Use these links to rapidly review the document
TABLE OF CONTENTS
TABLE OF CONTENTS
Table of Contents
Filed Pursuant to Rule 424(b)(5)
Registration No. 333-234353
The information in this preliminary prospectus supplement is not complete and may be changed. A registration statement relating to these securities has been
filed with the Securities and Exchange Commission and is effective. This preliminary prospectus supplement and the accompanying prospectus are not an offer to sell these securities and they are not
soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED NOVEMBER 18, 2019
Prospectus Supplement
(To Prospectus Dated November 14, 2019)

Shares of Common Stock
We are offering shares of common stock, at a public offering price of
$ per share.
Our
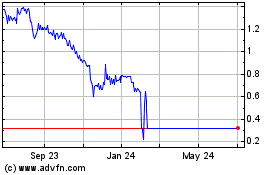
common stock is listed on the Nasdaq Capital Market under the symbol "NVIV." On November 15, 2019, the last reported sale price of our common stock on the Nasdaq Capital
Market was $0.43 per share.
Each
purchaser in this offering will be required, as a condition to such purchase, to execute a subscription agreement pursuant to which they will (i) agree, effective until
5:00 p.m. New York City time on November 22, 2019, not to sell, dispose or otherwise transfer, directly or indirectly any shares of our common stock that they own or control as of the
closing of this offering and (ii) agree to vote at our next stockholder meeting the shares of our common stock that they own or control as of the closing of this offering in favor of all of the
proposals presented to our stockholders in our preliminary proxy statement on Schedule 14A, filed with the Securities and Exchange Commission on November 8, 2019.
As
of September 23, 2019, the aggregate market value of our outstanding common stock held by non-affiliates was approximately $5,022,686, which was calculated based on 9,301,271
shares of outstanding common stock held by non-affiliates and a price per share of $0.54. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell, pursuant to the
registration statement of which this prospectus supplement forms a part, securities in a public primary offering with a value exceeding one-third of the aggregate market value of our common
stock held by non-affiliates in any 12-month period, so long as the aggregate market value of our outstanding common stock held by non-affiliates remains below $75 million. During the 12
calendar months prior to and including the date of this prospectus supplement, we have not offered or sold any securities pursuant to General Instruction I.B.6 of Form S-3.
We
have engaged H.C. Wainwright & Co., LLC, or the placement agent, to act as our exclusive placement agent in connection with this offering. The placement agent has
agreed to use its reasonable best efforts to arrange for the sale of the securities offered by this prospectus supplement. The placement agent is not purchasing or selling any of the securities we are
offering and the placement agent is not required to arrange the purchase or sale of any specific number of securities or dollar amount. We have agreed to pay to the placement agent the placement agent
fees set forth in the table below, which assumes that we sell all of the securities offered by this prospectus supplement. All sales will be evidenced by subscription agreements between us and the
investors. There is no arrangement for funds to be received in escrow, trust or similar arrangement. There is no minimum offering requirement. We will bear all costs associated with the offering. See
"Plan of Distribution" on page S-20 of this prospectus supplement for more information regarding these arrangements.
Investing in the offered securities involves a high degree of risk. See "Risk Factors" beginning on page S-7 of this prospectus supplement
for a discussion of information that you should consider before investing in our securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these
securities or passed upon the adequacy or accuracy of this prospectus supplement or the accompanying prospectus. Any representation to the contrary is a criminal offense.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Per Share
|
|
Total
|
|
|
|
Public offering price
|
|
$
|
|
$
|
|
|
|
Placement agent's fees(1)
|
|
$
|
|
$
|
|
|
|
Proceeds, before expenses, to us
|
|
$
|
|
$
|
|
|
-
(1)
-
We
have agreed to reimburse the placement agent for certain of its expenses. In addition, we have agreed to issue to the placement agent warrants to purchase
shares of our common stock. See "Plan of Distribution" on page S-20 of this prospectus supplement for a description of the compensation payable to the placement agent.
Delivery
of the securities is expected on or about , 2019, subject to satisfaction of certain conditions.
H.C. Wainwright & Co.
The date of this prospectus supplement is , 2019.
Table of Contents
TABLE OF CONTENTS
PROSPECTUS SUPPLEMENT
PROSPECTUS
Table of Contents
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is in two parts. The first part is the prospectus supplement, including the documents incorporated by reference, which describes
the specific terms of this offering. The second part, the accompanying prospectus, including the documents incorporated by reference, provides more general information. Generally, when we refer to
this prospectus, we are referring to both parts of this document combined. Before you invest, you should carefully read this prospectus supplement, the accompanying prospectus, all information
incorporated by reference herein and therein, as well as the additional information described under "Where You Can Find Additional Information" on page S-27 of this prospectus supplement. These
documents contain information you should consider when making your investment decision. This prospectus supplement may add, update or change information contained in the accompanying prospectus. To
the extent there is a conflict between the information contained in this prospectus supplement, on the one hand, and the information contained in the accompanying prospectus or any document
incorporated by reference therein filed prior to the date of this prospectus supplement, on the other hand, you should rely on the
information in this prospectus supplement. If any statement in one of these documents is inconsistent with a statement in another document having a later date—for example, a document filed
after the date of this prospectus supplement and incorporated by reference in this prospectus supplement and the accompanying prospectus—the statement in the document having the later date
modifies or supersedes the earlier statement.
You
should rely only on the information contained or incorporated by reference in this prospectus supplement, the accompanying prospectus and in any free writing prospectuses we may
provide to you in connection with this offering. We have not, and the placement agent has not, authorized any other person to provide you with any information that is different. If anyone provides you
with different or inconsistent information, you should not rely on it. We are offering to sell, and seeking offers to buy, our securities only in jurisdictions where offers and sales are permitted.
The distribution of this prospectus supplement and the offering of securities covered hereby in certain jurisdictions may be restricted by law. Persons outside the United States who come into
possession of this prospectus supplement must inform themselves about, and observe any restrictions relating to, the offering of securities covered hereby and the distribution of this prospectus
supplement outside the United States. This prospectus supplement does not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities
offered by this prospectus supplement by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
We
further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference in this
prospectus supplement or the accompanying prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties
to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made.
Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
Unless
the context otherwise requires, the terms "InVivo," "the Company," "our company," "we," "us," "our" and similar names refer collectively to InVivo Therapeutics Holdings Corp. and
its subsidiaries.
All
trademarks, trade names and service marks appearing in this prospectus supplement, the accompanying prospectus or the documents incorporated by reference herein or therein are the
property of their respective owners. Use or display by us of other parties' trademarks, trade dress or products is not intended to and does not imply a relationship with, or endorsements or
sponsorship of, us by the trademark or trade dress owner. Solely for convenience, trademarks, tradenames and service marks referred to in this prospectus supplement, the accompanying prospectus or the
documents incorporated by reference herein or therein appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not
assert, to the fullest extent under applicable law, our rights or that the applicable owner will not assert its rights, to these trademarks and trade names.
S-1
Table of Contents
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights certain information about us, this offering and selected information contained elsewhere in or
incorporated by reference into this prospectus supplement, the accompanying prospectus and the information incorporated by reference herein and therein. This summary is not complete and does not
contain all of the information that you should consider before deciding whether to invest in our securities. For a more complete understanding of our company and this offering, we encourage you to
read and consider carefully the entire prospectus supplement and the accompanying prospectus, including "Risk Factors" beginning on page S-7 of this prospectus supplement, along with our
consolidated financial statements and notes to those consolidated financial statements and the other documents incorporated by reference in this prospectus supplement and the accompanying
prospectus.
Business Overview
Overview
We are a research and clinical-stage biomaterials and biotechnology company with a focus on treatment of spinal cord injuries, or SCIs. Our
approach to treating acute SCIs is based on our investigational Neuro-Spinal Scaffold™ implant, a bioresorbable polymer scaffold that is
designed for implantation at the site of injury within a spinal cord and is intended to treat acute SCI. The Neuro-Spinal Scaffold implant incorporates
intellectual property licensed under an exclusive, worldwide license from Boston Children's Hospital, or BCH, and the Massachusetts Institute of Technology, or MIT. We also plan to evaluate other
technologies and therapeutics that may be complementary to our development of the Neuro-Spinal Scaffold implant or offer the potential to bring us
closer to our goal of redefining the life of the SCI patient.
The
current standard of care for acute management of spinal cord injuries focuses on preventing further injury to the spinal cord. However, the current standard of care does not address
repair of the spinal cord.
Our Clinical Program
We currently have one clinical development program for the treatment of acute SCI.
Neuro-Spinal Scaffold Implant for acute SCI
Our Neuro-Spinal Scaffold implant is an investigational bioresorbable polymer scaffold that is
designed for implantation at the site of injury within a spinal cord. The Neuro-Spinal Scaffold implant is intended to promote appositional, or
side-by-side, healing by supporting the surrounding tissue after injury, minimizing expansion of areas of necrosis, and providing a biomaterial substrate for the body's own healing/repair processes
following injury. We believe this form of appositional healing may spare white matter, increase neural sprouting, and diminish post-traumatic cyst formation.
The
Neuro-Spinal Scaffold implant is composed of two biocompatible and bioresorbable polymers that are cast to form a highly porous
investigational product:
-
•
-
Poly lactic-co-glycolic acid, a polymer that is widely used in resorbable sutures and provides the biocompatible support for Neuro-Spinal
Scaffold implant; and
-
•
-
Poly-L-Lysine, a positively charged polymer commonly used to coat surfaces in order to promote cellular attachment.
Because
of the complexity of SCIs, it is likely that multi-modal therapies will be required to maximize positive outcomes in SCI patients. In the future, we may attempt to further
enhance the performance of our Neuro-Spinal Scaffold implant by multiple combination strategies involving electrostimulation devices, additional
biomaterials, drugs approved by the U.S. Food and Drug
S-2
Table of Contents
Administration,
or FDA, or growth factors. We expect the Neuro-Spinal Scaffold implant to be regulated by the FDA as a Class III medical device.
Completed Pilot Study
We conducted an early feasibility human pilot study, as the initial phase of a larger pivotal study, of our Neuro-Spinal
Scaffold under our approved Investigational Device Exemption, or IDE, application for the treatment of complete, traumatic acute SCI. The study was intended to assess the
safety and feasibility of the Neuro-Spinal Scaffold for the treatment of complete thoracic functional SCI, as well as to gather preliminary evidence of
the clinical effectiveness of the Neuro-Spinal Scaffold.
The
pilot study was initially approved for five subjects in up to six clinical sites across the United States, and was later modified to increase the number of allowable clinical sites
to up to 20 and to permit enrollment of up to 10 subjects. The pilot study was initially staggered such that each patient that met the eligibility criteria would be followed for three months prior to
enrolling the next patient in the study. In December 2014, the FDA approved an expedited enrollment plan that allowed us to continue enrolling patients more rapidly barring any significant safety
issues. We enrolled five subjects in the pilot study between October 2014 and September 2015. The FDA approved conversion of this pilot study to a pivotal probable benefit study, which we refer to as
The INSPIRE Study, that includes data from the patients enrolled in the pilot study.
The INSPIRE Study
Our Neuro-Spinal Scaffold implant has been studied in The INSPIRE Study: the "InVivo Study of
Probable Benefit of the Neuro- Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury," under an
IDE application for the treatment of neurologically complete thoracic traumatic acute SCI. We commenced an FDA-approved pilot study in 2014 that the FDA approved converting into The INSPIRE Study in
January 2016. As of December 31, 2017, we had implanted our Neuro-Spinal Scaffold implant in a total of 19 patients in The INSPIRE Study, 16 of
whom reached the six-month primary endpoint visit, and three of whom died. In July 2017, after the third patient death, enrollment of patients in The INSPIRE Study was placed on hold as we engaged
with the FDA to address the patient deaths. We subsequently closed enrollment in The INSPIRE Study and will follow the remaining active subjects until completion. Following discussions with the FDA,
in March 2018, we received FDA approval for a randomized controlled trial to supplement the existing clinical evidence for the Neuro-Spinal Scaffold
implant that we obtained from The INSPIRE Study. We refer to this as the INSPIRE 2.0 Study.
The
purpose of The INSPIRE Study, which was the original study, was to evaluate whether the Neuro-Spinal Scaffold implant is safe and
demonstrates probable benefit for the treatment of complete T2-T12 neurological level of injury, or NLI, SCI. The primary endpoint was defined as the proportion of patients achieving an improvement of
at least 1 AIS grade at six months post-implantation. Additional endpoints included measurements of pain, sensory and motor scores, bladder and bowel function, Spinal Cord Independence Measure (a
disability scale for patients with SCI), and quality of life. The INSPIRE Study included an Objective Performance Criterion, or OPC, which is a measure of study success used in clinical studies
designed to demonstrate safety and probable benefit in support of a Humanitarian Device Exemption, or HDE, approval. At the time enrollment of patients in The INSPIRE Study was placed on hold, the OPC
was defined as 25% or more of the patients in the study demonstrating an improvement of at least 1 AIS grade at the six-month post-implantation visit.
