CALCULATION OF REGISTRATION FEE
|
Title
of Each Class Of Securities to be Registered
|
Amount
to be
Registered
(1)
|
Proposed
Maximum
Aggregate
Offering
Price
per
share (2)
|
Proposed
Maximum
Aggregate
Offering
Price (3)
|
Amount
of
Registration
fee(4)
|
|
Class A Common
Stock, no par value
|
110,000,000
|
$
0.0401
|
$
4,411,000
|
$
512.56
|
(1) In
the event of a stock split, stock dividend, or similar transaction
involving the common stock, the number of shares registered shall
automatically be increased to cover the additional shares of common
stock issuable pursuant to Rule 416 under the Securities
Act. The amount of shares to be registered represents
the Company’s good faith estimate of the number of shares
that the registrant may issue pursuant to a Letter Agreement with
the selling security holder.
(2) The
proposed maximum offering price per share and the proposed maximum
aggregate offering price have been estimated solely for the purpose
of calculating the amount of the registration fee in accordance
with Rules 457(c) and 457(h) under the Securities Act of 1933. Our
common stock is quoted under the symbol “KBLB” on
the OTC Markets, Inc. OTCQB. As of October 31, 2014, the
last sale reported price was $0.0401 per share. Accordingly, the
registration fee is $512.56 based on $0.0401 per
share.
(3)
This amount represents the maximum aggregate market value of common
stock which may be put to the selling shareholder by the registrant
pursuant to the terms and conditions of a Letter Agreement between
the selling shareholder and the registrant.
(4)
previously paid.
The registrant hereby amends this registration statement on such
date or dates as may be necessary to delay its effective date until
the registrant shall file a further amendment which specifically
states that this registration statement shall thereafter become
effective in accordance with section 8(a) of the securities act of
1933 or until the registration statement shall become effective on
such date as the commission, acting pursuant to said section 8(a),
may determine.
EXPLANATORY NOTE
We are filing this Amendment No. 9 to our registration
statement on Form S-1 filed on June 2, 2015, (File No. 333-199820)
to conform the disclosure herein to our Annual Report on Form
10-K for the year ending December 31, 2016, which was filed on
March 22, 2017.
Pursuant to Section 84001 of the Fixing America’s Surface
Transportation Act (the “FAST Act”), the Company elects
to incorporate by reference all documents subsequently filed by the
Company pursuant to Sections 13(a), 13(c), 14 or 15(d) of the
Exchange Act. The Company will (i) provide to each
person, including any beneficial owner, to whom a prospectus is
delivered, a copy of any or all of the reports or documents that
have been incorporated by reference in the prospectus contained in
the registration statement but not delivered with the prospectus;
(ii) provide these reports or documents upon written or oral
request; (iii) provide these reports or documents at no cost to the
requester; (iv) state the name, address, telephone number, and
email address, if any, to which the request for these reports or
documents must be made; and (v) state the Company’s Website
address, including the uniform resource locator (URL) where the
incorporated reports and other documents may be
accessed.
The information in this prospectus is not complete and may be
changed. These securities may not be sold by the selling
stockholders publicly until the registration statement filed with
the Securities and Exchange Commission is effective. This
prospectus is not an offer to sell these securities and no offer to
buy these securities is being solicited in any state where the
offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED MAY 4, 2017
AMENDMENT NO. 9 TO PRELIMINARY PROSPECTUS
KRAIG BIOCRAFT LABORATORIES, INC.
110,000,000
shares of Class A Common Stock
This
prospectus relates to the resale of up to 110,000,000 shares of the
common stock of Kraig Biocraft Laboratories, Inc., a Wyoming
corporation, which shares will be offered and sold by the selling
shareholder, Calm Seas Capital, LLC, a Nevada limited liability
company (“Calm Seas”), pursuant to a “put
right” under a letter agreement for an equity line financing,
that we entered into with Calm Seas on October 2, 2014 (the
“Letter Agreement”). As of May 4, 2017, of the
110,000,000 shares of common stock initially registered, 38,824,427
shares of common stock remain available for registration. The
Letter Agreement permits us to “put” up to an aggregate
of $7,500,000 in shares of our Class A common stock to Calm Seas
during a two year period ending on the second anniversary of
the effective date of the registration statement covering the
resale of the put shares. We will not receive any
proceeds from the sale of these shares of our Class A common stock.
However, we will receive proceeds from the sale of securities
pursuant to our exercise of the put right offered by Calm Seas
under the Letter Agreement. We will bear all costs
associated with this registration.
Calm
Seas is an “underwriter” within the meaning of the
Securities Act of 1933, as amended (the “Securities
Act”) in connection with the resale of our Class A common
stock sold to it by our exercise of the put right under the Letter
Agreement. Each month we may put up to $200,000 of our
Class A common stock under the Letter Agreement, which will
purchase such shares at a price per share equal to 80% of the
lowest price of our Class A common stock during the five
consecutive trading days immediately following the date the notice
of our election to put shares pursuant to the Letter Agreement is
delivered to Calm Seas (the date of delivery of such notice is
referred to as the “put
date”). Notwithstanding the aggregate $200,000
ceiling for monthly puts under the Letter Agreement, if both we and
Calm Seas agree, we may submit one or more additional puts during
any given month to the extent we need additional capital for our
operations and/or our product development. We can only
submit such additional put(s) if Calm Seas Capital agrees to
it. Furthermore, the additional put is subject to the
aggregate $7,500,000 limitation of this offering. The
additional put allows us to obtain additional capital in the event
that our product development proceeds quicker than we
expect.
We will
automatically withdraw our put notice to Calm Seas if the lowest
price used to determine the purchase price of the put shares is not
at least equal to seventy-five percent (75%) of the average closing
“bid” price for our Class A common stock for the ten
(10) trading days prior to the put date.
Our shares of Class A common stock are traded on the OTC Markets,
Inc. OTCQB under the symbol “KBLB.” On
May 4,
2017
, the closing sale price
of our common stock was $0.07 per share.
This investment involves a high degree of risk. You should purchase
shares only if you can afford a complete loss. See "Risk Factors"
beginning on page 8.
Our
principal executive offices are located at 2723 South State St.,
Suite 150, Ann Arbor, Michigan. Our telephone number is
(734)619-8066.
Neither the Securities and Exchange Commission nor any state
securities commission has approved or disapproved of these
securities or determined if this prospectus is truthful or
complete. Any representation to the contrary is a criminal
offense.
The date of this amendment is May 4, 2017
TABLE OF CONTENTS
|
|
|
|
Prospectus
Summary
|
5
|
|
Summary Financial
Data
|
7
|
|
Risk
Factors
|
8
|
|
Use of
Proceeds
|
15
|
|
Selling
Shareholder
|
16
|
|
Plan of
Distribution
|
19
|
|
Description of
Securities
|
20
|
|
Interests of Named
Experts and Counsel
|
21
|
|
Description of
Business
|
21
|
|
Legal
Proceedings
|
27
|
|
Market For Common
Equity and Related Stockholder Matters
|
28
|
|
Available
Information
|
28
|
|
Financial
Statements
|
29
|
|
Management
Discussion and Analysis of Financial Condition and Results of
Operations
|
51
|
|
Changes In and
Disagreements with Accounts on Accounting and Financial
Disclosure
|
52
|
|
Directors,
Executive Officers, Promoters and Control Persons
|
57
|
|
Executive
Compensation
|
58
|
|
Security Ownership
of Certain Beneficial Owners and Management
|
60
|
|
Certain
Relationships and Related Transactions
|
60
|
|
Disclosure of
Commission Position of Indemnification for Securities Act
Liabilities
|
61
|
ABOUT THIS PROSPECTUS
You should rely only on the information contained in this
prospectus. We have not, and the selling security holder
has not, authorized anyone to provide you with different
information. If anyone provides you with different
information, you should not rely on it. We are not, and
the selling security holder is not, making an offer to sell these
securities in any jurisdiction where the offer or sale is not
permitted. You should assume that the information
contained in this prospectus is accurate only as of the date on the
front cover of this prospectus. Our business, financial
condition, results of operations and prospects may have changed
since that date. In this prospectus, “Kraig”,
“Kraig Biocraft” “KBLB”, “the
Company”, “we”, “us” and
“our” refer to Kraig Biocraft Laboratories, Inc., a
Wyoming corporation, unless the context otherwise
requires.
PROSPECTUS SUMMARY
This
summary highlights selected information contained elsewhere in this
prospectus. This summary does not contain all the
information that you should consider before investing in the common
stock. You should carefully read the entire prospectus,
including “Risk Factors,” “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations” and the Condensed Financial Statements, before
making an investment decision
.
About Our Company
We are
Kraig Biocraft Laboratories, Inc., a corporation organized under
the laws of Wyoming on April 25, 2006. We were organized
to develop high strength, protein-based fibers, using recombinant
DNA technology, for commercial applications in both the specialty
fiber and technical textile industries. Specialty fibers
are engineered for specific uses that require exceptional strength,
heat resistance and/or chemical resistance. The
specialty fiber market is dominated by two synthetic fiber
products: aramid fibers and ultra-high molecular weight
polyethylene fiber. Examples of these synthetic fibers
include Kevlar® and Spectra®. The technical
textile industry involves products for both industrial and consumer
products, such as filtration fabrics, medical textiles (e.g.,
sutures and artificial ligaments), safety and protective clothing
and fabrics used in military and aerospace applications (e.g.,
high-strength composite materials).
We
entered into license agreements with University of Notre Dame and
the University of Wyoming that give us the use of certain
intellectual property for developing transgenic silkworms to
produce spider silk fibers in commercially viable
quantities. We are using these genetic engineering
technologies to develop fibers with greater strength, resiliency
and flexibility for use in our target markets, namely the specialty
fiber and technical textile industries.
We are
currently in the research and development stage of our development,
which is to develop a transgenic silkworm that can produce a
recombinant spider silk fiber by inserting genetic sequences into
ordinary silkworms using patented genetic engineering technology
under our license and collaboration agreements with the University
of Norte Dame and the University of Wyoming. The proceeds from
the offering, as described below, will be used to fund this
research and development and to increase the scale of our
production of genetically endeared silks. We anticipate this stage
in our development may be complete by December 31,
2017.
As of
the date of this prospectus, the Company has generated $31,858 from
our development activities. As of December 31, 2016, we
have an accumulated deficit since inception of
$23,385,979. As of December 31, 2016, we had $298,859 in
cash. Our cash balance is not sufficient to advance our
research and development obligations under our agreements with The
Universities of Norte Dame and Wyoming. Both
universities have indicated to us that they desire to continue
their respective collaborative efforts with us, with the
expectation that we will be able to raise capital under the Equity
Line Financing to fund our research and development
efforts. We have also received a going concern opinion
from our independent registered accounting firm in its audit report
dated March 22, 2017, for our fiscal year ended December 31,
2016.
The
proceeds from the equity line financing will be used for working
capital including employee salaries, the costs of our research and
development obligations under the agreement with the University of
Notre Dame, the costs related to our operation as a public company
(primarily, legal and accounting fees) as well as legal fees for
securing our intellectual property, rent and telecommunications
(phone, fax and Internet). Consequently, we believe that
it is highly likely that we will use all $7,500,000 of the proceeds
we expect to raise from the equity line financing under the Letter
Agreement. We believe the results of our research and
development efforts, if successful, will help increase our stock
price and, therefore, reduce the number of shares we will need to
put to Calm Seas in order to raise the full $7,500,000 in gross
proceeds we are seeking to raise under the equity line financing
under the Letter Agreement. In the event that we do not
have positive results from our research and development during the
24 month term of the equity line financing, we believe it will be
unlikely that we will raise the full $7,500,000 in gross proceeds
under the equity line financing. In the event that we
raise substantially less than the maximum proceeds that we expect
to raise under the equity line financing by the expiration of its
24 month term, we will seek to raise additional capital from other
investors.
Where You Can Find Us
Our
principal executive offices are located at 2723 South State St.,
Suite 150, Ann Arbor, Michigan. Our telephone number is
(734)619-8066.
The Offering
This
prospectus relates to the resale of up to 110,000,000 shares of our
Class A common stock that may be issued to Calm Seas pursuant to a
“put right” under the Letter Agreements, that we
entered into with Calm Seas. As of May 4, 2017, of the
110,000,000 shares of common stock initially registered, 38,824,427
shares of common stock remain available for
registration.
For the
purpose of determining the number of shares of common stock to be
offered by this prospectus, we have assumed that we will issue not
more than 110,000,000 shares pursuant to the exercise of our put
right under the Letter Agreement, although the number of shares
that we will actually issue pursuant to that put right may be
significantly less than 110,000,000, depending on the trading price
of our Class A common stock.
The
Letter Agreement with Calm Seas provides that over a 24 month
period we may put to Calm Seas up to an aggregate of $7,500,000 in
shares of our Class A common stock for a purchase price equal to
80% of the lowest price of our Class A common stock during the five
consecutive trading days immediately following the date we deliver
notice to Calm Seas of our election to put shares pursuant to the
Letter Agreement. We may only put shares twice during
each calendar month, unless Calm Seas accepts an additional put (as
described below). The dollar value that we will be
permitted to put each month pursuant to the Letter Agreement will
be the lesser of: (A) the product of (i) 200% of the average daily
volume in the US market of our Class A common stock for the ten
trading days prior to the date we deliver our put notice to Calm
Seas multiplied by (ii) the average of the daily closing prices for
the ten (10) trading days immediately preceding the date we deliver
our put notice to Calm Seas, or (B) $200,000 with regard to the
Letter Agreement. We will automatically withdraw our put
notice to Calm Seas if the lowest price used to determine the
purchase price of the put shares is not at least equal to
seventy-five percent (75%) of the average closing “bid”
price for our Class A common stock for the ten (10) trading days
prior to the date we deliver our put notice to Calm
Seas.
On the
seventh business day after we deliver our put notice to it, Calm
Seas will purchase the number of shares set forth in the put notice
at the dollar value set forth in the put notice by delivering such
amount to us by wire transfer.
Notwithstanding
the aggregate $200,000 ceiling for monthly puts under the Letter
Agreement, as described above, we may at any time request Calm Seas
to purchase shares in excess of such ceiling, either as a part of a
monthly put or as an additional put(s) during such
month. If Calm Seas, in its sole discretion, accepts
such request to purchase additional shares, then we may include the
put for additional shares in our monthly put request or submit an
additional put for such additional shares in accordance with the
procedure set forth above.
Calm
Seas has indicated that it will resell those shares in the open
market, resell our shares to other investors through negotiated
transactions, or hold our shares in its portfolio. This
prospectus covers the resale of our stock by Calm Seas either in
the open market or to other investors through negotiated
transactions. Calm Seas’ obligations under the Letter
Agreement are not transferrable and this registration statement
does not cover sales of our common stock by transferees of Calm
Seas.
Except
as described above, there are no other conditions that must be met
in order for Calm Seas to be obligated to purchase the shares set
forth in the put notice.
The
Letter Agreement will terminate when any of the following events
occur:
|
●
|
Calm
Seas has purchased an aggregate of $7,500,000 of our Class A common
stock; or
|
|
●
|
The
second anniversary of the effective date of the registration
statement covering the Equity Line Financing with Calm Seas under
the Letter Agreement.
|
As we
draw down on the Equity Line Financing, shares of our Class A
common stock will be sold into the market by Calm Seas. The
sale of these additional shares could cause our stock price to
decline. In turn, if the stock price declines and we issue
more puts, more shares will come into the market, which could cause
a further drop in the stock price. You should be aware that
there is an inverse relationship between the market price of our
Class A common stock and the number of shares to be issued under
the Equity Line Financing. If our stock price declines, we
will be required to issue a greater number of shares under the
Equity Line Financing. We have no obligation to utilize the
full amount available under the Equity Line Financing.
Terms of the Offering
|
Class A
common stock offered:
|
Up to
110,000,000 shares of Class A common stock, no par value, to be
offered for resale by Calm Seas.
|
|
|
|
|
Class A
common stock outstanding before this offering:
|
676,903,348 shares
|
|
|
|
|
Common
stock to be outstanding after this offering:
|
786,903,348
shares
|
|
|
|
|
Use of
proceeds:
|
We will
not receive any proceeds from the sale of the shares of Class A
common stock. However, we will receive proceeds from the Equity
Line Financing. See “Use of Proceeds”.
|
|
|
|
|
Risk
factors:
|
An
investment in our Class A common stock involves a high degree of
risk. See “Risk Factors” beginning on page 4 of
this prospectus.
|
|
|
|
|
OTC
Markets symbol:
|
“KBLB”
|
As
of the date of this filing there are 783,046,190 outstanding shares
of Class A common stock, which includes 71,175,573 issued share
under this registration. 38,824,427 shares of Class A common
stock remain available for registration under this
offering.
SUMMARY FINANCIAL DATA
The
following table provides summary financial statement data of Kraig
Biocraft Laboratories, Inc. The financial data have been
derived from our audited financial statements. The data set forth
below should be read in conjunction with “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations,” our financial statements and the related notes
included in this prospectus.
|
|
For
the Year
Ended
December
31,
2016
|
For
the Year
Ended
December
31,
2015
|
|
|
|
|
|
|
|
|
|
Revenue
|
$
31,858
|
$
--
|
|
|
|
|
|
Total Operating
Expenses
|
$
2,977,462
|
$
2,054,539
|
|
|
|
|
|
Loss from
Operations
|
$
(2,945,604
)
|
$
(2,054,539
)
|
|
|
|
|
|
Net
loss
|
$
(3,073,843
)
|
$
(2,133,793
)
|
|
|
|
|
|
Loss Per Share
– Basic and Diluted
|
$
(0.00
)
|
$
(0.00
)
|
|
|
|
|
|
BALANCE
SHEET DATA:
|
|
|
|
|
|
|
|
Cash
|
$
298,859
|
$
238,188
|
|
|
|
|
|
Total
assets
|
$
383,659
|
$
304,937
|
|
|
|
|
|
Total Current
liabilities – related party
|
$
2,230,910
|
$
1,732,040
|
|
|
|
|
|
Total Current
Liabilities
|
$
2,744,472
|
$
2,201,873
|
|
|
|
|
|
Stockholders’
Deficit
|
$
(2,360,813
)
|
$
(1,896,936
)
|
RISK FACTORS
An investment in our common stock involves a high degree of risk.
You should carefully consider the risks described below and the
other information in this prospectus before investing in our common
stock. If any of the following risks occur, our business, operating
results and financial condition could be seriously harmed. Please
note that throughout this prospectus, the words “we”,
“our” or “us” refer to the Company and its
subsidiary not to the selling stockholders.
Risk Related to Our Company
The report of the independent registered public accounting firm on
our 2016 and 2015 financial statements contains a going concern
qualification.
The
report of the independent registered public accounting firm
covering our financial statements for the years ended December 31,
2016 and December 31, 2015 stated that certain factors, including
that we have a working capital and shareholder deficit and raised
substantial doubt as to our ability to continue as a going
concern. Because we are not yet producing revenue, we
are dependent upon raising capital to continue our
business. If we are unable to raise capital, we may not
be able to continue as a going concern.
We may be unable to maintain an effective system of internal
controls and accurately report our financial results or prevent
fraud, which may cause our current and potential stockholders to
lose confidence in our financial reporting and adversely impact our
business and our ability to raise additional funds in the
future.
Effective
internal controls are necessary for us to provide reliable
financial statements and effectively prevent fraud. We
have no internal accounting staff. As we noted in our
annual report on Form 10-K for the year ended December 31, 2016, we
reported that our internal control over financial reporting are not
effective for the purposes for which it is intended because we had
material weaknesses, as described below. Though we have
taken some steps to address our material weaknesses in our internal
control over financial reporting, including education of management
of disclosure requirements and financial reporting
controls, we still have not eliminated the material weakness
in our internal controls over financial reporting. If we
cannot provide reliable financial statements or prevent fraud, our
operating results and our reputation could be harmed as a result,
causing stockholders and/or prospective investors to lose
confidence in management and making it more difficult for us to
raise additional capital in the future.
In our
“Management's Annual Report on Internal Control Over
Financial Reporting” that appeared in our annual report on
Form 10-K for the year ended December 31, 2016, we reported that
our internal control over financial reporting was not effective for
the purposes for which it is intended based on the following
material weaknesses:
|
●
|
Lack of internal audit function
. During 2016, the Company,
upon review of the independent auditors, made some adjustments to
its financial statements, including, adjusting salary amounts and
the related tax accruals, allocating the “related party
gain” to be donated capital and adding the liability due to
our attorney that should have been recorded. Management
believes that the foregoing is due to the fact that the Company
lacks qualified resources to perform the internal audit functions
properly and that the scope and effectiveness of the internal audit
function are yet to be developed. Specifically, the reporting
mechanism between the Company’s accounting personnel, the
Board of Directors and the CEO was not effective, therefore
resulting in the delay of recording and reporting.
|
|
|
|
|
●
●
|
No Segregation of Duties Ineffective controls over financial
reporting
: As of December 31, 2016, we had no full-time
employees with the requisite expertise in the key functional areas
of finance and accounting; since inception, we have retained
contract accountants to serve in that role on an as needed basis.
As a result, there is a lack of proper segregation of duties
necessary to insure that all transactions are accounted for
accurately and in a timely manner.
Lack of a functioning audit committee
: Due to a lack of a
majority of independent members and a lack of a majority of outside
directors on our board of directors, and no audit committee has
been elected, the oversight in the establishment and monitoring of
required internal controls and procedures is
inadequate.
Written Policies & Procedures
: Due to lack of written
policies and procedures for accounting and financial reporting, the
Company did not establish a formal process to close our books
monthly and account for all transactions.
|
As
reported in our most recent Annual Report, we had hired a payroll
service firm to manage all payroll functions including tax
withholdings. We will take the following remediation steps to help
address our material weaknesses in our internal control over
financial reporting:
|
|
1.
|
We will
continue to educate our management personnel to increase its
ability to comply with the disclosure requirements and financial
reporting controls;
|
|
|
2.
|
We will
increase management oversight of accounting and reporting functions
in the future; and
|
|
|
3.
|
As soon
as we can raise sufficient capital or our operations generate
sufficient cash flow, we will hire personnel to handle our
accounting and reporting functions.
|
We do
not expect to remediate the weaknesses in our internal controls
over financial reporting until the time when we start to
commercialize a recombinant fiber (and, therefore, may have
sufficient cash flow for hiring personnel to handle our accounting
and reporting functions).
We currently do not have any patent rights in the products we are
seeking to develop and we currently license the genetic sequences
and genetic engineering technology we need to develop our
products. If any third party challenges our claim to
intellectual property rights in the fiber products we are seeking
to develop or the intellectual property rights that we license, our
business may be materially harmed
We have
no patents or design patents on any of the fiber products we are
seeking to develop. It is possible that the fiber
products we are seeking to develop could be imitated or directly
manufactured and sold by a competitor. In addition, some
or all of our research, development ideas and proposed products may
be covered by patent rights held by some other
entity. In that event, we could incur devastating
liability and be forced to cease operations.
We
entered into intellectual property licensing agreements with Notre
Dame and the University of Wyoming. Pursuant to these
licensing agreements, we have obtained certain exclusive rights to
use intellectual property and genetic sequences owned by these
universities. However, we have no guarantee of the
viability of these intellectual property rights or the rights that
we have licensed do not infringe on the legal rights of third
parties. The intellectual property rights that we have
licensed could be challenged or voided or that the licensed
intellectual property is worthless and without
utility. We may also need to license additional
intellectual property from persons or entities in order to
successfully complete our research and development, and we cannot
be certain that we would be able to enter into a license agreement
with such persons or entities. In which event our
operations will be adversely affected and our prospects negatively
affected.
The 110,000,000 shares we are registering for resale hereunder may
not be enough to access the full $7,500,000 available under our
equity line agreement with Calm Seas Capital, LLC
We have
elected to register for resale 110 million shares of our Class A
Common Stock under our equity line agreement with Calm Seas. By
choosing to register this number of shares, we may not be able to
receive the full $7,500,000 available to us under the Calm Seas
agreement unless our stock price were to increase to at
approximately $0.068 per share from its price of $0.04 per share as
of the date of this Registration Statement. In the event
we are not able to receive the full $7,500,000 from Calm Seas, we
may have to seek capital from other sources to fund our operations
which may not be available to us.
Whether
our stock price will reach $0.068 is dependent on a number of
factors such as the progress of our business and commercialization
of our Monster Silk™ and whether sales of our Class A Common
Stock by Calm Seas after we put stock to them have the effect of
depressing our stock price below $0.068 even if there are positive
developments in our business.
Existing stockholders could experience substantial dilution upon
the issuance of Class A common stock pursuant to an equity line we
have with Calm Seas Capital.
Under
the Equity Line Financing set forth in the Letter Agreement with
Calm Seas Capital, we may put up to $200,000 of our Class A common
stock to Calm Seas per month. Notwithstanding the
$200,000 ceiling for monthly puts under the Letter Agreement, if
both we and Calm Seas agree, we may submit one or more additional
puts during any given month to the extent we need additional
capital for our operations and/or our product
development. When we exercise our put Calm Seas will
purchase such shares at a price per share equal to 80% of the
lowest price of our Class A common stock during the five
consecutive trading days immediately following the put date (as
defined on page 2 of this prospectus). Our equity line
with Calm Seas Capital contemplates our future possible issuance of
up to an aggregate 110,000,000 shares of our Class A common stock
as a result of this prospectus, subject to certain
restrictions. As of May 4, 2017, of the 110,000,000
shares of common stock initially registered, 38,824,427 shares of
common stock remain available for registration. Our existing
stockholders will be significantly diluted if the terms and
conditions of the equity line are satisfied, and we choose to
exercise our put rights to the fullest extent permitted and sell
all of the 110,000,000 shares of our common stock to Calm Seas
Capital. Additionally, if we are unable to raise $7,500,000 in
proceeds from the sale of the entire 110,000,000 shares to Calm
Seas Capital under the equity line financing, we will seek funding
from other capital sources. In such cases, we expect
that we would have to issue a significant number of additional
shares that would further dilute existing
shareholders.
We may not successfully manage any growth that we may
experience.
Our
future success will depend upon not only product development but
also on the expansion of our operations and the effective
management of any such growth, which will place a significant
strain on our management and on our administrative, operational,
and financial resources. To manage any such growth, we must expand
our facilities, augment our operational, financial and management
systems, and hire and train additional qualified personnel. If we
are unable to manage our growth effectively, our business would be
harmed as our growth could be adversely affected by such
mismanagement.
Our initial development of recombinant silk fiber from the
transgenic silkworm and other product development programs depend
upon third-party researchers who are outside our
control.
We
depend upon independent researchers and collaborators, such as
universities and their staff, to conduct our development of a
transgenic silkworm and recombinant silk polymers, such as spider
silk. Such researchers and collaborators perform
services under agreements with us. Such agreements are often
standard-form agreements typically not subject to extensive
negotiation. These researchers or collaborators are not
our employees, and in general we cannot control the amount or
timing of resources that they devote to our product development
programs. These researchers and collaborators may not assign
as great a priority to our programs or pursue them as diligently as
we would if we were undertaking such programs ourselves. If outside
collaborators fail to devote sufficient time and resources to our
transgenic silkworm development and our product development
programs, or if their performance is substandard, our introduction
of protein based fiber products will be delayed or may not result
at all. These researchers and collaborators may also
have relationships with other commercial entities, some of whom may
compete with us.
If conflicts arise with our collaborators, they may act in their
self-interests, which may be adverse to our interests.
Conflicts
may arise in our collaborations we have entered into or may enter
into due to one or more of the following:
|
●
|
disputes
with respect to payments that we believe are due under a
collaboration agreement;
|
|
●
|
disagreements
with respect to ownership of intellectual property
rights;
|
|
●
|
unwillingness
on the part of a collaborator to keep us informed regarding the
progress of its development and commercialization activities, or to
permit public disclosure of these activities;
|
|
●
|
delay
of a collaborator’s development or commercialization efforts
with respect to our product development; or
|
|
●
|
termination
or non-renewal of the collaboration.
|
In
addition, in our collaborations, we may be required to agree not to
conduct independently, or with any third party, any research that
is competitive with the research conducted under our
collaborations. Our collaborations may have the effect of limiting
the areas of research that we may pursue, either alone or with
others. Our collaborators, however, may be able to develop, either
alone or with others, products in related fields that are
competitive with the products or potential products that are the
subject of these collaborations.
If we lose the services of key management personnel, we may not be
able to execute our business strategy effectively.
