As filed with the Securities and Exchange Commission on February
25, 2020
Registration No.
333-
UNITED STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
___________________
FORM S-1
REGISTRATION STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
___________________
EDESA BIOTECH, INC.
(Exact
name of registrant as specified in its charter)
___________________
|
British
Columbia, Canada
|
2836
|
N/A
|
|
(State or other
jurisdiction of incorporation or
organization)
|
(Primary Standard
IndustrialClassification Code Number)
|
(I.R.S.
EmployerIdentification No.)
|
100 Spy Court
Markham, ON L3R 5H6 Canada
(289) 800-9600
(Address,
including zip code, and telephone number, including area code, of
registrant’s principal executive offices)
___________________
Kathi Niffenegger
Chief Financial Officer
Edesa Biotech, Inc.
100 Spy Court
Markham, ON L3R 5H6 Canada
(289) 800-9600
(Name,
address, including zip code, and telephone number, including area
code, of agent for service)
___________________
Copies to:
|
Jonathan Friedman
Stubbs Alderton & Markiles, LLP
15260 Ventura Boulevard, 20th Floor
Sherman Oaks, California 91403
(818) 444-4500
|
___________________
Approximate date of commencement of proposed sale to the public: As
soon as practicable after this registration statement becomes
effective.
If any
of the securities being registered on this Form are to be offered
on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933 check the following box:
☒
If this
Form is filed to register additional securities for an offering
pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement
number of the earlier effective registration statement for the same
offering. ☐
If this
Form is a post-effective amendment filed pursuant to Rule 462(c)
under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier
effective registration statement for the same offering.
☐
If this
Form is a post-effective amendment filed pursuant to Rule 462(d)
under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier
effective registration statement for the same offering.
☐
Indicate
by check mark whether the registrant is a large accelerated filer,
an accelerated filer, a non-accelerated filer, smaller reporting
company, or an emerging growth company. See the definitions of
“large accelerated filer,” “accelerated
filer,” “smaller reporting company,” and
“emerging growth company” in Rule 12b-2 of the Exchange
Act.
|
Large accelerated
filer ☐
|
Accelerated filer
☐
|
|
Non-accelerated
filer ☒
|
Smaller reporting
company ☒
|
|
|
Emerging growth
company ☒
|
If an emerging growth company, indicate by check mark if the
registrant has elected not to use the extended transition period
for complying with any new or revised financial accounting
standards provided pursuant to Section 7(a)(2)(B) of the Securities
Act. ☒
___________________
CALCULATION OF REGISTRATION FEE
|
Title of Each
Class ofSecurities to be Registered(1)
|
|
Proposed Maximum
Offering Price Per Share
|
Proposed Maximum
Aggregate Offering Price
|
Amount of
Registration Fee
|
|
Common shares, no
par value
|
1,016,036(2)
|
$4.80(3)
|
$4,876,972.80(3)
|
$633.03
|
|
Common shares, no
par value
|
677,358(2)
|
$4.00(3)
|
$2,709,432.00(3)
|
$351.68
|
|
Common shares, no
par value
|
12,364(2)
|
$3.20(3)
|
$39,564.80(3)
|
$5.14
|
|
Common shares, no
par value
|
1,897,030
|
$3.58(4)
|
$6,791,367.40(4)
|
$881.52
|
|
Total
|
3,602,788
|
|
$14,417,337.00
|
$1,871.37
|
(1)
Pursuant
to Rule 416 under the Securities Act, the securities being
registered hereunder include such indeterminate number of
additional common shares as may be issued after the date hereof as
a result of stock splits, stock dividends or similar
transactions.
(2)
Represents
Common Shares issuable upon the exercise of warrants by the selling
shareholders named herein.
(3)
Estimated
solely for the purpose of calculating the registration fee in
accordance with Rule 457(o) under the Securities Act of 1933, as
amended, based on the price at which the warrants may be
exercised.
(4)
Estimated solely for the purpose of computing the amount of the
registration fee. In accordance with Rule 457(c) under the
Securities Act of 1933, as amended, the maximum price per share and
maximum aggregate offering price are based on the average of the
$3.6535 (high) and $3.50 (low) sale price of the registrant’s
Common Shares as reported on The Nasdaq Capital Market on February
21, 2020, which date is within five business days prior to filing
this Registration Statement.
The Registrant hereby amends this registration statement on such
date or dates as may be necessary to delay its effective date until
the Registrant shall file a further amendment which specifically
states that this registration statement shall thereafter become
effective in accordance with Section 8(a) of the Securities Act of
1933 or until the registration statement shall become effective on
such date as the Commission, acting pursuant to said Section 8(a),
may determine.
The information in this prospectus is not complete and may be
changed. The selling
shareholders may not sell these securities until the registration
statement filed with the Securities and Exchange Commission is
effective. This prospectus is not an offer to sell these securities
and is not soliciting an offer to buy these securities in any
jurisdiction where the offer or sale is not
permitted.
Subject to Completion, Dated February 25, 2020
Edesa Biotech, Inc.
PRELIMINARY PROSPECTUS
3,602,788 Common Shares
We are registering an aggregate of 3,602,788 common shares, no par
value (“Common Shares”), for resale by certain of our
shareholders identified in this prospectus. The 3,602,788 Common
Shares include (i) 1,016,036
Common Shares underlying outstanding
Class A Purchase Warrants exercisable at $4.80 per share (subject
to customary adjustments for share splits and dividends),
(ii) 677,358
Common Shares underlying outstanding
Class B Purchase Warrants exercisable at $4.00 per share (subject
to customary adjustments for share splits and dividends) and (iii)
12,364 Common Shares underlying outstanding warrants issued to
representatives of a Placement Agent,
exercisable at $3.20 per share
(subject to customary adjustments for share splits and
dividends)(the “Placement Agent Warrants”), which Class
A Purchase Warrants, Class B Purchase Warrants and Placement Agent
Warrants were acquired from us on January 8, 2020.
The
remaining 1,897,030 Common Shares being registered for resale by
certain of the selling shareholders were acquired from us on June
7, 2019 upon the completion of our business combination with Edesa
Biotech Research, Inc., a company organized under the laws of the
province of Ontario. We are not selling any
securities under this prospectus and we will not receive any
proceeds from the resale of the Common Shares by the selling shareholders. Any proceeds received
by us from the exercise of the warrants will be used for general
corporate purposes.
The selling shareholders may offer our Common Shares from time to
time in a number of different methods and at varying prices. For
more information on possible methods of offer and sale by the
selling shareholders, please see the section entitled “Plan
of Distribution” beginning on page 25 of this
prospectus.
We have agreed to bear all of the expenses incurred in connection
with the registration of these shares. The selling shareholders
will pay or assume discounts, commissions, fees of underwriters,
selling brokers or dealer managers, if any, incurred for the resale
of our Common Shares.
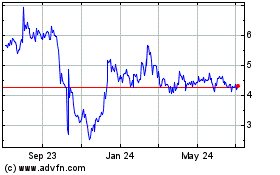
Our Common Shares are listed on the Nasdaq Capital Market under the
symbol “EDSA.” The last reported sale price of our
Common Shares on February 21, 2020 was $3.60 per
share.
We are an “emerging growth company” as that term is
used in the Jumpstart Our Business Startups Act of 2012 and, as
such, we have elected to comply with certain reduced public company
reporting requirements for this prospectus and future filings. See
“Prospectus Summary – Implications of Being an Emerging
Growth Company.”
You should read this prospectus, together with additional
information described under the headings “Incorporation of
Certain Information by Reference” and “Where You Can
Find More Information,” carefully before you invest in our
securities.
Investing in our securities involves a high degree of risk. These
risks are described in the “Risk Factors” section on
page 6 of this prospectus. You should also consider the risk
factors described or referred to in any documents incorporated by
reference in this prospectus, and in an applicable prospectus
supplement, before investing in our securities.
Neither the Securities and Exchange Commission nor any state
securities commission has approved or disapproved of these
securities or determined if this prospectus is truthful or
complete. Any representation to the contrary is a criminal
offense.
The date of this prospectus is 2020.
TABLE OF CONTENTS
|
|
Page
|
|
PROSPECTUS
SUMMARY
|
1
|
|
THE
OFFERING
|
3
|
|
RISK
FACTORS
|
6
|
|
CAUTIONARY NOTE
REGARDING FORWARD-LOOKING STATEMENTS AND INDUSTRY DATA
|
7
|
|
USE OF
PROCEEDS
|
8
|
|
SELLING
SHAREHOLDERS
|
8
|
|
CERTAIN TAX
MATTERS
|
17
|
|
PLAN
OF DISTRIBUTION
|
25
|
|
DESCRIPTION OF
SECURITIES
|
27
|
|
LEGAL
MATTERS
|
31
|
|
EXPERTS
|
31
|
|
WHERE
YOU CAN FIND ADDITIONAL INFORMATION
|
31
|
|
INCORPORATION BY
REFERENCE OF CERTAIN DOCUMENTS
|
32
|
______________
ABOUT THIS PROSPECTUS
You should rely only on the information contained in this
prospectus or in any related free writing prospectus filed by us
with the Securities and Exchange Commission, or the SEC. We have
not authorized anyone to provide you with any information or to
make any representation not contained in this prospectus or
incorporated by reference. We do not take any responsibility for,
and can provide no assurance as to the reliability of, any
information that others may provide to you. This prospectus is not
an offer to sell or an offer to buy securities in any jurisdiction
where offers and sales are not permitted. The information in this
prospectus is accurate only as of its date, regardless of the time
of delivery of this prospectus or any sale of Common Shares. You
should not assume that the information contained in this prospectus
or any prospectus supplement or free writing prospectus is accurate
as of any date other than the date on the front cover of those
documents, or that the information contained in any document
incorporated by reference is accurate as of any date other than the
date of the document incorporated by reference, regardless of the
time of delivery of this prospectus or any sale of a security. Our
business, financial condition, results of operations and prospects
may have changed since those dates.
We have not done anything that would permit a public offering of
the Common Shares or possession or distribution of this prospectus
in any jurisdiction where action for that purpose is required,
other than in the United States. Persons outside the United States
who come into possession of this prospectus must inform themselves
about, and observe any restrictions relating to, the offering of
Common Shares and the distribution of this prospectus outside of
the United States.
It is
important for you to read and consider all of the information
contained in this prospectus in making your investment decision. To
understand the offering fully and for a more complete description
of the offering you should read this entire document
carefully.
|
|
|
|
|
|
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere or
incorporated by reference in this prospectus and does not contain
all of the information you should consider in making your
investment decision. You should read this summary together with the
more detailed information, including our financial statements and
the related notes, contained or incorporated by reference in this
prospectus. You should carefully consider, among other things, the
matters discussed in “Risk Factors” included elsewhere
in this prospectus, the sections titled “Risk Factors”
and “Management’s Discussion and Analysis of Financial
Condition and Results of Operations” and our financial
statements and related notes, each included in our Annual Report on
Form 10-K for the year ended September 30, 2019, filed with the SEC
on December 12, 2019, which is incorporated by reference herein,
and the section entitled “Management’s Discussion and
Analysis of Financial Condition and Results of Operations”
and our unaudited consolidated interim financial statements and
related notes, each included in our Quarterly Report on Form 10-Q
filed with the SEC on February 13, 2020, which is incorporated by
reference herein, before making an investment decision. You should
also read and consider the information in the documents to which we
have referred you in “Where You Can Find Additional
Information” and “Incorporation of Certain Information
by Reference.” As used in this prospectus,
“Edesa,” “the Company,” “we,”
“us,” and “our” refer to Edesa Biotech,
Inc. and our consolidated subsidiaries, except where the context
otherwise requires.
Business Overview
We are a biopharmaceutical company focused on
acquiring, developing and commercializing clinical-stage drugs for
dermatological and gastrointestinal indications with clear unmet
medical needs. Our lead product candidate, EB01, is an
sPLA2
inhibitor for the topical treatment of
chronic allergic contact dermatitis (ACD), a common, potentially
debilitating condition and occupational illness. EB01 employs a
novel, non-steroidal mechanism of action and in two clinical
studies has demonstrated statistically significant improvement of
multiple symptoms in ACD patients. Our investigational new
drug (IND) application for EB01 was accepted by the U.S. Food and
Drug Administration (FDA) in November 2018 and we initiated patient
enrollment for a Phase 2B clinical study evaluating EB01 in October
2019.
We also intend to expand the utility of our
sPLA2
inhibitor technology, which forms the
basis for EB01, across multiple indications. For example, in
September 2019, we received approval from Health Canada to
begin a proof-of-concept clinical study of EB02, an
sPLA2
inhibitor, as a potential treatment
for patients with hemorrhoids disease (HD). In addition to EB01 and
EB02, we plan to expand our portfolio with drug candidates to treat
other skin and gastrointestinal conditions.
Competitive Strengths
We
believe that we possess a number of competitive strengths that
position us to become a leading biopharmaceutical company focused
on dermatological and gastrointestinal diseases,
including:
●
Novel pipeline addressing
large underserved markets. Our
product candidates are novel clinical-stage compounds that have
significant scientific rationale for effectiveness. By initially
targeting large markets that have significant unmet medical needs,
we believe that we can drive adoption of new products and improve
our competitive position. For example, we believe that the novel,
non-steroidal mode of action of our lead product candidates will be
an appealing alternative for managing the symptoms of ACD and HD.
These diseases impact millions of people in the United States and
Canada, and can have significant effects on patients’ quality
of life and, in the case of many chronic ACD patients and their
employers, significant workplace-related costs and
limitations.
●
Intellectual property
protection and market exclusivity. We have opportunities to develop our competitive
position through patents, trade secrets, technical know-how and
continuing technological innovation. We have exclusive license
rights in our target indications to multiple patents and pending
patent applications in the United States and in various foreign
jurisdictions. In addition to patent protection, we intend to
utilize trade secrets and market exclusivity afforded to a New
Chemical Entity, where applicable, to enhance or maintain our
competitive position.
●
Experienced management and
drug development capabilities. Our leadership team possesses core capabilities in
dermatology, gastrointestinal medicine, drug development and
commercialization, chemistry, manufacturing and controls, public
company management and finance. Our founder and Chief Executive
Officer, Pardeep Nijhawan, MD, FRCPC, AGAF, is a board-certified
gastroenterologist and hepatologist with a successful track record
of building life science businesses, including Medical Futures,
Inc., which was sold to Tribute Pharmaceuticals in 2015. In
addition to our internal capabilities, we have also established a
network of key opinion leaders, contract research organizations,
contract manufacturing organizations and consultants. As a result,
we believe we are well positioned to efficiently develop novel
dermatological and gastrointestinal treatments.
|
|
|
|
|
|
|
|
|
|
|
|
Our
business strategy is to develop and commercialize innovative drug
products that address unmet medical needs for large, underserved
markets where there is limited competition. Key elements of our
strategy include:
●
Establish EB01 as the leading
treatment for chronic ACD. Our
primary goal is to obtain regulatory approval for EB01 and
commercialize EB01 for use in the treatment of ACD. Based on
promising clinical trial results in which patients treated with
EB01 experienced statistically significant improvements of their
symptoms with minimal side effects, we initiated a Phase 2B
clinical study evaluating EB01 in the United
States.
●
Selectively targeting
additional indications within the areas of dermatology and
gastroenterology. In addition
to our ACD program, we plan to efficiently generate
proof-of-concept data for other programs where the inhibition of
sPLA2
activity may have a therapeutic
benefit. For example, given sufficient funding, we are planning a
clinical study to evaluate EB02 for internal
hemorrhoids.
●
In-license promising product
candidates. We are applying our
cost-effective development approach to advance and expand our
pipeline. Our current product candidates are in-licensed from
academic institutions or other pharmaceutical companies, and we
plan to continue to identify, evaluate and potentially obtain
rights to and develop additional assets. Our objective is to
maintain a well-balanced portfolio with product candidates across
various stages of development. In general, we seek to identify
product candidates and technology that represent a novel
therapeutic approach to dermatological and gastrointestinal
diseases, are supported by compelling science, target an unmet
medical need, and provide a meaningful commercial opportunity. We
do not currently intend to invest significant capital in basic
research, which can be expensive and
time-consuming.
●
Capture the full commercial
potential of our product candidates. If our product candidates are successfully
developed and approved, we may build commercial infrastructure
capable of directly marketing the products in North America and
potentially other major geographies of strategic interest. We also
plan to evaluate strategic licensing arrangements with
pharmaceutical companies for the commercialization of our drugs,
where applicable, such as in territories where a partner may
contribute additional resources, infrastructure and
expertise.
Corporate Information
We were incorporated in
the Province of British Columbia, Canada in 2007 and we operate
through our wholly-owned subsidiaries, Edesa Biotech Research,
Inc., an Ontario corporation incorporated in 2015, formerly known
as Edesa Biotech Inc., which we acquired on
June 7, 2019, and Stellar Biotechnologies, Inc., a California
corporation organized September 9, 1999 and acquired on April 12,
2010. Our Common Shares are traded on The Nasdaq Capital Market
under the symbol “EDSA”. Our executive offices are
located at 100 Spy Court, Markham, Ontario L3R 5H6 Canada and our
telephone number at this location is (289) 800-9600. Our website address
is www.edesabiotech.com. The information contained on, or that can
be accessed through, our website is not a part of this prospectus.
Our trademarks and trade names include, but may not be limited to,
“Edesa Biotech,” and the Edesa
logo.
|
|
|
|
|
|
|
|
|
|
|
|
THE
OFFERING
The following summary contains basic information about the offering
and the securities the selling shareholders are offering and is not
intended to be complete. It does not contain all the information
that is important to you. For a more complete understanding of the
securities the selling shareholders are offering, please refer to
the section of this prospectus titled “Description of
Securities.”
|
|
|
|
Common
Shares being offered by the selling shareholders
|
|
|
The selling shareholders are offering up to 3,602,788 Common
Shares, including (i) 1,016,036 Common Shares underlying outstanding Class A
Purchase Warrants exercisable at $4.80 per share (subject to
customary adjustments for share splits and dividends), (ii) 677,358
Common Shares underlying outstanding Class B Purchase Warrants
exercisable at $4.00 per share (subject to customary adjustments
for share splits and dividends) and (iii) 12,364 Common Shares
underlying outstanding warrants issued to representatives of
Brookline
Capital Markets, a division of Arcadia Securities, LLC,
exercisable at $3.20 per share
(subject to customary adjustments for share splits and
dividends)(the “Placement Agent Warrants”).