The
FDA approved the enrollment of up to 30 patients in The INSPIRE Study so that there would be at least 20 evaluable patients at the primary endpoint analysis, accounting for events
such as screen failures or deaths that would prevent a patient from reaching the primary endpoint visit. Of the 19 patients implanted in The INSPIRE Study, 16 patients have reached the six-month
primary endpoint
S-3
Table of Contents
visit.
Of these 16, seven had improved from complete AIS A SCI to incomplete SCI (two patients to AIS C and five patients to AIS B) at the six-month primary endpoint visit and nine had not
demonstrated improvement at that visit. Three of the seven patients who improved were assessed to have AIS B SCI at the six-month primary endpoint and were later assessed to have improved to
AIS C SCI at the 12 or 24-month visits. Two of the 16 patients were initially assessed to have improved from complete AIS A SCI to incomplete AIS B SCI, but each was later assessed to
have reverted to complete AIS A SCI prior to the six-month examination. One of these two was then assessed at the six-month visit to have improved again to AIS B and the other remained AIS A. Since we
have closed enrollment, the target of enrolling 20 evaluable patients into The INSPIRE Study will not be reached.
The
FDA had previously recommended that we include a randomized, concurrent control arm in The INSPIRE Study. Acting on the FDA's recommendation, we proposed and received approval for
the INSPIRE 2.0 Study (described below) to supplement the existing clinical evidence for the Neuro-Spinal Scaffold implant. In addition, as 1 source of
comparator data, we completed the Contemporary Thoracic SCI Registry Study, or the CONTEMPO Registry Study. The CONTEMPO Registry Study utilized existing databases and registries to develop a
historical comparator that, to the extent possible, matched patients to those patients enrolled in The INSPIRE Study. The CONTEMPO Registry Study was designed to provide comprehensive natural history
benchmarks for The INSPIRE Study results that included SCI patients with similar baseline characteristics treated since 2006. The CONTEMPO Registry Study included data from the Christopher &
Dana Reeve Foundation North American Clinical Trials Network Registry, or NACTN, as well as the Model Systems Registry and the European Multicenter Study about Spinal Cord Injury, or EMSCI. We
announced top-line findings from CONTEMPO in March 2018 from a total of 170 patients from the 3 registries consisting of: 12 individuals from NACTN, 64 from EMSCI, and 94 from the Model Systems
Registry. AIS conversion rates at approximately 6 months post-injury varied from 16.7%—23.4% across the 3 registries. In 2 of the registries, there was a skew of the patient
population to low (T10- T12) thoracic injuries, representing 46-47% of the registry population. This compares to just 4 out of 16 patients (25%) in follow-up in the INSPIRE study with low thoracic
injuries. Patients with low thoracic injuries are known to have the best prognoses, and the conversion rates were the highest in the low thoracic group in all 3 registries and the INSPIRE study. When
all 3 registries were normalized to the INSPIRE patient population distribution across T2-T5, T6-T9 and T10-T12 injury groups, the normalized conversion rate for CONTEMPO registries ranged from
15.5%-20.6%. We cannot be certain what additional information or studies will be required by the FDA to approve our HDE submission.
INSPIRE 2.0 Study
Our Neuro-Spinal Scaffold implant has been approved to be studied under our approved IDE in the
INPSIRE 2.0 Study, which is titled the "Randomized, Controlled, Single-blind Study of Probable Benefit of the Neuro-Spinal Scaffold™ for
Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury as Compared to Standard of
Care." The purpose of the INSPIRE 2.0 Study is to assess the overall safety and probable benefit of the Neuro-Spinal Scaffold for the treatment of
neurologically complete thoracic traumatic acute SCI. The INSPIRE 2.0 Study is designed to enroll 10 subjects into each of the two study arms, which we refer to as the Scaffold Arm and the Comparator
Arm. Patients in the Comparator Arm will receive the standard of care, which is spinal stabilization without dural opening or myelotomy. The INSPIRE 2.0 Study is a single blind study, meaning that the
patients and assessors are blinded to treatment assignments. The FDA approved the enrollment of up to 35 patients in this study so that there would be at least 20 evaluable patients (10 in each study
arm) at the primary endpoint analysis, accounting for events such as screen failures or deaths that would prevent a patient from reaching the primary endpoint visit. We expect to conduct the INSPIRE
2.0 Study at up to 25 sites in the United States. Enrolling patients in the INSPIRE 2.0 Study requires the approvals of the institutional review boards, or IRBs, at each clinical site. We estimate
that enrollment in the INSPIRE 2.0 Study will be complete in the fourth quarter of 2020, with the final
S-4
Table of Contents
patient
enrolled in the INSPIRE 2.0 study reaching their six-month primary endpoint visit in the second quarter of 2021. As of November 1, 2019, the first five patients in the INSPIRE 2.0 Study
have been enrolled and 14 sites are open.
The
primary endpoint is defined as the proportion of patients achieving an improvement of at least one AIS grade at six months post-implantation. Assessments of AIS grade are at hospital
discharge, three months, six months, 12 months and 24 months. The definition of study success for INSPIRE 2.0 is that the difference in the proportion of subjects who demonstrate an
improvement of at least one grade on AIS assessment at the six-month primary endpoint follow-up visit between the Scaffold Arm and the Comparator Arm must be equal to or greater than 20%. In one
example, if 50% of subjects in the Scaffold Arm have an improvement of AIS grade at the six-month primary endpoint and 30% of subjects in the Comparator Arm have an improvement, then the difference in
the proportion of subjects who demonstrated an improvement is equal to 20% (50% minus 30% equals 20%) and the definition of study success would be met. In another example, if 40% of subjects in the
Scaffold Arm have an improvement of AIS grade at the six-month primary endpoint and 30% of subjects in the Comparator Arm have an improvement, then the difference in the proportion of subjects who
demonstrated an improvement is equal to 10% (40% minus 30% equals 10%) and the definition of study success would not be met. Additional endpoints include measurements of changes in NLI, sensory levels
and motor scores, bladder, bowel and sexual function, pain, Spinal Cord Independence Measure, and quality of life.
Although
The INSPIRE Study is structured with the OPC as the primary component for demonstrating probable benefit, the OPC is not the only variable that the FDA would evaluate when
reviewing a future HDE application. Similarly, while our INSPIRE 2.0 Study is structured with a definition of study success requiring a minimum difference between study arms in the proportion of
subjects achieving improvement, that success definition is not the only factor that the FDA would evaluate in the future HDE application. Approval is not guaranteed if the OPC is met for The INSPIRE
Study or the definition of study success is met for the INSPIRE 2.0 Study, and even if the OPC or definition of study success are not met, the FDA may approve a medical device if probable benefit is
supported by a comprehensive review of all clinical endpoints and preclinical results, as demonstrated by the sponsor's body of evidence.
In
2016, the FDA accepted our proposed HDE modular shell submission and review process for the Neuro-Spinal Scaffold implant. The HDE
modular shell is comprised of three modules: a preclinical studies module, a manufacturing module, and a clinical data module. As part of its review process, the FDA reviews each module, which are
individual sections of the HDE submission, on a rolling basis. Following the submission of each module, the FDA reviews and provides feedback, typically within 90 days, allowing the applicant
to receive feedback and potentially resolve any deficiencies during the review process. Upon receipt of all three modules, which constitutes the complete HDE submission, the FDA makes a filing
decision that may trigger the review clock for an approval decision. We submitted the first module in March 2017 and received feedback in June 2017. We plan to submit an updated first module in the
fourth quarter of 2019. The HDE submission will not be complete until the manufacturing and clinical modules are also submitted.
Corporate Information
We were incorporated on April 2, 2003, under the name of Design Source, Inc. On October 26, 2010, we acquired the business
of InVivo Therapeutics Corporation, which was founded in 2005, and we are continuing the existing business operations of InVivo Therapeutics Corporation as our wholly-owned subsidiary.
Our
principal executive offices are located in leased premises at One Kendall Square, Suite B14402, Cambridge, Massachusetts 02139. Our telephone number is (617) 863-5500.
We maintain a website at www.invivotherapeutics.com. Information contained on, or accessible through, our website is not a part of, and is not incorporated by reference into, this prospectus
supplement or the accompanying prospectus.
S-5
Table of Contents
THE OFFERING
|
|
|
|
|
Securities offered by us:
|
|
shares of our common stock
|
|
Common stock outstanding after this offering
|
|
shares of common stock.
|
|
Sale restriction and voting agreements
|
|
Each purchaser in this offering will be required, as a condition to such purchase, to execute a subscription agreement
pursuant to which they will (i) agree, effective until 5:00 p.m. New York City time on November 22, 2019, not to sell, dispose or otherwise transfer, directly or indirectly any shares of our common stock that they own or control as of
the closing of this offering and (ii) agree to vote at our next stockholder meeting the shares of our common stock that they own or control as of the closing of this offering in favor of all of the proposals presented to our stockholders in our
preliminary proxy statement on Schedule 14A, filed with the Securities and Exchange Commission on November 8, 2019.
|
|
Use of proceeds
|
|
We intend to use the net proceeds from this offering for working capital and general corporate purposes. See "Use of
Proceeds" on page S-12 of this prospectus supplement.
|
|
Risk factors
|
|
See "Risk Factors" beginning on page S-7 of this prospectus supplement and the other information included or
incorporated by reference elsewhere in this prospectus supplement and the accompanying prospectus, for a discussion of factors you should carefully consider before deciding to invest in our securities.
|
|
Nasdaq Capital Market symbol
|
|
Our common stock is listed on the Nasdaq Capital Market under the symbol "NVIV."
|
The
number of shares of common stock to be outstanding immediately after this offering is based on 9,519,570 shares of our common stock outstanding as of October 31, 2019, and
excludes:
-
•
-
7,672,887 shares of common stock issuable upon the exercise of warrants outstanding as of October 31, 2019 at a weighted average
exercise price of $4.78 per share;
-
•
-
124,890 shares of common stock issuable upon the exercise of options at a weighted average exercise price of $35.15 per share and 7,500 shares
of common stock issuable upon vesting of restricted stock units outstanding as of October 31, 2019 pursuant to our stock incentives plans, which we refer to collectively as the Incentive Plans;
-
•
-
6,146 shares of common stock available for future awards under the Incentive Plans and for future issuance our 401(k) plan as of
October 31, 2019; and
-
•
-
7,923 shares of common stock reserved for future sale under our employee stock purchase plan as of October 31, 2019.
S-6
Table of Contents
RISK FACTORS
An investment in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you
should consider carefully the risks and uncertainties described below and under the section captioned "Risk Factors" contained in our most recent
Quarterly Report on Form 10-Q for the quarterly period ended September 30,
2019 and other filings we make with the Securities and Exchange Commission, or SEC, from time to time, which are incorporated by reference herein in their entirety, together with other
information in this prospectus supplement, the accompanying prospectus and the information incorporated by reference herein and therein and in any free writing prospectus that we may authorize for use
in connection with this offering. If any of these risks actually occurs, our business, financial condition, results of operations or cash flow could suffer materially. In such event, the trading price
of our common stock could decline and you might lose all or part of your investment.
Risks Related to This Offering
We have broad discretion over the use of our cash and cash equivalents, including the net proceeds we receive
in this offering, and may not use them effectively.
Our management has broad discretion to use our cash and cash equivalents, including the net proceeds we receive in this offering, to fund our
operations and could spend these funds in ways that do not improve our results of operations or enhance the value of our common stock. The failure by our management to apply these funds effectively
could result in financial losses that could have a material adverse effect on our business, cause the price of our common stock to decline and delay the development of our product candidates. Pending
their use to fund operations, we may invest our cash and cash equivalents in a manner that does not produce income or that loses value.
Even if this offering is successful, we will need additional funding to continue our operations. If we are
unable to raise capital when needed, we could be forced to delay, reduce, or eliminate our product development programs or commercialization efforts.
Even if this offering is successful, we will not have sufficient cash resources to continue our business operations beyond the second quarter of
2020. Our cash resources will not be sufficient to complete clinical development of our Neuro-Spinal Scaffold implant, including the resources needed to
complete enrollment in our INSPIRE 2.0 Study and to reach submission of the HDE application to the FDA. In addition, we expect that our expenses will increase in connection with our ongoing
activities, particularly as we conduct our INSPIRE 2.0 Study, and as we seek regulatory approval for our Neuro-Spinal Scaffold implant. If we obtain
regulatory approval for any of our current or future product candidates, we expect to incur significant commercialization expenses related to manufacturing, marketing, sales, and distribution.
Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise additional capital, we may seek to engage in one or more
potential transactions, such as the sale of our company, a strategic partnership with one or more parties or the licensing, sale or divestiture of some of our assets or proprietary technologies, or we
may be forced to cease our operation entirely. There can be no assurance that we will be able to enter into such a transaction or transactions on a timely basis or on terms that are favorable to us.
If we are unable to raise capital when needed or on attractive terms, or should we engage in one or more potential strategic transactions, we could be forced to delay, reduce, or eliminate our
research and development programs or any future commercialization efforts. If we determine to change our business strategy or to seek to engage in a strategic transaction, our future business,
prospects, financial position and operating results could be significantly different than those in
historical periods or projected by our management. Because of the significant uncertainty regarding these events, we are not able to accurately predict the impact of any potential changes in our
existing business strategy.
S-7
Table of Contents
Our
future funding requirements, both near- and long-term, will depend on many factors, including, but not limited to:
-
•
-
the scope, progress, results, and costs of preclinical development, laboratory testing, and clinical trials for our Neuro-Spinal Scaffold implant and
any other product candidates that we may develop or acquire, including our INSPIRE 2.0 Study;
-
•
-
future clinical trial results of our Neuro-Spinal Scaffold implant;
-
•
-
the timing of, and the costs involved in, obtaining regulatory approvals for the Neuro-Spinal
Scaffold implant, and the outcome of regulatory review of the Neuro-Spinal Scaffold implant;
-
•
-
the cost and timing of future commercialization activities for our products if any of our product candidates are approved for marketing,
including product manufacturing, marketing, sales, and distribution costs;
-
•
-
the revenue, if any, received from commercial sales of our product candidates for which we receive marketing approval;
-
•
-
the cost of having our product candidates manufactured for clinical trials in preparation for regulatory approval and in preparation for
commercialization;
-
•
-
the cost and delays in product development as a result of any changes in regulatory oversight applicable to our product candidates;
-
•
-
our ability to establish and maintain strategic collaborations, licensing, or other arrangements and the financial terms of such agreements;
-
•
-
the cost and timing of establishing sales, marketing, and distribution capabilities;
-
•
-
the costs involved in preparing, filing, prosecuting, maintaining, defending, and enforcing our intellectual property portfolio;
-
•
-
the efforts and activities of competitors and potential competitors;
-
•
-
the effect of competing technological and market developments; and
-
•
-
the extent to which we acquire or invest in businesses, products, and technologies.
Identifying
potential product candidates and conducting preclinical testing and clinical trials is a time-consuming, expensive, and uncertain process that takes years to complete, and we
may never generate the necessary data or results required to obtain regulatory approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success.
Our commercial revenues, if any, will be derived from sales of products that we do not expect to be commercially available for several years, if at all. Accordingly, we will need to continue to rely
on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all and if we are not successful in raising additional
capital, we may not be able to continue as a going concern.
There is substantial doubt about our ability to continue as a going concern, which will affect our ability to
obtain future financing and may require us to curtail our operations.
Our consolidated financial statements as of September 30, 2019 were prepared under the assumption that we will continue as a going
concern. At September 30, 2019, we had cash and cash equivalents of $8.1 million. We estimate that our existing cash resources, together with the anticipated proceeds of this offering,
will be sufficient to fund our operations into the second quarter of 2020. This estimate is based on assumptions that may prove to be wrong; expenses could prove to be significantly higher, leading to
a more rapid consumption of our existing resources.
S-8
Table of Contents
Our
ability to continue as a going concern will depend on our ability to obtain additional equity or debt financing, attain further operating efficiencies, reduce or contain
expenditures, and, ultimately, to generate revenue.
Our
independent registered public accounting firm expressed substantial doubt as to our ability to continue as a going concern in its report dated April 1, 2019 included in our
Annual report on Form 10-K as filed with the SEC on April 1, 2019. Our management has determined that there continues to be substantial doubt regarding our ability to continue as a going
concern. Even if this offering is successful, we expect that there will continue to be substantial doubt about our ability to continue as a going concern. If we are unable to continue as a going
concern, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our audited consolidated
financial statements, and it is likely that investors will lose all or part of their investment. If we seek additional financing to fund our business activities in the future and there remains
substantial doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable terms or at
all.
You may experience future dilution as a result of future equity offerings.
In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or
exchangeable for our common stock at prices that may not be the same as the public offering price for the shares in this offering. We may sell shares or other securities in any other offering at
prices that are less than the price paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders.
The subscription agreements to be executed by purchasers in this offering could result in the authorization
by our stockholders of the issuance of additional shares of common stock, which could result in your experiencing further dilution.
Each
purchaser in this offering will be required, as a condition to such purchase, to execute a subscription agreement pursuant to which they will (i) agree,
effective until 5:00 p.m. New York City time on November 22, 2019, not to sell, dispose or otherwise transfer, directly or indirectly any shares of our common stock that they own or
control as of the closing of this offering and (ii) agree to vote at our next stockholder meeting the shares of our common stock that they own or control as of the closing of this offering in
favor of all of the proposals presented to our stockholders in our preliminary proxy statement on Schedule 14A, filed with the Securities and Exchange Commission on November 8, 2019. If
the requisite stockholders vote for approval of such an amendment of our articles of incorporation, our board of directors will have the right to issue the additional shares of common stock authorized
through such amendment without any additional stockholder approval. Such additional shares of common stock could be issued and sold at prices that are lower than the public offering price for the
shares in this offering, which would result additional dilution of your investment beyond the dilution you will experience immediately upon purchasing securities in this offering.
S-9
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement, the accompanying prospectus and the information incorporated by reference herein and therein, contain and
incorporate "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange
Act of 1934, as amended, or the Exchange Act. These statements include statements made regarding our commercialization strategy, future operations, cash requirements and liquidity, capital
requirements, and other statements on our business plans and strategy, financial position, and market trends. In some cases, you can identify forward-looking statements by terms such as "may,"
"might," "will," "should," "believe," "plan," "intend," "anticipate," "target," "estimate," "expect," and other similar expressions. These forward-looking statements are subject to risks and
uncertainties that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements, including factors such as our ability to raise
substantial additional capital to finance our planned operations and to continue as a going concern; our ability to execute our strategy and business plan; our ability to obtain regulatory approvals
for our products, including the Neuro-Spinal Scaffold™; our ability to successfully commercialize our current and future product candidates,
including the Neuro-Spinal Scaffold; the progress and timing of our development programs; market acceptance of our products; our ability to retain
management and other key personnel; our ability to promote, manufacture, and sell our products, either directly or through collaborative and other arrangements with third parties; and other factors
detailed under "Risk Factors" in this prospectus supplement, our most recent Annual Report on Form 10-K and our most recent Quarterly Report on Form 10-Q. These forward-looking
statements are only predictions, are uncertain, and involve substantial known and unknown risks, uncertainties, and other factors which may cause our actual results, levels of activity, or performance
to be materially different from any future results, levels of activity, or performance expressed or implied by these forward-looking statements. Such factors include, among others, the
following:
-
•
-
our limited operating history and history of net losses;
-
•
-
our ability to raise substantial additional capital to finance our planned operations and to continue as a going concern;
-
•
-
our ability to complete the INSPIRE 2.0 Study to support our existing Humanitarian Device Exemption application;
-
•
-
our ability to execute our strategy and business plan;
-
•
-
our ability to obtain regulatory approvals for our current and future product candidates, including our Neuro-Spinal
Scaffold implant;
-
•
-
our ability to successfully commercialize our current and future product candidates, including our Neuro-Spinal
Scaffold implant;
-
•
-
the progress and timing of our current and future development programs;
-
•
-
our ability to successfully open, enroll and complete clinical trials and obtain and maintain regulatory approval of our current and future
product candidates;
-
•
-
our ability to protect and maintain our intellectual property and licensing arrangements;
-
•
-
our reliance on third parties to conduct testing and clinical trials;
-
•
-
market acceptance and adoption of our current and future technology and products;
-
•
-
our ability to promote, manufacture and sell our current and future products, either directly or through collaborative and other arrangements
with third parties; and
-
•
-
our ability to attract and retain key personnel.
S-10
Table of Contents
We
cannot guarantee future results, levels of activity, or performance. You should not place undue reliance on these forward-looking statements, which speak only as of the respective
dates as of which they were made. You are cautioned that these forward-looking statements are only predictions and are subject to risks, uncertainties and assumptions that are referenced in the
section of this prospectus supplement entitled "Risk Factors." You should also carefully review the risk factors and cautionary statements described in the other documents we file from time to time
with the SEC, specifically our most recent Annual Report on Form 10-K, our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K. Except as required by applicable
law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to reflect actual results, later events or
circumstances, or to reflect the occurrence of unanticipated events.
S-11
Table of Contents
USE OF PROCEEDS
We estimate that the net proceeds from this offering will be approximately $1,245,123 million after deducting the placement agent fees
and estimated offering expenses payable by us, based on an assumed public offering price of $0.43 per share, which is equal to the last reported sale price per share of our common stock on the Nasdaq
Capital Market on November 15, 2019. The public offering price per share will be determined between us, the placement agent and investors based on market conditions at the time of pricing and
may be at a discount to the current market price of our common stock.
We
intend to use the net proceeds from this offering for working capital and general corporate purposes. We cannot predict with certainty all of the particular uses for the net proceeds
to be received upon the completion of this offering. Accordingly, our management will have broad discretion and flexibility in applying the net proceeds from the sale of securities sold pursuant to
this prospectus. Pending the uses described above, we intend to invest the net proceeds from this offering in a variety of capital preservation investments, including short-term, investment-grade and
interest-bearing instruments.
S-12
Table of Contents
DIVIDEND POLICY
We have never declared or paid cash dividends. We do not intend to pay cash dividends on our common stock for the foreseeable future, but
currently intend to retain any future earnings to fund the development and growth of our business. The payment of cash dividends, if any, on our common stock, will rest solely within the discretion of
our board of directors and will depend, among other things, upon our earnings, capital requirements, financial condition, and other relevant factors.
S-13
Table of Contents
CAPITALIZATION
The following table sets forth our cash and cash equivalents, as well as our capitalization, as of September 30, 2019 as
follows:
-
•
-
on an actual basis; and
-
•
-
on an as adjusted basis to reflect the assumed issuance and sale in this offering of 3,893,555 shares of common stock at an assumed public
offering price of $0.43 per share of common stock, which is equal to the last reported sale price per share of our common stock on the Nasdaq Capital Market on November 15, 2019, after
deducting placement agent fees and estimated offering expenses payable by us.
You
should read this information together with our consolidated financial statements and related notes incorporated by reference in this prospectus supplement and the accompanying
prospectus.
|
|
|
|
|
|
|
|
|
|
|
As of September 30, 2019
|
|
|
|
(Unaudited)
|
|
|
|
Actual
|
|
As Adjusted
|
|
|
|
(in thousands, except
share amounts)
|
|
|
Cash and cash equivalents
|
|
$
|
8,136
|
|
$
|
9,382
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders' equity
|
|
|
|
|
|
|
|
|
Common stock, $0.00001 par value—25,000,000 shares authorized; 9,519,570 shares issued and outstanding, actual; 14,170,733 shares issued and
outstanding as adjusted
|
|
|
1
|
|
|
1
|
|
|
Additional paid-in capital
|
|
|
223,644
|
|
|
224,889
|
|
|
Accumulated deficit
|
|
|
(215,788
|
)
|
|
(215,788
|
)
|
|
|
|
|
|
|
|
|
|
|
Total stockholders' equity
|
|
|
7,857
|
|
|
9,102
|
|
|
|
|
|
|
|
|
|
|
|
Total capitalization
|
|
$
|
11,144
|
|
$
|
12,389
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The
information above is based on 9,519,570 shares of our common stock outstanding as of September 30, 2019, and excludes:
-
•
-
7,673,130 shares of common stock issuable upon the exercise of warrants outstanding as of September 30, 2019 at a weighted average
exercise price of $4.78 per share;
-
•
-
124,890 shares of common stock issuable upon the exercise of options at a weighted average exercise price of $35.15 per share and 7,500 shares
of common stock issuable upon vesting of restricted stock units outstanding as of September 30, 2019 pursuant to the Incentive Plans;
-
•
-
6,146 shares of common stock available for future awards under the Incentive Plans and for future issuance our 401(k) plan as of
September 30, 2019; and
-
•
-
7,923 shares of common stock reserved for future sale under our employee stock purchase plan as of September 30, 2019.
