As filed with the Securities and Exchange Commission on July 12, 2017
Registration No. 333-213908
UNITED STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
POST-EFFECTIVE
AMENDMENT NO. 1 TO
FORM S-3 ON FORM S-1
REGISTRATION STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
Galena Biopharma, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
Delaware
|
|
2834
|
|
20-8099512
|
|
(State or other jurisdiction
of incorporation or organization)
|
|
(Primary Standard Industrial
Classification Code Number)
|
|
(I.R.S. Employer
Identification Number)
|
2000 Crow Canyon Place, Suite 380
San Ramon, CA 94583
(855) 855-4253
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Stephen F. Ghiglieri
Interim Chief Executive Officer & Chief Financial Officer
Galena Biopharma, Inc.
2000 Crow Canyon Place, Suite 380
San Ramon, CA 94583
(855) 855-4253
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
|
|
|
|
|
|
|
|
Thomas J. Knapp
Interim General Counsel & Corporate Secretary
Galena Biopharma, Inc.
2000
Crow Canyon Place, Suite 380
San Ramon, CA 94583
(855) 855-4253
|
|
Thomas Pollock, Esq.
Paul Hastings LLP
101
California Street
San Francisco, CA 94111
(415) 856-7047
|
As soon as practicable after this Registration Statement becomes effective.
(Approximate date of commencement of proposed sale to the public)
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act
of 1933, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to
Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act
registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective
amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a
non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company”
in Rule 12b-2 of the Exchange Act. (Check one):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Large accelerated filer
|
|
☐
|
|
|
|
|
|
Accelerated filer
|
|
☐
|
|
|
|
Non-accelerated filer
|
|
☐
|
|
|
|
(Do not check if a smaller reporting company)
|
|
Smaller reporting company
|
|
☒
|
|
|
|
Emerging growth company
|
|
☐
|
|
|
|
|
|
|
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period
for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The Registrant has previously
filed:
|
|
•
|
|
a registration statement on Form S-3 originally filed with the Securities and Exchange Commission (“
SEC
”) on September 30, 2016, SEC File No. 333-213908 (as amended,
“Registration
Statement 213908”
);
|
|
|
•
|
|
a registration statement on Form S-3 originally filed with the SEC on September 2, 2016, SEC File No. 333-213493 (as amended,
“Registration Statement 213493”
);
|
|
|
•
|
|
a registration statement on Form S-3 originally filed with the SEC on December 4, 2015, SEC File No. 333-208331 (as amended,
“Registration Statement 208331”
); and
|
|
|
•
|
|
a registration statement on Form S-3 originally filed with the SEC on December 4, 2015, SEC File No. 333-208330 (as amended,
“Registration Statement 208330”
and, together with Registration
Statement 213908, Registration Statement 213493 and Registration Statement 208331, the
“Registration Statements”
).
|
The Registrant is not currently eligible to use a Form S-3 registration statement due to its failure to timely file certain Current Reports on Form 8-K.
Pursuant to Rule 429 under the Securities Act, the prospectus included in this Post-Effective Amendment No. 1 to Form S-3 on Form S-1 is a combined prospectus, and this registration statement constitutes a post-effective amendment to
each of the Registration Statements and such post-effective amendments will become effective concurrently with the effectiveness of this post-effective amendment in accordance with Section 8(c) of the Securities Act. The Registration Statements
registered, in relevant part, and this Post-Effective Amendment is being filed to continue the registration of:
Registration Statement 213908
|
|
•
|
|
the offer and sale by certain selling stockholders of up to 700,000 shares of common stock issuable upon the exercise of outstanding warrants registered pursuant to the prospectus filed with the SEC under Rule 424(b)(3)
on October 20, 2016;
|
Registration Statement 213493
|
|
•
|
|
the offer and sale by certain selling stockholders of up to 100,000 shares of common stock issuable upon the exercise of outstanding warrants registered pursuant to the prospectus filed with the SEC under Rule 424(b)(3)
on October 12, 2016;
|
Registration Statement 208331
|
|
•
|
|
the offer and sale of up to 151,655 shares of common stock issuable upon the exercise of outstanding warrants originally registered pursuant to the prospectus filed with the SEC under Rule 424(b)(5) on December 18,
2012 and subsequently registered pursuant to a prospectus filed with the SEC under Rule 424(b)(3) on December 23, 2015;
|
|
|
•
|
|
the offer and sale of up to 700,320 shares of common stock issuable upon the exercise of outstanding warrants originally registered pursuant to the prospectus filed with the SEC under Rule 424(b)(5) on March 13,
2015 and subsequently registered pursuant to a prospectus filed with the SEC under Rule 424(b)(3) on December 23, 2015;
|
|
|
•
|
|
the offer and sale of up to 198,608 shares of common stock issuable upon the exercise of outstanding warrants originally registered pursuant to a prospectus filed with the SEC under Rule 424(b)(5) on September 13,
2013 and subsequently registered pursuant to a prospectus filed with the SEC under Rule 424(b)(3) on December 23, 2015;
|
Registration
Statement 208330
|
|
•
|
|
the offer and sale of 682,159 shares of common stock issuable upon the exercise of outstanding warrants registered pursuant to a prospectus filed with the SEC under Rule 424(b)(5) on January 7, 2016; and
|
|
|
•
|
|
the offer and sale of 17,000,000 shares of common stock issuable upon the exercise of outstanding warrants registered pursuant to a prospectus filed with the SEC under Rule 424(b)(5) on February 9, 2017.
|
On October 31, 2016, the Registrant announced a reverse stock split of its shares of common stock at a ratio of 1-for-20, which became
effective on November 11, 2016. The Registrant’s common stock commenced trading on a split-adjusted basis on November 14, 2016. Unless otherwise stated, all shares and price per share numbers set forth above are presented after giving
effect to the reverse stock split.
All filing fees payable in connection with the registration of these securities were previously paid in connection with
the filing of the Registration Statements.
The Registrant hereby amends
this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in
accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission acting pursuant to said Section 8(a) may
determine.
The information in this prospectus is not complete and may be changed. These
securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any
jurisdiction where the offer or sale is not permitted.
Subject to Completion, dated July 11, 2017
|
|
|
|
|
|
|
PROSPECTUS
|
|

|
|
|
Galena Biopharma, Inc.
18,732,742 Shares of Common Stock Issuable Upon Exercise of Outstanding Warrants
800,000 Shares of Common Stock for Resale by Selling Stockholders
This prospectus relates to:
|
|
•
|
|
the issuance by Galena Biopharma, Inc., or Galena, of up to 151,655 shares of our common stock, par value $0.0001 per share, or Common Stock, at a purchase price of $10.32 per share upon the exercise of outstanding
warrants issued in December 2012, which we refer to as the December 2012 Warrants;
|
|
|
•
|
|
the issuance by Galena of up to 198,608 shares of Common Stock at a purchase price of $50.00 per share upon the exercise of outstanding warrants issued in September 2013, which we refer to as the September 2013
Warrants;
|
|
|
•
|
|
the issuance by Galena of up to 700,320 shares of Common Stock at a purchase price of $41.60 per share upon the exercise of outstanding warrants issued in March 2015, which we refer to as the March 2015 Warrants;
|
|
|
•
|
|
the issuance by Galena of up to 682,159 shares of Common Stock at a purchase price of $28.40 per share upon the exercise of outstanding warrants issued in January 2016, which we refer to as the January 2016 Warrants;
|
|
|
•
|
|
the issuance by Galena of up to 17,000,000 shares of Common Stock at a purchase price of $1.10 per share upon the exercise of outstanding warrants issued in February 2017, which we refer to as the February 2017
Warrants;
|
|
|
•
|
|
the offer and sale by certain selling stockholders of up to 700,000 shares of Common Stock issuable upon the exercise of outstanding warrants issued in July 2016, which we refer to as the July 2016 Warrants; and
|
|
|
•
|
|
the offer and sale by certain selling stockholders of up to 100,000 shares of Common Stock issuable upon the exercise of outstanding warrants issued in May and June of 2016, which we refer to as the JGB Warrants.
|
We will not receive any proceeds from the resale of any of the shares of Common Stock by the selling stockholders. The selling stockholders
may sell the shares of Common Stock pursuant to this prospectus in a number of different ways and at varying prices. See “Plan of Distribution” for more information about how the selling stockholders may sell the shares of Common Stock
pursuant to this prospectus. We may receive proceeds from our issuance of Common Stock upon exercise of the December 2012 Warrants, the September 2013 Warrants, the March 2015 Warrants, the January 2016 Warrants, the February 2017 Warrants, the July
2016 Warrants and the JGB Warrants, which we collectively refer to as the Warrants. If all of the Warrants are exercised for cash (meaning we issue the maximum possible number of shares of Common Stock upon exercise of the Warrants), we will receive
gross cash proceeds of approximately $88,662,107. There can be no assurance that any Warrant holder will exercise Warrants, especially since, as of the date of this prospectus, none of the Warrants are in-the-money.
No underwriter or other person has been engaged to facilitate the sale of the shares of Common Stock in this offering. We will bear all costs, expenses and
fees in connection with the registration of such securities.
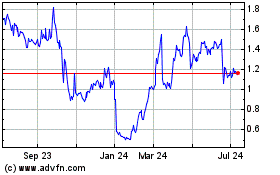
Our Common Stock is traded on The NASDAQ Capital Market under the symbol “GALE.” On
July 10, 2017, the last reported sales price for our Common Stock was $0.5659 per share. Galena has not established a trading market for the Warrants.
You should read this prospectus and any information incorporated by reference herein carefully before you invest.
Investing in our Common Stock involves a high degree of risk. You should review carefully the risks and uncertainties described in the section entitled
“Risk Factors” referenced on page 4 of this prospectus as well as any other risk factors and other information contained in any other document that is incorporated by reference herein or therein.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this
prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is
, 2017.
TABLE OF CONTENTS
i
ABOUT THIS PROSPECTUS
You should rely only on the information contained in or incorporated by reference into this prospectus. We have not authorized anyone to provide you with
information different from that contained in this prospectus or incorporated by reference herein. No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus. You must not
rely on any unauthorized information or representation.
This prospectus is an offer to sell only the securities offered hereby, but only under
circumstances and in jurisdictions where it is lawful to do so. You should assume that the information in this prospectus is accurate only as of the date on the front of the document and that any information we have incorporated by reference is
accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus or any sale of a security.
The distribution of this prospectus and the issuance of the securities in certain jurisdictions may be restricted by law. Persons outside the United States
who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the issuance of the securities and the distribution of this prospectus outside the United States. This prospectus does not
constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, the securities offered by this prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer or
solicitation.
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission, or SEC. Please carefully
read both this prospectus together with the additional information described below under the section entitled “Incorporation of Certain Information by Reference.”
Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus to “Galena,” “the Company,”
“we,” “us,” “our,” and similar references refer to Galena Biopharma, Inc. and its consolidated subsidiaries, Apthera, Inc., or “Apthera,” and Mills Pharmaceuticals, LLC, or “Mills.”
ii
SUMMARY
This summary highlights selected information contained elsewhere in this prospectus or incorporated by reference in this prospectus. This summary is not
complete and does not contain all of the information that you need to consider in making your investment decision. You should carefully read the entire prospectus, including the risks of investing in our Common Stock discussed under the section
entitled “Risk Factors” contained in this prospectus and under similar headings in the other documents that are incorporated by reference into this prospectus. You should also carefully read the information incorporated by reference into
this prospectus, including our financial statements and related notes and the exhibits to the registration statement of which this prospectus forms a part
.
The Company
Galena Biopharma, Inc. is a
biopharmaceutical company developing hematology and oncology therapeutics that address unmet medical needs. Galena’s pipeline consists of multiple mid- to late-stage clinical assets, including our hematology asset, GALE-401, and our novel
cancer immunotherapy programs including NeuVax™ (nelipepimut-S), GALE-301 and GALE-302. GALE-401 is a controlled release version of the approved drug anagrelide for the treatment of elevated platelets in patients with myeloproliferative
neoplasms. GALE-401 has completed a Phase 2 clinical trial and, subject to finalizing the supply of the new formulation and the internal work to prepare the Phase 3 trial for initiation, the asset is ready to advance into a pivotal trial in patients
with essential thrombocythemia (ET). NeuVax is currently in multiple investigator-sponsored Phase 2 clinical trials in breast cancer. GALE-301 and GALE-302 have completed early stage trials in ovarian, endometrial and breast cancers.
On January 31, 2017, Galena announced that it is in the process of evaluating strategic alternatives focused on maximizing stockholder value. Potential
strategic alternatives that may be explored or evaluated as part of this review include continuing to advance the clinical programs as a stand-alone entity, a sale of Galena, a business combination, merger or reverse merger, and a license or other
disposition of corporate assets of Galena. There is no set timetable for this process and there can be no assurance that this process will result in a transaction. While we are working through the strategic alternatives process, management has
significantly reduced the staffing levels and certain operational expenses to preserve cash, although work remains ongoing to advance our two core clinical programs, GALE-401 and NeuVax™ (nelipepimut-S), and maintain their value.
On October 31, 2016, we announced a reverse stock split of our shares of Common Stock at a ratio of 1-for-20, which became effective on November 11,
2016. Our Common Stock commenced trading on a split-adjusted basis on November 14, 2016. Unless otherwise stated, all shares and price per share numbers set forth in this prospectus are presented after giving effect to the reverse stock split.
For a complete description of our business, financial condition, results of operations and other important information, we refer you to our filings with
the SEC that are incorporated by reference in this prospectus, including our most recent Annual Report on Form 10-K. For instructions on how to find copies of these documents, see the sections entitled “Where You Can Find More
Information” and “Incorporation of Certain Information by Reference.”
Company Information
We were incorporated as Argonaut Pharmaceuticals, Inc. in Delaware on April 3, 2006 and changed our name to RXi Pharmaceuticals Corporation on
November 28, 2006. On September 26, 2011, we changed our company name from RXi Pharmaceuticals Corporation to Galena Biopharma, Inc.
Our
principal executive offices are located at 2000 Crow Canyon Place, Suite 380, San Ramon, California 94583, and our phone number is (855) 855-4253. Our website address is
www.galenabiopharma.com
. Except for the documents incorporated by
reference in this prospectus, the information contained on our website is not part of this prospectus and should not be relied upon in connection with making an investment decision.