The
remaining 1,897,030 Common Shares being registered for resale by
certain of the selling shareholders were acquired from us on June
7, 2019 upon the completion of our business combination with Edesa
Biotech Research, Inc.
|
|
|
|
|
|
|
|
|
|
|
Warrant
exercisability and expiration
|
|
|
The Class A Purchase Warrants will be exercisable at any time on or
after July 8, 2020 (the “Class A Purchase Warrant Initial
Exercise Date”), at an exercise price of $4.80 per share and
will expire on the third anniversary of the Class A Purchase
Warrant Initial Exercise Date. The Class B Purchase Warrants will
be exercisable at any time on or after July 8, 2020 (the
“Class B Purchase Warrant Initial Exercise Date”), at
an exercise price of $4.00 per share and will expire on the four
month anniversary of the Class B Purchase Warrant Initial Exercise
Date. The Placement Agent Warrants will be exercisable at any time
on or after July 6, 2020 (the “Placement Agent Warrant
Initial Exercise Date”), at an exercise price of $3.20 per
share and will expire on the fifth anniversary of the Placement
Agent Warrant Initial Exercise Date.
|
|
|
|
|
|
|
|
|
|
|
Common
shares outstanding prior to this offering
|
|
|
8,859,159
shares
|
|
|
|
|
|
|
|
|
|
|
Common shares to be outstanding after this offering
|
|
|
10,564,917
shares
|
|
|
|
|
|
|
|
|
|
|
Use of
Proceeds
|
|
|
All proceeds from the sale of the Common Shares under this
prospectus will be for the account of the selling shareholders. We
will not receive any proceeds from the sale of our Common Shares
offered pursuant to this prospectus. Any proceeds received by us
from the exercise of the warrants will be used for general
corporate purposes, which may include working capital, capital
expenditures and research and development expenses. See the section
entitled “Use of Proceeds” in this
prospectus.
|
|
|
|
|
|
|
|
|
|
|
Nasdaq Capital Market trading symbol
|
|
|
“EDSA”
|
|
|
|
|
|
|
|
|
|
|
Listing
|
|
|
Our Common Shares are listed for trading on the Nasdaq Capital
Market. There is no established trading market for the warrants and
we do not intend to list the warrants on any exchange or other
trading or quotation system.
|
|
|
|
|
|
|
|
|
|
|
Risk Factors
|
|
|
See “Risk Factors” on page 6 of this prospectus to read
about factors you should consider before buying Common
Shares.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The
number of our Common Shares to be outstanding after the offering is
based on 8,859,159 of our Common Shares outstanding as of February
21, 2020 and excludes:
●
671,891
of our Common Shares issuable upon exercise of outstanding options
granted under our equity incentive plans at a weighted average
exercise price of $3.27 per share;
●
481,256 of
our Common Shares available for issuance or future grant pursuant
to our equity incentive plan; and
●
48,914
of our Common Shares issuable upon exercise of outstanding warrants
at a weighted average exercise price of $11.19 per
share.
Implications of Being an Emerging Growth Company
As
a company with less than $1.07 billion in revenue during our
last fiscal year, we qualify as an “emerging growth
company” as defined in the Jumpstart Our Business Startups
Act, or JOBS Act, enacted in April 2012. An “emerging
growth company” may take advantage of reduced reporting
requirements that are otherwise applicable to public companies.
These provisions include, but are not limited to:
●
not
being required to comply with the auditor attestation requirements
of Section 404(b) of the Sarbanes-Oxley Act;
●
reduced
disclosure obligations regarding executive compensation in our
periodic reports, proxy statements and registration statements;
and
●
exemptions
from the requirements of holding a nonbinding advisory vote on
executive compensation and shareholder approval of any golden
parachute payments not previously approved.
We
may take advantage of these provisions until September 30, 2021.
However, if certain events occur prior to September 30, 2021,
including if we become a “large accelerated filer,” our
annual gross revenues exceed $1.07 billion or we issue more
than $1.0 billion of non-convertible debt in any three-year
period, we will cease to be an emerging growth company before such
date.
We
have elected to take advantage of certain of the reduced disclosure
obligations and may elect to take advantage of other reduced
reporting requirements in future filings. As a result, the
information that we provide to our shareholders may be different
than the information you might receive from other public reporting
companies in which you hold equity interests.
Description of Business Combination Transaction
On
June 7, 2019, we completed a business combination with Edesa
Biotech Research, Inc., formerly known as Edesa Biotech Inc.
(“Edesa Research”), a company organized under the laws
of the province of Ontario, in accordance with the terms of a Share
Exchange Agreement, dated March 7, 2019, by and among us, Edesa
Research and the shareholders of Edesa Research. At the closing of
the transaction, we acquired the entire issued share capital of
Edesa Research, with Edesa Research becoming a wholly owned
subsidiary of ours. Also on June 7, 2019, in connection with and
following the completion of the business combination, we effected a
1-for-6 reverse split of our Common Shares and changed our name to
“Edesa Biotech, Inc.” At the closing of the
transaction, the Edesa Research shareholders exchanged their shares
for 88% of our outstanding shares on a fully diluted
basis.
At
the closing of the transaction, the Edesa Research shareholders
received 6,249,780 of our Common Shares in exchange for the capital
shares of Edesa Research and the holders of unexercised Edesa
Research share options immediately prior to the closing of the
transaction were issued replacement share options
(“Replacement Options”) to purchase an aggregate of
297,422 of our Common Shares. On July 26, 2019, pursuant to
the post-closing adjustment contemplated by the Share Exchange
Agreement, we issued an additional 366,234 of our Common Shares to
the Edesa Research shareholders and the holders of unexercised
Edesa Research stock options immediately prior to the closing of
the transaction were issued 17,701 additional Replacement Options
to purchase our Common Shares. Following the completion of the
transactions contemplated by the Share Exchange Agreement and the
reverse split, there were approximately 7,504,468, of our Common
Shares issued and outstanding and approximately 7,876,292 of our
Common Shares outstanding on a fully-diluted basis, and the former
Edesa Research shareholders and option holders owned approximately
6,931,137 of our Common Shares on a fully-diluted basis, or 88% of
our Common Shares on a fully-diluted basis, and our shareholders
and option holders prior to the transactions contemplated by the
Share Exchange Agreement owned approximately 945,155 of our Common
Shares on a fully-diluted basis, or 12% of our Common Shares on a
fully-diluted basis. 1,897,030 Common Shares acquired by the Edesa
Research shareholders in the business combination transaction are
being registered for resale under this prospectus.
|
|
|
|
|
|
|
|
|
|
|
|
Description of Offering and Private Placement with Selling
Shareholders
On
January 6, 2020, we entered into a Securities Purchase Agreement
(the “Securities Purchase Agreement”) with certain
United States resident investors and Subscription Agreements (the
“Subscription Agreements”) with certain non-U.S.
investors providing for the issuance and sale by us of an
aggregate of 1,354,961 of our Common Shares, in a registered direct
offering (the “Offering”). In a concurrent private
placement (the “Private Placement”), we agreed to sell
to such investors (i) Class A Purchase Warrants to purchase an
aggregate of up to 1,016,036 Common Shares, or 0.75 of a Common
Share for each Common Share purchased in the Offering (the
“Class A Purchase Warrants”), and (ii) Class B Purchase
Warrants to purchase an aggregate of up to 677,358 Common Shares,
or 0.50 of a Common Share for each Common Share purchased in the
offering (the “Class B Purchase Warrants,” and together
with the Class A Purchase Warrants, the “Purchase
Warrants”). The price per Common Share and associated
Purchase Warrants was (i) $3.20 for investors other than investors
that are officers, directors, employees or consultants of the
company and (ii) $4.11 for each investor that is an officer,
director, employee or consultant of the company. The closing of the
Offering and concurrent Private Placement occurred on January 8,
2020. The Class A Purchase Warrants and Class B Purchase Warrants
will be exercisable as described in the table above. The exercise
price and number of Common Shares issuable upon the exercise of the
Purchase Warrants will be subject to adjustment in the event of any
share dividends and splits, reverse share split, recapitalization,
reorganization or similar transaction. Subject to limited
exceptions, a holder of Purchase Warrants will not have the right
to exercise any portion of its Purchase Warrants if the holder,
together with its affiliates, would beneficially own in excess of
9.99% of the number of Common Shares outstanding immediately after
giving effect to such exercise (the “Beneficial Ownership
Limitation”); provided, however, that upon 61 days’
prior notice to us, the holder may increase the Beneficial
Ownership Limitation, provided that in no event shall the
Beneficial Ownership Limitation exceed 9.99%.
Brookline
Capital Markets, a division of Arcadia Securities, LLC
(“Brookline”), acted as placement agent in the United
States in connection with the Offering and Private Placement
pursuant to a Financial Advisory Agreement between us and Brookline
dated November 5, 2019, as amended. Upon the closing of the
Offering and Private Placement, Brookline received a placement
agent fee of $207,475, which equals 6.5% of the gross proceeds from
sales arranged by Brookline (or 3.5% in the case of sales to
investors introduced by the company). Brookline did not receive any
cash placement fee with respect to non-U.S. investors. As
additional compensation, we issued to Brookline the Placement Agent
Warrants to purchase 12,364 Common Shares, which equals 1.25% of
the number of Common Shares sold in the Offering to investors
introduced by Brookline. Brookline did not receive any warrant
compensation for securities issued to non-U.S investors. The
company also reimbursed Brookline $55,000 for certain expenses
incurred by Brookline in connection with the Offering.
The
Financial Advisory Agreement provides that, for a period of nine
(9) months from the closing date of the Offering, Brookline has a
right of first refusal to act as a co-manager for any financing of
the company by means of a fully marketed public offering, with no
less than 20% of the total fees paid to the
underwriters.
We
received gross proceeds of approximately $4.36 million from
the sale of these securities, before deducting placement agent fees
and offering expenses, and excluding the exercise of any
warrants.
For
a detailed description of the transactions contemplated by the
Share Exchange Agreement, Financial Advisory Agreement, Securities
Purchase Agreement, Subscription Agreements, Purchase Warrants and
Placement Agent Warrants with the selling shareholders and the
securities issued or issuable pursuant thereto, see the section
captioned “Selling Shareholders” in this
prospectus.
We
filed the registration statement on Form S-1, of which this
prospectus forms a part, (i) to fulfill our contractual
obligations under the Securities Purchase Agreement, Subscription
Agreements and Placement Agent Warrants with the selling
shareholders to provide for the resale by the selling shareholders
of the Common Shares underlying the Purchase Warrants and Placement
Agent Warrants and (ii) to provide liquidity to certain
shareholders of the company that acquired Common Shares in our
business combination transaction with Edesa Research.
|
|
|
|
|
|
RISK FACTORS
Investing in our Common Shares involves a high degree of
risk. Investors should carefully consider the risks
described in the filings incorporated by reference in this
prospectus and any prospectus supplement, including our Annual
Report on Form 10-K for the transition period from January 1, 2019
to September 30, 2019 filed with the SEC, before deciding whether
to invest in our securities. We expect to update the risk factors
from time to time in the periodic and current reports that we file
with the SEC after the date of this prospectus. These updated risk
factors will be incorporated by reference in this prospectus. The
risks described in our filings incorporated by reference are not
the only ones we face. Our business,
financial condition and results of operations could be materially
and adversely affected by any or all of these risks or by
additional risks and uncertainties not presently known to us or
that we currently deem immaterial that may adversely affect us in
the future. In such case, the trading price of our Common
Shares could decline and you could lose all or part of your
investment. Our actual results could differ materially from those
anticipated in the forward-looking statements made throughout this
prospectus and in the documents incorporated by reference as a
result of different factors, including the risks we face described
in the filings incorporated by reference.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference in this
prospectus contain certain statements that constitute
forward-looking statements within the meaning of Section 27A of the
Securities Act of 1933, as amended, or Securities Act, and Section
21E of the Securities Exchange Act of 1934, as amended, or Exchange
Act. These statements involve known and unknown risks,
uncertainties and other important factors that may cause our actual
results, performance or achievements to be materially different
from any future results, performances or achievements expressed or
implied by the forward looking statements. These forward looking
statements include, but are not limited to, those concerning the
following:
●
our ability to fund
our planned operations and implement our business
plan;
●
the scope, number,
progress, duration, cost, results and timing of clinical trials and
nonclinical studies of our current or future product
candidates;
●
our ability to
raise sufficient funds to support the development and potential
commercialization of our product candidates;
●
the outcomes and
timing of regulatory reviews, approvals or other
actions;
●
our ability to
obtain marketing approval for our product candidates and otherwise
execute our business plan;
●
our ability to
establish and maintain licensing, collaboration or similar
arrangements on favorable terms and whether and to what extent we
retain development or commercialization responsibilities under any
new licensing, collaboration or similar arrangement;
●
the success of any
other business, product or technology that we acquire or in which
we invest;
●
our ability to
maintain, expand and defend the scope of our intellectual property
portfolio;
●
our ability to
manufacture any approved products on commercially reasonable
terms;
●
our ability to
establish a sales and marketing organization or suitable
third-party alternatives for any approved product;
●
the number and
characteristics of product candidates and programs that we
pursue;
●
the attraction and
retention of qualified employees and personnel;
●
future acquisitions
or investments in complementary companies or technologies;
and
●
our ability to
comply with evolving legal standards and regulations pertaining to
our industry.
In some cases, you can identify forward-looking statements by terms
such as “anticipates,” “believes”,
“could”, “estimates”,
“expects”, “intends”, “may”,
“plans”, “potential”,
“predicts”, “projects”,
“should”, “will”, “would” as
well as similar expressions. Forward-looking statements reflect our
current views with respect to future events, are based on
assumptions and are subject to risks, uncertainties and other
important factors. We discuss many of these risks, uncertainties
and other important factors in greater detail under the heading
“Risk Factors” contained in this prospectus and in our
most recent annual report on Form 10-K, as well as any amendments
thereto reflected in subsequent filings with the SEC. Given these
risks, uncertainties and other important factors, you should not
place undue reliance on these forward-looking statements. Also,
these forward-looking statements represent our estimates and
assumptions only as of the date such forward-looking statements are
made. Except as required by law, we assume no obligation to update
any forward-looking statements publicly, or to reflect facts and
circumstances after the date of this prospectus. Before deciding to
purchase our securities, you should carefully read this prospectus,
together with the information incorporated herein by reference as
described under the heading “Incorporation by Reference
of
Certain Documents,” completely and with the
understanding that our actual future results may be materially
different from what we expect.
This
prospectus and the documents incorporated by reference in this prospectus also refer to estimates
and other statistical data made by independent parties and by us
relating to market size and growth and other data about our
industry. This data involves a number of assumptions and
limitations, and you are cautioned not to give undue weight to such
estimates. In addition, projections, assumptions and estimates of
our future performance and the future performance of the markets in
which we operate are necessarily subject to a high degree of
uncertainty and risk.
USE OF PROCEEDS
We will not receive any proceeds from the resale of our Common
Shares by the selling shareholders. We cannot predict when or if
the Purchase Warrants or Placement Agent Warrants will be
exercised, and it is possible that the Purchase Warrants and/or
Placement Agent Warrants may expire and never be exercised. Any
proceeds received by us from the exercise of such warrants will be
used for general corporate purposes, which may include working
capital, capital expenditures, and research and development
expenses.
We have not yet determined the amount of net proceeds to be used
specifically for any of the foregoing purposes. Accordingly, our
management will have significant discretion and flexibility in
applying the net proceeds from the exercise of the Purchase
Warrants and/or Placement Agent Warrants. Pending any use, as
described above, we intend to invest the net proceeds in
high-quality, short-term, interest-bearing securities.
The selling shareholders will pay any discounts, commissions, fees
of underwriters, selling brokers or dealer managers and expenses
incurred by the selling shareholders for brokerage, accounting, tax
or legal services or any other expenses incurred by the selling
shareholders in disposing of the shares. We will bear all other
costs, fees and expenses incurred in effecting the registration of
the shares covered by this prospectus, including, without
limitation, all registration and filing fees, printing fees, Nasdaq
listing fees and fees and expenses of our counsel and our
accountants.
SELLING SHAREHOLDERS
Description of Transactions with Selling Shareholders
Description of Business Combination Transaction
On June 7, 2019, we completed a business combination with Edesa
Biotech Research, Inc., formerly known as Edesa Biotech Inc.
(“Edesa Research”), a company organized under the laws
of the province of Ontario, in accordance with the terms of a Share
Exchange Agreement, dated March 7, 2019, by and among us, Edesa
Research and the shareholders of Edesa Research. At the closing of
the transaction, we acquired the entire issued share capital of
Edesa Research, with Edesa Research becoming a wholly owned
subsidiary of ours. Also on June 7, 2019, in connection with and
following the completion of the business combination, we effected a
1-for-6 reverse split of our Common Shares and changed our name to
“Edesa Biotech, Inc.” At the closing of the
transaction, the Edesa Research shareholders exchanged their shares
for 88% of our outstanding shares on a fully diluted
basis.
At the closing of the transaction, the Edesa Research shareholders
received 6,249,780 of our Common Shares in exchange for the capital
shares of Edesa Research and the holders of unexercised Edesa
Research share options immediately prior to the closing of the
transaction were issued replacement share options
(“Replacement Options”) to purchase an aggregate of
297,422 of our Common Shares. On July 26, 2019, pursuant to
the post-closing adjustment contemplated by the Share Exchange
Agreement, we issued an additional 366,234 of our Common Shares to
the Edesa Research shareholders and the holders of unexercised
Edesa Research stock options immediately prior to the closing of
the transaction were issued 17,701 additional Replacement Options
to purchase our Common Shares. Following the completion of the
transactions contemplated by the Share Exchange Agreement and the
reverse split, there were approximately 7,504,468, of our Common
Shares issued and outstanding and approximately 7,876,292 of our
Common Shares outstanding on a fully-diluted basis, and the former
Edesa Research shareholders and option holders owned approximately
6,931,137 of our Common Shares on a fully-diluted basis, or 88% of
our Common Shares on a fully-diluted basis, and our shareholders
and option holders prior to the transactions contemplated by the
Share Exchange Agreement owned approximately 945,155 of our Common
Shares on a fully-diluted basis, or 12% of our Common Shares on a
fully-diluted basis. 1,897,030 Common Shares acquired by the Edesa
Research shareholders in the business combination transaction are
being registered for resale under this prospectus.
Description of Offering and Private Placement with Selling
Shareholders
On January 6, 2020, we entered into a Securities Purchase Agreement
(the “Securities Purchase Agreement”) with certain
United States resident investors and Subscription Agreements (the
“Subscription Agreements”) with certain non-U.S.
investors providing for the issuance and sale by us of an
aggregate of 1,354,961 of our Common Shares, in a registered direct
offering (the “Offering”). In a concurrent private
placement (the “Private Placement”), we agreed to sell
to such investors (i) Class A Purchase Warrants to purchase an
aggregate of up to 1,016,036 Common Shares, or 0.75 of a Common
Share for each Common Share purchased in the Offering (the
“Class A Purchase Warrants”), and (ii) Class B Purchase
Warrants to purchase an aggregate of up to 677,358 Common Shares,
or 0.50 of a Common Share for each Common Share purchased in the
offering (the “Class B Purchase Warrants,” and together
with the Class A Purchase Warrants, the “Purchase
Warrants”). The price per Common Share and associated
Purchase Warrants was (i) $3.20 for investors other than investors
that are officers, directors, employees or consultants of the
company and (ii) $4.11 for each investor that is an officer,
director, employee or consultant of the company. The closing of the
Offering and concurrent Private Placement occurred on January 8,
2020. The Class A Purchase Warrants and Class B Purchase Warrants
will be exercisable as described under the heading
“The Offering” on page 3. The exercise
price and number of Common Shares issuable upon the exercise of the
Purchase Warrants will be subject to adjustment in the event of any
share dividends and splits, reverse share split, recapitalization,
reorganization or similar transaction. Subject to limited
exceptions, a holder of Purchase Warrants will not have the right
to exercise any portion of its Purchase Warrants if the holder,
together with its affiliates, would beneficially own in excess of
9.99% of the number of Common Shares outstanding immediately after
giving effect to such exercise (the “Beneficial Ownership
Limitation”); provided, however, that upon 61 days’
prior notice to us, the holder may increase the Beneficial
Ownership Limitation, provided that in no event shall the
Beneficial Ownership Limitation exceed 9.99%.
