Filed Pursuant to Rule 424(b)(5)
Registration No. 333-231537
PROSPECTUS SUPPLEMENT
(To Prospectus dated June 5, 2019)

UP TO $7,000,000 SHARES OF COMMON STOCK
We have entered into an At-The-Market Offering
Agreement (the “Offering Agreement”) with H.C. Wainwright & Co., LLC, as sales agent (“Wainwright”),
relating to shares of our common stock, par value $0.001, offered by this prospectus supplement and the accompanying prospectus.
In accordance with the terms of the Offering Agreement, we may offer and sell up to $7,000,000 of our common stock from time to
time through Wainwright.
Our common stock is traded on the Nasdaq
Capital Market under the symbol “BPTH.” The last reported sale price of our common stock on the Nasdaq Capital Market
on July 10, 2020 was $5.11 per share.
Sales of our common stock, if any, under
this prospectus supplement and the accompanying prospectus may be made by any method permitted by law deemed to be an “at-the-market”
offering as defined in Rule 415 of the Securities Act of 1933 (the “Securities Act”), including without limitation
sales made directly on or through the Nasdaq Capital Market, the trading market for our common stock, or any other existing trading
market in the United States for our common stock, sales made to or through a market maker other than on an exchange or otherwise,
directly to Wainwright as principal in negotiated transactions at market prices prevailing at the time of sale or at prices related
to such prevailing market prices, and/or in any other method permitted by law. If we and Wainwright agree on a method of distribution
other than sales of shares of our common stock into the Nasdaq Capital Market or another existing trading market at market prices,
we will file a further prospectus supplement providing all information about such offering as required by Rule 424(b) under
the Securities Act. Wainwright is not required to sell any certain number of shares or dollar amount of our common stock, but will
act as a sales agent and use commercially reasonable efforts to sell on our behalf all of the shares of common stock requested
to be sold by us, consistent with its normal trading and sales practices, subject to the terms of the Offering Agreement.
Wainwright will be entitled to compensation
at a commission rate of 3.0% of the gross sales price per share sold. In connection with the sale of our common stock on our behalf,
Wainwright will be deemed to be an “underwriter” within the meaning of the Securities Act, and the compensation of
Wainwright will be deemed to be underwriting commissions or discounts. Please see “Plan of Distribution” on page S-12
for further information relating to the compensation arrangements with Wainwright. We have also agreed to provide indemnification
and contribution to Wainwright with respect to certain liabilities, including liabilities under the Securities Act or the Exchange
Act of 1934, as amended (the “Exchange Act”).
There is no arrangement for funds to be
received in any escrow, trust or similar arrangement.
As of the date of this prospectus supplement,
the aggregate market value of our outstanding common stock held by non-affiliates was $22,093,492.52, based on 3,691,857 shares
of outstanding common stock, of which 3,657,863 shares are held by non-affiliates, and the last reported sale price of our common
stock of $6.04 per share on May 19, 2020. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell
securities in a primary offering with a value exceeding more than one-third of our public float in any 12-month period so long
as our public float remains below $75,000,000. We have not sold any securities pursuant to General Instruction 1.B.6 of Form S-3
during the 12 calendar month period that ends on and includes the date hereof.
Investing in our securities involves
a high degree of risk. You should review carefully the risks and uncertainties described in the section titled “Risk Factors”
on page S-5 of this prospectus supplement and under similar headings in the other documents that are incorporated by reference
into this prospectus supplement.
Neither the Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or passed on the adequacy or accuracy of this
prospectus supplement or the accompanying prospectus. Any representation to the contrary is a criminal offense.
H.C. Wainwright & Co.
Prospectus supplement dated July 13,
2020
TABLE OF CONTENTS
Prospectus Supplement
Prospectus
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying
prospectus are part of a registration statement that we filed with the U.S. Securities and Exchange Commission utilizing a “shelf”
registration process. This document is in two parts. The first part is this prospectus supplement, which describes the terms of
this offering and also adds to and updates information contained in the accompanying prospectus as well as the documents incorporated
by reference into this prospectus supplement and the accompanying prospectus. The second part, the accompanying prospectus, gives
more general information about securities we may offer from time to time, some of which does not apply to this offering. This prospectus
supplement and the accompanying prospectus incorporate by reference important business and financial information about us that
is not included in or delivered with this prospectus supplement and the accompanying prospectus.
You should rely only on the information
we have provided or incorporated by reference in this prospectus supplement or in the accompanying prospectus. If information in
this prospectus supplement is inconsistent with the accompanying prospectus or any document incorporated by reference therein filed
prior to the date of this prospectus supplement, you should rely on this prospectus supplement; provided that if any statement
in one of these documents is inconsistent with a statement in another document having a later date—for example, a document
incorporated by reference in the accompanying prospectus—the statement in the document having the later date modifies or
supersedes the earlier statement. We have not authorized anyone to provide you with different information. No dealer, salesperson
or other person is authorized to give any information or to represent anything not contained in this prospectus or the applicable
prospectus supplement. You must not rely on any unauthorized information or representation. You should assume that the information
in this prospectus supplement and accompanying prospectus is accurate only as of the dates on the front of the respective document
and that any information we have incorporated by reference is accurate only as of the date of the document incorporated by reference.
Our business, financial condition, results of operations and prospects may have changed since those dates. You should read this
prospectus supplement, the accompanying prospectus and the documents incorporated by reference in this prospectus supplement and
the accompanying prospectus when making your investment decision.
Unless the context requires otherwise, references
in this prospectus supplement or the accompanying prospectus to “we,” “our,” “us,” “the
Company” and “Bio-Path” refer to Bio-Path Holdings, Inc. and its wholly-owned subsidiary. Bio-Path Holdings, Inc.’s
wholly-owned subsidiary, Bio-Path, Inc., is sometimes referred to herein as “Bio-Path Subsidiary.”
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights selected information
contained elsewhere in this prospectus supplement, in the accompanying prospectus or in documents incorporated by reference. This
summary does not contain all of the information that you should consider before making an investment decision. This prospectus
supplement and the accompanying prospectus include or incorporate by reference information about this offering, our business and
our financial and operating data. You should carefully read the entire prospectus supplement, the accompanying prospectus, including
under the sections titled “Risk Factors” included therein, and the documents incorporated by reference into this prospectus
supplement and the accompanying prospectus, before making an investment decision.
Our Company
We are a clinical and preclinical stage
oncology focused RNAi nanoparticle drug development company utilizing a novel technology that achieves systemic delivery for target
specific protein inhibition for any gene product that is over-expressed in disease. Our drug delivery and antisense technology,
called DNAbilize®, is a platform that uses P-ethoxy, which is a deoxyribonucleic acid (DNA) backbone modification that is intended
to protect the DNA from destruction by the body’s enzymes when circulating in vivo, incorporated inside of a lipid bilayer
having neutral charge. We believe this combination allows for high efficiency loading of antisense DNA into non-toxic, cell-membrane-like
structures for delivery of the antisense drug substance into cells. In vivo, the DNAbilize® delivered antisense drug substances
are systemically distributed throughout the body to allow for reduction or elimination of target proteins in blood diseases and
solid tumors. Through testing in numerous animal studies and treatment in over 70 patients, the Company’s DNAbilize®
drug candidates have demonstrated an excellent safety profile. DNAbilize® is a registered trademark of the Company.
Using DNAbilize® as a platform for drug
development and manufacturing, we currently have four drug candidates in development to treat at least five different cancer disease
indications. Our lead drug candidate, prexigebersen (pronounced prex” i je ber’ sen), initially started the efficacy
portion of a Phase 2 clinical trial for untreated or de novo acute myeloid leukemia (“AML”) patients in combination
with low-dose cytarabine (“LDAC”). Subsequently, a second cohort of untreated AML patients was added to the clinical
trial for treatment with prexigebersen in combination with decitabine. On March 6, 2019, we announced intended Stage 2 amendments
to this Phase 2 clinical trial to (i) add high risk myelodysplastic syndrome (“MDS”) patients to the untreated
AML cohort being treated with prexigebersen in combination with decitabine, (ii) close the prexigebersen in combination with
LDAC treatment cohort, (iii) add a cohort of relapsed/refractory AML and high risk MDS patients for treatment with prexigebersen
and decitabine and (iv) following successful completion of a safety segment treating AML and MDS patients with prexigebersen
in combination with decitabine, add venetoclax to the treatment combination treating all patient cohorts with prexigebersen in
combination with decitabine and venetoclax. On November 26, 2019, we announced successful completion of safety testing of
the treatment combination of prexigebersen and decitabine in AML and MDS patients in Stage 2 of this Phase 2 clinical trial. In
addition, preclinical efficacy studies for the triple combination treatment of prexigebersen, decitabine and venetoclax in AML
have been successfully completed. Based on the successful completion of these studies, we submitted the amendment for the Stage
2 Phase 2 clinical trial to add the triple combination treatment comprised of prexigebersen, decitabine and venetoclax to the U.S.
Food and Drug Administration (“FDA”). The FDA requested that the treatment of MDS patients with the triple combination
of prexigebersen, decitabine and venetoclax be conducted in a separate clinical trial. As a result, the amendment was modified
and resubmitted to the FDA for approval. The Company is awaiting approval of the modified amendment, which is expected by summer
2020. Prexigebersen has also been planned for a safety portion of a Phase 2a clinical trial for chronic myeloid leukemia (“CML”)
accelerated and blast crisis patients in combination with dasatinib. However, due to declining patient population, the Company
will not pursue this program any further. Finally, a modified product named prexigebersen-A, Bio-Path’s fourth drug candidate,
has shown to enhance chemotherapy efficacy in preclinical solid tumor models. Prexigebersen-A incorporates the same drug substance
as prexigebersen but has a slightly modified formulation designed to enhance nanoparticle properties. In late 2019, we filed an
Investigational New Drug (“IND”) application to initiate a Phase 1 clinical trial of prexigebersen-A in patients with
solid tumors, including ovarian and uterine, pancreatic and breast cancer. Ovarian cancer is one of the most common type of gynecologic
malignancies, with approximately 50% of all cases occurring in women older than 63 years. This trial is expected to commence after
the IND has been cleared by the FDA, which we currently anticipate being in 2020.
Our second drug candidate, Liposomal
Bcl-2 (“BP1002”), targets the protein Bcl-2, which is responsible for driving cell survival in up to 60% of all
cancers. On November 21, 2019, we announced that the FDA cleared an IND application for BP1002. An initial Phase 1
clinical trial will evaluate the ability of BP1002 to treat refractory/relapsed lymphoma and chronic lymphocytic leukemia
patients. The Phase 1 clinical trial is expected to be conducted at several leading cancer centers, including The University
of Texas MD Anderson Cancer Center (“MD Anderson”), the Georgia Cancer Center and the Sarah Cannon Research
Institute.
Our third drug candidate, Liposomal STAT3
(“BP1003”), targets the STAT3 protein and is currently in IND enabling studies as a potential treatment of pancreatic
cancer, non-small cell lung cancer (NSCLC) and AML. Preclinical models have shown BP1003 to inhibit cell viability and STAT3 protein
expression in NSCLC and AML cell lines. Further, BP1003 successfully penetrated pancreatic tumors and significantly enhanced the
efficacy of gemcitabine, a treatment for patients with advanced pancreatic cancer, in a pancreatic cancer patient derived tumor
model. Our lead indication for BP1003 is pancreatic cancer due to the severity of this disease and the lack of effective, life-extending
treatments. For example, pancreatic adenocarcinoma is projected to be the second most lethal cancer behind lung cancer by 2030.
Typical survival for a metastatic or advanced patient is about six to 15 months from diagnosis. We expect to complete several IND
enabling studies of BP1003 in 2020. If those studies are successful, we expect to file an IND in late 2020 for the first-in-humans
Phase 1 study of BP1003 in patients with refractory/metastatic solid tumors, including pancreatic, NSCLC and colorectal cancers.