1
The Offering
|
|
|
|
|
Securities Offered
|
|
This prospectus relates to:
• the issuance by Galena of up to 151,655 shares of Common Stock at a purchase price of
$10.32 per share upon the exercise of the December 2012 Warrants;
• the issuance by Galena of up to 198,608 shares of Common Stock at a purchase price of
$50.00 per share upon the exercise of the September 2013 Warrants;
• the issuance by Galena of up to 700,320 shares of Common Stock at a purchase price of
$41.60 per share upon the exercise of the March 2015 Warrants;
• the issuance by Galena of up to 682,159 shares of Common Stock at a purchase price of
$28.40 per share upon the exercise of the January 2016 Warrants;
• the issuance by Galena of up to 17,000,000 shares of Common Stock at a purchase price of
$1.10 per share upon the exercise of the February 2017 Warrants;
• the offer and sale by certain selling stockholders of up to 700,000 shares of Common
Stock issuable upon the exercise of the July 2016 Warrants; and
• the offer and sale by certain selling stockholders of up to 100,000 shares of Common
Stock issuable upon the exercise of the JGB Warrants.
|
|
|
|
|
Use of Proceeds
|
|
We intend to use the net proceeds from any exercise of Warrants in our evaluation of strategic alternatives, to help fund certain of our clinical trials of our product candidates, to augment our working capital and for general
corporate purposes. General corporate purposes may include, but are not limited to, payments relating to the settlement and defense costs related to the government investigations, possible settlement and defense costs related to pending shareholder
lawsuits, discontinuation of our commercial operations, winding down the PRESENT Trial, repayment of existing or future indebtedness and financing of capital expenditures. There can be no assurance that any Warrant holder will exercise Warrants,
especially since, as of the date of this prospectus, none of the Warrants are in-the-money. In addition, we will not receive any proceeds from the resale pursuant to this prospectus of Common Stock that may be obtained upon the exercise of warrants
by the selling stockholders. See the section entitled “Use of Proceeds.”
|
|
|
|
|
Shares Outstanding Before this Offering
|
|
As of July 10, 2017, there were 37,505,644 shares of our Common Stock outstanding.
|
|
|
|
|
Shares Outstanding After Completion of this
Offering
|
|
Assuming that all of the Warrants are exercised in full, we expect 56,681,058 shares of Common Stock will be outstanding immediately after completion of this offering.
|
|
|
|
|
Fees and Expenses
|
|
We will pay the fees and expenses we incur related to this offering.
|
|
|
|
|
The NASDAQ Capital Market Symbol
|
|
Our Common Stock is listed on The NASDAQ Capital Market under the symbol “GALE.”
|
|
|
|
|
Risk Factors
|
|
You should be aware that there are risks associated with investing in our Common Stock, including, but not limited to, the risks referenced in the section entitled “Risk Factors” beginning on page 4 of this
prospectus, including the risk factors disclosed in our most recent Annual Report on Form 10-K, and any updates in our subsequent Quarterly Reports on Form 10-Q. You should carefully read and consider these risk factors together with all
of the other information included in or incorporated by reference into this prospectus before you decide to exercise the Warrants to purchase shares of Common Stock.
|
2
|
|
|
|
|
Dividend Policy
|
|
We do not anticipate paying any cash dividends on our Common Stock.
|
|
|
|
|
Dilution
|
|
The exercise price of certain of the Warrants is subject to anti-dilution adjustments in certain circumstances. See “Description of Our Securities.”
|
The number of shares of Common Stock shown above to be outstanding after this offering is based on 37,148,316 shares
outstanding as of March 31, 2017 and excludes the following:
|
|
•
|
|
33,750 shares held in treasury;
|
|
|
•
|
|
557,872 shares of our Common Stock subject to outstanding options having a weighted-average exercise price of $37.68 per share;
|
|
|
•
|
|
483,017 shares of our Common Stock reserved for issuance in connection with future awards under our 2016 stock incentive plan;
|
|
|
•
|
|
16,882 shares of our Common Stock reserved for future sale under our employee stock purchase plan; and
|
|
|
•
|
|
19,569,751 shares of our Common Stock subject to outstanding warrants (other than the Warrants) having a weighted-average exercise price of $4.62 per share.
|
3
RISK FACTORS
Investing in shares of our Common Stock involves a high degree of risk. Before making an investment decision, you should carefully consider the risks
described under “Risk Factors” in our most recent Annual Report on Form 10-K, as well as any updates to those risk factors in our subsequent Quarterly Reports on Form 10-Q, together with all of the other information appearing in
or incorporated by reference into this prospectus, before deciding whether to purchase any of the Common Stock being offered. The risks described in these documents are not the only ones we face, but those that we consider to be material. There may
be other unknown or unpredictable economic, business, competitive, regulatory or other factors that could have material adverse effects on our future results. Past financial performance may not be a reliable indicator of future performance, and
historical trends should not be used to anticipate results or trends in future periods. Our business, financial condition or results of operations could be materially adversely affected by any of these risks. The trading price of shares of our
Common Stock could decline due to any of these risks, and you may lose all or part of your investment. Please also read carefully the section entitled “Disclosure Regarding Forward-Looking Statements.”
4
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
Some of the information contained in this prospectus may include forward-looking statements that reflect our current views with respect to our development
programs, business strategy, business plan, financial performance and other future events. All statements other than statements of historical fact are statements that could be deemed forward-looking statements, including any projections of financing
needs, revenue, expenses, earnings or losses from operations, or other financial items, any statements of the plans, strategies and objectives of management for future operations including any statements regarding the strategic alternative process
and its outcomes, any statements concerning product research, development and commercialization plans and timelines, any statements regarding safety and efficacy of product candidates, any statements of expectation or belief and any statements of
assumptions underlying any of the foregoing. These statements include forward-looking statements both with respect to us, specifically, and our industry, in general. Statements that include the words “expect,” “intend,”
“plan,” “believe,” “project,” “estimate,” “may,” “should,” “anticipate,” “will” and similar statements of a future or forward-looking nature identify forward-looking
statements for purposes of the federal securities laws and otherwise. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions
regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent
uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. There are or will be important factors that could cause actual results to differ materially from those indicated in these
statements. These factors also include, but are not limited to, those factors set forth in the sections entitled “Risk Factors,” “Legal Proceedings,” “Management’s Discussion and Analysis of Financial Condition and
Results of Operations,” “Quantitative and Qualitative Disclosures About Market Risk” and “Controls and Procedures” in our most recent Annual Report on Form 10-K and our subsequent Quarterly Reports on Form 10-Q, all of which
you should review carefully.
Given these uncertainties, you should not place undue reliance on these forward-looking statements. You should read this
prospectus and the documents incorporated by reference in this prospectus with the understanding that our actual future results may be materially different from what we expect. Except as required by law, we do not undertake any obligation to update
or revise any forward-looking statements contained in this prospectus or such other documents, whether as a result of new information, future events or otherwise.
5
USE OF PROCEEDS
The gross proceeds that we receive from this offering will depend upon the number of Warrants exercised and the exercise price of the Warrants exercised. If
all of the Warrants are exercised for cash (meaning we issue the maximum possible number of shares of Common Stock upon exercise of the Warrants), we will receive gross cash proceeds of approximately $88,662,107. There can be no assurance that any
Warrant holder will exercise Warrants, especially since, as of the date of this prospectus, none of the Warrants are in-the-money. In addition, we will not receive any proceeds from the resale pursuant to this prospectus of Common Stock that may be
obtained upon the exercise of Warrants by the selling stockholders.
We currently intend to use the net proceeds, if any, from any exercise of Warrants in
our evaluation of strategic alternatives, to help fund certain of our clinical trials of our product candidates, to augment our working capital and for general corporate purposes. General corporate purposes may include, but are not limited to,
payments relating to the settlement and defense costs related to the government investigations, possible settlement and defense costs related to pending shareholder lawsuits, discontinuation of our commercial operations, winding down the PRESENT
Trial, repayment of existing or future indebtedness and financing of capital expenditures.
We have not determined the amounts we plan to spend on any of
the areas listed above or the timing of these expenditures. As a result, our management will have broad discretion to allocate the net proceeds from any exercise of Warrants.
6
DIVIDEND POLICY
Our business requires significant funding. We currently plan to invest all available funds and any future earnings in our business and do not anticipate
paying any cash dividends on our Common Stock in the foreseeable future. We currently are prohibited by the terms of our outstanding indebtedness from paying dividends on our Common Stock, except with the prior consent of our lenders.
7
DILUTION
Our net tangible book deficit as of March 31, 2017 was approximately ($833,000), or ($0.02) per share of Common Stock. Net tangible book value (deficit)
per share is calculated by subtracting our total liabilities from our total tangible assets, which is total assets less intangible assets, and dividing this amount by the number of shares of Common Stock outstanding. After giving effect to the
exercise of the Warrants for the underlying 19,532,742 shares of Common Stock for an aggregate exercise price of $88,662,107, and after deducting any offering expenses payable by us, we would have had a net tangible book value as of March 31,
2017 of approximately $87,699,107, or $1.55 per share of Common Stock. This represents an immediate increase in net tangible book value of $1.57 per share to our existing stockholders and an immediate increase in net tangible book value to our
existing stockholders and to the holders of the February 2017 Warrants, and an immediate dilution in net tangible book value to the holders of the December 2012 Warrants, the September 2013 Warrants, the March 2015 Warrants, the January 2016
Warrants, the July 2016 Warrants and the JGB Warrants. The following table illustrates this dilution:
|
|
|
|
|
|
|
|
|
|
|
Exercise Price of the December 2012 Warrants
|
|
|
|
|
|
$
|
10.32
|
|
|
Exercise Price of the September 2013 Warrants
|
|
|
|
|
|
$
|
50.00
|
|
|
Exercise Price of the March 2015 Warrants
|
|
|
|
|
|
$
|
41.60
|
|
|
Exercise Price of the January 2016 Warrants
|
|
|
|
|
|
$
|
28.40
|
|
|
Exercise Price of the July 2016 Warrants
|
|
|
|
|
|
$
|
13.00
|
|
|
Exercise Price of the JGB Warrants
|
|
|
|
|
|
$
|
8.60
|
|
|
Exercise Price of the February 2017 Warrants
|
|
|
|
|
|
$
|
1.10
|
|
|
Net tangible book value (deficit) per share as of March 31, 2017
|
|
($
|
0.02
|
)
|
|
|
|
|
|
Increase per share attributable to the exercise of all the Warrants
|
|
$
|
1.57
|
|
|
|
|
|
|
As adjusted net tangible book per share after the exercise of all the Warrants
|
|
|
|
|
|
$
|
1.55
|
|
|
Dilution in net tangible book value per share to investors exercising December 2012
Warrants
|
|
|
|
|
|
$
|
8.77
|
|
|
Dilution in net tangible book value per share to investors exercising September 2013
Warrants
|
|
|
|
|
|
$
|
48.45
|
|
|
Dilution in net tangible book value per share to investors exercising March 2015 Warrants
|
|
|
|
|
|
$
|
40.05
|
|
|
Dilution in net tangible book value per share to investors exercising January 2016
Warrants
|
|
|
|
|
|
$
|
26.85
|
|
|
Dilution in net tangible book value per share to investors exercising July 2016 Warrants
|
|
|
|
|
|
$
|
11.45
|
|
|
Dilution in net tangible book value per share to investors exercising JGB Warrants
|
|
|
|
|
|
$
|
7.05
|
|
|
Increase in net tangible book value per share to investors exercising February 2017
Warrants
|
|
|
|
|
|
$
|
0.45
|
|
The number of shares of Common Stock shown above to be outstanding after this offering is based on 37,148,316 shares
outstanding as of March 31, 2017 and excludes as of such date:
|
|
•
|
|
33,750 shares held in treasury;
|
|
|
•
|
|
557,872 shares of our Common Stock subject to outstanding options having a weighted-average exercise price of $37.68 per share;
|
|
|
•
|
|
483,017 shares of our Common Stock reserved for issuance in connection with future awards under our 2016 stock incentive plan;
|
|
|
•
|
|
16,882 shares of our Common Stock reserved for future sale under our employee stock purchase plan; and
|
|
|
•
|
|
19,569,751 shares of our Common Stock subject to outstanding warrants (other than the Warrants) having a weighted-average exercise price of $4.62 per share.
|
To the extent our outstanding options and warrants (other than the Warrants) are exercised, you may experience further dilution. The above illustration of
dilution per share to investors participating in this offering assumes no
8
exercise of outstanding options or outstanding warrants to purchase shares of our Common Stock (other than the Warrants). The illustration also assumes no further issuance of shares of our Common
Stock in payment of contingent consideration to holders of our outstanding contingent value rights or others. The exercise of outstanding options and warrants having an exercise price less than the exercise prices of the Warrants, or our payment to
our contingent value rights holders or others of Common Stock valued at less than the offering price of shares in this offering, would further increase dilution to investors in this offering.
While the above table assumes full exercise of all of the Warrants, there can be no assurance that any Warrant holder will exercise Warrants, especially
since, as of the date of this prospectus, none of the Warrants are in-the-money.
9
THE OFFERING
This prospectus relates to:
|
|
•
|
|
the issuance by Galena of up to 151,655 shares of Common Stock at a purchase price of $10.32 per share upon the exercise of the December 2012 Warrants;
|
|
|
•
|
|
the issuance by Galena of up to 198,608 shares of Common Stock at a purchase price of $50.00 per share upon the exercise of the September 2013 Warrants;
|
|
|
•
|
|
the issuance by Galena of up to 700,320 shares of Common Stock at a purchase price of $41.60 per share upon the exercise of the March 2015 Warrants;
|
|
|
•
|
|
the issuance by Galena of up to 682,159 shares of Common Stock at a purchase price of $28.40 per share upon the exercise of the January 2016 Warrants;
|
|
|
•
|
|
the issuance by Galena of up to 17,000,000 shares of Common Stock at a purchase price of $1.10 per share upon the exercise of the February 2017 Warrants;
|
|
|
•
|
|
the offer and sale by certain selling stockholders of up to 700,000 shares of Common Stock issuable upon the exercise of the July 2016 Warrants; and
|
|
|
•
|
|
the offer and sale by certain selling stockholders of up to 100,000 shares of Common Stock issuable upon the exercise of the JGB Warrants.
|
The closing price of our Common Stock on July 10, 2017 was $0.5659. In addition, there were 37,505,644 shares of our Common Stock outstanding as of
July 10, 2017. If all of the Warrants are exercised (meaning we issue the maximum possible number of shares of Common Stock upon exercise of all of the Warrants as described above), 57,038,386 shares of Common Stock would be outstanding.
10
SELLING STOCKHOLDERS
Selling Stockholders with Respect to Common Stock Issuable Pursuant to the July 2016 Warrants
On July 7, 2016, we entered into a Securities Purchase Agreement (the “
Purchase Agreement
”) with CVI Investments, Inc., Sabby
Volatility Warrant Master Fund, Ltd. and Sabby Healthcare Master Fund, Ltd. (the “
2016 Investors
”), providing for the issuance and sale by us to the 2016 Investors of an aggregate of approximately $12,600,000 of our
registered and unregistered securities. Pursuant to the Purchase Agreement, we agreed, among other things, to issue and sell to the 2016 Investors an aggregate of 1,400,000 shares of our Common Stock at a purchase price per share of $0.45 in a
registered direct offering and 700,000 July 2016 Warrants in a concurrent private placement. We closed on the sale of these securities on July 13, 2016.
