Filed
Pursuant to Rule 424(b)(2)
Registration No. 333-202466
Prospectus supplement
(To prospectus dated April 18, 2016)
15,533,981 shares

Common stock
BioCryst Pharmaceuticals, Inc. is offering 15,533,981 shares of its common stock.
Our common stock is listed on the NASDAQ Global Select Market under the symbol “BCRX.”
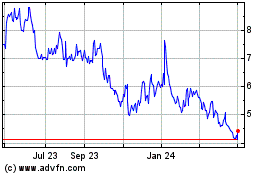
On September 12, 2017, the last reported sale price of our common stock on the NASDAQ Global Select Market was $5.23 per share.
|
|
|
Per share
|
|
|
Total
|
|
|
Public offering price
|
|
$
|
5.150
|
|
|
$
|
80,000,002
|
|
|
Underwriting discounts and commissions
(1)
|
|
$
|
0.309
|
|
|
$
|
4,800,000
|
|
|
Proceeds to BioCryst, before expenses
|
|
$
|
4.841
|
|
|
$
|
75,200,002
|
|
|
____________
(1) We have agreed to reimburse the underwriters for certain FINRA-related expenses. See
“Underwriting.”
|
We have granted the underwriters an option for a period
of 30 days to purchase up to 2,330,097 additional shares of our common stock at the public offering price less the underwriting
discounts and commissions.
Investing in our common stock involves risks. See
“Risk factors” on page S-8 of this prospectus supplement and in the documents incorporated by reference into this
prospectus supplement and the accompanying prospectus for a discussion of the factors you should carefully consider before deciding
to purchase shares of our common stock.
Neither the Securities and Exchange Commission nor
any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this
prospectus supplement or the accompanying prospectus. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares on or about September 15, 2017.
Joint book-running managers
Co-manager
H.C. Wainwright & Co.
September 12, 2017
Table of
contents
|
Prospectus Supplement
|
|
Page
|
|
About this prospectus supplement
|
|
S-ii
|
|
Forward-looking statements
|
|
S-iii
|
|
Prospectus supplement summary
|
|
|
S-1
|
|
The offering
|
|
|
S-7
|
|
Risk factors
|
|
|
S-8
|
|
Use of proceeds
|
|
|
S-29
|
|
Dilution
|
|
|
S-30
|
|
Price range of common stock and dividend policy
|
|
|
S-31
|
|
Underwriting
|
|
|
S-32
|
|
Legal matters
|
|
|
S-37
|
|
Experts
|
|
|
S-37
|
|
Where you can find more information
|
|
|
S-38
|
|
Incorporation of certain documents by reference
|
|
|
S-38
|
|
|
|
|
|
|
Prospectus
|
|
|
Page
|
|
About this Prospectus
|
|
|
i
i
|
|
Prospectus Summary
|
|
|
1
|
|
Risk Factors
|
|
|
2
|
|
Information Regarding Forward-Looking Statements
|
|
|
3
|
|
Use of Proceeds
|
|
|
5
|
|
Description of Common Stock, Preferred Stock
and Depositary Shares
|
|
|
6
|
|
Description of Stock Purchase Contracts
|
|
|
9
|
|
Description of Warrants
|
|
|
10
|
|
Description of Units
|
|
|
12
|
|
Plan of Distribution
|
|
|
13
|
|
Legal Matters
|
|
|
16
|
|
Experts
|
|
|
16
|
|
Where You Can Find More Information
|
|
|
17
|
|
Incorporation of Certain Documents by Reference
|
|
|
17
|
About this
prospectus supplement
This document is part of a registration
statement that we filed with the Securities and Exchange Commission (the “SEC”) using a "shelf" registration
process and consists of two parts. The first part is the prospectus supplement, which describes the specific terms of this offering
of shares of our common stock and also adds to and updates information contained in the accompanying prospectus and the documents
incorporated by reference into this prospectus supplement and the accompanying prospectus. The second part is the accompanying
prospectus, or the base prospectus, dated April 18, 2016, including the documents incorporated by reference therein, which provides
more general information, some of which may not apply to this offering. Generally, when we refer to this prospectus, we are referring
to both parts of this document combined. You should read both this prospectus supplement and the accompanying prospectus, together
with the additional information described under the caption “Where you can find more information” below.
Neither we nor the underwriters
have authorized anyone to provide you with information different from that contained in or incorporated by reference into this
prospectus supplement, the accompanying prospectus or any free writing prospectus prepared by us or on our behalf. We and the
underwriters take no responsibility for, and can provide no assurances as to the reliability of, any information other than the
information contained in or incorporated by reference into this prospectus supplement, the accompanying prospectus or any free
writing prospectus prepared by us or on our behalf. Neither we nor the underwriters are offering to sell, nor seeking offers to
buy, shares of our common stock in any jurisdiction where an offer or sale is prohibited. You should assume that the information
appearing in or incorporated by reference into this prospectus supplement, the accompanying prospectus or any free writing prospectus
prepared by us or on our behalf is accurate or complete only as of their respective dates or on the date or dates which are specified
in such documents, and that any information in documents that we have incorporated by reference is accurate or complete only as
of the date of such document incorporated by reference. Our business, financial condition, liquidity, results of operations and
prospects may have changed since those dates. Management estimates are derived from publicly available information, our knowledge
of our industry and assumptions based on such information and knowledge, which we believe to be reasonable. In addition, assumptions
and estimates of our and our industry's future performance are necessarily subject to a high degree of uncertainty and risk due
to a variety of factors, including those described in “Risk factors” in this prospectus supplement, the accompanying
prospectus and in our Annual Report on Form 10-K for the fiscal year ended December 31, 2016 and Quarterly Reports on Form 10-Q
for the quarters ended March 31, 2017 and June 30, 2017, each of which is incorporated by reference into this prospectus supplement.
These and other important factors could cause our future performance to differ materially from our assumptions and estimates.
See "Forward-looking statements."
If the information set forth
in this prospectus supplement, on the one hand, differs in any way from the information set forth in the accompanying prospectus
or in a document which is incorporated by reference herein or therein that was filed with the SEC before the date of this prospectus
supplement, on the other hand, you should rely on the information set forth in this prospectus supplement. If any statement in
one of these documents conflicts with a statement in another document having a later date (for example, a document incorporated
by reference in this prospectus supplement or in the accompanying prospectus), the statement in the document having the later
date modifies or supersedes the earlier statement.
Unless otherwise mentioned or
unless the context requires otherwise, all references in this prospectus supplement and the accompanying prospectus to “BioCryst,”
the “Company,” “we,” “us” and “our” refer to BioCryst Pharmaceuticals, Inc. together
with its consolidated subsidiaries.
Forward-looking
statements
This prospectus supplement and
the accompanying prospectus, including the information we incorporate by reference, contain forward-looking statements within
the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the
Securities Exchange Act of 1934, as amended (the “Exchange Act”), which are subject to the “safe harbor”
created in Section 21E. All statements other than statements of historical facts contained in this prospectus supplement, the
accompanying prospectus and the information we incorporate herein and therein by reference are forward-looking statements. These
forward-looking statements can generally be identified by the use of words such as “may,” “will,” “intends,”
“plans,” “believes,” “anticipates,” “expects,” “estimates,” “predicts,”
“potential,” the negative of these words or similar expressions. Statements that describe our future plans, strategies,
intentions, expectations, objectives, goals or prospects are also forward-looking statements. These forward-looking statements
include, but are not limited to, statements about:
|
•
|
the preclinical development,
clinical development, commercialization, or post-marketing studies of our product candidates and products, including
our hereditary angioedema (“HAE”) program, peramivir, galidesivir, and early stage discovery programs;
|
|
|
|
|
•
|
the potential funding from our contracts with
the Biomedical Advanced Research and Development Authority (the “BARDA/HHS”) and the National Institute of Allergy
and Infectious Diseases (“NIAID/HHS”) for the development of galidesivir;
|
|
|
|
|
•
|
the potential for government stockpiling orders
of peramivir, additional regulatory approvals of peramivir, or milestones, royalties or profit from sales of peramivir by
us or our partners;
|
|
|
|
|
•
|
the potential use of peramivir as a treatment
for H1N1, H5N1, and H7N9 or other strains of influenza;
|
|
|
|
|
•
|
the implementation of our business model, strategic
plans for our business, products, product candidates and technology;
|
|
|
|
|
•
|
our ability to establish and maintain collaborations
or out-license rights to our product candidates and products;
|
|
|
|
|
•
|
plans, programs, progress and potential success
of our collaborations, including Seqirus UK Limited (“SUL”) for peramivir, Mundipharma International Holdings
Limited (“Mundipharma”) for forodesine, and Shionogi & Co., Ltd. (“Shionogi”) and Green Cross
Corporation (“Green Cross”) for peramivir in their territories;
|
|
|
|
|
•
|
the ability of our wholly-owned subsidiary, JPR
Royalty Sub LLC (“Royalty Sub”), to service its payment obligations in respect of its PhaRMA Senior Secured 14.0%
Notes due 2020 (the “PhaRMA Notes”), and our ability to benefit from our equity interest in Royalty Sub;
|
|
|
|
|
•
|
the foreign currency hedge agreement entered
into by us in connection with the issuance by Royalty Sub of the PhaRMA Notes;
|
|
|
|
|
•
|
the scope of protection we are able to establish
and maintain for intellectual property rights covering our product candidates and technology;
|
|
|
|
|
•
|
our ability to operate our business without infringing
the intellectual property rights of others;
|
|
|
|
|
•
|
estimates of our expenses, revenues, capital
requirements, annual cash utilization, and our needs for additional financing;
|
|
|
|
|
•
|
our ability to continue as a going concern;
|
|
|
|
|
•
|
the timing or likelihood of regulatory filings
or regulatory agreements, deferrals and approvals;
|
|
|
|
|
•
|
our ability to raise additional capital to fund
our operations or repay our recourse debt obligations;
|
|
|
|
|
•
|
our ability to comply with the covenants as set
forth in the agreements governing our debt obligations;
|
|
|
|
|
|
|
•
|
our financial performance; and
|
|
|
|
|
•
|
competitive companies, technologies and our industry.
|
These statements relate to future
events or to our future financial performance and involve known and unknown risks, uncertainties and other important factors that
may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements
expressed or implied by these forward-looking statements. Factors that may cause actual results to differ materially from current
expectations include, among other things, those listed under “Risk factors” and elsewhere in this prospectus supplement,
the accompanying prospectus and the documents incorporated by reference herein and therein. Any forward-looking statement reflects
our current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating
to our operations, results of operations, industry and future growth. Except as required by law, we assume no obligation to update
or revise these forward-looking statements for any reason, even if new information becomes available in the future.
Discussions containing these
forward-looking statements are also included in “Management’s Discussion and Analysis of Financial Condition and Results
of Operations” incorporated by reference from our most recent Annual Report on Form 10-K, our Quarterly Reports on Form
10-Q for the quarters ended March 31, 2017 and June 30, 2017 and our Current Reports on Form 8-K, as well as any amendments we
make to those filings with the SEC.
|
|
|
|
|
|
Prospectus
supplement summary
|
|
|
|
|
|
|
|
This summary highlights information
contained elsewhere or incorporated by reference in this prospectus supplement, the accompanying prospectus or any free writing
prospectus prepared by us or on our behalf and does not contain all of the information that you should consider before investing
in shares of our common stock. You should read this entire prospectus supplement and the accompanying prospectus carefully, including
the “Risk factors” section of this prospectus supplement beginning on page S-8 and the consolidated financial statements
and related notes and other information incorporated by reference in this prospectus supplement and the accompanying prospectus,
before making an investment decision.
|
|
|
|
|
|
|
|
BioCryst
Pharmaceuticals, Inc.
|
|
|
|
|
|
|
|
We are a biotechnology company
that designs, optimizes and develops novel small molecule drugs that block key enzymes involved in the pathogenesis of diseases.
We focus on the treatment of rare diseases in which significant unmet medical needs exist and that align with our capabilities
and expertise. We integrate the disciplines of biology, crystallography, medicinal chemistry and computer modeling to discover
and develop small molecule pharmaceuticals through the process known as structure-guided drug design.
|
|
|
|
|
|
|
|
Drugs
and drug candidates
|
|
|
|
|
|
|
|
Set forth below is a description
of our main drugs and drug candidates that are commercially available and/or in development.
|
|
|
|
|
|
|
|
Drug/Drug
Candidate
|
|
Drug
Class
|
|
Therapeutic
Area(s)
|
|
Phase
|
|
Rights
|
|
|
|
RAPIVAB
®
(peramivir injection)
|
|
Intravenous Neuraminidase
Inhibitor
|
|
Acute uncomplicated
Influenza
|
|
Approved
(US & Canada)
sNDA being reviewed
for a pediatric indication
|
|
Seqirus (worldwide,
except Japan, Taiwan, Korea and Israel)
BioCryst retains full
U.S. Government stockpiling rights
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ALPIVAB
TM
(peramivir injection)
|
|
Intravenous Neuraminidase
Inhibitor
|
|
Acute uncomplicated
Influenza
|
|
MAA filed and being
reviewed by the EMA
|
|
Seqirus (worldwide,
except Japan, Taiwan, Korea and Israel)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RAPIACTA
®
(peramivir injection)
|
|
Intravenous Neuraminidase
Inhibitor
|
|
Uncomplicated seasonal
influenza
|
|
Approved
(Japan & Taiwan)
|
|
Shionogi
(Japan & Taiwan)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PERAMIFLU
®
(peramivir injection)
|
|
Intravenous Neuraminidase
Inhibitor
|
|
Uncomplicated seasonal
influenza
|
|
Approved
(Korea)
|
|
Green Cross
(Korea)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BCX7353
|
|
Oral Serine Protease
Inhibitor Targeting Kallikrein (intended to be a once-daily treatment)
|
|
HAE
|
|
Phase 2
|
|
BioCryst
(worldwide)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other 2
nd
generation
HAE compounds
|
|
Oral Serine Protease
Inhibitors Targeting Kallikrein
|
|
HAE and other indications
|
|
Preclinical
|
|
BioCryst
(worldwide)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Galidesivir
(formerly
BCX4430)
|
|
RNA dependent-RNA
Polymerase Inhibitor
|
|
Broad spectrum antiviral
for 20 RNA viruses, including Ebola, Marburg, and Zika
|
|
Phase 1
|
|
BioCryst
(worldwide)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forodesine
|
|
Oral Purine Nucleoside
Phosphorylase Inhibitor
|
|
Oncology-PTCL
|
|
Approved (Japan)
|
|
Mundipharma
(worldwide)
|
|
|
|
|
|
|
|
Hereditary Angioedema
(“HAE”) Program:
|
|
|
|
|
|
|
|
HAE is a rare, severely debilitating and potentially fatal genetic condition that occurs
in approximately 1 in 50,000 people. HAE symptoms include recurrent episodes of edema in various locations, including the hands,
feet, face, genitalia and airway. Airway swelling is particularly dangerous and can lead to death by asphyxiation. In addition,
patients often have bouts of severe abdominal pain, nausea and vomiting caused by swelling in the intestinal wall. By inhibiting
plasma kallikrein, our HAE drug candidates suppress bradykinin production. Bradykinin is the mediator of acute swelling attacks
in HAE patients.
|
|
|
|
|
|
|
|
BCX7353
|
|
|
|
|
|
|
|
BCX 7353 is a second generation
HAE compound and our lead molecule that is being developed as a once-daily (QD) oral therapy for the prevention of HAE attacks
(prophylaxis), as well as an acute therapy for HAE attacks. We have recently completed our Phase 2 prophylaxis program (with the
completion of APeX-1) and are in the process of scheduling meetings with U.S. and E.U. regulators to finalize our plans for late-stage
development, and to file regulatory applications for commercialization in those regions.
|
|
|
|
|
|
|
|
APeX-1
Phase 2 Trial in HAE
|
|
|
|
|
|
|
|
In August 2016, we announced
that we had dosed the first subject in the Phase 2 APeX-1 clinical trial of BCX7353 for the orally administered once-daily (QD)
prophylactic treatment of HAE. APeX-1 was a multi-part, Phase 2, randomized, double-blind, placebo-controlled, dose ranging trial
to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of BCX7353 as a preventative treatment to
eliminate or reduce the frequency of angioedema attacks in HAE patients.
|
|
|
|
|
|
|
|
On February 27, 2017, we reported
statistically significant and clinically meaningful reductions in attack frequency from our first interim analysis (part 1) of
the multi-part APeX-1 clinical trial in HAE patients. On May 25, 2017, we announced positive results from a second interim analysis
of our APeX-1 clinical trial. This second interim analysis was a composite of pooled data from Parts 1 and 2 of the clinical trial.
|
|
|
|
|
|
|
|
On September 5, 2017, we announced results from the Phase 2 APeX-1 clinical trial. This
final analysis evaluated data from all patients in Parts 1, 2 and 3 of the trial. The primary efficacy endpoint of APeX-1 was the
number of angioedema attacks. Efficacy analyses were conducted for HAE attacks reported over the entire dosing interval (Days 1
through 28) and during the dosing period in which plasma concentrations of BCX7353 should be at steady-state conditions (Days 8
through 28). Secondary efficacy endpoints included severity and duration of angioedema attacks and measures of health-related quality
of life. Safety was characterized through evaluation of adverse events and laboratory testing. Pharmacokinetics and pharmacodynamic
effects were assessed through measurement of plasma drug levels and kallikrein inhibition.
|
|
|
|
|
|
|
|
Final results from APeX-1 are
summarized below:
|
|
|
|
|
|
|
|
Seventy-five subjects were randomized
and included in the final analysis of pooled data from Parts 1, 2 and 3: 7 at 62.5 mg, 14 at 125 mg, 14 at 250 mg and
18 at 350 mg of BCX7353 QD; and 22 placebo. The qualifying attack rate was approximately 1/week. Baseline characteristics
were generally well balanced across the treatment groups. Compliance with daily study drug dosing for 28 days was excellent (≥ 98%
across all treatment groups).
|
|
|
|
|
|
|
|
|
|
|
|
Subjects recorded angioedema
symptoms in a diary; diary records were reviewed and attacks adjudicated by an independent expert group. The primary endpoint
of the trial was the number of HAE attacks. The pre-specified per-protocol (“PP”) final analysis included data on
a total of 67 subjects with Type 1 or Type 2 HAE completing > 90% of planned study drug doses. The percentage reductions by
treatment group in the mean rate of angioedema attacks for the pre-defined effective dosing period (weeks 2 through 4) in BCX7353
treated subjects are indicated in the Table below. Results from a pre-planned analysis of peripheral and abdominal attacks are
also shown. Similar results to those shown were seen in the analysis of weeks 1 through 4 and the intent-to-treat population (“ITT”).
|
|
|
|
|
|
|
|
|
|
Percentage
change in attacks vs placebo (p-Value)
Per
protocol population, weeks 2-4 of treatment
|
|
|
|
|
N
|
All
Attacks
|
Peripheral
Attacks
|
Abdominal
Attacks
|
|
|
|
BCX7353
350 mg
|
14
|
-58%
(p < 0.001)
|
-90%
(p < 0.001)
|
-5%
(p=0.884)
|
|
|
|
BCX7353
250 mg
|
12
|
-46%
(p=0.006)
|
-66%
(p=0.005)
|
-13%
(p=0.700)
|
|
|
|
BCX7353
125 mg
|
13
|
-73%
(p < 0.001)
|
-79%
(p < 0.001)
|
-63%
(p=0.048)
|
|
|
|
BCX7353
62.5 mg
|
7
|
-7%
(p=0.715)
|
-25%
(p=0.371)
|
+22%
(p=0.578)
|
|
|
|
|
|
|
|
The 125 mg dose level showed
statistically significant and similar benefit for all attacks, and also when split into abdominal attacks and peripheral attacks.
In contrast, at the 250 mg and 350 mg dose levels, there was no statistically significant effect for abdominal attacks, despite
strong and statistically significant effects on peripheral attacks. Based on these findings, it is likely that subjects in the
250 mg and 350 mg arms recorded transient drug-related abdominal AEs as HAE attack symptoms in their diary. As expected,
the lowest dose tested (62.5 mg QD) showed no statistically significant differences in attack rates (total, or when split into
abdominal and peripheral) compared with placebo. The range of doses studied and associated results complete the dose response
evaluation required to inform Phase 3 dose selection.
|
|
|
|
|
|
|
|
An analysis of the proportion of subjects achieving levels of percent reduction in attacks
was also performed; this analysis compared on-study attack rate to qualifying attack rate for each subject. Response levels were
defined as reductions of ≥50%, ≥70%, or ≥90%. Results for all dose groups indicated that the dose group with the highest
proportion of responders using each definition was the 125 mg group, and was 4 to 5 times greater than the proportion of responders
in the placebo group. The proportion of responders in the 62.5 mg group was similar to placebo.
|
|
|
|
|
|
|
|
In addition, we performed an exploratory ad hoc analysis of the placebo, 62.5 mg
QD and 125
mg QD dose groups to examine
the relationship of change in attack rate to dose and the effect of
achieving target drug
levels. The 125 mg group had a mean change in
attack rate of -0.73 per week (improvement from qualifying) compared to -0.07
attacks per week for placebo and -0.24 attacks per week for the 62.5 mg QD dose group. The effect of achieving the target
drug level (equivalent to 4 times EC50 against plasma kallikrein) at trough measured at steady state on study day 15 was
also explored for subjects randomized to the placebo, 62.5 mg and 125 mg levels.
