Table of Contents
Filed Pursuant to Rule 424(b)(5)
Registration Statement No. 333-224031
PROSPECTUS SUPPLEMENT
(To Prospectus dated April 6, 2018)
1,713,064 Shares

Common Stock
We are offering 1,713,064 shares of our
common stock, par value $0.0001 per share (the “Common Stock”), at a price of $2.21 pursuant to this prospectus
supplement. In a concurrent private placement, we are also selling warrants to purchase up to a total of 1,713,064 shares of Common
Stock (the “Warrants”). Each Warrant is being sold at a price of $0.125 per underlying warrant share and will
be exercisable at an exercise price of $2.21 per share. The Warrants and the shares of Common Stock issuable upon the exercise
of the Warrants (the “Warrant Shares”) are not being registered under the Securities Act of 1933, as amended
(the “Securities Act”), pursuant to the registration statement of which this prospectus supplement and the accompanying
base prospectus form a part, nor are such Warrants and Warrant Shares being offered pursuant to such prospectus supplement and
base prospectus. The Warrants are being offered pursuant to the exemption provided in Section 4(a)(2) of the Securities Act and
Rule 506(b) promulgated thereunder. The Warrants are not and will not be listed for trading on any national securities exchange.
Each purchaser will be an “accredited investor” as such term is defined in Rule 501(a) under the Securities Act.
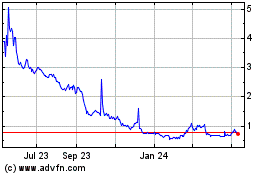
Our Common Stock is listed on The Nasdaq
Capital Market under the symbol “PHIO.” The closing price of our Common Stock on March 30, 2020, as reported by Nasdaq,
was $2.21 per share.
As of March 31, 2020, the aggregate
market value of our outstanding common equity held by non-affiliates was $26,918,250 based on 2,867,851 shares of Common
Stock then outstanding, of which 2,833,500 shares were held by non-affiliates, and a closing sale price on the Nasdaq Capital
Market of $9.50 on February 4, 2020. As of the date of this prospectus supplement, we have sold $2,740,005 of securities pursuant to
General Instruction I.B.6. of Form S-3 during the prior 12 calendar month period that ends on, and includes, the date of this
prospectus (but excludes this offering).
We have retained H.C. Wainwright &
Co., LLC to act as our exclusive placement agent in connection with the securities offered by this prospectus supplement and the
accompanying prospectus. The placement agent has agreed to use its reasonable best efforts to sell the securities offered by this
prospectus supplement and the accompanying prospectus. We have agreed to pay the placement agent the placement agent fees set forth
in the table below, which assumes that we sell all of the securities we are offering.
Investing in our securities involves
a high degree of risk. Before making any investment in these securities, you should consider carefully the risks and uncertainties
in the section entitled “Risk Factors” beginning on page S-6 of this prospectus supplement and page 2
of the accompanying prospectus, and in the other documents that are incorporated by reference and any related free writing prospectus.
Neither the Securities and Exchange
Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy
of this prospectus supplement or the accompanying prospectus. Any representation to the contrary is a criminal offense.
|
|
|
Per Share
|
|
|
Total
|
|
|
Offering price
|
|
$
|
2.21000
|
|
|
$
|
3,785,871.44
|
|
|
Placement agent fees(1)
|
|
$
|
0.16575
|
|
|
$
|
283,940.36
|
|
|
Proceeds, before expenses, to us(2)
|
|
$
|
2.04425
|
|
|
$
|
3,501,931.08
|
|
|
(1)
|
We have also agreed to pay the placement agent a management fee of 1%, reimburse the placement agent for certain of its expenses and to grant warrants to purchase shares of common stock to the placement agent as described under the “Plan of Distribution” on page S-9 of this prospectus supplement.
|
|
(2)
|
The amount of the offering proceeds to us presented in this table does not include proceeds from the sale of the warrants in the concurrent private placement or exercise of the warrants in cash, if any.
|
Delivery of the shares of Common Stock
will be made on or about April 2, 2020.
H.C. Wainwright & Co.
The date of this prospectus supplement
is March 31, 2020
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying
prospectus form part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission (the “SEC”),
under the Securities Act, using a “shelf” registration or continuous offering process. This document is in two parts.
The first part is this prospectus supplement, which describes the specific terms of this offering and certain other matters and
may add, update or change information in the accompanying prospectus, including the documents incorporated by reference into this
prospectus supplement. The second part is the accompanying prospectus dated April 6, 2018 including the documents incorporated
by reference therein, which provides you with general information about securities we may offer from time to time, some of which
may not apply to this offering. Generally, when we refer to this prospectus, we are referring to both parts of this document combined.
To the extent there is a conflict between the information contained in this prospectus supplement, on the one hand, and the information
contained in the accompanying prospectus, on the other hand, you should rely on the information in this prospectus supplement.
These documents contain important information you should consider when making your investment decision.
You should rely only on the information
provided in this prospectus supplement and the accompanying prospectus, including any information incorporated by reference, and
in any free writing prospectus that we have authorized for use in connection with this offering. We have not, and the placement
agent has not, authorized anyone to provide you with any other information. The information contained in this prospectus supplement
and the accompanying prospectus speaks only as of the date set forth on the cover page and may not reflect subsequent changes in
our business, financial condition, results of operations and prospects.
We are not, and the placement agent is
not, making offers to sell these securities in any jurisdiction in which an offer or solicitation is not authorized or permitted
or in which the person making such offer or solicitation is not qualified to do so or to any person to whom it is unlawful to make
such an offer or solicitation. You should read this prospectus supplement, the accompanying prospectus, including any information
incorporated by reference, and any free writing prospectus that we have authorized for use in connection with this offering, in
their entirety before making an investment decision. You should also read and consider the information in the documents to which
we have referred you in the sections entitled “Where You Can Find More Information” and “Incorporation of Certain
Information by Reference.”
In this prospectus supplement and accompanying
prospectus, unless otherwise noted, (1) the term “Phio” refers to Phio Pharmaceuticals Corp. and our subsidiary, MirImmune,
LLC and (2) the terms “Company,” “we,” “us” and “our” refer to the ongoing business
operations of Phio and MirImmune, LLC, whether conducted through Phio or MirImmune, LLC.
PROSPECTUS SUPPLEMENT SUMMARY
The following summary highlights certain
information contained elsewhere or incorporated by reference in this prospectus supplement and the accompanying prospectus. This
summary provides an overview of selected information and does not contain all of the information you should consider in making
your investment decision. Therefore, you should read the entire prospectus supplement and the accompanying prospectus, and the
documents incorporated by reference herein carefully before investing in our securities. Investors should carefully consider the
information set forth under “Risk Factors” beginning on page S-6 of this prospectus supplement and the financial statements
and other information incorporated by reference in this prospectus.
Overview
Phio
Pharmaceuticals Corp. is a biotechnology company developing the next generation of immuno-oncology therapeutics based on our
self-delivering RNAi (“INTASYL™”) therapeutic platform. The Company's efforts are focused on
silencing tumor-induced suppression of the immune system through our proprietary INTASYL™ platform with utility in
immune cells and/or the tumor micro-environment. Our goal is to develop powerful
INTASYL™ therapeutic compounds that can weaponize immune effector cells to overcome tumor immune escape, thereby
providing patients a powerful new treatment option that goes beyond current treatment modalities.
Our development efforts are based on our
broadly patented INTASYL™ technology platform. Our INTASYL™ compounds do not require a delivery vehicle to penetrate
into tissues and cells and are designed to “silence” or down-regulate, the expression of a specific gene which is over-expressed
in cancer. We believe that our INTASYL™ platform uniquely positions the Company in the field of immuno-oncology because of
this and the following reasons:
|
|
·
|
Efficient uptake of INTASYL™ to immune cells obviating the need for facilitated delivery (mechanical or formulation);
|
|
|
·
|
Can target multiple genes (i.e. multiple immunosuppression pathways) in a single therapeutic entity;
|
|
|
·
|
Gene silencing by INTASYL™ has been shown to have a sustained,
or long-term, effect in vivo;
|
|
|
·
|
Favorable clinical safety profile of INTASYL™ with local administration;
and
|
|
|
·
|
Can be readily manufactured under current good manufacturing practices.
|
The self-delivering
nature of our compounds makes INTASYL™ ideally suited for use with
adoptive cell transfer (“ACT”) treatments and direct therapeutic use. ACT consists of the infusion of immune
cells with antitumor properties. These cells can be derived from unmodified (i.e. naturally occurring) immune cells, immune cells
isolated from resected tumors, or genetically engineered immune cells recognizing tumor neoantigen/neoepitope cells.
Currently, ACT
therapies for the treatment of solid tumors face several hurdles. Multiple inhibitory mechanisms restrain immune cells used in
ACT from effectively eradicating tumors, including immune checkpoints, reduced cell fitness and cell persistence. Furthermore,
the immunosuppressive tumor micro-environment (the “TME”) can pose a formidable barrier to immune cell infiltration
and function.
Phio has developed
a product platform based on our INTASYL™ technology that allows easy,
precise, rapid, and selective non-genetically modified programming of ACT cells (ex vivo, during manufacturing) and of the
TME (in vivo, by local application), resulting in improved immunotherapy.
Adoptive Cell Transfer
ACT includes a number of different types
of immunotherapy treatments. These treatments use immune cells, that are grown in a lab to large numbers, followed by administering
them to the body to fight the cancer cells. Sometimes, immune cells that naturally recognize a tumor are used, while other times
immune cells are modified or “engineered” to make them recognize and kill the cancer cells. There are several types
of ACT, including: a.) non-engineered cell therapy in which immune cells are grown from the patient’s tumor or blood, such
as tumor infiltrating lymphocytes (“TILs”), or from donor blood or tissue such as natural killer (“NK”)
cells, dendritic cells (“DC”) and macrophages, and b.) engineered immune cells that are genetically modified
to recognize specific tumor proteins and to remain in an activated state (such as T cell receptor technology (“TCRs”),
chimeric antigen receptor (“CAR”) T cells, or CAR-NK cells).
In
ACT, immune cells are isolated from patients, donors or retrieved from allogeneic immune cell banks. The immune cells are then
expanded and modified before being returned and used to treat the patient. We believe our INTASYL™ compounds are ideally
suited to be used in combination with ACT, in order to make these immune cells more effective.
Our approach to
immunotherapy builds on well-established methodologies of ACT and involves the treatment of immune cells with our INTASYL™ compounds
while they are grown in the lab and before administering them to the patient. Because our INTASYL™ compounds
do not require a delivery vehicle to penetrate into the cells, we are able to enhance the function of these cells (for example,
by inhibiting the expression of immune checkpoint genes) by merely adding our INTASYL™ compounds
during the expansion process and without the need for genetic engineering. After enhancing these cells ex vivo, they
are returned to the patient for treatment.
Our method introduces an important step
in the ex vivo processing of immune cells. This step uses our INTASYL™ technology to reduce or eliminate
the expression of genes that make the immune cells less effective. For example, with our INTASYL™ compounds, we can reduce
the expression of immunosuppressive receptors or proteins by the therapeutic immune cells, potentially enabling them to overcome
tumor resistance mechanisms and thus improving their ability to destroy the tumor cells. In
various types of immune cells tested to date, INTASYL™ treatment results in potent silencing while maintaining close to 100%
transfection efficiency and nearly full cell viability.
One of the main issues with ACT is that
the cells are very susceptible to the cancer signals that turn down the immune response and continuous activation of these cells
causes them to become exhausted. These factors, among others, may reduce their efficacy and lifespan. A technology that can reprogram
the immune cells used in ACT, such as with INTASYL™ technology, is of key interest now in the current immuno-oncology world.
In comparison to other technologies available, reprogramming cells with INTASYL™ does not require genetic engineering, its
use is not limited to specific cell types and can be easily integrated with cell manufacturing approaches.
We currently have two product candidates
that are being developed for use in ACT, PH-762 and PH-804. PH-762, our most advanced program and lead pipeline compound, targets
the checkpoint protein PD-1, a checkpoint protein on immune cells. PD-1 normally acts as a type of “off switch” that
helps keep the T cells from attacking other cells in the body. T cells are immune cells that protect the body from cancer cells
and are important for the activation of immune cells to fight infection. Our second pipeline compound, PH-804, targets the suppressive
immune receptor TIGIT, which is a checkpoint protein present on T cells and NK cells.
Data developed in-house and with our collaborators,
which include both leading academic centers and corporate institutions, has shown that PH-762 can elicit PD-1 checkpoint blockade
by silencing PD-1 receptor expression resulting in enhanced T cell activation and tumor cytotoxicity. We have also shown with studies
completed with our collaborators that PH-804 can silence the expression of TIGIT in NK cells and T cells, overcoming their exhaustion
and thereby becoming “weaponized.”
Recent data shown by the Company as well
as with our collaborators, Iovance Biotherapeutics, Inc. and the Karolinska Institutet, at the 2019 Society for Immunotherapy of
Cancer annual meeting further supports the application of INTASYL™ technology in immunotherapy of cancer. PH-762 has shown
to silence the expression of checkpoint molecule PD-1 in target human T cells in a potent and durable manner suitable for both
ACT and intra-tumoral injection, and increases function of patient derived TILs for ACT. The application of INTASYL™ compounds
to novel immuno-oncology targets was shown by the silencing of BRD4, a regulator of gene expression impacting cell differentiation
and function, by a BRD4 targeting INTASYL™ compound in human T cells during expansion for ACT, which has the potential to
confer superior anti-tumor activity.
