As filed with the Securities and Exchange Commission on April 3, 2017
Registration No. 333 -215331
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
IMMUNOCELLULAR THERAPEUTICS, LTD.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
|
|
Delaware
|
|
2834
|
|
93-1301885
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(Primary Standard Industrial
Classification Code Number)
|
|
(I.R.S. Employer
Identification No.)
|
23622 Calabasas Road, Suite 300
Calabasas, California 91302
(818) 264-2300
(Address, including zip code and telephone number, including area code, of registrant’s principal place of business)
Anthony Gringeri, Ph.D.
ImmunoCellular Therapeutics, Ltd.
23622 Calabasas Road, Suite 300
Calabasas, California 91302
(818) 264-2300
(Name, address, including zip code and telephone number, including area code, of agent for service)
Copies to:
|
|
|
|
|
|
|
Glen Y. Sato
John T. McKenna
J. Carlton Fleming
Cooley LLP
3175 Hanover Street
Palo Alto, California 94304
(650) 843-5000
|
|
Barry I. Grossman
Sarah E. Williams
Ellenoff Grossman & Schole LLP
1345 Avenue of the Americas, 11
th
Floor
New York, New York 10105
(212) 370-1300
|
|
|
|
|
Approximate date of commencement of proposed sale to the public
: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, check the following box:
¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective Registration Statement for the same offering:
¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, please check the following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering:
¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering:
¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act:
|
|
|
|
|
|
|
|
|
|
|
Large accelerated filer
|
|
¨
|
|
Accelerated filer
|
|
¨
|
|
Non-accelerated filer
|
|
¨
(Do not check if a smaller reporting company)
|
|
Smaller reporting company
|
|
þ
|
__________________________________________________________________________________________
CALCULATION OF REGISTRATION FEE
|
|
|
|
|
|
|
|
|
|
|
|
|
Title of each class of
securities to be registered
|
Proposed
maximum
aggregate
offering price(1)
|
|
Amount of
registration fee(6)
|
|
Units, each consisting of (a) one share of Series B 6.0% Mandatorily Convertible Preferred Stock, par
value $0.0001, stated value $1,000 per share, and (b) one Series A Warrant to purchase shares of Common
Stock, par value $0.0001 per share
|
$10,000,000
|
|
$1,159
|
|
Series B 6.0% Mandatorily Convertible Preferred Stock, par value $0.0001, stated value $1,000 per share(2)(3)
|
—
|
|
—
|
|
Series A Warrants to Purchase Common Stock, par value $0.0001 per share(2)(3)
|
—
|
|
—
|
|
Common Stock, par value $0.0001 per share, issuable under the Series B 6.0% Mandatorily Convertible Preferred Stock(2)(3)
|
—
|
|
—
|
|
Common Stock, par value $0.0001 per share, issuable under the Series A Warrants(3)(4)(5)
|
$9,330,000
|
|
$1,082
|
|
Total:
|
$19,330,000
|
|
$2,241
|
|
|
|
(1)
|
Estimated solely for the purpose of calculating the amount of the registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended (Securities Act).
|
|
(2)
|
Pursuant to Rule 457(i) of and existing interpretations under the Securities Act, no separate registration fee is required for the Preferred Stock, Series A Warrants and Common Stock because the Preferred Stock, Series A Warrants and Common Stock are being registered at the same time as the Units.
|
|
(3)
|
Pursuant to Rule 416, the securities being registered hereunder include such indeterminate number of additional securities as may be issuable to prevent dilution resulting from stock splits, stock dividends or similar transactions.
|
|
(4)
|
No fee pursuant to Rule 457(g).
|
|
(5)
|
Estimated in accordance with Rule 457(c) under the Securities Act solely for the purpose of calculating the registration fee on the basis of $3.11, the average of the high and low prices of the Registrant’s Common Stock as reported on the NYSE MKT on March 30, 2017.
|
|
(6)
|
Calculated pursuant to Rule 457(o) based on an estimate of the total proposed maximum aggregate offering price. The Registrant previously paid $2,318 with the initial filing of this registration statement.
|
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant files a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and we are not soliciting an offer to purchase these securities in any jurisdiction where the offer or sale is not permitted.