Our
future success depends in a large part upon the continued service
of our founder and sole officer and director, Kim
Thompson. Mr. Thompson is critical to our overall
management as well as the development of our technology, our
culture and our strategic direction. We do not maintain a
key-person life insurance policy on Mr. Thompson. The
loss of Mr. Thompson would materially harm our
business.
As our business grows, we will need to hire highly skilled
personnel and, if we are unable to retain or motivate hire
additional qualified personnel, we may not be able to grow
effectively.
Our
performance will be largely dependent on the talents and efforts of
highly skilled individuals. Our future success depends on our
continuing ability to identify, hire, develop, motivate, and retain
highly skilled personnel for all areas of our company.
Despite the current economic conditions, competition in our
industry for qualified employees remains intense as the skills we
require in our employees are highly specialized. We
compete with companies in the biotechnology and pharmaceutical
industries that seek to retain scientists with genetic engineering
experience and expertise. Competition for qualified
individuals remains intense despite the current economic
conditions, which have somewhat softened demand for qualified
personnel. However, we expect that over the longer term we
will continue to face stiff competition and may not be able to
successfully recruit or retain such personnel. Attracting and
retaining qualified personnel will be critical to our
success.
If we are unable to establish sales and marketing capabilities or
enter into agreements with third parties to sell and market any
products we may develop, we may not be able to generate product
revenue.
We do
not currently have an organization for the sales, marketing and
distribution of any fiber products that we expect to
develop. In order to market any products that may be
developed, we must build our sales, marketing, managerial and other
non-technical capabilities or make arrangements with third parties
to perform these services. In addition, we have no experience in
developing, training or managing a sales force and will incur
substantial additional expenses in doing so. The cost of
establishing and maintaining a sales force may exceed its cost
effectiveness. Furthermore, we will compete with many
companies that currently have extensive and well-funded marketing
and sales operations. Our marketing and sales efforts
may be unable to compete successfully against these
companies. If we are unable to establish adequate sales,
marketing and distribution capabilities, whether independently or
with third parties, we may not be able to generate product revenue
and may not become profitable.
We may be unable to generate significant revenues and may never
become profitable.
The
Company did not have revenues in 2015 and generated $31,858 in
revenue in 2016, making it difficult to predict when we will
generate significant revenues or be profitable. We expect to incur
significant research and development costs for the foreseeable
future. We may not be able to successfully market fiber
products we produce in the future that will generate significant
revenues. In addition, any revenues that we may generate may
be insufficient for us to become profitable.
In
particular, potential investors should be aware that we have not
proven that we can:
|
●
|
raise
sufficient additional capital in the public and/or private markets
to continue the development of the transgenic silkworm or
demonstrate the ability to produce commercial volumes of
recombinant silk fibers;
|
|
●
|
develop
and manufacture specialty fibers achieve market
acceptance;
|
|
●
|
develop
and maintain relationships with key vendors that will be necessary
to optimize the market value of the fibers we develop;
|
|
●
|
maintain
relationships with strategic partners that will be necessary to
manufacture the fibers we develop or develop relationships with
potential strategic partners which may license or distribute fiber
products that we develop;
|
|
●
|
respond
effectively to competitive pressures; or
|
|
●
|
recruit
and build a management team to accomplish our business
plan.
|
If we
are unable to accomplish these goals, our business is unlikely to
succeed.
As a result of our limited operating history, we may not be able to
correctly estimate our future operating expenses, which could lead
to cash shortfalls.
We have
a limited operating history from which to evaluate our
business. The Company did not have revenues in 2015 and
generated $31,858 in revenue in 2016, making it difficult to
predict when we will generate significant revenues or be
profitable. Our failure to develop a transgenic silkworm
would have a material adverse effect on our ability to continue
operating. Accordingly, our prospects must be considered in
light of the risks, expenses, and difficulties frequently
encountered by companies in an early stage of development. We
may not be successful in addressing such risks, and the failure to
do so could have a material adverse effect on our business,
operating results and financial condition.
Because
of this limited operating history and because of the emerging
nature of our fiber product we are producing, our historical
financial data is of limited value in estimating future operating
expenses. Our budgeted expense levels are based in part on
our expectations concerning future revenues. However, our
ability to generate any revenues depends largely on the market
acceptance of the fibers we develop, which is difficult to forecast
accurately.
Our
operating results may fluctuate as a result of a number of factors,
many of which are outside of our control. For these reasons,
comparing our operating results on a period-to-period basis may not
be meaningful, and you should not rely on our past results as any
indication of our future performance. Our quarterly and
annual expenses are likely to increase substantially over the next
several years depending upon the level of fiber development
activities. Our operating results in future quarters may
fall below expectations. Any of these events could adversely
impact our business prospects and make it more difficult to raise
additional equity capital at an acceptable price per
share.
We have limited intellectual property protection in overseas
markets, which could affect our ability to grow our markets and
increase our revenue.
The
intellectual property that we licensed from Notre Dame and the
University of Wyoming is covered by a series of US patents and US
patent applications with limited or no international patent
protection. Overseas competitors could be using the same
technology that we have licensed, which would affect our ability to
expand our markets beyond the United States. We are
aware that laboratories and potential competitors overseas are
using the “piggyback” gene splicing technology for the
genetic modification of silkworm. Such limited overseas
intellectual property could affect our ability to introduce fiber
products in overseas markets or effectively compete in such
markets.
The patents underlying our license agreements could expire prior to
our commercializing our specialty fibers, which would result in the
loss of our competitive edge and could negatively impact our
revenues and results of operations.
The
patent rights that we license could expire before we are ready to
market or commercialize any fiber product, or while we are still in
research and development of proposed products. In which
event the patents would be worthless and would not protect us from
potential competitors who would then have low barriers to entry and
who would be in a position to compete more effectively with
us.
Our license agreements restrict us from developing products for
certain markets.
Some,
but not all, of the gene sequences that we have licensed from Notre
Dame and the University of Wyoming are covered by restrictions in
the licensing agreement which preclude their use by us for sporting
goods and medical applications.
Our management has no previous experience in developing, marketing
or selling recombinant fiber which may have a negative effect
on our ability to develop or sell our products.
Our
current management has no previous experience in developing,
marketing or selling recombinant fiber and the other products that
we intend to develop and market. Additionally, our
current management has no experience in the business of scientific
research and development, which is critical to our
success. The inexperience of our management may
negatively affect our ability to succeed in developing, marketing
and/or distributing our proposed products.
We are unprepared for technological changes in our industry, which
could result in our products being obsolete or replaced by better
technology
.
The
industry in which we participate
is subject to rapid business and
technological changes. The business, technology,
marketing, legal and regulatory changes that could occur may have a
material adverse impact on us. New inventions and
product innovations may make our proposed products
obsolete. Other researches may develop and patent
technologies which make our line of research
obsolete. We may not have the financial or technical
ability to keep up with its competitors.
Our business is based on unproven scientific research and makes our
business highly risky
.
We are
engaging in research and development of new recombinant silk
fibers. Due to the speculative nature of this scientific
research, our chances of success are speculative and we cannot be
certain that we will succeed in developing new fibers or that our
use of novel transgenic methods will be successful. An
investment in us, therefore, is highly speculative and
risky.
The fibers we develop could expose us to product liability claims
and government regulation, which could have a negative impact on
our results of operations
.
The
fibers we are seeking to develop may subject us to product
liability claims if widely used, including but not limited to
design defect, environmental hazards, quality control, and
durability of product. This potential liability is
increased by virtue of the fact that our products in development
may be used as protective and safety materials. There is
tremendous potential liability to any person who is injured by, or
while using, one of our products. As a manufacturer, we
may be strictly liable for any damage caused by our
products. This liability might not be covered by
insurance, or may exceed any coverage that we may
obtain.
Additionally,
our products, if successfully developed, will be produced by means
of genetic engineering. These transgenic methods may
carry inherent environmental risks and the production of the
products may therefore also be heavily regulated by the
government. We may face changes in governmental
regulation policies and practices which could have a significant
adverse effect on us and our ability to develop, produce and market
any products.
Our operations would be negatively affected by any dispute with our
partner Universities or by labor unrest (such as disputes, strikes
or lockouts) between such Universities and its academic
staff
.
We have
signed intellectual property, sponsored research and collaborative
research agreements with one or more universities. The
continued cooperation of university(s), as well as the cooperation
of other institutions and or universities is essential for our
success of the Company. In the event of a material dispute with the
university(s), such a dispute could create a cessation of
operations for a period of time that could be detrimental to our
operations and survival. Additionally, in the event of a
material dispute between such universities and its employees could
create a cessation of operations for period of time that could be
detrimental to our product development.
Unforeseen circumstances may require us to use the proceeds from
the Equity Line Financing in a manner not set forth in the Use of
Proceeds section of this prospectus
.
Management
intends to use the proceeds from this offering, in part, to pay off
some of unpaid salary we owe to our Chief Executive Officer (which
we have accounted for as an accrued expense and which amount bears
interest at the rate of three percent per annum and is due on
demand by our Chief Executive Officer) and accounts payable,
including contractual obligations associated with this
offering. However, results of our research and
development may not go as we hope and we may have to conduct
further research and development that we currently do not expect
that we will have to do. In such event, the funds used
for these purposes will require us to raise additional
capital.
Our competitors are larger competitors with greater financial
resources than we have and we may face increased competition due to
the low barriers of entry to our industry
.
We
compete directly with numerous other companies with similar product
lines and/or distribution that have extensive capital, resources,
market share, and brand recognition. There are few
barriers to entry on the industry in which we
compete. This creates the strong possibility of new
competitors emerging, and of others succeeding in developing the
same or similar fibers that we are trying to
develop. The effects of this increased competition may
be materially adverse to us and our stockholders.
We may face various governmental regulation, which could increase
our costs and lower our future profitability
.
Governmental
regulation regarding import/export, taxes, transgenic, scientific
research and university based research, biological research;
transgenic product manufacture and distribution, environmental
regulation and packaging requirements may be adverse to our
operations, research and development, revenues, and potential
profit. We are especially at risk from governmental
restriction and regulations related to the development of materials
by use of transgenic organisms. Federal and state
regulations impose strict regulation on the use, storage, and
transportation of such transgenic organisms. Such rules
impose severe penalties on us for any breach of regulations, for
any spill, release, or contamination caused while the substances
are under our direct or indirect ownership or
control. We are not aware of any such breach of
governmental regulation, or of any spill, release, or
contamination. If such a release, or other regulatory
breach does occur in the future, the resulting clean-up costs,
and/or fines and penalties, would cause a material negative effect
on the Company and its financial future. In that event,
investors could expect to lose their entire
investment.
Risks Related to Our Stock
We may need to raise additional capital by sales of our Class A
common stock, which may adversely affect the market price of our
Class A common stock and your rights in us may be
reduced.
We
expect to continue to incur product development and selling,
general and administrative costs, and in order to satisfy our
funding requirements, we will need to sell additional equity
securities, in transactions similar in size and scope to our Equity
Line Financing covered by this prospectus. Such
additional sales of equity securities may be subject to
registration rights. The sale or the proposed sale of
substantial amounts of our Class A common stock in the public
markets may adversely affect the market price of our Class A common
stock and our stock price may decline substantially. Our
stockholders may experience substantial dilution and a reduction in
the price that they are able to obtain upon sale of their shares.
Also, new equity securities issued may have greater rights,
preferences or privileges than our existing Class A common
stock.
There is no assurance of an established public trading
market.
A
regular trading market for our Class A common stock may not be
sustained in the future. FINRA has enacted changes that limit
quotation on the OTCQB to securities of issuers that are current in
their reports filed with the SEC. The OTCQB is an inter-dealer,
over-the-counter quotation medium that provides significantly less
liquidity than a listing on the NASDAQ Stock Markets or other
national securities exchange. Quotes for stocks included on the
OTCQB are not listed in the financial sections of newspapers as are
those for the NASDAQ Stock Market. Therefore, prices for securities
traded solely on the OTCQB may be difficult to obtain and holders
of Class A common stock may be unable to resell their securities at
or near their original offering price or at any price. Market
prices for our Class A common stock will be influenced by a number
of factors, including:
|
●
|
the
issuance of new equity securities pursuant to a future
offering;
|
|
●
|
competitive
developments;
|
|
●
|
variations
in quarterly operating results;
|
|
●
|
change
in financial estimates by securities analysts;
|
|
●
|
the
depth and liquidity of the market for our Class A common
stock;
|
|
●
|
investor
perceptions of our company and the technologies industries
generally; and
|
|
●
|
general
economic and other national conditions.
|
Our Class A common stock is considered “a penny stock”
and, as a result, it may be difficult to trade a significant number
of shares of our Class A common stock.
The
Securities and Exchange Commission (“SEC”) has adopted
regulations that generally define “penny stock” to be
an equity security that has a market price of less than $5.00 per
share, subject to specific exemptions. Since our Class A common
stock has been eligible for quotation on the OTCQB, the market
price of our Class A common stock has been less than $5.00 per
share. As a result of our prior private placements, we will
have increased the number of shares outstanding by almost
ten-fold. Consequently, it is likely that the market price
for our Class A common stock will remain less than $5.00 per share
for the foreseeable future and therefore may be a “penny
stock” according to SEC rules. This designation requires any
broker or dealer selling these securities to disclose certain
information concerning the transaction, obtain a written agreement
from the purchaser and determine that the purchaser is reasonably
suitable to purchase the securities. These rules may restrict
the ability of brokers or dealers to sell our Class A common stock
and may affect the ability of investors hereunder to sell their
shares. In addition, because our Class A common stock is traded on
the OTC Markets, investors may find it difficult to obtain accurate
quotations of the stock and may experience a lack of buyers to
purchase such stock or a lack of market makers to support the stock
price.
We do not intend to pay dividends.
We have
never declared or paid any cash dividends on our securities. We
currently intend to retain our earnings for funding growth and,
therefore, do not expect to pay any dividends in the foreseeable
future.
Risk Factors Related to the Equity Line Financing and this
Offering
We are registering an aggregate of 110,000,000 shares of Class A
Common Stock to be issued under the Equity Line
Financing. The sale of such shares could depress the
market price of our Class A common stock.
We are
registering an aggregate of 110,000,000 shares of our Class A
common stock under the registration statement of which this
prospectus forms a part for issuance pursuant to the Equity Line
Financing. The sale of these shares into the public market by Calm
Seas could depress the market price of our common stock. As of
May, 2017, of the 110,000,000 shares of common stock initially
registered, 38,824,427 shares of common stock remain available for
registration. As of May 4, 2017there were 783,046,190 shares
of our common stock issued and outstanding.
We may not have access to the full amount under the Equity
Line.
As of
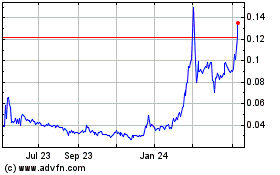
May 4, 2017, the closing market price of our common stock was
$0.07. There is no assurance that the market price of our Class A
common stock will increase substantially in the near future. The
entire commitment under the Equity Line Financing is $7,500,000 as
of the date of this prospectus. We would need to
maintain the market price of our Class A common stock at
approximately $0.172 in order to have access to the full amount
under the Equity Line Financing. Since January 1,
2012, the lowest and the highest prices at which our Class A common
stock has been trading were $0.017 and $0.13 per share,
respectively. We expect that, initially, the resale by Calm
Seas Capital of the shares of our Class A common stock that we will
sell to them under our Equity Line Financing will cause our stock
to decrease. However, if we are able to report positive
developments in our research and development efforts, we expect
that such announcements will cause our stock price to increase
sufficiently to a price per share above the price we need to allow
us to obtain $7,500,000 gross proceeds under the Equity Line
Financing. If we achieve our research and development
goals, we expect that the announcement of such achievements will
have a significant positive impact on the price of our Class A
common stock. Technology research and development is
very risky. We cannot be certain that we will achieve
our research and development goals. Any setbacks in our
research and development activities may cause our stock price to
drop, in which event we would probably not be able to raise
$7,500,000 under our Equity Line Financing. In the event
that we raise substantially less than the maximum proceeds we
expect to raise under the Equity Line Financing by the expiration
of their respective terms, we will seek to raise funds from other
capital sources. Alternatively, if our research yields
some promising or positive results, we may seek a corporate partner
in a joint venture or licensing arrangement in which we would seek
to negotiation an upfront licensing fee and/or capital investment
from such corporate partner. We have not yet
identified any corporate partners for any such joint venture or
licensing arrangement.
Calm Seas will pay less than the then-prevailing market price for
our Class A common stock.
The
Class A common stock to be issued to Calm Seas pursuant to the
Letter Agreement will be purchased at a twenty percent discount to
the lowest price of our Class A common stock during the five
consecutive trading days immediately following the date we deliver
to Calm Seas a notice of our election to put shares to it pursuant
to the Letter Agreement. Calm Seas has a financial incentive to
sell our Class A common stock immediately upon receiving the shares
to realize the profit equal to the difference between the
discounted price and the market price. If Calm Seas sells the
shares, the price of our Class A common stock could decrease. If
our stock price decreases, Calm Seas may have a further incentive
to sell the shares of our Class A common stock that it holds. These
sales may have a further impact on our stock price.
There may not be sufficient trading volume in our Class A common
stock to permit us to generate adequate funds from the exercise of
our put.
The
Letter Agreement provides that the dollar value that we will be
permitted to put to Calm Seas at each drawdown will be the lesser
of: (A) 200% of the average daily volume in the OTCQB of the Class
A common stock for the ten trading days prior to the date we
deliver to Calm Seas a notice of our put, multiplied by the average
of the ten daily closing prices immediately preceding the date we
deliver a put notice to Calm Seas, or (B) $100,000.
We will automatically
withdraw our put notice to Calm Seas if the lowest price used to
determine the purchase price of the put shares is not at least
equal to seventy-five percent (75%) of the average closing bid
price for our Class A common stock for the ten (10) trading days
prior to the put date. If the average daily trading
volume in our Class A common stock is too low, it is possible that
we would exercise a put for less than $100,000, which may not
provide adequate funding for our planned operations.
The selling shareholder may engage in hedging transactions, other
than short sales, which may result in broker-dealers or other
financial institutions engaging in short sales for their own
account and not for the benefit of the selling security holder,
which may cause a steep decline of our share price.
In
connection with the distribution of the Class A common stock or
otherwise, the selling shareholder may enter into hedging
transactions, other than short sales, with broker-dealers or other
financial institutions. In connection with such hedging
transactions, broker-dealers or other financial institutions may,
for their own account and not for the benefit of Calm Seas
Capital, engage in short sales of shares in the course of
hedging the positions they assume with the selling
shareholder. If there are significant short sales of our
stock by such broker-dealers or other financial institutions, the
price decline that would result from this activity will cause our
share price to decline which in turn may cause long holders of our
stock to sell their shares thereby contributing to sales of stock
in the market. If there is an imbalance on the sell side
of the market our stock the price will decline. It is not possible
to predict if the circumstances where by a short sales could
materialize or to what our share price could drop. In some
companies that have been subjected to short sales their stock price
has dropped to near zero. We cannot provide any assurances that
this situation will not happen to us.
Shares eligible for future sale by our current shareholders may
adversely affect our stock price.
Sales
of substantial amounts of Class A common stock, including shares
issued upon the exercise of outstanding options and warrants, under
Securities and Exchange Commission Rule 144 or otherwise could
adversely affect the prevailing market price of our common stock
and could impair our ability to raise capital at that time through
the sale of our securities.
If we fail to remain current on our reporting requirements, we
could be removed from the OTCQB, which would limit the ability of
Broker-Dealers to sell our securities and the ability of
shareholders to sell their securities in the secondary
market.
Companies
trading on the OTCQB, such as Kraig Biocraft Laboratories, must be
current in their reports under Section 13 of the Exchange Act, in
order to maintain price quotation privileges on the OTCQB. If
we fail to remain current on our reporting requirements, we could
be removed from the OTCQB. As a result, the market liquidity
for our securities could be adversely affected by limiting the
ability of broker-dealers to sell our securities and the ability of
shareholders to sell their securities in the secondary
market.
USE OF PROCEEDS
We will
not receive any proceeds from the sale of common stock offered by
Calm Seas. However, we will receive proceeds from the sale of
our common stock to Calm Seas pursuant to the put right under the
Letter Agreement. We intend to use the proceeds from our exercise
of the put options pursuant to the Letter Agreement for working
capital.
The
proceeds from the Equity Line Financing will be used for working
capital including employee salaries, the costs of our research and
development obligations under the agreement with the University of
Notre Dame, the cost of increasing our production, the costs
relating to commercializing our products, the costs related to
our operation as a public company (primarily, legal and accounting
fees) as well legal fees for securing our intellectual property,
rent and telecommunications (phone, fax and
Internet). Consequently, we believe that it is highly
likely that we will use all $7,500,000 of the proceeds we expect to
raise from the equity line financing. We also expect to
be able to raise the full $7,500,000 from the Equity Line
Financing. We believe that positive results of our
research and development efforts under our arrangement with the
University of Notre Dame will help increase our stock price and,
therefore, reduce the number of shares we will need to put to Calm
Seas in order to raise the full $7,500,000 in gross proceeds we are
seeking to raise under the equity line financing.
In the
event we are unable to raise the full $7,500,000 from the equity
line financing, we would use the proceeds in the following
priority: (i) research and development expenses, (ii) employee
salaries, cost of benefits and payroll taxes, (iii) rent and
telecommunications, (iv) legal expenses (both SEC and intellectual
property) and accounting expenses, press release and EDGAR filing
services and transfer agent expenses, (v) postage/shipping, office
equipment and office supplies, (vi) auto and travel expenses, (vii)
increasing production and commercialization of our products, (viii)
payments for a portion of the amount owed by the Company to its CEO
for the transfer of intellectual property to the Company, which has
been recorded in the financial statements as royalty payments due
to a related party, and (ix) reserves to cover expenses due to
contingent events.
In the
event that we raise substantially less than the maximum proceeds we
expect to raise under the equity line financing by the expiration
of their respective terms, we will seek to raise additional capital
from other investors.
SELLING SHAREHOLDER
Other than as described below, none of the Selling Stockholders nor
any of their affiliates has held any position or office with, been
employed by or otherwise has had any material relationship with us
or our affiliates during the three years prior to the date of this
prospectus. Unless otherwise indicated below, none of the Selling
Stockholders are broker-dealers or affiliates of a broker-dealer
within the meaning of Section 3 of the Securities Exchange
Act.
This prospectus relates to the offering and sale, from time to
time, of up to 110,000,000 shares of our common stock held by the
stockholders named in the table below. As of
May 4,
2017
, of the 110,000,000 shares of
common stock initially registered, 38,824,427 shares of common
stock remain available for registration.
The
table below lists the selling stockholders and other information
regarding the beneficial ownership (as determined under Section
13(d) of the Securities Exchange Act of 1934, as amended, and the
rules and regulations thereunder) of our common stock with respect
to the securities held by each of the selling stockholders. The
second column lists the number of shares of common stock
beneficially owned by each of the selling stockholders as of May 4,
2017. The third column lists the shares of common stock being
offered by this prospectus by the selling stockholders and does not
take into account any ownership caps or limitations. The fourth
column assumes the sale of all of the shares offered by the selling
stockholders pursuant to this prospectus. However, the selling
stockholders may sell all, some or none of their shares in this
offering. See “Plan of Distribution.”
We estimate that our costs and expenses of registering the shares
listed herein for resale will be approximately
$
23,873.91.
On
September 14, 2009, we entered into the Amended Letter Agreement
with Calm Seas to raise up to $1,000,000 through an equity line
financing. On June 28, 2011 we entered into the Letter
Agreement with Calm Seas to raise up to $1,500,000 through an
equity line financing. On April 30, 2013, we entered
into the Letter Agreement with Calm Seas to raise up to $2,500,000
through an equity line financing. On October 2, 2014, we entered
into the Letter Agreement with Calm Seas to raise up to $7,500,000
through an equity line financing. Except as described above, to our
knowledge Calm Seas has not had a material relationship with us
during the last three years, other than as an owner of our common
stock or other securities.
During
the fiscal year 2016, the Company exercised a total of nine puts
under the Calm Seas equity line for an aggregate amount of
$1,025,000. For seven puts, the Company’s stock price
declined in the five-week price declined by approximately 3.6% to
34.9%. For two puts, the Company’s stock price
increased in the five-week period by approximately 5.1% to
65%.
During
fiscal year 2015, the Company exercised a total of seven puts under
the Calm Seas equity line for an aggregate amount of $750,000 and
the Company’s stock price in year 2015 ranged from $0.024 to
$0.052. For the seven puts, the Company’s stock price
declined in the five-week price declined by approximately 8.2% to
40.3%.
During
fiscal year 2014, the Company exercised a total of ten puts under
the Calm Seas equity line for an aggregate amount of $1,150,000 and
the Company’s stock price in year 2014 ranged from $0.037 to
$0.067. Of the ten puts, the Company’s stock price remained
stable in the five-week period following one put, our stock price
declined by approximately 2.6% to 19.8% in the five-week period
following five puts, and our stock price increased by approximately
1.2% to 9.1% during the five-week period following four
puts.
During
fiscal year 2013, the Company exercised a total of twelve puts
under the Calm Seas equity line for an aggregate amount of
$1,050,000 and the Company’s stock price in year 2013 ranged
from $0.036 to $0.136. Of the twelve puts, the Company’s
stock price remained stable in the five-week period following three
puts, our stock price declined by approximately 13% to 25% in the
five-week period following four puts, and our stock price increased
by approximately 14% to 86% during the five-week period following
five puts.
During
fiscal year 2012, the Company exercised a total of five puts under
the Calm Seas equity line for an aggregate amount of $425,000 and
the Company’s stock price in year 2012 ranged from $0.03 to
$0.108. Of the five puts, the Company’s stock price remained
stable in the five-week period following three puts, our stock
price declined by 17% in the five-week period following one put,
and our stock price increased by approximately 100% during the
five-week period following one put.
During
fiscal year 2011, the Company exercised a total of seven puts under
the Calm Seas equity line for an aggregate amount of $700,000 and
the Company’s stock price in year 2011 ranged from $0.06 to
$0.161. Of the seven puts, the Company’s stock price remained
stable in the five-week period following two puts, our stock price
declined by approximately 20% in the four-week period following
three puts, and our stock price increased by approximately 25% to
30% during the four to five weeks after two puts.
During
fiscal year 2010, the Company exercised a total of five puts under
the Calm Seas equity line for an aggregate amount of $245,000 and
the Company’s stock price in year 2010 ranged from $0.01 to
$0.24. Of the five puts, the Company’s stock price
declined by approximately 30% in the four-week period following one
put, the stock price increased between 15% and 400% over the
four-week period following the four puts.
In
addition, in fiscal year 2010, there were a total of two issuances
for a total of 6,548,620 shares to Calm Seas as a result of the
conversion of twelve convertible notes acquired by Calm Seas and a
total of one issuance of 6,000,000 shares as a result of the
exercise of twelve warrants held by Calm Seas. Following the
aforementioned 3 issuances to Calm Seas Capital, the
Company’s stock price remained flat in the four-week period
following two issuances, and the Company’s stock price
declined by approximately 20% in the four-week period subsequent to
one issuance. In 2010 there was one issuance to Calm Seas Capital
for a total of 4,000,000 shares as the result of the
Company’s sale of stock to Calm Seas Capital for proceeds of
$28,632. Our review of the four-week period following that sale of
stock shows that our stock price made no significant
movement.
Our
capital raises through our equity line financings have been
exclusively with Calm Seas Capital. We have not entered into
any financing agreements with any affiliate of Calm Seas Capital.
We have not issued any put to any affiliate of Calm Seas Capital,
nor have we issued any stock in exchange for financing with any
affiliate of Calm Seas Capital.
An
equity line financing, such as our past equity lines with Calm Seas
Capital by its nature, increases the number of outstanding shares
and places downward pressure on stock price. We have reviewed
our past equity lines with Calm Seas Capital and have not
identified any direct correlation between the issuance
of shares to Calm Seas Capital pursuant to the puts
under the past equity lines and changes in our stock price
following such issuance. However, in aggregate, our stock
price has declined since its highs in 2010 and during the same time
period we have been issuing shares pursuant to the puts to Calm
Seas Capital under our past equity lines. Such puts do tend to put
downward pressure on stock prices. Some portion of the
reduction in our share price since 2010, which we cannot estimate,
can be attributed to the past equity line
financings. However, the Company believes the downward
pressure on our stock price since its high 2010 is also related to
fundamental factors concerning our path to commercialization and
the fact that we have not yet commercialized our Monster
Silk™ product.
