DermTech’s Melanoma Test Included in Two Continuing Medical Education (“CME”) Sessions at 2020 Fall Clinical Virtual Gr...
December 11 2020 - 9:00AM
Business Wire
DermTech, Inc. (NASDAQ: DMTK) (“DermTech”), a leader in
precision dermatology enabled by a non-invasive skin genomics
platform, announced today its inclusion in two CME sessions, at the
Fall Clinical Dermatology Virtual Grand Rounds (the “FCVGR”) and
the 23rd Annual Mount Sinai Winter Symposium “Advances in Medical
and Surgical Dermatology” (the “Mount Sinai Symposium”).
The FCVGR presentation was led by Laura K Ferris, MD, PhD of the
University of Pittsburgh Department of Dermatology on December 2.
The Mount Sinai Symposium presentation was led by George Han, MD,
Chairman of the Department of Dermatology at Mount Sinai Beth
Israel, on December 4, 2020.
Fall Clinical Dermatology Virtual Grand Rounds
The co-directors of the Fall Clinical Dermatology Conference
created the Virtual Grand Round series to help meet the continued
need for CME accreditation during uncertain times and maintain the
educational opportunities that would traditionally be available at
an in-person conference.
Dr. Ferris led the CME presentation “Gene Expression Profiling
for Melanoma Diagnosis,” during the “Evolving Concepts in
Dermatology Part XVIII,” session of the FCVGR. The presentation is
available for viewing here.
Dr. Ferris commented in her presentation, “New clinical research
demonstrates that 78% of lesions that are Pigmented Lesion Assay
positive and therefore demonstrate genomic atypia in all cases,
also have features of atypia or melanoma histopathologically.”
Mount Sinai Winter Symposium
The Mount Sinai Symposium is specifically designed to equally
update the practicing dermatologists, cosmetic surgeons and other
healthcare professionals on the latest advances in medical and
surgical dermatology.
Dr. Han, who serves as the Director of Teledermatology for the
Department of Dermatology at the Icahn School of Medicine at Mount
Sinai, led the CME presentation in the session “Using Genomics for
Melanoma Diagnosis.”
Dr. Han commented: “The current paradigm of evaluating pigmented
lesions leaves much to be desired, both in our approach to lesions
in clinical practice as well as in obtaining and evaluating
biopsies for accurate diagnoses. There is great potential in using
genomics to improve our approach to pigmented lesions—and with
DermTech’s Pigmented Lesion Assay, we can now offer our patients a
non-invasive test that improves our current sensitivity towards
diagnosing melanoma. That, combined with the fact that we can
utilize telemedicine to bring this test into our patients' homes
and potentially catch melanomas earlier, gives us the ability to
finally make an impact on the diagnosis and mortality rate of
melanoma.”
About DermTech:
DermTech is the leading genomics company in dermatology and is
creating a new category of medicine, precision dermatology, enabled
by our non-invasive skin genomics platform. DermTech’s mission is
to transform the practice of dermatology through more accurate
diagnosis and treatment, and the elimination of unnecessary
surgery, leading to improved patient care and lower costs. DermTech
provides genomic analysis of skin samples collected non-invasively
using an adhesive patch rather than a scalpel. DermTech markets and
develops products that facilitate the early detection of skin
cancers, and is developing products that assess inflammatory
diseases and customize drug treatments. For additional information
on DermTech, please visit DermTech’s investor relations site at:
www.DermTech.com.
Forward-looking Statements
This press release includes “forward-looking statements” within
the meaning of the “safe harbor” provisions of the Private
Securities Litigation Reform Act of 1995. The expectations,
estimates, and projections of DermTech may differ from its actual
results and consequently, you should not rely on these
forward-looking statements as predictions of future events. Words
such as “expect,” “estimate,” “project,” “budget,” “forecast,”
“anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,”
“believes,” “predicts,” “potential,” “continue,” and similar
expressions are intended to identify such forward-looking
statements. These forward-looking statements include, without
limitation, expectations with respect to: the performance, patient
benefits, cost-effectiveness, commercialization and adoption of
DermTech’s products, including the Pigmented Lesion Assay, and the
market opportunity therefor. These forward-looking statements
involve significant risks and uncertainties that could cause the
actual results to differ materially from the expected results. Most
of these factors are outside of the control of DermTech and are
difficult to predict. Factors that may cause such differences
include, but are not limited to: (1) the outcome of any legal
proceedings that may be instituted against DermTech; (2) DermTech’s
ability to obtain additional funding to develop and market its
products; (3) the existence of favorable or unfavorable clinical
guidelines for DermTech’s tests; (4) the reimbursement of
DermTech’s tests by Medicare and private payors; (5) the ability of
patients or healthcare providers to obtain coverage of or
sufficient reimbursement for DermTech’s products; (6) DermTech’s
ability to grow, manage growth and retain its key employees; (7)
changes in applicable laws or regulations; (8) the market adoption
and demand for DermTech’s products and services together with the
possibility that DermTech may be adversely affected by other
economic, business, and/or competitive factors; and (9) other risks
and uncertainties included in (x) the “Risk Factors” section of the
most recent Quarterly Report on Form 10-Q filed by DermTech with
the Securities and Exchange Commission (the “SEC”), and (y) other
documents filed or to be filed by DermTech with the SEC. DermTech
cautions that the foregoing list of factors is not exclusive. You
should not place undue reliance upon any forward-looking
statements, which speak only as of the date made. DermTech does not
undertake or accept any obligation or undertaking to release
publicly any updates or revisions to any forward-looking statements
to reflect any change in its expectations or any change in events,
conditions, or circumstances on which any such statement is
based.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20201211005009/en/
DermTech Sarah Dion sdion@dermtech.com 858.450.4222
Crowe PR Sarah Gallagher sgallagher@crowepr.com 224.406.4709
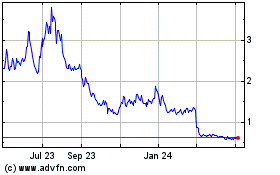
DermTech (NASDAQ:DMTK)
Historical Stock Chart
From Mar 2024 to Apr 2024
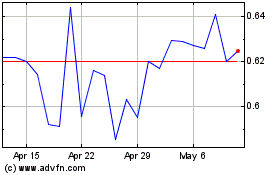
DermTech (NASDAQ:DMTK)
Historical Stock Chart
From Apr 2023 to Apr 2024