Advaxis Recognized as One of New Jersey’s “Best Places to Work 2017”
April 27 2017 - 8:00AM
Business Wire
Advaxis, Inc. (NASDAQ:ADXS), a biotechnology company developing
cancer immunotherapies, was named to NJBIZ’s 2017 Best Places to
Work in New Jersey list, as compiled and ranked by the Best
Companies Group (BCG). Advaxis, which has grown from about 10
employees to more than 100 in the last three years, was among the
honorees at an awards reception and ceremony on Wednesday, April
26.
Companies on this prestigious list stand out for prioritizing
their employees’ professional growth and quality of life, as judged
on a critical review of policies, practices, employee demographics
and an anonymous employee survey. Advaxis ranked 34th of the top
100 employers across the state.
BCG evaluates each nominated company’s policies, practices and
demographics. As part of the nomination and review process, BCG
confidentially surveys employees about how they view workplace
benefits and policies, professional growth and well-being and their
overall experience at the company. To qualify for consideration,
more than 70 percent of employees must complete the survey. The
initial evaluation and employee survey scores were then combined to
determine the top companies and their rankings.
“With so many prestigious companies, institutions and
organizations throughout the state, we are honored to be named
among the Best Places to Work in New Jersey,” said Sara M.
Bonstein, Executive Vice President and Chief Financial Officer of
Advaxis. “Advaxis has been able to grow thanks to our talented
workforce, and our continued success is a result of our employees’
commitment to providing much needed therapeutic treatments for
hard-to-treat cancers. Fostering a positive and engaging
environment that promotes recognition, growth opportunities and
great benefits has always been a priority for Advaxis, and we thank
our staff, BCG and NJBIZ for this award.”
The awards reception and ceremony hosted by NJBIZ was held at
iPlay America’s Event Center in Freehold, NJ. For more information
on the 2017 Best Places to Work in New Jersey program, please visit
www.BestPlacestoWorkinNJ.com. To learn more about Advaxis, visit
www.advaxis.com and connect on Twitter, LinkedIn, Facebook, and
YouTube.
About Advaxis, Inc.
Located in Princeton, N.J., Advaxis, Inc. is a biotechnology
company developing multiple cancer immunotherapies based on its
proprietary Lm Technology™. The Lm Technology, using
bioengineered live attenuated Listeria monocytogenes (Lm)
bacteria, is the only known cancer immunotherapy agent shown in
preclinical studies to both generate cancer fighting T cells
directed against cancer antigens and neutralize Tregs and
myeloid-derived suppressor cells (MDSCs) that protect the tumor
microenvironment from immunologic attack and contribute to tumor
growth. Advaxis' lead Lm Technology immunotherapy,
axalimogene filolisbac, targets HPV-associated cancers and is in
clinical trials for three potential indications: Phase 3 in
invasive cervical cancer, Phase 2 in head and neck cancer, and
Phase 2 in anal cancer. The FDA has granted axalimogene filolisbac
orphan drug designation for each of these three clinical settings,
as well as Fast Track designation for adjuvant therapy for HRLACC
patients and a SPA for the Phase 3 AIM2CERV trial in HRLACC
patients. Axalimogene filolisbac has also been classified as an
advanced therapy medicinal product for the treatment of cervical
cancer by the EMA’s CAT. Advaxis has two additional immunotherapy
products: ADXS-PSA in prostate cancer and ADXS-HER2 in HER2
expressing solid tumors, in human clinical development. In
addition, Advaxis and Amgen are developing ADXS-NEO, a preclinical
investigational cancer immunotherapy treatment designed to activate
a patient's immune system to respond against the unique mutations,
or neoepitopes, contained in and identified from each individual
patient's tumor, with plans to enter the clinic in 2017.
Advaxis Forward-Looking Statement
This press release contains forward-looking statements,
including, but not limited to, statements regarding Advaxis’
ability to develop the next generation of cancer immunotherapies,
and the safety and efficacy of Advaxis’ proprietary immunotherapy,
axalimogene filolisbac. These forward-looking statements are
subject to a number of risks including the risk factors set forth
from time to time in Advaxis’ SEC filings including, but not
limited to, its report on Form 10-K for the fiscal year ended
October 31, 2016, which is available at http://www.sec.gov.
Any forward-looking statements set forth in this presentation
speak only as of the date of this presentation. We do not intend to
update any of these forward-looking statements to reflect events or
circumstances that occur after the date hereof other than as
required by law.
You are cautioned not to place undue reliance on any
forward-looking statements.
View source
version on businesswire.com: http://www.businesswire.com/news/home/20170427005814/en/
Company:Advaxis, Inc.Noelle Heber, 609-250-7575Sr.
Director, Corporate Communications and Government
Affairsheber@advaxis.comorMedia:JPA Health
CommunicationsDavid Connolly, 617-657-1301dconnolly@jpa.com
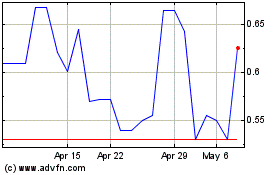
Ayala Pharmaceuticals (QX) (USOTC:ADXS)
Historical Stock Chart
From Mar 2024 to Apr 2024
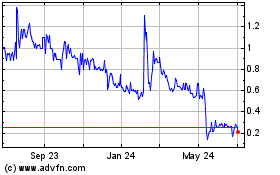
Ayala Pharmaceuticals (QX) (USOTC:ADXS)
Historical Stock Chart
From Apr 2023 to Apr 2024