U.S. to Invest $1.2 Billion to Secure Potential Coronavirus Vaccine From AstraZeneca, Oxford University--Update
May 21 2020 - 2:05PM
Dow Jones News
By Denise Roland
The U.S. government has agreed to hand AstraZeneca PLC up to
$1.2 billion to secure the supply of a potential coronavirus
vaccine that could be ready as early as October.
Under the deal, the government will bankroll a 30,000-person
vaccine trial in the U.S. starting in the summer, plus the ramp-up
of manufacturing capacity to make at least 300 million doses. The
first doses will be ready in the fall should the vaccine prove
effective, it said.
Alex Azar, the Health and Human Services secretary, called the
deal a "major milestone" in the administration's effort --
code-named "Operation Warp Speed" -- to make a safe, effective
vaccine widely available to Americans by 2021.
The vaccine in question was developed by the University of
Oxford's Jenner Institute and is one of a small group of candidates
already being tested in humans. Others include vaccines from Pfizer
Inc. and Moderna Inc. AstraZeneca, under a licensing deal with
Oxford, has responsibility for manufacturing the university's
vaccine, and has promised to sell the vaccine without making a
profit during the pandemic.
Governments around the world are counting on an effective
vaccine against Covid-19 to defeat a virus that has killed hundreds
of thousands of people and devastated the global economy. But to
guarantee that doses are ready as soon as possible, companies must
ramp up manufacturing capacity significantly before clinical trials
provide solid proof that the vaccines work -- a costly exercise
more viable with financial support from governments and other
funders.
The U.S. government has moved fast to secure supply deals with
vaccine makers, although the AstraZeneca deal is its biggest by
far. It has also awarded Johnson & Johnson $456 million to ramp
up U.S. production of the drugmaker's potential vaccine to 300
million doses, with the first of those available by early 2021
should the shot prove effective.
It has also awarded Moderna $483 million to ramp up production
of its candidate and another $30 million to support research into a
potential vaccine from France's Sanofi SA, though those deals don't
commit either company to manufacture a set number of doses in the
U.S. It is doing those deals through its Biomedical Advanced
Research and Development Authority division, or Barda, which was
set up in 2006 to prepare for biologic threats such as pandemics
and bioterrorism.
Still, those deals are no guarantee that the U.S. will have a
working vaccine within months. Many promising drugs and vaccines
falter during clinical trials.
Earlier this week, the U.K. government agreed to pay AstraZeneca
GBP65.5 million ($79 million) to secure 100 million doses for its
population, with 30 million of those ready as early as September.
That deal relates purely to manufacturing and doesn't include any
clinical trial funding.
AstraZeneca says it is in talks with several other governments,
as well as nonprofits like the international vaccine alliance,
Gavi, and the Coalition for Epidemic Preparedness Innovations on
deals that would further boost production.
Oxford started a 1,100-person study in April. If that goes well,
the U.K. trial will expand to include around 10,000 participants
starting in June, according to an AstraZeneca spokesman.
Its vaccine has progressed quickly, in part because it uses a
technology that has been deployed in earlier vaccines developed by
the university. It uses an inactivated chimpanzee virus containing
the genetic sequence for the "spike protein" found on the new
coronavirus.
In a small animal study, not yet peer-reviewed, it appeared to
stop the virus from spreading to the lungs, protecting the
inoculated monkeys from developing pneumonia. It was unclear
whether the vaccine stopped infection entirely, however, as the
vaccinated monkeys tested positive for virus in their noses.
Write to Denise Roland at Denise.Roland@wsj.com
(END) Dow Jones Newswires
May 21, 2020 13:50 ET (17:50 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
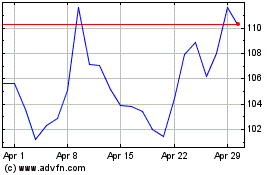
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Mar 2024 to Apr 2024
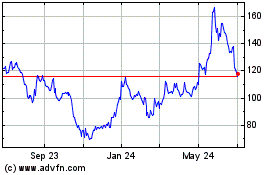
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Apr 2023 to Apr 2024