The 2016 Investors are offering under this prospectus up to 700,000 shares of our Common Stock issuable by us upon the exercise of the July 2016 Warrants (the
“
July 2016 Warrant Shares
”), the terms of which are described in this prospectus under the caption “Description of Our Securities—Warrants—The July 2016 Warrants.” The 2016 Investors may, from time to
time, offer and sell pursuant to this prospectus any or all of the July 2016 Warrant Shares. The 2016 Investors may sell some, all or none of the July 2016 Warrant Shares, to the extent they exercise the July 2016 Warrants. We do not know how long
the 2016 Investors will hold the July 2016 Warrant Shares before selling them, and we currently have no agreements, arrangements or understandings with the 2016 Investors regarding the sale of any of the July 2016 Warrant Shares or the exercise of
the July 2016 Warrants.
The following table presents information regarding the 2016 Investors and the shares that they may offer and sell from time to
time under this prospectus. The table is prepared based on information supplied to us by the 2016 Investors and reflects their holdings as of July 11, 2017. None of the 2016 Investors, nor any affiliate of a 2016 Investor, has held a
position or office, or had any other material relationship, with us or any of our predecessors or affiliates. Beneficial ownership is determined in accordance with Rule 13d-3(d) promulgated by the SEC under the Exchange Act, except the percentages
set forth in the following table do not take into account contractual limitations in the Warrants that limit beneficial ownership to specified percentages. See footnotes (2), (5) and (7) to the table below. The percentage of shares beneficially
owned prior to the offering is based on 37,505,644 shares of our Common Stock actually outstanding as of July 10, 2017.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling Stockholder
|
|
Shares
Beneficially
Owned Before
this
Offering
|
|
|
Percentage
of
Outstanding
Shares
Beneficially
Owned
Before
this
Offering
|
|
|
No. of Shares
Offered by
Selling
Stockholder
Upon
Exercise of
Warrants
|
|
|
Percentage
of
Outstanding
Shares
Beneficially
Owned
After
this
Offering
|
|
|
CVI Investments, Inc. (1)
|
|
|
3,583,550
|
(2)
|
|
|
8.7
|
%
|
|
|
350,000
|
(3)
|
|
|
7.9
|
%
|
|
Sabby Healthcare Master Fund, Ltd. (4)
|
|
|
4,876,057
|
(5)
|
|
|
12.1
|
%
|
|
|
250,000
|
(6)
|
|
|
11.5
|
%
|
|
Sabby Volatility Warrant Master Fund, Ltd. (4)
|
|
|
1,637,285
|
(7)
|
|
|
4.2
|
%
|
|
|
100,000
|
(8)
|
|
|
4.0
|
%
|
|
(1)
|
Heights Capital Management, Inc. is the investment manager to CVI Investments, Inc. and as such may exercise voting and dispositive power over these shares of Common Stock.
|
|
(2)
|
Based on information supplied to us on July 11, 2017, this number includes shares of Common Stock that CVI Investments, Inc. has the right to acquire pursuant to 2,980,000 February 2017 Warrants,
136,363 January 2016 Warrants, 350,000 July 2016 Warrants, 80,000 March 2015 Warrants and 37,187 September 2013 Warrants. The terms of the Warrants include a blocker provision that restricts exercise or conversion, as applicable,
to the extent the securities beneficially owned by a 2016 Investor and its affiliates would represent beneficial ownership in excess of 4.99% (4.9% with respect to the December
|
11
|
|
2012 Warrants) of shares of our Common Stock outstanding immediately after giving effect to such exercise or conversion, subject to the holder’s option, on 61 days’ prior notice to us,
to increase or decrease this beneficial ownership limitation not to exceed 9.99% of shares of our Common Stock immediately after giving effect to such exercise or conversion.
|
|
(3)
|
Represents the shares of Common Stock underlying the July 2016 Warrants held by CVI Investments, Inc.
|
|
(4)
|
Sabby Management, LLC serves as the investment manager of Sabby Healthcare Master Fund, Ltd. and Sabby Volatility Warrant Master Fund, Ltd., and as such may exercise voting and dispositive power over these shares of
Common Stock. In addition, Hal Mintz, as Manager of Sabby Management, LLC, also has voting and dispositive power over these shares of Common Stock. Sabby Management, LLC and Hal Mintz do not directly own any shares of Common Stock. Each of Sabby
Management, LLC and Hal Mintz disclaims beneficial ownership over the securities listed except to the extent of their pecuniary interest therein.
|
|
(5)
|
Based on information supplied to us on July 11, 2017, this number includes the shares of Common Stock that Sabby Healthcare Master Fund, Ltd. has the right to acquire pursuant to 6,084 listed calls expiring
January 19, 2018, 1,666,667 February 2017 Warrants, 250,000 July 2016 Warrants, 106,300 January 2016 Warrants, 75,000 March 2015 Warrants and 836,936 shares of Common Stock that are currently purchasable upon exercise of certain other Common
Stock purchase warrants held by the Sabby Healthcare Master Fund, Ltd. The terms of the different Warrants include a blocker provision that restricts exercise or conversion, as applicable, to the extent the securities beneficially owned by a 2016
Investor and its affiliates would represent beneficial ownership in excess of 4.99% (4.9% with respect to the December 2012 Warrants) of shares of our Common Stock outstanding immediately after giving effect to such exercise or conversion, subject
to the holder’s option, on 61 days’ prior notice to us, to increase or decrease this beneficial ownership limitation not to exceed 9.99% of shares of our Common Stock immediately after giving effect to such exercise or conversion.
|
|
(6)
|
Represents the shares of Common Stock underlying the July 2016 Warrants held by Sabby Healthcare Master Fund, Ltd.
|
|
(7)
|
Based on information supplied to us on July 11, 2017, this number includes the shares of Common Stock that Sabby Volatility Warrant Master Fund, Ltd. has the right to acquire pursuant to 833,333 February 2017
Warrants, 100,000 July 2016 Warrants, 30,000 January 2016 Warrants and 56,200 March 2015 Warrants. The terms of the different Warrants include a blocker provision that restricts exercise or conversion, as applicable, to the extent the securities
beneficially owned by a 2016 Investor and its affiliates would represent beneficial ownership in excess of 4.99% (4.9% with respect to the December 2012 Warrants) of shares of our Common Stock outstanding immediately after giving effect to such
exercise or conversion, subject to the holder’s option, on 61 days’ prior notice to us, to increase or decrease this beneficial ownership limitation not to exceed 9.99% of shares of our Common Stock immediately after giving effect to such
exercise or conversion.
|
|
(8)
|
Represents the shares of Common Stock underlying the July 2016 Warrants held by Sabby Volatility Warrant Master Fund, Ltd.
|
Selling Stockholders with Respect to Common Stock Issuable Pursuant to the JGB Warrants
On May 10, 2016, Galena entered into a Securities Purchase Agreement (the “
Securities Purchase Agreement
”), with JGB (Cayman)
Newton Ltd. (“
JGB
”) pursuant to which Galena sold to JGB, at a 6.375% original issue discount, a $25,530,000 Senior Secured Debenture (as subsequently amended, the “
Debenture
”) and 100,000 JGB Warrants
to assignees and affiliates of JGB. Upon the closing of the sale of the Debenture, JGB received Series A JGB Warrants to purchase 50,000 shares of Common Stock at an exercise price of $30.20 per share, exercisable for five years from
issuance. In accordance with the terms of the Securities Purchase Agreement, JGB also received Series B JGB Warrants to purchase 50,000 shares of Common Stock in connection with Galena’s announcement of the results of the interim analysis of
its PRESENT Trial in June 2016. The Series B JGB Warrants have an exercise price of $8.60 per share, 120% of the volume-weighted average price of the Common Stock on the date of the announcement of the results of the interim analysis. In
connection with an amendment to the Securities Purchase Agreement on August 22, 2016, the exercise price of the Series A JGB Warrants was reduced to $8.60 per share.
12
The selling stockholders with respect to the Common Stock issuable pursuant to the JGB Warrants (the
“
JGB Investors
”) are offering under this prospectus up to 100,000 shares of our Common Stock issuable by us to assignees and affiliates of JGB (Cayman) Newton Ltd. upon the exercise of the Series A and Series B JGB Warrants
(the “
JGB Warrant Shares
”), the terms of which are described in this prospectus under the caption “Description of Our Securities—Warrants—The JGB Warrants.” The JGB Investors may, from time to time, offer
and sell pursuant to this prospectus any or all of the JGB Warrant Shares. The JGB Investors may sell some, all or none of their JGB Warrant Shares. We do not know how long the JGB Investors will hold the JGB Warrant Shares before selling them, and
we currently have no agreements, arrangements or understandings with the JGB Investors regarding the sale of any of the JGB Warrant Shares or the exercise of the JGB Warrants.
The following table presents information regarding the JGB Investors and the shares that they may offer and sell from time to time under this prospectus. The
table is prepared based on information supplied to us by the JGB Investors, and reflects their holdings as of July 10, 2017. None of the JGB Investors, nor any affiliate of a selling stockholder, has held a position or office, or had any other
material relationship, with us or any of our predecessors or affiliates other than with respect to the Debenture. Beneficial ownership is determined in accordance with Rule 13d-3(d) promulgated by the SEC under the Exchange Act. The percentage of
shares beneficially owned prior to the offering is based on 37,505,644 shares of our Common Stock actually outstanding as of July 10, 2017.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling Stockholder
|
|
Shares
Beneficially
Owned
Before this
Offering
|
|
|
Percentage
of
Outstanding
Shares
Beneficially
Owned
Before this
Offering
|
|
|
No. of Shares
Offered by
Selling Stockholder
Upon Exercise of
Warrants
|
|
|
Percentage
of
Outstanding
Shares
Beneficially
Owned
After this
Offering
|
|
|
JGB Capital Offshore Ltd (1)
|
|
|
100,000
|
|
|
|
*
|
|
|
|
19,470
|
|
|
|
*
|
|
|
JGB Capital LP (1)
|
|
|
100,000
|
|
|
|
*
|
|
|
|
3,230
|
|
|
|
*
|
|
|
JGB Partners LP (1)
|
|
|
100,000
|
|
|
|
*
|
|
|
|
77,300
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTALS
|
|
|
|
|
|
|
|
|
|
|
100,000
|
|
|
|
|
|
|
(1)
|
Shares beneficially owned before this offering reflect all shares beneficially owned by JGB Capital Offshore Ltd., JGB Capital LP and JGB Partners LP. The investment advisor to each of JGB Capital Offshore Ltd., JGB
Capital LP and JGB Partners LP is JGB Management Inc. and has voting and investment discretion over the shares in such capacity. The President of JGB Management Inc. is Brett Cohen. Brett Cohen disclaims beneficial ownership of the securities held
by the JGB Investors. The shares beneficially owned before this offering do not reflect shares of our Common Stock that JGB (Cayman) Newton Ltd. may elect to receive in satisfaction of the outstanding principal and accrued interest under the
Debenture.
|
13
PLAN OF DISTRIBUTION
Shares of Common Stock Issuable Upon Exercise of the Warrants
We are offering an aggregate of up to 18,732,742 shares of our Common Stock issuable upon the exercise of:
|
|
•
|
|
the December 2012 Warrants;
|
|
|
•
|
|
the September 2013 Warrants;
|
|
|
•
|
|
the March 2015 Warrants;
|
|
|
•
|
|
the January 2016 Warrants; and
|
|
|
•
|
|
the February 2017 Warrants,
|
in each case pursuant to the terms of the respective warrant agreements, which
are incorporated by reference into this prospectus. See the sections entitled “Where You Can Find More Information” and “Incorporation of Certain Information by Reference.”
Selling Stockholders (The July 2016 Warrants and the JGB Warrants)
The selling stockholders are offering an aggregate of up to 800,000 shares of Common Stock issuable upon the exercise of the July 2016 Warrants and the JGB
Warrants. The July 2016 Warrants are exercisable until July 13, 2021 at an exercise price of $13.00 per share of Common Stock. The JGB Warrants are exercisable until November 10, 2021 at an exercise price of $8.60 per share of Common
Stock. Each selling stockholder of the shares offered hereby and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their securities covered hereby on the NASDAQ Capital Market or any other stock
exchange, market or trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices. A selling stockholder may use any one or more of the following methods when selling securities:
|
|
•
|
|
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
|
•
|
|
block trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction;
|
|
|
•
|
|
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
|
•
|
|
an exchange distribution in accordance with the rules of the applicable exchange;
|
|
|
•
|
|
privately negotiated transactions;
|
|
|
•
|
|
settlement of short sales;
|
|
|
•
|
|
in transactions through broker-dealers that agree with the selling stockholders to sell a specified number of such securities at a stipulated price per security;
|
|
|
•
|
|
through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
|
14
|
|
•
|
|
a combination of any such methods of sale; or
|
|
|
•
|
|
any other method permitted pursuant to applicable law.
|
The selling stockholders may also sell securities
under Rule 144 under the Securities Act, if available, rather than under this prospectus.
Broker-dealers engaged by the selling stockholders may arrange
for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling stockholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be
negotiated, but in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2440; and in the case of a principal transaction a markup or markdown in compliance with FINRA IM-2440.
15
DESCRIPTION OF OUR SECURITIES
General
Under our Certificate of Incorporation, our
authorized capital stock consists of 355,000,000 shares, all which includes:
|
|
•
|
|
350,000,000 shares of Common Stock, par value $0.0001 per share, and
|
|
|
•
|
|
5,000,000 shares of preferred stock, par value $0.0001 per share.
|
Common Stock
As of July 10, 2017, there were 37,505,644 shares of our Common Stock outstanding and 33,750 shares of Common Stock held in treasury.
Each holder of our Common Stock is entitled to one vote for each share of Common Stock held on all matters submitted to a vote of stockholders. Common
stockholders are not entitled to cumulative voting in the election of directors by our Certificate of Incorporation. This means that the holders of a majority of the shares voted will be able to elect all of the directors then standing for election.
Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of our Common Stock are entitled to receive dividends out of assets legally available at the times and in the amounts that
our board of directors may determine from time to time.
Upon our liquidation or dissolution, whether voluntary or involuntary, the holders of our Common
Stock will be entitled to receive all assets available for distribution, subject to any preferential rights of any then outstanding preferred stock. Holders of our Common Stock have no preemptive or conversion rights or other subscription rights.
There are no redemption or sinking fund provisions applicable to the Common Stock. All outstanding shares of Common Stock are fully paid and nonassessable, and the shares of Common Stock to be issued under this prospectus supplement, when they are
paid for, will be fully paid and nonassessable.
Our Common Stock is listed on The NASDAQ Capital Market under the symbol “GALE.” The transfer
agent of our Common Stock is Computershare Trust Company, N.A.