Preliminary Prospectus
SUBJECT TO COMPLETION, DATED APRIL 3, 2017
10,000 Units
Each Consisting of
One Share of Series B 6.0% Mandatorily Convertible Preferred Stock and
Series A Warrants to Purchase 300 Shares of Common Stock
We are offering up to 10,000 units, or “Units,” each consisting of: (i) one share of our Series B 6.0% Mandatorily Convertible Preferred Stock, par value $0.0001 per share, with a stated value of $1,000.00 per share, or “Preferred Stock,” which is initially convertible into 4,329,004 shares of our common stock, par value $0.0001 per share, or “Common Stock;” assuming a conversion price of $2.31, which is equal to 87.5% of the lowest volume weighted average trading price of the Common Stock during the five trading days ending March 29, 2017, and (ii) one Series A Warrant to purchase 300 shares of our Common Stock. The Units will not be issued or certificated. The Preferred Stock and Series A Warrants are immediately separable and will be issued separately, but will be purchased together as a unit in this offering. Each Series A Warrant will have an initial exercise price per share equal to the last reported sale price of our Common Stock as of the closing of the trading day immediately preceding the pricing of this offering, will be exercisable immediately after issuance and will expire five years from the date of issuance. The exercise price of the Series A Warrants is subject to weighted-average anti-dilution adjustments if we issue or are deemed to issue additional shares of our common stock at a price per share less than the then effective exercise price, subject to certain exceptions. Such adjustment will not affect the number of shares of our common stock issuable upon the exercise of the Series A Warrants.
The Preferred Stock is convertible into shares of Common Stock by dividing the stated value of the Preferred Stock by the conversion price. The conversion price is equal to the lesser of: (i) $ per share of Common Stock, referred to as the “Set Price;” and (ii) 87.5% of the lowest volume weighted average trading price of the Common Stock during the five trading days ending on, and including the date of delivery of a notice of conversion, subject to adjustment as provided for in the Certificate of Designation.
Investing in our securities involves a high degree of risk. See the section titled "
Risk Factors
" beginning on page 8 of this prospectus. You should carefully consider these risk factors, as well as the information contained in this prospectus, before you invest.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
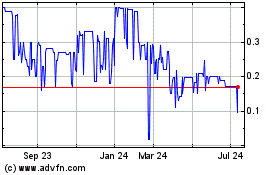
Our Common Stock is listed on the NYSE MKT under the symbol “IMUC.” The last reported sale price of our Common Stock on March 29, 2017 was $3.08 per share. There is no established public trading market for the Units, the Series B Preferred Stock or the Series A Warrants and we do not expect a market to develop. In addition, we do not intend to list the Units, the
Series B Preferred Stock or the Series A Warrants on the NYSE MKT, any other national securities exchange or any other nationally recognized trading system.
Maxim Group LLC, which we refer to as the “representative,” has agreed to act as the representative of the underwriters in connection with this offering. The underwriters may engage one or more selected dealers in this offering.
|
|
|
|
|
|
|
|
|
|
|
Per Unit
|
|
Total
|
|
Public offering price
|
$
|
|
|
|
|
Underwriter discount
|
$
|
|
|
|
|
Proceeds, before expenses, to us(1)
|
$
|
|
|
|
|
|
|
|
(1)
|
We estimate the total expenses of this offering payable by us, excluding the underwriting discount, will be approximately $ .
|
The above summary of offering proceeds to us does not give effect to any exercise of the warrants being issued in this offering. Delivery of the Preferred Stock and the Series A Warrants is expected to be made on or before , 2017 subject to customary closing conditions.