Our DNAbilize® technology-based products
are available for out-licensing or partnering. We intend to apply our drug delivery technology template to new disease-causing
protein targets to develop new nanoparticle antisense RNAi drug candidates. We have a new product identification template in place
to define a process of scientific, preclinical, commercial and intellectual property evaluation of potential new drug candidates
for inclusion into our drug product development pipeline. As we expand, we will look at indications where a systemic delivery is
needed and antisense RNAi nanoparticles can be used to slow, reverse or cure a disease, either alone or in combination with another
drug. On September 25, 2019, we announced that the United States Patent and Trademark Office (“USPTO”) issued
a patent for claims related to DNAbilize®, including its use in the treatment of cancers, autoimmune diseases and infectious
diseases. This is the second patent issued to the Company.
We have certain intellectual property as
the basis for our current drug products in clinical development, prexigebersen, prexigebersen-A, BP1002 and BP1003. We are developing
RNAi antisense nanoparticle drug candidates based on our own patented technology to treat cancer and autoimmune disorders where
targeting a single protein may be advantageous and result in reduced patient adverse effects as compared to small molecule inhibitors
with off-target and non-specific effects. We have composition of matter and method of use intellectual property for the design
and manufacture of antisense RNAi nanoparticle drug products.
To date, COVID-19’s impact on our
operations has been limited to the inability to travel to clinical trial sites and clinical trial sites not allowing nonessential
personnel on site for the purpose of monitoring activity. We anticipate COVID-19 may have an effect on patient recruiting in the
near term as social distancing mandates are in effect. We believe these operational issues can be managed through remote monitoring
capabilities currently being developed.
Corporate Information
The Company was incorporated in May 2000
as a Utah corporation. In February 2008, Bio-Path Subsidiary completed a reverse merger with the Company, which at the time
was traded over the counter and had no current operations. The prior name of the Company was changed to Bio-Path Holdings, Inc.
and the directors and officers of Bio-Path Subsidiary became the directors and officers of Bio-Path Holdings, Inc. On March 10,
2014, our common stock ceased trading on the OTCQX and commenced trading on the Nasdaq Capital Market under the ticker symbol “BPTH.”
Effective December 31, 2014, we changed our state of incorporation from Utah to Delaware through a statutory conversion pursuant
to the Utah Revised Business Corporation Act and the Delaware General Corporation Law.
On February 8, 2018, we effected a
reverse stock split of our outstanding shares of common stock at a ratio of 1-for-10, and our common stock began trading on the
split-adjusted basis on the Nasdaq Capital Market at the commencement of trading on February 9, 2018. In addition, on January 17,
2019, we effected a reverse stock split of our outstanding shares of common stock at a ratio of 1-for-20, and our common stock
began trading on the split-adjusted basis on the Nasdaq Capital Market at the commencement of trading on January 18, 2019.
All common stock share and per share amounts in this prospectus supplement and in the accompanying prospectus have been adjusted
to give effect to both the 1-for-10 reverse stock split and the 1-for-20 reverse stock split, retrospectively.
Our principal executive offices are located
at 4710 Bellaire Boulevard, Suite 210, Bellaire, Texas 77401, and our telephone number is (832) 742-1357. Our Internet address
is www.biopathholdings.com. None of the information on our website forms a part of, or incorporated by reference into, this
prospectus supplement or the accompanying prospectus.
The Offering
|
Common stock offered by us
|
|
Shares of our common stock having an aggregate offering price of up to $7,000,000.
|
|
|
|
|
|
Common stock to be outstanding immediately after this offering (1)
|
|
Up to 5,061,720 shares, assuming sales of 1,369,863 shares of our common stock in this offering at an offering price of $5.11 per share, which was the last reported sale price of our common stock on the Nasdaq Capital Market on July 10, 2020. The actual number of shares issued will vary depending on the sales price under this offering.
|
|
|
|
|
|
Plan of Distribution
|
|
Sales of our common stock, if any, under this prospectus supplement and
the accompanying prospectus may be made by any method permitted by law deemed to be an “at-the-market” offering as
defined in Rule 415 of the Securities Act, including without limitation sales made directly on the Nasdaq Capital Market,
on any other existing trading market for the common stock in the United States. Wainwright is not required to sell any certain
number of shares or dollar amount of our common stock, but will act as a sales agent and use commercially reasonable efforts to
sell on our behalf all of the shares of common stock requested to be sold by us, consistent with its normal trading and sales
practices, subject to the terms of the Offering Agreement. See “Plan of Distribution” on page S-12.
|
|
|
|
|
|
Use of proceeds
|
|
We currently expect to use the net proceeds from this offering for working
capital and general corporate purposes. See “Use of Proceeds” on page S-10.
|
|
|
|
|
|
Risk factors
|
|
An investment in our company involves a high degree of risk. Please refer to the sections titled “Risk Factors,” “Special Note Regarding Forward-Looking Statements” and other information included or incorporated by reference in this prospectus supplement and the accompanying prospectus for a discussion of factors you should carefully consider before investing our securities.
|
|
|
|
|
|
Nasdaq Capital Market Symbol
|
|
“BPTH”
|
(1) The number of shares of common stock to be outstanding
after this offering is based on 3,691,857 shares of our common stock outstanding as of June 30, 2020, which excludes as of
such date:
|
|
·
|
267,321 shares of common stock reserved for issuance upon the exercise of outstanding options granted under our equity incentive plans with a weighted average exercise price of $20.98 per share;
|
|
|
|
|
|
|
·
|
407,893 additional shares of common stock reserved for future issuance under our 2017 Stock Incentive Plan; and
|
|
|
|
|
|
|
·
|
858,698 shares of common stock issuable upon exercise of outstanding warrants with a weighted average exercise price of $18.67 per share.
|
RISK FACTORS
An investment in our company involves
a high degree of risk. Before you make a decision to invest in our securities, you should consider carefully the risks described
below, as well as the risks described in or incorporated by reference in this prospectus supplement and the accompanying prospectus,
including the risks and uncertainties discussed under the section titled “Risk Factors” in our most recent Annual Report
on Form 10-K and any subsequent Quarterly Reports on Form 10-Q or Current Reports on Form 8-K, and all other documents
incorporated by reference into this prospectus supplement and accompanying prospectus, as updated by our subsequent filings under
the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
Any of these risks could have a material
adverse effect on our business, prospects, financial condition and results of operations. In any such case, the trading price of
our securities could decline and you could lose all or part of your investment. Additional risks not presently known to us or that
we currently deem immaterial may also adversely affect our business operations.
Resales of our common stock in the
public market following the offering may cause its market price to fall.
We will issue common
stock from time to time in connection with this offering. This issuance from time to time of these new shares of our common stock,
or our ability to issue these shares of common stock in this offering, could result in resales of our common stock by our current
stockholders concerned about the potential dilution of their holdings. If our stockholders sell substantial amounts of our common
stock in the public market following this offering, the market price of our common stock could fall.
The common stock
offered hereby will be sold in “at-the-market” offerings, and investors who buy shares at different times will likely
pay different prices.
Investors who purchase
shares in this offering at different times will likely pay different prices. As a result, investors may experience different outcomes
in their investment results. We will have discretion, subject to market demand, to vary the timing, prices and numbers of shares
sold, and there is no minimum or maximum sales price. Investors may experience a decline in the value of their shares as a result
of share sales made at prices lower than the prices they paid.
The actual number
of shares of common stock we will issue under the Offering Agreement, at any one time or in total, is uncertain.
Subject to certain
limitations in the Offering Agreement and compliance with applicable law, we have the discretion to deliver a sales notice to Wainwright
as our sales agent at any time throughout the term of the Offering Agreement. The number of shares that are sold by Wainwright
after delivering a sales notice will fluctuate based on the market price of our common stock during the sales period and limits
we set with Wainwright. Because the price per share of each share sold will fluctuate based on the market price of our common stock
during the sales period, it is not possible at this stage to predict the number of shares that will be ultimately issued.
You will experience immediate and
substantial dilution in the net tangible book value per share of the common stock you purchase.
Since the price per share of our common
stock being offered is substantially higher than the net tangible book value per share of our common stock, you will suffer substantial
dilution in the net tangible book value of the common stock you purchase in this offering. Based on an assumed offering price of
$5.11 per share, which was the last reported sale price of our common stock on the Nasdaq Capital Market on July 10, 2020,
if you purchase shares of common stock in this offering, you will suffer immediate and substantial dilution of approximately $0.25
per share in the net tangible book value of the common stock. See the section titled “Dilution” in this prospectus
supplement for a more detailed discussion of the dilution you will incur if you purchase common stock in this offering. In addition,
we have a significant number of stock options and warrants outstanding. To the extent that outstanding stock options or warrants
have been or may be exercised or other shares issued, you may experience further dilution.
Furthermore, to the extent we need to raise
additional capital in the future and we issue additional shares of common stock or securities convertible or exchangeable for our
common stock, our then-existing stockholders may experience dilution and the new securities may have rights senior to those of
our common stock offered in this offering.
There may be future sales of our securities
or other dilution of our equity, which may adversely affect the market price of our common stock.
With limited exceptions, we are generally
not restricted from issuing additional common stock, including any securities that are convertible into or exchangeable for, or
that represent the right to receive, common stock. The market price of our common stock could decline as a result of sales of common
stock or securities that are convertible into or exchangeable for, or that represent the right to receive, common stock after this
offering or the perception that such sales could occur.
Our management has significant flexibility
in using the net proceeds of this offering.
We currently intend generally to use the
net proceeds from this offering for working capital and general corporate purposes. Our management will have significant flexibility
in applying the net proceeds of this offering. Management’s failure to use these funds effectively would have an adverse
effect on the value of our common stock and could make it more difficult and costly to raise funds in the future.
We are a clinical stage biotechnology
company with no significant revenue. We have incurred significant operating losses since our inception, and we expect to incur
losses for the foreseeable future and may never achieve profitability.
We have incurred significant operating losses
since our inception. As of March 31, 2020, we had an accumulated deficit of $59.7 million. To date, we have not generated
any revenue from the sale of our drug candidates and we do not expect to generate any revenue from sales of our drug candidates
for the foreseeable future. We expect to continue to incur significant operating losses and we anticipate that our losses may increase
substantially as we expand our drug development programs and commercialization efforts.
To achieve profitability, we must successfully
develop and obtain regulatory approval for one or more of our drug candidates and effectively commercialize any drug candidates
we develop. Even if we succeed in developing and commercializing one or more of our drug candidates, we may not be able to generate
sufficient revenue and we may never be able to achieve or sustain profitability.
We will continue to require substantial
additional capital for the foreseeable future. If we are unable to raise additional capital when needed, we may be forced to delay,
reduce or eliminate our drug development programs and commercialization efforts.
We expect to continue to incur significant
operating expenses in connection with our ongoing activities, including conducting clinical trials, manufacturing and seeking regulatory
approval of our drug candidates, prexigebersen, BP1002, BP1003 and prexigebersen-A. In addition, if we obtain regulatory approval
of one or more of our drug candidates, we expect to incur significant commercialization expenses related to product sales, marketing,
manufacturing and distribution.
As of March 31, 2020, we had $17.9
million in cash on hand, compared to $20.4 million as of December 31, 2019. Our ongoing future capital requirements will depend
on numerous factors, including:
|
|
·
|
the rate of progress, results and costs of completion of ongoing clinical trials of our drug candidates;
|
|
|
·
|
the rate of progress, results and costs of completion of the ongoing preclinical testing of prexigebersen, BP1002, BP1003 and
prexigebersen-A;
|
|
|
·
|
the size, scope, rate of progress, results and costs of completion of any potential future clinical trials and preclinical
tests of our drug candidates that we may initiate;
|
|
|
·
|
the costs to obtain adequate supply of the compounds necessary for our drug candidates;
|
|
|
·
|
the costs of obtaining regulatory approval of our drug candidates;
|
|
|
·
|
the scope, prioritization and number of drug development programs we pursue;
|
|
|
·
|
the costs for preparing, filing, prosecuting, maintaining and enforcing our intellectual property rights and defending intellectual
property-related claims;
|
|
|
·
|
the extent to which we acquire or in-license other products and technologies and the costs to develop those products and technologies;
|
|
|
·
|
the costs of future commercializing activities, including product sales, marketing, manufacturing and distribution, of any
of our drug candidates or other products for which marketing approval has been obtained;
|
|
|
·
|
our ability to establish strategic collaborations and licensing or other arrangements on terms favorable to us; and
|
|
|
·
|
competing technological and market developments.
|
Any additional fundraising efforts may divert
our management from their day to day activities, which may adversely affect our ability to develop and commercialize our drug candidates.