Warrants
The following summaries of the material terms and provisions of the warrants are not complete and are subject to, and qualified in their entirety by, the
respective provisions of the Warrants, the forms of which have been incorporated by reference into the registration statement of which this prospectus is part:
The December 2012 Warrants
Form
. The
December 2012 Warrants were issued as individual warrant agreements to the investors.
Exercisability
. Each December 2012 Warrant may be exercised
at any time and from time to time on or after December 21, 2012 and through and including December 21, 2017. The December 2012 Warrants are exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed
exercise notice and payment in full of the exercise price in immediately available funds for the number of shares of Common Stock purchased upon such exercise. If a registration statement registering the issuance of the shares of Common Stock
underlying the December 2012 Warrants under the Securities Act is not effective or available, the holder may, in its sole discretion, elect to exercise the December 2012 Warrants through a cashless exercise, in which case the holder would receive
upon such exercise the net number of shares of Common Stock determined according to the formula set forth in the December 2012 Warrants. No fractional shares of Common Stock will be issued in connection with the exercise of a December 2012 Warrants.
16
Exercise Limitation
. A holder will not have the right to exercise any portion of the December 2012
Warrants if the holder (together with its affiliates) would beneficially own in excess of 4.9% of the number of shares of our Common Stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in
accordance with the terms of the December 2012 Warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon at least 61 days’ prior notice from the holder to us.
Exercise Price
. The December 2012 Warrants are exercisable for a total of 151,655 shares at an exercise price of $10.32 per share. The exercise price
is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Stock and also upon any distributions of assets, including
cash, stock or other property to our stockholders.
Weighted Average Anti-Dilution Protection
. In the event that we, during the time the 2012
Warrants are outstanding, sell or grant any option to purchase, or sell or grant any right to reprice, or otherwise dispose of or issue (or announce any offer, sale, grant or any option to purchase or other disposition) any Common Stock or Common
Stock equivalents entitling any person to acquire shares of Common Stock, at an effective price per share less than the then exercise price of the 2012 Warrants (such issuances collectively, a “
Dilutive Issuance
”), then the
exercise price of the 2012 Warrants will be reduced by multiplying the exercise price by a fraction, the numerator of which is the number of shares of Common Stock issued and outstanding immediately prior to the Dilutive Issuance plus the number of
shares of Common Stock which the offering price for such Dilutive Issuance would purchase at the then exercise price, and the denominator of which shall be the sum of the number of shares of Common Stock issued and outstanding immediately prior to
the Dilutive Issuance plus the number of shares of Common Stock so issued or issuable in connection with the Dilutive Issuance.
Transferability
.
Subject to applicable laws, the December 2012 Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange
Listing
. The December 2012 Warrants are not listed on The NASDAQ Capital Market, any other national securities exchange or any other nationally recognized trading system.
Fundamental Transactions
. In the event of a “fundamental transaction,” as described in the December 2012 Warrants
and generally including any merger or consolidation with or into another entity, as a result of which the holders of our outstanding voting securities as of immediately prior to such merger or consolidation hold less than a majority of the
outstanding voting securities of the surviving or successor entity as of immediately after such merger or consolidation or a sale, transfer or other disposition of all or substantially all our property, assets or business to another person or
entity, the holders of the December 2012 Warrants will be entitled to receive upon exercise of the December 2012 Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the December
2012 Warrants immediately prior to such fundamental transaction. In addition, in the event of any Fundamental Transaction the holder of the December 2012 Warrants has the right, in lieu of receiving the consideration described in the preceding
sentence, to require us to purchase the December 2012 Warrant for an amount of cash based on the value of the remaining unexercised portion of the December 2012 Warrants determined in accordance with the Black Scholes option pricing model.
Rights as a Stockholder
. Except as otherwise provided in the December 2012 Warrants or by virtue of such
holder’s ownership of shares of our Common Stock, the holder of a December 2012 Warrant does not have the rights or privileges of a holder of our Common Stock, including any voting rights, until the holder exercises the December 2012 Warrant.
The September 2013 Warrants
Form.
The September 2013 Warrants were issued under a warrant agreement entered into between us and the transfer agent.
Exercisability.
Each September 2013 Warrant may be exercised at any time and from time to time on or after September 18, 2013 and through and
including September 18, 2018. The September 2013 Warrants are exercisable,
17
at the option of each holder, in whole or in part by delivering to us a warrant certificate or book-entry warrant, a duly executed election to purchase the shares underlying the September 2013
Warrants to be exercised and payment in full of the exercise price in immediately available funds for the number of shares of Common Stock purchased upon such exercise. If a registration statement registering the issuance or the resale of the shares
of Common Stock underlying the September 2013 Warrants under the Securities Act is not effective or available, the holder may, in its sole discretion, elect to exercise the September 2013 Warrant through a cashless exercise, in which case the holder
would receive upon such exercise the net number of shares of Common Stock determined according to the formula set forth in the September 2013 Warrant. No fractional shares of Common Stock will be issued in connection with the exercise of a September
2013 Warrant. In lieu of fractional shares, we will pay the holders an amount in cash equal to the fractional amount multiplied by the current market price of our Common Stock or round up to the next whole share.
Exercise Limitation.
A holder will not have the right to exercise any portion of the September 2013 Warrant if the holder (together with affiliates)
would beneficially own in excess of 4.99% of the number of shares of our Common Stock outstanding immediately after giving effect to the exercise, as such percentage of ownership is determined in accordance with the terms of the September 2013
Warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon at least 61 days’ prior notice from the holder to us.
Exercise Price
. The September 2013 Warrants are exercisable for a total of 198,608 shares at an exercise price of $50.00 per share. The exercise price
is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Stock and also upon any distribution of assets, including
cash, stock or other property, to our stockholders.
Transferability.
Subject to applicable laws, the September 2013 Warrants may be offered for
sale, sold, transferred or assigned without our consent.
Exchange Listing.
The September 2013 Warrants are not listed on The NASDAQ Capital
Market, any other national securities exchange or any other nationally recognized trading system.
Fundamental Transactions.
In the event of a
“fundamental transaction,” as described in the September 2013 Warrants and generally including any merger or consolidation with or into another entity, as a result of which the holders of our outstanding voting securities as of immediately
prior to such merger or consolidation hold less than a majority of the outstanding voting securities of the surviving or successor entity as of immediately after such merger or consolidation or a sale, transfer or other disposition of all or
substantially all our property, assets or business to another person or entity, the holders of the September 2013 Warrants will be entitled to receive upon exercise of the September 2013 Warrants the kind and amount of securities, cash or other
property that the holders would have received had they exercised the September 2013 Warrant immediately prior to such fundamental transactions. In addition in the event of any fundamental transaction the holder of the September 2013 Warrant has the
right, in lieu of receiving the consideration described in the preceding sentence, to require us to purchase the September 2013 Warrant for an amount of cash based on the value of the remaining unexercised portion of the September 2013 Warrant
determined in accordance with the Black Scholes option pricing model.
Rights as a Stockholder.
Except as otherwise provided in the September 2013
Warrants or by virtue of such holders’ ownership of shares of our Common Stock, the holder of a September 2013 Warrant does not have the rights or privileges of a holder of our Common Stock, including any voting rights, until the holder
exercises the September 2013 Warrant.
Warrant Agent.
Computershare Trust Company, N.A. acts as warrant agent for the September 2013 Warrants.
The March 2015 Warrants
Form.
The March
2015 Warrants were issued under a warrant agreement entered into between us and the warrant agent.
18
Exercisability
. Each March 2015 Warrant may be exercised at any time and from time to time on or
after March 18, 2015 and through and including March 18, 2020. The March 2015 Warrants are exercisable, at the option of each holder, in whole or in part by delivering to us a warrant certificate or book-entry warrant, a duly executed
election to purchase the shares underlying the March 2015 Warrants to be exercised and payment in full of the exercise price within three Trading Days in available funds for the number of shares of Common Stock purchased upon such exercise. If a
registration statement registering the issuance or the resale of the shares of Common Stock underlying the March 2015 Warrants under the Securities Act is not effective or available, the holder may, in its sole discretion, elect to exercise the
March 2015 Warrant through a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of Common Stock determined according to the formula set forth in the March 2015 Warrant. No fractional shares of
Common Stock will be issued in connection with the exercise of a March 2015 Warrant. In lieu of fractional shares, we will pay the holders an amount in cash equal to the fractional amount multiplied by the current market price of our Common Stock or
round up to the next whole share.
Exercise Limitation
. A holder will not have the right to exercise any portion of the March 2015 Warrant if the
holder (together with affiliates) would beneficially own in excess of 4.99% of the number of shares of our Common Stock outstanding immediately after giving effect to the exercise, as such percentage of ownership is determined in accordance with the
terms of the March 2015 Warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon at least 61 days’ prior notice from the holder to us.
Exercise Price.
The March 2015 Warrants are exercisable for a total of 700,320 shares at an exercise price of $41.60 per share. The exercise price is
subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Stock and also upon any distribution of assets, including cash,
stock or other property, to our stockholders.
Transferability
. Subject to applicable laws, the March 2015 Warrants may be offered for sale, sold,
transferred or assigned without our consent.
Exchange Listing
. The March 2015 Warrants are not listed on The NASDAQ Capital Market, any other
national securities exchange or any other nationally recognized trading system.
Fundamental Transactions
. In the event of a “fundamental
transaction,” as described in the March 2015 Warrants and generally including any merger or consolidation with or into another entity, as a result of which the holders of our outstanding voting securities as of immediately prior to such merger
or consolidation hold less than a majority of the outstanding voting securities of the surviving or successor entity as of immediately after such merger or consolidation or a sale, transfer or other disposition of all or substantially all our
property, assets or business to another person or entity, the holders of the March 2015 Warrants will be entitled to receive upon exercise of the March 2015 Warrants the kind and amount of securities, cash or other property that the holders would
have received had they exercised the March 2015 Warrant immediately prior to such fundamental transactions. In addition in the event of any fundamental transaction the holder of the March 2015 Warrant has the right, in lieu of receiving the
consideration described in the preceding sentence, to require us to purchase the March 2015 Warrant for an amount of cash backed on the value of the remaining unexercised portion of the March 2015 Warrant determined in accordance with the Black
Scholes option pricing model.
Rights as a Stockholder
. Except as otherwise provided in the March 2015 Warrants or by virtue of such holders’
ownership of shares of our Common Stock, the holder of a March 2015 Warrant does not have the rights or privileges of a holder of our Common Stock, including any voting rights, until the holder exercises the March 2015 Warrant.
Warrant Agent
. Computershare Trust Company, N.A. acts as warrant agent for the March 2015 Warrants.
The January 2016 Warrants
Form
. The
January 2016 Warrants were issued under a warrant agreement entered into between us and the warrant agent.
19
Exercisability
. The January 2016 Warrants will expire on January 12, 2021. The January 2016
Warrants are exercisable, at the option of each holder, in whole or in part by delivering to us a warrant certificate or book-entry warrant, a duly executed election to purchase the shares underlying the January 2016 Warrants to be exercised and
payment in full of the exercise price within three Trading Days in available funds for the number of shares of Common Stock purchased upon such exercise. If a registration statement registering the issuance or the resale of the shares of Common
Stock underlying the January 2016 Warrants under the Securities Act is not effective or available, the holder may, in its sole discretion, elect to exercise the January 2016 Warrant through a cashless exercise, in which case the holder would receive
upon such exercise the net number of shares of Common Stock determined according to the formula set forth in the January 2016 Warrant. No fractional shares of Common Stock will be issued in connection with the exercise of a January 2016 Warrant. In
lieu of fractional shares, we will pay the holders an amount in cash equal to the fractional amount multiplied by the current market price of our Common Stock or round up to the next whole share.
Exercise Limitation
. A holder will not have the right to exercise any portion of the January 2016 Warrant if the holder (together with affiliates)
would beneficially own in excess of 4.99% of the number of shares of our Common Stock outstanding immediately after giving effect to the exercise, as such percentage of ownership is determined in accordance with the terms of the January 2016
Warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon at least 61 days’ prior notice from the holder to us.
Exercise Price.
The January 2016 Warrants are exercisable for a total of 682,159 shares at an exercise price of $28.40 per share. The exercise price is
subject to adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Stock and also upon any distribution of assets, including cash, stock or
other property, to our stockholders.
Transferability
. Subject to applicable laws, the January 2016 Warrants may be offered for sale, sold,
transferred or assigned without our consent.
Exchange Listing
. The January 2016 Warrants are not listed on The NASDAQ Capital Market, any other
national securities exchange or any other nationally recognized trading system.
Fundamental Transactions
. In the event of a “fundamental
transaction,” as described in the January 2016 Warrants and generally including any merger or consolidation with or into another entity, as a result of which the holders of our outstanding voting securities as of immediately prior to such
merger or consolidation hold less than a majority of the outstanding voting securities of the surviving or successor entity as of immediately after such merger or consolidation or a sale, transfer or other disposition of all or substantially all our
property, assets or business to another person or entity, the holders of the January 2016 Warrants will be entitled to receive upon exercise of the January 2016 Warrants the kind and amount of securities, cash or other property that the holders
would have received had they exercised the January 2016 Warrant immediately prior to such fundamental transactions. In addition in the event of any fundamental transaction the holder of the January 2016 Warrant has the right, in lieu of receiving
the consideration described in the preceding sentence, to require us to purchase the January 2016 Warrant for an amount of cash backed on the value of the remaining unexercised portion of the January 2016 Warrant determined in accordance with the
Black Scholes option pricing model.
Rights as a Stockholder
. Except as otherwise provided in the January 2016 Warrants or by virtue of such
holders’ ownership of shares of our Common Stock, the holder of a January 2016 Warrant does not have the rights or privileges of a holder of our Common Stock, including any voting rights, until the holder exercises the January 2016 Warrant.
Warrant Agent
. Computershare Trust Company, N.A. acts as warrant agent for the January 2016 Warrants.
The JGB Warrants
Form
. The Series A and
Series B JGB Warrants were issued as individual warrant agreements.
20
Exercisability
. Each of the Series A and Series B JGB Warrants became exercisable on
November 10, 2016 and will expire on November 10, 2021. During the applicable exercise period, the JGB Warrants will be exercisable, at the option of the holder, in whole or in part by delivering to us a duly executed exercise notice and
payment in full of the exercise price in immediately available funds for the number of shares of Common Stock purchased upon such exercise. If a registration statement registering the resale of the shares of Common Stock underlying the JGB Warrants
under the Securities Act of 1933, as amended, is not effective or available, the holder may, in its sole discretion, elect to exercise a JGB Warrant through a cashless exercise, in which case the holder would receive upon such exercise the net
number of shares of Common Stock determined according to the formula set forth in the JGB Warrants. No fractional shares of Common Stock will be issued in connection with the exercise of a JGB Warrant. In lieu of a fractional share, we will pay the
holder an amount in cash equal to the fractional amount multiplied by the current market price of our Common Stock or round up to the next whole share.
