PART I
ITEM 1.
BUSINESS
General Development of Business
Sangui BioTech, Inc. (
“
SBT
”
) was incorporated in Delaware on August 2, 1996 and began operations in October 1996. Shortly after the formation of SBT, the shareholders of SanguiBioTech AG (
“
Sangui GmbH
”
) and GlukoMediTech AG (
“
Gluko AG
”
) agreed to a share swap in which all of the outstanding shares held by the shareholders would be exchanged for shares of SBT, thereby making Sangui GmbH and Gluko AG wholly owned subsidiaries of SBT. In August 1997, a publicly held company, Citadel Investment System, Inc., a Colorado corporation (
“
Citadel
”
), acquired one hundred percent (100%) of the outstanding common shares of Sangui BioTech, Inc., and as a result, Sangui BioTech, Inc. became a wholly owned subsidiary of Citadel. Thereafter, Citadel changed its name to Sangui BioTech International, Inc. (the
“
Company
”
or
“
SGBI
”
).
Until the end of its fiscal year 2003, SGBI's business operations were conducted through the wholly owned subsidiaries. During the first quarter of the 2003 fiscal year, SBT sold its assets, and commenced a wind-down of its U.S. business operations. SBT was merged with and into SGBI effective December 31, 2002. Gluko AG was merged with Sangui GmbH effective June 30, 2003.
Sangui BioTech GmbH, (
“
Sangui GmbH
”
) develops hemoglobin-based artificial oxygen carriers for use as blood additives, blood volume substitutes and variant products thereof. Sangui GmbH has also developed an anti-aging cosmetic line and a number of related products aimed at improving oxygen supply to the skin. Enhanced oxygen supply is the key to improved wound healing; therefore the Company has extended its product portfolio to contain wound pads and other wound management products. The facilities of Sangui GmbH are located on the premises of the Forschungs- und Entwicklungszentrum of the University of Witten/Herdecke, Witten, Germany.
In December 2010, Sangui GmbH established a joint venture company with SanderStrothmann GmbH of Georgsmarienhuette, Germany, under the name of SastoMed GmbH. This enterprise was in charge of obtaining the CE mark certification authorizing the distribution of one of SGBI
’
s products in the member states of the European Union. Effective December 31, 2015, Sangui GmbH sold its stake in Sastomed GmbH to SanderStrohmann GmbH.
On or about June 18, 2018, Sangui GmbH together with Sastomed GmbH founded Sangui Know-how- und Patentverwertungsgesellschaft mbH & Co. KG (
“
Sangui KG
”
). Sangui KG is a limited partnership. On June 22, 2018, Sangui KG acquired all the rights in the license agreement made on December 17, 2010 between Sastomed GmbH and Sangui GmbH.
Given the Company
’
s business strength is primarily in research and product development, we have decided to partner with established distribution entities who license our marketable products, or those products that are close to market entry, for sale to end users. In pursuit of this strategy we have licensed the most promising product, a hemoglobin based wound spray technology to Sastomed GmbH, a former joint venture of SGBI, for distribution in several European, Latin American and Asian countries. In addition, we are entering the preclinical testing of hemoglobin based artificial oxygen carriers aiming at the remediation of ischemic conditions in human patients.
To date, neither SGBI nor its subsidiaries has had profitable operations. The Company has never been profitable, and through June 30, 2018, SGBI's accumulated deficit has exceeded $37.1 million. The Company may continue to incur substantial losses over the next several years as it pursues its development, marketing and market entry efforts, testing activities and other growth operations. No assurance can be given that our programs will be successful.
Business of the Company
Our mission is the development of novel and proprietary pharmaceutical, medical and cosmetic products. We develop our products through our German subsidiaries, Sangui GmbH and our limited partnership Sangui KG. Currently, we are seeking to market and sell our products through partnerships with industry partners worldwide.
Our focus has been the development of oxygen carriers capable of providing oxygen transport in humans in the event of acute and/or chronic lack of oxygen due to arterial occlusion, anemia or blood loss whether due to surgery, trauma, or other causes, as well as in the case of chronic wounds.
We have thus far focused our development and commercialization efforts on such artificial oxygen carriers by reproducing and synthesizing polymers out of native hemoglobin of defined molecular sizes.
In addition, we have developed external applications of oxygen transporters, in the medical and cosmetic fields, in the form of sprays for the healing of chronic wounds and of gels and emulsions for the regeneration of the skin.
A wound dressing spray we developed that shows outstanding properties in the support of wound healing is being distributed by SastoMed GmbH, under the Granulox brand name. Sastomed GmbH was initially a joint venture company in which SGBI held 25% ownership. We sold that 25% stake to our partner in the joint venture effective as of December 31, 2015.
We also market a wound dressing that shows outstanding wound healing support properties, which we call Chitoskin. SanguiGmbH holds the distribution rights for these Chitoskin wound pads for the European Union and various other countries. A European patent has been granted for the production and use of Chitoskin wound pads.
Our current key business focuses are: (a) selling our existing cosmetics and wound management products through distribution partners, or by way of direct sale, to end users; (b) identifying additional industrial and distribution partners for our patents, production techniques, and products; and, (c) obtaining the additional certifications on our products in development.
Sangui GmbH is ISO 9001:2000 (General Quality Management System) and ISO 13485:2003 (Quality Management System Medical Products) certified, and is subject to audits on a regular basis.
Products of the Company
Artificial Oxygen Carriers
We have developed several products based on polymers of purified natural porcine hemoglobin with oxygen carrying abilities that are similar to those of native hemoglobin. These are (1) oxygen carrying blood additives, and (2) oxygen carrying blood volume substitutes.
In December 1997, we decided that porcine hemoglobin should be used as the basic material for artificial oxygen carriers. In March 1999, we decided which hemoglobin hyperpolymer would go into preclinical investigation, that glutaraldehyde would be utilized as a cross linker, and further that the polymer hemoglobin be chemically masked to prevent protein interaction in blood plasma. The fine adjustment of the molecular formula of the artificial oxygen carriers, optimized for laboratory scale production, was finalized in the summer of 2000.
The experiments completed in our laboratories demonstrated that it is possible to polymerize hemoglobins isolated from porcine blood resulting in huge soluble molecules, so-called hyperpolymers. In August 2000, we finalized our work on the pharmaceutical formulation of the
oxygen carrier for laboratory scale. In February 2001, a pilot production in a laboratory scale was carried out in our clean room. The resulting product was successfully applied in animal tests, moreover, single volunteers underwent pilot self-experiments.
The blood additives and blood substitute projects were halted in 2003 due to the lack of financing for the pre-clinical test phase.
During the first quarter of our 2013 financial year, the European Patent Office granted a patent based on Sangui
’
s application (1299457)
“
Mammalian hemoglobin compatible with blood plasma, cross-linked and conjugated with polyalkylene oxides as artificial medical oxygen carriers, production and use thereof.
”
During the third quarter of our 2013 financial year, the company had a feasibility study prepared by external experts inquiring into market potentials and further preclinical and clinical development requirements. The study came to the conclusion that an approval of Sangui's hemoglobin hyperpolymers as a blood additive appears possible, expedient, and promising.
During the fourth quarter of our 2014 financial year, the company filed a patent application aimed at significantly expanding the protection of our hemoglobin formulations. It encompassed a greater array of ischemic conditions of the human body, an example of which would be the case of severe dysfunctions of the lung.
During the first quarter of our 2015 financial year, we began, together with Excellence Cluster Cardio-Pulmonary System (ECCPS) and TransMIT Gesellschaft für Technologietransfer mbH (TransMIT), to investigate therapeutic approaches to treating septic shock and acute respiratory distress syndrome (ARDS). The approach adopted by Sangui, ECCPS and TransMIT presupposes that self-perpetuating septic shock, that has so far been highly resistant to treatment, can be interrupted by Sangui's artificial hemoglobin-based oxygen carrier, which would ultimately lower mortality rates. The preclinical trials commenced at ECCPS were investigating the effect of various hemoglobin preparations on the oxygen supply of a number of organs in septic shock models and ARDS.
Also, during the first quarter of our 2015 financial year, we were notified that the period for objection against European Patent EP 2550973 (
“
Wound Spray
”
) elapsed without any objection being raised. The patent, therefore, has become effective.
During the second quarter of our 2015 financial year, the first phase of preclinical trials was concluded successfully. It was successfully demonstrated that applying an oxygen-carrying liquid (the hemoglobin hyperpolymer formulation SBT102) in the abdomen did significantly improve the oxygen supply to the intestines. The restoration of intestinal oxygenation should have an impact on tissue integrity and ultimately on patient survival.
During the third quarter of our 2015 financial year, the preclinical trials were concluded successfully, and the final results did fully confirm the interim results obtained in the second quarter.
According to regulatory requirements, all drugs must complete preclinical and clinical trials before approval (Government Regulation; No Assurance of Product Approval, see Certain Business Risks below) and market launch. Our management believes that the European and United States FDA approval process will take at a minimum several years to complete.
Our most promising potential product in the area of artificial oxygen carriers, the blood additive is still in an early development stage. In the pursuit of these projects we will need to obtain substantial additional capital to continue their development.
The blood additives project was halted in the second quarter of our financial year 2016 due to the lack of financing the further authorization.
Nano Formulations for the Regeneration of the Skin
Healthy skin is supplied with oxygen both from the inside, by way of the blood circulation, as well as through diffusion from the outside. A lack of oxygen will cause degenerative alterations, ranging from premature aging, to surface damage, and even as extensive as causing open wounds. The cause for the lack of oxygen may be a part of the normal aging process, but it may also be caused by burns, radiation, trauma, or a medical condition. Impairment of the blood flow, for example caused by diabetes mellitus or by chronic venous insufficiency, can also lead to insufficient oxygen supply and the resulting skin damage.
Our nano-emulsion-based preparations have been designed to support the regeneration of the skin by improving its oxygen supply. The products were thoroughly tested by an independent research institute and received top marks for skin moisturizing, and enhanced skin elasticity.
Sales of these preparations had remained at a low levels for years. After the end of the 2015 fiscal year we decided to discontinue our operations in this particular segment and to abandon the patent protection for this range of products.
Chitoskin Wound Pads
Usually, normal (
“
primary
”
) wounds tend to heal over a couple of days without leaving scars following a certain sequence of phases. Burns and certain diseases impede the normal wound healing process, resulting in large, hardly healing (
“
secondary
”
) wounds which only close by growing new tissue from the bottom. Wound dressings serve to safeguard the wound with its highly sensitive new granulation tissue from mechanical damage as well as from infection. Using the natural polymer chitosan, our Chitoskin wound dressings show outstanding properties in supporting wound healing.
It is our strategy to find industry partners ready to acquire or license this product range as a whole.
Hemoglobin Based Wound Spray Technology
Sangui GmbH has developed a novel medical product aimed at the healing of chronic wounds. Chronic wounds are a medical problem of increasing importance as they originate from widespread and increasingly common risk factors such as diabetes and obesity, as well as other personal lifestyle choices like smoking. A lack of oxygen supply to the cells in the wound ground is the main reason why these wounds lose their ability to self-heal. Based on our concept of artificial oxygen carriers, our Hemospray wound spray product bridges the watery wound surface and permits an enhanced afflux of oxygen to the wound ground.
In December 2010, Sangui GmbH established a joint venture company with SanderStrothmann GmbH of Georgsmarienhuette, Germany. Under the name of SastoMed GmbH this enterprise was in charge of obtaining the CE mark certification authorizing the distribution of the Hemospray wound spray in the member states of the European Union. Sangui GmbH has granted SastoMed GmbH global distribution rights for its Hemospray product.
The basic terms of the licensing contract agreement are that Sangui GmbH is awarded a fixed licensing fee as a percentage of the external revenues received from sales of the Granulox product (based on SastoMed selling prices). The percentage ranges in the uppermost zone of what is usually granted in the pharmaceutical and medical products industries and thus well above the average licensing rate of 7.5% of sales revenues as calculated by market analysts. In addition and complementing this basic agreement, the percentage will be permanently increased by one fourth of the current rate as soon as cumulated sales revenues at SastoMed have exceeded
€
50,000,000.
In September 2011, the Mexican Health Authorities registered the entire current range of Sangui wound management products and thus granted the authorization to apply and sell these products on a nationwide level.
On April 5, 2012, SastoMed GmbH notified Sangui GmbH that the wound spray product was granted a certification as class III medical product. The CE mark according to sections 6 and 7 of the German Medical Devices Act authorizes production, distribution and sales of the product in all member countries of the European Union. Sales of the product by SastoMed GmbH under the brand name
“
Granulox
”
started in Germany on April 16, 2012.
In December 2012, actual distribution of the product was initiated in Mexico under the management of SastoMed GmbH and their local distribution partner Bio-Mac Pharma. International distribution has been expanded since then through cooperation agreements with local distribution partners in the Benelux countries and South Eastern Europe.
In May 2013, the Company declared in the course of the filing of its nine-month report on form 10-QSB that it now expects the Granulox market entry phase to last longer than initially expected.
Since December 2013, international distribution outside Germany was initiated in collaboration with local partners in more than 40 countries in Europe and Latin American.
Effective December 31, 2015, Sangui GmbH sold its 25 % stake in Sastomed GmbH to SanderStrohmann GmbH. Also effective December 31, 2015, SanderStrohmann GmbH increased the capital of Sastomed GmbH by
€
500,000 to strengthen the capital base of Sastomed GmbH.
With effect from June 18, 2018 Sangui GmbH together with Sastomed GmbH founded Sangui Know-How- und Patentverwertungsgesellschaft mbH & Co. KG (
“
Sangui KG
”
). Sangui KG is a limited partnership. The Sangui GmbH has agreed as a fully liable compliant with 99.8% and the Sastomed GmbH as a limited partner with 0.2%. On June 22, 2018, Sangui KG has acquired all rights in the license agreement concluded on December 17, 2010 with Sastomed GmbH from Sangui GmbH.
Nevertheless, all material content of the existing license agreement remains unchanged even after the transition from Sangui GmbH to Sangui KG. This also applies to the pledging of the European patent EP 1485120 concerning the Hemo2 spray to the Sastomed GmbH.
It has to be noted, however, that Granulox sales by our distribution partner SastoMed GmbH have become more volatile and declining from time to time. We remain confident, however, that SastoMed will be able to considerably increase its sales along with more international markets entering actual distribution of the product.
Patents and Proprietary Rights
The Company seeks patent protection for all of its research and development projects, and all the most important modifications and improvements thereto. As of June 30, 2018 SanguiBioTech GmbH had been granted patents from three patent families, furthermore, it has applied for additional patents. Two of the patents have been filed in the United States of America (US), and three as international patent applications with the European Patent Office (EP). Validation of granted EP patents in all cases includes Germany, France, Great Britain, Italy, and Spain. Below are listed the most important of the rights held by the Company.
In the fiscal year, a patent in the USA expired.
1. Hemoglobin-Hyperpolymers
|
|
|
US 7,005,414
EP 1 294 386
|
“
Synthetic oxygen transport made from cross-linked modified human or porcine hemoglobin with improved properties, method for a preparation thereof from purified material and use thereof
”
(patents granted, end of duration 2020)
|
|
|
|
|
DE 10 2013 014 651
EP 14758344
US SN 14 569,846
|
“
Compositions for Improved Tissue Oxygenation by Peritoneal Ventilation
”
(patents pending)
|
2. Wound Management
|
|
|
EP 1 485 120
|
“
Use of one or more natural or modified oxygen carriers, devoid of plasma and cellular membrane constituents, for externally treating open, in particular chronic wounds
”
(patent granted, end of duration 2022)
|
Manufacturing, Marketing and Distribution
Manufacturing, marketing and distribution are not core competencies of the company. It is our strategy, therefore to outsource such business processes to external partners. In selecting and mandating them special attention is being paid to their experience, reputation and standing including the required quality management systems and certifications.