Placebo subjects were assigned a zero drug level value. Subjects were categorized as
achieving or not achieving the target level and the proportion of subjects in each of
these two categories who had responses of reductions in ≥50%, ≥70%, or ≥90% in attack rate was compared and the
odds ratios calculated. The odds for subjects achieving ≥50%, ≥70%, or ≥90%
responses if the target drug level was met or exceeded were 5.6, 12.9 and 26.4 times
higher respectively than for subjects who did not meet or exceed target drug
level.
|
|
|
|
|
|
|
|
A significant increase in the
proportion of attack-free subjects was observed in the 125 mg QD dose group compared to placebo (46% versus 10%, p= 0.033). Furthermore,
a clinically important and statistically significant improvement in patient quality of life total score, measured using the AE-QoL
(Quality of Life) instrument, was seen in the 125 mg QD group compared to placebo (p < 0.001). The mean improvement in the
125 mg QD group was more than four times the minimum clinically important difference.
|
|
|
|
|
|
|
|
Oral BCX7353 once-daily for 28 days was generally safe and well tolerated in
subjects with HAE. No new clinically significant safety findings were seen in Part 3 of the trial. Overall, there was one
serious adverse event of moderate gastrointestinal infection that was determined by the investigator not to be drug-related.
As previously reported, study drug was discontinued before day 28 in three subjects in the BCX7353 350 mg treatment arm
(unrelated pre-existing liver disorder; related gastroenteritis with liver disorder; and related vomiting/abdominal cramps).
The most common treatment-emergent AEs in descending order of frequency were the common cold, headache, diarrhea, nausea and
abdominal pain. Gastrointestinal AEs
|
|
|
|
|
|
|
|
|
|
|
|
were infrequent at the 125 mg and 62.5 mg dose levels, and there were no clinically
significant laboratory abnormalities at these dose levels, though increases in liver enzymes were observed in several
subjects at higher dose levels. Adverse events
in the gastrointestinal
system organ class
were more frequent in the 350 mg QD
and 250 mg QD dose groups compared to placebo. Elevated liver enzymes were reported in several subjects, and an analysis of liver enzyme safety tests including alanine aminotransferase (ALT) levels showed that of 4 subjects who had elevations of ALT to more than or equal to 3 times the upper limit of normal (ULN), all 4 had prior
exposure to androgens, 3 were in the 350 mg QD group, 1 was in the 250 mg QD group, and 3 had baseline (prior to study drug administration) elevations in ALT of close
to or greater than 3 times the ULN.
|
|
|
|
|
|
|
|
Steady state BCX7353 plasma levels
and kallikrein inhibition levels were consistent with previous analyses. Steady state trough drug levels (24 hours
after dosing) exceeded the proposed target threshold for efficacy of 4 times the 50% effective concentration
(EC
50
) of 9ng/ml in 0%, 64%, 100% and 100% of subjects at the 62.5 mg, 125 mg, 250 mg and 350 mg dose levels,
respectively.
|
|
|
|
|
|
|
|
We believe the observed efficacy, dose response, PK, safety and tolerability
profile of BCX7353 strongly support its advancement into Phase 3 development. We intend to interact with the U.S. Food
and Drug Administration (“FDA”) and European Medicines Agency (“EMA”) during the fourth
quarter of 2017 to finalize the Phase 3 program required to support New Drug Application (“NDA”) and
Marketing Authorization Application (“MAA”) submissions.
|
|
|
|
|
|
|
|
In addition, the successful
results achieved in our APeX-1 clinical trial satisfied a vesting criterion associated with previously issued performance-based
stock options. In association with this vesting, we incurred approximately $3.2 million of non-cash stock compensation expense.
|
|
|
|
|
|
|
|
ZENITH-1
Trial
|
|
|
|
|
|
|
|
On August 2, 2017, we announced
the dosing of the first subject into ZENITH-1, a clinical trial studying up to three dosage strengths of a liquid formulation
of BCX7353 given as a single oral dose for the acute treatment of angioedema attacks in patients with HAE. ZENITH-1 is a randomized,
double-blind, placebo-controlled, adaptive dose-ranging trial of the efficacy, safety and tolerability of BCX7353 for treatment
of acute angioedema attacks, and will enroll up to 60 subjects with HAE. Blinded study drug is being dosed as an oral liquid after
onset of symptoms, for up to 3 attacks in each subject, with each subject receiving both BCX7353 (for 2 attacks) and placebo (for
one attack) in a randomized sequence. The trial is structured with up to 3 consecutive cohorts testing single doses of 750 mg
(from 12 to 36 subjects), 500 mg (up to 12 subjects) and 250 mg (up to 12 subjects), starting with 750 mg. Efficacy assessments
include patient-reported composite visual analogue scale (“VAS”) scores, patient global assessment, change in symptoms,
and use of rescue medication. Treatment effect will be assessed on accumulating results, beginning after 12 subjects have completed
study in the first cohort (750 mg), by comparing the proportion of BCX7353-treated and placebo-treated attacks which have a stable
or improved composite VAS at 4 hours post dose. Once a treatment effect is demonstrated, enrollment at the 500 mg dose level will
commence. If treatment effect at the 500 mg dose level is similar to 750 mg dose level, the 250 mg dose cohort will be enrolled.
|
|
|
|
|
|
|
|
Infectious disease programs
|
|
|
|
|
|
|
|
Peramivir
injection (RAPIVAB
®
, RAPIACTA
®
, PERAMIFLU
®
, ALPIVAB
TM
)
|
|
|
|
|
|
|
|
Peramivir is an intravenous
neuraminidase inhibitor approved in the United States, Canada, Japan, Korea and Taiwan for the treatment of patients with influenza.
Influenza is a seasonal virus with the highest infection rates generally observed in colder months. In countries for which peramivir
is commercially available, influenza occurs primarily during the September to April timeframe. Peramivir is available commercially
under the trade names RAPIVAB
®
, RAPIACTA
®
, and PERAMIFLU
®
.
|
|
|
|
|
|
|
|
Peramivir was most recently
approved in Canada in January 2017, and was approved in the United States in December 2014, in each case for the treatment of
acute uncomplicated influenza in patients 18 years and older who have been symptomatic for no more than two days. On June 17,
2015, we announced that we licensed RAPIVAB (peramivir injection) for the treatment of influenza to CSL Limited (“CSL”),
a global biopharmaceutical company. RAPIVAB is being commercialized by a subsidiary of CSL called Seqirus UK Limited (“SUL”
or “Seqirus”), which specializes in influenza prevention through the supply of seasonal and pandemic influenza vaccine
to global markets. Under the terms of the agreement, SUL obtained worldwide
|
|
|
|
|
|
|
|
|
|
|
|
rights to commercialize RAPIVAB, with the exception
of Japan, Korea, Taiwan, for which peramivir is commercialized by Shionogi & Co. Ltd. and Green Cross Corporation. BioCryst
retained all rights to pursue pandemic stockpiling orders for RAPIVAB from the U.S. Government, while SUL is responsible for government
stockpiling outside the U.S.
|
|
|
|
|
|
|
|
In January 2017, we announced
that the EMA has accepted the filing of our peramivir injection MAA for treatment of
symptoms typical of influenza in adults 18 years and older. If the MAA is approved, Seqirus will commercialize peramivir as ALPIVAB
TM
in the European Union. The acceptance of the MAA begins the review process by the EMA under the centralized licensing procedure
for all 28 member states of the European Union, Norway and Iceland.
|
|
|
|
|
|
|
|
In addition, we also have submitted
a supplemental New Drug Application (“sNDA”) for a RAPIVAB label expansion for pediatric patients aged 2-17 years
in the United States. The sNDA has been classified by the FDA as a priority review and has a Prescription Drug User Fee Act (“PDUFA”)
goal date for a decision by the end of September 2017.
|
|
|
|
|
|
|
|
RAPIVAB was developed under
a $234.8 million contract from the Biomedical Advanced Research and Development Authority within the United States Department
of Health and Human Services (“BARDA/HHS”), which expired on June 30, 2014.
|
|
|
|
|
|
|
|
Galidesivir (formerly
BCX4430) broad spectrum anti-viral
|
|
|
|
|
|
|
|
Galidesivir is a broad-spectrum antiviral (“BSAV”) research program and is
currently being developed under contracts with the National Institute of Allergy and Infectious Diseases (“NIAID/HHS”)
and BARDA/HHS. The NIAID/HHS contract totals $39.5 million and the BARDA/HHS contract totals $39.1 million in available funding.
The total amount of NIAID/HHS and BARDA/HHS funding obligated under awarded options is $39.5 million and $20.6 million, respectively.
The objective of our BSAV program is to develop galidesivir as a broad-spectrum therapeutic for viruses that pose a threat to national
health and security. The primary focus of the program is treatment of hemorrhagic fever viruses. NIAID/HHS funding has supported
galidesivir’s development as a treatment for Marburg virus and Ebola virus. In March 2014, galidesivir was featured in an
online
Nature
publication depicting successful efficacy results in animal models of infection with Marburg virus and Ebola
virus. Galidesivir has been shown to be active against more than 20 RNA viruses in nine different families, including filoviruses,
togaviruses, bunyaviruses, arenaviruses, paramyxoviruses, coronaviruses and flaviviruses. In tests conducted at USAMRIID,
galidesivir protected animals against parenteral exposures to Marburg, Ebola and Rift Valley Fever viruses and from exposures to
aerosolized Marburg virus, an experimental condition designed to mimic an exposure scenario that could result during a bioterrorist
attack. In other experiments conducted at Utah State University and Harvard University, respectively, galidesivir protected animals
exposed to yellow fever virus and Zika virus.
|
|
|
|
|
|
|
|
In August 2016, we reported the results of the Phase 1 clinical study to evaluate the
safety, tolerability and pharmacokinetics of escalating doses of galidesivir administered via i.m. injection in healthy subjects.
In this study, we determined that galidesivir i.m. was generally safe and well tolerated, and that exposure was dose-proportional
and supported the continued development of this BSAV drug candidate for serious emerging viral infections.
|
|
|
|
|
|
|
|
A number of preclinical studies
have been conducted with galidesivir. On December 23, 2014, we announced results from a successful proof-of-concept study of galidesivir
for the treatment of experimental Ebola virus infection in Rhesus macaques, conducted at USAMRIID. On March 7, 2016, results from
a preclinical study of our antiviral galidesivir in interferon-receptor-deficient mice infected with Zika virus were presented
at a World Health Organization conference in Geneva, Switzerland. A number of additional studies of galidesivir in the same mouse
model have been conducted at Utah State University. On October 29, 2016, galidesivir nonclinical results from a Zika virus infection
model were presented in a late-breaker scientific session at ID Week by Dr. James B. Whitney, PhD, Assistant Professor of Medicine,
Harvard Medical School, and Principal Investigator in the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical
Center in Boston.
|
|
|
|
|
|
|
|
After discussions with the FDA, NIAID/HHS and BARDA/HHS, we have delayed the initiation of the
galidesivir i.v. Phase 1 clinical trial. Based upon ongoing conversations, we expect the next step in galidesivir’s development
will be to conduct an additional nonclinical efficacy study in a delayed treatment setting in Ebola virus disease before finalizing
the Phase 1 clinical trial protocol design.
|
|
|
|
|
|
|
|
|
|
|
|
Financial outlook
for 2017
|
|
|
|
|
|
|
|
Based upon our development plans, expected operations and our awarded government contracts,
we had previously indicated that we expect 2017 operating cash usage to be in the range of $30 to $50 million, and expect our total
2017 operating expenses to be in the range of $53 to $73 million. We continue to believe this range to be appropriate, and currently
project that our 2017 operating cash usage and total 2017 operating expenses will be in the upper-half of our guidance range. Our
operating expense range excludes equity-based compensation expense due to the difficulty in accurately projecting this expense
as it is significantly impacted by the volatility and price of our stock, as well as vesting of our outstanding performance-based
stock options. Our operating cash forecast excludes any impact of non-routine cash outflows or inflows. Our ability to remain within
our operating expense and operating cash target ranges is subject to multiple factors, including unanticipated or additional general
development and administrative costs and other factors described under the “Risk factors” section located elsewhere
in this prospectus supplement, the accompanying prospectus and in the documents incorporated herein by reference.
|
|
|
|
|
|
|
|
________________________
|
|
|
|
|
|
|
|
We are a Delaware corporation
originally founded in 1986. Our principal executive offices are located at 4505 Emperor Blvd., Suite 200, Durham, North Carolina
27703, and our telephone number is (919) 859-1302. Our website is located at http://www.biocryst.com. The information on our website
is not incorporated by reference into this prospectus supplement or the accompanying prospectus.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The
offering
|
|
|
|
|
|
|
|
|
|
|
Common
stock offered
|
15,533,981 shares of common stock
|
|
|
|
|
|
|
|
|
|
Option to purchase additional shares
|
Up to 2,330,097 shares of common stock
|
|
|
|
|
|
|
|
|
|
Common stock to be outstanding after the offering
|
96,000,681 shares of common stock
|
|
|
|
|
|
|
|
|
|
Use of proceeds
|
We intend to use the net proceeds of this offering for general corporate purposes, which may include funding
the global launch preparation of BCX7353; expanding our global awareness and commercial and manufacturing efforts; expanding our
infrastructure, including commercial and manufacturing, to benefit BCX7353 and the rest of our programs; supporting the initiation
of the Phase 3 clinical trial for BCX7353, the long term safety study, and all remaining preclinical and clinical studies to support
an NDA filing for BCX7353; funding the exploratory Phase 2 trial for acute treatment with BCX7353; and advancing our early-stage
programs into clinical trials. See “Use of Proceeds.”
|
|
|
|
|
|
|
|
|
|
NASDAQ global select market symbol
|
BCRX
|
|
|
|
|
|
|
|
|
|
Dividend
policy
|
We have never paid cash
dividends and do not anticipate paying cash dividends in the foreseeable future.
|
|
|
|
|
|
|
|
|
|
Risk
factors
|
See “Risk factors” beginning on page
S-8 and the other information included in, or incorporated by reference into, this prospectus supplement and the accompanying
prospectus for a discussion of certain factors you should carefully consider before deciding to invest in shares of our common
stock.
|
|
|
|
|
|
|
|
|
The number of shares of our common stock to be outstanding after this offering is based on 80,466,700 shares
outstanding as of September 8, 2017 and excludes:
|
|
|
|
|
|
|
|
•
|
13,400,694 shares of common
stock issuable upon the exercise of stock options outstanding under our Stock Incentive Plan as of August 31, 2017, at a weighted
average exercise price of $6.18 per share;
|
|
|
|
|
|
|
|
|
•
|
219,490 shares of common stock issuable upon
the vesting of restricted stock units outstanding as of August 31, 2017; and
|
|
|
|
|
|
|
|
|
•
|
1,592,532 additional shares of common stock reserved
for issuance under our Stock Incentive Plan and 330,270 additional shares of common stock reserved for issuance under our
Employee Stock Purchase Plan as of February 28, 2017.
|
|
|
|
|
|
|
|
|
Except as otherwise
noted, all information in this prospectus supplement assumes no exercise of the outstanding stock options, no vesting of the outstanding
restricted stock units and no exercise of the underwriters’ option to purchase additional shares of our common stock.
|
|
|
|
|
|
Risk factors
An investment in our common
stock involves risks. You should consider carefully all of the information that is included or incorporated by reference in this
prospectus supplement and the accompanying prospectus before investing in our common stock. In particular, you should evaluate
the uncertainties and risks referred to or described below, which may adversely affect our business, financial condition, liquidity,
results of operations, or prospects, along with all of the other information included in our other filings with the SEC, before
deciding to buy our common stock.
Risks relating to our business
We have incurred losses
since our inception, expect to continue to incur such losses, and may never be profitable.
Since our inception, we have
not achieved sustained profitability. We expect to incur additional losses for the foreseeable future, and our losses could increase
as our research and development efforts progress. We expect that such losses will fluctuate from quarter to quarter and losses
and fluctuations may be substantial.
To become profitable, we, or
our collaborative partners, must successfully manufacture and develop product candidates, receive regulatory approval, and successfully
commercialize and/or enter into profitable agreements with other parties. It could be several years, if ever, before we receive
significant revenue from any current or future license agreements or revenues directly from product sales.
Because of the numerous risks
and uncertainties associated with developing our product candidates and their potential for commercialization, we are unable to
predict the extent of any future losses. Even if we do achieve profitability, we may not be able to sustain or increase profitability
on a quarterly or annual basis. If we are unable to achieve and sustain profitability, the market value of our common stock will
likely decline.
Our success depends upon
our ability to advance our products through the various stages of development, especially through the clinical trial process.
To receive the regulatory approvals
necessary for the sale of our product candidates, we or our partners must demonstrate through preclinical studies and clinical
trials that each product candidate is safe and effective. The development process and related regulatory process are complex and
uncertain. Because of the cost and duration of clinical trials, we may decide to discontinue development of product candidates
that are unlikely to show good results in the clinical trials, unlikely to help advance a product to the point of a meaningful
collaboration, or unlikely to have reasonable commercial potential. We may suffer significant setbacks in pivotal pre-clinical
studies and clinical trials (e.g. galidesivir, BCX7353, other kallikrein inhibitors and our other rare disease product candidates),
even after earlier clinical trials show promising results. The development of our product candidates, including our clinical trials,
may not be adequately designed or executed, which could affect the potential outcome and analysis of study results. Any of our
product candidates may produce undesirable side effects in humans. The pre-clinical and clinical data from our product candidates
could cause us or regulatory authorities to interrupt, delay, modify or halt preclinical or clinical trials of a product candidate.
Undesirable or inconclusive data or side effects in humans could also result in the FDA or foreign regulatory authorities refusing
to approve the product candidate for any targeted indications. In addition, the FDA or other regulatory agencies may determine
that study data from our product candidates necessitates additional studies or study designs which differ from our planned development
strategy, and regulatory agencies may also require patient monitoring and testing or may implement restrictions or other conditions
on our development activities, any of which could materially impact the cost and timing of our planned development strategy. We,
our partners, the FDA or foreign regulatory authorities may suspend or terminate clinical trials at any time if we or they believe
the trial participants face unacceptable health risks. Clinical trials may fail to demonstrate that our product candidates are
safe or effective and have acceptable commercial viability. Regulatory authorities may interrupt, delay or halt clinical trials
for a product candidate for any number of reasons.
Our ability to successfully
complete clinical trials is dependent upon many factors, including but not limited to:
|
•
|
our ability to find suitable
clinical sites and investigators to enroll patients;
|
|
|
|
|
|
|
|
|
|
•
|
the ability to maintain contact with patients
to provide complete data after treatment;
|
|
|
|
|
•
|
our product candidates may not prove to be either
safe or effective;
|
|
|
|
|
•
|
clinical protocols or study procedures may not
be adequately designed or followed by the investigators;
|
|
|
|
|
•
|
formulation improvements may not work as expected,
which could negatively impact commercial demand for our product candidates;
|
|
|
|
|
•
|
manufacturing or quality control problems could
affect the supply of product candidates for our trials; and
|
|
|
|
|
•
|
delays or changes in our planned development
strategy, the regulations or guidelines, or other unexpected conditions or requirements of government agencies.
|
Clinical trials are lengthy
and expensive. We or our partners incur substantial expense for, and devote significant time to, preclinical testing and clinical
trials, yet we cannot be certain that the tests and trials will ever result in the commercial sale of a product. For example,
clinical trials require adequate supplies of drug and sufficient patient enrollment. Lack of adequate drug supply or delays in
patient enrollment can result in increased costs and longer development times. Even if we or our partners successfully complete
clinical trials for our product candidates, we or our partners might not file the required regulatory submissions in a timely
manner and may not receive regulatory approval for the product candidates.
We focus on rare diseases,
which may create additional risks and challenges.
Because we focus on developing
drugs as treatments for rare diseases, we may seek orphan drug, breakthrough therapy or fast track designations for our product
candidates in the United States or the equivalent designations elsewhere in the world. Often, regulatory agencies have broad discretion
in determining whether or not to grant such designations. We cannot guarantee that we will be able to receive orphan drug status
from the FDA or equivalent regulatory designations elsewhere. We also cannot guarantee that we will obtain breakthrough therapy
or fast track designation, which may provide certain potential benefits such as more frequent meetings with the FDA to discuss
the development plan, intensive guidance on an efficient drug development program, and potential eligibility for rolling review
or priority review. Even if we are successful in obtaining any such designation by the FDA or other regulatory agency for our
product candidates, such designations may not lead to faster development or regulatory review or approval, and it does not increase
the likelihood that our product candidates will receive marketing approval. We may not be able to obtain or maintain such designations
for our product candidates, and our competitors may obtain these designations for their product candidates, which could impact
our ability to develop and commercialize our product candidates or compete with such competitors, which may adversely impact our
business, financial condition or results of operations.
Although we have received Sakigake
designation for BCX7353 in Japan, we may not experience a faster development, review or approval process compared to the conventional
process.
Our clinical trials may
not adequately show that our product candidates are safe or effective.
Progression of our product candidates
through the clinical development process is dependent upon our trials indicating our product candidates have adequate safety and
efficacy in the patients being treated by achieving pre-determined safety and efficacy endpoints according to the clinical trial
protocols. Failure to achieve any of these endpoints in any of our programs, including BCX7353, galidesivir and our other rare
disease product candidates, could result in delays in our trials or require the performance of additional unplanned trials. This
could result in delays in the development of our product candidates and could result in significant unexpected costs or the termination
of programs.
If our development collaborations
with third parties, such as our development partners and contract research organizations, fail, the development of our product
candidates will be delayed or stopped.