Of the
total proceeds received from the Calm Seas equity lines, the
Company paid to the University of Notre Dame, under the
Intellectual Property/ Collaborative Research Agreement, research
and development costs of $177,800 in 2011, $315,990 in 2012,
$428,394 in 2013, 439,536 in 2014, $432,008 in 2015, and $397,136
in 2016. In addition, the Company has paid to the
University of Notre Dame for reimbursement of costs associated with
the filing and maintaining of patent applications relating to the
technology developed within the University laboratories for which
the Company has exclusive commercialization rights; $110,736 in
2013, $81,546 in 2014, $170,068 in 2015, and $107,801 in 2016. The
Company utilized the remaining proceeds for general corporate
purposes such as payroll, rent and legal expenses, etc. With the
funds from the equity lines, the Company was able to continue its
endeavor to build its intellectual property portfolio and advance
its technology with the goal of commercialization, which the
Company believes will ultimately drive up the value of our stock
price.
Except
as described above, to our knowledge Calm Seas has not had a
material relationship with us during the last three years, other
than as an owner of our common stock or other
securities.
Beneficial
Ownership of Class A Common Shares Prior to this
Offering
|
Number
of Shares
to
be Sold
|
Beneficial
Ownership of Class A Common Shares after this Offering
|
|
Selling
Shareholder
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calm Seas Capital,
LLC. (2)
|
110,000,000
|
13.9
%
|
38,824,427
|
0
|
0
%
|
|
377 S. Nevada
St.
|
|
|
|
|
|
|
Carson City, NV
89703
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
110,000,000
|
13.9
%
|
38,824,427
|
0
|
0
%
|
|
(1)
|
Based
on 786,903,348 shares of Class A common stock outstanding after the
completion of the offering.
|
|
(2)
|
Calm
Seas Capital, LLC is a Nevada limited liability company.
Michael McCarthy is the managing member of Calm Seas with
voting and investment power over the shares.
|
Equity Line Financing
The
selling security holder is reselling shares of our Class A common
stock sold to it by our exercise of the put right under the Letter
Agreement. Each month we may put up to an aggregate of
$200,000 of our Class A common stock to Calm Seas, which will
purchase such shares at a price per share equal to 80% of the
lowest price of our Class A common stock during the five
consecutive trading days immediately following the date the notice
of our election to put shares pursuant to the Letter Agreement is
delivered to Calm Seas (the date of delivery of such notice is
referred to as the “put
date”). Notwithstanding the $200,000 ceiling for
each monthly put, if both we and Calm Seas agree, we may submit one
or more additional puts during any given month to the extent we
need additional capital for our operations and/or our product
development. We can only submit such additional put(s)
if Calm Seas Capital agrees to it. Furthermore, the
additional put is subject to the $7,500,000 limitation of this
offering. The additional put allows us to obtain
additional capital in the event that our product development
proceeds quicker than we expect.
We will
automatically withdraw our put notice to Calm Seas if the lowest
price used to determine the purchase price of the put shares is not
at least equal to seventy-five percent (75%) of the average closing
bid price for our Class A common stock for the ten (10) trading
days prior to the put date.
The
selling shareholder will not receive any compensation, fees or
commissions under the Letter Agreement.
We
expect to be able to raise the full $7,500,000 from the Equity Line
Financing available as of the date of this
prospectus. We believe that if we achieve positive
results from our research and development efforts those results may
help increase our stock price and, therefore, reduce the number of
shares we will need to put to Calm Seas in order to raise the full
$7,500,000 in gross proceeds we are seeking to raise under the
Equity Line Financing.
Our
equity line with Calm Seas Capital contemplates our future possible
issuance of up to an aggregate 110,000,000 shares of our Class A
common stock as a result of this registration statement, subject to
certain restrictions. Currently, we believe it is likely we will
need to draw the full amount available under this equity line prior
to the expiration of the equity line. If the terms and conditions
of the equity line are satisfied, and we choose to exercise our put
rights to the fullest extent permitted and sell all of the
110,000,000 shares of our common stock to Calm Seas Capital, the
ownership by our existing non-affiliate stockholders will be
diluted by approximately 7.1% based on 549,054,423 shares of Class
A common stock held by non-affiliates on May 4, 2017. If we issue
all of the shares under the equity line, we would
have 786,903,348 shares issued and outstanding, of which
542,383,085 shares will be held by non-affiliates, resulting in a
10.6% increase in the public float. We expect that the initial
issuance of the shares under the equity line and subsequent resale
by Calm Seas Capital will probably cause our share price to
decrease. However, we also expect that with a substantial amount of
the proceeds from the equity line being spent on research and
development activities we may be able to offset such downward
pressure on our stock price with announcements of our ongoing
research and development. If our research and development efforts
are positive, we would expect that the announcement of such result
would cause our share price to increase. Conversely, if our
research and development efforts fail to produce positive results,
we would expect that such result would cause a significant decrease
in our stock price. As of May 4, 2017, of the 110,000,000
shares of common stock initially registered, 38,824,427 shares of
common stock remain available for registration.
In the
event that we raise substantially less than the maximum proceeds we
expect to raise under the Equity Line Financing by the expiration
of their respective terms, we will seek to raise additional capital
from other sources.
Bridge Investment
Pursuant
to the 2009 Letter Agreement, Calm Seas Capital made a Bridge
Investment in the Company in the aggregate amount of $120,000, of
which $100,000 was paid promptly after the 2009 Letter Agreement
was signed in July 2009 and the remaining $20,000 was paid in late
September 2009. In this Bridge Investment, Calm Seas
Capital purchased (i) twelve convertible debentures, each in the
principal amount of $10,000 (the “Bridge Debentures”)
and (ii) twelve warrants each exercisable for the purchase of
500,000 shares (the “Bridge Warrants”). In
February and April 2010, Calm Seas Capital has converted $115,000
of the principal amount of the debentures into shares of our Class
A common stock, and $5,000 of convertible debt remained
outstanding. At December 31, 2010, pursuant to the agreement, all
outstanding principal and accrued interest on the convertible debt
was due, and the conversion rights of the holder terminated. In
addition, on October 4, 2010, the Company issued 5,177,801 shares
in connection with the cashless exercise of the 6,000,000 Bridge
Warrants. In 2013, the note holder forgave the $5,000 convertible
note balance along with accrued interest of $1,775.
PLAN OF DISTRIBUTION
The
purpose of this prospectus is to permit the selling shareholder to
offer and sell up to an aggregate of 110,000,000 shares at such
times and at such places as it chooses. The decision to sell
any shares is within the sole discretion of the holder
thereof.
The
distribution of the Class A common stock by the selling shareholder
may be effected from time to time in one or more transactions.
Any of the Class A common stock may be offered for sale, from
time to time, by the selling shareholder, or by permitted
transferees or successors of the selling shareholder, or otherwise,
at prices and on terms then obtainable, at fixed prices, at prices
then prevailing at the time of sale, at prices related to such
prevailing prices, or in negotiated transactions at negotiated
prices or otherwise.
The
Class A common stock may be sold by one or more of the
following:
|
●
|
On the
OTCQB or any other national common stock exchange or automated
quotation system on which our Class A common stock is traded, which
may involve transactions solely between a broker-dealer and its
customers which are not traded across an open market and block
trades.
|
|
●
|
Through
one or more dealers or agents (which may include one or more
underwriters), including, but not limited to:
|
|
|
-
Block
trades in which the broker or dealer as principal and resale by
such broker or dealer for its account pursuant to this
prospectus.
|
|
|
-
Purchases
by a broker or dealer as principal and resale by such broker or
dealer for its account pursuant to this prospectus.
|
|
●
|
Ordinary
brokerage transactions.
|
|
●
|
Directly
to one or more purchasers.
|
|
●
|
A
combination of these methods.
|
Calm
Seas and any broker-dealers who act in connection with the sale of
its shares are “underwriters” within the meaning of the
Securities Act, and any discounts, concessions or commissions
received by them and profit on any resale of the shares as
principal may be deemed to be underwriting discounts, concessions
and commissions under the Securities Act.
In
connection with the distribution of the Class A common stock or
otherwise, the selling shareholder may enter into hedging
transactions with broker-dealers or other financial institutions.
In connection with such transactions, broker-dealers or other
financial institutions may, for their own account and not for the
benefit of the selling shareholder, engage in short sales of shares
in the course of hedging the positions they assume with the
selling shareholder. Under the Letter Agreement, the selling
shareholder cannot sell shares short. The selling shareholder may
enter into options or other transactions with broker-dealers or
other financial institutions which require the delivery to such
broker-dealers or other financial institutions of the Class A
common stock, which shares such broker-dealers or financial
institutions may resell pursuant to this prospectus, as
supplemented or amended to reflect that transaction. The selling
shareholder may also pledge the common stock registered hereunder
to a broker-dealer or other financial institution and, upon a
default, such broker-dealer or other financial institution may
affect sales of the pledged shares pursuant to this prospectus, as
supplemented or amended to reflect such transaction.
The
selling shareholder or its underwriters, dealers or agents may sell
the Class A common stock to or through underwriters, dealers or
agents, and such underwriters, dealers or agents may receive
compensation in the form of discounts or concessions allowed or
re-allowed. Underwriters, dealers, brokers or other agents engaged
by the selling shareholder may arrange for other such persons to
participate. Any fixed public offering price and any discounts and
concessions may be changed from time to time. Underwriters, dealers
and agents who participate in the distribution of the common stock
may be deemed to be underwriters within the meaning of the
Securities Act, and any discounts or commissions received by them
or any profit on the resale of shares by them may be deemed to be
underwriting discounts and commissions thereunder. The proposed
amounts of the Class A common stock, if any, to be purchased by
underwriters and the compensation, if any, of underwriters, dealers
or agents will be set forth in a prospectus
supplement.
Unless
granted an exemption by the Commission from Regulation M under the
Exchange Act, or unless otherwise permitted under Regulation M, the
selling shareholder will not engage in any stabilization
activity in connection with our Class A common stock, will furnish
each broker or dealer engaged by the selling shareholder and each
other participating broker or dealer the number of copies of this
prospectus required by such broker or dealer, and will not bid for
or purchase any Class A common stock of our or attempt to induce
any person to purchase any of the Class A common stock other than
as permitted under the Exchange Act.
We will
not receive any proceeds from the sale of these shares of Class A
common stock offered by the selling shareholder. We shall use our
best efforts to prepare and file with the Commission such
amendments and supplements to the registration statement and this
prospectus as may be necessary to keep such registration statement
effective and to comply with the provisions of the Securities Act
with respect to the disposition of the common stock covered by the
registration statement for the period required to effect the
distribution of such common stock.
We have
agreed to pay all expenses of the registration of the shares of
common stock; provided, however, that the Selling Stockholder will
pay all underwriting discounts and selling commissions, if
any.
In
order to comply with certain state securities laws, if applicable,
the common stock will be sold in such jurisdictions only through
registered or licensed brokers or dealers. In certain states the
shares of common stock may not be sold unless they have been
registered or qualify for sale in such state or an exemption from
registration or qualification is available and is complied
with.
DESCRIPTION OF SECURITIES
General
Our
original articles of incorporation authorized 60,000,000 shares of
Class A common stock, 25,000,000 shares of Class B common stock
with no par value per share and 10,000,000 shares of preferred
stock with no par value per share. On March 18, 2009, we amended
our articles of incorporation to provide for unlimited authorized
shares, no par value, of Class A common stock and Class B common
stock, and preferred stock. There are no provisions in our charter
or by-laws that would delay, defer or prevent a change in our
control.
Common Stock
As of
May 4, 2017, 783,046,190 shares of common stock Class A were issued
and outstanding and held by 30 shareholders of record, and we had
no shares of Class B common issued and outstanding. Holders of our
common stock are entitled to one vote for each share on all matters
submitted to a stockholder vote.
Holders of Class A common stock and Class B common stock do not
have cumulative voting rights
.
Holders
of a majority of the shares of both classes of common stock voting
for the election of directors can elect all of the directors.
Holders of both classes of our common stock representing a majority
of the voting power of our capital stock issued and outstanding and
entitled to vote, represented in person or by proxy, are necessary
to constitute a quorum at any meeting of our stockholders. A vote
by the holders of a majority of our outstanding shares is required
to effectuate certain fundamental corporate changes such as
liquidation, merger or an amendment to our Articles of
Incorporation.
Although
there are no provisions in our charter or by-laws that may delay,
defer or prevent a change in control, we are authorized, without
shareholder approval, to issue shares of preferred stock that may
contain rights or restrictions that could have this
effect.
Holders
of both classes of common stock are entitled to share in all
dividends that the board of directors, in its discretion, declares
from legally available funds. In the event of liquidation,
dissolution or winding up, each outstanding share entitles its
holder to participate pro rata in all assets that remain after
payment of liabilities and after providing for each class of stock,
if any, having preference over the common stock. Holders of both
classes of our common stock have no pre-emptive rights, no
conversion rights and there are no redemption provisions applicable
to our common stock.
Preferred Stock
Our
Board of Directors has the authority, without further action by the
shareholders, to issue from time to time the preferred stock in one
or more series for such consideration and with such relative
rights, privileges, preferences and restrictions that the Board may
determine. The preferences, powers, rights and restrictions of
different series of preferred stock may differ with respect to
dividend rates, amounts payable on liquidation, voting rights,
conversion rights, redemption provisions, sinking fund provisions
and purchase funds and other matters. The issuance of preferred
stock could adversely affect the voting power or other rights of
the holders of common stock.
Effective
December 17, 2013, the Company amended its Articles of
Incorporation to designate Series A of the Company’s
preferred stock, no par value. Under the amendment, there are two
shares of Series A preferred stock authorized. The holder of each
share of the Series A preferred stock is entitled to vote together
with the holders of the Company’s common stock on all matters
upon which the Company’s stockholders may
vote.
Each
share of Series A preferred stock is entitled to 200,000,000 votes
on all such matters. Each share of Series A Preferred Stock is
convertible into one share of the Company’s common stock at
the holder’s option. On December 19, 2013, the Company
issued two shares of Series A preferred stock to Kim Thompson, the
Company’s founder and CEO.
The
shares of Series A preferred were issued to Mr. Thompson in
exchange for an agreement to extend to October, 30, 2014 the date
on which the Company would pay certain debts owed to Mr.
Thompson. As part of the transaction, Mr. Thompson also
agreed to forgive $30,000 which the Company owed to him as
compensation. In connection with the transaction, the Company
incurred a loss on settlement of debt of $5,187,800.
Dividends
Since
inception we have not paid any cash dividends on our Class A
common stock. We currently do not anticipate paying any cash
dividends in the foreseeable future on our common stock, when
issued pursuant to this offering. Although we intend to retain our
earnings, if any, to finance the exploration and growth of our
business, our Board of Directors will have the discretion to
declare and pay dividends in the future. Payment of dividends in
the future will depend upon our earnings, capital requirements, and
other factors, which our Board of Directors may deem
relevant.
INTERESTS OF NAMED EXPERTS AND COUNSEL
No
expert or counsel named in this prospectus as having prepared or
certified any part of this prospectus or having given an opinion
upon the validity of the securities being registered or upon other
legal matters in connection with the registration or offering of
the common stock was employed on a contingency basis, or had, or is
to receive, in connection with the offering, a substantial
interest, direct or indirect, in the registrant or any of its
parents or subsidiaries. Nor was any such person connected with the
registrant or any of its parents or subsidiaries as a promoter,
managing or principal underwriter, voting trustee, director,
officer, or employee.
Legal Matters
Certain legal matters with respect to the shares of common stock
offered hereby will be passed upon for us by Hunter
Taubman Fischer & Li LLC, 1450 Broadway,
26
th
Floor, New York, New York
10018.
Experts
The
financial statements for the years ended December 31, 2016 and 2015
included in this prospectus and the registration statement have
been audited by M&K CPAS, PLLC, to the extent and for the
periods set forth in its report appearing elsewhere herein and in
the registration statement, and are included in reliance upon such
report given upon the authority of the said firm as expert in
auditing and accounting.
DESCRIPTION OF BUSINESS
Overview
Kraig Biocraft Laboratories, Inc. is a corporation organized under
the laws of Wyoming on April 25, 2006. We were organized to develop
high strength fibers using recombinant DNA technology, for
commercial applications in both the specialty fiber and technical
textile industries. Specialty fibers are engineered for specific
uses that require exceptional strength, flexibility, heat
resistance and/or chemical resistance. The specialty fiber market
is exemplified by two synthetic fiber products: aramid fibers and
ultra-high molecular weight polyethylene fiber. The technical
textile industry involves products for both industrial and consumer
products, such as filtration fabrics, medical textiles (e.g.,
sutures and artificial ligaments), safety and protective clothing
and fabrics used in military and aerospace applications (e.g.,
high-strength composite materials).
We are using genetic engineering technologies to develop fibers
with greater strength, resiliency and flexibility for use in our
target markets, namely the textile, specialty fiber and technical
textile industries.
Collaborative Research and Licensing
In 2006, the Company entered into a licensing agreement with the
University Of Wyoming, which granted the Company the exclusive
global rights to use and commercialize patented genetic sequences
in silkworm. In exchange for this license the University
of Wyoming received $10,000 cash payment and the University of
Wyoming Foundation received 17,050,000 shares of the
Company’s common stock. Under the terms of the licensing
agreement, the Company is obligated to provide annual license fees
of $10,000 and support the University research with $13,700
annually. No royalties are required. This agreement has
remained unchanged since 2006. The Company has not
signed any other agreements with the University of
Wyoming.
In 2007, the Company entered into the first of a series of
collaborative research agreements with the University of Notre
Dame (“Notre Dame”). The Company is contractually
obligated to financially support the ongoing research and
development of transgenic silkworms and the creation of recombinant
silk fibers. In exchange, the Company has an option to obtain the
exclusive global commercialization rights to the technology
developed pursuant to the research effort.
The distinction between the successive collaborative research
agreements with the University of Notre Dame has been the level of
financial support that the Company has agreed to
provide. The trend has been for an increase in financial
support for the research and development in nearly every successive
agreement. In June 2012, we entered into the
Intellectual Property / Collaborative Research Agreement with Notre
Dame (“2012 Notre Dame Research Agreement”). On March
4, 2015 we entered into a new Intellectual Property / Collaborative
Research Agreement with Notre Dame extending the agreement through
March 2016 (“2015 Notre Dame Research Agreement”).
Under the 2015 Notre Dame Research agreement the Company will
provide approximately $534,000 in financial support.
In 2011, the Company exercised its option to obtain the global
commercialization rights to the technology developed under the
collaborative research agreements with Notre Dame. That
has resulted in a separate license agreement with Notre
Dame. Pursuant to that license agreement, Notre Dame has
filed an international patent application and numerous national
patent applications on technology relating to the creation and use
of recombinant spider silks. The license agreement
obligates the Company to reimburse the University for costs
associated with the filing, prosecuting and maintaining of such
patents and patent applications. In exchange for the
rights to commercialization, Notre Dame has received 2,200,000
shares of the Company’s common stock and the Company has
agreed to pay Notre Dame royalties of 2% of the Company’s
gross sales of the licensed products and 10% of any sublicensing
fees received by the Company on licensed technology. The Company
has also agreed to pay to Notre Dame $50,000 a year, which is
credited toward any royalties that are due. The $50,000 payment to
Notre Dame is not owed for any year in which the Company is
sponsoring research within Notre Dame.
On
October 15, 2013, the Company entered into an intellectual property
agreement with a scientific researcher relating to the development
of new recombinant silk fibers. Under the terms of that
agreement the scientific researcher will transfer to the Company
his rights to intellectual property, inventions and trade secrets
which the researcher develops relating to recombinant
silk. The researcher will receive 8,000,000 warrants of
the Company’s stock, exercisable 24 months from the date of
the agreement. The researcher will also receive
additional warrants when and if the researcher develops advanced
recombinant silk fibers for the Company’s
use. Under the terms of the agreement the researcher
will receive 10,000,000 warrants in the event that he develops a
new recombinant silk fiber with certain performance
characteristics, and another 10,000,000 warrants if he develops a
second recombinant silk fiber with certain
characteristics. If the consultant performs the contract
in good faith the consultant will be entitled to an additional
8,000 warrants. The warrants described above all
contain a cashless exercise provision and are exercisable on the 24
month anniversary of the date on which they were issuable under the
agreement.
On
February 17, 2014, the Company entered into two consulting
agreements with two consultants for independent technical expertise
to further the Company’s business plans and scientific
research and development. As consideration for the
services performed, the Company agrees to issue the following to
each of the consultants:
|
●
|
Within
30 days of the date of this agreement, a warrant for six hundred
thousand shares of the Company’s common stock to be
exercisable on the 14 month anniversary of this agreement for a
period of 12 months with a cashless exercise provision. Such
warrant has been issued as of the date of this
prospectus.
|
|
●
|
Within
30 days of the date of this agreement, a warrant for one million
shares of the Company’s common stock to be exercisable on the
20 month anniversary of this agreement for a period of 12 months
with a cashless exercise provision. Such warrant has been issued as
of the date of this prospectus.
|
|
●
|
Within
30 days of the date of this agreement, a warrant for two million
shares of the Company’s common stock to be exercisable on the
32 month anniversary of this agreement for a period of 12 months
with a cashless exercise provision. Such warrant has been issued as
of the date of this prospectus.
|
|
●
|
Based
on the consultants reaching two sets of benchmarks, two separate
warrants for one million five hundred thousand shares of the
Company’s common stock to be exercisable on the 28 month
anniversary of this agreement for a period of 12 months with a
cashless exercise provision. Such warrant has not been issued as of
the date of this prospectus.
|
|
●
|
On the
three year anniversary, assuming the consultant acted in good faith
and the Company’s board of directors approval, a warrant for
one million five hundred thousand shares of the Company’s
common stock to be exercisable on the 28 month anniversary of this
agreement for a period of 12 months with a cashless exercise
provision. Such warrant has not been issued as of the date of this
prospectus.
|
As of
the date hereof, the Company has issued a total of 7,200,000
warrants under the foregoing two consulting
agreements.
On
October 2, 2014, the Company entered into a letter agreement for an
equity line of financing up to $7,500,000 (the “Letter
Agreement”) with Calm Seas Capital, LLC (“Calm
Seas”).
Under
the Letter Agreement, over a 24 month period from the effective
date of a registration statement covering shares issuable to Calm
Seas (the "Effective Date") we may put to Calm Seas up to an
aggregate of $7,500,000 in shares of our Class A common stock for a
purchase price equal to 80% of the lowest price of our Class A
common stock during the five consecutive trading days immediately
following the date we deliver notice to Calm Seas of our election
to put shares pursuant to the Letter Agreement. We may put shares
bi-monthly. The dollar value that will be permitted for each put
pursuant to the Letter Agreement will be the lesser of: (A) the
product of (i) 200% of the average daily volume in the US market of
our Class A common stock for the ten trading days prior to the date
we deliver our put notice to Calm Seas multiplied by (ii) the
average of the daily closing prices for the ten (10) trading days
immediately preceding the date we deliver our put notice to Calm
Seas, or (B) $100,000. We will automatically withdraw our put
notice to Calm Seas if the lowest closing bid price used to
determine the purchase price of the put shares is not at least
equal to seventy-five percent (75%) of the average closing
“bid” price for our Class A common stock for the ten
(10) trading days prior to the date we deliver our put notice to
Calm Seas. Notwithstanding the $100,000 ceiling for each bi-monthly
put, as described above, we may at any time request Calm Seas to
purchase shares in excess of such ceiling, either as a part of
bi-monthly puts or as an additional put(s) during such month. If
Calm Seas, in its sole discretion, accepts such request to purchase
additional shares, then we may include the put for additional
shares in our monthly put request or submit an additional put for
such additional shares in accordance with the procedure set forth
above.
The
Letter Agreement will terminate when any of the following events
occur:
●
Calm Seas has purchased an aggregate of $7,500,000 of our Class A
common stock; or
●
The second anniversary from the Effective Date.
As of
May 4, 2017, 71,175,573 shares of common stock were issued pursuant
to the Letter Agreement.
On June
22, 2015, the Company entered into an agreement with a consultant
pursuant to which the consultant would provide investor relations
services. The agreement commenced on June 22, 2015 and continued
until December 16, 2015. As agreed in the agreement and as a
consideration for the services performed, on June 22, 2015, the
Company issued the consultant a three year warrant to purchase
15,000,000 shares of common stock which carries a cashless exercise
provision with a fair value of $590,335.
On
December 30, 2015, the Company entered into a cooperative agreement
for the research and pilot production of hybrid silkworms in
Vietnam. Under this agreement the Company will establish a
subsidiary in Vietnam where it will develop and produce hybrid
silkworms. As of December 31, 2016, the subsidiary was not yet
established and no work has been performed in Vietnam for the year
ended December 31, 2016. The Company delayed the announcement of
this agreement until late in February, 2016. This additional time
was used to confirm this agreement with higher level authorities
and outside review.
The Market
We are focusing our work on the creation of new fibers with unique
properties including fibers with potential high performance and
technical fiber applications. The performance fiber market is
exemplified by two classes of product: aramid fibers, and
ultra-high molecular weight polyethylene fiber. These products
service the need for materials with high strength, resilience, and
flexibility. Because these synthetic performance fibers are
stronger and tougher than steel, they are used in a wide variety of
military, industrial, and consumer applications.
Among the users of performance fibers are the military and police,
which employ them for ballistic protection. The materials are also
used for industrial applications requiring superior strength and
toughness, i.e. critical cables and abrasion/impact resistant
components. Performance fibers are also employed in safety
equipment, high strength composite materials for the aero-space
industry and for ballistic protection by the defense
industry.
The global market for technical textiles has been estimated at
greater than $133 billion (1).
These
are industrial materials which have become essential products for
both industrial and consumer applications. The market for technical
textiles can be defined as consisting of:
|
●
|
Textiles
used in Defense and Military;
|
|
●
|
Safe
and Protective Clothing;
|
|
●
|
Textiles
used in Transportation;
|
|
●
|
Textiles
used in Buildings;
|
|
●
|
Composites
with Textile Structure;
|
|
●
|
Functional
and Sportive Textiles.
|
We
believe that the superior mechanical characteristics of the next
generation of protein-based polymers (in other words, genetically
engineered silk fibers), will open up new applications for the
technology. The materials which we are working to produce are many
times tougher and stronger than steel. These fibers are often
referred to as “super fibers.”
(1)
https://globenewswire.com/news-release/2015/08/04/757406/10144484/en/Global-Technical-Textiles-Market-to-Reach-US-160-38-Billion-owing-to-Innovative-Product-Development-Transparency-Market-Research.html
The Product
Certain
fibers produced in nature possess unique mechanical properties in
terms of strength, resilience and flexibility.
Comparison of the Properties of Spider Silk and Steel
|
|
|
Material Toughness1
|
|
|
Tensile Strength2
|
|
|
Weight3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dragline
spider silk
|
|
120,000-160,000
|
|
|
1,100-2,900
|
|
|
1.18-1.36
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Steel
|
|
2,000-6,000
|
|
|
300-2,000
|
|
|
7.84
|
|
_______________
1
Measured by the energy required to break a continuous
filament, expressed in joules per kilogram (J/kg). A .357 caliber
bullet has approximately 925 joules of kinetic energy at
impact.
2
Tensile strength refers to the greatest longitudinal
stress the fiber can bear, measured by force over area in units of
newtons per square meter. The measurement here is in millions of
pascals.
3
In grams per cubic centimeter of
material.
This
comparison table was the result of research performed by Randolph
Lewis, Ph.D. at the University of Wyoming. Such work was summarized
in an article entitled “Spider Silk: Ancient Ideas for New
Biomaterials” which was published in Chemicals Review, volume
106, issue 9, pages 3672 – 3774. The measurements in joules
in the table above are a conversion from Dr. Lewis’
measurements in newtons/meter squared.
We
believe that the genetically engineered protein-based fibers we
seek to produce have properties that are in some ways superior to
the materials currently available in the marketplace. For example,
as noted above, the ability of spider silk to absorb in excess of
100,000 joules of kinetic energy makes it a potentially ideal
material for structural blast protection.
Production
of this material in commercial quantities holds the potential of a
life-saving ballistic resistant material, which is lighter,
thinner, more flexible, and tougher than steel. Other applications
for spider silk based recombinant fibers include use as structural
material and for any application in which light weight and high
strength are required. We believe that fibers made with recombinant
protein-based polymers will make significant inroads into the
specialty fiber and technical textile markets.
