Abbott Labs Gets FDA Approval for MitraClip for Leaky Heart Valves
July 12 2018 - 9:52AM
Dow Jones News
By Chris Wack
Abbott Laboratories (ABT) has received approval from the Food
and Drug Administration for a next-generation version of its
leading MitraClip heart valve repair device used to repair a leaky
mitral valve without open-heart surgery.
The Abbott Park, Ill.-based company said in a release Thursday
that it received CE Mark for the next-generation device earlier
this year, allowing for sale of the devices in the European Union
and other countries that recognize the regulatory designation.
The next-generation MitraClip system provides cardiologists with
advanced steering, navigation and positioning capabilities for the
clip, making it easier to use in difficult anatomies, Abbott
said.
Abbott recently began enrollment in the MitraClip EXPAND
clinical study, a prospective study evaluating the safety and
performance of the new MitraClip system in a contemporary
real-world setting.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
July 12, 2018 09:37 ET (13:37 GMT)
Copyright (c) 2018 Dow Jones & Company, Inc.
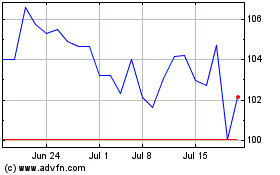
Abbott Laboratories (NYSE:ABT)
Historical Stock Chart
From Mar 2024 to Apr 2024
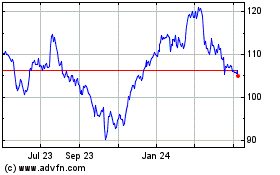
Abbott Laboratories (NYSE:ABT)
Historical Stock Chart
From Apr 2023 to Apr 2024