We rely heavily upon third parties
for many important stages of our product candidate development, including but not limited to:
|
•
|
discovery of compounds that
cause or enable biological reactions necessary for the progression of the disease or disorder, called enzyme targets;
|
|
|
|
|
•
|
licensing or designing of enzyme inhibitors for
development as product candidates;
|
|
|
|
|
•
|
execution of certain preclinical studies and
late-stage development for our compounds and product candidates;
|
|
|
|
|
•
|
management of our clinical trials, including
medical monitoring and data management;
|
|
|
|
|
•
|
execution of additional toxicology studies that
may be required to obtain approval for our product candidates;
|
|
|
|
|
•
|
formulation improvement strategies and methods;
and
|
|
|
|
|
•
|
manufacturing the starting materials and drug
substance required to formulate our products and the product candidates to be used in our clinical trials, toxicology studies
and any potential commercial product.
|
Our failure to engage in successful
collaborations at any one of these stages would greatly impact our business. If we do not license enzyme targets or inhibitors
from academic institutions or from other biotechnology companies on acceptable terms, our drug development efforts would suffer.
Similarly, if the contract research organizations that conduct our initial or late-stage clinical trials, conduct our toxicology
studies, manufacture our starting materials, drug substance and product candidates or manage our regulatory function breached
their obligations to us or perform their services inconsistent with industry standards and not in accordance with the required
regulations, this would delay or prevent both the development of our product candidates and the availability of any potential
commercial product.
If we lose our relationship
with any one or more of these parties, we could experience a significant delay in both identifying another comparable provider
and then contracting for its services. We may be unable to retain an alternative provider on reasonable terms, if at all. Even
if we locate an alternative provider, it is likely that this provider may need additional time to respond to our needs and may
not provide the same type or level of service as the original provider. In addition, any provider that we retain will be subject
to applicable FDA current Good Laboratory Practices, current Good Manufacturing Practices (“cGMP”) and current Good
Clinical Practices, and comparable foreign standards. We do not have control over compliance with these regulations by these providers.
Consequently, if these practices and standards are not adhered to by these providers, the development and commercialization of
our product candidates could be delayed. If any of the foregoing risks are realized, our business, financial condition and results
of operations could be materially adversely affected.
Because we have limited
manufacturing experience, we depend on third-party manufacturers to manufacture our product, product candidates and the materials
for our product candidates. Often, especially early in the development and commercialization process, we have only one source
for manufacturing. If we cannot rely on existing third-party manufacturers, we will be required to incur significant costs and
potential delays in finding new third-party manufacturers.
We have limited manufacturing
experience and only a small scale manufacturing facility. We currently rely upon a very limited number of third-party manufacturers
to manufacture the materials required for our product, product candidates and most of the preclinical and clinical quantities
of our product candidates. We depend on these third-party manufacturers to perform their obligations in a timely manner and in
accordance with applicable governmental regulations. Our third-party manufacturers, which may be the only manufacturer we have
engaged for a particular product, may encounter difficulties with meeting our requirements, including but not limited to problems
involving:
|
•
|
inconsistent production yields;
|
|
|
|
|
•
|
product liability claims or recalls of commercial
product;
|
|
|
|
|
|
|
|
|
|
•
|
difficulties in scaling production to commercial
and validation sizes;
|
|
|
|
|
•
|
interruption of the delivery of materials required
for the manufacturing process;
|
|
|
|
|
•
|
scheduling of plant time with other vendors or
unexpected equipment failure;
|
|
|
|
|
•
|
potential catastrophes that could strike their
facilities or have an effect on infrastructure;
|
|
|
|
|
•
|
potential impurities in our drug substance or
products that could affect availability of product for our clinical trials or future commercialization;
|
|
|
|
|
•
|
poor quality control and assurance or inadequate
process controls; and
|
|
|
|
|
•
|
lack of compliance or cooperation with regulations
and specifications or requests set forth by the FDA or other foreign regulatory agencies, particularly associated with peramivir,
BCX7353, galidesivir and our early stage compounds.
|
These contract manufacturers
may not be able to manufacture the materials required for our product candidates at a cost or in quantities necessary to make
them commercially viable. We also have no control over whether third-party manufacturers breach their agreements with us or whether
they may terminate or decline to renew agreements with us. To date, our third-party manufacturers have met our manufacturing requirements,
but they may not continue to do so. Furthermore, changes in the manufacturing process or procedure, including a change in the
location where the drug is manufactured or a change of a third-party manufacturer, may require prior review and approval in accordance
with the FDA’s cGMP and comparable foreign requirements. This review may be costly and time-consuming and could delay or
prevent the launch of a product. The FDA or similar foreign regulatory agencies may at any time implement new standards, or change
their interpretation and enforcement of existing standards for manufacture, packaging or testing of products. If we or our contract
manufacturers are unable to comply, we or they may be subject to regulatory action, civil actions or penalties any of which could
be costly to the Company and could result in a delay or shortage of product.
If we are unable to maintain
current manufacturing or other contract relationships, or enter into new agreements with additional manufacturers on commercially
reasonable terms, or if there is poor manufacturing performance or failure to comply with any regulatory agency on the part of
any of our third-party manufacturers, we may not be able to complete development of, seek timely approval of, or market, our product
candidates.
Our raw materials, drug substances,
and product candidates are manufactured by a limited group of suppliers, including some at a single facility. If any of these
suppliers were unable to produce these items, this could significantly impact our supply of product candidate material for further
preclinical testing and clinical trials.
We face intense competition,
and if we are unable to compete effectively, the demand for our products, if any, may be reduced.
The biotechnology and pharmaceutical
industries are highly competitive and subject to rapid and substantial technological change. There are many companies seeking
to develop products for the same indications that we currently target. Our competitors in the United States and elsewhere are
numerous and include, among others, major multinational pharmaceutical and chemical companies and specialized biotechnology firms.
Most of these competitors have greater resources than we do, including greater financial resources, larger research and development
staffs and more experienced marketing and manufacturing organizations. In addition, most of our competitors have greater experience
than we do in conducting clinical trials and obtaining FDA and other regulatory approvals. Accordingly, our competitors may succeed
in obtaining FDA or other regulatory approvals of product candidates more rapidly than we do. Companies that complete clinical
trials, obtain required regulatory approvals, and commence commercial sale of their drugs before we do may achieve a significant
competitive advantage, including patent and FDA exclusivity rights that would delay our ability to market products. We face, and
will continue to face, competition in the licensing of potential product candidates for desirable disease targets, licensing of
desirable product candidates, and development and marketing of our product candidates from academic institutions, government agencies,
research institutions and biotechnology and pharmaceutical companies. Competition may also arise from, among other things:
|
•
|
other drug development technologies;
|
|
|
|
|
|
|
•
|
methods of preventing or reducing the incidence
of disease, including vaccines; and
|
|
|
|
|
•
|
new small molecule or other classes of therapeutic
agents.
|
Developments by others
may render our product candidates or technologies obsolete or noncompetitive.
We are performing research on
or developing products for the treatment of several rare disorders, including HAE, as well as developing broad spectrum antivirals
for use as medical countermeasures. We expect to encounter significant competition for any of the pharmaceutical products we are
developing and plan to develop. Companies that complete clinical trials, obtain required funding or government support, obtain
required regulatory approvals and commence commercial sales or stockpiling orders of their products before their competitors may
achieve a significant competitive advantage. Such is the case with the current neuraminidase inhibitors marketed by GSK and Roche
for influenza; CINRYZE®, KALBITOR® and FIRAZYR®, marketed by Shire Pharmaceuticals, Inc. (“Shire”) for
HAE; BERINERT® and HAEGARDA®, marketed by CSL for HAE; and RUCONEST®, marketed by Pharming Healthcare, Inc. (“Pharming”)
for HAE.
Further, several pharmaceutical and biotechnology firms have announced efforts in HAE
and in other therapeutic areas where we have discovery and development efforts ongoing. Notably, prophylactic treatment for HAE
is becoming increasingly competitive with the recent approval of CSL’s HAEGARDA, Shire’s positive Phase III data for
the monoclonal antibody, lanadelumab, and Pharming’s completion of a Phase II HAE prophylaxis trial for RUCONEST. Additionally,
KalVista Pharmaceuticals, Inc. (KVD818) and Attune Pharmaceuticals, Inc. (ATN-249) have oral candidates for HAE prophylaxis in
Phase I development. Therapeutic products with potentially promising data to treat Ebola include Mapp Biopharmaceutical, Inc.’s
ZMapp (antibody-based) and Gilead Sciences, Inc.’s product currently under development (small molecule), both of which have
been used in Ebola infected patients. Shionogi also recently announced positive Phase III data for S033188, an oral treatment for
influenza. If one or more of our competitors’ products or programs are successful, the market for our products may be reduced
or eliminated.
Compared to us, many of our
competitors and potential competitors have substantially greater:
|
•
|
capital resources;
|
|
|
|
|
•
|
research and development resources, including
personnel and technology;
|
|
|
|
|
•
|
regulatory experience;
|
|
|
|
|
•
|
preclinical study and clinical testing experience;
|
|
|
|
|
•
|
manufacturing and marketing experience; and
|
|
|
|
|
•
|
production facilities.
|
Any of these competitive factors
could impede our funding efforts, render technology and product candidates noncompetitive or eliminate or reduce demand for our
product candidates.
We face risks related
to our government-funded programs; if BARDA/HHS or NIAID/HHS were to eliminate, reduce or delay funding from our contracts, this
would have a significant negative impact on the programs associated with such funding and could have a significant negative impact
on our revenues and cash flows.
Our projections of revenues
and incoming cash flows are substantially dependent upon BARDA/HHS and NIAID/HHS reimbursement for the costs related to our galidesivir
program. If BARDA/HHS or NIAID/HHS were to eliminate, reduce or delay the funding for these programs or disallow some of our incurred
costs, we would have to obtain additional funding for continued development or regulatory registration for these product candidates
or significantly reduce or stop the development effort.
In contracting with BARDA/HHS
and NIAID/HHS, we are subject to various U.S. Government contract requirements, including general clauses for a cost-reimbursement
research and development contract, which may limit our reimbursement or if we are found to be in violation could result in contract
termination. If the U.S.
Government terminates any of its contracts with us for its convenience, or if we default by failing to perform in accordance with the contract schedule and terms, significant negative impact on our cash flows and operations could result.
Our government contracts
with BARDA/HHS and NIAID/HHS have special contracting requirements, which create additional risks of reduction or loss of funding.
We have completed work under
a contract with BARDA/HHS for the development of our neuraminidase inhibitor, RAPIVAB. We also have entered into contracts with
BARDA/HHS and NIAID/HHS for the development of galidesivir as a treatment for diseases caused by RNA pathogens, including Marburg
virus disease and Ebola virus disease. In contracting with these government agencies, we are subject to various U.S. Government
contract requirements, including general clauses for a cost-reimbursement research and development contract, which may limit our
reimbursement or, if we are found to be in violation, could result in contract termination.
U.S. Government contracts typically
contain a number of extraordinary provisions that would not typically be found in commercial contracts and which may create a
disadvantage and additional risks to us as compared to competitors that do not rely on U.S. Government contracts.
These risks include the ability
of the U.S. Government to unilaterally:
|
•
|
terminate or reduce the scope
of our contract with or without cause;
|
|
|
|
|
•
|
interpret relevant regulations (federal acquisition
regulation clauses);
|
|
|
|
|
•
|
require performance under circumstances which
may not be favorable to us;
|
|
|
|
|
•
|
require an in process review where the U.S. Government
will review the project and its options under the contract;
|
|
|
|
|
•
|
control the timing and amount of funding, which
impacts the development progress of our programs; and
|
|
|
|
|
•
|
audit and object to our contract-related costs
and fees, including allocated indirect costs.
|
Our government contracts
with BARDA/HHS and NIAID/HHS have termination and audit provisions which create additional risks to us.
The U.S. Government may terminate
its contracts with us either for its convenience or if we default by failing to perform in accordance with the contract schedule
and terms. Termination for convenience provisions generally enable us to recover only our costs incurred or committed, and settlement
expenses and profit on the work completed prior to termination. Termination does not permit these recoveries under default provisions.
In the event of termination or upon expiration of a contract, the U.S. Government may dispute wind-down and termination costs
and may question prior expenses under the contract and deny payment of those expenses. Should we choose to challenge the U.S.
Government for denying certain payments under a contract, such a challenge could subject us to substantial additional expenses
which we may or may not recover. Further, if the U.S. Government terminates its contracts with us for its convenience, or if we
default by failing to perform in accordance with the contract schedule and terms, significant negative impact on our cash flows
and operations could result.
As a U.S. Government contractor,
we are required to comply with applicable laws, regulations and standards relating to our accounting practices and are subject
to periodic audits and reviews. As part of any such audit or review, the U.S. Government may review the adequacy of, and our compliance
with, our internal control systems and policies, including those relating to our purchasing, property, estimating, compensation
and management information systems. Audits conducted by the U.S. Government for the completed BARDA/HHS peramivir contract have
been performed and concluded through fiscal 2009; all subsequent fiscal years are still open and auditable. Audits under the active
BARDA/HHS and NIAID/HHS galidesivir contracts may occur at the election of the U.S. Government and have been concluded through
fiscal 2013. Based on the results of its audits, the U.S. Government may adjust our contract-related costs and fees, including
allocated indirect costs. This adjustment could impact the amount of revenues reported on a historic basis and could impact our
cash flows under the contracts prospectively. In addition, in the event BARDA/HHS or NIAID/HHS determines that certain costs and
fees were unallowable or determines that the allocated indirect cost rate was higher than the actual indirect cost rate, BARDA/HHS
or NIAID/HHS would be entitled to recoup any overpayment from us as a
result. In addition, if an audit or review uncovers any improper or illegal activity, we may be subject to civil and criminal penalties and administrative sanctions, including termination of our contracts, forfeiture of profits, suspension of payments, fines and suspension or prohibition from doing business with the U.S. Government. We could also suffer serious harm to our reputation if allegations of impropriety were made against us. In addition, under U.S. Government purchasing regulations, some of our costs may not be reimbursable or allowed under our contracts. Further, as a U.S. Government contractor, we are subject to an increased risk of investigations, criminal prosecution, civil fraud, whistleblower lawsuits and other legal actions and liabilities as compared to private sector commercial companies.
If we fail to reach milestones
or to make annual minimum payments or otherwise breach our obligations under our license agreements, our licensors may terminate
our agreements with them and seek additional remedies.
If we are unable or fail to
meet payment obligations, performance milestones relating to the timing of regulatory filings, product supply obligations, post
approval commitments for RAPIVAB, or development and commercial diligence obligations; are unable or fail to make milestone payments
or material data use payments in accordance with applicable provisions; or fail to pay the minimum annual payments under our respective
licenses, our licensors may terminate the applicable license or seek other available remedies. As a result, our development of
the respective product candidate or commercialization of the product would cease.
If we fail to obtain additional
financing or acceptable partnership arrangements, we may be unable to complete the development and commercialization of our product
candidates or continue operations.
As our programs advance, our
costs are likely to increase. Our current and planned discovery activities, pre-clinical and clinical trials, the related development,
manufacturing, regulatory approval process requirements, and the additional personnel resources and testing required for supporting
the development of our product candidates will consume significant capital resources. Our expenses, revenues and cash utilization
rate could vary significantly depending on many factors, including: our ability to raise additional capital; the development progress
of our collaborative agreements for our product candidates; the amount of funding we receive from NIAID/HHS and BARDA/HHS for
galidesivir or from other new partnerships with third parties for the development of our product candidates, including BCX7353
and our other rare disease product candidates; the commercial success of peramivir achieved by our partners; the amount or profitability
of any orders for peramivir or galidesivir by any government agency or other party; the progress and results of our current and
proposed clinical trials for our most advanced product candidates, including BCX7353 and our other rare disease product candidates;
the progress made in the manufacture of our lead products and the progression of our other programs.
We expect that we will be required
to raise additional capital to complete the development and commercialization of our current product candidates and we may seek
to raise capital at any time. Additional funding, whether through additional sales of securities, additional borrowings, or collaborative
arrangements with partners, including governmental agencies in general and from any BARDA/HHS or NIAID/HHS contract specifically,
may not be available when needed or on terms acceptable to us. The issuance of preferred or common stock or convertible securities,
with terms and prices significantly more favorable than those of the currently outstanding common stock, could have the effect
of diluting or adversely affecting the holdings or rights of our existing stockholders. Additional borrowings may subject us to
more restrictive covenants than are currently applicable to us under our September 23, 2016, Senior Credit Facility with an affiliate
of MidCap Financial Services, LLC, as administrative agent (the “Senior Credit Facility”). In addition, collaborative
arrangements may require us to transfer certain material rights to such corporate partners. Insufficient funds or lack of an acceptable
partnership may require us to delay, scale-back or eliminate certain of our research and development programs.
In order to continue future operations and continue our drug development programs, we
will be required to raise additional capital. In addition to seeking strategic partnerships, transactions and government funding,
we may decide to access the equity or debt markets, incur additional borrowings, or seek other sources to meet liquidity needs.
Our ability to raise additional capital may be limited and may greatly depend upon the success of ongoing development related to
our current drug development programs, including post approval studies for RAPIVAB, the progress, timeline and ultimate outcome
of our kallikrein inhibitors, including the BCX7353 program (including, but not limited to, formulation progress, Phase 3
trials, long-term human safety studies, and the timing of carcinogenicity or other required studies), the progress of our other
rare disease product candidates, funding for and continued successful development of galidesivir, and the progress of our early
discovery programs. In addition, constriction and volatility in the equity and debt markets may restrict our future flexibility to raise capital when such needs arise. Furthermore, we have exposure to many different industries, financing partners and counterparties, including commercial banks, investment banks and partners (which include investors, licensing partners, and the U.S. Government) which may be unstable or may become unstable in the current economic and political environment. Any such instability may impact these parties? ability to fulfill contractual obligations to us or they might limit or place burdensome conditions upon future transactions with us. Also, it is possible that suppliers may be negatively impacted. Any such unfavorable outcomes in our current programs or unfavorable economic conditions could place severe downward pressure on the price of our common stock and may decrease opportunities to raise capital in the capital or credit markets, and further could reduce the return available on invested corporate cash, which, if severe and sustained, could have a material and adverse impact on our results of operations and cash flows and limit our ability to continue development of our product candidates.
We may not be able to
continue as a going concern if we do not obtain additional capital.
We have sustained operating
losses for the majority of our corporate history and expect that our 2017 expenses will exceed our 2017 revenues. We expect to
continue to incur operating losses and negative cash flows until revenues reach a level sufficient to support ongoing operations.
Our liquidity needs will be
largely determined by the success of operations in regards to the progression of our product candidates in the future. Our plans
to alleviate the doubt regarding our ability to continue as a going concern primarily include our ability to control the timing
and spending on our research and development programs and raising additional funds through equity financings. We also may consider
other plans to fund operations including: (1) securing or increasing U.S. Government funding of our programs, including obtaining
procurement contracts; (2) out-licensing rights to certain of our products or product candidates, pursuant to which the we would
receive cash milestones; (3) raising additional capital through debt financings or from other sources; (4) obtaining additional
product candidate regulatory approvals, which would generate revenue, milestones and cash flow; (5) reducing spending on one or
more research and development programs by discontinuing development; and/or (6) restructuring operations to change our overhead
structure.
There can be no assurance that
any of our plans will be successful or that additional capital will be available to us on reasonable terms, or at all, when needed.
If we are unable to obtain sufficient additional capital, we may be forced to curtail operations, delay or stop ongoing clinical
trials, cease operations altogether or file for bankruptcy.
If we fail to successfully
commercialize or establish collaborative relationships to commercialize certain of our product candidates, or if any partner terminates
or fails to perform its obligations under agreements with us, potential revenues from commercialization of our product candidates
could be reduced, delayed or eliminated.
Our business strategy is to
increase the asset value of our product candidate portfolio. We believe this is best achieved by retaining full product rights
or through collaborative arrangements with third parties as appropriate. As needed, potential third-party relationships could
include preclinical development, clinical development, regulatory approval, marketing, sales and distribution of our product candidates.
Currently, we have established
collaborative relationships with Mundipharma for the development and commercialization of forodesine and with each of Shionogi
and Green Cross for the development and commercialization of peramivir in Japan, Taiwan and South Korea. Most recently we have
established a collaborative relationship with Seqirus UK Limited for RAPIVAB on a worldwide basis other than Israel, Japan, Korea
and Taiwan. The process of establishing and implementing collaborative relationships is difficult, time-consuming and involves
significant uncertainty, including:
|
•
|
our partners may seek to renegotiate
or terminate their relationships with us due to unsatisfactory commercial, regulatory or clinical results, including post
approval clinical commitments, a change in business strategy, a change of control or other reasons;
|
|
|
|
|
•
|
our contracts for collaborative arrangements
may expire;
|
|
|
|
|
•
|
our partners may choose to pursue alternative
technologies, including those of our competitors;
|
|
|
|
|
|
|
|
|
|
•
|
we may have disputes with a partner that could
lead to litigation or arbitration;
|
|
|
|
|
•
|
we do not have day to day control over the activities
of our partners and have limited control over their decisions;
|
|
|
|
|
•
|
our ability to generate future event payments
and royalties from our partners depends upon their abilities to establish the safety and efficacy of our product candidates,
obtain regulatory approvals and achieve market acceptance of products developed from our product candidates;
|
|
|
|
|
•
|
we or our partners may fail to properly initiate,
maintain or defend our intellectual property rights, where applicable, or a party may utilize our proprietary information
in such a way as to invite litigation that could jeopardize or potentially invalidate our proprietary information or expose
us to potential liability;
|
|
|
|
|
•
|
we or our partners may not devote sufficient
capital or resources towards our product candidates; and
|
|
|
|
|
•
|
we or our partners may not comply with applicable
government regulatory requirements.
|
If we or our partners fail to
fulfill our responsibilities in a timely manner, or at all, our commercialization efforts related to that collaboration could
be reduced, delayed or terminated, or it may be necessary for us to assume responsibility for activities that would otherwise
have been the responsibility of our partner. If we are unable to establish and maintain collaborative relationships on acceptable
terms, we may have to delay or discontinue further development of one or more of our product candidates, undertake commercialization
activities at our own expense or find alternative sources of funding. Any delay in the development or commercialization of our
product candidates would severely affect our business, because if our product candidates do not progress through the development
process or reach the market in a timely manner, or at all, we may not receive additional future event payments and may never receive
milestone, product sales or royalty payments.