Sole Book-Running Manager
Maxim Group LLC
The date of this prospectus is , 2017.
TABLE OF CONTENTS
We and the underwriter have not authorized anyone to provide any information or to make any representations other than those contained in or incorporated by reference in this prospectus or in any free writing prospectuses prepared by or on behalf of us or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in or incorporated by reference in this prospectus is accurate only as of its date regardless of the time of delivery of this prospectus or of any sale of Units, Preferred Stock, Series A Warrants or Common Stock.
To the extent there is a conflict between the information contained in this prospectus, on the one hand, and the information contained in any document incorporated by reference filed with the Securities and Exchange Commission (SEC) before the date of this prospectus, on the other hand, you should rely on the information in this prospectus. If any statement in a document incorporated by reference is inconsistent with a statement in another document incorporated by reference having a later date, the statement in the document having the later date modifies or supersedes the earlier statement.
Neither we nor the underwriter has done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons who come into possession of this prospectus and any free writing prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus and any free writing prospectus applicable to that jurisdiction.
This prospectus and the documents incorporated by reference in this prospectus contain market data and industry statistics and forecasts that are based on independent industry publications and other publicly available information. Although we believe that these sources are reliable, we do not guarantee the accuracy or completeness of this information and we have not independently verified this information. Although we are not aware of any misstatements regarding the market and industry data presented or incorporated by reference in this prospectus, these estimates involve risks and uncertainties and are subject to change based on
various factors, including those discussed under the heading “Risk Factors” and any related free writing prospectus. Accordingly, investors should not place undue reliance on this information.
PROSPECTUS SUMMARY
This summary highlights certain information about us, this offering and selected information contained elsewhere in this prospectus and in the documents incorporated by reference. This summary is not complete and does not contain all of the information that you should consider before deciding whether to invest in our securities. For a more complete understanding of our company and this offering, we encourage you to read and consider carefully the more detailed information contained in or incorporated by reference in this prospectus, including the information contained under the heading “Risk Factors” beginning on page 8 of this prospectus, and the information included in any free writing prospectus that we have authorized for use in connection with this offering.
Throughout this prospectus, the terms “we,” “us,” “our,” and “our company” refer to ImmunoCellular Therapeutics, Ltd.
Company Overview
ImmunoCellular Therapeutics, Ltd. is a clinical-stage biotechnology company that is developing immune-based therapies for the treatment of cancers. Immunotherapy is an emerging approach to treating cancer in which a patient’s own immune system is stimulated to target tumor antigens, which are molecular signals that the immune system uses to identify foreign bodies. While some other cancer immunotherapies target only a single cancer antigen, our technology can elicit an immune response against several antigens. Our clinical stage cancer immunotherapy programs are also distinguished by the fact that they target cancer stem cells (CSCs), which are the primary drivers of tumor growth and disease recurrence. Our most advanced product candidate, ICT-107, recently began phase 3 testing in which we anticipate randomizing approximately 542 patients at approximately 120 clinical sites in the U.S., Canada and Europe. In addition, we have a portfolio of other potential therapeutic immunotherapies using our proprietary approach to treating cancer.