We expect that
PH-762 can be ready to enter into the clinic with a partner in ACT therapy in the second half of 2020 and we are developing PH-804
with the aim to enter the clinic with a partner in ACT in 2021.
Tumor Micro-Environment
The TME is the environment that surrounds
and feeds a tumor, including normal cells, blood vessels, immune cells and the extracellular matrix. A tumor can change the microenvironment
and the microenvironment can affect how a tumor grows and spreads and can create an immunosuppressive microenvironment that inhibits
the immune system’s natural ability to recognize and destroy tumor cells. This attracts immunosuppressive cells, induces
and activates immune checkpoint expression and excludes and exhausts T cells. Reprogramming different components of the TME may
overcome its resistance to immunotherapy.
Such reprogramming of the TME by INTASYL™
compounds through direct local administration into the tumor, could potentially become an important form of (neo)adjuvant therapy.
We believe that this will also show that our contributions with our INTASYL™ compounds
in immuno-oncology are not limited to use with a cell therapy platform. Additionally, the Company has shown in a clinical setting
that its INTASYL™ compounds are safe and well-tolerated following
local administration.
Our INTASYL™ compounds being developed
for use in ACT, are also being developed for use directly towards the TME, including PH-762 and PH-804. We are also working on
other relevant compounds for TME targets, such as PH-790, an INTASYL™ compound targeting PD-L1. PD-L1 is a protein that keeps
immune cells from attacking nonharmful cells in the body. If cancer cells have large amounts of PD-L1, this “tricks”
the immune system into not recognizing and attacking the tumor. Our approach with PH-790 is to block the PD-L1 protein, which may
prevent cancer cells from inactivating T cells and attack the cancer.
Our collaborative research agreement with
Gustave Roussy, a leading comprehensive cancer center in France, concentrates on determining the feasibility of our INTASYL™ platform
to target the TME via intra-tumoral injection. An in vivo study completed with Gustave Roussy demonstrated that
an INTASYL™ compound delivered via intra-tumoral injection showed
silencing of gene expression with our INTASYL™ compounds with greater
than 90% reduction of the target gene expression in a mouse model of melanoma.
Recent in vivo studies
performed by the Company showed that intra-tumoral injections of a mouse version of PH-804 reduced the tumor growth in colorectal
carcinoma tumor bearing mice, which was shown to be correlated with the silencing of TIGIT messenger RNA (“mRNA”)
expression and an increase in cytotoxic effector T cells in the TME.
The Company expects to move PH-762 for intra-tumoral
injection into the clinical development stage in 2021.
For
additional information about the Company, please refer to other documents we have filed with the Securities and Exchange Commission
and that are incorporated by reference into this prospectus, as listed under the heading “Incorporation of Certain Information
by Reference.”
Corporate Information
We were incorporated
in the state of Delaware in 2011 as RXi Pharmaceuticals Corporation. On November 19, 2018, the Company changed its name to Phio
Pharmaceuticals Corp., to reflect its transition from a platform company to one that is fully committed to developing groundbreaking
immuno-oncology therapeutics. Our executive offices are located at 257 Simarano Drive, Suite 101, Marlborough, MA 01752, and our
telephone number is (508) 767-3861. The Company’s website address is http://www.phiopharma.com. Our website and the
information contained on that site, or connected to that site, is not part of or incorporated by reference into this prospectus.
THE OFFERING
|
|
|
|
|
Common stock offered by this prospectus supplement:
|
|
1,713,064 shares of Common Stock.
|
|
|
|
|
Offering price:
|
|
$2.21 per share of Common Stock.
|
|
|
|
|
Common stock outstanding after this offering:
|
|
2,382,497 shares.1
|
|
|
|
|
Concurrent private placement:
|
|
We are offering 1,713,064 shares of our Common Stock in this
offering pursuant to this prospectus supplement and the accompanying base prospectus and a securities purchase agreement at a
price of $2.21 per share. In a concurrent private placement, we are also selling to investors, at a purchase price of $0.125
per underlying share, Warrants to purchase an additional 100% of the number of shares of Common Stock purchased in this
offering. Each Warrant will be exercisable for one share of Common Stock at an exercise price of $2.21 per share. The
Warrants will be immediately exercisable and expire five and one-half years following the date of issuance. The Warrants and
the shares of Common Stock issuable upon the exercise of the Warrants, or the Warrant Shares, are not being registered under
the Securities Act, pursuant to the registration statement of which this prospectus supplement and the accompanying base
prospectus form a part nor are such Warrants and Warrant Shares being offered pursuant to such prospectus supplement and base
prospectus and are being offered pursuant to an exemption provided in Section 4(a)(2) of the Securities Act and Rule 506(b)
promulgated thereunder. The Warrants are not and will not be listed for trading on any national securities exchange. Each
purchaser will be an “accredited investor” as such term is defined in Rule 501(a) under the Securities Act. See
"Private Placement Transaction and Warrants.”
|
|
|
|
|
Use of proceeds:
|
|
We intend to use the proceeds from this offering to fund the development of the Company’s immuno-oncology program, for other research and development activities and for general working capital needs. See “Use of Proceeds.”
|
|
|
|
|
Risk factors:
|
|
You should read the “Risk Factors” section of this prospectus supplement for a discussion of factors to consider carefully before deciding to invest in shares of our securities.
|
|
|
|
|
Nasdaq Capital Market symbol:
|
|
Our Common Stock is listed on the Nasdaq Capital Market under the symbol “PHIO.”
|
1 The number of shares of Common Stock to be outstanding
after this offering is based on 669,433 shares of Common Stock outstanding as of December 31, 2019 and excludes:
|
|
·
|
197,056 shares of Common issued on February 6, 2020, along with warrants to purchase an additional 197,056 shares of Common
Stock at an exercise price of $8.71 per share;
|
|
|
·
|
993,633 shares of Common Stock and pre-funded warrants to purchase an aggregate of 1,006,367 shares of Common Stock issued
on February 13, 2020, along with warrants to purchase an additional 2,300,000 shares of Common Stock at an exercise price of $4.00
per share;
|
|
|
·
|
2,649 shares of Common Stock issuable upon the exercise of stock options outstanding as of December 31, 2019, having a weighted
average exercise price of $3,298.90 per share;
|
|
|
·
|
14,945 shares of common stock issuable upon the vesting of restricted stock units outstanding as of December 31, 2019;
|
|
|
·
|
480,035 shares of Common Stock issuable upon the exercise of warrants outstanding as of December 31, 2019, having a weighted
average exercise price of $55.58 per share;
|
|
|
·
|
An aggregate of 53,864 shares of Common Stock reserved for future issuance under our 2012 Long-Term Incentive Plan as of December
31, 2019; and
|
|
|
·
|
An aggregate of 8,084 shares of Common Stock reserved for future issuance under our Employee Stock Purchase Plan as of December,
2019.
|
Unless otherwise indicated, all information in this prospectus
supplement assumes no exercise of the Warrants to be issued to the investors in the concurrent private placement and no exercise
of the warrants to be issued as compensation to the placement agent for this offering.
RISK FACTORS
Investing in our securities involves
a high degree of risk. Before investing in our securities, you should carefully consider the risks, uncertainties and assumptions
described below, discussed under the heading “Risk Factors” included in our most recent Annual Report on Form 10-K
for the year ended December 31, 2019, as revised or supplemented by subsequent filings, which are on file with the SEC and are
incorporated herein by reference, and which may be amended, supplemented or superseded from time to time by other reports we file
with the SEC in the future. Our business, financial condition, results of operations and future growth prospects could be materially
and adversely affected by any of these risks. In these circumstances, the market price of our Common Stock could decline, and you
may lose all or part of your investment.
Risks Related to this Offering
We have broad discretion in how we
use the net proceeds of this offering, and we may not use these proceeds effectively or in ways with which you agree.
Our management will have broad discretion
as to the application of the net proceeds of this offering and could use them for purposes other than those contemplated at the
time of the offering. Our stockholders may not agree with the manner in which our management chooses to allocate and spend the
net proceeds. Moreover, our management may use the net proceeds for corporate purposes that may not increase the market price of
our common stock.
The offering price was set by our Board of Directors and
does not necessarily indicate the actual or market value of our common stock.
Our Board of Directors approved the offering
price and other terms of this offering after considering, among other things: the number of shares authorized in our certificate
of incorporation; the current market price of our common stock; trading prices of our common stock over time; the volatility of
our common stock; our current financial condition and the prospects for our future cash flows; the availability of and likely cost
of capital of other potential sources of capital; and market and economic conditions at the time of the offering. The offering
price is not intended to bear any relationship to the book value of our assets or our past operations, cash flows, losses, financial
condition, net worth or any other established criteria used to value securities. The offering price may not be indicative of the
fair value of the common stock.
You may experience further dilution if we issue additional
equity securities in future fundraising transactions.
To raise additional capital, we may in
the future offer additional shares of our Common Stock or other securities convertible into or exchangeable for our Common Stock
at prices that may not be the same as the price per share in this offering. We may sell shares or other securities in any other
offering at a price per share that is less than the price per share paid by investors in this offering, and investors purchasing
shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell
additional shares of our Common Stock, or securities convertible or exchangeable into Common Stock, in future transactions may
be higher or lower than the price per share paid by investors in this offering. Further, the exercise of outstanding stock options
and warrants may result in further dilution of your investment.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING
STATEMENTS
This prospectus supplement contains forward-looking
statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified
by words such as “intends,” “believes,” “anticipates,” “indicates,” “plans,”
“expects,” “suggests,” “may,” “would,” “should,” “potential,”
“designed to,” “will,” “ongoing,” “estimate,” “forecast,” “predict,”
“could,” and similar references, although not all forward-looking statements contain these words. Forward-looking statements
are neither historical facts nor assurances of future performance. These statements are based only on our current beliefs, expectations
and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends,
the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent
uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Risks
that could cause actual results to vary from expected results expressed in our forward-looking statements include, but are not
limited to:
|
|
·
|
our business and operations may be materially and adversely affected by the recent coronavirus outbreak;
|
|
|
·
|
our product candidates are in an early stage of development and may fail or experience significant delays or may never advance to the clinic, which may materially and adversely impact our business;
|
|
|
·
|
we are dependent on collaboration partners for the successful development of our adoptive cell therapy product candidates;
|
|
|
·
|
the approach we are taking to discover and develop novel therapeutics using RNAi may never lead to marketable products;
|
|
|
·
|
a number of different factors could prevent us from advancing into clinical development, obtaining regulatory approval, and ultimately commercializing our product candidates on a timely basis, or at all;
|
|
|
·
|
the FDA could impose a unique regulatory regime for our therapeutics;
|
|
|
·
|
we may be unable to protect our intellectual property rights licensed from other parties; our intellectual property rights may be inadequate to prevent third parties from using our technologies or developing competing products; and we may need to license additional intellectual property from others;
|
|
|
·
|
we are subject to significant competition and may not be able to compete successfully;
|
|
|
·
|
if we fail to attract, hire and retain qualified personnel, we may not be able to design, develop, market or sell our products or successfully manage our business;
|
|
|
·
|
future financing may be obtained through, and future development efforts may be paid for by, the issuance of debt or equity, which may have an adverse effect on our stockholders or may otherwise adversely affect our business; and
|
|
|
·
|
the price of our common stock has been and may continue to be volatile.
|
Our actual results and financial condition
may differ materially from those indicated in the forward-looking statements as a result of the foregoing factors, including those
identified in this prospectus under the heading “Risk Factors,” for the reasons described elsewhere in this prospectus
and in other filings the Company periodically makes with the Securities and Exchange Commission. Therefore, you should not rely
unduly on any of these forward-looking statements. Forward-looking statements contained in this prospectus speak as of the date
hereof and Phio does not undertake to update any of these forward-looking statements to reflect a change in its views or events
or circumstances that occur after the date of this report.
USE OF PROCEEDS
We estimate that the net proceeds from
the sale of the shares of Common Stock that we are offering will be approximately $3.3 million, after deducting the placement agent
fees and estimated offering expenses payable by us and excluding any proceeds we may receive upon exercise of the warrants being
offered in the concurrent private placement.
We intend to use the net proceeds from
this offering to fund the development of the Company’s immuno-oncology program, for other research and development activities
and for general working capital needs. We may also use a portion of the net proceeds to acquire or invest in complementary businesses,
products and technologies or to fund the development of any such complementary businesses, products or technologies that we may
acquire in a stock-based acquisition. We have no current plans for any such acquisitions.
PLAN OF DISTRIBUTION
Pursuant to an engagement letter agreement
dated January 31, 2020, we have engaged H.C. Wainwright & Co., LLC, (“Wainwright” or the “placement
agent”) to act as our exclusive placement agent in connection with this offering of our shares of Common Stock pursuant
to this prospectus supplement and accompanying prospectus. Under the terms of the engagement agreement, the placement agent has
agreed to be our exclusive placement agent, on a reasonable best efforts basis, in connection with the issuance and sale by us
of our shares of Common Stock in this takedown from our shelf registration statement. The terms of this offering were subject to
market conditions and negotiations between us, the placement agent and prospective investors. The engagement agreement does not
give rise to any commitment by the placement agent to purchase any of our shares of common stock, and the placement agent will
have no authority to bind us by virtue of the engagement agreement. Further, the placement agent does not guarantee that it will
be able to raise new capital in any prospective offering. The placement agent may engage sub-agents or selected dealers to assist
with the offering.
