if we continue to use stock-based compensation. In the event
we undertake an unusual transaction, such as an acquisition,
securities offering, or file a registration statement, we would
expect these fees to substantially increase during that
period.
Depreciation and Amortization
During the year ended December 31, 2019, we had depreciation and
amortization of $159,424, compared to $180,640 in the year ended
December 31, 2018. The depreciation and amortization expense in
2019 was related to the sale of the two Audi vehicles as a
repayment to Nicole Breen. The depreciation and amortization
expense in 2018 was related to the purchases of a house and
condominium in La Veta, Colorado and two trademarks acquired from
Copalix (PTY) LTD.
Interest Income
Interest
income decreased from $9,338 to $0 for the year ended December 31,
2019 compared to the same period in 2018. Our interest income in
2018 primarily as a result of a credit received at closing for the
purchase of the property located in La Veta, Colorado.
Interest Expense
Interest expense decreased slightly from ($12,179)
to ($11,672) for the year ended December 31, 2019 compared to the
same period in 2018. Our interest expense primarily relates to
interest on a convertible note and short-term loans.
Other Income
In
2019, we had other income of $1,016, compared to $155,701 in 2018.
The other income in 2019 related to a refund from a merchant. The
other income in 2018 related to settlement payment of $155,000 we
received from an insurance company related to a fire near one of
our properties in La Veta, Colorado, and loan discount of $121,475
on the settlement between Sangre AT, LLC and Craig W.
Clark.
Impairment Expense
In
2019, we had impairment expense of $0, compared to $321,614 in
2018. The impairment expense in 2018 related to the appraised value
of the property located at 1390 Mountain Valley Road purchased for
$1,200,000 on February 16, 2018.
Loss on Deposit
In 2019, we had loss on deposit of $100,000,
compared to $110,000 in 2018. The loss on deposit for 2019 period
was related to the termination of the
exclusive license and assignment agreement between us and Yissum
Research Development Company. The loss on deposit for 2018 period was related to
the non-refundable deposit amount of $110,000 for
the Lake Erie Project in
Westfield, New York.
Loss on Extinguishment of Debt
During
the year ended December 31, 2019, we had a loss on extinguishment
of debt of $0, compared to $1,064,720 in the year ended December
31, 2018. The loss on extinguishment of debt in 2018 was related to
the $475,000 principle amount promissory note issued by us to the
seller of property that was paid in full. The loss on
extinguishment of debt was recorded based on the fair value of the
consideration paid and the carrying value of the note payable on
the settlement date of January 17, 2018.
Other Expense
During
2019, we had other expense of $1,956, compared to $9,004 in 2018.
In 2019, the other expense primarily related to credit card finance
charges, and in the 2018 the other expense primarily related to
credit card finance charges.
Liquidity and Capital Resources
Introduction
During
the years ended December 31, 2019 and 2018, because of our
operating losses, we did not generate positive operating cash
flows. Our cash on hand as of December 31, 2019 was $2,509 and our
monthly cash flow burn rate was approximately $40,000. Our cash on
hand was primarily proceeds from the sales of our securities. We
currently do not believe we will be able to satisfy our cash needs
from operations for many years to come.
Our
cash, current assets, total assets, current liabilities, and total
liabilities as of December 31, 2019 and 2018, respectively, are as
follows:
|
|
|
|
|
|
Cash
|
$ 2,509
|
$ 70,608
|
$ (68,099)
|
|
Total Current
Assets
|
126,310
|
491,939
|
(365,629)
|
|
Total
Assets
|
1,952,612
|
3,020,989
|
(1,068,377)
|
|
Total Current
Liabilities
|
752,970
|
259,362
|
493,608
|
|
Total
Liabilities
|
$ 752,970
|
$ 259,362
|
493,608
|
Our
current assets decreased by $365,629 as of December 31, 2019 as
compared to December 31, 2018, primarily due to decreases in cash,
deposits, and prepaid expenses. The decrease in our total assets
between the two periods was primarily attributed to a decrease in
our vehicles and higher accumulated depreciation of our
assets.
Our
current liabilities and total liabilities increased by $493,608, as
of December 31, 2019 as compared to December 31, 2018. This
increase in liabilities as of December 31, 2019 was primarily
related to increases in our accrued officer compensation, accrued
expenses, accrued interest, notes payable and notes payable,
related parties compared to December 31, 2018.
In
order to repay our obligations in full or in part when due, we will
be required to raise significant capital from other sources. There
is no assurance, however, that we will be successful in these
efforts.
Cash Requirements
We
had cash available as of December 31, 2019 of $2,509 and $70,608 on
December 31, 2018. Based on our revenues, cash on hand and current
monthly burn rate of approximately $40,000, we will need to
continue borrowing from our shareholders and other related parties,
and/or raise money from the sales of our securities, to fund
operations.
Sources and Uses of Cash
Operations
We
had net cash used in operating activities of $1,110,597 for the
year ended December 31, 2019, as compared to $3,181,303 for the
year ended December 31, 2018. In 2019, the net cash used in
operating activities consisted primarily of our net loss of
($27,529,679), offset by estimated fair value of stock-based
compensation of $22,770,662, estimated fair of shares issued for
services of $2,578,250, depreciation and amortization of $159,423,
and impairment of construction in progress of $499,695 adjusted by
an increases in accounts receivable of $801, accrued expenses of
$141,800, and a decreases in prepaid expenses and other assets of
$298,331 and accounts payable of $28,278. In 2018, the net cash
used in operating activities consisted primarily of our net loss of
($29,315,007) and gain of settlement of debt of ($121,475), offset
by estimated fair value of stock-based compensation of $21,201,397,
estimated fair of shares issued for services of $4,041,575,
impairment of property of $321,614, loss on debt extinguishment of
$1,064,720, loss of deposit of $110,000, and depreciation and
amortization of $180,640, adjusted by an increases in prepaid
expenses and other assets of $498,311, accounts receivable of $21,
accounts payable of $11,849, and a decrease in accrued expenses of
$178,335.
Investments
In
2019, we had net cash used in investing activities of $2,979,
consisting entirely of purchases of property and equipment. In
2018, we had net cash used in investing activities of $876,481,
consisting of purchases of property and equipment of $826,481 and
purchase of intangible assets of $50,000.
Financing
Our
net cash provided by financing activities for the year ended
December 31, 2019 was $1,046,086, compared to $3,967,214 for the
year ended December 31, 2018. For the period in 2019, our financing
activities related to proceeds from the sale of common stock of
$573,000, proceeds from notes payable, related party of $305,823,
and proceeds from notes payable of $250,850, offset by repayments
on notes payable of ($83,587). For the period in 2018, our
financing activities related to proceeds from the sale of common
stock of $5,023,401 and proceeds from notes payable of $7,000,
offset by repayments on notes payable of ($1,063,187).
Off Balance Sheet Arrangements
We have no off-balance sheet arrangements.
The
below discussion and analysis should be read in conjunction with
our financial statements included as part of this Offering
Statement.
Three Months Ended September 30, 2020 Compared
to Three Months Ended September 30, 2019
Results of
Operations
|
|
Three Months Ended
September 30,
|
|
|
|
|
|
Revenue
|
$-
|
$-
|
|
|
|
|
|
Operating
expenses:
|
|
|
|
|
|
|
|
General
and administrative
|
75,550
|
80,249
|
|
Professional
fees
|
109,927
|
6,065,896
|
|
Depreciation
and amortization
|
29,775
|
40,756
|
|
Total
operating expenses
|
215,252
|
6,186,901
|
|
|
|
|
|
Net
operating loss
|
(215,252)
|
(6,186,901)
|
|
|
|
|
|
Other
Expense
|
|
|
|
Interest
expense
|
(8,329)
|
(3,225)
|
|
Loss
on deposit
|
-
|
(100,000)
|
|
Gain
on sale of fixed asset
|
46,948
|
-
|
|
|
|
|
|
Net
loss
|
$(176,633)
|
$(6,290,126)
|
|
|
|
|
|
Other
Comprehensive Loss
|
(13)
|
(25)
|
|
|
|
|
|
Comprehensive
Loss
|
$(176,646)
|
$(6,290,151)
|
Operating Loss; Net
Loss
Our comprehensive loss decreased by $6,113,505, from ($6,290,151)
to ($147,646), from the three months ended September 30, 2019
compared to the three months ended September 30, 2020. Our
operating loss decreased by $5,971,649, from ($6,186,901) to
($215,252) for the same period. The decrease in net loss compared
to the same period of the prior year is primarily a result of
decreases in professional fees and general and administrative
expenses and a gain on sale of fixed asset, offset slightly by an
increase in our interest expense. These changes are detailed
below.
Revenue
We have not had any revenues since our inception. We are a company
focused on the medical cannabis sector. In the short-term we plan
to conduct Sangre’s Cannabis Genomic Study over the next 5
years and process those result, and in the long-term is to be a
company focused on purchasing land and building commercial grade
“Cultivation Centers” to consult, assist, manage &
lease to licensed dispensary owners and organic grow operators on a
contract basis, with a concentration on the legal and medical
marijuana (Cannabis) sector. Our long-term plan is to become a True
“Seed-to-Sale” company providing infrastructure,
financial solutions and real estate options in this new emerging
market, worldwide. We plan to make our brand global and therefore
we will look for opportunities to conduct future research,
marketing, import and exporting, and manufacturing of any
proprietary products on an international level.
General and Administrative
Expenses
General and administrative expenses decreased by $4,699, from
$80,249 for the three months ended September 30, 2019 to $75,550
for the three months ended September 30, 2020, primarily due to
decreases in travel, facilities maintenance, and charitable
contribution expenses.
Professional
Fees
Our professional fees decreased by $5,955,969 during the three
months ended September 30, 2020 compared to the three months ended
September 30, 2019. Our professional fees were $109,927 for the
three months ended September 30, 2020 and $6,065,896 for the three
months ended September 30, 2019. These fees are largely related to
fees paid for legal and accounting services, along with
compensation to independent contractors, and decreased
significantly primarily as a result of a decrease in the value of
stock-based compensation awards due to our lower stock price. We
expect these fees to vary quarter-to-quarter as our business and
stock price fluctuate if we continue to use stock-based
compensation. In the event we undertake an unusual transaction,
such as an acquisition, securities offering, or file a registration
statement, we would expect these fees to substantially increase
during that period.
Depreciation and
Amortization
During the three months ended September 30, 2020 we had
depreciation and amortization expense of $29,775, compared to
$40,756 in the three months ended September 30, 2019. Our
depreciation and amortization expense primarily relates to our
property and trademark acquisitions.
Interest
Expense
Interest expense increased from $3,225 to $8,329 for the three
months ended September 30, 2019 compared to the same period in
2020. Our interest expense primarily relates to notes payable from
attorney and related parties.
Loss on
Deposit
We had a loss on deposit of $0 during the three months ended
September 30, 2020 compared to $100,000 during the three months
ended September 30, 2019. Our loss on deposit during the three
months ended September 30, 2019 related the termination of the
exclusive license and assignment agreement between us and Yissum
Research Development Company. The second installment of the license
fee of $400,000, due on May 1, 2019, was not paid, and the first
installment of $100,000 was recorded as a loss on deposit due to
the termination.
Gain on Sale of Fixed
Asset
During the three months ended September 30, 2020, we had a gain on
sale of fixed asset of $46,948, compared to $0 for the three months
ended September 30, 2019. The gain on sale of fixed asset during
the three months ended September 30, 2020 related to the sale of a
condominium we owned in La Veta, Colorado.
Nine Months Ended September 30,
2020 Compared to Nine Months Ended September 30,
2019
Results of
Operations
|
|
Nine
months Ended
September
30,
|
|
|
|
|
|
Revenue
|
$-
|
$-
|
|
|
|
|
|
Operating
expenses:
|
|
|
|
|
|
|
|
General and
administrative
|
224,233
|
390,937
|
|
Professional
fees
|
3,540,739
|
23,979,599
|
|
Depreciation and
amortization
|
106,498
|
122,172
|
|
Total operating
expenses
|
3,871,470
|
24,492,708
|
|
|
|
|
|
Net operating
loss
|
(3,871,470)
|
(24,492,708)
|
|
|
|
|
|
Other
Expense
|
|
|
|
Interest
expense
|
(30,959)
|
(5,624)
|
|
Other
income
|
-
|
1,017
|
|
Loss on
deposit
|
-
|
(100,000)
|
|
Gain on sale of
fixed asset
|
46,948
|
-
|
|
Other
expense
|
-
|
(1,956)
|
|
|
|
|
|
Net
loss
|
$(3,855,481)
|
$(24,599,261)
|
|
|
|
|
|
Other Comprehensive
Loss
|
(638)
|
(546)
|
|
|
|
|
|
Comprehensive
Loss
|
$(3,856,119)
|
$(24,599,807)
|
Operating Loss; Net
Loss
Our comprehensive loss decreased by $20,743,688, from ($24,599,807)
to ($3,856,119), from the nine months ended September 30, 2019
compared to the nine months ended September 30, 2020. Our operating
loss decreased by $20,621,238, from ($24,492,708) to ($3,871,470)
for the same period. The decrease in operating loss and net loss
compared to the same period of the prior year is primarily a result
of decreases in professional fees and general and administrative
expenses, offset slightly by an increase in our interest expense.
These changes are detailed below.
Revenue
We have not had any revenues since our inception. We are company
focused on the medical cannabis sector. In the short-term we plan
to conduct Sangre’s Cannabis Genomic Study over the next 5
years and process those result, and in the long-term is to be a
company focused on purchasing land and building commercial grade
“Cultivation Centers” to consult, assist, manage &
lease to licensed dispensary owners and organic grow operators on a
contract basis, with a concentration on the legal and medical
marijuana (Cannabis) sector. Our long-term plan is to become a True
“Seed-to-Sale” company providing infrastructure,
financial solutions and real estate options in this new emerging
market, worldwide. We plan to make our brand global and therefore
we will look for opportunities to conduct future research,
marketing, import and exporting, and manufacturing of any
proprietary products on an international level.
General and Administrative
Expenses
General and administrative expenses decreased by $166,704, from
$390,937 for the nine months ended September 30, 2019 to $224,233
for the nine months ended September 30, 2020, primarily due to
decreases in travel, facilities maintenance, and charitable
contribution expenses.
Professional
Fees
Our professional fees decreased by $20,438,860 during the nine
months ended September 30, 2020 compared to the nine months ended
September 30, 2019. Our professional fees were $3,540,739 for the
nine months ended September 30, 2020 and $23,979,599 for the nine
months ended September 30, 2019. These fees are largely related to
fees paid for legal and accounting services, along with
compensation to independent contractors, and decreased
significantly primarily as a result of a decrease in the value of
stock-based compensation awards due to our lower stock price. We
expect these fees to vary quarter-to-quarter as our business and
stock price fluctuate if we continue to use stock-based
compensation. In the event we undertake an unusual transaction,
such as an acquisition, securities offering, or file a registration
statement, we would expect these fees to substantially increase
during that period.
Depreciation and
Amortization
During the nine months ended September 30, 2020 we had depreciation
and amortization expense of $106,498, compared to $122,172 in the
nine months ended September 30, 2019. Our depreciation and
amortization expense primarily relates to our property and
trademark acquisitions.
Interest
Expense
Interest expense increased from $5,614 to $30,959 for the nine
months ended September 30, 2019 compared to the same period in
2020. Our interest expense primarily relates to notes payable from
attorney and related parties.
Other
Income
Other income during the nine months ended September 30, 2020 was
$0, compared to $1,017 for the nine months ended September 30,
2019. Our other income for the nine months ended September 30,
2019, related to a refund from a merchant.
Loss on
Deposit
We had a loss on deposit of $0 during the nine months ended
September 30, 2020 compared to $100,000 during the nine months
ended September 30, 2019. Our loss on deposit during the nine
months ended September 30, 2019 related the termination of the
exclusive license and assignment agreement between us and Yissum
Research Development Company. The second installment of the license
fee of $400,000, due on May 1, 2019, was not paid, and the first
installment of $100,000 was recorded as a loss on deposit due to
the termination.
Gain on Sale of Fixed
Asset
During the nine months ended September 30, 2020, we had a gain on
sale of fixed asset of $46,948, compared to $0 for the nine months
ended September 30, 2019. The gain on sale of fixed asset during
the three months ended September 30, 2020 related to the sale of a
condominium we owned in La Veta, Colorado.
Other
Expense
Other expense decreased from $1,956 to $0 for the nine months ended
September 30, 2019 compared to the same period in 2020. Our other
expense for the nine months ended September 30, 2019, relates to
bank service charges.
Liquidity and Capital
Resources
Introduction
During the nine months ended September 30, 2020, because of our
operating losses, we did not generate positive operating cash
flows. Our cash on hand as of September 30, 2020 was $166,463 and
our monthly cash flow burn rate was approximately $45,000. Our cash
on hand was primarily proceeds from the sales of our securities and
from the sale of the condominium we owned in La Veta, Colorado. We
currently do not believe we will be able to satisfy our cash needs
from our revenues for many years to come.
Our cash, current assets, total assets, current liabilities, and
total liabilities as of September 30, 2020 and December 31, 2019,
respectively, are as follows:
|
|
|
|
|
|
|
|
|
|
|
Cash
|
$ 166,463
|
$ 2,509
|
$ 163,954
|
|
Total Current
Assets
|
382,625
|
126,310
|
256,315
|
|
Total
Assets
|
1,985,787
|
1,952,612
|
33,175
|
|
Total Current
Liabilities
|
982,599
|
752,970
|
229,629
|
|
Total
Liabilities
|
$ 982,599
|
$ 752,970
|
$ 229,629
|
Our total assets increased by $33,175 as of September 30, 2020 as
compared to December 31, 2019. The increase in our total assets
between the two periods was primarily attributed to increases in
our cash and deposits, partially offset by decreases in our land,
buildings and property and equipment, net (due to depreciation) at
September 30, 2020 compared to December 31,
2019.
Our current liabilities and total liabilities increased by
$229,629, as of September 30, 2020 as compared to December 31,
2019. This increase was due to increases in accounts payable,
accrued officer compensation, notes payable, related party, accrued
expenses, and accrued interest, partially offset by a decrease in
notes payable.
In order to pay our obligations in full or in part when due, we
will be required to raise capital from other sources. There is no
assurance, however, that we will be successful in these
efforts.
Cash
Requirements
We had cash available of $166,463 and $2,509 as of September 30,
2020 and December 31, 2019, respectively. Based on our revenues,
cash on hand and current monthly burn rate of approximately
$45,000, we will need to continue borrowing from our shareholders
and other related parties, and/or raise money from the sales of our
securities, to fund operations.
Sources and Uses of
Cash
Operations
We had net cash used in operating activities of $279,631 for the
nine months ended September 30, 2020, as compared to $1,052,279 for
the nine months ended September 30, 2019. For the period in 2020,
the net cash used in operating activities consisted primarily of
our net loss of ($3,855,481), adjusted by estimated fair value of
stock-based compensation of $2,044,911, estimated value of shares
issued for services of $1,365,200, depreciation and amortization of
$106,498, imputed interest on RP loans of $13,945, and gain on sale
of fixed asset of ($46,948) and adjusted by an increase in assets
of prepaid expenses and other assets of $92,361, and increases in
liabilities of accounts payable of $52,174 and accrued expenses of
$132,431. For the period in 2019, the net cash used in operating
activities consisted primarily of our net loss of ($24,599,261),
offset by estimated fair value of stock-based compensation of
$21,209,062, estimated value of shares issued for services of
$1,909,929, and depreciation and amortization of $122,172, and
adjusted by a decrease in prepaid expenses and other assets of
$305,707, a decrease in accounts payable of $91,452, and an
increase in accrued expenses of $92,365.
Investments
For the nine months ended September 30, 2020, we cash flows used in
investing activities of $163,590, which was all attributable to the
condominium we sold in La Veta, Colorado. For the period in 2019,
the net cash used in investing activities of $2,979, with the
entire amount related to purchases of property and
equipment.
Financing
Our net cash provided by financing activities for the nine months
ended September 30, 2020 was $280,024, compared to $997,636 for the
nine months ended September 30, 2019. For the period in 2020, our
financing activities related to proceeds from the sale of common
stock of $195,000, proceeds from notes payable of $10,200, proceeds
from notes payable-related party of $63,100, and stock payable of
$40,000, partially offset by repayments on notes payable of
$28,276. For the period in 2019, our financing activities related
to proceeds from the sale of common stock of $498,001, proceeds
from notes payable of $230,812, and proceeds from notes
payable-related party of $268,823.
Off Balance Sheet
Arrangements
We have no off
balance sheet arrangements.
Penny
Stock Rules / Section 15(g) of the Exchange Act
Our
shares may be considered penny stock covered by Section 15(g) of
the Securities Exchange Act of 1934, as amended (the "Exchange
Act"), and Rules 15g-1 through 15g-6 promulgated thereunder. They
impose additional sales practice requirements on broker/dealers who
sell our securities to persons other than established customers and
accredited investors who are generally institutions with assets in
excess of $5,000,000 or individuals with net worth in excess of
$1,000,000 (including spouse's net worth and may include the fair
market value of home furnishings and automobiles, but excluding
from the calculation the value any primary residence and the
related amount of any indebtedness on primary residence up to the
fair market value of the primary residence (any indebtedness that
exceeds the fair market value of the primary residence must be
deducted from net worth calculation)) or annual income exceeding
$200,000 or $300,000 jointly with their spouses.
Rule
15g-1 exempts a number of specific transactions from the scope of
the penny stock rules. Rule 15g-2 declares unlawful broker/dealer
transactions in penny stocks unless the broker/dealer has first
provided to the customer a standardized disclosure
document.
Rule
15g-3 provides that it is unlawful for a broker/dealer to engage in
a penny stock transaction unless the broker/dealer first discloses
and subsequently confirms to the customer current quotation prices
or similar market information concerning the penny stock in
question.
Rule
15g-4 prohibits broker/dealers from completing penny stock
transactions for a customer unless the broker/dealer first
discloses to the customer the amount of compensation or other
remuneration received as a result of the penny stock
transaction.
Rule
15g-5 requires that a broker/dealer executing a penny stock
transaction, other than one exempt under Rule 15g-1, disclose to
its customer, at the time of or prior to the transaction,
information about the sales person’s
compensation.
Rule
15g-6 requires broker/dealers selling penny stocks to provide their
customers with monthly account statements.
Rule
15g-9 requires broker/dealers to approved the transaction for the
customer’s account; obtain a written agreement from the
customer setting forth the identity and quantity of the stock being
purchased; obtain from the customer information regarding his
investment experience; make a determination that the investment is
suitable for the investor; deliver to the customer a written
statement for the basis for the suitability determination and that
it is unlawful to effect the transaction without written
authorization for the transaction from the customer.
The
application of the penny stock rules may affect your ability to
resell your shares due to broker-dealer reluctance to undertake the
above-described regulatory burdens.
SECURITIES
BEING OFFERED
Our
authorized capital stock consists of 200,000,000 shares of common
stock, par value $0.001, and 20,000,000 shares of preferred stock,
par value $0.001. As of November 30, 2020, there are 112,972,685
shares of our common stock issued and outstanding, held by
approximately 266 shareholders of record. There are no shares of
our preferred stock outstanding as of the date of this
filing.
Units. We
are offering 40,000,000 Units under this Offering, with each Unit
consisting of one (1) share of our Common Stock and one (1) warrant
to purchase (1) share of our Common Stock, at a purchase price of
$1.00 per Unit. The exercise price on the warrant included in each
Unit will be $1.50 per share and the warrant cannot be exercised by the holder
until at least twelve months have passed from the date of issuance.
The warrants will expire two years from the issuance
date.
Common Stock. Each shareholder of our
common stock is entitled to a pro rata share of cash distributions
made to shareholders, including dividend payments. The holders of
our common stock are entitled to one vote for each share of record
on all matters to be voted on by shareholders. There is no
cumulative voting with respect to the election of our directors or
any other matter. Therefore, the holders of more than 50% of the
shares voted for the election of those directors can elect all of
the directors. The holders of our common stock are entitled to
receive dividends when and if declared by our Board of Directors
from funds legally available therefore. Cash or stock dividends are
at the sole discretion of our Board of Directors. In the event of
our liquidation, dissolution or winding up, the holders of common
stock are entitled to share ratably in all assets remaining
available for distribution to them after payment of our liabilities
and after provision has been made for each class of stock, if any,
having any preference in relation to our common stock. Holders of
shares of our common stock have no conversion, preemptive or other
subscription rights, and there are no redemption provisions
applicable to our common stock.
Warrants. The warrants included as part
of each Unit offered hereunder will be a warrant to purchase shares
of our common stock at an exercise price equal to 150% of the
amount the investor pays for the Unit. For example, if an investor
purchases Units at a price of $1.00 per Unit, then the exercise
price on the warrant issued with the Unit will be $1.50 per share.
The warrants issued as part of each Unit will expire two (2)
years after the date of issuance and cannot be exercised until at
least twelve months have passed from the date of
issuance.
Dividend Policy. We have never issued
any dividends under WEED, Inc and do not expect to pay any stock
dividend or any cash dividends on our common stock in the
foreseeable future. We currently intend to retain our earnings, if
any, for use in our business. Any dividends declared on our common
stock in the future will be at the discretion of our Board of
Directors and subject to any restrictions that may be imposed by
our lenders.
Liquidation Rights. In the event of a
voluntary or involuntary liquidation, dissolution or winding up of
our company, the holders of our common stock will be entitled to
share ratably on the basis of the number of shares held in any of
the assets available for distribution after we have paid in full
all of our debts and after the holders of all outstanding preferred
stock, if any, have received their liquidation preferences in
full.
Exclusive Forum Provision. In the event
of litigation with an investor that participates in the Offering,
the subscription agreement for the sale of the Units contains an
exclusive forum provision that states any litigation much be filed
exclusively in the state and federal courts sitting in Pima County,
Arizona. However, notwithstanding this provision, this choice of
forum provision does not preclude or contract the scope of
exclusive federal or concurrent jurisdiction for any actions
brought under the Securities Act or the Exchange Act and does not
apply to claims arising under the federal securities laws.
Accordingly, our exclusive forum provision will not relieve us of
our duties to comply with the federal securities laws and the rules
and regulations thereunder, and our compliance with these laws,
rules, and regulations cannot be waived by us or an
investor.
Transfer Agent. The transfer agent for
our common stock is Pacific Stock Transfer Company, 6725 Via Austi
Pkwy #300, Las Vegas, NV 89119.
INTEREST
OF NAMED EXPERTS AND COUNSEL
Law
Offices of Craig V. Butler serves as our legal counsel in
connection with this offering. The principal of the Law Offices of
Craig V. Butler, Mr. Craig V. Butler owns 1,300,000 shares of our
common stock as of November 30, 2020.
DESCRIPTION
OF BUSINESS
General
We are
a USA-based fully reporting public company currently specializing
in the medicinal cannabis space. We are a multi-national,
multi-faceted, vertically-integrated organization. We are
structured as a holding company doing business through our
divisions, wholly-owned subsidiaries, and strategically placed
collaborative partners to achieve and promote a global brand. We
are dedicated to global goals and outreach across the full spectrum
of the cannabis industry to find treatments, therapies and medical
cures utilizing the Cannabaceae plant family. We do not grow,
harvest, produce, or sell any substance in violation of US Federal
law under The Federal Controlled Substances Act, and we meet all
standards of international law for WEED, Inc. and its subsidiaries
in foreign locations.
Currently, we are
either working or planning for several different business
opportunities in the cannabis space. Our first business opportunity
is through our wholly-owned subsidiary, Sangre AT, LLC
(“Sangre”), where we are focused on the development and
application of cannabis-derived compounds for the treatment of
human disease. To that end Sangre, is working on a planned
five-year Cannabis Genomic Study to complete a genetic blueprint of
the Cannabis plant genus, by creating a global genomic
classification of the entire plant. Second, we are under contract
to purchase a golf course property located in Westfield, New York.
We own the unlimited water extraction rights from Lake Erie to the
property already, but need to raise at least $500,000 to acquire
the property. If we are successful in acquiring the property we
plan to utilize the property to access the hemp and infused
beverage markets. Third, we have established WEED Australia Ltd.
and The Cannabis Institute of Australia (C.I.A.) in Australia for
the purpose of conducting cannabis and hemp research and
potentially developing products in Australia. C.I.A. is a
non-profit entity formed for the purpose of conducting cannabis and
hemp research and potentially developing products in Australia for
domestic research and development of products, services and
educational purposes to all 7 States and territories including
Tasmania. . Fourth, we have an arrangement with Professor Elka
Touitou to assist us with cannabis research and studies in Israel.