We do not have a great
deal of experience in commercializing our products or technologies, and our future revenue generation is uncertain.
We do not have a great deal
of experience in commercializing our product candidates or technologies. We currently have limited marketing and commercial capability,
no direct or third-party sales force and limited distribution capabilities. We may be unable to establish or sufficiently increase
these capabilities for products we currently, or plan to, commercialize. In addition, our revenue from collaborative agreements
may be dependent upon the status of our preclinical and clinical programs.
Our ability to receive revenue
from products we commercialize presents several risks, including:
|
•
|
we or our collaborators may
fail to successfully complete clinical trials, or satisfy post-marketing commitments, sufficient to obtain and keep FDA marketing
approval;
|
|
|
|
|
•
|
many competitors are more experienced and have
significantly more resources, and their products could reach the market faster, be more cost effective or have a better efficacy
or tolerability profile than our product candidates;
|
|
|
|
|
•
|
we may fail to employ a comprehensive and effective
intellectual property strategy, which could result in decreased commercial value of our Company and our products;
|
|
|
|
|
•
|
we may fail to employ a comprehensive and effective
regulatory strategy, which could result in a delay or failure in commercialization of our products;
|
|
|
|
|
•
|
our ability to successfully commercialize our
products is affected by the competitive landscape, which cannot be fully known at this time;
|
|
|
|
|
•
|
reimbursement is constantly changing, which could
greatly affect usage of our products; and
|
|
|
|
|
•
|
future revenue from product sales would depend
on our ability to successfully complete clinical studies, obtain regulatory approvals, and manufacture, market and commercialize
our approved drugs.
|
Commercialization of peramivir
by our partners is subject to the potential commercialization risks described herein and numerous additional risks. Any potential
revenue benefits to us in the form of milestone payments, royalties or other consideration are highly speculative.
Commercialization success of
peramivir is uncertain and is subject to all the risks and uncertainties disclosed in our other risk factors relating to drug
development and commercialization. In addition, commercialization of peramivir products is subject to further risks and may be
negatively impacted by a number of factors, including, but not limited to, the following:
|
•
|
peramivir may not prove to
be adequately safe and effective for market approval in markets other than the United States, Canada, Japan, Korea and Taiwan;
|
|
|
|
|
•
|
necessary funding for post-marketing commitments
and further development of peramivir may not be available timely, at all, or in sufficient amounts;
|
|
|
|
|
•
|
flu prevention or pandemic treatment concerns
may not materialize at all, or in the near future;
|
|
|
|
|
•
|
advances in flu vaccines or other antivirals,
including competitive i.v. antivirals, could substantially replace potential demand for peramivir;
|
|
|
|
|
•
|
a limited number of governmental entities are
expected to be the primary potential stockpiling customers for peramivir and if we are not successful at marketing peramivir
to these entities for any reason, we will not receive substantial revenues from stockpiling orders;
|
|
|
|
|
•
|
government and third party payors may not provide
sufficient coverage or reimbursement which would negatively impact the demand for peramivir;
|
|
|
|
|
•
|
we may not be able to supply commercial material
to our partners and our partners may not be able to maintain or establish sufficient and acceptable commercial manufacturing,
either directly or through third-party manufacturers;
|
|
|
|
|
•
|
the commercial demand and acceptance for peramivir
by healthcare providers and by patients may not be sufficient to result in substantial revenues of peramivir to our partners
and may result in little to no milestones or royalties to us;
|
|
|
|
|
•
|
effectiveness of marketing and commercialization
efforts for peramivir by our partners;
|
|
|
|
|
•
|
market satisfaction with existing alternative
therapies;
|
|
|
|
|
•
|
perceived efficacy relative to other available
therapies;
|
|
|
|
|
•
|
disease prevalence;
|
|
|
|
|
•
|
cost of treatment;
|
|
|
|
|
•
|
pricing and availability of alternative products;
|
|
|
|
|
•
|
marketing and sales activities of competitors;
|
|
|
|
|
•
|
shifts in the medical community to new treatment
paradigms or standards of care; and
|
|
|
|
|
•
|
relative convenience and ease of administration.
|
We are subject to various
federal and state laws related to RAPIVAB and other products under development and, if we or our partners do not comply with these
regulations, we could face substantial penalties.
Our or our partners’ activities
related to RAPIVAB, or any of our other products under development and following their regulatory approval, are subject to regulatory
and law enforcement authorities in addition to the FDA, including the Federal Trade Commission, the Department of Justice, and
state and local governments. In
the case of our collaboration with SUL, although SUL is responsible for RAPIVAB marketing and
commercialization efforts, we continue to carry certain risks associated with RAPIVAB because we hold the RAPIVAB NDA. For example,
we are responsible for reporting adverse drug experiences, we have responsibility for certain post-approval studies, we may have
responsibilities and costs related to a recall or withdrawal of RAPIVAB from sale, we may incur liability associated with RAPIVAB
manufacturing contracted by us or in support of any of our partners, we are required to maintain records and provide data and
reports to regulatory agencies related to RAPIVAB (e.g. risk evaluation and mitigation strategies, track and trace requirements,
adverse events), and we may incur certain promotional regulatory and government pricing risks, all of which could have a material
adverse impact on our operations and financial condition.
In addition, we are subject
to the federal physician sunshine act and certain similar physician payment and drug pricing transparency legislation in various
states. We are also subject to various federal and state laws pertaining to health care “fraud and abuse,” including
both federal and state anti-kickback and false claims laws. These laws regulate our or our partners’ operations, sales and
marketing practices, price reporting, and relationships with physicians and other customers and third-party payors. Anti-kickback
laws generally prohibit a manufacturer from soliciting, offering, receiving, or paying any remuneration to generate business,
including the purchase or prescription of a particular drug. Although the specific provisions of these laws vary, their scope
is generally broad and there may be no regulations, guidance or court decisions that clarify how the laws apply to particular
industry practices. False claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented for
payment to third party payors (including Medicare and Medicaid) claims for reimbursement or services that are false or fraudulent,
claims for items or services not provided as claimed, or claims for medically unnecessary items or services. The sunshine provisions
apply to manufacturers with products reimbursed under certain government programs and require those manufacturers to disclose
annually to the federal government certain payments made to physicians (defined to include doctors, dentists, optometrists, podiatrists
and chiropractors) and teaching hospitals, as well as, ownership and investment interests held by physicians (as defined above)
and their immediate family members. State laws may also require disclosure of pharmaceutical pricing information and marketing
expenditures. Although we seek to comply with these statutes, it is possible that our practices, or those of our partners, might
be challenged under health care fraud and abuse, anti-kickback, false claims or similar laws. Violations of the physician sunshine
act and similar state legislation or the fraud and abuse laws may be punishable by civil or criminal sanctions, including fines
and civil monetary penalties, and future exclusion from participation in government healthcare programs.
We have a number of outstanding
post-marketing commitments to the FDA that we retain, despite our partnership with SUL, which we may not complete successfully
or on time for any number of reasons, including but not limited to lack of funds to complete the studies and insufficient interest
by appropriate sites, investigators or study subjects. For example, as a condition of the approval of RAPIVAB, we are required
to complete a pediatric patient study of RAPIVAB and to submit the final results of this clinical trial to the FDA. Depending
on the FDA’s evaluation of the data from this clinical trial, we may be unable to expand the indication for RAPIVAB or we
may be required to include specific warnings or limitations on dosing this product, which could negatively impact sales of RAPIVAB
and negatively impact our relationship with our partner. We may be subject to penalties if we fail to comply with post-approval
legal and regulatory requirements and our products could be subject to continual recordkeeping and reporting requirements, review
and periodic inspections by the FDA and other regulatory bodies. Regulatory approval of a product may be subject to limitations
on the indicated uses for which the product may be marketed or to the other restrictive conditions of approval that limit our
ability to promote, sell or distribute a product. Furthermore, the approval of RAPIVAB and any other future product candidates
may be subject to requirements for costly post-marketing testing and surveillance to monitor its safety or efficacy.
Advertising and promotion are
subject to stringent FDA rules and oversight and as the holder of the NDA we may be held responsible for any advertising and promotion
conducted by our partner that is not in compliance with the rules and regulations. In particular, the claims in all promotional
materials and activities must be consistent with the FDA approvals for approved products, and must be appropriately substantiated
and fairly balanced with information on the safety risks and limitations of the products. Adverse event information concerning
approved products must be reviewed and as the NDA holder of RAPIVAB we are required to make expedited and periodic adverse event
reports to the FDA and other regulatory authorities.
In addition, the research, manufacturing,
distribution, sale and promotion of products are potentially subject to regulation by various federal, state and local authorities
in addition to the FDA, including the Centers for Medicare and Medicaid Services, other divisions of the U.S. Department of Health
and Human Services, the
U.S. Department of Justice and individual U.S. Attorney offices within the Department of Justice, and
state and local governments. Until we can successfully transfer the pricing responsibilities to our partner, we remain responsible
for pricing and rebate programs. Pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus
Budget Reconciliation Act of 1990, as amended, and the Veterans Health Care Act of 1992, as amended. If products are made available
to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply.
All of these activities are also potentially subject to federal and state healthcare false claims and fraud and abuse laws, as
well as consumer protection and unfair competition laws.
If our operations with respect
to RAPIVAB or our other products that are subject to healthcare laws and regulations are found to be in violation of any of the
healthcare fraud and abuse laws described above or any other governmental regulations that apply to us, we may be subject to penalties,
including civil and criminal penalties, damages, fines and the curtailment or restructuring of our operations. Any penalties,
damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and
our financial results. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these
laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend
against it, could cause us to incur significant legal expenses and divert our management's attention from the operation of our
business. Moreover, achieving and sustaining compliance with all applicable federal and state fraud and abuse laws may be costly.
We and our partners may
be subject to new legislation, regulatory proposals and healthcare payor initiatives that may increase our costs of compliance
and adversely affect our or our partners’ ability to market our products, including RAPIVAB, obtain collaborators and raise
capital.
The Patient Protection and Affordable
Care Act, or PPACA, made extensive changes to the delivery of health care in the U.S. The PPACA included numerous provisions that
affect pharmaceutical companies, some of which became effective immediately and others of which have taken effect over the past
several years. For example, the PPACA expanded health care coverage to the uninsured through private health insurance reforms
and an expansion of Medicaid. The PPACA also imposed substantial costs on pharmaceutical manufacturers, such as an increase in
liability for rebates paid to Medicaid, new drug discounts that must be offered to certain enrollees in the Medicare prescription
drug benefit, an annual fee imposed on all manufacturers of brand prescription drugs in the U.S., and an expansion of an existing
program requiring pharmaceutical discounts to certain types of hospitals and federally subsidized clinics. The PPACA also contains
cost containment measures that could reduce reimbursement levels for health care items and services generally, including pharmaceuticals.
It also required reporting and public disclosure of payments and other transfers of value provided by pharmaceutical companies
to physicians and teaching hospitals.
We expect that the current presidential
administration and U.S. Congress will likely continue to seek to modify, repeal, or otherwise invalidate all, or certain provisions
of, the PPACA. There is still significant uncertainty with respect to the impact that the current presidential administration
and the U.S. Congress may have on the PPACA, if any, and any changes will likely take time to unfold. As such, we cannot predict
what effect the PPACA or other healthcare reform initiatives that may be adopted in the future will have on our business.
We cannot predict what effect
the PPACA or other healthcare reform initiatives that may be adopted in the future will have on our business. The continuing efforts
of the government, insurance companies, managed care organizations and other payors of health care services to contain or reduce
costs of health care could result in decreased net revenues from our pharmaceutical products and decrease potential returns from
our development efforts. In addition, pharmaceutical and device manufacturers are also required to report and disclose certain
payments and transfers of value to, and investment interests held by, physicians and their immediate family members during the
preceding calendar year. Failure to submit required information may result in civil monetary penalties for payments, transfers
of value or ownership or investment interests not reported in an annual submission. Compliance with the PPACA and state laws with
similar provisions is difficult and time consuming, and companies that do not comply with these state laws face civil penalties.
Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities
could be subject to challenge under one or more of such laws. Such a challenge could have a material adverse effect on our business,
financial condition, results of operations and growth prospects.
In addition, there have been
a number of other legislative and regulatory proposals aimed at changing the pharmaceutical industry. In particular, legislation
has been enacted in certain states and at a federal level that requires development of an electronic pedigree to track and trace
each prescription drug at the saleable unit
level through the
distribution system. Compliance with these electronic pedigree requirements may increase our operational expenses and impose
significant administrative burdens. In addition, our compliance may be deemed insufficient and we could face a material
adverse effect on our business, financial condition, results of operations and growth prospects. As a result of these
and other new proposals, we may determine to change our current manner of operation, provide additional benefits or change
our contract arrangements, any of which could have a material adverse effect on our business, financial condition and results
of operations.
Adequate coverage and reimbursement
in the U.S. and other markets is critical to the commercial success of RAPIVAB or any other product that we might bring to market.
Recently in the U.S. there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their
marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among other things, reform
government program reimbursement methodologies. Third-party payors are increasingly challenging the prices charged for medical
products and services and, in some cases, imposing restrictions on the coverage of particular drugs. Many third-party payors negotiate
the price of medical services and products and develop formularies which establish pricing and reimbursement levels. Exclusion
of a product from a formulary can lead to its sharply reduced usage in the third-party payor’s patient population. The process
for obtaining coverage can be lengthy and costly, and we expect that it could take several months before a particular payor initially
reviews our product and makes a decision with respect to coverage. For example, third-party payors may require cost-benefit analysis
data from us in order to demonstrate the cost-effectiveness of RAPIVAB or any other product we might bring to market. For any
individual third-party payor, we may not be able to provide data sufficient to gain reimbursement on a similar or preferred basis
to competitive products, or at all which may have a material adverse effect on our business, financial condition and results of
operations.
There are risks related
to the potential government use or sale of peramivir (RAPIVAB).
United States Government use
or sale of RAPIVAB in emergency situations, or otherwise, may result in the use of RAPIVAB outside of its approved use. To the
extent that RAPIVAB is used as a treatment for influenza by the U.S. Government or peramivir by any other government entity, there
can be no assurance that it will prove to be generally safe, well-tolerated and effective. Such government use of RAPIVAB/peramivir
may create certain liabilities for us or our partners in the case of government use outside of the U.S. There is no assurance
that we or our manufacturers will be able to fully meet the demand for peramivir in the event of additional orders. Further, we
may not achieve a favorable price for additional orders of RAPIVAB in the U.S. or peramivir in any other country. Our competitors
may develop products that could compete with or replace peramivir. We may face competition in markets where we have no existing
intellectual property protection or are unable to successfully enforce our intellectual property rights.
There is no assurance that the
non-U.S. partnerships that we have entered into for peramivir will result in any order for peramivir in those countries. There
is no assurance that peramivir will be approved for any use or will achieve market approval in additional countries. In the event
that any emergency use or market approval is granted, there is no assurance that any government order or commercialization of
peramivir in any countries will be substantial or will be profitable to us. In addition, the sale of peramivir, emergency use
or other use of peramivir in any country may create certain liabilities for us and our partners.
If we or our partners
do not obtain and maintain governmental approvals for our product candidates under development, we or our partners will not be
able to sell these potential products, which would significantly harm our business because we will receive no revenue.
We or our partners must obtain
regulatory approval before marketing or selling our future product candidates. If we or our partners are unable to receive regulatory
approval and do not market or sell our future product candidates, we will never receive any revenue from such product sales. In
the United States, we or our partners must obtain FDA approval for product candidates that we intend to commercialize. The process
of preparing for and obtaining FDA approval may be lengthy and expensive, and approval is never certain. Products distributed
abroad are also subject to foreign government regulation and export laws of the United States. Because of the risks and uncertainties
in biopharmaceutical development, our product candidates could take a significantly longer time to gain regulatory approval than
we expect or may never gain approval. If the FDA delays regulatory approval of our product candidates, our management’s
credibility, our value and our operating results may suffer. Even if the FDA or foreign regulatory agencies approve a product
candidate, the approval may limit the indicated uses for a product candidate and/or may require post-approval studies.
The FDA regulates, among other
things, the record keeping and storage of data pertaining to potential pharmaceutical products. We currently store most of our
preclinical research data, our clinical data and our manufacturing data at our facility. While we do store duplicate copies of
most of our clinical data offsite and a significant portion of our data is included in regular backups of our systems, we could
lose important data if our facility incurs damage, or if our vendor data systems fail, suffer damage or are destroyed. If we receive
approval to market our potential products, whether in the United States or internationally, we will continue to be subject to
extensive regulatory requirements. These requirements are wide ranging and govern, among other things:
|
•
|
adverse drug experience reporting
regulations;
|
|
|
|
|
•
|
product promotion;
|
|
|
|
|
•
|
product manufacturing, including good manufacturing
practice requirements; and
|
|
|
|
|
•
|
product changes or modifications.
|
Our failure to comply with existing
or future regulatory requirements, or our loss of, or changes to, previously obtained approvals, could have a material adverse
effect on our business because we will not receive product or royalty revenues if we or our partners do not receive approval of
our products for marketing.
Royalties and milestone
payments from Shionogi under our license agreement with Shionogi (the “Shionogi Agreement”) will be required to be
used by Royalty Sub to service its obligations under its PhaRMA Notes, and generally will not be available to us for other purposes
until Royalty Sub has repaid in full its obligations under the PhaRMA Notes.
In March 2011, our wholly-owned
subsidiary Royalty Sub issued $30.0 million in aggregate principal amount of PhaRMA Notes. The PhaRMA Notes are secured principally
by (i) certain royalty and milestone payments under the Shionogi Agreement, pursuant to which Shionogi licensed from us the rights
to market peramivir in Japan and Taiwan, (ii) rights to certain payments under a Japanese yen/U.S. dollar foreign currency hedge
arrangement put into place by us in connection with the issuance of the PhaRMA Notes and (iii) the pledge by us of our equity
interest in Royalty Sub. Payments from Shionogi to us on non-governmental sales under the Shionogi Agreement will generally not
be available to us for other purposes until Royalty Sub has repaid in full its obligations under the PhaRMA Notes. Accordingly,
these funds will be required to be dedicated to Royalty Sub’s debt service and not available to us for product development
or other purposes. As of September 1, 2014, the payments from Shionogi were insufficient for Royalty Sub to service its obligations
under the PhaRMA Notes, resulting in an event of default with respect to the PhaRMA Notes. As a result of this event of default,
the holders of the PhaRMA Notes may be able to pursue acceleration of the PhaRMA Notes and foreclose on the collateral securing
the PhaRMA Notes and our equity interest in Royalty Sub and may exercise other remedies available to them under the indenture
or other documents related to the PhaRMA Notes. In such event, we may not realize the benefit of future royalty payments
that might otherwise accrue to us following repayment of the PhaRMA Notes, we may incur legal costs and we might otherwise
be adversely affected.
Because an event of default
has occurred under the PhaRMA Notes, the holders of the PhaRMA Notes may be able to pursue acceleration of the PhaRMA Notes and
foreclose on the collateral securing the PhaRMA Notes and our equity interest in Royalty Sub, in which case we may not realize
the benefit of future royalty payments that might otherwise accrue to us following repayment of the PhaRMA Notes and we could
otherwise be adversely affected.
Royalty Sub’s ability
to service its payment obligations in respect of the PhaRMA Notes, and our ability to benefit from our equity interest in Royalty
Sub, is subject to numerous risks. Royalty Sub’s ability to service the PhaRMA Notes may be adversely affected by, among
other things, changes in or any termination of our relationship with Shionogi, reimbursement, regulatory, manufacturing and/or
intellectual property issues, product returns, product recalls, product liability claims and allegations of safety issues, as
well as other factors. As Royalty Sub has been unable to service its obligations under the PhaRMA Notes and an event of default
has occurred under the PhaRMA Notes, the holders of the PhaRMA Notes may be able to pursue acceleration of the PhaRMA Notes and
foreclose on the collateral securing the PhaRMA Notes and our equity interest in Royalty Sub and may exercise other remedies
available to them under the indenture or other documents related to the PhaRMA Notes. In such event, we may not realize
the benefit of future royalty payments that might otherwise accrue to us following repayment of the PhaRMA Notes, we may incur
legal costs and we might otherwise be adversely affected.
We may be required to
pay significant premiums under the foreign currency hedge arrangement entered into by us in connection with the issuance of the
PhaRMA Notes. In addition, because our potential obligations under the foreign currency hedge are marked to market, we may experience
additional quarterly volatility in our operating results and cash flows attributable to the foreign currency hedge arrangement.
In connection with the issuance
by Royalty Sub of the PhaRMA Notes, we entered into a foreign currency hedge arrangement to hedge certain risks associated with
changes in the value of the Japanese yen relative to the U.S. dollar. Under the foreign currency hedge agreement, we may be required
to pay an annual premium in the amount of $2.0 million in each May continuing through May 2020. Such payment will be required
if, in May of the relevant year, the spot rate of exchange for Japanese yen-U.S. dollars (determined in accordance with the foreign
currency hedge arrangement) is such that the U.S. dollar is worth 100 yen or less. We will be required to mark to market our potential
obligations under the currency hedge and post cash collateral, which may cause us to experience additional quarterly volatility
in our operating results and cash flows as a result. Additionally, we may be required to pay significant premiums or a termination
fee under the foreign currency hedge agreement entered into by us in connection with the issuance of the PhaRMA Notes. We are
required to maintain a foreign currency hedge at 100 yen per dollar under the agreements governing the PhaRMA Notes.