The placement agent proposes to arrange
for the sale of the shares we are offering pursuant to this prospectus supplement and accompanying prospectus to one or more institutional
or accredited investors through securities purchase agreements directly between the purchasers and us. We will only sell to such
investors who have entered into the securities purchase agreement with us.
We expect to deliver the shares of our
common stock being offered pursuant to this prospectus supplement on or about April 2, 2020.
We have agreed to pay the placement agent
a total cash fee equal to 7.5% of the gross proceeds of this offering. We will also pay the placement agent $90,000 for non-accountable
expenses, a management fee equal to 1.0% of the gross proceeds raised in the offering and $12,900 for clearing fees. We estimate
the total expenses payable by us for this offering will be approximately $470,000, which amount includes the placement agent’s
fees and reimbursable expenses. In addition, we have agreed to issue to the placement agent warrants to purchase up to 7.5% of
the aggregate number of shares of common stock sold in this offering (128,480 shares). The placement agent warrants will have
substantially the same terms as the warrants issued to the investors in this offering, except that the placement agent warrants
will have an exercise price equal to $2.9188, or 125% of the offering price per share and Warrant (combined) and will be exercisable
for five years from the effective date of this offering. Pursuant to FINRA Rule 5110(g), the placement agent warrants and any
shares issued upon exercise of the placement agent warrants shall not be sold, transferred, assigned, pledged, or hypothecated,
or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic
disposition of the securities by any person for a period of 180 days immediately following the date of effectiveness or commencement
of sales of this offering, except the transfer of any security: (i) by operation of law or by reason of our reorganization; (ii)
to any FINRA member firm participating in the offering and the officers or partners thereof, if all securities so transferred
remain subject to the lock-up restriction set forth above for the remainder of the time period; (iii) if the aggregate amount
of our securities held by the placement agent or related persons do not exceed 1% of the securities being offered; (iv) that is
beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no participating member manages
or otherwise directs investments by the fund and the participating members in the aggregate do not own more than 10% of the equity
in the fund; or (v) the exercise or conversion of any security, if all securities remain subject to the lock-up restriction set
forth above for the remainder of the time period.
We have granted the placement agent a twelve-month
right of first refusal to act as our exclusive underwriter or placement agent for any further capital raising transactions undertaken
by us.
We also have granted the placement agent
a tail cash fee equal to 7.5% of the gross proceeds and warrants to purchase shares of common stock equal to 7.5% of the aggregate
number of shares of common stock sold in any offering, within twelve months following the termination of the engagement letter,
to investors whom the placement agent contacted or introduced to us directly or indirectly in connection with this offering.
We have agreed to indemnify the placement
agent and specified other persons against certain liabilities relating to or arising out of the placement agent’s activities
under the placement agency agreement and to contribute to payments that the placement agent may be required to make in respect
of such liabilities.
The placement agent may be deemed to be
an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit
realized on the resale of the securities sold by it while acting as principal might be deemed to be underwriting discounts or commissions
under the Securities Act. As an underwriter, the placement agent would be required to comply with the requirements of the Securities
Act and the Exchange Act, including, without limitation, Rule 415(a)(4) under the Securities Act and Rule 10b-5 and Regulation
M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of shares of common stock and
warrants by the placement agent acting as principal. Under these rules and regulations, the placement agent:
|
|
·
|
|
may not engage in any stabilization activity in connection with our securities; and
|
|
|
·
|
|
may not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until it has completed its participation in the distribution.
|
From time to time, the placement agent
may provide in the future various advisory, investment and commercial banking and other services to us in the ordinary course
of business, for which they have received and may continue to receive customary fees and commissions. The placement agent acted
as our exclusive placement agent in connection with the registered direct offering we consummated in February 2020 and our underwriter
in connection with the public offering we consummated in November 2019, for which it received compensation. However, except as
disclosed in this prospectus, we have no present arrangements with the placement agent for any further services.
PRIVATE PLACEMENT TRANSACTION AND WARRANTS
In a concurrent private placement, we are
selling to each of the investors in this offering for consideration of $0.125 per underlying warrant share, a Warrant to purchase
an additional 100% of the number of shares purchased in this offering. The aggregate number of shares of Common Stock exercisable
pursuant to the Warrants is 1,713,064. The Warrants will be exercisable at an exercise price of $2.21 per share, subject to certain
adjustments, will be immediately exercisable and expire five and one-half years following the date of issuance.
The exercise price and number of shares
of common stock issuable upon the exercise of the Warrants will be subject to adjustment in the event of any stock dividend and
split, reverse stock split, recapitalization, reorganization or similar transaction, as described in the Warrants.
The Warrants and the Warrant Shares are
not being registered under the Securities Act pursuant to the registration statement of which this prospectus supplement and the
accompanying base prospectus form a part and are not being offered pursuant to this prospectus supplement and the accompanying
base prospectus. The Warrants and the Warrant Shares are being offered pursuant to the exemption provided in Section 4(a)(2) of
the Securities Act and Rule 506(b) promulgated thereunder.
After the exercise date of the Warrants,
if and only if there is no effective registration statement registering the applicable shares of Common Stock, or no current prospectus
available for such shares, the resale of the shares of Common Stock issuable upon exercise of the Warrants, the purchasers may
exercise the Warrants by means of a “cashless exercise.”
All purchasers are required to be “accredited
investors” as such term is defined in Rule 501(a) under the Securities Act.
LEGAL MATTERS
Certain legal matters relating to the issuance
of the securities offered by this prospectus will be passed upon for us by Gibson, Dunn & Crutcher LLP, San Francisco, California.
EXPERTS
The
consolidated financial statements as of December 31, 2019 and 2018 and for each of the two years in the period ended December 31,
2019 incorporated by reference in this prospectus have been so incorporated in reliance on the report of BDO USA, LLP, an independent
registered public accounting firm, and have been incorporated in this prospectus in reliance upon such report and given on the
authority of said firm as experts in auditing and accounting.
Where
You Can Find More Information
We
are required to file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and
copy any document filed by us at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Our filings
with the SEC are also available to the public at the SEC’s Internet web site at http://www.sec.gov. Copies
of certain information filed by us with the SEC are also available on our website at www.phiopharma.com. Our website is
not a part of this prospectus and is not incorporated by reference in this prospectus, and you should not consider the contents
of our website in making an investment decision with respect to our common stock.
We
have filed a registration statement, of which this prospectus is a part, covering the securities offered hereby. As allowed by
SEC rules, this prospectus does not include all of the information contained in the registration statement and the included exhibits,
financial statements and schedules. You are referred to the registration statement, the included exhibits, financial statements
and schedules for further information. You should review the information and exhibits in the registration statement for
further information about us and our subsidiaries and the securities we are offering. Statements in this prospectus concerning
any document we filed as an exhibit to the registration statement or that we otherwise filed with the SEC are not intended to be
comprehensive and are qualified by reference to these filings. You should review the complete document to evaluate these statements.
INCORPORATION OF CERTAIN INFORMATION
BY REFERENCE
The SEC allows us to “incorporate
by reference” the information we have filed with them, which means that we can disclose important information to you by referring
you to those documents. The information we incorporate by reference is an important part of this prospectus supplement, and information
that we file later with the SEC will automatically update and supersede this information. The documents we are incorporating by
reference are:
|
|
·
|
|
Our Current Reports on Form 8-K, filed with the SEC on January 10, 2020, January 14, 2020, February 6, 2020, February 10, 2020, February 13, 2020 and March 12, 2020; and
|
|
|
·
|
|
The description of our common stock contained in our registration statement on Form 8-A12B filed with the SEC on February 7, 2014, including any amendment or report filed for the purpose of updating such description.
|
All
documents we file with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, except as to any portion of
any report or document that is not deemed filed under such provisions, (1) on or after the date of filing of the registration
statement containing this prospectus and prior to the effectiveness of the registration statement and (2) on or after the
date of this prospectus until the earlier of the date on which all of the securities registered hereunder have been sold or the
registration statement of which this prospectus is a part has been withdrawn, shall be deemed incorporated by reference in this
prospectus and to be a part of this prospectus from the date of filing of those documents and will be automatically updated and,
to the extent described above, supersede information contained or incorporated by reference in this prospectus and previously filed
documents that are incorporated by reference in this prospectus.
Nothing in this prospectus shall be deemed
to incorporate information furnished but not filed with the SEC pursuant to Item 2.02, 7.01 or 9.01 of Form 8-K.
Upon written or oral request, we will provide
without charge to each person, including any beneficial owner, to whom a copy of the prospectus is delivered a copy of any or all
of the reports or documents incorporated by reference herein (other than exhibits to such documents, unless such exhibits are specifically
incorporated by reference herein). You may request a copy of these filings, at no cost, by writing or telephoning us at the following
address: Phio Pharmaceuticals Corp., 257 Simarano Drive, Suite 101, Marlborough, Massachusetts 01752 Attention: Investor Relations,
telephone: (508) 767-3861. We maintain a website at www.phiopharma.com. You may access our definitive proxy
statements on Schedule 14A, annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on
Form 8-K and periodic amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange
Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with,
or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not incorporated by reference
in, and is not part of, this prospectus. We have not authorized any one to provide you with any information that differs from that
contained in this prospectus. Accordingly, you should not rely on any information that is not contained in this prospectus. You
should not assume that the information in this prospectus is accurate as of any date other than the date of the front cover of
this prospectus.
PROSPECTUS
$100,000,000
RXi Pharmaceuticals Corporation

Common Stock
Preferred Stock
Debt Securities
Warrants
Units
We may offer and sell an indeterminate
number of shares of our common stock and preferred stock, debt securities, warrants and/or units from time to time under this prospectus.
We will describe in a prospectus supplement or sales agreement prospectus the securities we are offering and selling, as well as
the specific terms of the securities.
We may offer these securities in amounts,
at prices and on terms determined at the time of offering. We may sell the securities directly to you, through agents we select,
or through underwriters and dealers we select. If we use agents, underwriters or dealers to sell the securities, we will name them
and describe their compensation in a prospectus supplement or sales agreement prospectus.
Our common stock trades on the NASDAQ Capital
Market under the symbol “RXII”. On March 28, 2018, the closing price for our common stock, as reported on the
NASDAQ Capital Market, was $3.56 per share.
Investing in our securities involves
certain risks. See “Risk Factors” beginning on Page 2 of this prospectus and in the applicable prospectus supplement
or sales agreement prospectus for certain risks you should consider. You should read the entire prospectus carefully before you
make your investment decision.
Neither the Securities and Exchange
Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus
is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is
April 6, 2018.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration
statement that we filed with the Securities and Exchange Commission, or SEC, utilizing a shelf registration process. Under the
shelf registration process, we may offer shares of our common stock and preferred stock, various series of debt securities, warrants
and units to purchase any of such securities with a total value of up to $100,000,000 from time to time under this prospectus at
prices and on terms to be determined by market conditions at the time of offering. This prospectus provides you with a general
description of the securities we may offer. Each time we offer a type or series of securities, we will provide a prospectus supplement
(which term includes, as applicable, the sales agreement prospectus filed with the registration statement of which this prospectus
forms a part) that will describe the specific amounts, prices and other important terms of the securities, including, to the extent
applicable:
|
|
•
|
|
designation or classification;
|
|
|
|
|
|
|
|
•
|
|
aggregate principal amount or aggregate offering price;
|
|
|
|
|
|
|
|
•
|
|
maturity;
|
|
|
|
|
|
|
|
•
|
|
original issue discount, if any;
|
|
|
|
|
|
|
|
•
|
|
rates and times of payment of interest, dividends or other payments, if any;
|
|
|
|
|
|
|
|
•
|
|
redemption, conversion, exchange, settlement or sinking fund terms, if any;
|
|
|
|
|
|
|
|
•
|
|
conversion, exchange or settlement prices or rates, if any, and, if applicable, any provisions for changes to or adjustments in the conversion, exchange or settlement prices or rates and in the securities or other property receivable upon conversion, exchange or settlement;
|
|
|
|
|
|
|
|
•
|
|
ranking;
|
|
|
|
|
|
|
|
•
|
|
restrictive covenants, if any;
|
|
|
|
|
|
|
|
•
|
|
voting or other rights, if any; and
|
|
|
|
|
|
|
|
•
|
|
important federal income tax considerations.
|
A prospectus supplement may include a
discussion of risks or other special considerations applicable to us or the offered securities. A prospectus supplement may also
add, update or change information in this prospectus. If there is any inconsistency between the information in this prospectus
and the applicable prospectus supplement, you must rely on the information in the prospectus supplement. Please carefully read
both this prospectus and the applicable prospectus supplement in their entirety together with additional information described
under the heading “Where You Can Find More Information” in this prospectus. This prospectus may not be used to offer
or sell any securities unless accompanied by a prospectus supplement.
The registration statement containing
this prospectus, including exhibits to the registration statement, provides additional information about us and the securities
offered under this prospectus. The registration statement can be read on the SEC’s website or at the SEC’s public reading
room mentioned under the heading “Where You Can Find More Information” in this prospectus.