While
the properties of spider silks are well known, there was no known
way to produce these fibers in commercial quantity. The spiders are
cannibalistic, and cannot be raised in concentrated
colonies.
Our Technology
While
scientists have been able to replicate the proteins that are the
building blocks of spider silk, the technological barrier that has
stymied production until now has been the inability to form these
proteins into a fiber with the desired mechanical characteristics
and to do so in a cost effective manner.
We have
licensed the right to use the patented genetic sequences and
genetic engineering technology developed in university
laboratories. The Company has been working collaboratively with
university laboratories to develop fibers with the mechanical
characteristics of spider silk. We are applying this proprietary
genetic engineering technology to domesticated silkworms, which are
already the most efficient commercial producers of
silk.
Our
technology builds upon the unique advantages of the domesticated
silkworm for this application. The silkworm is ideally suited to
produce recombinant protein fiber because it is already an
efficient commercial and industrial producer of protein based
polymers. Forty percent (40%) of the caterpillars’ weight is
devoted to the silk glands. The silk glands produce large volumes
of protein, called fibroin, which are then spun into a composite
protein thread (silk).
We are
working to use our genetic engineering technology to create
recombinant silk polymers. On September 29, 2010, we jointly
announced with the University of Notre Dame the success of our
collaborative research with the University in creating
approximately twenty different strains of transgenic silkworm which
produce recombinant silk polymers. In April 2011, we entered into a
licensing agreement with Sigma-Aldrich which provides us the use of
Sigma-Aldrich’s zinc finger technology to accelerate and
enhance our product development.
A part
of our intellectual property portfolio is the exclusive right to
use certain patented spider silk gene sequences in silkworm. Under
the Exclusive License Agreement with The University of Wyoming, we
have obtained certain exclusive rights to use numerous genetic
sequences which are the subject of US patents.
The
introduction of the gene sequence, in the manner employed by us,
results in a germline transformation and is therefore
self-perpetuating. This technology is in essence a protein
expression platform which has other potential applications
including diagnostics and pharmaceutical production.
The Company
Kraig
Biocraft Laboratories, Inc. (Kraig) is a Wyoming corporation. Our
shares are traded on the OTCQB under the ticker
symbol:
KBLB.
There are
783,046,190 shares of common stock issued and outstanding as of May
4, 2017. Kim Thompson, our founder and CEO, owns
approximately 29.96% of the issued and outstanding common
shares. There are 2 shares of super voting preferred stock issued
and outstanding as of May 4, 2017. Kim Thompson owns 100% of the
issued and outstanding super voting preferred shares.
The
inventor of our technology concept, Kim Thompson, is the founder of
Kraig Biocraft Laboratories, Inc. Our protein expression system is,
in concept, scalable, cost effective, and capable of producing a
wide range of proteins and materials.
On
April 8, 2011, Kraig and Sigma-Aldrich Co., an Illinois corporation
(“Sigma”) entered into a License and Option Agreement.
Under the terms of the agreement, Sigma will provide Kraig with its
proprietary genetic engineering tools and expertise in zinc finger
nuclease to enable Kraig to significantly accelerate its product
development. In addition to providing the customized tools and
technological know-how, Sigma has granted Kraig an option for a
commercial license to use the technology in the textile, technical
textile and biomedical markets. Sigma will be creating customized
zinc fingers for Kraig's use in its development of spider silk
polymers and technical textiles.
In
September 2010 the Company announced that it had succeeded in
introducing spider silk DNA in silkworm with the result that the
transgenic silkworm were producing new recombinant silk fibers.
These fibers are a combination of natural silkworm silk proteins
and proteins that the silkworms are making as a result of the
introduction of the spider silk DNA. The Company announced that it
had created approximately twenty different transgenic silkworm
strains producing recombinant silk.
We
entered into an intellectual property and collaborative research
agreement with the University of Notre Dame in 2007. That agreement
was subsequently extended and expanded to include research and
development of certain platform technologies with potential
applications for diagnostics and pharmaceutical production. On
March 20, 2010, the Company extended its agreement with Notre Dame
through February 28, 2011. Pursuant to these agreements the genetic
work has been conducted primarily within Notre Dame’s
laboratories. In June 2012, we entered into the Intellectual
Property / Collaborative Research Agreement with Notre Dame
(“2012 Notre Dame Research Agreement”). On March 4,
2015 we entered into a new Intellectual Property / Collaborative
Research Agreement with University of Notre Dame extending the
agreement through March 2016 (“2015 Notre Dame Research
Agreement”). Under the 2015 Notre Dame Research agreement the
Company will provide approximately $534,000 in financial support.
On September 20, 2015 this agreement was amended to increase the
total funding by approximately $179,000. In February 2016 this
agreement was extended to July 31, 2016. In August 2016 this
agreement was extended to December 31, 2016. In May 2017 this
agreement was extended to June 30, 2017. For the year ended
December 31, 2016 and 2015, respectively, the Company paid $397,136
and $432,008 in research and development fees.
We also
entered into an intellectual property and sponsored research
agreement with the University of Wyoming in 2006.
License Agreements/Intellectual Property
We have obtained certain rights to use a number of university
created, and patented, spider silk proteins, gene sequences and
methodologies.
Between 2010 and 2014 the University of Notre Dame filed
approximately 12 patent applications pursuant to our intellectual
property and collaborative research agreement. Under the
terms of that agreement the Company has an option for the exclusive
commercial rights to that technology. The Company has notified the
University of its exercise of that option. These patent
applications include coverage in the United States, Europe, Korea,
Vietnam, Brazil, India, China, Australia, Japan, and
Canada. As of the date hereof, all of these patents were
pending applications and none have been issued.
We do not own any patents. In 2014, six trademarks were issued to
the Company which it intends to use for product branding in the
future. The details of such trademarks are set forth in the
following table:
|
Marks
|
Registered Owner
|
Country
|
Status
|
|
Monster
SilkTM
|
Kraig
Biocraft Laboratories
|
United
States of America
|
issued
|
|
SpiderpillarTM
|
Kraig
Biocraft Laboratories
|
United
States of America
|
issued
|
|
SpilkTM
|
Kraig
Biocraft Laboratories
|
United
States of America
|
issued
|
|
Monster
WormTM
|
Kraig
Biocraft Laboratories
|
United
States of America
|
issued
|
|
Spider
WormTM
|
Kraig
Biocraft Laboratories
|
United
States of America
|
issued
|
|
Spider
MothTM
|
Kraig
Biocraft Laboratories
|
United
States of America
|
issued
|
License Agreement with Notre Dame University
In 2011, the Company exercised its option to obtain the global
commercialization rights to the technology developed under the
collaborative research agreements with Notre Dame. On October
28, 2011, the Company entered into a license agreement with the
University of Notre Dame. Under the agreement, the Company received
exclusive and non-exclusive rights to certain spider silk
technologies including commercial rights with the right to
sublicense such intellectual property.
In consideration of the licenses granted under the Agreement, the
Company agreed to issue to the University of Notre Dame 2,200,000
shares of its common stock and to pay a royalty of 2% of net
sales.
The
Agreement has a term of 20 years which can be extended on an annual
basis after that. It can be terminated by the University of Notre
Dame if the Company defaults on its obligations under the Agreement
and fails to cure such default within 90 days of a written notice
by the university. The Company can terminate the Agreement upon a
90 day written notice subject to payment of a termination fee of
$5,000 if the termination takes place within 2 years after its
effectiveness, $10,000 if the termination takes place within 4
years after its effectiveness, and $20,000 if the Agreement is
terminated after 4 years.
Exclusive License Agreement with University of Wyoming
In May 2006, we entered into a license agreement with the
University of Wyoming, pursuant to which we have licensed the right
to commercialize the production by silkworms of certain synthetic
and natural spider silk proteins and the genetic sequencing for
such spider silk proteins. These spider silk proteins and genetic
sequencing are covered by patents held by the University of
Wyoming. Our license allows us only to use silkworms to produce the
licensed proteins and genetic sequencing. We have the right to
sublicense the intellectual property that we license from the
University of Wyoming. Our license agreement with the University of
Wyoming requires that we pay licensing and research fees to the
university in exchange for an exclusive license in our field of use
for certain university-developed intellectual property including
patented spider silk gene sequences. Pursuant to the agreement, we
issued 17,500,000 shares of our Class A common stock to the
University Foundation. Our license agreement with the University of
Wyoming will continue until the later of (i) expiration of the
last-to-expire patent we license from the University of Wyoming
under this license agreement in such country or (ii) ten years from
the date of first commercial sale of a licensed product in such
country. There are no royalties payable to the University of
Wyoming under the terms of our agreement with them.
We anticipate paying all accrued fees to, or making alternative
arrangements with the University within the next twelve months. If
we fail to make such payment the University of Wyoming could
terminate our license agreement. We anticipate that such a
termination would result in a loss of three to nine months of
research time and result in increased research and development
costs in the range of $30,000 to $140,000.
Research and Development
On September 29, 2010 we announced that we had achieved our
longstanding goal of producing new silk fibers composed of
recombinant proteins. The Company intends to turn our technology to
the development and production of high performance
polymers
.
During the fiscal years ended December 31, 2016 and 2015, we have
spent approximately 7,500 hours and 7,000 hours, respectively,
on research and development activities, which consisted primarily
of laboratory research on genetic engineering by our outside
consultants pursuant to our collaborative research agreement with
the University of Notre Dame.
Employees
As of
the date of this filing, we have six (6) employees including Kim
Thompson, our sole officer and director and Jonathan R. Rice, our
Chief Operating Officer. We plan to hire more persons on as-needed
basis.
Property
Starting in September of 2015, we rent office space at 2723 South
State Street, Suite 150, Ann Arbor, Michigan 48104, which is our
principal place of business. We pay an annual rent of $2,112 for
conference facilities, mail, fax, and reception services located at
our principal place of business.
Starting in February of 2015, we rent additional office space in
East Lansing, Michigan and currently pay a monthly rent of $432 for
office space, conference facilities, mail, fax, and reception
services.
Starting in July of 2016, we rent factory space in South Bend,
Indiana with a monthly rent of $670.
Starting
in February of 2017, we rent property in Texas for mulberry
production with a monthly rent of $960
Legal Proceedings
We may
from time to time become a party to various legal or administrative
proceedings arising in the ordinary course of our
business.
To the
knowledge of our management, we are currently not a party to any
material legal or administrative proceedings and are not aware of
any pending or threatened legal or administrative proceedings
against us.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER
MATTERS
Our common stock trades on the OTCQB system under the symbol
“
KBLB
.”
Our CUSIP number is 50075W. On May 4, 2017, the closing bid price
of our Common Stock was $0.07 per share.
The following table sets forth the high and low trade information
for our common stock for each quarter during the past two years.
The prices reflect inter-dealer quotations, do not include retail
mark-ups, markdowns or commissions and do not necessarily reflect
actual transactions.
|
|
|
|
|
Fourth Quarter
2016
|
$
0.04
|
$
0.056
|
|
Third Quarter
2016
|
$
0.019
|
$
0.095
|
|
Second Quarter
2016
|
$
0.014
|
$
0.025
|
|
First Quarter
2016
|
$
0.022
|
$
0.027
|
|
|
|
|
|
Fourth Quarter
2015
|
$
0.02
|
$
0.04
|
|
Third Quarter
2015
|
$
0.02
|
$
0.04
|
|
Second Quarter
2015
|
$
0.03
|
$
0.06
|
|
First Quarter
2015
|
$
0.02
|
$
0.04
|
Holders
As of May 4, 2017 in accordance with our transfer agent records, we
had 30 record holders of our Class A common stock.
This number excludes
any estimate by us of the number of beneficial owners of shares
held in street name, the accuracy of which cannot be
guaranteed.
Transfer Agent and Registrar
Our
transfer agent is Olde Monmouth Stock Transfer Co., Inc., 200
Memorial Parkway, Atlantic Highlands, NJ 07716 and its
phone number is (732) 872-2727.
AVAILABLE INFORMATION
We have
filed with the SEC a registration statement on Form S-1 under the
Securities Act with respect to the Class A common stock offered
hereby. This prospectus, which constitutes part of the registration
statement, does not contain all of the information set forth in the
registration statement and the exhibits and schedule thereto,
certain parts of which are omitted in accordance with the rules and
regulations of the SEC. For further information regarding our
Class A common stock and our company, please review the
registration statement, including exhibits, schedules and reports
filed as a part thereof. Statements in this prospectus
as to the contents of any contract or other document filed as an
exhibit to the registration statement, set forth the material terms
of such contract or other document but are not necessarily
complete, and in each instance reference is made to the copy
of such document filed as an exhibit to the registration statement,
each such statement being qualified in all respects by such
reference.
We are
also subject to the informational requirements of the Exchange Act
which requires us to file reports, proxy statements and other
information with the SEC. Such reports, proxy statements and other
information along with the registration statement, including the
exhibits and schedules thereto, may be inspected at public
reference facilities of the SEC at 100 F Street N.E.,
Washington D.C. 20549. Copies of such material can be
obtained from the Public Reference Section of the SEC at prescribed
rates. You may call the SEC at 1-800-SEC-0330 for further
information on the operation of the public reference room. Because
we file documents electronically with the SEC, you may also obtain
this information by visiting the SEC’s Internet website at
http://www.sec.gov.
FINANCIAL STATEMENTS
Kraig Biocraft Laboratories, Inc.
Kraig Biocraft Laboratories, Inc.
CONTENTS
|
PAGE
|
30
|
REPORT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
|
|
|
|
|
|
PAGE
|
31
|
BALANCE SHEETS AS
OF DECEMBER 31, 2016 AND DECEMBER 31, 2015.
|
|
|
|
|
|
PAGE
|
32
|
STATEMENTS OF
OPERATIONS FOR THE YEARS ENDED DECEMBER 31, 2016 AND DECEMBER 31,
2015.
|
|
|
|
|
|
PAGE
|
34
|
STATEMENTS OF CASH
FLOWS FOR THE YEARS ENDED DECEMBER 31, 2016 AND DECEMBER 31,
2015.
|
|
|
|
|
|
PAGES
|
34
|
STATEMENT OF
CHANGES IN STOCKHOLDERS’ DEFICIT FOR THE PERIOD FROM DECEMBER
31, 2013 TO DECEMBER 31, 2015.
|
|
|
|
|
|
PAGES
|
35
|
NOTES TO FINANCIAL
STATEMENTS.
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
To the
Board of Directors and
Stockholders
of Kraig Biocraft Laboratories, Inc.
We hereby consent to the inclusion in this Registration Statement
on Form S-1 of our report dated April 26, 2017, of Kraig Biocraft
Laboratories, Inc. relating to the audit of the financial
statements for the period ending December 31, 2016 and the
reference to our firm under the caption “Experts” in
the Registration Statement.
/s/ M&K CPAS, PLLC
www.mkacpas.com
Houston, Texas
April 26, 2017
|
Kraig Biocraft Laboratories, Inc.
|
|
Balance Sheets
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets
|
|
|
|
Cash
|
$
298,859
|
$
238,188
|
|
|
31,858
|
-
|
|
|
1,324
|
645
|
|
|
332,041
|
238,833
|
|
|
|
|
|
Property
and Equipment, net
|
51,618
|
66,104
|
|
|
|
|
|
Total Assets
|
$
383,659
|
$
304,937
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' DEFICIT
|
|
|
|
|
|
Current Liabilities
|
|
|
Accounts
payable and accrued expenses
|
$
513,562
|
$
469,833
|
Note
payable - related party
|
50,000
|
-
|
Royalty
agreement payable - related party
|
65,292
|
65,292
|
Accounts
payable and accrued expenses - related party
|
2,115,618
|
1,666,748
|
|
Total Current Liabilities
|
2,744,472
|
2,201,873
|
|
|
|
|
|
Commitments and Contingencies
|
|
|
|
|
|
|
|
Stockholders' Deficit
|
|
|
|
Preferred
stock Series A, no par value;
|
|
|
2
and 2 shares issued and outstanding, respectively
|
5,217,800
|
5,217,800
|
|
Common
stock Class A, no par value; unlimited shares
authorized,
|
|
|
773,627,964
and 708,068,385 shares issued and outstanding,
respectively
|
12,958,757
|
10,801,942
|
|
Common
stock Class B, no par value; unlimited shares
authorized,
|
|
|
no
shares issued and outstanding
|
-
|
-
|
|
Common
Stock Issuable, 5,778,633 and 1,122,311 shares,
respectively
|
279,754
|
22,000
|
|
Additional
paid-in capital
|
2,568,855
|
2,373,458
|
|
Accumulated
Deficit
|
(23,385,979
)
|
(20,312,136
)
|
|
|
|
|
|
Total Stockholders' Deficit
|
(2,360,813
)
|
(1,896,936
)
|
|
|
|
|
|
Total Liabilities and Stockholders' Deficit
|
$
383,659
|
$
304,937
|
See
accompanying notes to financial statements
|
Kraig Biocraft Laboratories, Inc.
|
|
Statements of Operations
|
|
|
|
|
|
|
|
|
Revenue
|
$
31,858
|
$
-
|
|
|
|
|
|
Operating Expenses
|
|
|
|
General
and Administrative
|
1,736,918
|
920,919
|
|
Professional
Fees
|
396,125
|
260,716
|
|
Officer's
Salary
|
447,283
|
440,896
|
|
Research
and Development
|
397,136
|
432,008
|
|
Total Operating Expenses
|
2,977,462
|
2,054,539
|
|
|
|
|
|
Loss from Operations
|
(2,945,604
)
|
(2,054,539
)
|
|
|
|
|
|
Other Income/(Expenses)
|
|
|
|
Gain
on forgiveness of debt
|
11,191
|
23,245
|
|
Loss
on disposal of fixed asset
|
-
|
(953
)
|
|
Interest
expense
|
(139,430
)
|
(101,546
)
|
|
Total Other Income/(Expenses)
|
(128,239
)
|
(79,254
)
|
|
|
|
|
|
Net (Loss) before Provision for Income Taxes
|
(3,073,843
)
|
(2,133,793
)
|
|
|
|
|
|
Provision for Income Taxes
|
-
|
-
|
|
|
|
|
|
Net (Loss)
|
$
(3,073,843
)
|
$
(2,133,793
)
|
|
|
|
|
|
Net Income (Loss) Per Share - Basic and Diluted
|
$
(0.00
)
|
$
(0.00
)
|
|
|
|
|
|
Weighted average number of shares outstanding
|
|
|
|
during the period - Basic and Diluted
|
744,284,497
|
685,836,581
|
See
accompanying notes to consolidated financial
statements
|
Kraig
Biocraft Laboratories, Inc.
|
|
Statements
of Cash Flows
|
|
|
For the years ended December 31,
|
|
|
|
|
|
|
|
|
|
Cash Flows From Operating Activities:
|
|
|
|
Net
Loss
|
$
(3,073,843
)
|
$
(2,133,793
)
|
|
Adjustments
to reconcile net loss to net cash used in operations
|
|
|
|
Depreciation
expense
|
16,974
|
15,419
|
|
Gain
on forgiveness of debt
|
11,191
|
(23,245
)
|
|
Loss
on disposal of fixed asset
|
-
|
953
|
|
Imputed
interest
|
1,425
|
-
|
|
Stock
issuable for services
|
32,850
|
-
|
|
Warrants
issued to consultants
|
1,356,230
|
590,335
|
|
Warrants
issued to related party
|
193,654
|
121,448
|
|
Changes
in operating assets and liabilities:
|
|
|
|
(Increase)
Decrease in prepaid expenses
|
(679
)
|
355
|
|
Increase
in accounts receivables, net
|
(31,858
)
|
-
|
|
(Decrease)
in accrued expenses and other payables - related party
|
498,640
|
489,718
|
|
(Decrease)
Increase in accounts payable
|
(16,425
)
|
(28,753
)
|
|
Net Cash Used In Operating Activities
|
(1,011,841
)
|
(967,563
)
|
|
|
|
|
|
Cash Flows From Investing Activities:
|
|
|
|
Purchase
of Fixed Assets and Domain Name
|
(2,488
)
|
(39,285
)
|
|
Net Cash Used In Investing Activities
|
(2,488
)
|
(39,285
)
|
|
|
|
|
|
Cash Flows From Financing Activities:
|
|
|
|
Proceeds
from Notes Payable - related party
|
50,000
|
-
|
|
Proceeds
from issuance of common stock
|
1,025,000
|
750,000
|
|
Net Cash Provided by Financing Activities
|
1,075,000
|
750,000
|
|
|
|
|
|
Net Increase in Cash
|
60,671
|
(256,848
)
|
|
|
|
|
|
Cash
at Beginning of Period
|
238,188
|
495,036
|
|
|
|
|
|
Cash at End of Period
|
$
298,859
|
$
238,188
|
|
|
|
|
|
Supplemental disclosure of cash flow information:
|
|
|
|
|
|
|
|
Cash
paid for interest
|
$
-
|
$
-
|
|
Cash
paid for taxes
|
$
-
|
$
-
|
|
|
|
|
|
Supplemental disclosure of non-cash investing and financing
activities:
|
|
|
|
Shares issued in connection with cashless warrants
exercise
|
$
1,131,007
|
$
238,342
|
|
Shares issuable in connection with cashless warrant
exercise
|
$
224,904
|
$
-
|
|
Settlement of accounts payable with stock issuance
|
$
808
|
$
755
|
See
accompanying notes to consolidated financial
statements
Kraig Biocraft
Laboratories, Inc.
|
Statement of Changes in Stockholders Deficit
|
For the years ended December 31, 2016 and 2015
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred
Stock - Series A
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31 ,2014
|
2
|
5,217,800
|
673,974,429
|
9,812,845
|
-
|
-
|
1,122,311
|
22,000
|
1,900,018
|
(18,178,345
)
|
(1,225,682
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant
5,000,000 warrants for services to related
party
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
121,448
|
-
|
121,448
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant
15,000,000 warrants for services to consultants
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
590,335
|
-
|
590,335
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of
4,200,000 warrants in exchange for stock
|
-
|
-
|
4,095,841
|
238,342
|
-
|
-
|
-
|
-
|
(238,342
)
|
-
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Settlement of
accounts payable with stock issuance
($0.03/share)
|
-
|
-
|
24,000
|
755
|
-
|
-
|
-
|
-
|
-
|
-
|
755
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock issued
for cash ($0.01/share)
|
-
|
-
|
29,974,115
|
750,000
|
-
|
-
|
-
|
-
|
-
|
-
|
750,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for
the year ended December 31, 2015
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(2,133,793
)
|
(2,133,793
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2015
|
2
|
$
5,217,800
|
708,068,385
|
$
10,801,942
|
-
|
$
-
|
1,122,311
|
$
22,000
|
$
2,373,458
|
$
(20,312,136
)
|
$
(1,896,936
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock issued
for cash ($0.0246/share)
|
-
|
-
|
41,626,276
|
1,025,000
|
-
|
-
|
-
|
-
|
-
|
-
|
1,025,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock issued
for services ($0.04380/share)
|
-
|
-
|
-
|
-
|
-
|
-
|
750,000
|
32,850
|
-
|
-
|
32,850
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Imputed
Interest
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
1,425
|
-
|
1,425
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant
6,000,000 warrants for services to related
party
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
125,053
|
-
|
125,053
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants
issued for services - related party
|
|
|
-
|
|
|
|
|
|
68,600
|
-
|
68,600
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants
issued for services
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
1,356,230
|
-
|
1,356,230
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of
27,500,000 warrants in exchange for stock
|
-
|
-
|
23,909,303
|
1,131,007
|
-
|
-
|
3,906,322
|
224,904
|
(1,355,911
)
|
-
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Settlement of
accounts payable with stock issuance
($0.03367/share)
|
-
|
-
|
24,000
|
808
|
-
|
-
|
-
|
-
|
-
|
-
|
808
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for
the year ended December 31, 2016
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(3,073,843
)
|
(3,073,843
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2016
|
2
|
$
5,217,800
|
773,627,964
|
$
12,958,757
|
-
|
$
-
|
5,778,633
|
$
279,754
|
$
2,568,855
|
$
(23,385,979
)
|
$
(2,360,813
)
|
See
accompanying notes to financial statements
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
NOTE 1 SUMMARY OF SIGNIFICANT ACCOUNTING
POLICIES AND ORGANIZATION
(A) Organization
Kraig
Biocraft Laboratories, Inc. (the "Company") was incorporated under
the laws of the State of Wyoming on April 25, 2006. The Company was
organized to develop high strength, protein based fiber, using
recombinant DNA technology, for commercial applications in the
textile and specialty fiber industries.
(B) Use of Estimates
In
preparing financial statements in conformity with generally
accepted accounting principles, management is required to make
estimates and assumptions that affect the reported amounts of
assets and liabilities and the disclosure of contingent assets and
liabilities at the date of the financial statements and revenues
and expenses during the reported period. Actual results
could differ from those estimates.
(C) Cash
For
purposes of the cash flow statements, the Company considers all
highly liquid investments with original maturities of three months
or less at the time of purchase to be cash
equivalents. There were no cash equivalents as of
December 31, 2016 or December 31, 2015.
(D) Loss Per Share
Basic
and diluted net loss per common share is computed based upon the
weighted average common shares outstanding as defined by FASB
Accounting Standards Codification No. 260, “Earnings per
Share.” During the years ended December 31, 2016
and 2015, warrants were not included in the computation of income/
(loss) per share because their inclusion is
anti-dilutive.
The
computation of basic and diluted loss per share during the years
ended December 31, 2016 and 2015 excludes the common stock
equivalents of the following potentially dilutive securities
because their inclusion would be anti-dilutive:
|
|
|
|
|
|
|
|
|
Stock Warrants
(Exercise price - $0.001/share)
|
47,800,000
|
34,000,000
|
|
Convertible
Preferred Stock
|
2
|
2
|
|
Total
|
47,800,002
|
345,000,002
|
(E) Research and Development Costs
The
Company expenses all research and development costs as incurred for
which there is no alternative future use. These costs also include
the expensing of employee compensation and employee stock based
compensation.
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
(F)
Income Taxes
The
Company accounts for income taxes under FASB Codification Topic
740-10-25 (“ASC 740-10-25”). Under ASC
740-10-25, deferred tax assets and liabilities are recognized for
the future tax consequences attributable to differences between the
financial statement carrying amounts of existing assets and
liabilities and their respective tax bases. Deferred tax
assets and liabilities are measured using enacted tax rates
expected to apply to taxable income in the years in which those
temporary differences are expected to be recovered or
settled. Under ASC 740-10-25, the effect on deferred tax
assets and liabilities of a change in tax rates is recognized in
income in the period that includes the enactment date.
The net
deferred tax liability in the accompanying balance sheets includes
the following amounts of deferred tax assets and
liabilities:
|
|
|
|
|
|
|
|
|
Expected income tax
recovery (expense) at the statutory rate of 34%
|
(1,045,001
)
|
$
(724,717
)
|
|
Tax effect of
expenses that are not deductible for income tax purposes (net of
other amounts deductible for tax purposes)
|
534,325
|
229,561
|
|
Change in valuation
allowance
|
510,677
|
495,156
|
|
|
|
|
|
Provision for
income taxes
|
$
-
|
$
-
|
|
|
|
|
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
The
components of deferred income taxes are as follows
:
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax
liability:
|
$
-
|
$
-
|
|
Deferred tax
asset
|
|
|
|
Net
Operating Loss Carryforward
|
2,813,989
|
3,324,665
|
|
Valuation
allowance
|
(2,813,989
|
(3,324,665
)
|
|
Net
deferred tax asset
|
-
|
-
|
|
Net
deferred tax liability
|
$
-
|
$
-
|
|
|
|
|
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
The
valuation allowance was established to reduce the deferred tax
asset to the amount that will more likely than not be
realized. This is necessary due to the Company’s
continued operating losses and the uncertainty of the
Company’s ability to utilize all of the net operating loss
carryforwards before they will expire through the year
2035.
The net
change in the valuation allowance for the year ended December 31,
2016 and 2015 was an increase of $510,677 and $495,156
respectively.