Our ability to raise additional funds will depend, in part, on the success of our product development activities and other factors
related to financial, economic and market conditions, many of which are beyond our control. There can be no assurance that we will
be able to raise additional capital when needed or on terms that are favorable to us, if at all. If adequate funds are not available
on a timely basis, we may be forced to:
|
|
·
|
delay, reduce the scope of or eliminate one or more of our drug development programs;
|
|
|
·
|
relinquish, license or otherwise dispose of rights to technologies, drug candidates or products that we would otherwise seek
to develop or commercialize ourselves at an earlier stage or on terms that are less favorable than might otherwise be available;
or
|
|
|
·
|
liquidate and dissolve the Company.
|
If our operating plans change, we may require
additional capital sooner than planned. Such additional financing may not be available when needed or on terms favorable to us.
In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe
we have sufficient funds for our current and future operating plan.
The trading price of our common stock
has been volatile and is likely to be volatile in the future.
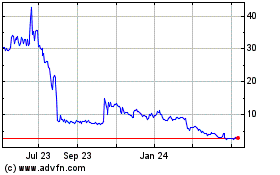
The trading price of our common stock has
been highly volatile. On March 10, 2014, our common stock commenced trading on The Nasdaq Capital Market, and there is a limited
history on which to gauge the volatility of our stock price on The Nasdaq Capital Market. From January 1, 2017 through June 30,
2020, our stock price has fluctuated from a low of $1.61 to a high of $270.00, after adjustment for reverse stock splits. The market
price for our common stock will be affected by a number of factors, including:
|
|
·
|
the denial or delay of regulatory approvals of our drug candidates or receipt of regulatory approval of competing products;
|
|
|
·
|
our ability to accomplish clinical, regulatory and other drug development milestones;
|
|
|
·
|
the ability of our drug candidates, if they receive regulatory approval, to achieve market success;
|
|
|
·
|
the performance of third-party manufacturers and suppliers;
|
|
|
·
|
developments with respect to patents and other intellectual property rights;
|
|
|
·
|
sales of common stock or other securities by us or our stockholders in the future;
|
|
|
·
|
additions or departures of key scientific or management personnel;
|
|
|
·
|
disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain
patent protection for our drug candidates;
|
|
|
·
|
trading volume of our common stock;
|
|
|
·
|
investor perceptions about us and our industry;
|
|
|
·
|
public reaction to our press releases, other public announcements and SEC and other filings;
|
|
|
·
|
the failure of analysts to cover us, or changes in analysts’ estimates or recommendations;
|
|
|
·
|
the failure by us to meet analysts’ projections or guidance;
|
|
|
·
|
general market conditions and other factors unrelated to our operating performance or the operating performance of our competitors;
and
|
|
|
·
|
other risk factors described elsewhere in our public filings.
|
The stock prices of
many companies in the biotechnology industry have experienced wide fluctuations that have often been unrelated to the operating
performance of these companies. Following periods of volatility in the market price of a company’s securities, securities
class action litigation often has been initiated against a company. If any class action litigation is initiated against us, we
may incur substantial costs and our management’s attention may be diverted from our operations, which could materially adversely
affect our business and financial condition.
SPECIAL NOTE REGARDING FORWARD-LOOKING
STATEMENTS
This prospectus supplement, the accompanying
prospectus and the documents incorporated by reference into this prospectus supplement and the accompanying prospectus contain
“forward-looking statements” within the meaning of Section 27A of the Securities Act and Section 21E of the
Exchange Act. Forward-looking statements can be identified by words such as “anticipate,” “expect,” “intend,”
“plan,” “believe,” “seek,” “estimate,” “project,” “goal,”
“strategy,” “future,” “likely,” “may,” “should,” “will”
and variations of these words and similar references to future periods, although not all forward-looking statements contain these
identifying words. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they
are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies,
projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate
to the future, they are subject to inherent risks, uncertainties, and changes in circumstances, including but not limited to risk
factors incorporated by reference under “Item 1A. Risk Factors” to Part I of our Annual Report on Form 10-K for the fiscal year ended December 31, 2019 and other factors described elsewhere in this prospectus supplement, the accompanying
prospectus or in our current and future filings with the SEC. As a result, our actual results may differ materially from those
expressed or forecasted in the forward-looking statements, and you should not rely on such forward-looking statements. You should
carefully read this prospectus supplement and the accompanying prospectus, together with the information incorporated by reference
herein and therein as described under the sections titled “Where You Can Find More Information,” completely and with
the understanding that our actual future results may be materially different from what we expect. We can give no assurances that
any of the events anticipated by the forward-looking statements will occur or, if any of them do, what impact they will have on
our results of operations and financial condition. Important factors that could cause our actual results and financial condition
to differ materially from those indicated in the forward-looking statements include, among others, the following:
|
|
·
|
the impact, risks and uncertainties related to COVID-19 and actions taken by governmental authorities or others in connection therewith;
|
|
|
·
|
our lack of significant revenue to date, our history of recurring operating losses and our expectation of future operating losses;
|
|
|
·
|
our need for substantial additional capital and our need to delay, reduce or eliminate our drug development and commercialization efforts if we are unable to raise additional capital;
|
|
|
·
|
the highly-competitive nature of the pharmaceutical and biotechnology industry and our ability to compete effectively;
|
|
|
·
|
the success of our plans to use collaboration arrangements to leverage our capabilities;
|
|
|
·
|
our ability to retain and attract key personnel;
|
|
|
·
|
the risk of misconduct of our employees, agents, consultants and commercial partners;
|
|
|
·
|
disruptions to our operations due to expansions of our operations;
|
|
|
·
|
the costs we would incur if we acquire or license technologies, resources or drug candidates;
|
|
|
·
|
risks associated with product liability claims;
|
|
|
·
|
our reliance on information technology systems and the liability or interruption associated with cyber-attacks or other breaches of our systems;
|
|
|
·
|
our ability to use net operating loss carryforwards;
|
|
|
·
|
provisions in our charter documents and state law that may prevent a change in control;
|
|
|
·
|
work slowdown or stoppage at government agencies could negatively impact our business;
|
|
|
·
|
our need to complete extensive clinical trials and the risk that we may not be able to demonstrate the safety and efficacy of our drug candidates;
|
|
|
·
|
risks that that our clinical trials may be delayed or terminated;
|
|
|
·
|
our ability to obtain domestic and foreign regulatory approval for our drug candidates;
|
|
|
·
|
changes in existing laws and regulations affecting the healthcare industry;
|
|
|
·
|
our reliance on third parties to conduct clinical trials for our drug candidates;
|
|
|
·
|
our ability to maintain orphan drug exclusivity for our drug candidates;
|
|
|
·
|
our reliance on third parties for manufacturing our clinical drug supplies;
|
|
|
·
|
risks associated with the manufacture of our drug candidates;
|
|
|
·
|
our ability to establish sales and marketing capabilities relating to our drug candidates;
|
|
|
·
|
market acceptance of our drug candidates;
|
|
|
·
|
third-party payor reimbursement practices;
|
|
|
·
|
our ability to adequately protect the intellectual property of our drug candidates;
|
|
|
·
|
infringement on the intellectual property rights of third parties;
|
|
|
·
|
costs and time relating to litigation regarding intellectual property rights;
|
|
|
·
|
our ability to adequately prevent disclosure by our employees or others of trade secrets and other proprietary information;
|
|
|
·
|
our need to raise additional capital;
|
|
|
·
|
the volatility of the trading price of our common stock;
|
|
|
·
|
our common stock being thinly traded;
|
|
|
·
|
our ability to issue shares of common or preferred stock without approval from our stockholders;
|
|
|
·
|
our ability to pay cash dividends;
|
|
|
·
|
costs and expenses associated with being a public company; and
|
|
|
·
|
our ability to maintain compliance with the listing standards of the Nasdaq Capital Market.
|
Any forward-looking statement made by us
in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein is based
only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to
publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise. However,
you should carefully review the risk factors set forth in other reports or documents we file from time to time with the SEC.
USE OF PROCEEDS
The amount of proceeds
from this offering will depend upon the number of shares of our common stock sold and the market price at which they are sold.
There can be no assurance that we will be able to sell any shares under or fully utilize the Offering Agreement with Wainwright
as a source of financing.
We will retain broad
discretion over the use of the net proceeds from the sale of the securities offered hereby. We currently intend to use the net
proceeds from this offering, if any, for working capital and general corporate purposes. The amount and timing of these expenditures
will depend on a number of factors, such as the timing, scope, progress and results of our research and development efforts, the
timing and progress of any partnership efforts and the competitive environment for our product candidates.
DILUTION
If you invest in our common stock, you will
experience immediate and substantial dilution to the extent of the difference between the public offering price of our common stock
in this offering and the adjusted net tangible book value per share of our common stock immediately after the offering.
Our net tangible book value per share is
determined by subtracting our total liabilities from our total tangible assets, which is total assets less intangible assets, and
dividing this amount by the number of shares of common stock outstanding. The historical net tangible book value of our common
stock as of March 31, 2020 was approximately $17.9 million, or $4.86 per share, based on 3,691,857 shares of common stock
outstanding at March 31, 2020.
After giving effect to our sale in this
offering of shares of our common stock in the aggregate amount of $7,000,000 at an assumed public offering price of $5.11 per share
(the last reported sale price of our common stock on the Nasdaq Capital Market on July 10, 2020), and after deducting the
sales agent commissions and our estimated offering expenses payable by us, our as adjusted net tangible book value as of March 31,
2020 would have been approximately $24.6 million, or $4.86 per share of common stock. While this would not result in an increase
in net tangible book value per share to existing stockholders, it would result in an immediate dilution of $0.25 per share to new
investors purchasing shares of common stock in this offering at the assumed public offering price. The following table illustrates
this dilution on a per share basis:
|
Assumed public offering price per share
|
|
|
|
|
|
$
|
5.11
|
|
|
Historical net tangible book value per share as of March 31, 2020
|
|
$
|
4.86
|
|
|
|
|
|
|
Increase in net tangible book value per share attributable to new investors
|
|
$
|
0.00
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As adjusted net tangible book value per share after this offering
|
|
|
|
|
|
$
|
4.86
|
|
|
|
|
|
|
|
|
|
|
|
|
Dilution per share to new investors
|
|
|
|
|
|
$
|
0.25
|
|
The table above assumes for illustrative
purposes that an aggregate of 1,369,863 shares of our common stock are sold at a price of $5.11 per share, the last reported sale
price of our common stock on the Nasdaq Capital Market on July 10, 2020, for aggregate gross proceeds of approximately $7,000,000.
The shares sold in this offering, if any, will be sold from time to time at various prices. An increase of $1.00 per share in the
price at which the shares are sold from the assumed public offering price of $5.11 per share shown in the table above, assuming
all of our common stock in the aggregate amount of approximately $7,000,000 is sold at that price, would increase the dilution
in net tangible book value per share to new investors in this offering to $1.02 per share, after deducting commissions and estimated
offering expenses payable by us. A decrease of $1.00 per share in the price at which the shares are sold from the assumed public
offering price of $5.11 per share shown in the table above, assuming all of our common stock in the aggregate amount of approximately
$7,000,000 is sold at that price, would not result in dilution in net tangible book value per share to new investors in this offering,
after deducting commissions and estimated offering expenses payable by us. This information is supplied for illustrative purposes
only.