Research and Development
Research and development are charged to operations as they are incurred. Legal fees and other direct costs incurred in obtaining and protecting patents are expensed as incurred. Research and development costs totaled $23,003 and $16,530 during the fiscal years ended June 30, 2018 and 2017, respectively.
Government Regulation
Sangui BioTech International, Inc. and its former United States subsidiaries are and were subject to governmental regulation under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, and other similar laws of general application, as to all of which we believe we and our subsidiaries were in material compliance.
Although it is believed that we, and our former United States subsidiaries have been in material compliance with all applicable governmental and environmental laws, rules, regulations and policies, and although no government concerns were put forward during the operation of or after the closing of the US operations, there can be no assurance that the business, financial condition, and our results of operations of and those of our subsidiaries will not be materially adversely affected by future government claims with regard to unlikely, but not impossible, infringements on these or other laws resulting from our former United States operations.
Additionally, the clinical testing, manufacture, promotion and sale of a significant majority of the products and technologies, if those products and technologies are to be offered and sold in the United States, are subject to extensive regulation by numerous governmental authorities in the United
States, principally the Federal Drug Administration (
“
FDA
”
), and corresponding state regulatory agencies. To the extent those products and technologies are to be offered and sold in markets other than the United States, the clinical testing, manufacture, promotion and sale of those products and technologies will be subject to similar regulation by corresponding foreign regulatory agencies. In general, the regulatory framework for biological health care products is more rigorous than for non-biological health care products. Generally, biological health care products must be shown to be safe, pure, potent and effective. There are numerous state and federal, foreign and international statutes and regulations that govern or influence the testing, manufacture, safety, effectiveness, labeling, storage, record keeping, approval, advertising, distribution and promotion of biological health care products. Non-compliance with applicable requirements can result in, among other things, fines, injunctions, seizures of products, total or partial suspension of product marketing, and failure of the government to grant pre-market approval, withdrawal of marketing approvals, product recall and criminal prosecution.
Competition
The market for our products and technologies is highly competitive, and we expect competition to increase. Experiments and clinical testing in the field of artificial oxygen carriers are being carried out by Alliance Pharmaceutical Corp. of San Diego, California. In the fields of anti-aging and anti-cellulite cosmetics, all major cosmetic vendors are actively marketing proprietary formulations. Leading wound management product providers include Johnson & Johnson, Bristol-Myers Squibb, Coloplast A/S of Denmark as well as BSNmedical, a former part of Beiersdorf AG.
Dependence on Major Customers
As of June 30, 2018 and June 30, 2017, the majority of revenues and trade receivables were generated by SastoMed GmbH, the licensee of the global distribution rights of the Sangui developed wound spray technology known as Granulox.
Human Resources
We consider our relations with our employees to be favorable. As of June 30, 2018, the Company, and our subsidiary, had one fulltime employee, who was not involved in research and development. For management, research and development purposes, the Company has consulting arrangements with five individuals and one related entity.
Dividends
We anticipate that we will use any funds available to finance our growth and that we will not pay cash dividends to stockholders in the foreseeable future.
Reports to Security Holders
Copies of our reports, as filed with the Securities and Exchange Commission, are available and may be viewed as filed at the SEC
’
s Public Reference Room at 450 Fifth Street, N.W., Washington D.C. 20549 or by calling 1-800-SEC-0330. Additionally they can be accessed and downloaded via the internet at http://www.sec.gov/cgi-bin/srch-edgar by simply typing in
“
Sangui Biotech International
”
or via the web links at the corporate website http://www.sanguibiotech.com.
ITEM 1A.
RISK FACTORS
Investment in our common stock involves significant risk. You should carefully consider the information described in the following risk factors, together with the other information appearing elsewhere in this report, before making an investment decision regarding our common stock. If any of the events or circumstances described in these risks actually occur, our business, financial conditions, results of operations and future growth prospects would likely be materially and adversely affected. In these circumstances, the market price of our common stock could decline, and you may lose all or a part of your investment in our common stock.
The risks and uncertainties described below are not the only ones facing the company, and there may be additional risks that are not presently known or are currently deemed immaterial. All of these risks may impair business operations.
The Company's present and proposed business operations will be highly speculative and subject to the same types of risks inherent in any new or unproven venture, as well as risk factors particular to the industries in which it will operate, as well as other significant risks not normally associated with investing in equity securities of United States companies, among other things, those types of risk factors outlined below.
Risks Related to Our Business
Global economic crisis could result in decreases in customer spending
We operate in competitive and evolving markets locally, nationally and globally. These markets are subject to rapid technological change and changes in demand. In seeking market acceptance, we will encounter competition from many sources, including other well-established and dominant larger providers. Many of these competitors have substantially greater financial, marketing and other resources than does Sangui. Our revenue could be materially adversely affected if we are unable to compete successfully with these other providers. The current economic climate has resulted in a decrease in customer spending.
There is uncertainty relating to the ability of the Company to enforce its rights under agreements
Many of our agreements are with foreign entities and are governed by the laws of foreign jurisdictions. If a partner breaches an agreement, then we will incur the additional costs of determining our rights and obligations under the agreement, under applicable foreign laws, and enforcing the agreement in a foreign jurisdiction. We also may face practical difficulties in enforcing any of our rights in such jurisdictions. We may not be able to enforce such rights or in the alternative may determine that it would be too costly to enforce such rights.
The Company may be subject to other third-party intellectual property rights claims
Companies in our industry often own large numbers of patents, copyrights, trademarks and trade secrets and frequently enter into litigation based on allegations of infringement or other violations of intellectual property rights. As we face increasing competition, the possibility of intellectual property rights claims against us grows. Our technologies may not be able to withstand third-party claims or rights against their use. Intellectual property claims, whether having merit or otherwise, could be time consuming and expensive to litigate or settle and could divert management resources and attention. If litigation is successfully brought by a third party against the Company in respect of intellectual property, we may be required to cease distributing or marketing certain products or obtain licenses from the holders of the intellectual property at material cost, redesign affected products in such a way as to avoid infringing intellectual property rights, any or all of which could materially adversely affect our business, financial condition and results of operations. If those
intellectual property rights are held by a competitor, we may be unable to obtain the intellectual property at any price, which could also adversely affect our competitive position. An adverse determination could also prevent us from offering its products. Any of these results could harm the Company
’
s business, financial condition and results of operations.
Licenses and Consents
The utilization or other exploitation of the products and services developed by our Company or its subsidiary may require us to obtain licenses or consents from the producers or other holders of patents, trademarks, copyrights or other similar rights. In the event we are unable, if so required, to obtain any necessary license or consent on terms, which we consider to be reasonable, we may be required to cease developing, utilizing, or exploiting products or technologies affected by those Intellectual Property rights. In the event the holders of such Intellectual Property rights challenge us, there can be no assurance that we will have the financial or other resources to defend any resulting legal action, which could be significant.
Technological Factors
The market for our products and technology is characterized by rapidly changing technology, which could result in product obsolescence or short product life cycles. Similarly, the industry is characterized by continuous development and introduction of new products and technology to replace outdated products and technology. Accordingly, the ability of us to compete will be dependent upon the ability to provide new and innovative products and technology. There can be no assurance that competitors will not develop technologies or products that render the proposed products and technology obsolete or less marketable. We will be required to adapt to technological changes in the industry and develop products and technology to satisfy evolving industry or customer requirements, any of which could require the expenditure of significant funds and resources, and we do not have a source or commitment for any such funds and resources. Development efforts relating to the technological aspects of the various products and technologies to be developed are not substantially completed. Accordingly, we will continue to refine and improve those products and technologies. Continued refinement and improvement efforts remain subject to the risks inherent in new product development, including unanticipated technical or other problems, which could result in material delays in product commercialization or significantly increased costs. In addition, there can be no assurance that those products and technologies will prove to be sufficiently reliable or durable in wide spread commercial application. The products or technologies to be developed will be the result of significant efforts, which may result in errors that become apparent subsequent to widespread commercial utilization. In such event, we would be required to modify such products or technologies and continue with additional research and development, which could delay our plans and cause us to incur additional cost.
The Company is subject to foreign business, political and economic disruption risks
We contract with various entities around the world. As a result, we are exposed to foreign business, political and economic risks, which could adversely affect our financial position and results of operations, including:
·
difficulties in managing relationships from abroad;
·
political and economic instability;
·
less developed infrastructures in some emerging economies and countries;
·
susceptibility to business interruption in foreign areas due to war, terrorist attacks, medical epidemics, changes in political regimes, and general interest rate and currency instability;
·
·
exposure to possible litigation or claims in foreign jurisdictions; and,
·
competition from foreign-based providers and the existence of protectionist laws and business practices that favor such providers.
Early stage of the Company and its products
We have generated limited revenue from operations, and may not generate any significant or sufficient revenue from its current operations to continue future operations. A very limited number of our products are currently in the marketplace. However, to achieve profitable operations, either alone or with others, we must successfully initiate and maintain sales and distribution of our products. The time frame necessary to achieve market success for any individual product is uncertain. There can be no assurance that our efforts will be successful, or that any of our products will prove to meet the anticipated levels of approval or effectiveness, or that we will be able to obtain and sustain customer, as well as distribution approval.
Our results can also be affected by the ability of competition to introduce new products that have advantages over our own, or the competition's ability to adjust its pricing to reduce any competitive advantage we may have. Results will also be affected by strategic decisions made by the management regarding product volume, mix, and timing of orders received during operations. See
“
Description of Business.
”
Uncertainty of future profitability
We will require the commitment of substantial resources to increase our advertising, marketing and distribution of our existing products. While we believe that the additional advertising, marketing and distribution will further enhance our profitability, there can be no assurance our products will meet the expectations and effectiveness required to be competitive in the market place, and that we will enter into arrangements for commercialization, market our products successfully, or achieve customer acceptance.
Future capital requirements; uncertainty of future funding
Substantial expenditures will be required to enable us to conduct existing product research, manufacturing, marketing and distribution of our products and Intellectual Property. We may need to raise additional capital to facilitate growth and support its longterm manufacturing, and marketing programs. We have no established bank-financing arrangements and until we have sufficient assets, capital, and inventory or accounts receivable, it is not anticipated that we will secure any bank financing in the near future. Therefore, it is likely that we may need to seek additional financing through subsequent future public or private sales of its securities, including equity securities. We may also seek funding for the manufacturing, and marketing of its products through strategic partnerships and other arrangements with corporate partners. There can be no assurance, however, that such collaborative arrangements or additional funds will be available when needed, or on terms acceptable to the Company, if at all. Any such additional financing may result in significant dilution to existing stockholders. If adequate funds are not available, we may be required to curtail one or more of our programs. Our future cash requirements will be affected by the revenue generated from the sale of our products, the costs of production and marketing, as well as relationships with corporate partners, changes in the focus and direction of our programs, competitive and technological advances, and other factors.
Substantial Doubt that the Company Can Continue as a Going Concern
We expect to continue to incur significant capital expenses in pursuing our business plan to market our products and expand our product line, while obtaining additional financing through stock offerings or other feasible financing alternatives. In order for us to continue its operations at its existing levels, we will require significant additional funds over the next twelve months. Therefore,
we are dependent on funds raised through equity or debt offerings. Additional financing may not be available on terms favorable to the Company, or at all. If these funds are not available, we may not be able to execute our business plan or take advantage of business opportunities. The ability to obtain such additional financing and to achieve our operating goals is uncertain. In the event that we cannot obtain additional capital or are not able to increase cash flow through the increase of sales, there is a substantial doubt of our being able to continue as a going concern.
Future Capital Needs and Uncertainty of Additional Funding
We believe that our cash position is insufficient to cover its financing requirements for the current fiscal year, and anticipate that substantial funds will be required in order to enact our development plans. We will require additional cash for: (i) payment of increased operating expenses; (ii) payment of development expenses; and (iii) further implementation of its business strategies. Such additional capital may be raised by additional public or private financing, as well as borrowings and other resources. To the extent that additional capital is received by the sale of equity or equity-related securities, the issuance of such securities will result in dilution to our shareholders. There can be no assurance that additional funding will be available on favorable terms, if at all. We may also seek arrangements with collaborative partners in order to gain additional funding, marketing assistance or other contributions. However, such arrangements may require us to relinquish rights or reduce its interests in certain of our technologies or product candidates. The inability to access the capital markets or obtain acceptable financing could have a material adverse effect on the results of operations and financial condition of the Company. Moreover, if funds are not available from any sources, we may not be able to continue to operate.
Dependence on others; manufacturing capabilities and limited distribution capabilities
An important element of our strategy for the marketing and release of its products is to enter into various arrangements with distribution and retail partners. The success and commercialization of our products will be dependent, in part, upon our ability to enter into such arrangements and upon the ability of these third parties to perform their responsibilities. Although we believe that parties to any such arrangements would have an economic motivation to succeed in performing their contractual responsibilities, the amount and timing of resources to be devoted to these activities may not be within the control of the Company. There can be no assurance that any such arrangements will be available on terms acceptable to us, if any at all, and that such parties will perform their obligations as expected, or that any revenue will be derived from such arrangements. If we are not able to enter into such arrangements, we could encounter delays in introducing our products into the market. See
“
Business.
”
We may be dependent on other manufacturers for the production and manufacturing of certain products. In the event that we are unable to obtain or retain the necessary manufacturer
’
s products on acceptable terms, we may not be able to continue to commercialize and market our products as planned. The manufacture of our products will be subject to current good manufacturing practices (
“
GMP
”
) requirements prescribed by the Company in order to meet the specifications and other standards prescribed by us to satisfy the anticipated and appropriate levels of effectiveness when in use. There can be no assurance that we will be able to i) obtain adequate supplies of its products in a timely fashion at acceptable quality and prices, ii) enter into arrangements for the manufacture of its products with manufacturers whose facilities and procedures comply with our GMP or other regulatory requirements, iii) or that manufacturers will continue to comply with such standards, or iv) that such manufacturers will be able to adequately supply us with our product needs. Our dependence upon others for the manufacture of its proposed products may adversely affect the Company's ability to develop and deliver products on a timely and competitive basis.
In addition, we do not now have, nor do we have current plans to acquire or obtain, the facilities, or personnel necessary to conduct our own full-scale distribution of its products. Consequently, we will have to rely on existing commercial distribution channels for the sale of our
products. There can be no assurance that we will be able to secure sufficient distribution of any of its products on acceptable terms.
Changes of prices for products
While the prices of our products are projected to be in line with those from market competitors, there can be no assurance that they will not decrease in the future. Competition may cause us to lower prices in the future. Moreover, it is difficult to raise prices even if internal costs increase.
Creditworthiness of distributors is an ongoing concern
We may not always be able to collect all of the funds owed to us by our distributors. Some distributors may experience financial difficulties, which may adversely impact our collection of accounts receivable.
C Corporation tax status
We are presently a C Corporation under the Internal Revenue Code of 1986. All items of income and loss of the Company are taxed first at the corporate level and any dividends distributed to shareholders are taxed at the shareholder level as well.
Limited current sales and marketing capability
Though we have key personnel with experience in sales, marketing and distribution to market our products, we must either retain and hire the necessary personnel to distribute and market our products or enter into collaborative arrangements or distribution agreements with third parties who will market such products or develop their own marketing and sales force with technical expertise and supporting distribution capability. There can be no assurance that we will be able to retain or hire the personnel with sufficient experience and knowledge to distribute and market our products or be able to enter into collaborative or distribution arrangements or develop our own sales force, or that such sales and marketing efforts, including the efforts of the companies with which we have entered into collaborative agreements, will be successful.