Effective
January 1, 2009, the Company adopted guidance regarding accounting
for uncertainty in income taxes. This guidance clarifies the
accounting for income taxes by prescribing the minimum recognition
threshold an income tax position is required to meet before being
recognized in the financial statements and applies to all federal
or state income tax positions. Each income tax position is assessed
using a two-step process. A determination is first made as to
whether it is more likely than not that the income tax position
will be sustained, based upon technical merits, upon examination by
the taxing authorities. If the income tax position is expected to
meet the more likely than not criteria, the benefit recorded in the
financial statements equals the largest amount that is greater than
50% likely to be realized upon its ultimate settlement. As of
December 31, 2016 and December 31, 2015 there were no amounts that
had been accrued in respect to uncertain tax
positions.
The Company’s U.S. federal income tax return for the fiscal
year ended December 31, 2013 was audited by the Internal Revenue
Service (“IRS”). The IRS made certain adjustments to
the Company’s prior year tax returns; however, the
adjustments did not result in any tax liability or required
payments.
(G) Derivative Financial Instruments
Fair
value accounting requires bifurcation of embedded derivative
instruments such as conversion features in convertible debt or
equity instruments, and measurement of their fair value for
accounting purposes. In determining the appropriate fair value, the
Company uses the Black-Scholes option-pricing model. In assessing
the convertible debt instruments, management determines if the
convertible debt host instrument is conventional convertible debt
and further if there is a beneficial conversion feature requiring
measurement. If the instrument is not considered conventional
convertible debt, the Company will continue its evaluation process
of these instruments as derivative financial
instruments.
Once
determined, derivative liabilities are adjusted to reflect fair
value at each reporting period end, with any increase or decrease
in the fair value being recorded in results of operations as an
adjustment to fair value of derivatives. In addition, the fair
value of freestanding derivative instruments such as warrants, are
also valued using the Black-Scholes option-pricing
model.
(H) Stock-Based Compensation
In
December 2004, the FASB issued FASB Accounting Standards
Codification No. 718,
Compensation – Stock
Compensation
. Under FASB Accounting Standards
Codification No. 718, companies are required to measure the
compensation costs of share-based compensation arrangements based
on the grant-date fair value and recognize the costs in the
financial statements over the period during which employees are
required to provide services. Share-based compensation arrangements
include stock options, restricted share plans, performance-based
awards, share appreciation rights and employee share purchase
plans. As such, compensation cost is measured on the
date of grant at their fair value. Such compensation
amounts, if any, are amortized over the respective vesting periods
of the option grant. The Company applies this statement
prospectively.
Equity
instruments (“instruments”) issued to other than
employees are recorded on the basis of the fair value of the
instruments, as required by FASB Accounting Standards Codification
No. 718. FASB Accounting Standards Codification No.
505,
Equity Based Payments to
Non-Employees
defines the measurement date and
recognition period for such instruments. In general, the
measurement date is when either a (a) performance commitment, as
defined, is reached or (b) the earlier of (i) the non-employee
performance is complete or (ii) the instruments are vested. The
measured value related to the instruments is recognized over a
period based on the facts and circumstances of each particular
grant as defined in the FASB Accounting Standards
Codification.
(I) Business Segments
The
Company operates in one segment and therefore segment information
is not presented.
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
(J) Recent Accounting
Pronouncements
In January 2016, the Financial Accounting Standards Board
(“FASB”) issued Accounting Standards Update (ASU)
2016-01, which amends the guidance in U.S. GAAP on the
classification and measurement of financial instruments. Changes to
the current guidance primarily affect the accounting for equity
investments, financial liabilities under the fair value option, and
the presentation and disclosure requirements for financial
instruments. In addition, the ASU clarifies guidance related to the
valuation allowance assessment when recognizing deferred tax assets
resulting from unrealized losses on available-for-sale debt
securities. The new standard is effective for fiscal years and
interim periods beginning after December 15, 2017, and upon
adoption, an entity should apply the amendments by means of a
cumulative-effect adjustment to the balance sheet at the beginning
of the first reporting period in which the guidance is effective.
Early adoption is not permitted except for the provision to record
fair value changes for financial liabilities under the fair value
option resulting from instrument-specific credit risk in other
comprehensive income. The Company is currently evaluating the
impact of adopting this guidance.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic
842) to increase transparency and comparability among organizations
by recognizing lease assets and lease liabilities on the balance
sheet and disclosing key information about leasing arrangements.
Topic 842 affects any entity that enters into a lease, with some
specified scope exemptions. The guidance in this Update supersedes
Topic 840, Leases. The core principle of Topic 842 is that a lessee
should recognize the assets and liabilities that arise from leases.
A lessee should recognize in the statement of financial position a
liability to make lease payments (the lease liability) and a
right-of-use asset representing its right to use the underlying
asset for the lease term. For public companies, the amendments in
this Update are effective for fiscal years beginning after December
15, 2018, including interim periods within those fiscal years. We
are currently evaluating the impact of adopting ASU No. 2016-02 on
our financial statements.
In March 2016, the FASB issued ASU 2016-08, Revenue from Contracts
with Customers (Topic 606): Principal versus Agent Considerations
(Reporting Revenue Gross versus Net) that clarifies how to apply
revenue recognition guidance related to whether an entity is a
principal or an agent. ASU 2016-08 clarifies that the analysis must
focus on whether the entity has control of the goods or services
before they are transferred to the customer and provides additional
guidance about how to apply the control principle when services are
provided and when goods or services are combined with other goods
or services. The effective date for ASU 2016-08 is the same as the
effective date of ASU 2014-09 as amended by ASU 2015-14, for annual
reporting periods beginning after December 15, 2017, including
interim periods within those years. The Company has not yet
determined the impact of ASU 2016-08 on its financial
statements.
In March 2016, the FASB issued ASU No. 2016-09, Compensation
– Stock Compensation, or ASU No. 2016-09. The areas for
simplification in this Update involve several aspects of the
accounting for share-based payment transactions, including the
income tax consequences, classification of awards as either equity
or liabilities, and classification on the statement of cash flows.
For public entities, the amendments in this Update are effective
for annual periods beginning after December 15, 2016, and interim
periods within those annual periods. Early adoption is permitted in
any interim or annual period. If an entity early adopts the
amendments in an interim period, any adjustments should be
reflected as of the beginning of the fiscal year that includes that
interim period. An entity that elects early adoption must adopt all
of the amendments in the same period. Amendments related to the
timing of when excess tax benefits are recognized, minimum
statutory withholding requirements, forfeitures, and intrinsic
value should be applied using a modified retrospective transition
method by means of a cumulative-effect adjustment to equity as of
the beginning of the period in which the guidance is adopted.
Amendments related to the presentation of employee taxes paid on
the statement of cash flows when an employer withholds shares to
meet the minimum statutory withholding requirement should be
applied retrospectively. Amendments requiring recognition of excess
tax benefits and tax deficiencies in the income statement and the
practical expedient for estimating expected term should be applied
prospectively. An entity may elect to apply the amendments related
to the presentation of excess tax benefits on the statement of cash
flows using either a prospective transition method or a
retrospective transition method. We are currently evaluating the
impact of adopting ASU No. 2016-09 on our financial
statements.
In April 2016, the FASB issued ASU 2016-10, Revenue from Contracts
with Customers (Topic 606): Identifying Performance Obligations and
Licensing, which provides further guidance on identifying
performance obligations and improves the operability and
understandability of licensing implementation guidance. The
effective date for ASU 2016-10 is the same as the effective date of
ASU 2014-09 as amended by ASU 2015-14, for annual reporting periods
beginning after December 15, 2017, including interim periods within
those years.
In May 2016,
the FASB issued ASU 2016-12 “Revenue from Contracts with
Customers (Topic 606) - Narrow-Scope Improvements and Practical
Expedients,” which amends the guidance on transition,
collectability, non-cash consideration, and the presentation of
sales and other similar taxes. ASU 2016-12 clarifies that, for a
contract to be considered completed at transition, all (or
substantially all) of the revenue must have been recognized under
legacy GAAP. In addition, ASU 2016-12 clarifies how an entity
should evaluate the collectability threshold and when an entity can
recognize nonrefundable consideration received as revenue if an
arrangement does not meet the standard’s contract criteria.
The standard allows for both retrospective and modified
retrospective methods of adoption.
The Company has not yet determined the impact of
ASU 2016-10 on its financial statements.
In June 2016, the FASB issued ASU 2016-13, "Measurement of Credit
Losses on Financial Statements," which requires companies to
measure credit losses utilizing a methodology that reflects
expected credit losses and requires consideration of a broader
range of reasonable and supportable information to inform credit
loss estimates. ASU 2016-13 is effective for annual reporting
periods, and interim periods therein, beginning after December 15,
2019 (fiscal year 2021 for the Company). The Company has not yet
determined the potential effects of the adoption of ASU 2016-13 on
its Financial Statements.
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
In August 2016, the FASB issued ASU 2016-15, "Classification of
Certain Cash Receipts and Cash Payments," which aims to eliminate
diversity in practice in how certain cash receipts and cash
payments are presented and classified in the statement of cash
flows under Topic 230, Statement of Cash Flows, and other Topics.
ASU 2016-15 is effective for annual reporting periods, and interim
periods therein, beginning after December 15, 2017 (fiscal year
2019 for the Company). The Company has not yet determined the
potential effects of the adoption of ASU 2016-15 on its Financial
Statements.
In
September 2015, the FASB issued ASU No. 2015-16, Business
Combinations (Topic 805) (“ASU 2015-16”). Topic 805
requires that an acquirer retrospectively adjust provisional
amounts recognized in a business combination, during the
measurement period. To simplify the accounting for adjustments made
to provisional amounts, the amendments in the Update require that
the acquirer recognize adjustments to provisional amounts that are
identified during the measurement period in the reporting period in
which the adjustment amount is determined. The acquirer is required
to also record, in the same period’s financial statements,
the effect on earnings of changes in depreciation, amortization, or
other income effects, if any, as a result of the change to the
provisional amounts, calculated as if the accounting had been
completed at the acquisition date. In addition an entity is
required to present separately on the face of the income statement
or disclose in the notes to the financial statements the portion of
the amount recorded in current-period earnings by line item that
would have been recorded in previous reporting periods if the
adjustment to the provisional amounts had been recognized as of the
acquisition date. ASU 2015-16 is effective for fiscal years
beginning December 15, 2015. The adoption of ASU 2015-016 is not
expected to have a material effect on the Company’s
consolidated financial statements.
In
August 2015, the FASB issued ASU No. 2015-14, Revenue from
Contracts with Customers (Topic 606): Deferral of the Effective
Date (“ASU 2015-14”). The amendment in this ASU defers
the effective date of ASU No. 2014-09 for all entities for one
year. Public business entities, certain not-for-profit entities,
and certain employee benefit plans should apply the guidance in ASU
2014-09 to annual reporting periods beginning December 15, 2017,
including interim reporting periods within that reporting period.
Earlier application is permitted only as of annual reporting
periods beginning after December 31, 2016, including interim
reporting periods with that reporting period.
In
April 2015, the Financial Accounting Standards Board
(“FASB”) issued Accounting Standards Update
(“ASU”) No. 2015-03, Interest–Imputation of
Interest (Subtopic 835-30) (“ASU 2015-03”), which
changes the presentation of debt issuance costs in financial
statements. ASU 2015-03 requires an entity to present such costs in
the balance sheet as a direct deduction from the related debt
liability rather than as an asset. Amortization of the costs will
continue to be reported as interest expense. It is effective for
annual reporting periods beginning after December 15, 2016. Early
adoption is permitted. The new guidance will be applied
retrospectively to each prior period presented. The Company is
currently in the process of evaluating the impact of adoption of
ASU 2015-03 on its balance sheets.
All
other newly issued accounting pronouncements but not yet effective
have been deemed either immaterial or not applicable.
(K)
Reclassification
The
2015 financial statements have been reclassified to conform to the
2016 presentation.
(L) Equipment
The
Company values property and equipment at cost and depreciates these
assets using the straight-line method over their expected useful
life. The Company uses a five year life for
automobiles.
In
accordance with FASB Accounting Standards Codification No.
360,
Property, Plant and
Equipment
, the Company carries long-lived assets at the
lower of the carrying amount or fair value. Impairment is evaluated
by estimating future undiscounted cash flows expected to result
from the use of the asset and its eventual disposition. If the sum
of the expected undiscounted future cash flow is less than the
carrying amount of the assets, an impairment loss is recognized.
Fair value, for purposes of calculating impairment, is measured
based on estimated future cash flows, discounted at a market rate
of interest.
There
were no impairment losses recorded during the years ended December
31, 2016 and 2015.
(M) Fair Value of Financial Instruments
We hold
certain financial assets, which are required to be measured at fair
value on a recurring basis in accordance with the Statement of
Financial Accounting Standard No. 157,
“Fair Value Measurements”
(“ASC Topic 820-10”). ASC Topic 820-10
establishes a fair value hierarchy that prioritizes the inputs to
valuation techniques used to measure fair value. The hierarchy
gives the highest priority to unadjusted quoted prices in active
markets for identical assets or liabilities (Level 1 measurements)
and the lowest priority to unobservable inputs (Level 3
measurements). ASC Topic 820-10 defines fair value as
the price that would be received to sell an asset or paid to
transfer a liability in an orderly transaction between market
participants on the measurement date. Level 1 instruments include
cash, account receivable, prepaid expenses, inventory and account
payable and accrued liabilities. The carrying values are assumed to
approximate the fair value due to the short term nature of the
instrument.
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
The
three levels of the fair value hierarchy under ASC Topic 820-10 are
described below:
|
°
|
Level 1
- Valuations based on quoted prices in active markets for identical
assets or liabilities that an entity has the ability to
access. We believe our carrying value of level 1
instruments approximate their fair value at December 31, 2016 and
December 31, 2015.
|
|
°
|
Level 2
- Valuations based on quoted prices for similar assets or
liabilities, quoted prices for identical assets or liabilities in
markets that are not active, or other inputs that are observable or
can be corroborated by observable data for substantially the full
term of the assets or liabilities.
|
|
°
|
Level 3
- Valuations based on inputs that are supported by little or no
market activity and that are significant to the fair value of the
assets or liabilities. We consider depleting assets, asset
retirement obligations and net profit interest liability to be
Level 3. We determine the fair value of Level 3
assets and liabilities utilizing various inputs, including NYMEX
price quotations and contract terms.
|
|
|
|
|
|
Level
1
|
$
-
|
$
-
|
|
Level
2
|
-
|
-
|
|
Level
3
|
-
|
-
|
|
Total
|
$
-
|
$
-
|
(N) Revenue Recognition
The Company’s revenues are generated primarily from contracts
with the U.S. Government. The Company performs work under the
cost-plus-fixed-fee contract.
Cost-plus-fixed-fee contracts—Revenue is recognized on
cost-plus-fixed-fee contracts with the U.S. Government on the basis
of partial performance equal to costs incurred plus an estimate of
applicable fees earned as the Company becomes contractually
entitled to reimbursement of costs and the applicable
fees
. Invoicing for
costs and applicable fees are reported to the U.S. Government on a
monthly basis and invoices are typically paid within 30
days.
For the year ended December 31, 2016, the Company recognized
$31,858 in revenue from the Government contract.
(
O) Concentration of Credit
Risk
The Company at times has cash in banks in excess of FDIC insurance
limits. At December 31, 2016 and December 31, 2015, the Company had
approximately $48,859 and $0, respectively in excess of FDIC
insurance limits.
At December 31, 2016 and December 31, 2015, the Company had a
concentration of accounts receivable of:
|
Customer
|
|
|
|
Customer
A
|
100
%
|
0
%
|
|
Customer
A
|
$
31,858
|
$
-
|
For the years ended December 31, 2016 and December 31, 2015, the
Company had a concentration of sales of:
|
Customer
|
|
|
|
Customer
A
|
100
%
|
0
%
|
|
Customer
A
|
$
31,858
|
$
-
|
For the years ended December 31, 2016 and December 31, 2015, the
Company booked $0 and $0 for doubtful accounts.
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
NOTE 2
GOING CONCERN
As
reflected in the accompanying financial statements, the Company has
a working capital deficiency of $2,412,431 and stockholders’
deficiency of $2,360,813 and used $1,011,841 of cash in operations
for the year ended December 31, 2016. This raises
substantial doubt about its ability to continue as a going
concern. The ability of the Company to continue as a
going concern is dependent on the Company’s ability to raise
additional capital and implement its business plan. The
financial statements do not include any adjustments that might be
necessary if the Company is unable to continue as a going
concern.
Management
believes that actions presently being taken to obtain additional
funding and implement its strategic plans provide the opportunity
for the Company to continue as a going concern.
NOTE 3 EQUIPMENT
At
December 31, 2016 and December 31, 2015, property and equipment,
net, is as follows:
|
|
|
|
|
Automobile
|
$
41,805
|
$
41,805
|
|
Laboratory Equipment
|
39,310
|
36,822
|
|
Office Equipment
|
6,466
|
6,466
|
|
Less: Accumulated
Depreciation
|
(35,963
)
|
(18,989
)
|
|
Total Property and Equipment,
net
|
$
51,618
|
$
66,104
|
Depreciation expense for the year ended December 31, 2016 and 2015
was $16,974 and $15,419 respectively.
NOTE 4 ACRRUED INTEREST – RELATED
PARTY
On June 6, 2016, the Company received $50,000 from a principal
stockholder. Pursuant to the terms of the loan, the
advance bears interest at 3%, is unsecured and due on demand. The
Company recorded accrued interest payable of $855 as of December
31, 2016.
In addition,
the Company recorded $1,425 as an in-kind contribution of interest
related to the loan for the year ended December 31,
2016.
On February 25, 2013, the Company received $150,000 from a
principal stockholder. Pursuant to the terms of the
loan, the advance bears interest at 3%, is unsecured and due on
demand. At December 31, 2013 the loan balance was
repaid.
For the Company’s fiscal
years ended December 31, 2016 and 2015, the Company recorded $0 and
$2,001 in
accrued interest
payable respectively.
NOTE 5 STOCKHOLDERS’
DEFICIT
(A) Common Stock Issued
for Cash
On June
16, 2015, the Company issued 4,675,811 share of common stock for
$150,000 ($0.03/share).
On July
9, 2015, the Company issued 3,731,343 share of common stock for
$100,000 ($0.026/share).
On
August 3, 2015, the Company issued 4,152,824 share of common stock
for $100,000 ($0.024/share).
On
September 28, 2015, the Company issued 4,166,667 share of common
stock for $100,000 ($0.024/share).
On
October 19, 2015, the Company issued 3,894,081 shares of common
stock for $100,000 ($0.026/share).
On
November 16, 2015, the Company issued 4,166,667 shares of common
stock for $100,000 ($0.024/share).
On
December 21, 2015, the Company issued 5,186,722 shares of common
stock for $100,000 ($0.019/share).
On
February 16, 2016 the Company issued 5,630,631 share of common
stock for $100,000 ($0.018/share).
On
March 28, 2016 the Company issued 5,411,255 share of common stock
for $100,000 ($0.018/share).
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
On
April 25, 2016 the Company issued 5,952,381 share of common stock
for $100,000 ($0.017/share).
On June
28, 2016 the Company issued 7,812,500 share of common stock for
$125,000 ($0.016/share).
On July
26, 2016 the Company issued 6,028,939 shares of common stock for
$150,000 ($0.025/share).
On
August 8, 2016 the Company issued 2,181,501 shares of common stock
for $100,000 ($0.046/share).
On
August 18, 2016 the Company issued 1,838,235 shares of common stock
for $100,000 ($0.054/share).
On
September 9, 2016 the Company issued 2,604,167 shares of common
stock for $100,000 ($0.038/share).
On
October 21, 2016 the Company issued 4,166,667 shares of common
stock for $150,000 ($0.036/per share).
(B)
Common Stock Issued for Services
Shares
issued for services as mentioned below were valued at the closing
price of the stock on the date of grant.
On
March 5, 2015, the Company issued 10,000 shares with a fair value
of $321 ($0.0321/share) to a consultant as consideration for
consulting fees owed from October 1, 2014 through February 28, 2015
of $10,000. The issuance of shares resulted in gain on settlement
of accounts payable of $9,679.
On
November 9, 2015, the Company issued 14,000 shares with a fair
value of $434 ($0.031/share) to a consultant as consideration for
consulting fees owed from March 1, 2015 through September 30, 2015
of $14,000. The issuance of shares resulted in gain on settlement
of accounts payable of $13,566.
On
April 4, 2016, the Company issued 12,000 shares with a fair value
of $296 ($0.0247/share) to a consultant as consideration for
consulting fees owed from October 1, 2015 through March 31, 2016 of
$6,000. The issuance of shares resulted in gain on settlement of
accounts payable of $5,704.
On
November 7, 2016, the Company issued 12,000 shares with a fair
value of $512 ($0.0427/share) to a consultant as consideration for
consulting fees owed from April 1, 2016 through October 31, 2016 of
$6,000. The issuance of shares resulted in gain on settlement of
accounts payable of $5,488.
On
December 4, 2016, the Company granted 750,000 shares valued at
$32,850 ($0.0438/share) to a consultant for services rendered. The
shares were issued subsequent to period end on January 25,
2017.
(C) Common
Stock Warrants
On
January 21, 2015, the Company issued 2,918,919 shares in connection
with the cashless exercise of the 3,000,000 warrants.
On
January 23, 2015, the Company issued 3-year warrant to purchase
2,000,000 shares of common stock at $0.001 per share to a related
party for services to be rendered. The warrants had a fair value of
$72,317, based upon the Black-Scholes option-pricing model on the
date of grant and were fully vested upon issuance and will be
exercisable on February 2, 2016, and for a period expiring on
January 19, 2018.
Grant
Date
|
Expected
dividends
|
0
%
|
|
Expected
volatility
|
88.13
%
|
|
Expected
term
|
|
|
Risk free
interest rate
|
1.33
%
|
|
Expected
forfeitures
|
0
%
|
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
On May
28, 2015, the Company issued 3-year warrant to purchase 3,000,000
shares of common stock at $0.001 per share to a related party for
services to be rendered. The warrants had a fair value of $117,503,
based upon the Black-Scholes option-pricing model on the date of
grant and vested on October 28, 2016, and will be exercisable
commencing on May 28, 2018, and for a period expiring on May 28,
2022. During the years ended December 31, 2016 and 2015, the
Company recorded $68,600 and $28,300 as an expense for such
warrants issued to related party.
Grant Date
|
Expected
dividends
|
0
%
|
|
Expected
volatility
|
77.49
%
|
|
Expected
term
|
|
|
Risk free
interest rate
|
1.24
%
|
|
Expected
forfeitures
|
0
%
|
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
On June
22, 2015, the Company issued 3-year warrant to purchase 15,000,000
shares of common stock at a price of $0.001 per share to a
consultant for services to be rendered. The warrants had a fair
value of $590,335, based upon the Black-Scholes option-pricing
model on the date of grant and were fully vested upon issuance and
will be exercisable commencing on December 28, 2015, and for a
period expiring on September 22, 2018 (See Note 6(
C)).
Grant Date
|
Expected
dividends
|
0
%
|
|
Expected
volatility
|
78.85
%
|
|
Expected
term
|
|
|
Risk free
interest rate
|
1.06
%
|
|
Expected
forfeitures
|
0
%
|
On July
2, 2015, the Company issued 1,176,922 shares in connection with the
cashless exercise of the 1,200,000 warrants
On
January 1, 2016, the Company issued 3-year warrant to purchase
6,000,000 shares of common stock at $0.001 per share to a related
party for services to be rendered. The warrants had a fair value of
$142,526, based upon the Black-Scholes option-pricing model on the
date of grant and vested on February 20, 2017, and will be
exercisable commencing on February 20, 2018, and for a period
expiring on February 20, 2021. During the years ended December 31,
2016, the Company recorded $125,053 as an expense for such warrants
issued to this related party.
Grant Date
|
Expected
dividends
|
0
%
|
|
Expected
volatility
|
78.58
%
|
|
Expected
term
|
|
|
Risk free
interest rate
|
1.32
%
|
|
Expected
forfeitures
|
0
%
|
On
April 7, 2016, the Company issued 958,506 shares in connection with
the cashless exercise of the 1,000,000 warrants.
On
April 7, 2016, the Company issued 958,506 shares in connection with
the cashless exercise of the 1,000,000 warrants
On May
5, 2016, the Company issued 7,627,907 shares in connection with the
cashless exercise of the 8,000,000 warrants.
On June
23, 2016, the Company issued 12,867,681 shares in connection with
the cashless exercise of the 13,500,000 warrants.
On
November 7, 2016, the Company issued 1,496,703 shares in connection
with the cashless exercise of the 1,500,000 warrants.
On
December 30, 2016, the Company recorded stock issuable of 1,953,161
shares in connection with the cashless exercise of the 1,500,000
warrants.
On
December 30, 2016, the Company recorded stock issuable of 1,953,161
shares in connection with the cashless exercise of the 1,500,000
warrants.
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
On July
26, 2016, the Company issued 4-year warrant to purchase 10,000,000
shares of common stock at $0.001 per share to a consultant for
services rendered. The warrants had a fair value of $365,157, based
upon the Black-Scholes option-pricing model on the date of grant
and are fully vested on the date granted. Warrants will become
exercisable on July 26, 2018, and for a period of 4 years expiring
on July 26, 2022. During the years ended December 31, 2016, the
Company recorded $365,157 as an expense for such warrants
issued.
|
Expected
dividends
|
0
%
|
|
Expected
volatility
|
93.6
%
|
|
Expected
term
|
|
|
Risk free
interest rate
|
1.01
%
|
|
Expected
forfeitures
|
0
%
|
On July
26, 2016, the Company issued 4-year warrant to purchase 8,000,000
shares of common stock at a price of $0.001 per share to a
consultant for services rendered. The warrants had a fair value of
$292,126, based upon the Black-Scholes option-pricing model on the
date of grant and are fully vested on the date granted. Warrants
will become exercisable on July 26, 2018, and for a period of 4
years expiring on July 26, 2022. During the years ended December
31, 2016, the Company recorded $292,126 as an expense for such
warrants issued.
|
Expected
dividends
|
0
%
|
|
Expected
volatility
|
93.60
%
|
|
Expected
term
|
|
|
Risk free
interest rate
|
1.01
%
|
|
Expected
forfeitures
|
0
%
|
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
On
October 2, 2016, the Company issued 2-year warrant to purchase
2,300,000 shares of common stock at an exercise price of $0.04 per
share to a consultant for services rendered. The warrants had a
fair value of $68,686, based upon the Black-Scholes option-pricing
model on the date of grant and are fully vested on the date
granted. Warrants will become exercisable on August 25, 2019, and
for a period of 2 years expiring on August 25, 2021. During the
years ended December 31, 2016, the Company recorded $68,686 as an
expense for such warrants issued (See Note 6(C)).
|
Expected
dividends
|
0
%
|
|
Expected
volatility
|
107.51
%
|
|
Expected
term
|
|
|
Risk free
interest rate
|
0.82
%
|
|
Expected
forfeitures
|
0
%
|
On
December 8, 2016 the company issued, the Company issued 4-year
warrant to purchase 15,000,000 shares of common stock at an
exercise price of $0.001 per share to a consultant for services
rendered. The warrants had a fair value of $630,259, based upon the
Black-Scholes option-pricing model on the date of grant and are
fully vested on the date granted. Warrants will be exercisable on
June 12, 2017, and for a period of 2 years expiring on December 8,
2019. During the years ended December 31, 2016, the Company
recorded $630,259 as an expense for warrants issued (See Note 6
(C)).
|
Expected
dividends
|
0
%
|
|
Expected
volatility
|
106.57
%
|
|
Expected
term
|
|
|
Risk free
interest rate
|
1.15
%
|
|
Expected
forfeitures
|
0
%
|
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
|
|
|
Weighted Average Exercise
Price
|
Weighted
Average Remaining Contractual Life (in Years)
|
|
Balance, December
31, 2014
|
18,200,000
|
$
0.001
|
2.1
|
|
Granted
|
20,000,000
|
|
|
|
Exercised
|
(4,200,000
)
|
|
|
|
Cancelled/Forfeited
|
-
|
|
|
|
Balance,
December 31, 2015
|
34,000,000
|
$
0.001
|
1.7
|
|
Granted
|
41,300,000
|
|
|
|
Exercised
|
(27,500,000
)
|
|
|
|
Cancelled/Forfeited
|
-
|
|
|
|
Balance,
December 31, 2016
|
47,800,000
|
$
0.001
|
3.8
|
|
|
|
|
|
|
Intrinsic
Value
|
$
2,557,300
|
|
|
For the year ended December 31, 2016, the following warrants were
outstanding:
Exercise
Price Warrants Outstanding
|
|
Weighted
Average Remaining Contractual Life
|
Aggregate
Intrinsic Value
|
|
|
|
|
|
|
$
0.001
|
45,500,000
|
4.1
|
$
2,434,250
|
|
$
0.04
|
2,300,000
|
5
|
$
123,050
|
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
For the year ended December 31, 2015, the following warrants were
outstanding:
Exercise
Price Warrants Outstanding
|
|
Weighted
Average Remaining Contractual Life
|
A
ggregate Intrinsic Value
|
|
|
|
|
|
|
$
0.001
|
34,000,000
|
1.7
|
$
842,000
|
(D) Amendment to Articles of Incorporation
On
February 16, 2009, the Company amended its articles of
incorporation to amend the number and class of shares the Company
is authorized to issue as follows:
|
●
|
Common
stock Class A, unlimited number of shares authorized, no par
value
|
|
●
|
Common
stock Class B, unlimited number of shares authorized, no par
value
|
|
●
|
Preferred
stock, unlimited number of shares authorized, no par
value
|
Effective
December 17, 2013, the Company amended its articles of
incorporation to designate a Series A no par value preferred
stock. Two shares of Series A Preferred stock have been
authorized.