Exercise Limitation
. A holder will not have the right to exercise any portion of a JGB Warrant if the holder (together with its affiliates) would
beneficially own in excess of 4.99% of the number of shares of our Common Stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the JGB Warrants. However, any
holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon at least 61 days’ prior notice from the holder to us.
Exercise Price.
Each of the Series A and Series B JGB Warrants is exercisable for a total of 100,000 shares at an exercise price of $8.60 per share.
The exercise price is subject to adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Stock and also upon any distribution of assets,
including cash, stock or other property, to our stockholders.
Transferability
. Subject to applicable laws, the JGB Warrants may be offered for
sale, sold, transferred or assigned without our consent.
Exchange Listing
. The JGB Warrants are not listed on The NASDAQ Capital Market, any other
national securities exchange or any other nationally recognized trading system.
Fundamental Transactions
. In the event of a “fundamental
transaction,” as described in the JGB Warrants and generally including any merger or consolidation with or into another entity, as a result of which the holders of our outstanding voting securities as of immediately prior to such merger or
consolidation hold less than a majority of the outstanding voting securities of the surviving or successor entity as of immediately after such merger or consolidation or a sale, transfer or other disposition of all or substantially all our property,
assets or business to another person or entity, the holders of the JGB Warrants will be entitled to receive upon exercise of the JGB Warrants the kind and amount of securities, cash or other property that the holders would have received had they
exercised the JGB Warrants immediately prior to such fundamental transaction.
Rights as a Stockholder
. Except as otherwise provided in the JGB
Warrants, the holder of a JGB Warrant does not have the rights or privileges of a holder of our Common Stock, including any voting rights, until the holder exercises the JGB Warrant.
The July 2016 Warrants
Form.
The July 2016
Warrants were issued as individual warrant agreements.
Exercisability
. The July 2016 Warrants are not exercisable until January 13, 2017 and
will expire on July 13, 2021. During the applicable exercise period, the July 2016 Warrants will be exercisable, at the option of the holder, in whole or in part by delivering to us a duly executed exercise notice and payment in full of the
exercise price in immediately available funds for the number of shares of Common Stock purchased upon such exercise. If a registration statement registering the resale of the shares of Common Stock underlying the July 2016 Warrants under the
Securities Act of 1933, as amended, is not effective or available, the holder may, in its sole discretion, elect to exercise a July 2016 Warrant through a cashless exercise, in which case the holder would receive upon such exercise the net number of
shares of Common Stock determined according to the formula set forth in the July 2016 Warrants.
21
No fractional shares of Common Stock will be issued in connection with the exercise of a July 2016 Warrant. In lieu of a fractional share, we will pay the holder an amount in cash equal to the
fractional amount multiplied by the current market price of our Common Stock or round up to the next whole share.
Exercise Limitation.
A holder
will not have the right to exercise any portion of a July 2016 Warrant if the holder (together with its affiliates) would beneficially own in excess of 4.99% of the number of shares of our Common Stock outstanding immediately after giving effect to
the exercise, as such percentage ownership is determined in accordance with the terms of the July 2016 Warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon at least 61 days’
prior notice from the holder to us.
Exercise Price.
The July 2016 Warrants are exercisable for a total of 700,000 shares at an exercise price of
$13.00 per share. The exercise price is subject to adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Stock and also upon any distribution
of assets, including cash, stock or other property, to our stockholders.
Transferability
. Subject to applicable laws, the July 2016 Warrants may
be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing
. The July 2016 Warrants are not listed on The NASDAQ
Capital Market, any other national securities exchange or any other nationally recognized trading system.
Fundamental Transactions
. In the event
of a “fundamental transaction,” as described in the July 2016 Warrants and generally including any merger or consolidation with or into another entity, as a result of which the holders of our outstanding voting securities as of immediately
prior to such merger or consolidation hold less than a majority of the outstanding voting securities of the surviving or successor entity as of immediately after such merger or consolidation or a sale, transfer or other disposition of all or
substantially all our property, assets or business to another person or entity, the holders of the July 2016 Warrants will be entitled to receive upon exercise of the July 2016 Warrants the kind and amount of securities, cash or other property that
the holders would have received had they exercised the July 2016 Warrants immediately prior to such fundamental transaction.
Pro Rata
Distributions
. In the event that we declare or make any dividend or other distribution of our assets (or rights to acquire our assets) to holders of shares of our Common Stock, by way of return of capital or otherwise (including, without
limitation, by way of a dividend, spin off, reclassification, corporate rearrangement, scheume of arrangement or other similar transaction), at any time after the issuance of the July 2016 Warrants, then the holders will be entitled to participate
in such distribution to the same extent they would have participated had they exercised the July 2016 Warrants immediately prior to the distribution.
Rights as a Stockholder
. Except as otherwise provided in the July 2016 Warrants, the holder of a July 2016 Warrant does not have the rights or
privileges of a holder of our Common Stock, including any voting rights, until the holder exercises the July 2016 Warrant.
The February 2017
Warrants
Form
. The February 2017 Warrants were issued under a warrant agreement entered into between us and the warrant agent.
Exercisability
. The February 2017 Warrants will expire on February 13, 2022. The February 2017 Warrants will be exercisable, at the option of each
holder, in whole or in part by delivering to us a warrant certificate or book-entry warrant, a duly executed election to purchase the shares underlying the February 2017 Warrants to be exercised and payment in full of the exercise price within three
Trading Days in available funds for the number of shares of Common Stock purchased upon such exercise. If a registration statement registering the issuance or the resale of the shares of Common Stock underlying the February 2017 Warrants under the
Securities Act is not effective or available, the holder may, in its sole discretion, elect to exercise the February 2017 Warrant through a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of
Common Stock
22
determined according to the formula set forth in the February 2017 Warrant. No fractional shares of Common Stock will be issued in connection with the exercise of a February 2017 Warrant. In lieu
of fractional shares, we will pay the holders an amount in cash equal to the fractional amount multiplied by the current market price of our Common Stock or round up to the next whole share.
Exercise Limitation
. A holder will not have the right to exercise any portion of the February 2017 Warrant if the holder (together with affiliates)
would beneficially own in excess of 4.99% of the number of shares of our Common Stock outstanding immediately after giving effect to the exercise, as such percentage of ownership is determined in accordance with the terms of the February 2017
Warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon at least 61 days’ prior notice from the holder to us.
Exercise Price.
The February 2017 Warrants are exercisable for a total of 17,000,000 shares at an exercise price of $1.10 per share. The exercise price
is subject to adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Stock and also upon any distribution of assets, including cash, stock or
other property, to our stockholders.
Transferability
. Subject to applicable laws, the February 2017 Warrants may be offered for sale, sold,
transferred or assigned without our consent.
Exchange Listing
. The February 2017 Warrants are not listed on The NASDAQ Capital Market, any other
national securities exchange or any other nationally recognized trading system.
Fundamental Transactions
. In the event of a “fundamental
transaction,” as described in the February 2017 Warrants and generally including any merger or consolidation with or into another entity, as a result of which the holders of our outstanding voting securities as of immediately prior to such
merger or consolidation hold less than a majority of the outstanding voting securities of the surviving or successor entity as of immediately after such merger or consolidation or a sale, transfer or other disposition of all or substantially all our
property, assets or business to another person or entity, the holders of the February 2017 Warrants will be entitled to receive upon exercise of the February 2017 Warrants the kind and amount of securities, cash or other property that the holders
would have received had they exercised the February 2017 Warrant immediately prior to such fundamental transactions. In addition in the event of any fundamental transaction the holder of the February 2017 Warrant has the right, in lieu of receiving
the consideration described in the preceding sentence, to require us to purchase the February 2017 Warrant for an amount of cash backed on the value of the remaining unexercised portion of the February 2017 Warrant determined in accordance with the
Black Scholes option pricing model.
Rights as a Stockholder
. Except as otherwise provided in the February 2017 Warrants or by virtue of such
holders’ ownership of shares of our Common Stock, the holder of a February 2017 Warrant does not have the rights or privileges of a holder of our Common Stock, including any voting rights, until the holder exercises the February 2017 Warrant.
Warrant Agent
. Computershare Trust Company, N.A. acts as warrant agent for the February 2017 Warrants.
Anti-Takeover Effects of Certain Provisions of Delaware Law and our Charter Documents
Certain provisions, summarized below, of Delaware law, along with certain provisions of our Certificate of Incorporation and our By-laws may have the effect of
delaying, deferring or discouraging another person from acquiring control of our Company. However, these provisions could have the effect of deferring hostile takeovers or delaying, discouraging or preventing attempts to acquire us, which could
deprive our stockholders of opportunities to sell their shares of Common Stock at prices higher than prevailing market prices.
Delaware Law
We are subject to the provisions of Section 203 of the General Corporation Law of the State of Delaware (the “
DGCL
”)
regulating corporate takeovers. In general, those provisions prohibit a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years following the date that the stockholder became an
interested stockholder, unless:
|
|
•
|
|
the transaction is approved by the board of directors before the date the interested stockholder attained that status;
|
23
|
|
•
|
|
upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the
time the transaction commenced, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned by (1) persons who are directors and also officers
and (2) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
|
|
|
•
|
|
on or after the date of the transaction, the transaction is approved by the board of directors and authorized at a meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of
the outstanding voting stock that is not owned by the interested stockholder.
|
In general, Section 203 of the DGCL defines a business
combination to include the following:
|
|
•
|
|
any merger or consolidation involving the corporation and the interested stockholder;
|
|
|
•
|
|
any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder;
|
|
|
•
|
|
subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder;
|
|
|
•
|
|
any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation owned by the interested stockholder; or
|
|
|
•
|
|
the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation.
|
In general, Section 203 of the DGCL defines an “interested stockholder” as any entity or person who, together with the person’s affiliates
and associates, owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
A Delaware corporation may opt out of this provision by express provision in its original certificate of incorporation or by amendment to its certificate of
incorporation or bylaws approved by its stockholders. However, we have not opted out of this provision. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire
our Company.
Our Certificate of Incorporation and By-laws
Our Certificate of Incorporation and By-laws include a number of provisions that may discourage, delay or prevent a merger, acquisition or other change in
control of Galena, even if such a change in control would be beneficial to our stockholders. These provisions include authorizing the board of directors to fix the number of directors, and establishing advance notice requirements for stockholder
proposals that can be acted on at stockholder meetings and nominations to the board of directors.
24
LEGAL MATTERS
The validity of the shares of Common Stock underlying the July 2016 Warrants has been passed upon by Thomas J. Knapp, Interim General Counsel of Galena
Biopharma, Inc. At the time of issuance of such opinion on October 15, 2016, Mr. Knapp was employed by Galena Biopharma, Inc., and as of September 30, 2016, he owned and had rights to acquire an aggregate of less than 0.1 % of
our Common Stock.
The validity of the shares of Common Stock underlying the JGB Warrants has been passed upon by Fredrikson & Byron P.A.,
Minneapolis, Minnesota.
The validity of the shares of Common Stock underlying the December 2012 Warrants, the September 2013 Warrants, the March 2015
Warrants, the January 2016 Warrants and the February 2017 Warrants has been passed upon by TroyGould PC, Los Angeles, California. At the time of issuance of such opinion on December 4, 2015, Sanford J. Hillsberg, the Chairman of our Company,
was an attorney with TroyGould PC, and further, as of September
30, 2015, TroyGould PC owned 63,941 shares of our Common Stock.
EXPERTS
Our consolidated financial statements appearing in our Annual Report on Form 10-K for the year ended December 31, 2016, and the effectiveness of our
internal control over financial reporting as of December 31, 2016, have been audited by Moss Adams LLP, an independent registered public accounting firm, as stated in their report, which is incorporated herein by reference. Our consolidated
financial statements have been so incorporated by reference in reliance upon Moss Adam LLP’s report given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We are a reporting company and file annual, quarterly and current reports, proxy statements and other information with the SEC. We have filed with the SEC a
registration statement on Form S-1 under the Securities Act of 1933, with respect to the Common Stock being offered under this prospectus. This prospectus does not contain all of the information set forth in the registration statement and the
exhibits to the registration statement. For further information with respect to us and the securities being offered under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration
statement. You may read and copy the registration statement, as well as our reports, proxy statements and other information, at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC
at 1-800-SEC-0330 for more information about the operation of the Public Reference Room. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file
electronically with the SEC. The SEC’s Internet site can be found at
http://www.sec.gov
. You can also obtain copies of materials we file with the SEC from our website found at
www.galenabiopharma.com
under “Investors.”
Except for the documents incorporated by reference in this prospectus, the information contained on our website is not part of this prospectus and should not be relied upon in connection with making an investment decision.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” information into this prospectus, which means that we can disclose important information to you by
referring you to another document filed separately with the SEC. The documents incorporated by reference into this prospectus contain important information that you should read about us.
The following documents are incorporated by reference into this prospectus:
|
|
a.
|
our Annual Report on Form 10-K for the fiscal year ended December 31, 2016, filed with the SEC on March 15, 2017;
|
|
|
b.
|
our Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2017, filed with the SEC on May 10, 2017;
|
25
|
|
c.
|
our Current Reports on Form 8-K, which were filed with the SEC on January 9, 2017, January 31, 2017, February 7, 2017, February 10, 2017, February 21,
2017, March 13, 2017, March 23, 2017, March 28, 2017, April 3, 2017, May 2, 2017, June 6, 2017, June 12, 2017, July 6, 2017 and July 11, 2017;
|
|
|
d.
|
our Proxy Statements on Schedule 14A, filed with the SEC on April 20, 2017, May 31, 2017 and June 9, 2017; and
|
|
|
e.
|
the description of our Common Stock set forth in our in our registration statement on Form 8-A (File No. 001-33958), filed with the SEC on February 8, 2008, including any amendments or reports filed for the
purpose of updating such description.
|
All documents subsequently filed by us (other than current reports furnished under Item 2.02 or
Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items unless such Form 8-K expressly provides to the contrary) with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Securities
Exchange Act of 1934, as amended, including those made after the date of the initial filing of the registration statement of which this prospectus forms a part and prior to effectiveness of such registration statement, until we file a
post-effective amendment that indicates the termination of the offering of the Common Stock made by this prospectus are deemed to be incorporated by reference into this prospectus. Such future filings will become a part of this prospectus from the
respective dates that such documents are filed with the SEC.
Any statement contained herein or in a document incorporated or deemed to be incorporated by
reference herein shall be deemed to be modified or superseded for purposes hereof to the extent that such statement contained herein or in any other subsequently filed document, which is also incorporated or deemed to be incorporated herein,
modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
You may obtain copies of any or all of the documents incorporated by reference in this prospectus from us free of charge on our website at
www.galenabiopharma.com
under “Investors” or by requesting them in writing or by telephone at the following address:
Galena Biopharma, Inc.
2000 Crow
Canyon Place, Suite 380
San Ramon, CA 94583
Attention: Investor Relations
Telephone: (855) 855-4253
26

GALENA BIOPHARMA, INC.