S-14
Table of Contents
DESCRIPTION OF SECURITIES WE ARE OFFERING
Common Stock
We have authorized 25,000,000 shares of capital stock, par value $0.00001 per share, all of which are shares of common stock. As of
October 31, 2019, there were 9,519,570 shares of common stock issued and outstanding. The authorized and unissued shares of common stock are available for issuance without further action by our
stockholders, unless such action is required by applicable law or the rules of any stock exchange on which our securities may be listed. Unless approval of our stockholders is so required, our board
of directors does not intend to seek stockholder approval for the issuance and sale of our common stock. All shares of common stock will, when issued,
be duly authorized, fully paid and non-assessable. Accordingly, the full price for the outstanding shares of common stock will have been paid at issuance and any holder of our common stock will not be
later required to pay us any additional money for such common stock.
The
holders of our common stock are entitled to one vote per share. Generally, all matters to be voted on by stockholders must be approved by a majority (or, in the case of election of
directors, by a plurality) of the votes entitled to be cast by all shares of common stock that are present in person or represented by proxy. Additionally, any alteration, amendment or appeal of any
provision of our bylaws would require the affirmative vote of the holders of at least 80% of the voting power of our then outstanding shares entitled to vote, voting together as a single class. Except
as otherwise provided by law, amendments to the articles of incorporation generally must be approved by a majority of the votes entitled to be cast by all outstanding shares of common stock. Our
articles of incorporation do not provide for cumulative voting in the election of directors. Our directors are divided into three classes. At each annual meeting of stockholders, directors elected to
succeed those directors whose terms expire are elected for a term of office to expire at the third succeeding annual meeting of stockholders after their election. The holders of our common stock are
entitled to receive ratably such dividends, if any, as may be declared by our board of directors out of legally available funds; however, the current policy of our board of directors is to retain
earnings, if any, for operations and growth. Upon liquidation, dissolution or winding-up, the holders of our common stock are entitled to share ratably in all assets that are legally available for
distribution after payment of our liabilities. The holders of our common stock have no preemptive, subscription, redemption or conversion rights.
The
foregoing description summarizes important terms of our capital stock, but is not complete. For the complete terms of our common stock, please refer to our articles of incorporation,
as amended, and our amended and restated bylaws, as may be amended from time to time.
The
transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company. Our common stock is listed on the Nasdaq Capital Market under the symbol
"NVIV."
Sale Restriction and Voting Agreements
Each purchaser in this offering will be required, as a condition to such purchase, to execute a subscription agreement pursuant to which they
will (i) agree, effective until 5:00 p.m. New York City time on November 22, 2019, not to sell, dispose or otherwise transfer, directly or indirectly any shares of our common
stock that they own or control as of the closing of this offering and (ii) agree to vote at our next stockholder meeting the shares of our common stock that they own or control as of the
closing of this offering in favor of all of the proposals presented to our stockholders in our preliminary proxy statement on Schedule 14A, filed with the Securities and Exchange Commission on
November 8, 2019. If the requisite stockholders vote for approval of such an amendment of our articles of incorporation, our board of directors will have the right to issue the additional
shares of common stock authorized through such amendment without any additional stockholder approval.
S-15
Table of Contents
MATERIAL U.S. FEDERAL TAX CONSIDERATIONS FOR NON-U.S. HOLDERS OF
OUR COMMON STOCK
The following discussion describes the material U.S. federal income and estate tax considerations relating to the acquisition, ownership and
disposition of our common stock acquired in this offering by a Non-U.S. Holder. For purposes of this discussion, the term "Non-U.S. Holder" means a beneficial owner (other than a partnership or
pass-through entity) of our common stock that is not, for U.S. federal income tax purposes:
-
•
-
an individual citizen or resident of the United States;
-
•
-
a corporation (or other entity treated as a corporation for U.S. federal income tax purposes), created or organized in or under the laws of the
United States, any state thereof or the District of Columbia;
-
•
-
an estate the income of which is subject to U.S. federal income taxation regardless of its source; or
-
•
-
a trust if (1) a U.S. court is able to exercise primary supervision over the administration of the trust and one or more U.S. persons
(within the meaning of Section 7701(a)(30) of the Code) have the authority to control all substantial decisions of the trust or (2) it has a valid election in effect under applicable
U.S. Treasury regulations to be treated as a U.S. person.
This
discussion is based on the current provisions of the Internal Revenue Code of 1986, as amended, or the Code, existing and proposed U.S. Treasury regulations promulgated thereunder,
and administrative rulings and court decisions in effect as of the date of this prospectus supplement, all of which are subject to change or differing interpretation, possibly with retroactive effect.
Any change or differing interpretation could alter the tax consequences to Non-U.S. Holders described in this prospectus supplement. No ruling has been or will be sought from the Internal Revenue
Service, or IRS, with respect to the matters discussed below, and there can be no assurance the IRS will not take a contrary position regarding the tax consequences of the acquisition, ownership or
disposition of our common stock described in this prospectus supplement, or that any such contrary position would not be sustained by a court.
We
assume in this discussion that the shares of our common stock will be held as capital assets (generally, property held for investment). This discussion does not address all aspects of
U.S. federal income and estate taxation, does not discuss the potential application of the Medicare contribution tax, the alternative minimum tax and does not deal with state or local taxes, U.S.
federal gift and estate tax laws, except as specifically provided below with respect to Non-U.S. Holders, or any non-U.S. tax consequences that may be relevant to a Non-U.S. Holder in light of that
Holder's particular circumstances. This discussion also does not consider any specific facts or circumstances that may apply to a Non-U.S. Holder and does not address the special tax rules applicable
to particular holders, such as:
-
•
-
financial institutions;
-
•
-
brokers or dealers in securities;
-
•
-
tax-exempt organizations;
-
•
-
pension plans;
-
•
-
regulated investment companies;
-
•
-
owners that hold our common stock as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment;
-
•
-
insurance companies;
S-16
Table of Contents
-
•
-
controlled foreign corporations, passive foreign investment companies, or corporations that accumulate earnings to avoid U.S. federal income
tax; and
-
•
-
certain U.S. expatriates.
In
addition, this discussion does not address the tax treatment of partnerships or other pass-through entities or persons who hold our common stock through partnerships or other entities
that are pass-through entities for U.S. federal income tax purposes. A partner in a partnership or other pass-through entity that will hold our common stock should consult his, her or its own tax
advisor regarding the tax consequences of the acquisition, ownership and disposition of our common stock through a partnership or other pass-through entity, as applicable.
The discussion of U.S. federal tax considerations is for information purposes only and is not tax advice. Prospective investors should consult their own tax
advisors regarding the U.S. federal, state, local and non-U.S. income and other tax considerations of acquiring, holding and disposing of our common stock.
Distributions
As discussed above, we currently anticipate that we will retain future earnings for the development, operation, and expansion of our business
and do not anticipate declaring or paying any cash dividends for the foreseeable future. In the event that we do make distributions on our common stock, those distributions generally will constitute
dividends for U.S. federal income tax purposes to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Distributions in
excess of our current and accumulated earnings and profits will constitute a tax-free return of the Non-U.S. Holder's investment, up to such Holder's tax basis in our common stock. Any remaining
excess will be treated as capital gain realized on the sale or exchange of our common stock as described below under the section titled "—Gain on Disposition of Our Common Stock"
Dividends
paid to a Non-U.S. Holder generally be subject to withholding of U.S. federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty
between the United States and the non-U.S. holder's country of residence. To obtain a reduced rate of withholding under a treaty, a non-U.S. holder generally will be required to provide the applicable
withholding agent with a properly executed IRS Form W-8BEN or IRS Form W-8BEN-E (or successor form) and satisfy applicable certification and other requirements. A Non-U.S. Holder that is
eligible for a reduced rate of U.S. withholding tax under an income tax treaty may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim with the IRS. Non-U.S.
Holders are urged to consult their own tax advisors regarding their entitlement to benefits under a relevant income tax treaty.
Dividends
that are treated as effectively connected with the Holder's conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are
attributable to a permanent establishment or fixed base that the holder maintains in the United States) are generally exempt from the 30% withholding tax if the Non-U.S. Holder satisfies applicable
certification and disclosure requirements. However, such U.S. effectively connected income is taxed on a net income basis at the same U.S. federal income tax rates applicable to United States persons
(as defined in the Code). Any U.S. effectively connected income received by a Non-U.S. Holder that is a corporation may also, under certain circumstances be subject to an additional "branch profits
tax," at a rate of 30% (or such lower rate as may be specified by an applicable treaty).
See
also the sections below titled "—Information Reporting and Backup Withholding" and "—Foreign Accounts" for additional withholding rules that may apply to
dividends paid to certain foreign financial institutions or non-financial foreign entities.
S-17
Table of Contents
Gain on Disposition of Our Common Stock
A Non-U.S. Holder generally will not be subject to U.S. federal income tax with respect to gain realized on a sale or other disposition of our
common stock unless:
-
•
-
the gain is effectively connected with the Non-U.S. Holder's conduct of a trade or business in the United States, and if an applicable income
tax treaty so provides, the gain is attributable to a permanent establishment or fixed base maintained by the Non-U.S. Holder in the United States; in these cases, the Non-U.S. Holder will be taxed on
a net income basis at the regular graduated rates and in the manner applicable to U.S. persons (as defined in the Code), and if the Non-U.S. Holder is a corporation, an additional branch profits tax
at a rate of 30%, or a lower rate as may be specified by an applicable income tax treaty, may also apply;
-
•
-
the Non-U.S. Holder is a nonresident alien present in the United States for 183 days or more in the taxable year of the disposition and
certain other requirements are met, in which case the Non-U.S. Holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty between the United States
and such holder's country of residence) on the net gain derived from the disposition, which may be offset by certain U.S.-source capital losses of the Non-U.S. Holder, if any; or
-
•
-
we are, or have been at any time during the five-year period preceding such disposition (or the Non-U.S. Holder's holding period of the common
stock , if shorter), a "U.S. real property holding corporation," unless our common stock is regularly traded on an established securities market and the Non-U.S. Holder held no more than 5% of our
outstanding common stock, directly or indirectly, during the shorter of the five-year period ending on the date of the disposition or the period that the Non-U.S. Holder held our common stock. If we
are determined to be a U.S. real property holding corporation and the foregoing exception does not apply, then the Non-U.S. Holder generally will be taxed on its net gain derived from the disposition
at the U.S. federal income tax rates applicable to United States persons (as defined in the Code). Generally, a corporation is a "U.S. real property holding corporation" if the fair market value of
its "U.S. real property interests" (as defined in the Code and applicable regulations) equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other
assets used or held for use in a trade or business. Although there can be no assurance, we believe that we are not currently, and we do not anticipate becoming, a "U.S. real property holding
corporation" for U.S. federal income tax purposes. No assurance can be provided that our common stock will be regularly traded on an established securities market for purposes of the rules described
above.
See
the sections titled "—Information Reporting and Backup Withholding" and "—Foreign Accounts" below for additional information regarding withholding rules that
may apply to proceeds of a disposition of our common stock paid to foreign financial institutions or non-financial foreign entities.
Federal Estate Tax
Common stock owned or treated as owned by an individual who is a non-U.S. holder (as specially defined for U.S. federal estate tax purposes) at
the time of death will be included in the individual's gross estate for U.S. federal estate tax purposes and, therefore, may be subject to U.S. federal estate tax, unless an applicable estate tax or
other treaty provides otherwise.
Information Reporting and Backup Withholding
We must report annually to the IRS and to each non-U.S. holder the gross amount of the distributions on our common stock paid to such holder and
the tax withheld, if any, with respect to such distributions. Non-U.S. holders may have to comply with specific certification procedures to
S-18
Table of Contents
establish
that the holder is not a U.S. person (as defined in the Code) in order to avoid backup withholding at the applicable rate, currently 24%, with respect to dividends on our common stock .
Generally, a Non-U.S. Holder will comply with such procedures if it provides a properly executed IRS Form W-8BEN or W-8BEN-E (or other applicable Form W-8) or otherwise meets documentary
evidence requirements for establishing that it is a Non-U.S. Holder, or otherwise establishes an exemption. Dividends paid to Non-U.S. Holders subject to withholding of U.S. federal income tax, as
described above under the heading "—Distributions," will generally be exempt from U.S. backup withholding.
Information
reporting and backup withholding generally will apply to the proceeds of a disposition of our common stock by a Non-U.S. Holder effected by or through the U.S. office of any
broker, U.S. or foreign, unless the holder certifies its status as a Non-U.S. Holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting
and backup withholding will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the United States through a non-U.S. office of a broker.
However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to
dispositions effected through a U.S. office of a broker. Non-U.S. Holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to
them.
Copies
of information returns may be made available to the tax authorities of the country in which the Non-U.S. Holder resides or is incorporated under the provisions of a specific
treaty or agreement.
Backup
withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a Non-U.S. Holder can be refunded or credited against the Non-U.S.
Holder's U.S. federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS.