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
NOTE 6 COMMITMENTS
AND CONTINGENCIES
On
March 18, 2010, the Company entered into an addendum to the
employment agreement whereby the Company will reimburse the
employee and his family for up to $20,000 of out of pocket medical
and dental care costs, including prescription costs or
co-pays.
On
November 10, 2010, the Company entered into an addendum to the
employment agreement, effective January 1, 2011 through the
December 31, 2015. The term of the agreement is a five
year period at an annual salary of $210,000. There is a
6% annual increase. For the year ending
December 31, 2015, the annual salary was $281,027. The
employee is also to receive a 20% bonus based on the annual based
salary. Any stock, stock options bonuses have to be
approved by the board of directors. On January 1, 2016 the
agreement renewed with the same terms for another 5 years with an
annual salary of $297,889 for the year ended December 31, 2016.
(See Note 8).
On
October 2, 2014, the Company entered into a letter agreement for an
equity line of financing up to $7,500,000 (the
“Letter Agreement”) with Calm Seas Capital, LLC
(“Calm Seas”).
Under
the Letter Agreement, over a 24 month period from the Effective
Date we may put to Calm Seas up to an aggregate of $7,500,000 in
shares of our Class A common stock for a purchase price equal to
80% of the lowest price of our Class A common stock during the five
consecutive trading days immediately following the date we deliver
notice to Calm Seas of our election to put shares pursuant to the
Letter Agreement. We may put shares
bi-monthly. The dollar value that will be
permitted for each put pursuant to the Letter Agreement
will be the lesser of: (A) the product of (i) 200% of the average
daily volume in the US market of our Class A common stock for the
ten trading days prior to the date we deliver our put notice to
Calm Seas multiplied by (ii) the average of the daily closing
prices for the ten (10) trading days immediately preceding the date
we deliver our put notice to Calm Seas, or (B)
$100,000. We will automatically withdraw our put notice
to Calm Seas if the lowest closing bid price used to determine the
purchase price of the put shares is not at least equal to
seventy-five percent (75%) of the average closing “bid”
price for our Class A common stock for the ten (10) trading days
prior to the date we deliver our put notice to Calm Seas.
Notwithstanding the $100,000 ceiling for each bi-monthly put,
as described above, we may at any time request Calm Seas to
purchase shares in excess of such ceiling, either as a part of
bi-monthly puts or as an additional put(s) during such
month. If Calm Seas, in its sole discretion, accepts
such request to purchase additional shares, then we may include the
put for additional shares in our monthly put request or submit an
additional put for such additional shares in accordance with the
procedure set forth above.
The
Letter Agreement will terminate when any of the following events
occur:
|
●
|
Calm
Seas has purchased an aggregate of $7,500,000 of our Class A common
stock; or
|
|
●
|
The
second anniversary from the Effective Date.
|
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
On
January 23, 2015, the board of directors appointed Mr. Jonathan R.
Rice as our Chief Operating Officer. Mr. Rice’s employment
agreement has a term of one year and can be terminated by either
the Company or Mr. Rice at any time. Under the employment
agreement, Mr. Rice is entitled to an annual cash compensation of
$120,000, which includes salary, health insurance, 401K retirement
plan contributions, etc. The Company also agreed to reimburse Mr.
Rice for his past educational expenses of approximately $11,000. In
addition, Mr. Rice will be issued a three-year warrant to purchase
2,000,000 shares of common stock of the Company at an exercise
price of $0.001 per share pursuant to the employment agreement.
Additionally, on May 28, 2015, the Company issued a three-year
warrant to purchase 3,000,000 shares of common stock of the Company
at an exercise price of $0.001 per share to Mr. Rice. The warrant
fully vests on October 28, 2016. For the year ended December 31,
2015, the Company recorded $121,448 for the warrants issued to Mr.
Rice. On January 14, 2016 the Company signed a new employment
agreement with Mr. Rice, our COO. The employment agreement has a
term of one year and can be terminated by either the Company or Mr.
Rice at any time. Under the employment agreement, Mr. Rice is
entitled to an annual cash compensation of $140,000, which includes
salary, health insurance, 401K retirement plan contributions, etc.
In addition, Mr. Rice will be issued a three-year warrant to
purchase 6,000,000 shares of common stock of the Company at an
exercise price of $0.001 per share pursuant to the employment
agreement. For the year ended December 31, 2016, the Company
recorded $193,652 for the warrants issued to Mr. Rice in
2016.
(A)License Agreement
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
On May
8, 2006, the Company entered into a license
agreement. Pursuant to the terms of the agreement, the
Company paid a non-refundable license fee of $10,000. The Company
will pay a license maintenance fee of $10,000 on the one year
anniversary of this agreement and each year
thereafter. The Company will pay an annual research fee
of $13,700 with first payment due January 2007, then on each
subsequent anniversary of the effective date commencing May 4,
2007. The annual research fees are accrued by the Company for
future payment. Pursuant to the terms of the agreement the Company
may be required to pay additional fees aggregating up to a maximum
of $10,000 a year for patent maintenance and prosecution relating
to the licensed intellectual property.
On
October 28, 2011, the Company entered into a license agreement with
the University of Notre Dame. Under the agreement, the Company
received exclusive and non-exclusive rights to certain spider silk
technologies including commercial rights with the right to
sublicense such intellectual property. In consideration of the
licenses granted under the agreement, the Company agreed to issue
to the University of Notre Dame 2,200,000 shares of its common
stock and to pay a royalty of 2% of net sales. On March
4, 2015, the Company entered into a new Intellectual Property /
Collaborative Research Agreement with Notre Dame extending the
duration of the agreement through March 2016. In February of 2016
this agreement was extended to July 31, 2016. Under the
agreement the Company will provide approximately $534,000 in
financial support. The license agreement has a term of 20 years
which can be extended on an annual basis after that. It can be
terminated by the University of Notre Dame if the Company defaults
on its obligations under the agreement and fails to cure such
default within 90 days of a written notice by the university. The
Company can terminate the agreement upon a 90 day written notice
subject to payment of a termination fee of $5,000 if the
termination takes place within 2 years after its effectiveness,
$10,000 if the termination takes place within 4 years after its
effectiveness and $20,000 if the Agreement is terminated after 4
years. The Company is currently working with the University of
Notre Dame to extend this contract, but no final agreement has been
signed as of the date of this report.
(B)Royalty and Research Agreements
On May
1, 2008 the Company entered into a five year consulting agreement
for research and development. Pursuant to the terms of the
agreement, the Company will be required to pay $1,000 per month, or
at the Company’s option, the consulting fee may be paid in
the form of Company common stock based upon the greater of $0.05
per share or the average of the closing price of the
Company’s shares over the five days preceding such stock
issuance. As of September 30, 2011, the Company had
accrued $17,000 of accounts payable for the services provided of
which was paid in common stock on July 1, 2009. As of
September 30, 2011 the Company issued 280,000 shares of common
stock in exchange for $14,000 of accounts payable for the services
performed. On March 19, 2014, the Company entered into a
five year consulting agreement for general advisor and consulting
services. As consideration for the services performed,
the Company agrees to pay the consultant a fee of $1,000 per
month. At the Company’s option, said consulting
fee may be paid to the consultant in the form of Company stock
based upon the greater of $0.50/share or the average of the closing
price of the Company’s common stock over the five days
preceding such stock issuance. On March 28, 2014, the
Company issued 44,000 shares of common stock as consideration for
consulting fees owed from September 1, 2012 through March 31, 2014.
On October 9, 2014 the Company issued 12,000 shares with a fair
value of $484 ($0.0403/share) to a consultant as consideration for
consulting fees owed from April 1, 2014 through September 30, 2014
of $12,000. The issuance of shares resulted in gain on
settlement of accounts payable of $11,516. The consultant also
received a bonus of 4,000 shares with a fair value of $161
($0.0403/share).During the years ended December 31, 2015 the
Company issued 24,000 shares with a fair value of $730
($0.03367/share) to a consultant as consideration for consulting
fees owed from October 1, 2014 through September 30, 2015 of
$12,000. The issuance resulted in gain on settlement of accounts
payable of 23,245. During the years ended December 31, 2016, the
Company issued 24,000 shares with a fair value of $808
($0.03367/share) to a consultant as consideration for consulting
fees owed from October 1, 2015 through October 31, 2016 of $12,000.
The issuance resulted in gain on settlement of accounts payable of
11,191 (See Note 5 (B)).
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
On
December 26, 2006, the Company entered into an addendum to the
intellectual property transfer agreement with Mr Thompson, its
CEO. In consideration of the Company issuing either
200,000 preferred shares with the following preferences; no
dividends and voting rights equal to 100 common shares per share of
preferred stock or the payment of $120,000, the officer agreed to
terminate the royalty payments due under the agreement and give
title to the exclusive license for the non-protective apparel use
of the intellectual property to the Company. On the date
of the agreement, the Company did not have any preferred stock
authorized with the required preferences. In accordance
with FASB Accounting Standards Codification No
480,
Distinguishing
Liabilities from Equity
, the Company determined that the
present value of the payment of $120,000 that was due on December
26, 2007, the one year anniversary of the addendum, should be
recorded as an accrued expense until such time as the Company has
the ability to assert that it has preferred shares
authorized. As of March 31, 2010, the Company has
recorded $120,000 in accrued expenses- related party. On
December 21, 2007 the officer extended the due date to July 30,
2008. On May 30, 2008 the officer extended the due date
to December 31, 2008. On October 10, 2008, the officer
extended the due date to the earlier of (a) March 30, 2010 or (b)
upon demand by the officer. The due date was extended to
March 31, 2011. On September 8, 2009, a payment of
$15,000 was paid to the officer. An additional payment of $10,000
was made on October 19, 2009 and December 1, 2009,
respectfully. Additionally, the accrued expenses are
accruing 7% interest per year. On January 15, 2010 an additional
payment of $10,000 was made. During the quarter ending
September 30, 2010 an additional payment of $8,000 was made. During
the quarter ending September 30, 2012 an additional payment of
$1,000 was made. During the year ended December 31,
2013, an additional payment of $1,280 was made. During
the year ended December 31, 2014, an additional loan of $572 was
made. As of December 31, 2016 and December 31,
2015, the outstanding balance is $65,292. As of December 31, 2016
the Company recorded interest expense and related accrued interest
payable of $1,959.
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
On June
6, 2012, the Company entered into a consulting agreement for
intellectual property and collaborative research and development
with an American university. The agreement covers
ongoing research and development work performed by the university
at the Company’s behest and with the Company’s
assistance. On March 4, 2015, the Company entered into a new
Intellectual Property / Collaborative Research Agreement with Notre
Dame extending the duration of the agreement through March
2016. Pursuant to the terms of the agreement, the Company will
be required to pay approximately $534,000 for research and
development over the two-year period. For the year ended December
31, 2016 and 2015, respectively, the company recorded $397,136 and
$432,008 in research and development fees. On September 20, 2015
this agreement was amended to increase the total funding by
approximately $179,000. In February 2016, this agreement was
extended to July 31, 2016. In August 2016 this agreement was
amended to increase the total funding by approximately $175,000 and
the duration of this agreement was extended to December 31,
2016. As of filing of this Annual Report the Compnay is still
negotiating a new Intellectual Property / Collaborative Research
Agreement.
On December 30, 2015, the Company entered into a cooperative
agreement for the research and pilot production of hybrid silkworms
in Vietnam. Under this agreement, the Company will establish
a subsidiary in Vietnam where it will develop and produce hybrid
silkworms. As of December 31, 2016, the subsidiary was not yet
established and no work has been performed in Vietnam for the year
ended December 31, 2016. The Company delayed the announcement of
this agreement until late in February, 2016. This additional time
was used to confirm this agreement with higher level authorities
and outside review
required by
related Vietnam authorities.
(C) Consulting Agreement
On July
9, 2013, the Company entered into an agreement with a consultant to
provide investor relations services in exchange for a warrant for
10,000,000 common shares at $.001 with a cashless provision and a
five year term.
On
September 30, 2013, the Company entered into a Collaborative Yarn
and Textile Development Agreement with a technical textile
manufacturing company. Pursuant to the terms of that
agreement, the Company has agreed to supply the technical textile
manufacturing company with sample quantities of the Company’s
recombinant spider silk for the purpose of developing and testing
new textiles which are made from, or which incorporate recombinant
spider silk. The agreement provides that the two
companies will jointly share, on an equal basis, any intellectual
property, including any utility patents, which are developed as a
result of this collaboration. Such intellectual property
potentially includes utility patents on textile
designs. The Company has agreed that it will pay half of
the cost associated with the filing and prosecution of utility
patents relating to intellectual property which is developed
through its collaboration with the technical textile manufacturing
company.
On
October 15, 2013, the Company entered into an intellectual property
agreement with a scientific researcher relating to the development
of new recombinant silk fibers. Under the terms of that
agreement, the scientific researcher will transfer to the Company
his rights to intellectual property, inventions and trade secrets
which the researcher develops relating to recombinant
silk. The researcher will receive 8,000,000 warrants of
the Company’s stock, exercisable 24 months from the date of
the agreement. The researcher will also receive
additional warrants when and if the researcher develops advanced
recombinant silk fibers for the Company’s
use. Under the terms of the agreement the researcher
will receive 10,000,000 warrants in the event that he develops a
new recombinant silk fiber with certain performance
characteristics, and another 10,000,000 warrants if he develops a
second recombinant silk fiber with certain
characteristics. If the consultant performs the contract
in good faith the consultant will be entitled to an additional
8,000 warrants. The warrants described in this note all
contain a cashless exercise provision and are exercisable on the 24
month anniversary of the date on which they were issuable under the
agreement. On July 26, 2016 the Company issued a warrant for
10,000,000 to the researcher in accordance with this agreement for
the development of a new recombinant silk fiber. On July 26,
2016 the Company issued a warrant for 8,000,000 to the researcher
upon reaching the 24 month of this agreement.
On
February 17, 2014, the Company entered into two consulting
agreements with two consultants for independent technical expertise
to further the Company’s business plans and scientific
research and development. As consideration for the
services performed, the Company agrees to issue the following to
each of the consultants:
|
|
●
|
Within
30 days of the date of this agreement, a warrant for six hundred
thousand shares of the Company’s common stock to be
exercisable on the 14 month anniversary of this agreement for a
period of 12 months with a cashless exercise
provision.
|
|
|
●
|
Within
30 days of the date of this agreement, a warrant for one million
shares of the Company’s common stock to be exercisable on the
20 month anniversary of this agreement for a period of 12 months
with a cashless exercise provision.
|
|
|
●
|
Within
30 days of the date of this agreement, a warrant for two million
shares of the Company’s common stock to be exercisable on the
32 month anniversary of this agreement for a period of 12 months
with a cashless exercise provision.
|
|
|
●
|
Based
on the consultants reaching two sets of benchmarks, two separate
warrants for one million five hundred thousand shares of the
Company’s common stock to be exercisable on the 28 month
anniversary of this agreement for a period of 12 months with a
cashless exercise provision.
|
|
|
●
|
On the
three year anniversary, assuming the consultant acted in good faith
and the Company’s board of directors approval, a warrant for
one million five hundred thousand shares of the Company’s
common stock to be exercisable on the 28 month anniversary of this
agreement for a period of 12 months with a cashless exercise
provision.
|
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
On June
22, 2015, the Company entered into an agreement with a consultant
to provide investor relations services until December 16,
2015. As consideration for the services performed, the
Company agrees to issue a 3-year warrant to purchase 15,000,000
shares of common stock at $0.001 per share with a cashless exercise
provision. On June 22, 2015, the company issued such warrant with a
fair value of $590,335 (See Note 5(C)).
On
November 11, 2015, the Company entered into an agreement with a
consultant to provide advisory services. As consideration for the
services performed, the Company agreed to pay the consultant
$10,000.
On
January 23, 2016, the Company entered into an agreement with a
consultant to provide investor relations services for four months.
As consideration for the services performed, the Company agrees to
pay $25,000 dollar monthly payments. During the course of that
contract, additional services were rendered for a consideration
amounting a total of $31,000. During the years ended December
31, 2016, the Company paid $131,000.
On
August 25, 2016, the Company entered into an agreement with a
consultant to provide consulting services in helping the Company
expand its operations. The agreement commenced on August 25, 2016
and will continue for 18 months. In return, the Company agrees to
issue a 2-year warrant to purchase 2,300,000 shares of common stock
at a price of $0.04 per share. On October 2, 2016, the company
issued such warrant with a fair value of $590,335 (See Note
5(C)).
On
December 1, 2016, the Company entered into an agreement with a
consultant to provide investor relations services for one
year. As consideration for the services performed, the
Company agrees to issue a 2-year warrant to purchase 15,000,000
shares of common stock at a price of $0.001 per share with a
cashless exercise provision. On December 8, 2016, the company
issued such warrant with a fair value of $630,564 (See Note
5(C)).
On
December 4, 2016, the Company entered into an agreement with a
consultant to provide investor relations services. The agreement
commenced on December 4, 2016 and will continue for twelve months.
As consideration for the services performed, the Company will issue
750,000 shares with a fair value of $32,850 ($0.0321/share) to this
consultant. For the year ended December 31, 2016, the Company
recorded 750,000 as common stock issuable. Shares were subsequently
issued on January 25, 2017 (See Note 8).
(D) Operating Lease Agreement
On
April 1, 2012, the Company executed a one-year non-cancelable
operating lease for its Laboratory space. The lease was
subsequently extended through March 31, 2014. On February 25, 2015,
the Company renewed its lease of a Laboratory. The lease is on a
month to month basis at an annual rate of $13,200. On
June 30, 2015, the Company terminated this lease.
The
Company rented office space at 120 N. Washington Square, Suite 805,
Lansing, Michigan 48950, which was its principal place of business.
The lease was on a month to month basis. The Company paid an annual
rent of $600 for conference facilities, mail, fax, and reception
services located at our principal place of business. On
September 1, 2015, the Company ended the lease of this
office.
Starting
in February of 2015, we rent additional office space in East
Lansing, Michigan. In July 2015, the Company signed a
new lease for its East Lansing, Michigan office
space. The Company pays an annual rent of $4,742 for
office space, conference facilities, mail, fax, and reception
services.
Starting
in September of 2015, we rent office space at 2723 South State
Street, Suite 150, Ann Arbor, Michigan 48104, which is our
principal place of business. We pay an annual rent of $2,028 for
conference facilities, mail, fax, and reception services located at
our principal place of business.
On
February 1, 2016 the Company signed a six (6) month lease extension
for its East Lansing office. The Company pays an annual rent of
$4,893 for office space, conference facilities, mail, fax, and
reception services.
On June
29, 2016 the Company signed a twelve (12) month lease for new
office space in Vietnam. The Company pays an annual rent of $2,329
for office space and reception services.
On July
19, 2016 the Company signed a month to month lease for a production
facility in Indiana. The Company pays a monthly rent of $670 for
office space light industrial manufacturing space.
Rent
expense for the year ended December 31, 2016 and 2015 was $9,845
and $12,832, respectively.
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
NOTE 7 RELATED
PARTY TRANSACTIONS
On
December 26, 2006, the Company entered into an addendum to the
intellectual property transfer agreement with Mr. Thompson, its
CEO. Pursuant to the addendum, the Company agreed to issue
either 200,000 preferred shares with the following preferences; no
dividends and voting rights equal to 100 common shares per share of
preferred stock or the payment of $120,000, the officer agreed to
terminate the royalty payments due under the agreement and give
title to the exclusive license for the non-protective apparel use
of the intellectual property to the Company. On the date
of the agreement, the Company did not have any preferred stock
authorized with the required preferences. In accordance with
FASB Accounting Standards Codification No. 480,
Distinguishing Liabilities from Equity
,
the Company determined that the present value of the payment of
$120,000 that was due on December 26, 2007, one year anniversary of
the addendum, should be recorded as an accrued expense until such
time as the Company has the ability to assert that it has preferred
shares authorized. As of March 31, 2010, the Company has
recorded $120,000 in royalty agreement payable- related
party. On December 21, 2007 the officer extended the due
date to July 30, 2008. On May 30, 2008, the officer
extended the due date to March 31, 2009. On October 10,
2008, the officer extended the due date to the earlier of (a) March
30, 2010 or (b) upon demand by the officer. On March 30, 2010,
the officer extended the due date to the earlier of (a) March 30,
2010 or (b) upon demand by the officer. On September 8,
2009, a payment of $15,000 was paid to the officer. On October 19,
2009 and December 1, 2009, $10,000 was paid to the officer
respectfully. An additional payment of $10,000 was made
on January 15, 2010. During the quarter ending September
30, 2010 an additional payment of $8,000 was made. During the year
ended December 31, 2012 an additional payment of $1,000 was made.
During the year ended December 31, 2013 an additional payment of
$1,280 was made. During the year ended December 31,
2014, an additional loan of $572 was made. As
of December 31, 2016 the outstanding balance is
$65,292. Additionally, the accrued expenses are accruing
7% interest per year. As of December 31, 2016, the
Company recorded interest expense and related accrued interest
payable of $1,959.
On
November 10, 2010, the Company entered into an addendum to the
employment agreement, with its CEO, effective January 1, 2011
through the March 31, 2016. The term of the agreement is a
five year period at an annual salary of $210,000. There
is a 6% annual increase. The employee is also to receive a 20%
bonus based on the annual based salary. Any stock, stock
options bonuses have to be approved by the board of directors. On
January 1, 2016, the agreement renewed with the same terms for
another 5 years with an annual salary of $297,889 for the year
ended December 31, 2016.
On
January 23, 2015, the board of directors appointed Mr. Jonathan R.
Rice as its Chief Operating Officer. The employment agreement has a
term of one year and can be terminated by either the Company or Mr.
Rice at any time. Under the employment agreement, Mr. Rice is
entitled to an annual cash compensation of $120,000, which includes
salary, health insurance, 401K retirement plan contributions, etc.
The Company also agreed to reimburse Mr. Rice for his past
educational expenses of approximately $11,000. In addition, Mr.
Rice was issued a three-year warrant to purchase 2,000,000 shares
of common stock of the Company at an exercise price of $0.001 per
share pursuant to the employment agreement. Additionally, on May
28, 2015, the Company issued a three-year warrant to purchase
3,000,000 shares of common stock of the Company at an exercise
price of $0.001 per share. The warrant fully vests on October 28,
2016. On January 14, 2016 the Company signed a new employment
agreement with Mr. Rice, the COO. The employment agreement has a
term of one year and can be terminated by either the Company or Mr.
Rice at any time. Under the employment agreement, Mr. Rice is
entitled to an annual cash compensation of $140,000, which includes
salary, health insurance, 401K retirement plan contributions, etc.
In addition, Mr. Rice will be issued a three-year warrant to
purchase 6,000,000 shares of common stock of the Company at an
exercise price of $0.001 per share pursuant to the employment
agreement. For the year ended December 31, 2016, the Company
recorded $193,654 for the warrants issued to Mr Rice.
On
August 4, 2016 the Company issued a bonus of $20,000 payable to Mr.
Rice if he remains employed with the Company through March 31,
2018.
On June 6, 2016, the Company borrowed $50,000 from a principal
stockholder. Pursuant to the terms of the loan, the loan
is unsecured carrying an annual interest at 3% and due on
demand.
The Company
recorded accrued interest payable of $855 as of December 31, 2016.
In addition, the Company recorded $1,425 as an in-kind contribution
of interest related to the loan for the year ended December 31,
2016.
As of
December 31, 2016 and December 31, 2015, there was $187,756 and
$148,019, respectively, included in accounts payable and accrued
expenses - related party, which is owed to the Company’s
Chief Executive Officer.
As of
December 31, 2016 there was $561,245 of accrued interest- related
party and $15,532 in shareholder loan interest – related
party included in accounts payable and accrued expenses –
related party, which is owed to the Company’s Chief Executive
officer.
As of
December 31, 2015, there was $426,054 of accrued interest- related
party and $12,718 in shareholder loan interest – related
party included in accounts payable and accrued expenses –
related party, which his owed to the Company’s Chief
Executive officer.
As of
December 31, 2016, the Company owes $1,392,041 in accrued salary to
principal stockholder, $20,000 to the Company’s COO, and $421
to its intern.
As of
December 31, 2015, the Company owes $1,094,153 in accrued salary to
principal stockholder and $1,748 to the Company’s
COO.
The
Company owes $65,292 in royalty payable to related party for the
year ended December 31, 2016 and December 31, 2015.
Kraig Biocraft Laboratories, Inc.
Notes to Financial Statements
As of December 31, 2016 and 2015
On May
28, 2015, the Company issued 3-year warrant for 3,000,000 shares to
a related party, with an exercise price of $0.001 per share. The
warrants were granted for services to be rendered. The warrants had
a fair value of $117,503, based upon the Black-Scholes
option-pricing model on the date of grant and vesting on October
28, 2016, and will be exercisable on May 28, 2018, and for a period
expiring on May 28, 2022. During the years ended of December 31,
2016 and 2015, the Company recorded $68,600 and $49,129 as an
expense for warrants issued to related party.
On
January 1, 2016, the Company issued 3-year warrant for 6,000,000
shares to a related party, with an exercise price of $0.001 per
share. The warrants were granted for services to be rendered. The
warrants had a fair value of $142,526, based upon the Black-Scholes
option-pricing model on the date of grant and vesting on February
20, 2017, and will be exercisable on February 20, 2018, and for a
period expiring on February 20, 2021. During the year ended
December 31, 2016, the Company recorded $125,053 as an expense for
warrants issued to related party.
NOTE 8 SUBSEQUENT EVENTS
In
preparing these financial statements, the Company has evaluated
events and transactions for potential recognition or disclosure
through March 22, 2017, the date the financial statements were
available to be issued.
On
Janaury 23, 2017, the Company signed a lease with a related party
for property in Texas. This property will be used to grow the
Company's mulberry trees. The Company pays $960 per month
starting on February 1, 2017.
On
January 25, 2017, the Company issued 750,000 shares of common stock
previously recorded as common stock issuable for the year end
December 31, 2016 (See Note 6 (C)).
On January 25, 2017, the Company issued 2,678,571
share of common stock for $150,000
($0.056/share).
On
February 6, 2017 the Company issued a warrant for 750,000 share of
common stock to a consultant for services rendered.
On
April 6, 2017 the Company issued 2,083,333 shares of common stock
for $100,000 ($0.048/share).
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
Caution Regarding Forward-Looking Information
Certain
statements contained herein, including, without limitation,
statements containing the words “believes”,
“anticipates”, “expects” and words of
similar import, constitute forward-looking statements. Such
forward-looking statements involve known and unknown risks,
uncertainties and other factors that may cause the actual results,
performance or achievements of the Company, or industry results, to
be materially different from any future results, performance or
achievements expressed or implied by such forward-looking
statements.
Such
factors include, among others, the following: international,
national and local general economic and market conditions:
demographic changes; the ability of the Company to sustain, manage
or forecast its growth; the ability of the Company to successfully
make and integrate acquisitions; raw material costs and
availability; new product development and introduction;
existing government regulations and changes in, or the failure to
comply with, government regulations; adverse publicity;
competition; the loss of significant customers or suppliers;
fluctuations and difficulty in forecasting operating results;
changes in business strategy or development plans; business
disruptions; the ability to attract and retain qualified personnel;
the ability to protect technology; and other factors referenced in
this and previous filings.