ICT-107, our lead product candidate, is a dendritic cell (DC) immunotherapy for the treatment of newly diagnosed glioblastoma multiforme (GBM), the most common and lethal type of brain cancer. ICT-107 is designed to activate a patient’s immune system to target six different tumor-associated antigens. ICT-107 has completed phase 2 testing with results reported in December 2013. Additional updated results were reported in June 2014 and November 2014. In November 2015, overall survival (OS) was additionally updated and reported. The phase 2 clinical trial was designed as a double-blind, placebo-controlled (2:1 randomized), multicenter evaluation of the safety and efficacy of ICT-107 in patients with newly diagnosed GBM. From January 2011 until September 2012, 124 patients were randomized to standard of care treatment plus ICT-107 or standard of care plus placebo (i.e. control). The most recent results are summarized in Table 1.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 1.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Overall Survival*
|
|
|
|
Median Overall Survival - in Months
|
|
|
|
|
|
Population
|
|
Patients Randomized
|
|
Treatment Group
|
|
Placebo Group
|
|
Difference
|
|
P Value
|
|
|
|
Intent to treat (ITT)
|
|
124
|
|
|
18.3
|
|
|
16.7
|
|
|
1.6
|
|
|
0.436
|
|
|
|
|
|
Per Protocol (PP) HLA-A2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MGMT Methylated
|
|
31
|
|
|
37.7
|
|
|
23.9
|
|
|
13.8
|
|
|
0.645
|
|
|
|
|
|
MGMT Unmethylated
|
|
38
|
|
|
15.8
|
|
|
11.8
|
|
|
4.0
|
|
|
0.326
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Progression Free Survival*
|
|
|
Median Progression Free Survival - in Months
|
|
|
|
|
|
Population
|
|
Patients Randomized
|
|
Treatment Group
|
|
Placebo Group
|
|
Difference
|
|
P Value
|
|
|
|
ITT
|
|
124
|
|
11.4
|
|
10.1
|
|
1.3
|
|
0.033
|
|
|
|
PP HLA-A2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MGMT Methylated
|
|
31
|
|
24.1
|
|
8.5
|
|
15.6
|
|
0.004
|
|
|
|
MGMT Unmethylated
|
|
38
|
|
10.5
|
|
6.0
|
|
4.0
|
|
0.364
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Overall survival data from October 2015; progression free survival from October 2014.
As reported in November 2015, ICT-107 treated patients had a numerical advantage in median OS of 1.6 months more than control patients in the intent-to-treat (ITT) population but the difference in survival between ICT-107 and control treated patients (the primary efficacy endpoint of the trial) did not reach statistical significance (p-value = 0.44). For Progression-Free Survival (PFS), an important secondary efficacy endpoint, the most updated results were reported in November 2014 when ICT-107 treated patients had a 1.3 month advantage in median PFS compared with control treated patients in the ITT population. This difference in PFS between ICT-107 and control treated patients reached statistical significance (p-value = 0.03). ICT-107 was generally well tolerated, with no imbalance in adverse events between the treated and control groups.
Patients in the phase 2 study were HLA-A1, A2, or dual A1/A2. HLA type refers to a person’s human leukocyte antigen status which corresponds to a family of genes that regulate the immune system. Though the ICT-107 immunotherapy is designed for all three of these HLA types, the most benefit and best immune responses were observed in patients who were HLA-A2 positive (about 50% of the GBM population in the US and Europe). Thus, the phase 3 includes only patients who are HLA-A2 positive. We analyzed HLA-A2 positive patients according to their MGMT gene status (unmethylated or methylated) which is a known predictor of responsiveness to standard of care chemotherapy. MGMT is a gene involved with DNA repair. As the standard of care chemotherapy in GBM works by damaging DNA, an active repair mechanism diminishes or precludes benefit from chemotherapy. MGMT unmethylated tumor cells can repair DNA damage while MGMT methylated cells cannot. While the subgroups we analyzed were small in size, and not powered to show statistical significance, the numeric advantages in favor of the ICT-107 treated patients were shown to be large and potentially clinically meaningful. Median OS for the HLA-A2 methylated MGMT per protocol (PP) population was 37.7 months for the ICT-107 patients and 23.9 months for the control group, representing a 13.8 month median OS numeric benefit for the ICT-107 treated group while not achieving statistical significance (p-value = 0.65). Median OS for the HLA-A2 unmethylated MGMT PP population was 15.8 months for ICT-107 patients and 11.8 months for the control group, representing a 4 month median OS numeric benefit for the ICT-107 treated group while not achieving statistical significance (p-value = 0.33).