18,732,742 Shares of Common Stock Issuable Upon Exercise of Outstanding
Warrants
800,000 Shares
of Common Stock for Resale by Selling Stockholders
PROSPECTUS
, 2017
We have not authorized any dealer, salesperson or other person to give any information or to make any representations not contained in this prospectus. You
must not rely on any unauthorized information. This prospectus is not an offer to sell these securities in any jurisdiction where an offer or sale is not permitted.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth all expenses payable by the Registrant in connection with the sale of the Common Stock being registered. All the amounts shown
are estimates except for the registration fee.
|
|
|
|
|
|
|
SEC registration fee
|
|
$
|
—*
|
|
|
Legal fees and expenses
|
|
$
|
100,000
|
|
|
Accounting fees and expenses
|
|
$
|
15,000
|
|
|
Filing Fees and miscellaneous expenses
|
|
$
|
25,000
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
140,000
|
|
|
|
|
|
|
|
Item 14. Indemnification of Directors and Officers
Section 145 of the General Corporation Law of the State of Delaware (the “
DGCL
”) provides that a corporation may indemnify any
person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, other than an action by or in the right of the
corporation, by reason of the fact that the person is or was a director, officer, employee or agent of the corporation or is or was serving at the corporation’s request as a director, officer, employee or agent of another corporation,
partnership, joint venture, trust or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with the action, suit or proceeding
if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe the
person’s conduct was unlawful. The power to indemnify applies to actions brought by or in the right of the corporation as well, but only to the extent of expenses, including attorneys’ fees, actually and reasonably incurred by the person
in connection with the defense or settlement of the action or suit if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation and except that no indemnification
shall be made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that a court of competent jurisdiction shall determine that such indemnity is
proper.
Section 145(g) of the DGCL provides that a corporation shall have the power to purchase and maintain insurance on behalf of its officers,
directors, employees and agents, against any liability asserted against and incurred by such persons in any such capacity.
Section 102(b)(7) of the
DGCL provides that a corporation may eliminate or limit the personal liability of a director to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, provided that such provision shall not eliminate or
limit the liability of a director (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation
of law, (iii) under Section 174 of the DGCL, or (iv) for any transaction from which the director derived an improper personal benefit. No such provision shall eliminate or limit the liability of a director for any act or omission
occurring prior to the date when such provision becomes effective.
Our Amended and Restated Certificate of Incorporation provides that our directors
shall not be liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director, except to the extent that the exculpation from liabilities is not permitted under the DGCL as in effect at the time such liability is
determined. In addition, our Amended and Restated Certificate of Incorporation provides that we shall, to the maximum extent permitted by the DGCL, indemnify each person who was or is a party or is threatened to be made a party to any threatened,
pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he is or was, or has agreed to become, our director or officer, or is or was serving, or has agreed to serve, at
our request, as a director, officer, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, including any employee benefit plan (all such persons being referred to hereafter
as an “
Indemnitee
”), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and
reasonably incurred by him or on his behalf in connection with such action, suit or proceeding and any appeal therefrom, if he acted in good faith and in a manner he reasonably believed to be in, or not opposed to, our best interests, and, with
respect to
II-1
any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful, and that we shall not indemnify an Indemnitee seeking indemnification in connection with any
action, suit, proceeding, claim or counterclaim, or part thereof, initiated by the Indemnitee unless the initiation thereof was approved by our board of directors.
Our Amended and Restated Certificate of Incorporation also provides that any indemnification under the preceding paragraph (unless ordered by a court) shall
be made by us only as authorized in the specific case upon a determination that indemnification is proper in the circumstances because such person has either met the applicable standard of conduct set forth in this paragraph and that the amount
requested has been actually and reasonably incurred. Such determination shall be made:
|
|
1.
|
by a majority vote of the directors who are not parties to such action, suit or proceeding, even though less than a quorum; or
|
|
|
2.
|
by a committee of such directors designated by a majority vote of such directors, even though less than a quorum; or
|
|
|
3.
|
if there are no such directors, or if such directors so direct, by independent legal counsel in a written opinion; or
|
|
|
4.
|
by the holders of our Common Stock.
|
Our Amended and Restated Certificate of Incorporation provides that we
may purchase and maintain insurance policies on behalf of our directors and officers against specified liabilities for actions taken in its capacities as such, including liabilities under the Securities Act. We have obtained directors’ and
officers’ liability insurance to cover liabilities our directors and officers may incur in connection with their services to us.
Item 15.
Recent Sales of Unregistered Securities
Since July 1, 2014, Galena has issued the following securities that were not registered under the
Securities Act of 1933, as amended, or the Securities Act:
Securities Purchase Agreement dated July 7, 2016
On July 7, 2016, Galena entered into a Securities Purchase Agreement (the “
Purchase Agreement
”) with certain investors (the
“
Investors
”) providing for the issuance and sale by the Company to the Investors of an aggregate of approximately $12,600,000 of registered and unregistered securities of the Company (the “
Offering
”).
Pursuant to the Purchase Agreement, the Company agreed, among other things, to issue and sell to the Investors an aggregate of 1,400,000 shares (the “
Shares
”) of the Company’s common stock, par value $0.0001 per share
(the “
Common Stock
”) at a purchase price per share of $9.00 in a registered direct offering and warrants to purchase up to 700,000 shares of common stock with an exercise price of $13.00 per share (the
“
Warrants
”) in a concurrent private placement. The warrants were initially exercisable six months and one day following issuance and have a term of five years from the date of issuance.
The Warrants were issued by the Company to the Investors in a transaction exempt from registration under the Securities Act in reliance on the exemptions
provided by Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder. The offering of the Warrants did not involve a public offering, and no general solicitation or advertisement was made in connection with the offering
of the Warrants.
July 6, 2016 Agreement to Settle Claims
On July 6, 2016, Galena agreed, in connection with a negotiated settlement of outstanding claims in a certain matter, to issue a total of 156,250 shares
of the Company’s common stock to five parties in a settlement. The parties in the litigation and settlement discussions were sophisticated persons represented by professional advisers. The Company issued the shares without registration in a
private placement in reliance on the exemption provided by Section 4(a)(2) of the Securities Act of 1933, as amended (the “
Securities Act
”), and in reliance on similar exemptions under applicable state laws.
December 3, 2016 Agreement to Settle Putative Class Action Lawsuit
On December 3, 2015, the Company also agreed in principal to resolve and settle the securities putative class action lawsuit, In re Galena Biopharma, Inc.
Securities Litigation, Civil Action No. 3:14-cv-00367-SI, against the Company, certain of the Company’s current and former officers and directors and other defendants in the United States District Court for the District of Oregon.
Following the hearing on June 23, 2016, on June 24, 2016, the U.S. District Court for the District of Oregon entered a final order and partial judgment in In re Galena Biopharma, Inc. Securities Litigation, granting final approval of the
settlement.
II-2
On the same day, the Court also issued an opinion and order awarding attorney’s fees of $4.5 million plus costs, which is paid out of the settlement funds. The agreement provides for a
settlement payment of $20 million to the class and the dismissal of all claims against the Company and the other defendants in connection with the consolidated federal securities class actions. Of the $20 million settlement payment to the class,
$16.7 million was paid by the Company’s insurance carriers and $2.3 million in cash was paid by the Company on July 1, 2016. Pursuant to the court order, the Company issued 24,003 shares of its common stock as part of the settlement on
July 6, 2016 without registration in reliance on an exemption provided by Section 3(a)(10) of the Securities Act.
Amendment Agreement dated
August 22, 2016 with Respect to Debenture Issued to JGB (Cayman) Newton Ltd.
On May 10, 2016, Galena entered into a Securities Purchase
Agreement (the “
Securities Purchase Agreement
”), with JGB (Cayman) Newton Ltd. (the “
Purchaser
”) pursuant to which the Company sold to Purchaser, at a 6.375% original issue discount, a $25,530,000
Senior Secured Debenture (the “
Initial Debenture
”) and warrants to purchase up to 100,000 shares of the Common Stock. Net proceeds to the Company from sale of the Initial Debenture and warrants, after payment of commissions
and legal fees, were approximately $23,400,000. The Initial Debenture contained no conversion features to shares of Common Stock. Unless noted otherwise, all per share prices and per share amounts discussed herein have been adjusted to take into
account the November 11, 2016 1-for-20 reverse split of the Common Stock (the “
Reverse Stock Split
”).
The Initial Debenture
carried an interest only period of six months following which the holder of the Initial Debenture had the right, at its option, to require the Company to redeem up to $1,100,000 of the outstanding principal amount of the Initial Debenture per
calendar month. The Company is required to promptly, but in any event no more than three trading days after the holder delivers a redemption notice to the Company, pay the applicable redemption amount in cash or, at the Company’s election and
subject to certain conditions, in shares of Common Stock. If the Company elects to pay the redemption amount in shares of Common Stock, then the shares will be delivered at the lesser of A) 7.5% discount to the average of the 3 lowest volume
weighted average prices over the prior 20 trading days or B) a 7.5% discount to the prior trading day’s volume weighted average price (the “
Stock Payment Price
”). Pursuant to the Initial Debenture, the Company may only
opt for payment in shares of Common Stock if certain equity conditions are met or waived, including, among others, that the volume weighted price of the Common Stock be at least $15.00 (the “
Original Minimum Price
Condition
”).
Under the Initial Debenture, if the interim analysis of the PRESENT Trial resulted in a discontinuation of the study (which it
did), the holder of the Initial Debenture had the right to require the Company to prepay in cash all, or any portion, of the outstanding principal amount of the Initial Debenture funded in cash by the holder on the closing date, plus all accrued and
unpaid interest. If the holder elected such prepayment of the Initial Debenture, then the number of shares subject to the warrants issued to the holder would have been reduced in proportion to the percentage of principal and accrued interest
required to be prepaid by the Company. Upon the closing of the sale of the Initial Debenture, the Purchaser received a Series A Warrant to purchase 50,000 shares of Common Stock at an exercise price of $30.20, exercisable for five years from
issuance (the “
Series A Warrant
”). In accordance with the terms of the Securities Purchase Agreement, the Purchaser also received a warrant to purchase 50,000 shares of Common Stock in connection with the Company’s
announcement of the results of the interim analysis of its PRESENT Trial in June 2016 (the “
Series B Warrant
”). The Series B Warrant has an exercise price of $8.60, 120% of the volume-weighted average price of the Common
Stock on the date of the announcement of the results of the interim analysis.
Armentum Partners, LLC (the “
Placement Agent
”)
acted as the placement agent in the offering of the Initial Debenture and the Company agreed to pay the Placement Agent a fee equal to 2% of the funds received from the sale of the Initial Debenture. The Company paid half of the placement fee upon
funding and the other half was paid at the time the PRESENT Trial was discontinued. The Debenture was not prepaid in connection with such discontinuation.
The Initial Debenture was amended and restated in its entirety on August 22, 2016 (as so amended, the “
Debenture
”) pursuant to an
Amendment Agreement, dated August 22, 2016, among the Company, the Purchaser and JGB Collateral LLC (the “
Amendment Agreement
”). Interest on the Debenture is payable at the end of each month based on the outstanding
principal. The Debenture matures on November 10, 2018, and accrues interest at 9% per year. In addition, on the maturity date of the Debenture (or such earlier date that the principal amount of the Debenture is paid in full by acceleration
or otherwise) a fixed amount, which shall be deemed interest under the Debenture, equal to $765,900, will be due and payable to the holder of the Debenture on such date in, at the option of the Company, cash and, subject to the same conditions for
the payment of interest in shares of Common Stock, shares of Common Stock or a combination of cash and Common Stock.
The Company’s obligations under
the Debenture are secured under a Security Agreement by a senior lien on all of the Company’s assets, including all of the Company’s interests in its consolidated subsidiaries.
II-3
After giving effect to the Amendment Agreement, the Debenture contains the following modified and/or
additional terms, among others:
|
|
•
|
|
With respect to interest accruing on the outstanding principal amount under the Debenture for the period prior to November 10, 2016, the Company was permitted to satisfy such interest payments in kind by adding
such amount to the outstanding principal.
|
|
|
•
|
|
The Purchaser can from time to time during the term of the Debenture require the Company to prepay in cash all or a portion of the outstanding principal plus accrued and unpaid interest (the “
Outstanding
Amount
”) on written notice to the Company, provided, that such prepayment amount shall not exceed the lesser of $18,500,000 and the Outstanding Amount. If the holder elects such prepayment of the Debenture, then the number of shares
subject to the warrants issued to the holder will be reduced in proportion to the percentage of principal and accrued interest required to be prepaid by the Company. In addition, the Company shall have the right to prepay in cash all (but not less
than all) of the Outstanding Amount (1) at any time after November 10, 2017, or (2) upon a “change of control” (as such term is used un the Debenture), in each case with a 10% premium on the Outstanding Amount.
|
|
|
•
|
|
The Purchaser shall continue to have the right, which commenced on November 10, 2016, to require the Company to redeem the Outstanding Amount, except that the maximum monthly amount of such redemptions was
increased from $1,100,000 to $1,500,000; provided, that if the trading price of Common Stock is at least $8.00 per share (as may be further adjusted appropriately for stock splits, combinations or similar events) during such calendar month, then
such monthly maximum redemption amount may be increased to $2,200,000 at the Purchaser’s election and if the Company has already elected to satisfy such redemptions in shares of Common Stock. In addition, notwithstanding the foregoing
limitations on the monthly redemption amount, the Purchaser may elect up to three times in any 12-month period to increase the monthly maximum to $2,500,000.
|
|
|
•
|
|
Among the various conditions that must be satisfied (or waived) in order for the Company to be able to elect to satisfy the monthly redemption amounts in shares of Common Stock, the Original Minimum Price Condition of
$15.00 was decreased to a volume-weighted average price of $4.00 per share (the “
Amended Minimum Price Condition
”).
|
|
|
•
|
|
Following November 10, 2016, the Purchaser may elect to convert any portion of the Outstanding Amount into shares of Common Stock at a fixed price of $12.00 per share (as adjusted appropriately for stock splits,
combinations or similar events).
|
|
|
•
|
|
Under the Initial Debenture, the Company was required to maintain a minimum of $24,000,000 of unencumbered cash in a restricted account as security for its obligations under the Initial Debenture. Such minimum amount
has been reduced to the lesser of $18,500,000 or the Outstanding Amount.
|
In addition, in accordance with the terms of the Amendment
Agreement, the exercise price of the Series A Warrant was reduced from $30.20 per share to $8.60 per share (as may be further adjusted appropriately for stock splits, combinations or similar events).