Professor Touitou was the Head of the Innovative Dermal,
Transdermal and Transmucosal Delivery Lab at the Institute of Drug
Research, The School of Pharmacy, HUJ, now retired but still has
HUJ clinical trial & independent studies/lab privileges.
Professor Touitou is an internationally renowned authority in the
field of drug delivery and design of new technologies for efficient
administration of drugs and development of new products.
Professor Touitou
has been involved in Cannabinoid research since 1988 at The Hebrew
University of Jerusalem, (HUJ) Jerusalem, Israel.
Our
current, short-term goals relate to the Cannabis Genomic Study and
the resulting development of a variety of new cannabis strains,
and, over the next few years, we plan to process those results in
order to become an international cannabis research and product
development company, with a globally-recognized brand focusing on
building and purchasing labs, land and building commercial grade
“Cultivation Centers”. We plan to consult, assist,
manage & lease to universities, state governments, licensed
dispensary owners and organic grow operators on a contract basis
with a concentration on the legal and medical cannabis
sector..
Our
long-term plan is to become a true “Seed-to-Sale”
global holding company providing infrastructure, financial
solutions, product development, and real estate options in this new
emerging market. Our long term growth may also come from the
acquisition of synergistic businesses, such as distilleries, to
make anything from infused beverages to super oxygenated water with
CBD and THC.
Our
website is www.WEEDincUSA.com.
Corporate History
We were
originally incorporated under the name Plae, Inc., in the State of
Arizona on August 20, 1999. At the time we operated under the name
Plae, Inc., no business was conducted. No books or records were
maintained and no meetings were held. In essence, nothing was done
after incorporation until Glenn E. Martin took possession of Plae,
Inc. in January 2005. On February 18, 2005, the corporate name was
changed to King Mines, Inc. and then subsequently changed to its
current name, United Mines, Inc., on March 30, 2005. No shares were
issued until the Company became United Mines, Inc. From 2005 until
2015, we were an exploration stage mineral exploration company that
owned a number of unpatented BLM mining claims and Arizona State
Land Department exploration leases. We still own 15
“unpatented” mining claims covering 3 mining properties
located in the historic gold mining territory of Southern Arizona,
but are not currently conducting any mining activities and have no
plans to in the immediate future.
On
November 26, 2014, our Board of Directors approved the
redomestication of our company from Arizona to Nevada (the
“Articles of Domestication”), and approved Articles of
Incorporation in Nevada, which differed from then-Articles of
Incorporation in Arizona, primarily by (a) changing our name from
United Mines, Inc. to WEED, Inc., (b) authorizing Twenty Million
(20,000,000) shares of preferred stock, with blank check rights
granted to our Board of Directors, and (c) authorizing Two Hundred
Million (200,000,000) shares of common stock (the “Nevada
Articles of Incorporation”). On December 19, 2014, the
holders of a majority of our outstanding common stock approved the
Articles of Domestication and the Nevada Articles of Incorporation
at a Special Meeting of Shareholders. On January 16, 2015, the
Articles of Domestication and the Nevada Articles of Incorporation
went effective with the Secretary of State of the State of Nevada.
On February 2, 2015, our name change to WEED, Inc., and a
corresponding ticker symbol change to “BUDZ” went
effective with FINRA and was reflected on the quotation of our
common stock on OTC Markets.
These
changes were effected in order to make our corporate name and
ticker symbol better align with our short-term and long-term
business focus, which in the short-term is to conduct a Cannabis
Genomic Study over the next 5 years, process those results, and in
the long-term to be an international cannabis research and product
development company, with a globally-recognized brand focusing on
building and purchasing labs, land and building commercial grade
“Cultivation Centers”. WEED plans to consult, assist,
manage & lease to universities, state governments, licensed
dispensary owners and worldwide organic grow operators on a
contract basis, with a concentration on the legal and medical
Cannabis sector. These operations are being conducted through our
primary wholly-owned subsidiary, Sangre AT, LLC, a Wyoming limited
liability company (“Sangre”), dba Sangre BioSciences.
Our long-term plan is to become a True “Seed-to-Sale”
global holding company providing infrastructure, financial
solutions, product development and real estate options in this new
emerging market. Our long term plans may also include acquisitions
of synergistic businesses, such as distilleries to make infused
beverages and/or super oxygenated water with CBD and
THC.
As of
December 31, 2019, in addition to Sangre, WEED Inc. had four other
wholly-owned subsidiaries, namely WEED Australia Ltd., an
Australian corporation, The Cannabis Institute of Australia, an
Australian non-profit corporation, WEED Israel Cannabis Ltd., an
Israeli corporation and WEED Hong Kong Limited, a Chinese
corporation. WEED Australia is registered as an unlisted public
company in Australia. All subsidiaries were formed to address
future anticipated global demand and to take advantage of countries
that have more developed laws related to cannabis legal at the
Federal level. In March 2019, we formed WEED Hong Kong Limited with
offices in Hong Kong for IP and worldwide branding
purposes.
Our
U.S. corporate offices are located at 4920 N. Post Trail, Tucson,
AZ 85750, telephone number (520) 818-8582.
Business Overview
General
Currently, we are
either working on or planning for several different business
opportunities in the cannabis space. Our first business opportunity
is through our wholly-owned subsidiary, Sangre AT, LLC
(“Sangre”), where we are focused on the development and
application of cannabis-derived compounds for the treatment of
human disease. To that end Sangre , is working on a planned
five-year Cannabis Genomic Study to complete a genetic blueprint of
the Cannabis plant genus, by creating a global genomic
classification of the entire plant. Second, we are under contract
to purchase a golf course property located in Westfield, New York.
We own the unlimited water extractions rights from Lake Erie to the
property already, but need to raise at least $500,000 to acquire
the property. If we are successful in acquiring the property we
plan to utilize the property to access the hemp and infused
beverage markets. Third, we have established WEED Australia Ltd.
and The Cannabis Institute of Australia (C.I.A.) in Australia for
the purpose of conducting cannabis and hemp research and
potentially developing products in Australia. C.I.A. is a
non-profit entity formed for the purpose of conducting cannabis and
hemp research and potentially developing products in Australia for
domestic research and development of products, services and
educational purposes to all 7 States and territories including
Tasmania. Fourth, we have an arrangement with Professor Elka
Touitou to assist us with cannabis research and studies in Israel.
Professor Touitou was the Head of the Innovative Dermal,
Transdermal and Transmucosal Delivery Lab at the Institute of Drug
Research, The School of Pharmacy, HUJ, now retired but still has
HUJ clinical trial & independent studies/lab privileges.
Professor Touitou is an internationally renowned authority in the
field of drug delivery and design of new technologies for efficient
administration of drugs and development of new products. Professor
Touitou has been involved in Cannabinoid research since 1988 at The
Hebrew University of Jerusalem, (HUJ) Jerusalem,
Israel.
Using
annotated genomic data and newly generated phenotypic data, WEED
plans to identify and isolate regions of the plant genome which are
related to growth, synthesis of desired molecules, and drought and
pest resistance. This complex data set would then be utilized in a
breeding program to generate and establish new hybrid cultivars
which exemplify the traits that are desired by the medical and
patient community. This breeding program would produce new seed
stocks and clones, which we plan on patenting. If successful this
intellectual property should generate immense value for the
Company. After developing a comprehensive understanding of the
annotated genome of a variety of cannabis strains and obtaining
intellectual property protection over the most promising strains,
we plan to move forward either independently or with strategic
partners to develop medicinal products for the treatment and
therapies for a multitude of human and animal
diseases.
Our
current, short-term goals relate to the Cannabis Genomic Study and
the resulting development of a variety of new cannabis strains,
and, over the next 5 years, we plan to process those results in
order to become an international cannabis research and product
development company, with a globally-recognized brands focusing on
building and purchasing labs, land and building commercial grade
“Cultivation Centers”. WEED plans to consult, assist,
manage & lease to universities, state governments, licensed
dispensary owners and organic grow operators on a contract basis
with a concentration on the legal and medical cannabis
sector.
Our
long-term plan is to become a true “Seed-to-Sale”
global holding company providing infrastructure, financial
solutions, product development, and real estate options in this new
emerging market thru acquisitions and strategic partnerships. Our
long term growth may also come from the acquisition of synergistic
businesses, such as distilleries, to make anything from infused
beverages to super oxygenated water with CBD and THC. Currently,
WEED, Inc. has formed WEED Australia Ltd., registered as an
unlisted public company in Australia to address this global demand.
We have also formed WEED Israel Cannabis Ltd., an Israeli
corporation, to address future global demand, and in March 2019,
WEED Israel Cannabis Ltd. was involved in the transaction with
Yissum discussed herein. In 2018, we formed a non-profit company in
Australia called The Cannabis Institute of Australia. To date this
company has been dormant.
As of December 31,
2018, the original Sangre’s research team leader is no longer
with the Company. However, we believe his departure will have
little to no impact on Sangre’s genomic study. Although Mr.
Williams was the team leader of the Sangre project, most of the
work on the study was conducted under contract by Industrial
Metagenomics in connection with a public university in Texas. The
leadership of Industrial Metagenomics is intact and will still be
conducting the study once we have secured sufficient financing. In
addition, we will plan to utilize top researchers from Israel.
WEED’s facilities manager is the only individual who is
currently located onsite at the Sangre BioSciences compound in La
Veta, Colorado. His duties also include caretaker for our 6,000 sq
ft corporate Colorado headquarters and our 3 bedroom condo in
Cucharas, Colorado to house personnel.
Cannabis
Genomic Study
After
more than 40 years of illicit, underground breeding programs, the
genetic integrity of Cannabis has been significantly degraded. Our
subsidiary, Sangre AT, LLC (“Sangre”) plans to use a
gene-based breeding program to root out inferior cultivars and
replace them with fully-validated and patentable cultivars which
produce consistent plant products for the medicinal markets. We
believe our unique gene-based breeding program will improve
cultivars and introduce integrity, stability, and quality to the
market in the following ways:
● Accelerated and optimized growth
rates; modern genomic resources will enhance traditional breeding
methods
● Generation of new cultivars,
accelerating and perfecting the art of selective
breeding
● Provide the ability to assay for
specific genes within the crop, which is critical to strain
tracking and market quality assurance
● Improve disease and drought
resistance
We
believe our gene-based breeding program will facilitate and
accelerate:
● Improved therapeutic
properties
● New therapies for migraines/chronic
pain, epilepsy, cancer, PTSD, chronic head injury, and
others
● Enhanced opportunities for new drug
discovery through collaborations with national and international
medical research/treatment centers, bio-pharma companies including
nutraceuticals and botanical companies
● Development and protection of
intellectual property on a global scale. WEED currently owns
several trademarks for WEED, WEED Rules!, Panama Red and Acapulco
Gold in in several countries in certain limited International
categories as placeholders for future growth.
The Research Plan
In
order to achieve the desired results outlined above, Sangre has
developed a research plan entitled the
“Cannabis Genomic Study.” The goal of the study is to
complete a global genomic classification of the Cannabis plant
genus. Once the classification is complete, the research team plans
to develop new cannabis strains that show the highest likelihood of
being successful in the treatment of a variety of human diseases,
test those strains and then work to produce those strains in a
medicinal form for the treatment of disease. The research plan will
be conducted using the following steps: Extraction, Purification,
Sequencing. Annotation, and Cloning
(micro-propagation).
Extraction:
The extraction of genomic DNA from cannabis is a complex process of
cell lysis and DNA recovery. Sangre has evaluated, updated, and
validated new methods for DNA recovery.
Purification:
Using next generation purification chemistries, the DNA is cleaned
and concentrated for downstream applications.
Sequencing:
The Cannabis DNA is sequenced using both the Illumina MiSeq and
MinIon instruments.
Annotation:
The genomic data is assembled and annotated using proprietary
bioinformatic systems and the data provided to the Sangre AgroTech
genetic breeders and cellular cloners.
Cloning:
Through this process, new, high-value cannabis strains are
developed.
The
objectives of the research plan are as follows:
Technical Objective
1: Using two next generation
sequencing platforms and proprietary bioinformatics programs, we
will sequence five cultivars of Cannabis, and generate fully
annotated genomic data.
Technical Objective
2: Using the selected cultivars, backcross and forward
hybridization studies will be
performed to produce a new generation of stock. The progeny of
these crosses will be grown, genetically finger-printed, and
introduced to the market under patent protection. Up-selection and
cultivation of cultivars for quality assurance.
Technical Objective
3: Genotypic and phenotypic measurements of the offspring
will be performed using Next
Generation Sequence Analysis, Genotyping, and Phenotyping analysis.
Product focus groups will evaluate new cultivars. Patent protection
will be initiated for new cultivars which meet product development
criteria.
Technical Objective
4: Utilize gene-driven breeding
of up-selected cultivars to initiate the generation of
“designer” cultivars for clinical
research.
Technical Objective
5: Market placement of selected, genetically enhanced
cultivars for the medicinal and bio-pharma markets.
Where We Are in the Research Plan
As noted above,
phase one of our planned five year “Cannabis Genomic
Study” includes “extraction technologies”. On
April 20, 2017, we, in connection with Industrial Metagenomics,
initiated the genomic study by extracting DNA from seven cannabis
strains in Tucson, Arizona. Sangre followed the initial extraction
with a second round of extractions in July 2017. So far, Industrial
Megagenomics, under their agreement with Sangre have designed,
tested, and developed standard operating procedures for efficient
DNA isolation and sequencing of Cannabis genomes. Extensive
bioinformatics analysis of repeatable and variable regions has been
performed on newly generated DNA, sequencing data of 26 landrace
cultivars and publicly available genomes to identify potential
biomarkers to type cannabis plants without the need for whole
genome sequencing. The developed biomarkers were prepared into a
package for patenting, however, we are waiting for funding to
perform in-vivo validation. We believe we will be able to finish
phase one of the trial within 9-12 months after we have secured
sufficient financing.
Under
the next phase of our genomic study, we plan to continue our
genetic studies in both the United States and Israel for phase 2,
moving directly to clinical human trials with our wholly owned
subsidiary, WEED Israel Cannabis Ltd. under the guidance currently
of Professor Elka Touitou, who has patented “Delivery
systems” to increase bioavailability with Hundreds of
formulas she developed at the Hebrew University of Jerusalem since
1988. In addition, Professor Elka has worked with World Class
scientists such as Dr. Raphael Mechoulam and many top echelon
scientists in Israel in Cannabis studies and Human Trials outside
of the scope of Patents and Formulas with WEED Inc. In February
2019, Glenn Martin, our CEO, and WEED Israel Managing Director,
Elliot Kwestel, met with Dr. Mechoulam in his office at HUJ to
discuss WEED Israel goals of attaining a high THC cultivar to
synthesis for controlled dosing purposes as discussed below. We are
ready to begin this phase, but need additional funding in order to
proceed with the planned clinical trials, as well as to continue on
to future phases of the genomic study.
After the planned
clinical trials, and assuming they are successful, then
in Phase 3, we plan
for WEED Israel Cannabis Ltd. to utilize our human clinical trials
with continuing genetic studies in Israel, not just at University
levels but to include separate studies at Israeli Hospitals and
clinics across Israel from Haifa to Tel Aviv, under Contract
Research Organizations (CROs) in order to begin final research
& developments from strain extractions to advance
manufacturing, refinement of raw product, scientifically backed, to
incorporate into Protocols and Procedures, QA/QC to meet EU
standards, TGA standards in Australia, and that will meet World
Class standards required by the FDA in the United States. This
strong pipeline and provenance will clarify and streamline
licensing standards to issue all vertically integrated licenses
needed in each jurisdiction that we enter in each country. We plan
to utilize the highest GMP standards for eventual sale to the
public, both for Domestic use and International
export.
Under
the continuation of Phase 3 and for Phase 4 – assuming our human
clinical trials proved to be successful, we plan for WEED
Israel Cannabis Ltd., once cleared and permitted by The Israeli
Ministry of Health, to begin to move to manufacturing of product
both for commercial pharmaceutical and non-pharmaceutical uses.
These above stated studies will include development of
“educational courses” for continuing education to
medical professionals to attract; Drs. PHDs, pharmacists,
pharmacist assistants and retail managers, pharmacy industry
service providers, pharmacy trade press, educators, government
officials, students & new graduates, other pharmaceutical &
health-related industry professionals.
In
addition, we plan for proprietary strains of cannabis genetics to
be imported & incorporated into our studies upon Ministry of
Health authorization or lead authorities globally for additional
research and evaluation to complete DNA sequencing, Pathogen
studies, Metabolic Studies, and MetaBolomics analysis adding to our
databases to increase efficiencies, worldwide. Our goal is to
achieve a 38% - 40% THC (tetrahydrocannabinol) plant, natural or
enhanced genetics, to synthesis THC and THC-A to separate THC
(pyschoactive) from THC-A (non-pyschoactive) to control dosing for
commercial release of products. We will conduct separate studies
and evaluations for Cannabidiol (CBD), a phytocannabinoid. CBD does
not have the same psychoactivity as THC. CBD is one of 113
currently identified cannabinoids in the Cannabaceae plant. CBD
accounts for up to 40% of plant extracts.
This also
requires the development of continuing “Education
Studies” to educate doctors and health practitioners on
proper use as stated above for increased bioavailability to achieve
consistent controlled dosing for patients medical requirements and
needs. This will require short and long term, continual studies to
supply proper medical advice and treatment to provide the highest
quality medical and legal use products for both humans and animal
conditions and diseases. We will look to supply constant and
continual medical information and products for preventative
treatments, therapies, with ultimate goal for eventual cures
utilizing the Cannabis plant and its potential for a panacea of
medical relief worldwide.
New York Property – Infused Beverage Industry
As
detailed elsewhere herein, we currently have an agreement in place
that allows us the option to acquire certain improved property
located in Westfield, New York from DiPaolo for a total purchase
price of Eight Hundred Thousand Dollars ($800,000). Under the terms
of the agreement, we still owe approximately $392,000 to acquire
the property. We have agreed to pay that amount in installment
payments of $10,000 per month for six months beginning February
2020, with a balloon payment of approximately $332,000 due on or
before August 3, 2020. We made the six $10,000 monthly payments but
were not able to pay the balloon payment by the August 3, 2020
deadline, but on July 31, 2020 we worked out an additional
extension with the bank. Under the terms of that agreement, we paid
$10,000 in exchange for an additional extension and we owed
approximately $332,000 to acquire the property. We agreed to pay
that amount in installment payments of $10,000 per month for six
months beginning September 2020, with a balloon payment of
approximately $272,000 due on or before February 1, 2021. These
payments are in addition to the approximately $480,000 in payments
we have already made. To date we have made the $10,000 payments for
September 2020 and October 2020. We need to raise funds in order to
make the remaining scheduled payments. In the event we make the
payments we will own the property. In the event we do not make the
payments, all monies we have paid are non-refundable and the bank
may acquire the property. The property is approximately 43 acres
and has unlimited water extraction rights from the State of New
York. If we are successful in acquiring the property, we plan to
use this property as our inroads to the New York hemp and infused
beverage markets in the future. In order to make the payments
required to acquire the property we must raise
funds.
WEED Australia Ltd.
WEED
Australia Ltd.’s corporate strategy is to become a leader in
cannabis and hemp research and development. To support this goal
WEED Australia Ltd. has assembled a highly qualified team of
well-respected Ph.D.’s, scientists, researchers and business
experts with the goal of establishing an export industry. WEED
Australia’s personnel has presented at several conferences
regarding the use of cannabis for medical purposes as well as for
other uses. We need additional funding in order to further WEED
Australia’s efforts to conduct research and development
activities in Australia.
WEED Israel Cannabis Ltd.
Through
our subsidiary, WEED Israel Cannabis, Ltd., we have an arrangement
with Professor Elka Touitou to assist us with cannabis research and
studies in Israel. Professor Touitou was the Head of the Innovative
Dermal, Transdermal and Transmucosal Delivery Lab at the Institute
of Drug Research, The School of Pharmacy, HUJ, now retired but
still has HUJ clinical trial & independent studies/lab
privileges. Professor Touitou is an internationally renowned
authority in the field of drug delivery and design of new
technologies for efficient administration of drugs and development
of new products. As noted above with our genomic study, there is a
strong possibility we do our initial clinic trials in Israel due to
a variety of factors, including the fact that Dr.Touitou is located
in Israel, and the fact that Israel has certain advancements in the
study of cannabis that we believe would be beneficial to our clinic
trial work. We need to raise additional funds in order to conduct
our planned operations in Israel.
Competitive Advantages
Sangre’s
research and development team works with next generation sequencing
(NGS) and emerging third generation instruments and has developed
the most advanced proprietary bioinformatics data systems
available. Sangre uses a unique two sequencing approach. One system
provides DNA reads of up to 300,000 base pair reads and an NGS
system which provides highly accurate short reads. This allows the
genomic data to be assembled in a scaffold construct; the long
reads forming the scaffold and the short reads providing highly
accurate verification and quality assurance of the genomic data.
This approach, together with the bioinformatics program,
facilitates a highly accurate construct of the Cannabis genome
which can be annotated and facilitate gene discovery and gene
location. Sangre combination of personnel, skill-sets, and data
analytics capabilities will allow us to accomplish our goals in
months, rather than years.
Using
annotated genomic data and newly generated phenotypic data, we plan
to identify and isolate regions of the genome which are related to
growth, synthesis of desired molecules, and environmental
compatibility. This complex data set will be utilized in a breeding
program to generate and establish new hybrid cultivars which
exemplify the traits that are desired by the medical community.
This breeding program will produce new seed stocks, clones,
cultivars, and intellectual property which will generate value for
the business organization. Eventual expansion to Israel will allow
us to include human clinical trials thru product development for
its domestic and international export markets.
Sangre
plans to develop a translational breeding program to establish a
new collection of Cannabis cultivars for the USA national market.
In Israel we plan to establish a unique 2nd new collection of
Cannabis Cultivars exclusive to WEED for the EU marketplace. Using
genetic screening technology and micro-propagation, cultivars can
be up-selected for specific traits and grown to address the needs
of consumers in the medicinal and drug discovery markets. The
combination of next generation genomics, selective hybridization,
and In Vitro cloning provide us with the tools to enhance new
cultivars of patentable Cannabis.
Marketing
We have
not developed a marketing plan and do not intend to until we are in
the latter stages of the Cannabis Genomic Study and believe we have
strains that are marketable for the treatment of disease. At that
time we plan to develop a marketing plan for our newly-developed
strains of Cannabis. We believe that if we are successful in
developing strains of Cannabis that effectively treat human
diseases then the market for our products will be a vibrant market.
We will continue to look to acquire companies with revenue and
companies or individuals with unique proprietary strains for future
growth. We believe securing intellectual property, where possible,
and branding are keys to long term financial success in the
emerging global cannabis and hemp industries.
Manufacturing
We are
not currently manufacturing any products and do not intend to do so
until we are in the latter stages of the Cannabis Genomic Study and
believe we have strains that are marketable for the treatment of
disease such that we could begin the manufacturing of such
products, either in-house or through relationships with third party
companies. We do not currently have any relationships with third
party companies for the manufacturing of any products.
Competition
The
cannabis industry, taken as a whole, is an emerging industry with
many new entrants, with some of them focused on research, some on
medicinal cannabis and others focused on cannabis for legal, adult
use, i.e. “recreational” use. We are currently focused
solely on the research and medicinal cannabis part of the industry.
Additionally, many cannabis companies are international companies
due to the restrictions on the cannabis industry in the
U.S.
At this
point in our development, we believe our competitors are those
companies that are attempting study and sequence cannabis DNA with
the goal of creating medicines from that research. We do not view
ourselves in competition with those companies currently growing
and/or selling cannabis for medicinal or recreational use since we
are primarily a research company at this stage. However, in the
future WEED looks provide both pharmaceutical grade medicinal
products along with non-pharmaceutical products, such as Acapulco
Gold suntan lotion as an example. We are aware of companies that
supply synthetic cannabinoids and cannabis extracts to researchers
for pre-clinical and clinical investigation. We are also aware of
various companies that cultivate cannabis plants with a view to
supplying herbal cannabis or non-pharmaceutical cannabis-based
formulations to patients. These activities have not been approved
by the FDA or the TGA in Australia.
We have
never endorsed or supported the idea of distributing or legalizing
crude herbal cannabis, or preparations derived from crude herbal
cannabis for medical use and do not believe our research to
hopefully create prescription cannabinoids are the same, and
therefore competitive, with crude herbal cannabis. We believe that
only a cannabinoid medication, one that is standardized in
composition, formulation and dose, administered by means of an
appropriate delivery system, and tested in properly controlled
pre-clinical and clinical studies, can meet the standards of
regulatory authorities around the world, including those of the
FDA. We believe that any cannabinoid medication must be subjected
to, and satisfy, such rigorous scrutiny through proper accredited
education and federal regulations.
As
cannabis has moved through the legalization process in North
America, research groups in Canada and the Unites States, along
with Israel, Australia, have initiated work on understanding the
Cannabis genome.
The
methods of competition for companies in the cannabis research
market segment revolve around a variety of factors, including, but
not limited to, experience of the company’s research team,
the facilities used by the company to conduct research, the
instrumentation used to sequence DNA, the company’s internal
research protocols, and the company’s relationship with those
in the scientific community.
Applying
those competitive factors to WEED, Inc.: our research team averages
over 15 years of experience (including peer-reviewed publications
and conference presentations), we have dedicated over 14,000 square
feet of research space to the resolution of cannabis genomics and
the development of new strains, our instrumentation is designed to
sequence large pieces of DNA (>25,000 bp - 10 times larger than
our typical competitors), and we use custom bioinformatics (DNA
sequence analysis software) not available to any other competitor
in the industry. We believe these factors, along with our strong
relationships in the industry and our unique validation protocols,
will allow us to measure up favorably when compared to our
competition.
Next Generation Sequencing
Next-generation
sequencing (NGS), introduced nearly ten years ago, is the catch-all
term used to describe several sequencing technologies
including:
● Illumina (Solexa)
sequencing
● Roche 454 sequencing
● Ion torrent: Proton / PGM
sequencing
● SOLiD sequencing
These
recent sequencing technologies allow scientists to sequence DNA and
RNA much more quickly and cheaply than the previously used Sanger
sequencing, and as such, have greatly expanded the study of
genomics and molecular biology. Numerous laboratories within the
Cannabis community are currently employing this
technology.
Colorado State University – Boulder
To the
best of our knowledge, Colorado State University – Boulder is
conducting a Cannabis Genomic Research Initiative, which is
currently seeking to describe the Cannabis genome. The data
generated through this effort is provided through the public domain
to growers in an effort to stimulate the production of new,
high-value stains of Cannabis.
Anandia Labs
Anandia
Labs is conducting work in the area of Cannabis genomics based on
sequence work which was completed in 2011. The sequencing work
conducted was based on “next generation sequencing”
technology and resulted in the generation of tens-of-thousands of
DNA segments that have yet to be completely and correctly
reassembled. Much of the sequence data that was generated through
their sequencing efforts has been placed into the public domain and
shared with other laboratories. In some instances, the data has
been found to be less than accurate.
Phylos Biosciences
Phylos
Biosciences is currently using DNA-based genetic fingerprinting to
establish relationships between strains and to assist in the
development of phenotypic databases to accelerate traditional
breeding programs. Phylos Biosciences has a primary goal of
bringing clarity to the Cannabis market and promote the generation
of IP held by individual growers. To the best of our knowledge,
Phylos Biosciences is not engaged in whole genome sequencing and is
not engaged in any genetic enhancements of the Cannabis strains.
They simply supply genetic
data to their customer base to more effectively drive the
traditional breeding process.
New West Genetics
New
West Genetics aims to improve and develop industrial hemp as a
viable crop for the United States. New West Genetics seeks to
exploit the diverse end uses of hemp and optimize the genetics of
hemp to create a lucrative crop to add to the rotation of US
farmers. Industrial hemp’s uses and potential are as great as
many major crops, if not more. We believe NWG is utilizing modern
sequencing technology and statistical genomics approaches to
understand these factors as they apply to hemp production in states
where it is legal to grow. Understanding the genotype to phenotype
map will be increasingly useful for expanding production of
hemp.