Foreign Accounts
Provisions of the Code commonly referred to as the Foreign Account Tax Compliance Act, or FATCA, generally impose a 30% withholding tax on
dividends on, and gross proceeds from the sale or other disposition of, common stock if paid to a non-U.S. entity unless (i) if the non-U.S. entity is a "foreign financial institution," the
non-U.S. entity undertakes certain due diligence, reporting, withholding, and certification obligations, (ii) if the non-U.S. entity is not a "foreign financial institution," the non-U.S.
entity identifies certain of its U.S. investors, if any, or (iii) the non-U.S. entity is otherwise exempt under FATCA.
Withholding
under FATCA generally applies to payments of dividends on our common stock. While withholding under FATCA may apply to payments of gross proceeds from a sale or other
disposition of our common stock, under recently proposed U.S. Treasury regulations, withholding on payments of gross proceeds is not required. Although such regulations are not final, applicable
withholding agents may rely on the proposed regulations until final regulations are issued. If withholding under FATCA is required on any payment related to our common stock, investors not otherwise
subject to withholding (or that otherwise would be entitled to a reduced rate of withholding) on such payment may be required to seek a refund or credit from the IRS. An intergovernmental agreement
between the United States and an applicable foreign country may modify the requirements described in this section. Non-U.S. holders should consult their own tax advisors regarding the possible
implications of FATCA on their investment in our common stock and the entities through which they hold our common stock.
The preceding discussion of material U.S. federal tax considerations is for information only. It is not tax advice. Prospective investors should consult their own
tax advisors regarding the particular U.S. federal, state, local and non-U.S. tax consequences of purchasing, holding and disposing of our common stock, including the consequences of any proposed
changes in applicable laws.
S-19
Table of Contents
PLAN OF DISTRIBUTION
We have engaged H.C. Wainwright & Co., LLC, which we refer to in this prospectus supplement as H.C. Wainwright or the
placement agent, to act as our exclusive placement agent to
solicit offers to purchase the securities offered by this prospectus. H.C. Wainwright is not purchasing or selling any securities, nor are they required to arrange for the purchase and sale of any
specific number or dollar amount of securities, other than to use their reasonable best efforts to arrange for the sale of the securities by us. Therefore, we may not sell the entire amount of the
securities being offered. There is no minimum amount of proceeds that is a condition to closing of this offering. We will enter into subscription agreements directly with institutional investors that
purchase our securities in this offering. H.C. Wainwright may engage one or more sub-placement agents or selected dealers to assist with the offering.
Fees and Expenses
The following table show the per share and total placement agent fees we will pay in connection with the sale of the securities in this
offering, assuming the purchase of all of the securities we are offering.
|
|
|
|
|
|
|
Per share placement agent cash fees
|
|
$
|
|
|
|
Total placement agent cash fees
|
|
$
|
|
|
We
have agreed to pay the placement agent a total cash fee equal to 7.5% of the gross proceeds of this offering and a management fee equal to 1% of the gross proceeds raised in this
offering. We will also pay the placement agent a non-accountable expense allowance of $35,000 and will reimburse the placement agent's legal fees, service fees and clearing expenses in an aggregate
amount of up to $115,000. We estimate the total offering expenses of this offering that will be payable by us, excluding the placement agent fees and expenses, will be approximately
$ .
After deducting the placement agent fees and our estimated offering expenses, we expect the net proceeds from this offering to be approximately $ .
Placement Agent Warrants
We have agreed to grant compensation warrants to H.C. Wainwright (the "Placement Agent Warrant") to purchase a number of shares of our common
stock equal to 6.5% of the aggregate number of shares of common stock sold to the investors in this offering. The Placement Agent Warrants will have an exercise price of $ and
will
terminate on the five year anniversary of the effective date of the offering. The Placement Agent Warrants are registered on the registration statement of which this prospectus is a part. Pursuant to
FINRA Rule 5110(g), the Placement Agent Warrants and any shares issued upon exercise of the Placement Agent Warrants shall not be sold, transferred, assigned, pledged, or hypothecated, or be
the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days
immediately following the date of effectiveness or commencement of sales of this offering, except the transfer of any security:
-
•
-
by operation of law or by reason of our reorganization;
-
•
-
to any FINRA member firm participating in the offering and the officers or partners thereof, if all securities so transferred remain subject to
the lock-up restriction set forth above for the remainder of the time period;
-
•
-
if the aggregate amount of our securities held by the placement agent or related persons do not exceed 1% of the securities being offered; or
S-20
Table of Contents
-
•
-
that is beneficially owned on a pro rata basis by all equity owners of an investment fund, provided that no participating member manages or
otherwise directs investments by the fund and the participating members in the aggregate do not own more than 10% of the equity in the fund;
and
the exercise or conversion of any security, if all securities remain subject to the lock-up restriction set forth above for the remainder of the time period.
Right of First Refusal
In addition, we have granted a right of first refusal to the placement agent pursuant to which it has the right to act as the exclusive advisor,
manager or underwriter or agent, as applicable, if we or our subsidiaries sell or acquire a business, finance any indebtedness using an agent, or raise capital through a public or private offering of
equity or debt securities at any time prior to the 12 month anniversary of the date of this prospectus.
Other Relationships
The placement agent may, from time to time, engage in transactions with or perform services for us in the ordinary course of its business and
may continue to receive compensation from us for such services.
Determination of Offering Price
The public offering price of the securities we are offering was negotiated between us and the investors, in consultation with the placement
agent based on the trading of our common stock prior to the offering, among other things. Other factors considered in determining the public offering price of the securities we are offering include
the history and prospects of our company, the stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment of our
management, general conditions of the securities markets at the time of the offering and such other factors as were deemed relevant.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company.
Nasdaq Listing
Our common stock is listed on the Nasdaq Capital Market under the symbol "NVIV." On November 15, 2019, the reported closing price per
share of our common stock on the Nasdaq Capital Market was $0.43.
Indemnification
We have agreed to indemnify the placement agent against certain liabilities, including liabilities under the Securities Act, or to contribute to
payments the placement agent may be required to make with respect to any of these liabilities.
Regulation M
The placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act and any fees received
by it and any profit realized on the sale of the securities by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. The placement agent
will be required to comply with the requirements of the Securities
S-21
Table of Contents
Act
and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of
our securities by the placement agent. Under these rules and regulations, the placement agent may not (i) engage in any stabilization activity in connection with our securities and
(ii) bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until they have completed their
participation in the distribution.
S-22
Table of Contents
LEGAL MATTERS
The validity of the securities offered by this prospectus supplement and the accompanying prospectus will be passed upon for us by Ballard
Spahr LLP, Las Vegas, Nevada, and certain other legal matters will be passed upon for us by Wilmer Cutler Pickering Hale and Dorr LLP, Boston, Massachusetts. Ellenoff Grossman &
Schole LLP, New York, New York, has acted as counsel for the underwriter in connection with certain legal matters related to this offering.
EXPERTS
The consolidated financial statements of InVivo Therapeutics Holdings Corp. and its subsidiaries as of December 31, 2018 and 2017 and for
each of the years in the two-year period ended December 31, 2018 incorporated in this prospectus supplement and the accompanying prospectus by reference from the InVivo Therapeutics Holdings
Corp.'s Annual Report on Form 10-K for the year ended December 31,
2018, have been audited by RSM US LLP, an independent registered public accounting firm, as stated in their report thereon (which report expresses an unqualified opinion and
includes an explanatory paragraph relating to InVivo Therapeutics Holdings Corp.'s ability to continue as a going concern), incorporated herein and therein by reference, and have been incorporated in
this prospectus supplement and the accompanying prospectus in reliance upon such report and upon the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to you on the
SEC's Internet site at www.sec.gov. Copies of certain information filed by us with the SEC are also available on our website at www.invivotherapeutics.com.
The information on our Internet website is not incorporated by reference in this prospectus or any prospectus supplement.
This
prospectus supplement is part of a registration statement that we filed with the SEC. This prospectus supplement does not contain all of the information included in the registration
statement, including certain exhibits and schedules. You should review the information and exhibits in the registration statement for further information about us and the securities we are offering.
Statements in this prospectus concerning any document we filed as an exhibit to the registration statement or that we otherwise filed with the SEC are not intended to be comprehensive and are
qualified by reference to these filings. You should review the complete document to evaluate these statements. You can obtain a copy of the registration statement and exhibits from the SEC's Internet
site.
INCORPORATION OF DOCUMENTS BY REFERENCE
The SEC allows us to incorporate by reference into this prospectus supplement and the accompanying prospectus information and reports that we
file with the SEC. This means that we can disclose important information to you by referring to other documents that contain that
information. Any information that we incorporate by reference is considered part of this prospectus supplement and the accompanying prospectus. The documents and reports that we list below are
incorporated by reference into this prospectus supplement and the accompanying prospectus, other than any portion of any such documents that are not deemed "filed" under the Exchange Act in accordance
with the Exchange Act and applicable SEC rules.
In
addition, all documents and reports which we file pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus supplement and prior to
the termination of the offering made hereby are incorporated by reference in this prospectus supplement and the accompanying prospectus as of the respective filing dates of these documents and
reports.
S-23
Table of Contents
We
have filed the following documents with the SEC. These documents are incorporated in this prospectus supplement and the accompanying prospectus by reference as of their respective
dates of filing:
-
(1)
-
Our
Annual Report on Form 10-K for the fiscal
year ended December 31, 2018, filed on April 1, 2019, including the information specifically incorporated by reference into the Annual Report on Form 10-K from our
definitive proxy statement for the 2019 Annual Meeting of Stockholders, filed on April 25,
2019;
-
(2)
-
Our
Quarterly Reports on Form 10-Q for the quarter ended March 31, 2019, filed on
May 10, 2019, for the quarter ended June 30, 2019, filed on
August 13, 2019, and for the quarter ended September 30, 2019,
filed on November 7, 2019;
-
(3)
-
Our
Current Reports on Form 8-K filed on January 4,
2019 (Item 8.01 only), January 14, 2019,
June 14, 2019,
July 5, 2019,
July 12, 2019,
July 19, 2019
September 27, 2019, and
November 12, 2019; and
-
(4)
-
The description of our common stock contained in our
Registration Statement on Form 8-A filed on April 15, 2015, including any amendments or reports filed for the purpose of updating such description.
You
may request a copy of these documents, which will be provided to you at no cost, by writing or telephoning us at:
InVivo
Therapeutics Holdings Corp.
One Kendall Square, Suite B14402
Cambridge, Massachusetts 02139
Attn: Investor Relations
(617) 863-5500
Statements
contained in documents that we file with the SEC and that are incorporated by reference in this prospectus supplement or the accompanying prospectus will automatically update
and supersede information contained in this prospectus supplement and the accompanying prospectus, including information in previously filed documents or reports that have been incorporated by
reference in this prospectus supplement or the accompanying prospectus, to the extent the new information differs from or is inconsistent with the old information. Any statement so modified or
superseded will not be deemed to be a part of this supplement or the accompanying prospectus, except as so modified or superseded. Because information that we later file with the SEC will update and
supersede previously incorporated information, you should look at all of the SEC filings that we incorporate by reference to
determine if any of the statements in this prospectus supplement or the accompanying prospectus or in any documents previously incorporated by reference have been modified or superseded.
S-24
Table of Contents
PROSPECTUS

INVIVO THERAPEUTICS HOLDINGS CORP.
$20,000,000
Common Stock
Warrants
Units
This prospectus relates to common stock, warrants and units that we may sell from time to time in one or more offerings up to a total dollar
amount of $20,000,000 on terms to be determined at the time of sale. We will provide specific terms of these securities in supplements to this prospectus. You should read this prospectus and any
supplement carefully before you invest. This prospectus may not be used to offer and sell securities unless accompanied by a prospectus supplement for those securities.
Our
common stock is listed on The Nasdaq Capital Market under the symbol "NVIV." On October 25, 2019, the last sales price of our common stock as reported on The Nasdaq Capital
Market was $0.44 per share.
These
securities may be sold directly by us, through dealers or agents designated from time to time, to or through underwriters or through a combination of these methods. See "Plan of
Distribution" in this prospectus. We may also describe the plan of distribution for any particular offering of these securities in any applicable prospectus supplement. If any agents, underwriters or
dealers are involved in the sale of any securities in respect of which this prospectus is being delivered, we will disclose their names and the nature of our arrangements with them in a prospectus
supplement. The net proceeds we expect to receive from any such sale will also be included in a prospectus supplement.
As
of September 15, 2019, the aggregate market value of our outstanding common stock held by non-affiliates was approximately $5,766,788, which was calculated based on 9,301,271
shares of outstanding common stock held by non-affiliates and a price per share of $0.62. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell, pursuant to the
registration statement of which this prospectus forms a part, securities in a public primary offering with a value exceeding one-third of the aggregate market value of our common stock held by
non-affiliates in any 12-month period, so long as the aggregate market value of our outstanding common stock held by non-affiliates remains below $75 million. During the 12 calendar months
prior to and including the date of this prospectus, we have not offered or sold any securities pursuant to General Instruction I.B.6 of Form S-3.