We incur significant costs as a result of our operating as a public company and our management is required to devote substantial time to new compliance initiatives
As a public company with substantial operations, we incur increased legal, accounting and other expenses. The costs of preparing and filing annual, quarterly and current reports, proxy statements and other information with the SEC and furnishing audited reports to shareholders is time-consuming and costly.
It will also be time-consuming, difficult and costly for us to develop and implement the internal controls and reporting procedures required by the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act. Certain members of our management have limited or no experience operating a company whose securities are listed on a national securities exchange or with the rules and reporting practices required by the federal securities laws and applicable to a publicly traded company. We will need to recruit, hire, train and retain additional financial reporting, internal control and other personnel in order to develop and implement appropriate internal controls and reporting procedures. If we are unable to comply with the internal controls requirements of the Sarbanes-Oxley Act, we may not be able to obtain the independent accountant certifications required by the Sarbanes-Oxley Act.
If we fail to establish and maintain an effective system of internal controls, we may not be able to report our financial results accurately. Any inability to report and file our financial
results accurately and timely could harm our business and adversely affect the trading price of our common stock
We are required to establish and maintain internal controls over financial reporting and disclosure controls and procedures and to comply with other requirements of the Sarbanes-Oxley Act and the rules promulgated by the SEC. At present, we have instituted internal controls, but it may take time to implement them fully as a public company. Our management, including our Chief Executive Officer and Chief Financial Officer, cannot guarantee that our internal controls and disclosure controls and procedures will prevent all possible errors. Because of the inherent limitations in all control systems, no system of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the company have been detected. These inherent limitations include the possibility that judgments in decision-making can be faulty and subject to simple error or mistake. Furthermore, controls can be circumvented by individual acts of some persons, by collusion of two or more persons, or by management override of the controls. The design of any system of controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, a control may become inadequate because of changes in conditions or the degree of compliance with policies or procedures may deteriorate. Because of inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and may not be detected.
Dependence on Key Personnel
Our future success will depend on the service of our key scientific personnel and, additionally, our ability to identify, hire and retain additional qualified personnel. There is intense competition for qualified personnel in this industry and there can be no assurance that we will be able to attract and retain personnel necessary for the development of the business. Because of the intense competition, there can be no assurance that we will be successful in adding technical personnel if needed to satisfy our staffing requirements. Failure to attract and retain key personnel could have a material adverse effect on the Company.
The Company and its subsidiary are dependent on the efforts and abilities of their senior management. The loss of various members from management could have a material adverse effect on the business and our prospects. There can be no assurance that upon the departure of key personnel from our business, or that of our subsidiary, that a suitable replacement will be available.
Conflicts of Interest; Related Party Transactions
The possibility exists that we may acquire or merge with a business or company in which the Company's executive officers, directors, beneficial owners or their affiliates may have an ownership interest. Although there is no formal bylaw, stockholder resolution or agreement authorizing any such transaction, corporate policy does not forbid it and such a transaction may occur if management deems it to be in the best interests of the Company and its stockholders, after consideration of all factors. A transaction of this nature would present a conflict of interest to those parties with a managerial position and/or an ownership interest in both the Company and the acquired entity, and may compromise management's fiduciary duties to the Company's stockholders. An independent appraisal of the acquired company may or may not be obtained in the event a related party transaction is contemplated. Furthermore, because management and/or beneficial owners of the Company's common stock may be eligible for finder's fees or other compensation related to potential acquisitions by the Company, such compensation may become a factor in negotiations regarding such potential acquisitions. It is the Company's intention that all future transactions be entered into on such terms as if negotiated at arm
’
s length, unless the Company is able to receive more favorable terms from a related party.
Market Acceptance
There can be no assurance that our products and technologies will achieve a significant degree of market acceptance, and that acceptance, if achieved, will be sustained for any significant period or that product life cycles will be sufficient (or substitute products developed) to permit us to achieve or sustain market acceptance which could have a material adverse effect on the business, financial condition, and results of operations.
Government Regulation; No Assurance of Product Approval
The clinical testing, manufacture, promotion, and sale of biotechnology and pharmaceutical products are subject to extensive regulation by numerous governmental authorities in the United States, principally the Federal Drug Administration (
“
FDA
”
), and corresponding state and foreign regulatory agencies prior to the introduction of those products. Management believes that many of the potential products will be regulated by the FDA, subject to the then current regulations of the FDA. Other federal and state statutes and regulations may govern or influence the testing, manufacture, safety, effectiveness, labeling, storage, record-keeping, approval, advertising, distribution and promotion of certain products developed. Non-compliance with applicable requirements can result in, among other things, fines, injunctions, seizure of products, suspensions of regulatory approvals, product recalls, operating restrictions, re-labeling costs, delays in sales, cessation of manufacture of products, the imposition of civil or criminal sanctions, total or partial suspension of product marketing, failure of the government to grant pre-market approval, withdrawal of marketing approvals and criminal prosecution.
The FDA's requirements include lengthy and detailed laboratory and clinical testing procedures, sampling activities and other costly and time-consuming procedures. In particular, human therapeutic products are subject to rigorous pre-clinical and clinical testing and other approval requirements by the FDA, and corresponding agencies in other countries. Although the time required for completing such testing and obtaining such approvals is uncertain, satisfaction of these requirements typically takes a number of years and varies substantially based on the type, complexity and novelty of each product. Neither us nor our subsidiary can accurately predict when product applications or submissions for FDA or other regulatory review may be submitted. Management has no experience in obtaining regulatory clearance on these types of products. The lengthy process of obtaining regulatory approval and ensuring compliance with applicable law requires the expenditure of substantial resources. Any delays or failure by us or our subsidiary to obtain regulatory approval and ensure compliance with appropriate standards could adversely affect the commercialization of such products, the ability of us to earn product or royalty revenue, and our results of operations, liquidity and capital resources.
Pre-clinical testing is generally conducted in laboratory animals to evaluate the potential safety and effectiveness of a drug. The results of these studies are submitted to the FDA, which must be approved before clinical trials can begin. Typically, clinical evaluation involves a time consuming and costly three-phase process. In Phase I, clinical trials are conducted with a small number of subjects to determine the early safety profile, the pattern of drug distribution and metabolism. In Phase II, clinical trials are conducted with groups of patients afflicted with a specific disease in order to determine preliminary efficacy, optimal dosages and expanded evidence of safety. In Phase III, large-scale, multi-center, comparative trials are conducted with patients afflicted with a target disease in order to provide enough data to demonstrate the efficacy and safety required by the FDA. The FDA closely monitors the progress of each of the three phases of clinical trials and may, at its discretion, re-evaluate, alter, suspend or terminate the testing based upon the data which have been accumulated to that point and its assessment of the risk/benefit ratio to the patient.
Clinical trials and the marketing and manufacturing of products are subject to the rigorous testing and approval processes of the FDA and foreign regulatory authorities. The process of obtaining FDA and other required regulatory approvals is lengthy and expensive. There can be no
assurance we will be able to obtain the necessary approvals to conduct clinical trials for the manufacturing and marketing of products, that all necessary clearances will be granted to us or one of our licensors for future products on a timely basis, or at all, or that FDA review or other actions will not involve delays adversely affecting the marketing and sale of the products. In addition, the testing and approval process with respect to certain new products which we may seek to introduce is likely to take a substantial number of years and involve the expenditure of substantial resources. There can be no assurance that pharmaceutical products currently in development will be cleared for marketing by the FDA. Failure to obtain any necessary approvals or failure to comply with applicable regulatory requirements could have a material adverse effect on the business, financial condition or results of operations. Further, future government regulation could prevent or delay regulatory approval of the products.
There can be no assurance as to the length of the clinical trial period or the number of patients the FDA will require to be enrolled in the clinical trials in order to establish the safety and effectiveness of the products. We may encounter significant delays or excessive costs in our efforts to secure necessary approvals, and regulatory requirements are evolving and uncertain. Future United States or foreign legislative or administrative acts could also prevent or delay regulatory approval of the products. If commercial regulatory approvals are obtained, they may include significant limitations on the indicated uses for which a product may be marketed. In addition, a marketed product is subject to continual FDA review. Later discovery of previously unknown problems or the failure to comply with the applicable regulatory requirements may result in restrictions on the marketing of a product, or even the removal of the product from the market, as well as possible civil or criminal sanctions. Failure to obtain marketing approval for any of our products under development on a timely basis, or FDA withdrawal of marketing approval once obtained, could have a material adverse effect on the business, financial condition and results of operations.
Any party that manufactures therapeutic or pharmaceutical products is required to adhere to applicable standards for manufacturing practices and to engage in extensive record keeping and reporting. Any of the manufacturing facilities are subject to periodic inspection by state and federal agencies, including the FDA and comparable agencies in foreign countries.
The effect of governmental regulation may be to delay the marketing of new products for a considerable period of time, to impose costly requirements on the activities or to provide a competitive advantage to other companies that compete with us. There can be no assurance that FDA or other regulatory approval for any products developed by us will be granted on a timely basis, if at all or, if granted, that compliance with regulatory standards will be maintained. Adverse clinical results by our products could have a negative impact on the regulatory process and timing. A delay in obtaining, or failure to obtain, regulatory approvals could preclude or adversely affect the marketing of products and the liquidity and capital resources. The extent of potentially adverse governmental regulation that might result from future legislation or administrative action cannot be predicted.
Additionally, we will be subject to regulatory authorities in Germany and other countries governing clinical trials and product sales. Even if FDA approval is obtained, approval of a product by the comparable regulatory authorities of other countries must be obtained prior to the commencement of marketing the product in those countries. The approval process varies from country to country and the time required may be longer or shorter than that required for FDA approval. The foreign regulatory approval process includes all of the risks associated with obtaining FDA approval set forth above, and approval by the FDA does not ensure approval by the health authorities of any other country. There can be no assurance that any foreign regulatory agency will approve any product submitted for review.
We are subject to various federal, state and local laws, regulations and recommendations relating to safe working conditions, laboratory and manufacturing practices, the experimental use of animals and the use and disposal of hazardous or potentially hazardous substances, including radioactive compounds and infectious disease agents, used in connection with its research work. The
extent and character of governmental regulation that might result from future legislation or administrative action cannot be accurately predicted.
Intense Competition
Competition in the biotechnology, pharmaceutical, and cosmetic industries is intense and is expected to increase. In the field of medical and cosmetic products we, and our subsidiary, compete directly with the research departments of biotechnology and pharmaceutical companies, chemical companies and, possibly, joint collaborations between chemical companies and research and academic institutions. Management is aware that other companies and businesses have developed and are in the process of developing technologies and products, which may be competitive with the products and technologies developed and offered by us. Eventually, this might include the field of blood additives where there is no known direct competition at present. The biotechnology and pharmaceutical industries continue to undergo rapid change. There can be no assurance that competitors have not or will not succeed in developing technologies and products that are more effective than any which have been or are being developed by us or which would render our technology and products obsolete. Many of our competitors have substantially greater experience, financial and technical resources and production, marketing and development capabilities than us. Accordingly, certain of those competitors may succeed in obtaining regulatory approval for products more rapidly or effectively.
Uncertainties Associated With Patents and Proprietary Rights
Our success may depend in part on the ability to obtain patents for our technologies and products, if any, resulting from the application of such technologies, to defend patents once obtained and to maintain trade secrets, both in the United States and in foreign countries.
Our success will also depend on avoiding the infringement of patents issued to competitors. There can be no assurance that we will be able to obtain patent protection for products based upon the technology. Moreover, there can be no assurance that any patents issued will not be challenged, invalidated or circumvented or that the rights granted there under would provide competitive advantages to us. Litigation, which could result in substantial cost may be necessary to enforce the patent and license rights or to determine the scope and validity of our and others' proprietary rights.
Due to the length of time and expense associated with bringing new products through development and the length of time required for the governmental approval process, the biotechnology and pharmaceutical industries have traditionally placed considerable importance on obtaining and maintaining patent and trade secret protection for significant new technologies, products and processes. The enforceability of patents issued to biotechnology and pharmaceutical firms can be highly uncertain. U.S. Federal court decisions establishing legal standards for determining the validity and scope of patents in the field are in transition. In addition, there can be no assurance that patents will be issued or, if issued, any such patents will afford us protection from infringing patents granted to others.
A number of biotechnology and pharmaceutical companies, and research and academic institutions, have developed technologies, filed patent applications or received patents on various technologies that may be related to our business. Some of these technologies, applications or patents may conflict with our technologies. Such conflicts could also limit the scope of the patents, if any, that we may be able to obtain or result in the denial of our patent applications.
Many of our competitors are, have, or are affiliated with companies having, substantially greater resources than us, and such competitors may be able to sustain the costs of complex patent litigation to a greater degree and for longer periods of time than us. Uncertainties resulting from the initiation and continuation of any patent or related litigation could have a material adverse effect on the ability of us to compete in the marketplace pending resolution of the disputed matters. Moreover, an adverse outcome could subject us to significant liabilities to third parties and require us to license
disputed rights from third parties or cease using the technology. In the event that third parties have or obtain rights to intellectual property or technology used or needed by us, there can be no assurance that any licenses would be available or would be available on terms reasonably acceptable to us.
We may rely on certain proprietary technologies, trade secrets, and know-how that are not patentable. Although we have taken steps to protect their unpatented trade secrets and technology, in part through the use of confidentiality agreements with their employees, consultants and certain of its contractors, there can be no assurance that: (i) these agreements will not be breached; (ii) we would have adequate remedies for any breach; or (iii) our proprietary trade secrets and know-how will not otherwise become known or be independently developed or discovered by competitors.
Risk of Product Liability; Potential Unavailability of Insurance
Our business will expose it to potential product liability risks that are inherent in the testing, manufacturing and marketing of human pharmaceutical and therapeutic products. We do not currently have product liability insurance, and there can be no assurance that we will be able to obtain or maintain such insurance on acceptable terms or, if obtained, that such insurance will be adequate to cover potential product liability claims or that a loss of insurance coverage or the assertion of a product liability claim or claims would not materially adversely affect the business, financial condition and results of operations. We face an inherent business risk of exposure to product liability and other claims in the event that the development or use of its technology or products is alleged to have resulted in adverse effects. Such risk exists even with respect to those products that are manufactured in licensed and regulated facilities or that otherwise possess regulatory approval for commercial sale. There can be no assurance that we will avoid significant product liability exposure.
While we have taken, and will continue to take, what it believes are appropriate precautions, there can be no assurance that it will avoid significant liability exposure. An inability to obtain product liability insurance at acceptable cost or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of products developed. A product liability claim could have a material adverse effect on the business, financial condition and results of operations.
Uncertainties Relating to Pricing and Third-Party Reimbursement
Our operating results may depend in part on the availability of adequate reimbursement for our products from third-party payers, such as government entities, private health insurers and managed care organizations. Third-party payers are increasingly seeking to negotiate the pricing of medical services and products. In some cases, third-party payers will pay or reimburse a user or supplier of a product for only a portion of the purchase price of the product. In the case of the products, payment or reimbursement by third-party payers of only a portion of the cost of such products could make such products less attractive, from a cost perspective, to users, suppliers and physicians. There can be no assurance that reimbursement, if available, will be adequate. Moreover, certain of the products may not be of the type generally eligible for third-party reimbursement. If adequate reimbursement levels are not provided by government entities or other third-party payers for the products, the business, financial condition and results of operations would be materially adversely affected. A number of legislative and regulatory proposals aimed at changing the United States
’
health care system have been proposed in recent years. While we cannot predict whether any such proposals will be adopted, or the effect that any such proposal may have on its business, such proposals, if enacted, could have a material adverse effect on the business, financial condition or results of operations.
Risk of Product Recall; Product Returns
Product recalls may be issued at our discretion, the FDA or other government agencies having regulatory authority for product sales and may occur due to disputed labeling claims, manufacturing issues, quality defects or other reasons. No assurance can be given that product recalls will not occur
in the future. Any product recall could materially adversely affect the business, financial condition or results of operations. There can be no assurance that future recalls or returns would not have a material adverse effect upon the business, financial condition and results of operations.