While
we do not believe any of the above companies or universities are
direct competitors of ours based on what we believe about their
work in the industry, they could be competitors for research
funding dollars. We are not aware of the financial situation of
many of the above companies and universities, but we will need to
raise substantial additional capital in order to fully-fund the
five year genomic study and the facilities to complete the study.
Most of the above companies and universities are likely better
financed than we are and we will need to raise substantial funds in
order to compete in the cannabis research industry.
Intellectual Property
On
March 1, 2019, we entered into an Exclusive License and Assignment
Agreement (the “Technology Agreement”) with Yissum
Research Development Company of the Hebrew University of Jerusalam,
Ltd., an entity organized in Israel (“Yissum”). Under
the terms of the Technology Agreement, Yissum agreed to grant an
exclusive license, and eventually assign, to us certain platform
technologies relating to different formulations for administration
and delivery of lipophilic compositions, (including cannabinoids)
(collectively, the “Technology”) invented and/or
developed by Prof. Elka Touitou at The Hebrew University of
Jerusalem, which technologies are more fully described in the
patent applications and/or patents listed in Appendix A to the
Technology Agreement.
Under the
Agreement, in exchange for an exclusive license to use the Existing
Technologies, we were to pay Yissum a total of USD$1,000,000 as
follows: (i) $100,000 within three (3) business days of signing the
Technology Agreement (which amount has been paid), (ii) $400,000 on
or before May 1, 2019, and (iii) $500,000 on or before December 31,
2019 (together, the “License Payments”). The grant of
the exclusive license and the transfer to us of the responsibility
for the administration and control of patent activities and patent
expenses related to the Existing Technologies was to occur after
the USD$400,000 payment due May 1, 2019. However, prior to that
payment, WEED terminated the agreement with Yissum. We do not
currently plan to revisit our agreement with Yissum in the future.
However, we do plan to continue to work with Professor Elka Touitou
of Hebrew University of Jerusalem, who remains our Chairperson for
our Israeli Scientific Advisory Board, to implement our research
and product development along with WEED Israel clinical
trials.
Additionally, we
consider certain elements of our Cannabis Genomic Study to be trade
secrets and we protect it as our intellectual property. In the
future, if we are successful in identifying certain Cannabis
strains as promising for the treatment of diseases we will seek to
patent those strains.
Government Regulation
As
of the end of February 2019, 33 states and the District of Columbia
allow its citizens to use medical marijuana. Voters in the states
of Colorado, Washington, Alaska, Oregon and the District of
Columbia were the first to approve ballot measures to legalize
cannabis for adult use. The state laws are in conflict with the
Federal Controlled Substances Act, which makes marijuana use and
possession illegal at the federal level. The prior administration
(President Obama) effectively stated that it is not an efficient
use of resources to direct law federal law enforcement agencies to
prosecute those lawfully abiding by state-designated laws allowing
the use and distribution of medical marijuana. However, the Trump
administration has indicated the potential for stricter enforcement
of the marijuana industry at the federal level, but to date there
has been very little in terms of action. There is no guarantee that
the Trump administration or future administrations will maintain
the low-priority enforcement of federal laws in the marijuana
industry that was adopted by the Obama administration. The Trump
administration or any new administration that follows could change
this policy and decide to enforce the federal laws strongly. Any
such change in the federal government’s enforcement of
current federal laws could cause significant financial damage to
our business and our shareholders.
Further,
and while we do not intend to harvest, distribute or sell cannabis,
if we conduct research with the cannabis plant or lease buildings
to growers of marijuana, etc., we could be deemed to be
participating in cannabis cultivation, which remains illegal under
federal law, and exposes us to potential criminal liability, with
the additional risk that our properties could be subject to civil
forfeiture proceedings.
Currently,
there are no approvals needed in order to sequence the cannabis
genome, which is what is currently being conducted by Sangre.
However, prior to doing any research into the medical applications
of the cannabis plant once the study is completed, we will need to
obtain medicinal cannabis and hemp research licenses from the State
of Colorado and New York State. Additionally, if we ever cultivate
and process cannabis plants, we will need cultivation and
processing licenses from the State of Colorado and New York State
which covers cannabis and hemp. These licenses will cost
approximately $1,000 to $5,000 per license, and likely take
approximately six months to 1 year to obtain.
Sangre Agreement
On
April 20, 2017, we entered into a Share Exchange Agreement with
Sangre AT, LLC, a Wyoming limited liability company, under which we
acquired all of the issued and outstanding limited liability
company membership units of Sangre in exchange for Five Hundred
Thousand (500,000) shares of our common stock, restricted in
accordance with Rule 144. As a result of this agreement, Sangre is
a wholly-owned subsidiary of WEED, Inc.
Employees
As of
December 31, 2019, we employed two people on a full time basis,
namely Glenn E. Martin and Nicole M. Breen. We also contract with Thomas Perry, and Tom Pool
as consultants on a full-time basis who work with Sangre. As of
December 31, 2019, WEED Israel Cannabis Ltd. had one consultant. As
of December 31, 2019, WEED Australia Ltd. had three consultants.
WEED Hong Kong Limited has hired; Ed Lehman of Lehman, Lee and Xu
as corporate counsel and Lehman, Lee and Xu Corporate Services
Limited as WEED HKs legal representative in China and Hong
Kong.
Le Veta, Colorado Properties
On July
26, 2017, we acquired property located in La Veta, Colorado in
order for Sangre to complete its 5-Year, $15+ million Cannabis
Genomic Study. The site includes a
10,000+ sq. ft. building that will house Sangre’s genomic
research facility, a 4,000+ square foot building for plant product
analytics and plant product extraction, a 3,500 sq. ft. corporate
office center, and 25 RV slots with full water and electric, which
we plan to convert into a series of small research pods. Under the
terms of the purchase agreement, we paid $525,000 down, including
25,000 shares of our common stock, and Sangre took immediate
possession of the property. Under the terms of the purchase we were
obligated to pay an additional $400,000 in cash and issue an
additional 75,000 shares of our common stock over the next two
years in order to pay the entire purchase price. On January
12, 2018, we entered into an Amendment No. 1 to the $475,000
principal amount promissory note issued by us to the seller of the
property, under which both parties agreed to amend the purchase and
the promissory note to allow us to payoff the note in full if we
paid $100,000 in cash on or before January 15, 2018 and issued the
seller 125,000 shares of common stock, restricted in accordance
with Rule 144, on before January 20, 2018. Through an escrow
process, we paid the seller $100,000 in cash and issued him 125,000
shares of common stock in accordance with the Amendment No. 1, in
exchange for a full release of the deed of trust that was securing
the promissory note, on January 17, 2018. As a result, the $475,000
principal promissory note issued to the seller is deemed
paid-in-full and fully satisfied and we own the property without
encumbrances. To date we have spent
$354,000 renovating the property and an additional $400,000 on
extraction and analytical lab equipment. Our plans to complete the
property renovations, at an estimated cost of $300,000, are
currently on hold pending future financing. We will need additional
extraction equipment and analytical lab equipment, totaling
approximately $700,000. We will need to raise additional funds in
order to complete the planned renovations and pay the purchase
price for the equipment. We currently have another $1.2 million
dollar property in La Veta, CO that was to house Sangre personnel,
on the market to sell in order to keep our options open and to fund
other operations, and we may or may not consummate a sale of the
property, depending on the timing of future
financing.
On
January 3, 2018, Sangre closed on the purchase of a condominium in
La Veta, Colorado. Sangre paid $140,000 in cash for the condominium
which is a three story condominium, with three bedrooms and three
bathrooms and is approximately 1,854 square feet. In February 2018,
we closed on the purchase of property, consisting of a home in La
Veta, Colorado to house company personnel and consultants for total
consideration approximating $1,200,000. The home has 5 bedrooms and
3 bathrooms. Under the terms of the purchase agreement, we paid
$150,000 down, entered into a note payable in the amount of
approximately $1,041,000. We secured a below-market interest rate
of 1.81% based on the short-term nature of the term. This note was
repaid on October 5, 2018. Sangre took immediate possession of the
property. We acquired these properties for the purpose of housing
personnel we believe are vital to the 5-year Cannabis Genomic
Study. La Veta, Colorado is a small town without many rentals, so
it became necessary to find more permanent housing in La Veta,
Colorado for those that will be working with Sangre on the
study.
New York Property
On
October 24, 2017, we entered into an amended Purchase and Sale
Agreement with Greg DiPaolo’s Pro Am Golf, LLC
(“DiPaolo”), under which we agreed to purchase certain
improved property located in Westfield, New York from DiPaolo for a
total purchase price of Eight Hundred Thousand Dollars ($800,000).
Under the terms of the agreement, we paid a Ten Thousand Dollar
($10,000) deposit on October 26, 2017, with the remaining purchase
price to be paid on or before the date closing date, which was
originally scheduled for February 1, 2018. On February 19, 2018, we
entered into a Second Addendum to the Purchase and Sale Agreement
extending the closing date to May 1, 2018 in exchange for payment
of $8,750. On May 1, 2018, we entered
into a Fourth Addendum and a Fifth Addendum to agreement amending
the “Closing Date” under the Agreement to August 1,
2018, in exchange for our payment of $50,000 as a non-refundable
deposit to be applied against the purchase price when the property
sale is completed and $10,000 for maintenance, tree removal and
other grounds keeping in order to prepare the golf course for the
2018 season. The property is approximately 43 acres and has
unlimited water extraction rights from the State of New York. We
had planned to use this property as our inroads to the New York
hemp and infused beverage markets in the future. Since the property
was in foreclosure it was put up for auction, which occurred on
July 1, 2019. At the auction, we were the winning bidder with a bid
of $597,000. Our prior deposit payment of $120,000 was credited
towards the purchase price, and the remaining $477,000, was finally
due on November 30, 2019, after several extensions (which cost us
total of $40,000 to obtain). We were not able to meet that
deadline, but in January 2020 we worked out an additional extension
with the bank. Under the terms of the new agreement, we still owe
approximately $392,000 to acquire the property. We have agreed to
pay that amount in installment payments of $10,000 per month for
six months beginning February 2020, with a balloon payment of
approximately $332,000 due on or before August 3, 2020. These
payments are in addition to the approximately $420,000 in payments
we had already made. We made the six $10,000 monthly payments but
were not able to pay the balloon payment by the August 3, 2020
deadline, but on July 31, 2020 we worked out an additional
extension with the bank. Under the terms of that agreement, we paid
$10,000 in exchange for an additional extension and we owed
approximately $332,000 to acquire the property. We agreed to pay
that amount in installment payments of $10,000 per month for six
months beginning September 2020, with a balloon payment of
approximately $272,000 due on or before February 1, 2021. These
payments are in addition to the approximately $480,000 in payments
we have already made. To date we have made the $10,000 payments for
September 2020 and October 2020. We need to raise funds in order to
make the remaining scheduled payments.
Employees
As of
December 31, 2019, we employed two people on a full time basis,
namely Glenn E. Martin and Nicole M. Breen. We also contract with Thomas Perry, and Tom Pool
as consultants on a full-time basis who work with Sangre. As of
December 31, 2019, WEED Israel Cannabis Ltd. had one consultant. As
of December 31, 2019, WEED Australia Ltd. had three consultants.
WEED Hong Kong Limited has hired; Ed Lehman of Lehman, Lee and Xu
as corporate counsel and Lehman, Lee and Xu Corporate Services
Limited as WEED HKs legal representative in China and Hong
Kong.
Available
Information
We are
a fully reporting issuer, subject to the Securities Exchange Act of
1934. Our Quarterly Reports, Annual Reports, and other filings can
be obtained from the SEC’s Public Reference Room at 100 F
Street, NE., Washington, DC 20549, on official business days during
the hours of 10 a.m. to 3 p.m. You may also obtain information on
the operation of the Public Reference Room by calling the
Commission at 1-800-SEC-0330. The Commission maintains an Internet
site that contains reports, proxy and information statements, and
other information regarding issuers that file electronically with
the Commission at http://www.sec.gov.
LEGAL
PROCEEDINGS
William Martin v. WEED, Inc. et al
On
January 19, 2018, we were sued in the United States District Court
for the District of Arizona (William Martin v. WEED, Inc..,
Case No. 4:18-cv-00027-RM) by the listed Plaintiff. We were served
with the Verified Complaint on January 26, 2018. The Complaint
alleges claims for breach of contract-specific performance, breach
of contract-damages, breach of the covenant of good faith and fair
dealing, conversion, and injunctive relief. In addition to the
Verified Complaint, we were served with an application to show
cause for a temporary restraining order. The Verified Complaint
alleges we entered into a contract with the Plaintiff on October 1,
2014 for the Plaintiff to perform certain consulting services for
the company in exchange for 500,000 shares of our common stock up
front and an additional 700,000 shares of common stock to be issued
on May 31, 2015. The Plaintiff alleges he completed the requested
services under the agreement and received the initial 500,000
shares of common stock, but not the additional 700,000 shares. The
request for injunctive relief asks the Court to Order us to issue
the Plaintiff 700,000 shares of our common stock, and possibly
include them in our previously-filed Registration Statement on Form
S-1, or, in the alternative, issue the shares and have them held by
the Court pending resolution of the litigation, or, alternatively,
sell the shares and deposit the sale proceeds in an account that
the Court will control. The hearing on the Temporary Restraining
Order occurred on January 29, 2018. On January 30, 2018, the Court
issued its ruling denying the application for a Temporary
Restraining Order. Currently, there is no further hearing scheduled
in this matter.
On
February 13, 2018, we filed an Answer to the Verified Complaint and
a Counterclaim. In the original Counterclaim we named William
Martin as the sole counter-defendant, and alleged, that based upon
William Martin’s representations and recommendation, WEED,
Inc. hired Michael Ryan as a consultant. We allege that William
Martin misrepresented, failed to disclose, and concealed facts from
us concerning the relationship between him and Michael Ryan. We are
seeking compensatory damages caused by William Martin’s
misrepresentation, failure to disclose, and
concealment.
On
February 15, 2018, we filed a Motion to Dismiss the Verified
Complaint. On February 23, 2018, we filed a Motion to Amend
Counterclaim to add W. Martin’s wife, Joanna Martin as a
counterdefendant. On March 9, 2018, William Martin filed a Motion
to Dismiss the Counterclaim. On March 12, 2018, William Martin
filed a Motion to Amend the Verified Complaint to, among other
things, add claims against Glenn Martin and Nicole and Ryan Breen.
On March 27, 2018, the Court granted both William Martin and WEED,
Inc.’s Motions to Amend. On March 27, 2018, we filed an
Amended Counterclaim adding Joanna Martin. On April 2, 2018, we
filed a Motion to Amend our Counterclaim to add a breach of
contract claim. On April 10, 2018, we filed an Answer to First
Amended Verified Complaint. On April 23, 2018, Glenn Martin and
Nicole and Ryan Breen filed their Answer to the First Amended
Complaint. On May 31, 2018, the Court issued an Order: (a) granting
our Motion to Dismiss thereby dismissing the claims for breach of
the covenant of good faith and fair dealing and the claim for
conversion, (b) denying William Martin’s Motion to Dismiss
the counterclaim as to the claims for fraudulent concealment and
fraudulent misrepresentation, but granting the Motion to Dismiss
only as to the claim for fraudulent nondisclosure, and (c) granting
our Motion to Amend our Counterclaim to add a breach of contract
claim. In our breach of contract claim, we allege William Martin
breached his Consulting Agreement with us by failing to perform
consulting services to us in a professional and timely manner using
the highest degree of skill, diligence, and expertise pursuant to
the Consulting Agreement. We are seeking an award of compensatory
damages caused by the breach of the Consulting Agreement, together
with attorney’s fees and costs. On June 1, 2018, William
Martin and his wife filed their Answer to the First Amended
Counterclaim. On June 1, 2018, William Martin and his wife filed
their Answer to the Second Amended Counterclaim.
The
parties have conducted discovery and disclosure, including the
production by WEED, Inc. of voluminous electronically stored
information and the depositions of William, Martin, Glenn E.
Martin, Michael Ryan, and Chris Richardson. No other depositions
are presently anticipated.
On
September 14, 2018, WEED, Inc. filed a Motion for Partial Summary
Judgment (MPSJ) seeking the dismissal of all remaining claims in
the First Amended Complaint. On November 26, 2018, plaintiff filed
an opposition to the motion for partial summary judgment, together
with a cross-motion for summary judgment on both plaintiff’s
claims and the Corporation’s counterclaims. Those motions
have been fully briefed. Originally, the Court set oral argument on
the motions for May 16, 2019, but that hearing has been postponed
by the Court due to health issues with Plaintiff’s counsel.
Subsequently,
Plaintiff’s counsel withdrew and consequently, William Martin
and his wife are unrepresented. By order of the Court, the Parties
participated in a judicial settlement conference August 21, 2019
with Magistrate Judge Thomas Ferraro, but the case did not settle.
On October 15, 2019, Judge Marquez heard oral argument on the
cross-motions for partial summary judgment. The judge took the
motions under considerations. On November 21, 2019, the Judge
issued a ruling, which (i) granted our motion for summary judgment
as to the Plaintiff’s claim for fraudulent transfer, and as a
result Glenn Martin, Nicole Breen, Ryan Breen and GEM Management
Group, LLC were dismissed from the lawsuit, (ii) denied our motion
to dismiss Plaintiff’s claims for breach of contract, and
(iii) granted Plaintiff’s motion to dismiss our claims for
fraudulent misrepresentation/concealment, which acted to dismiss
our claims against the Plaintiffs. As a result of this
ruling, the remaining claim in the lawsuit was one for breach of
contract against WEED, Inc. On March 5, 2020, the Plaintiffs filed
a Motion to Dismiss the remaining counts in the lawsuit, without
prejudice. On March 5, 2020, we filed a Response to the Motion to
Dismiss stating that we did not object to the Plaintiff’s
motion. As a result, on March 10, 2020, the Court entered an Order
dismissing Plaintiff’s remaining counts in the Complaint
without prejudice. The only remaining claims relate to awards for
attorneys’ fees, with the Parties motions
pending.
Travis Nelson v. WEED, Inc.
On February 5, 2018, we were sued in Huerfano
County, Colorado District Court (Travis Nelson v.
WEED, Inc., et al., Case No.
18CV30003) by the listed Plaintiff. After we successfully pursued
motions to dismiss Plaintiff’s two initial Complaints, the
Court issued an Order on October 1, 2018 granting Plaintiff
permission to file a Second Amended Complaint, which was then filed
on October 22, 2018. The Second Amended Complaint includes three
claims: 1) breach of fiduciary duty/shareholder derivative action;
2) a claim under Colorado’s Organized Crime Control Act; and
3) a wrongful discharge claim. We have answered the Second Amended
Complaint, denying all allegations and alleging that the decision
not to offer employment to Nelson, the core factual dispute in this
case, was the result of pre-employment background checks that
showed Nelson had an extensive, violent criminal history. The
parties exchanged Initial Disclosures on November 11, 2018. We
still have a motion pending with the Court that seeks
attorneys’ fees in the amount of $53,000 for the expense of
defending the first two Complaints. On January 31, 2019, Plaintiff
submitted an Offer of Judgment under Colorado Statute
§13-17-202 offering to dismiss the case in exchange for
payment of $100,000. The Company has rejected this offer. Plaintiff
served us with written discovery that we responded to in March
2019. The parties attended mediation in April 2019, but the
case did not settle. Due to concerns related to the COVID-19
pandemic the case had mainly remained static during 2020 until
mid-summer. On July 3, 2020, Plaintiff offered to dismiss the case
in exchange for payment of $10,000. We rejected the offer. On July
21, 2020, the Parties filed a Joint Stipulation for Dismissal of
all claims with Prejudice. On July 27, 2020, the Court issued its
Order granting the Stipulation for Dismissal with Prejudice. The
lawsuit is now terminated and all claims against us have been
dismissed with prejudice.
In the
ordinary course of business, we are from time to time involved in
various pending or threatened legal actions. The litigation process
is inherently uncertain and it is possible that the resolution of
such matters might have a material adverse effect upon our
financial condition and/or results of operations. However, in the
opinion of our management, other than as set forth herein, matters
currently pending or threatened against us are not expected to have
a material adverse effect on our financial position or results of
operations.
MARKET
PRICE OF AND DIVIDENDS ON THE REGISTRANT’S COMMON EQUITY AND
RELATED STOCKHOLDER MATTERS
Our
stock is quoted on the OTC Markets’ “OTCQB” tier
under the symbol “BUDZ.” We were originally quoted
over-the-counter on November 2009. As of November 30, 2020, we had
112,972,685 shares of our common stock outstanding. The following
table sets forth the high and low bid information for each quarter
within the two most recent fiscal years, as estimated based on
information on OTC Markets. The information reflects prices between
dealers, and does not include retail markup, markdown, or
commission, and may not represent actual transactions.
|
|
|
|
|
Fiscal
Year
Ended
December
31,
|
|
|
|
|
|
|
|
|
|
|
|
2019
|
|
First
Quarter
|
$1.78
|
$1.02
|
|
|
Second
Quarter
|
$1.03
|
$0.57
|
|
|
|
Third
Quarter
|
$0.68
|
$0.40
|
|
|
Fourth
Quarter
|
$0.44
|
$0.30
|
|
|
|
|
|
|
2018
|
|
First
Quarter
|
$14.71
|
$3.43
|
|
|
|
Second
Quarter
|
$6.04
|
$4.45
|
|
|
|
Third
Quarter
|
$4.23
|
$2.81
|
|
|
|
Fourth
Quarter
|
$2.64
|
$1.05
|
As of
November 6, 2020, our common stock closed at $0.29 per share, as
quoted on OTC Markets.
The
Securities Enforcement and Penny Stock Reform Act of 1990 requires
additional disclosure relating to the market for penny stocks in
connection with trades in any stock defined as a penny stock. The
Commission has adopted regulations that generally define a penny
stock to be any equity security that has a market price of less
than $5.00 per share, subject to a few exceptions which we do not
meet. Unless an exception is available, the regulations require the
delivery, prior to any transaction involving a penny stock, of a
disclosure schedule explaining the penny stock market and the risks
associated therewith.
Currently, the only
options to purchase WEED, Inc. common stock we have outstanding is
an option to purchase 4,000,000 shares held by Mr. Glenn E. Martin
and an option to purchase 2,000,000 shares held by Nicole M. Breen.
We do not have any or ever had any convertible debentures
outstanding that permit the holder to convert the outstanding
obligation into shares of our common stock.
The
number of holders of record of shares of our common stock is Two
Sixty Seven (267).
There
have been no cash dividends declared on our common stock. Dividends
are declared at the sole discretion of our Board of
Directors.
We have
not adopted any stock option or stock bonus plans.
DIRECTORS,
EXECUTIVE OFFICERS, PROMOTERS, AND CONTROL PERSONS
The
following table sets forth the names and ages of the current
directors and executive officers of the Company, the principal
offices and positions with the Company held by each person and the
date such person became a director or executive officer of the
Company. The executive officers of the Company are elected annually
by the Board of Directors. The directors serve one-year terms until
their successors are elected. The executive officers serve terms of
one year or until their death, resignation or removal by the Board
of Directors. Unless described below, there are no family
relationships among any of the directors and officers.
|
Name
|
|
Age
|
|
Position(s)
|
|
|
|
|
|
|
|
Glenn
E. Martin
|
|
66
|
|
President, Chief
Executive Officer, Chief Financial Officer and a
Director
|
|
|
|
|
|
|
|
Nicole
M. Breen
|
|
43
|
|
Secretary,
Treasurer and a Director
|
Glenn E. Martin was appointed as our
President, Chief Executive Officer and Chief Financial Officer on
September 30, 2014. Mr. Martin has been a Director since January 1,
2005. Mr. Martin was our President from 2005 until 2012.
Between
July 2012 and September 2014, there was a dispute with our Board of
Directors and Mr. Martin remained on the Board of Directors but was
no longer our Chief Executive Officer or Chief Financial Officer.
During this time he was still involved with our company and was
reinstated to those positions in September 2014. Prior to
joining United Mines, Mr. Martin has served in an executive
capacity with several different companies. From 1988 through the
fall of 1992, Mr. Martin was Executive Director of World Trade
Center, Tucson, a subsidiary of the former Twin Towers in New York
City. In this position he oversaw the day to day operation,
including projects, programs, and seminars for the U.S. Dept. of
Commerce associate office in the W.T.C., Tucson promoting D.O.C.
programs, servicing clients for both the D.O.C. and Small Business
administration. During his tenure with World Trade Center he served
as speaker for international trade seminars and the AIESEC (U.S)
National Leadership Seminars. Member; Hong Kong Trade Association
1988 to present. Member; Society of Mining, Metallurgy &
Exploration (2008) Guest speaker at Inaugural HKBAH Annual Event in
May 2010 & member of Hong Kong Business Association of Hawaii
(2010)
During
our fiscal years ended December 31, 2018 and December 31, 2017, Mr.
Martin received $254,331 and $78,000, respectively, in cash
compensation for his services. Mr. Martin did not receive shares of
our common stock as compensation for the years ended December 31,
2018 and December 31, 2017, December 31, 2016 and December 31,
2015. As of January 28, 2020, Mr. Martin owned, beneficially-owned,
or controlled an aggregate of 55,841,078 shares of our common
stock. Mr. Martin has not sold any shares of his stock since
inception in January 2005.
Nicole M. Breen, was appointed as our
Secretary and Treasurer on September 30, 2014. Ms. Breen has been a
Director since January 1, 2005. Ms. Breen was our Secretary and
Treasurer from 2005 until 2012. Between July 2012
and September 2014, there was a dispute with our Board of Directors
and Ms. Breen remained on the Board of Directors but was no longer
our Secretary and Treasurer. During this time she was still
involved with our company and was reinstated to those positions in
September 2014. From June 2000 to 2012 she served as the
Managing Associate of GEM Management Group, LLC specializing in
acquiring mineral rights and mining properties, along with
servicing administration requirements for the company. All Ms.
Breen’s current work in the Cannabis industry is done on our
behalf. In this position, she oversees as corporate secretary,
recording secretary and the day-to-day treasury operations of the
company. Ms. Breen received her Bachelor of Science in Physical
Education in Education, with a minor in Elementary Education, from
the University of Arizona.
During our fiscal years ended December 31,
2018 and December 31, 2017, Ms. Breen received $57,000 in cash
compensation for her services. Ms. Breen did not receive
shares of our common stock as compensation for the years ended
December 31, 2018 and December 31, 2017, December 31, 2016 and
December 31, 2015. As January 28, 2020, Ms. Breen owned,
beneficially-owned, or controlled an aggregate of 23,385,826 shares
of our common stock.
Nicole
Marie Breen is a related party as Glenn E. Martin’s
daughter.
EXECUTIVE
COMPENSATION
The
Summary Compensation Table shows certain compensation information
for services rendered in all capacities for the fiscal years ended
December 31, 2019, 2018 and 2017. Other than as set forth herein,
no executive officer’s salary and bonus exceeded $100,000 in
any of the applicable years. The following information includes the
dollar value of base salaries, bonus awards, the number of stock
options granted and certain other compensation, if any, whether
paid or deferred.
|
SUMMARY
COMPENSATION TABLE
|
|
Name and
Principal Position
|
Year
|
Salary ($)
|
Bonus ($)
|
Stock Awards ($)
|
Option Awards ($)
|
Non-Equity Incentive Plan Compensation ($)
|
Change in Pension
Value and Nonqualified Deferred Compensation Earnings ($)
|
All Other
Compensation ($)
|
Total ($)
|
|
Glenn
E. Martin
President,
CEO, CFO(1)
|
2019
2018
2017
|
96,000
80,000
56,174
|
-0-
-0-
-0-
|
-0-
-0-
-0-
|
-0-
-0-
-0-
|
-0-
-0-
-0-
|
-0-
-0-
-0-
|
-0-
-0-
-0-
|
96,000
80,000
56,174
|
|
Nicole
M. Breen, Secretary and Treasurer(2)
|
2019
2018
2017
|
79,500
52,000
23,000
|
-0-
5,000
-0-
|
-0-
-0-
-0-
|
-0-
-0-
-0-
|
-0-
-0-
-0-
|
-0-
-0-
-0-
|
-0-
-0-
-0-
|
79,500
57,000
23,000
|
(1)
Mr. Martin was
appointed President, Chief Executive Officer, and Chief Financial
Officer on September 30, 2014.
(2)
Ms. Breen was
appointed Secretary and Treasurer on September 30,
2014.