Investing in these securities involves certain risks. See "Risk Factors" included in any accompanying prospectus supplement and in the documents
incorporated by reference in
this prospectus for a discussion of the factors you should carefully consider before deciding to purchase these securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these
securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is November 14, 2019.
Table of Contents
TABLE OF CONTENTS
i
Table of Contents
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission, or the SEC, using a "shelf"
registration process. Under this shelf registration process, we may sell any combination of the securities described in this prospectus in one or more offerings up to a total dollar amount of
$20,000,000.
This
prospectus provides you with a general description of the securities we may offer. Each time we sell securities, we will provide a prospectus supplement that will contain specific
information about the securities being offered and the terms of that offering. The prospectus supplement may also add to, update or change information contained in this prospectus. You should read
both this prospectus and any prospectus supplement together with the additional information described under the heading "Where You Can Find More Information" on page 3 of this prospectus
carefully before making an investment decision.
You
should rely only on the information contained or incorporated by reference in this prospectus or any applicable prospectus supplement. We have not authorized any other person to
provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not making an offer to sell these securities in any
jurisdiction where the offer or sale is not permitted.
You
should not assume that the information appearing in this prospectus or any applicable prospectus supplement is accurate as of any date other than the date on the front cover of this
prospectus or the applicable prospectus supplement, or that the information contained in any document incorporated by reference is accurate as of any date other than the date of the document
incorporated by reference, regardless of the time of delivery of this prospectus or any prospectus supplement or any sale of a security. Our business, financial condition, results of operations and
prospects may have changed since such dates.
Unless
the context otherwise requires, the terms "InVivo," "the Company," "our company," "we," "us," "our" and similar names refer collectively to InVivo Therapeutics Holdings Corp. and
its subsidiaries.
1
Table of Contents
ABOUT INVIVO THERAPEUTICS HOLDINGS CORP.
We
are a research and clinical-stage biomaterials and biotechnology company with a focus on treatment of spinal cord injuries, or SCIs. Our mission is to redefine the life of the SCI
patient, and we seek to develop treatment options intended to provide meaningful improvement in patient outcomes following SCI. Our approach to treating acute SCIs is based on our investigational
Neuro-Spinal Scaffold™ implant, a bioresorbable polymer scaffold that is designed for implantation at the site of injury within a spinal cord and is intended to treat acute SCI. The
Neuro-Spinal Scaffold implant incorporates intellectual property licensed under an exclusive, worldwide license from Boston Children's Hospital, or BCH, and the Massachusetts Institute of Technology,
or MIT. We also plan to evaluate other technologies and therapeutics that may be complementary to our development of the Neuro-Spinal Scaffold implant or offer the potential to bring us closer to our
goal of redefining the life of the SCI patient.
InVivo
Therapeutics Corporation was incorporated on November 28, 2005 under the laws of the State of Delaware and on October 26, 2010 completed a reverse merger transaction
with and became a wholly-owned subsidiary of InVivo Therapeutics Holdings Corporation, a company incorporated under the laws of the State of Nevada.
Our
principal executive offices are located at One Kendall Square, Suite B14402, Cambridge, Massachusetts 02139, and our telephone number is (617) 863-5500. Our worldwide web
address is www.invivotherapeutics.com. The information on our web site is not incorporated by reference into this prospectus and should not be considered to be part of this prospectus.
2
Table of Contents
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to you on the
SEC's Internet site at www.sec.gov. Copies of certain information filed by us with the SEC are also available on our website at www.invivotherapeutics.com.
The information on our Internet website is not incorporated by reference in this prospectus or any prospectus supplement.
This
prospectus is part of a registration statement that we filed with the SEC. This prospectus does not contain all of the information included in the registration statement, including
certain exhibits and schedules. You should review the information and exhibits in the registration statement for further information about us and the securities we are offering. Statements in this
prospectus concerning any document we filed as an exhibit to the registration statement or that we otherwise filed with the SEC are not intended to be comprehensive and are qualified by reference to
these filings. You should review the complete document to evaluate these statements. You can obtain a copy of the registration statement and exhibits from the SEC's Internet site.
3
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING INFORMATION
This
prospectus and each prospectus supplement contains and incorporates "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended
(the "Securities Act"), and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). These statements include statements made regarding our commercialization
strategy, future operations, cash requirements and liquidity, capital requirements, and other statements on our business plans and strategy, financial position, and market trends. In some cases, you
can identify forward-looking statements by terms such as "may," "might," "will," "should," "believe," "plan," "intend," "anticipate," "target," "estimate," "expect," and other similar expressions.
These forward-looking statements are subject to risks and uncertainties that could cause actual results or events to differ materially from those expressed or implied by the forward-looking
statements, including factors such as our ability to raise substantial additional capital to finance our planned operations and to continue as a going concern; our ability to execute our strategy and
business plan; our ability to obtain regulatory approvals for our products, including the Neuro-Spinal Scaffold™; our ability to
successfully commercialize our current and future product candidates, including the Neuro-Spinal Scaffold; the progress and timing of our development
programs; market acceptance of our products; our ability to retain management and other key personnel; our ability to promote, manufacture, and sell our products, either directly or through
collaborative and other arrangements with third parties; and other factors detailed under "Risk Factors" in our most recent Annual Report on Form 10-K and our most recent Quarterly Report on
Form 10-Q. These forward looking statements are only predictions, are uncertain, and involve substantial known and unknown risks, uncertainties, and other factors which may cause our actual
results, levels of activity, or performance to be materially different from any future results, levels of activity, or performance expressed or implied by these forward looking statements. Such
factors include, among others, the following:
-
•
-
our limited operating history and history of net losses;
-
•
-
our ability to raise substantial additional capital to finance our planned operations and to continue as a going concern;
-
•
-
our ability to complete the INSPIRE 2.0 Study to support our existing Humanitarian Device Exemption application;
-
•
-
our ability to execute our strategy and business plan;
-
•
-
our ability to obtain regulatory approvals for our current and future product candidates, including our Neuro-Spinal
Scaffold implant;
-
•
-
our ability to successfully commercialize our current and future product candidates, including our Neuro-Spinal
Scaffold implant;
-
•
-
the progress and timing of our current and future development programs;
-
•
-
our ability to successfully open, enroll and complete clinical trials and obtain and maintain regulatory approval of our current and future
product candidates;
-
•
-
our ability to protect and maintain our intellectual property and licensing arrangements;
-
•
-
our reliance on third parties to conduct testing and clinical trials;
-
•
-
market acceptance and adoption of our current and future technology and products;
-
•
-
our ability to promote, manufacture and sell our current and future products, either directly or through collaborative and other arrangements
with third parties; and
-
•
-
our ability to attract and retain key personnel.
4
Table of Contents
We
cannot guarantee future results, levels of activity, or performance. You should not place undue reliance on these forward looking statements, which speak only as of the date of this
prospectus. You are cautioned that these forward-looking statements are only predictions and are subject to risks, uncertainties and assumptions that are referenced in the section of any accompanying
prospectus supplement entitled "Risk Factors." You should also carefully review the risk factors and cautionary statements described in the other documents we file from time to time with the SEC,
specifically our most recent Annual Report on Form 10-K, our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K. Except as required by applicable law, including
the securities laws of the United States, we do not intend to update any of the forward looking statements to conform these statements to reflect actual results, later events or circumstances, or to
reflect the occurrence of unanticipated events.
5
Table of Contents
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to "incorporate" into this prospectus information and reports that we file with the SEC. This means that we can disclose
important information to you by referring to other documents that contain that information. Any information that we incorporate by reference is considered part of this prospectus. The documents and
reports that we list below are incorporated by reference into this prospectus, other than any portion of any such documents that are not deemed "filed" under the Exchange Act in accordance with the
Exchange Act and applicable SEC rules.
In
addition, all documents and reports which we file pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus and prior to the
termination of the offering made hereby are incorporated by reference in this prospectus as of the respective filing dates of these documents and reports.
We
have filed the following documents with the SEC. These documents are incorporated herein by reference as of their respective dates of filing:
-
(1)
-
Our
Annual Report on Form 10-K for the fiscal
year ended December 31, 2018, filed on April 1, 2019, including the information specifically incorporated by reference into the Annual Report on Form 10-K from our
definitive proxy statement for the 2019 Annual Meeting of Stockholders, filed on April 25,
2019;
-
(2)
-
Our
Quarterly Reports on Form 10-Q for the quarter ended March 31, 2019, filed on
May 10, 2019 and for the quarter ended June 30, 2019, filed on
August 13, 2019;
-
(3)
-
Our
Current Reports on Form 8-K filed on January 4,
2019 (Item 8.01 only), January 14, 2019,
June 14, 2019,
July 5, 2019,
July 12, 2019,
July 19, 2019 and
September 27, 2019;
-
(4)
-
All
of our filings pursuant to the Exchange Act after the date of filing the initial registration statement and prior to the effectiveness of the registration
statement; and
-
(5)
-
The description of our common stock contained in our
Registration Statement on Form 8-A filed on April 15, 2015, including any amendments or reports filed for the purpose of updating such description.
You
may request a copy of these documents, which will be provided to you at no cost, by writing or telephoning us at:
InVivo
Therapeutics Holdings Corp.
One Kendall Square, Suite B14402
Cambridge, Massachusetts 02139
Attn: Investor Relations
(617) 863-5500
Statements
contained in documents that we file with the SEC and that are incorporated by reference in this prospectus will automatically update and supersede information contained in
this prospectus, including information in previously filed documents or reports that have been incorporated by reference in this prospectus, to the extent the new information differs from or is
inconsistent with the old information. Any statement so modified or superseded will not be deemed to be a part of this prospectus or any prospectus supplement, except as so modified or superseded.
Because information that we later file with the SEC will update and supersede previously incorporated information, you should look at all of the SEC filings that we incorporate by reference to
determine if any of the statements in this prospectus or any prospectus supplement or in any documents previously incorporated by reference have been modified or superseded.
6
Table of Contents
USE OF PROCEEDS
We
currently intend to use the estimated net proceeds from the sale of these securities to conduct our INSPIRE 2.0 clinical trial and for other business development activities, as well
as for working capital and for general corporate purposes, which may include the following:
-
•
-
the research, development and pre-clinical and clinical trials for our product candidates;
-
•
-
the acquisition of other companies, businesses, products or technologies;
-
•
-
the repayment and refinancing of debt;
-
•
-
capital expenditures;
-
•
-
working capital; and
-
•
-
any other purpose that we may specify in any prospectus supplement.
We
have not yet determined the amount of net proceeds to be used specifically for any of the foregoing purposes. Accordingly, our management will have significant discretion and
flexibility in applying the net proceeds from the sale of these securities. Pending any use, as described above, we intend to invest the net proceeds in high-quality, short-term, interest-bearing
securities. Our plans to use the estimated net proceeds from the sale of these securities may change, and if they do, we will update this information in a prospectus supplement.
7
Table of Contents
THE SECURITIES WE MAY OFFER
The descriptions of the securities contained in this prospectus, together with the applicable prospectus supplements, summarize the material
terms and provisions of the various types of securities that we may offer. We will describe in the applicable prospectus supplement relating to any securities the particular terms of the securities
offered by that prospectus supplement. If we so indicate in the applicable prospectus supplement, the terms of the securities may differ from the terms we have summarized below. We will also include
in the prospectus supplement information, where applicable, about material United States federal income tax considerations relating to the securities, and the securities exchange, if any, on which the
securities will be listed.
We
may sell from time to time, in one or more offerings:
-
•
-
common stock;
-
•
-
warrants to purchase common stock or units;
-
•
-
units comprised of common stock and warrants; or
-
•
-
any combination of the foregoing securities.
In
this prospectus, we refer to the common stock, warrants and units collectively as "securities." The total dollar amount of all securities that we may issue will not exceed
$20,000,000.
This
prospectus may not be used to consummate a sale of securities unless it is accompanied by a prospectus supplement.
8
Table of Contents
DESCRIPTION OF COMMON STOCK
The
following is a description of the material terms and provisions of our common stock. It may not contain all the information that is important to you. You can access complete
information by referring to our articles of incorporation and bylaws, which are filed as exhibits to the registration statement of which this prospectus forms a part.
Under
our articles of incorporation, as amended, we have authority to issue 25,000,000 shares of common stock, par value $0.00001 per share. As of September 15, 2019, there were
9,313,070 shares of common stock issued and outstanding. All shares of common stock will, when issued, be duly authorized, fully paid and nonassessable. Accordingly, the full price for the outstanding
shares of common stock will have been paid at issuance and any holder of our common stock will not be later required to pay us any additional money for such common stock.