The above discussion and table are based
on 3,691,857 shares of our common stock outstanding as of March 31, 2020, which excludes as of such date:
|
|
·
|
166,008 shares of common stock reserved for issuance upon the exercise of outstanding options granted under our equity incentive plans with a weighted average exercise price of $30.66 per share;
|
|
|
|
|
|
|
·
|
509,206 additional shares of common stock reserved for future issuance under our 2017 Stock Incentive Plan; and
|
|
|
|
|
|
|
·
|
858,698 shares of common stock issuable upon exercise of outstanding warrants with a weighted average exercise price of $18.67 per share.
|
The above illustration of dilution per
share to investors participating in this offering assumes no exercise of outstanding options to purchase our common stock or
outstanding warrants to purchase shares of our common stock. To the extent that any of these outstanding options or warrants
are exercised or we issue additional shares under our equity incentive plans, there will be further dilution to new
investors. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even
if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is
raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further
dilution to our stockholders.
PLAN OF DISTRIBUTION
We have entered into the Offering Agreement
with Wainwright, under which we may issue and sell from time to time shares of our common stock having an aggregate offering price
of not more than $7,000,000 through Wainwright as our sales agent. Sales of the common stock, if any, will be made by any method
permitted by law deemed to be an “at-the-market offering” as defined in Rule 415 promulgated under the Securities
Act. If we and Wainwright agree on any method of distribution other than sales of shares of our common stock into the Nasdaq Capital
Market or another existing trading market in the United States at market prices, we will file a further prospectus supplement providing
all information about such offering as required by Rule 424(b) under the Securities Act.
Wainwright will offer our common stock at
prevailing market prices subject to the terms and conditions of the Offering Agreement as agreed upon by us and Wainwright. We
will designate the number of shares which we desire to sell, the time period during which sales are requested to be made, any limitation
on the number of shares that may be sold in one day and any minimum price below which sales may not be made. Subject to the terms
and conditions of the Offering Agreement, Wainwright will use its commercially reasonable efforts consistent with its normal trading
and sales practices and applicable law and regulations to sell on our behalf all of the shares of common stock requested to be
sold by us. We or Wainwright may suspend the offering of the common stock being made through Wainwright under the Offering Agreement
upon proper notice to the other party.
Settlement for sales of common stock will
occur on the second business day or such shorter settlement cycle as may be in effect under the Exchange Act from time to time,
following the date on which any sales are made, or on some other date that is agreed upon by us and Wainwright in connection with
a particular transaction, in return for payment of the net proceeds to us. Sales of our common stock as contemplated in this prospectus
will be settled through the facilities of The Depository Trust Company or by such other means as we and Wainwright may agree upon.
There is no arrangement for funds to be received in an escrow, trust or similar arrangement.
We will pay Wainwright in cash, upon each
sale of our shares of common stock pursuant to the Offering Agreement, a commission equal to 3.0% of the gross proceeds from each
sale of shares of our common stock. Because there is no minimum offering amount required as a condition to this offering, the actual
total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. Pursuant to the terms
of the Offering Agreement, we agreed to reimburse Wainwright for the reasonable fees and expenses of its legal counsel incurred
in connection with entering into the transactions contemplated by the Offering Agreement in an amount not to exceed $50,000 in
the aggregate. Additionally, pursuant to the terms of the Offering Agreement, we agreed to reimburse Wainwright for the documented
fees and costs of its legal counsel reasonably incurred in connection with Wainwright’s ongoing diligence, drafting and other
filing requirements arising from the transactions contemplated by the Offering Agreement in an amount not to exceed $2,500 in the
aggregate per calendar quarter. We estimate that the total expenses of the offering payable by us, excluding commissions payable
to Wainwright under the Offering Agreement, will be approximately $112,500. We will disclose in our Annual Reports on Form 10-K
and Quarterly Reports on Form 10-Q, as applicable, the number of shares of our common stock sold through Wainwright under
the Offering Agreement, the net proceeds to us and the compensation paid by us with respect to sales under the Offering Agreement
during the relevant quarter.
In connection with the sales of common stock
on our behalf, Wainwright will be deemed to be an “underwriter” within the meaning of the Securities Act, and the compensation
paid to Wainwright will be deemed to be underwriting commissions or discounts. We have agreed in the Offering Agreement to provide
indemnification and contribution to Wainwright against certain liabilities, including liabilities under the Securities Act.
The offering of our shares of common stock
pursuant to the Offering Agreement will terminate upon the earlier of the (i) sale of all of our shares of common stock provided
for in this prospectus supplement or (ii) termination of the Offering Agreement as permitted therein.
To the extent required by Regulation M,
Wainwright will not engage in any market making activities involving our shares of common stock while the offering is ongoing under
this prospectus supplement.
From time to time, Wainwright may
provide in the future various advisory, investment and commercial banking and other services to us in the ordinary course of
business, for which they have received and may continue to receive customary fees and commissions. Wainwright served as our
exclusive placement agent in connection with our registered direct offerings we consummated in July 2016,
September 2018, January 2019, March 2019 and November 2019, and as the underwriter in connection with our
underwritten public offering that we consummated on January 17, 2019, and Wainwright received compensation for each such
offering. However, except as disclosed in this prospectus supplement, we have no present arrangements with Wainwright for any
further services.
This summary of the material provisions
of the Offering Agreement does not purport to be a complete statement of its terms and conditions. We will file a copy of the Offering
Agreement with the SEC on a Current Report on Form 8-K within the time required by the Exchange Act.
This prospectus in electronic format may
be made available on a website maintained by Wainwright and Wainwright may distribute this prospectus electronically.
The transfer agent for our common stock
to be issued in this offering is American Stock Transfer & Trust Company, LLC.
LEGAL MATTERS
The validity of the issuance of the securities
offered hereby will be passed upon for us by Winstead PC, Houston, Texas. Certain legal matters will be passed upon for Wainwright
by Sichenzia Ross Ference LLP, New York, New York.
EXPERTS
The consolidated financial statements as
of December 31, 2019 and 2018 and for the years then ended incorporated by reference in this prospectus supplement have been
so incorporated in reliance on the report of BDO USA, LLP, an independent registered public accounting firm, incorporated herein
by reference, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration
statement on Form S-3 under the Securities Act with respect to the securities being offered hereby. This prospectus supplement
and the accompanying prospectus, which constitute a part of the registration statement, do not contain all of the information set
forth in the registration statement or the exhibits and schedules filed therewith. For further information about us and the securities
offered hereby, we refer you to the registration statement and the exhibits filed thereto. Statements contained in this prospectus
supplement and the accompanying prospectus regarding the contents of any contract or any other document that is filed as an exhibit
to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to
the full text of such contract or other document filed as an exhibit to the registration statement.
We file annual, quarterly and current reports,
proxy statements and other information with the SEC. The SEC maintains an Internet site that contains reports, proxy and information
statements, and other information regarding issuers that file electronically with the SEC, including us. The SEC’s Internet
site can be found at http://www.sec.gov. In addition, we make available on or through our Internet site copies of these
reports as soon as reasonably practicable after we electronically file or furnished them to the SEC. Our Internet site can be found
at http://www.biopathholdings.com. The information contained in, or that can be accessed through, our website is not incorporated
by reference in, and is not part of, this prospectus supplement or the accompanying prospectus.
INFORMATION INCORPORATED BY REFERENCE
We are incorporating by reference into this
prospectus supplement and the accompanying prospectus certain information that we file with the SEC, which means that we are disclosing
important information to you by referring you to those documents. The information incorporated by reference is deemed to be part
of this prospectus supplement and the accompanying prospectus, except for information incorporated by reference that is superseded
by information contained in this prospectus supplement and the accompanying prospectus. This means that you must look at all of
the SEC filings that we incorporate by reference to determine if any statements in this prospectus supplement, the accompanying
prospectus or any document previously incorporated by reference have been modified or superseded. This prospectus supplement incorporates
by reference the documents set forth below that we have previously filed with the SEC:
Any information in any of the foregoing
documents will automatically be deemed to be modified or superseded to the extent that information in this prospectus supplement
or in a later filed document that is incorporated or deemed to be incorporated herein by reference modifies or replaces such information.
We also incorporate by reference all documents
we subsequently file in the future pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of
this prospectus supplement until the termination of the offering. These documents include periodic reports, such as Annual Reports
on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K (except, in any such case, the portions
furnished and not filed pursuant to Item 2.02, Item 7.01 or otherwise), as well as any proxy statements.
We will provide to each person, including
any beneficial owner, to whom a prospectus supplement and accompanying prospectus are delivered, without charge upon written or
oral request, a copy of any or all of the documents that are incorporated by reference into this prospectus supplement and the
accompanying prospectus but not delivered with the prospectus supplement and accompanying prospectus, including exhibits which
are specifically incorporated by reference into such documents. You may request a copy of these filings at no cost, by writing
to or telephoning us at the following address:
Bio-Path Holdings, Inc.
Attention: Secretary
4710 Bellaire Boulevard, Suite 210
Bellaire, Texas 77401
(832) 742-1357
PROSPECTUS

$125,000,000
COMMON STOCK
PREFERRED STOCK
WARRANTS
UNITS
We may from time to time offer and sell
up to $125,000,000 of common stock, preferred stock, warrants to purchase common stock or preferred stock or any combination of
the foregoing, either individually or in units, at prices and on terms described in one or more supplements to this prospectus.
We may also offer securities as may be issuable upon conversion, redemption, repurchase, exchange or exercise of any securities
registered hereunder, including any applicable anti-dilution provisions.
This prospectus provides the general terms
of the securities we may offer and the general manner in which these securities will be offered. Each time we offer to sell securities,
we will provide specific terms related to such offers in a supplement to this prospectus. The prospectus supplements may also add,
update or change information contained in this prospectus. Before you invest, you should carefully read this prospectus and the
applicable prospectus supplement, as well the documents incorporated by referenced in this prospectus. This prospectus may not
be used to consummate a sale of securities unless accompanied by the applicable prospectus supplement.
Our common stock is currently listed on
the Nasdaq Capital Market under the symbol “BPTH.” On May 14, 2019, the last reported sales price per share of our
common stock on the Nasdaq Capital Market was $18.00.
We will sell these securities directly,
through agents, dealers or underwriters as designated from time to time, or through a combination of these methods. For additional
information on the methods of sale, you should refer to the section titled “Plan of Distribution” in this prospectus.
If any agents, dealers or underwriters are involved in the sale of these securities, the applicable prospectus supplement will
set forth the names of the agents, dealers or underwriters and any applicable fees, commissions or discounts. The price to the
public of such securities and the net proceeds that we expect to receive from such sale will also be set forth in a supplement
to this prospectus.
Investing in our securities involves
a high degree of risk. Before making an investment decision, you should review carefully and consider all of the information set
forth in this prospectus, the applicable prospectus supplement and the documents incorporated by reference in this prospectus and
applicable prospectus supplement. See “Risk Factors” on page 4 of this prospectus and under similar headings in the
other documents that are incorporated by reference into this prospectus.
Neither the Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful
or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is June
5, 2019
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration
statement on Form S-3 that we filed with the Securities and Exchange Commission (the “SEC”) using a “shelf”
registration process. Under this shelf registration statement process, we may from time to time sell common stock, preferred stock,
warrants to purchase common stock or preferred stock or any combination of the foregoing, either individually or in units, in one
or more offerings up to an offering amount of $125,000,000. This prospectus provides you with a general description of the securities
we may offer and the general manner in which these securities will be offered.
Each time we offer securities hereunder,
we will provide specific terms related to such offering in a supplement to this prospectus.
The prospectus supplements may add, update
or change any of the information contained in this prospectus or in the documents that we have incorporated by reference into this
prospectus. We urge you to read carefully this prospectus and the applicable prospectus supplement, together with the information
incorporated herein by reference as described under the sections titled “Where You Can Find More Information” and “Information
Incorporated by Reference” below. If there is any inconsistency between the information in this prospectus and any prospectus
supplement, you should rely on the information contained in that prospectus supplement.
This prospectus may not be used to consummate
a sale of securities unless it is accompanied by a prospectus supplement.
