Cassava Sciences Announces Lead Drug Candidate PTI-125 Is Assigned the Chemical Drug Name ‘sumifilam’ by USAN
August 24 2020 - 8:15AM

Cassava Sciences, Inc. (Nasdaq: SAVA), a clinical-stage
biotechnology company focused on Alzheimer’s disease, today
announced that its lead drug candidate PTI-125 has been assigned
the chemical drug name sumifilam by the United States Adopted Names
(USAN) Council. Future references to PTI-125 will be sumifilam. A
commercial brand name for sumifilam is expected to be announced at
a future date. Sumifilam is an investigational new drug and has not
been approved for any indication.
Sumifilam is the first of a new class of drugs
that bind filamin proteins. For this reason, USAN is expected to
grant this class of drugs its own name stem, i.e., -filam. Any
future drug by any company that targets filamin protein is expected
to incorporate the -filam name stem as part of its drug name.
“A very famous author once asked, what’s in a
name?” said Remi Barbier, President & CEO. “With apologies to
that author, that which we call sumifilam by any other name would
still elicit palpable excitement for an absolutely new type of drug
therapy for Alzheimer’s disease.”
About USANThe United States
Adopted Names (USAN) Council is responsible for selecting simple,
informative and unique nonproprietary drug names. The USAN Council
establishes logical nomenclature classifications based on
pharmacological or chemical relationships. The
USAN Council (USANC) is comprised of volunteers nominated
to the Council based on relevant knowledge, experience and interest
in pharmacology or medicinal chemistry. In addition to one
member-at-large and a U.S. Food and Drug Administration (FDA)
liaison, the USAN council consists of one representative from The
American Medical Association (AMA), the United States Pharmacopeia
(USP) and the American Pharmacists Association (APhA).
About Alzheimer's
Disease Alzheimer’s disease is a progressive brain
disorder that destroys memory and thinking skills. Currently, there
are no drug therapies to halt Alzheimer’s disease, much less
reverse its course. In the U.S. alone, approximately 5.8 million
people are currently living with Alzheimer’s disease, and
approximately 487,000 people age 65 or older developed Alzheimer’s
in 2019.1 The number of people living with Alzheimer’s disease is
expected to grow dramatically in the years ahead, resulting in a
growing social and economic burden.2
About Sumifilam (previously known as
PTI-125)Cassava Sciences’ lead therapeutic product
candidate is for the treatment of Alzheimer’s disease.
Sumifilam is a proprietary, small molecule (oral) drug that
restores the normal shape and function of altered filamin A (FLNA),
a scaffolding protein, in the brain. Altered FLNA in the brain
disrupts the normal function of neurons, leading to Alzheimer’s
pathology, neurodegeneration and neuroinflammation. The underlying
science is published in peer-reviewed scientific journals,
including Journal of Neuroscience, Neurobiology of Aging, Journal
of Biological Chemistry, Neuroimmunology and Neuroinflammation and
Journal of Prevention of Alzheimer’s Disease.
About SavaDxSavaDx is Cassava
Sciences’ investigational diagnostic to detect Alzheimer’s disease.
The goal of SavaDx is to make the detection of Alzheimer’s as
simple as getting a blood test, possibly years before the
appearance of any overt clinical symptoms.
About Cassava Sciences,
Inc.Cassava Sciences’ mission is to discover and
develop innovations for chronic, neurodegenerative
conditions. Over the past 10 years, Cassava Sciences has combined
state-of-the-art technology with new insights in neurobiology to
develop novel solutions for Alzheimer’s disease. Both sumifilam and
SavaDx are substantially funded by research grant awards from the
National Institutes of Health. Cassava Sciences owns worldwide
development and commercial rights to its research programs in
Alzheimer’s disease, and related technologies, without royalty
obligations to any third-party.
For more information, please visit:
https://www.CassavaSciences.com
For More Information Contact:
Eric Schoen, Chief Financial Officer
Cassava Sciences, Inc.
eschoen@CassavaSciences.com
(512) 501-2450
1, 2 Source: Alzheimer’s Association. 2019 Alzheimer’s Disease
Facts and Figures. Available online at:
https://www.alz.org/media/documents/alzheimers-facts-and-figures-2019-r.pdf
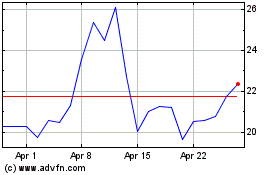
Cassava Sciences (NASDAQ:SAVA)
Historical Stock Chart
From Mar 2024 to Apr 2024
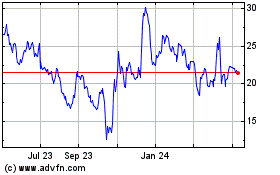
Cassava Sciences (NASDAQ:SAVA)
Historical Stock Chart
From Apr 2023 to Apr 2024