Risks of International Sales and Operations
Our results of operations are subject to fluctuations in the value of the Euro against the U.S. Dollar due to our German subsidiary. Although management will monitor exposure to currency fluctuations, there can be no assurance that exchange rate fluctuations will not have a material adverse effect on the results of operations or financial condition. In the future, we could be required to sell its products in other currencies, which would make the management of currency fluctuations more difficult and expose us to greater risks in this regard.
Our products will be subject to numerous foreign government standards and regulations that are continually being amended. Although we will endeavor to satisfy foreign technical and regulatory standards, there can be no assurance that our products will comply with foreign government standards and regulations, or changes thereto, or that it will be cost effective for us to redesign its products to comply with such standards or regulations. The inability of us to design or redesign products to comply with foreign standards could have a material adverse effect on our business, financial condition and results of operations.
Lack of Commercial Manufacturing and Marketing Experience
We have not yet manufactured products in commercial quantities. The Company and its manufacturing contractors and partners will be engaged in manufacturing pharmaceutical products which will be subject to stringent regulatory requirements. No assurance can be given that we, on a timely basis, will be able to make the transition from manufacturing clinical trial quantities to commercial production quantities successfully or be able to arrange for contract manufacturing. The Company and our subsidiary have limited experience in the sales, marketing and distribution of products. There can be no assurance that we will be able to establish sales, marketing and distribution capabilities or make arrangements with collaborators, licensees or others to perform such activities or that such effort will be successful.
The manufacture of the products involves a number of steps and requires compliance with stringent quality control specifications imposed by us and by the FDA or similar regulatory bodies under the law of the respective countries. Typically, our products can only be manufactured in a facility that has undergone a satisfactory inspection by the FDA or similar regulatory bodies under the law of the respective countries. For these reasons, we would not be able to quickly replace its manufacturing capacity if one of our manufacturing contractors or partners were unable to use their manufacturing facilities as a result of a fire, natural disaster, equipment failure or other difficulty, or if such facilities are deemed not in compliance with the FDA's Good Manufacturing Practice (
“
GMP
”
) requirements and the non-compliance could not be rapidly rectified. The inability or reduced capacity to manufacture their products would have a material adverse effect on our business and results of operations.
We have entered and may enter into arrangements with contract manufacturing companies to expand its production capacities in order to satisfy requirements for its products, or to attempt to improve manufacturing efficiency. If we choose to contract for manufacturing services and encounters delays or difficulties in establishing relationships with manufacturers to produce, package and distribute its finished products, clinical trials, market introduction and subsequent sales of such products would be adversely affected. Further, contract manufacturers must also operate in compliance with the FDA's GMP requirements; failure to do so could result in, among other things, the disruption of product supplies.
Currently, we have our products manufactured by contract manufacturers in Germany. No assurance can be given, that the vendors will be willing or able to produce the products in the required quality or quantities or at prices which will enable us to sell the end products as requested by its customers.
Risks resulting from investing and financing activities
We may from time to time in the ordinary course of business carry out certain investing or financing transactions including extending loans to non-related third parties or the purchase of treasury stocks. Such transactions are subject to certain risks including but not limited to the inability of borrowers to redeem interest and principal of such loans, the inability of the company to capitalize on collaterals provided by the borrowers if any, or the devaluation of the treasury stock. In the event that one or more of these risks actually occur, the company may be confronted with the situation that it in turn may not be able to refinance its ongoing operations.
Hazardous Materials and Environmental Matters
Our research and development processes involve the controlled storage, use and disposal of hazardous materials. We are subject to federal, state and local laws and regulations governing the use, generation, manufacturing, storage, handling, and disposal of such materials and certain waste products. Although we do not currently manufacture commercial quantities of product candidates, we produce limited quantities of such products for clinical or preclinical trials or comparable laboratory testing necessary for research or product development and we may eventually intend to manufacture commercial quantities of our products. Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by the ISO 9001:2000 (General Quality Management System) and ISO 13485:2003 (Medical Products Quality Management System), the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result, and any such liability could exceed our resources. There can be no assurance that we will not be required to incur significant costs to comply with current or future environmental laws and regulations nor that the operations, business or assets will not be materially or adversely affected by current or future environmental laws or regulations.
Fluctuations in Foreign Currency Exchange Rates could have an Adverse Impact
Because a portion of our total income is derived from international operations that are conducted in foreign currencies, changes in value of these foreign currencies relative to the US dollar may affect our results of operation and financial position. If for any reason exchange or price controls or other restriction on the conversion of foreign currencies were imposed, our business could be adversely affected.
Risks Related to Our Securities
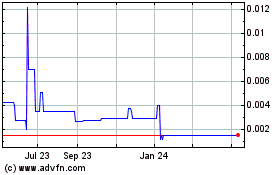
Trading and limited market
At the present time, the Company
’
s common stock is traded on the OTCQB under the symbol SGBI. There is currently a limited public market for the Common Stock and there can be no assurance that an active trading market will develop or, if one does develop, that it will be maintained. If such a market arise, the possibility or actual sale into the market of shares of the Company's Common Stock may adversely affect prevailing market prices for the Company's Common Stock and could impair the Company's ability to raise capital through the sale of its equity securities. In order to qualify for resale of our Common Stock under Rule 144, certain criteria must be met. There is no assurance that investors will be able to rely on its provisions of Rule 144 now or in the future.
No dividends
No cash dividends have been paid. Payment of dividends on the Common Stock is within the discretion of the Board of Directors, is subject to state law, and will depend upon the Company's earnings, if any, its capital requirements, financial condition and other relevant factors.
Possible volatility of stock price
The market price of the Company
’
s securities is likely to be highly volatile. Factors such as the market acceptance of the Company's products, success of distribution channels or its competitors, announcements of technological innovations or new commercial products by the Company or its competitors, developments in trademark, patent or other proprietary rights of the Company or its competitors, and fluctuations in the Company's operating results may have a significant effect on the market price of the Common Stock. In addition, the stock market has experienced and continues to experience extreme price and volume fluctuations which have affected the market price of many companies and which have often been unrelated to the operating performance of these companies. These broad market fluctuations, as well as general economic and political conditions, may adversely affect the market price, if a market develops, of the Common Stock. See
“
Description of Capital Stock
”
.
We are subject to the periodic reporting requirements of the Securities Exchange Act of 1934, which require us to incur audit fees and legal fees in connection with the preparation of such reports. These additional costs could reduce or eliminate our ability to earn a profit
We are required to file periodic reports with the Securities and Exchange Commission pursuant to the Securities Exchange Act of 1934 and the rules and regulations promulgated thereunder. In order to comply with these requirements, our independent registered public accounting firm has to review our financial statements on a quarterly basis and audit our financial statements on an annual basis. Moreover, our legal counsel will have to review and assist in the preparation of such reports. The costs charged by these professionals for such services cannot be accurately predicted at this time because factors such as the number and type of transactions that we engage in and the complexity of our reports cannot be determined at this time and will have a major effect on the amount of time to be spent by our auditors and attorneys. However, the incurrence of such costs will obviously be an expense to our operations and thus have a negative effect on our ability to meet our overhead requirements and earn a profit. We may be exposed to potential risks resulting from new requirements under Section 404 of the Sarbanes-Oxley Act of 2002. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock, if a market ever develops, could drop significantly.
We do not have a sufficient number of employees to segregate responsibilities and may be unable to afford increasing our staff or engaging outside consultants or professionals to overcome our lack of employees. During the course of our testing, we may identify other deficiencies that we may not be able to remediate in time to meet the deadline imposed by the Sarbanes-Oxley Act for compliance with the requirements of Section 404. In addition, if we fail to achieve and maintain the adequacy of our internal controls, as such standards are modified, supplemented or amended from time to time, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act. Moreover, effective internal controls, particularly those related to revenue recognition, are necessary for us to produce reliable financial reports and are important to help prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results
could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock, if a market ever develops, could drop significantly.
Management believes that these reporting obligations will increase the Company
’
s annual legal and accounting costs by an estimated $25,000 and $30,000, respectively.
Penny stock regulations
If the Company's stock is below $5.00 per share, or the Company does not have $2,000,000 in net tangible assets, or is not listed on an exchange or on the NASDAQ National Market System, among other conditions, the Company's shares may be subject to a rule promulgated by the Securities and Exchange Commission (the
“
SEC
”
) that imposes additional sales practice requirements on brokerdealers who sell such securities to persons other than established customers and institutional accredited investors. For transactions covered by the rule, the brokerdealer must make a special suitability determination for the purchaser and receive the purchaser's written consent to the transaction prior to the sale. Furthermore, if the price of the Company's stock is below $5.00, and does not meet the conditions set forth above, sales of the Company's stock in the secondary market will be subject to certain additional new rules promulgated by the SEC. These rules generally require, among other things, that brokers engaged in secondary trading of stock provide customers with written disclosure documents, monthly statements of the market value of penny stocks, disclosure of the bid and asked prices, and disclosure of the compensation to the brokerdealer and disclosure of the sales person working for the brokerdealer. These rules and regulations may affect the ability of brokerdealers to sell the Company's securities, thereby limiting the liquidity of the Company's securities. They may also affect the ability of shareholders to resell their securities in the secondary market.
Future sales of shares of our common stock by our shareholders could cause our stock price to decline.
Future sales of shares of our common stock could adversely affect the prevailing market price of our stock. If our significant shareholders sell a large number of shares, or if we issue a large number of shares, the market price of our stock could decline. Moreover, the perception in the public market that shareholders might sell shares of our stock could depress the market for our shares. If such shareholders sell substantial amounts of our common stock in the public market, such sales could create a circumstance commonly referred to as an
“
overhang
”
, in anticipation of which the market price of our common stock could fall. The existence of an overhang, whether or not sales have occurred or are occurring, also could make it more difficult for us to raise additional financing through the sale of equity or equity-related securities in the future at a time and price we deem reasonable or appropriate.
We may issue additional shares of our common stock or debt securities to raise capital or complete acquisitions, which would reduce the equity interest of our shareholders.
Although we have no commitments as of the date of this prospectus to issue our securities, we may issue a substantial number of additional shares of our common stock or debt securities to complete a business combination or to raise capital. The issuance of additional shares of our common stock may significantly reduce the equity interest of our existing shareholders and adversely affect prevailing market prices for our common stock.
ITEM 2.
PROPERTIES
The Company leases its office and laboratory facilities on a month-to-month basis and is housed in approximately 3,050 square feet based in the Forschungs und Entwicklungszentrum of the University Witten/Herdecke, Germany. Rent expense was approximately $32,018 and $45,831 during the years ended June 30, 2018 and 2017, respectively.
ITEM 3.
LEGAL PROCEEDINGS
We are not a party to any pending legal proceeding. No federal, state or local governmental agency is presently contemplating any proceeding against the Company. No director, executive officer or affiliate of the Company or owner of record or beneficiary of more than five percent of the Company's common stock is a party adverse to the Company or has a material interest adverse to the Company in any proceeding.
PART II
ITEM 5.
MARKET FOR REGISTRANT
’
S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
As of June 30, 2018, our common stock was traded on the OTCQB Venture Marketplace under the symbol SGBI as well as on the OTC markets of the Berlin and Hamburg-Hanover stock exchanges in Germany.
The following table sets forth the high and low closing prices for shares of SGBI common stock for the fiscal periods noted, as reported by OTCQB. Quotations reflect inter-dealer prices, without retail mark-up, markdown or commissions and may not represent actual transactions.
|
|
|
|
|
|
|
|
|
|
Common Stock
Closing Prices (US$)
|
|
|
|
High
|
|
Low
|
|
2018
|
|
$
|
|
$
|
|
Quarter ended September 2017
|
|
0.040
|
|
0.022
|
|
Quarter ended December 2017
|
|
0.080
|
|
0.019
|
|
Quarter ended March 2018
|
|
0.065
|
|
0.020
|
|
Quarter ended June 2018
|
|
0.040
|
|
0.015
|
|
2017
|
|
$
|
|
$
|
|
Quarter ended September 2016
|
|
|
0.01
|
|
|
0.0027
|
|
Quarter ended December 2016
|
|
0.045
|
|
0.06
|
|
Quarter ended March 2017
|
|
0.039
|
|
0.02
|
|
Quarter ended June 2017
|
|
0.0045
|
|
0.022
|
In addition to freely tradable shares, SGBI has numerous shares of common stock outstanding that could be sold pursuant to Rule 144. In general, under Rule 144, subject to the satisfaction of certain other conditions, a person, including one of our affiliates, who has beneficially owned restricted shares of common stock for at least one year is entitled to sell, in certain brokerage transactions, within any three-month period, a number of shares that does not exceed the greater of 1% of the total number of outstanding shares of the same class, or the average weekly trading volume during the four calendar weeks immediately preceding the sale. A person who presently is not and who has not been an affiliate for at least three months immediately preceding the sale and who has beneficially owned the shares of common stock for at least six months is entitled to sell such shares under Rule 144 without regard to any of the volume limitations described above.
Holders
On June 30, 2018, the number of record holders of the Company's common stock was approximately 869.
Dividends
The company did not pay any cash dividends during the past two fiscal years and does not contemplate paying dividends in the foreseeable future.
Securities Authorized For Issuance Under Equity Compensation Plans
The following table sets forth information as of June 30, 2018, with respect to our equity compensation plans previously approved by stockholders and equity compensation plans not previously approved by stockholders.
|
|
|
|
|
|
|
|
|
|
|
Equity Compensation Plan Information
|
|
Plan Category
[1]
|
|
Number of securities to
be issued upon exercise
of outstanding options,
warrants and rights
|
|
Weighted average
exercise price of
outstanding options,
warrants and rights
|
|
Number of securities
remaining available for
future issuance under
equity compensation
plans (excluding
securities reflected in
column (a))
|
|
|
|
(a)
|
|
(b)
|
|
(c)
|
|
Equity compensation plans approved by stockholders
|
|
-
|
|
$
|
0.00
|
|
-
|
|
Equity compensation plans not approved by stockholders
|
|
-
|
|
|
0.00
|
|
-
|
|
Total
|
|
-
|
|
$
|
0.00
|
|
-
|
[1]
On October 22, 2008 the Company adopted the 2008 Amended and Restated Long-Term Equity Incentive Plan, whereby the Board was authorized to issue up to 10,000,000 shares of common stock (including incentive stock options) to certain eligible employees, directors, and consultants of the Company or its subsidiaries.
By the end of our 2013 financial year all shares available under the Plan had been issued.
Issuer Purchases of Equity Securities
The Company holds 53,756 shares of its common stock. The treasury stock is valued using the Treasury Stock Method at $19,387.
Recent Sales of Unregistered Securities
In August, September and October, 2017, the Company issued 3,570,000 restricted shares of its common stock upon receipt of a capital contribution of approximately $ 88,925 in cash to one individual. No underwriters were used. The securities were sold pursuant to an exemption from registration provided by Regulation S and Section 4(2) of the Securities Act of 1933. The certificate representing the shares contained a restricted legend.
In May 2018, the Company issued 3,500,000 restricted shares of its common stock upon receipt of capital contributions of approximately $65,563 in cash to three individuals. No underwriters were used. The securities were sold pursuant to an exemption from registration provided by Regulation S and Section 4(2) of the Securities Act of 1933. The certificate representing the shares contained a restricted legend.
Sales of Unregistered Securities Subsequent to the Period Covered by this Report
Subsequent to the year ended June 30, 2018, the Company issued 1,000,000 shares of its common stock for cash to one individual at a stock price of $0.02054 No underwriters were used. The securities were sold pursuant to an exemption from registration provided by Regulation S and Section 4(2) of the Securities Act of 1933. The certificate representing the shares contained a restricted legend.