You should rely only on the information
we have provided or incorporated by reference in this prospectus and the applicable prospectus supplement. We have not authorized
anyone to provide you with different information. No dealer, salesperson or other person is authorized to give any information
or to represent anything not contained in this prospectus or the applicable prospectus supplement. You must not rely on any unauthorized
information or representation. This prospectus or any applicable supplement to this prospectus do not constitute an offer to sell
or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus
or any applicable supplement to this prospectus constitute an offer to sell or the solicitation of an offer to buy securities in
any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction. You should assume
that the information in this prospectus is accurate only as of the date on the front of the document and that any information we
have incorporated by reference is accurate only as of the date of the document incorporated by reference. Our business, financial
condition, results of operations and prospects may have changed since those dates.
This prospectus contains summaries of certain
provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information.
All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein
have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus
is a part, and you may obtain copies of those documents as described below under the section titled “Where You Can Find More
Information.”
Unless the context requires otherwise, references
in this prospectus to “we,” “our,” “us,” “the Company” and “Bio-Path”
refer to Bio-Path Holdings, Inc. and its wholly-owned subsidiary. Bio-Path Holdings, Inc.’s wholly-owned subsidiary, Bio-Path,
Inc., is sometimes referred to herein as “Bio-Path Subsidiary.”
PROSPECTUS
SUMMARY
This prospectus summary highlights
selected information contained elsewhere in this prospectus or in documents incorporated by reference. This summary does not contain
all of the information that you should consider before making an investment decision. You should carefully read the entire prospectus,
the applicable prospectus supplement, including under the section titled “Risk Factors” and the documents incorporated
by reference into this prospectus, before making an investment decision.
Our Company
We are a clinical and preclinical stage
oncology focused RNAi nanoparticle drug development company utilizing a novel technology that achieves systemic delivery for target
specific protein inhibition for any gene product that is over-expressed in disease. Our drug delivery and antisense technology,
called DNAbilize®, is a platform that uses P-ethoxy, which is a deoxyribonucleic acid (DNA) backbone modification that is intended
to protect the DNA from destruction by the body’s enzymes when circulating in vivo, incorporated inside of a neutral
charged lipid bilayer. We believe this combination allows for high efficiency loading of antisense DNA into non-toxic, cell-membrane-like
structures for delivery of the antisense drug substance into cells. In vivo, the DNAbilize® delivered antisense drug
substances are systemically distributed throughout the body to allow for reduction or elimination of target proteins in blood diseases
and solid tumors. Through testing in numerous animal studies and treatment in over 70 patients, the Company’s DNAbilize®
drug candidates have demonstrated an excellent safety profile. DNAbilize® is a registered trademark of the Company.
Using DNAbilize® as a platform for drug
development and manufacturing, we currently have three antisense drug candidates in development to treat at least five different
cancer disease indications. Our lead drug candidate, prexigebersen (pronounced prex” i je ber’ sen), is in the efficacy
portion of a Phase 2 clinical trial for acute myeloid leukemia (AML) in combination with low-dose cytarabine (LDAC) and in combination
with decitabine. On March 6, 2019, we announced intended amendments to this Phase 2 clinical trial to, among other things, add
prexigebersen in combination with decitabine for myelodysplastic syndrome and close prexigebersen in combination with LDAC. In
addition, preclinical efficacy studies are underway for triple combination prexigebersen, decitabine and Venclexta in AML. Prexigebersen
is also being studied in the safety portion of a Phase 2a clinical trial for chronic myeloid leukemia in combination with dasatinib.
Prexigebersen was shown to enhance chemotherapy efficacy in preclinical solid tumor models, such as ovarian cancer, and we intend
to file an Investigational New Drug (IND) application for prexigebersen in solid tumors in 2019.
Our second drug candidate, Liposomal Bcl-2
(“BP1002”), targets the protein Bcl-2, which is responsible for driving cell survival in up to 60% of all cancers.
We are currently preparing an IND application for BP1002 after completing additional IND enabling studies. We intend to initiate
a Phase 1 clinical trial of BP1002 in refractory/relapsed lymphoma and chronic lymphocytic leukemia patients once we receive approval
from the U.S. Food and Drug Administration (FDA).
Our third drug candidate, Liposomal Stat3
(“BP1003”), targets the Stat3 protein and is currently in preclinical development as a potential treatment of pancreatic
cancer, non-small cell lung cancer (NSCLC) and AML. Preclinical models have shown BP1003 to inhibit cell viability and STAT3 protein
expression in NSCLC and AML cell lines. Further, BP1003 successfully penetrated pancreatic tumors and significantly enhanced the
efficacy of gemcitabine, a treatment for patients with advanced pancreatic cancer, in a pancreatic patient derived tumor model.
Our lead indication for BP1003 is pancreatic cancer due to the severity of this disease and the lack of effective, life-extending
treatments. We intend to complete IND enabling studies of BP1003 in 2019 and to file an IND application for a Phase 1 clinical
trial of BP1003 for the treatment of solid tumors, including pancreatic cancer in 2020.
Our DNAbilize® technology-based products
are available for out-licensing or partnering. We intend to apply our drug delivery technology template to new disease-causing
protein targets as a means to develop new nanoparticle antisense RNAi drug candidates. We have a new product identification template
in place to define a process of scientific, preclinical, commercial and intellectual property evaluation of potential new drug
candidates for inclusion into our drug product development pipeline. As we expand, we will look at indications where a systemic
delivery is needed and antisense RNAi nanoparticles can be used to slow, reverse or cure a disease, either alone or in combination
with another drug. On July 19, 2017, we announced that the United States Patent and Trademark Office issued a notice of allowance
for claims related to DNAbilize®, including its use in the treatment of cancers, autoimmune diseases and infectious diseases.
We have certain intellectual property as
the basis for our current drug products in clinical development, specifically prexigebersen, BP1002 and BP1003. We are developing
RNAi antisense nanoparticle drug candidates based on our own patented technology to treat cancer and autoimmune disorders where
targeting a single protein may be advantageous and result in reduced patient adverse effects as compared to small molecule inhibitors
with off-target and non-specific effects. We have composition of matter and method of use intellectual property for the design
and manufacture of antisense RNAi nanoparticle drug products.
Corporate Information
The Company was incorporated in May 2000
as a Utah corporation. In February 2008, Bio-Path Subsidiary completed a reverse merger with the Company, which at the time was
traded over the counter and had no current operations. The prior name of the Company was changed to Bio-Path Holdings, Inc. and
the directors and officers of Bio-Path Subsidiary became the directors and officers of Bio-Path Holdings, Inc. On March 10, 2014,
our common stock ceased trading on the OTCQX and commenced trading on the Nasdaq Capital Market under the ticker symbol “BPTH.”
Effective December 31, 2014, we changed our state of incorporation from Utah to Delaware through a statutory conversion pursuant
to the Utah Revised Business Corporation Act and the Delaware General Corporation Law. Our principal executive offices are located
at 4710 Bellaire Boulevard, Suite 210, Bellaire, Texas 77401, and our telephone number is (832) 742-1357.
On February 8, 2018, we effected a reverse
stock split of our outstanding shares of common stock at a ratio of 1-for-10, and our common stock began trading on the split-adjusted
basis on the Nasdaq Capital Market at the commencement of trading on February 9, 2018. In addition, on January 17, 2019, we effected
a reverse stock split of our outstanding shares of common stock at a ratio of 1-for-20, and our common stock began trading on the
split-adjusted basis on the Nasdaq Capital Market at the commencement of trading on January 18, 2019. All common stock share and
per share amounts in this prospectus have been adjusted to give effect to both the 1-for-10 reverse stock split and the 1-for-20
reverse stock split, retrospectively.
THE OFFERING
We may offer common stock, preferred stock,
warrants to purchase common stock or preferred stock or any combination of the foregoing, either individually or in units, in one
or more offerings, with an aggregate initial offering price not to exceed $125,000,000. This prospectus describes the general terms
that may apply to the securities to be offered and the specific terms of any particular securities that we offer will be described
in a separate supplement to this prospectus, including, to the extent applicable:
|
|
•
|
designation or classification;
|
|
|
•
|
aggregate offering price;
|
|
|
•
|
rates and times of payment of dividends, if any;
|
|
|
•
|
redemption, conversion, exchange or sinking fund terms, if any;
|
|
|
•
|
restrictive covenants, if any;
|
|
|
•
|
voting or other rights, if any;
|
|
|
•
|
conversion, exchange and exercise prices, if any; and
|
|
|
•
|
important federal income tax considerations.
|
We may offer and sell the securities directly,
through agents, dealers or underwriters as designated from time to time, or through a combination of these methods. The applicable
prospectus supplement will include any required information about the agents, dealers or underwriters we use and the applicable
fees, commissions or discounts we may pay them for their services.
This prospectus may not be used to consummate
a sale of securities unless it is accompanied by a prospectus supplement.
RISK
FACTORS
An investment in our company involves a
high degree of risk. Before you make a decision to invest in our securities, you should consider carefully the risks described
in or incorporated by reference in this prospectus, including the risks and uncertainties discussed under the section titled “Risk
Factors” in our most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q or Current Reports
on Form 8-K, and all other documents incorporated by reference into this prospectus, as updated by our subsequent filings under
the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the risk factors and other information contained
in the applicable prospectus supplement.
Any of these risks could have a material
adverse effect on our business, prospects, financial condition and results of operations. In any such case, the trading price of
our securities could decline and you could lose all or part of your investment. Additional risks not presently known to us or that
we currently deem immaterial may also adversely affect our business operations.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated
by reference into this prospectus contain “forward-looking statements” within the meaning of Section 27A of the Securities
Act of 1933, as amended (the “Securities Act”), and Section 21E of the Exchange Act. Forward-looking statements can
be identified by words such as “anticipate,” “expect,” “intend,” “plan,” “believe,”
“seek,” “estimate,” “project,” “goal,” “strategy,” “future,”
“likely,” “may,” “should,” “will” and variations of these words and similar references
to future periods, although not all forward-looking statements contain these identifying words. Forward-looking statements are
neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and
assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the
economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent risks,
uncertainties, and changes in circumstances, including but not limited to risk factors contained in or incorporated by reference
under the section titled, “Risk Factors.” As a result, our actual results may differ materially from those expressed
or forecasted in the forward-looking statements, and you should not rely on such forward-looking statements. You should carefully
read this prospectus and any applicable supplement to this prospectus, together with the information incorporated herein by reference
as described under the section titled “Where You Can Find More Information,” completely and with the understanding
that our actual future results may be materially different from what we expect. We can give no assurances that any of the events
anticipated by the forward-looking statements will occur or, if any of them do, what impact they will have on our results of operations
and financial condition. Forward-looking statements include, but are not limited to, statements about:
|
|
•
|
our lack of significant revenue to date, our history of recurring operating losses and our expectation of future operating losses;
|
|
|
•
|
our need for substantial additional capital and our need to delay, reduce or eliminate our drug development and commercialization efforts if we are unable to raise additional capital;
|
|
|
•
|
the highly-competitive nature of the pharmaceutical and biotechnology industry and our ability to compete effectively;
|
|
|
•
|
the success of our plans to use collaboration arrangements to leverage our capabilities;
|
|
|
•
|
our ability to retain and attract key personnel;
|
|
|
•
|
the risk of misconduct of our employees, agents, consultants and commercial partners;
|
|
|
•
|
disruptions to our operations due to expansions of our operations;
|
|
|
•
|
the costs we would incur if we acquire or license technologies, resources or drug candidates;
|
|
|
•
|
risks associated with product liability claims;
|
|
|
•
|
our reliance on information technology systems and the liability or interruption associated with cyber-attacks or other breaches of our systems;
|
|
|
•
|
our ability to use net operating loss carryforwards;
|
|
|
•
|
provisions in our charter documents and state law that may prevent a change in control;
|
|
|
•
|
work slowdown or stoppage at government agencies could negatively impact our business
|
|
|
•
|
our need to complete extensive clinical trials and the risk that we may not be able to demonstrate the safety and efficacy of our drug candidates;
|
|
|
•
|
risks that that our clinical trials may be delayed or terminated;
|
|
|
•
|
our ability to obtain domestic and foreign regulatory approval for our drug candidates;
|
|
|
•
|
changes in existing laws and regulations affecting the healthcare industry;
|
|
|
•
|
our reliance on third parties to conduct clinical trials for our drug candidates;
|
|
|
•
|
our ability to maintain orphan drug exclusivity for our drug candidates;
|
|
|
•
|
our reliance on third parties for manufacturing our clinical drug supplies;
|
|
|
•
|
risks associated with the manufacture of our drug candidates;
|
|
|
•
|
our ability to establish sales and marketing capabilities relating to our drug candidates;
|
|
|
•
|
market acceptance of our drug candidates;
|
|
|
•
|
third-party payor reimbursement practices;
|
|
|
•
|
our ability to adequately protect the intellectual property of our drug candidates;
|
|
|
•
|
infringement on the intellectual property rights of third parties;
|
|
|
•
|
costs and time relating to litigation regarding intellectual property rights;
|
|
|
•
|
our ability to adequately prevent disclosure by our employees or others of trade secrets and other proprietary information;
|
|
|
•
|
the volatility of the trading price of our common stock;
|
|
|
•
|
our common stock being thinly traded;
|
|
|
•
|
our ability to issue shares of common or preferred stock without approval from our stockholders;
|
|
|
•
|
our ability to pay cash dividends;
|
|
|
•
|
costs and expenses associated with being a public company;
|
|
|
•
|
a material weakness identified in our internal controls over financial reporting; and
|
|
|
•
|
our ability to maintain compliance with the listing standards of the Nasdaq Capital Market.
|
Any forward-looking statement made by us
in this prospectus, any applicable supplement to this prospectus and the documents incorporated by reference into this prospectus
is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation
to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise. However,
you should carefully review the risk factors set forth in other reports or documents we file from time to time with the SEC.