We have not authorized any broker-dealer,
salesperson or other person to give any information or to make any representation other than those contained or incorporated by
reference in this prospectus and the accompanying prospectus supplement. You must not rely upon any information or representation
not contained or incorporated by reference in this prospectus or the accompanying prospectus supplement. This prospectus and the
accompanying prospectus supplement do not constitute an offer to sell or the solicitation of an offer to buy securities, nor do
this prospectus and the accompanying prospectus supplement constitute an offer to sell or the solicitation of an offer to buy securities
in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation. The information contained in this
prospectus and the accompanying prospectus supplement speaks only as of the date set forth on the cover page and may not reflect
subsequent changes in our business, financial condition, results of operations and prospects even though this prospectus and any
accompanying prospectus supplement is delivered or securities are sold on a later date.
PROSPECTUS SUMMARY
In this prospectus, unless the context
otherwise requires, (1) the term “RXi” refers to RXi Pharmaceuticals Corporation and our subsidiary, MirImmune,
LLC and (2) the terms “Company,” “we,” “us” and “our” refer to the ongoing
business operations of RXi and MirImmune, LLC, whether conducted through RXi or MirImmune, LLC.
Overview
RXi Pharmaceuticals Corporation is a biotechnology
company focused on discovering and developing immuno-oncology therapeutics to treat cancer based on our self-delivering RNAi, or
sd-rxRNA®, platform. Our sd-rxRNA compounds do not require a delivery
vehicle to penetrate the cell and are designed to “silence,” or down-regulate, the expression of a specific gene that
may be over-expressed in a disease condition. We believe that this provides RXi with a distinct advantage in adoptive cell therapy,
the Company’s initial focus and approach to immuno-oncology.
Prior to the acquisition of MirImmune
Inc., or MirImmune, in January 2017, the Company’s principal activities consisted of the preclinical and clinical development
of our sd-rxRNA compounds and topical immunotherapy agent in the areas of dermatology and ophthalmology. In January 2018, after
a thorough review of its business operations, development programs and financial resources, the Company made a strategic decision
to focus solely on immuno-oncology to accelerate growth and support a potential return on investment for its stockholders. The
Company’s business strategy will focus on the discovery and development of immuno-oncology therapeutics utilizing our proprietary
sd-rxRNA technology. The Company plans to complete its current ongoing clinical trials in dermatology and ophthalmology with RXI-109
and Samcyprone™ and intends to seek a partner and/or out-license these programs
to continue the clinical development and commercialization. The goal of any such transaction would be to allow the Company to monetize
these clinical assets to further fund ongoing and future development work in our immuno-oncology programs and extend our financial
runway.
For additional information about our Company,
please refer to other documents we have filed with the SEC and that are incorporated by reference into this prospectus, as listed
under the heading “Incorporation of Certain Information by Reference.”
Our offices are located at 257 Simarano
Drive, Suite 101, Marlborough, Massachusetts and our telephone number is (508) 767-3861. Additional information about RXi
can be found on our website, at www.rxipharma.com, and in our periodic and current reports filed with the SEC. Copies of
our current and periodic reports filed with the SEC are available at the SEC Public Reference Room at 100 F Street, N.E., Washington,
D.C. 20549, and online at www.sec.gov and our website at www.rxipharma.com. No portion of our website is incorporated
by reference into this prospectus.
RISK FACTORS
Before making an investment decision,
you should carefully consider the risks described under “Risk Factors” in the applicable prospectus supplement, together
with all of the other information appearing in this prospectus or incorporated by reference into this prospectus and any applicable
prospectus supplement, in light of your particular investment objectives and financial circumstances. Our business, financial condition
or results of operations could be materially adversely affected by any of these risks. The trading price of our securities could
decline due to any of these risks, and you may lose all or part of your investment. This prospectus and the incorporated documents
also contain forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those
anticipated in these forward-looking statements as a result of certain factors, including the risks mentioned elsewhere in this
prospectus. You should also consider the risks, uncertainties and assumptions discussed under the heading “Risk Factors”
included in our most recent Annual Report on Form 10-K, which is on file with the SEC and is incorporated herein by reference,
and which may be amended, supplemented or superseded from time to time by other reports we file with the SEC in the future.
We are not in compliance with the
Nasdaq continued listing requirements. If we are unable to comply with the continued listing requirements of the Nasdaq Capital
Market, our Common Stock could be delisted, which could affect our Common Sock’s market price and liquidity and reduce our
ability to raise capital.
Our common stock is listed on the Nasdaq
Capital Market. The listing rules of the Nasdaq Capital Market require the Company to meet certain minimum requirements, including
at least $2.5 million of stockholders’ equity. As of December 31, 2017, we failed to meet this required level of stockholders’
equity and, on March 29, 2017, we received a notice from Nasdaq regarding our non-compliance. We believe that if we are able to
execute a successful offering, we will be able to provide sufficient stockholders’ equity to enable us to regain compliance
with the Nasdaq listing rules. However, there can be no assurance that Nasdaq will accept our proposed compliance plan or that
we will not fail to satisfy other Nasdaq listing criteria, such as the minimum bid requirement.
If we fail to comply with the Nasdaq Capital
Market’s continued listing standards, we may be delisted and our common stock will trade, if at all, only on the over-the-counter
market, such as the OTC Bulletin Board or OTCQX market, and then only if one or more registered broker-dealer market makers comply
with quotation requirements. In addition, delisting of our common stock could depress our stock price, substantially limit liquidity
of our common stock and materially adversely affect our ability to raise capital on terms acceptable to us, or at all.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING
STATEMENTS
This prospectus contains forward-looking
statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified
by words such as “intends,” “believes,” “anticipates,” “indicates,” “plans,”
“expects,” “suggests,” “may,” “should,” “potential,” “designed
to,” “will” and similar references, although not all forward-looking statements contain these words. Forward-looking
statements are neither historical facts nor assurances of future performance. These statements are based only on our current beliefs,
expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events
and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject
to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our
control. Risks that could cause actual results to vary from expected results expressed in our forward-looking statements include,
but at not limited to:
|
|
•
|
|
our ability to obtain sufficient financing to develop our product candidates;
|
|
|
•
|
|
expected ongoing significant research and development expenses without a current source of revenue, which may lead to uncertainty as to our ability to continue as a going concern;
|
|
|
•
|
|
dilution that could be caused by future financing transactions or future issuances of capital stock in strategic transactions;
|
|
|
•
|
|
our strategic focus on immuno-oncology;
|
|
|
•
|
|
the novel and unproven approach associated with our RNAi technology;
|
|
|
•
|
|
our limited experience as a company in immuno-oncology;
|
|
|
•
|
|
identifying and developing product candidates, including whether we are able to commence clinical trials in humans or obtain approval for our product candidates;
|
|
|
•
|
|
our dependence on the success of our product candidates, which may not receive regulatory approval or be successfully commercialized;
|
|
|
•
|
|
factors could prevent us from obtaining regulatory approval or commercializing our product candidates on a timely basis, or at all;
|
|
|
•
|
|
FDA regulation of our therapeutics;
|
|
|
•
|
|
our reliance on in-licensed technologies and the potential need for additional intellectual property rights in the future;
|
|
|
•
|
|
our ability protect our intellectual property rights and the adequacy of our intellectual property rights;
|
|
|
•
|
|
competitive risks, including the risks associated with competing against companies in the immuno-oncology space with significantly greater resources;
|
|
|
•
|
|
our reliance on third parties for the manufacture of our clinical product candidates;
|
|
|
•
|
|
potential product liability claims;
|
|
|
•
|
|
pricing regulations, third-party reimbursement practices or healthcare reform initiatives;
|
|
|
•
|
|
our ability to attract, hire and retain qualified personnel;
|
|
|
•
|
|
effectiveness of our internal control over financial reporting; and
|
|
|
•
|
|
volatility of our common stock.
|
Our actual results and financial condition
may differ materially from those indicated in the forward-looking statements as a result of the foregoing factors, as well as those
identified in this prospectus under the heading “Risk Factors” and in other filings the Company periodically makes
with the Securities and Exchange Commission. Therefore, you should not rely on any of these forward-looking statements. Forward-looking
statements contained in this prospectus speak as of the date hereof and the Company does not undertake to update any of these forward-looking
statements to reflect a change in its views or events or circumstances that occur after the date of this report.
DESCRIPTION OF SECURITIES
We may offer shares of our common stock
and preferred stock, various series of debt securities, warrants, and units to purchase any such securities with a total value
of up to $100,000,000 from time to time under this prospectus at prices and on terms to be determined by market conditions at the
time of offering. Each time we offer a type or series of securities, we will provide a prospectus supplement that will describe
the specific amounts, prices and other important terms of the securities.
Common Stock.
We may issue shares of our common stock
from time to time. Holders of our common stock are entitled to one vote per share for the election of directors and on all other
matters that require stockholder approval. Subject to any preferential rights of any outstanding preferred stock, in the event
of our liquidation, dissolution or winding up, holders of our common stock are entitled to share ratably in the assets remaining
after payment of liabilities and the liquidation preferences of any outstanding preferred stock. Our common stock does not carry
any redemption rights or any preemptive rights enabling a holder to subscribe for, or receive shares of, any class of our common
stock or any other securities convertible into shares of any class of our common stock.
We are authorized to issue 100,000,000
shares of common stock, par value $0.0001 per share, of which 2,699,962 shares were issued and outstanding as of March 28,
2018.
Our common stock is listed on the NASDAQ
Capital Market under the symbol “RXII”. The transfer agent and registrar for our common stock is Computershare Trust
Company, N.A.
Preferred Stock.
We may issue shares of our preferred stock
from time to time, in one or more series. Under our certificate of incorporation, our board of directors has the authority, without
further action by stockholders, to designate up to 10,000,000 shares of preferred stock in one or more series and to fix the rights,
preferences, privileges, qualifications and restrictions granted to or imposed upon the preferred stock, including dividend rights,
conversion rights, voting rights, rights and terms of redemption, liquidation preference and sinking fund terms, any or all of
which may be greater than the rights of the common stock.
If we issue preferred stock, we will fix
the rights, preferences, privileges, qualifications and restrictions of the preferred stock of each series that we sell under this
prospectus and applicable prospectus supplements in the certificate of designations relating to that series. If we issue preferred
stock, we will incorporate by reference into the registration statement of which this prospectus is a part the form of any certificate
of designations that describes the terms of the series of preferred stock we are offering before the issuance of the related series
of preferred stock. We urge you to read the prospectus supplement related to any series of preferred stock we may offer, as well
as the complete certificate of designations that contains the terms of the applicable series of preferred stock.
Debt Securities.
The paragraphs below describe the general
terms and provisions of the debt securities we may issue. When we offer to sell a particular series of debt securities, we will
describe the specific terms of the securities in a supplement to this prospectus, including any additional covenants or changes
to existing covenants relating to such series. The prospectus supplement also will indicate whether the general terms and provisions
described in this prospectus apply to a particular series of debt securities. You should read the actual indenture if you do not
fully understand a term or the way we use it in this prospectus or applicable prospectus supplement.
We may offer senior or subordinated debt
securities. Each series of debt securities may have different terms. The senior debt securities will be issued under one or more
senior indentures, dated as of a date prior to such issuance, between us and a trustee, as amended or supplemented from time to
time. We will refer to any such indenture throughout this prospectus as the “senior indenture.” Any subordinated debt
securities will be issued under one or more separate indentures, dated as of a date prior to such issuance, between us and a trustee,
as amended or supplemented from time to time. We will refer to any such indenture throughout this prospectus as the “subordinated
indenture” and to the trustee under the senior or subordinated indenture as the “trustee.” The senior indenture
and the subordinated indenture are sometimes collectively referred to in this prospectus as the “indentures.” The indentures
will be subject to and governed by the Trust Indenture Act of 1939, as amended. We included copies of the forms of the indentures
as exhibits to our registration statement and they are incorporated into this prospectus by reference.
If we issue debt securities at
a discount from their principal amount, then, for purposes of calculating the aggregate initial offering price of the offered
securities issued under this prospectus, we will include only the initial offering price of the debt securities and not the principal
amount of the debt securities.
We have summarized below the material
provisions of the indentures and the debt securities, or indicated which material provisions will be described in the related prospectus
supplement. The prospectus supplement relating to any particular securities offered will describe the specific terms of the securities,
which may be in addition to or different from the general terms summarized in this prospectus. Because the summary in this prospectus
and in any prospectus supplement does not contain all of the information that you may find useful, you should read the documents
relating to the securities that are described in this prospectus or in any applicable prospectus supplement. Please read “Where
You Can Find More Information” in this prospectus to find out how you can obtain a copy of those documents. Except as otherwise
indicated, the terms of the indentures are identical. As used under this caption, the term “debt securities” includes
the debt securities being offered by this prospectus and all other debt securities issued by us under the indentures.
General
The indentures:
|
|
•
|
|
do not limit the amount of debt securities that we may issue;
|
|
|
•
|
|
allow us to issue debt securities in one or more series;
|
|
|
•
|
|
do not require us to issue all of the debt securities of a series at the same time;
|
|
|
•
|
|
allow us to reopen a series to issue additional debt securities without the consent of the holders of the debt securities of such series; and
|
|
|
•
|
|
provide that the debt securities will be unsecured, except as may be set forth in the applicable prospectus supplement.
|
Unless we give you different information
in the applicable prospectus supplement, the senior debt securities will be unsubordinated obligations and will rank equally with
all of our other unsecured and unsubordinated indebtedness. Payments on the subordinated debt securities will be subordinated to
the prior payment in full of all of our senior indebtedness, as described under “Description of the Debt Securities—Subordination”
and in the applicable prospectus supplement.