Our Senior Credit Facility
contains restrictions that limit our flexibility in operating our business. We may be required to make a prepayment or repay the
outstanding indebtedness earlier than we expect if a prepayment event or an event of default occurs, including a material adverse
change with respect to us, which could have a material adverse effect on our business.
The Senior Credit Facility contains
various covenants that limit our ability to engage in specified types of transactions. These covenants limit our ability to, among
other things:
|
•
|
convey, sell, lease, license,
transfer or otherwise dispose of certain parts of our business or property;
|
|
|
|
|
•
|
change the nature of our business;
|
|
|
|
|
•
|
liquidate or dissolve;
|
|
|
|
|
•
|
enter into certain change in control or acquisition
transactions;
|
|
|
|
|
•
|
incur or assume certain debt;
|
|
|
|
|
•
|
grant certain types of liens on our assets;
|
|
|
|
|
•
|
modify, liquidate or transfer assets in certain
collateral accounts;
|
|
|
|
|
•
|
pay dividends or make certain distributions to
our stockholders;
|
|
|
|
|
•
|
make certain investments;
|
|
|
|
|
•
|
enter into material transactions with affiliates;
and
|
|
|
|
|
•
|
modify existing debt or collaboration arrangements.
|
The restrictive covenants contained
in the Senior Credit Facility could cause us to be unable to pursue business opportunities that we or our stockholders may consider
beneficial without the lender’s permission or without repaying all Senior Credit Facility obligations.
A breach of any of these covenants
could result in an event of default under the Senior Credit Facility. An event of default will also occur if, among other things,
a material adverse change in our business, operations or condition occurs, which could potentially include negative results in
clinical trials, or a material impairment of the prospect of our repayment of any portion of the amounts we owe under the Senior
Credit Facility occurs. In the case of a continuing event of default under the agreement, the lender could elect to declare all
amounts outstanding to be immediately due and payable, proceed against the collateral in which we granted to the lender a security
interest under the Senior Credit Facility, or otherwise exercise the rights of a secured creditor.
Amounts outstanding under the
Senior Credit Facility are secured by substantially all of our assets and those of our subsidiaries, excluding certain specified
assets but including proceeds from those assets.
If we fail to adequately
protect or enforce our intellectual property rights or secure rights to patents of others, the value of those rights would diminish.
Our success will depend in part
on our ability and the abilities of our partners to obtain, protect and enforce viable intellectual property rights including
but not limited to trade name, trademark and patent protection for our Company and its products, methods, processes and other
technologies we may license or develop, to preserve our trade secrets, and to operate without infringing the proprietary rights
of third parties both domestically and abroad. The patent position of biotechnology and pharmaceutical companies is generally
highly uncertain, involves complex legal and factual questions and has recently been the subject of much litigation. Neither the
United States Patent and Trademark Office (“USPTO”), the Patent Cooperation Treaty offices, nor the courts of the
United States and other jurisdictions have consistent policies nor predictable rulings regarding the breadth of claims allowed
or the degree of protection afforded under many biotechnology and pharmaceutical patents.
Further, we may not have worldwide patent
protection for all of our product candidates and our intellectual property rights may not be legally protected or enforceable
in all countries throughout the world. In some jurisdictions, some of our product candidates in certain programs, including our
HAE program, may have short or no composition of matter patent life and we may therefore rely on orphan drug exclusivity or data
exclusivity. There can be no assurance that we will obtain orphan drug exclusivity or data exclusivity in every jurisdiction.
Further, in some jurisdictions, we may rely on formulation patents or method of use patents. Both the ability to achieve issuance
and the enforcement of formulation and method of use patents can be highly uncertain and can vary from jurisdiction to jurisdiction,
and such patents may therefore not adequately prevent competitors and potential infringers in some jurisdictions. The validity,
scope, enforceability and commercial value of the rights protected by such patents, therefore, is highly uncertain.
We also rely on trade secrets
to protect technology in cases when we believe patent protection is not appropriate or obtainable. However, trade secrets are
difficult to protect. If we cannot maintain the confidentiality of our technology and other confidential information in connection
with our collaborators and advisors, our ability to receive patent protection or protect our proprietary information may be imperiled.
We may be involved in
lawsuits to protect or enforce our patents, the patents of our partners or our other intellectual property rights, which could
be expensive, time consuming and unsuccessful.
Competitors may infringe or
otherwise violate our patents, the patents of our licensors or our other intellectual property rights. To counter infringement
or unauthorized use, we may be required to file legal claims, which can be expensive and time-consuming and unsuccessful. An adverse
result in any litigation or defense proceeding could put one or more of our patents at risk. Our success depends in part on avoiding
the infringement of other parties’ patents and other intellectual property rights as well as avoiding the breach of any
licenses relating to our technologies and products. In the United States, patent applications filed in recent years are confidential
for 18 months, while older applications are not published until the patent issues. As a result, avoiding patent infringement may
be difficult and we may inadvertently infringe third-party patents or proprietary rights. These third parties could bring claims
against us, our partners or our licensors that even if resolved in our favor, could cause us to incur substantial expenses and,
if resolved against us, could additionally cause us to pay substantial damages. Further, if a patent infringement suit were brought
against us, our partners or our licensors, we or they could be forced to stop or delay research, development, manufacturing or
sales of any infringing product in the country or countries covered by the patent we infringe, unless we can obtain a license
from the patent holder. Such a license may not be available on acceptable terms, or at all, particularly if the third party is
developing or marketing a product competitive with the infringing product. Even if we, our partners or our licensors were able
to obtain a license, the rights may be nonexclusive, which would give our competitors access to the same intellectual property.
If we or our partners are unable
or fail to adequately initiate, protect, defend or enforce our intellectual property rights in any area of commercial interest
or in any part of the world where we wish to seek regulatory approval for our products, methods, processes and other technologies,
the value of the product candidates to produce revenue would diminish. Additionally, if our products, methods, processes, and
other technologies or our commercial use of such products, processes, and other technologies, including but not limited to any
trade name, trademark or commercial strategy infringe the proprietary rights of other parties, we could incur substantial costs.
The USPTO and the patent offices of other jurisdictions have issued to us a number of patents
for our various inventions and we
have in-licensed several patents from various institutions. We have filed additional patent applications and provisional patent
applications with the USPTO. We have filed a number of corresponding foreign patent applications and intend to file additional
foreign and U.S. patent applications, as appropriate. We have also filed certain trademark and trade name applications worldwide.
We cannot assure you as to:
|
•
|
the degree and range of protection
any patents will afford against competitors with similar products;
|
|
|
|
|
•
|
if and when patents will issue;
|
|
|
|
|
•
|
if patents do issue we cannot be sure that we
will be able to adequately defend such patents and whether or not we will be able to adequately enforce such patents; or
|
|
|
|
|
•
|
whether or not others will obtain patents claiming
aspects similar to those covered by our patent applications.
|
If the USPTO or other foreign
patent office upholds patents issued to others or if the USPTO grants patent applications filed by others, we may have to:
|
•
|
obtain licenses or redesign
our products or processes to avoid infringement;
|
|
|
|
|
•
|
stop using the subject matter claimed in those
patents; or
|
|
|
|
|
•
|
pay damages.
|
We may initiate, or others may
bring against us, litigation or administrative proceedings related to intellectual property rights, including proceedings before
the USPTO or other foreign patent office. Any judgment adverse to us in any litigation or other proceeding arising in connection
with a patent or patent application could materially and adversely affect our business, financial condition and results of operations.
In addition, the costs of any such proceeding may be substantial whether or not we are successful.
Our success is also dependent
upon the skills, knowledge and experience, none of which is patentable, of our scientific and technical personnel. To help protect
our rights, we require all employees, consultants, advisors and partners to enter into confidentiality agreements that prohibit
the disclosure of confidential information to anyone outside of our company and require disclosure and assignment to us of their
ideas, developments, discoveries and inventions. These agreements may not provide adequate protection for our trade secrets, know-how
or other proprietary information in the event of any unauthorized use or disclosure or the lawful development by others of such
information, and if any of our proprietary information is disclosed, our business will suffer because our revenues depend upon
our ability to license or commercialize our product candidates and any such events would significantly impair the value of such
product candidates.
Our failure to comply
with data protection laws and regulations could lead to government enforcement actions and significant penalties against us and
adversely impact our operating results.
European Union (“EU”)
Member States, Switzerland and other countries have adopted data protection laws and regulation, which impose significant compliance
obligations. For example, the EU Data Protection Directive, as implemented into national laws by the EU Member States, imposes
strict obligations and restrictions on the ability to collect, analyze and transfer personal data, including health data from
clinical trials and adverse event reporting. Data protection authorities from the different EU Member States may interpret
the EU Data Protection Directive and national laws differently, which adds to the complexity of processing personal data in the
European Union, and guidance on implementation and compliance practices is often updated or otherwise revised. Our failure
to comply with these laws and regulations could lead to government enforcement actions and significant penalties against us and
adversely impact our operating results.
We are subject to litigation, which could result in losses or unexpected expenditure
of time and resources.
From time to time, we may be
called upon to defend ourselves against lawsuits relating to our business. Due to the inherent uncertainties in litigation,
we cannot accurately predict the ultimate outcome of any such proceedings. An unfavorable outcome in any such proceedings
could have an adverse impact on our business, financial condition and results of operations. If our stock price is volatile,
we may become involved in securities
class action lawsuits in the future. Any litigation in the future, regardless of its
merits, could result in substantial costs and a diversion of management’s attention and resources that are needed to successfully
run our business.
We face an inherent risk
of liability in the event that the use or misuse of our products results in personal injury or death and our product liability
insurance coverage may be insufficient.
If the use or misuse of peramivir,
forodesine or any other regulatory body-approved products we or a partner may sell in the future harms people, we may be subject
to costly and damaging product liability claims brought against us by consumers, healthcare providers, pharmaceutical companies,
third-party payors or others. The use of our product candidates in clinical trials, including post marketing clinical studies,
could also expose us to product liability claims. We cannot predict all of the possible harms or side effects that may result
from the use of our products or the testing of product candidates and, therefore, the amount of insurance coverage we currently
may not be adequate to cover all liabilities or defense costs we might incur. A product liability claim or series of claims brought
against us could give rise to a substantial liability that could exceed our resources. Even if claims are not successful, the
costs of defending such claims and potential adverse publicity could be harmful to our business.
We face an inherent risk of
product liability exposure related to the testing of our product candidates in human clinical trials and will face even greater
risks upon any commercialization by us of our product candidates. We have product liability insurance covering our clinical trials.
Clinical trial and product liability insurance is becoming increasingly expensive. As a result, we may be unable to obtain sufficient
insurance or increase our existing coverage at a reasonable cost to protect us against losses that could have a material adverse
effect on our business. An individual may bring a product liability claim against us if one of our products or product candidates
causes, or is claimed to have caused, an injury or is found to be unsuitable for consumer use. Any product liability claim brought
against us, with or without merit, could result in:
|
•
|
liabilities that substantially
exceed our product liability insurance, which we would then be required to pay from other sources, if available;
|
|
|
|
|
•
|
an increase of our product liability insurance
rates or the inability to maintain insurance coverage in the future on acceptable terms, or at all;
|
|
|
|
|
•
|
withdrawal of clinical trial volunteers or patients;
|
|
|
|
|
•
|
damage to our reputation and the reputation of
our products, resulting in lower sales;
|
|
|
|
|
•
|
regulatory investigations that could require
costly recalls or product modifications;
|
|
|
|
|
•
|
litigation costs; and
|
|
|
|
|
•
|
the diversion of management’s attention
from managing our business.
|
Insurance coverage is
increasingly more costly and difficult to obtain or maintain.
While we currently have insurance
for our business, property, directors and officers, and our products, insurance is increasingly more costly and narrower in scope,
and we may be required to assume more risk in the future. If we are subject to claims or suffer a loss or damage in excess of
our insurance coverage, we will be required to bear any loss in excess of our insurance limits. If we are subject to claims or
suffer a loss or damage that is outside of our insurance coverage, we may incur significant uninsured costs associated with loss
or damage that could have an adverse effect on our operations and financial position. Furthermore, any claims made on our insurance
policies may impact our ability to obtain or maintain insurance coverage at reasonable costs or at all.
If our facility incurs
damage or power is lost for a significant length of time, our business will suffer.
We store clinical and stability
samples at our facility that could be damaged if our facility incurs physical damage or in the event of an extended power failure.
We have backup power systems in addition to backup generators to maintain power to all critical functions, but any loss of these
samples could result in significant delays in our drug development process.
In addition, we store most of
our preclinical and clinical data at our facilities. Duplicate copies of most critical data are secured off-site. Any significant
degradation or failure of our computer systems could cause us to inaccurately calculate or lose our data. Loss of data could result
in significant delays in our drug development process and any system failure could harm our business and operations.
A significant disruption
in our information technology systems or a cyber-security breach could adversely affect our business.
We are increasingly dependent
on information technology systems to operate our business. Like other companies in our industry, our networks and infrastructure
may be vulnerable to cyber-attacks or intrusions, including by computer hackers, foreign governments, foreign companies or competitors,
or may be breached by employee error, malfeasance or other disruption. A breakdown, invasion, corruption, destruction or interruption
of critical information technology systems could negatively impact operations. If our systems are damaged, fail to function
properly or otherwise become unavailable, we may incur substantial costs to repair or replace them, and we may experience loss
of critical data and interruptions or delays in our ability to perform critical functions, which could adversely affect our business,
financial condition or results of operations. Any compromise of our data security could also result in a violation of applicable
privacy and other laws, significant legal and financial exposure, damage to our reputation, loss or misuse of the information
and a loss of confidence in our data security measures, which could harm our business. There can be no assurance that our efforts
to protect our data and information technology systems will prevent breakdowns or breaches in our systems, or those of third parties
with which we do business, and any such events could adversely affect our business.
If we fail to retain our
existing key personnel or fail to attract and retain additional key personnel, the development of our product candidates and commercialization
of our products and the related expansion of our business will be delayed or stopped.
We are highly dependent upon
our senior management and scientific team, the unexpected loss of whose services might impede the achievement of our development
and commercial objectives. Competition for key personnel with the experience that we require is intense and is expected to continue
to increase. Our inability to attract and retain the required number of skilled and experienced management, commercial, operational
and scientific personnel will harm our business because we rely upon these personnel for many critical functions of our business.
If because of our use
of hazardous materials, we violate any environmental controls or regulations that apply to such materials, we may incur substantial
costs and expenses in our remediation efforts.
Our research and development
involves the controlled use of hazardous materials, chemicals and various radioactive compounds. We are subject to federal, state
and local laws and regulations governing the use, storage, handling and disposal of these materials and some waste products. Accidental
contamination or injury from these materials could occur. In the event of an accident, we could be liable for any damages that
result and any liabilities could exceed our resources. Compliance with environmental laws and regulations or a violation of such
environmental laws and regulations could require us to incur substantial unexpected costs, which would materially and adversely
affect our results of operations.
Risks relating
to investing in our common stock
Our existing principal
stockholders hold a substantial amount of our common stock and may be able to influence significant corporate decisions, which
may conflict with the interest of other stockholders.
Several of our stockholders
own greater than 5% of our outstanding common stock. Our top ten stockholders own more than 50% of BioCryst and can individually,
and as a group, influence our operations based upon their concentrated ownership. These stockholders, if they act together, may
be able to influence the outcome of matters requiring approval of the stockholders, including the election of our directors and
other corporate actions.
Our stock price has been,
and is likely to continue to be, highly volatile, which could cause the value of an investment in our common stock to decline
significantly.
The market prices for securities
of biotechnology companies in general have been highly volatile and may continue to be highly volatile in the future. Moreover,
our stock price has fluctuated frequently, and these
fluctuations are often not related to our financial results. For the twelve
months ended June 30, 2017, the 52-week range of the market price of our stock was from $2.82 to $9.25 per share. The following
factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our
common stock:
|
•
|
announcements of technological
innovations or new products by us or our competitors;
|
|
|
|
|
•
|
developments or disputes concerning patents or
proprietary rights;
|
|
|
|
|
•
|
additional dilution through sales of our common
stock or other derivative securities;
|
|
|
|
|
•
|
status of new or existing licensing or collaborative
agreements and government contracts;
|
|
|
|
|
•
|
announcements relating to the status of our programs;
|
|
|
|
|
•
|
developments and announcements regarding new
and virulent strains of influenza;
|
|
|
|
|
•
|
we or our partners achieving or failing to achieve
development milestones;
|
|
|
|
|
•
|
publicity regarding actual or potential medical
results relating to products under development by us or our competitors;
|
|
|
|
|
•
|
publicity regarding certain public health concerns
for which we are or may be developing treatments;
|
|
|
|
|
•
|
regulatory developments in both the United States
and foreign countries;
|
|
|
|
|
•
|
public concern as to the safety of pharmaceutical
products;
|
|
|
|
|
•
|
actual or anticipated fluctuations in our operating
results;
|
|
|
|
|
•
|
changes in financial estimates or recommendations
by securities analysts;
|
|
|
|
|
•
|
changes in the structure of healthcare payment
systems, including developments in price control legislation;
|
|
|
|
|
•
|
announcements by us or our competitors of significant
acquisitions, strategic partnerships, joint ventures or capital commitments;
|
|
|
|
|
•
|
additions or departures of key personnel or members
of our board of directors;
|
|
|
|
|
•
|
purchases or sales of substantial amounts of
our stock by existing stockholders, including officers or directors;
|
|
|
|
|
•
|
economic and other external factors or other
disasters or crises; and
|
|
|
|
|
•
|
period-to-period fluctuations in our financial
results.
|
Future sales and issuances
of securities may dilute your ownership interest and cause our stock price to decline.
Future sales of our common stock
by current stockholders into the public market could cause the market price of our stock to fall. As of August 31, 2017, there
were 80,465,497 shares of our common stock outstanding. We may from time to time issue securities in relation to a license arrangement,
collaboration, merger or acquisition. We may also sell, for our own account, shares of common stock or other equity securities,
from time to time at prices and on terms to be determined at the time of sale.
As of August 31, 2017, there
were 13,620,184 stock options and restricted stock units outstanding, 1,592,532 shares available for issuance under our Amended
and Restated Stock Incentive Plan, and 330,270 shares available for issuance under our Employee Stock Purchase Plan. In addition,
we could also make equity compensation grants outside of our Stock Incentive Plan. The shares underlying existing stock options,
restricted stock units and possible future stock options, stock appreciation rights and stock awards have been registered pursuant
to registration statements on Form S-8.
If some or all of such shares
are sold or otherwise issued into the public market over a short period of time, our current stockholders’ ownership interests
may be diluted and the value of all publicly traded shares is likely to decline, as the market may not be able to absorb those
shares at then-current market prices. Additionally, such sales and issuances may make it more difficult for us to sell equity
securities or equity-related securities in the future at a time and price that our management deems acceptable, or at all.
In March 2017, we entered into
a Registration Rights Agreement with entities affiliated with Baker Bros. Advisors LP (the “Baker Entities”) to provide
that, if requested, we will register the shares of our common stock beneficially owned by the Baker Entities for resale under
the Securities Act. Our registration obligations pursuant to the Registration Rights Agreement cover all shares then held or thereafter
acquired by the Baker Entities, for up to ten years, and include our obligation to facilitate certain underwritten public offerings
of our common stock by the Baker Entities in the future. On May 10, 2017, we filed a registration statement on Form S-3 with respect
to 11,710,951 shares of common stock held by the Baker Entities. If the Baker Entities, by exercising their underwriting rights
or otherwise, sell a large number of our shares, or the market perceives that the Baker Entities intend to sell a large number
of our shares, this could adversely affect the market price of our common stock.
We have anti-takeover
provisions in our corporate charter documents that may result in outcomes with which you do not agree.
Our board of directors has the
authority to issue up to 4,800,000 shares of undesignated preferred stock and to determine the rights, preferences, privileges
and restrictions of those shares without further vote or action by our stockholders. The rights of the holders of any preferred
stock that may be issued in the future may adversely affect the rights of the holders of common stock. The issuance of preferred
stock could make it more difficult for third parties to acquire a majority of our outstanding voting stock.
In addition, our certificate
of incorporation provides for staggered terms for the members of the board of directors and supermajority approval of the removal
of any member of the board of directors and prevents our stockholders from acting by written consent. Our certificate also requires
supermajority approval of any amendment of these provisions. These provisions and other provisions of our by-laws and of Delaware
law applicable to us could delay or make more difficult a merger, tender offer or proxy contest involving us.
We have broad discretion
in the use of the net proceeds from this offering and may not use them effectively.
Management will have broad discretion
in the application of the net proceeds, including any of the purposes described in “Use of Proceeds.” The failure
by our management to apply these funds effectively could have a material adverse effect on our business.
We have never paid dividends
on our common stock and do not anticipate doing so in the foreseeable future.
We have never paid cash dividends
on our stock. We currently intend to retain all future earnings, if any, for use in the operation of our business. Accordingly,
we do not anticipate paying cash dividends on our common stock in the foreseeable future.
Use of proceeds
We estimate that we will receive net proceeds of approximately $74.4 million from the
sale of shares of our common stock offered by us in this offering, after deducting underwriting discounts and commissions
and estimated offering expenses payable by us. If the underwriters exercise their option to purchase additional shares in full,
we estimate that the net proceeds to us will be approximately $85.5 million, after deducting underwriting discounts and
commissions and estimated offering expenses payable by us. We currently intend to use the net proceeds from the sale of the common
stock in this offering for general corporate purposes, which may include funding the global launch preparation of BCX7353; expanding
our global awareness and commercial and manufacturing efforts; expanding our infrastructure, including commercial and manufacturing,
to benefit BCX7353 and the rest of our programs; supporting the initiation of the Phase 3 clinical trial for BCX7353, the long
term safety study, and all remaining preclinical and clinical studies to support an NDA filing for BCX7353; funding the exploratory
Phase 2 trial for acute treatment with BCX7353; and advancing our early-stage programs into clinical trials.