We decided to pursue phase 3 testing of ICT-107 in HLA-A2 patients on the basis of the updated phase 2 ICT-107 trial data, post-phase 2 discussions with U.S. and European regulators and consultation with GBM key opinion leaders.
In addition to focusing only on HLA-A2 patients, we made several changes to the phase 3 protocol based on the phase 2 results and analysis.
|
|
|
|
•
|
An anergy test was added to patient screening. This test seeks to identify patients with a properly functioning immune system, which is an important consideration when testing an immune-based therapy.
|
|
|
|
|
•
|
More doses are included in the phase 3 protocol. Patients are dosed until they progress or run out of treatment or placebo. In the first year, after standard of care surgery and chemoradiation, patients receive four induction doses in the first month and then monthly maintenance doses thereafter. The phase 3 design now includes 15 doses in the first year if the patient does not progress compared to seven doses in the phase 2 design. The intent is to give patients the opportunity to mount an immune response to treatment.
|
|
|
|
|
•
|
An updated progression assessment is included. Progression will now be assessed using the iRANO criteria. This methodology is an update from the RANO criteria utilized in the phase 2 trial. Because dosing stops once a patient has progressed, accurate progression assessment is important for keeping patients on the trial as long as possible.
|
|
|
|
|
•
|
Monocytes will be used as the control in phase 3. In the phase 2 trial, activated dendritic cells were used as the control. These cells are potentially more immunogenic than the precursor monocyte cells.
|
The phase 3 design was submitted to the U.S. FDA and we received Special Protocol Assessment (SPA) agreement in August 2015. The Company submitted a protocol amendment to FDA in December 2016 and plans to submit to regulatory agencies in Canada and the European countries participating in the trial during the first quarter of 2017. This amendment is designed to improve the speed of randomization from the original protocol by resolving certain issues identified in the first several months of operating the trial. In February 2017, the FDA responded to our protocol amendment, indicating that in order to proceed with the protocol amendment, we would not be able to take advantage of the benefits of the previously granted SPA. The FDA did not indicate that the trail would be placed on clinical hold if we implement the amended protocol and we intend to work with the FDA in an effort to clarify the rationale for the protocol amendment and attempt to maintain the SPA. In any event, based on the response we plan to implement the amended protocol and proceed with the Phase 3 trial of ICT-107, with or without the SPA in effect, as the FDA further indicated that we could re-propose a SPA for the amended study.
Patient screening began in November 2015 in the U.S. We anticipate that it will take until mid-2019 to randomize approximately 542 patients and that the trial will complete by about mid-2021. The final analysis will be performed after at least 387 OS events have been observed and at least 50% of subjects with the methylated MGMT gene have died. As of December 31, 2016, we had 59 active trial sites in the U.S. and two in Canada. The first patient in the trial was treated on June 6, 2016 and 14 patients have been randomized to date. In addition, our initial clinical trial applications have been approved by regulatory authorities in the Netherlands, the U.K., Spain, Austria and Switzerland, and we are in discussions with regulatory authorities in Germany, Italy and France.
There are currently two interim analyses to be conducted by the Independent Data Monitoring Committee (DMC). The first is a futility assessment that will occur when 30% of the required OS events have been observed. We estimate that the triggering condition for this assessment will occur approximately mid-2019. The second is an efficacy assessment that will occur when 67% of the required OS events have been observed. We estimate that the triggering condition for this assessment will occur approximately in the first quarter of 2020. The trial is being conducted in the U.S., Canada, and Europe and we are working with the major cancer cooperative groups in each region to ensure sufficient and timely access to qualifying patients.