Each indenture provides that we may, but
need not, designate more than one trustee under an indenture. Any trustee under an indenture may resign or be removed and a successor
trustee may be appointed to act with respect to the series of debt securities administered by the resigning or removed trustee.
If two or more persons are acting as trustee with respect to different series of debt securities, each trustee shall be a trustee
of a trust under the applicable indenture separate and apart from the trust administered by any other trustee. Except as otherwise
indicated in this prospectus, any action described in this prospectus to be taken by each trustee may be taken by each trustee
with respect to, and only with respect to, the one or more series of debt securities for which it is trustee under the applicable
indenture.
The prospectus supplement for each offering
will provide the following terms, where applicable:
|
|
•
|
|
the title of the debt securities and whether they are senior or subordinated;
|
|
|
•
|
|
the aggregate principal amount of the debt securities being offered, the aggregate principal amount of the debt securities outstanding as of the most recent practicable date and any limit on their aggregate principal amount, including the aggregate principal amount of debt securities authorized;
|
|
|
•
|
|
the price at which the debt securities will be issued, expressed as a percentage of the principal and, if other than the principal amount thereof, the portion of the principal amount thereof payable upon declaration of acceleration of the maturity thereof or, if applicable, the portion of the principal amount of such debt securities that is convertible into common stock or preferred stock or the method by which any such portion shall be determined;
|
|
|
•
|
|
if convertible, the terms on which such debt securities are convertible, including the initial conversion price or rate and the conversion period and any applicable limitations on the ownership or transferability of common stock or preferred stock received on conversion;
|
|
|
•
|
|
the date or dates, or the method for determining the date or dates, on which the principal of the debt securities will be payable;
|
|
|
•
|
|
the fixed or variable interest rate or rates of the debt securities, or the method by which the interest rate or rates is determined;
|
|
|
•
|
|
the date or dates, or the method for determining the date or dates, from which interest will accrue;
|
|
|
•
|
|
the dates on which interest will be payable;
|
|
|
•
|
|
the record dates for interest payment dates, or the method by which we will determine those dates;
|
|
|
•
|
|
the persons to whom interest will be payable;
|
|
|
•
|
|
the basis upon which interest will be calculated if other than that of a 360-day year of twelve 30-day months;
|
|
|
•
|
|
any make-whole amount, which is the amount in addition to principal and interest that is required to be paid to the holder of a debt security as a result of any optional redemption or accelerated payment of such debt security, or the method for determining the make-whole amount;
|
|
|
•
|
|
the place or places where the principal of, and any premium, or make-whole amount, and interest on, the debt securities will be payable;
|
|
|
•
|
|
where the debt securities may be surrendered for registration of transfer or conversion or exchange;
|
|
|
•
|
|
where notices or demands to or upon us in respect of the debt securities and the applicable indenture may be served;
|
|
|
•
|
|
the times, prices and other terms and conditions upon which we may redeem the debt securities;
|
|
|
•
|
|
any obligation we have to redeem, repay or purchase the debt securities pursuant to any sinking fund or analogous provision or at the option of holders of the debt securities, and the times and prices at which we must redeem, repay or purchase the debt securities as a result of such an obligation;
|
|
|
•
|
|
the currency or currencies in which the debt securities are denominated and payable if other than United States dollars, which may be a foreign currency or units of two or more foreign currencies or a composite currency or currencies and the terms and conditions relating thereto, and the manner of determining the equivalent of such foreign currency in United States dollars;
|
|
|
•
|
|
whether the principal of, and any premium, or make-whole amount, or interest on, the debt securities of the series are to be payable, at our election or at the election of a holder, in a currency or currencies other than that in which the debt securities are denominated or stated to be payable, and other related terms and conditions;
|
|
|
•
|
|
whether the amount of payments of principal of, and any premium, or make-whole amount, or interest on, the debt securities may be determined according to an index, formula or other method and how such amounts will be determined;
|
|
|
•
|
|
whether the debt securities will be in registered form, bearer form or both and (1) if in registered form, the person to whom any interest shall be payable, if other than the person in whose name the security is registered at the close of business on the regular record date for such interest, or (2) if in bearer form, the manner in which, or the person to whom, any interest on the security shall be payable if otherwise than upon presentation and surrender upon maturity;
|
|
|
•
|
|
any restrictions applicable to the offer, sale or delivery of securities in bearer form and the terms upon which securities in bearer form of the series may be exchanged for securities in registered form of the series and vice versa if permitted by applicable laws and regulations;
|
|
|
•
|
|
whether any debt securities of the series are to be issuable initially in temporary global form and whether any debt securities of the series are to be issuable in permanent global form with or without coupons and, if so, whether beneficial owners of interests in any such permanent global security may or shall be required to exchange their interests for other debt securities of the series, and the manner in which interest shall be paid;
|
|
|
•
|
|
the identity of the depositary for securities in registered form, if such series are to be issuable as a global security;
|
|
|
•
|
|
the date as of which any debt securities in bearer form or in temporary global form shall be dated if other than the original issuance date of the first security of the series to be issued;
|
|
|
•
|
|
the applicability, if any, of the defeasance and covenant defeasance provisions described in this prospectus or in the applicable indenture;
|
|
|
•
|
|
whether and under what circumstances we will pay any additional amounts on the debt securities in respect of any tax, assessment or governmental charge and, if so, whether we will have the option to redeem the debt securities in lieu of making such a payment;
|
|
|
•
|
|
whether and under what circumstances the debt securities being offered are convertible into common stock or preferred stock, as the case may be, including the conversion price or rate or manner or calculation thereof;
|
|
|
•
|
|
the circumstances, if any, specified in the applicable prospectus supplement, under which beneficial owners of interests in the global security may obtain definitive debt securities and the manner in which payments on a permanent global debt security will be made if any debt securities are issuable in temporary or permanent global form;
|
|
|
•
|
|
any provisions granting special rights to holders of securities upon the occurrence of such events as specified in the applicable prospectus supplement;
|
|
|
•
|
|
if the debt securities of such series are to be issuable in definitive form only upon receipt of certain certificates or other documents or satisfaction of other conditions, then the form and/or terms of such certificates, documents or conditions;
|
|
|
•
|
|
the name of the applicable trustee and the nature of any material relationship with us or any of our affiliates, and the percentage of debt securities of the class necessary to require the trustee to take action;
|
|
|
•
|
|
any deletions from, modifications of, or additions to our events of default or covenants and any change in the right of any trustee or any of the holders to declare the principal amount of any of such debt securities due and payable;
|
|
|
•
|
|
applicable CUSIP numbers; and
|
|
|
•
|
|
any other terms of such debt securities not inconsistent with the provisions of the applicable indenture.
|
We may issue debt securities at a discount
below their principal amount and provide for less than the entire principal amount thereof to be payable upon declaration of acceleration
of the maturity of the debt securities. We refer to any such debt securities throughout this prospectus as “original issue
discount securities.” The applicable prospectus supplement will describe the United States federal income tax consequences
and other relevant considerations applicable to original issue discount securities.
We also may issue indexed debt securities.
Payments of principal of, and premium and interest on, indexed debt securities are determined with reference to the rate of exchange
between the currency or currency unit in which the debt security is denominated and any other currency or currency unit specified
by us, to the relationship between two or more currencies or currency units or by other similar methods or formulas specified in
the prospectus supplement.
Except as described under “—Merger,
Consolidation or Sale of Assets” or as may be set forth in any prospectus supplement, the debt securities will not contain
any provisions that (1) would limit our ability to incur indebtedness or (2) would afford holders of debt securities
protection in the event of (a) a highly leveraged or similar transaction involving us, or (b) a change of control or
reorganization, restructuring, merger or similar transaction involving us that may adversely affect the holders of the debt securities.
In the future, we may enter into transactions, such as the sale of all or substantially all of our assets or a merger or consolidation,
that may have an adverse effect on our ability to service our indebtedness, including the debt securities, by, among other things,
substantially reducing or eliminating our assets.
Neither the Delaware General Corporation
Law nor our governing instruments define the term “substantially all” as it relates to the sale of assets. Additionally,
Delaware cases interpreting the term “substantially all” rely upon the facts and circumstances of each particular case.
Consequently, to determine whether a sale of “substantially all” of our assets has occurred, a holder of debt securities
must review the financial and other information that we have disclosed to the public.
We will provide you with more information
in the applicable prospectus supplement regarding any deletions, modifications, or additions to the events of default or covenants
that are described below, including any addition of a covenant or other provision providing event risk or similar protection.
Payment
Unless we give you different information
in the applicable prospectus supplement, the principal of, and any premium, or make-whole amount, and interest on, any series of
the debt securities will be payable at the corporate trust office of the trustee. We will provide you with the address of the trustee
in the applicable prospectus supplement. We may also pay interest by mailing a check to the address of the person entitled to it
as it appears in the applicable register for the debt securities or by wire transfer of funds to that person at an account maintained
within the United States.
All monies that we pay to a paying agent
or a trustee for the payment of the principal of, and any premium, or make-whole amount, or interest on, any debt security will
be repaid to us if unclaimed at the end of two years after the obligation underlying payment becomes due and payable. After funds
have been returned to us, the holder of the debt security may look only to us for payment, without payment of interest for the
period which we hold the funds.
Denomination, Interest, Registration
and Transfer
Unless otherwise described in the applicable
prospectus supplement, the debt securities of any series will be issuable in denominations of $1,000 and integral multiples of
$1,000.
Subject to the limitations imposed upon
debt securities that are evidenced by a computerized entry in the records of a depository company rather than by physical delivery
of a note, a holder of debt securities of any series may:
|
|
•
|
|
exchange them for any authorized denomination of other debt securities of the same series and of a like aggregate principal amount and kind upon surrender of such debt securities at the corporate trust office of the applicable trustee or at the office of any transfer agent that we designate for such purpose; and
|
|
|
•
|
|
surrender them for registration of transfer or exchange at the corporate trust office of the applicable trustee or at the office of any transfer agent that we designate for such purpose.
|
Every debt security surrendered for registration
of transfer or exchange must be duly endorsed or accompanied by a written instrument of transfer satisfactory to the applicable
trustee or transfer agent. Payment of a service charge will not be required for any registration of transfer or exchange of any
debt securities, but we or the trustee may require payment of a sum sufficient to cover any tax or other governmental charge payable
in connection therewith. If in addition to the applicable trustee, the applicable prospectus supplement refers to any transfer
agent initially designated by us for any series of debt securities, we may at any time rescind the designation of any such transfer
agent or approve a change in the location through which any such transfer agent acts, except that we will be required to maintain
a transfer agent in each place of payment for such series. We may at any time designate additional transfer agents for any series
of debt securities.
Neither we, nor any trustee, will be required to:
|
|
•
|
|
issue, register the transfer of or exchange debt securities of any series during a period beginning at the opening of business 15 days before the day that the notice of redemption of any debt securities selected for redemption is mailed and ending at the close of business on the day of such mailing;
|
|
|
•
|
|
register the transfer of or exchange any debt security, or portion thereof, so selected for redemption, in whole or in part, except the unredeemed portion of any debt security being redeemed in part; and
|
|
|
•
|
|
issue, register the transfer of or exchange any debt security that has been surrendered for repayment at the option of the holder, except the portion, if any, of such debt security not to be so repaid.
|
Merger, Consolidation or Sale of
Assets
The indentures provide that we may, without
the consent of the holders of any outstanding debt securities, (1) consolidate with, (2) sell, lease or convey all or
substantially all of our assets to, or (3) merge with or into, any other entity provided that:
|
|
•
|
|
either we are the continuing entity, or the successor entity, if other than us, assumes the obligations (A) to pay the principal of, and any premium (or make-whole amount) and interest on, all of the debt securities and (B) to duly perform and observe all of the covenants and conditions contained in each indenture;
|
|
|
•
|
|
after giving effect to the transaction, there is no event of default under the indentures and no event which, after notice or the lapse of time, or both, would become such an event of default, occurs and continues; and
|
|
|
•
|
|
an officers’ certificate and legal opinion covering such conditions are delivered to each applicable trustee.
|
Covenants
Existence. Except as permitted
under “Debt Securities—Merger, Consolidation or Sale of Assets,” the indentures require us to do or cause to
be done all things necessary to preserve and keep in full force and effect our existence, rights and franchises. However, the indentures
do not require us to preserve any right or franchise if we determine that any right or franchise is no longer desirable in the
conduct of our business.
Provision of financial information.
The indentures require us to (1) within 15 days of each of the respective dates by which we are required to file our annual
reports, quarterly reports and other documents with the SEC, file with the trustee copies of the annual report, quarterly report
and other documents that we file with the SEC under Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended,
or the Exchange Act, (2) file with the trustee and the SEC any additional information, documents and reports regarding compliance
by us with the conditions and covenants of the indentures, as required, (3) within 30 days after the filing with the trustee,
mail to all holders of debt securities, as their names and addresses appear in the applicable register for such debt securities,
without cost to such holders, summaries of any documents and reports required to be filed by us pursuant to (1) and (2) above,
and (4) supply, promptly upon written request and payment of the reasonable cost of duplication and delivery, copies of such
documents to any prospective holder.