Brookline Capital Markets, a division of Arcadia Securities, LLC
(“Brookline”), acted as placement agent in the United
States in connection with the Offering and Private Placement
pursuant to a Financial Advisory Agreement between us and Brookline
dated November 5, 2019, as amended. Upon the closing of the
Offering and Private Placement, Brookline received a placement
agent fee of $207,475, which equals 6.5% of the gross proceeds from
sales arranged by Brookline (or 3.5% in the case of sales to
investors introduced by the company). Brookline did not receive any
cash placement fee with respect to non-U.S. investors. As
additional compensation, we issued to Brookline the Placement Agent
Warrants to purchase 12,364 Common Shares, which equals 1.25% of
the number of Common Shares sold in the Offering and concurrent
Private Placement to investors introduced by Brookline. Brookline
did not receive any warrant compensation for securities issued to
non-U.S investors. The company also reimbursed Brookline $55,000
for certain expenses incurred by Brookline in connection with the
Offering.
The Financial Advisory Agreement provides that, for a period of
nine (9) months from the closing date of the Offering, Brookline
has a right of first refusal to act as a co-manager for any
financing of the company by means of a fully marketed public
offering, with no less than 20% of the total fees paid to the
underwriters.
We received gross proceeds of approximately $4.36 million from
the sale of these securities, before deducting placement agent fees
and offering expenses, and excluding the exercise of any
warrants.
We filed the registration statement on Form S-1, of which this
prospectus forms a part, (i) to fulfill our contractual
obligations under the Securities Purchase Agreement, Subscription
Agreements and Placement Agent Warrants with the selling
shareholders to provide for the resale by the selling shareholders
of the Common Shares underlying the Purchase Warrants and Placement
Agent Warrants and (ii) to provide liquidity to certain
shareholders of the company that acquired Common Shares in our
business combination transaction with Edesa Research.
The resale registration statement, of which this prospectus is a
part, when declared effective by the SEC, permits the resale into
the market from time to time over an extended period of the Common
Shares underlying the Purchase Warrants and Placement Agent
Warrants and the resale of a portion of the Common Shares held by
certain of the selling shareholders that were acquired in our
business combination transaction with Edesa Research.
When we refer to the selling shareholders in this prospectus, we
mean those persons listed in the table below, as well as the
permitted transferees, pledgees, donees, assignees, successors and
others who later come to hold any of the selling
shareholders’ interests other than through a public
sale.
The selling shareholders may from time to time offer and sell
pursuant to this prospectus any or all of the Common Shares set
forth in the following table. There is no requirement for the
selling shareholders to sell their shares, and we do not know when,
or if, or in what amount the selling shareholders may offer the
Common Shares for sale pursuant to this prospectus.
The table below has been prepared based on the information
furnished to us by the selling shareholders as of February 7, 2020.
The selling shareholders identified below may have sold,
transferred or otherwise disposed of some or all of their shares
since the date on which the information in the following table is
presented in transactions exempt from or not subject to the
registration requirements of the Securities Act. Information
concerning the selling shareholders may change from time to time
and, if necessary, we will supplement this prospectus accordingly.
We are unable to confirm whether the selling shareholders will in
fact sell any or all of their Common Shares.
To our knowledge and except as noted below, none of the selling
shareholders has, or within the past three years has had, any
material relationships with us or any of our affiliates. Each
selling shareholder who is also an affiliate of a broker dealer, as
noted below, has represented that: (1) the selling shareholder
purchased in the ordinary course of business and (2) at the time of
purchase of the securities being registered for resale, the selling
shareholder had no agreements or understandings, directly or
indirectly, with any person to distribute the
securities.
Beneficial ownership for the purposes of this table is determined
in accordance with the rules and regulations of the SEC. These
rules generally provide that a person is the beneficial owner
of securities if such person has or shares the power to vote or
direct the voting thereof, or to dispose or direct the disposition
thereof or has the right to acquire such powers within 60 days.
Common Shares subject to options or warrants that are currently
exercisable or exercisable within 60 days of February 21, 2020, are
deemed to be outstanding and beneficially owned by the person
holding the options or warrants. These shares, however, are not
deemed outstanding for the purposes of computing the percentage
ownership of any other person.
Percentage of beneficial ownership is based on 8,859,159 common
shares outstanding as of February 21, 2020.
|
|
Common Shares
Beneficially Owned Prior to Offering
|
|
Common Shares
Beneficially Owned After Offering
|
|
Selling
Shareholders
|
|
|
Number of
Common Shares Being Offered
|
|
|
|
Firstfire Global
Opportunities Fund LLC (1)
|
68,360
|
*
|
68,360
|
-
|
-
|
|
KBB Asset
Management (2)
|
105,470
|
1.2%
|
58,595
|
46,875
|
*
|
|
RRSJ Associates
(3)
|
105,470
|
1.2%
|
58,595
|
46,875
|
*
|
|
Laurence Lytton
(4)
|
70,313
|
*
|
39,063
|
31,250
|
*
|
|
Warberg WF VII LP
(5)
|
28,125
|
*
|
15,625
|
12,500
|
*
|
|
William Cassano
(6)
|
35,157
|
*
|
19,532
|
15,625
|
*
|
|
Intracoastal
Capital LLC (7)
|
78,125
|
*
|
78,125
|
-
|
-
|
|
Robert Masters
(8)
|
70,313
|
*
|
39,063
|
31,250
|
*
|
|
AG Family LP
(9)
|
140,625
|
1.6%
|
78,125
|
62,500
|
*
|
|
Pichon Family Trust
(10)
|
35,157
|
*
|
19,532
|
15,625
|
*
|
|
Lagom LLC
(11)
|
140,625
|
1.6%
|
78,125
|
62,500
|
*
|
|
Michael Mullins
(12)
|
10,548
|
*
|
5,860
|
4,688
|
*
|
|
Josiah Austin
(13)
|
70,312
|
*
|
39,062
|
31,250
|
*
|
|
Craig Effron
(14)
|
35,157
|
*
|
19,532
|
15,625
|
*
|
|
Bruce Conway
(15)
|
63,282
|
*
|
35,157
|
28,125
|
*
|
|
Stephen Mut
(16)
|
35,157
|
*
|
19,532
|
15,625
|
*
|
|
Jennifer A.
Duncan’s Inheritors Trust (17)
|
35,157
|
*
|
19,532
|
15,625
|
*
|
|
Nicholas Finegold
(18)
|
105,470
|
1.2%
|
58,595
|
46,875
|
*
|
|
YJP International
Limited (19)
|
56,250
|
*
|
31,250
|
25,000
|
*
|
|
Starlight
Investment Holdings Limited (20)
|
84,375
|
*
|
46,875
|
37,500
|
*
|
|
James Clancey
(21)
|
14,063
|
*
|
7,813
|
6,250
|
*
|
|
John Moore
(22)
|
17,580
|
*
|
9,767
|
7,813
|
*
|
|
Tomsat Investment
& Trading Co. Inc. (23)
|
70,313
|
*
|
39,063
|
31,250
|
*
|
|
Stanford Ventures
LLC (24)
|
175,782
|
2.0%
|
97,657
|
78,125
|
*
|
|
Bigger Capital
Fund, LP (25)
|
58,595
|
1.2%
|
58,595
|
-
|
-
|
|
Hill Blalock, Jr.
(26)
|
35,157
|
*
|
19,532
|
15,625
|
*
|
|
VI LLC
(27)
|
70,313
|
*
|
39,063
|
31,250
|
*
|
|
Catalysis Partners,
LLC (28)
|
70,313
|
*
|
39,063
|
31,250
|
*
|
|
Lee Revocable Trust
(29)
|
35,157
|
*
|
19,532
|
15,625
|
*
|
|
Joel Levine
(30)
|
17,813
|
*
|
7,813
|
10,000
|
*
|
|
William Nimrod
(31)
|
14,063
|
*
|
7,813
|
6,250
|
*
|
|
The Hewett Fund LP
(32)
|
88,595
|
*
|
58,595
|
30,000
|
*
|
|
Richard Maulit
(33)
|
7,032
|
*
|
3,907
|
3,125
|
*
|
|
Lorin Johnson
(34)
|
23,361
|
*
|
10,655
|
12,706
|
*
|
|
Frank and Dorothy
Oakes Family Trust (35)
|
14,040
|
*
|
1,523
|
12,517
|
*
|
|
Kathi Niffenegger
Trust (36)
|
37,289
|
*
|
1,523
|
35,766
|
*
|
|
Gary Koppenjan
(37)
|
20,219
|
*
|
1,523
|
18,696
|
*
|
|
Lumira Capital II,
L.P. (38)
|
2,154,874
|
23.9%
|
672,208
|
1,482,666
|
14.0%
|
|
Lumira Capital II
(International), L.P. (39)
|
199,262
|
2.2%
|
62,160
|
137,102
|
1.3%
|
|
Jeff McLean
(40)
|
140,625
|
1.6%
|
78,125
|
62,500
|
*
|
|
Bruce and Bonny
Jean MacDonald (41)
|
70,313
|
*
|
39,063
|
31,250
|
*
|
|
James Gary Paterson
(42)
|
70,313
|
*
|
39,063
|
31,250
|
*
|
|
10379085 Canada
Inc. (43)
|
710,375
|
8.0%
|
222,098
|
488,277
|
4.6%
|
|
2248618 Ontario
Inc. (44)
|
35,157
|
*
|
19,532
|
15,625
|
*
|
|
Caitlin McCain
(45)
|
10,548
|
*
|
5,860
|
4,688
|
*
|
|
K. Jessa Medicine
Prof. Corporation (46)
|
14,063
|
*
|
7,813
|
6,250
|
*
|
|
Michael Brooks
(47)
|
188,227
|
2.1%
|
2,285
|
185,942
|
1.7%
|
|
York-Cav
Enterprises Inc. (48)
|
9,663
|
*
|
3,045
|
6,618
|
*
|
|
Geoffrey Christie
(49)
|
21,467
|
*
|
9,760
|
11,707
|
*
|
|
Paul Pay
(50)
|
38,463
|
*
|
3,045
|
35,418
|
*
|
|
Ronnie Tarter
(51)
|
14,063
|
*
|
7,813
|
6,250
|
*
|
|
Kassum Properties
Canada Limited (52)
|
35,157
|
*
|
19,532
|
15,625
|
*
|
|
Sean
McDonald (53)
|
18,551
|
*
|
4,311
|
14,240
|
*
|
|
Inveready
Innvierte Biotech II, S.C.R. S.A. (54)
|
531,986
|
6.0%
|
159,596
|
372,390
|
3.5%
|
|
Pardeep Nijhawan
Medical Professional Corporation (55)
|
2,127,594
|
24.0%
|
643,601
|
1,483,993
|
14.0%
|
|
Pardeep
Nijhawan (56)
|
580,287
|
6.5%
|
161,194
|
419,093
|
4.0%
|
|
The
Digestive Health Clinic Inc. (57)
|
224,094
|
2.5%
|
67,229
|
156,865
|
1.5%
|
|
1968160
Ontario Inc. (58)
|
371,727
|
4.2%
|
111,519
|
260,208
|
2.5%
|
|
William
Buchanan, Jr. (59)
|
2,312
|
*
|
2,312
|
-
|
-
|
|
Harris
Lydon (60)
|
4,064
|
*
|
4,064
|
-
|
-
|
|
Scott
A. Katzmann (61)
|
2,196
|
*
|
2,196
|
-
|
-
|
|
Graham
Powis (62)
|
1,541
|
*
|
1,541
|
-
|
-
|
|
Andrew
Daniels (63)
|
1,360
|
*
|
1,360
|
-
|
-
|
|
Patrick
Sturgeon (64)
|
205
|
*
|
205
|
-
|
-
|
|
Westley
McGeoghegan (65)
|
293
|
*
|
293
|
-
|
-
|
|
Dan
Weston (66)
|
293
|
*
|
293
|
-
|
-
|
|
Henry
Moore (67)
|
53
|
*
|
53
|
-
|
-
|
|
Matthew
Hoban (68)
|
47
|
|
47
|
-
|
-
|
(1)
Consists of (i)
41,016 Common Shares issuable upon
exercise of Class A Warrants; and (ii) 27,344
Common Shares issuable upon exercise
of Class B Warrants. Eli Fireman, as Managing Member of
Firstfire Global Opportunities Fund LLC, has sole voting and
dispositive
power over all such shares.
(2)
Consists of (i)
46,875 Common Shares; (ii) 35,157 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 23,438 Common Shares issuable upon exercise of
Class B Warrants. Steven Segal, as Managing Member, has sole
control and investment direction over the reported securities that
are held by KBB Asset Management. As a result, Steven Segal may be
deemed to have beneficial ownership over such
securities.
(3)
Consists of (i)
46,875 Common Shares; (ii) 35,157 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 23,438 Common Shares issuable upon exercise of
Class B Warrants. Ralph Finerman, as Partner, has sole voting and
dispositive
power over all such shares.
(4)
Consists of (i)
31,250 Common Shares; (ii) 23,438 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 15,625 Common Shares issuable upon exercise of
Class B Warrants.
(5)
Consists of (i)
12,500 Common Shares; (ii) 9,375 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 6,250 Common Shares issuable upon exercise of
Class B Warrants. Daniel Warsh, as manager, has sole voting and
dispositive
power over all such shares.
(6)
Consists of (i)
15,625 Common Shares; (ii) 11,719 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 7,813 Common Shares issuable upon exercise of
Class B Warrants.
(7)
Consists of (i)
46,875 Common Shares issuable upon
exercise of Class A Warrants; and (ii) 31,250 Common Shares issuable upon exercise of
Class B Warrants. Mitchell P. Kopin and Daniel B. Asher, each of
whom are managers of Intracoastal Capital LLC
(“Intracoastal”), have shared voting and investment
discretion over the securities reported herein that are held by
Intracoastal. As a result, each of Mr. Kopin and Mr. Asher may be
deemed to have beneficial ownership (as determined under Section
13(d) of the Securities Exchange Act of 1934, as amended) of the
securities reported herein that are held by
Intracoastal.
(8)
Consists of (i)
31,250 Common Shares; (ii) 23,438 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 15,625 Common Shares issuable upon exercise of
Class B Warrants.
(9)
Consists of (i)
62,500 Common Shares; (ii) 46,875 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 31,250 Common Shares issuable upon exercise of
Class B Warrants. Thomas Satterfield, Jr., as managing partner of
the general partner of AG Family LP, has sole voting and
dispositive
power over all such shares.
(10)
Consists of (i)
15,625 Common Shares; (ii) 11,719 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 7,813 Common Shares issuable upon exercise of
Class B Warrants. Wayne M. Pichon, as trustee of the Pichon Family
Trust, has sole voting and dispositive power over
all such shares.
(11)
Consists of (i)
62,500 Common Shares; (ii) 46,875 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 31,250 Common Shares issuable upon exercise of
Class B Warrants. Ray Nimrod and Marika Lindholm, as managing
members of Lagom, LLC, exercise shared voting and dispositive power over
all such shares.
(12)
Consists of (i)
4,688 Common Shares; (ii) 3,516 Common
Shares issuable upon exercise of Class A Warrants; and (iii)
2,344 Common Shares issuable upon
exercise of Class B Warrants.
(13)
Consists of (i)
31,250 Common Shares; (ii) 23,437 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 15,625 Common Shares issuable upon exercise of
Class B Warrants.
(14)
Consists of (i)
15,625 Common Shares; (ii) 11,719 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 7,813 Common Shares issuable upon exercise of
Class B Warrants.
(15)
Consists of (i)
28,125 Common Shares; (ii) 21,094 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 14,063 Common Shares issuable upon exercise of
Class B Warrants.
(16)
Consists of (i)
15,625 Common Shares; (ii) 11,719 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 7,813 Common Shares issuable upon exercise of
Class B Warrants.
(17)
Consists of (i)
15,625 Common Shares; (ii) 11,719 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 7,813 Common Shares issuable upon exercise of
Class B Warrants. Jennifer Duncan, as trustee, has sole voting and
dispositive
power over all such shares.
(18)
Consists of (i)
46,875 Common Shares; (ii) 35,157 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 23,438 Common Shares issuable upon exercise of
Class B Warrants.
(19)
Consists of (i)
25,000 Common Shares; (ii) 18,750 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 12,500 Common Shares issuable upon exercise of
Class B Warrants. Young Soo Park, as Director of YJP International
Limited, has sole voting and dispositive power over
all such shares.
(20)
Consists of (i)
37,500 Common Shares; (ii) 28,125 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 18,750 Common Shares issuable upon exercise of
Class B Warrants. Nicola Hodge, as Director of Starlight Investment
Holdings Limited, has sole voting and dispositive power over
all such shares.
(21)
Consists of (i)
6,250 Common Shares; (ii) 4,688 Common
Shares issuable upon exercise of Class A Warrants; and (iii)
3,125 Common Shares issuable upon
exercise of Class B Warrants.
(22)
Consists of (i)
7,813 Common Shares; (ii) 5,860 Common
Shares issuable upon exercise of Class A Warrants; and (iii)
3,907 Common Shares issuable upon
exercise of Class B Warrants.
(23)
Consists of (i)
31,250 Common Shares; (ii) 23,438 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 15,625 Common Shares issuable upon exercise of
Class B Warrants. Thomas Satterfield, Jr., as President, has sole
voting and dispositive power over
all such shares.
(24)
Consists of (i)
78,125 Common Shares; (ii) 58,594 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 39,063 Common Shares issuable upon exercise of
Class B Warrants. Hartley Wasko, as VP, has sole voting and
dispositive
power over all such shares.
(25)
Consists of (i)
35,157 Common Shares issuable upon
exercise of Class A Warrants; and (ii) 23,438 Common Shares issuable upon exercise of
Class B Warrants. Michael Bigger, as managing member of the general
partner of Bigger Capital Fund LP, has sole voting and dispositive power over
all such shares.
(26)
Consists of (i)
15,625 Common Shares; (ii) 11,719 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 7,813 Common Shares issuable upon exercise of
Class B Warrants.
(27)
Consists of (i)
31,250 Common Shares; (ii) 23,438 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 15,625 Common Shares issuable upon exercise of
Class B Warrants. Timothy Cohen, as managing member, has sole
voting and dispositive power over
all such shares.
(28)
Consists of (i)
31,250 Common Shares; (ii) 23,438 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 15,625 Common Shares issuable upon exercise of
Class B Warrants. John Francis, as Managing Member, has sole voting
and dispositive power over
all such shares.
(29)
Consists of (i)
15,625 Common Shares; (ii) 11,719 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 7,813 Common Shares issuable upon exercise of
Class B Warrants. Frank Lee, as Tustee of the Lee Revocable Trust,
has sole voting and dispositive power over
all such shares.
(30)
Consists of (i)
10,000 Common Shares; (ii) 4,688 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 3,125 Common Shares issuable upon exercise of
Class B Warrants.