ITEM 6.
SELECTED FINANCIAL DATA
As a
“
smaller reporting company
”
(as defined by §229.10(f)(1)), we are not required to provide the information required by this Item.
ITEM 7.
MANAGEMENT
’
S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenue, expenses, and related disclosure of contingent assets and liabilities. We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our consolidated financial statements. Actual results may differ from these estimates under different assumptions or conditions.
CRITICAL ACCOUNTING POLICIES: Our significant accounting policies are described in Note 1 to the consolidated financial statements for the year ended June 30, 2018. The following are our critical accounting policies:
Revenue Recognition
The Company derives revenue primarily from licensing fees on sales of its wound spray product.
The Company is presently entitled to royalties on net sales of the wound spray product. The licensing fees are invoiced on a quarterly basis and are recognized as revenues during the quarter for which the sales were reported by the licensee.
The Company recognizes revenues when: (i) persuasive evidence of a sales arrangement exists, (ii) the sales terms are fixed and determinable, (iii) title and risk of loss have transferred, and (iv) collectability is reasonably assured, generally when products are shipped to the customer, except in situations in which title passes upon receipt of the products by the customer. In this case, revenues are recognized upon notification that customer receipt has occurred. The Company does not have customer acceptance provisions, but it does provide its customers a limited right of return. As warranted the Company accrues an estimated amount for sales returns and allowances at the time of sale based on its ability to estimate sales returns and allowances using historical information. Shipping and handling fees are included as part of net sales. The related freight costs and supplies associated with shipping products to customers are included as a component of cost of goods sold.
Research and Development
Research and development costs are charged to operations as they are incurred. Legal fees and other direct costs incurred in obtaining and protecting patents are expensed as incurred. Research
and development costs totaled $23,003 and $16,530 during the fiscal years ended June 30, 2018 and 2017, respectively.
Foreign Currency Translation
The functional currency of the Company
’
s Sangui GmbH and Sangui KG subsidiaries is the local currency, the Euro. Accordingly, assets and liabilities of the subsidiary are translated into U.S. dollars at period-end exchange rates. Sales and expenses are translated at the average exchange rates in effect for the period. The resulting translation gains or losses are recorded as a component of accumulated other comprehensive income in the consolidated statement of stockholders
’
equity (deficit). For the periods ending June 30, 2018 and 2017, the Company recognized foreign currency translation loss of ($29,306) and loss of ($562).
The exchange rates used to calculate values and results for the years ended June 30, 2018 and 2017 were as follows (USD):
|
|
|
|
|
|
Year-end Rates
|
|
Average Period Rates
|
|
June 30, 2018
|
0.856494
|
|
0.838296
|
|
June 30, 2017
|
0.876355
|
|
0.9177
|
FINANCIAL POSITION
Our current assets increased by $8,256, or 9%, from $93,773 at June 30, 2017 to $102,029 at June 30, 2018. The increase is primarily attributable to an increase in accounts receivables
.
Our net property and equipment $-0- at June 30, 2018 and at June 30, 2017. There were no investments in net property and equipment during the 2018 fiscal year.
We funded our operations primarily through sales of unregistered securities. The Company
’
s stockholders
’
deficit was increased from $260,523 at June 30, 2017 to $351,144 as of June 30, 2018. The primary reason for the increase in the deficit was the net loss of $201,810 and the loss on foreign currency translation of $29,306 offset by issuance of common stock totalling approximately $154,488.
REVENUES. Revenues increased 14% to $77,152 during the year ended June 30, 2018 from $67,653 in the previous fiscal year. This increase is due
amongst other things
to a rise in income from royalties due from sales of the wound spray product. We incurred Cost of Sales totaling $62 during the 2018 fiscal year, a 91% decrease from the prior year.
RESEARCH AND DEVELOPMENT. Research and development expenses increased to $23,003 during the year ended June 30, 2018 from $16,530 during the 2017 fiscal year. This increase is due
to higher fees for patent applications
during the 2018 fiscal year.
OTHER OPERATING EXPENSES. Professional fees for 2018 decreased to $121,478 from $225,901 in 2017 due to decreased legal fees. General and administrative expenses decreased by $674, due to a decrease in rent expenses. Overall Total Operating Expenses decreased by $98,624 or 26%.
OTHER INCOME (EXPENSE). Other Income (expense)
decreased
by $5,049 from $12,807 in 2017 to $7,758 in 2018. The
decrease
relates to a
decrease
in interest expense of $904, and an
increase
on losses on foreign currency exchange of $4,145.
NET LOSS. As a result of the above and other factors, the Company's consolidated net loss attributable to common stockholders was $ 201,810 or ($0.00) per common share in 2018, as compared to $308,980 or ($0.00) per common share in 2017.
LIQUIDITY AND CAPITAL RESOURCES
For the year ended June 30, 2018, net cash used in operating activities
decreased
to $253,761 from $323,103 for the year ended June 30, 2017, primarily related to the reduced net loss for the year offset by loss on equity investment and note receivable.
For the year ended June 30, 2018, net cash provided by financing activities
decreased
from $307,590 received during the fiscal year 2017 to net proceeds of $244,602 received in fiscal year 2018. The decrease came about due to a reduction of proceeds arising common stock issued offset by an increase of related party note payables.
We had net working capital deficit of $ 351,144 at June 30, 2018, compared to a deficit of $260,523 at June 30, 2017. The Company had a decrease in cash and prepaid expenses when compared to prior year. While accounts receivable and Tax refunds increased from prior year, there was an overall increase in accrued expenses, in related party payables, note payables and notes payables related parties when compared to prior year.
The Company incurred a net loss applicable to common stockholders of $201,810 and used cash in operating activities of $253,761 for the year ended June 30, 2018. These and other conditions raise substantial doubt about the Company's ability to continue as a going concern. The Company expects to continue to incur significant capital expenses in pursuing its business plan to market its products and expand its product line, while obtaining additional financing through stock offerings or other feasible financing alternatives. In order for the Company to continue its operations at its existing levels, the Company will require significant additional funds over the next twelve months. Therefore, the Company is dependent on funds raised through equity or debt offerings. Additional financing may not be available on terms favorable to the Company, or at all. If these funds are not available, the Company may not be able to execute its business plan or take advantage of business opportunities. The ability of the Company to obtain such additional financing and to achieve its operating goals is uncertain. In the event that the Company does not obtain additional capital or is not able to increase cash flow through the increase of sales, there is a substantial doubt of its being able to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Currently, it is the core strategy of the Company to license its technologies to industry partners. The current state of the sales efforts, in particular with regard to the Granulox product, distributed by our former joint venture partner, SastoMed, GmbH, has induced management to believe that income from these agreements can reasonably be anticipated to begin during the 2019 fiscal year. The Company will need substantial additional funding to fulfill its business plan. The Company intends to explore financing sources for its future development activities. No assurance can be given that these efforts will be successful.
ITEM 7A.
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As a
“
smaller reporting company
”
(as defined by §229.10(f)(1)), we are not required to provide the information required by this item.
ITEM 8.
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
SANGUI BIOTECH INTERNATIONAL, INC.
AUDIT REPORT OF INDEPENDENT ACCOUNTANTS
AND
CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2018 and 2017
SANGUI BIOTECH INTERNATIONAL, INC.
Table of Contents
Page
Report of Independent Registered Public Accounting Firm
F-1
Consolidated Balance Sheets
–
June 30, 2018 and 2017
F-3
Consolidated Statements of Operations and Comprehensive Loss for the years ended
June 30, 2018 and 2017
F-4
Consolidated Statements of Stockholders
’
Equity (Deficit) for the years ended
June 30, 2018 and 2017
F-5
Consolidated Statements of Cash Flows for the years ended June 30, 2018 and 2017
F-6
Notes to Consolidated Financial Statements
F-7
_______________________________________
29
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Sangui Biotech International, Inc.:
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Sangui Biotech International, Inc. (
“
the Company
”
) as of June 30, 2018 and 2017, the related consolidated statements of operations and comprehensive income (loss), stockholders
’
equity (deficit), and cash flows for each of the years in the two-year period ended June 30, 2018 and the related notes (collectively referred to as the
“
financial statements
”
). In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of June 30, 2018 and 2017, and the results of its operations and its cash flows for each of the years in the two-year period ended June 30, 2018, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph Regarding Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company
’
s management. Our responsibility is to express an opinion on the Company
’
s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (
“
PCAOB
”
) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company
’
s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Sadler, Gibb & Associates, LLC
We have served as the Company
’
s auditor since 2010.
Salt Lake City, UT
September 28, 2018
office: 801.783.2950
fax: 801.783.2960
www.sadlergibb.com 2455 East Parleys Way Suite 320, Salt Lake City, UT 84109
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SANGUI BIOTECH INTERNATIONAL, INC.
|
|
Consolidated Balance Sheets
|
|
|
|
|
|
|
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30,
|
|
June 30,
|
|
|
|
|
|
2018
|
|
2017
|
|
CURRENT ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
20,943
|
|
$
|
56,990
|
|
|
Tax refunds receivable
|
|
|
2,143
|
|
|
3,183
|
|
|
Accounts receivable, net
|
|
|
49,107
|
|
|
468
|
|
|
Note receivable, related party
|
|
|
7,062
|
|
|
6,470
|
|
|
Prepaid expenses and other assets
|
|
|
22,774
|
|
|
26,662
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Current Assets
|
|
102,029
|
|
|
93,773
|
|
|
|
|
|
|
|
|
|
|
|
PROPERTY AND EQUIPMENT, Net
|
|
|
-
|
|
|
-
|
|
|
|
TOTAL ASSETS
|
|
$
|
102,029
|
|
$
|
93,773
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES
|
|
|
|
|
|
|
Accounts payable and accrued expenses
|
$
|
189,739
|
|
$
|
188,855
|
|
|
Accrued interest - related party
|
|
|
19,088
|
|
|
12,214
|
|
|
Note payable
|
|
|
40,025
|
|
|
39,118
|
|
|
Notes payable - related party
|
|
|
204,321
|
|
|
114,109
|
|
|
|
Total Current Liabilities
|
|
453,173
|
|
|
354,296
|
|
|
|
|
|
|
|
|
|
|
|
COMMITMENTS & CONTINGENCIES
|
|
|
-
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS' EQUITY (Deficit)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock, no par value; 10,000,000 shares
|
|
|
|
|
|
|
|
authorized, -0- shares issued and outstanding
|
$
|
-
|
|
$
|
-
|
|
|
Common stock, no par value; 250,000,000 shares authorized
|
|
|
|
|
|
|
|
191,951,503 and 184,881,503 shares issued and
|
|
|
|
|
|
|
|
191,897,747 and 184,827,747 shares outstanding respectively
|
|
32,864,356
|
|
|
32,709,868
|
|
|
Additional paid-in capital
|
|
|
4,513,328
|
|
|
4,513,328
|
|
|
Treasury stock, at cost
|
|
|
(19,387)
|
|
|
(19,387)
|
|
|
Accumulated other comprehensive income
|
|
92,921
|
|
|
122,227
|
|
|
Accumulated deficit
|
|
|
(37,180,108)
|
|
|
(36,978,298)
|
|
|
Total Sangui Biotech International, Inc.'s Stockholder's equity
|
|
271,110
|
|
|
347,738
|
|
|
Non-controlling interest
|
|
|
(622,254)
|
|
|
(608,261)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Stockholders' Equity (Deficit)
|
|
(351,144)
|
|
|
(260,523)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT)
|
$
|
102,029
|
|
$
|
93,773
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SANGUI BIOTECH INTERNATIONAL, INC.
|
|
Consolidated Statements of Operations and Comprehensive Income (Loss)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Year Ended
|
|
|
|
|
June 30,
|
|
|
|
|
2018
|
|
2017
|
|
|
|
|
|
|
|
|
|
|
REVENUES
|
|
|
|
|
|
|
|
|
Product sales
|
|
$
|
77,152
|
|
$
|
67,653
|
|
COST OF SALES
|
|
|
62
|
|
|
693
|
|
|
|
|
|
|
|
|
|
|
GROSS MARGIN
|
|
|
77,090
|
|
|
66,960
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
23,003
|
|
|
16,530
|
|
|
Professional fees
|
|
|
121,478
|
|
|
225,901
|
|
|
General and administrative
|
|
|
140,654
|
|
|
141,328
|
|
Total Operating Expenses
|
|
|
285,135
|
|
|
383,759
|
|
OPERATING LOSS
|
|
|
(208,045)
|
|
|
(316,799)
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME (EXPENSE)
|
|
|
|
|
|
|
|
|
Gain (Loss) of foreign exchange
|
|
|
549
|
|
|
(3,596)
|
|
|
Interest expense
|
|
|
(8,307)
|
|
|
(9,211)
|
|
Total other income (expense)
|
|
|
(7,758)
|
|
|
(12,807)
|
|
|
|
|
|
|
|
|
|
LOSS BEFORE INCOME TAXES AND NON-CONTROLLING INTEREST
|
|
|
(215,803)
|
|
|
(329,606)
|
|
|
|
|
|
|
|
|
|
|
|
Provision for income taxes
|
|
|
-
|
|
|
-
|
|
NET LOSS BEFORE NON-CONTROLLING INTEREST
|
|
|
(215,803)
|
|
|
(329,606)
|
|
|
|
|
|
|
|
|
|
|
|
Less: Net loss attributable to non-controlling interest
|
|
|
13,993
|
|
|
20,626
|
|
|
|
|
|
|
|
|
|
|
NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS
|
|
$
|
(201,810)
|
|
$
|
(308,980)
|
|
|
|
|
|
|
|
|
|
|
OTHER COMPREHENSIVE INCOME (LOSS)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustments
|
|
|
(29,306)
|
|
|
(562)
|
|
COMPREHENSIVE INCOME (LOSS)
|
|
$
|
(245,109)
|
|
$
|
(330,168)
|
|
|
|
|
|
|
|
|
|
|
|
BASIC AND DILUTED LOSS PER SHARE
|
|
$
|
(0.00)
|
|
$
|
(0.00)
|
|
|
|
|
|
|
|
|
|
|
|
BASIC AND DILUTED WEIGHTED AVERAGE
|
|
|
|
|
|
|
|
|
NUMBER OF SHARES OUTSTANDING
|
|
|
188,204,782
|
|
|
178,461,623
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SANGUI BIOTECH INTERNATIONAL, INC.
|
|
Consolidated Statements of Stockholders' Equity (Deficit)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
|
|
|
Other
|
|
Non-
|
|
|
|
|
|
|
|
|
Common Stock
|
|
Paid-In
|
|
Treasury
|
|
Comprehensive
|
|
controlling
|
|
Accumulated
|
|
|
|
|
Shares
|
|
Amount
|
|
Capital
|
|
Stock
|
|
Income (Loss)
|
|
Interest
|
|
Deficit
|
|
Total
|
|
Balance, June 30, 2016
|
165,372,503
|
|
$
|
32,392,657
|
|
$
|
4,513,328
|
|
$
|
(19,387)
|
|
$
|
122,789
|
|
$
|
(587,635)
|
|
$
|
(36,669,318)
|
|
$
|
(247,566)
|
|
Common shares issued for cash
|
18,675,000
|
|
|
307,590
|
|
|
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
307,590
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for services
|
834,000
|
|
|
9,621
|
|
|
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
9,621
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Currency translation adjustment
|
-
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
(562)
|
|
|
-
|
|
|
-
|
|
|
(562)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the year ended June 30, 2017
|
-
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
(20,626)
|
|
|
(308,980)
|
|
|
(329,606)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2017
|
184,881,503
|
|
$
|
32,709,868
|
|
$
|
4,513,328
|
|
$
|
(19,387)
|
|
$
|
122,227
|
|
$
|
(608,261)
|
|
$
|
(36,978,298)
|
|
$
|
(260,523)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for cash
|
7,070,000
|
|
$
|
154,488
|
|
$
|
-
|
|
$
|
-
|
|
$
|
-
|
|
$
|
-
|
|
$
|
-
|
|
$
|
154,488
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Currency translation adjustment
|
-
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
(29,306)
|
|
|
-
|
|
|
-
|
|
|
(29,306)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the year ended June 30, 2018
|
-
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
-
|
|
|
(13,993)
|
|
|
(201,810)
|
|
|
(215,803)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, June 30, 2018
|
191,951,503
|
|
$
|
32,864,356
|
|
$
|
4,513,328
|
|
$
|
(19,387)
|
|
$
|
92,921
|
|
$
|
(622,254)
|
|
$
|
(37,180,108)
|
|
|
(351,144)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
|
|
|
|
|
|
|
|
|
|
|
SANGUI BIOTECH INTERNATIONAL, INC.
