AstraZeneca's Prostate Cancer Supplemental Drug Gets U.S. Approval
January 20 2020 - 2:46AM
Dow Jones News
By Sabela Ojea
AstraZeneca PLC (AZN.LN) said Monday that its supplemental new
drug application for Lynparza has been accepted and granted
priority review in the U.S. by the Food and Drug Administration for
patients with metastatic castration-resistant prostate cancer.
The pharmaceutical company said that the Prescription Drug User
Fee Act date is set for the second quarter of 2020.
The London-listed company said that Lynparza is also aimed at
patients with deleterious or suspected deleterious germline or
somatic homologous recombination repair gene mutations, which have
progressed following prior treatment with a new hormonal agent.
Results of the PROfound trial showed Lynparza significantly
reduced the risk of disease progression or death by 66%, it
added.
Prostate cancer is the second-most common cancer in men, with an
estimated 1.3 million new cases diagnosed world-wide in 2018 and it
is associated with a significant mortality rate, AstraZeneca
noted.
Write to Sabela Ojea at sabela.ojea@wsj.com; @sabelaojeaguix
(END) Dow Jones Newswires
January 20, 2020 02:31 ET (07:31 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
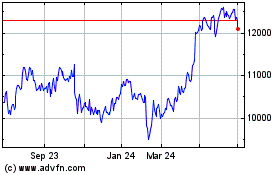
Astrazeneca (LSE:AZN)
Historical Stock Chart
From Mar 2024 to Apr 2024
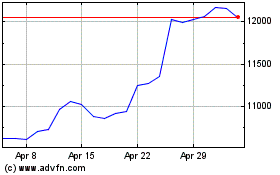
Astrazeneca (LSE:AZN)
Historical Stock Chart
From Apr 2023 to Apr 2024