FDA Seeks to Reduce Nicotine in Cigarettes to Nonaddictive Levels--2nd Update
July 28 2017 - 2:21PM
Dow Jones News
By Jennifer Maloney
U.S. health officials said Friday they are considering new
standards that would require tobacco companies to reduce nicotine
in cigarettes to nonaddictive levels, as part of a sweeping review
of industry regulations.
Shares of major tobacco companies tumbled on the move by the
Federal Drug Administration. Marlboro maker Altria Group Inc. fell
10%, while British American Tobacco PLC, maker of Camel cigarettes,
tumbled 8.5%.
The agency said it was adopting a harm-reduction strategy that
seeks to balance regulation of existing products and encourage
development of "new products that may be less dangerous than
cigarettes." Among the issues it will examine is the role of
menthol and other flavors in tobacco products.
The FDA will consider an exemption for premium cigars, and will
allow more time for makers of reduced-risk products to submit
product applications under the agency's recently implemented rules,
FDA Commissioner Scott Gottlieb said in a press conference.
Mr. Gottlieb said the agency would look at regulation to "render
cigarettes minimally addictive."
"Cigarettes will likely remain incredibly toxic," he said. "We
may be able to reach a day when the most harmful products will no
longer be capable of addicting our kids."
Altria, British American Tobacco and Philip Morris International
didn't immediately respond to requests to comment.
A spokesman for Imperial Brands said, "We note the FDA's
announcement relating to its strategic direction but until the
eventual development of specific proposals, it's too early to
understand the practical implications."
Mr. Gottlieb said the FDA's new approach would consider "a
continuum of risk for nicotine delivery," from combustible products
such as cigarettes to nicotine-replacement therapies such as
nicotine patches and gums. Cigarette smoke, he said, not nicotine,
is what causes cancer, heart disease and lung disease.
"The problem is not just nicotine, the problem is the delivery
mechanism, " Mr. Gottlieb said. Cigarettes kill 480,000 people in
the U.S. each year, according to the Centers for Disease Control
and Prevention.
British American Tobacco's U.S. subsidiary, Reynolds American
Inc., said it was encouraged by Mr. Gottlieb's comments on the
relative risks of different tobacco products.
"These principles have long been the core of our efforts in
transforming the tobacco industry, as evidenced by our existing
leadership in the snus [tobacco pouches] and vapor categories," a
Reynolds spokesman said. "We are well prepared and look forward to
participating in a thorough process to develop a comprehensive plan
for tobacco and nicotine regulation."
Some tobacco analysts were skeptical. Citigroup analyst Adam
Spielman said he didn't believe the FDA will be able to lower
nicotine to nonaddictive levels because defining that level will be
difficult, it would be hard to make cigarettes with a very low
level of nicotine and there would be huge political opposition.
"We don't believe the proposal to reduce nicotine in cigarettes
dramatically is practical," said Mr. Spielman, adding that Friday's
tobacco stock drops are unjustified.
E-cigarettes and vaping devices that were on the market in
August 2016 won't be subject to review until 2021 or 2022,
depending on the type of product. The agency also will consider the
potential impact of a black market for cigarettes.
Some antismoking advocates and public health officials been
urging the FDA and CDC to provide the public with information on
the relative risks of tobacco products. Until now, U.S. health
officials have stuck with an abstinence-only message to the
public.
The FDA's shift comes as the agency considers whether to approve
new marketing claims for new heat-not-burn devices, smokeless
tobacco and other products. In April, Reynolds American, which was
recently acquired by BAT, submitted applications to market six
styles of Camel Snus tobacco pouches as being less harmful than
cigarettes. Philip Morris also has an application pending for a new
device that heats tobacco instead of burning it.
Saabira Chaudhuri contributed to this article
Write to Jennifer Maloney at jennifer.maloney@wsj.com
(END) Dow Jones Newswires
July 28, 2017 14:06 ET (18:06 GMT)
Copyright (c) 2017 Dow Jones & Company, Inc.
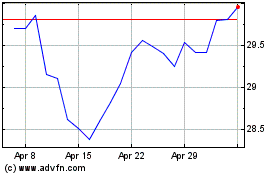
British American Tobacco (NYSE:BTI)
Historical Stock Chart
From Mar 2024 to Apr 2024
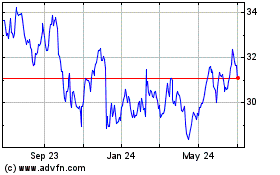
British American Tobacco (NYSE:BTI)
Historical Stock Chart
From Apr 2023 to Apr 2024