|
|
Consolidated Statements of Cash Flows
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Year Ended
|
|
|
|
|
|
June 30,
|
|
|
|
|
|
2018
|
|
2017
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
|
|
|
|
|
|
|
Net loss
|
$
|
(215,803)
|
|
$
|
(329,606)
|
|
|
Adjustments to reconcile net loss to net cash
|
|
|
|
|
|
|
|
used by operating activities:
|
|
|
|
|
|
|
|
|
Common stock issued for services
|
|
-
|
|
|
9,620
|
|
|
|
Gain on foreign currency exchange transactions
|
|
99
|
|
|
3,169
|
|
|
Changes in operating assets and liabilities
|
|
|
|
|
|
|
|
|
Trade accounts receivable
|
|
(48,628)
|
|
|
48
|
|
|
|
Prepaid expenses and other current assets
|
|
4,282
|
|
|
4,294
|
|
|
|
Tax refunds receivable
|
|
1,113
|
|
|
929
|
|
|
|
Accounts payable and accrued expenses
|
|
(1,254)
|
|
|
(14,546)
|
|
|
|
Related party accounts payable
|
|
6,873
|
|
|
3,792
|
|
|
|
Related party advances
|
|
(443)
|
|
|
(803)
|
|
|
|
|
Net Cash Used in Operating Activities
|
|
(253,761)
|
|
|
(323,103)
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
|
|
|
|
|
|
|
|
Proceeds from related party note payable
|
|
90,113
|
|
|
-
|
|
|
|
Proceeds from common stock issued for cash
|
|
154,489
|
|
|
307,590
|
|
|
|
|
Net Cash Provided by Financing Activities
|
|
244,602
|
|
|
307,590
|
|
|
|
|
|
|
|
|
|
|
|
EFFECTS OF EXCHANGE RATES
|
|
(26,888)
|
|
|
2,428
|
|
|
|
|
|
|
|
|
|
|
|
|
NET CHANGE IN CASH
|
|
(36,047)
|
|
|
(13,085)
|
|
|
CASH AT BEGINNING OF PERIOD
|
|
56,990
|
|
|
70,074
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH AT END OF PERIOD
|
$
|
20,943
|
|
$
|
56,989
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOW INFORMATION
|
|
|
|
|
|
|
|
CASH PAID FOR:
|
|
|
|
|
|
|
|
|
Interest
|
$
|
1,647
|
|
$
|
1,542
|
|
|
|
|
|
|
|
|
|
|
|
|
NON CASH INVESTING AND FINANCING ACTIVITIES
|
$
|
-
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
F-3
SANGUI BIOTECH INTERNATIONAL, INC.
Notes to Consolidated Financial Statements
June 30, 2018 and 2017
NOTE 1
–
ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Organization and Nature of Business
Sangui Biotech International, Inc. (
“
the Company
”
) was incorporated in Colorado in 1995. Since 2003 when a comprehensive restructuring of the group was completed, all operations have been carried out by Sangui BioTech GmbH, its 90% owned subsidiary which is headquartered in Witten, Germany. Sangui Biotech International, Inc., (
“
the Parent Company
”
) acts as a holding company whose purpose it is to secure financing and access to the capital markets. Effective from June 18, 2018 Sangui BioTech GmbH together with Sastomed GmbH founded Sangui Know-How- und Patentverwertungsgesellschaft mbH & Co. KG (
“
Sangui KG
”
). Sangui KG is a limited partnership, with Sangui BioTech GmbH as the general partner (owing 99.8%) and Sastomed GmbH as a limited partner (owning 0.2%).
Sangui BioTech GmbH is engaged in the development of technologies aimed at improved supply of oxygen to the human body such as wound management products in particular a wound spray based on natural hemoglobin, wound dressings based on Chitosan (a natural polymer), artificial oxygen carriers (external applications of hemoglobin, blood substitutes and blood additives) and cosmetics. The cosmetics products are currently being sold via the Company
’
s internet shop, yielding a small amount of revenues. Otherwise, the Company does not produce nor market its products. It has adopted the strategy to license its technologies to industry partners in exchange for royalties. In the pursuit of this strategy, the Company established a joint venture company in December 2010 for the purposes of marketing and selling the wound spray product in Germany and of preparing its market entry in several other European countries and Mexico. As consideration for the license, the Company is paid royalties on all sales of this product and is entitled to a 25 percent share of all future profits of the joint venture. Effective December 31, 2017 the Company sold its 25% share of the joint venture to its co-partner. On June 22, 2018, Sangui KG has acquired all rights in the license agreement concluded on December 17, 2010 with Sastomed GmbH from Sangui BioTec GmbH.
Going Concern
The Company incurred a net loss attributable to common stockholders of $ 201,810 and used cash in operating activities of $253,761 for the year ended June 30, 2018. These and other conditions raise substantial doubt about the Company's ability to continue as a going concern. The Company expects to continue to incur significant capital expenses in pursuing its business plan to market its products and expand its product line; however, obtaining additional financing through stock offerings or other feasible financing alternatives may be difficult or even impossible. In order for the Company to continue operating at its existing levels, it will require significant additional funds over the next twelve months. Therefore, the Company is dependent on funds raised through equity or debt offerings. The Company plans to continue to raise necessary capital through both notes payable, as well as stock sales.
Additional financing may not be available on terms favorable to the Company or at all. If these funds are not available the Company may not be able to execute its business plan or take advantage of business opportunities. The Company
’
s ability to obtain such additional financing and to achieve its operating goals is uncertain. In the event that the Company does not obtain additional capital or is not able to increase cash flow through the increase of sales, there is a substantial doubt of its being able to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Principles of Consolidation
The consolidated financial statements include the accounts of Sangui BioTech International, Inc., its 90% owned foreign subsidiaries, Sangui BioTech GmbH and Sangui KG. All intercompany accounts and transactions have been eliminated in consolidation.
NOTE 1
–
ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the respective reporting period. As future events and their effects cannot be determined with precision, actual results could differ from those estimates. Significant estimates made by management are, among others, the realization of receivables, inventories, long-lived assets, and valuation allowance on deferred tax assets.
Due to the current dependence of Sangui on the revenue from the license agreement with Sastomed GmbH, the management places the highest priority on the sales development in this area in order to be able to recognize potential risks in good time and to take appropriate measures if necessary. These measures include regular and ad hoc discussions with the licensee about its planned business development.
Risks and Uncertainties
The Company's line of future pharmaceutical and cosmetic products (artificial oxygen carriers or blood substitute and additives) as well as other medical products being developed by Sangui BioTech GmbH, are deemed as medical devices or biologics, and as such are governed by the Federal Food and Drug and Cosmetics Act and by the regulations of state agencies and various foreign government agencies. The pharmaceutical products will be subject to stringent regulatory requirements because they are in vivo products for humans. The Company and its subsidiaries have limited experience in obtaining regulatory clearance on these types of products. Therefore, the Company could be subject to risks of delays in obtaining or failing to obtain regulatory clearance.
Financial Instruments
Pursuant to ASC 820,
Fair Value Measurements and Disclosures
, an entity is required to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument
’
s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 820 prioritizes the inputs into three levels that may be used to measure fair value:
Level 1
Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2
Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3
Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.The Company
’
s financial instruments consist principally of cash, accounts and notes receivable, accounts payable and accrued liabilities, notes payable and amounts due to related parties. We believe that the recorded values of all of our other financial instruments approximate their current fair values because of their nature and respective maturity dates or durations.
NOTE 1
–
ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Foreign Currency Translation
The functional currency of the Company
’
s Sangui GmbH subsidiary and Sangui KG subsidiary is the local currency, the Euro. Accordingly, assets and liabilities of the subsidiary are translated into U.S. dollars at period-end exchange rates. All equity account balances have been translated at the historical rates. Revenues and expenses are translated at the average exchange rates in effect for the period. The resulting translation gains or losses are recorded as a component of accumulated other comprehensive income in the consolidated statement of stockholders
’
equity. For the years ended June 30, 2018 and 2017, the Company recognized a loss on translation adjustment in the amount of $29,306 and a loss of $562, respectively. Gains of $549
respectively
losses of $3,596 resulting from foreign currency transactions as of June 30, 2018 and 2017.
The exchange rates used to calculate values and results of operations for the years ended June 30, 2018 and 2017, were as follows:
|
|
|
|
|
|
Year-end Rates
|
|
Average Period Rates
|
|
June 30, 2018
|
0.856494
|
|
0.838296
|
|
June 30, 2017
|
0.876355
|
|
0.917966
|
Pursuant to ASC 830-20-35,
Foreign Currency Matters
, the Company accounts for the translation of transactions denominated in foreign currencies in the Parent Company
’
s books as transaction gains (losses) recognized in General & Administrative expenses.
Cash and Cash Equivalents
The Company considers highly liquid investments with insignificant interest rate risk and original maturities to the Company of three months or less to be cash equivalents. The Company maintains its cash in uninsured bank accounts in Germany. Cash and cash equivalents include time deposits for which the Company has no requirements for compensating balances. The Company has not experienced any losses in its uninsured bank accounts. The Company had no cash equivalents outstanding as of June 30, 2018 and 2017.
Property and Equipment
Property and equipment are recorded at cost and are depreciated or amortized using the straight-line method over the expected useful lives, which range from three to five years. Leasehold improvements are amortized using the straight-line method over the lesser of the estimated useful lives of the assets or the related lease terms. Depreciation expense for the years ended June 30, 2018 and 2017 was $0 and $0, respectively. Expenditures for normal maintenance and routine repairs are charged to expense, and significant improvements are capitalized. The cost and related accumulated depreciation of assets are removed from the accounts upon retirement or other disposition; any resulting gain or loss is reflected in the statement of operations.
Impairment of Long-Lived Assets
Long-lived assets, including property and equipment and certain identifiable intangibles to be held and used are reviewed by the management of the Company for impairment whenever events or changes in circumstances indicate that the carrying value of an asset or asset group may not be recoverable. On a regular basis and at least annually, the Company evaluates whether events and circumstances have occurred that indicate possible impairment and relies on a number of factors, including business plans, economic projections, and anticipated future cash flows. Measurement of the amount of impairment, if any, is based upon the difference between the asset
’
s carrying value and estimated fair value. As of June 30, 2018 and 2017, management of the Company believes that no impairment has been indicated. There can be no assurance, however, that market conditions will not change or demand for the Company's products will continue which could result in impairment of long-lived assets in the future.
NOTE 1
–
ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Revenue Recognition
The Company derives revenue primarily from licensing fees on sales of its wound spray product as well as from the sale of its cosmetics products.
The wound spray technology is licensed to an entity, in which the Company held a 25 percent equity interest, as a Joint Venture (See Note 2). Effective December 31, 2015 the Company sold its 25 % share of the joint venture to its co-partner. The Company presently is entitled to royalties on net sales of the wound spray product. Licensing fees are invoiced on a quarterly basis and are recognized as revenue during the quarter that the sales were reported by the licensee.
The Company
’
s product sales (amounting to less than 10% of total revenues in the current year) are generated via online orders, with credit card payment. The Company recognizes revenues when: (i) persuasive evidence of a sales arrangement exists, (ii) the sales terms are fixed and determinable, (iii) title and risk of loss have transferred, and (iv) collectability is reasonably assured
—
generally when products are shipped to the customer, except in situations in which title passes upon receipt of the products by the customer. In this case, revenues are recognized upon notification that customer receipt has occurred. The Company does not have customer acceptance provisions, but it does provide its customers a limited right of return. The Company accrues an estimated amount for sales returns and allowances at the time of sale, based on its ability to estimate sales returns and allowances using historical information. Shipping and handling fees are included as part of net sales. The related freight costs and supplies associated with shipping products to customers are included as a component of cost of goods sold.
Trade Accounts Receivable
Accounts receivable are reflected at estimated net realizable value, do not bear interest and generally require collateral. The Company maintains an allowance for doubtful accounts based upon a variety of factors. The Company reviews all open accounts and provides specific reserves for customer collection issues when it believes a loss is probable.. The reserve estimate includes consideration of such factors as the length of time receivables are past due, the financial condition of the customer, and historical experience. The Company also records a reserve for all customers, excluding those that have been specifically reserved for, based upon evaluation of historical losses which exceeded the specific reserves the Company had established. For the years ended June 30, 2018 and 2017, the Company recognized bad debt expense in the amounts of $0 and $0, respectively.
Inventory
Inventory is stated at the lower of cost (computed on a first-in, first-out basis) or market value. Provisions to value the inventory at the lower of the actual cost to purchase or manufacture the inventory, or the current estimated market value of the inventory, are based upon assumptions about future demand and market conditions. The Company also performs evaluations of inventory and records a provision or impairment for estimated excess and obsolete items based upon demand, and any other known factors at the time. As of June 30, 2018 and 2017, all inventory balances had been reserved against in full.
Concentration of Credit Risk
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivable. The Company performs ongoing credit evaluations of its customers and maintains allowances for potential credit losses. Sales to the Company's largest customer represents over 95% of the Company's revenues.
NOTE 1
–
ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Sales Tax Collected from Customers
As a part of the Company
’
s normal course of business, sales taxes are collected from customers. Sales taxes collected are remitted, in a timely manner, to the appropriate governmental tax authority on behalf of the customer. The Company
’
s policy is to present revenue and costs net of sales taxes.
Income Taxes
The Company accounts for income taxes in accordance with ASC 740. Deferred tax assets and liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be realized or settled. The effect on deferred income tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Deferred income tax assets are reviewed for recoverability and the Company records a valuation allowance to reduce deferred income tax assets when it is more likely than not that such deferred tax assets will not be realized.
The Company has foreign subsidiaries formed or acquired to conduct or support its business outside the United States. The Company provides for income taxes, net of applicable foreign tax credits, on temporary differences in its investment in foreign subsidiaries which are not considered to be permanently invested outside of the United States.
The Company adopted ASC 740 which defines the threshold for recognizing the benefits of tax return positions in the financial statements as
“
more-likely-than-not
”
to be sustained by the taxing authority. A tax position that meets the
“
more-likely-than-not
”
criterion shall be measured at the largest amount of benefit that is more than 50 percent likely of being realized upon ultimate settlement. ASC 740 also provides guidance on derecognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures. ASC 740 applies to all tax positions accounted for under ASC 740. Estimated interest and penalties related to the underpayment of income taxes are recorded as a component of provision for income taxes in the consolidated statements of operations. For the years ended
June 30, 2018 and 2017, the Company did not recognize any such interest or penalties, nor were any interest fees or penalties accrued as of June 30, 2018 and 2017.
Research and Development
Research and development costs are charged to operations as they are incurred. Legal fees and other direct costs incurred in obtaining and protecting patents are also expensed as incurred, due to the uncertainty with
respect to future cash flows resulting from the patents. Research and development costs totaled $23,003 and $16,530 during the fiscal years ended June 30, 2018 and 2017, respectively.