In
addition, as of September 15, 2019:
-
•
-
there were outstanding warrants to purchase an aggregate of up to 7,673,130 shares of our common stock at a weighted average exercise price of
$4.78 per share;
-
•
-
there were an aggregate of 124,890 shares of our common stock subject to outstanding stock options at a weighted average exercise price of
$35.15 per share;
-
•
-
7,500 shares of our common stock were issuable upon vesting of outstanding restricted stock units;
-
•
-
4,275 shares of our common stock were reserved for future issuances under our incentive compensation plans and 401(k) plan; and
-
•
-
7,923 shares of our common stock reserved for future issuance under our employee stock purchase plan.
The
holders of common stock are entitled to one vote per share on all matters submitted to a vote of the stockholders, including the election of directors. Generally, all matters to be
voted on by stockholders must be approved by a majority (or, in the case of election of directors, by a plurality) of the votes entitled to be cast by all shares of common stock that are present in
person or represented by proxy. Except as otherwise provided by law, amendments to the articles of incorporation generally must be approved by a majority of the votes entitled to be cast by all
outstanding shares of common stock. Our articles of incorporation do not provide for cumulative voting in the election of directors. The holders of common stock will be entitled to such cash dividends
as may be declared from time to time by the Board from funds available. The holders of common stock have no preferential or preemptive right and no subscription, redemption or conversion privileges
with respect to the issuance of additional shares of our common stock. Upon liquidation, dissolution or winding up of the Company, the holders of common stock will be entitled to receive pro rata all
assets available for distribution to such holders after payment of our liabilities.
Registrar and Transfer Agent
The registrar and transfer agent for our common stock is Continental Stock Transfer & Trust Company.
Trading Market
Our common stock is listed on The Nasdaq Capital Market under the symbol "NVIV."
9
Table of Contents
DESCRIPTION OF WARRANTS
We may issue warrants for the purchase of common stock or units. Warrants may be issued independently or together with common stock or units,
and the warrants may be attached to or separate from such securities. We may issue warrants directly or under a warrant agreement to be entered into between us and a warrant agent. We will name any
warrant agent in the applicable prospectus supplement. Any warrant agent will act solely as our agent in connection with the warrants of a particular series and will not assume any obligation or
relationship of agency or trust for or with any holders or beneficial owners of warrants.
The
following is a description of the general terms and provisions of any warrants we may issue and may not contain all the information that is important to you. You can access complete
information by referring to the applicable prospectus supplement. In the applicable prospectus supplement, we will
describe the terms of the warrants and any applicable warrant agreement, including, where applicable, the following:
-
•
-
the title of the warrants;
-
•
-
the offering price and aggregate number of warrants offered;
-
•
-
the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security;
-
•
-
the date on and after which the warrants and the related securities will be separately transferable;
-
•
-
any information with respect to book-entry procedures;
-
•
-
in the case of warrants to purchase common stock or units, the number of shares of common stock or units, as the case may be, purchasable upon
the exercise of one warrant and the price at which these securities may be purchased upon such exercise;
-
•
-
the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreement and the warrants;
-
•
-
the terms of any rights to redeem or call the warrants;
-
•
-
any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants;
-
•
-
the dates on which the right to exercise the warrants will commence and expire;
-
•
-
the manner in which the warrant agreement and warrants may be modified;
-
•
-
a discussion of any material U.S. federal income tax considerations of holding or exercising the warrants;
-
•
-
the terms of the securities issuable upon exercise of the warrants; and
-
•
-
any other specific terms, preferences, rights or limitations of or restrictions on the warrants.
10
Table of Contents
DESCRIPTION OF UNITS
The following description, together with the additional information we include in any applicable prospectus supplement, summarizes the material
terms and provisions of the units that we may offer under this prospectus. Units may be offered independently or together with common stock and warrants offered by any prospectus supplement, and may
be attached to or separate from those securities.
While
the terms we have summarized below will generally apply to any future units that we may offer under this prospectus, we will describe the particular terms of any series of units
that we may offer in more detail in the applicable prospectus supplement. The terms of any units offered under a prospectus supplement may differ from the terms described below.
We
will incorporate by reference into the registration statement of which this prospectus is a part the form of unit agreement, including a form of unit certificate, if any, that
describes the terms of the series of units we are offering before the issuance of the related series of units. The following summaries of material provisions of the units and the unit agreements are
subject to, and qualified in their entirety by reference to, all the provisions of the unit agreement applicable to a particular series of units. We urge you to read the applicable prospectus
supplements related to the units that we sell under this prospectus, as well as the complete unit agreements that contain the terms of the units.
General
We may issue units consisting of common stock and warrants. Each unit will be issued so that the holder of the unit is also the holder of each
security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that
the securities included in the unit may not be held or transferred separately, at any time, or at any time before a specified date.
We
will describe in the applicable prospectus supplement the terms of the series of units, including the following:
-
•
-
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those
securities may be held or transferred separately;
-
•
-
any provisions of the governing unit agreement that differ from those described below; and
-
•
-
any provisions for the issuance, payment, settlement, transfer, or exchange of the units or of the securities comprising the units.
The
provisions described in this section, as well as those described under "Description of Common Stock," and "Description of Warrants," will apply to each unit and to the common stock
and warrants included in each unit, respectively.
Issuance in Series
We may issue units in such amounts and in such numerous distinct series as we determine.
Enforceability of Rights by Holders of Units
Each unit agent will act solely as our agent under the applicable unit agreement and will not assume any obligation or relationship of agency or
trust with any holder of any unit. A single bank or trust company may act as unit agent for more than one series of units. A unit agent will have no duty or responsibility in case of any default by us
under the applicable unit agreement or unit, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us.
11
Table of Contents
Any
holder of a unit, without the consent of the related unit agent or the holder of any other unit, may enforce by appropriate legal action its rights as holder under any security included in the
unit.
Title
We, the unit agent, and any of their agents may treat the registered holder of any unit certificate as an absolute owner of the units evidenced
by that certificate for any purposes and as
the person entitled to exercise the rights attaching to the units so requested, despite any notice to the contrary.
12
Table of Contents
CERTAIN ANTI-TAKEOVER AND INDEMNIFICATION PROVISIONS OF
OUR ARTICLES OF INCORPORATION, BY-LAWS AND NEVADA LAW
Anti-Takeover Effects of Provisions of Nevada State Law
We may be or in the future we may become subject to Nevada's control share laws. A corporation is subject to Nevada's control share law if it
has more than 200 stockholders, at least 100 of whom are stockholders of record and residents of Nevada, and if the corporation does business in Nevada, including through an affiliated corporation.
This control share law may have the effect of discouraging corporate takeovers. We currently have less than 100 stockholders of record who are residents of Nevada.
The
control share law focuses on the acquisition of a "controlling interest," which means the ownership of outstanding voting shares that would be sufficient, but for the operation of
the control share law, to enable the acquiring person to exercise the following proportions of the voting power of the corporation in the election of directors: (1) one-fifth or more but less
than one-third; (2) one-third or more but less than a majority; or (3) a majority or more. The ability to exercise this voting power may be direct or indirect, as well as individual or
in association with others.
The
effect of the control share law is that an acquiring person, and those acting in association with that person, will obtain only such voting rights in the control shares as are
conferred by a resolution of the stockholders of the corporation, approved at a special or annual meeting of stockholders. The control share law contemplates that voting rights will be considered only
once by the other stockholders. Thus, there is no authority to take away voting rights from the control shares of an acquiring person once
those rights have been approved. If the stockholders do not grant voting rights to the control shares acquired by an acquiring person, those shares do not become permanent non-voting shares. The
acquiring person is free to sell the shares to others. If the buyer or buyers of those shares themselves do not acquire a controlling interest, the shares are not governed by the control share law.
If
control shares are accorded full voting rights and the acquiring person has acquired control shares with a majority or more of the voting power, a stockholder of record, other than
the acquiring person, who did not vote in favor of approval of voting rights, is entitled to demand fair value for such stockholder's shares.
In
addition to the control share law, Nevada has a business combination law, which prohibits certain business combinations between Nevada corporations and "interested stockholders" for
two years after the interested stockholder first becomes an interested stockholder, unless the corporation's board of directors approves the combination in advance. For purposes of Nevada law, an
interested stockholder is any person who is: (a) the beneficial owner, directly or indirectly, of 10% or more of the voting power of the outstanding voting shares of the corporation, or
(b) an affiliate or associate of the corporation and at any time within the previous two years was the beneficial owner, directly or indirectly, of 10% or more of the voting power of the
then-outstanding shares of the corporation. The definition of "business combination" contained in the statute is sufficiently broad to cover virtually any kind of transaction that would allow a
potential acquirer to use the corporation's assets to finance the acquisition or otherwise to benefit its own interests rather than the interests of the corporation and its other stockholders.
The
effect of Nevada's business combination law is to potentially discourage parties interested in taking control of the Company from doing so if it cannot obtain the approval of our
board of directors.
Anti-Takeover Effects of Provisions of Our Articles of Incorporation and Bylaws
Our articles of incorporation provide for a classified board of directors. This provision could prevent a party who acquires control of a
majority of our outstanding common stock from obtaining
13
Table of Contents
control
of the board until our second annual stockholders meeting following the date the acquirer obtains the controlling stock interest. The classified board provision could have the effect of
discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us and could increase the likelihood that incumbent directors will retain their positions. In
addition, under our amended and restated bylaws, directors may be removed only for cause and only by the affirmative vote of the holders of at least 80% of the voting power of our then outstanding
shares of capital stock entitled to vote generally in the election of directors, voting together as a single class.
Our
amended and restated bylaws also provide that stockholders may only act at meetings of stockholders and not by written consent in lieu of a stockholders' meeting. Our amended and
restated bylaws provide that stockholders may not call a special meeting of stockholders. Rather, only the Chairman of our Board, the President or the Board of Directors pursuant to a resolution
approved by a majority of the entire Board of Directors are able to call special meetings of stockholders. Our amended and restated bylaws also provide that stockholders may only conduct business at
special meetings of stockholders that was specified in the notice of the meeting. These provisions may discourage another person or entity from making a tender offer, even if it acquired a majority of
our outstanding voting stock, because the person or entity could only take action at a duly called stockholders' meeting relating to the business specified in the notice of meeting and not by written
consent.
Indemnification of Directors and Officers
Nevada Revised Statutes ("NRS") Sections 78.7502 and 78.751 provide us with the power to indemnify any of our directors, officers,
employees and agents. The person entitled to indemnification must have conducted himself in good faith, and must reasonably believe that his conduct was in, or not opposed to, our best interests. In a
criminal action, the director, officer, employee or agent must also not have had reasonable cause to believe that his conduct was unlawful. In addition, any of our directors, officers, employees or
agents are entitled to indemnification if such person is successful on the merits or otherwise in defense of any threatened, pending or completed action, suit or proceeding, whether civil, criminal,
administrative or investigative against actual and reasonable expenses incurred in connection with defending such action.
Under
NRS Section 78.751, advances for expenses may be made by agreement if the director or officer affirms in writing to repay the expenses if it is determined that such officer
or director is not entitled to be indemnified.
Our
bylaws include an indemnification provision under which we have the power to indemnify our directors, officers, former directors and officers, employees and other agents (including
heirs and personal representatives) against all costs, charges and expenses actually and reasonably incurred, including an amount paid to settle an action or satisfy a judgment to which a director or
officer is made a party by reason of being or having been a director or officer of the Company. Our bylaws further provide for the advancement of all expenses incurred in connection with a proceeding
upon receipt of an undertaking by or on behalf of such person to repay such amounts unless it is determined
that the party is entitled to be indemnified under our bylaws. No advance will be made by the Company to a party if it is determined that the party acted in bad faith. These indemnification rights are
contractual, and as such will continue as to a person who has ceased to be a director, officer, employee or other agent, and will inure to the benefit of the heirs, executors and administrators of
such a person. Unless our articles are amended to provide for greater liability, neither our directors nor officers are individually liable to us or our stockholders or creditors for any act or
omission as a director or officer unless it is proven that: (i) such act or omission constituted a breach of such director's or officer's fiduciary duties; and (ii) such breach involved
intentional misconduct, fraud or a knowing violation of law. These provisions may be sufficiently broad to indemnify such persons for
14
Table of Contents
liabilities
arising under the Securities Act, in which case such provisions are against public policy as expressed in the Securities Act and are therefore unenforceable.
We
maintain an insurance policy on behalf of our directors and officers, covering certain liabilities which may arise as a result of the actions of the directors and officers.
We
have entered into an indemnification agreement with each of our officers and directors pursuant to which they will be indemnified by us, subject to certain limitations, for any
liabilities incurred by them in connection with their role as officers and/or directors of the Company.