As of the date of this prospectus supplement, we cannot specify with certainty all of the particular uses
of the proceeds from this offering. As a result, our management will retain broad discretion in the allocation and use of the net
proceeds from this offering. Pending these uses, we intend to invest the net proceeds from this offering in short-term, interest-bearing
investment grade securities, certificates of deposit or direct or guaranteed obligations of the U.S. government.
Dilution
As of June 30, 2017, our net tangible book value was approximately $33.1 million, or
approximately $0.41 per share of common stock. Net tangible book value per share represents the amount of our total assets, excluding
deferred collaboration expenses, less total liabilities, excluding deferred collaboration revenues, divided by the 80,428,121 shares
of our common stock outstanding as of June 30, 2017.
Investors participating in this offering will incur immediate, substantial dilution. After giving effect to
our receipt of approximately $74.4 million of estimated net proceeds (after deducting underwriting discounts and commissions and
estimated offering expenses payable by us) from our sale of common stock in this offering, our as adjusted net tangible book value
as of June 30, 2017 would have been $107.5 million, or $1.12 per share. This amount represents an immediate increase in net tangible
book value of $0.71 per share of our common stock to existing stockholders and an immediate dilution in net tangible book value
of $4.03 per share of our common stock to new investors purchasing shares of common stock in this offering.
The following table illustrates
this dilution on a per share basis:
|
Public
offering price per share
|
|
$ 5.15
|
|
Net tangible book value per share as of June
30, 2017
|
$ 0.41
|
|
|
Increase in net tangible book value per share
attributable to new investors
|
$ 0.71
|
|
|
Net tangible book
value per share as of June 30, 2017 after giving effect to this offering
|
|
$ 1.12
|
|
Dilution
in net tangible book value per share to new investors
|
|
$ 4.03
|
If the underwriters’ option to purchase additional shares is exercised in full, the as adjusted net
tangible book value per share after giving effect to this offering would be $1.21 per share, which amount represents an immediate
increase in as adjusted net tangible book value of $0.80 per share of our common stock to existing stockholders and an immediate
dilution in net tangible book value of $3.94 per share of our common stock to new investors purchasing shares of common stock in
this offering.
The above discussion and table
are based on 80,428,121 shares of our common stock outstanding as of June 30, 2017 and exclude:
|
•
|
13,412,549 shares of common
stock issuable upon the exercise of stock options outstanding under our Stock Incentive Plan as of June 30, 2017, at a weighted
average exercise price of $6.19 per share;
|
|
|
|
|
•
|
219,574 shares of common stock issuable upon
the vesting of restricted stock units outstanding under our Stock Incentive Plan as of June 30, 2017; and
|
|
|
|
|
|
|
•
|
1,580,593 additional shares of common stock reserved
for issuance under our Stock Incentive Plan and 363,646 additional shares of common stock reserved for issuance under our
Employee Stock Purchase Plan as of June 30, 2017.
|
To the extent that outstanding
options have been or may be exercised or other shares issued, there may be further dilution to investors. In addition, we may
choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient
funds for our current or future operating plans. To the extent that we raise additional capital by issuing equity securities or
convertible debt, your ownership may be further diluted.
Price range
of common stock and dividend policy
Our common stock is listed on
the NASDAQ Global Select Market under the symbol “BCRX.” The following table sets forth, for the periods indicated,
the range of high and low sales prices for our common stock, as reported by the NASDAQ Global Select Market.
|
|
|
High
|
|
Low
|
|
Year ended December 31,
2015
|
|
|
|
|
|
|
|
|
|
1
st
Quarter
|
|
$
|
12.71
|
|
|
$
|
7.85
|
|
|
2
nd
Quarter
|
|
$
|
16.43
|
|
|
$
|
8.50
|
|
|
3
rd
Quarter
|
|
$
|
16.83
|
|
|
$
|
10.26
|
|
|
4
th
Quarter
|
|
$
|
12.88
|
|
|
$
|
8.01
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2016
|
|
|
|
|
|
|
|
|
|
1
st
Quarter
|
|
$
|
10.24
|
|
|
$
|
1.63
|
|
|
2
nd
Quarter
|
|
$
|
4.03
|
|
|
$
|
2.49
|
|
|
3
rd
Quarter
|
|
$
|
5.80
|
|
|
$
|
2.82
|
|
|
4
th
Quarter
|
|
$
|
7.56
|
|
|
$
|
3.75
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ending December 31, 2017
|
|
|
|
|
|
|
|
|
|
1
st
Quarter
|
|
$
|
9.25
|
|
|
$
|
4.20
|
|
|
2
nd
Quarter
|
|
$
|
8.80
|
|
|
$
|
5.02
|
|
|
3
rd
Quarter (through September 12, 2017)
|
|
$
|
6.22
|
|
|
$
|
3.95
|
|
The last reported sale price of our common stock on the NASDAQ Global
Select Market on September 12, 2017 was $5.23 per share. As of September 12, 2017 there were approximately 178 holders of record
of our common stock.
We have
never paid cash dividends and do not anticipate paying cash dividends in the foreseeable future.
Underwriting
We are offering the shares of
common stock described in this prospectus supplement through a number of underwriters. J.P. Morgan Securities LLC and Barclays
Capital Inc. are acting as book-running managers of the offering and as representatives of the underwriters. We have entered into
an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, we have agreed
to sell to the underwriters, and each underwriter has severally agreed to purchase, at the public offering price less the underwriting
discounts and commissions set forth on the cover page of this prospectus supplement, the number of shares of common stock listed
next to its name in the following table:
|
Name
|
Number of
Shares
|
|
J.P. Morgan Securities LLC
|
9,475,728
|
|
Barclays Capital Inc.
|
5,126,213
|
|
H.C. Wainwright & Co., LLC
|
932,040
|
|
Total
|
15,533,981
|
The underwriters are committed
to purchase all the shares of common stock offered by us if they purchase any shares. The underwriting agreement also provides
that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may also be increased or the offering
may be terminated.
The underwriters propose to
offer the shares of common stock directly to the public at the public offering price set forth on the cover page of this prospectus
supplement and to certain dealers at that price less a concession not in excess of $0.1854
per share. After the offering of the shares to the public, the offering price and other selling terms may be changed by the underwriters.
Sales of shares made outside of the United States may be made by affiliates of the underwriters.
The underwriters have an option to buy up to 2,330,097 additional shares of
common stock from us to cover sales of shares by the underwriters which exceed the number of shares specified in the table
above. The underwriters have 30 days from the date of this prospectus supplement to exercise this option to purchase
additional shares. If any shares are purchased with this option to purchase additional shares, the underwriters will purchase
shares in approximately the same proportion as shown in the table above. If any additional shares of common stock are
purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being
offered.
The underwriting fee is equal
to the public offering price per share of common stock less the amount paid by the underwriters to us per share of common stock.
The underwriting fee is $0.309 per share.
The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters assuming
both no exercise and full exercise of the underwriters’ option to purchase additional shares.
|
|
|
|
Without
exercise
of option to
purchase
additional shares
|
|
|
|
With
full
exercise of
option to
purchase
additional shares
|
|
Per Share
|
|
$
|
0.309
|
|
|
$
|
0.309
|
|
Total
|
|
$
|
4,800,000
|
|
|
$
|
5,520,000
|
We estimate that the total expenses of this offering, including registration, filing
and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and commissions, will
be approximately $800,000. We have agreed to reimburse the underwriters for all expenses related to qualification of our common
stock under states securities laws and the clearing of this offering with the Financial Industry Regulatory Authority. In addition,
we have granted to J.P. Morgan Securities LLC the right to participate in any public offering of our common stock until March 21,
2018, subject to certain limitations.
A prospectus supplement in electronic
format may be made available on the web sites maintained by one or more underwriters, or selling group members, if any, participating
in the offering. The underwriters may agree to allocate a number of shares to underwriters and selling group members for sale
to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and
selling group members that may make Internet distributions on the same basis as other allocations.
We have agreed, subject to limited exceptions, that we will not (i) offer, pledge, announce
the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell,
grant any option, right or warrant to purchase or otherwise dispose of, directly or indirectly, or file with the SEC a registration
statement under the Securities Act relating to, any shares of our common stock or securities convertible into or exchangeable or
exercisable for any shares of our common stock, or publicly disclose the intention to make any offer, sale, pledge, disposition
or filing, or (ii) enter into any swap or other arrangement that transfers all or a portion of the economic consequences associated
with the ownership of any shares of common stock or any such other securities (regardless of whether any of these transactions
are to be settled by the delivery of shares of common stock or such other securities, in cash or otherwise), in each case without
the prior written consent of J.P. Morgan Securities LLC and Barclays Capital Inc. for a period of 60 days after the date of this
prospectus supplement (the “Restricted Period”), other than (A) the shares of our common stock to be sold hereunder
(B) shares and options to purchase shares of common stock issued pursuant to our existing equity compensation plans and (C) any
shares of our common stock issued upon the exercise of options or the vesting of restricted stock units granted under our existing
management incentive plans.
Our directors and executive officers have entered into lock-up agreements with the underwriters
prior to the commencement of this offering pursuant to which each of these persons, with limited exceptions, for a period of 60
days after the date of this prospectus supplement, may not, without the prior written consent of J.P. Morgan Securities LLC and
Barclays Capital Inc., (1) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract
to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or
dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable
for our common stock (including, without limitation, common stock or such other securities which may be deemed to be beneficially
owned by such directors and executive officers in accordance with the rules and regulations of the SEC and securities which may
be issued upon exercise of a stock option or warrant) or (2) enter into any swap or other agreement that transfers, in whole
or in part, any of the economic consequences of ownership of the common stock or such other securities, whether any such transaction
described in clause (1) or (2) above is to be settled by delivery of common stock or such other securities, in cash or otherwise,
or (3) make any demand for or exercise any right with respect to the registration of any shares of our common stock or any security
convertible into or exercisable or exchangeable for our common stock. Each of the lock-up agreements contain certain exceptions,
including the establishment of a contract or plan meeting the requirements of Rule 10b5-1 under the Exchange Act for the transfer
or sale of shares of common stock after the expiration of a period of 60 days after the date of this prospectus supplement, provided
that such plan does not provide for the sale of common stock during the Restricted Period and, provided further, that no filing
by any party under the Exchange Act, or other public announcement regarding the establishment of such plan, shall be required or
shall be voluntarily made during the Restricted Period; the transfer or sale of shares of common stock pursuant to a 10b5-1 plan
that has been entered into by certain of our executive officers prior to the date hereof and provided any filing required or voluntarily
made under the Exchange Act shall note that such transaction was conducted pursuant to a pre-established 10b5-1 plan; the disposition
of shares of common stock to the Company for the purpose of covering tax liabilities and/or the exercise price in connection with
the exercise of options to purchase shares of common stock or the vesting of restricted stock units or shares of restricted stock,
in each case awarded pursuant to our existing equity compensation plans, provided that any required filing under the Exchange Act
shall clearly indicate the circumstances of such disposition; or with respect or with respect to the exercise and sale of up to
54,250 shares of our common stock subject to options held by one individual which would otherwise expire during the Restricted
Period.
We have agreed to indemnify
the underwriters against certain liabilities, including liabilities under the Securities Act.
Our common stock is listed on
the NASDAQ Global Select Market under the symbol “BCRX.”
In connection with this offering,
the underwriters may engage in stabilizing transactions, which involves making bids for, purchasing and selling shares of common
stock in the open market for the purpose of preventing or retarding a decline in the market price of the common stock while this
offering is in progress. These stabilizing transactions may include making short sales of the common stock, which involves the
sale by the underwriters of a greater number of shares of common stock than they are required to purchase in this offering, and
purchasing shares of common stock on the open market to cover positions created by short sales. Short sales may be “covered”
shorts, which are short positions in an amount not greater than the underwriters’ option to purchase additional shares referred
to above, or may be “naked” shorts, which are short positions in excess of that amount. The underwriters may close
out any covered short position either by exercising their option to purchase additional shares, in whole or in part, or by purchasing
shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares
available for purchase in the open market compared to the price at which the underwriters may purchase shares through the option
to purchase additional shares. A naked short position is more likely to be created if the underwriters are concerned that there
may be downward pressure on the price of the common stock in the open market that could adversely affect investors who purchase
in this offering. To the extent that the underwriters create a naked short position, they will purchase shares in the open market
to cover the position.
The underwriters have advised
us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain
or otherwise affect the price of the common stock, including the imposition of penalty bids. This means that if the representatives
of the underwriters purchases common stock in the open market in stabilizing transactions or to cover short sales, the representatives
can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
These activities may have the
effect of raising or maintaining the market price of the common stock or preventing or retarding a decline in the market price
of the common stock, and, as a result, the price of the common stock may be higher than the price that otherwise might exist in
the open market. If the underwriters commence these activities, they may discontinue them at any time. The underwriters may carry
out these transactions on the NASDAQ Global Select Market, in the over-the-counter market or otherwise.
In addition, in connection with
this offering certain of the underwriters (and selling group members) may engage in passive market making transactions in our
common stock on the NASDAQ Stock Market prior to the pricing and completion of this offering. Passive market making consists of
displaying bids on the NASDAQ Stock Market no higher than the bid prices of independent market makers and making purchases at
prices no higher than these independent bids and effected in response to order flow. Net purchases by a passive market maker on
each day are generally limited to a specified percentage of the passive market maker’s average daily trading volume in the
common stock during a specified period and must be discontinued when such limit is reached. Passive market making may cause the
price of our common stock to be higher than the price that otherwise would exist in the open market in the absence of these transactions.
If passive market making is commenced, it may be discontinued at any time.
Other than in the United States,
no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus
supplement in any jurisdiction where action for that purpose is required. The securities offered by this prospectus supplement
may not be offered or sold, directly or indirectly, nor may this prospectus supplement or any other offering material or advertisements
in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances
that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this
prospectus supplement comes are advised to inform themselves about and to observe any restrictions relating to the offering and
the distribution of this prospectus supplement. This prospectus supplement does not constitute an offer to sell or a solicitation
of an offer to buy any securities offered by this prospectus supplement in any jurisdiction in which such an offer or a solicitation
is unlawful.
Certain of the underwriters and their affiliates have provided in the past to us and
our affiliates and may provide from time to time in the future certain commercial banking, financial advisory, investment banking
and other services for us and such affiliates in the ordinary course of their business, for which they have received and may continue
to receive customary fees and commissions. In addition, from time to time, certain of the underwriters and their affiliates may
effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long
or short positions in our debt or equity securities or loans, and may do so in the future. The underwriters and certain of their
affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express
independent research views in
respect of such securities or instruments and may at any time hold, or recommend to clients that
they acquire, long and/or short positions in such securities and instruments.
Notice to
Prospective Investors in the European Economic Area
In relation to each Member State
of the European Economic Area (each, a “Relevant Member State”), no offer of shares may be made to the public in that
Relevant Member State other than:
|
A.
|
to any legal entity which
is a qualified investor as defined in the Prospectus Directive;
|
|
|
|
|
B.
|
to fewer than 100 or, if the Relevant Member
State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than
qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining
the prior consent of the representatives; or
|
|
|
|
|
C.
|
in any other circumstances falling within Article
3(2) of the Prospectus Directive, provided that no such offer of shares shall require the Company or the representatives to
publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of
the Prospectus Directive.
|
Each person in a Relevant Member
State who initially acquires any shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed
that it is a “qualified investor” within the meaning of the law in that Relevant Member State implementing Article
2(1)(e) of the Prospectus Directive. In the case of any shares being offered to a financial intermediary as that term is
used in Article 3(2) of the Prospectus Directive, each such financial intermediary will be deemed to have represented, acknowledged
and agreed that the shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have
they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any shares
to the public other than their offer or resale in a Relevant Member State to qualified investors as so defined or in circumstances
in which the prior consent of the representatives has been obtained to each such proposed offer or resale.
The Company, the representatives
and their affiliates will rely upon the truth and accuracy of the foregoing representations, acknowledgements and agreements.
This prospectus supplement has
been prepared on the basis that any offer of shares in any Relevant Member State will be made pursuant to an exemption under the
Prospectus Directive from the requirement to publish a prospectus for offers of shares. Accordingly, any person making or intending
to make an offer in that Relevant Member State of shares which are the subject of the offering contemplated in this prospectus
supplement may only do so in circumstances in which no obligation arises for the Company or any of the underwriters to publish
a prospectus pursuant to Article 3 of the Prospectus Directive in relation to such offer. Neither the Company nor the underwriters
have authorized, nor do they authorize, the making of any offer of shares in circumstances in which an obligation arises for the
Company or the underwriters to publish a prospectus for such offer.
For the purpose of the above
provisions, the expression “an offer to the public” in relation to any shares in any Relevant Member State means the
communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so
as to enable an investor to decide to purchase or subscribe the shares, as the same may be varied in the Relevant Member State
by any measure implementing the Prospectus Directive in the Relevant Member State and the expression “Prospectus Directive”
means Directive 2003/71/EC (including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member States)
and includes any relevant implementing measure in the Relevant Member State and the expression “2010 PD Amending Directive”
means Directive 2010/73/EU.
Notice to
Prospective Investors in the United Kingdom
In addition, in the United Kingdom,
this prospectus supplement is being distributed only to, and is directed only at, and any offer subsequently made may only be
directed at persons who are “qualified investors” (as defined in the Prospectus Directive) (i) who have professional
experience in matters relating to investments falling within Article 19 (5) of the Financial Services and Markets Act 2000 (Financial
Promotion) Order 2005, as amended (the “Order”) and/or (ii) who are high net worth companies (or persons to whom it
may otherwise be lawfully
communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred
to as “relevant persons”).
Any person in the United Kingdom
that is not a relevant person should not act or rely on the information included in this prospectus supplement or use it as basis
for taking any action. In the United Kingdom, any investment or investment activity that this prospectus supplement relates to
may be made or taken exclusively by relevant persons. Any person in the United Kingdom that is not a relevant person should not
act or rely on this prospectus supplement or any of its contents.
Notice to
Prospective Investors in Canada
The shares of common stock may
be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National
Instrument 45-106
Prospectus Exemptions
or subsection 73.3(1) of the
Securities Act
(Ontario), and are permitted
clients, as defined in National Instrument 31-103
Registration Requirements, Exemptions and Ongoing Registrant Obligations
.
Any resale of the shares of common stock must be made in accordance with an exemption from, or in a transaction not subject to,
the prospectus requirements of applicable securities laws.
Securities legislation in certain
provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus supplement
(including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised
by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory.
The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory
for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of
National Instrument 33-105
Underwriting Conflicts
(
NI 33-105
), the underwriters are not required to comply with
the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Legal
matters
The validity of the shares of
common stock offered by this prospectus supplement will be passed upon for us by Gibson, Dunn & Crutcher LLP. Certain legal
matters in connection with this offering will be passed upon for the underwriters by Latham & Watkins LLP, San Diego, California.
Experts
Ernst & Young LLP, independent
registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K
for the year ended December 31, 2016, and the effectiveness of our internal control over financial reporting as of December 31,
2016, as set forth in their reports, which are incorporated by reference in this prospectus and elsewhere in the registration
statement. Our financial statements and our management’s assessment of the effectiveness of internal control over financial
reporting as of December 31, 2016 are incorporated by reference in reliance on Ernst & Young LLP’s reports, given on
their authority as experts in accounting and auditing.
Where
you can find more information
We file electronically with
the SEC our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statements and other
information. We make available on or through our website, http://www.biocryst.com, free of charge, copies of these filings as
soon as reasonably practicable after we electronically file them with or furnish them to the SEC. The information on our website
is not incorporated by reference into this prospectus supplement. You can also request copies of such documents by contacting
our Investor Relations Department at 4505 Emperor Blvd., Suite 200, Durham, North Carolina 27703 or sending an email to investorrelations@biocryst.com.
You may read and copy any document we file at the SEC’s Public Reference Room at 100 F Street NE, Washington, D.C. 20549.
You can also obtain copies of this information by mail from the Public Reference Room of the SEC at prescribed rates. You may
obtain information on the operation of the Public Reference Room by calling the SEC at (800) SEC-0330.
The SEC also maintains a website
that contains reports, proxy statements and other information about issuers, like BioCryst, that file electronically with the
SEC. The address of that site is http://www.sec.gov. Unless specifically listed below under “Incorporation of Certain Documents
by Reference” the information contained on the SEC website is not incorporated by reference into this prospectus supplement.
We have filed with the SEC a
registration statement on Form S-3 that registers the securities we are offering. The registration statement, including the attached
exhibits and schedules, contains additional relevant information about us and our securities. The rules and regulations of the
SEC allow us to omit certain information included in the registration statement from this prospectus supplement.
Incorporation
of certain documents by reference
The SEC allows us to “incorporate
by reference” information into this prospectus supplement. This means that we can disclose important information to you
by referring you to another document filed separately with the SEC. The information incorporated by reference is considered to
be part of this prospectus supplement, except for any information that is superseded by information that is included directly
in this document.