(31)
Consists of (i)
6,250 Common Shares; (ii) 4,688 Common
Shares issuable upon exercise of Class A Warrants; and (iii)
3,125 Common Shares issuable upon
exercise of Class B Warrants.
(32)
Consists of (i)
30,000 Common Shares; (ii) 35,157 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 23,438 Common Shares issuable upon exercise of
Class B Warrants. Martin Chopp, as General Partner, has sole voting
and dispositive power over
all such shares.
(33)
Consists of (i)
3,125 Common Shares; (ii) 2,344 Common
Shares issuable upon exercise of Class A Warrants; and (iii)
1,563 Common Shares issuable upon
exercise of Class B Warrants for which Richard Maulit has
sole voting and dispositive power over
all such shares.
(34)
Consists of (i)
8,524 Common Shares; (ii) 6,393 Common
Shares issuable upon exercise of Class A Warrants; (iii)
4,262 Common Shares issuable upon
exercise of Class B Warrants and (iv) 4,182 Common Shares
issuable upon exercise of options that are exercisable within sixty
days of February 21, 2020. Lorin Johnson is a director of the
Company and has served in such capacity since June
2019.
(35)
Consists of (A)(i)
6,165 Common Shares and (ii) 5,134 Common Shares issuable upon
exercise of options that are exercisable within sixty days of
February 21, 2020 held by Frank Oakes and (B)(i) 1,218 Common
Shares; (ii) 914 Common Shares
issuable upon exercise of Class A Warrants; and (iii) 609
Common Shares issuable upon exercise
of Class B Warrants held by Frank and Dorothy Oakes Family
Trust for which each of Frank Oakes and Dorothy Oakes have voting
and dispositive power over
all such shares. Frank Oakes has been a director of the company
since April 2010 and served as its Chairman of the Board until June
2019. From 1999 to 2019, Mr. Oakes also served as the President and
Chief Executive Officer of the company’s legacy operating
subsidiary, which he founded.
(36)
Consists of (A)
34,548 Common Shares issuable upon exercise of options that are
exercisable within sixty days of February 21, 2020 held by Kathi
Niffenegger and (B) (i) 1,218 Common Shares; (ii) 914 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 609 Common
Shares issuable upon exercise of Class B Warrants held by
the Kathi Niffenegger Trust for which Kathi Niffenegger has sole
voting and dispositive power over
all such shares. Kathi Niffenegger serves as CFO of the
Company, and has served in such capacity since 2013.
(37)
Consists of (i)
1,220 Common Shares; (ii) 914 Common
Shares issuable upon exercise of Class A Warrants; (iii) 609
Common Shares issuable upon exercise
of Class B Warrants and (iv) 17,476 Common Shares issuable
upon exercise of options exercisable within sixty days of February
21, 2020.
Gary Koppenjan has served as Vice President, Investor
Relations and Communications of the Company since June 2019 and
prior to this, served as the Company’s Senior Director of
Investor Relations and Communications.
(38)
Consists of (A)(i)
1,993,970 Common Shares; (ii) 96,542 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 64,362 Common Shares issuable upon exercise of
Class B Warrants held by Lumira Capital
II, L.P. and (B)(i) 184,382 Common Shares; (ii) 8,928
Common Shares issuable upon exercise
of Class A Warrants; and (iii) 5,952 Common Shares issuable upon exercise of
Class B Warrants held by Lumira Capital
II (International), L.P., an affiliate of Lumira Capital II, L.P.
Lumira Capital GP, L.P., the general partners of which are Lumira
GP Inc. and Lumira GP Holdings Co., is the general partner of each
of Lumira Capital II, L.P. and Lumira Capital II (International),
L.P. Each of Lumira Capital II, L.P. and Lumira Capital II
(International), L.P. is managed by Lumira Capital Investment
Management Inc. Each of Lumira Capital GP, L.P., Lumira
GP Inc., Lumira GP Holdings Co. and Lumira Capital Investment
Management Inc. may be deemed to beneficially own the shares held
by Lumira Capital II, L.P. and Lumira Capital II (International),
L.P. Peter van der Velden is an executive
officer of Lumira GP Inc., Lumira GP Holdings Co. and Lumira
Capital Investment Management Inc. Mr. van der Velden has served as
a director of the Company since June 2019, having previously served
as a director of the company’s principal operating
subsidiary, Edesa Biotech Research, Inc., since September 2017. Mr.
van der Velden holds 4,182 Common Shares issuable upon
exercise of options exercisable within sixty days of February 21,
2020. Please also see
footnote (39).
(39)
Consists of (A)(i)
1,993,970 Common Shares; (ii) 96,542 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 64,362 Common Shares issuable upon exercise of
Class B Warrants held by Lumira Capital
II, L.P. and (B)(i) 184,382 Common Shares; (ii) 8,928
Common Shares issuable upon exercise
of Class A Warrants; and (iii) 5,952 Common Shares issuable upon exercise of
Class B Warrants held by Lumira Capital
II (International), L.P., an affiliate of Lumira Capital II, L.P.
Lumira Capital GP, L.P., the general partners of which are Lumira
GP Inc. and Lumira GP Holdings Co., is the general partner of each
of Lumira Capital II, L.P. and Lumira Capital II (International),
L.P. Each of Lumira Capital II, L.P. and Lumira Capital II
(International), L.P. is managed by Lumira Capital Investment
Management Inc. Each of Lumira Capital GP, L.P., Lumira
GP Inc., Lumira GP Holdings Co. and Lumira Capital Investment
Management Inc. may be deemed to beneficially own the shares held
by Lumira Capital II, L.P. and Lumira Capital II (International),
L.P. Peter van der Velden is an executive officer
of Lumira GP Inc., Lumira GP Holdings Co. and Lumira Capital
Investment Management Inc. Mr. van der Velden has served as a
director of the Company since June 2019, having previously served
as a director of the company’s principal operating
subsidiary, Edesa Biotech Research, Inc., since September 2017. Mr.
van der Velden holds 4,182 Common Shares issuable upon
exercise of options exercisable within sixty days of February 21,
2020. Please also see
footnote (38).
(40)
Consists of (i)
62,500 Common Shares; (ii) 46,875 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 31,250 Common Shares issuable upon exercise of
Class B Warrants.
(41)
Consists of (i)
31,250 Common Shares; (ii) 23,438 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 15,625 Common Shares issuable upon exercise of
Class B Warrants.
(42)
Consists of (i)
31,250 Common Shares; (ii) 23,438 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 15,625 Common Shares issuable upon exercise of
Class B Warrants.
(43)
Consists of (i)
690,843 Common Shares; (ii) 11,719 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 7,813 Common Shares issuable upon exercise of
Class B Warrants. Voting and investment
power over the shares held by 10379085 Canada Inc. is exercised by
an investment committee of PCRI Inc., the parent of 10379085 Canada
Inc.
(44)
Consists of (i)
15,625 Common Shares; (ii) 11,719 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 7,813 Common Shares issuable upon exercise of
Class B Warrants. Michael DeGasperis, as President, has sole voting
and dispositive power over
all such shares.
(45)
Consists of (i)
4,688 Common Shares; (ii) 3,516 Common
Shares issuable upon exercise of Class A Warrants; and (iii)
2,344 Common Shares issuable upon
exercise of Class B Warrants.
(46)
Consists of (i)
6,250 Common Shares; (ii) 4,688 Common
Shares issuable upon exercise of Class A Warrants; and (iii)
3,125 Common Shares issuable upon
exercise of Class B Warrants. Karim Jessa, as President, has
sole voting and dispositive power over
all such shares.
(47)
Consists of
(i)1,827 Common Shares; (ii) 1,371 Common Shares issuable upon exercise of
Class A Warrants; (iii) 914 Common
Shares issuable upon exercise of Class B Warrants and (iv)
184,115 Common Shares issuable upon exercise of options exercisable
within sixty days of February 21, 2020. Michael Brooks serves as
the Company’s President, a position he has
held since June 2019. Prior to this, Mr. Brooks served as Vice
President of Corporate Development and Strategy for the
company’s principal operating subsidiary, Edesa Biotech
Research, Inc., since January 2015.
(48)
Consists of (A)
4,182 Common Shares issuable upon exercise of options exercisable
within sixty days of February 21, 2020 held by Carlo Sistilli and
(B)(i) 2,436 Common Shares; (ii) 1,827 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 1,218 Common Shares issuable upon exercise of
Class B Warrants held by York-Cav Enterprises Inc for which Carlo
Sistilli, as President and Director, has sole voting and
dispositive
power over all such shares. Carlo Sistilli has served as a
director of the Company since June 2019.
(49)
Consists of (i)
7,807 Common Shares; (ii) 5,856 Common
Shares issuable upon exercise of Class A Warrants; and (iii)
3,904 Common Shares issuable upon
exercise of Class B Warrants.
(50)
Consists of (i)
2,436 Common Shares; (ii) 1,827 Common
Shares issuable upon exercise of Class A Warrants; (iii)
1,218 Common Shares issuable upon
exercise of Class B Warrants and (iv) 32,982
Common Shares issuable upon exercise of options exercisable within
sixty days of February 21, 2020. Paul Pay has been a
member of the Company’s board of directors since June 2019,
having previously served as a director of the Company’s
principal operating subsidiary, Edesa Biotech Research, Inc., since
its founding in January 2015.
(51)
Consists of (i)
6,250 Common Shares; (ii) 4,688 Common
Shares issuable upon exercise of Class A Warrants; and (iii)
3,125 Common Shares issuable upon
exercise of Class B Warrants.
(52)
Consists of (i)
15,625 Common Shares; (ii) 11,719 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 7,813 Common Shares issuable upon exercise of
Class B Warrants. Kassum Properties Canada Limited is managed by
The Six One Family Trust. Zool Kassum, as trustee of the The Six
One Family Trust, has sole voting and dispositive power over
all such shares.
(53)
Consists of (i)
14,369 Common Shares and (ii) 4,182 Common Shares issuable upon
exercise of options exercisable within sixty days of February 21,
2020. Sean MacDonald has been the
Company’s Chairman of the Board since June 2019, having
previously served as a director of the Company’s principal
operating subsidiary, Edesa Biotech Research, Inc., since September
2017.
(54)
Consists of 531,986 Common Shares. Voting and investment power over
the shares held by Inveready Innvierte Biotech II, S.C.R. S.A is
exercised by its board of directors.
(55)
Consists of (i)
2,116,024 Common Shares; (ii) 6,942 Common Shares issuable upon exercise of
Class A Warrants; and (iii) 4,628 Common Shares issuable upon exercise of
Class B Warrants for which held by Pardeep Nijhawan Medical
Professional Corporation for which Pardeep Nijhawan has sole voting
and dispositive power over
all such shares. Pardeep Nijhawan has served as a director
of the Company since June 2019 and serves as the Company’s
Chief Executive Officer and Secretary. Dr. Nijhawan previously
founded and led the company’s principal operating subsidiary,
Edesa Biotech Research, Inc., since January 2015. Please
also see footnotes 56 and 57.
(56)
Consists of (i)
537,312 Common Shares, and (ii) 42,975 Common Shares issuable upon
exercise of options exercisable within sixty days of February 21,
2020. Pardeep Nijhawan
has served as a director of the Company since June 2019 and serves
as the Company’s Chief Executive Officer and Secretary.
Dr.
Nijhawan previously founded and led the company’s principal
operating subsidiary, Edesa Biotech Research, Inc., since January
2015. Please also see footnotes 55 and 57.
(57)
Consists of 224,094
Common Shares held by The Digestive
Health Clinic Inc. for which Pardeep Nijhawan has sole
voting and dispositive power over
all such shares. Pardeep Nijhawan has served as a director
of the Company since June 2019 and serves as the Company’s
Chief Executive Officer and Secretary. Dr. Nijhawan previously
founded and led the company’s principal operating subsidiary,
Edesa Biotech Research, Inc., since January 2015. Please
also see footnotes 55 and 56.
(58)
Consists of 371,727
Common Shares held by 1968160 Ontario
Inc. for which Nidhi Nijhawan has sole voting and
dispositive
power over all such shares.
(59)
Consists of 2,312
Common Shares issuable upon exercise
of Placement Agent Warrants. The selling shareholder is an affiliate of
Brookline, a broker-dealer and placement agent, and at the time of
the acquisition of securities by the selling shareholder, such
selling shareholder did not have any arrangements or understandings
with any person to distribute such securities.
(60)
Consists of 4,064
Common Shares issuable upon exercise
of Placement Agent Warrants. The selling shareholder is an affiliate of
Brookline, a broker-dealer and placement agent, and at the time of
the acquisition of securities by the selling shareholder, such
selling shareholder did not have any arrangements or understandings
with any person to distribute such securities.
(61)
Consists of 2,196
Common Shares issuable upon exercise
of Placement Agent Warrants. The selling shareholder is an affiliate of
Brookline, a broker-dealer and placement agent, and at the time of
the acquisition of securities by the selling shareholder, such
selling shareholder did not have any arrangements or understandings
with any person to distribute such securities.
(62)
Consists of 1,541
Common Shares issuable upon exercise
of Placement Agent Warrants. The selling shareholder is an affiliate of
Brookline, a broker-dealer and placement agent, and at the time of
the acquisition of securities by the selling shareholder, such
selling shareholder did not have any arrangements or understandings
with any person to distribute such securities.
(63)
Consists of 1,360
Common Shares issuable upon exercise
of Placement Agent Warrants. The selling shareholder is an affiliate of
Brookline, a broker-dealer and placement agent, and at the time of
the acquisition of securities by the selling shareholder, such
selling shareholder did not have any arrangements or understandings
with any person to distribute such securities.
(64)
Consists of 205
Common Shares issuable upon exercise
of Placement Agent Warrants. The selling shareholder is an affiliate of
Brookline, a broker-dealer and placement agent, and at the time of
the acquisition of securities by the selling shareholder, such
selling shareholder did not have any arrangements or understandings
with any person to distribute such securities.
(65)
Consists of 293
Common Shares issuable upon exercise
of Placement Agent Warrants. The selling shareholder is an affiliate of
Brookline, a broker-dealer and placement agent, and at the time of
the acquisition of securities by the selling shareholder, such
selling shareholder did not have any arrangements or understandings
with any person to distribute such securities.
(66)
Consists of 293
Common Shares issuable upon exercise
of Placement Agent Warrants. The selling shareholder is an affiliate of
Brookline, a broker-dealer and placement agent, and at the time of
the acquisition of securities by the selling shareholder, such
selling shareholder did not have any arrangements or understandings
with any person to distribute such securities.
(67)
Consists of 53
Common Shares issuable upon exercise
of Placement Agent Warrants. The selling shareholder is an affiliate of
Brookline, a broker-dealer and placement agent, and at the time of
the acquisition of securities by the selling shareholder, such
selling shareholder did not have any arrangements or understandings
with any person to distribute such securities.
(68)
Consists of 47
Common Shares issuable upon exercise
of Placement Agent Warrants. The selling shareholder is an affiliate of
Brookline, a broker-dealer and placement agent, and at the time of
the acquisition of securities by the selling shareholder, such
selling shareholder did not have any arrangements or understandings
with any person to distribute such securities.
CERTAIN TAX MATTERS
Certain U.S. Federal Income Tax Considerations
The
following discussion is a summary of certain U.S. federal income
tax issues that may be relevant to a U.S. Holder (as defined
herein) and non-U.S. Holder (as defined herein), holding and
disposing of the Common Shares. Additional tax issues may exist
that are not addressed in this discussion and that could affect the
U.S. federal income tax treatment of the acquisition, holding and
disposition of the Common Shares.
This
section is based on the U.S. Tax Code, its legislative history,
existing and proposed regulations, published rulings by the United
States Internal Revenue Service (IRS) and court decisions, all as
currently in effect. These authorities are subject to change,
possibly on a retroactive basis. The discussion applies, unless
indicated otherwise, only to U.S. Holders and certain non-U.S.
Holders who hold Common Shares as capital assets within the meaning
of Section 1221 of the U.S. Tax Code (generally, as property held
for investment) and use the U.S. dollar as their functional
currency. It does not address special classes of holders that may
be subject to different treatment under the U.S. Tax Code, such
as:
●
financial institutions, insurance
companies, underwriters, real estate
investment trusts, or regulated investment
companies;
●
controlled
foreign corporations or passive foreign investment companies under
the U.S. Tax Code;
●
dealers
and traders in securities;
●
persons
holding Common Shares as part of a hedge, straddle, conversion or
other integrated transaction;
●
persons
that acquired Common Shares as compensation for
services;
●
partnerships
or other entities classified as partnerships for U.S. federal
income tax purposes;
●
persons
liable for the alternative minimum tax;
●
tax-exempt
organizations, qualified retirement plans, individual retirement
accounts, or other tax-deferred accounts;
●
certain
U.S. expatriates or former long-term residents of the United
States;
●
persons
that are required to accelerate the recognition of any item of
gross income with respect to the Common Shares as a result of such
income being recognized on an applicable financial statement;
or
●
persons
holding Common Shares that own or are deemed to own 10 per cent or
more (by vote or value) of the company’s shares.
United States Federal Income Taxation
As used
below, a “U.S. Holder” is a beneficial owner of Common
Shares that is, for U.S. federal income tax purposes, (i) a citizen
or resident alien individual of the United States, (ii) a
corporation (or an entity treated as a corporation) created or
organized under the law of the United States, any State thereof or
the District of Columbia, (iii) an estate the income of which is
subject to U.S. federal income tax without regard to its source or
(iv) a trust if (1) a court within the United States is able to
exercise primary supervision over the administration of the trust,
and one or more United States persons have the authority to control
all substantial decisions of the trust, or (2) the trust has a
valid election in effect under applicable U.S. Treasury Regulations
to be treated as a United States person. For purposes of this
discussion, a “non-U.S. Holder” is a beneficial owner
of Common Shares that is (i) a nonresident alien individual, (ii) a
corporation (or an entity treated as a corporation) created or
organized in or under the law of a country other than the United
States or a political subdivision thereof or (iii) an estate or
trust that is not a U.S. Holder. If a partnership (including for
this purpose any entity treated as a partnership for U.S. federal
tax purposes) is a beneficial owner of Common Shares, the U.S.
federal tax treatment of a partner in the partnership generally
will depend on the status of the partner and the activities of the
partnership. A holder of Common Shares that is a partnership and
partners in that partnership should consult their own tax advisers
regarding the U.S. federal income tax consequences of holding and
disposing of Common Shares. We have not sought a ruling from the
IRS or an opinion of counsel as to any U.S. federal income tax
consequence described herein. The IRS may disagree with the
description herein, and its determination may be upheld by a court.
This discussion does not address U.S. federal tax laws other than
those pertaining to U.S. federal income taxation (such as estate or
gift tax laws), nor does it address any aspects of U.S. state or
local or non-U.S. taxation.
This
summary is based upon certain understandings and assumptions with
respect to the business, assets and holders, including that the
company is not, does not expect to become, nor at any time has been
a controlled foreign corporation as defined in Section 957 of the
U.S. Tax Code (“CFC”). The company believes that it is
not and has never been a CFC, and does not expect to become a CFC.
In the event that one or more of such understandings and
assumptions proves to be inaccurate, the following summary may not
apply and material adverse U.S. federal income tax consequences may
result to U.S. Holders.