USE
OF PROCEEDS
Unless otherwise indicated in the applicable
prospectus supplement, we expect to use the net proceeds from the sale of securities offered pursuant to this prospectus for working
capital and general corporate purposes. Until the net proceeds are used for these purposes, we may deposit them in interest-bearing
accounts or invest them in short-term marketable securities.
DESCRIPTION
OF CAPITAL STOCK
The following description of our common
stock and preferred stock is a summary. It is not complete and is subject to and qualified in its entirety by our certificate of
incorporation and first amended and restated bylaws, each of which is incorporated by reference into this prospectus.
See the sections titled “Where You Can Find More Information” and “Information Incorporated by Reference.”
As of the date of this prospectus, our certificate of incorporation authorizes us to issue 200,000,000 shares of common stock,
par value $0.001 per share, and 10,000,000 shares of preferred stock, par value $0.001 per share. As of May 7, 2019, there were
2,831,356 shares of common stock issued and outstanding and no shares of preferred stock issued and outstanding.
Common Stock
Holders of common stock
are entitled to one vote for each share held in the election of directors and on all other matters submitted to a vote of stockholders.
Cumulative voting of shares of common stock is prohibited. Accordingly, holders of a majority of the shares of common stock entitled
to vote in any election of directors may elect all of the directors standing for election.
Subject to the prior
rights of the holders of any outstanding preferred stock, holders of common stock are entitled to receive dividends when, as and
if declared by our board of directors out of funds legally available therefor. Upon the liquidation, dissolution or winding up
of our company, the holders of common stock are entitled to receive ratably the assets of our company remaining after payment of
all liabilities and payment to holders of preferred stock if such preferred stock has an involuntary liquidation preference over
the common stock. Holders of common stock have no preemptive, subscription, redemption or conversion rights. The outstanding shares
of common stock are, and the shares offered by us in this offering will be, when issued and paid for, validly issued, fully paid
and nonassessable.
As of March 31, 2019,
there were approximately 227 holders of record of our common stock.
Preferred Stock
Our board of directors is authorized, without
any further notice to or action of the stockholders, to issue up to 10,000,000 shares of preferred stock in one or more series.
Our board of directors is further authorized, subject to limitations prescribed by law, to fix by resolution or resolutions the
designations, powers, preferences and rights, and the qualifications, limitations or restrictions thereof, of any wholly unissued
series of our preferred stock, including without limitation authority to fix by resolution or resolutions the dividend rights,
dividend rate, conversion rights, voting rights, rights and terms of redemption (including sinking fund provisions), redemption
price or prices, and liquidation preferences of any such series, and the number of shares constituting any such series and the
designation thereof, or any of the foregoing. The board of directors is further authorized to increase (but not above the total
number of authorized shares of the class) or decrease (but not below the number of shares of any such series then outstanding)
the number of shares of any series, the number of which was fixed by it, subsequent to the issuance of shares of such series then
outstanding, subject to the powers, preferences and rights, and the qualifications, limitations and restrictions thereof stated
in our certificate of incorporation or the resolution of our board of directors originally fixing the number of shares of such
series. If the number of shares of any series is so decreased, then the shares constituting such decrease shall resume the status
which they had prior to the adoption of the resolution originally fixing the number of shares of such series. As of the date of
this prospectus, no such shares had been designated.
The following briefly summarizes the material
terms of preferred stock that we may offer, other than pricing and related terms disclosed in a prospectus supplement. You should
read the particular terms of any series of preferred stock that we offer which we will describe in more detail in the applicable
prospectus supplement relating to such series. You should also read the more detailed provisions of our certificate of incorporation
and the statement with respect to shares relating to each particular series of preferred stock for provisions that may be important
to you. The statement with respect to shares relating to each particular series of preferred stock offered by the applicable prospectus
supplement and this prospectus will be filed as an exhibit to a document incorporated by reference in the prospectus. The prospectus
supplement will also state whether any of the terms summarized below do not apply to the series of preferred stock being offered.
Rank. A series of preferred stock
will have the rank set forth in the relevant certificate of designation and described in the prospectus supplement relating to
the applicable series.
Voting Rights. The holders of shares
of a series of preferred stock will have the voting rights provided by the applicable certificate of designation and as required
by applicable law. These voting rights will be described in the relevant prospectus supplement.
Dividends. The certificate of designation
setting forth the terms of a series of preferred stock may provide that the holders of that series are entitled to receive dividends,
when, as and if authorized by our board of directors out of funds legally available for dividends, before any declaration or payment
of any dividends on securities ranking junior to such series relating to dividends. The rates and dates of payment of dividends
and any other terms applicable to the dividends will be set forth in the applicable certificate of designation and described in
the prospectus supplement relating to the relevant series. To the extent provided in the certificate of designation, dividends
will be payable to the holders of record of our preferred stock as they appear on our books on the record dates fixed by our board
of directors. Dividends on any series of our preferred stock may be cumulative or noncumulative and payable in cash or in kind.
Conversion and Exchange. The certificate
of designation setting forth the terms of a series of our preferred stock may provide for, and the prospectus supplement for the
applicable series of preferred stock may describe, the terms, if any, on which shares of that series are convertible into or exchangeable
for shares of our common stock or securities of a third party.
Redemption. If so specified in the
certificate of designation setting forth the terms of a series of our preferred stock, which will be described in the applicable
prospectus supplement, a series of our preferred stock may be redeemable at our or the holder’s option and/or may be mandatorily
redeemed partially or in whole.
Liquidation Preference. Upon any
voluntary or involuntary liquidation, dissolution or winding up of our company, the holders of each series of our preferred stock
may be entitled to receive distributions upon liquidation. Those distributions will be made before any distribution is made on
any securities ranking junior to such series relating to liquidation. The terms and conditions of those distributions to the relevant
series of our preferred stock will be set forth in the applicable certificate of designation and described in the relevant prospectus
supplement.
Our board of directors may cause shares
of preferred stock to be issued in public or private transactions for any proper corporate purposes, and such issuance could adversely
affect the voting rights of the holders of our common stock. The issuance of our preferred stock could also affect the likelihood
that the holders of common stock will receive dividends or distributions upon liquidation. In addition, the rights of the holders
of the preferred stock offered may be adversely affected by the rights of the holders of any shares of our preferred stock that
may be issued in the future. The preferred stock could have the effect of acting as an anti-takeover device to prevent a change
in control of our company.
Unless the particular prospectus supplement
states otherwise, the holders of each series of our preferred stock will not have any preemptive or subscription rights to acquire
more of our capital stock.
The transfer agent, registrar, dividend
disbursing agent and redemption agent for shares of each series of preferred stock will be named in the prospectus supplement relating
to such series.
Limitation on Liability and Indemnification of Officers and
Directors
Our certificate of incorporation and first
amended and restated bylaws provide for indemnification of our officers and directors to the fullest extent permitted by Delaware
law. Our certificate of incorporation and first amended and restated bylaws limit the liability of our directors for monetary damages
to the fullest extent permitted by Delaware law. We maintain directors’ and officers’ liability insurance.
Anti-Takeover Effects of Provisions of Our Certificate of
Incorporation, Our Bylaws and Delaware Law
Some provisions of Delaware law and our
certificate of incorporation and our first amended and restated bylaws contain provisions that could have the effect of delaying,
deterring or preventing another party from acquiring or seeking to acquire control of us. These provisions are intended to discourage
certain types of coercive takeover practices and inadequate takeover bids and to encourage anyone seeking to acquire control of
us to negotiate first with our board of directors. However, these provisions may also delay, deter or prevent a change in control
or other takeover of our company that our stockholders might consider to be in their best interests, including transactions that
might result in a premium being paid over the market price of our common stock and also may limit the price that investors are
willing to pay in the future for our common stock. These provisions may also have the effect of preventing changes in our management.
Our certificate of incorporation and first
amended and restated bylaws include anti-takeover provisions that:
|
|
•
|
authorize our board of directors, without further action by the stockholders, to issue shares of preferred stock in one or more series, and with respect to each series, to fix the number of shares constituting that series and establish the rights and other terms of that series;
|
|
|
•
|
establish advance notice procedures for stockholders to submit nominations of candidates for election to our board of directors and other proposals to be brought before a stockholders meeting;
|
|
|
•
|
provide that our first amended and restated bylaws may be amended by our board of directors without stockholder approval;
|
|
|
•
|
limit our stockholders’ ability to call special meetings of stockholders;
|
|
|
•
|
allow our directors to establish the size of the board of directors by action of the board, subject to a minimum of three members;
|
|
|
•
|
provide that vacancies on our board of directors or newly created directorships resulting from an increase in the number of our directors may be filled only by a majority of directors then in office, even though less than a quorum; and
|
|
|
•
|
do not give the holders of our common stock cumulative voting rights with respect to the election of directors.
|
Business Combinations
Section 203 of the Delaware General Corporation
Law provides that we may not engage in certain “business combinations” with any “interested stockholder”
for a three-year period following the time that the person became an interested stockholder, unless:
|
|
•
|
prior to the time that person became an interested stockholder, our board of directors approved either the business combination or the transaction which resulted in the person becoming an interested stockholder;
|
|
|
•
|
upon consummation of the transaction which resulted in the person becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding certain shares; or
|
|
|
•
|
at or subsequent to the time the person became an interested stockholder, the business combination is approved by the board of directors and by the affirmative vote of at least 66 2/3% of the outstanding voting stock which is not owned by the interested stockholder.
|
Generally, a business combination includes
a merger, consolidation, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder.
Subject to certain exceptions, an interested stockholder is a person who, together with that person’s affiliates and associates,
owns, or within the previous three years owned, 15% or more of our voting stock. The statute could prohibit or delay mergers or
other takeover or change in control attempts with respect to us and, accordingly, may discourage attempts to acquire us.
Listing
Our common stock is listed for trading on
the Nasdaq Capital Market under the symbol “BPTH.”
Transfer Agent and Registrar
The transfer agent and registrar for our
common stock is American Stock Transfer & Trust Company, LLC, 6201 15th Avenue, Brooklyn, New York 11219. Its phone
number is (800) 937-5449.