Additional covenants. The applicable
prospectus supplement will set forth any additional covenants relating to any series of debt securities.
Events of Default, Notice and Waiver
Unless the applicable prospectus supplement
states otherwise, when we refer to “events of default” as defined in the indentures with respect to any series of debt
securities, we mean:
|
|
•
|
|
default in the payment of any installment of interest on any debt security of such series continuing for 30 days;
|
|
|
•
|
|
default in the payment of principal of, or any premium, or make-whole amount, on any debt security of such series for five business days at its stated maturity;
|
|
|
•
|
|
default in making any sinking fund payment as required for any debt security of such series for five business days;
|
|
|
•
|
|
default in the performance or breach of any covenant or warranty in the debt securities or in the indenture continuing for 60 days after written notice as provided in the applicable indenture, but not of a covenant added to the indenture solely for the benefit of a series of debt securities issued thereunder other than such series;
|
|
|
•
|
|
bankruptcy, insolvency or reorganization, or court appointment of a receiver, liquidator or trustee of the Company or any significant subsidiary of the Company; and
|
|
|
•
|
|
any other event of default provided with respect to a particular series of debt securities.
|
When we use the term “significant
subsidiary,” we refer to the meaning ascribed to such term in Rule 1-02 of Regulation S-X promulgated under the Securities
Act of 1933, as amended, or Securities Act.
If an event of default occurs and is continuing
with respect to debt securities of any series outstanding, then the applicable trustee or the holders of 25% or more in principal
amount of the debt securities of that series will have the right to declare the principal amount of all the debt securities of
that series to be due and payable. If the debt securities of that series are original issue discount securities or indexed securities,
then the applicable trustee or the holders of 25% or more in principal amount of the debt securities of that series will have the
right to declare the portion of the principal amount as may be specified in the terms thereof to be due and payable. However, at
any time after such a declaration of acceleration has been made, but before a judgment or decree for payment of the money due has
been obtained by the applicable trustee, the holders of at least a majority in principal amount of outstanding debt securities
of such series or of all debt securities then outstanding under the applicable indenture may rescind and annul such declaration
and its consequences if:
|
|
•
|
|
we have deposited with the applicable trustee all required payments of the principal, any premium, or make-whole amount, interest and, to the extent permitted by law, interest on overdue installment of interest, plus applicable fees, expenses, disbursements and advances of the applicable trustee; and
|
|
|
•
|
|
all events of default, other than the non-payment of accelerated principal, or a specified portion thereof, and any premium, or make-whole amount, have been cured or waived.
|
The indentures also provide that the holders
of at least a majority in principal amount of the outstanding debt securities of any series or of all debt securities then outstanding
under the applicable indenture may, on behalf of all holders, waive any past default with respect to such series and its consequences,
except a default:
|
|
•
|
|
in the payment of the principal, any premium, or make-whole amount, or interest;
|
|
|
•
|
|
in respect of a covenant or provision contained in the applicable indenture that cannot be modified or amended without the consent of the holders of the outstanding debt security that is affected by the default; or
|
|
|
•
|
|
in respect of a covenant or provision for the benefit or protection of the trustee, without its express written consent.
|
The indentures require each trustee to
give notice to the holders of debt securities within 90 days of a default unless such default has been cured or waived. However,
the trustee may withhold notice if specified persons of such trustee consider such withholding to be in the interest of the holders
of debt securities. The trustee may not withhold notice of a default in the payment of principal, any premium or interest on any
debt security of such series or in the payment of any sinking fund installment in respect of any debt security of such series.
The indentures provide that holders of
debt securities of any series may not institute any proceedings, judicial or otherwise, with respect to such indenture or for any
remedy under the indenture, unless the trustee fails to act for a period of 60 days after the trustee has received a written request
to institute proceedings in respect of an event of default from the holders of 25% or more in principal amount of the outstanding
debt securities of such series, as well as an offer of indemnity reasonably satisfactory to the trustee. However, this provision
will not prevent any holder of debt securities from instituting suit for the enforcement of payment of the principal of, and any
premium, or make-whole amount, and interest on, such debt securities at the respective due dates thereof.
The indentures provide that, subject to
provisions in each indenture relating to its duties in the case of a default, a trustee has no obligation to exercise any of its
rights or powers at the request or direction of any holders of any series of debt securities then outstanding under the indenture,
unless the holders have offered to the trustee reasonable security or indemnity. The holders of at least a majority in principal
amount of the outstanding debt securities of any series or of all debt securities then outstanding under an indenture shall have
the right to direct the time, method and place of conducting any proceeding for any remedy available to the applicable trustee,
or of exercising any trust or power conferred upon such trustee. However, a trustee may refuse to follow any direction which:
|
|
•
|
|
is in conflict with any law or the applicable indenture;
|
|
|
•
|
|
upon a good faith determination of a responsible officer of the trustee, may involve the trustee in personal liability; or
|
|
|
•
|
|
upon a good faith determination of a responsible officer of the trustee, may be unduly prejudicial to the holders of debt securities of the series not joining the proceeding.
|
Within 120 days after the close of each
fiscal year, we will be required to deliver to each trustee a certificate, signed by one of our several specified officers, stating
whether or not that officer has knowledge of any default under the applicable indenture. If the officer has knowledge of any default,
the notice must specify the nature and status of the default.
Modification of the Indentures
The indentures provide that modifications
and amendments may be made only with the consent of the affected holders of at least a majority in principal amount of all outstanding
debt securities issued under that indenture. However, no such modification or amendment may, without the consent of the holders
of the debt securities affected by the modification or amendment
|
|
•
|
|
change the stated maturity of the principal of, or any premium, or make-whole amount, on, or any installment of principal of or interest on, any such debt security;
|
|
|
•
|
|
reduce the principal amount of, the rate or amount of interest on or any premium, or make-whole amount, payable on redemption of any such debt security;
|
|
|
•
|
|
reduce the amount of principal of an original issue discount security that would be due and payable upon declaration of acceleration of the maturity thereof or would be provable in bankruptcy, or adversely affect any right of repayment of the holder of any such debt security;
|
|
|
•
|
|
change the place of payment or the coin or currency for payment of principal of, or any premium, or make-whole amount, or interest on, any such debt security;
|
|
|
•
|
|
impair the right to institute suit for the enforcement of any payment on or with respect to any such debt security;
|
|
|
•
|
|
reduce the percentage in principal amount of any outstanding debt securities necessary to modify or amend the applicable indenture with respect to such debt securities, to waive compliance with particular provisions thereof or defaults and consequences thereunder or to reduce the quorum or voting requirements set forth in the applicable indenture; and
|
|
|
•
|
|
modify any of the foregoing provisions or any of the provisions relating to the waiver of particular past defaults or covenants, except to increase the required percentage to effect such action or to provide that some of the other provisions may not be modified or waived without the consent of the holder of such debt security.
|
The holders of a majority in aggregate
principal amount of the outstanding debt securities of each series may, on behalf of all holders of debt securities of that series,
waive, insofar as that series is concerned, our compliance with material restrictive covenants of the applicable indenture.
We and our respective trustee may make
modifications and amendments of an indenture without the consent of any holder of debt securities for any of the following purposes:
|
|
•
|
|
to evidence the succession of another person to us as obligor under such indenture;
|
|
|
•
|
|
to add to our covenants for the benefit of the holders of all or any series of debt securities or to surrender any right or power conferred upon us in such indenture;
|
|
|
•
|
|
to add events of default for the benefit of the holders of all or any series of debt securities;
|
|
|
•
|
|
to add or change any provisions of an indenture (1) to change or eliminate restrictions on the payment of principal of, or premium, or make-whole amount, or interest on, debt securities in bearer form, or (2) to permit or facilitate the issuance of debt securities in uncertificated form, provided that such action shall not adversely affect the interests of the holders of the debt securities of any series in any material respect;
|
|
|
•
|
|
to change or eliminate any provisions of an indenture, provided that any such change or elimination shall become effective only when there are no debt securities outstanding of any series created prior thereto which are entitled to the benefit of such provision;
|
|
|
•
|
|
to secure the debt securities;
|
|
|
•
|
|
to establish the form or terms of debt securities of any series;
|
|
|
•
|
|
to provide for the acceptance of appointment by a successor trustee or facilitate the administration of the trusts under an indenture by more than one trustee;
|
|
|
•
|
|
to cure any ambiguity, defect or inconsistency in an indenture, provided that such action shall not adversely affect the interests of holders of debt securities of any series issued under such indenture; and
|
|
|
•
|
|
to supplement any of the provisions of an indenture to the extent necessary to permit or facilitate defeasance and discharge of any series of such debt securities, provided that such action shall not adversely affect the interests of the holders of the outstanding debt securities of any series.
|
Voting
The indentures provide that in determining
whether the holders of the requisite principal amount of outstanding debt securities of a series have given any request, demand,
authorization, direction, notice, consent or waiver under the indentures or whether a quorum is present at a meeting of holders
of debt securities:
|
|
•
|
|
the principal amount of an original issue discount security that shall be deemed to be outstanding shall be the amount of the principal thereof that would be due and payable as of the date of such determination upon declaration of acceleration of the maturity thereof;
|
|
|
•
|
|
the principal amount of any debt security denominated in a foreign currency that shall be deemed outstanding shall be the United States dollar equivalent, determined on the issue date for such debt security, of the principal amount or, in the case of an original issue discount security, the United States dollar equivalent on the issue date of such debt security of the amount determined as provided in the preceding bullet point;
|
|
|
•
|
|
the principal amount of an indexed security that shall be deemed outstanding shall be the principal face amount of such indexed security at original issuance, unless otherwise provided for such indexed security under such indenture; and
|
|
|
•
|
|
debt securities owned by us or any other obligor upon the debt securities or by any affiliate of ours or of such other obligor shall be disregarded.
|
The indentures contain provisions
for convening meetings of the holders of debt securities of a series. A meeting will be permitted to be called at any time by
the applicable trustee, and also, upon request, by us or the holders of at least 25% in principal amount of the outstanding
debt securities of such series, in any such case upon notice given as provided in such indenture. Except for any consent that
must be given by the holder of each debt security affected by the modifications and amendments of an indenture described
above, any resolution presented at a meeting or adjourned meeting duly reconvened at which a quorum is present may be adopted
by the affirmative vote of the holders of a majority of the aggregate principal amount of the outstanding debt securities of
that series represented at such meeting.
Notwithstanding the preceding paragraph,
except as referred to above, any resolution relating to a request, demand, authorization, direction, notice, consent, waiver or
other action that may be made, given or taken by the holders of a specified percentage, which is less than a majority of the aggregate
principal amount of the outstanding debt securities of a series, may be adopted at a meeting or adjourned meeting duly reconvened
at which a quorum is present by the affirmative vote of such specified percentage.
Any resolution passed or decision taken
at any properly held meeting of holders of debt securities of any series will be binding on all holders of such series. The quorum
at any meeting called to adopt a resolution, and at any reconvened meeting, will be persons holding or representing a majority
in principal amount of the outstanding debt securities of a series. However, if any action is to be taken relating to a consent
or waiver which may be given by the holders of at least a specified percentage in principal amount of the outstanding debt securities
of a series, the persons holding such percentage will constitute a quorum.
Notwithstanding the foregoing provisions,
the indentures provide that if any action is to be taken at a meeting with respect to any request, demand, authorization, direction,
notice, consent, waiver or other action that such indenture expressly provides may be made, given or taken by the holders of a
specified percentage in principal amount of all outstanding debt securities affected by such action, or of the holders of such
series and one or more additional series:
|
|
•
|
|
there shall be no minimum quorum requirement for such meeting; and
|
|
|
•
|
|
the principal amount of the outstanding debt securities of such series that vote in favor of such request, demand, authorization, direction, notice, consent, waiver or other action shall be taken account in determining whether such request, demand, authorization, direction, notice, consent, waiver or other action has been made, given or taken under such indenture.
|
Subordination
Unless otherwise provided in the applicable
prospectus supplement, subordinated securities will be subject to the following subordination provisions.
Upon any distribution to our creditors
in a liquidation, dissolution or reorganization, the payment of the principal of and interest on any subordinated securities will
be subordinated to the extent provided in the applicable indenture in right of payment to the prior payment in full of all senior
debt. However, our obligation to make payments of the principal of and interest on such subordinated securities otherwise will
not be affected. No payment of principal or interest will be permitted to be made on subordinated securities at any time if a default
on senior debt exists that permits the holders of such senior debt to accelerate its maturity and the default is the subject of
judicial proceedings or we receive notice of the default. After all senior debt is paid in full and until the subordinated securities
are paid in full, holders of subordinated securities will be subrogated to the rights of holders of senior debt to the extent that
distributions otherwise payable to holders of subordinated securities have been applied to the payment of senior debt. The subordinated
indenture will not restrict the amount of senior debt or other indebtedness of the Company and its subsidiaries. As a result of
these subordination provisions, in the event of a distribution of assets upon insolvency, holders of subordinated securities may
recover less, ratably, than our general creditors.