GIVEN
THE COMPLEXITY OF THE TAX LAWS AND BECAUSE THE TAX CONSEQUENCES TO
ANY PARTICULAR SHAREHOLDER MAY BE AFFECTED BY MATTERS NOT DISCUSSED
HEREIN, SHAREHOLDERS ARE URGED TO CONSULT THEIR OWN TAX ADVISORS
WITH RESPECT TO THE SPECIFIC TAX CONSEQUENCES OF THE ACQUISITION,
OWNERSHIP AND DISPOSITION OF COMMON SHARES, INCLUDING THE
APPLICABILITY AND EFFECT OF STATE, LOCAL AND NON-U.S. TAX LAWS, AS
WELL AS U.S. FEDERAL TAX LAWS.
Taxation of Dividends
U.S. Holders
In
general, subject to the passive foreign investment company (PFIC)
rules discussed below, a distribution on the Common Shares will
constitute a dividend for U.S. federal income tax purposes to the
extent that it is made from the company’s current or
accumulated earnings and profits as determined under U.S. federal
income tax principles. If a distribution exceeds the current and
accumulated earnings and profits of the company, it will generally
be treated as a non-taxable reduction of basis to the extent of the
U.S. Holder’s tax basis in the Common Shares on which it is
paid, and to the extent it exceeds that basis it will be treated as
capital gain. The company has not and does not plan to maintain
calculations of earnings and profits under U.S. federal income tax
principles. Accordingly, it is unlikely that U.S. Holders will be
able to establish that a distribution by the company is in excess
of its current and accumulated earnings and profits (as computed
under U.S. federal income tax principles). Therefore, a U.S. Holder
should expect that a distribution by the company will generally be
taxable in its entirety as a dividend to U.S. Holders for U.S.
federal income tax purposes even though the distribution may be
treated in whole or in part as a non-taxable distribution for
Canadian tax purposes.
The
gross amount of any dividend on the Common Shares (which will
include the amount of any Canadian taxes withheld with respect to
such dividend) generally will be subject to U.S. federal income tax
as foreign source dividend income, and will not be eligible for the
corporate dividends received deduction. The amount of a dividend
paid in Canadian dollars will be its value in U.S. dollars based on
the prevailing spot market exchange rate in effect on the day the
U.S. Holder receives the dividend. A U.S. Holder will have a tax
basis in any distributed Canadian dollars equal to their U.S.
dollar value on the date of receipt, and any gain or loss realized
on a subsequent conversion or other disposition of such Canadian
dollars generally will be treated as U.S. source ordinary income or
loss. If dividends paid in Canadian dollars are converted into U.S.
dollars on the date they are received by a U.S. Holder, the U.S.
Holder generally should not be required to recognize foreign
currency gain or loss in respect of the dividend
income.
Subject
to certain exceptions for short-term and hedged positions, as well
as the PFIC rules, a dividend that a non-corporate U.S. Holder
receives on the Common Shares will generally be subject to a
maximum federal income tax rate of 20% if the dividend is a
“qualified dividend.” A dividend on the Common Shares
will be a qualified dividend if (i) either (a) the Common Shares
are readily tradable on an established market in the United States
or (b) we are eligible for the benefits of a comprehensive income
tax treaty with the United States that the Secretary of the
Treasury determines is satisfactory for purposes of these rules and
that includes an exchange of information program, and (ii) we were
not, in the year prior to the year the dividend was paid, and are
not, in the year the dividend is paid, a PFIC. The Common Shares
are listed on The Nasdaq Capital Market, which should be treated as
an established securities market in the United States. In any
event, the U.S.-Canada Income Convention (the Treaty) satisfies the
requirements of clause (i)(b), we are incorporated in and tax
resident of Canada and should be entitled to the benefits of the
Treaty. Based on our audited financial statements, income tax
returns and relevant market and shareholder data, we believe that
we likely will not be classified as a PFIC in the September 30,
2019 taxable year. There can be no assurance, however, that the
company has not been classified as a PFIC in any prior taxable year
or that the company will not be considered to be a PFIC for any
particular year in the future because PFIC status is factual in
nature, depends upon factors not wholly within the company’s
control, generally cannot be determined until the close of the
taxable year in question, and is determined annually. Accordingly,
no assurance can be made that a dividend paid, if any, would be a
“qualified dividend.” In addition, as described in the
section below entitled “Passive Foreign Investment Company
Rules,” if we were a PFIC in a year while a U.S. Holder held
Common Shares, and if the U.S. Holder has not made a qualified
electing fund election effective for the first year the U.S. Holder
held the Common Shares, such Common Shares remain an interest in a
PFIC for all future years or until such an election is made. The
IRS takes the position that such rule will apply for purposes of
determining whether the Common Shares are an interest in a PFIC in
the year a dividend is paid or in the prior year, even if we do not
satisfy the tests to be a PFIC in either of those years. Even if
dividends on the Common Shares would otherwise be eligible for
qualified dividend treatment, in order to qualify for the reduced
qualified dividend tax rates, a non-corporate U.S. Holder must hold
the Common Shares on which a dividend is paid for more than 60 days
during the 120-day period beginning 60 days before the ex-dividend
date, disregarding for this purpose any period during which the
non-corporate U.S. Holder has an option to sell, is under a
contractual obligation to sell or has made (and not closed) a short
sale of substantially identical stock or securities, is the grantor
of an option to buy substantially identical stock or securities or,
pursuant to U.S. Treasury regulations, has diminished such
holder’s risk of loss by holding one or more other positions
with respect to substantially similar or related property. In
addition, to qualify for the reduced qualified dividend tax rates,
the non-corporate U.S. Holder must not be obligated to make related
payments with respect to positions in substantially similar or
related property. Payments in lieu of dividends from short sales or
other similar transactions will not qualify for the reduced
qualified dividend tax rates.
A
non-corporate U.S. Holder that receives an extraordinary dividend
(generally, any dividend that is in excess of 10% of the holder's
adjusted basis in the Common Shares on which the dividend is paid)
that is eligible for the reduced qualified dividend rates must
treat any loss on the sale of the Common Shares as a long-term
capital loss to the extent of the dividend. For purposes of
determining the amount of a non-corporate U.S Holder’s
deductible investment interest expense, a dividend is treated as
investment income only if the non-corporate U.S. Holder elects to
treat the dividend as not eligible for the reduced qualified
dividend tax rates. Special limitations on foreign tax credits with
respect to dividends subject to the reduced qualified dividend tax
rates apply to reflect the reduced rates of tax.
The
U.S. Treasury has announced its intention to promulgate rules
pursuant to which non-corporate U.S. Holders of stock of non-U.S.
corporations, and intermediaries through which the stock is held,
will be permitted to rely on certifications from issuers to
establish that dividends are treated as qualified dividends.
Because those procedures have not yet been issued, it is not clear
whether we will be able to comply with them.
Non-corporate
U.S. Holders of Common Shares are urged to consult their own tax
advisers regarding the availability of the reduced qualified
dividend tax rates with respect to dividends, if any, received on
the Common Shares in the light of their own particular
circumstances.
Any
Canadian withholding tax imposed on dividends received with respect
to the Common Shares will be treated as a foreign income tax
eligible for credit against a U.S. Holder’s U.S. federal
income tax liability, subject to generally applicable limitations
under U.S. federal income tax law. For purposes of computing those
limitations under current law, which must be calculated separately
for specific categories of income, a dividend generally will
constitute foreign source “passive category income” or,
in the case of certain holders, “general category
income.” A U.S. Holder will be denied a foreign tax credit
with respect to Canadian income tax withheld from dividends
received with respect to the Common Shares to the extent the U.S.
Holder has not held the Common Shares for at least 16 days of the
30-day period beginning on the date which is 15 days before the
ex-dividend date or to the extent the U.S. Holder is under an
obligation to make related payments with respect to substantially
similar or related property. Any days during which a U.S. Holder
has substantially diminished its risk of loss on the Common Shares
are not counted toward meeting the 16-day holding period required
by the statute. The rules relating to the determination of the
foreign tax credit are complex, and U.S. Holders are urged to
consult with their own tax advisers to determine whether and to
what extent they will be entitled to foreign tax credits as well as
with respect to the determination of the foreign tax credit
limitation. Alternatively, any Canadian withholding tax may be
taken as a deduction against taxable income, provided the U.S.
Holder takes a deduction and not a credit for all foreign income
taxes paid or accrued in the same taxable year. In general, special
rules will apply to the calculation of foreign tax credits in
respect of dividend income that is subject to preferential rates of
U.S. federal income tax.
Non-U.S. Holders
A
dividend paid to a non-U.S. Holder of the Common Shares will
generally not be subject to U.S. federal income tax unless the
dividend is effectively connected with the conduct of trade or
business by the non-U.S. Holder within the United States (and is
attributable to a permanent establishment or fixed base the
non-U.S. Holder maintains in the United States if an applicable
income tax treaty so requires as a condition for the non-U.S.
Holder to be subject to U.S. taxation on a net income basis on
income from the Common Shares). A non-U.S. Holder generally will be
subject to tax on an effectively connected dividend in the same
manner as a U.S. Holder. A corporate non-U.S. Holder under certain
circumstances may also be subject to an additional “branch
profits tax,” the rate of which may be reduced pursuant to an
applicable income tax treaty.
Taxation of Capital Gains
U.S. Holders
Subject
to the PFIC rules discussed below, on a sale or other taxable
disposition of the Common Shares, a U.S. Holder will recognize
capital gain or loss in an amount equal to the difference between
the U.S. Holder’s adjusted basis in the Common Shares and the
amount realized on the sale or other disposition, each determined
in U.S. dollars. Such capital gain or loss will be long-term
capital gain or loss if at the time of the sale or other taxable
disposition the Common Shares have been held for more than one
year. In general, any adjusted net capital gain of an individual is
subject to a maximum federal income tax rate of 20%. Capital gains
recognized by corporate U.S. Holders generally are subject to U.S.
federal income tax at the same rate as ordinary income. The
deductibility of capital losses is subject to
limitations.
Any
gain a U.S. Holder recognizes generally will be U.S. source income
for U.S. foreign tax credit purposes, and, subject to certain
exceptions, any loss will generally be a U.S. source loss. If a
Canadian tax is paid on a sale or other disposition of the Common
Shares, the amount realized will include the gross amount of the
proceeds of that sale or disposition before deduction of the
Canadian tax. The generally applicable limitations under U.S.
federal income tax law on crediting foreign income taxes may
preclude a U.S. Holder from obtaining a foreign tax credit for any
Canadian tax paid on a sale or other disposition of the Common
Shares. The rules relating to the determination of the foreign tax
credit are complex, and U.S. Holders are urged to consult with
their own tax advisers regarding the application of such rules.
Alternatively, any Canadian tax paid on the sale or other
disposition of the Common Shares may be taken as a deduction
against taxable income, provided the U.S. Holder takes a deduction
and not a credit for all foreign income taxes paid or accrued in
the same taxable year.
Non-U.S. Holders
A
non-U.S. Holder will not be subject to U.S. federal income tax on
gain recognized on a sale or other disposition of Common Shares
unless (i) the gain is effectively connected with the conduct of
trade or business by the non-U.S. Holder within the United States
(and is attributable to a permanent establishment or fixed base the
non-U.S. Holder maintains in the United States if an applicable
income tax treaty so requires as a condition for the non-U.S.
Holder to be subject to U.S. taxation on a net income basis on
income from the Common Shares), or (ii) in the case of a non-U.S.
Holder who is an individual, the holder is deemed present in the
United States for 183 or more days in the taxable year of the sale
or other disposition and certain other conditions apply. Any
effectively connected gain of a corporate non-U.S. Holder may also
be subject under certain circumstances to an additional
“branch profits tax,” the rate of which may be reduced
pursuant to an applicable income tax treaty.
Passive Foreign Investment Company Rules
A
special set of U.S. federal income tax rules applies to a foreign
corporation that is a PFIC for U.S. federal income tax purposes. As
noted above, based on our audited financial statements, income tax
returns, and relevant market data, we believe that we likely will
not be classified as a PFIC in the September 30, 2019 taxable year.
There can be no assurance, however, that the company has not been
classified as a PFIC in any prior taxable year or that the company
will not be considered to be a PFIC for any particular year in the
future because PFIC status is factual in nature, depends upon
factors not wholly within the company’s control, generally
cannot be determined until the close of the taxable year in
question, and is determined annually.
In
general, a non-US corporation is a PFIC if in any taxable year
either (i) at least 75% of its gross income is “passive
income” or (ii) at least 50% of the quarterly average value
of its assets is attributable to assets that produce or are held to
produce “passive income.” In applying these tests, the
company generally is treated as holding its proportionate share of
the assets and receiving its proportionate share of the income of
any other corporation in which the company owns at least 25% by
value of the shares. Passive income for this purpose generally
includes dividends, interest, royalties, rent and capital gains,
but generally does not include certain rents and royalties derived
in an active business. Passive assets are those assets that are
held for production of passive income or do not produce income at
all. Thus, cash will be a passive asset. Interest, including
interest on working capital, is treated as passive income for
purposes of the income test. Without taking into account the value
of its goodwill, more than 50% of the company’s assets by
value would be passive so that the company would be a PFIC under
the asset test. Based upon its current operations, its goodwill
(the value of which is based on our belief of the estimated fair
market value of the company in excess of book value) will likely be
attributable to its activities that will generate active income
and, to such extent, should be treated as an active asset. The
determination of whether a foreign corporation is a PFIC is a
factual determination made annually and is therefore subject to
change. Subject to exceptions pursuant to certain elections that
generally require the payment of tax, once stock in a foreign
corporation is stock in a PFIC in the hands of a particular
shareholder that is a United States person, it remains stock in a
PFIC in the hands of that shareholder.
If we
are treated as a PFIC, contrary to the tax consequences described
in “Taxation of Dividends” and “Taxation of
Capital Gains” above, a U.S. Holder that does not make an
election described in the succeeding two paragraphs would be
subject to special rules with respect to (i) any gain realized on a
sale or other disposition of Common Shares (for purposes of these
rules, a disposition of Common Shares includes many transactions on
which gain or loss is not realized under general U.S. federal
income tax rules) and (ii) any “excess distribution” by
the company to the U.S. Holder (generally, any distribution during
a taxable year in which distributions to the U.S. Holder on the
Common Shares exceed 125% of the average annual taxable
distributions (whether actual or constructive and whether or not
out of earnings and profits) the U.S. Holder received on the Common
Shares during the preceding three taxable years or, if shorter, the
U.S. Holder’s holding period for the Common Shares). Under
those rules, (i) the gain or excess distribution would be allocated
ratably over the U.S. Holder’s holding period for the Common
Shares, (ii) the amount allocated to the taxable year in which the
gain or excess distribution is realized would be taxable as
ordinary income in its entirety and not as capital gain, would be
ineligible for the reduced qualified dividend rates, and could not
be offset by any deductions or losses, and (iii) the amount
allocated to each prior year, with certain exceptions, would be
subject to tax at the highest tax rate in effect for that year, and
the interest charge generally applicable to underpayments of tax
would be imposed in respect of the tax attributable to each of
those years.
The
special PFIC rules described above will not apply to a U.S. Holder
if the U.S. Holder makes a timely election, which remains in
effect, to treat the company as a “qualified electing
fund” (QEF) in the first taxable year in which the U.S.
Holder owns Common Shares and the company is a PFIC and if the
company complies with certain requirements. Instead, a shareholder
of a QEF generally is currently taxable on a pro rata share of the
company’s ordinary earnings and net capital gain as ordinary
income and long-term capital gain, respectively. Neither that
ordinary income nor any actual dividend from the company would
qualify for the 20% maximum federal income tax rate on dividends
described above if the company is a PFIC in the taxable year the
ordinary income is realized or the dividend is paid or in the
preceding taxable year. A QEF election cannot be made unless the
company provides U.S. Holders the information and computations
needed to report income and gains pursuant to a QEF election. The
company expects that it will not provide this information. It is,
therefore, likely that U.S. Holders would not be able to make a QEF
election in any year the company is a PFIC.
In lieu
of a QEF election, a U.S. Holder of stock in a PFIC that is
considered marketable stock could elect to mark the stock to market
annually, recognizing as ordinary income or loss each year an
amount equal to the difference as of the close of the taxable year
between the fair market value of the stock and the U.S.
Holder’s adjusted basis in the stock. Losses would be allowed
only to the extent of net mark-to-market gain previously included
in income by the U.S. Holder under the election for prior taxable
years. A U.S. Holder’s adjusted basis in Common Shares will
be adjusted to reflect the amounts included or deducted with
respect to the mark-to-market election. If the mark-to-market
election were made, the rules set forth in the second preceding
paragraph would not apply for periods covered by the election. A
mark-to-market election will not apply during any later taxable
year in which the company does not satisfy the tests to be a PFIC.
In general, the Common Shares will be marketable stock if the
Common Shares are traded, other than in de minimis quantities, on
at least 15 days during each calendar quarter on a national
securities exchange that is registered with the SEC or on a
designated national market system or on any exchange or market that
the Treasury Department determines to have rules sufficient to
ensure that the market price accurately represents the fair market
value of the stock. Under current law, the mark-to-market election
may be available to U.S. Holders of Common Shares because the
Common Shares are listed on The Nasdaq Capital Market, which should
constitute a qualified exchange for this purpose, although there
can be no assurance that the Common Shares will be “regularly
traded” for purposes of the mark-to-market
election.
If we
are treated as a PFIC, each U.S. Holder generally will be required
to file a separate annual information return with the IRS with
respect to the company (and any lower-tier PFICs). A failure to
file this return will suspend the statute of limitations with
respect to any tax return, event, or period to which such report
relates (potentially including with respect to items that do not
relate to a U.S. Holder’s investment in the Common Shares).
Given the complexities of the PFIC rules and their potentially
adverse tax consequences, U.S. Holders of Common Shares are urged
to consult their tax advisers about the PFIC rules.
Medicare Surtax on Net Investment Income
Non-corporate
U.S. Holders whose income exceeds certain thresholds generally will
be subject to 3.8% surtax on their “net investment
income” (which generally includes, among other things,
dividends on, and capital gain from the sale or other taxable
disposition of, the Common Shares). Absent an election to the
contrary, if a QEF election is available and made, QEF inclusions
will not be included in net investment income at the time a U.S.
Holder includes such amounts in income, but rather will be included
at the time distributions are received or gains are recognized.
Non-corporate U.S. Holders should consult their own tax advisors
regarding the possible effect of such tax on their ownership and
disposition of the Common Shares, in particular the applicability
of this surtax with respect to a non-corporate U.S. Holder that
makes a QEF or mark-to-market election in respect of their Common
Shares.
Information Reporting and Backup Withholding
Dividends
paid on, and proceeds from the sale or other disposition of, Common
Shares to a U.S. Holder generally will be subject to information
reporting requirements and may be subject to backup withholding
unless the U.S. Holder provides an accurate taxpayer identification
number or otherwise establishes an exemption. The amount of any
backup withholding collected from a payment to a U.S. Holder will
be allowed as a credit against the U.S. Holder’s U.S. federal
income tax liability and may entitle the U.S. Holder to a refund,
provided certain required information is furnished to the Internal
Revenue Service on a timely basis. A non-U.S. Holder generally will
be exempt from these information reporting requirements and backup
withholding tax but may be required to comply with certain
certification and identification procedures in order to establish
its eligibility for exemption.