Basic and Diluted Loss per Common Share
Basic loss per common share excludes dilution and is computed by dividing loss available to common stockholders by the weighted average number of common shares outstanding during the period of computation. Diluted loss per share gives effect to all potential dilutive common shares outstanding during the period of compensation. The computation of diluted loss per share does not assume conversion, exercise or contingent exercise of securities that would have an antidilutive effect on earnings. As of June 30, 2018 and 2017, the Company had no potentially dilutive securities that would affect the loss per share if they were to be included in the loss per share.
NOTE 1
–
ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Comprehensive Loss
Total comprehensive loss represents the net change in stockholders' equity during a period from sources other than transactions with stockholders and as such, includes net loss. For the Company, the components of other comprehensive loss are the changes in the cumulative foreign currency translation adjustments.
Segments of an Enterprise and Related Information
The Company adopted ASC 280, "Disclosures about Segments of an Enterprise and Related Information." ASC 280 establishes standards for the way public companies report information about segments of their business in their annual financial statements and requires them to report selected segment information in their quarterly reports issued to stockholders. It also requires entity-wide disclosures about the products and services an entity provides, the material countries in which it holds assets and reports revenues and its major customers, if any. As of June 30, 2018 and 2017, the Company has one business segment, which is includes the manufacturing and sales of its wound treatment and cosmetic products as well as the licensing of business partners to do the same.
Non-controlling Interests
On June 11, 2008, the Company
’
s wholly-owned German subsidiary, Sangui Biotech GmbH (
“
GmbH
”
) issued 11,400 shares of its previously unissued common stock for cash proceeds of $1,140,759. These shares amount to 10 percent of the GmbH
’
s total outstanding common stock, which totaled 113,800 shares of as June 30, 2018 and 2017, respectively. The Company accounts for these non-controlling interests pursuant to ASC 810 whereby gains or losses in a subsidiary with a non-controlling interest are allocated to the non-controlling interest based on the ownership percentage of the non-controlling interest, even if that allocation results in a deficit non-controlling interest balance.
As stated above, effective June 18, 2018 GmbH founded Sangui KG as a limited partnership. As a result, the business activity and operations of Sangui KG are included in those of GmbH and are therefore subject to the same non-controlling interests accounting guidance as that of GmbH, adjusted for GmbH's 0.2% non-controlling interest in Sangui KG. Due to the fact that there were relatively few days in the period
between its founding and the end of the business year, the business activity of Sangui KG were negligible.
Recent Accounting Pronouncements
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606) (
“
ASU 2014-09
”
), which amends the existing accounting standards for revenue recognition. ASU 2014-09 is based on principles that govern the recognition of revenue at an amount an entity expects to be entitled when products are transferred to customers.
Subsequently, the FASB has issued the following standards related to ASU 2014-09: ASU No. 2016-08, Revenue from Contracts with Customers (Topic 606): Principal versus Agent Considerations (
“
ASU 2016-08
”
); ASU No. 2016-10, Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing (
“
ASU 2016-10
”
); ASU No. 2016-12, Revenue from Contracts with Customers (Topic 606): Narrow-Scope Improvements and Practical Expedients (
“
ASU 2016-12
”
); and ASU No. 2016-20, Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers (
“
ASU 2016-20
”
). The Company must adopt ASU 2016-08, ASU 2016-10, ASU 2016-12 and ASU 2016-20 with ASU 2014-09 (collectively, the
“
new revenue standards
”
).
The new revenue standards may be applied retrospectively to each prior period presented or retrospectively with the cumulative effect recognized as of the date of adoption. The Company will adopt the new revenue standards in its first quarter of fiscal year 2019. The new revenue
standards are not expected to have a material impact on the amount and timing of revenue recognized in the Company
’
s consolidated financial statements.
NOTE 1
–
ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Financial Statement Reclassifications
The Company has reclassified certain prior-year account balances in order to comply with current-period classifications and increase comparability.
NOTE 2
–
INVESTMENT IN JOINT VENTURE
During December 2010, the Company
’
s subsidiary Sangui BioTech GmbH established a joint venture company with SanderStrothmann GmbH, under the name of SastoMed GmbH (
“
the Joint Venture
”
). The Company owned 25 percent of the Joint Venture and accounted for its interest in the Joint Venture using the equity method of accounting. The Company invested $8,508 in the Joint Venture during the year ended June 30, 2011. The Company has written the investment down to $0 for its share of the Joint Venture
’
s losses, amounting to $112,819 during the calendar year ending December 31, 2014.
The Company entered into an agreement which is cancellable by either party, with 14 days written notice. The agreement includes payments to its joint venture partner (SastoMed GmbH) by the Company of $7,760 per month for research and development consulting services. The agreement was terminated September 30, 2017.
On June 9, 2015, the Company entered into a note payable with the Joint Venture for 32,863 Euros. The note payable accrues interest at 4% annum and is due June 30, 2018.
Effective December 31, 2015 the company sold its interest in the SastoMed joint venture for Euro 6,250, resulting in a gain reported in Other Income of $6,936. The sale of the joint venture terminated the relationship with SastoMed however, the licensing agreement is still in effect. Accordingly the note payable of 34,282 Euros, which was previously recorded as a related party note payable, is now reclassified as non-related party note payable. As of June 30, 2018 and 2017 the balance due was $40,025 and $39,119.
NOTE 3
–
PROPERTY AND EQUIPMENT
Property and equipment consist of the following at June 30, 2018 and 2017:
|
|
|
|
|
|
|
|
June 30,
|
|
|
2018
|
|
2017
|
|
Technical and laboratory equipment
|
$
|
641,326
|
|
$
|
641,326
|
|
Leasehold improvements
|
|
285,189
|
|
|
285,189
|
|
Office equipment and furniture
|
|
311,371
|
|
|
311,371
|
|
Total property and equipment
|
|
1,237,886
|
|
|
1,237,886
|
|
Less accumulated depreciation and amortization
|
|
(1,237,886)
|
|
|
(1,237,886)
|
|
Total property and equipment, net
|
$
|
-
|
|
$
|
-
|
NOTE 4
–
RELATED PARTY TRANSACTIONS
As of June 30, 2018 and 2017, the Company has recorded $19,088 and $12,214, respectively, in accounts payable to related parties for accrued interest from the related party loans payable below.
Related Party Loans Payable
Prior to 2016, the Company entered into a note payable with a Company Director for 100,000 Euros ($116,755 as of June 30, 2018). The note payable accrues interest at 5 percent per annum, is due on June 30, 2019 and is unsecured. As of June 30, 2018, the note has an accrued interest balance of $18,335.
On December 12, 2017 a Company Director advanced an amount of 25,000 Euros ($29,189 as of June 30, 2018) to the Company. The loan is due on demand, accrues interest annually at 2% and is unsecured. As of June 30, 2018, the note has an accrued interest balance of $320.
On January 19, 2018 a Company Director advanced an amount of 25,000 Euros ($29,189 as of June 30, 2018) to the Company. The loan is due on demand, accrues interest annually at 2% and is unsecured. As of June 30, 2018, the note has an accrued interest balance of $259.
On March 13, 2018 a Company Director advanced an amount of 25,000 Euros ($29,189 as of June 30, 2018) to the Company. The loan is due on demand, accrues interest annually at 2% and is unsecured. As of June 30, 2018, the note has an accrued interest balance of $174.
NOTE 5
–
STOCKHOLDERS' EQUITY
Preferred Stock
The Company is authorized to issue 10,000,000 shares of preferred stock. The authorized preferred shares are non-voting and the Board of Directors has not designated any liquidation value or dividend rates. During the financial years ended June 30, 2018 and 2017 no shares of preferred stock were issued or outstanding.
Common Stock
The Company is authorized to issue 250,000,000 shares of common stock with no par value. The holders of the Company's common stock are entitled to one vote for each share held of record on all matters to be voted on by those stockholders.
Common Stock Issuances
During the year ended June 30, 2018 the Company issued 7,070,000 shares of common stock for cash at an average of $0.0219 per share, yielding total cash proceeds of $154,488.
During the year ended June 30, 2017 the Company issued 834,000 shares of common stock for services at an average of $0.0115 per share for a total cost of $9,620. In addition, the Company issued 18,675,000 shares of common stock for cash at an average of $0.0165 per share, yielding total cash proceeds of $307,590. The shares issued for services were valued at the trading price of the common stock on the date the shares were issued.
On May 11, 2015, the Company entered into an equity purchase agreement (the
“
EPA
”
) with an unrelated investor (
“
the Investor
”
). The EPA is a put option contract wherein, at the Company
’
s sole discretion, up
to $5,000,000 of common stock may be sold to the Investor for a period of 3 years ending May 2018. A note payable was entered into as consideration to the Investor for execution of the EPA.
On August 14, 2015, the company issued 43,189 shares of common stock to the investor in pursuit of the EPA yielding total cash proceeds of $1,500 which was received by the company on September 15, 2015. The EPA expired during the year and no additional common shares were issued.
Stock Options
From time to time, the Company may issue stock options pursuant to various agreements and other contemporary agreements. At June 30, 2018 and 2017, and during the years ended June 30, 2018 and 2017, no options were issued or outstanding.
NOTE 6 - INCOME TAX PROVISION
Treasury Shares
The Company holds 53,756 of its common stock as treasury stock, which is valued at cost of $19,387, at June 30, 2018 and 2017.
The Company
’
s provision for income taxes was $-0- and $-0- for the years ended June 30, 2018 and 2017 respectively, since the Company incurred net operating losses through June 30, 2018.
Income tax expense for the years ended June 30, 2018 and 2017 differed from the amounts computed by applying the U.S. federal income tax rate of 21 percent as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30,
|
|
June 30,
|
|
|
|
2018
|
|
2017
|
|
Income tax benefit at U.S. federal statutory rates
|
$
|
(42,380)
|
$
|
(64,886)
|
|
Effect of:
|
|
|
|
|
|
Changes in derivative liability
|
|
-
|
|
-
|
|
Loss on sale of asset
|
|
-
|
|
-
|
|
Increase (decrease) in valuation allowance
|
|
42,380
|
|
64,886
|
|
Provision for income taxes
|
$
|
-
|
$
|
-
|
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets at June 30, 2018 and 2017 are presented below:
|
|
|
|
|
|
|
|
June 30,
|
|
June 30,
|
|
|
|
2018
|
|
2017
|
|
Deferred tax assets
|
|
|
|
|
|
Net operating losses
|
$
|
(7,789,678)
|
|
(7,747,298)
|
|
Common stock issued for services
|
|
130,273
|
|
130,273
|
|
Debt issued for financing costs
|
|
10,500
|
|
10,500
|
|
Impairment of related parties receivables
|
|
269,541
|
|
269,541
|
|
Change in derivative liabilities
|
|
5,892
|
|
5,892
|
|
Gain on sale of assets
|
|
1,456
|
|
1,456
|
|
Increase (decrease) in valuation allowances
|
|
7,372,017
|
|
7,329,637
|
|
Net deferred taxes
|
$
|
-
|
$
|
-
|
As of June 30, 2018, the Company had net operating loss carryforwards of approximately $35.1million which is available to offset future taxable federal, state and foreign income. The federal and state carryforward amounts expire in varying amounts between 2018 and 2030. The foreign net operating loss carryforwards do not have an expiration period.
NOTE 6 - INCOME TAX PROVISION (CONTINUED)
The Company has evaluated its uncertain tax positions and determined that any required adjustments for unrecognized tax benefits would not have a material impact on the Company
’
s balance sheet, income statement, or statement of cash flows.
The Company
’
s tax filings for 2012 through 2017 remain subject to examination by tax authorities for federal income tax purposes and by other major taxing jurisdictions to which we are subject. The Company has identified potential penalties for the late filing of reports to taxing authorities. The Company believes that it is more likely than not the penalties will be waived and accordingly has not accrued the penalties in the financial statements.
NOTE 7
–
COMMITMENTS AND CONTINGENCIES
Indemnities and Guarantees
During the normal course of business, the Company has made certain indemnities and guarantees under which it may be required to make payments in relation to certain transactions. These indemnities include certain agreements with the Company's officers, under which the Company may be required to indemnify such person for liabilities arising out of their employment relationship. The duration of these indemnities and guarantees varies and, in certain cases, is indefinite. The majority of these indemnities and guarantees do not provide for any limitation of the maximum potential future payments the Company could be obligated to make. Historically, the Company has not been obligated to make significant payments for these obligations. The Company has recorded a reserve for indemnities and guarantees of $-0- as of June 30, 2018 and 2017.
Leases
The Company leases office facilities from an unrelated third party at Euro 2,491 per month. The office lease contract is maintained on a month-to-month basis.
The Company also leases an automobile under an operating lease. The lease provides for a lease payment of 669 Euros per month beginning August 2015 expiring July 2018.
NOTE 8
–
STOCK-BASED COMPENSATION
The Company has applied the disclosure provisions of ASC 718 for the years ended June 30, 2018 and 2017. There were no common shares or stock options outstanding, issued or granted to employees during these reporting periods.
On April 28, 2004, the Company adopted the 2004 Employee Stock Incentive Plan (
“
the Plan
”
). Under the terms of this plan the Board was authorized to issue up to 1,000,000 shares of common stock to certain eligible employees of the Company or its subsidiaries. All of these shares were issued pursuant to the plan prior to June 30, 2007. On September 22, 2008 the Company adopted the 2008 Amended and Restated Long-Term Equity Incentive Plan, whereby the Board was authorized to issue up to 10,000,000 shares of common stock (including incentive stock options) to certain eligible employees, directors, and/or consultants of the Company or its subsidiaries. During the years ended June 30, 2018 and 2017, respectively, the Company issued no shares pursuant to this Plan. All shares available under the 2008 Long-Term Equity Incentive Plan had been issued as of June 30, 2018.
NOTE 9
–
NOTE PAYABLE
On June 9, 2015, the Company entered into a note payable with the Joint Venture for 32,863 Euros (see note 2). The note payable accrues interest at 4% annum and is due June 30, 2018. Accordingly the note payable and accrued interest of 34,282 Euros, which was previously recorded as a related party note payable, is now reclassified as non-related party note payable. The principal and accrued interest of the note at June 30, 2018 was $40,025.
NOTE 10
–
SUBSEQUENT EVENTS
Subsequent to the year ended June 30, 2018, the Company issued 1,000,000 shares of common stock for cash at $0.02054 per share to one investor, yielding total cash proceeds of $20,537.
On September 10, 2018 a Company Director advanced an amount of 25,000 Euros ($29,189; converted with exchange rate of June 30, 2018) to the Company. The loan is due on demand, accrues interest annually at 2% and is unsecured.
F-7
ITEM 9.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A.
CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
As of the date of the end of the period covered by this report, our Chief Executive Officer and Chief Financial Officer conducted an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures, as required by Exchange Act Rule 13a-15. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were not effective as of the end of the period covered by this report to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified by the SEC
’
s rules and forms.
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC
’
s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed under the Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and our Chief Financial Officer, to allow timely decisions regarding required disclosure.
Management
’
s Annual Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Exchange Act Rule 13a-15(f). Management conducted an evaluation of the effectiveness of the internal control over financial reporting as of June 30, 2018, using the criteria established in
Internal Control
–
Integrated Framework
(2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a control deficiency, or combination of control deficiencies, that results in more than a remote likelihood that a material misstatement of the annual or interim financial statements will not be prevented or detected. Based on the evaluation of the effectiveness of the internal controls over financial reporting as of June 30, 2018, management has concluded that our internal controls over financial reporting were not effective as of the end of the period covered by this report.