The following
table sets forth certain information concerning outstanding stock
awards held by the Named Executive Officers on December 31,
2019:
|
|
Option Awards
|
Stock Awards
|
|
Name
|
Number
of Securities Underlying Unexercised Options
(#)
Exercisable
|
Number
of Securities Underlying Unexercised Options
(#)
Unexercisable
|
Equity
Incentive Plan Awards: Number of Securities Underlying Unexercised
Unearned Options
(#)
|
Option
Exercise Price
($)
|
Option
Expiration Date
|
Number
of Shares or Units of Stock That Have Not Vested
(#)
|
Market
Value of Shares or Units of Stock That Have Not Vested
($)
|
Equity
Incentive Plan Awards: Number of Unearned Shares, Units or Other
Rights That Have Not Vested
(#)
|
Equity Incentive Plan Awards: Market or Payout Value of Unearned
Shares, Units or Other Rights That Have Not Vested
($)
|
|
|
|
|
|
|
|
|
|
|
|
|
Glenn
E. Martin
|
1,333,333
|
2,666,667
|
-0-
|
10.55
|
2/1/2028
|
-0-
|
-0-
|
-0-
|
-0-
|
|
Nicole
M. Breen
|
666,666
|
1,333,334
|
-0-
|
10.55
|
2/1/2028
|
-0-
|
-0-
|
-0-
|
-0-
|
Outstanding Equity Awards at Fiscal Year-End
On
February 1, 2018, we granted Mr. Glenn Martin a Non-Qualified Stock
Option to purchase up to Four Million (4,000,000) shares of our
common stock at $10.55 per share, with the options vesting 33 1/3%
on August 1, 2018, 33 1/3% on February 1, 2019 and 33 1/3rd % on
February 1, 2020. The options expire ten years from the date of
grant.
On
February 1, 2018, we granted Ms. Nicole Breen a Non-Qualified Stock
Option to purchase up to Two Million (2,000,000) shares of our
common stock at $10.55 per share, with the options vesting 33 1/3%
on August 1, 2018, 33 1/3% on February 1, 2019 and 33 1/3rd % on
February 1, 2020. The options expire ten years from the date of
grant.
Aggregated Option Exercises
There
were no options exercised by any officer or director of our company
during our twelve month period ended December 31,
2019.
Long-Term Incentive Plan
Currently,
our company does not have a long-term incentive plan in favor of
any director, officer, consultant or employee of our
company.
On
January 23, 2018, our Board of Directors agreed to enter into an
Amended and Restated Employment Agreement with Glenn E. Martin.
Under the new agreement, Mr. Martin will serve as our President and
Chief Executive Officer for a five (5) year term in exchange for a
base salary of $1,500 per week, which will be increased to $120,000
annually in the event we raise an aggregate of $2,000,000 during
the term of the agreement. The agreement went effective beginning
February 1, 2018. Additionally, we agreed to grant Mr. Martin One
Million (1,000,000) shares of our restricted common stock on
February 1, 2018 pursuant to the terms of a Restricted Stock
Agreement, with the shares subject to certain restrictions on
transfer which expire on 33% of the shares on February 1, 2019, 66%
of the shares on February 1, 2020 and 100% of the shares on
February 1, 2021. We also agreed to issue Mr. Martin a
Non-Qualified Stock Option on February 1, 2018 to purchase up to
Four Million (4,000,000) shares of our common stock at $10.55 per
share, with the options vesting 33 1/3% on August 1, 2018, 33 1/3%
on February 1, 2019 and 33 1/3rd % on February 1, 2020. The options
expire ten years from the date of grant. As a result of the Amended
and Restated Employment Agreement with Mr. Martin, he is no longer
entitled to the Seven Million (7,000,000) shares of our common
stock as annual salary, or the One Million (1,000,000) shares of a
yet-to-be-created class of Series B Preferred Stock if we become
fully-reporting, which were both set forth in his prior employment
agreement. On December 19, 2018, Mr. Martin requested termination
of his Restricted Stock Agreement dated February 1, 2018 and
requested that the grants of restricted stock therein be forfeited.
As a result, we terminated his Restricted Stock Agreement and the
grants of stock thereunder immediately. At the time of the
termination none of the transfer restrictions on the shares had
been lifted and Mr. Martin never received the shares.
On
January 23, 2018, our Board of Directors agreed to enter into an
Amended and Restated Employment Agreement with Nicole M. Breen.
Under the new agreement, Ms. Breen will serve as our Secretary and
Treasurer for a five (5) year term in exchange for a base salary of
$1,000 per week. The agreement went effective beginning February 1,
2018. Additionally, we agreed to grant Ms. Breen Five Hundred
Thousand (500,000) shares of our restricted common stock on
February 1, 2018 pursuant to the terms of a Restricted Stock
Agreement, with the shares subject to certain restrictions on
transfer which expire on 33% of the shares on February 1, 2019, 66%
of the shares on February 1, 2020 and 100% of the shares on
February 1, 2021. We also agreed to issue Ms. Breen a Non-Qualified
Stock Option on February 1, 2018 to purchase up to Two Million
(2,000,000) shares of our common stock at $10.55 per share, with
the options vesting 33 1/3% on August 1, 2018, 33 1/3% on February
1, 2019 and 33 1/3rd % on February 1, 2020. The options expire ten
years from the date of grant. As a result of the Amended and
Restated Employment Agreement with Ms. Breen, she is no longer
entitled to the One Million (1,000,000) shares of our common stock
as annual salary, or the One Hundred Thousand (100,000) shares of a
yet-to-be-created class of Series B Preferred Stock if we become
fully-reporting, which were both set forth in her prior employment
agreement. On December 19, 2018, Ms. Breen requested termination of
her Restricted Stock Agreement dated February 1, 2018 and requested
that the grants of restricted stock therein be forfeited. As a
result, we terminated her Restricted Stock Agreement and the grants
of stock thereunder immediately. At the time of the termination
none of the transfer restrictions on the shares had been lifted and
Ms. Breen never received the shares.
When
our Board of Directors approved the employments agreements they
resolved to create a new series of preferred stock to be entitled
“Series B Convertible Preferred Stock” with the
following rights and preferences: (i) no dividend rights; (ii) no
liquidation preference over the Company’s common stock; (iii)
conversion rights into shares of common stock at a ratio of 20
shares of common stock for each share of Series V Convertible
Preferred Stock; (iv) no redemption rights; (v) no call rights by
the Company; and (vi) voting rights on an “as
converted” basis on all matters properly brought before our
common stockholders for a vote.
Long-Term Incentive
Plans. We do not provide its officers or employees with pension,
stock appreciation rights, long-term incentive or other plans and
has no intention of implementing any of these plans for the
foreseeable future.
Employee Pension,
Profit Sharing or other Retirement Plans. We do not have a defined
benefit, pension plan, profit sharing or other retirement plan,
although it may adopt one or more of such plans in the
future.
Compensation
of Directors
Our
directors did not receive any compensation for their services as
directors during the fiscal year ended December 31, 2019, 2018 or
2017.
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The
following table sets forth, as of November 30, 2020, certain
information with respect to our equity securities owned of record
or beneficially by (i) each Officer and Director of the Company;
(ii) each person who owns beneficially more than 5% of each class
of the Company’s outstanding equity securities; and (iii) all
Directors and Executive Officers as a group.
|
Title
of Class
|
|
Name
and Address
of
Beneficial Owner (1)
|
Amount
and Nature of
Beneficial
Ownership
|
|
|
Common
Stock
|
|
Glenn E. Martin
(3)(4)
|
55,841,078
|
49.43%
|
|
Common
Stock
|
|
Nicole M. Breen
(3)(5)
|
22,864,309
|
20.24%
|
|
|
|
|
|
|
Common
Stock
|
|
All Directors and
Officers
As a Group (2
persons)
|
78,705,387
|
69.67%
|
(1)
Unless otherwise
indicated, based on 112,972,685 shares of common stock issued and
outstanding. Shares of common stock subject to options or warrants
currently exercisable, or exercisable within 60 days, are deemed
outstanding for purposes of computing the percentage of the person
holding such options or warrants, but are not deemed outstanding
for the purposes of computing the percentage of any other
person.
(2)
Indicates one of
our officers or directors.
(3)
Unless indicated
otherwise, the address of the shareholder is WEED, Inc., at 4920 N.
Post Trail, Tucson, AZ 85750.
(4)
Includes 80,666
shares of common stock held in the name of Tanque Verde Valley
Missionary Society, an entity controlled by Mr.
Martin.
(5)
Includes shares
held in the name of Ms. Breen’s husband, Ryan Breen, as well
as 305,505 shares of common stock held in the name of GEM
Management Group, LLC, an entity controlled by Ms. Breen, and an
aggregate of 15,927 shares of common stock held in the name of Ms.
Breen’s children.
The
issuer is not aware of any person who owns of record, or is known
to own beneficially, ten percent or more of the outstanding
securities of any class of the issuer, other than as set forth
above. The issuer is not aware of any person who controls the
issuer as specified in Section 2(a)(1) of the 1940 Act. There are
no classes of stock other than common stock issued or outstanding.
The Company does not have an investment advisor.
There
are no current arrangements which will result in a change in
control.
CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS
Employment Agreements
On
January 23, 2018, our Board of Directors agreed to enter into an
Amended and Restated Employment Agreement with Glenn E. Martin.
Under the new agreement, Mr. Martin will serve as our President and
Chief Executive Officer for a five (5) year term in exchange for a
base salary of $1,500 per week, which will be increased to $120,000
annually in the event we raise an aggregate of $2,000,000 during
the term of the agreement. The agreement went effective beginning
February 1, 2018. Additionally, we agreed to grant Mr. Martin One
Million (1,000,000) shares of our restricted common stock on
February 1, 2018 pursuant to the terms of a Restricted Stock
Agreement, with the shares subject to certain restrictions on
transfer which expire on 33% of the shares on February 1, 2019, 66%
of the shares on February 1, 2020 and 100% of the shares on
February 1, 2021. We also agreed to issue Mr. Martin a
Non-Qualified Stock Option on February 1, 2018 to purchase up to
Four Million (4,000,000) shares of our common stock at $10.55 per
share, with the options vesting 33 1/3% on August 1, 2018, 33 1/3%
on February 1, 2019 and 33 1/3rd % on February 1, 2020. The options
expire ten years from the date of grant. As a result of the Amended
and Restated Employment Agreement with Mr. Martin, he is no longer
entitled to the Seven Million (7,000,000) shares of our common
stock as annual salary, or the One Million (1,000,000) shares of a
yet-to-be-created class of Series B Preferred Stock if we become
fully-reporting, which were both set forth in his prior employment
agreement. On December 19, 2018, Mr. Martin requested termination
of his Restricted Stock Agreement dated February 1, 2018 and
requested that the grants of restricted stock therein be forfeited.
As a result, we terminated his Restricted Stock Agreement and the
grants of stock thereunder immediately. At the time of the
termination none of the transfer restrictions on the shares had
been lifted and Mr. Martin never received the shares.
On
January 23, 2018, our Board of Directors agreed to enter into an
Amended and Restated Employment Agreement with Nicole M. Breen.
Under the new agreement, Ms. Breen will serve as our Secretary and
Treasurer for a five (5) year term in exchange for a base salary of
$1,000 per week. The agreement went effective beginning February 1,
2018. Additionally, we agreed to grant Ms. Breen Five Hundred
Thousand (500,000) shares of our restricted common stock on
February 1, 2018 pursuant to the terms of a Restricted Stock
Agreement, with the shares subject to certain restrictions on
transfer which expire on 33% of the shares on February 1, 2019, 66%
of the shares on February 1, 2020 and 100% of the shares on
February 1, 2021. We also agreed to issue Ms. Breen a Non-Qualified
Stock Option on February 1, 2018 to purchase up to Two Million
(2,000,000) shares of our common stock at $10.55 per share, with
the options vesting 33 1/3% on August 1, 2018, 33 1/3% on February
1, 2019 and 33 1/3rd % on February 1, 2020. The options expire ten
years from the date of grant. As a result of the Amended and
Restated Employment Agreement with Ms. Breen, she is no longer
entitled to the One Million (1,000,000) shares of our common stock
as annual salary, or the One Hundred Thousand (100,000) shares of a
yet-to-be-created class of Series B Preferred Stock if we become
fully-reporting, which were both set forth in her prior employment
agreement. On December 19, 2018, Ms. Breen requested termination of
her Restricted Stock Agreement dated February 1, 2018 and requested
that the grants of restricted stock therein be forfeited. As a
result, we terminated her Restricted Stock Agreement and the grants
of stock thereunder immediately. At the time of the termination
none of the transfer restrictions on the shares had been lifted and
Ms. Breen never received the shares.
Long-Term Incentive
Plans. We do not provide its officers or employees with pension,
stock appreciation rights, long-term incentive or other plans and
has no intention of implementing any of these plans for the
foreseeable future.
Employee Pension,
Profit Sharing or other Retirement Plans. We do not have a defined
benefit, pension plan, profit sharing or other retirement plan,
although it may adopt one or more of such plans in the
future.
Notes Payable
On
various dates, we received advances from our Chief Executive
Officer, Glenn Martin, and our Secretary, Nicole Breen. Mr. Martin
and Ms. Breen own approximately 51% and 21% of our common stock,
respectively. The unsecured interest bearing loans at 5% are due on
demand. As of December 31, 2019, owed Mr. Martin $0 and Ms. Breen
$212,000 under these notes.
Lease of Real Property
We
lease our executive offices from Glenn E. Martin, our President, on
a month-to-month basis at a monthly rent of $1,000, which began on
April 1, 2017.
DISCLOSURE
OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT
LIABILITIES
Section
15 of our Articles of Incorporation provides that, to the fullest
extent permitted by law, no director or officer shall be personally
liable to the corporation or its shareholders for damages for
breach of any duty owed to the corporation or its
shareholders.
Section
16 of our Articles of Incorporation provides that, to the fullest
extent permitted by the General Corporation Law of the State of
Nevada we will indemnify our officers and directors from and
against any and all expenses, liabilities, or other
matters.
Article
IX of our Bylaws further addresses indemnification of our directors
and officers and allows us to indemnify our directors in the event
they meet certain criteria in terms of acting in good faith and in
an official capacity within the scope of their duties, when such
conduct leads them to be involved in a legal action.
Insofar
as indemnification for liabilities arising under the Securities Act
of 1933 (the “Securities Act”) may be permitted to
directors, officers and controlling persons of the small business
issuer pursuant to the foregoing provisions, or otherwise, the
small business issuer has been advised that in the opinion of the
Securities and Exchange Commission such indemnification is against
public policy as expressed in the Act and is, therefore,
unenforceable.
AVAILABLE
INFORMATION
We have
filed with the SEC a Form 1-A for a Tier 2 offering pursuant to
Regulation A (Regulation A+) under the Securities Act of 1933, as
amended, to sell the Units. This Offering Statement, which
constitutes a part of the Form 1-A, does not contain all of the
information set forth in the Form 1-A or the exhibits filed
therewith. For further information about us, our common stock and
the Selling Shareholders, reference is made to our filings with the
SEC since we are subject to the reporting requirements of the
Securities Exchange Act of 1934, as amended. Statements contained
in this Offering Statement regarding the contents of any contract
or any other document that is filed as an exhibit to this Offering
Statements are not necessarily complete, and in each instance we
refer you to the copy of such contract or other document filed as
an exhibit to our filings. A copy of our filings with the SEC may
be inspected without charge at the public reference room maintained
by the SEC, located at 100 F Street, NE, Washington, DC 20549, and
copies of all or any part of the registration statement may be
obtained from that office upon the payment of the fees prescribed
by the SEC. Please call the SEC at 1-800-SEC-0330 for further
information about the public reference room. The SEC also maintains
a website that contains reports, proxy and information statements
and other information regarding registrants that file
electronically with the SEC. The address of the website is
www.sec.gov.
EXPERTS
The
financial statements of WEED, Inc. as of December 31, 2019 and
December 31, 2018 and for the years ended December 31, 2019 and
December 31, 2018, have been included herein in reliance upon the
reports of M&K CPAS, PLLC., independent registered public
accounting firm, appearing elsewhere herein, and upon the authority
of said firm as experts in accounting and auditing.
FINANCIAL
STATEMENTS
|
Index
to Financial Statements
|
|
|
|
|
|
Independent
Auditors’
Report
|
F-1
|
|
Consolidated
Balance Sheets of WEED, Inc. as of December 31, 2019 and
2018
|
F-2
|
|
Consolidated
Statements of Operations of WEED, Inc. for the Years Ended December
31, 2019 and 2018
|
F-3
|
|
Consolidated
Statements of Changes in Stockholders’ Equity of WEED, Inc.
for the Years Ended December 31, 2019 and 2018
|
F-4
|
|
Consolidated
Statements of Cash Flows of WEED, Inc. for the Years Ended December
31, 2019 and 2018
|
F-5
|
|
Notes
to Financial Statements
|
F-6
|
|
|
|
Consolidated
Balance Sheets of WEED, Inc. as of September 30, 2020 and December
31, 2019
|
F-22
|
|
Consolidated
Statement of Operations of WEED, Inc. for the Three and Nine Months
Ended September 30, 2020 and September 30, 2019
|
F-23
|
|
Consolidated
Statements of Changes in Stockholders’ Equity of WEED, Inc.
for the Nine Months Ended September 30, 2020 and September 30,
2019
|
F-24
|
|
Consolidated
Statement of Cash Flows of WEED, Ind. For the Nine Months Ended
September 30, 2020 and September 30, 2019
|
F-25
|
|
Notes to Financial
Statements
|
F-26
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
To the Board of Directors and
Stockholders of WEED, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of
WEED, Inc. (the Company) as of December 31, 2019 and 2018 and the
related consolidated statements of operations and comprehensive
income, consolidated statements of changes in stockholders’
equity, and consolidated and combined statements of cash flows for
each of the years in the two-year period ending December 31, 2019
and 2018 and the related notes (collectively referred to as the
financial statements). In our opinion, the consolidated financial
statements present fairly, in all material respects, the
consolidated financial position of the Company as of December 31,
2019 and 2018 and the results of its consolidated operations and
its cash flows, in conformity with accounting principles generally
accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the
Company’s management. Our responsibility is to express an
opinion on the Company’s financial statements based on our
audits. We are a public accounting firm registered with the Public
Company Accounting Oversight Board (United States) (PCAOB) and are
required to be independent with respect to the Company in
accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and
the PCAOB.
We conducted our audits in accordance with the standards of the
PCAOB. Those standards require that we plan and perform the audits
to obtain reasonable assurance about whether the consolidated
financial statements are free of material misstatement, whether due
to error or fraud. The Company is not required to have, nor were we
engaged to perform, an audit of its internal control over financial
reporting. As part of our audits, we are required to obtain an
understanding of internal control over financial reporting, but not
for the purpose of expressing an opinion on the effectiveness of
the Company’s internal control over financial reporting.
Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of
material misstatement of the financial statements, whether due to
error or fraud, and performing procedures that respond to those
risks. Such procedures included examining, on a test basis,
evidence regarding the amounts and disclosures in the financial
statements. Our audits also included evaluating the accounting
principles used and significant estimates made by management, as
well as evaluating the overall presentation of the financial
statements. We believe that our audits provide a reasonable basis
for our opinion.
The accompanying financial statements have been prepared assuming
that the Company will continue as a going concern. As discussed in
Note 2 of the consolidated financial statements, the Company
suffered a net loss from operations and has a net capital
deficiency, which raises substantial doubt about its ability to
continue as a going concern. Management’s plans regarding
those matters are also described in Note 2. The consolidated
financial statements do not include any adjustments that might
result from the outcome of this uncertainty.
/s/ M&K CPAS, PLLC
We have served as the Company’s auditor since
2017.
Houston, TX
March 30, 2020
|
WEED, INC.
|
|
CONSOLIDATED BALANCE SHEETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
CURRENT
ASSETS:
|
|
|
|
Cash
|
$ 2,509
|
$ 70,608
|
|
Accounts
Receivable
|
822
|
21
|
|
Prepaid
expenses
|
30,979
|
71,290
|
|
Deposits
|
92,000
|
350,020
|
|
|
|
|
|
TOTAL CURRENT
ASSETS
|
126,310
|
491,939
|
|
|
|
|
|
Land
|
136,400
|
136,400
|
|
|
|
|
|
Building
|
1,887,802
|
1,887,802
|
|
Computers &
Equipment
|
73,681
|
570,397
|
|
Vehicle
|
0
|
105,132
|
|
Leasehold
improvements
|
5,000
|
5,000
|
|
|
2,102,883
|
2,704,731
|
|
|
|
|
|
Less: Accumulated
depreciation
|
(322,498)
|
(224,198)
|
|
|
|
|
|
Property and
equipment, net
|
1,780,385
|
2,480,533
|
|
|
|
|
|
Trademark
|
50,000
|
50,000
|
|
Less: Accumulated
amortization
|
(4,083)
|
(1,483)
|
|
Trademark,
net
|
45,917
|
48,517
|
|
|
|
|
|
TOTAL
ASSETS
|
$ 1,952,612
|
$ 3,020,989
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’
EQUITY
|
|
|
|
|
|
|
|
CURRENT
LIABILITIES
|
|
|
|
Accounts
payable
|
$ 212,181
|
$ 240,459
|
|
Accrued
Expense
|
10,000
|
-
|
|
Accrued officer
compensation
|
122,250
|
-
|
|
Accrued
interest
|
16,453
|
6,903
|
|
Notes payable,
related parties
|
224,100
|
12,000
|
|
Notes
payable
|
167,263
|
-
|
|
Due to
Officer
|
723
|
-
|
|
|
|
|
|
TOTAL CURRENT
LIABILITIES
|
752,970
|
259,362
|
|
|
|
|
|
TOTAL
LIABILITIES
|
752,970
|
259,362
|
|
|
|
|
|
STOCKHOLDERS’
EQUITY
|
|
|
|
Common stock,
$0.001 par value, 200,000,000 authorized, 109,262,685 and
105,950,685 issued and outstanding,
|
109,263
|
105,951
|
|
Additional paid-in
capital
|
76,660,712
|
50,695,721
|
|
Subscription
payable
|
356,250
|
356,250
|
|
Accumulated
deficit
|
(75,925,974)
|
(48,396,295)
|
|
Accumulated other
comprehensive loss:
|
|
|
|
Foreign currency
translation
|
(609)
|
-
|
|
|
|
|
|
TOTAL
STOCKHOLDERS’ EQUITY
|
1,199,642
|
2,761,627
|
|
|
|
|
|
TOTAL LIABILITIES
& STOCKERHOLDERS’
|
$ 1,952,612
|
$ 3,020,989
|
WEED, INC.
CONSOLIDATED
STATEMENTS OF OPERATIONS AND COMPREHENSIVE
INCOME
(UNAUDITED)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
REVENUE
|
$ -
|
-
|
|
|
|
|
|
OPERATING
EXPENSES
|
|
|
|
General and
administrative expenses
|
969,913
|
1,358,178
|
|
Professional
fees
|
26,287,730
|
26,866,800
|
|
Depreciation &
amortization
|
159,424
|
180,640
|
|
|
|
|
|
Total operating
expenses
|
27,417,067
|
28,405,618
|
|
|
|
|
|
NET OPERATING
LOSS
|
(27,417,067)
|
(28,405,618)
|
|
|
|
|
|
OTHER INCOME
(EXPENSE)
|
|
|
|
Interest
income
|
-
|
9,338
|
|
Interest
expense
|
(11,672)
|
(12,179)
|
|
Other
income
|
1,016
|
155,701
|
|
Loss on
deposit
|
(100,000)
|
(110,000)
|
|
Loss on
extinguishment of debt
|
-
|
(1,064,720)
|
|
Gain on
extinguishment of debt
|
-
|
121,475
|
|
Other
expense
|
(1,956)
|
(9,004)
|
|
|
|
|
|
TOTAL OTHER
EXPENSE, NET
|
(112,612)
|
(909,389)
|
|
|
|
|
|
NET
LOSS
|
$ (27,529,679)
|
(29,315,007)
|
|
|
|
|
|
OTHER COMPREHENSIVE
LOSS
|
(609)
|
-
|
|
|
|
|
|
COMPREHENSIVE
LOSS
|
(27,530,288)
|
(29,315,007)
|
|
|
|
|
|
WEIGHTED AVERAGE
NUMBER OF COMMON SHARES
|
|
|
|
|
|
|
|
Outstanding - basic
and fully diluted
|
107,649,127
|
103,168,018
|
|
|
|
|
|
Net loss per share
- basic and fully diluted
|
$ 0.26
|
(0.28)
|
WEED, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’
EQUITY
For the Year Ended December 31, 2019
(UNAUDITED)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December
31, 2017
|
100,861,235
|
$ 100,861
|
$ 19,139,868
|
$ $200,770
|
$ (19,081,288)
|
$
|
$ 360,211
|
|
|
|
|
|
|
|
|
|
|
Common stock sold
for cash
|
3,899,450
|
3,900
|
4,794,651
|
|
|
|
4,798,551
|
|
|
|
|
|
|
|
|
|
|
Shares issued for
warrant exercises
|
150,000
|
150
|
224,850
|
|
|
|
225,000
|
|
|
|
|
|
|
|
|
|
|
Common stock issued
for debt settlement
|
125,000
|
125
|
1,449,875
|
|
|
|
1,450,000
|
|
|
|
|
|
|
|
|
|
|
Coomon stock issued
for services
|
915,000
|
915
|
2,932,705
|
155,480
|
|
|
3,089,100
|
|
|
|
|
|
|
|
|
|
|
Vesting of employee
stock options
|
|
|
21,284,610
|
|
|
|
21,284,610
|
|
|
|
|
|
|
|
|
|
|
Vesting of employee
stock comp
|
|
|
869,162
|
|
|
|
869,162
|
|
|
|
|
|
|
|
|
|
|
Net
loss
|
|
|
|
|
(29,315,007)
|
|
(29,315,007)
|
|
|
|
|
|
|
|
|
|
|
Balance,
December 31, 2018
|
105,950,685
|
$ 105,951
|
$ 50,695,721
|
356,250
|
$ (48,396,295)
|
$
|
$ 2,761,627
|
|
|
|
|
|
|
|
|
|
|
Common stock sold
for cash
|
1,065,000
|
1,065
|
571,935
|
|
|
|
573,000
|
|
|
|
|
|
|
|
|
|
|
Common stock
returned
|
(220,000)
|
(220))
|
220
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
Common stock issued
for services
|
2,467,000
|
2,467
|
2,575,783
|
|
|
|
2,578,250
|
|
|
|
|
|
|
|
|
|
|
Vesting of employee
stock options
|
|
|
22,770,662
|
|
|
|
22,770,662
|
|
|
|
|
|
|
|
|
|
|
Related party gain
on sale of automobile
|
|
|
46,391
|
|
|
|
46,391
|
|
|
|
|
|
|
|
|
|
|
Net
loss
|
|
|
|
|
(27,529,679)
|
|
(27,529,679)
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive
income, net
|
|
|
|
|
|
(609)
|
(609)
|
|
|
|
|
|
|
|
|
|
|
Balance,
December 31, 2019
|
109,262,685
|
$ 109,263
|
$ 76,660,712
|
$ 356,250
|
$ (75,925,974)
|
(609)
|
$ 1,199,642
|
|
WEED, INC.
|
|
CONSOLIDATED AND COMBINED STATEMENTS OF CASH
FLOWS
|
|
For the
Year Ended December 31, 2019 and December 31, 2018
|
|
(UNAUDITED)
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM OPERATING
ACTIVITIES
|
|
|
|
|
|
|
|
Net loss
|
$ (27,529,679)
|
$ (29,315,007)
|
|
Adjustments to
reconcile net loss used in operating activities:
|
|
|
|
Depreciation and
amortization
|
159,423
|
180,640
|
|
Gain on settlement
of debt
|
-
|
(121,475)
|
|
Loss on
deposit
|
-
|
110,000
|
|
Impairment of
CIP
|
499,695
|
321,614
|
|
Estimated fair
value of stock based compensation
|
22,770,662
|
21,201,397
|
|
Estimated fair
value of shares issued for services
|
2,578,250
|
4,041,575
|
|
Loss on debt
extinguishment
|
-
|
1,064,720
|
|
Decrease (increase)
in assets
|
|
|
|
Accounts
Receivable
|
(801)
|
(21)
|
|
Prepaid expenses
and other assets
|
298,331
|
(498,311)
|
|
Increase (decrease)
in liabilities
|
|
|
|
Accounts
Payable
|
(28,278)
|
11,849
|
|
Accrued
expenses
|
141,800
|
(178,335)
|
|
|
|
|
|
NET CASH USED IN
OPERATING ACTIVITIES
|
(1,110,597)
|
(3,181,303)
|
|
|
|
|
|
CASH FLOWS FROM INVESTING
ACTIVITIES
|
|
|
|
Purchases of
property and equipment
|
(2,979)
|
(826,481)
|
|
Purchase of
intangible assets
|
-
|
(50,000)
|
|
|
|
|
|
NET CASH USED IN
INVESTING ACTIVITIES
|
(2,979)
|
(876,481)
|
|
|
|
|
|
CASH FLOWS FROM FINANCING
ACTIVITIES
|
|
|
|
|
|
|
|
Proceeds from notes
payable - related party
|
305,823
|
7,000
|
|
Proceeds from the
sale of common stock
|
573,000
|
5,023,401
|
|
Proceeds on notes
payable
|
250,850
|
-
|
|
Repayments on notes
payable
|
(83,587)
|
(1,063,187)
|
|
|
|
|
|
NET CASH PROVIDED
BY FINANCING ACTIVITIES
|
1,046,086
|
3,967,214
|
|
|
|
|
|
NET CHANGE IN
CASH
|
(67,490)
|
(90,570)
|
|
|
|
|
|
EFFECT OF EXCHANGE
RATE ON CASH
|
(609)
|
-
|
|
|
|
|
|
CASH, BEGINNING OF
PERIOD
|
70,608
|
161,178
|
|
|
|
|
|
CASH, END OF
PERIOD
|
$ 2,509
|
$ 70,608
|
|
|
|
|
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW
INFORMATION
|
|
|
|
|
|
|
|
Cash paid during the year ended
December 31:
|
|
|
|
|
|
|
|
Income
taxes
|
$ -
|
$ -
|
|
Interest
paid
|
$ -
|
$ -
|
|
|
|
|
|
Non-cash investing and financing
activities:
|
|
|
|
|
|
|
|
Gain on related
party transaction
|
$ 93,000
|
$ -
|
|
Mortgage issued for
acquisition of land and property
|
$ -
|
$ 1,040,662
|
|
Shares issued from
subscription payable
|
$ -
|
$ 200,770
|
|
Values of shares
issued o pay off note payable
|
$ -
|
$ 385,281
|
WEED,
INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019 and 2018
Note 1 – Nature of Business and Significant Accounting
Policies
Nature of Business
WEED, Inc. (the “Company”), (formerly United Mines,
Inc.) was incorporated under the laws of the State of Arizona on
August 20, 1999 (“Inception Date”) as Plae, Inc. to
engage in the exploration of gold and silver mining properties. On
November 26, 2014, the Company was renamed from United Mines, Inc.
to WEED, Inc. and was repurposed to pursue a business involving the
purchase of land, and building Commercial Grade “Cultivation
Centers” to consult, assist, manage & lease to Licensed
Dispensary owners and organic grow operators on a contract basis,
with a concentration on the legal and medical marijuana sector. The
Company’s plan is to become a True “Seed-to-Sale”
company providing infrastructure, financial solutions and real
estate options in this new emerging market. The Company, under
United Mines, was formerly in the process of acquiring mineral
properties or claims located in the State of Arizona, USA. The name
was previously changed on February 18, 2005 to King Mines, Inc. and
then subsequently changed to United Mines, Inc. on March 30, 2005.