On December 14, 2016, the Company and the Purchaser entered into a waiver (the “
First Waiver
”) pursuant to which, as contemplated
by the Debenture, the Purchaser waived with respect to the calendar months of December 2016, January 2017, February 2017 and March 2017 (collectively, the “
First Specified Months
”) the Amended Minimum Price
Condition, provided that, among other things, with respect to the First Specified Months, the volume weighted average price of the Common Stock was not less than $1.00 and the Company’s cash on hand exceeded the outstanding principal amount of
the Debenture by $10 million. Furthermore, the First Waiver set out a monthly amount to be redeemed for each of the First Specified Months equal to $1,500,000 and amended the Debenture to require the Company to withdraw all cash and/or cash
equivalents in excess of $18,500,000 from certain accounts and deposit such funds into an account in a form acceptable to the Purchaser, to be executed by the Company, U.S. Bank, N.A. and SVB Asset Management such that the Company requires the prior
written consent of the Purchaser for certain withdrawals. The First Waiver amends the Debenture to grant the Purchaser the right to redeem any portion of the outstanding principal amount of the Debenture in Common Stock if the price per share of
Common Stock on a principal trading market at any point in time of any trading day exceeds the closing price per share of the Common Stock on the immediately preceding trading day by more than 25%.
On April 1, 2017, the Company and Purchaser entered into a waiver (the “
Second Waiver
”) pursuant to which, as contemplated by the
Debenture, the Purchaser waived with respect to the calendar months of April 2017, May 2017, June 2017, July 2017, August 2017 and September 2017 (collectively, the “
Second Specified Months
”) the Amended
Minimum Price Condition, provided that, among other things, with respect to the Second Specified Months, the volume weighted average price of the
II-4
Common Stock is not less than $0.30 and the Company’s cash on hand exceeds the outstanding principal amount of the Debenture by $10 million.
On May 1, 2017, the Purchaser, the Company and the guarantors of the Company’s obligations under the Debenture entered into an amendment agreement
(the “
2017 Amendment Agreement
”) pursuant to which the Purchaser may, from time to time, at the Purchaser’s option waive the Amended Minimum Price Condition; provided, however, the Purchaser cannot waive the Amended
Minimum Price Condition to the extent that the resulting Stock Payment Price would be less than $0.35 per share as a result of any such waiver (the “
Minimum Stock Payment Price Condition
”). The 2017 Amendment Agreement
further provides that, in the event of any Equity Conditions Failure (as such term is defined in the Debenture) that is not, or cannot be as a result of the 2017 Amendment Agreement, waived by the Purchaser, the Company shall honor the holder
redemption amounts in cash or, at the Company’s election, with the prior written consent of the Purchaser, deliver aggregate consideration in shares of Common Stock and cash in satisfaction of the applicable holder redemption amount as follows:
(i) the number of shares of Common Stock equal to the quotient obtained by dividing such holder redemption amount and $0.35 (each such share having a deemed value per share at the Stock Payment Price that would have been in effect but for the
Minimum Stock Payment Price Condition of $0.35 per share) and (ii) cash equal to the difference between the holder redemption amount and the aggregate deemed value of the shares of Common Stock delivered in clause (i).
As of July 10, 2017, (i) there were 37,505,644 shares of Common Stock outstanding and (ii) 9,131,868 shares of Common Stock had been issued by
the Company pursuant to the terms of the Debenture. Assuming all the shares issuable pursuant to the terms of the Debenture subsequent to July 10, 2017 are issued at a Stock Payment Price of $0.35, the lowest Stock Payment Price permitted under
the Minimum Stock Price Payment Condition, the Company estimates that the maximum number of shares of Common Stock that the Company could issue pursuant to the terms of the Debenture subsequent to July 10, 2017 is 45,000,000.
The Debenture, warrants and shares of Common Stock issued (or to be issued) to the Purchaser pursuant to the Debenture were sold to the Purchaser in reliance
on the exemption from registration under Section 4(a)(2) of the Securities Act of 1933, as amended (the “
Securities Act
”) and the Regulation D (Rule 506) promulgated under the Securities Act. The Common Stock to be
issued upon exercise, if any, of the Series A Warrant will be sold in reliance on the exemption from registration under Section 4(a)(2) of the Securities Act. The Purchaser has represented that it was an accredited investor (as defined by Rule
501 promulgated under the Securities Act) at the time it was offered the Debenture, the shares of Common Stock issuable pursuant to the Debenture and the warrants, and that it will be an accredited investor on each date it exercises the Series A
Warrant and the Series B Warrant. The Company did not engage in any general solicitation in connection with the sale of securities pursuant to the Securities Purchase Agreement. The Sale of Securities Pursuant to the Securities Purchase Agreement
did not, and the sale of Common Stock upon the exercise of the warrants will not, involve a public offering.
Item 16. Exhibits and Financial
Statement Schedules
(a) Exhibits
The
exhibits to the registration statement are listed in the Exhibit Index attached hereto and incorporated by reference herein.
(b) Financial
Statement Schedules
All financial statement schedules are omitted because they are not applicable, the required information is not present in
amounts sufficient to require submission of such schedules, or the information is included in the Registrant’s financial statements or notes thereto.
Item 17. Undertakings
|
|
(a)
|
The undersigned Registrant hereby undertakes:
|
|
|
(1)
|
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
|
|
|
(i)
|
To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
|
|
|
(ii)
|
To reflect in the prospectus any facts or events arising after the effective date of the registration statement
(or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement.
|
II-5
|
|
Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any
deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a
20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
|
|
|
(iii)
|
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
|
Provided
,
however
, that Paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) of this section do not apply if the
registration statement is on Form S-1 and the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the Registrant pursuant to Section 13 or
Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement.
|
|
(2)
|
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and
the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
|
|
|
(3)
|
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
|
|
|
(b)
|
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise,
the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for
indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted
by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate
jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933and will be governed by the final adjudication of such issue.
|
|
|
(c)
|
The undersigned registrant hereby undertakes that:
|
|
|
(1)
|
For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a
form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
|
|
|
(2)
|
For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the
securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
|
II-6
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this Registration Statement to be signed on its behalf by the
undersigned, thereunto duly authorized in the City of San Ramon, State of California, on July 11, 2017.
|
|
|
|
|
GALENA BIOPHARMA, INC.
|
|
|
|
|
By:
|
|
/s/ Stephen F. Ghiglieri
|
|
|
|
Stephen F. Ghiglieri
Interim Chief Executive
Officer & Chief Financial Officer
|
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS
, that each person whose signature appears below constitutes and appoints Stephen F. Ghiglieri and Thomas J. Knapp
and each or any one of them, as his true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments (including
post-effective amendments) to this Registration Statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents,
and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and
confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on
the dates indicated.
|
|
|
|
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
/s/ Stephen F. Ghiglieri
Stephen F. Ghiglieri
|
|
Interim Chief Executive Officer & Chief Financial Officer
(Principal Executive and Financial Officer and Principal Accounting Officer)
|
|
July 11, 2017
|
|
|
|
|
|
/s/ William Ashton
William Ashton
|
|
Director
|
|
July 11, 2017
|
|
|
|
|
|
/s/ Richard Chin, M.D.
Richard Chin, M.D.
|
|
Director
|
|
July 11, 2017
|
|
|
|
|
|
/s/ Irving M. Einhorn
Irving M. Einhorn
|
|
Director
|
|
July 11, 2017
|
|
|
|
|
|
/s/ Stephen S. Galliker
Stephen S. Galliker
|
|
Director
|
|
July 11, 2017
|
|
|
|
|
|
/s/ Mary Ann Gray, Ph.D.
Mary Ann Gray, Ph.D.
|
|
Director
|
|
July 11, 2017
|
|
|
|
|
|
/s/ Sanford J. Hillsberg
Sanford J. Hillsberg
|
|
Director
|
|
July 11, 2017
|
|
|
|
|
|
/s/ Rudolph Nisi, M.D.
Rudolph Nisi, M.D.
|
|
Director
|
|
July 11, 2017
|
INDEX TO EXHIBITS
|
|
|
|
|
|
|
|
|
|
|
Exhibit
Number
|
|
Description
|
|
Registrant’s
Form
|
|
Date Filed with the
SEC
|
|
Exhibit
Number
|
|
|
|
|
|
|
|
2.1
|
|
Unit Purchase Agreement, dated as of January 12, 2014, between Galena Biopharma, Inc. and Mills Pharmaceuticals, LLC
|
|
10-Q
|
|
March 17, 2014
|
|
2.1
|
|
|
|
|
|
|
|
3.1
|
|
Amended and Restated Certificate of Incorporation of Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation), amended as of February 10, 2017
|
|
10-K
|
|
March 15, 2017
|
|
3.1
|
|
|
|
|
|
|
|
3.2
|
|
Certificate of Ownership and Merger
|
|
8-K
|
|
September 26, 2011
|
|
3.1
|
|
|
|
|
|
|
|
3.3
|
|
Amended and Restated By-Laws of Galena Biopharma, Inc.
|
|
10-K
|
|
March 15, 2017
|
|
3.3
|
|
|
|
|
|
|
|
4.1
|
|
Form of Common Stock Certificate
|
|
10-K
|
|
March 15, 2017
|
|
4.1
|
|
|
|
|
|
|
|
4.2
|
|
Form of Warrant Agreement by and Galena Biopharma, Inc., Computershare Inc. and Computershare Trust Company, N.A.
|
|
8-K
|
|
September 18, 2013
|
|
4.1
|
|
|
|
|
|
|
|
4.3
|
|
Form of Warrant Agreement by and among Galena Biopharma, Inc., Computershare Inc. and Computershare Trust Company, N.A.
|
|
8-K
|
|
January 7, 2016
|
|
4.1
|
|
|
|
|
|
|
|
4.4
|
|
Form of Warrant, to be issued by Galena Biopharma, Inc. to the Investors on July 13, 2016
|
|
8-K
|
|
July 8, 2016
|
|
4.1
|
|
|
|
|
|
|
|
4.5
|
|
Form of Warrant Agreement, including the Form of Warrant, issued by Galena Biopharma, Inc. to the Investors on February 13, 2017
|
|
8-K
|
|
February 10, 2017
|
|
4.1
|
|
|
|
|
|
|
|
4.6
|
|
9% Original Issues Discount Senior Secured Debenture of Galena Biopharma, Inc.
|
|
10-Q
|
|
May 10, 2016
|
|
4.1
|
|
|
|
|
|
|
|
4.7
|
|
Securities Purchase Agreement dated May 10, 2016 between Galena Biopharma, Inc. and Purchasers
|
|
10-Q
|
|
August 9, 2016
|
|
10.1
|
|
|
|
|
|
|
|
4.8
|
|
Waiver dated April 1, 2017 to the Securities Purchase Agreement, dated as of May 10, 2016 by and between Galena Biopharma Inc. and JGB Newton, Ltd.
|
|
8-K
|
|
April 3, 2017
|
|
10.1
|
|
|
|
|
|
|
|
4.9
|
|
Waiver dated December 14, 2016 to the Securities Purchase Agreement, dated as of May 10, 2016 by and between Galena Biopharma Inc. and JGB Newton, Ltd.
|
|
8-K
|
|
February 7, 2017
|
|
10.3
|
|
|
|
|
|
|
|
4.10
|
|
Amended and Restated 9% Original Issue Discount Senior Secured Debenture Due November 10, 2018, issued to JGB (Cayman) Newton Ltd. as of August 22, 2016
|
|
8-K
|
|
August 23, 2016
|
|
4.1
|
|
|
|
|
|
|
|
4.11
|
|
Series A Common Stock Purchase Warrant assigned to JGB (Cayman) Newton Ltd.
|
|
10-Q
|
|
May 10, 2016
|
|
4.2
|
|
|
|
|
|
|
|
4.12
|
|
Series B Common Stock Purchase Warrant assigned to JGB (Cayman) Newton Ltd.
|
|
10-Q
|
|
May 10, 2016
|
|
4.3
|
|
|
|
|
|
|
|
4.13
|
|
Subsidiary Guarantee dated May 10, 2016 between Galena Biopharma, Inc. and JGB Collateral LLC
|
|
10-Q
|
|
August 9, 2016
|
|
10.2
|
|
|
|
|
|
|
|
4.14
|
|
Registration Rights Agreement dated May 10, 2016 between Galena Biopharma, Inc. and Purchasers
|
|
10-Q
|
|
August 9, 2016
|
|
10.3
|
|
|
|
|
|
|
|
4.15
|
|
Security Agreement dated May 10, 2016 between Galena Biopharma, Inc. and JGB Collateral LLC
|
|
10-Q
|
|
August 9, 2016
|
|
10.4
|
|
|
|
|
|
|
|
4.16
|
|
Amendment Agreement between Galena Biopharma, Inc. and JGB (Cayman) Newton Ltd. dated August 22, 2016
|
|
8-K
|
|
August 23, 2016
|
|
10.1
|
|
|
|
|
|
|
|
4.17
|
|
Form of Securities Purchase Agreement, dated as of July 7, 2016, by and among Galena Biopharma, Inc. and the Investors
|
|
8-K
|
|
July 8, 2016
|
|
10.2
|
|
|
|
|
|
|
|
4.18
|
|
First Amendment to Securities Purchase Agreement, dated as of July 12, 2016, by and between Galena Biopharma, Inc. and each purchaser identified on the signature pages therein, amending the Securities Purchase Agreement, dated as of
July 7, 2016, by and among the Company and the purchasers named therein
|
|
8-K
|
|
July 18, 2016
|
|
10.1
|
|
|
|
|
|
|
|
4.19
|
|
Form of Common Stock Purchase Warrant issued in April 2011
|
|
8-K
|
|
April 15, 2011
|
|
4.1
|
|
|
|
|
|
|
|
4.20
|
|
Form of December 2012 Warrant
|
|
8-K
|
|
December 19, 2012
|
|
4.1
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.21
|
|
Form of warrants granted on May 8, 2013 under the Loan and Security Agreement
|
|
10-Q
|
|
May 9, 2013
|
|
10.7
|
|
|
|
|
|
|
|
4.22
|
|
Warrant Agreement, dated as of March 18, 2015, by and among Galena Biopharma, Inc., Computershare, Inc. and Computershare Trust Company, N.A.
|
|
10-Q
|
|
August 6, 2015
|
|
4.1
|
|
|
|
|
|
|
|
4.23
|
|
Registration Rights Agreement, dated January 12, 2014, between Galena Biopharma, Inc. and each former owner of membership units of Mills Pharmaceuticals, LLC
|
|
10-Q
|
|
March 17, 2014
|
|
4.9
|
|
|
|
|
|
|
|
4.24
|
|
Form of Contingent Value Rights Agreement among Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation), Computershare Trust Company, N.A., Computershare Inc., and Robert E Kennedy, dated April 13, 2011
|
|
8-K
|
|
April 14, 2011
|
|
10.1
|
|
|
|
|
|
|
|
4.25
|
|
First Amendment to Contingent Value Rights Agreement among Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation), Computershare Trust Company, N.A., Computershare Inc., and Robert E Kennedy, dated February 15, 2012
|
|
10-K
|
|
March 28, 2012
|
|
10.2
|
|
|
|
|
|
|
|
4.26
|
|
Amendment Agreement dated May 1, 2017 between Galena Biopharma, Inc. and JGB (Cayman) Newton Ltd.