DESCRIPTION
OF WARRANTS
The following description, together with
the additional information we include in the applicable prospectus supplement, summarizes the material terms and provisions of
the warrants that we may offer under this prospectus. We may issue warrants for the purchase of shares of common stock and/or preferred
stock. We may issue warrants independently or together with shares of common stock and/or shares of preferred stock, and the warrants
may be attached to or separate from these securities. While the terms summarized below will apply generally to any warrants that
we may offer, we will describe the particular terms of any series of warrants in more detail in the applicable prospectus supplement.
The terms of any warrants offered under a prospectus supplement may differ from the terms described below.
Before the issuance of a series of warrants,
the form of warrant agreement, including a form of warrant certificate, if applicable, that describes the terms of the particular
series of warrants we are offering will be filed as an exhibit to the registration statement of which this prospectus is a part,
or incorporated by reference from reports that we file with the SEC. The following summaries of material provisions of the warrants
are subject to, and qualified in their entirety by reference to, all the provisions of the warrant agreement and warrant certificate
applicable to the particular series of warrants that we may offer under this prospectus and the applicable prospectus supplement.
We urge you to read the applicable prospectus supplements related to the particular series of warrants that we may offer under
this prospectus, as well as any related free writing prospectuses, and the complete warrant agreements and warrant certificates
that contain the terms of the warrants.
General
We will describe in the applicable prospectus
supplement the terms of the warrants that may be offered, including the following, where applicable:
|
|
•
|
the title of the warrants;
|
|
|
•
|
the aggregate number of the warrants offered;
|
|
|
•
|
the price or prices at which the warrants will be issued;
|
|
|
•
|
the securities, which may include shares of any class or series of common stock or preferred stock, for which the warrants are exercisable;
|
|
|
•
|
in the case of warrants to purchase common stock or preferred stock, the number of shares of common stock or preferred stock, as the case may be, purchasable upon the exercise of one warrant and the price at which these shares may be purchased upon such exercise;
|
|
|
•
|
the terms of any rights to redeem or call the warrants;
|
|
|
•
|
the date on and after which the warrants and the related securities will be separately transferable;
|
|
|
•
|
the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreements and the warrants;
|
|
|
•
|
the terms of any rights to force the exercise of the warrants;
|
|
|
•
|
the dates on which the right to exercise the warrants will commence and the date on which the right will expire;
|
|
|
•
|
the minimum or maximum number of warrants that may be exercised at any one time;
|
|
|
•
|
the manner in which the warrant agreements and warrants may be modified;
|
|
|
•
|
any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants;
|
|
|
•
|
information relating to book-entry procedures;
|
|
|
•
|
the listing of the warrants on a securities exchange or automated quotation system;
|
|
|
•
|
whether the warrants may be sold separately or with other securities as parts of units;
|
|
|
•
|
a discussion of material United States federal income tax considerations of holding or exercising the warrants; and
|
|
|
•
|
any other terms of the warrants, including terms, procedures and limitations relating to the exchange and exercise of the warrants.
|
Before exercising their warrants, the holders
of warrants will not have any of the rights of the holders of the securities purchasable upon such exercise, including the right
to receive dividends, if any, or, payments upon our liquidation, dissolution or winding up or to exercise voting rights, if any.
Exercise of Warrants
Each warrant will entitle the holder to
purchase the securities that we specify in the applicable prospectus supplement at the exercise price that we describe in the applicable
prospectus supplement. Unless we otherwise specify in the applicable prospectus supplement, the holders of the warrants may exercise
the warrants at any time up to the specified time on the expiration date that we set forth in the applicable prospectus supplement.
After the close of business on the expiration date, unexercised warrants will become void.
Unless we otherwise specify in the applicable
prospectus supplement, the holders of the warrants may exercise the warrants by delivering the warrant certificate representing
the warrants to be exercised together with specified information, and paying the required amount to us or to the warrant agent
in immediately available funds, or, if provided in the applicable prospectus supplement, by cashless exercise. We will set forth
in the warrant agreement, on the reverse side of the warrant certificate and in the applicable prospectus supplement the information
that the holder of the warrant will be required to deliver to the warrant agent in connection with the exercise of the warrant.
Unless we otherwise specify in the applicable
prospectus supplement, upon receipt of the required exercise price and the warrant certificate properly completed and duly executed
at the corporate trust office of the warrant agent or any other office indicated in the applicable prospectus supplement, we will
issue and deliver the securities purchasable upon such exercise. If fewer than all of the warrants represented by the warrant certificate
are exercised, then we may issue a new warrant certificate for the remaining amount of warrants. If we so indicate in the applicable
prospectus supplement, the holders of the warrants may surrender securities as all or part of the exercise price for warrants.
Enforceability of Rights by Holders of Warrants
Any warrant agent will act solely as our
agent under the applicable warrant agreement and will not assume any obligation or relationship of agency or trust with any holder
of any warrant. A single bank or trust company may act as warrant agent for more than one issue of warrants. A warrant agent will
have no duty or responsibility in case of any default by us under the applicable warrant agreement or warrant, including any duty
or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a warrant may,
without the consent of any related warrant agent or the holder of any other warrant, enforce by appropriate legal action its right
to exercise, and receive the securities purchasable upon exercise of, its warrants.
Warrant Adjustments
Unless the applicable prospectus supplement
states otherwise, the exercise price of, and the number of securities covered by, a warrant to purchase shares of common stock
or preferred stock will be adjusted proportionately if we subdivide or combine common stock or preferred stock, as applicable.
Warrant Agreement Will Not Be Qualified Under Trust Indenture
Act
No warrant agreement will be qualified as
an indenture, and no warrant agent will be required to qualify as a trustee, under the Trust Indenture Act. Therefore, holders
of warrants issued under a warrant agreement will not have the protection of the Trust Indenture Act with respect to their warrants.
Currently Outstanding Warrants
As of May 7, 2019, we had issued and outstanding
warrants to purchase 256,564 shares of our common stock with a weighted exercise price of $37.43 per share.
DESCRIPTION
OF UNITS
The following description, together with
the additional information we may include in any applicable prospectus supplements, summarizes the material terms and provisions
of the units that we may offer under this prospectus. While the terms we have summarized below will apply generally to any units
that we may offer under this prospectus, we will describe the particular terms of any series of units in more detail in the applicable
prospectus supplement. The terms of any units offered under a prospectus supplement may differ from the terms described below.
However, no prospectus supplement will fundamentally change the terms that are set forth in this prospectus or offer a security
that is not registered and described in this prospectus at the time of its effectiveness.
Before the issuance of units, the form of
unit agreement that describes the terms of the series of units we are offering, and any supplemental agreements, will be filed
as an exhibit to the registration statement of which this prospectus is a part, or incorporated by reference from reports that
we file with the SEC. The following summaries of material terms and provisions of the units are subject to, and qualified in their
entirety by reference to, all the provisions of the unit agreement and any supplemental agreements applicable to a particular series
of units. We urge you to read the applicable prospectus supplements related to the particular series of units that we sell under
this prospectus, as well as any related free writing prospectuses, and the complete unit agreement and any supplemental agreements
that contain the terms of the units.
General
We may issue units consisting of common
stock, preferred stock and/or warrants in any combination. Each unit will be issued so that the holder of the unit is also the
holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each
included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may not
be held or transferred separately, at any time or at any time before a specified date.
We will describe in the applicable prospectus
supplement the terms of the series of units, including:
|
|
•
|
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately;
|
|
|
•
|
any provisions of the governing unit agreement that differ from those described below; and
|
|
|
•
|
any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units.
|
The provisions described in this section,
as well as those described under “Description of Capital Stock” and “Description of Warrants” will apply
to each unit and to any common stock, preferred stock and/or warrant included in each unit, respectively.
Issuance in Series
We may issue units in such amounts and in
numerous distinct series as we determine.
Enforceability of Rights by Holders of Units
Any unit agent will act solely as our agent
under the applicable unit agreement and will not assume any obligation or relationship of agency or trust with any holder of any
unit. A single bank or trust company may act as unit agent for more than one series of units. A unit agent will have no duty or
responsibility in case of any default by us under the applicable unit agreement or unit, including any duty or responsibility to
initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a unit may, without the consent of the
related unit agent or the holder of any other unit, enforce by appropriate legal action its rights as holder under any security
included in the unit.
LEGAL
OWNERSHIP OF SECURITIES
We can issue securities in registered form
or in the form of one or more global securities. We describe global securities in greater detail below. We refer to those persons
who have securities registered in their own names on the books that we or any applicable trustee, depositary or warrant or unit
agent maintain for this purpose as the “holders” of those securities. These persons are the legal holders of the securities.
We refer to those persons who, indirectly through others, own beneficial interests in securities that are not registered in their
own names as “indirect holders” of those securities. As we discuss below, indirect holders are not legal holders and
investors in securities issued in book-entry form or in street name will be indirect holders.
Book-Entry Holders
We may issue securities in book-entry form
only, as we will specify in the applicable prospectus supplement. This means securities may be represented by one or more global
securities registered in the name of a financial institution that holds them as depositary on behalf of other financial institutions
that participate in the depositary’s book-entry system. These participating institutions, which are referred to as participants,
in turn, hold beneficial interests in the securities on behalf of themselves or their customers.
Only the person in whose name a security
is registered is recognized as the holder of that security. Securities issued in global form will be registered in the name of
the depositary or its participants. Consequently, for securities issued in global form, we will recognize only the depositary as
the holder of the securities, and we will make all payments on the securities to the depositary. The depositary passes along the
payments it receives to its participants, which in turn pass the payments along to their customers who are the beneficial owners.
The depositary and its participants do so under agreements they have made with one another or with their customers; they are not
obligated to do so under the terms of the securities.
As a result, investors in a book-entry security
will not own securities directly. Instead, they will own beneficial interests in a global security, through a bank, broker or other
financial institution that participates in the depositary’s book-entry system or holds an interest through a participant.
As long as the securities are issued in global form, investors will be indirect holders, and not holders, of the securities.
Street Name Holders
We may terminate a global security or issue
securities in non-global form. In these cases, investors may choose to hold their securities in their own names or in “street
name.” Securities held by an investor in street name would be registered in the name of a bank, broker or other financial
institution that the investor chooses, and the investor would hold only a beneficial interest in those securities through an account
he or she maintains at that institution.
For securities held in street name, we or
any applicable trustee or depositary will recognize only the intermediary banks, brokers and other financial institutions in whose
names the securities are registered as the holders of those securities, and we or any such trustee or depositary will make all
payments on those securities to them. These institutions pass along the payments they receive to their customers who are the beneficial
owners, but only because they agree to do so in their customer agreements or because they are legally required to do so. Investors
who hold securities in street name will be indirect holders, not holders, of those securities.
Legal Holders
Our obligations, as well as the obligations
of any applicable trustee and of any third parties employed by us or a trustee, run only to the legal holders of the securities.
We do not have obligations to investors who hold beneficial interests in global securities, in street name or by any other indirect
means. This will be the case whether an investor chooses to be an indirect holder of a security or has no choice because we are
issuing the securities only in global form.
For example, once we make a payment or give
a notice to the holder, we have no further responsibility for the payment or notice even if that holder is required, under agreements
with depositary participants or customers or by law, to pass it along to the indirect holders but does not do so.
Special Considerations for Indirect Holders
If you hold securities through a bank, broker
or other financial institution, either in book-entry form or in street name, you should check with your own institution to find
out:
|
|
•
|
how it handles securities payments and notices;
|
|
|
•
|
whether it imposes fees or charges;
|
|
|
•
|
how it would handle a request for the holders’ consents, if ever required;
|
|
|
•
|
whether and how you can instruct it to send you securities registered in your own name so you can be a registered holder, if that is permitted in the future;
|
|
|
•
|
how it would exercise rights under the securities if there were a default or other event triggering the need for holders to act to protect their interests; and
|
|
|
•
|
if the securities are in book-entry form, how the depositary’s rules and procedures will affect these matters.
|
Global Securities
A global security is a security that represents
one or any other number of individual securities held by a depositary. Generally, all securities represented by the same global
securities will have the same terms.