The Company trades on the OTC Pink Sheets under the stock symbol:
BUDZ.
On April 20, 2017, the Company acquired Sangre AT, LLC, a Wyoming
company doing business as Sangre AgroTech. (“Sangre”).
Sangre is a plant genomic research and breeding company comprised
of top-echelon scientists with extensive expertise in genomic
sequencing, genetics-based breeding, plant tissue culture, and
plant biochemistry, utilizing the most advanced sequencing and
analytical technologies and proprietary bioinformatics data systems
available. No work is being conducted now until further funds are
available.
The accompanying financial statements have been prepared in
conformity with accounting principles generally accepted in the
United States of America. These statements reflect all adjustments,
consisting of normal recurring adjustments, which in the opinion of
management are necessary for fair presentation of the information
contained therein.
The Company has a calendar year end for reporting
purposes.
Basis of Presentation:
The accompanying condensed consolidated balance sheet at December
31, 2019, has been derived from audited consolidated financial
statements and the unaudited condensed consolidated financial
statements as of June 30, 2019 and 2018 ( the “financial
statements”), have been prepared in accordance with
accounting principles generally accepted in the United States of
America (“GAAP”) for interim financial information and
with the instructions to Form 10-Q and Article 8 of Regulation S-X.
Accordingly, they do not include all of the information and
footnotes required by GAAP for complete financial statements and
should be read in conjunction with the audited consolidated
financial statements and related footnotes included in our
Registration Statement on Form S-1 for the year ended December 31,
2019 (the “2019 Annual Report”), filed with the
Securities and Exchange Commission (the “SEC”). It is
management’s opinion, however, that all material adjustments
(consisting of normal recurring adjustments), have been made which
are necessary for a fair financial statements presentation. The
condensed consolidated financial statements include all material
adjustments (consisting of normal recurring accruals) necessary to
make the condensed consolidated financial statements not misleading
as required by Regulation S-X, Rule 10-01.
Principles of Consolidation
The accompanying consolidated financial statements include the
accounts of the following entities, all of which are under common
control and ownership:
|
|
|
State
of
|
|
|
|
Abbreviated
|
|
Name of
Entity
|
|
Incorporation
|
|
Relationship
(1)
|
|
Reference
|
|
WEED,
Inc.
|
|
Nevada
|
|
Parent
|
|
WEED
|
|
Sangre
AT, LLC (2)
|
|
Wyoming
|
|
Subsidiary
|
|
Sangre
|
|
|
(1)
|
Sangre
is a wholly-owned subsidiary of WEED, Inc.
|
|
|
(2)
|
Sangre
AT, LLC is doing business as Sangre AgroTech.
|
The consolidated financial statements herein contain the operations
of the wholly-owned subsidiary listed above. All significant
inter-company transactions have been eliminated in the preparation
of these financial statements. The parent company, WEED and
subsidiary, Sangre will be collectively referred to herein as the
“Company”, or “WEED”. The Company’s
headquarters are located in Tucson, Arizona and its operations are
primarily within the United States, with minimal operations in
Australia.
These statements reflect all adjustments, consisting of normal
recurring adjustments, which in the opinion of management are
necessary for fair presentation of the information contained
therein.
Note 1 – Nature of Business and Significant Accounting
Policies (continued)
Use of Estimates
The preparation of financial statements in conformity with
accounting principles generally accepted in the United States
requires management to make estimates and assumptions that affect
the reported amounts of assets and liabilities, and the disclosure
of contingent assets and liabilities at the date of the financial
statements, and the reported amounts of revenues and expenses
during the reporting period. Actual results could differ from those
estimates.
Fair Value of Financial Instruments
Under FASB ASC 820-10-05, the Financial Accounting Standards Board
establishes a framework for measuring fair value in generally
accepted accounting principles and expands disclosures about fair
value measurements. This Statement reaffirms that fair value is the
relevant measurement attribute. The adoption of this standard did
not have a material effect on the Company’s financial
statements as reflected herein. The carrying amounts of cash,
prepaid expenses and accrued expenses reported on the balance sheet
are estimated by management to approximate fair value primarily due
to the short term nature of the instruments.
Impairment of Long-Lived Assets
Long-lived assets held and used by the Company are reviewed for
possible impairment whenever events or circumstances indicate the
carrying amount of an asset may not be recoverable or is impaired.
Recoverability is assessed using undiscounted cash flows based upon
historical results and current projections of earnings before
interest and taxes. Impairment is measured using discounted cash
flows of future operating results based upon a rate that
corresponds to the cost of capital. Impairments are recognized in
operating results to the extent that carrying value exceeds
discounted cash flows of future operations.
Basic and Diluted Loss Per Share
The basic net loss per common share is computed by dividing the net
loss by the weighted average number of common shares outstanding.
Diluted net loss per common share is computed by dividing the net
loss adjusted on an “as if converted” basis, by the
weighted average number of common shares outstanding plus potential
dilutive securities. For the periods presented, potential dilutive
securities had an anti-dilutive effect and were not included in the
calculation of diluted net loss per common share.
Stock-Based Compensation
Under FASB ASC 718-10-30-2, all share-based payments to employees,
including grants of employee stock options, to be recognized in the
income statement based on their fair values. Pro forma disclosure
is no longer an alternative.
Revenue Recognition
On January 1, 2018, the Company adopted the new revenue recognition
standard ASU 2014-09, “Revenue from Contracts with Customers
(Topic 606)”, using the cumulative effect (modified
retrospective) approach. Modified retrospective adoption requires
entities to apply the standard retrospectively to the most current
period presented in the financial statements, requiring the
cumulative effect of the retrospective application as an adjustment
to the opening balance of retained earnings at the date of initial
application. No cumulative-effect adjustment in retained earnings
was recorded as the Company’s has no historical revenue. The
impact of the adoption of the new standard was not material to the
Company’s condensed consolidated financial statements for the
year ended December 31, 2019. The Company expects the impact to be
immaterial on an ongoing basis.
The primary change under the new guidance is the requirement to
report the allowance for uncollectible accounts as a reduction in
net revenue as opposed to bad debt expense, a component of
operating expenses. The adoption of this guidance did not have an
impact on our condensed consolidated financial statements, other
than additional financial statement disclosures. The guidance
requires increased disclosures, including qualitative and
quantitative disclosures about the nature, amount, timing and
uncertainty of revenue and cash flows arising from contracts with
customers.
The Company operates as one reportable segment.
Sales on fixed price contracts are recorded when services are
earned, the earnings process is complete or substantially complete,
and the revenue is measurable and collectability is reasonably
assured. Provisions for discounts and rebates to customers,
estimated returns and allowances, and other adjustments are
provided for in the same period the related sales are recorded. The
Company will defer any revenue from sales in which payment has been
received, but the earnings process has not occurred. Sales have not
yet commenced.
Advertising and Promotion
All costs associated with advertising and promoting products are
expensed as incurred. These expenses were $3,450 and $998 for the
years ended December 31, 2019 and 2018.
Note 1 – Nature of Business and Significant Accounting
Policies (continued)
Recently Issued Accounting Pronouncements
In August 2018, the FASB issued ASU 2018-15 Accounting for
Implementation Costs Related to Cloud Computing or Hosting
Arrangements. This standard provides authoritative guidance
intended to address a customer’s accounting for
implementation costs incurred in a cloud computing arrangement that
is a service contract. This guidance aligns the requirements for
capitalizing implementation costs incurred in a hosting arrangement
that is a service contract with the requirements for capitalizing
implementation costs incurred to develop or obtain internal-use
software. The guidance also requires presentation of the
capitalized implementation costs in the statement of financial
position and in the statement of cash flows in the same line item
that a prepayment for the fees of the associated hosting
arrangement would be presented, and the expense related to the
capitalized implementation costs to be presented in the same line
item in the statement of operations as the fees associated with the
hosting element (service) of the arrangement. This guidance is
effective for annual periods beginning after December 15,
2019, including interim periods within those annual periods, with
early adoption permitted. We are currently evaluating the potential
impact on our financial position, results of operations and
statement of cash flows upon adoption of this guidance. We do not
expect this guidance to have a significant impact, or potential
significant impact, to our unaudited condensed consolidated interim
financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases. The standard
requires lessees to recognize lease assets and lease liabilities on
the consolidated balance sheet and requires expanded disclosures
about leasing arrangements. We plan to adopt the standard on
January 1, 2019. We are currently assessing the impact that the new
standard will have on our consolidated financial statements, which
will consist primarily of a balance sheet gross up of our operating
leases to show equal and offsetting lease assets and lease
liabilities.
The Company adopted the new lease guidance effective January 1,
2019 using the modified retrospective transition approach,
applying the new standard to all of its leases existing at the date
of initial application which is the effective date of
adoption. Consequently, financial information will not be
updated and the disclosures required under the new standard will
not be provided for dates and periods before January 1,
2019. We elected the package of practical expedients which
permits us to not reassess (1) whether any expired or existing
contracts are or contain leases, (2) the lease classification for
any expired or existing leases, and (3) any initial direct costs
for any existing leases as of the effective date. We did not elect
the hindsight practical expedient which permits entities to use
hindsight in determining the lease term and assessing impairment.
The adoption of the lease standard did not change our previously
reported consolidated statements of operations and did not result
in a cumulative catch-up adjustment to opening equity. As of
December 31, 2019, the adoption of the standard had no impact on
the Company, as there were no leases in place longer than 12
months.
In June 2018, the FASB issued Accounting Standards Update
(“ASU”) 2018-07, Compensation – Stock
Compensation (Topic 718) Improvements to Nonemployee Share-Based
Payment Accounting. This ASU
expands the scope of Topic 718 to include share-based payment
transactions for acquiring goods and services from nonemployees.
The amendments in this ASU has now become effective beginning
January 1, 2019, and early adoption is permitted. We do not
anticipate that this ASU will have a material effect on our
consolidated financial statements.
Note 2 – Going Concern
As shown in the accompanying financial statements, the Company has
no revenues, incurred net losses from operations resulting in an
accumulated deficit of $75,925,974 and negative working capital of
626,660 at December 31, 2019. These factors raise substantial doubt
about the Company’s ability to continue as a going concern.
Management is actively pursuing new products and services to begin
generating revenues. In addition, the Company is currently seeking
additional sources of capital to fund short term operations. The
Company, however, is dependent upon its ability to secure equity
and/or debt financing and there are no assurances that the Company
will be successful; therefore, without sufficient financing it
would be unlikely for the Company to continue as a going
concern.
The financial statements do not include any adjustments that might
result from the outcome of any uncertainty as to the
Company’s ability to continue as a going concern. The
financial statements also do not include any adjustments relating
to the recoverability and classification of recorded asset amounts
or amounts and classifications of liabilities that might be
necessary should the Company be unable to continue as a going
concern.
Note 3 – Related Party
Notes Payable
From time to time, the Company has received short term loans from
officers and directors as disclosed in Note 7 below. The Company
has a total of $224,100 and $12,000 of note payable on the
consolidated balance sheet as of December 31, 2019 and 2018,
respectively. During the year ended December 31, 2019, the Company
transferred the ownership of the 2017 Audi Q7 and Audi A4, valued
at $46,609, to Nicole Breen as a repayment for her loans. $93,000
was recorded as a forgiveness on notes payable, and $46,391
recorded to additional paid-in capital.
Services
Nicole M. Breen receives $1,500 a week in cash compensation for her
services rendered to the Company.
Glenn E. Martin receives $8,000 a month in cash compensation for
his services rendered to the Company.
Capital Contributions
The Company imputed interest on non-interest bearing, related party
loans, resulting in a total of $0 and $0 of contributed capital
during the years ended December 31, 2019 and 2018,
respectively.
Common Stock Issued for Bartered Assets
On January 18, 2017, the Company exchanged 66,000 units, consisting
of 66,000 shares of common stock and warrants to purchase 66,000
shares of common stock at an exercise price of $3.00 per share,
exercisable until January 18, 2018, in exchange for a 2017 Audi Q7
and a 2017 Audi A4 driven by the Officers. The total fair value
received, based on the market price of the stock at $4.02 per
share, was allocated to the $105,132 purchase price of the vehicles
and the $160,188 excess value of the common stock and warrants was
expensed as stock-based compensation. During the year ended
December 31, 2019, the Company transferred the ownership of the two
Audi vehicles, valued at $46,609, to Nicole Breen as a repayment
for her loans. $93,000 was recorded as a forgiveness on notes
payable, and $46,391 recorded to additional paid-in
capital.
Common Stock
On August 1, 2017, the Company granted 150,000 shares of common
stock to Mary Williams, a principal of Sangre AT, LLC, for services
performed. The fair value of the common stock was $154,500 based on
the closing price of the Company’s common stock on the date
of grant.
On January 7, 2017, the Company granted 50,000 shares of common
stock to Pat Williams. PhD, a principal of Sangre AT, LLC, for
services performed. The total fair value of the common stock was
$210,250 based on the closing price of the Company’s common
stock on the date of grant.
A total of $122,250 and $0 of officer compensation was unpaid and
outstanding at December 31, 2019 and 2018,
respectively.
Stock Options Issued for Services – related party
(2019)
On February 1, 2018, in connection with executive employment
agreements, the Company granted non-qualified options to purchase
an aggregate of 6,000,000 shares of the Company’s common
stock at the exercise price of $10.55 per share. The options shall
become exercisable at the rate of 1/3 upon the six-month
anniversary, 1/3 upon the one-year anniversary and 1/3 upon the
second anniversary of the grant. The options were valued at
$45,987,970 using the Black-Scholes option pricing model. The
Company recognized expense of approximately, $15,329,323 relating
to these options for the year ended December 31, 2019.
Note 4 – Fair Value of Financial Instruments
Under FASB ASC 820-10-5, fair value is defined as the price that
would be received to sell an asset or paid to transfer a liability
in an orderly transaction between market participants at the
measurement date (an exit price). The standard outlines a valuation
framework and creates a fair value hierarchy in order to increase
the consistency and comparability of fair value measurements and
the related disclosures. Under GAAP, certain assets and liabilities
must be measured at fair value, and FASB ASC 820-10-50 details the
disclosures that are required for items measured at fair
value.
The Company has certain financial instruments that must be measured
under the new fair value standard. The Company’s financial
assets and liabilities are measured using inputs from the three
levels of the fair value hierarchy. The three levels are as
follows:
Level
1 - Inputs are unadjusted quoted prices in active markets for
identical assets or liabilities that the Company has the ability to
access at the measurement date.
Level
2 - Inputs include quoted prices for similar assets and liabilities
in active markets, quoted prices for identical or similar assets or
liabilities in markets that are not active, inputs other than
quoted prices that are observable for the asset or liability (e.g.,
interest rates, yield curves, etc.), and inputs that are derived
principally from or corroborated by observable market data by
correlation or other means (market corroborated
inputs).
Level
3 - Unobservable inputs that reflect our assumptions about the
assumptions that market participants would use in pricing the asset
or liability.
The following schedule summarizes the valuation of financial
instruments at fair value on a recurring basis in the balance
sheets as of December 31, 2019 and 2018, respectively:
Fair Value Measurements at December 31, 2018
|
|
|
|
|
|
Assets
|
|
|
|
|
Cash
|
$ 70,608
|
$ -
|
$ -
|
|
Total
assets
|
$ 70,608
|
$ -
|
$ -
|
|
Liabilities
|
|
|
|
|
Notes payable,
related parties
|
|
|
|
|
Notes
payable
|
$ -
|
$ 12,000
|
$ -
|
|
Total
liabilities
|
$ -
|
$ 12,000
|
$ -
|
|
|
$ 70,608
|
$ 12,000
|
$ -
|
Fair Value Measurements at December 31, 2019
|
|
|
|
|
|
Assets
|
|
|
|
|
Cash
|
$ 2,509
|
$ -
|
$ -
|
|
Total
assets
|
$ 2,509
|
$ -
|
$ -
|
|
Liabilities
|
|
|
|
|
Notes payable,
related parties
|
|
$ 224,100
|
|
|
Notes
payable
|
$ -
|
$ 167,263
|
$ -
|
|
Total
liabilities
|
$ -
|
$ 391,363
|
$ -
|
|
|
$ 2,509
|
$ 391,363
|
$ -
|
The fair values of our related party debts are deemed to
approximate book value and are considered Level 2 inputs as defined
by ASC Topic 820-10-35.
There were no transfers of financial assets or liabilities between
Level 1, Level 2 and Level 3 inputs for the years ended December
31, 2019 and 2018, respectively.
Note 5 – Investment in Land and Property
On July 26, 2017, the Company closed on the purchase of property,
consisting of a home, recreational facility and RV park located at
5535 State Highway 12 in La Veta, Colorado to be developed into a
bioscience center. The home has 4 Bedrooms and 2 Baths, and the
recreational facility has showers, laundry, and reception area with
an additional equipment barn attached, in addition to another
facility with 9,500 square feet. The RV Park has 24 sites with full
hook-ups including water, sewer, and electric, which the Company
plans to convert into a series of small research pods. Under the
terms of the purchase agreement, the Company paid $525,000 down,
including 25,000 shares of our common stock, and Sangre took
immediate possession of the property. Under the terms of the
original purchase agreement, the Company was obligated to pay an
additional $400,000 in cash and issue an additional 75,000 shares
of our common stock over the next two years in order to pay the
entire purchase price. On January 12, 2018, the Company entered
into an Amendment No. 1 to the $475,000 principal amount promissory
note issued by the Company to the seller of the property, under
which both parties agreed to amend the purchase and the promissory
note to allow the Company to pay off the note in full if it paid
$100,000 in cash on or before January 15, 2018 and issued the
seller 125,000 shares of common stock, restricted in accordance
with Rule 144, on before January 20, 2018. Through an escrow
process, the Company paid the seller $100,000 in cash and issued
him 125,000 shares of common stock in accordance with the Amendment
No. 1, in exchange for a full release of the deed of trust that was
securing the promissory note, on January 17, 2018. As a result, the
$475,000 principal promissory noteissued to the seller was deemed
paid-in-full and fully satisfied and the Company owned the property
without encumbrances as of that date. The Company recorded a loss
on extinguishment of debt of approximately $1,065,000 based on the
fair value of the consideration paid and the carrying value of the
note payable on the settlement date. The total purchase price was
as follows:
|
|
|
|
Consideration:
|
|
|
Common stock
payment of 25,000 shares (1)
|
$ 30,000
|
|
Cash payment of
down payment
|
50,000
|
|
Cash paid at
closing
|
44,640
|
|
Short term
liabilities assumed and paid at closing (2)
|
5,360
|
|
Note payable
(3)
|
475,000
|
|
Total
purchase price
|
$ 1,005,000
|
|
|
(1)
|
Consideration
consisted of an advance payment of 25,000 shares of the
Company’s common stock valued at $30,000 based on the closing
price of the Company’s common stock on the July 18, 2017 date
of grant.
|
|
|
(2)
|
Purchaser’s
shares of closing costs, including the seller’s prepaid
property taxes.
|
|
|
(3)
|
As
noted above, the note was settled with a payment of $100,000 and
the issuance of 125,000 shares of common stock.
|
In January 2018, the Company closed on the purchase of property,
consisting of a condominium in La Veta, Colorado to house Company
personnel and consultants for total consideration approximating
$140,000, which was paid in cash at the time of closing. Thehome
has 3 bedrooms and 2.5 baths. Sangre took immediate possession of
the property. La Veta, Colorado is a small town and rental or
short-term housing is very difficult to obtain. The Company
personnel and consultants are no longer residing at the property,
and it is currently vacant.
In February 2018, the Company closed on the purchase of property,
consisting of a home in La Veta, Colorado to house Company
personnel and consultants for total consideration approximating
$1,200,000. The home has 5 Bedrooms and 3 Baths. Under the terms of
the purchase agreement, the Company paid $150,000 down, entered
into a note payable in the amount of approximately $1,041,000 (see
Note 8). The Company secured a below-market interest rate of 1.81%
based on the short-termnature of the term (due on August 15, 2018).
Sangre took immediate possession of the property. La Veta, Colorado
is a small town and rental or short-term housing is very difficult
to obtain. The Company personnel and consultants are no longer
residing at the property, and it is currently vacant. On October
10, 2018, a payment of $750,000 was made to Craig W. Clark to pay
off the note payable, and a loan discount of $125,475 was given to
the Company which was recorded as a gain.
A settlement payment of $155,000 was received from an insurance
company related to a fire near one of our properties in La Veta,
Colorado.
On June 25, 2019, the Company received $60,000 from Lex Seabre in
exchange for 120,000 shares of common stock of the Company. The
$60,000 was paid as a deposit for the Sugar Hill golf course
property auction.
On June 28, 2019, the Company received a loan of $12,000 from
Nicole Breen. The $12,000 was paid as a deposit for the Sugar Hill
golf course property auction.
On September 25, 2019, the Company received $20,000 from Lex Seabre
in exchange for 100,000 shares of common stock of the Company. The
$20,000 was paid as a deposit for the additional 60-day extension
for the Sugar Hill golf course property purchase.
Note 6 – Property and Equipment
Property and equipment consist of the following at December 31,
2019 and 2018, respectively:
|
|
|
|
|
|
|
|
|
Property
improvements
|
$ 5,000
|
$ 5,000
|
|
Automobiles
|
0
|
105,132
|
|
Office
equipment
|
4,933
|
4,933
|
|
Furniture &
Fixtures
|
2,979
|
0
|
|
Lab
equipment
|
65,769
|
65,769
|
|
Construction in
progress
|
0
|
499,695
|
|
Property
(1)
|
1,887,802
|
1,887,802
|
|
Property and
equipment, gross
|
1,966,483
|
2,568,331
|
|
Less accumulated
depreciation
|
(322,498)
|
(224,198)
|
|
Property and
equipment, net
|
$ 1,643,985
|
2,344,133
|
|
|
(1)
|
In
2018, the Company purchased two properties in La Veta, Colorado.
The property located on 169 Valley Vista was purchased for
$140,000, and the property located on 1390 Mountain Valley Road was
purchased for $1,200,000 (see Note 8).
|
Depreciation and amortization expense totaled $159,424 and $180,640
for the years ended December 31, 2019 and 2018,
respectively.
During the year ended December 31, 2019, the Company transferred
the ownership of the 2017 Audi Q7 and Audi A4, valued at $46,609,
to Nicole Breen as a repayment for her loans. $93,000 was recorded
as a forgiveness on notes payable, and $46,391 recorded to
additional paid-in capital.
Construction in progress in the amount of $499,695 was fully
impaired due to the Company may not receive funds to complete the
research facility center project. There was no work performed in
2019.
Note 7 – Intangible Assets
In accordance with FASB ASC 350, “Intangibles-Goodwill and
Other”, the Company evaluates the recoverability of
identifiable intangible assets whenever events or changes in
circumstances indicate that an intangible asset’s carrying
amount may not be recoverable. The impairment loss would be
calculated as the amount by which the carrying value of the asset
exceeds its fair value. The US and Europe trademarks were acquired
for $40,000 and $50,000, respectively, for the year ended December
31, 2018. Trademarks are initially measured based on their fair
value and amortized by 10 and 25 years.
Amortization expense totaled $2,600 and $1,483 for the years ended
December 31, 2019 and 2018, respectively.
Note 8 – Notes Payable, Related Parties
Notes payable, related parties consist of the following at December
31, 2019 and 2018, respectively:
|
|
|
|
|
On April 12, 2010,
the Company received an unsecured, non-interest-bearing loan in the
amount of $2,000, due on demand from Robert Leitzman. Interest is
being imputed at the Company’s estimated borrowing rate, or
10% per annum. The largest aggregate amount outstanding was $2,000
during the periods ended December 31, 2019 and December 31, 2018.
Mr. Leitzman owns less than 1% of the Company’s common stock,
however, the Mr. Leitzman is deemed to be a related party given the
non-interest-bearing nature of the loan and the materiality of the
debt at the time of origination.
|
2,000
|
2,000
|
|
|
|
|
|
Over various dates
in 2011 and 2012, the Company received unsecured loans in the
aggregate amount of $10,000, due on demand, bearing interest at
10%, from Sandra Orman. The largest aggregate amount outstanding
was $10,000 during the periods ended December 31, 2019 and December
31, 2018. Mrs. Orman owns less than 1% of the Company’s
common stock, however, Mrs. Orman is deemed to be a related party
given the nature of the loan and the materiality of the debt at the
time of origination.
|
10,000
|
10,000
|
|
|
|
|
|
Over various dates
from April to December 2019, the company received a total of
$305,100 of advances, bearing interest at 5%, from Nicole Breen. A
detailed list of advances and repayments follows. On October 28,
2019, the company’s vehicles valued at $93,000 were used as a
repayment.
|
212,100
|
0
|
|
|
|
|
|
Notes payable,
related parties
|
$ 224,100
|
$ 12,000
|
The Company recorded interest expense in the amount of $9,264 and
$12,179 for the years ended December 31, 2019 and 2018,
respectively, including imputed interest expense in the amount of
$0 and $0 during such periods related to notes payable, related
parties.