|
|
8-K
|
|
May 2, 2017
|
|
10.1
|
|
|
|
|
|
|
|
5.1
|
|
Opinion of Thomas J. Knapp, Esq. in connection with Registration Statement 333-213908
|
|
S-3
|
|
September 30, 2016
|
|
5.1
|
|
|
|
|
|
|
|
5.2
|
|
Opinion of Fredrikson & Byron, P.A. in connection with Registration Statement 333-213493
|
|
S-3
|
|
September 2, 2016
|
|
5.1
|
|
|
|
|
|
|
|
5.3
|
|
Opinion of TroyGould PC in connection with Registration Statement 333-208330
|
|
S-3
|
|
December 4, 2015
|
|
5.1
|
|
|
|
|
|
|
|
5.4
|
|
Opinion of TroyGould PC in connection with Registration Statement 333-208331
|
|
S-3
|
|
December 4, 2015
|
|
5.1
|
|
|
|
|
|
|
|
10.1
|
|
Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) Amended and Restated 2007 Incentive Plan
|
|
Schedule 14A
|
|
April 23, 2010
|
|
Annex A
|
|
|
|
|
|
|
|
10.2
|
|
Amendment to Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) Amended and Restated 2007 Incentive Plan
|
|
Schedule 14A
|
|
May 31, 2011
|
|
Annex A
|
|
|
|
|
|
|
|
10.3
|
|
Galena Biopharma, Inc. 2016 Incentive Plan
|
|
8-K
|
|
August 22, 2016
|
|
10.3
|
|
|
|
|
|
|
|
10.4
|
|
Form of Incentive Stock Option
|
|
10-Q
|
|
August 8, 2015
|
|
10.1
|
|
|
|
|
|
|
|
10.5
|
|
Form of Nonstatutory Stock Option
|
|
10-Q
|
|
August 8, 2015
|
|
10.2
|
|
|
|
|
|
|
|
10.6
|
|
Patent and Technology License Agreement, dated September 11, 2006, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson Foundation for the
Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.)
|
|
10-Q
|
|
August 15, 2011
|
|
10.1
|
|
|
|
|
|
|
|
10.7
|
|
Amendment No. 1 to Patent and Technology License Agreement, dated December 21, 2007, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson
Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.)
|
|
10-Q
|
|
August 15, 2011
|
|
10.2
|
|
|
|
|
|
|
|
10.8
|
|
Amendment No. 2 to Patent and Technology License Agreement, dated September 3, 2008, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson
Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.)
|
|
10-Q
|
|
August 15, 2011
|
|
10.3
|
|
|
|
|
|
|
|
10.9
|
|
Amendment No. 3 to Patent and Technology License Agreement, dated July 8, 2009, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson
Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.)
|
|
10-Q
|
|
August 15, 2011
|
|
10.4
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.10
|
|
Amendment No. 4 to Patent and Technology License Agreement, dated February 11, 2010, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson
Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.)
|
|
10-Q
|
|
August 15, 2011
|
|
10.5
|
|
|
|
|
|
|
|
10.11
|
|
Amendment No. 5 to Patent and Technology License Agreement, dated January 10, 2011, by and among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center, the Henry M. Jackson
Foundation for the Advancement of Military Medicine, Inc., and Apthera, Inc. (formerly Advanced Peptide Therapeutics, Inc.)
|
|
10-Q
|
|
August 15, 2011
|
|
10.6
|
|
|
|
|
|
|
|
10.12
|
|
Scientific Advisory Agreement between Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) and George E. Peoples, Ph.D., dated May 1, 2011 as amended through November 1, 2017
|
|
10-K
|
|
March 12, 2013
|
|
10.19
|
|
|
|
|
|
|
|
10.13
|
|
Exclusive License Agreement, dated as of July 11, 2011, by and among The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) and its
wholly-owned subsidiary, Apthera, Inc.
|
|
8-K
|
|
September 21, 2011
|
|
10.1
|
|
|
|
|
|
|
|
10.14
|
|
Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) Employee Stock Purchase Plan
|
|
Schedule 14A
|
|
April 23, 2010
|
|
Annex B
|
|
|
|
|
|
|
|
10.15
|
|
License Agreement, effective as of April 30, 2009, between Kwangdong Pharmaceutical Co., Ltd. and Apthera, Inc.
|
|
10-K
|
|
March 28, 2012
|
|
10.45
|
|
|
|
|
|
|
|
10.16
|
|
Amendment No. 1 to License Agreement, dated as of January 13, 2012, by and among Apthera, Inc., Kwangdong Pharmaceutical Co., Ltd., and Galena Biopharma, Inc.
|
|
10-K
|
|
March 28, 2012
|
|
10.46
|
|
|
|
|
|
|
|
10.17
|
|
Form of Amendment to Stock Options Granted under Galena Biopharma, Inc. (formerly RXi Pharmaceuticals Corporation) 2007 Incentive Plan, entered into in April 2011 by Galena Biopharma, Inc. with all directors of Galena Biopharma,
Inc., as of April 1, 2011, and Mark J. Ahn, Ph.D.
|
|
10-Q
|
|
August 15, 2011
|
|
10.2
|
|
|
|
|
|
|
|
10.18
|
|
License and Supply Agreement, effective December 3, 2012, between Galena Biopharma, Inc. and ABIC Marketing Limited, a subsidiary of Teva Pharmaceuticals
|
|
10-K
|
|
March 12, 2013
|
|
10.43
|
|
|
|
|
|
|
|
10.19
|
|
Loan and Security Agreement dated May 8, 2013 among Galena Biopharma, Inc., Apthera, Inc., Oxford Finance LLC and the Lenders
|
|
10-Q
|
|
May 9, 2013
|
|
10.6
|
|
|
|
|
|
|
|
10.20
|
|
At Market Issuance Sales Agreement dated May 24, 2013 between Galena Biopharma, Inc. and Maxim Group LLC
|
|
8-K
|
|
May 31, 2013
|
|
10.2
|
|
|
|
|
|
|
|
10.21
|
|
At Market Issuance Sales agreements dated May 24, 2013 between Galena Biopharma, Inc. and MLV & Co. LLC
|
|
8-K
|
|
May 31, 2013
|
|
10.1
|
|
|
|
|
|
|
|
10.22
|
|
Form of warrants granted on May 8, 2013 under the Loan and Security Agreement
|
|
10-Q
|
|
May 9, 2013
|
|
10.7
|
|
|
|
|
|
|
|
10.23
|
|
License and Development Agreement, dated January 13, 2014, between Galena Biopharma, Inc. and Dr. Reddy’s Laboratories, Ltd.
|
|
10-Q
|
|
March 17, 2014
|
|
10.36
|
|
|
|
|
|
|
|
10.24
|
|
Exclusive License Agreement, dated as of December 20, 2013, between Mills Pharmaceuticals, LLC and BioVascular, Inc.
|
|
10-Q
|
|
March 17, 2014
|
|
10.37
|
|
|
|
|
|
|
|
10.25
|
|
License and Supply Agreement dated as of July 17, 2014 between Galena Biopharma, Inc. and MonoSol Rx, LLC
|
|
10-Q
|
|
August 11, 2014
|
|
10.1
|
|
|
|
|
|
|
|
10.26
|
|
Purchase Agreement, dated as of November 18, 2014, by and between Galena Biopharma, Inc. and Lincoln Park Capital Fund, LLC
|
|
8-K
|
|
November 20, 2014
|
|
10.1
|
|
|
|
|
|
|
|
10.27
|
|
Amendment dated August 8, 2016 to the Purchase Agreement, dated as of November 18, 2014, by and between Galena Biopharma, Inc. and Lincoln Park Capital Fund, LLC
|
|
10-Q
|
|
November 9, 2016
|
|
10.1
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.28
|
|
Separation and Consulting Agreement, dated as of June 24, 2015, by and between Galena Biopharma, Inc. and Margaret Kivinski, and General Release, dated as of June 24, 2015, by Margaret Kivinski
|
|
10-Q
|
|
August 6, 2015
|
|
10.4
|
|
|
|
|
|
|
|
10.29
|
|
Employment Offer Letter effective June 25, 2015, between Galena Biopharma, Inc. and Thomas J. Knapp
|
|
10-Q
|
|
August 6, 2015
|
|
10.5
|
|
|
|
|
|
|
|
10.30
|
|
Employment Agreement, dated as of October 30, 2015, between Galena Biopharma, Inc. and Bijan Nejadnik, M.D.
|
|
10-K
|
|
March 10, 2016
|
|
10.33
|
|
|
|
|
|
|
|
10.31
|
|
Asset Purchase Agreement, dated November 19, 2015, between Galena Biopharma, Inc. and Sentynl Therapeutics Inc.
|
|
10-K
|
|
March 10, 2016
|
|
10.34
|
|
|
|
|
|
|
|
10.32
|
|
Amendment, dated as of December 16, 2015, to License and Supply Agreement dated as of July 17, 2014 between Galena Biopharma, Inc. and MonoSol Rx, LLC
|
|
10-K
|
|
March 10, 2016
|
|
10.35
|
|
|
|
|
|
|
|
10.33
|
|
Asset Purchase Agreement, dated December 17, 2015, between Galena Biopharma, Inc. and Midatech Pharma PLC
|
|
10-K
|
|
March 10, 2016
|
|
10.36
|
|
|
|
|
|
|
|
10.34
|
|
Separation Agreement and Releases, dated December 31, 2015, between Galena Biopharma, Inc. and Ryan Dunlap
|
|
10-K
|
|
March 10, 2016
|
|
10.37
|
|
|
|
|
|
|
|
10.35
|
|
Amendment, dated December 31, 2015, to Employment Offer Letter effective June 25, 2015, between Galena Biopharma, Inc. and Thomas J. Knapp
|
|
10-K
|
|
March 10, 2016
|
|
10.38
|
|
|
|
|
|
|
|
10.36
|
|
Form of Undertaking re Advancement of Expenses between Galena Biopharma, Inc. and certain of its Existing or Former Directors and Executive officers
|
|
10-K
|
|
March 10, 2016
|
|
10.39
|
|
|
|
|
|
|
|
10.37
|
|
Placement Agency Agreement, dated as of July 7, 2016, by and between Galena Biopharma, Inc. and Raymond James & Associates, Inc.
|
|
8-K
|
|
July 8, 2016
|
|
10.1
|
|
|
|
|
|
|
|
10.38
|
|
Severance Agreement, dated as of August 22, 2016, between Galena Biopharma, Inc. and John T. Burns
|
|
8-K
|
|
August 22, 2016
|
|
10.1
|
|
|
|
|
|
|
|
10.39
|
|
Second Amendment to Employment Agreement, dated as of August 22, 2016, between Galena Biopharma, Inc. and Bijan Nejadnik
|
|
8-K
|
|
August 22, 2016
|
|
10.2
|
|
|
|
|
|
|
|
10.40
|
|
Second Amendment to Offer Letter between Galena Biopharma, Inc. and Thomas J. Knapp
|
|
8-K
|
|
October 6, 2016
|
|
10.1
|
|
|
|
|
|
|
|
10.41
|
|
Employment Agreement between Galena Biopharma, Inc. and Stephen Ghiglieri dated November 1, 2016
|
|
8-K
|
|
November 3, 2016
|
|
99.2
|
|
|
|
|
|
|
|
10.42
|
|
Separation Agreement and General Release between Mark W. Schwartz and Galena Biopharma, Inc. executed on January 31, 2017
|
|
10-Q
|
|
May 10, 2017
|
|
10.1
|
|
|
|
|
|
|
|
10.43
|
|
Amendment to Employment Agreement between Galena Biopharma, Inc. and Stephen Ghiglieri, dated as of February 21, 2017
|
|
8-K
|
|
February 21, 2017
|
|
10.1
|
|
|
|
|
|
|
|
10.44
|
|
Retention Agreement between Stephen Ghiglieri and Galena Biopharma, Inc. dated February 23, 2017
|
|
10-Q
|
|
May 10, 2017
|
|
10.3
|
|
|
|
|
|
|
|
10.45
|
|
Retention Agreement between Bijan Nejadnik and Galena Biopharma, Inc. dated February 23, 2017
|
|
10-Q
|
|
May 10, 2017
|
|
10.4
|
|
|
|
|
|
|
|
10.46
|
|
Retention Agreement between John Burns and Galena Biopharma, Inc. dated February 23, 2017
|
|
10-Q
|
|
May 10, 2017
|
|
10.5
|
|
|
|
|
|
|
|
10.47
|
|
Retention Agreement between Thomas J. Knapp and Galena Biopharma, Inc. dated February 23, 2017
|
|
10-Q
|
|
May 10, 2017
|
|
10.6
|
|
|
|
|
|
|
|
10.48
|
|
Second Amendment to the Purchase Agreement, dated as of February 6, 2017, by and between Galena Biopharma, Inc. and Lincoln Park Capital Fund, LLC
|
|
8-K
|
|
February 7, 2017
|
|
10.1
|
|
|
|
|
|
|
|
10.49
|
|
Third Amendment to Offer Letter between Galena Biopharma, Inc. and Thomas J. Knapp, dated as of January 31, 2017
|
|
8-K
|
|
February 7, 2017
|
|
10.2
|
|
|
|
|
|
|
|
21.1
|
|
Subsidiaries of the Registrant
|
|
10-K
|
|
March 15, 2017
|
|
21.1
|
|
|
|
|
|
|
|
23.1*
|
|
Consent of Moss Adams LLP, Independent Registered Public Accounting Firm
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23.2
|
|
Consent of Thomas J. Knapp, Esq. in connection with Registration Statement 333-213908 (included in Exhibit 5.1 hereof)
|
|
S-3
|
|
September 30, 2016
|
|
23.1
|
|
|
|
|
|
|
|
23.3
|
|
Consent of Fredrikson & Byron, P.A. in connection with Registration Statement 333-213493 (included in Exhibit 5.2 hereof)
|
|
S-3
|
|
September 2, 2016
|
|
23.1
|
|
|
|
|
|
|
|
23.4
|
|
Consent of TroyGould PC in connection with Registration Statement 333-208330 (included in Exhibit 5.3 hereof)
|
|
S-3
|
|
December 4, 2015
|
|
23.1
|
|
|
|
|
|
|
|
23.5
|
|
Opinion of TroyGould PC in connection with Registration Statement 333-208331 (included in Exhibit 5.4 hereof)
|
|
S-3
|
|
December 4, 2015
|
|
23.1
|
|
|
|
|
|
|
|
24.1*
|
|
Powers of Attorney (included on signature page hereof)
|
|
|
|
|
|
|
SELLAS Life Sciences (NASDAQ:SLS)
Historical Stock Chart
From Mar 2024 to Apr 2024
SELLAS Life Sciences (NASDAQ:SLS)
Historical Stock Chart
From Apr 2023 to Apr 2024