Given
these uncertainties, readers of this prospectus and investors are
cautioned not to place undue reliance on such forward-looking
statements.
Plan of Operations
During
the next twelve months, we expect to take the following steps in
connection with the further development of our business and the
implementation of our plan of operations:
|
●
|
|
We have
spent approximately $397,136 between January 2016 and December 2016
on collaborative research and development of high strength polymers
at the University of Notre Dame. We expect to spend approximately
$35,000 per month between January 2017 and March 2017 on
collaborative research and development of high strength polymers at
the University of Notre Dame. With this funding we plan
to accelerate both our microbiology and selective breeding programs
as well as providing more resources for our material testing
protocols. If our financing allows, management will give
strong consideration to accelerating the pace of spending on
research and development within the University of Notre
Dame’s laboratories.
|
|
●
|
|
We
expect to spend approximately $13,700 on collaborative research and
development of high strength polymers and spider silk protein at
the University of Wyoming over the next twelve months. This level
of research spending at the university is also a requirement of our
licensing agreement with them.
|
|
●
|
|
We will
actively consider pursuing collaborative research opportunities
with other university laboratories in the area of high strength
polymers. If our financing will allow, management will give strong
consideration to increasing the depth of our research to include
polymer production technologies that are closely related to our
core research
|
|
●
|
|
We will
consider buying an established revenue producing company in a
compatible business, in order to broaden our financial base and
facilitate the commercialization of our products. We expect to use
a combination of stock and cash for any such purchase.
|
|
●
|
|
We will
also actively consider pursuing collaborative research
opportunities with both private and university laboratories in
areas of research which overlap the company’s existing
research and development. One such potential area for collaborative
research which the company is considering is protein expression
platforms. If our financing will allow, management will give strong
consideration to increasing the breadth of our research to include
protein expression platform technologies.
|
|
●
|
|
We plan
to actively pursue collaborative research and product testing,
opportunities with companies in the biotechnology, materials,
textile and other industries.
|
|
●
|
|
We plan
to actively pursue collaborative commercialization, marketing and
manufacturing opportunities with companies in the textile and
material sectors for the fibers we developed and for any new
polymers that we created in 2016.
|
|
●
|
|
We plan
to actively pursue the development of commercial scale production
of our recombinant materials including Monster SilkTM and
Dragon SilkTM.
|
Limited Operating History
We have not previously demonstrated that we will be able to expand
our business through an increased investment in our research and
development efforts. We cannot guarantee that the research and
development efforts described in this filing will be successful.
Our business is subject to risks inherent in growing an enterprise,
including limited capital resources, risks inherent in the research
and development process and possible rejection of our products in
development.
If financing is not available on satisfactory terms, we may be
unable to continue expanding our operations. Equity financing will
result in a dilution to existing shareholders.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS
ON ACCOUNTING AND FINANCIAL DISCLOSURE
N/A
Results
of Operations for the Years ended December 31, 2016 and
2015.
Our
revenue, operating expenses, and net loss from operations for the
years ended December 31, 2016 as compared to the year ended
December 31, 2015, were as follows – some balances on the
prior period’s combined financial statements have been
reclassified to conform to the current period presentation:
|
|
|
|
|
|
|
|
|
|
|
|
NET
REVENUES
|
$
31,858
|
$
-
|
$
31,858
|
100
%
|
|
OPERATING
EXPENSES:
|
|
|
|
|
|
General and
Administrative
|
$
1,736,918
|
$
920,919
|
$
815,999
|
88.61
%
|
|
Professional
Fees
|
$
396,125
|
$
260,716
|
$
135,409
|
51.94
%
|
|
Officer's
Salary
|
$
447,283
|
$
440,896
|
$
6,387
|
1.45
%
|
|
Research and
Development
|
$
397,136
|
$
432,008
|
$
(34,872
)
|
(8.07
%)
|
|
Total
operating expenses
|
$
2,977,462
|
$
2,054,539
|
$
922,923
|
44.92
%
|
|
Loss
from operations
|
$
(2,945,604
)
|
$
(2,054,539
)
|
$
(891,065
)
|
(43.37
%)
|
|
Gain on forgiveness
of debt
|
$
11,191
|
$
23,245
|
$
(12,054
)
|
(51.86
)
|
|
Loss on disposal of
fixed asset
|
-
|
$
(953
)
|
$
(953
)
|
(100
%)
|
|
Interest
expense
|
$
(139,430
)
|
$
(101,546
)
|
$
37,884
|
37.31
%
|
|
Net
Loss
|
$
(3,073,843
)
|
$
(2,133,793
)
|
$
940,050
|
44.06
%
|
12
Net Revenues
: During
the year ended December 31, 2016, we realized $31,858 of revenues
from our business. During the year ended December 31, 2015, we
realized $0 of revenues from our business. The change in revenues
between the years ended December 31, 2016 and 2015 was $31,858 or
100%. This increase was related to our first contract with
the US Defense Department.
Research and development
expenses:
During year ended December 31, 2016 we
incurred $397,136 research and development expenses. During
year ended December 31, 2015 we incurred $432,008 of research and
development expenses, a decrease of $34,872 or 8.07% compared with
the same period in 2016. The research and development expenses are
attributable to the research and development with the Notre Dame
University; this increase was due to the timing of research related
activity and related charges and the hiring of an additional lab
team member.
Professional
Fees:
During year ended December 31 2016, we
incurred $396,125 professional expenses, which increased by
$135,409 or 51.94% from $260,716 for year ended December 31,
2015. The increase in professional fees expense was
attributable to increased expenses related to investor relations
services during year ended December 31, 2016.
Officers
Salary:
During year ended December 31, 2016,
officers’ salary expenses increased to $447,283 or 1.45% from
$440,896 for year ended December 31, 2015.
General and Administrative
Expense
: General and administrative expenses increased by
$815,999 or 88.61% to $1,736,918 for year ended December 31, 2016
from $920,919 for year ended December 31, 2015. Our general
and administrative expenses for year ended December 31, 2016
consisted of consulting fees of $23,457 and other general and
administrative expenses (which includes expenses such as Auto,
Business Development, SEC Filing, Investor Relations, General
Office, warrant Compensation) of $1,685,825, Travel of $16,548,
office salary of $11,088 for a total of $1,736,918. Our general and
administrative expenses for year ended December 31, 2015 consisted
of salaries and benefits of $3,570, consulting fees of $34,000, and
other general and administrative expenses (which includes expenses
such as: Auto, Business Development, SEC Filing, Investor
Relations, General Office, warrant Compensation of $866,825, and
travel of $16,524 for a total of $920,919. The primary reason for
the increase in comparing year ended December 31, 2016 to the
corresponding period for 2015 was mainly due to general business
expenses and warrants issuances for services.
Interest Expense
: Interest expense increased by
$37,884 to $139,430 for the year ended December 31, 2016 from
$101,546 for the year ended December 31, 2015. The increase
was primarily due to interest on the loans.
Net Loss
: Net loss
increased by $940,050, or 44.06%, to a net loss of $3,073,843 for
the year ended December 31, 2016 from a net loss of $2,133,793 for
the year ended December 31, 2015. This increase in net loss
was driven primarily by increases in research and development,
warrant compensation and professional fees.
Capital Resources, Liquidity and Management Plans
Our
financial statements have been presented on the basis that we have
a going concern, which contemplates the realization of assets and
satisfaction of liabilities in the normal course of
business. As presented in the financial statements, we
incurred a net loss of $3,073,843 during the year ended December
31, 2016, and losses are expected to continue in the near
term. The accumulated deficit is $23,385,979 at December 31,
2016. Refer to Note 5 for our discussion of stockholder
deficit. We have been funding our operations through private
loans and the sale of common stock in private placement
transactions. Refer to Note 4 and Note 5 in the condensed
financial statements for our discussion of notes payable and shares
issued, respectively. Our cash resources are insufficient to meet
our planned business objectives without additional
financing. These and other factors raise substantial doubt
about our ability to continue as a going concern. The
accompanying financial statements do not include any adjustments to
reflect the possible future effects on the recoverability and
classification of assets or the amounts and classification of
liabilities that may result from the possible inability of our
company to continue as a going concern.
Management
anticipates that significant additional expenditures will be
necessary to develop and expand our business before significant
positive operating cash flows can be achieved. Our ability to
continue as a going concern is dependent upon our ability to raise
additional capital and to ultimately achieve sustainable revenues
and profitable operations. At December 31, 2016, we had
$298,859 of cash on hand. These funds are insufficient to
complete our business plan and as a consequence, we will need to
seek additional funds, primarily through the issuance of debt or
equity securities for cash to operate our business. No
assurance can be given that any future financing will be available
or, if available, that it will be on terms that are satisfactory to
us. Even if we are able to obtain additional financing, it may
contain undue restrictions on our operations, in the case of debt
financing or cause substantial dilution for our stock holders, in
case or equity financing.
Management has
undertaken steps as part of a plan to improve operations with the
goal of sustaining our operations for the next twelve months and
beyond. These steps include (a) raising additional capital
and/or obtaining financing; (b) controlling overhead and expenses;
and (c) executing material sales or research contracts. There
can be no assurance that the Company can successfully accomplish
these steps and it is uncertain that the Company will achieve a
profitable level of operations and obtain additional
financing. There can be no assurance that any additional
financing will be available to the Company on satisfactory terms
and conditions, if at all. As of the date of this Report, we
have not entered into any formal agreements regarding the
above.
In the
event the Company is unable to continue as a going concern, the
Company may elect or be required to seek protection from its
creditors by filing a voluntary petition in bankruptcy or may be
subject to an involuntary petition in bankruptcy. To date,
management has not considered this alternative, nor does management
view it as a likely occurrence.
Cash,
total current assets, total assets, total current liabilities and
total liabilities as of December 31, 2016 as compared to December
31, 2015, were as follows:
|
|
|
|
|
Cash
|
$
298,859
|
$
238,188
|
|
Accounts
receivable
|
$
31,858
|
$
-
|
|
Prepaid
Expenses
|
$
1,324
|
$
645
|
|
Total current
assets
|
$
332,041
|
$
238,833
|
|
Total
assets
|
$
383,659
|
$
304,937
|
|
Total current
liabilities
|
$
2,744,472
|
$
2,201,873
|
|
Total
liabilities
|
$
2,744,472
|
$
2,201,873
|
At December
31, 2016, we had a working capital deficit of $2,412,431, compared
to a working capital deficit of $1,963,040 at December 31,
2015. Current liabilities increased to $2,744,472 at December
31, 2016 from $2,201,873 at December 31, 2015, primarily as a
result of accounts payable and accrued compensation.
For the
year ended December 31, 2016, net cash used in operations of
$1,011,841 was the result of a net loss of $3,073,843 offset by
depreciation expense of $16,974, gain on forgiveness of debt of
$11,191, warrants issued to related parties of $193,654, increase
in prepaid expenses of $679, an increase of accrued expenses and
other payables-related party of $498,640 and a decrease in accounts
payable of $16,425. For the year ended December 31, 2015, net
cash used in operations of $967,563 was the result of a net loss of
$2,133,793 offset by depreciation expense of $15,419, gain on
forgiveness of debt of $23,245, loss on disposal of fixed assets of
$953, warrants issued to related parties of $121,448, warrants
issued to consultants of $590,335, decrease in prepaid expenses of
$355 an increase of accrued expenses and other payables-related
party of $489,719 and a decrease in accounts payable of
$28,753.
Net
cash used in our investing activities were $2,488 and $39,285 for
the year ended December 31, 2016 and December 31, 2015,
respectively. Our investing activities for the years ended
December 31, 2016 and 2015 are attributable to purchases of fixed
assets.
Our
financing activities resulted in a cash inflow of $1,075,000 for
the years ended December 31, 2016, which is represented by
$1,025,000 proceeds from issuance of common stock and $50,000
proceeds from shareholder note payable. Our financing activities
resulted in cash inflow of $750,000 for the year ended December 31,
2015, which is represented by $750,000 proceeds from issuance of
common stock.
Off-Balance Sheet Arrangements
The Company does not have any off-balance sheet arrangements that
have or are reasonably likely to have a current or future effect on
the Company's financial condition, changes in financial condition,
revenues or expenses, results of operations, liquidity, capital
expenditures or capital resources that are material to
investors.
Critical Accounting Policies
Our financial statements and related public financial information
are based on the application of accounting principles generally
accepted in the United States (“GAAP”). GAAP requires
the use of estimates; assumptions, judgments and subjective
interpretations of accounting principles that have an impact on the
assets, liabilities, and revenue and expense amounts reported.
These estimates can also affect supplemental information contained
in our external disclosures including information regarding
contingencies, risk and financial condition. We believe our use of
estimates and underlying accounting assumptions adhere to GAAP and
are consistently and conservatively applied. We base our estimates
on historical experience and on various other assumptions that we
believe to be reasonable under the circumstances. Actual results
may differ materially from these estimates under different
assumptions or conditions. We continue to monitor significant
estimates made during the preparation of our financial
statements.
Our significant accounting policies are summarized in Note 1 of our
financial statements. While all these significant accounting
policies impact its financial condition and results of operations,
we view certain of these policies as critical. Policies determined
to be critical are those policies that have the most significant
impact on our financial statements and require management to use a
greater degree of judgment and estimates. Actual results may differ
from those estimates. Our management believes that given current
facts and circumstances, it is unlikely that applying any other
reasonable judgments or estimate methodologies would cause effect
on our results of operations, financial position or liquidity for
the periods presented in this
prospectus
.
Recent Accounting Pronouncements
In January 2016, the Financial Accounting Standards Board
(“FASB”) issued Accounting Standards Update (ASU)
2016-01, which amends the guidance in U.S. GAAP on the
classification and measurement of financial instruments. Changes to
the current guidance primarily affect the accounting for equity
investments, financial liabilities under the fair value option, and
the presentation and disclosure requirements for financial
instruments. In addition, the ASU clarifies guidance related to the
valuation allowance assessment when recognizing deferred tax assets
resulting from unrealized losses on available-for-sale debt
securities. The new standard is effective for fiscal years and
interim periods beginning after December 15, 2017, and upon
adoption, an entity should apply the amendments by means of a
cumulative-effect adjustment to the balance sheet at the beginning
of the first reporting period in which the guidance is effective.
Early adoption is not permitted except for the provision to record
fair value changes for financial liabilities under the fair value
option resulting from instrument-specific credit risk in other
comprehensive income. The Company is currently evaluating the
impact of adopting this guidance.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic
842) to increase transparency and comparability among organizations
by recognizing lease assets and lease liabilities on the balance
sheet and disclosing key information about leasing arrangements.
Topic 842 affects any entity that enters into a lease, with some
specified scope exemptions. The guidance in this Update supersedes
Topic 840, Leases. The core principle of Topic 842 is that a lessee
should recognize the assets and liabilities that arise from leases.
A lessee should recognize in the statement of financial position a
liability to make lease payments (the lease liability) and a
right-of-use asset representing its right to use the underlying
asset for the lease term. For public companies, the amendments in
this Update are effective for fiscal years beginning after December
15, 2018, including interim periods within those fiscal years. We
are currently evaluating the impact of adopting ASU No. 2016-02 on
our financial statements.
In March 2016, the FASB issued ASU 2016-08, Revenue from Contracts
with Customers (Topic 606): Principal versus Agent Considerations
(Reporting Revenue Gross versus Net) that clarifies how to apply
revenue recognition guidance related to whether an entity is a
principal or an agent. ASU 2016-08 clarifies that the analysis must
focus on whether the entity has control of the goods or services
before they are transferred to the customer and provides additional
guidance about how to apply the control principle when services are
provided and when goods or services are combined with other goods
or services. The effective date for ASU 2016-08 is the same as the
effective date of ASU 2014-09 as amended by ASU 2015-14, for annual
reporting periods beginning after December 15, 2017, including
interim periods within those years. The Company has not yet
determined the impact of ASU 2016-08 on its financial
statements.
In March 2016, the FASB issued ASU No. 2016-09, Compensation
– Stock Compensation, or ASU No. 2016-09. The areas for
simplification in this Update involve several aspects of the
accounting for share-based payment transactions, including the
income tax consequences, classification of awards as either equity
or liabilities, and classification on the statement of cash flows.
For public entities, the amendments in this Update are effective
for annual periods beginning after December 15, 2016, and interim
periods within those annual periods. Early adoption is permitted in
any interim or annual period. If an entity early adopts the
amendments in an interim period, any adjustments should be
reflected as of the beginning of the fiscal year that includes that
interim period. An entity that elects early adoption must adopt all
of the amendments in the same period. Amendments related to the
timing of when excess tax benefits are recognized, minimum
statutory withholding requirements, forfeitures, and intrinsic
value should be applied using a modified retrospective transition
method by means of a cumulative-effect adjustment to equity as of
the beginning of the period in which the guidance is adopted.
Amendments related to the presentation of employee taxes paid on
the statement of cash flows when an employer withholds shares to
meet the minimum statutory withholding requirement should be
applied retrospectively. Amendments requiring recognition of excess
tax benefits and tax deficiencies in the income statement and the
practical expedient for estimating expected term should be applied
prospectively. An entity may elect to apply the amendments related
to the presentation of excess tax benefits on the statement of cash
flows using either a prospective transition method or a
retrospective transition method. We are currently evaluating the
impact of adopting ASU No. 2016-09 on our financial
statements.
In April 2016, the FASB issued ASU 2016-10, Revenue from Contracts
with Customers (Topic 606): Identifying Performance Obligations and
Licensing, which provides further guidance on identifying
performance obligations and improves the operability and
understandability of licensing implementation guidance. The
effective date for ASU 2016-10 is the same as the effective date of
ASU 2014-09 as amended by ASU 2015-14, for annual reporting periods
beginning after December 15, 2017, including interim periods within
those years. In May 2016, the FASB issued ASU 2016-12
“Revenue from Contracts with Customers (Topic 606) -
Narrow-Scope Improvements and Practical Expedients,” which
amends the guidance on transition, collectability, non-cash
consideration, and the presentation of sales and other similar
taxes. ASU 2016-12 clarifies that, for a contract to be considered
completed at transition, all (or substantially all) of the revenue
must have been recognized under legacy GAAP. In addition, ASU
2016-12 clarifies how an entity should evaluate the collectability
threshold and when an entity can recognize nonrefundable
consideration received as revenue if an arrangement does not meet
the standard’s contract criteria. The standard allows for
both retrospective and modified retrospective methods of adoption.
The Company has not yet determined the impact of ASU 2016-10 on its
financial statements.
In June 2016, the FASB issued ASU 2016-13, "Measurement of Credit
Losses on Financial Statements," which requires companies to
measure credit losses utilizing a methodology that reflects
expected credit losses and requires consideration of a broader
range of reasonable and supportable information to inform credit
loss estimates. ASU 2016-13 is effective for annual reporting
periods, and interim periods therein, beginning after December 15,
2019 (fiscal year 2021 for the Company). The Company has not yet
determined the potential effects of the adoption of ASU 2016-13 on
its Financial Statements.
In August 2016, the FASB issued ASU 2016-15, "Classification of
Certain Cash Receipts and Cash Payments," which aims to eliminate
diversity in practice in how certain cash receipts and cash
payments are presented and classified in the statement of cash
flows under Topic 230, Statement of Cash Flows, and other Topics.
ASU 2016-15 is effective for annual reporting periods, and interim
periods therein, beginning after December 15, 2017 (fiscal year
2019 for the Company). The Company has not yet determined the
potential effects of the adoption of ASU 2016-15 on its Financial
Statements.
In September, 2015, the FASB issued ASU No. 2015-16, Business
Combinations (Topic 805) (“ASU 2015-16”). Topic 805
requires that an acquirer retrospectively adjust provisional
amounts recognized in a business combination, during the
measurement period. To simplify the accounting for adjustments made
to provisional amounts, the amendments in the Update require that
the acquirer recognize adjustments to provisional amounts that are
identified during the measurement period in the reporting period in
which the adjustment amount is determined. The acquirer is required
to also record, in the same period’s financial statements,
the effect on earnings of changes in depreciation, amortization, or
other income effects, if any, as a result of the change to the
provisional amounts, calculated as if the accounting had been
completed at the acquisition date. In addition an entity is
required to present separately on the face of the income statement
or disclose in the notes to the financial statements the portion of
the amount recorded in current-period earnings by line item that
would have been recorded in previous reporting periods if the
adjustment to the provisional amounts had been recognized as of the
acquisition date. ASU 2015-16 is effective for fiscal years
beginning December 15, 2015. The adoption of ASU 2015-016 is not
expected to have a material effect on the Company’s
consolidated financial statements.
In August, 2015, the FASB issued ASU No. 2015-14, Revenue from
Contracts with Customers (Topic 606): Deferral of the Effective
Date (“ASU 2015-14”). The amendment in this ASU defers
the effective date of ASU No. 2014-09 for all entities for one
year. Public business entities, certain not-for-profit entities,
and certain employee benefit plans should apply the guidance in ASU
2014-09 to annual reporting periods beginning December 15, 2017,
including interim reporting periods within that reporting period.
Earlier application is permitted only as of annual reporting
periods beginning after December 31, 2016, including interim
reporting periods with that reporting period.
In April 2015, the Financial Accounting Standards Board
(“FASB”) issued Accounting Standards Update
(“ASU”) No. 2015-03, Interest–Imputation of
Interest (Subtopic 835-30) (“ASU 2015-03”), which
changes the presentation of debt issuance costs in financial
statements. ASU 2015-03 requires an entity to present such costs in
the balance sheet as a direct deduction from the related debt
liability rather than as an asset. Amortization of the costs will
continue to be reported as interest expense. It is effective for
annual reporting periods beginning after December 15, 2016. Early
adoption is permitted. The new guidance will be applied
retrospectively to each prior period presented. The Company is
currently in the process of evaluating the impact of adoption of
ASU 2015-03 on its balance sheets.
All other newly issued accounting pronouncements but not yet
effective have been deemed either immaterial or not
applicable
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, financings, or
other relationships with unconsolidated entities or other persons,
also known as “special purpose entities”
(SPEs).
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL
PERSONS
Our
executive officers and sole director as of the date of the
Prospectus is as follows:
|
NAME
|
|
AGE
|
|
POSITION
|
|
DATE APPOINTED
|
|
|
|
|
|
|
|
|
|
Kim
Thompson
|
|
55
|
|
President,
Chief Executive Officer, Chief Financial Officer, and
Director
|
|
April
25, 2006
|
|
Jonathan
R. Rice
|
|
37
|
|
Chief
Operating Officer
|
|
January
20, 2015
|
The
following summarizes the occupation and business experience during
the past five years for our officers and sole
director.
KIM THOMPSON
Mr.
Thompson was a founder of the California law firm of Ching &
Thompson which was founded in 1997 where he focused primarily on
commercial litigation. He has been a partner in the Illinois law
firm of McJessy, Ching & Thompson since 2004 where he also
emphasizes commercial and civil rights litigation. Mr. Thompson
received his bachelor’s degree in applied economics from
James Madison College, Michigan State University, and his Juris
Doctorate from the University of Michigan. He is the named inventor
or co-inventor on a number of provisional patent applications
including inventions relating to biotechnology and mechanics. Mr.
Thompson is the inventor of the technology concept that lead to the
forming of the Company. We believe that Mr. Thompson is well suited
to serve as our director because of his knowledge of biotechnology,
legal expertise and background in economics.
JONANTHAN R. RICE
Jonathan
R. Rice had worked at Ultra Electronics, Adaptive Materials Inc., a
Michigan company (“UEA”) since 2002. At UEA, he worked
as the Director of Advanced Technologies, where he was responsible
for new products development and commercialization. He was also the
Corporate Facility Security Officer for UEA since 2006, where Mr.
Rice ensured UEA’s compliance with federal regulations under
the National Industrial Security Program Operating Manual and
completed its annual security audit. During 2004 through 2007 while
working as an Engineering Manager at UEA, Mr. Rice, among other
things, led the design and development of multiple fuel cell and
power management systems, established a team to identify and
eliminate production and performance limitation, authored technical
progress and final reports for customers and provided training to
military personnel on use of fuel cell systems. From 2002 through
2005, Mr. Rice had also served as UEA’s Production Manager in
charge of developing manufacturing process and techniques and
sourcing the production equipment for UEA’s products. Mr.
Rice graduated from Michigan Technological University in 2002 with
a degree of Bachelors of Science Chemical Engineering. Mr. Rice
recieved his Masters of Business Administration from Michigan State
University in April 2016.
Term of Office
Our directors are appointed for a one-year term to hold office
until the next annual general meeting of our shareholders or until
removed from office in accordance with our bylaws. Our officers are
appointed by our board of directors and hold office until removed
by the board. Mr. Thompson is employed as the CEO and CFO of the
company pursuant to a five year employment contract.
Our officers and director have not filed any bankruptcy petition,
been convicted of or been the subject of any criminal proceedings
or the subject of any order, judgment or decree involving the
violation of any state or federal securities laws within the past
ten (10) years.
Our sole director was appointed for a one-year term to hold office
until the next annual general meeting of our shareholders or until
removed from office in accordance with our bylaws. Our officers
were appointed by our board of directors and holds office until
removed by the board.
Committees
Because
our Board of Directors currently consists of only one member, no
board committees have been formed as of the filing of this
prospectus. All audit committee functions are performed by Mr. Kim
Thompson, as the sole member of our Board of Directors and he is
the largest shareholder of the Company and the Company’s
Chief Executive Officer and President. Mr. Thompson does not
qualify as an “audit committee financial expert” within
the applicable definition of the Securities and Exchange
Commission.
Code of Ethics
The
Company has adopted a Code of Ethics applicable to its Chief
Executive Officer and Chief Financial Officer. This Code of Ethics
was previously filed as an exhibit to our annual report on Form
10-KSB on March 26, 2008.
EXECUTIVE COMPENSATION
The following summary compensation table sets forth all
compensation awarded to, earned by, or paid to the named executive
officer during the years ended December 31, 2016 and 2015 in all
capacities for the accounts of our executive, including the Chief
Executive Officer (CEO) and Chief Financial Officer
(CFO):
SUMMARY COMPENSATION TABLE
|
Name and principal position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock Awards
($)
|
Option Awards
($)
|
Non-Equity Incentive Plan Compensation
($)
|
Nonqualified Deferred Compensation Earnings
($)
|
All Other Compensation
($)
|
|
Total
($)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kim
Thompson
President,
CEO, CFO and Director
|
2016
|
$297,889
|
$59,578
|
$0
|
$0
|
$0
|
$0
|
$44,609
|
(1)
|
$402,076
|
|
2015
|
$281,027
|
$56,205
|
$0
|
$0
|
$0
|
$0
|
$37,580
|
(2)
|
$374,812
|
|
Jonathan
R. Rice COO
|
2016
|
$125,393
|
$24,000
|
$0
|
$125,053
|
$0
|
$0
|
$17,215
|
(3)
|
$291,661
|
|
2015
|
$97,915
|
$4,000
|
$0
|
$121,448
|
$0
|
$0
|
$13,814
|
(4)
|
$237,117
|
|
1)
|
In
2016, Kim Thompson received $29,613 in medical insurance and
medical reimbursement pursuant to an employment agreement entered
into with us. In 2016, Kim Thompons received $14,996 in
reimbursement for office and travel related expenses.
|
|
2)
|
In
2015, Kim Thompson received $37,580 in medical insurance and
medical reimbursement pursuant to an employment agreement entered
into with us.
|
|
3)
|
In
2016, Jonathan Rice received $10,925 in medical insurance and
medical reimbursement, $1,040 in phone service expenses, and $
5,250 in tuition reimbursements pursuant to an employment agreement
entered into with us.
|
|
4)
|
In
2015, Jonathan Rice received $7,604 in medical insurance and
medical reimbursement, $960 in phone service expenses, and $
5,250 in tuition reimbursements pursuant to an employment agreement
entered into with us.
|
Employment Agreements
On
November 10, 2010 the Company entered into a five-year employment
agreement with the Company’s Chairman, Chief Executive
Officer and Chief Financial Officer, effective as of January
1, 2011. The agreement renews annually so that at all times, the
term of the agreement is five years. Pursuant to this agreement,
the Company will pay an annual base salary of $210,000 for the
period January 1, 2011 through December 31, 2011. Base pay will be
increased each January 1st, for the subsequent twelve month periods
by six percent. The officer will also be entitled to life,
disability, health and dental insurance as well as an annual bonus
in an amount equal to 20% of the base salary. The agreement also
calls for the retention of the executive as a consultant following
the termination of employment with compensation during such
consultancy based upon the Company reaching certain
milestones:
a. Upon
the expiration or termination of this agreement for any reason, or
by either party, Company agrees that it will employ Executive as a
consultant for a period of four (4) years and at a rate of $4,500
per month.
b. In
the event that Company achieves gross sales of five million dollars
($5,000,000) or more, or one million dollars ($1,000,000) or more
in net income, in any year during the term of this agreement, or
upon the Company’s achieving an average market capitalization
over a 240 consecutive calendar day period, in excess of
$70,000,000 during the term of this agreement, then the consulting
period will be for five (5) years and the consulting rate will be
increased to $5,500 per month.
c. In
the event that Company achieves gross sales of ten million dollars
($10,000,000) or more, or two million dollars ($2,000,000) or more
in net income, in any year during the term of this agreement, or
upon the Company’s achieving an average market capitalization
over a 240 consecutive calendar day period, in excess of
$90,000,000 during the term of this agreement, then the consulting
period will be for six (6) years and the consulting rate will be
increased to $7,500 per month.