Note 9 – Notes Payable
Note payable consist of the following at December 31, 2019 and
2018, respectively:
|
|
|
|
|
On July 26, 2017,
the Company issued a $475,000 note payable, bearing interest at 5%
per annum, to A.R. Miller (“Miller Note”) pursuant to
the purchase of land and property in La Veta, Colorado. The note is
to be paid in four consecutive semi-annual installments in the
amount of $118,750 plus accrued interest commencing on January 26,
2018 and continuing on the 26th day of July and the 26th day of
January each year until the debt is repaid on July 26, 2019. The
note carries a late fee of $5,937.50 in the event any installment
payment is more than 30 days late, and upon default the interest
rate shall increase to 12% per annum. During the three months ended
March 31, 2018, the Company issued 125,000 shares of common stock,
valued at $1,450,000 based on the closing price on the measurement
date. Accordingly, the Company recorded a loss on extinguishment of
$1,064,719.
|
$ -
|
$ -
|
|
|
|
|
|
On February 16,
2018, the Company issued a $1,040,662 note payable, bearing
interest at 1.81% per annum (the low interest rate was due to the
short-term nature of the note – six months. See Note 6), to
Craig and Carol Clark (“Clark Note”) pursuant to the
purchase of land and property in La Veta, Colorado. The note is to
be paid in consecutive monthly installments in the amount of
$5,000, including accrued interest commencing on March 15, 2018 and
continuing through August 15, 2018. The note carries a late fee of
3% in the event any installment payment is more than 10 days late,
and upon default the interest rate shall increase to 10% per annum.
As of September 12, 2018, a total of $171,300 was paid to the note
holder. On October 9, 2018, the Company enteredinto a settlement
agreement with the note holder to pay the settlement payment of
$750,000. The Company had already paid $650,000 by September 27,
2018 and made the remaining payment of $100,000 on October 10,
2018. The Company recorded a gain on extinguishment of
$121,475.
|
|
|
|
|
|
|
|
On August 5, 2019,
the Company entered into a promissory note, whereby the Company
promises to pay Snell & Wilmer L.L.P the principal amount of
$250,000, bearing interest at 2.5% per annum. The note is to be
paid in consecutive monthly installments in theamount of $25,000,
including accrued interest commencing on August 30, 2019, until the
final balloon payment is paid on January 30, 2020. The promissory
note is secured by the Deed of Trust, Assignment of Leases and
Rents, Security Agreement and Fixture Filing with respect to the
real property owned by Sangre located on 1390 Mountain Valley Road,
La Veta, Colorado 81055. As of December 31, 2019, $83,588 has been
paid to Snell & Wilmer.
|
$ 166,412
|
|
|
|
|
|
|
On various dates,
the Company received advances from consultant, Patrick Brodnik,
bearing 0% interest.
|
$ 850
|
|
|
|
|
|
|
|
$ 167,262
|
$ -
|
The Company recognized interest expense of $2,408 and $0 related to
the note payables for the year ended December 31, 2019 and 2018,
respectively.
Note 10 – Commitments and Contingencies
On November 8, 2016, the Company entered into an agreement with
Gregory DiPaolo’s Pro Am Golf, LLC to acquire improved
property located in Westfield, New York. The total purchase price
of $1,600,000 is to be paid with a deposit of 50,000 shares of
common stock, followed by cash of $1,250,000 and 300,000 shares of
the Company’s common stock to be delivered at closing. The
deposit of 50,000 shares issued as a deposit was $42,500 based on
the closing price of the Company’s common stock on the date
of grant. Subsequently, we entered into an amended Purchase and
Sale Agreement on October 24, 2017, under which we amended the
total purchase price to Eight Hundred Thousand Dollars ($800,000)
and forfeited our previous deposit of stock. Under the terms of the
amended agreement, we paid an additional Ten Thousand Dollar
($10,000) deposit on October 26, 2017, with the remaining purchase
price to be paid on or before the date closing date, which was
scheduled on May 1, 2018. The property is approximately 43 acres
and has unlimited water extraction rights from the State of New
York. We had planned to use this property as our inroads to the New
York hemp and infused beverage markets in the future. Since the
property was in foreclosure it was put up for auction, which
occurred on July 1, 2019. At the auction, we were the winning
bidder with a bid of $597,000. Our prior deposit payment of
$120,000 was credited towards the purchase price, and the remaining
$477,000, was finally due on November 30, 2019, after several
extensions (which cost us total of $40,000 to obtain). At the end
ofDecember 31, 2019, a total of $92,00 was issued as a deposit for
the property. We were not able to meet that deadline, but in
January 2020 we worked out an additional extension with the bank.
Under the terms of the new agreement, we still owe approximately
$392,000 to acquire the property. We have agreed to pay that amount
in installment payments of $10,000 per month for six months
beginning February 2020, with a balloon payment of approximately
$332,000 due on or before August 3, 2020. To date we have paid the
$10,000 payments for February 2020 and March 2020, and plan to pay
the April 2020 payment on time. We need to raise funds in order to
make the remaining scheduled payments.
On January 19, 2018, the Company was sued in the United States
District Court for the District of Arizona
( William Martin v. WEED,
Inc.. , Case No.
4:18-cv-00027-RM) by the listed Plaintiff. The Company was served
with the Verified Complaint on January 26, 2018. The Complaint
alleges claims for breach of contract-specific performance, breach
of contract-damages, breach of the covenant of good faith and fair
dealing, conversion, and injunctive relief. In addition to the
Verified Complaint, the Company was served with an application to
show cause for a temporary restraining order. The Verified
Complaint alleges the Company entered into a contract with the
Plaintiff on October 1, 2014 for the Plaintiff to perform certain
consulting services for the Company in exchange for 500,000 shares
of its common stock up front and an additional 700,000 shares of
common stock to be issued on May 31, 2015. The Plaintiff alleges he
completed the requested services under the agreement and received
the initial 500,000 shares of common stock, but not the additional
700,000 shares. The request for injunctive relief asks the Court to
Order the Company to issue the Plaintiff 700,000 shares of its
common stock, and possibly include them in its Registration
Statement on Form S-1, or, in the alternative, issue the shares and
have them held by the Court pending resolution of the litigation,
or, alternatively, sell the shares and deposit the sale proceeds in
an account that the Court will control. The hearing on the
Temporary Restraining Order occurred on January 29, 2018. On
January 30, 2018, the Court issued its ruling denying the
application for a Temporary Restraining Order. Currently, there is
no further hearing scheduled in this matter. On
February 13, 2018, the Company filed an Answer to the Verified
Complaint and Counterclaim. On February 15, 2018, the Company filed
a Motion to Dismiss the Verified Complaint. On February 23, 2018,
the Company filed a Motion to Amend Counterclaim to add W.
Martin’s wife, Joanna Martin as a counterdefendant. On March
9, 2018, William Martin filed a Motion to Dismiss the Counterclaim.
On March 12, 2018, William Martin filed a Motion to Amend the
Verified Complaint to, among other things, add claims against Glenn
Martin and Nicole and Ryan Breen. On March 27, 2018, the Court
granted both William Martin and WEED, Inc.’s Motions to
Amend. On March 27, 2018, the Company filed an Amended Counterclaim
adding Joanna Martin. On April 2, 2018, the Company filed a Motion
to Amend our Counterclaim to add a breach of contract claim. On
April 10, 2018, the Company filed an Answer to First Amended
Verified Complaint. On April 23, 2018, Glenn Martin and Nicole and
Ryan Breen filed their Answer to the First Amended Complaint. On
May 31, 2018, the Court issued an Order: (a) granting the
Company’s Motion to Dismiss thereby dismissing the
Plaintiff’s claims for breach of the covenant of good faith
and fair dealing and the claim for conversion, (b) denying William
Martin’s Motion to Dismiss the counterclaim as to the claims
for fraudulent concealment and fraudulent misrepresentation, but
granting the Motion to Dismiss only as to the claim for fraudulent
nondisclosure, and (c) granting the Company’s Motion to Amend
its Counterclaim to add a breach of contract claim. On June 1,
2018, William Martin and his wife filed their Answer to the First
Amended Counterclaim. On June 1, 2018, William Martin and his wife
filed their Answer to the Second Amended Counterclaim. In addition
to the above pleadings and motions, the parties have exchanged
disclosure statements and served and responded to written
discovery. The Company denies the Plaintiff’s allegations in
the Verified Complaint in their entirety and plan to vigorously
defend against this lawsuit. Due to the loss not being probable, no
accrual has been recorded for the 700,000 shares of common stock
the Plaintiff alleges he is owed under his agreement with the
Company.
Travis Nelson v. Sangre AgroTech, LLC, et al. (Huerfeno County
Colorado District Court, Case No. 2018CV30003, filed on February 5,
2018). Mr. Travis Nelson, formerly a member of the subsidiary
Sangre AgroTech, LLC, filed this action alleging wrongful discharge
in retaliation for whistleblower activity purportedly related to
insider trading, fraud and unlawful interstate transportation of
plant genetics. After a motion to dismiss was granted in part, Mr.
Nelson filed a second amended complaint asserting revised claims
for breach of fiduciary duty, wrongful discharge, and violation of
the Colorado organized crime control act. Mr. Nelson has alleged
lost wages in the amount of $600,000, unspecified losses related to
whistleblower allegations, plus costs and attorneys’ fees. In
his initial disclosures, Mr. Nelson alleges damages of $10,000,000.
On January 31, 2019, Mr. Nelson submitted an offer of judgement in
the amount of $100,000. That offer was rejected by the Corporation.
Court-ordered mediation was conducted on April 24, 2019, but the
matter was not resolved. By order dated February 4, 2020, the court
scheduled trial for October 5, 2020. The Corporation denies
liability as to all claims. Inasmuch as an unfavorable outcome is
neither probable nor remote within the meaning of the ABA Statement
of Policy referred to in the last paragraph of this letter, we
decline to express an opinion concerning the likely outcome of this
matter or the liability of the Corporation, if any, associated
therewith.
Note 10 – Commitments and Contingencies
(Continued)
Material Definitive Agreements
On May 1, 2018, the Company entered into a Fourth Addendum and
Fifth Addendum to that certain Purchase and Sale Agreement between
the Company and Greg DiPaolo’s Pro Am Golf, LLC, amending the
“Closing Date” under the Agreement to August 1, 2018,
in exchange for the Company paying $50,000 as a non-refundable
deposit to be applied against the purchase price once the property
sale is completed and $10,000 for maintenance, tree removal and
other grounds keeping in order to prepare the golf course for the
2018 season.
On July 23, 2018, the Company entered into a Sixth Addendum,
extending the “Closing Date” to November 1, 2018, in
exchange for the Company paying an additional $50,000 as a
non-refundable deposit to be applied against the purchase
price.
On July 1, 2019, the Greg DiPaolo’s Pro Am Golf, LLC property
was in foreclosure, and it was put up for auction. At the auction,
we were the winning bidder with a bid of $597,000. Our prior
deposit payment of $120,000 was credited towards the purchase
price, and the remaining $477,000, was finally due on November 30,
2019, after several extensions (which cost us total of $40,000 to
obtain). At the end of December 31, 2019, a total of $92,000 was
issued as a deposit for the property. We were not able to meet that
deadline, but in January 2020 we worked out an additional extension
with the bank. Under the terms of the new agreement, we still owe
approximately $392,000 to acquire the property. We have agreed to
pay that amount in installment payments of $10,000 per month for
six months beginning February 2020, with a balloon payment of
approximately $332,000 due on or before August 3, 2020. To date we
have paid the $10,000 payments for February 2020 and March 2020,
and plan to pay the April 2020 payment on time. We need to raise
funds in order to make the remaining scheduled
payments.
On May 21, 2018, the Company entered into a Trademark Purchase
Agreement with Copalix Pty Ltd., a private South African company,
to acquire U.S. Trademark Registration No. 4,927,872 for the WEED
TM mark, in exchange for USD$40,000.
On July 27, 2018, the Company entered into a Trademark Purchase
Agreement with Copalix Pty Ltd., to acquire European Community
Trademark Registration No. 11953387 for WEED Registered Mark in
exchange for USD$10,000.
Note 11 – Stockholders’ Equity
Preferred Stock
On December 5, 2014, the Company amended the Articles of
Incorporation, pursuant to which 20,000,000 shares of “blank
check” preferred stock with a par value of $0.001 were
authorized. No series of preferred stock has been designated to
date.
Common Stock
On December 5, 2014, the Company amended the Articles of
Incorporation, and increased the authorized shares to 200,000,000
shares of $0.001 par value common stock.
2019 Common Stock Activity
Common Stock Sales (2019)
During the year ended December 31, 2019, the Company issued
1,065,000 shares of common stock for proceeds of
$573,000.
Common Stock Issued for Services (2019)
During the year ended December 31, 2019, the Company agreed to
issue an aggregate of 2,467,000 shares of common stock to
consultants for services performed. The total fair value of common
stock was $2,578,250 based on the closing price of the
Company’s common stock earned on the measurement date. Shares
valued at $121,650 were issued during the year ended December 31,
2019 and services will be performed in 2020 and has been included
in unamortized stock-based compensation.
Common Stock Cancellations
During the year ended December 31, 2019, the Company cancelled a
total of 220,000 shares of common stock valued at $0 previously
granted to consultants, David Johnson, and Avigor Gordon, for
non-performance of services. The cancellation was accounted as a
repurchase for no consideration.
2018 Common Stock Activity
Common Stock Sales (2018)
During the year ended December 31, 2018, the Company issued
3,899,450 shares of common stock for proceeds of $4,798,550. In
connection with certain of the share issuances, the Company issued
warrants to purchase an aggregate of $1,927,500 shares of the
Company’s common stock. The warrants to purchase 462,500
shares have an exercise price of $5.00 per share, exercisable on
various dates through March 2019. Warrants to purchase 215,000
shares have an exercise price of $12.50 per share and are
exercisable on various dates through January 2020. The warrants to
purchase $1,250,000 shares have an exercise price of $6.00 per
share, exercisable on various dates through June 2019. The proceeds
received were allocated $3,361,832 to common stock and $1,436,718
to warrants on a relative fair value basis. On January 12, 2018, a
warrant holder exercised warrants to purchase 150,000 shares of
common stock at a price of $1.50 in exchange for proceeds of
$225,000.
Common Stock Issued for Services (2018)
During the year ended December 31, 2018, the Company agreed to
issue an aggregate of 915,000 shares of common stock to consultants
for services performed. The total fair value of common stock was
$3,042,940 based on the closing price of the Company’s common
stock earned on the measurement date. Shares valued at $200,400
were issued at December 31, 2018 and services will be performed in
2019 and has been included in unamortized stock-based
compensation.
Note 12 – Common Stock Warrants and Options
Common Stock Warrants Granted (2019)
No common stock warrants were granted during the year ended
December 31, 2019.
Common stock warrants granted consist of the following at December
31, 2019 and 2018, respectively:
|
2019
|
|
2018
|
|
Issuance
|
|
Warrant
|
|
|
|
# of
Common
|
|
Issuance
|
|
Warrant
|
|
|
|
# of
Common
|
|
Date
|
|
#
|
|
Name
|
|
Stock
Warrants
|
|
Date
|
|
#
|
|
Name
|
|
Stock
Warrants
|
|
|
|
|
|
|
|
|
|
1/5/2018
|
|
1029
|
|
Lex
Seabre
|
|
100,000.00
|
|
Total
|
|
|
|
|
|
-
|
|
1/21/2018
|
|
1031
|
|
Roger
Forsyth
|
|
100,000.00
|
|
|
|
|
|
|
|
|
|
1/23/2018
|
|
1032
|
|
Roger
Forsyth
|
|
100,000.00
|
|
|
|
|
|
|
|
|
|
2/9/2018
|
|
1033
|
|
Lawrence
Wesigal
|
|
15,000.00
|
|
|
|
|
|
|
|
|
|
3/19/2018
|
|
1034
|
|
Donald
Steinberg
|
|
150,000.00
|
|
|
|
|
|
|
|
|
|
3/15/2018
|
|
1035
|
|
Donald
Harrington
|
|
12,500.00
|
|
|
|
|
|
|
|
|
|
4/26/2018
|
|
1036
|
|
Roger
Seabre
|
|
100,000.00
|
|
|
|
|
|
|
|
|
|
4/26/2018
|
|
1037
|
|
Michael
Kirk Wines
|
|
100,000.00
|
|
|
|
|
|
|
|
|
|
5/7/2018
|
|
1038
|
|
Donald
Steinberg
|
|
400,000.00
|
|
|
|
|
|
|
|
|
|
5/15/2018
|
|
1039
|
|
Roger
Seabre
|
|
200,000.00
|
|
|
|
|
|
|
|
|
|
6/13/2018
|
|
1040
|
|
Blue
Ridge Enterprises
|
|
450,000.00
|
|
|
|
|
|
|
|
|
|
6/26/2018
|
|
1041
|
|
Dianna
Steinberg
|
|
200,000.00
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
1,927,500.00
|
Note 12 – Common Stock Warrants and Options
(Continued)
A summary of the Company’s outstanding common stock warrants
is as follows as of December 31, 2019:
|
Issuance
|
|
|
|
|
|
|
Date
|
#
|
Name
|
Document
|
|
|
|
12/31/2017
|
|
|
|
1,973,333
|
|
|
|
|
|
|
|
|
|
1/2/2018
|
1009
|
Exercise - Edward
Matkoff
|
Subscription
Agreement
|
-50,000
|
$ 3.00
|
|
1/5/2018
|
1029
|
Lex
Seabre
|
Subscription
Agreement
|
100,000
|
$ 5.00
|
|
1/21/2018
|
1031
|
Roger
Forsyth
|
Subscription
Agreement
|
100,000
|
$ 12.50
|
|
1/23/2018
|
1010
|
Expired - Sandra
Hogan
|
Subscription
Agreement
|
-2,000
|
$ 3.00
|
|
1/23/2018
|
1032
|
Roger
Forsyth
|
Subscription
Agreement
|
100,000
|
$ 12.50
|
|
2/9/2018
|
1033
|
Lawrence
Wesigal
|
Subscription
Agreement
|
15,000
|
$ 12.50
|
|
3/19/2018
|
1034
|
Donald
Steinberg
|
Subscription
Agreement
|
150,000
|
$ 5.00
|
|
3/15/2018
|
1035
|
Donald
Harrington
|
Subscription
Agreement
|
12,500
|
$ 5.00
|
|
4/20/2017
|
1015
|
Expired - Lex
Seabre
|
Subscription
Agreement
|
-375,000
|
$ 3.00
|
|
4/20/2017
|
1020
|
Expired - Lex
Seabre
|
Subscription
Agreement
|
-125,000
|
$ 3.00
|
|
4/26/2018
|
1036
|
Roger
Seabre
|
Subscription
Agreement
|
100,000
|
$ 5.00
|
|
4/26/2018
|
1037
|
Michael Kirk
Wines
|
Subscription
Agreement
|
100,000
|
$ 5.00
|
|
5/7/2018
|
1038
|
Donald
Steinberg
|
Subscription
Agreement
|
400,000
|
$ 6.00
|
|
5/15/2018
|
1039
|
Roger
Seabre
|
Subscription
Agreement
|
200,000
|
$ 6.00
|
|
6/13/2018
|
1040
|
Blue Ridge
Enterprises
|
Subscription
Agreement
|
450,000
|
$ 6.00
|
|
6/16/2017
|
1019
|
Expired - Black
Mountain Equities
|
Debt Exchange
Agreement
|
-70,000
|
$ 3.00
|
|
6/26/2018
|
1041
|
Dianna
Steinberg
|
Subscription
Agreement
|
200,000
|
$ 6.00
|
|
12/31/2018
|
|
|
|
3,278,833
|
|
|
1/5/2018
|
1029
|
Expired - Lex
Seabre
|
Subscription
Agreement
|
-100,000
|
$ 5.00
|
|
2/9/2018
|
1033
|
Expired - Lawrence
Wesigal
|
Subscription
Agreement
|
-15,000
|
$ 12.50
|
|
3/19/2018
|
1034
|
Expired - Donald
Steinberg
|
Subscription
Agreement
|
-150,000
|
$ 5.00
|
|
3/15/2018
|
1035
|
Expired - Donald
Harrington
|
Subscription
Agreement
|
-12,500
|
$ 5.00
|
|
4/26/2018
|
1036
|
Expired -Roger
Seabre
|
Subscription
Agreement
|
-100,000
|
$ 5.00
|
|
4/26/2018
|
1037
|
Expired -Michael
Kirk Wines
|
Subscription
Agreement
|
-100,000
|
$ 5.00
|
|
5/7/2018
|
1038
|
Expired -Donald
Steinberg
|
Subscription
Agreement
|
-400,000
|
$ 6.00
|
|
5/15/2018
|
1039
|
Expired -Roger
Seabre
|
Subscription
Agreement
|
-200,000
|
$ 6.00
|
|
6/13/2018
|
1040
|
Expired -Blue Ridge
Enterprises
|
Subscription
Agreement
|
-450,000
|
$ 6.00
|
|
6/26/2018
|
1041
|
Expired -Dianna
Steinberg
|
Subscription
Agreement
|
-200,000
|
$ 6.00
|
|
5/25/2017
|
1016
|
Expired -Russ
Karlen
|
Subscription
Agreement
|
-100,000
|
$ 3.00
|
|
5/25/2017
|
1017
|
Expired -Eric
Karlen
|
Subscription
Agreement
|
-20,000
|
$ 3.00
|
|
5/31/2017
|
1018
|
Expired -Matt
Turner
|
Subscription
Agreement
|
-20,000
|
$ 3.00
|
|
5/31/2017
|
1022
|
Expired -Rodger
Seabre
|
Subscription
Agreement
|
-300,000
|
$ 3.00
|
|
7/7/2017
|
1021
|
Expired - Rodger
Seabre
|
Subscription
Agreement
|
-200,000
|
$ 3.00
|
|
8/2/2017
|
1026
|
Expired - Rodger
Seabre
|
Subscription
Agreement
|
-100,000
|
$ 3.00
|
|
9/5/2017
|
1023
|
Expired - Harry
Methewson #1
|
Subscription
Agreement
|
-40,000
|
$ 3.00
|
|
9/24/2017
|
1024
|
Expired - Harry
Methewson #2
|
Subscription
Agreement
|
-133,000
|
$ 3.00
|
|
9/29/2017
|
1025
|
Expired - A2Z
Inc.
|
Subscription
Agreement
|
-300,000
|
$ 3.00
|
|
10/24/2017
|
1027
|
Expired - Salvatore
Rutigliano
|
Subscription
Agreement
|
-13,3333
|
$ 3.00
|
|
11/10/2017
|
1028
|
Expired - Roger
Seabre
|
Subscription
Agreement
|
-125,000
|
$ 3.00
|
|
12/31/2019
|
|
|
|
200,000
|
|
Common Stock Warrants Expired (2019)
A total of 3,078,833 warrants expired during the year ended
December 31, 2019.
Warrants Exercised (2019)
No warrants were exercised during the year ended December 31,
2019.
Note 12 – Common Stock Warrants and Options
(Continued)
2018 Common Stock Warrant Activity
Common Stock Warrants Granted (2018)
See Note 10 for details on warrants issued during the year ended
December 31, 2018.
Common Stock Warrants Exercised (2018)
On January 12, 2018, a warrant holder exercised warrants to
purchase 150,000 shares of common stock at a price of $1.50 in
exchange for proceeds of $225,000.
Common Stock Warrants Expired (2018)
A total of 572,000 warrants expired during the year ended December
31, 2018.
Common Stock Options (2018)
On February 1, 2018, in connection with executive employment
agreements, the Company granted non-qualified options to purchase
an aggregate of 6,000,000 shares of the Company’s common
stock at the exercise price of $10.55 per share. The options shall
become exercisable at the rate of 1/3 upon the six-month
anniversary, 1/3 upon the one-year anniversary and 1/3 upon the
second anniversary of the grant. The options expire ten years from
the date of grant. The options were valued at $45,753,000 using the
Black-Scholes option pricing model. The Company recognized expense
of approximately $22,770,662 and $21,201,397 relating to these
options during the year ended December 31, 2019 and December 31,
2018, respectively.
The assumptions used in the Black-Scholes model are as
follows:
|
|
|
For the period ended
December 31,2019
|
|
Risk-free
interest rate
|
|
1.75%
|
|
Expected
dividend yield
|
|
0%
|
|
Expected
lives
|
|
10.0
years
|
|
Expected
volatility
|
|
200%
|
A summary of the Company’s stock option activity and related
information is as follows:
|
|
For
the Year EndedDecember 31, 2019 and 2018
|
|
|
|
|
|
|
|
|
|
Outstanding at the
beginning of period
|
$ -
|
$ -
|
|
Granted
|
6,000,000
|
$ 10.55
|
|
Exercised/Expired/Cancelled
|
-
|
-
|
|
Outstanding at the
end of period
|
6,000,000
|
$ 10.55
|
|
Exercisable at the
end of period
|
1,250,000
|
$ 10.55
|
Note 13 – Income Tax
The Company accounts for income taxes under FASB ASC 740-10, which
requires use of the liability method. FASB ASC 740-10-25 provides
that deferred tax assets and liabilities are recorded based on the
differences between the tax bases of assets and liabilities and
their carrying amounts for financial reporting purposes, referred
to as temporary differences.
For the years ended December 31, 2019 and 2018, the Company
incurred a net operating loss and, accordingly, no provision for
income taxes has been recorded. In addition, no benefit for income
taxes has been recorded due to the uncertainty of the realization
of any tax assets. At December 31, 2019 and December 31, 2018, the
Company had approximately $10,786,000 and $8,179,000 of federal net
operating losses, respectively. The net operating loss carry
forwards, if not utilized, will begin to expire in
2031.
The components of the Company’s deferred tax asset are as
follows:
|
|
|
|
|
Deferred tax
assets:
|
|
|
|
Net operating loss
carry forwards
|
59,276,171
|
31,276,171
|
|
Net deferred tax
assets before valuation allowance
|
59,276,171
|
31,276,171
|
|
Less: Valuation
allowance
|
(59,276,171)
|
(31,276,171)
|
|
Net deferred tax
assets
|
-
|
-
|
The 2017 Act reduces the corporate tax rate from 34% to 21% for tax
years beginning after December 31, 2017.
Based on the available objective evidence, including the
Company’s history of losses, management believes it is more
likely than not that the net deferred tax assets will not be fully
realizable. Accordingly, the Company provided for a full valuation
allowance against its net deferred tax assets at December 31, 2019
and 2018, respectively.
Note 14 – Subsequent Events
Common Stock Sales
On January 29, 2020, the Company sold 50,000 shares of common stock
in exchange for total proceeds of $15,000.
On February 18, 2020, the Company sold 100,000 shares of common
stock in exchange for total proceeds of $25,000.
On February 18, 2020, the Company sold 100,000 shares of common
stock in exchange for total proceeds of $15,000.
Common Stock Issued for Services
On January 29, 2020, the Company issued 200,000 shares to the
Robert Wolkin in exchange for services rendered to the
Company.
On January 29, 2020, the Company issued 600,000 shares to the Craig
Butler in exchange for services rendered to the
Company.
On January 29, 2020, the Company issued 100,000 shares to the Soul
Singh in exchange for services rendered to the
Company.
On January 29, 2020, the Company issued 30,000 shares to the
Beverly Weiss in exchange for services rendered to the
Company.
On February 18, 2020, the Company issued 100,000 shares to the Sal
Rutigliano in exchange for services rendered to the
Company.
On February 18, 2020, the Company issued 100,000 shares to the
Eilers Law Group in exchange for services rendered to the
Company.
On February 18, 2020, the Company issued 24,000 shares to the
Advance CFO Solutions in exchange for services rendered to the
Company.
On February 18, 2020, the Company issued 44,800 shares to the
Insight Performance Consulting in exchange for services rendered to
the Company.
On February 18, 2020, the Company issued 11,200 shares to the JB
Henriksen in exchange for services rendered to the
Company.