Under
U.S. federal income tax law and U.S. Treasury Regulations, certain
categories of U.S. Holders must file information returns with
respect to their investment in, or involvement in, a foreign
corporation. U.S. Holders are urged to consult with their own tax
advisors concerning such reporting requirements.
Reporting Obligations of Individual Owners of Foreign Financial
Assets
Section
6038D of the U.S. Internal Revenue Code generally requires U.S.
individuals (and possibly certain entities that have U.S.
individual owners) to file IRS Form 8938 if they hold certain
“specified foreign financial assets,” the aggregate
value of which exceeds $50,000 on the last day of the year or
$75,000 at any time during the year. The definition of specified
foreign financial assets includes not only financial accounts
maintained in foreign financial institutions, but also, unless held
in accounts maintained by a financial institution, any stock or
security issued by a non-US. person, any financial instrument or
contract held for investment that has an issuer or counterparty
other than a U.S. person and any interest in a foreign entity.
Persons who are required to report foreign financial assets and
fail to do so may be subject to substantial penalties.
Foreign Account Tax Compliance Act
Under
certain circumstances, the company or its paying agent may be
required, pursuant to the Foreign Account Tax Compliance Act and
the regulations promulgated thereunder (“FATCA”), to
withhold U.S. tax at a rate of 30% on all or a portion of payments
of dividends or other corporate distributions to U.S. Holders which
are treated as “foreign pass-thru payments” made on or
after the date that is two years after the issuance of final
treasury regulations providing a definition of foreign pass-thru
payments are published, if such payments are not in compliance with
FATCA. Such regulations have not yet been issued. The rules
regarding FATCA and “foreign pass-thru payments,
“including the treatment of proceeds from the disposition of
the Ordinary Shares, are not completely clear, and further guidance
is expected from the IRS that would clarify how FATCA might apply
to dividends or other amounts paid on or with respect to the Common
Shares.
Canadian Federal Income Tax Consequences
The
following summary of the material Canadian federal income tax
consequences is stated in general terms and is not intended to be
legal or tax advice to any particular shareholder. Each shareholder
or prospective shareholder is urged to consult his or her own tax
advisor regarding the tax consequences of his or her purchase,
ownership and disposition of Common Shares. The tax consequences to
any particular holder of Common Shares will vary according to the
status of that holder as an individual, trust, corporation or
member of a partnership, the jurisdiction in which that holder is
subject to taxation, the place where that holder is resident and,
generally, according to that holder’s particular
circumstances.
This
summary is applicable only to holders who are resident in the
United States for income tax purposes, have never been resident in
Canada for income tax purposes, deal at arm’s length with the
company, hold their Common Shares as capital property and who will
not use or hold the Common Shares in carrying on business in
Canada. Special rules, which are not discussed in this summary, may
apply to a United States holder that is an issuer that carries on
business in Canada and elsewhere.
This
summary is based upon the provisions of the Income Tax Act (Canada)
and the regulations thereunder (collectively, the Tax Act or ITA)
and the Canada-United States Tax Convention (the Tax Convention) at
the date of this prospectus and the current administrative
practices of the Canada Revenue Agency. This summary does not take
into account provincial income tax consequences. The comments in
this summary that are based on the Tax Convention are applicable to
U.S. Holders only if they qualify for benefits under the Tax
Convention. Management urges each holder to consult his own tax
advisor with respect to the income tax consequences applicable to
him in his own particular circumstances.
Non-Resident Holders
The
summary below is restricted to the case of a holder (a Holder) of
one or more Common Shares who for the purposes of the Tax Act is a
non- resident of Canada, holds his Common Shares as capital
property and deals at arm’s length with the
company.
Dividends
A
Holder will be subject to Canadian withholding tax (Part XIII Tax)
equal to 25%, or such lower rates as may be available under an
applicable tax treaty, of the gross amount of any dividend paid or
deemed to be paid on his Common Shares. The company will be
required to withhold the applicable amount of Part XIII Tax from
each dividend so paid and remit the withheld amount directly to the
Receiver General for Canada for the account of the
Holder.
Disposition of Common Shares
A
Holder who disposes of Common Shares, including by deemed
disposition on death, will not be subject to Canadian tax on any
capital gain thereby realized unless the Common Share constituted
“taxable Canadian property” as defined by the Tax Act.
Generally, a common share of a public corporation will not
constitute taxable Canadian property of a Holder unless he held the
Common Share as capital property used by him carrying on a business
in Canada, or he, persons with whom he did not deal at arm’s
length or partnerships in which he or persons with whom he did not
deal at arm’s length held an interest, alone or together held
or held options to acquire, at any time within the 60 months
preceding the disposition, 25% or more of the issued shares of any
class of the capital shares of the company and at any time during
the 60 months preceding the disposition more than 50% of the value
of the common shares is derived from, or from an interest in,
Canadian real estate, including Canadian resource or timber
resource properties.
Holders Resident in the United States
A
Holder who is a resident of the United States and realizes a
capital gain on disposition of Common Shares that was taxable
Canadian property will, if qualified for benefits under the Tax
Convention, generally be exempt from Canadian tax thereon unless
(a) more than 50% of the value of the Common Shares is derived
from, or from an interest in, Canadian real estate, including
Canadian natural resource properties, (b) the Common Shares formed
part of the business property of a permanent establishment that the
Holder has or had in Canada within the 12 months preceding
disposition, or (c) the Holder (i) was a resident of Canada at any
time within the ten years immediately preceding the disposition,
and for a total of 120 months during any period of 20 consecutive
years, preceding the disposition, (ii) owned the Common Shares when
he ceased to be resident in Canada, and (iii) the common shares
were not subject to a deemed disposition on the Holder’s
departure from Canada.
Inclusion in Taxable Income
A
Holder who is subject to Canadian tax in respect of a capital gain
realized on disposition of Common Shares must include one half of
the capital gain (“taxable capital gain”) in computing
his taxable income earned in Canada. The Holder may, subject to
certain limitations, deduct one half of any capital loss
(“allowable capital loss”) arising on disposition of
taxable Canadian property from taxable capital gains realized in
the year of disposition in respect to taxable Canadian property
and, to the extent not so deductible, from such taxable capital
gains of any of the three preceding years or any subsequent
year.
Subject
to certain exceptions, a non-resident person who disposes of
taxable Canadian property must notify the Canada Revenue Agency
either before or after the disposition (within ten days of the
disposition).
PLAN OF DISTRIBUTION
Subject to any restrictions on trading in a
selling shareholer's applicable jurisdiction, each selling
shareholder and any of their pledgees, assignees and
successors-in-interest may, from time to time, sell any or all of
their Common Shares included in the registration statement of which
this prospectus is a part, on the Nasdaq Capital Market or any
other stock exchange, market or trading facility on which the
Common Shares are traded or in private transactions.
These
dispositions may be at fixed prices, at prevailing market prices at
the time of sale, at prices related to the prevailing market price,
at varying prices determined at the time of sale, or at negotiated
prices.
A selling shareholder may use any one or more of the following
methods when selling securities:
●
ordinary
brokerage transactions and transactions in which the broker-dealer
solicits purchases;
block
trades in which the broker-dealer will attempt to sell the
securities as agent but may position and resell a portion of the
block as principal to facilitate the transaction;
purchases
by a broker-dealer as principal and resale by the broker-dealer for
its account;
an
exchange distribution in accordance with the rules of the
applicable exchange;
privately
negotiated transactions;
settlement
of short sales;
in
transactions through broker-dealers that agree with the selling
shareholder to sell a specified number of such securities at a
stipulated price per security;
through
the writing or settlement of options or other hedging transactions,
whether through an options exchange or otherwise;
a
combination of any such methods of sale; or
●
any
other method permitted pursuant to applicable law.
The selling shareholders may, from time to time, pledge or grant a
security interest in some or all of the Common Shares owned by them
and, if they default in the performance of their secured
obligations, the pledgees or secured parties may offer and sell the
Common Shares, from time to time, under this prospectus, or under
an amendment to this prospectus under Rule 424(b)(3) or other
applicable provision of the Securities Act amending the list of
selling shareholders to include the pledgee, transferee or other
successors in interest as selling shareholders under this
prospectus. The selling shareholders also may transfer the Common
Shares in other circumstances, in which case the transferees,
pledgees or other successors in interest will be the selling
beneficial owners for purposes of this prospectus.
In connection with the sale of our Common Shares or interests
therein, the selling shareholders may enter into hedging
transactions with broker-dealers or other financial institutions,
which may in turn engage in short sales of the Common Shares in the
course of hedging the positions they assume. The selling
shareholders may also sell Common Shares short and deliver these
securities to close out their short positions, or loan or pledge
the Common Shares to broker-dealers that in turn may sell these
securities. The selling shareholders may also enter into option or
other transactions with broker-dealers or other financial
institutions or the creation of one or more derivative securities
which require the delivery to such broker-dealer or other financial
institution of shares offered by this prospectus, which shares such
broker-dealer or other financial institution may resell pursuant to
this prospectus (as supplemented or amended to reflect such
transaction).
The aggregate proceeds to the selling shareholders from the sale of
the Common Shares offered by them will be the purchase price of the
Common Shares less discounts or commissions, if any. Each of the
selling shareholders reserves the right to accept and, together
with their agents from time to time, to reject, in whole or in
part, any proposed purchase of Common Shares to be made directly or
through agents. We will not receive any of the proceeds from this
offering. Upon any exercise of the warrants by payment of cash,
however, we will receive the exercise price of the
warrants.
The selling shareholders also may resell all or a portion of the
shares in open market transactions in reliance upon Rule 144 under
the Securities Act of 1933, provided that they meet the criteria
and conform to the requirements of that rule.
The selling shareholders and any underwriters, broker-dealers or
agents that participate in the sale of the Common Shares or
interests therein may be “underwriters” within the
meaning of Section 2(11) of the Securities Act. Any discounts,
commissions, concessions or profit they earn on any resale of the
shares may be underwriting discounts and commissions under the
Securities Act. Selling shareholders who are
“underwriters” within the meaning of Section 2(11)
of the Securities Act will be subject to the prospectus delivery
requirements of the Securities Act.
To the extent required, Common Shares to be sold, the names of the
selling shareholders, the respective purchase prices and public
offering prices, the names of any agents, dealer or underwriter,
any applicable commissions or discounts with respect to a
particular offer will be set forth in an accompanying prospectus
supplement or, if appropriate, a post-effective amendment to the
registration statement that includes this prospectus.
In order to comply with the securities laws of some states, if
applicable, the Common Shares may be sold in these jurisdictions
only through registered or licensed brokers or dealers. In
addition, in some states the Common Shares may not be sold unless
it has been registered or qualified for sale or an exemption from
registration or qualification requirements is available and is
complied with.
We have advised the selling shareholders that the anti-manipulation
rules of Regulation M under the Exchange Act may apply to sales of
shares in the market and to the activities of the selling
shareholders and their affiliates. In addition, to the extent
applicable we will make copies of this prospectus (as it may be
supplemented or amended from time to time) available to the selling
shareholders for the purpose of satisfying the prospectus delivery
requirements of the Securities Act. The selling shareholders may
indemnify any broker-dealer that participates in transactions
involving the sale of the shares against certain liabilities,
including liabilities arising under the Securities
Act.
We have agreed to indemnify the selling shareholders against
liabilities, including liabilities under the Securities Act and
state securities laws, relating to the registration of the shares
offered by this prospectus.
We have agreed with the selling shareholders to keep the
registration statement of which this prospectus constitutes a part
effective until the earlier of (1) such time as all of the
shares covered by this prospectus have been disposed of pursuant to
and in accordance with such registration statement or (2) the
date on which all of the shares may be sold without restriction
pursuant to Rule 144 of the Securities Act.
At the time a particular offer of shares is made, if required, a
prospectus supplement will be distributed that will set forth the
number of shares being offered and the terms of the offering,
including the name of any underwriter, dealer or agent, the
purchase price paid by any underwriter, any discount, commission
and other item constituting compensation, any discount, commission
or concession allowed or reallowed or paid to any dealer, and the
proposed selling price to the public.
Once
sold under the registration statement of which this prospectus is a
part, the Common Shares will be freely tradable in the United
States in the hands of persons other than our
affiliates.
Listing
Our Common Shares are listed on the Nasdaq Capital Market under the
symbol “EDSA.”
Transfer Agent and Registrar
The transfer agent and registrar for our Common Shares is
Computershare Investor Services Inc. located at 100 University
Avenue, 8th Floor, Toronto, Ontario M5J 2Y1, and its telephone
number is 1-800-564-6253.
DESCRIPTION OF SECURITIES
The selling shareholders are offering 3,602,788 of our Common
Shares. The following is a brief description of the securities the
selling shareholders are offering. This summary does not purport to
be complete in all respects. The following summary description of
our capital shares is based on the provisions of our Notice of
Articles and Articles. This information is qualified entirely by
reference to the applicable provisions of our Articles and the
British Columbia Business Corporations
Act. For information on how to
obtain copies of our Notice of Articles and Articles, which are
exhibits to the registration statement of which this prospectus is
a part, see “Where You Can Find Additional
Information.”
Description of Capital Shares
We are authorized to issue an unlimited number of common shares, no
par value, and preferred shares, no par value. As of February 21,
2020, there were 8,859,159 Common Shares outstanding and no
preferred shares outstanding.
Common Shares
The holders of our Common Shares are entitled to one vote for each
share held of record on all matters submitted to a vote of the
shareholders. Our shareholders do not have cumulative voting rights
in the election of directors. Subject to preferences that may be
applicable to any outstanding preferred shares, the holders of
Common Shares are entitled to receive ratably only those dividends
as may be declared by our board of directors out of legally
available funds. Upon our liquidation, dissolution or winding up,
holders of our Common Shares are entitled to share ratably in all
assets remaining after payment of liabilities and the liquidation
preferences of any outstanding preferred shares. Holders of Common
Shares have no preemptive or other subscription or conversion
rights. There are no redemption or sinking fund provisions
applicable to our Common Shares. Common Shares outstanding, and to
be issued, are, and will be, fully paid and non-assessable.
Additional shares of authorized Common Shares may be issued, as
authorized by our board of directors from time to time, without
shareholder approval, except as may be required by applicable stock
exchange requirements.
Certain Provisions of Our Charter Documents and British Columbia
Law
Anti-takeover Provisions of our Articles
In addition to the board of directors’ ability to issue
preferred shares, our Articles, as amended, contain other
provisions that are intended to enhance the likelihood of
continuity and stability in the composition of our board of
directors and which may have the effect of delaying, deferring or
preventing a future takeover or change in control of our Company
unless such takeover or change in control is approved by our board
of directors. These provisions include a supermajority vote
requirement for business combinations.
Advance Notice Procedures for Shareholder Proposals
Effective October 31, 2013, our board of directors adopted an
advance notice policy (the “Advance Notice Policy”)
with immediate effect for the purpose of providing our
shareholders, directors and management with a clear framework for
nominating our directors in connection with any annual or special
meeting of shareholders. The Advance Notice Policy was approved by
the shareholders at our annual meeting on February 13,
2014.
Purpose of the Advance Notice Policy. Our directors are committed to: (i) facilitating
an orderly and efficient annual general or, where the need arises,
special meeting, process; (ii) ensuring that all shareholders
receive adequate notice of the director nominations and sufficient
information with respect to all nominees; and (iii) allowing
shareholders to register an informed vote having been afforded
reasonable time for appropriate deliberation. The purpose of the
Advance Notice Policy is to provide our shareholders, directors and
management with a clear framework for nominating directors. The
Advance Notice Policy fixes a deadline by which holders of record
of our Common Shares must submit director nominations to the
Company prior to any annual or special meeting of shareholders and
sets forth the information that a shareholder must include in the
notice to the Company for the notice to be in proper written form
in order for any director nominee to be eligible for election at
any annual or special meeting of shareholders.
Terms of the Advance Notice Policy. The Advance Notice Policy provides that advance
notice to the Company must be made in circumstances where
nominations of persons for election to our board of directors are
made by shareholders of the Company other than pursuant to: (i) a
"proposal" made in accordance with Division 7 of Part 5 of the
British Columbia Business Corporations Act, or the Act; or (ii) a
requisition of the shareholders made in accordance with section 167
of the Act. Among other things, the Advance Notice Policy fixes a
deadline by which holders of record of our Common Shares must
submit director nominations to the secretary of the Company prior
to any annual or special meeting of shareholders and sets forth the
specific information that a shareholder must include in the written
notice to the secretary of the Company for an effective nomination
to occur. No person will be eligible for election as a director of
the Company unless nominated in accordance with the provisions of
the Advance Notice Policy.
In the case of an annual meeting of shareholders, notice to the
Company must be made not less than 30 nor more than 65 days prior
to the date of the annual meeting; provided, however, that in the
event that the annual meeting is to be held on a date that is less
than 50 days after the date on which the first public announcement
of the date of the annual meeting was made, notice may be made not
later than the close of business on the 10th day following such
public announcement.
In the case of a special meeting of shareholders (which is not also
an annual meeting), notice to the Company must be made not later
than the close of business on the 15th day following the day on
which the first public announcement of the date of the special
meeting was made.
Our board of directors may, in its sole discretion, waive any
requirement of the Advance Notice Policy.
Provisions of British Columbia Law Governing Business
Combinations
All provinces of Canada have adopted National Instrument 62-104
entitled “Take-Over Bids and Issuer
Bids” and related forms
to harmonize and consolidate take-over bid and issuer bid regimes
nationally (“NI 62-104”). The Canadian Securities
Administrators, or CSA, have also issued National Policy 62-203
entitled “Take-Over Bids and Issuer
Bids” (the
“National Policy”) which contains regulatory guidance
on the interpretation and application of NI 62-104 and on the
conduct of parties involved in a bid. The National Policy and NI
62-104 are collectively referred to as the “Bid
Regime.” The National Policy does not have the force of law,
but is an indication by the CSA of what the intentions and desires
of the regulators are in the areas covered by their policies.
Unlike some regimes where the take-over bid rules are primarily
policy-driven, in Canada the regulatory framework for take-over
bids is primarily rules-based, which rules are supported by
policy.
A “take-over bid” or “bid” is an offer to
acquire outstanding voting or equity securities of a class made to
any person who is in one of the provinces of Canada or to any
securityholder of an offeree issuer whose last address as shown on
the books of a target is in such province, where the securities
subject to the offer to acquire, together with the securities
“beneficially owned” by the offeror, or any other
person acting jointly or in concert with the offeror, constitute in
the aggregate 20% or more of the outstanding securities of that
class of securities at the date of the offer to acquire. For the
purposes of the Bid Regime, a security is deemed to be
“beneficially owned” by an offeror as of a specific
date if the offeror is the beneficial owner of a security
convertible into the security within 60 days following that date,
or has a right or obligation permitting or requiring the offeror,
whether or not on conditions, to acquire beneficial ownership of
the security within 60 days by a single transaction or a series of
linked transactions. Offerors are also subject to early warning
requirements, where an offeror who acquires “beneficial
ownership of”, or control or direction over, voting or equity
securities of any class of a reporting issuer or securities
convertible into, voting or equity securities of any class of a
target that, together with the offeror’s securities, would
constitute 10% or more of the outstanding securities of that class
must promptly publicly issue and file a news release containing
certain prescribed information, and, within two business days, file
an early warning report containing substantially the same
information as is contained in the news release.