The term “senior debt” will
be defined in the applicable indenture as the principal of and interest on, or substantially similar payments to be made by us
in respect of, other outstanding indebtedness, whether outstanding at the date of execution of the applicable indenture or subsequently
incurred, created or assumed. The prospectus supplement may include a description of additional terms implementing the subordination
feature.
No restrictions will be included in any
indenture relating to subordinated securities upon the creation of additional senior debt.
If this prospectus is being delivered
in connection with the offering of a series of subordinated securities, the accompanying prospectus supplement or the information
incorporated in this prospectus by reference will set forth the approximate amount of senior debt outstanding as of the end of
our most recent fiscal quarter.
Discharge, Defeasance and Covenant
Defeasance
Unless otherwise indicated in the applicable
prospectus supplement, the indentures allow us to discharge our obligations to holders of any series of debt securities issued
under any indenture when:
|
|
•
|
|
either (1) all securities of such series have already been delivered to the applicable trustee for cancellation; or (2) all securities of such series have not already been delivered to the applicable trustee for cancellation but (A) have become due and payable, (B) will become due and payable within one year, or (C) if redeemable at our option, are to be redeemed within one year, and we have irrevocably deposited with the applicable trustee, in trust, funds in such currency or currencies, currency unit or units or composite currency or currencies in which such debt securities are payable, an amount sufficient to pay the entire indebtedness on such debt securities in respect of principal and any premium, or make-whole amount, and interest to the date of such deposit if such debt securities have become due and payable or, if they have not, to the stated maturity or redemption date;
|
|
|
•
|
|
we have paid or caused to be paid all other sums payable; and
|
|
|
•
|
|
an officers’ certificate and an opinion of counsel stating the conditions to discharging the debt securities have been satisfied has been delivered to the trustee.
|
Unless otherwise indicated in the applicable
prospectus supplement, the indentures provide that, upon our irrevocable deposit with the applicable trustee, in trust, of an amount,
in such currency or currencies, currency unit or units or composite currency or currencies in which such debt securities are payable
at stated maturity, or government obligations, or both, applicable to such debt securities, which through the scheduled payment
of principal and interest in accordance with their terms will provide money in an amount sufficient to pay the principal of, and
any premium, or make-whole amount, and interest on, such debt securities, and any mandatory sinking fund or analogous payments
thereon, on the scheduled due dates therefor, the issuing company may elect either:
|
|
•
|
|
to defease and be discharged from any and all obligations with respect to such debt securities; or
|
|
|
•
|
|
to be released from its obligations with respect to such debt securities under the applicable indenture or, if provided in the applicable prospectus supplement, its obligations with respect to any other covenant, and any omission to comply with such obligations shall not constitute an event of default with respect to such debt securities.
|
Notwithstanding the above, we may not
elect to defease and be discharged from the obligation to pay any additional amounts upon the occurrence of particular events of
tax, assessment or governmental charge with respect to payments on such debt securities and the obligations to register the transfer
or exchange of such debt securities, to replace temporary or mutilated, destroyed, lost or stolen debt securities, to maintain
an office or agency in respect of such debt securities, or to hold monies for payment in trust.
The indentures only permit us to establish
the trust described in the paragraph above if, among other things, it has delivered to the applicable trustee an opinion of counsel
to the effect that the holders of such debt securities will not recognize income, gain or loss for United States federal income
tax purposes as a result of such defeasance or covenant defeasance and will be subject to United States federal income tax on the
same amounts, in the same manner and at the same times as would have been the case if such defeasance or covenant defeasance had
not occurred. Such opinion of counsel, in the case of defeasance, will be required to refer to and be based upon a ruling received
from or published by the Internal Revenue Service or a change in applicable United States federal income tax law occurring after
the date of the indenture. In the event of such defeasance, the holders of such debt securities would be able to look only to such
trust fund for payment of principal, any premium, or make-whole amount, and interest.
When we use the term “government
obligations,” we mean securities that are:
|
|
•
|
|
direct obligations of the United States or the government that issued the foreign currency in which the debt securities of a particular series are payable, for the payment of which its full faith and credit is pledged; or
|
|
|
•
|
|
obligations of a person controlled or supervised by and acting as an agency or instrumentality of the United States or other government that issued the foreign currency in which the debt securities of such series are payable, the payment of which is unconditionally guaranteed as a full faith and credit obligation by the United States or such other government, which are not callable or redeemable at the option of the issuer thereof and shall also include a depository receipt issued by a bank or trust company as custodian with respect to any such government obligation or a specific payment of interest on or principal of any such government obligation held by such custodian for the account of the holder of a depository receipt. However, except as required by law, such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the government obligation or the specific payment of interest on or principal of the government obligation evidenced by such depository receipt.
|
Unless otherwise provided in the applicable
prospectus supplement, if after we have deposited funds and/or government obligations to effect defeasance or covenant defeasance
with respect to debt securities of any series, (1) the holder of a debt security of such series is entitled to, and does,
elect under the terms of the applicable indenture or the terms of such debt security to receive payment in a currency, currency
unit or composite currency other than that in which such deposit has been made in respect of such debt security, or (2) a
conversion event occurs in respect of the currency, currency unit or composite currency in which such deposit has been made, the
indebtedness represented by such debt security will be deemed to have been, and will be, fully discharged and satisfied through
the payment of the principal of, and premium, or make whole amount, and interest on, such debt security as they become due out
of the proceeds yielded by converting the amount so deposited in respect of such debt security into the currency, currency unit
or composite currency in which such debt security becomes payable as a result of such election or such cessation of usage based
on the applicable market exchange rate.
When we use the term “conversion
event,” we mean the cessation of use of:
|
|
•
|
|
a currency, currency unit or composite currency both by the government of the country that issued such currency and for the settlement of transactions by a central bank or other public institutions of or within the international banking community;
|
|
|
•
|
|
the European Currency Unit both within the European Monetary System and for the settlement of transactions by public institutions of or within the European Communities; or
|
|
|
•
|
|
any currency unit or composite currency other than the European Currency Unit for the purposes for which it was established.
|
Unless otherwise provided in the applicable
prospectus supplement, all payments of principal of, and any premium, or make-whole amount, and interest on, any debt security
that is payable in a foreign currency that ceases to be used by its government of issuance shall be made in United States dollars.
In the event that (1) we effect covenant
defeasance with respect to any debt securities and (2) those debt securities are declared due and payable because of the occurrence
of any event of default, the amount in the currency, currency unit or composite currency in which such debt securities are payable,
and government obligations on deposit with the applicable trustee, will be sufficient to pay amounts due on such debt securities
at the time of their stated maturity but may not be sufficient to pay amounts due on such debt securities at the time of the acceleration
resulting from such event of default. However, the issuing company would remain liable to make payments of any amounts due at the
time of acceleration.
If a trustee
or paying agent is unable to apply any money in accordance with the foregoing paragraphs describing discharge and defeasance
by reason of any order or judgment of any court or governmental authority enjoining, restraining or otherwise prohibiting
such application, then the obligations under the indentures and such securities from which we have been discharged or
released pursuant to the foregoing shall be revived and reinstated as though no deposit had occurred with respect to such
securities, until such time as the trustee or paying agent is permitted to apply all money held in trust with respect to such
securities in accordance with the foregoing; provided, that if we make any payment of principal of or any premium or interest
on any such security following such reinstatement of its obligations, we shall be subrogated to the rights (if any) of the
holders of such securities to receive such payment from the money so held in trust.
The applicable prospectus supplement may
further describe the provisions, if any, permitting such defeasance or covenant defeasance, including any modifications to the
provisions described above, with respect to the debt securities of or within a particular series.
Conversion Rights
The terms and conditions, if any, upon
which the debt securities are convertible into common stock or preferred stock will be set forth in the applicable prospectus supplement.
The terms will include whether the debt securities are convertible into shares of common stock or preferred stock, the conversion
price, or manner of calculation thereof, the conversion period, provisions as to whether conversion will be at the issuing company’s
option or the option of the holders, the events requiring an adjustment of the conversion price and provisions affecting conversion
in the event of the redemption of the debt securities and any restrictions on conversion.
Global Securities
The debt securities of a series may be
issued in whole or in part in the form of one or more global securities that will be deposited with, or on behalf of, a depository
identified in the applicable prospectus supplement relating to such series. Global securities, if any, issued in the United States
are expected to be deposited with The Depository Trust Company, or DTC, as depository. We may issue global securities in either
registered or bearer form and in either temporary or permanent form. We will describe the specific terms of the depository arrangement
with respect to a series of debt securities in the applicable prospectus supplement relating to such series. We expect that unless
the applicable prospectus supplement provides otherwise, the following provisions will apply to depository arrangements.
All interests in global securities will
be subject to the operations and procedures of the depository for such global securities or its nominee. We provide the following
summaries of those operations and procedures solely for the convenience of investors. Once a global security is issued, we expect
that the depository for such global security or its nominee will credit on its book-entry registration and transfer system the
respective principal amounts of the individual debt securities represented by such global security to the accounts of participants
that have accounts with such depository. Such accounts shall be designated by the underwriters, dealers or agents with respect
to such debt securities or by us if we offer such debt securities directly. Ownership of beneficial interests in such global security
will be limited to participants with the depository or persons that may hold interests through those participants.
We expect that, under procedures established
by DTC, ownership of beneficial interests in any global security for which DTC is the depository will be shown on, and the transfer
of that ownership will be effected only through, records maintained by DTC or its nominee, with respect to beneficial interests
of participants with the depository, and records of participants, with respect to beneficial interests of persons who hold through
participants with the depository. Neither we nor the trustee will have any responsibility or liability for any aspect of the records
of DTC or for maintaining, supervising or reviewing any records of DTC or any of its participants relating to beneficial ownership
interests in the debt securities.
So long as the depository for a global
security or its nominee is the registered owner of such global security, such depository or such nominee, as the case may be, will
be considered the sole owner or holder of the debt securities represented by the global security for all purposes under the applicable
indenture. Except as described below or in the applicable prospectus supplement, owners of beneficial interest in a global security
will not be entitled to have any of the individual debt securities represented by such global security registered in their names,
will not receive or be entitled to receive physical delivery of any such debt securities in definitive form and will not be considered
the owners or holders thereof under the applicable indenture. Beneficial owners of debt securities evidenced by a global security
will not be considered the owners or holders thereof under the applicable indenture for any purpose, including with respect to
the giving of any direction, instructions or approvals to the trustee under the indenture. Accordingly, each person owning a beneficial
interest in a global security with respect to which DTC is the depository must rely on the procedures of DTC and, if such person
is not a participant with the depository, on the procedures of the participant through which such person owns its interests, to
exercise any rights of a holder under the applicable indenture.
Payments of principal of, and any premium, or make-whole amount,
and interest on, individual debt securities represented by a global security registered in the name of a depository or its nominee
will be made to or at the direction of the depository or its nominee, as the case may be, as the registered owner of the global
security under the applicable indenture. Under the terms of the applicable indenture, we and the trustee may treat the persons
in whose name debt securities, including a global security, are registered as the owners thereof for the purpose of receiving such
payments. Consequently, neither we nor the trustee have or will have any responsibility or liability for the payment of such amounts
to beneficial owners of debt securities including principal, any premium, or make-whole amount, or interest. We believe, however,
that it is currently the policy of DTC to immediately credit the accounts of relevant participants with such payments, in amounts
proportionate to their respective holdings of beneficial interests in the relevant global security as shown on the records of DTC
or its nominee. We also expect that payments by participants to owners of beneficial interests in such global security held through
such participants will be governed by standing instructions and customary practices, as is the case with securities held for the
account of customers in bearer form or registered in street name, and will be the responsibility of such participants. Redemption
notices with respect to any debt securities represented by a global security will be sent to the depository or its nominee. If
less than all of the debt securities of any series are to be redeemed, we expect the depository to determine the amount of the
interest of each participant in such debt securities to be redeemed to be determined by lot. Neither we, the trustee, any paying
agent nor the security registrar for such debt securities will have any responsibility or liability for any aspect of the records
relating to or payments made on account of beneficial ownership interests in the global security for such debt securities or for
maintaining any records with respect thereto.
Neither we nor the trustee will be liable
for any delay by the holders of a global security or the depository in identifying the beneficial owners of debt securities, and
we and the trustee may conclusively rely on, and will be protected in relying on, instructions from the holder of a global security
or the depository for all purposes. The rules applicable to DTC and its participants are on file with the SEC.
If a depository for any debt securities
is at any time unwilling, unable or ineligible to continue as depository and we do not appoint a successor depository within 90
days, we will issue individual debt securities in exchange for the global security representing such debt securities. In addition,
we may at any time and in our sole discretion, subject to any limitations described in the applicable prospectus supplement relating
to such debt securities, determine not to have any of such debt securities represented by one or more global securities and in
such event will issue individual debt securities in exchange for the global security or securities representing such debt securities.
Individual debt securities so issued will be issued in denominations of $1,000 and integral multiples of $1,000.
The debt securities of a series may also
be issued in whole or in part in the form of one or more bearer global securities that will be deposited with a depository, or
with a nominee for such depository, identified in the applicable prospectus supplement. Any such bearer global securities may be
issued in temporary or permanent form. The specific terms and procedures, including the specific terms of the depositary arrangement,
with respect to any portion of a series of debt securities to be represented by one or more bearer global securities will be described
in the applicable prospectus supplement.
No Recourse
There is no recourse under any obligation,
covenant or agreement in the applicable indenture or with respect to any security against any of our or our successor’s past,
present or future stockholders, employees, officers or directors.