The Company entered into Memorandum of Sale agreement for the Sugar
Hill property with M&T Bank and the Referee to make payment of
$10,000 per month commencing on February 1, 2020 and continuing on
the 1st of
each month until July 1, 2020 with a balloon payment of $332,167.73
on August 3, 2020.
|
WEED, INC.
|
|
|
|
CONSOLIDATED BALANCE
SHEETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ASSETS
|
|
|
|
CURRENT
ASSETS:
|
|
|
|
Cash
|
$ 166,463
|
$ 2,509
|
|
Accounts
Receivable
|
822
|
822
|
|
Prepaid
expenses
|
33,340
|
30,979
|
|
Deposits
|
182,000
|
92,000
|
|
|
|
|
|
TOTAL CURRENT
ASSETS
|
382,625
|
126,310
|
|
|
|
|
|
Land
|
124,708
|
136,400
|
|
|
|
|
|
Building
|
1,759,292
|
1,887,802
|
|
Computers &
Equipment
|
73,681
|
73,681
|
|
Leasehold
improvements
|
5,000
|
5,000
|
|
|
1,962,681
|
2,102,883
|
|
|
|
|
|
Less: Accumulated
depreciation
|
(403,486)
|
(322,498)
|
|
|
|
|
|
Property and equipment,
net
|
1,559,195
|
1,780,385
|
|
|
|
|
|
Trademark
|
50,000
|
50,000
|
|
Less: Accumulated
amortization
|
(6,033)
|
(4,083)
|
|
Trademark,
net
|
43,967
|
45,917
|
|
|
|
|
|
TOTAL
ASSETS
|
$ 1,985,787
|
$ 1,952,612
|
|
|
|
|
|
LIABILITIES AND
STOCKHOLDERS’ EQUITY
|
|
|
|
|
|
|
|
CURRENT
LIABILITIES
|
|
|
|
Accounts
payable
|
$ 264,355
|
$ 212,181
|
|
Accrued
expense
|
15,500
|
10,000
|
|
Accrued officer
compensation
|
238,750
|
122,250
|
|
Accrued
interest
|
26,884
|
16,453
|
|
Notes payable, related
parties
|
287,200
|
224,100
|
|
Notes
payable
|
149,187
|
167,263
|
|
Due to
officer
|
723
|
723
|
|
|
|
|
|
TOTAL CURRENT
LIABILITIES
|
982,599
|
752,970
|
|
|
|
|
|
TOTAL
LIABILITIES
|
982,599
|
752,970
|
|
|
|
|
|
STOCKHOLDERS’
EQUITY
|
|
|
|
Common stock, $0.001
par value, 200,000,000 authorized, 112,672,685 and 109,262,685
issued and outstanding, respectively
|
112,673
|
109,263
|
|
Additional paid-in
capital
|
80,247,358
|
76,660,712
|
|
Subscription
payable
|
425,250
|
356,250
|
|
Accumulated
deficit
|
(79,781,455)
|
(75,925,974)
|
|
Accumulated other
comprehensive loss:
|
|
|
|
Foreign currency
translation
|
(638)
|
(609)
|
|
|
|
|
|
TOTAL
STOCKHOLDERS’ EQUITY
|
1,003,188
|
1,199,642
|
|
|
|
|
|
TOTAL LIABILITIES &
STOCKERHOLDERS’ EQUITY
|
$ 1,985,787
|
$ 1,952,612
|
The accompanying
notes are an integral part of the condensed consolidated financial
statements
|
WEED, INC.
|
|
|
|
CONSOLIDATED STATEMENTS OF OPERATIONS
AND COMPREHENSIVE INCOME
|
|
(UNAUDITED)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
REVENUE
|
$ -
|
-
|
|
|
|
|
|
|
|
|
|
OPERATING
EXPENSES
|
|
|
|
|
|
General and
administrative expenses
|
75,550
|
80,249
|
224,233
|
390,937
|
|
Professional
fees
|
109,927
|
6,065,896
|
3,540,739
|
23,979,599
|
|
Depreciation &
amortization
|
29,775
|
40,756
|
106,498
|
122,172
|
|
|
|
|
|
|
|
Total operating
expenses
|
215,252
|
6,186,901
|
3,871,470
|
24,492,708
|
|
|
|
|
|
|
|
NET OPERATING
LOSS
|
(215,252)
|
(6,186,901)
|
(3,871,470)
|
(24,492,708)
|
|
|
|
|
|
|
|
OTHER INCOME
(EXPENSE)
|
|
|
|
|
|
Interest
income
|
-
|
-
|
-
|
-
|
|
Interest
expense
|
(8,329)
|
(3,225)
|
(30,959)
|
(5,614)
|
|
Other
income
|
-
|
-
|
-
|
1,017
|
|
Loss on
deposit
|
-
|
(100,000)
|
-
|
(100,000)
|
|
Loss on extinguishment
of debt
|
-
|
-
|
-
|
-
|
|
Gain on Sale of Fixed
Asset
|
46,948
|
-
|
46,948
|
-
|
|
Other
expense
|
-
|
-
|
-
|
(1,956)
|
|
|
|
|
|
|
|
TOTAL OTHER EXPENSE,
NET
|
38,619
|
(103,225)
|
15,989
|
(106,553)
|
|
|
|
|
|
|
|
NET LOSS
|
$ (176,633)
|
(6,290,126)
|
(3,855,481)
|
(24,599,261)
|
|
|
|
|
|
|
|
OTHER COMPREHENSIVE
LOSS
|
(13)
|
(25)
|
(638)
|
(546)
|
|
|
|
|
|
|
|
COMPREHENSIVE
LOSS
|
(176,646)
|
(6,290,151)
|
(3,856,119)
|
(24,599,807)
|
|
|
|
|
|
|
|
WEIGHTED AVERAGE NUMBER
OF COMMON SHARES
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding - basic and
fully diluted
|
113,792,468
|
107,659,860
|
111,874,656
|
107,168,557
|
|
|
|
|
|
|
|
Net loss per share -
basic and fully diluted
|
$ (0.0016)
|
(0.06)
|
(0.03)
|
(0.23)
|
The accompanying
notes are an integral part of the condensed consolidated financial
statements
|
WEED, INC.
|
|
|
|
CONSOLIDATED AND COMBINED STATEMENTS
OF CHANGES IN STOCKHOLDERS’ EQUITY
(DEFICIT)
|
|
|
|
For the Nine Months Ended September
30, 2020
|
|
(UNAUDITED)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31,
2019
|
109,262,685
|
109,263
|
76,660,712
|
356,250
|
(75,925,974)
|
(609.00)
|
1,199,642
|
|
|
|
|
|
|
|
|
|
|
Common stock sold for
cash
|
150,000
|
150
|
39,850
|
55,000
|
-
|
-
|
95,000
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for
services
|
1,310,000
|
1,310
|
906,390
|
-
|
-
|
-
|
907,700
|
|
|
|
|
|
|
|
|
|
|
Vesting of employee
stock options
|
-
|
-
|
2,015,911
|
-
|
-
|
-
|
2,015,911
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
-
|
-
|
-
|
-
|
(3,104,335)
|
-
|
(3,104,335)
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive
income, net
|
-
|
-
|
-
|
-
|
-
|
(114)
|
(114)
|
|
|
|
|
|
|
|
|
|
|
Balance, March 31, 2020
|
110,722,685
|
110,723
|
79,622,863
|
411,250
|
(79,030,309)
|
(723)
|
1,113,804
|
|
|
|
|
|
|
|
|
|
|
Common stock sold for
cash
|
200,000
|
200
|
54,800
|
(15,000)
|
-
|
-
|
40,000
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for
services
|
1,000,000
|
1,000
|
399,000
|
-
|
-
|
-
|
400,000
|
|
|
|
|
|
|
|
|
|
|
Imputed Interest on RP
Loans
|
-
|
-
|
12,198
|
-
|
-
|
-
|
12,198
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
-
|
-
|
-
|
-
|
(574,513)
|
-
|
(574,513)
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive
income, net
|
-
|
-
|
-
|
-
|
-
|
98
|
98
|
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2020
|
111,922,685
|
111,923
|
80,088,861
|
396,250
|
(79,604,822)
|
(625)
|
991,587
|
|
|
|
|
|
|
|
|
|
|
Common stock sold for
cash
|
500,000
|
500
|
99,500
|
-
|
-
|
-
|
100,000
|
|
|
|
|
|
|
|
|
|
|
Common stock
returned
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for
services
|
250,000
|
250
|
57,250
|
29,000
|
-
|
-
|
86,500
|
|
|
|
|
|
|
|
|
|
|
Vesting of employee
stock options
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
|
|
|
|
|
|
|
|
|
Vesting of employee
stock comp
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
|
|
|
|
|
|
|
|
|
Audit
Adjustment
|
-
|
-
|
1,747
|
-
|
-
|
-
|
1,747
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
-
|
-
|
-
|
-
|
(176,633)
|
-
|
(176,633)
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive
income, net
|
-
|
-
|
-
|
-
|
-
|
(13)
|
(13)
|
|
|
|
|
|
|
|
|
|
|
Balance, September 30, 2020
|
112,672,685
|
112,673
|
80,247,358
|
425,250
|
(79,781,455)
|
(638)
|
1,003,188
|
The accompanying
notes are an integral part of the condensed consolidated financial
statements
|
WEED, INC.
|
|
|
|
CONSOLIDATED AND COMBINED STATEMENTS
OF CASH FLOWS
|
|
|
|
For the Nine Months Ended September
30, 2020 and September 30, 2019
|
|
(UNAUDITED)
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM
OPERATING ACTIVITIES
|
|
|
|
|
|
|
|
Net loss
|
$ (3,855,481)
|
$ (24,599,261)
|
|
|
|
|
|
Adjustments to
reconcile net loss used in operating
activities:
|
|
|
|
Depreciation and
amortization
|
106,498
|
122,172
|
|
Gain on sale of fixed
asset
|
(46,948)
|
-
|
|
Gain on settlement of
debt
|
-
|
-
|
|
Loss on
deposit
|
-
|
-
|
|
Impairment of
CIP
|
-
|
-
|
|
Imputed Interest on RP
Loans
|
13,945
|
-
|
|
Estimated fair value of
stock based compensation
|
2,044,911
|
21,209,062
|
|
Estimated fair value of
shares issued for services
|
1,365,200
|
1,909,929
|
|
Loss on debt
extinguishment
|
-
|
-
|
|
Decrease (increase) in
assets
|
|
|
|
Accounts
Receivable
|
-
|
(801)
|
|
Prepaid expenses and
other assets
|
(92,361)
|
305,707
|
|
Increase (decrease) in
liabilities
|
|
|
|
Accounts
Payable
|
52,174
|
(91,452)
|
|
Accrued
expenses
|
132,431
|
92,365
|
|
|
|
|
|
NET CASH USED IN
OPERATING ACTIVITIES
|
(279,631)
|
(1,052,279)
|
|
|
|
|
|
CASH FLOWS FROM
INVESTING ACTIVITIES
|
|
|
|
Purchases of property
and equipment
|
-
|
(2,979)
|
|
Purchase of intangible
assets
|
-
|
-
|
|
Procceds from sale of
fixed asset
|
163,590
|
-
|
|
|
|
|
|
NET CASH USED IN
INVESTING ACTIVITIES
|
163,590
|
(2,979)
|
|
|
|
|
|
CASH FLOWS FROM
FINANCING ACTIVITIES
|
|
|
|
|
|
|
|
Stock
Payable
|
40,000
|
-
|
|
Proceeds from notes
payable - related party
|
63,100
|
268,823
|
|
Proceeds from the sale
of common stock
|
195,000
|
498,001
|
|
Proceeds on notes
payable
|
10,200
|
230,812
|
|
Repayments on notes
payable
|
(28,276)
|
-
|
|
|
|
|
|
NET CASH PROVIDED BY
FINANCING ACTIVITIES
|
280,024
|
997,636
|
|
|
|
|
|
NET CHANGE IN
CASH
|
163,983
|
(57,622)
|
|
|
|
|
|
EFFECT OF EXCHANGE RATE
ON CASH
|
(29)
|
(546)
|
|
|
|
|
|
CASH, BEGINNING OF
PERIOD
|
2,509
|
70,608
|
|
|
|
|
|
CASH, END OF
PERIOD
|
$ 166,463
|
$ 12,440
|
|
|
|
|
|
SUPPLEMENTAL DISCLOSURE
OF CASH FLOW INFORMATION
|
|
|
|
|
|
|
|
Cash paid during the
year ended December 31:
|
|
|
|
|
|
|
|
Income
taxes
|
$ -
|
$ -
|
|
Interest
paid
|
$ -
|
$ -
|
|
|
|
|
|
Non-cash investing and
financing activities:
|
|
|
|
|
|
|
|
Shares issued from
subscription payable
|
$ 40,000
|
$ -
|
The accompanying
notes are an integral part of the condensed consolidated financial
statements
WEED, INC.
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
September 30, 2020
Note 1 – Nature of Business and
Significant Accounting Policies
Nature of Business
WEED, Inc. (the
“Company”), (formerly United Mines, Inc.) was
incorporated under the laws of the State of Arizona on August 20,
1999 (“Inception Date”) as Plae, Inc. to engage in the
exploration of gold and silver mining properties. On November 26,
2014, the Company was renamed from United Mines, Inc. to WEED, Inc.
and was repurposed to pursue a business involving the purchase of
land, and building Commercial Grade “Cultivation
Centers” to consult, assist, manage & lease to Licensed
Dispensary owners and organic grow operators on a contract basis,
with a concentration on the legal and medical marijuana sector. The
Company’s plan is to become a True “Seed-to-Sale”
company providing infrastructure, financial solutions and real
estate options in this new emerging market. The Company, under
United Mines, was formerly in the process of acquiring mineral
properties or claims located in the State of Arizona, USA. The name
was previously changed on February 18, 2005 to King Mines, Inc. and
then subsequently changed to United Mines, Inc. on March 30, 2005.
The Company trades on the OTC Pink Sheets under the stock symbol:
BUDZ.
On April 20, 2017,
the Company acquired Sangre AT, LLC, a Wyoming company doing
business as Sangre AgroTech (“Sangre”). Sangre is a
plant genomic research and breeding company comprised of
top-echelon scientists with extensive expertise in genomic
sequencing, genetics-based breeding, plant tissue culture, and
plant biochemistry, utilizing the most advanced sequencing and
analytical technologies and proprietary bioinformatics data systems
available. No work is being conducted now until further funds are
available.
The accompanying
financial statements have been prepared in conformity with
accounting principles generally accepted in the United States of
America. These statements reflect all adjustments, consisting of
normal recurring adjustments, which in the opinion of management
are necessary for fair presentation of the information contained
therein.
The Company has a
calendar year end for reporting purposes.
Basis of
Presentation:
The accompanying
condensed consolidated balance sheet at December 31, 2019, has been
derived from audited consolidated financial statements and the
unaudited condensed consolidated financial statements as of
September 30, 2020 and 2019 ( the “financial
statements”), have been prepared in accordance with
accounting principles generally accepted in the United States of
America (“GAAP”) for interim financial information and
with the instructions to Form 10-Q and Article 8 of Regulation S-X.
Accordingly, they do not include all of the information and
footnotes required by GAAP for complete financial statements and
should be read in conjunction with the audited consolidated
financial statements and related footnotes included in our
Registration Statement on Form S-1 for the year ended December 31,
2019 (the “2019 Annual Report”), filed with the
Securities and Exchange Commission (the “SEC”). It is
management’s opinion, however, that all material adjustments
(consisting of normal recurring adjustments), have been made which
are necessary for a fair financial statements presentation. The
condensed consolidated financial statements include all material
adjustments (consisting of normal recurring accruals) necessary to
make the condensed consolidated financial statements not misleading
as required by Regulation S-X, Rule 10-01.
Principles of
Consolidation
The accompanying
consolidated financial statements include the accounts of the
following entities, all of which are under common control and
ownership:
|
|
|
State of
|
|
|
|
Abbreviated
|
|
Name of
Entity
|
|
Incorporation
|
|
Relationship
(1)
|
|
Reference
|
|
WEED, Inc.
|
|
Nevada
|
|
Parent
|
|
WEED
|
|
Sangre AT, LLC
(2)
|
|
Wyoming
|
|
Subsidiary
|
|
Sangre
|
|
|
(1)
|
Sangre is a
wholly-owned subsidiary of WEED, Inc.
|
|
|
(2)
|
Sangre AT, LLC is
doing business as Sangre AgroTech.
|
Note 1 – Nature of Business and
Significant Accounting Policies (continued)
The consolidated
financial statements herein contain the operations of the
wholly-owned subsidiary listed above. All significant inter-company
transactions have been eliminated in the preparation of these
financial statements. The parent company, WEED and subsidiary,
Sangre will be collectively referred to herein as the
“Company”, or “WEED”. The Company’s
headquarters are located in Tucson, Arizona and its operations are
primarily within the United States, with minimal operations in
Australia.
These statements
reflect all adjustments, consisting of normal recurring
adjustments, which in the opinion of management are necessary for
fair presentation of the information contained
therein.
Use of Estimates
The preparation of
financial statements in conformity with accounting principles
generally accepted in the United States requires management to make
estimates and assumptions that affect the reported amounts of
assets and liabilities, and the disclosure of contingent assets and
liabilities at the date of the financial statements, and the
reported amounts of revenues and expenses during the reporting
period. Actual results could differ from those
estimates.
Fair Value of Financial
Instruments
Under FASB ASC
820-10-05, the Financial Accounting Standards Board establishes a
framework for measuring fair value in generally accepted accounting
principles and expands disclosures about fair value measurements.
This Statement reaffirms that fair value is the relevant
measurement attribute. The adoption of this standard did not have a
material effect on the Company’s financial statements as
reflected herein. The carrying amounts of cash, prepaid expenses
and accrued expenses reported on the balance sheet are estimated by
management to approximate fair value primarily due to the short
term nature of the instruments.
Impairment of Long-Lived
Assets
Long-lived assets
held and used by the Company are reviewed for possible impairment
whenever events or circumstances indicate the carrying amount of an
asset may not be recoverable or is impaired. Recoverability is
assessed using undiscounted cash flows based upon historical
results and current projections of earnings before interest and
taxes. Impairment is measured using discounted cash flows of future
operating results based upon a rate that corresponds to the cost of
capital. Impairments are recognized in operating results to the
extent that carrying value exceeds discounted cash flows of future
operations.
Basic and Diluted Loss Per
Share
The basic net loss
per common share is computed by dividing the net loss by the
weighted average number of common shares outstanding. Diluted net
loss per common share is computed by dividing the net loss adjusted
on an “as if converted” basis, by the weighted average
number of common shares outstanding plus potential dilutive
securities. For the periods presented, potential dilutive
securities had an anti-dilutive effect and were not included in the
calculation of diluted net loss per common share.
Stock-Based
Compensation
Under FASB ASC
718-10-30-2, all share-based payments to employees, including
grants of employee stock options, to be recognized in the income
statement based on their fair values. Pro forma disclosure is no
longer an alternative.
Revenue
Recognition
On January 1, 2018,
the Company adopted the new revenue recognition standard ASU
2014-09, “Revenue from Contracts with Customers (Topic
606)”, using the cumulative effect (modified retrospective)
approach. Modified retrospective adoption requires entities to
apply the standard retrospectively to the most current period
presented in the financial statements, requiring the cumulative
effect of the retrospective application as an adjustment to the
opening balance of retained earnings at the date of initial
application. No cumulative-effect adjustment in retained earnings
was recorded as the Company’s has no historical revenue. The
impact of the adoption of the new standard was not material to the
Company’s condensed consolidated financial statements for the
three and nine months ended September 30, 2020. The Company expects
the impact to be immaterial on an ongoing basis.
The primary change
under the new guidance is the requirement to report the allowance
for uncollectible accounts as a reduction in net revenue as opposed
to bad debt expense, a component of operating expenses. The
adoption of this guidance did not have an impact on our condensed
consolidated financial statements, other than additional financial
statement disclosures. The guidance requires increased disclosures,
including qualitative and quantitative disclosures about the
nature, amount, timing and uncertainty of revenue and cash flows
arising from contracts with customers.
Note 1 – Nature of Business and
Significant Accounting Policies (continued)
The Company
operates as one reportable segment.
Sales on fixed
price contracts are recorded when services are earned, the earnings
process is complete or substantially complete, and the revenue is
measurable and collectability is reasonably assured. Provisions for
discounts and rebates to customers, estimated returns and
allowances, and other adjustments are provided for in the same
period the related sales are recorded. The Company will defer any
revenue from sales in which payment has been received, but the
earnings process has not occurred. Sales have not yet
commenced.
Advertising and
Promotion
All costs
associated with advertising and promoting products are expensed as
incurred. These expenses were $0 and $3,450 for the nine months
ended September 30, 2020 and 2019.
Recently Issued Accounting
Pronouncements
In August 2018, the
FASB issued ASU 2018-15 Accounting for Implementation Costs
Related to Cloud Computing or Hosting Arrangements. This standard
provides authoritative guidance intended to address a
customer’s accounting for implementation costs incurred in a
cloud computing arrangement that is a service contract. This
guidance aligns the requirements for capitalizing implementation
costs incurred in a hosting arrangement that is a service contract
with the requirements for capitalizing implementation costs
incurred to develop or obtain internal-use software. The guidance
also requires presentation of the capitalized implementation costs
in the statement of financial position and in the statement of cash
flows in the same line item that a prepayment for the fees of the
associated hosting arrangement would be presented, and the expense
related to the capitalized implementation costs to be presented in
the same line item in the statement of operations as the fees
associated with the hosting element (service) of the arrangement.
This guidance is effective for annual periods beginning after
December 15, 2019, including interim periods within those
annual periods, with early adoption permitted. We are currently
evaluating the potential impact on our financial position, results
of operations and statement of cash flows upon adoption of this
guidance. We do not expect this guidance to have a significant
impact, or potential significant impact, to our unaudited condensed
consolidated interim financial statements.
In February 2016,
the FASB issued ASU 2016-02, Leases. The standard requires lessees
to recognize lease assets and lease liabilities on the consolidated
balance sheet and requires expanded disclosures about leasing
arrangements. We adopted the standard, effective January 1, 2019,
and the new standard has no material impact on our consolidated
financial statements. We are currently assessing the impact that
the new standard will have on our consolidated financial
statements, which will consist primarily of a balance sheet gross
up of our operating leases to show equal and offsetting lease
assets and lease liabilities.
The Company adopted
the new lease guidance effective January 1, 2019 using
the modified retrospective transition approach, applying the
new standard to all of its leases existing at the date of initial
application which is the effective date of
adoption. Consequently, financial information will not be
updated and the disclosures required under the new standard will
not be provided for dates and periods before January 1,
2019. We elected the package of practical expedients which
permits us to not reassess (1) whether any expired or existing
contracts are or contain leases, (2) the lease classification for
any expired or existing leases, and (3) any initial direct costs
for any existing leases as of the effective date. We did not elect
the hindsight practical expedient which permits entities to use
hindsight in determining the lease term and assessing impairment.
The adoption of the lease standard did not change our previously
reported consolidated statements of operations and did not result
in a cumulative catch-up adjustment to opening equity. As of
September 30, 2020, the adoption of the standard had no impact on
the Company, as there were no leases in place longer than 12
months.
In June 2018, the
FASB issued Accounting Standards Update (“ASU”)
2018-07, Compensation – Stock Compensation (Topic 718)
Improvements to Nonemployee Share-Based Payment Accounting. This
ASU expands the scope of Topic 718 to include share-based payment
transactions for acquiring goods and services from nonemployees.
The amendments in this ASU became effective beginning January 1,
2019, and it does not have a material effect on our consolidated
financial statements.
Note 2 – Going
Concern
As shown in the
accompanying financial statements, the Company has no revenues,
incurred net losses from operations resulting in an accumulated
deficit of $79,781,455 and negative working capital of $599,974 at
September 30, 2020. These factors raise substantial doubt about the
Company’s ability to continue as a going concern. Management
is actively pursuing new products and services to begin generating
revenues. In addition, the Company is currently seeking additional
sources of capital to fund short term operations. The Company,
however, is dependent upon its ability to secure equity and/or debt
financing and there are no assurances that the Company will be
successful; therefore, without sufficient financing it would be
unlikely for the Company to continue as a going
concern.
The financial
statements do not include any adjustments that might result from
the outcome of any uncertainty as to the Company’s ability to
continue as a going concern. The financial statements also do not
include any adjustments relating to the recoverability and
classification of recorded asset amounts or amounts and
classifications of liabilities that might be necessary should the
Company be unable to continue as a going concern.
Note 3 – Related
Party
Notes Payable
From time to time,
the Company has received short term loans from officers and
directors as disclosed in Note 7 below. The Company has a total of
$287,200 and $224,100 of note payable on the consolidated balance
sheet as of September 30, 2020 and December 31, 2019, respectively.
On October 28, 2019, the Company transferred the ownership of the
2017 Audi Q7 and Audi A4, valued at $46,609, to Nicole Breen as a
repayment for her loans. $93,000 was recorded as a forgiveness on
notes payable, and $46,391 recorded to additional paid-in capital.
In March 2020, the Company received $22,000 loan from Nicole Breen,
and from April 1, 2020 to June 30, 2020, the Company received an
additional $37,500 loan from Nicole Breen. From August 1, 2020 to
September 30, 2020, the Company received $3,600 loan from Nicole
Breen.
Services
Nicole M. Breen
receives $1,500 a week in cash compensation for her services
rendered to the Company.
Glenn E. Martin
receives $8,000 a month in cash compensation for his services
rendered to the Company.
Capital
Contributions
The Company imputed
interest on non-interest bearing, related party loans, resulting in
a total of $0 and $0 of contributed capital during the nine months
ended September 30, 2020 and 2019, respectively.
Common Stock Issued for Bartered
Assets
On January 18,
2017, the Company exchanged 66,000 units, consisting of 66,000
shares of common stock and warrants to purchase 66,000 shares of
common stock at an exercise price of $3.00 per share, exercisable
until January 18, 2018, in exchange for a 2017 Audi Q7 and a 2017
Audi A4 driven by the Officers. The total fair value received,
based on the market price of the stock at $4.02 per share, was
allocated to the $105,132 purchase price of the vehicles and the
$160,188 excess value of the common stock and warrants was expensed
as stock-based compensation. On October 28, 2019, the Company
transferred the ownership of the two Audi vehicles, valued at
$46,609, to Nicole Breen as a repayment for her loans. $93,000 was
recorded as a forgiveness on notes payable, and $46,391 recorded to
additional paid-in capital.
Common Stock
On August 1, 2017,
the Company granted 150,000 shares of common stock to Mary
Williams, a principal of Sangre AT, LLC, for services performed.
The fair value of the common stock was $154,500 based on the
closing price of the Company’s common stock on the date of
grant.
On January 7, 2017,
the Company granted 50,000 shares of common stock to Pat Williams.
PhD, a principal of Sangre AT, LLC, for services performed. The
total fair value of the common stock was $210,250 based on the
closing price of the Company’s common stock on the date of
grant.
A total of $238,750
and $122,250 of officer compensation was unpaid and outstanding at
September 30, 2020 and 2019, respectively.
Stock Options Issued for Services
– related party
On February 1,
2018, in connection with executive employment agreements, the
Company granted non-qualified options to purchase an aggregate of
6,000,000 shares of the Company’s common stock at the
exercise price of $10.55 per share. The options shall become
exercisable at the rate of 1/3 upon the six-month anniversary, 1/3
upon the one-year anniversary and 1/3 upon the second anniversary
of the grant. The options were valued at $45,987,970 using the
Black-Scholes option pricing model. The Company recognized expense
of approximately, $2,015,911 relating to these options during the
nine months ended September 30, 2020.
Note 4 – Fair Value of Financial
Instruments
Under FASB ASC
820-10-5, fair value is defined as the price that would be received
to sell an asset or paid to transfer a liability in an orderly
transaction between market participants at the measurement date (an
exit price). The standard outlines a valuation framework and
creates a fair value hierarchy in order to increase the consistency
and comparability of fair value measurements and the related
disclosures. Under GAAP, certain assets and liabilities must be
measured at fair value, and FASB ASC 820-10-50 details the
disclosures that are required for items measured at fair
value.
Note 4 – Fair Value of Financial
Instruments (continued)
The Company has
certain financial instruments that must be measured under the new
fair value standard. The Company’s financial assets and
liabilities are measured using inputs from the three levels of the
fair value hierarchy. The three levels are as follows:
Level 1 - Inputs
are unadjusted quoted prices in active markets for identical assets
or liabilities that the Company has the ability to access at the
measurement date.
Level 2 - Inputs
include quoted prices for similar assets and liabilities in active
markets, quoted prices for identical or similar assets or
liabilities in markets that are not active, inputs other than
quoted prices that are observable for the asset or liability (e.g.,
interest rates, yield curves, etc.), and inputs that are derived
principally from or corroborated by observable market data by
correlation or other means (market corroborated
inputs).