In addition, where an offeror is required to file an early warning
report or a further report as described and the offeror acquires or
disposes of beneficial ownership of, or the power to exercise
control or direction over, an additional 2% or more of the
outstanding securities of the class, or disposes of beneficial
ownership of outstanding securities of the class below 10%, the
offeror must issue an additional press release and file a new early
warning report. Any material change in a previously filed early
warning report also triggers the issuance and filing of a new press
release and early warning report. During the period commencing on
the occurrence of an event in respect of which an early warning
report is required and terminating on the expiry of one business
day from the date that the early warning report is filed, the
offeror may not acquire or offer to acquire beneficial ownership of
any securities of the class in respect of which the early warning
report was required to be filed or any securities convertible into
securities of that class. This requirement does not apply to an
offeror that has beneficial ownership of, or control or direction
over, securities that comprise 20% of more of the outstanding
securities of the class.
Related party transactions, issuer bids and insider bids are
subject to additional regulation that may differ depending on the
particular jurisdiction of Canada in which it occurs.
Description of Outstanding Warrants to Purchase Common Shares
pursuant to which the Warrant Shares may be Issued
The following summary of certain terms and provisions of the Class
A Purchase Warrants, Class B Purchase Warrants and Placement Agent
Warrants is not complete and is subject to, and qualified in its
entirety by, the provisions of the warrants, the forms of which are
filed as exhibits to our Current Report on Form 8-K filed with the
SEC on January 6, 2020.
On January 8, 2020, we completed the Offering and Private
Placement. In the Private Placement, we sold to investors (i) Class
A Purchase Warrants to purchase an aggregate of up to 1,016,553
Common Shares, or 0.75 of a Common Share for each Common Share
purchased in the Offering, and (ii) Class B Purchase Warrants to
purchase an aggregate of up to 677,703 Common Shares, or 0.50 of a
Common Share for each Common Share purchased in the
offering.
Exercisability. The Class A
Purchase Warrants will be exercisable at any time on or after July
8, 2020, the six (6) month anniversary the date of issuance (the
“Class A Purchase Warrant Initial Exercise Date”), at
an exercise price of $4.80 per share and will expire and cease to
be exercisable on the third anniversary of the Class A Purchase
Warrant Initial Exercise Date. The Class B Purchase Warrants will
be exercisable at any time on or after July 8, 2020, the six (6)
month anniversary the date of issuance (the “Class B Purchase
Warrant Initial Exercise Date”), at an exercise price of
$4.00 per share and will expire and cease to be exercisable on the
four month anniversary of the Class B Purchase Warrant Initial
Exercise Date. The Placement Agent
Warrants will be exercisable at any time on or after July 6, 2020
(the “Placement Agent Warrant Initial Exercise Date”),
at an exercise price of $3.20 per share and will expire on the
fifth anniversary of the Placement Agent Warrant Initial Exercise
Date. The Purchase Warrants and
the Placement Agent Warrants will be exercisable, at the option of
each holder, in whole or in part by delivering to us a duly
executed exercise notice and, at any time a registration statement
registering the issuance of Common Shares underlying the Purchase
Warrants and/or Placement Agent Warrants, as applicable, under the
Securities Act is effective and available for the issuance of such
shares, or an exemption from registration under the Securities Act
is available for the issuance of such shares, by payment in full in
immediately available funds for the number of Common Shares
purchased upon such exercise. If a registration statement
registering the issuance of the Common Shares underlying the
Purchase Warrants or Placement Agent Warrants, as applicable, under
the Securities Act is not effective or available and an exemption
from registration under the Securities Act is not available for the
issuance of such shares, the holder may, in its sole discretion,
elect to exercise the Purchase Warrant or Placement Agent Warrants,
as applicable, through a cashless exercise, in which case the
holder would receive upon such exercise the net number of Common
Shares determined according to the formula set forth in the
Purchase Warrants and Placement Agent Warrants. No fractional
Common Shares will be issued in connection with the exercise of a
Purchase Warrant or Placement Agent Warrant. In lieu of fractional
shares, we shall, at our election, either pay a cash adjustment in
respect of such final fraction in an amount equal to such fraction
multiplied by the exercise price or round up to the next whole
share.
Exercise Limitation. A holder
will not have the right to exercise any portion of a Purchase
Warrant or Placement Agent Warrant if the holder (together with its
affiliates) would beneficially own in excess of 9.99% of the number
of Common Shares outstanding immediately after giving effect to the
exercise, as such percentage ownership is determined in accordance
with the terms of the Purchase Warrants and Placement Agent
Warrants. However, any holder may increase or decrease such
percentage, provided that any increase will not be effective until
the 61st day after such election.
Exercise Price Adjustment. The
exercise prices of the Purchase Warrants and Placement Agent
Warrants are subject to appropriate adjustment in the event of
certain stock dividends and distributions, stock splits, stock
combinations, reclassifications or similar events affecting our
Common Shares.
Transferability. Subject to
applicable laws, the Purchase Warrants and Placement Agent Warrants
may be offered for sale, sold, transferred or assigned without our
consent.
Exchange Listing. There is no
established trading market for the Purchase Warrants and Placement
Agent Warrants and we do not expect a market to develop. In
addition, we do not intend to apply for the listing of the Purchase
Warrants or Placement Agent Warrants on any national securities
exchange or other trading market. Without an active trading market,
the liquidity of the Purchase Warrants and Placement Agent Warrants
will be limited.
Fundamental Transactions. If,
at any time while the Purchase Warrants and/or Placement Agent
Warrants are outstanding, (1) we consolidate or merge with or into
another entity in which we are not the surviving entity; (2) we
sell, lease, assign, convey or otherwise transfer all or
substantially all of our assets; (3) any tender offer or exchange
offer (whether completed by us or a third party) is completed
pursuant to which holders of a majority of our outstanding Common
Shares tender or exchange their shares for securities, cash or
other property; (4) we effect any reclassification of our Common
Shares or compulsory share exchange pursuant to which outstanding
Common Shares are effectively converted or exchange for other
securities, cash or property or (5) any transaction is consummated
whereby any person or entity acquires more than 50% of our
outstanding Common Shares (each, a “Fundamental
Transaction”), then upon any subsequent exercise of a
Purchase Warrant or Placement Agent Warrant, as applicable, the
holder thereof will have the right to receive the same amount and
kind of securities, cash or other property as it would have been
entitled to receive upon the occurrence of such Fundamental
Transaction if it had been, immediately prior to such Fundamental
Transaction, the holder of the number of Common Shares then
issuable upon exercise of the Purchase Warrant or Placement Agent
Warrant, as applicable. If a Fundamental Transaction occurs, then
the successor entity will succeed to, and be substituted for us,
and may exercise every right and power that we may exercise and
will assume all of our obligations under the Purchase Warrants and
Placement Agent Warrants with the same effect as if such successor
entity had been named in the Purchase Warrant or Placement Agent
Warrant itself.
Rights as a Stockholder. Except
as otherwise provided in the Purchase Warrants or Placement Agent
Warrants or by virtue of such holder’s ownership of our
Common Shares, the holder of a Purchase Warrant or Placement Agent
Warrant does not have the rights or privileges of a holder of our
Common Shares, including any voting rights, until the holder
exercises the Purchase Warrant or Placement Agent Warrant, as
applicable.
Resale/Registration Rights. We
are required within 45 calendar days of the Offering to file a
registration statement providing for the resale of the Common
Shares issued and issuable upon the exercise of the Purchase
Warrants. We are required to use commercially reasonable efforts to
cause such registration to become effective within 75 days of the
closing of the offering, subject to certain exceptions, and to keep
such registration statement effective at all times until no
investor owns any Purchase Warrants or shares issuable upon
exercise thereof. We filed this registration statement on
Form S-1, of which this prospectus forms a part, to
fulfill our contractual obligations under the Securities Purchase
Agreement, Subscription Agreements and Placement Agent Warrants
with the selling shareholders to provide for the resale by the
selling shareholders of the Common Shares underlying the Purchase
Warrants and Placement Agent Warrants.
With respect to non-U.S. investors, the Common Shares underlying
the Purchase Warrants will be subject to restrictions on resale in
accordance with applicable foreign laws. A form of the subscription
agreement with non-U.S. investors is included as an exhibit to our
Current Report on Form 8-K that was previously filed with the SEC
on January 6, 2020 and incorporated by reference into the
Registration Statement of which this prospectus forms a part. See
“Where You Can Find Additional Information”
below.
LEGAL MATTERS
The validity of the securities offered by this prospectus is being
passed upon for us by Fasken Martineau
DuMoulin, LLP, Toronto, Ontario, Canada, and certain other
matters are being passed upon for us by Stubbs Alderton &
Markiles, LLP, Sherman Oaks, California.
EXPERTS
The balance sheets of Edesa Biotech, Inc. as of September 30, 2019
and December 31, 2018 and the related consolidated statements of
operations and comprehensive loss, changes in shareholders’
equity and cash flows for the nine-month period ended September 30,
2019 and year ended December 31, 2018 incorporated in this
prospectus by reference to the Annual Report on Form 10-K for
the transition period ended September 30, 2019 have been so
incorporated in reliance on the report of MNP LLP, an independent
registered public accounting firm, given on the authority of said
firm as experts in auditing and accounting.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed a registration statement on Form S-1 with the SEC
covering the Common Shares that the selling shareholders are
offering by this prospectus. This prospectus does not include all
of the information contained in the registration statement. You
should refer to the registration statement and its exhibits for
additional information. Whenever we make reference in this
prospectus to any of our contracts, agreements or other documents,
the references are not necessarily complete and you should refer to
the exhibits filed or documents incorporated by reference as part
of the registration statement for copies of the actual contract,
agreement or other document.
We file annual, quarterly and other periodic reports, proxy
statements and other information with the Securities and Exchange
Commission. You can read our Securities and Exchange Commission
filings, including this registration statement, over the Internet
at the Securities and Exchange Commission’s website at
www.sec.gov. You may also read and copy any document we file with
the Securities and Exchange Commission at its public reference
facilities at 100 F Street NE, Washington, D.C. 20549. You may also
obtain copies of these documents at prescribed rates by writing to
the Public Reference Section of the Securities and Exchange
Commission at 100 F Street NE, Washington, D.C. 20549. Please call
the Securities and Exchange Commission at 1-800-SEC-0330 for
further information on the operation of the public reference
facilities.
Our Internet address is www.edesabiotech.com. There we make
available free of charge, on or through the investor relations
section of our website, annual reports on Form 10-K, quarterly
reports on Form 10-Q, current reports on Form 8-K and amendments to
those reports filed pursuant to Section 13(a) or 15(d) of the
Exchange Act as soon as reasonably practicable after we
electronically file such material with the Securities and Exchange
Commission. The information found on our website is not part of
this prospectus and investors should not rely on any such
information in deciding whether to invest.
INCORPORATION BY REFERENCE OF CERTAIN DOCUMENTS
The SEC allows us to incorporate by reference the information we
file with it, which means that we can disclose important
information to you by referring you to another document that we
have filed separately with the SEC. You should read the information
incorporated by reference because it is an important part of this
prospectus. We incorporate by reference the following information
or documents that we have filed with the SEC (Commission File No.
001-37619):
●
Our Annual Transition
Report on Form 10-K for the nine-month period ended September 30,
2019 (filed on December 12, 2019);
●
our Quarterly
Report on Form 10-Q for the quarter ended December 31, 2019, filed
February 13, 2020;
●
Our Current Reports on
Form 8-K, dated January 6, 2020 (filed on January 6, 2020) and
dated January 8, 2020 (filed on January 9, 2020) ;
and
●
The description of
our Common Shares contained in our Registration Statement on Form
8-A filed with the SEC on November 3, 2015, including any amendment
or report filed for the purpose of updating such
description.
Any information in any of the foregoing documents will
automatically be deemed to be modified or superseded to the extent
that information in this prospectus or in a later filed document
that is incorporated or deemed to be incorporated herein by
reference modifies or replaces such information.
We also incorporate by reference any future filings (other than
current reports furnished under Item 2.02 or Item 7.01 of
Form 8-K and exhibits filed on such form that are related to
such items) made with the SEC pursuant to Sections 13(a),
13(c), 14 or 15(d) of the Exchange Act, including all such reports
filed after the date of the initial registration statement and
prior to effectiveness of the registration statement, until we file
a post-effective amendment that indicates the termination of the
offering of the securities made by this prospectus. Information in
such future filings updates and supplements the information
provided in this prospectus. Any statements in any such future
filings will automatically be deemed to modify and supersede any
information in any document we previously filed with the SEC that
is incorporated or deemed to be incorporated herein by reference to
the extent that statements in the later filed document modify or
replace such earlier statements.
We will furnish without charge to each person to whom a copy of
this prospectus is delivered, upon written or oral request, a copy
of the documents that have been incorporated by reference into this
prospectus, including exhibits to these documents. You should
direct any requests for copies to: Investor Relations, Edesa
Biotech, Inc., 100 Spy Court, Markham, Ontario L3R 5H6 Canada;
telephone number (289) 800-9600.
Edesa Biotech, Inc.
3,602,788 Common Shares
PROSPECTUS
, 2020
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM
13.
Other
Expenses of Issuance and Distribution
We will bear all expenses of registration incurred in connection
with this offering. The selling shareholders whose shares are being
registered will bear all selling and other expenses. The following
table itemizes the expenses in connection with the offering. All
the amounts shown are estimates except the SEC registration
fee.
|
|
|
|
SEC Registration
fee
|
$1,872
|
|
Legal fees and
expenses
|
40,000
|
|
Accounting fees and
expenses
|
10,000
|
|
Transfer agent fees
and expenses
|
1,000
|
|
Printing fees and
expenses
|
1,000
|
|
Miscellaneous fees
and expenses
|
1,000
|
|
Total
|
$54,872
|
ITEM
14.
Indemnification
of Directors and Officers
Subject
to the British Columbia Business
Corporations Act, or “the Act”, our directors, former
directors and alternate directors and their heirs and legal
personal representatives are indemnified against any judgment,
penalty or fine awarded or imposed in, or an amount paid in
settlement of, a stipulated legal or investigative proceeding, as
set forth in our Articles. In addition, our Articles provide that
we may, subject to any restrictions in the Act, indemnify any
person.
Under the Act, we may indemnify (a) a current or former
director or officer of the Company; (b) a current or former
director or officer of another corporation at a time when that
corporation is or was an affiliate of the Company; (c) a
current or former director or officer of another corporation who
holds or held such position at the request of the Company; or
(d) an individual who at the request of the Company, is or
was, or holds or held a position equivalent to that of, a director,
or officer of a partnership, trust, joint venture or other
unincorporated entity (collectively, an “Eligible
Party”). In certain circumstances an Eligible Party will
include the heirs and personal or other legal representatives of an
Eligible Party. We may indemnify an Eligible Party against any
Eligible Penalty (defined below) to which the Eligible Party is or
may be liable. After the final disposition of an Eligible
Proceeding (defined below), we may pay all Expenses (defined below)
actually and reasonably incurred by the Eligible Party in
connection with such Proceeding (defined below) and must pay all
such Expenses actually and reasonably incurred by the Eligible
Party in connection with such Proceeding if the Eligible Party has
not been reimbursed for those Expenses and is wholly successful on
the merits or otherwise in the outcome of the Proceeding, or is
substantially successful on the merits in the outcome of the
Proceeding. Among other circumstances, we shall not indemnify or
cover the Expenses of an Eligible Party if the Eligible Party did
not act honestly and in good faith with a view to the best
interests of the Company or if the Eligible Party (other than in
connection with a civil Proceeding) did not have reasonable grounds
for believing that the Eligible Party’s conduct in respect of
which the Proceeding was brought was lawful. Further, we cannot
indemnify or cover the Expenses of an Eligible Party in respect of
any Proceeding brought by or on behalf of the Company against an
Eligible Party. The Supreme Court of British Columbia may, among
other things, on the applications of a corporation or an Eligible
Party, order indemnification by the Company of any liability or
expense incurred by an Eligible Party.
“Eligible Penalty” means a judgment, penalty or fine
awarded or imposed in, or an amount paid in settlement of, an
Eligible Proceeding.
“Eligible Proceeding” means any legal proceeding or
investigative action, whether current, threatened, pending or
completed (each, a “Proceeding”), in which an Eligible
Party, or any of the Eligible Party’s heirs and personal or
other legal representatives (i) is or may be joined as a
party, or (ii) is or may be liable for or in respect of a
judgment, penalty or fine in, or Expenses related to, such
Proceeding, in each case by reason of the Eligible Party’s
being or having been a director or officer of, or holding or having
held a position equivalent to that of a director or officer of, the
Company, or is or was a director or officer of any corporation at a
time when the corporation is or was an affiliate of the Company, or
another entity at the Company’s request.
“Expenses” includes costs, charges and expenses,
including legal and other fees, but does not include judgments,
penalties, fines or amounts paid in settlement of a
Proceeding.
We have also entered into separate indemnification agreements with
each of our directors and executive officers, which are intended to
indemnify our directors and executive officers to the fullest
extent permitted under the laws of the Province of British
Columbia, subject to certain exceptions. Our obligations under such
separate indemnification agreements are in addition to our
indemnification obligations under the Act and our charter
documents.
We maintain a directors’ and officers’ liability
insurance policy, which insures directors and officers of the
Company and its subsidiaries for losses as a result of claims based
upon the directors’ and officers’ acts or omissions,
including liabilities arising under the Securities Act. The policy
also reimburses us for payments made pursuant to the indemnity
provisions under the Act and our charter documents.
Insofar as indemnification for liabilities arising under the
Securities Act may be permitted to directors, officers or persons
controlling the Registrant pursuant to the foregoing provisions,
the Registrant has been informed that in the opinion of the U.S.
Securities and Exchange Commission such indemnification is against
public policy as expressed in the Securities Act and is therefore
unenforceable.
ITEM
15.
Recent
Sales of Unregistered Securities.
Set forth below is information regarding our securities granted in
the three years preceding the filing of this registration statement
that were not registered under the Securities Act.