Each security issued in book-entry form
will be represented by a global security that we deposit with and register in the name of a financial institution or its nominee
that we select. The financial institution that we select for this purpose is called the depositary. Unless we specify otherwise
in the applicable prospectus supplement, The Depository Trust Company, New York, New York, known as DTC, will be the depositary
for all securities issued in book-entry form.
A global security may not be transferred
to or registered in the name of anyone other than the depositary, its nominee or a successor depositary, unless special termination
situations arise. We describe those situations below under the section titled “Special Situations When a Global Security
Will Be Terminated” in this prospectus. As a result of these arrangements, the depositary, or its nominee, will be the sole
registered owner and holder of all securities represented by a global security, and investors will be permitted to own only beneficial
interests in a global security. Beneficial interests must be held by means of an account with a broker, bank or other financial
institution that in turn has an account with the depositary or with another institution that does. Thus, an investor whose security
is represented by a global security will not be a holder of the security, but only an indirect holder of a beneficial interest
in the global security.
If the prospectus supplement for a particular
security indicates that the security will be issued in global form only, then the security will be represented by a global security
at all times unless and until the global security is terminated. If termination occurs, we may issue the securities through another
book-entry clearing system or decide that the securities may no longer be held through any book-entry clearing system.
Special Considerations for Global Securities
The rights of an indirect holder relating
to a global security will be governed by the account rules of the investor’s financial institution and of the depositary,
as well as general laws relating to securities transfers. We do not recognize an indirect holder as a holder of securities and
instead deal only with the depositary that holds the global security.
If securities are issued only in the form
of a global security, an investor should be aware of the following:
|
|
•
|
an investor cannot cause the securities to be registered in his or her name, and cannot obtain non-global certificates for his or her interest in the securities, except in the special situations we describe below;
|
|
|
•
|
an investor will be an indirect holder and must look to his or her own bank or broker for payments on the securities and protection of his or her legal rights relating to the securities, as we describe above;
|
|
|
•
|
an investor may not be able to sell interests in the securities to some insurance companies and to other institutions that are required by law to own their securities in non-book-entry form;
|
|
|
•
|
an investor may not be able to pledge his or her interest in a global security in circumstances where certificates representing the securities must be delivered to the lender or other beneficiary of the pledge in order for the pledge to be effective;
|
|
|
•
|
the depositary’s policies, which may change from time to time, will govern payments, transfers, exchanges and other matters relating to an investor’s interest in a global security. We and any applicable trustee have no responsibility for any aspect of the depositary’s actions or for its records of ownership interests in a global security. We and the trustee also do not supervise the depositary in any way;
|
|
|
•
|
the depositary may, and we understand that DTC will, require that those who purchase and sell interests in a global security within its book-entry system use immediately available funds, and your broker or bank may require you to do so as well; and
|
|
|
•
|
financial institutions that participate in the depositary’s book-entry system, and through which an investor holds its interest in a global security, may also have their own policies affecting payments, notices and other matters relating to the securities
|
There may be more than one financial intermediary
in the chain of ownership for an investor. We do not monitor and are not responsible for the actions of any of those intermediaries.
Special Situations When a Global Security Will Be Terminated
In a few special situations described below,
the global security will terminate and interests in it will be exchanged for physical certificates representing those interests.
After that exchange, the choice of whether to hold securities directly or in street name will be up to the investor. Investors
must consult their own banks or brokers to find out how to have their interests in securities transferred to their own name, so
that they will be direct holders. We have described the rights of the holders and street name investors above.
Unless we provide otherwise in the applicable
prospectus supplement, the global security will terminate when the following special situations occur:
|
|
•
|
if the depositary notifies us that it is unwilling, unable or no longer qualified to continue as depositary for that global security and we do not appoint another institution to act as depositary within 90 days;
|
|
|
•
|
if we notify any applicable trustee that we wish to terminate that global security; or
|
|
|
•
|
if an event of default has occurred with regard to securities represented by that global security and has not been cured or waived.
|
The applicable prospectus supplement may
also list additional situations for terminating a global security that would apply only to the particular series of securities
covered by the applicable prospectus supplement. When a global security terminates, the depositary, and not we or any applicable
trustee, is responsible for deciding the names of the institutions that will be the initial direct holders.
PLAN
OF DISTRIBUTION
We may sell the securities covered by this
prospectus from time to time in one or more offerings. Registration of the securities covered by this prospectus does not mean,
however, that those securities will necessarily be offered or sold.
We may sell our securities, separately or
together, in any one or more of the following ways:
|
|
•
|
directly to investors, including through a specific bidding, auction or other process;
|
|
|
•
|
to investors through agents;
|
|
|
•
|
to or through brokers or dealers;
|
|
|
•
|
to the public through underwriting syndicates led by one or more managing underwriters;
|
|
|
•
|
in “at the market” offerings, within the meaning of Rule 415(a)(4) of the Securities Act or through a market maker or into an existing trading market on an exchange or otherwise;
|
|
|
•
|
to one or more underwriters acting alone for resale to investors or to the public; and
|
|
|
•
|
through any combination of the foregoing.
|
Sales of securities may be effected from
time to time in one or more transactions, including negotiated transactions:
|
|
•
|
at a fixed price or prices, which may be changed;
|
|
|
•
|
at market prices prevailing at the time of sale;
|
|
|
•
|
at prices related to prevailing market prices; or
|
We will describe the method of distribution
of the securities and the terms of the offering in the prospectus supplement. Any discounts or concessions allowed or re-allowed
or paid to dealers may be changed from time to time.
If underwriters are used in the sale of
any securities, the securities will be acquired by the underwriters for their own account and may be resold from time to time in
one or more transactions described above. The securities may be either offered to the public through underwriting syndicates represented
by managing underwriters, or directly by underwriters. Generally, the underwriters’ obligations to purchase the securities
will be subject to conditions precedent and the underwriters will be obligated to purchase all of the securities if they purchase
any of the securities. We may use underwriters with whom we have a material relationship. We will describe in the prospectus supplement,
naming the underwriter, the nature of any such relationship.
We may authorize underwriters, dealers or
agents to solicit offers by certain purchasers to purchase the securities from us at the public offering price set forth in the
prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future.
The contracts will be subject only to those conditions set forth in the prospectus supplement, and the prospectus supplement will
set forth any commissions we pay for solicitation of these contracts.
We may enter into derivative transactions
with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. If
the applicable prospectus supplement indicates, in connection with those derivative transactions, the third parties may sell securities
covered by this prospectus and the applicable prospectus supplement, including in short sale transactions. If so, the third party
may use securities pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings
of stock, and may use securities received from us in settlement of those derivatives to close out any related open borrowings of
stock. The third party in such sale transactions will be an underwriter and will be identified in the applicable prospectus supplement
or in a post-effective amendment.
Underwriters, dealers and agents may be
entitled to indemnification by us against certain civil liabilities, including liabilities under the Securities Act, or to contribution
with respect to payments made by the underwriters, dealers or agents, under agreements between us and the underwriters, dealers
and agents.
We may grant underwriters who participate
in the distribution of securities an option to purchase additional securities to cover over-allotments, if any, in connection with
the distribution.
Underwriters, dealers or agents may receive
compensation in the form of discounts, concessions or commissions from us or our purchasers, as their agents in connection with
the sale of securities. These underwriters, dealers or agents may be considered to be underwriters under the Securities Act. As
a result, discounts, commissions or profits on resale received by the underwriters, dealers or agents may be treated as underwriting
discounts and commissions. The prospectus supplement will identify any such underwriter, dealer or agent and describe any compensation
received by them from us. Any initial public offering price and any discounts or concessions allowed or re-allowed or paid to dealers
may be changed from time to time.
Unless otherwise specified in the related
prospectus supplement, all securities we offer, other than common stock, will be new issues of securities with no established trading
market. Any underwriters may make a market in these securities, but will not be obligated to do so and may discontinue any market
making at any time without notice. Any common stock sold pursuant to a prospectus supplement will be listed on the Nasdaq Capital
Market or other principal market for our common stock. We may apply to list any series of preferred stock or warrants on an exchange,
but we are not obligated to do so. Therefore, there may not be liquidity or a trading market for any such series of preferred stock
or such warrants.
Any underwriter may engage in over-allotment
transactions, stabilizing transactions, short-covering transactions and penalty bids in accordance with Regulation M under the
Exchange Act. Over-allotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions
permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. Short covering
transactions involve purchases of the securities in the open market after the distribution is completed to cover short positions.
Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer
are purchased in a covering transaction to cover short positions. Those activities may cause the price of the securities to be
higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time. We make no
representation or prediction as to the direction or magnitude of any effect that such transactions may have on the price of the
securities. For a description of these activities, see the information under the section titled “Underwriting” or “Plan
of Distribution” in the applicable prospectus supplement.
Underwriters, broker-dealers or agents who
may become involved in the sale of the common stock may engage in transactions with and perform other services for us in the ordinary
course of their business for which they receive compensation.
LEGAL
MATTERS
The validity of the issuance of the securities
offered hereby will be passed upon for us by Winstead PC, Houston, Texas. The validity of any securities will be passed upon for
any underwriters or agents by counsel that we will name in the applicable prospectus supplement.
EXPERTS
The consolidated financial statements as
of December 31, 2018 and 2017 and for the years then ended incorporated by reference in this prospectus have been so incorporated
in reliance on the report of BDO USA, LLP, an independent registered public accounting firm, incorporated herein by reference,
given on the authority of said firm as experts in auditing and accounting.
WHERE
YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports,
proxy statements and other information with the SEC. The SEC maintains an Internet site that contains reports, proxy and information
statements, and other information regarding issuers that file electronically with the SEC, including us. The SEC’s Internet
site can be found at http://www.sec.gov. In addition, we make available on or through our Internet site copies of these
reports as soon as reasonably practicable after we electronically file or furnished them to the SEC. Our Internet site can be found
at http://www.biopathholdings.com. The information contained in, or that can be accessed through, our website is not
incorporated by reference in, and is not part of, this prospectus.
INFORMATION
INCORPORATED BY REFERENCE
We are incorporating by reference into this
prospectus certain information that we file with the SEC, which means that we are disclosing important information to you by referring
you to those documents. The information incorporated by reference is deemed to be part of this prospectus, except for information
incorporated by reference that is superseded by information contained in this prospectus. This means that you must look at all
of the SEC filings that we incorporate by reference to determine if any statements in the prospectus or any document previously
incorporated by reference have been modified or superseded. This prospectus incorporates by reference the documents set forth below
that we have previously filed with the SEC:
|
|
•
|
the description of our common stock contained in our registration statement on Form 8-A filed with the SEC on March 5, 2014, as updated by our Current Report on Form 8-K filed with the SEC on January 6, 2015.
|
Any information in any of the foregoing
documents will automatically be deemed to be modified or superseded to the extent that information in this prospectus or in a later
filed document that is incorporated or deemed to be incorporated herein by reference modifies or replaces such information.
We also incorporate by reference all documents
we file in the future pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act (i) after the date of the filing of the
registration statement of which this prospectus is a part and prior to the effectiveness of such registration statement or (ii)
after the date of this prospectus and until the offering of the securities made by this prospectus is terminated. These documents
include periodic reports, such as Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K (except,
in any such case, the portions furnished and not filed pursuant to Item 2.02, Item 7.01 or otherwise), as well as any proxy statements.
We will provide to each person, including
any beneficial owner, to whom a prospectus is delivered, without charge upon written or oral request, a copy of any or all of the
documents that are incorporated by reference into this prospectus but not delivered with the prospectus, including exhibits which
are specifically incorporated by reference into such documents. You may request a copy of these filings at no cost, by writing
to or telephoning us at the following address:
Bio-Path Holdings, Inc.
Attention: Secretary
4710 Bellaire Boulevard, Suite 210
Bellaire, Texas 77401
(832) 742-1357

UP TO $7,000,000 SHARES OF COMMON STOCK
H.C. Wainwright & Co.
July 13, 2020
Bio Path (NASDAQ:BPTH)
Historical Stock Chart
From Mar 2024 to Apr 2024
Bio Path (NASDAQ:BPTH)
Historical Stock Chart
From Apr 2023 to Apr 2024