This prospectus supplement includes
by reference the documents listed below that we have previously filed with the SEC and that are not included in or delivered with
this document. They contain important information about us and our financial condition.
|
•
|
Our Annual Report on Form
10-K for the year ended December 31, 2016, filed with the SEC on February 27, 2017;
|
|
|
|
|
•
|
The information in our Definitive Proxy Statement,
filed with the SEC on April 12, 2017, set forth under the captions “Items to be Voted on — 1. Election of Directors,”
“Executive Officers,” “Section 16(a) Beneficial Ownership Reporting Compliance,” “Corporate
Governance,” “Compensation Discussion and Analysis,” “Summary Compensation Table,” “Grants
of Plan-Based Awards in 2016,” “Outstanding Equity Awards at December 31, 2016,” “2016 Option
Exercises and Stock Vested,” “Potential Payments Upon Termination or Change in Control,” “2016 Director
Compensation,” “Compensation Committee Interlocks and Insider Participation,” “Compensation Committee
Report,” “Equity Compensation Plan Information,” “Security Ownership of Certain Beneficial Owners
and Management,” “Certain Relationships and Related Transactions,” and “Items to be Voted on —
2. Ratification of Appointment of Independent Registered Public Accountants”;
|
|
|
|
|
•
|
Our Quarterly Reports on form 10-Q for the quarters
ended March 31, 2017 and June 30, 2017, filed with the SEC on May 8, 2017 and August 8, 2017, respectively;
|
|
|
|
|
•
|
Our Current Reports on Form 8-K filed with the
SEC on February 27, 2017, March 15, 2017, March 17, 2017, May 30, 2017 and August 2, 2017; and
|
|
|
|
|
•
|
The description of our common stock contained
in our Registration Statement on Form 8-A (File No. 000-23186) filed with the SEC on January 8, 1994, including any amendment
or reports filed for the purpose of updating such description.
|
All documents filed by us pursuant
to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus supplement and prior to the termination
of this offering shall be deemed to be incorporated by reference herein and to be a part of this prospectus supplement from the
date of filing of such documents. We are not, however, incorporating by reference any documents or portions thereof, whether specifically
listed above or filed in the future, that are not deemed “filed” with the SEC, including any information furnished
under Item 2.02 or Item 7.01 of any Current Report on Form 8-K and exhibits filed on such form that are related to such items.
Any statement contained in a document incorporated by reference herein shall be deemed to be modified or superseded for purposes
of this prospectus supplement to the extent that a statement contained herein or in any other subsequently filed document which
also is or is deemed to be incorporated by reference herein modifies or supersedes such statement. Any statement so modified or
superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus supplement.
You can obtain any of the documents
incorporated by reference in this prospectus supplement from us without charge, excluding any exhibits to those documents unless
the exhibit is specifically incorporated by reference as an exhibit to this prospectus supplement by requesting them in writing
or by telephone from us at the following address and telephone number:
Investor
Relations
BioCryst
Pharmaceuticals, Inc.
4505
Emperor Blvd., Suite 200
Durham,
North Carolina 27703
(919)
859-7910
Neither we nor the underwriters
have authorized anyone to provide you with information different from that contained or incorporated by reference in this prospectus
supplement, the accompanying prospectus or any free writing prospectus prepared by us or on our behalf. We and the underwriters
take no responsibility for, or can provide no assurances as to the reliability of, any information other than the information
contained or incorporated by reference in this prospectus supplement, the accompanying prospectus or any free writing prospectus
prepared by us or on our behalf. If you are in a jurisdiction where offers to sell, or solicitations of offers to purchase, the
securities offered by this document are unlawful, or if you are a person to whom it is unlawful to direct these types of activities,
then the offer presented in this document does not extend to you.
PROSPECTUS

$150,000,000
Common
Stock
Preferred Stock
Depositary Shares
Stock Purchase Contracts
Warrants
Units
By this prospectus, we may from
time to time offer securities to the public. We will provide specific terms of these securities in supplements to this prospectus.
You should read this prospectus, the applicable prospectus supplement, and the information incorporated by reference in this prospectus
and the applicable prospectus supplement carefully before you invest.
Our common stock, par value
$0.01 per share, trades on the NASDAQ Global Select Market under the symbol “BCRX.”
We have not authorized anyone
else to make additional representations or to provide you with information other than information provided or incorporated by
reference in this prospectus or any prospectus supplement. We take no responsibility for, and can provide no assurances as to
the reliability of, any other information that others may give you or representations that others may make. We are not making
or soliciting an offer of any securities other than the securities described in this prospectus and any prospectus supplement.
We are not making or soliciting an offer of these securities in any state or jurisdiction where the offer is not permitted or
in any circumstances in which such offer or solicitation is unlawful. You should not assume that the information contained or
incorporated by reference in this prospectus or any prospectus supplement is accurate as of any date other than the date on the
front of those documents.
______________
Investing in these securities
involves a high degree of risk. See “Risk Factors” on page 2 of this prospectus, in the applicable prospectus supplement
we will deliver with this prospectus and in the documents incorporated herein and therein by reference.
______________
The securities may be sold by
us to or through underwriters or dealers, directly to purchasers or through agents designated from time to time, or through a
combination of these methods. For additional information on the methods of sale, you should refer to the section entitled “Plan
of Distribution” in this prospectus. If any underwriters are involved in the sale of any securities with respect to which
this prospectus is being delivered, the names of such underwriters and any applicable discounts or commissions and over-allotment
options will be set forth in a prospectus supplement. The price to the public of such securities and the net proceeds we expect
to receive from such sale will also be set forth in a prospectus supplement. This prospectus may not be used to sell any securities
unless accompanied by a prospectus supplement.
Neither the Securities and
Exchange Commission nor any state securities commission has approved or disapproved of these securities, or passed upon the adequacy
or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date
of this prospectus is April 18, 2016.
Table of
contents
Page
|
ABOUT THIS PROSPECTUS
|
i
i
|
|
PROSPECTUS SUMMARY
|
1
|
|
RISK FACTORS
|
2
|
|
INFORMATION REGARDING FORWARD-LOOKING STATEMENTS
|
3
|
|
USE OF PROCEEDS
|
5
|
|
DESCRIPTION OF COMMON STOCK, PREFERRED STOCK
AND DEPOSITARY SHARES
|
6
|
|
DESCRIPTION OF STOCK PURCHASE CONTRACTS
|
9
|
|
DESCRIPTION OF WARRANTS
|
10
|
|
DESCRIPTION OF UNITS
|
12
|
|
PLAN OF DISTRIBUTION
|
13
|
|
LEGAL MATTERS
|
16
|
|
EXPERTS
|
16
|
|
WHERE YOU CAN FIND MORE INFORMATION
|
17
|
|
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
|
17
|
About this
prospectus
This prospectus is part of a
registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or the SEC, using a “shelf”
registration or continuous offering process. Under this registration statement, we may sell any combination of the securities
described in this prospectus from time to time, either separately or in units, in one or more offerings. Together, these offerings
may total up to $150.0 million.
This prospectus provides you
with a general description of the securities we may offer. Each time we sell securities, we will provide a prospectus supplement
containing specific information about the terms of that offering. That prospectus supplement may add, update or change information
contained in this prospectus and will also include the following information:
|
•
|
the type and amount of securities
that we propose to sell;
|
|
•
|
the public offering price
of the securities;
|
|
|
|
|
•
|
the names of any underwriters, agents or dealers
through or to which the securities will be sold;
|
|
|
|
|
•
|
any compensation of those underwriters, agents
or dealers;
|
|
|
|
|
•
|
information about any securities exchanges or
automated quotation systems on which the securities will be listed or traded;
|
|
|
|
|
•
|
any risk factors applicable to the securities
that we propose to sell; and
|
|
|
|
|
•
|
any other material information about the offering
and sale of the securities.
|
If there is any inconsistency
between the information in this prospectus and any prospectus supplement, you should rely on the information in the prospectus
supplement. You should read both this prospectus and any prospectus supplement together with the additional information described
under the heading “Where You Can Find More Information.” The registration statement containing this prospectus, including
the exhibits to the registration statement, provides additional information about us and the securities offered under this prospectus.
The registration statement, including the exhibits, can be read at the SEC’s website or at the SEC’s offices referenced
under the heading “Where You Can Find More Information.”
All references to “Company,”
“we,” “our,” or “us” refer solely to BioCryst Pharmaceuticals, Inc. and not to the persons
who manage us or sit on our Board of Directors. All trade names used in this prospectus are either our registered trademarks or
trademarks of their respective holders.
Prospectus
summary
This summary highlights information
contained elsewhere or incorporated by reference into this prospectus. Because it is a summary, it does not contain all of the
information that you should consider before investing in our securities. You should read this entire prospectus carefully, including
the section entitled “Risk Factors” and the documents that we incorporate by reference into this prospectus, before
making an investment decision.
Business
of BioCryst Pharmaceuticals, Inc.
We are a biotechnology company
that designs, optimizes and develops novel small molecule drugs that block key enzymes involved in
the
pathogenesis of diseases.
We focus on the treatment of rare diseases in which significant unmet medical needs exist and
that align with our capabilities and expertise. We integrate the disciplines of biology, crystallography, medicinal chemistry
and computer modeling to discover and develop small molecule pharmaceuticals through the process known as structure-guided drug
design. Structure-guided drug design is a drug discovery approach by which we design synthetic compounds from detailed structural
knowledge of the active sites of enzyme targets associated with particular diseases. We use X-ray crystallography, computer modeling
of molecular structures and advanced chemistry techniques to focus on the three-dimensional molecular structure and active site
characteristics of the enzymes that control cellular biology. Enzymes are proteins that act as catalysts for many vital biological
reactions. Our goal generally is to design a compound that will fit in the active site of an enzyme and thereby prevent its catalytic
activity.
We are a Delaware corporation
originally founded in 1986. Our principal executive offices are located at 4505 Emperor Blvd. Suite 200, Durham, North Carolina
27703, and our telephone number is (919) 859-1302. For more information about us, please visit our website at http://www.biocryst.com.
The information on our web site is not incorporated by reference into this prospectus.
Risk
factors
Investing in our securities
involves risks. Our business is influenced by many factors that are difficult to predict and beyond our control and that involve
uncertainties that may materially affect our business, results of operations, financial condition or cash flows, or the value
of these securities. These risks and uncertainties are described in the risk factors section of the documents that are incorporated
by reference in this prospectus. Any subsequent prospectus supplement may contain a discussion of additional risks applicable
to an investment in us and the particular type of securities we are offering under such prospectus supplement. You should carefully
consider all of the information contained in or incorporated by reference in this prospectus and in the applicable prospectus
supplement before you invest in our securities.
Information
regarding forward-looking statements
This prospectus and any subsequent
prospectus supplement, including the information we incorporate by reference, contain forward-looking statements within the meaning
of Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which are subject to the “safe
harbor” created in Section 21E. All statements other than statements of historical facts contained in this prospectus, any
subsequent prospectus supplement and the information we incorporate by reference are forward-looking statements. These forward-looking
statements can generally be identified by the use of words such as “may,” “will,” “intends,”
“plans,” “believes,” “anticipates,” “expects,” “estimates,” “predicts,”
“potential,” the negative of these words or similar expressions. Statements that describe our future plans, strategies,
intentions, expectations, objectives, goals or prospects are also forward-looking statements. These forward-looking statements
include, but are not limited to, statements about:
|
•
|
the initiation, timing, progress
and results of our preclinical testing, clinical trials, and to the extent applicable, post-marketing commitments, for our
HAE product candidates, RAPIVAB, BCX4430, and our other research and development efforts;
|
|
|
|
|
•
|
the further preclinical development, clinical
development, commercialization, or post-marketing studies by either us or partners of our product candidates and products,
including our HAE program, RAPIVAB, BCX4430, and early stage discovery programs;
|
|
|
|
|
•
|
the potential funding from our contracts
with
the Biomedical Advanced Research and Development Authority within the United States Department of Health and Human Services
(“BARDA/HHS”) and the National Institute of Allergy and Infectious Diseases within the United States Department
of Health and Human Services (“NIAID/HHS”) for the development of BCX4430
;
|
|
|
|
|
•
|
the potential for government stockpiling orders
of RAPIVAB, additional regulatory approvals of RAPIVAB or milestones royalties or profit from commercial sales of RAPIVAB
by us or our partners;
|
|
|
|
|
•
|
the potential use of RAPIVAB as a treatment for
H1N1, H5N1, and H7N9 or other strains of influenza;
|
|
|
|
|
•
|
the implementation of our business model, strategic
plans for our business, products, product candidates and technology;
|
|
|
|
|
•
|
our ability to establish and maintain collaborations
or out-license rights to our drug candidates;
|
|
|
|
|
•
|
plans, programs, progress and potential success
of our collaborations, including Sequirus UK Limited (“SUL”) for RAPIVAB, Mundipharma International Holdings Limited
(“Mundipharma”) for forodesine and Shionogi & Co., Ltd. (“Shionogi”) and Green Cross Corporation
(“Green Cross”) for peramivir in their territories;
|
|
|
|
|
•
|
JPR Royalty Sub LLC’s (“Royalty Sub”)
ability to service its payment obligations in respect of the PhaRMA Notes, and our ability to benefit from our equity interest
in Royalty Sub;
|
|
|
|
|
•
|
the foreign currency hedge agreement entered
into by us in connection with the issuance by Royalty Sub of the PhaRMA Notes;
|
|
|
|
|
•
|
the scope of protection we are able to establish
and maintain for intellectual property rights covering our products, product candidates and technology;
|
|
|
|
|
•
|
our ability to operate our business without infringing
the intellectual property rights of others;
|
|
|
|
|
•
|
estimates of our expenses, revenues, capital
requirements and our needs for additional financing;
|
|
|
|
|
•
|
the timing or likelihood of regulatory filings
or regulatory agreements, deferrals and approvals;
|
|
|
|
|
•
|
our ability to raise additional capital to fund
our operations;
|
|
|
|
|
•
|
our financial performance; and
|
|
|
|
|
•
|
competitive companies, technologies and our industry.
|
These statements relate to future
events or to our future financial performance and involve known and unknown risks, uncertainties and other important factors that
may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements
expressed or implied by these forward-looking statements. Factors that may cause actual results to differ materially from current
expectations include, among other things, those listed under “Risk Factors” and elsewhere in this prospectus, any
subsequent prospectus supplement and the documents incorporated by reference. Any forward-looking statement reflects our current
views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our operations,
results of operations, industry and future growth. Except as required by law, we assume no obligation to update or revise these
forward-looking statements for any reason, even if new information becomes available in the future.
Discussions containing these
forward-looking statements are also contained in “Management’s Discussion and Analysis of Financial Condition and
Results of Operations” incorporated by reference from our most recent Annual Report on Form 10-K, our Quarterly Reports
on Form 10-Q for the quarters ended since our most recent Annual Report, our Current Reports on Form 8-K, as well as any future
amendments we make to those filings or future filings with the SEC.
Use
of proceeds
Except as otherwise described
in the applicable prospectus supplement, the net proceeds we expect to receive from the sale of any securities offered hereunder
will be added to our general funds and used for general corporate purposes, which may include, but are not limited to:
|
•
|
funding
development, manufacturing and regulatory activities for avoralstat;
|
|
|
|
|
•
|
funding development,
manufacturing and regulatory activities for BCX7353;
|
|
|
|
|
•
|
funding development,
manufacturing and regulatory activities for other second generation HAE compounds;
|
|
|
|
|
•
|
post-approval commitments
such as the pediatric study for RAPIVAB™;
|
|
|
|
|
•
|
pre-launch commercial
activities for the HAE market;
|
|
|
|
|
•
|
the advancement of
development activities on other rare disease targets;
|
|
|
|
|
•
|
funding our research
and development efforts;
|
|
|
|
|
•
|
capital expenditures
and enhancing our laboratory facilities; and
|
|
|
|
|
•
|
general working capital
needs.
|
We may also use a portion of
the net proceeds to acquire or invest in businesses, assets, products and technologies that are complementary to our own, although
we are not currently contemplating or negotiating any such acquisitions or investments.
The amount and timing of these
expenditures will depend on a number of factors, including the progress of our research and development efforts and amounts received
under our existing and any future government contracts and collaboration arrangements, as well as the amount of cash used in our
operations. Accordingly, our management will have broad discretion in the application of these proceeds, and investors will be
relying on the judgment of our management with regard to the use of these proceeds. Pending application of the net proceeds as
described above, we intend to invest the net proceeds in investment grade interest bearing instruments.
Description
of common stock, preferred stock and depositary shares
The following summary description
of our capital stock summarizes general terms and provisions that apply to the capital stock. Because this is only a summary,
it does not contain all of the information that may be important to you. This summary is subject to and qualified in its entirety
by reference to our restated certificate of incorporation, as amended, by-laws, as amended, and the rights agreement, as amended,
each of which are on file with the SEC. See “Where You Can Find More Information.”
Authorized and Outstanding
Capital Stock
Our authorized capital stock
consists of 200,000,000 shares of common stock, par value $0.01 per share, and 5,000,000 shares of preferred stock, par value
$0.01 per share, of which 200,000 shares are designated Series B Junior Participating Preferred Stock with a par value of $0.001
per share. On February 24, 2016, there were 73,629,816 shares of common stock outstanding and no shares of preferred stock outstanding.
Common Stock
Holders of our common stock
are entitled to one vote per share on all matters submitted to a vote of stockholders and may not cumulate votes for the election
of directors. Common stockholders have the right to receive dividends as and when declared by the Board of Directors from funds
legally available therefor, subject to any preferential dividend rights of any preferred stock then outstanding. Upon our dissolution
or liquidation, common stockholders are entitled to receive all assets legally available for distribution to stockholders, subject
to any preferential rights of any preferred stock then outstanding. Holders of common stock have no preemptive rights and have
no rights to convert their common stock into any other securities.
Preferred Stock
Preferred stock may be issued
from time to time in one or more series, each such series to have such terms as determined by our Board of Directors. Our Board
of Directors has the authority to determine and fix such voting powers, full or limited, or no voting powers, and such designations,
preferences and relative participating, optional or other special rights, and qualifications, limitations or restrictions thereof,
including without limitation dividend rights, conversion rights, redemption privileges and liquidation preferences, without further
vote or action by our stockholders. We will distribute a prospectus supplement with regard to each particular series of preferred
stock that will describe the terms and provisions of that series of preferred stock. The rights of the holders of any preferred
stock that may be issued may adversely affect the rights of the holders of common stock. The issuance of preferred stock could
make it more difficult for third parties to acquire a majority of our outstanding voting stock.
Anti-Takeover Provisions
Our certificate of incorporation
provides for staggered terms for the members of the board of directors and supermajority approval of the removal of any member
of the board of directors and prevents our stockholders from acting by written consent. Our certificate also requires supermajority
approval of any amendment of these provisions. These provisions and other provisions of our by-laws and of Delaware law applicable
to us could delay or make more difficult a merger, tender offer or proxy contest involving us.
Depositary Shares
We may, at our option, elect
to offer fractional shares of preferred stock, rather than full shares of preferred stock. If we exercise this option, we will
issue to the public receipts for depositary shares, and each of these depositary shares will represent a fraction, to be set forth
in the applicable prospectus supplement, of a share of a particular series of preferred stock.
The shares of any series of
preferred stock underlying the depositary shares will be deposited under a deposit agreement between us and a bank or trust company
selected by us. The depositary will have its principal office in the United States and a combined capital and surplus of at least
$50,000,000. Subject to the terms of the deposit agreement, each owner of a depositary share will be entitled, in proportion to
the applicable fraction of a share of preferred stock underlying the depositary share, to all the rights and preferences of the
preferred stock underlying that depositary share. Those rights may include dividend, voting, redemption, conversion and liquidation
rights.
The depositary shares will be
evidenced by depositary receipts issued under a deposit agreement. Depositary receipts will be distributed to those persons purchasing
the fractional shares of preferred stock underlying the depositary shares, in accordance with the terms of the offering. The following
description of the material terms of the deposit agreement, the depositary shares and the depositary receipts is only a summary
and you should refer to the forms of the deposit agreement and depositary receipts that will be filed with the SEC in connection
with the offering of the specific depositary shares.
Pending the preparation of definitive
engraved depositary receipts, the depositary, upon our written order, may issue temporary depositary receipts substantially identical
to the definitive depositary receipts but not in definitive form. These temporary depositary receipts would entitle their holders
to all the rights of definitive depositary receipts. Temporary depositary receipts would be exchangeable for definitive depositary
receipts at our expense.
Dividends and Other Distributions
.
The depositary will distribute all cash dividends or other cash distributions received with respect to the underlying stock to
the record holders of depositary shares in proportion to the number of depositary shares owned by those holders.
If there were a distribution
other than in cash, the depositary would distribute property received by it to the record holders of depositary shares that are
entitled to receive the distribution, unless the depositary determines that it is not feasible to make the distribution. If this
occurs, the depositary, with our approval, would sell the property and distribute the net proceeds from the sale to the applicable
holders.
Withdrawal of Underlying
Preferred Stock
. Unless we provide otherwise in a prospectus supplement, holders may surrender depositary receipts at the
principal office of the depositary and, upon payment of any unpaid amount due to the depositary, would be entitled to receive
the number of whole shares of underlying preferred stock and all money and other property represented by the related depositary
shares. We will not issue any partial shares of preferred stock. If the holder delivers depositary receipts evidencing a number
of depositary shares that represent more than a whole number of shares of preferred stock, the depositary will issue a new depositary
receipt evidencing the excess number of depositary shares to that holder.
Redemption of Depositary
Shares
. If a series of preferred stock represented by depositary shares were subject to redemption, the depositary shares
would be redeemed from the proceeds received by the depositary resulting from the redemption, in whole or in part, of that series
of underlying stock held by the depositary. The redemption price per depositary share would be equal to the applicable fraction
of the redemption price per share payable with respect to that series of underlying stock. Whenever we redeem shares of underlying
stock that are held by the depositary, the depositary will redeem, as of the same redemption date, the number of depositary shares
representing the shares of underlying stock so redeemed. If fewer than all the depositary shares are to be redeemed, the depositary
shares to be redeemed will be selected by lot or proportionately, as may be determined by the depositary.