The
November 10, 2010 employment agreement replaced the prior agreement
dated April 26, 2006 and the Chief executive subsequently waved all
warrants and milestone based compensation to which he would have
been entitled under the April 26, 2006 agreement.
COO
On January 20, 2015, the Company entered into an at-will employment
agreement with Mr. Jonathan R. Rice, its Chief Operating Officer
(the “COO Employment Agreement”). The COO Employment
Agreement has a term of one year and can be terminated by either
the Company or Mr. Rice at any time. Under the COO Employment
Agreement, Mr. Rice is entitled to an annual cash compensation of
$120,000, which includes salary, health insurance, 401K retirement
plan contributions, etc. The Company also agreed to reimburse Mr.
Rice for his past educational expenses of approximately $11,000. In
addition, on January 23, 2015, Mr. Rice was issued a three-year
warrant to purchase 2,000,000 shares of common stock of the Company
at an exercise price of $0.001 per share pursuant to the COO
Employment Agreement. Additionally, on May 28, 2015, the
Company issued a three-year warrant to purchase 3,000,000 shares of
common stock of the Company at an exercise price of $0.001 per
share. The warrant fully vests on October 28,
2016. For the twelve months ended the Company recorded
$121,448 for the warrants issued to related party.
On January 1, 2016, the Company entered into an at-will employment
agreement with Mr. Jonathan R. Rice, its Chief Operating Officer
(the “COO Employment Agreement”). The COO Employment
Agreement has a term of one year and can be terminated by either
the Company or Mr. Rice at any time. Under the COO Employment
Agreement, Mr. Rice is entitled to an annual cash compensation of
$140,000, which includes salary, health insurance, 401K retirement
plan contributions, etc. In addition, on March 30, 2016, Mr. Rice
was issued a three-year warrant to purchase 6,000,000 shares of
common stock of the Company at an exercise price of $0.001 per
share pursuant to the COO Employment Agreement. Additionally,
on August 4, 2016, the Company issued a performance retention bonus
to Mr. Rice of $20,000 which is payable on March 31,
2018. For the twelve months ended the Company recorded
$125,053 for the warrants issued to related party.
Outstanding Equity Awards
None.
Long-Term Incentive Plan (“LTIP”) Awards
Table.
There
were no awards made to a named executive officer in the last
completed fiscal year under any LTIP
Compensation of Directors
Directors
are permitted to receive fixed fees and other compensation for
their services as directors. The Board of Directors has the
authority to fix the compensation of directors. No amounts have
been paid to, or accrued to, directors in such
capacity.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND
MANAGEMENT
The
following table provides the names and addresses of each person
known to us to own more than 5% of our outstanding shares of common
stock and Series A Preferred Stock as of the date of this
prospectus and by the officers and directors, individually and as a
group. Except as otherwise indicated, all shares are owned
directly.
|
Title of Class
|
Name and Address of Beneficial Owner Owner
|
Amount
and Nature of Beneficial
|
|
Percent of Class (1)
|
|
|
|
|
|
|
|
Class A
Common Stock
|
Kim
Thompson
|
233,991,767
|
(2)
|
29.96%
|
|
2723 South State St Suite 150
|
|
|
|
|
Ann
Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
Class A
Common Stock
|
Jonathan
R. Rice
|
11,000,000
|
(3)
|
1.41%
|
|
2723 South State St Suite 150
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
Class A
Common Stock
|
All
executive officers and directors as a group (2 Person)
|
244,991,767
|
|
31.37%
|
|
|
|
|
|
|
Series
A Preferred Stock
|
Kim
Thompson
|
2
|
|
100%
|
|
2723 South State St Suite 150
|
|
|
|
|
Ann Arbor, MI 48104
|
|
|
|
|
|
|
|
|
|
Series
A Preferred Stock
|
All
executive officers and directors as a group (1 Person)
|
2
|
|
100%
|
_______________
* less than 1%
(1) The percent of class is based on
783,046,190 shares of our Class A common stock
issued and outstanding as of the date of this
prospectus.
(2) Such shares include 2 shares of common stock that may be issued
upon conversion of the Series A Preferred Stock that is owned by
Mr. Thompson.
(3) These are shares are shares of common stock that may be issued
upon exercise of a warrant that is owned by Mr. Rice; in addition,
Mr. Rice owns a warrant that is not exercisable until May 2018,
that can be exercised to purchase up to 3,000,000 shares and a
warrant that is not exercisable until February 2017, that can be
exercised to purchase up to 6,000,000 shares.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
On December 19, 2013, the Company issued to the CEO two shares of
Series A Preferred Stock, with each share entitles the CEO to
200,000,000 votes on all matters. Each share of Series A
Preferred Stock is convertible into one share of common stock and
has the same right to normal dividends as a common share but has no
other right to distributions. Such shares of Series A Preferred
Stock was issued to the CEO in consideration for the CEO’s
agreement to extend the Company’s repayment of the debts owed
to him to October 30, 2014 and to forgive $30,000 compensation that
the Company owed to him. On March 25, 2015, with
agreement of the CEO not to request repayment before July 1, 2015,
the Company extended the repayment period to begin no sooner than
July 31, 2016.
As of December 31, 2016 and December 31, 2015, the Company
owed $1,392,041 and $1,094,153, respectively, in accrued
salary and accrued payroll taxes to principal stockholder. As of
December 31, 2016, no accrued salary has been converted to Class A
Common Stock.
As of December 31, 2016 and December 31, 2015, there was $187,756
and $148,019, respectively, included in accounts payable and
accrued expenses - related party, which was owed to the
Company’s Chief Executive Officer.
As of December 31, 2015, there was $426,054 of accrued interest-
related party and $12,718 in shareholder loan interest –
related party included in accounts payable and accrued expenses
– related party, which was owed to the Company’s Chief
Executive officer.
As of December 31, 2016, there was $561,245 of accrued interest-
related party and $15,531 in shareholder loan interest –
related party included in accounts payable and accrued expenses
– related party, which was owed to the Company’s Chief
Executive officer.
The
Company owes $65,292 in royalty payable to related party for the
year ended December 31, 2016 and December 31, 2015.
On June
6, 2016 the Company received $50,000 from a principal
stockholder. Pursuant to the terms of the loan, the
advance bears interest at 3%, is unsecured and due on demand. The
Company recorded accrued interest payable of $855 as of December
31, 2016. In addition, the Company recorded $1,425 as an in-kind
contribution of interest related to the loan for the year ended
December 31, 2016.
Other than the above transactions or as otherwise set forth in this
report or in any reports filed by the Company with the SEC, there
have been no related party transactions, or any other transactions
or relationships required to be disclosed pursuant to Item 404 of
Regulation S-K. The Company is currently not a subsidiary of any
company.
Director Independence
We are
not subject to listing requirements of any national securities
exchange and, as a result, we are not at this time required to have
our board comprised of a majority of “independent
Directors.” Mr. Kim Thompson, our Chief Executive Officer,
Chief Financial Officer and President, is our sole director. Mr.
Thompson does not qualify as independent directors under Rule 10A-3
of the Securities Exchange Act of 1934 and as defined under the
rules and regulations of NASDAQ.
DISCLOSURE OF COMMISSION POSITION OF INDEMNIFICATION FOR SECURITIES
ACT LIABILITIES
The
General Corporation Law of Wyoming provides that directors,
officers, employees or agents of Wyoming corporations
are entitled, under certain circumstances, to be indemnified
against expenses (including attorneys' fees) and other liabilities
actually and reasonably incurred by them in connection with any
suit brought against them in their capacity as a director, officer,
employee or agent, if they acted in good faith and in a manner they
reasonably believed to be in or not opposed to the best interests
of the corporation, and with respect to any criminal action or
proceeding, if they had no reasonable cause to believe their
conduct was unlawful. This statute provides that directors,
officers, employees and agents may also be indemnified against
expenses (including attorneys' fees) actually and reasonably
incurred by them in connection with a derivative suit brought
against them in their capacity as a director, if they acted in good
faith and in a manner they reasonably believed to be in or not
opposed to the best interests of the corporation, except that no
indemnification may be made without court approval if such person
was adjudged liable to the corporation.
Our
Certificate of Incorporation provides that we shall indemnify any
and all persons whom we shall have power to indemnify to the
fullest extent permitted by the Wyoming Corporate Law. Article VII
of our by-laws provides that we shall indemnify our authorized
representatives to the fullest extent permitted by the Wyoming Law.
Our by-laws also permit us to purchase insurance on behalf of any
such person against any liability asserted against such person and
incurred by such person in any capacity, or out of such person's
status as such, whether or not we would have the power to indemnify
such person against such liability under the foregoing provision of
the by-laws.
We have
been advised that in the opinion of the Securities and Exchange
Commission indemnification for liabilities arising under the
Securities Act is against public policy as expressed in the
Securities Act, and is, therefore, unenforceable. In the event that
a claim for indemnification against such liabilities is asserted by
one of our directors, officers, or controlling persons in
connection with the securities being registered, we will, unless in
the opinion of our legal counsel the matter has been settled by
controlling precedent, submit the question of whether such
indemnification is against public policy to a court of appropriate
jurisdiction. We will then be governed by the court’s
decision.
PART II – INFORMATION NOT REQUIRED IN THE
PROSPECTUS
ITEM. 13 OTHER EXPENSES OF ISSUANCE AND
DISTRIBUTION.
|
Securities
and Exchange Commission registration fee
|
$573.91
|
|
Transfer
Agent Fees
|
$300.00
|
|
Accounting
fees and expenses
|
$4,500.00
|
|
Legal
fees and expenses
|
$18,500.00
|
|
Total
|
$23,873.91
|
All
amounts are estimates other than the Commission’s
registration fee. We are paying all expenses of the offering listed
above. No portion of these expenses will be borne by the selling
shareholders. The selling shareholders, however, will pay any other
expenses incurred in selling their common stock, including any
brokerage commissions or costs of sale.
ITEM. 14 INDEMNIFICATION OF DIRECTORS AND
OFFICERS.
The
General Corporation Law of Wyoming provides that directors,
officers, employees or agents of Wyoming corporations
are entitled, under certain circumstances, to be indemnified
against expenses (including attorneys' fees) and other liabilities
actually and reasonably incurred by them in connection with any
suit brought against them in their capacity as a director, officer,
employee or agent, if they acted in good faith and in a manner they
reasonably believed to be in or not opposed to the best interests
of the corporation, and with respect to any criminal action or
proceeding, if they had no reasonable cause to believe their
conduct was unlawful. This statute provides that directors,
officers, employees and agents may also be indemnified against
expenses (including attorneys' fees) actually and reasonably
incurred by them in connection with a derivative suit brought
against them in their capacity as a director, if they acted in good
faith and in a manner they reasonably believed to be in or not
opposed to the best interests of the corporation, except that no
indemnification may be made without court approval if such person
was adjudged liable to the corporation.
Our
Certificate of Incorporation provides that we shall indemnify any
and all persons whom we shall have power to indemnify to the
fullest extent permitted by the Wyoming Corporate Law. Article VII
of our by-laws provides that we shall indemnify our authorized
representatives to the fullest extent permitted by the Wyoming Law.
Our by-laws also permit us to purchase insurance on behalf of any
such person against any liability asserted against such person and
incurred by such person in any capacity, or out of such person's
status as such, whether or not we would have the power to indemnify
such person against such liability under the foregoing provision of
the by-laws.
We have
been advised that in the opinion of the Securities and Exchange
Commission indemnification for liabilities arising under the
Securities Act is against public policy as expressed in the
Securities Act, and is, therefore, unenforceable. In the event that
a claim for indemnification against such liabilities is asserted by
one of our directors, officers, or controlling persons in
connection with the securities being registered, we will, unless in
the opinion of our legal counsel the matter has been settled by
controlling precedent, submit the question of whether such
indemnification is against public policy to a court of appropriate
jurisdiction. We will then be governed by the court’s
decision.
ITEM. 15 RECENT SALES OF UNREGISTERED
SECURITIES.
On
January 28, 2014 the Company issued 3,537,736 shares of common
stock for $150,000 ($0.04/share).
On
February 2, 2014, the Company issued 9,821,429 shares in connection
with the exercise of 10,000,000 warrants.
On
February 17, 2014, the Company issued to two consultants the
following warrants in consideration of their services: (i) warrants
that are exercisable on the 14th month anniversary of the
issuance date for an aggregate of 1,200,000 shares of common stock,
(ii) warrants that are exercisable on the 20th month
anniversary of the issuance date for an aggregate of 2,000,000
shares of common stock, and (iii) warrants that are exercisable on
the 32nd month anniversary of the issuance of the issuance
date for an aggregate of 4,000,000 shares of common
stock.
On
February 18, 2014 the Company issued 3,409,091 shares of common
stock for $150,000 ($0.04/share).
On
March 12, 2014 the Company issued 2,551,020 shares of common stock
for $100,000 ($0.04/share).
On
March 28, 2014, the Company renewed and extended a consulting
agreement and has agreed to issue 44,000 shares of common stock as
consideration for consulting fees owed from June 1, 2012 through
March 31, 2014.
On
April 7, 2014 the Company issued 2,212,389 shares of common stock
for $100,000 ($0.04/share).
On
April 22, 2014 the Company issued 2,173,913 shares of common stock
for $100,000 ($0.04/share).
On May
5, 2014, the Company issued 1,800,000 shares of common stock valued
at $111,600 ($0.062/share) to a consultant.
On June
10, 2014, the Company issued 3,409,091 shares of common stock
valued at $150,000 ($0.04/share).
On June
24, 2014, the company issued to a consultant a warrant to purchase
3,000,000 shares of common stock.
On July
7, 2014, the Company issued 2,212,389 share of common stock for
$100,000 ($0.05/share).
On
August 6, 2014, the Company issued 2,272,727 share of common stock
for $100,000 ($0.04/share).
On
September 3, 2014, the Company issued 2,450,980 share of common
stock for $100,000 ($0.04/share).
On
September 22, 2014, the Company issued 2,821,670 share of common
stock for $100,000 ($0.04/share).
On
October 9, 2014, the Company issued 12,000 shares with a fair value
of $484 ($0.0403/share) to a consultant for research and
development services performed from April 1, 2014 through September
30, 2014 and a bonus of 4,000 shares with a fair value of
$161.
On January 21, 2015, the Company issued 2,918,919 shares in
connection with the cashless exercise of the 3,000,000
warrants.
On January 23, 2015, the Company issued to its COO a warrant to
purchase 2,000,000 shares of common stock as compensation for his
services pursuant to an employment agreement dated January 19,
2015.
On March 5, 2015, the Company issued 10,000 shares with a fair
value of $321 ($0.0321/share) to a consultant as consideration for
consulting fees owed from October 1, 2014 through February 28, 2015
of $10,000. The issuance of shares resulted in gain on
settlement of accounts payable of $9,679.
On May 28, 2015, the Company issued 3-year warrant for
3,000,000 shares to a related party, with an exercise price of
$0.001 per share.
On June 16, 2015, the Company issued 4,675,811 share of common
stock for $150,000 ($0.03/share).
On June 22, 2015, the Company issued 3-year warrant for
15,000,000 shares to a consultant, with an exercise price of $0.001
per share.
On July 2, 2015, the Company issued 588,461 shares and 588,461
shares of common stock for consulting services to two consultants
respectively.
On July 9, 2015, the Company issued 3,731,343 share of common stock
for $100,000 ($0.03/share).
On August 3, 2015, the Company issued 4,152,824 shares of common
stock for $100,000 ($0.025/share).
O
n
September 28, 2015, the Company issued 4,166,667 share of common
stock for $100,000 ($0.024/share).
On October 19, 2015, the Company issued 3,894,081 shares of common
stock for $100,000 ($0.026/share).
On November 9, 2015, the Company issued 14,000 shares with a fair
value of $434 ($0.031/share) to a consultant as consideration for
consulting fees owed from March 1, 2015 through September 30, 2015
of $14,000. The issuance of shares resulted in gain on
settlement of accounts payable of $13,556.
On February 16, 2016, the Company issued 5,630,631 shares of common
stock for $100,000 ($0.018/share).
On March 29, 2016, the Company issued 5,411,255 shares of common
stock for $100,000 ($0.018/share).
On April 4, 2016, the Company issued 12,000 shares with a fair
value of $302 ($0.0252/share) to a consultant as consideration for
consulting fees owed from October 1, 2015 through March 31, 2016 of
$6,000. The issuance of shares resulted in gain on settlement of
accounts payable of $5,698.
On April 7, 2016, the Company issued 1,917,012 shares in connection
with the cashless exercise of 1,200,000 warrants.
On April 25, 2016 the Company issued 5,952,381 share of common
stock for $100,000 ($0.017/share).
On May 4, 2016, the Company issued 7,627,907 shares in connection
with the cashless exercise of 8,000,000 warrants.
These
issuances were made in reliance upon exemptions from the
registration requirements of the Securities Act of 1933, as
amended, pursuant to Section 4(a)(2) and/or Regulation D
promulgated thereunder.
ITEM. 16 EXHIBITS AND FINANCIAL STATEMENT
SCHEDULES.
|
EXHIBIT NUMBER
|
|
DESCRIPTION
|
|
|
|
|
|
3.1
|
|
Articles
of Incorporation (1)
|
|
|
|
|
|
3.2
|
|
Articles
of Amendment (3)
|
|
|
|
|
|
3.3
|
|
Articles
of Amendment, filed with the Wyoming Secretary of State on November
15, 2013 (6)
|
|
|
|
|
|
3.2
|
|
Articles
of Amendment, filed with the Wyoming Secretary of State on December
17, 2013 (7)
|
|
|
|
|
|
3.3
|
|
By-Laws
(1)
|
|
|
|
|
|
4.1
|
|
Form of
Warrant issued Mr. Jonathan R. Rice (12)
|
|
|
|
|
|
5.1
|
|
Opinion
of Hunter Taubman Fischer, LLC *
|
|
|
|
|
|
10.1
|
|
Employment
Agreement, dated November 10, 2010, by and between Kraig Biocraft
Laboratories, Inc. and Kim Thompson (8)
|
|
|
|
|
|
10.2
|
|
Securities
Purchase Agreement between Kraig Biocraft Laboratories and Worth
Equity Fund, L.P. and Mutual Release (1)
|
|
|
|
|
|
10.3
|
|
Securities
Purchase Agreement between Kraig Biocraft Laboratories and Lion
Equity (1)
|
|
|
|
|
|
10.4
|
|
Amended
Letter Agreement, dated September 14, 2009, by and between Kraig
Biocraft Laboratories and Calm Seas Capital, LLC (3)
|
|
|
|
|
|
10.5
|
|
Exclusive
License Agreement, effective as of May 8, 2006, by and between The
University of Wyoming and Kraig Biocraft Laboratories, Inc.
(2)
|
|
|
|
|
|
10.6
|
|
Addendum
to the Founder’s Stock Purchase and Intellectual Property
Transfer Agreement, dated December 26, 2006, and the
Founder’s Stock Purchase and Intellectual Property Transfer
Agreement dated April 26, 2006 (3)
|
|
|
|
|
|
10.7
|
|
Intellectual
Property/Collaborative Research Agreement, dated March 20, 2010, by
and between Kraig Biocraft Laboratories and The University of Notre
Dame du Lac. (2)
|
|
|
|
|
|
10.8
|
|
Letter
Agreement, dated June 28, 2011, by and between Kraig Biocraft
Laboratories and Calm Seas Capital, LLC (4)
|
|
|
|
|
|
10.9
|
|
Letter
Agreement, dated April 30, 2013, by and between Kraig Biocraft
Laboratories and Calm Seas Capital, LLC (5)
|
|
|
|
|
|
10.10
|
|
Letter
Agreement, dated October 2, 2014, by and between Kraig Biocraft
Laboratories and Calm Seas Capital, LLC (9)
|
|
|
|
|
|
10.11
|
|
License
Agreement, dated October 28, 2011, between the Company and
University of Notre Dame du Lac. (11)
|
|
|
|
|
|
10.12
|
|
Intellectual
Property / Collaborative Research Agreement, dated June 6, 2012,
between the Company and University of Notre Dame du Lac.
(11)
|
|
|
|
|
|
10.13
|
|
Collaborative
Yarn and Textile Development Agreement, dated September 30, 2013,
between the Company and Warwick Mills, Inc. (11)
|
|
|
|
|
|
10.14
|
|
Employment
Agreement, dated January 19, 2015, between the Company and Mr.
Jonathan R. Rice. (10)
|
|
|
|
|
|
10.15
|
|
Intellectual
Property / Collaborative Research Agreement, dated March 4, 2015,
between the Company and University of Notre Dame du Lac.
(12)
|
|
|
|
|
|
|
|
Consent
of M&K CPAs*
|
|
|
|
|
|
23.2
|
|
Consent
of Hunter Taubman Fischer, LLC, contained in Exhibit
5.1
|
(1) Incorporated
by reference to our Registration Statement on Form SB-2 (Reg. No.
333-146316) filed with the SEC on September 26, 2007.
(2)
Incorporated by reference to our Annual Report on Form 10-K for the
year ended December 31, 2009 filed with the SEC on April 15,
2010.
(3)
Incorporated by reference to our Registration Statement on Form S-1
(Reg. No. 333-162316) filed with the SEC on October 2,
2009.
(4)
Incorporated by reference to our Current Report on Form 8-K filed
with the SEC on June 29, 2011.
(5)
Incorporated by reference to our Quarterly Report on Form 10-Q
filed with the SEC on May 15, 2013.
(6)
Incorporated by reference to our Current Report on Form 8-K filed
with the SEC on November 22, 2013.
(7)
Incorporated by reference to our Current Report on Form 8-K filed
with the SEC on December 19, 2013.
(8)
Incorporated by reference to our Registration Statement on Form S-1
(Reg. No. 333-175936) filed with the SEC on August 1,
2011.
(9)Incorporated
by reference to our Amendment No. 1 to Registration Statement on
Form S-1/A (Reg. No. 333-199820) filed with the SEC on January 7,
2015.
(10)
Incorporated by reference to our Current Report on Form 8-K filed
with the SEC on January 21, 2015.
(11)
Incorporated by reference to our Amendment No. 2 to Registration
Statement on Form S-1/A (Reg. No. 333-199820) filed with the SEC on
January 30, 2015.
(12)
Incorporated by reference to our Annual Report on Form 10-K for the
year ended December 31, 2014 filed with the SEC on March 31,
2015.
* Filed
herewith
ITEM 17. UNDERTAKINGS.
The
undersigned registrant hereby undertakes:
(1) To
file, during any period in which offers or sales are being made, a
post-effective amendment to this registration
statement:
i. To
include any prospectus required by section 10(a)(3) of the
Securities Act of 1933;
ii. To
reflect in the prospectus any facts or events arising after the
effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the
aggregate, represent a fundamental change in the information set
forth in the registration statement. Notwithstanding the foregoing,
any increase or decrease in volume of securities offered (if the
total dollar value of securities offered would not exceed that
which was registered) and any deviation from the low or high end of
the estimated maximum offering range may be reflected in the form
of prospectus filed with the Commission pursuant to Rule
424(b) if, in the aggregate, the changes in volume and price
represent no more than 20% change in the maximum aggregate offering
price set forth in the "Calculation of Registration Fee" table in
the effective registration statement.
iii. To
include any material information with respect to the plan of
distribution not previously disclosed in the registration statement
or any material change to such information in the registration
statement;
(2) That,
for the purpose of determining any liability under the Securities
Act of 1933, each such post-effective amendment shall be deemed to
be a new registration statement relating to the securities offered
therein, and the offering of such securities at that time shall be
deemed to be the initial bona fide offering thereof.
(3) To
remove from registration by means of a post-effective amendment any
of the securities being registered which remain unsold at the
termination of the offering.
(4) Insofar
as indemnification for liabilities arising under the Securities Act
of 1933 may be permitted to directors, officers and controlling
persons of the registrant pursuant to the foregoing provisions, or
otherwise, the registrant has been advised that in the opinion of
the Securities and Exchange Commission such indemnification is
against public policy as expressed in the Act and is,
therefore, unenforceable. In the event that a claim for
indemnification against such liabilities (other than the payment by
the registrant of expenses incurred or paid by a director, officer
or controlling person of the registrant in the successful defense
of any action, suit or proceeding) is asserted by such director,
officer or controlling person in connection with the securities
being registered, the registrant will, unless in the opinion of its
counsel the matter has been settled by controlling precedent,
submit to a court of appropriate jurisdiction the question whether
such indemnification by it is against public policy as expressed in
the Act and will be governed by the final adjudication of such
issue.
(5) That,
for the purpose of determining liability under the Securities Act
of 1933 to any purchaser:
i.
Each prospectus filed pursuant to Rule 424(b) as part of a
registration statement relating to an offering, other than
registration statements relying on Rule 430B or other than
prospectuses filed in reliance on Rule 430A, shall be deemed to be
part of and included in the registration statement as of the date
it is first used after effectiveness. Provided, however,
that no statement made in a registration statement or prospectus
that is part of the registration statement or made in a document
incorporated or deemed incorporated by reference into the
registration statement or prospectus that is part of the
registration statement will, as to a purchaser with a time of
contract of sale prior to such first use, supersede or modify any
statement that was made in the registration statement or prospectus
that was part of the registration statement or made in
any such document immediately prior to such date of first
use.
(6) That, for purposes of determining any liability under the
Securities Act of 1933, each filing of the registrant's annual
report pursuant to section 13(a) or section 15(d) of the Securities
Exchange Act of 1934 (and, where applicable, each filing of
an
employee benefit
plan's annual report pursuant to section 15(d) of the
Securities Exchange Act of 1934) that is incorporated by
reference in the registration statement shall be deemed to be a new
registration statement relating to the securities offered therein,
and the offering of such securities at that time shall be deemed to
be the initial bona fide offering thereof.
SIGNATURES
In
accordance with the requirements of the Securities Act of 1933, the
registrant certifies that it has reasonable grounds to believe that
it meets all of the requirements for filing on Form S-1/A and
authorized this registration statement to be signed on its behalf
by the undersigned on May 4, 2017.
|
|
KRAIG BIOCRAFT LABORATORIES, INC.
|
|
|
|
|
|
|
|
May 4,
2017
|
By:
|
/s/ Kim
Thompson
|
|
|
|
|
Kim
Thompson
|
|
|
|
|
President,
Chief Executive Officer
|
|
|
|
|
Principal
Financial and Accounting Officer
and
Chairman of the Board of Directors
|
|
Pursuant
to the requirements of the Securities Act of 1933, this
registration statement has been signed by the following person(s)
in the capacities and on the dates indicated:
|
|
KRAIG BIOCRAFT LABORATORIES, INC.
|
|
|
|
|
|
|
|
May 4,
2017
|
By:
|
/s/ Kim
Thompson
|
|
|
|
|
Kim
Thompson
|
|
|
|
|
President,
Chief Executive Officer,
Principal
Financial and Accounting Officer
and
Chairman of the Board of Directors
|
|
|
|
|
|
|
Kraig Biocraft Laborator... (QB) (USOTC:KBLB)
Historical Stock Chart
From Mar 2024 to Apr 2024
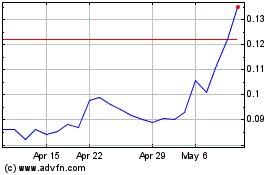
Kraig Biocraft Laborator... (QB) (USOTC:KBLB)
Historical Stock Chart
From Apr 2023 to Apr 2024