Description of Private Placement With The Selling
Stockholders
On January 6, 2020, we entered into a Securities Purchase Agreement
(the “Securities Purchase Agreement”) with certain
United States resident investors and Subscription Agreements (the
“Subscription Agreements”) with certain non-U.S.
investors providing for the issuance and sale by us of an
aggregate of 1,354,691 of our common shares, in a registered direct
offering (the “Offering”). In a concurrent private
placement (the “Private Placement”), we agreed to sell
to such investors (i) Class A Purchase Warrants to purchase an
aggregate of up to 1,016,036 Common Shares, or 0.75 of a Common
Share for each Common Share purchased in the Offering (the
“Class A Purchase Warrants”), and (ii) Class B Purchase
Warrants to purchase an aggregate of up to 677,358 Common Shares,
or 0.50 of a Common Share for each Common Share purchased in the
offering (the “Class B Purchase Warrants,” and together
with the Class A Purchase Warrants, the “Purchase
Warrants”). The price per Common Share and associated
Purchase Warrants was (i) $3.20 for investors other than investors
that are officers, directors, employees or consultants of the
company and (ii) $4.11 for each investor that is an officer,
director, employee or consultant of the company. The closing of the
Offering and concurrent Private Placement occurred on January 8,
2020. The Class A Purchase
Warrants will be exercisable at any time on or after July 8, 2020,
the six (6) month anniversary of the closing date of the Private
Placement (the “Class A Purchase Warrant Initial Exercise
Date”), at an exercise price of $4.80 per share and will
expire on the third anniversary of the Class A Purchase Warrant
Initial Exercise Date. The Class B Purchase Warrants will be
exercisable at any time on or after July 8, 2020, the six (6) month
anniversary of the closing date of the Private Placement (the
“Class B Purchase Warrant Initial Exercise Date”), at
an exercise price of $4.00 per share and will expire on the four
month anniversary of the Class B Purchase Warrant Initial Exercise
Date. The exercise price and
number of common shares issuable upon the exercise of the Purchase
Warrants will be subject to adjustment in the event of any share
dividends and splits, reverse share split, recapitalization,
reorganization or similar transaction. Subject to limited
exceptions, a holder of Purchase Warrants will not have the right
to exercise any portion of its Purchase Warrants if the holder,
together with its affiliates, would beneficially own in excess of
9.99% of the number of common shares outstanding immediately after
giving effect to such exercise (the “Beneficial Ownership
Limitation”); provided, however, that upon 61 days’
prior notice to us, the holder may increase the Beneficial
Ownership Limitation, provided that in no event shall the
Beneficial Ownership Limitation exceed 9.99%.
We received gross proceeds of approximately $4.36 million from
the sale of these securities, before deducting placement agent fees
and offering expenses, and excluding the exercise of any
warrants.
Brookline Capital Markets, a division of Arcadia Securities, LLC
(“Brookline”), acted as placement agent in the United
States in connection with the Offering and Private Placement
pursuant to a Financial Advisory Agreement between us and Brookline
dated November 5, 2019, as amended. Upon the closing of the
Offering and Private Placement, Brookline received a placement
agent fee equal to 6.5% of the gross proceeds from sales arranged
by Brookline (or 3.5% in the case of sales to investors introduced
by the company, or Company Investors). Brookline did not receive
any cash placement fee with respect to non-U.S. investors. As
additional compensation, the company issued to Brookline a warrant
to purchase 12,364 Common Shares, which is equal to 1.25% of the
number of Common Shares sold in the Offering and concurrent Private
Placement to investors introduced by Brookline (the
“Brookline Warrant”). The Brookline Warrant has a term
of five years and is exercisable at a price of $3.20 per share.
Brookline did not receive any warrant compensation for securities
issued to non-U.S investors. The company also reimbursed Brookline
$55,000 for certain expenses incurred by Brookline.
The Purchase Warrants, Warrant Shares, Brookline Warrant and the
Common Shares issuable upon exercise of the Brookline Warrant were
offered pursuant to an exemption from the registration requirement
of the Securities Act provided in Section 4(a)(2) of the Securities
Act and Rule 506(b) promulgated thereunder.
Description of Business Combination Transaction
On June 7, 2019, we completed a business combination with Edesa
Biotech Research, Inc., formerly known as Edesa Biotech Inc.
(“Edesa Biotech Research”), in accordance with the
terms of the Share Exchange Agreement, dated March 7, 2019, by and
among us, Edesa Biotech Research and the shareholders of Edesa
Biotech Research. At the closing of the transaction, we acquired
the entire issued share capital of Edesa Biotech Research, with
Edesa Biotech Research becoming a wholly-owned subsidiary of
ours.
At the closing of the transaction, Edesa Biotech Research
shareholders received 6,249,780 of our common shares in exchange
for the capital shares of Edesa Biotech Research and the holders of
unexercised Edesa Biotech Research share options immediately prior
to the closing of the transaction were issued replacement share
options (“Replacement Options”) to purchase an
aggregate of 297,422 of our common shares. On July 26,
2019, pursuant to the post-closing adjustment contemplated by
the Share Exchange Agreement, we issued an additional 366,234 of
our common shares to the Edesa Biotech Research shareholders and
the holders of unexercised Edesa Biotech Research stock options
immediately prior to the closing of the transaction were issued
17,701 additional Replacement Options to purchase our common
shares.
Our common shares issued in the exchange transaction were issued in
a transaction exempt from registration under Regulation S
promulgated under the Securities Act, because the offer and sale of
such securities was made to non-U.S. persons (as that term is
defined in Regulation S under the Securities Act) in an offshore
transaction.
Description of Warrant Exercise Agreement
On May 24, 2018, we entered into a Warrant Exercise Agreement
pursuant to which warrant holders exercised warrants to purchase
1,122,076 of our common shares at an exercise price of $2.65 per
share. In consideration, we issued 1,122,076 Series A Warrants and
2,244,152 Series B Warrants. In connection with the Warrant
Exercise Agreement, we also issued 78,545 Series A Warrants to H.C.
Wainwright & Co., LLC. We received gross proceeds of
approximately $3 million. Our issuance of our warrants was made in
reliance on Section 4(a)(2) of the Securities Act.
Issuance of Performance Shares
On June 26, 2017, we issued an aggregate of 54,834 performance
shares remaining under our performance share plan to our President,
CEO and Chairman, a director of the company and another eligible
participant in the plan. Our issuance of performance shares was
made in reliance on Section 4(a)(2) of the Securities
Act.
ITEM
16.
Exhibits
and Financial Statement Schedules
(a)
The
exhibits listed under the caption “Exhibit Index”
immediately preceding the signature page are filed herewith or
incorporated by reference herein.
(b)
All
schedules have been omitted because the information required to be
set forth in the schedules is either not applicable or is shown in
the financial statements or notes thereto incorporated by reference
herein.
(a)
The
undersigned registrant hereby undertakes:
(1)
To
file, during any period in which offers or sales are being made, a
post-effective amendment to this registration
statement:
(i)
To
include any prospectus required by Section 10(a)(3) of the
Securities Act;
(ii)
To
reflect in the prospectus any facts or events arising after the
effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the
aggregate, represent a fundamental change in the information set
forth in the registration statement. Notwithstanding the foregoing,
any increase or decrease in volume of securities offered (if the
total dollar value of securities offered would not exceed that
which was registered) and any deviation from the low or high end of
the estimated maximum offering range may be reflected in the form
of prospectus filed with the SEC pursuant to Rule 424(b) if, in the
aggregate, the changes in volume and price represent no more than a
20% change in the maximum aggregate offering price set forth in the
“Calculation of Registration Fee” table in the
effective registration statement; and
(iii)
To
include any material information with respect to the plan of
distribution not previously disclosed in the registration statement
or any material change to such information in the registration
statement; provided,
however, that paragraphs
(1)(i), (1)(ii) and (1)(iii) do not apply if the information
required to be included in a post-effective amendment by those
paragraphs is contained in reports filed with or furnished to the
Commission by the Registrant pursuant to Section 13 or Section
15(d) of the Securities Exchange Act of 1934, as amended (the
“Exchange Act”), that are incorporated by reference in
this registration statement.
(2)
That,
for the purpose of determining any liability under the Securities
Act, each such post-effective amendment shall be deemed to be a new
registration statement relating to the securities offered therein,
and the offering of such securities at that time shall be deemed to
be the initial bona fide offering thereof.
(3)
To
remove from registration by means of a post-effective amendment any
of the securities being registered which remain unsold at the
termination of the offering.
(4)
That,
for the purpose of determining liability under the Securities Act
to any purchaser each prospectus filed pursuant to Rule 424(b) as
part of a registration statement relating to an offering, other
than registration statements relying on Rule 430B or other than
prospectuses filed in reliance on Rule 430A, shall be deemed to be
part of and included in the registration statement as of the date
it is first used after effectiveness; provided, however, that no
statement made in a registration statement or prospectus that is
part of the registration statement or made in a document
incorporated or deemed incorporated by reference into the
registration statement or prospectus that is part of the
registration statement will, as to a purchaser with a time of
contract of sale prior to such first use, supersede or modify any
statement that was made in the registration statement or prospectus
that was part of the registration statement or made in any such
document immediately prior to such date of first use.
(b)
The Registrant hereby
undertakes that, for purposes of determining any liability under
the Securities Act, each filing of the Registrant’s annual
report pursuant to Section 13(a) or 15(d) of the Exchange Act
(and, where applicable, each filing of an employee benefit
plan’s annual report pursuant to Section 15(d) of the
Exchange Act) that is incorporated by reference in this
Registration Statement shall be deemed to be a new registration
statement relating to the securities offered therein, and the
offering of such securities at that time shall be deemed to be the
initial bona fide offering
thereof.
(c)
Insofar as indemnification for liabilities arising under the
Securities Act may be permitted to directors, officers and
controlling persons of the Registrant pursuant to the
indemnification provisions described herein, or otherwise, the
Registrant has been advised that in the opinion of the Securities
and Exchange Commission such indemnification is against public
policy as expressed in the Securities Act and is, therefore,
unenforceable. In the event that a claim for indemnification
against such liabilities (other than the payment by the Registrant
of expenses incurred or paid by a director, officer or controlling
person of the Registrant in the successful defense of any action,
suit or proceeding) is asserted by such director, officer or
controlling person in connection with the securities being
registered, the Registrant will, unless in the opinion of its
counsel the matter has been settled by controlling precedent,
submit to a court of appropriate jurisdiction the question whether
such indemnification by it is against public policy as expressed in
the Securities Act and will be governed by the final adjudication
of such issue.
EXHIBITS
|
Exhibit
|
|
Incorporated by Reference
|
Filed
|
|
Number
|
Exhibit Description
|
Form
|
File Number
|
Exhibit
|
Filing Date
|
Herewith
|
|
|
|
|
|
|
|
|
|
|
Financial Advisory Agreement, dated November 5, 2019, between Edesa
Biotech, Inc. and Brookline Capital Markets, a division of Arcadia
Securities, LLC
|
8-K
|
001-37619
|
1.1
|
January
6, 2020
|
|
|
|
Amendment to Financial Advisory Agreement, dated December 20, 2019,
between Edesa Biotech, Inc. and Brookline Capital Markets, a
division of Arcadia Securities, LLC
|
8-K
|
001-37619
|
1.2
|
January
6, 2020
|
|
|
|
Share Exchange Agreement, dated as of March 7, 2019, by and between
Stellar Biotechnologies Inc., Edesa Biotech Inc. and the Edesa
Shareholders
|
8-K
|
001-37619
|
2.1
|
March
8, 2019
|
|
|
|
Certificate
of Incorporation of the Company, dated June 12, 2007
|
20-F
|
000-54598
|
1(a)
|
February
3, 2012
|
|
|
|
Certificate
of Amendment of the Company, dated April 15, 2008
|
20-F
|
000-54598
|
1(b)
|
February
3, 2012
|
|
|
|
Certificate
of Continuation of the Company, dated November 25,
2009
|
20-F
|
000-54598
|
1(c)
|
February
3, 2012
|
|
|
|
Certificate
of Change of Name of the Company, dated April 7, 2010
|
20-F
|
000-54598
|
1(f)
|
February
3, 2012
|
|
|
|
Amended and Restated Articles of the Company, dated April 9,
2018
|
8-K
|
001-37619
|
3.1
|
April
11, 2018
|
|
|
|
Certificate of Change of Name of the Company, dated June 7,
2019
|
10-K
|
001-37619
|
3.6
|
December
12, 2019
|
|
|
|
Notice of Articles of the Company, dated June 7, 2019
|
8-K
|
001-37619
|
3.1
|
June 10, 2019
|
|
|
|
Specimen
of common share certificate
|
S-3
|
333-233567
|
4.1
|
August
30, 2019
|
|
|
|
Form of Class A Purchase Warrant issued to investors
|
8-K
|
001-37619
|
4.1
|
January
6, 2020
|
|
|
|
Form of Class B Purchase Warrant to issued to
investors
|
8-K
|
001-37619
|
4.2
|
January 6, 2020
|
|
|
|
Form
of Warrant issued to Brookline Capital Markets, a division of
Arcadia Securities, LLC
|
8-K
|
001-37619
|
4.3
|
January
6, 2020
|
|
|
|
Form of
Warrant
|
S-1
|
333-224314
|
4.2
|
May 8,
2018
|
|
|
|
Opinion
of Fasken Martineau DuMoulin, LLP
|
|
|
|
|
X
|
|
|
Patent
Assignment and Royalty Agreement between the Company and Frank
Oakes, dated August 6, 2002
|
20-F
|
000-54598
|
8(a)
|
February
3, 2012
|
|
|
|
Advance
Notice Policy, adopted October 31, 2013
|
10-K
|
000-54598
|
10.14
|
November
14, 2014
|
|
|
|
Form of
Securities Purchase Agreement
|
S-1
|
333-224314
|
10.21
|
May 8,
2018
|
|
|
|
Employment Agreement by and between the Company and Kathi
Niffenegger, dated June 7, 2019
|
8-K
|
001-37619
|
10.1
|
June
10, 2019
|
|
|
|
Employment
Agreement by and between the Company and Pardeep Nijhawan,
dated June 14, 2019
|
8-K/A
|
001-37619
|
10.2
|
June
20, 2019
|
|
|
|
Employment
Agreement by and between the Company and Michael Brooks, dated
June 14, 2019
|
8-K/A
|
001-37619
|
10.3
|
June
20, 2019
|
|
|
|
Form of
Indemnification Agreement, by and between the Company and each of
its directors and executive officers
|
8-K/A
|
001-37619
|
10.4
|
June
20, 2019
|
|
|
|
Fixed
Share Option Plan dated December 18, 2013
|
10-K
|
000-54598
|
10.11
|
November
14, 2014
|
|
|
|
2017
Incentive Compensation Plan
|
8-K
|
001-37619
|
10.1
|
March
29, 2017
|
|
|
|
2019
Equity Incentive Compensation Plan
|
8-K
|
001-37619
|
10.1
|
October
25, 2019
|
|
|
|
Lease, dated as of January 1, 2017, by and between the Registrant
and 1968160 Ontario Inc.
|
8-K
|
001-37619
|
10.1
|
August
30, 2019
|
|
|
|
Exclusive
License Agreement, dated as of June 29, 2016, by and between the
Registrant and Yissum Research Development Company
|
8-K
|
001-37619
|
10.2
|
August
30, 2019
|
|
|
|
First Amendment to Exclusive License Agreement, dated April 3,
2017, by and between the Registrant and Yissum Research Development
Company
|
8-K
|
001-37619
|
10.3
|
August
30, 2019
|
|
|
|
Second
Amendment to Exclusive License Agreement, dated May 7, 2017, by and
between the Registrant and Yissum Research Development
Company
|
8-K
|
001-37619
|
10.4
|
August
30, 2019
|
|
|
|
Exclusive
License Agreement, dated as of June 15, 2016, by and between the
Registrant and Cipher Pharmaceuticals Inc.
|
8-K
|
001-37619
|
10.5
|
August
30, 2019
|
|
|
|
License
and Development Agreement, dated as of August 27, 2017, by and
between the Registrant and Pendopharm, a division of Pharmascience
Inc.
|
8-K
|
001-37619
|
10.6
|
August
30, 2019
|
|
|
|
Form of Securities Purchase Agreement between Edesa Biotech, Inc.
and certain investors
|
8-K
|
001-37619
|
10.1
|
January
6, 2020
|
|
|
|
Form of Subscription Agreement between Edesa Biotech, Inc. and
certain investors
|
8-K
|
001-37619
|
10.2
|
January
6, 2020
|
|
|
|
Subsidiaries of Edesa Biotech, Inc.
|
10-K
|
001-37619
|
21
|
December
12, 2019
|
|
|
|
Consent
of MNP LLP
|
|
|
|
|
X
|
|
|
Consent
of Fasken Martineau DuMoulin, LLP (included in Exhibit
5.1)
|
|
|
|
|
X
|
|
|
Consent
of Stubbs, Alderton & Markiles, LLP
|
|
|
|
|
X
|
|
|
Power
of Attorney (included on signature page)
|
|
|
|
|
X
|
* All schedules and
exhibits to the Share Exchange Agreement have been omitted pursuant
to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule
and/or exhibit will be furnished to the Securities and Exchange
Commission upon request.
@ Management contract or compensatory plan or
arrangement.
+ Portions
of this exhibit have been omitted pursuant to Rule 601(b)(10)(iv)
of Regulation S-K.
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, the registrant
has duly caused this registration statement to be signed on its
behalf by the undersigned, thereunto duly authorized, in the City
of Markham, Province of Ontario, on February 25, 2020.
|
|
EDESA
BIOTECH, INC.
|
|
|
|
|
|
|
|
|
By:
|
/s/ Michael
Brooks
|
|
|
|
|
Michael
Brooks
|
|
|
|
|
President
|
|
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose
signature appears below hereby constitutes and appoints Pardeep
Nijhawan and Kathi Niffenegger, and each of them, as his or her
true and lawful attorney-in-fact and agent with full
power of substitution, for him or her in any and all capacities, to
sign any and all amendments to this registration statement
(including post-effective amendments or any abbreviated
registration statement and any amendments thereto filed pursuant to
Rule 462(b) increasing the number of securities for which
registration is sought), and to file the same, with all exhibits
thereto and other documents in connection therewith, with the
Securities and Exchange Commission, granting unto said
attorney-in-fact and agent full power
and authority to do and perform each and every act and thing
requisite and necessary to be done in connection therewith, as
fully for all intents and purposes as he or she might or could do
in person, hereby ratifying and confirming all that said
attorney-in-fact and agent, or his
substitute, may lawfully do or cause to be done by virtue
hereof.
SIGNATURES
Pursuant to the requirements of the Securities Act, this
Registration Statement has been signed below by the following
persons in the capacities and on the dates indicated.
|
Signature
|
Title
|
Date
|
|
/s/ Pardeep
Nijhawan
|
Director, Chief
Executive Officer and Corporate
(Principal
Executive Officer)
|
February 25,
2020
|
|
Pardeep
Nijhawan
|
|
|
|
|
|
|
|
/s/ Kathi
Niffenegger
|
Chief Financial
Officer
(Principal
Financial and Accounting Officer)
|
February 25,
2020
|
|
Kathi
Niffenegger
|
|
|
|
|
|
|
|
/s/ Lorin
Johnson
|
Director
|
February 25,
2020
|
|
Lorin
Johnson
|
|
|
|
|
|
|
|
/s/ Sean
McDonald
|
Director
|
February 25,
2020
|
|
Sean
McDonald
|
|
|
|
|
|
|
|
/s/ Frank
Oakes
|
Director
|
February 25,
2020
|
|
Frank
Oakes
|
|
|
|
|
|
|
|
/s/ Paul
Pay
|
Director
|
February 25,
2020
|
|
Paul
Pay
|
|
|
|
|
|
|
|
/s/ Carlo
Sistilli
|
Director
|
February 25,
2020
|
|
Carlo
Sistilli
|
|
|
|
|
|
|
|
/s/ Peter van der
Velden
|
Director
|
February 25,
2020
|
|
Peter van der
Velden
|
|
|
Edesa Biotech (NASDAQ:EDSA)
Historical Stock Chart
From Mar 2024 to Apr 2024
Edesa Biotech (NASDAQ:EDSA)
Historical Stock Chart
From Apr 2023 to Apr 2024