Level 3 -
Unobservable inputs that reflect our assumptions about the
assumptions that market participants would use in pricing the asset
or liability.
The following
schedule summarizes the valuation of financial instruments at fair
value on a recurring basis in the balance sheets as of September
30, 2020 and 2019, respectively:
Fair
Value Measurements at December 31, 2019
|
|
|
|
|
|
Assets
|
|
|
|
|
Cash
|
$ 2,509
|
$ -
|
$ -
|
|
Total
assets
|
$ 2,509
|
$ -
|
$ -
|
|
Liabilities
|
|
|
|
|
Notes payable, related
parties
|
|
$ 224,100
|
|
|
Notes
payable
|
$ -
|
$ 167,263
|
$ -
|
|
Total
liabilities
|
$ -
|
$ 391,363
|
$ -
|
|
|
$ 2,509
|
$ 391,363
|
$ -
|
Fair Value Measurements at September 30,
2020
|
|
|
|
|
|
Assets
|
|
|
|
|
Cash
|
$ 166,463
|
$ -
|
$ -
|
|
Total
assets
|
166,463
|
$ -
|
$ -
|
|
Liabilities
|
|
|
|
|
Notes payable, related
parties
|
|
$ 287,200
|
|
|
Notes
payable
|
$ -
|
$ 149,187
|
$ -
|
|
Total
liabilities
|
$ -
|
$ 436,387
|
$ -
|
|
|
$ 166,463
|
$ 436,387
|
$ -
|
The fair values of
our related party debts are deemed to approximate book value and
are considered Level 2 inputs as defined by ASC Topic
820-10-35.
There were no
transfers of financial assets or liabilities between Level 1, Level
2 and Level 3 inputs for the nine months ended September 30, 2020
and the year ended December 31, 2019.
Note 5 – Investment in Land and
Property
On July 26, 2017,
the Company closed on the purchase of property, consisting of a
home, recreational facility and RV park located at 5535 State
Highway 12 in La Veta, Colorado to be developed into a bioscience
center. The home has 4 Bedrooms and 2 Baths, and the recreational
facility has showers, laundry, and reception area with an
additional equipment barn attached, in addition to another facility
with 9,500 square feet. The RV Park has 24 sites with full hook-ups
including water, sewer, and electric, which the Company plans to
convert into a series of small research pods. Under the terms of
the purchase agreement, the Company paid $525,000 down, including
25,000 shares of our common stock, and Sangre took immediate
possession of the property. Under the terms of the original
purchase agreement, the Company was obligated to pay an additional
$400,000 in cash and issue an additional 75,000 shares of our
common stock over the next two years in order to pay the entire
purchase price. On January 12, 2018, the Company entered into an
Amendment No. 1 to the $475,000 principal amount promissory note
issued by the Company to the seller of the property, under which
both parties agreed to amend the purchase and the promissory note
to allow the Company to pay off the note in full if it paid
$100,000 in cash on or before January 15, 2018 and issued the
seller 125,000 shares of common stock, restricted in accordance
with Rule 144, on before January 20, 2018. Through an escrow
process, the Company paid the seller $100,000 in cash and issued
him 125,000 shares of common stock in accordance with the Amendment
No. 1, in exchange for a full release of the deed of trust that was
securing the promissory note, on January 17, 2018. As a result, the
$475,000 principal promissory note issued to the seller was deemed
paid-in-full and fully satisfied and the Company owned the property
without encumbrances as of that date. The Company recorded a loss
on extinguishment of debt of approximately $1,065,000 based on the
fair value of the consideration paid and the carrying value of the
note payable on the settlement date. The total purchase price was
as follows:
|
|
|
|
Consideration:
|
|
|
Common stock payment of
25,000 shares (1)
|
$ 30,000
|
|
Cash payment of down
payment
|
50,000
|
|
Cash paid at
closing
|
44,640
|
|
Short term liabilities
assumed and paid at closing (2)
|
5,360
|
|
Note payable
(3)
|
475,000
|
|
Total purchase price
|
$ 1,005,000
|
|
|
(1)
|
Consideration
consisted of an advance payment of 25,000 shares of the
Company’s common stock valued at $30,000 based on the closing
price of the Company’s common stock on the July 18, 2017 date
of grant.
|
|
|
(2)
|
Purchaser’s
shares of closing costs, including the seller’s prepaid
property taxes.
|
|
|
(3)
|
As noted above, the
note was settled with a payment of $100,000 and the issuance of
125,000 shares of common stock.
|
In January 2018,
the Company closed on the purchase of property, consisting of a
condominium in La Veta, Colorado to house Company personnel and
consultants for total consideration approximating $140,000, which
was paid in cash at the time of closing. The home has 3 bedrooms
and 2.5 baths. Sangre took immediate possession of the property. La
Veta, Colorado is a small town and rental or short-term housing is
very difficult to obtain.
In February 2018,
the Company closed on the purchase of property, consisting of a
home in La Veta, Colorado to house Company personnel and
consultants for total consideration approximating $1,200,000. The
home has 5 Bedrooms and 3 Baths. Under the terms of the purchase
agreement, the Company paid $150,000 down, entered into a note
payable in the amount of approximately $1,041,000 (see Note 8). The
Company secured a below-market interest rate of 1.81% based on the
short-term nature of the term (due on August 15, 2018). Sangre took
immediate possession of the property. La Veta, Colorado is a small
town and rental or short-term housing is very difficult to obtain.
The Company personnel and consultants are no longer residing at the
property, and it is currently vacant. On October 10, 2018, a
payment of $750,000 was made to Craig W. Clark to pay off the note
payable, and a loan discount of $125,475 was given to the Company
which was recorded as a gain.
A settlement
payment of $155,000 was received from an insurance company related
to a fire near one of our properties in La Veta,
Colorado.
On June 25, 2019,
the Company received $60,000 from Lex Seabre in exchange for
120,000 shares of common stock of the Company. The $60,000 was paid
as a deposit for the Sugar Hill golf course property
auction.
On June 28, 2019,
the Company received a loan of $12,000 from Nicole Breen. The
$12,000 was paid as a deposit for the Sugar Hill golf course
property auction.
On September 25,
2019, the Company received $20,000 from Lex Seabre in exchange for
100,000 shares of common stock of the Company. The $20,000 was paid
as a deposit for the additional 60-day extension for the Sugar Hill
golf course property purchase.
Note 5 – Investment in Land and Property
(continued)
The Company entered
into Memorandum of Sale agreement for the Sugar Hill property with
M&T Bank and the Referee to make payment of $10,000 per month
commencing on February 1, 2020 and continuing on the 1st of each month
until January 1, 2021 with a balloon payment of $272,167.73 on
February 1, 2021.
The property
located on 169 Valley Vista was sold for $175,000 on September 25,
2020, and $46,948 was recorded as gain on the sale. The Company
received a check in the amount of $153,809.03 for the sale on
September 25, 2020, and the check was deposited into a new bank
account set up under Sangre AT, LLC. Due to the check was not
deposited until October 2, 2020, $153,809 was recorded to
Undeposited Funds as of September 30, 2020.
Note 6 – Property and
Equipment
Property and
equipment consist of the following at September 30, 2020 and
December 31, 2019, respectively:
|
|
|
|
|
|
|
|
|
Property
improvements
|
$ 5,000
|
$ 5,000
|
|
Automobiles
|
0
|
0
|
|
Office
equipment
|
4,933
|
4,933
|
|
Furniture &
Fixtures
|
2,979
|
2,979
|
|
Lab
equipment
|
65,769
|
65,769
|
|
Construction in
progress
|
0
|
0
|
|
Property
(1)
|
1,759,292
|
1,887,802
|
|
Property and equipment,
gross
|
1,837,973
|
1,966,483
|
|
Less accumulated
depreciation
|
(403,486)
|
(322,498)
|
|
Property and equipment,
net
|
$ 1,434,487
|
$ 1,643,985
|
|
|
(1)
|
In 2018, the
Company purchased two properties in La Veta, Colorado. The property
located on 169 Valley Vista was purchased for $140,000, and the
property located on 1390 Mountain Valley Road was purchased for
$1,200,000 (see Note 8). The property located on 169 Valley Vista
was sold for $175,000 on September 25, 2020 and $46,948 was
recorded as gain on the sale.
|
Depreciation and
amortization expense totaled $106,498 and $122,172 for the years
ended September 30, 2020 and 2019, respectively.
On October 28,
2019, the Company transferred the ownership of the 2017 Audi Q7 and
Audi A4, valued at $46,609, to Nicole Breen as a repayment for her
loans. $93,000 was recorded as a forgiveness on notes payable, and
$46,391 recorded to additional paid-in capital.
Construction in
progress in the amount of $499,695 was fully impaired due to the
Company may not receive funds to complete the research facility
center project. There was no work performed in 2019 and
2020.
Note 7 – Intangible
Assets
In accordance with
FASB ASC 350, “Intangibles-Goodwill and Other”, the
Company evaluates the recoverability of identifiable intangible
assets whenever events or changes in circumstances indicate that an
intangible asset’s carrying amount may not be recoverable.
The impairment loss would be calculated as the amount by which the
carrying value of the asset exceeds its fair value. The US and
Europe trademarks were acquired for $40,000 and $50,000,
respectively, for the year ended December 31, 2018. Trademarks are
initially measured based on their fair value and amortized by 10
and 25 years.
Amortization
expense totaled $1,950 and $1,950 for the nine months ended
September 30, 2020 and 2019, respectively.
Note 8 – Notes Payable, Related
Parties
Notes payable,
related parties consist of the following at September 30, 2020 and
December 31, 2019, respectively:
|
|
|
|
|
On April 12, 2010,
the Company received an unsecured, non-interest-bearing loan in the
amount of $2,000, due on demand from Robert Leitzman. Interest is
being imputed at the Company’s estimated borrowing rate, or
10% per annum. The largest aggregate amount outstanding was $2,000
during the periods ended December 31, 2019 and December 31, 2018.
Mr. Leitzman owns less than 1% of the Company’s common stock,
however, the Mr. Leitzman is deemed to be a related party given the
non-interest-bearing nature of the loan and the materiality of the
debt at the time of origination.
|
2,000
|
2,000
|
|
|
|
|
|
Over various dates
in 2011 and 2012, the Company received unsecured loans in the
aggregate amount of $10,000, due on demand, bearing interest at
10%, from Sandra Orman. The largest aggregate amount outstanding
was $10,000 during the periods ended December 31, 2019 and December
31, 2018. Mrs. Orman owns less than 1% of the Company’s
common stock, however, Mrs. Orman is deemed to be a related party
given the nature of the loan and the materiality of the debt at the
time of origination.
|
10,000
|
10,000
|
|
|
|
|
|
Over various dates
from April 2019 to September 2020, the company received a total of
$368,200 of advances, bearing interest at 5%, from Nicole Breen. A
detailed list of advances and repayments follows. On October 28,
2019, the company’s vehicles valued at $93,000 were used as a
repayment.
|
275,200
|
212,100
|
|
|
|
|
|
Notes payable,
related parties
|
$ 287,200
|
$ 224,100
|
The Company
recorded interest expense in the amount of $19,530 and $5,614 for
the nine months ended September 30, 2020 and 2019, respectively,
including imputed interest expense in the amount of $0 and $0
during such periods related to notes payable, related
parties.
Note 9 – Notes
Payable
Note payable
consist of the following at September 30, 2020 and December 31,
2019, respectively:
|
|
|
|
|
On July 26, 2017,
the Company issued a $475,000 note payable, bearing interest at 5%
per annum, to A.R. Miller (“Miller Note”) pursuant to
the purchase of land and property in La Veta, Colorado. The note is
to be paid in four consecutive semi-annual installments in the
amount of $118,750 plus accrued interest commencing on January 26,
2018 and continuing on the 26th day of July and the 26th day of
January each year until the debt is repaid on July 26, 2019. The
note carries a late fee of $5,937.50 in the event any installment
payment is more than 30 days late, and upon default the interest
rate shall increase to 12% per annum. During the three months ended
March 31, 2018, the Company issued 125,000 shares of common stock,
valued at $1,450,000 based on the closing price on the measurement
date. Accordingly, the Company recorded a loss on extinguishment of
$1,064,719.
|
$ -
|
$ -
|
|
|
|
|
On February 16,
2018, the Company issued a $1,040,662 note payable, bearing
interest at 1.81% per annum (the low interest rate was due to the
short-term nature of the note – six months. See Note 6), to
Craig and Carol Clark (“Clark Note”) pursuant to the
purchase of land and property in La Veta, Colorado. The note is to
be paid in consecutive monthly installments in the amount of
$5,000, including accrued interest commencing on March 15, 2018 and
continuing through August 15, 2018. The note carries a late fee of
3% in the event any installment payment is more than 10 days late,
and upon default the interest rate shall increase to 10% per annum.
As of September 12, 2018, a total of $171,300 was paid to the note
holder. On October 9, 2018, the Company entered into a settlement
agreement with the note holder to pay the settlement payment of
$750,000. The Company had already paid $650,000 by September 27,
2018 and made the remaining payment of $100,000 on October 10,
2018. The Company recorded a gain on extinguishment of
$121,475.
On August 5, 2019, the Company entered into a promissory note,
whereby the Company promises to pay Snell & Wilmer L.L.P the
principal amount of $250,000, bearing interest at 2.5% per annum.
The note is to be paid in consecutive monthly installments in the
amount of $25,000, including accrued interest commencing on August
30, 2019, until the final balloon payment is paid on January 30,
2020. The promissory note is secured by the Deed of Trust,
Assignment of Leases and Rents, Security Agreement and Fixture
Filing with respect to the real property owned by Sangre located on
1390 Mountain Valley Road, La Veta, Colorado 81055. As of September
30, 2020, $111,864 has been paid to Snell &
Wilmer.
|
$ 138,136
|
166,412
|
|
|
|
|
|
On various dates,
the Company received advances from consultant, Patrick Brodnik,
bearing 5% interest.
|
$ 11,051
|
851
|
|
|
|
|
|
|
$ 149,187
|
$ 167,263
|
The Company
recognized interest expense of $11,429 and $494.95 related to the
note payables for the nine months ended September 30, 2020 and
2019, respectively.
Note 10 – Commitments and
Contingencies
On November 8,
2016, the Company entered into an agreement with Gregory
DiPaolo’s Pro Am Golf, LLC to acquire improved property
located in Westfield, New York. The total purchase price of
$1,600,000 is to be paid with a deposit of 50,000 shares of common
stock, followed by cash of $1,250,000 and 300,000 shares of the
Company’s common stock to be delivered at closing. The
deposit of 50,000 shares issued as a deposit was $42,500 based on
the closing price of the Company’s common stock on the date
of grant. Subsequently, we entered into an amended Purchase and
Sale Agreement on October 24, 2017, under which we amended the
total purchase price to Eight Hundred Thousand Dollars ($800,000)
and forfeited our previous deposit of stock. Under the terms of the
amended agreement, we paid an additional Ten Thousand Dollar
($10,000) deposit on October 26, 2017, with the remaining purchase
price to be paid on or before the date closing date, which was
scheduled on May 1, 2018. The property is approximately 43 acres
and has unlimited water extraction rights from the State of New
York. We had planned to use this property as our inroads to the New
York hemp and infused beverage markets in the future. Since the
property was in foreclosure it was put up for auction, which
occurred on July 1, 2019. At the auction, we were the winning
bidder with a bid of $597,000. Our prior deposit payment of
$120,000 was credited towards the purchase price, and the remaining
$477,000, was finally due on November 30, 2019, after several
extensions (which cost us total of $40,000 to obtain). At the end
of September 30, 2020, a total of $182,000 was issued as a deposit
for the property. We were not able to meet that deadline, but in
January 2020 we worked out an additional extension with the bank.
Under the terms of the new agreement, we still owe approximately
$392,000 to acquire the property. We have agreed to pay that amount
in installment payments of $10,000 per month for six months
beginning February 2020, with a balloon payment of approximately
$332,000 due on or before August 3, 2020. We were not able to pay
the balloon payment by the August 3, 2020 deadline, but on July 31,
2020 we worked out an additional extension with the bank. Under the
terms of that agreement, we paid $10,000 in exchange for an
additional extension and we owed approximately $332,000 to acquire
the property. We agreed to pay that amount in installment payments
of $10,000 per month for six months beginning September 2020, with
a balloon payment of approximately $272,000 due on or before
February 1, 2021. These payments are in addition to the
approximately $480,000 in payments we have already
made.
On January 19,
2018, the Company was sued in the United States District Court for
the District of Arizona ( William
Martin v. WEED, Inc.), Case No. 4:18-cv-00027-RM) by the
listed Plaintiff. The Company was served with the Verified
Complaint on January 26, 2018. The Complaint alleges claims for
breach of contract-specific performance, breach of
contract-damages, breach of the covenant of good faith and fair
dealing, conversion, and injunctive relief. In addition to the
Verified Complaint, the Company was served with an application to
show cause for a temporary restraining order. The Verified
Complaint alleges the Company entered into a contract with the
Plaintiff on October 1, 2014 for the Plaintiff to perform certain
consulting services for the Company in exchange for 500,000 shares
of its common stock up front and an additional 700,000 shares of
common stock to be issued on May 31, 2015. The Plaintiff alleges he
completed the requested services under the agreement and received
the initial 500,000 shares of common stock, but not the additional
700,000 shares. The request for injunctive relief asks the Court to
Order the Company to issue the Plaintiff 700,000 shares of its
common stock, and possibly include them in its Registration
Statement on Form S-1, or, in the alternative, issue the shares and
have them held by the Court pending resolution of the litigation,
or, alternatively, sell the shares and deposit the sale proceeds in
an account that the Court will control. The hearing on the
Temporary Restraining Order occurred on January 29, 2018. On
January 30, 2018, the Court issued its ruling denying the
application for a Temporary Restraining Order. Currently, there is
no further hearing scheduled in this matter. On February 13, 2018, the Company filed
an Answer to the Verified Complaint and Counterclaim. On February
15, 2018, the Company filed a Motion to Dismiss the Verified
Complaint. On February 23, 2018, the Company filed a Motion to
Amend Counterclaim to add W. Martin’s wife, Joanna Martin as
a counterdefendant. On March 9, 2018, William Martin filed a Motion
to Dismiss the Counterclaim. On March 12, 2018, William Martin
filed a Motion to Amend the Verified Complaint to, among other
things, add claims against Glenn Martin and Nicole and Ryan Breen.
On March 27, 2018, the Court granted both William Martin and WEED,
Inc.’s Motions to Amend. On March 27, 2018, the Company filed
an Amended Counterclaim adding Joanna Martin. On April 2, 2018, the
Company filed a Motion to Amend our Counterclaim to add a breach of
contract claim. On April 10, 2018, the Company filed an Answer to
First Amended Verified Complaint. On April 23, 2018, Glenn Martin
and Nicole and Ryan Breen filed their Answer to the First Amended
Complaint. On May 31, 2018, the Court issued an Order: (a) granting
the Company’s Motion to Dismiss thereby dismissing the
Plaintiff’s claims for breach of the covenant of good faith
and fair dealing and the claim for conversion, (b) denying William
Martin’s Motion to Dismiss the counterclaim as to the claims
for fraudulent concealment and fraudulent misrepresentation, but
granting the Motion to Dismiss only as to the claim for fraudulent
nondisclosure, and (c) granting the Company’s Motion to Amend
its Counterclaim to add a breach of contract claim. On June 1,
2018, William Martin and his wife filed their Answer to the First
Amended Counterclaim. On June 1, 2018, William Martin and his wife
filed their Answer to the Second Amended Counterclaim. In addition
to the above pleadings and motions, the parties have exchanged
disclosure statements and served and responded to written
discovery. The Company denies the Plaintiff’s allegations in
the Verified Complaint in their entirety and plan to vigorously
defend against this lawsuit. Due to the loss not being probable, no
accrual has been recorded for the 700,000 shares of common stock
the Plaintiff alleges he is owed under his agreement with the
Company.
Travis Nelson v.
Sangre AgroTech, LLC, et al. (Huerfeno County Colorado District
Court, Case No. 2018CV30003, filed on February 5, 2018). Mr. Travis
Nelson, formerly a member of the subsidiary Sangre AgroTech, LLC,
filed this action alleging wrongful discharge in retaliation for
whistleblower activity purportedly related to insider trading,
fraud and unlawful interstate transportation of plant genetics.
After a motion to dismiss was granted in part, Mr. Nelson filed a
second amended complaint asserting revised claims for breach of
fiduciary duty, wrongful discharge, and violation of the Colorado
organized crime control act. Mr. Nelson has alleged lost wages in
the amount of $600,000, unspecified losses related to whistleblower
allegations, plus costs and attorneys’ fees. In his initial
disclosures, Mr. Nelson alleges damages of $10,000,000. On January
31, 2019, Mr. Nelson submitted an offer of judgement in the amount
of $100,000. That offer was rejected by the Corporation.
Court-ordered mediation was conducted on April 24, 2019, but the
matter was not resolved. By order dated February 4, 2020, the court
scheduled trial for October 5, 2020. The Corporation denies
liability as to all claims. Inasmuch as an unfavorable outcome is
neither probable nor remote within the meaning of the ABA Statement
of Policy referred to in the last paragraph of this letter, we
decline to express an opinion concerning the likely outcome of this
matter or the liability of the Corporation, if any, associated
therewith.
Material Definitive
Agreements
On May 1, 2018, we
entered into a Fourth Addendum and a Fifth Addendum to agreement
amending the “Closing Date” under the Agreement to
August 1, 2018, in exchange for our payment of $50,000 as a
non-refundable deposit to be applied against the purchase price
when the property sale is completed and $10,000 for maintenance,
tree removal and other grounds keeping in order to prepare the golf
course for the 2018 season. The property is approximately 43 acres
and has unlimited water extraction rights from the State of New
York. We had planned to use this property as our inroads to the New
York hemp and infused beverage markets in the future. Since the
property was in foreclosure it was put up for auction, which
occurred on July 1, 2019. At the auction, we were the winning
bidder with a bid of $597,000. Our prior deposit payment of
$120,000 was credited towards the purchase price, and the remaining
$477,000, was finally due on November 30, 2019, after several
extensions (which cost us total of $40,000 to obtain). We were not
able to meet that deadline, but in January 2020 we worked out an
additional extension with the bank. Under the terms of that
agreement, we owed approximately $392,000 to acquire the property.
We agreed to pay that amount in installment payments of $10,000 per
month for six months beginning February 2020, with a balloon
payment of approximately $332,000 due on or before August 3, 2020.
We were not able to pay the balloon payment by the August 3, 2020
deadline, but on July 31, 2020 we worked out an additional
extension with the bank. Under the terms of that agreement, we paid
$10,000 in exchange for an additional extension and we owed
approximately $332,000 to acquire the property. We agreed to pay
that amount in installment payments of $10,000 per month for six
months beginning September 2020, with a balloon payment of
approximately $272,000 due on or before February 1, 2021. These
payments are in addition to the approximately $480,000 in payments
we have already made.
Note 10 – Commitments and Contingencies
(continued)
On May 21, 2018,
the Company entered into a Trademark Purchase Agreement with
Copalix Pty Ltd., a private South African company, to acquire U.S.
Trademark Registration No. 4,927,872 for the WEED TM mark, in
exchange for USD$40,000.
On July 27, 2018,
the Company entered into a Trademark Purchase Agreement with
Copalix Pty Ltd., to acquire European Community Trademark
Registration No. 11953387 for WEED Registered Mark in exchange for
USD$10,000.
Note 11 – Stockholders’
Equity
Preferred Stock
On December 5,
2014, the Company amended the Articles of Incorporation, pursuant
to which 20,000,000 shares of “blank check” preferred
stock with a par value of $0.001 were authorized. No series of
preferred stock has been designated to date.
Common Stock
On December 5,
2014, the Company amended the Articles of Incorporation, and
increased the authorized shares to 200,000,000 shares of $0.001 par
value common stock.
2020 Common
Stock Activity
Common Stock Sales
(2020)
During the quarter
ended September 30, 2020, the Company issued 850,000 shares of
common stock for proceeds of $235,000. 200,000 shares valued at
$40,000 were not issued at September 30, 2020, and such amount has
been included in subscriptions payable.
Common Stock Issued for Services
(2020)
During the nine
months ended September 30, 2020, the Company agreed to issue an
aggregate of 2,560,000 shares of common stock to consultants for
services performed. The total fair value of common stock was
$1,365,200 based on the closing price of the Company’s common
stock earned on the measurement date. 100,000 shares valued at
$29,000 were not issued at September 30, 2020, and such amount has
been included in subscriptions payable.
Common Stock
Cancellations
No common stocks
were cancelled during the quarter ended September 30,
2020.
2019 Common
Stock Activity
Common Stock Sales
(2019)
During the year ended December 31, 2019, the Company issued
1,065,000 shares of common stock for proceeds of
$573,000
Common Stock Issued for Services
(2019)
During the year
ended December 31, 2019, the Company agreed to issue an aggregate
of 2,467,000 shares of common stock to consultants for services
performed. The total fair value of common stock was $2,578,250
based on the closing price of the Company’s common stock
earned on the measurement date. Shares valued at $121,650 were
issued at December 31, 2019 and services will be performed in 2020
and has been included in unamortized stock-based
compensation.
Common Stock Cancellations
(2019)
During the year
ended December 31, 2019, the Company cancelled a total of 220,000
shares of common stock valued at $0 previously granted to
consultants, David Johnson, and Avigor Gordon, for non-performance
of services. The cancellation was accounted as a repurchase for no
consideration.
Note 12 – Common Stock Warrants and
Options
Common Stock Warrants Granted
(2020)
No common stock
warrants were granted during the nine months ended September 30,
2020 and December 31, 2019.
Note 12 – Common Stock Warrants and
Options (continued)
Common Stock Warrants Expired
(2020)
A total of 200,000
warrants expired during the nine months ended September 30,
2020.
Warrants Exercised
(2020)
No warrants were
exercised during the nine months ended September 30,
2020.
2019 Common
Stock Warrant Activity
Common Stock Warrants Granted
(2019)
No common stock
warrants were granted during the year ended December 31,
2019.
Common Stock Warrants Exercised
(2019)
No warrants were
exercised during the year ended December 31, 2019.
Common Stock Warrants Expired
(2019)
A total of
3,078,833 warrants expired during the year ended December 31,
2019.
Common Stock Options
(2019)
On February 1,
2018, in connection with executive employment agreements, the
Company granted non-qualified options to purchase an aggregate of
6,000,000 shares of the Company’s common stock at the
exercise price of $10.55 per share. The options shall become
exercisable at the rate of 1/3 upon the six-month anniversary, 1/3
upon the one-year anniversary and 1/3 upon the second anniversary
of the grant. The options expire ten years from the date of grant.
The options were valued at $45,753,000 using the Black-Scholes
option pricing model. The Company recognized expense of
approximately $22,770,662 relating to these options during the year
ended December 31, 2019.
The assumptions
used in the Black-Scholes model are as follows:
|
|
|
For the period
ended
September 30,
2020
|
|
Risk-free interest
rate
|
|
1.75%
|
|
|
|
|
|
Expected dividend
yield
|
|
0%
|
|
|
|
|
|
Expected
lives
|
|
10.0
years
|
|
|
|
|
|
Expected
volatility
|
|
200%
|
A summary of the
Company’s stock option activity and related information is as
follows:
|
|
For the Nine months ended
September 30,
2020
|
|
|
|
|
|
|
|
|
|
Outstanding at the
beginning of period
|
$ -
|
$ -
|
|
Granted
|
6,000,000
|
10.55
|
|
Exercised/Expired/Cancelled
|
-
|
-
|
|
Outstanding at the end
of period
|
6,000,000
|
$ 10.55
|
|
Exercisable at the end
of period
|
1,250,000
|
$ 10.55
|
Note 13 – Subsequent
Events
On October 8, 2020,
the Company issued 100,000 shares of common stock to Michael Kirk
Wines in exchange for total proceeds of $20,000.
On October 8, 2020,
the Company issued 100,000 shares of common stock to Wendy Seabre
in exchange for total proceeds of $20,000.
On October 8, 2020,
the Company issued 100,000 shares of common stock to Michael Peskin
for services performed.
WEED (QB) (USOTC:BUDZ)
Historical Stock Chart
From Mar 2024 to Apr 2024
WEED (QB) (USOTC:BUDZ)
Historical Stock Chart
From Apr 2023 to Apr 2024