Voting
. Upon receipt
of notice of any meeting at which the holders of the underlying stock are entitled to vote, the depositary will mail the information
contained in the notice to the record holders of the depositary shares underlying the preferred stock. Each record holder of the
depositary shares on the record date, which will be the same date as the record date for the underlying stock, will be entitled
to instruct the depositary as to the exercise of the voting rights pertaining to the amount of the underlying stock represented
by that holder's depositary shares. The depositary will then try, as far as practicable, to vote the number of shares of preferred
stock underlying those depositary shares in accordance with those instructions, and we will agree to take all actions which may
be deemed necessary by the depositary to enable the depositary to do so. The depositary will not vote the underlying shares to
the extent it does not receive specific instructions from the holders of depositary shares underlying the preferred stock.
Conversion of Preferred Stock
.
If the prospectus supplement relating to the depositary shares provides that the deposited preferred stock is convertible into
or exchangeable for common stock or preferred stock of another series of BioCryst or securities of any third party, the following
will apply. The depositary shares, as such, will not be convertible into or exchangeable for any securities of BioCryst or any
third party. Rather, any holder of the depositary shares may surrender the related depositary receipts to the depositary with
written instructions to instruct us to cause conversion or exchange of the preferred stock represented by the depositary shares
into or for whole shares of common stock or shares of another series of preferred stock of BioCryst or securities of the relevant
third party, as applicable. Upon receipt of those instructions and any amounts payable by the holder in connection with the conversion
or exchange, we will cause the conversion or exchange using the same procedures as those provided for conversion or exchange of
the deposited preferred stock. If only some of the depositary shares are to be converted or exchanged, a new depositary receipt
or receipts will be issued for any depositary shares not to be converted or exchanged.
Amendment and Termination
of the Depositary Agreement
. The form of depositary receipt evidencing the depositary shares and any provision of the deposit
agreement may be amended at any time by agreement between us and the depositary. However, any amendment which materially and adversely
alters the rights of the holders of depositary shares will not be effective unless the amendment has been approved by the holders
of at least a majority of the depositary shares then outstanding. The deposit agreement may be terminated by us or by the depositary
only if (a) all outstanding depositary shares have been redeemed or converted or exchanged for any other securities into which
the underlying preferred stock is convertible or exchangeable or (b) there has been a final distribution of the underlying stock
in connection with our liquidation, dissolution or winding up and the underlying stock has been distributed to the holders of
depositary receipts.
Charges of Depositary
.
We will pay all transfer and other taxes and governmental charges arising solely from the existence of the depositary arrangements.
We will also pay charges of the depositary in connection with the initial deposit of the underlying stock and any redemption of
the underlying stock. Holders of depositary receipts will pay other transfer and other taxes and governmental charges and those
other charges, including a fee for any permitted withdrawal of shares of underlying stock upon surrender of depositary receipts,
as are expressly provided in the deposit agreement to be for their accounts.
Reports
. The depositary
will forward to holders of depositary receipts all reports and communications from us that we deliver to the depositary and that
we are required to furnish to the holders of the underlying stock.
Limitation on Liability
.
Neither we nor the depositary will be liable if either of us is prevented or delayed by law or any circumstance beyond our control
in performing our respective obligations under the deposit agreement. Our obligations and those of the depositary will be limited
to performance in good faith of our respective duties under the deposit agreement. Neither we nor the depositary will be obligated
to prosecute or defend any legal proceeding in respect of any depositary shares or underlying stock unless satisfactory indemnity
is furnished. We and the depositary may rely upon written advice of counsel or accountants, or upon information provided by persons
presenting underlying stock for deposit, holders of depositary receipts or other persons believed to be competent and on documents
believed to be genuine.
Resignation and Removal of
Depositary
. The depositary may resign at any time by delivering notice to us of its election to resign. We may remove the
depositary at any time. Any resignation or removal will take effect upon the appointment of a successor depositary and its acceptance
of the appointment. The successor depositary must be appointed within 60 days after delivery of the notice of resignation or removal
and must be a bank or trust company having its principal office in the United States and having a combined capital and surplus
of at least $50,000,000.
Description
of stock purchase contracts
The following is a general description
of the terms of the stock purchase contracts we may issue from time to time. Particular terms of any stock purchase contracts
we offer will be described in the prospectus supplement relating to such stock purchase contracts. Material U.S. federal income
tax considerations applicable to the stock purchase contracts will also be discussed in the applicable prospectus supplement.
You should refer to the form of stock purchase contract and stock purchase certificate that we will file with the SEC in connection
with the offering of the specific stock purchase contracts for more complete information.
We may issue stock purchase
contracts, including contracts obligating holders to purchase from us, and obligating us to sell to holders, a specified number
of shares of common stock, preferred stock or depositary shares at a future date. The consideration per share of common stock,
preferred stock or depositary shares may be fixed at the time that the stock purchase contracts are issued or may be determined
by reference to a specific formula set forth in the stock purchase contracts. Any stock purchase contract may include anti-dilution
provisions to adjust the number of shares issuable pursuant to such stock purchase contract upon the occurrence of certain events.
The applicable prospectus supplement
will describe the terms of any stock purchase contracts in respect of which this prospectus is being delivered, including, to
the extent applicable, the following:
|
•
|
whether the stock purchase
contracts obligate the holder or us to purchase or sell, or both purchase and sell, the securities subject to purchase under
the stock purchase contract, and the nature and amount of each of those securities, or the method of determining those amounts;
|
|
|
|
|
•
|
whether the stock purchase contracts are to be
prepaid or not;
|
|
|
|
|
•
|
whether the stock purchase contracts will be
issued as part of a unit and, if so, the other securities comprising the unit;
|
|
|
|
|
•
|
whether the stock purchase contracts are to be
settled by delivery, or by reference or linkage to the value, performance, or level of the securities subject to purchase
under the stock purchase contract;
|
|
|
|
|
•
|
any acceleration, cancellation, termination,
or other provisions relating to the settlement of the stock purchase contracts; and
|
|
|
|
|
•
|
whether the stock purchase contracts will be
issued in full registered or global form.
|
Description
of warrants
We may issue warrants to purchase
our preferred stock, depositary shares or common stock or any combination thereof. Warrants may be issued independently or together
with any other securities in the form of units, and may be attached to, or separate from, such securities. The terms of any warrants
to be issued and a description of the material provisions of the applicable warrant agreement will be set forth in the applicable
prospectus supplement. Each series of warrants will be issued under a separate warrant agreement to be entered into between us
and a bank or trust company, as warrant agent. You should refer to the form of warrant agreement and warrant that we file with
the SEC in connection with the offering of the specific warrants for more complete information.
The prospectus supplement will
describe the terms of any warrants being offered, including:
|
•
|
the title and the aggregate
number of warrants;
|
|
|
|
|
•
|
the price or prices at which the warrants will
be issued;
|
|
|
|
|
•
|
the currency or currencies in which the price
of the warrants will be payable;
|
|
|
|
|
•
|
the securities or other rights, including rights
to receive payment in cash or securities based on the value, rate or price of one or more specified commodities, currencies,
securities or indices, or any combination of the foregoing, purchasable upon exercise of the warrants;
|
|
|
|
|
•
|
the price at which, and the currency or currencies
in which, the securities or other rights purchasable upon exercise of such warrants may be purchased;
|
|
|
|
|
•
|
the periods during which, and places at which,
the warrants are exercisable;
|
|
|
|
|
•
|
the date or dates on which the warrants shall
commence and the date or dates on which the warrants will expire;
|
|
|
|
|
•
|
the terms of any mandatory or optional call provisions;
|
|
|
|
|
•
|
the price or prices, if any, at which the warrants
may be redeemed at the option of the holder or will be redeemed upon expiration;
|
|
|
|
|
•
|
whether the warrants will be sold separately
or with other securities as part of a unit;
|
|
|
|
|
•
|
if applicable, the designation and terms of the
securities with which the warrants are issued and the number of warrants issued with each such security;
|
|
|
|
|
•
|
if applicable, the date on and after which the
warrants and the related securities will be separately transferable;
|
|
|
|
|
•
|
any provisions for the adjustment of the number
or amount of securities receivable upon exercise of warrants;
|
|
|
|
|
•
|
the identity of the warrant agent;
|
|
|
|
|
•
|
the exchanges, if any, on which the warrants
may be listed;
|
|
|
|
|
•
|
the maximum or minimum number of warrants which
may be exercised at any time;
|
|
|
|
|
•
|
if applicable, a discussion of any material United
States federal income tax considerations;
|
|
|
|
|
•
|
whether the warrants shall be issued in book-entry
form; and
|
|
•
|
any other terms of the warrants,
including terms, procedures and limitations relating to the exchange and exercise of the warrants.
|
Description
of units
We may issue units consisting
of one or more of the other securities described in this prospectus in any combination, as described in a prospectus supplement.
We may issue units in one or more series, which will be described in a prospectus supplement. We will issue the units or hybrid
securities under one or more unit agreements, each referred to as a unit agreement, to be entered into between us and a bank or
trust company, as unit agent. You should refer to the form of unit agreement and unit certificate that we file with the SEC in
connection with the offering of the specific units for more complete information.
The applicable
prospectus supplement will describe:
|
•
|
the designation and the terms
of the units and of the securities constituting the units, including whether and under what circumstances the securities comprising
the units may be traded separately;
|
|
|
|
|
•
|
any additional terms of the governing unit agreement;
|
|
|
|
|
•
|
any additional provisions for the issuance, payment,
settlement, transfer or exchange of the units or of the preferred stock, common stock, stock purchase contracts, depositary
shares or warrants constituting the units; and
|
|
|
|
|
•
|
any applicable United States federal income tax
consequences.
|
Plan
of distribution
We may sell the securities being
offered hereby at prices and under terms then prevailing, at prices related to the then current market price or in negotiated
transactions from time to time in one or more of the following ways:
|
•
|
directly
to one or more purchasers;
|
|
|
|
|
•
|
through one or more
underwriters on a firm commitment or best-efforts basis;
|
|
|
|
|
•
|
through broker-dealers, who may act as agents
or principals, including a block trade in which a broker or dealer so engaged will attempt to sell as agent but may position
and resell a portion of the block as principal to facilitate the transaction;
|
|
|
|
|
•
|
through agents;
|
|
|
|
|
•
|
through remarketing
firms;
|
|
|
|
|
•
|
in privately negotiated
transactions; or
|
|
|
|
|
•
|
in any combination
of these methods of sale.
|
We will
set forth in a prospectus supplement the terms of the offering of securities, including:
|
•
|
the name or names of any underwriters,
dealers or agents;
|
|
|
|
|
•
|
the number of securities and purchase price of
the securities being offered and the proceeds we will receive from the sale;
|
|
|
|
|
•
|
any underwriting discounts and commissions or
agency fees and other items constituting underwriters’ or agents’ compensation;
|
|
|
|
|
•
|
any over-allotment options under which underwriters
may purchase additional securities from us;
|
|
|
|
|
•
|
any delayed delivery arrangements;
|
|
|
|
|
•
|
any discounts or concessions allowed or re-allowed
or paid to dealers; and
|
|
|
|
|
•
|
any securities exchange
on which the securities may be listed.
|
The distribution of the securities
may be effected from time to time in one or more transactions at a fixed price or prices, which may be changed, at market prices
prevailing at the time of sale, at prices related to the prevailing market prices or at negotiated prices.
We may designate agents who
agree to use their reasonable efforts to solicit purchases for the period of their appointment or to sell securities on a continuing
basis. Agents may receive compensation in the form of commissions, discounts or concessions from us. Agents may also receive compensation
from the purchasers of the securities for whom they sell as principals. Each particular agent will receive compensation in amounts
negotiated in connection with the sale, which might be in excess of customary commissions. Agents and any other participating
broker-dealers may be deemed to be “underwriters” within the meaning of Section 2(11) of the Securities Act of 1933
(the “Securities Act”) in connection with sales of the securities. Accordingly, any commission, discount or concession
received by them and any profit on the resale of the securities purchased by them may be deemed to be underwriting discounts or
commissions under the Securities Act. We have not entered into any agreements, understandings or arrangements with any underwriters
or broker-dealers regarding the sale of their securities. As of the date of this prospectus, there are no special selling arrangements
between any broker-dealer or other person and us. No period of time has been fixed within which the securities will be offered
or sold.
If required under applicable
state securities laws, we will sell the securities only through registered or licensed brokers or dealers. In addition, in some
states, we may not sell securities unless they have been registered or qualified for sale in the applicable state or an exemption
from the registration or qualification requirement is available and complied with.
If we use underwriters for a
sale of securities, the underwriters will acquire the securities for their own account. The underwriters may resell the securities
in one or more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined
at the time of sale. The obligations of the underwriters to purchase the securities will be subject to the conditions set forth
in the applicable underwriting agreement. We may change from time to time any initial public offering price and any discounts
or concessions the underwriters allow or re-allow or pay to dealers. We may use underwriters with whom we have a material relationship.
We will describe in the prospectus supplement naming the underwriter the nature of any such relationship.
We may use a remarketing firm
to offer to sell the securities in connection with a remarketing arrangement upon their purchase. Remarketing firms will act as
principals for their own account or as agents for us. These remarketing firms will offer or sell the securities pursuant to the
terms of the securities. A prospectus supplement will identify any remarketing firm and the terms of its agreement, if any, with
us and will describe the remarketing firm’s compensation. Remarketing firms may be deemed to be underwriters in connection
with the securities they remarket.
If we offer and sell securities
through a dealer, we or an underwriter will sell the securities to the dealer, as principal. The dealer may then resell the securities
to the public at varying prices to be determined by the dealer at the time of resale. Any such dealer may be deemed to be an underwriter
of the securities so offered and sold. The name of the dealer and the terms of the transactions will be set forth in the applicable
prospectus supplement.
We may also sell securities
directly to one or more purchasers without using underwriters or agents. Underwriters, dealers and agents that participate in
the distribution of the securities may be underwriters as defined in the Securities Act and any discounts or commissions they
receive from us and any profit on their resale of the securities may be treated as underwriting discounts and commissions under
the Securities Act.
We will identify in the applicable
prospectus supplement any underwriters, dealers or agents and will describe their compensation. We may have agreements with the
underwriters, dealers and agents to indemnify them against specified civil liabilities, including liabilities under the Securities
Act. Underwriters, dealers and agents may engage in transactions with or perform services for us in the ordinary course of their
businesses.
We may authorize agents, dealers
or underwriters to solicit offers to purchase securities at the public offering price under delayed delivery contracts. The terms
of these delayed delivery contracts, including when payment for and delivery of the securities sold will be made under the contracts
and any conditions to each party’s performance set forth in the contracts, will be described in the applicable prospectus
supplement. The compensation received by underwriters, agents or dealers soliciting purchases of securities under delayed delivery
contracts will be described in the applicable prospectus supplement.
We may enter into derivative
or other hedging transactions with third parties, or sell securities not covered by this prospectus to third parties in privately
negotiated transactions. If the applicable prospectus supplement indicates, in connection with those derivatives, the third parties
may sell securities covered by this prospectus and the applicable prospectus supplement, including in short sale transactions.
If so, the third party may use securities pledged by us or borrowed from us or others to settle those sales or to close out any
related open borrowings of stock, and may use securities received from us in settlement of those derivatives to close out any
related open borrowings of stock. We may also loan or pledge securities covered by this prospectus and the applicable prospectus
supplement to third parties, who may sell the loaned securities or, in an event of default in the case of a pledge, sell the pledged
securities pursuant to this prospectus and the applicable prospectus supplement.
Unless otherwise specified in
the related prospectus supplement, all securities we offer, other than common stock, will be new issues of securities with no
established trading market. Any underwriters may make a market in these securities, but will not be obligated to do so and may
discontinue any market making at any time without notice. We may apply to list any series of securities on an exchange, but we
are not obligated to do so. Therefore, no assurance can be given as to the liquidity of, or the trading market for, any series
of securities.
Any underwriter may engage in
overallotment, stabilizing transactions, short covering transactions and penalty bids in accordance with Regulation M under the
Exchange Act. Overallotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions
permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. Short covering
transactions involve purchases of the securities in the open market after the distribution is completed to cover short positions.
Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the
dealer are purchased in a covering transaction to cover short positions. Those activities may cause the price of the securities
to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time. These
transactions may be effected on The NASDAQ Global Select Market or otherwise.
Any underwriters who are qualified
market makers on The NASDAQ Global Select Market may engage in passive market making transactions in the common stock on The NASDAQ
Global Select Market in accordance with Rule 103 of Regulation M, during the business day before the pricing of the offering,
before the commencement of offers or sales of the common stock. Passive market makers must comply with applicable volume and price
limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price
not in excess of the highest independent bid for such security; if all independent bids are lowered below the passive market maker’s
bid, however, the passive market maker’s bid must then be lowered when certain purchase limits are exceeded.
We will bear all costs, expenses
and fees in connection with the registration of the securities, as well as the expense of all commissions and discounts, if any,
attributable to sales of the securities by us.
Legal
matters
Gibson, Dunn & Crutcher
LLP has rendered an opinion with respect to the validity of the securities being offered by this prospectus. We have filed this
opinion as an exhibit to the registration statement of which this prospectus is a part. If counsel for any underwriters passes
on legal matters in connection with an offering made by this prospectus, we will name that counsel in the prospectus supplement
relating to that offering.
Experts
Ernst & Young LLP, independent
registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K
for the year ended December 31, 2015, and the effectiveness of our internal control over financial reporting as of December 31,
2015, as set forth in their reports, which are incorporated by reference in this prospectus and elsewhere in the registration
statement. Our financial statements are incorporated by reference in reliance on Ernst & Young LLP's reports, given on their
authority as experts in accounting and auditing.
Where
you can find more information
We file electronically with
the SEC our annual reports on Form 10-K, quarterly interim reports on Form 10-Q, current reports on Form 8-K, proxy statements
and other information. We make available on or through our website, http://www.biocryst.com, free of charge, copies of these filings
as soon as reasonably practicable after we electronically file them with or furnish them to the SEC. The information on our website
is not incorporated by reference into this prospectus. You can also request copies of such documents by contacting our Investor
Relations Department at 4505 Emperor Blvd., Suite 200, Durham, North Carolina 27703 or sending an email to investorrelations@biocryst.com.
You may read and copy any document we file at the SEC’s Public Reference Room at 100 F Street NE, Washington, D.C. 20549.
You can also obtain copies of this information by mail from the Public Reference Room of the SEC at prescribed rates. You may
obtain information on the operation of the Public Reference Room by calling the SEC at (800) SEC-0330.
The SEC also maintains an Internet
site that contains reports, proxy and information statements, and other information about issuers, like BioCryst, that file electronically
with the SEC. The address of that site is http://www.sec.gov. Unless specifically listed below under “Incorporation of Certain
Documents by Reference” the information contained on the SEC website is not incorporated by reference into this prospectus.
We have filed with the SEC a
registration statement on Form S-3 that registers the securities we are offering. The registration statement, including the attached
exhibits and schedules, contains additional relevant information about us and our securities. The rules and regulations of the
SEC allow us to omit certain information included in the registration statement from this prospectus.
Incorporation
of certain documents by reference
The SEC allows us to “incorporate
by reference” information into this prospectus. This means that we can disclose important information to you by referring
you to another document filed separately with the SEC. The information incorporated by reference is considered to be part of this
prospectus, except for any information that is superseded by information that is included directly in this document.
This prospectus includes by
reference the documents listed below that we have previously filed with the SEC and that are not included in or delivered with
this document. They contain important information about us and our financial condition.
|
•
|
Our Annual Report on Form
10-K for the year ended December 31, 2015, filed with the SEC on February 26, 2016;
|
|
|
|
|
•
|
Our Current Reports on Form 8-K filed with the
SEC on January 8, 2016 and February 8, 2016; and
|
|
|
|
|
•
|
The description of our common stock which is
contained in our Registration Statement on Form 8-A (File No. 000-23186) filed with the SEC on January 7, 1994, together with
the amendment thereto filed with the SEC on March 14, 1994, including any other amendment or reports filed for the purpose
of updating such description.
|
All documents filed by us pursuant
to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act on or after the date of this Amendment No. 1 and prior to its effectiveness
and on or after the date of this prospectus and prior to the termination of our offering of securities shall be deemed to be incorporated
by reference herein and to be a part of this prospectus from the date of filing of such documents, excluding any information furnished
under Item 2.02 or Item 7.01 of any Current Report on Form 8-K and exhibits filed on such form that are related to such items.
Any statement contained in a document incorporated by reference herein shall be deemed to be modified or superseded for purposes
of this prospectus to the extent that a statement contained herein or in any other subsequently filed document which also is or
is deemed to be incorporated by reference herein modifies or supersedes such statement. Any statement so modified or superseded
shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
You can obtain any of the documents
incorporated by reference in this prospectus from us without charge, excluding any exhibits to those documents unless the exhibit
is specifically incorporated by reference as an exhibit to this prospectus. You can obtain documents incorporated by reference
in this prospectus at no cost by requesting them in writing or by telephone from us at the following address:
Investor
Relations
BioCryst Pharmaceuticals, Inc.
4505 Emperor Blvd., Suite 200
Durham, North Carolina 27703
(919) 859-1302
We have not authorized anyone
else to make additional representations or to provide you with information other than information provided or incorporated by
reference in this prospectus or any prospectus supplement. We take no responsibility for, and can provide no assurances as to
the reliability of, any other information that others may give you or representations that others may make. If you are in a jurisdiction
where offers to sell, or solicitations of offers to purchase, the securities offered by this document are unlawful, or if you
are a person to whom it is unlawful to direct these types of activities, then the offer presented in this document does not extend
to you.
15,533,981 shares

Common
stock
Prospectus
supplement
J.P. Morgan
Barclays
H.C. Wainwright & Co.
September 12, 2017
BioCryst Pharmaceuticals (NASDAQ:BCRX)
Historical Stock Chart
From Mar 2024 to Apr 2024
BioCryst Pharmaceuticals (NASDAQ:BCRX)
Historical Stock Chart
From Apr 2023 to Apr 2024