15
Table of Contents
FORMS OF SECURITIES
Each unit and warrant will be represented either by a certificate issued in definitive form to a particular investor or by one or more global
securities representing the entire issuance of securities. Unless the applicable prospectus supplement provides otherwise, certificated securities in definitive form and global securities will be
issued in registered form. Definitive securities name you or your nominee as the owner of the security, and in order to transfer or exchange these
securities or to receive payments other than interest or other interim payments, you or your nominee must physically deliver the securities to the registrar, paying agent or other agent, as
applicable. Global securities name a depositary or its nominee as the owner of the units or warrants represented by these global securities. The depositary maintains a computerized system that will
reflect each investor's beneficial ownership of the securities through an account maintained by the investor with its broker/dealer, bank, trust company or other representative, as we explain more
fully below.
Global Securities
We may issue the units and warrants in the form of one or more fully registered global securities that will be deposited with a depositary or
its nominee identified in the applicable prospectus supplement and registered in the name of that depositary or nominee. In those cases, one or more global securities will be issued in a denomination
or aggregate denominations equal to the portion of the aggregate principal or face amount of the securities to be represented by global securities. Unless and until it is exchanged in whole for
securities in definitive registered form, a global security may not be transferred except as a whole by and among the depositary for the global security, the nominees of the depositary or any
successors of the depositary or those nominees.
If
not described below, any specific terms of the depositary arrangement with respect to any securities to be represented by a global security will be described in the prospectus
supplement relating to those securities. We anticipate that the following provisions will apply to all depositary arrangements.
Ownership
of beneficial interests in a global security will be limited to persons, called participants, that have accounts with the depositary or persons that may hold interests through
participants. Upon the issuance of a global security, the depositary will credit, on its book-entry registration and transfer system, the participants' accounts with the respective principal or face
amounts of the securities beneficially owned by the participants. Any dealers, underwriters or agents participating in the distribution of the securities will designate the accounts to be credited.
Ownership of beneficial interests in a global security will be shown on, and the transfer of ownership interests will be effected only through, records maintained by the depositary, with respect to
interests of participants, and on the records of participants, with respect to interests of persons holding through participants. The laws of some states may require that some purchasers of securities
take physical delivery of these securities in definitive form. These laws may impair your ability to own, transfer or pledge beneficial interests in global securities.
So
long as the depositary, or its nominee, is the registered owner of a global security, that depositary or its nominee, as the case may be, will be considered the sole owner or holder
of the securities represented by the global security for all purposes under the applicable indenture, deposit agreement, purchase contract, warrant agreement or purchase unit agreement. Except as
described below, owners of beneficial interests in a global security will not be entitled to have the securities represented by the global security registered in their names, will not receive or be
entitled to receive physical delivery of
the securities in definitive form and will not be considered the owners or holders of the securities under the applicable indenture, deposit agreement, purchase contract, purchase unit agreement or
warrant agreement. Accordingly, each person owning a beneficial interest in a global security must rely on the procedures of the depositary for that global security and, if that person is not a
participant, on the procedures of the participant through which the person owns its interest, to
16
Table of Contents
exercise
any rights of a holder under the applicable unit agreement or warrant agreement. We understand that under existing industry practices, if we request any action of holders or if an owner of a
beneficial interest in a global security desires to give or take any action that a holder is entitled to give or take under the applicable unit agreement or warrant agreement, the depositary for the
global security would authorize the participants holding the relevant beneficial interests to give or take that action, and the participants would authorize beneficial owners owning through them to
give or take that action or would otherwise act upon the instructions of beneficial owners holding through them.
Any
payments to holders with respect to warrants or units represented by a global security registered in the name of a depositary or its nominee will be made to the depositary or its
nominee, as the case may be, as the registered owner of the global security. None of us, or any warrant agent, unit agent or other agent of ours, or any agent of any warrant agent or unit agent will
have any responsibility or liability for any aspect of the records relating to payments made on account of beneficial ownership interests in the global security or for maintaining, supervising or
reviewing any records relating to those beneficial ownership interests.
We
expect that the depositary for any of the securities represented by a global security, upon receipt of any payment to holders of principal, premium, interest or other distribution of
underlying securities or other property on that registered global security, will immediately credit participants' accounts in amounts proportionate to their respective beneficial interests in that
global security as shown on the records of the depositary. We also expect that payments by participants to owners of beneficial interests in a global security held through participants will be
governed by standing customer instructions and customary practices, as is now the case with the securities held for the accounts of customers or registered in "street name," and will be the
responsibility of those participants.
If
the depositary for any of the securities represented by a global security is at any time unwilling or unable to continue as depositary or ceases to be a clearing agency registered
under the Exchange Act, and a successor depositary registered as a clearing agency under the Exchange Act is not appointed by us within 90 days, we will issue securities in definitive form in
exchange for the global security that had been held by the depositary. Any securities issued in definitive form in exchange for a global security will be registered in the name or names that the
depositary gives to the relevant warrant agent, unit agent or other relevant agent of ours or theirs. It is expected that the depositary's instructions will be based upon directions received by the
depositary from participants with respect to ownership of beneficial interests in the global security that had been held by the depositary.
17
Table of Contents
PLAN OF DISTRIBUTION
We may sell the securities being offered hereby in one or more of the following ways from time to
time:
-
•
-
directly to investors;
-
•
-
through agents to the public or to investors;
-
•
-
directly to agents;
-
•
-
to one or more underwriters or dealers for resale to the public or to investors;
-
•
-
in "at the market offerings," within the meaning of Rule 415(a)(4) of the Securities Act, to or through a market maker or into an
existing trading market, or an exchange or otherwise; or
-
•
-
through a combination of any of these methods of sale.
The
securities that we distribute by any of these methods may be sold, in one or more transactions, at:
-
•
-
a fixed price or prices, which may be changed;
-
•
-
market prices prevailing at the time of sale;
-
•
-
prices related to prevailing market prices; or
-
•
-
negotiated prices.
We
will set forth in a prospectus supplement the terms of the offering of our securities, including:
-
•
-
the name or names of any agents or underwriters;
-
•
-
the purchase price of our securities being offered and the proceeds we will receive from the sale;
-
•
-
any over-allotment options under which underwriters may purchase additional securities from us;
-
•
-
any agency fees or underwriting discounts and commissions and other items constituting agents' or underwriters' compensation;
-
•
-
any public offering price;
-
•
-
any discounts or concessions allowed or reallowed or paid to dealers; and
-
•
-
any securities exchanges on which such common stock may be listed.
Underwriters
Underwriters, dealers and agents that participate in the distribution of the securities may be underwriters as defined in the Securities Act and
any discounts or commissions they receive from us and any profit on their resale of the securities may be treated as underwriting discounts and commissions under the Securities Act. We will identify
in the applicable prospectus supplement any underwriters, dealers or agents and will describe their compensation. We may have agreements with the underwriters, dealers and agents to indemnify them
against specified civil liabilities, including liabilities under the Securities Act. Underwriters, dealers and agents may engage in transactions with or perform services for us or our subsidiaries in
the ordinary course of their businesses.
If
we use underwriters for a sale of securities, the underwriters will acquire the securities for their own account. The underwriters may resell the securities in one or more
transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale. The obligations of the underwriters to purchase the securities
will be subject to the conditions set forth in the applicable underwriting agreement. The underwriters will be obligated to purchase all the
18
Table of Contents
securities
offered if they purchase any of the securities offered. We may change from time to time any initial public offering price and any discounts or concessions the underwriters allow or reallow
or pay to dealers. We may use underwriters with whom we have a material relationship. We will describe in the prospectus supplement naming the underwriters the nature of any such relationship.
If
indicated in the applicable prospectus supplement, we will authorize underwriters or other persons acting as our agents to solicit offers by particular institutions to purchase
securities from us at the public offering price set forth in such prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on the date or dates stated in such
prospectus supplement. Each delayed delivery contract will be for an amount no less than, and the aggregate principal amounts of securities sold under delayed delivery contracts shall be not less nor
more than, the respective amounts stated in the applicable prospectus supplement. Institutions with which such contracts, when authorized, may be made include commercial and savings banks, insurance
companies, pension funds, investment
companies, educational and charitable institutions and others, but will in all cases be subject to our approval. The obligations of any purchaser under any such contract will be subject to the
conditions that (a) the purchase of the securities shall not at the time of delivery be prohibited under the laws of any jurisdiction in the United States to which the purchaser is subject, and
(b) if the securities are being sold to underwriters, we shall have sold to the underwriters the total principal amount of the securities less the principal amount thereof covered by the
contracts. The underwriters and such other agents will not have any responsibility in respect of the validity or performance of such contracts.
Agents
We may designate agents who agree to use their reasonable efforts to solicit purchases for the period of their appointment or to sell securities
on a continuing basis.
Direct Sales
We may also sell securities directly to one or more purchasers without using underwriters or agents. We may also make direct sales through
subscription rights distributed to our shareholders on a pro rata basis, which may or may not be transferable. In any distribution of subscription rights to shareholders, if all of the underlying
securities are not subscribed for, we may then sell the unsubscribed securities directly to third parties or may engage the services of one or more underwriters, dealers or agents, including standby
underwriters, to sell the unsubscribed securities to third parties.
Trading Markets and Listing of Securities
Unless otherwise specified in the applicable prospectus supplement, each class or series of securities will be a new issue with no established
trading market, other than our common stock, which is listed on The Nasdaq Capital Market. We may elect to list any other class or series of securities on any exchange, but we are not obligated to do
so. It is possible that one or more underwriters may make a market in a class or series of securities, but the underwriters will not be obligated to do so and may discontinue any market making at any
time without notice. We cannot give any assurance as to the liquidity of the trading market for any of the securities.
Stabilization Activities
In connection with an offering, an underwriter may purchase and sell securities in the open market. These transactions may include short sales,
stabilizing transactions and purchases to cover positions created by short sales. Shorts sales involve the sale by the underwriters of a greater number of securities than they are required to purchase
in the offering. "Covered" short sales are sales made in an amount not greater than the underwriters' option to purchase additional securities from us, if any, in the offering. If the underwriters
have an over-allotment option to purchase additional securities from
19
Table of Contents
us,
the underwriters may close out any covered short position by either exercising their over-allotment option or purchasing securities in the open market. In determining the source of securities to
close out the covered short position, the underwriters may consider, among other things, the price of securities available for purchase in the open market as compared to the price at which they may
purchase securities through the over-allotment option. "Naked" short sales are any sales in excess of such option or where the underwriters do not have an over-allotment option. The underwriters must
close out any naked short position by purchasing securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward
pressure on the price of the securities in the open market after pricing that could adversely affect investors who purchase in the offering.
Accordingly,
to cover these short sales positions or to otherwise stabilize or maintain the price of the securities, the underwriters may bid for or purchase securities in the open
market and may impose penalty bids. If penalty bids are imposed, selling concessions allowed to syndicate members or other broker-dealers participating in the offering are reclaimed if securities
previously distributed in the offering are repurchased, whether in connection with stabilization transactions or otherwise. The effect of these transactions may be to stabilize or maintain the market
price of the securities at a level above that which might otherwise prevail in the open market. The impositions of a penalty bid may also effect the price of the securities to the extent that it
discourages resale of the securities. The magnitude or effect of any stabilization or other transactions is uncertain. These transactions may be effected on The Nasdaq Capital Market or otherwise and,
if commenced, may be discontinued at any time.
20
Table of Contents
EXPERTS
The consolidated financial statements of InVivo Therapeutics Holdings Corp. and its subsidiaries as of December 31, 2018 and 2017 and for
each of the years in the two-year period ended December 31, 2018 incorporated in this Prospectus by reference from the InVivo Therapeutics Holdings Corp.'s
Annual Report on Form 10-K for the year ended December 31,
2018, have been audited by RSM US LLP, an independent registered public accounting firm, as stated in their report thereon (which report expresses an unqualified opinion and
includes an explanatory paragraph relating to InVivo Therapeutics Holdings Corp.'s ability to continue as a going concern), incorporated herein by reference, and have been incorporated in this
Prospectus and Registration Statement in reliance upon such report and upon the authority of such firm as experts in accounting and auditing.
LEGAL MATTERS
Unless the applicable prospectus supplement indicates otherwise, the validity of the securities in respect of which this prospectus is being
delivered will be passed upon by Ballard Spahr LLP, Las Vegas, Nevada, and certain other legal matters will be passed upon for us by Wilmer Cutler Pickering Hale and Dorr LLP, Boston,
Massachusetts.
21
Table of Contents
Shares of Common Stock
InVivo Therapeutics Holdings Corp.

Prospectus Supplement
H.C. Wainwright & Co.
, 2019
InVivo Therapeutics (NASDAQ:NVIV)
Historical Stock Chart
From Mar 2024 to Apr 2024
InVivo Therapeutics (NASDAQ:NVIV)
Historical Stock Chart
From Apr 2023 to Apr 2024