As a result of management
’
s assessment, management has determined that there is a material weakness due to the lack of segregation of duties. In order to address and resolve this weakness we will endeavor to locate and appoint additional qualified personnel to the board of directors and pertinent officer positions as our financial means allow. To date, our limited financial resources have not allowed us to hire the additional personnel necessary to address this material weakness.
Additionally, as a result of management
’
s assessment, management has determined that there is a significant deficiency with regard to the lack of a backup process for electronic financial information. There is no stored backup offsite or in a media safe, and as such, there are no regularly run test restorations of said financial information. In order to address and resolve this deficiency we are currently
34
researching the options available given our financial means to have a regularly scheduled and dependable offsite backup of our Company records.
Lastly, the Company has not instituted specific anti-fraud controls. While management found no evidence of fraudulent activity, the chief accounting officer has access to both accounting records and corporate assets, principally the operating bank account. Management believes this exposure to potential fraudulent activity is not significant either to the operations of the company or to the financial reporting; however, management is in the process of instituting controls specifically designed to address this material weakness, so as to prevent and detect
—
on a timely basis
—
any potential loss due to fraudulent activity.
This Annual Report does not include an attestation report of the company's registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by the company's registered public accounting firm pursuant to rules of the Securities and Exchange Commission that permit the company to provide only management's report in this annual report.
Changes in Internal Control Over Financial Reporting
There has been no change in our internal control over financial reporting that occurred during our last fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
The term
“
internal control over financial reporting
”
is defined as a process designed by, or under the supervision of, the registrant
’
s principal executive and principal financial officers, or persons performing similar functions, and effected by the registrant
’
s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
(a) Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the registrant;
(b) Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the registrant are being made only in accordance with authorizations of management and directors of the registrant; and
(c) Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the registrant
’
s assets that could have a material effect on the financial statements.
ITEM 9B.
OTHER INFORMATION
None.
35
PART III
ITEM 10.
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Identity of directors and executive officers:
The following table sets forth the names and ages of the current directors and executive officers of Sangui BioTech International, Inc., their principal offices and positions and the date each such person became a director or executive officer. Our executive officers are elected annually by the Board of Directors. Our directors serve one-year terms or until their successors are elected. The executive officers serve terms of one year or until their death, resignation or removal by the Board of Directors. There are no family relationships between any of the directors and executive officers. In addition, there was no arrangement or understanding between any executive officer and any other person pursuant to which any person was selected as an executive officer.
The directors as of June 30, 2018 were as follows
|
|
|
|
|
Name
|
Age
|
Position with the Company
|
Director Since
|
|
Hubertus Schmelz
|
62
|
Non-Executive Director
|
Dec 18, 2008
|
|
|
|
|
|
|
Thomas Striepe
|
56
|
CEO, CFO & Director
|
Feb 7, 2005
|
None of the Directors are related to one another. None of the independent Directors has a business or professional relationship with SGBI and/or the other Directors and substantial shareholders of SGBI, except as follows:
Since January 2004, the subsidiary of the Company has an agreement with Hubertus Schmelz under which the latter serves as a Managing Director on an hourly basis.
The day-to-day operations of SGBI are entrusted to the Executive Directors of SGBI.
The business and working experience of the Directors and key Executive Officers of SGBI as of June 30, 2018, are set out below:
THOMAS STRIEPE, is the Vice President Accounting and Controlling at Treukonzept Finance GmbH, Hamburg, Germany, a financial services company. Prior to joining Treukonzept Finance GmbH in 2004, he held management positions in the accounting departments of several German and international corporations. He holds an MBA from Hamburg University.
HUBERTUS SCHMELZ, is the General Manager of SanguiBioTech GmbH. He was appointed to this position effective December 16, 2003. Prior to joining Sangui he acted as a legal and business consultant. During the last decade prior to 2000 he was entrusted with numerous business development projects by the German Treuhandanstalt in restructuring the economy of Eastern Germany. After having studied law he acted as legal counsel in several positions.
There are no arrangements or understandings between any of the directors or executive officers, or any other person or person pursuant to which they were selected as directors and/or officers.
Significant Employees
All but one individuals serving as scientific or administrative staff have been engaged on the basis of consulting agreements. They include non-disclosure and exclusivity sections and secure the
36
ongoing cooperation. Key personnel the expertise and abilities of which would be difficult to replace, includes Dr. Harald Poetzschke.
Directorships
No Director of the Company or person nominated or chosen to become a Director holds any other directorship in any company with a class of securities registered pursuant to section 12 of the Exchange Act or subject to the requirements of section 15(d) of such Act or any other company registered as an investment company under the Investment Company Act of 1940.
Family Relationships
There are no family relationships between any of the directors, officers or employees of the Company.
Involvement in Certain Legal Proceedings
During the past ten years, no present director, executive officer or person nominated to become a director or an executive officer of the Company has been or filed:
1.
A petition under the Federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing;
2.
Such person was convicted in a criminal proceeding or is a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses);
3.
Such person was the subject of any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from, or otherwise limiting, the following activities:
i.
Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
ii.
Engaging in any type of business practice; or
iii.
Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of Federal or State securities laws or Federal commodities laws;
4.
Such person was the subject of any order, judgment or decree, not subsequently reversed, suspended or vacated, of any Federal or State authority barring, suspending or otherwise limiting for more than 60 days the right of such person to engage in any activity described in
37
paragraph (f)(3)(i) of this section, or to be associated with persons engaged in any such activity;
5.
Such person was found by a court of competent jurisdiction in a civil action or by the Commission to have violated any Federal or State securities law, and the judgment in such civil action or finding by the Commission has not been subsequently reversed, suspended, or vacated;
6.
Such person was found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any Federal commodities law, and the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended or vacated;
7.
Such person was the subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of:
i.
Any Federal or State securities or commodities law or regulation; or
ii.
Any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or
iii.
Any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
8.
Such person was the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
Promoters and Control Persons
None.
38
Audit Committee and Audit Committee Financial Expert
The Company has no separately designated standing audit committee or another committee performing similar functions. The Board of Directors acts as the audit committee. None of the directors qualifies as an Audit Committee Financial Expert.
Material Changes to the Method by Which the Shareholders May Recommend Nominees to the Board of Directors
None.
Section 16 (a) Beneficial Ownership Compliance
Section 16(a) of the Securities Exchange Act of 1934 requires the Company's executive officers, directors and persons who own more than ten percent of the Company's Common Stock, to file initial reports of beneficial ownership on Form 3, changes in beneficial ownership on Form 4 and an annual statement of beneficial ownership on Form 5, with the SEC. Such executive officers, directors and greater than ten percent shareholders are required by SEC rules to furnish the Company with copies of all such forms that they have filed.
Based solely upon a review of copies of the reports filed, we believe that during the year ended June 30, 2018, all executive officers, directors and persons who own more than ten percent of the Company's Common Stock are in compliance with such regulations.
Code of Ethics
As of the date of this report the Company has not adopted a code of ethics.
Audit Committee and Audit Committee Financial Expert
Our board of directors is comprised of two directors, none of which is an outside independent director, and as of the date hereof we have not established an audit committee. Accordingly, our board of directors presently performs the functions that would customarily be undertaken by an audit committee.
ITEM 11.
EXECUTIVE COMPENSATION AND OTHER INFORMATION
Summary Compensation Table
The table below summarizes all compensation awarded to, earned by, or paid to our Officers for all services rendered in all capacities to us for the fiscal periods indicated.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Salary
|
|
|
|
Stock
|
|
Option
|
|
Total
|
|
Name and Principal Position
|
|
Year
|
|
($)
(1)
|
|
Bonus ($)
|
|
Awards ($)
|
|
Awards ($)
|
|
($)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thomas Striepe
Chief Executive Officer
Chief Financial Officer
|
|
2018
2017
|
|
19,253
(3)
|
|
-
-
|
|
-
-
|
|
-
-
|
|
19,253
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hubertus Schmelz
(2)
|
|
2018
2017
|
|
-
-
|
|
-
-
|
|
-
-
|
|
-
-
|
|
-
-
|
(1)
All figures are expressed in United States Dollars (
“
USD
”
); for the German management personnel, the EURO or DM was converted to USD using the average exchange rate of the period July 1 through June 30 for each year.
(2)
Hubertus Schmelz serves as the Chief Executive Officer of the Company
’
s 90% owned subsidiary, Sangui Biotech, GMBH.
(3)
See Item 13 below, Certain Relationships and Related Transactions, and Director Independence.
Narrative Disclosure to Summary Compensation Table
There are no other employment contracts, compensatory plans or arrangements, including payments to be received from the Company with respect to any executive officer, that would result in payments to such person because of his or her resignation, retirement or other termination of employment with the Company, or its subsidiary, any change in control, or a change in the person
’
s responsibilities following a change in control of the Company.
There are no agreements or understandings for any executive officer to resign at the request of another person. None of our executive officers acts or will act on behalf of or at the direction of any other person.
Outstanding Equity Awards at Fiscal Year-End Table and Narrative
The Company had no outstanding equity awards at fiscal year-end.
Compensation of Directors
There was no compensation paid to any director who was a Named Executive Officer during the fiscal year ended June 30, 2018.
Other Contracts
None.
ITEM 12.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Securities Authorized for Issuance under Equity Compensation Plans
No securities have been authorized for issuance as part of any Equity Compensation Plan.
39
Security Ownership of Certain Beneficial Owners
The following table sets forth, as of June 30, 2018, certain information concerning ownership of shares of Common Stock by any person who is the beneficial owner of more than 5% of the issued and outstanding Common Stock of the Company.
|
|
|
|
|
|
|
|
|
|
Title of
Class
|
Name and
Address of
Beneficial
Owner
|
Amount and
Nature of
Beneficial
Owner
|
Percent of
Class
|
|
|
|
|
|
|
Common Stock
|
Wolfgang Jensen
Am Berge 9
21376 Eyendorf
Germany
|
20,026,800
|
10.4%
|
|
|
|
|
|
|
Common Stock
|
Hubertus Schmelz
Alfred Herrhausen Street 44
58455 Witten
Germany
|
18,806,481
|
9.8%
|
|
|
|
|
|
|
Common Stock
|
SastoMed GmbH
Brüsseler Straße 2
49124 Georgsmarienhütte
Germany
|
8,406,838
|
4.4%
|
Security Ownership of Management
The following table sets forth, as of June 30, 2018, certain information concerning ownership of shares of Common Stock by each director of the Company and by all executive officers and directors of the Company as a group:
|
|
|
|
|
|
|
|
|
|
Title of
Class
|
Name and
Address of
Beneficial
Owner
|
Amount and
Nature of
Beneficial
Owner
(1)
|
Percent of
Class
|
|
|
|
|
|
|
Common Stock
|
Thomas Striepe
Alfred Herrhausen Street 44
58455 Witten
Germany
|
1,350,000
|
0.7%
|
|
|
|
|
|
|
Common Stock
|
Hubertus Schmelz
Alfred Herrhausen Street 44
58455 Witten
Germany
|
18,806,481
|
9.8%
|
|
|
|
|
|
|
Common Stock
|
All Officers and Directors as a Group (2 persons)
|
20,156,481
|
10.5%
|
Percentages are calculated on the basis of 191,951,503 shares issued on June 30, 2018.
Changes in Control
To the best of the Company
’
s knowledge there are no present arrangements or pledges of the Company's securities, which may result in a change in control of the Company.
ITEM 13.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Transactions with related persons
Except as otherwise disclosed below, no Director, substantial shareholder or Executive Officer of SGBI was or is an interested party in any transaction undertaken by SGBI or its subsidiary within the last two years.
The Company has an agreement with the Company's former President and CEO, pursuant to which he is entitled to three percent royalties of gross revenues earned with any product based on his inventions. No royalties were outstanding, paid or earned in fiscal years 2018 and 2017.
As of June 30, 2018 and 2017, the Company has recorded $2,779 and $0, respectively, in accounts payable to related parties for services performed by Company officers and directors.
Related Party Loans Payable
On March 6, 2015, the Company entered into a note payable with a family member of a Company Director for 100,000 Euros. The note is adapted period to period. On May 31, 2016 the note payable and accrued interest were transferred to a Company Director. The note payable accrues interest at 5 percent per annum, is due on June 30, 2019 and is unsecured. On December 12, 2017, January 19, 2018 and March 13, 2018, the Director granted three additional loans of 25,000 Euros each to the Company. The interest rate is uniformly 2.0% per annum. They are unsecured. The balance of both note and loan principal and interest is $
223,410
at June 30, 2018.
Consulting Contract with Thomas Striepe.
The Company signed a consulting contract with Thomas Striepe, covering certain administrational services on April 01, 2018.
Parents
Not applicable.
Promoters and Control Persons
Not applicable.
40
ITEM 14.
PRINCIPAL ACCOUNTANT FEES AND SERVICES
Independent Registered Public Accountants
The Company
’
s independent accountants for the fiscal year ended June 30, 2018 and 2017 were Sadler, Gibb & Associates, LLC.
(a)
Audit Fees
. For the fiscal year ended 2018, the aggregate fees billed by Sadler, Gibb & Associates for services rendered for the audits of the annual financial statements and the review of the financial statements included in the quarterly reports on Form 10-Q or services provided in connection with the statutory and regulatory filings or engagements for those fiscal years were $25,500
|
|
|
|
(in $)
|
2018
|
2017
|
|
Audit Fees
|
25,500
|
27,950
|
|
Audit related fees
|
-0-
|
-0-
|
|
Tax fees
|
-0-
|
-0-
|
|
Other fees
|
-0-
|
-0-
|
(b)
Audit-Related Fees
. For the fiscal year ended 2018 and 2017 fees billed by Sadler, Gibb & Associates were an aggregate $0 for any audit-related services other than as set forth in paragraph (a) above.
(c)
Tax Fees
. For the fiscal years ended 2018 and 2017 Sadler, Gibb & Associates did not bill any fees for tax compliance services. The auditors did not provide tax-planning advice for the fiscal years ended 2018 and 2017.
(d)
All Other Fees
. None.
41
PART IV
ITEM 15.
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) Index to Exhibits
|
|
|
|
|
|
3.1
|
Articles of Incorporation of the Company
(1)
|
|
3.2
|
Bylaws of the Company
(1)
|
|
3.3
|
Amended and Restated Articles of Incorporation of the Company
(2)
|
|
3.4
|
Amended and Restated Bylaws of the Company
(2)
|
|
21.1
|
Subsidiaries of the Company, filed herewith
|
|
31.01
|
Certification of CEO Pursuant to Rule 13a-14(a) and 15d-14(a), filed herewith
|
|
31.02
|
Certification of CFO Pursuant to Rule 13a-14(a) and 15d-14(a), filed herewith
|
|
32.01
|
Certification Pursuant to Section 1350 of Title 18 of the United States Code, filed herewith
|
Notes:
(1)
Previously filed as an exhibit to the report on Form 8-K, filed on or about April 4, 2000, and incorporated herein by reference
(2)
Previously filed as an exhibit to the report on Form 10-Q, filed on February 25, 2009, and incorporated herein by reference
42
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
SANGUI BIOTECH INTERNATIONAL, INC.
|
|
|
/s/ Thomas Striepe
Thomas Striepe
Chief Executive Officer
Principal Executive Officer
Chief Financial Officer
Principal Financial Officer
|
September 28, 2018
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
|
|
|
Signatures
|
Title
|
Date
|
|
/s/ Thomas Striepe
Thomas Striepe
|
Director
|
September 28, 2018
|
|
|
|
|
|
/
s/ Hubertus Schmelz
Hubertus Schmelz
|
Director
|
September 28, 2018
|
43
Sangui Biotech (CE) (USOTC:SGBI)
Historical Stock Chart
From Mar 2024 to Apr 2024
Sangui Biotech (CE) (USOTC:SGBI)
Historical Stock Chart
From Apr 2023 to Apr 2024