Warrants
We may issue warrants for the purchase
of common stock, preferred stock and/or debt securities in one or more series, from time to time. We may issue warrants independently
or together with common stock, preferred stock and/or debt securities, and the warrants may be attached to or separate from those
securities.
If we issue warrants, they will be
evidenced by warrant agreements or warrant certificates issued under one or more warrant agreements, which are contracts
between us and the holders of the warrants or an agent for the holders of the warrants. We urge you to read the prospectus
supplement related to any series of warrants we may offer, as well as the complete warrant agreement and warrant certificate
that contain the terms of the warrants. If we issue warrants, forms of warrant agreements and warrant certificates relating
to warrants for the purchase of common stock, preferred stock and debt securities will be incorporated by reference into the
registration statement of which this prospectus is a part from reports we would subsequently file with the SEC.
We had 1,408,000 warrants outstanding
as of March 28, 2018.
Certain of our warrants are listed on
the NASDAQ Capital Market under the symbol “RXIIW”. The transfer agent and registrar for those warrants are Computershare
Trust Company, N.A.
Units
We may issue units comprised of shares
of common stock, shares of preferred stock, debt securities and warrants in any combination. We may issue units in such amounts
and in as many distinct series as we wish. This section outlines certain provisions of the units that we may issue. If we issue
units, they may be issued under one or more unit agreements to be entered into between us and a bank or other financial institution,
as unit agent. The information described in this section may not be complete in all respects and is qualified entirely by reference
to the unit agreement with respect to the units of any particular series. The specific terms of any series of units offered will
be described in the applicable prospectus supplement. If so described in a particular supplement, the specific terms of any series
of units may differ from the general description of terms presented below. We urge you to read any prospectus supplement related
to any series of units we may offer, as well as the complete unit agreement and unit certificate that contain the terms of the
units. If we issue units, forms of unit agreements and unit certificates relating to such units will be incorporated by reference
as exhibits to the registration statement, which includes this prospectus.
Each unit that we may issue will be issued
so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the
rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that
the securities included in the unit may not be held or transferred separately, at any time or at any time before a specified date.
The applicable prospectus supplement may describe:
|
|
•
|
|
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately;
|
|
|
•
|
|
any provisions of the governing unit agreement;
|
|
|
•
|
|
the price or prices at which such units will be issued;
|
|
|
•
|
|
the applicable United States federal income tax considerations relating to the units;
|
|
|
•
|
|
any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units; and
|
|
|
•
|
|
any other terms of the units and of the securities comprising the units.
|
The provisions described in this subsection,
as well as those described under “Description of Securities” will apply to the securities included in each unit, to
the extent relevant and as may be updated in any prospectus supplements.
USE OF PROCEEDS
We will retain broad discretion over the
use of the net proceeds from the sale of our securities offered hereby. Except as described in any prospectus supplement, we currently
anticipate using the net proceeds from the sale of our securities offered hereby primarily for general corporate purposes, which
include, but are not limited to, funding our preclinical and clinical development, other research and development activities and
for general and administrative expenses. We may also use a portion of the net proceeds to pay off outstanding indebtedness, if
any, and/or acquire or invest in complementary businesses, products and technologies. Further, from time to time we may evaluate
acquisition opportunities and engage in related discussions with other companies.
Pending the use of the net proceeds, we
intend to invest the net proceeds in short-term, interest-bearing, investment-grade securities.
RATIO OF EARNINGS TO FIXED CHARGES
If we offer debt securities and/or preferred
equity securities under this prospectus, then we will, if required at that time, provide a ratio of earnings to fixed charges and/or
ratio of combined fixed charges and preference dividends to earnings, respectively, in the applicable prospectus supplement for
such offering.
PLAN OF DISTRIBUTION
We may sell the securities covered by
this prospectus from time to time in one or more offerings. Registration of the securities covered by this prospectus does not
mean, however, that those securities will necessarily be offered or sold.
We may sell the securities separately
or together:
|
|
•
|
|
through one or more underwriters or dealers in a public offering and sale by them;
|
|
|
•
|
|
directly to investors; or
|
|
|
•
|
|
through agents.
|
We may sell the securities from time to
time:
|
|
•
|
|
in one or more transactions at a fixed price or prices, which may be changed from time to time;
|
|
|
•
|
|
at market prices prevailing at the times of sale;
|
|
|
•
|
|
at prices related to such prevailing market prices; or
|
|
|
•
|
|
at negotiated prices.
|
We will describe the method of distribution
of the securities and the terms of the offering in the prospectus supplement. Any discounts or concessions allowed or re-allowed
or paid to dealers may be changed from time to time.
If underwriters are used in the sale of
any securities, the securities will be acquired by the underwriters for their own account and may be resold from time to time in
one or more transactions described above. The securities may be either offered to the public through underwriting syndicates represented
by managing underwriters, or directly by underwriters. Generally, the underwriters’ obligations to purchase the securities
will be subject to conditions precedent and the underwriters will be obligated to purchase all of the securities if they purchase
any of the securities. We may use underwriters with whom we have a material relationship. We will describe in the prospectus supplement,
naming the underwriter, the nature of any such relationship.
We may authorize underwriters, dealers
or agents to solicit offers by certain purchasers to purchase the securities from us at the public offering price set forth in
the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the
future. The contracts will be subject only to those conditions set forth in the prospectus supplement, and the prospectus supplement
will set forth any commissions we pay for solicitation of these contracts.
We may enter into derivative transactions
with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. If
the applicable prospectus supplement indicates, in connection with those derivatives, the third parties may sell securities covered
by this prospectus and the applicable prospectus supplement, including in short sale transactions. If so, the third party may use
securities pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings of stock,
and may use securities received from us in settlement of those derivatives to close out any related open borrowings of stock. The
third party in such sale transactions will be an underwriter and will be identified in the applicable prospectus supplement or
in a post-effective amendment.
Underwriters, dealers and agents may be
entitled to indemnification by us against certain civil liabilities, including liabilities under the Securities Act, or to contribution
with respect to payments made by the underwriters, dealers or agents, under agreements between us and the underwriters, dealers
and agents.
We may grant underwriters who participate
in the distribution of securities an option to purchase additional securities to cover over-allotments, if any, in connection with
the distribution.
Underwriters, dealers or agents may receive
compensation in the form of discounts, concessions or commissions from us or our purchasers, as their agents in connection with
the sale of securities. These underwriters, dealers or agents may be considered to be underwriters under the Securities Act. As
a result, discounts, commissions or profits on resale received by the underwriters, dealers or agents may be treated as underwriting
discounts and commissions. The prospectus supplement will identify any such underwriter, dealer or agent and describe any compensation
received by them from us. Any initial public offering price and any discounts or concessions allowed or re-allowed or paid to dealers
may be changed from time to time.
Unless otherwise specified in the related
prospectus supplement, all securities we offer, other than common stock, will be new issues of securities with no established trading
market. Any underwriters may make a market in these securities, but will not be obligated to do so and may discontinue any market
making at any time without notice. Any common stock sold pursuant to a prospectus supplement will be listed for trading on the
NASDAQ Global Market or other principal market for our common stock. We may apply to list any series of debt securities, preferred
stock, warrants or units on an exchange, but we are not obligated to do so. Therefore, there may not be liquidity or a trading
market for any series of securities.
Any underwriter may engage in over-allotment
transactions, stabilizing transactions, short-covering transactions and penalty bids in accordance with Regulation M under the
Exchange Act. Over-allotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions
permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. Short covering
transactions involve purchases of the securities in the open market after the distribution is completed to cover short positions.
Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer
are purchased in a covering transaction to cover short positions. Those activities may cause the price of the securities to be
higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time. We make no
representation or prediction as to the direction or magnitude of any effect that such transactions may have on the price of the
securities. For a description of these activities, see the information under the heading “Underwriting” or “Plan
of Distribution” in the applicable prospectus supplement.
Underwriters, broker-dealers or agents
who may become involved in the sale of the common stock may engage in transactions with and perform other services for us in the
ordinary course of their business for which they receive compensation.
LEGAL MATTERS
The legality of the issuance of the securities
being offered hereby and the binding nature of any debt securities, warrants or units being offered hereby is being passed upon
by Gibson, Dunn & Crutcher LLP, San Francisco, California. The legality of the securities for any underwriters, dealers
or agents will be passed upon by counsel as may be specified in the applicable prospectus supplement.
EXPERTS
The consolidated financial statements
as of December 31, 2017 and 2016 and for each of the two years in the period ended December 31, 2017 incorporated by reference
in this Prospectus have been so incorporated in reliance on the report of BDO USA, LLP, an independent registered public accounting
firm (the report on the financial statements contains an explanatory paragraph regarding the Company’s ability to continue
as a going concern) incorporated by reference, given on the authority of said firm as experts in auditing and accounting.
INCORPORATION OF CERTAIN INFORMATION
BY REFERENCE
The SEC allows us to incorporate by reference
into this prospectus the information contained in other documents we file with the SEC, which means that we can disclose important
information to you by referring you to those documents. Any statement contained in any document incorporated or deemed to be incorporated
by reference herein shall be deemed to be modified or superseded, for purposes of this prospectus, to the extent that a statement
contained in or omitted from this prospectus, or in any other subsequently filed document that also is or is deemed to be incorporated
by reference herein, modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed, except
as so modified or superseded, to constitute a part of this prospectus. We incorporate by reference the documents listed below which
have been filed by us:
|
|
•
|
|
Our Annual Report on Form 10-K for the year ended December 31, 2017, filed with the SEC on March 26, 2018;
|
|
|
•
|
|
Our Quarterly Reports on Form 10-Q/A for the periods ended March 31, 2017, June 30, 2017 and September 30, 2017 that we filed with the SEC on March 26, 2018;
|
|
|
•
|
|
Our Current Reports on Form 8-K, filed with the SEC on January 5, 2018, January 24, 2018, March 26, 2018 and March 29, 2018; and
|
|
|
•
|
|
The description of our common stock contained in our registration on Form 8-A12B (File No. 001-36304) filed with the SEC on February 7, 2014, including any amendment or report filed for the purpose of updating such description.
|
All documents we file with the SEC pursuant
to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, except as to any portion of any report or documents that is not deemed
filed under such provisions, (1) on or after the date of filing of the registration statement containing this prospectus and
prior to the effectiveness of the registration statement and (2) on or after the date of this prospectus until the earlier
of the date on which all of the securities registered hereunder have been sold or the registration statement of which this prospectus
is a part has been withdrawn, shall be deemed incorporated by reference in this prospectus and to be a part of this prospectus
from the date of filing of those documents and will be automatically updated and, to the extent described above, supersede information
contained or incorporated by reference in this prospectus and previously filed documents that are incorporated by reference in
this prospectus.
Nothing in this prospectus shall be deemed
to incorporate information furnished but not filed with the SEC pursuant to Item 2.02, 7.01 or 9.01 of Form 8-K.
Upon written or oral request, we
will provide without charge to each person to whom a copy of the prospectus is delivered a copy of the documents incorporated
by reference herein (other than exhibits to such documents, unless such exhibits are specifically incorporated by reference herein).
You may request a copy of these filings, at no cost, by writing or telephoning us at the following address: RXi Pharmaceuticals
Corporation, 257 Simarano Drive, Suite 101, Marlborough, Massachusetts 01752 Attention: Investor Relations, telephone: (508) 767-3861.
We maintain a website at http://www.rxipharma.com. The information contained in, or that can be accessed through, our website
is not incorporated by reference in, and is not part of, this prospectus. We have not authorized any one to provide you with any
information that differs from that contained in this prospectus. Accordingly, you should not rely on any information that is not
contained in this prospectus. You should not assume that the information in this prospectus is accurate as of any date other than
the date of the front cover of this prospectus.
WHERE YOU CAN FIND MORE INFORMATION
We are required to file annual, quarterly
and special reports, proxy statements and other information with the SEC. You may read and copy any document filed by us at the
SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330
for further information on the public reference room. Our filings with the SEC are also available to the public at the SEC’s
Internet web site at http://www.sec.gov.
We have filed a registration statement,
or the Registration Statement, of which this prospectus is a part, covering the securities offered hereby, or the Registration
Statement. As allowed by SEC rules, this prospectus does not include all of the information contained in the Registration Statement
and the included exhibits, financial statements and schedules. You are referred to the Registration Statement, the included exhibits,
financial statements and schedules for further information. This prospectus is qualified in its entirety by such other information.
We are subject to the information and
periodic reporting requirements of the Exchange Act and, in accordance therewith, file periodic reports, proxy statements and other
information with the SEC. Such periodic reports, proxy statements and other information are available for inspection and copying
at the public reference room and website of the SEC referred to above. We maintain a website at www.rxipharma.com. The reference
to our website address does not constitute incorporation by reference of the information contained on our website, and you should
not consider the contents of our website in making an investment decision with respect to our securities.
* * *
1,713,064 Shares

Common Stock
PROSPECTUS SUPPLEMENT
H.C. Wainwright & Co.
March 31, 2020
Phio Pharmaceuticals (NASDAQ:PHIO)
Historical Stock Chart
From Mar 2024 to Apr 2024
Phio Pharmaceuticals (NASDAQ:PHIO)
Historical Stock Chart
From Apr 2023 to Apr 2024