Moderna Developing Vaccine Booster Shot Against Virus Strain First Identified in South Africa -- Update
January 25 2021 - 12:05PM
Dow Jones News
By Peter Loftus
Moderna Inc. said Monday its Covid-19 vaccine appeared to be
protective against emerging variants of the coronavirus in
laboratory tests, but as a precaution it will start testing whether
a booster shot improves immune responses and is developing a new
vaccine targeting the strain first identified in South Africa.
The company said its vaccine produced immune-system agents known
as neutralizing antibodies against emerging virus variants tested,
including strains first identified in the U.K. and South
Africa.
These new strains appear to spread more easily from person to
person, and there are signs that the U.K. variant is more deadly
than earlier forms of the virus.
Moderna said its vaccine induced antibody production against the
strain first identified in the U.K., known as B.1.1.7, at levels
comparable to prior variants. Yet antibodies decreased sixfold
against the strain first identified in South Africa, known as
B.1.351, according to a paper posted on the preprint server
bioRxiv, which hasn't yet gone through the standard peer-review
process. Researchers from both Moderna and the National Institutes
of Health's Vaccine Research Center conducted the analyses.
Even with the decrease, Moderna said, the vaccine-induced
antibody response against the B.1.35 variant remained above levels
that are expected to be protective. The company said it expects its
standard, two-dose vaccine to be protective against the emerging
strains to date.
It will test, however, whether adding a booster dose of its
original vaccine can boost antibody levels against emerging viral
strains.
The company is also developing a new version of the vaccine that
targets more specifically the mutations in the South African
variant and will test whether giving that as a booster shot induces
a better immune response.
The company plans to start within a couple of months a Phase 1
study of the booster shot aimed at the South African variant.
"In the event that this virus continues to mutate in this
direction, and a year from now is still circulating in some way, we
think it's prudent that we have tools like a booster vaccine to
address that," Moderna President Stephen Hoge said in an
interview.
Moderna said it expects that its booster shot -- whether the
original vaccine or the one targeting the variant first identified
in South Africa -- could be given in combination with other
vaccines.
The coronavirus variant first identified in South Africa has
several mutations in its so-called spike protein, which is found on
the surface of the virus. Moderna's vaccine and most others are
designed around the spike protein or its genetic code as a way to
induce an immune response to the virus, so mutations in the protein
have the potential to hurt the performance of a vaccine.
Moderna's modified vaccine is designed to trigger the production
of the spike protein specific to the South African variant, which
in turn induces an immune response.
The company believes it can quickly design and manufacture the
modified vaccine and have it ready for initial human testing at a
faster pace than its work last year on the original vaccine. Last
year, it shipped the first batch for testing about six weeks after
selecting the initial design, and the first human study started
within two months.
"I believe we should be able to go even faster now," Chief
Executive Stephane Bancel said last week during an interview for
the WSJ Executive Membership Series. "If in the future we encounter
a mutation that requires a new vaccine, we could very quickly, in a
matter of a month or so, get a new vaccine out."
Mr. Bancel said he didn't think regulators would require such a
vaccine to go through the full series of human studies since it
would have the same underlying gene-based technology as the
original vaccine.
Moderna's move points to the potential that Covid-19 vaccines
will have to be modified -- and possibly given as repeat doses to
people previously vaccinated -- to address a changing virus.
"We may have to begin thinking about this like influenza
vaccines and start rolling out regular annual vaccinations," with
modified vaccines that target different strains, said Peter Hotez,
dean of the National School of Tropical Medicine at Baylor College
of Medicine in Texas.
Pfizer Inc. and BioNTech SE, who make the only other Covid-19
vaccine authorized for use in the U.S., are continuing to run lab
studies of their vaccine against new variants, a Pfizer spokeswoman
said.
Last week, Pfizer and BioNTech said their vaccine appeared to be
protective against the U.K. virus strain, based on lab studies.
"The virus is evolving and it's getting fitter and better at
what it needs to do," said Dr. Hoge, Moderna's president. "And both
the South African and the U.K. strains, we're seeing clearly
increases in transmission, and the potential for infectivity."
Moderna shares were ahead 11% at $145.87 in morning trading
Monday.
--Jared S. Hopkins contributed to this article.
Write to Peter Loftus at peter.loftus@wsj.com
(END) Dow Jones Newswires
January 25, 2021 11:50 ET (16:50 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
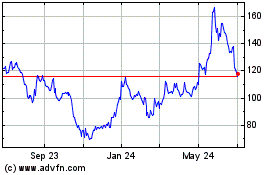
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Mar 2024 to Apr 2024
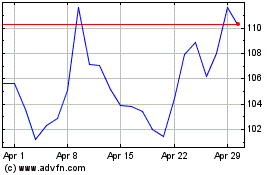
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Apr 2023 to Apr 2024