In addition to ICT-107, we are also developing two other therapeutic DC immunotherapies: ICT-140 for ovarian cancer and ICT-121 for recurrent GBM. ICT-140 targets seven tumor-associated antigens expressed on ovarian cancer cells. Some of the antigens utilized in ICT-140 were also used in ICT-107. We filed an investigational new drug (IND) application for ICT-140 at the end of 2012 and the IND was allowed by the FDA in January 2013. We subsequently modified the design of the trial and amended the IND to reflect these changes in May 2013 and September 2014. These amendments were allowed by the FDA shortly after the submissions. During the interim time period, we upgraded our generalized DC immunotherapy manufacturing process to bring it to a phase 3 and commercial ready state. We plan to use this improved process to manufacture clinical supplies for the ICT-140 trial. Currently, we are holding the initiation of this trial until we can find a partner to share expenses or until we have secured sufficient financial resources to complete the ICT-107 phase 3 program.
ICT-121 specifically targets CD133, a CSC marker that is overexpressed in a wide variety of solid tumors, including ovarian, pancreatic, and breast cancers. We began screening patients in September 2013 for a single-site phase 1 trial in recurrent GBM. Originally it was our intention to enroll 20 patients at one site. However, during 2014, we determined that enrollment could be accelerated if additional sites were added to the study. In 2015 we added five sites and made modifications in the screening criteria to facilitate enrollment. As of July 21, 2016, the trial was fully enrolled. The trial was closed in March 2017 and the final results should be available within three to four months.
In September 2014, we licensed from the California Institute of Technology (Caltech) the exclusive rights to novel technology for the development of Stem-to-T-cell immunotherapies for the treatment of cancer. The technology originated from the labs of David Baltimore, Ph.D., Nobel Laureate and President Emeritus at Caltech, and utilizes the patient’s own hematopoietic stem cells to create antigen-specific killer T cells to treat cancer. We plan to utilize this technology to expand and
complement our DC-based cancer immunotherapy platform, with the goal of developing new immunotherapies that kill cancer cells in a highly directed and specific manner and that can function as monotherapies or in combination therapy approaches.
Caltech’s technology potentially addresses the challenge, and limitation, that TCR (T cell receptor) technologies have faced of generating a limited immune response and having an unknown persistence in the patient’s body. We believe that by inserting DNA that encodes T cell receptors into hematopoietic stem cells rather than into T cells, the immune response can be transformed into a durable and more potent response that could effectively treat solid tumors. This observation has been verified in animal models by investigators at Caltech and the National Cancer Institute.
In March 2017, we announced the successful completion of the first milestone of our Stem-to-T-cell program; the sequencing of a selected TCR, that will become the basis for the product development program. In November 2015, we entered into a sponsored research agreement with The University of Texas MD Anderson Cancer Center with the goal of identifying a TCR sequence. In addition, in 2015 we acquired an option from Stanford University to evaluate certain technology related to the identification of TCRs that could prove useful in supporting our Stem-to-T-Cell research efforts. In March 2017, a TCR sequence for our Stem-to-T-Cell program became available.
In January of 2016, we entered into a sponsored research agreement with the University of Maryland, Baltimore (UMB). As part of this collaboration, UMB researchers are undertaking three projects to explore potential enhancements to our dendritic cell and Stem-to-T-Cell immunotherapy platforms.
Autologous cell-based therapies must be manufactured separately for each patient. Consequently, the manufacturing costs are typically higher than other types of therapies that are not patient-specific. Our DC immunotherapy manufacturing process produces multiple doses for a patient from a single manufacturing run utilizing a single apheresis from the patient. Each manufacturing run takes three days to complete. In addition, the immunotherapy is stored frozen in liquid nitrogen making the logistics of shipping and administration to the patient easier than that for cell therapies that must be shipped fresh and administered to the patient within hours of manufacture.
While we believe that we have a promising technology portfolio of multiple clinical-stage candidates, we do not currently anticipate that we will generate any revenues from either product sales or licensing in the foreseeable future. We have financed the majority of our prior operations through the sales of securities and believe that we may access grants and awards to supplement future sales of securities. On September 18, 2015, the Company received an award in the amount of $19.9 million from the California Institute of Regenerative Medicine (CIRM) to partially fund our phase 3 trial of ICT-107. The award provided for a $4.0 million project initial payment, which was received during the fourth quarter of 2015, and up to $15.9 million in future milestone payments that are primarily dependent on patient enrollment and randomization in the ICT-107 phase 3 trial. In June 2016, the terms of the award from CIRM were amended to (i) increase the project initial payment by $1.5 million, which we received on July 18, 2016, and (ii) reduce the potential future milestone payments by a corresponding $1.5 million. The potential total amount of the award from CIRM remains at $19.9 million. Under the terms of the CIRM award, we are obligated to share future ICT-107 related revenue with CIRM. The percentage of revenue sharing is dependent on the amount of the award we receive and whether the revenue is from product sales or license fees. The maximum revenue sharing amount we may be required to pay to CIRM is equal to nine times the total amount awarded and received. We have the option to decline any and all amounts awarded by CIRM. As an alternative to revenue sharing, we have the option to convert the award to a loan, which option must be exercised on or before ten (10) business days after the FDA notifies us that it has accepted our application for marketing authorization. In the event we exercise our right to convert the award to a loan, we will be obligated to repay the loan within ten (10) business days of making such election, including interest at the rate of the three-month LIBOR rate (0.92% as of December 31, 2016) plus 25% per annum.
The estimated cost of completing the development of any of the current or potential immunotherapy candidates will require us to raise additional capital, generate additional capital from the uncertain exercise of outstanding warrants, or enter into collaboration agreements with third parties. There can be no assurances that we will be able to obtain any additional funding, or if such funding is available, that the terms will be favorable. In addition, collaborations with third parties may not be
available to us and may require us to surrender rights to many of our products, which may reduce the potential share of returns in any licensed products. If we are unable to raise sufficient capital or secure collaborations with third parties, we will not be able to further develop our product candidates.
Recent Developments
We are undertaking an evaluation of strategic alternatives for our immuno-oncology research and development pipeline and technology platform, which may include a potential merger, consolidation, reorganization or other business combination, as well as the sale of the company or the company’s assets. We engaged Roth Capital Partners, LLC as our exclusive advisor in connection with the strategic evaluation. While we evaluate strategic alternatives, we plan to continue to advance our research and development strategies, including the execution of our phase 3 registration trial for ICT-107 in patients with newly diagnosed glioblastoma.
In March 2017, we announced the signing of a non-binding letter of intent with Memgen, LLC to exclusively negotiate the terms to possibly establish an immuno-oncology strategic collaboration focused on conducting clinical trials combining the companies’ respective cancer immunotherapy product candidates. The discussions pertain to our DC-based immunotherapy product candidates, ICT-107 and ICT-140, and Memgen’s ISF35, a viral cancer immunotherapy encoding an optimized version of CD40 ligand. Combining DC-based and viral oncology immunotherapeutic approaches could provide a novel way to stimulate CD40 to possibly induce a potent, specific and effective anti-tumor response. Insights from these combination trials, if successful, could also lead to later combination trials with other immune-oncology technologies, including checkpoint inhibitors.
Company Information
We filed our original Certificate of Incorporation with the Secretary of State of Delaware on March 20, 1987 under the name Redwing Capital Corp. On June 16, 1989, we changed our name to Patco Industries, Ltd. and conducted an unrelated business under that name until 1994. On January 30, 2006, we amended our Certificate of Incorporation to change our name to Optical Molecular Imaging, Inc. in connection with our merger on January 31, 2006 with Spectral Molecular Imaging, Inc. The acquisition was accounted for as a reverse merger, with Spectral Molecular Imaging deemed to be the accounting acquirer and Optical Molecular Imaging deemed to be the legal acquirer. As such, the consolidated financial statements herein reflect the historical activity of Spectral Molecular Imaging since its inception on February 25, 2004. On November 2, 2006, we amended our Certificate of Incorporation to change our name to ImmunoCellular Therapeutics, Ltd. to reflect the disposition of our Spectral Molecular Imaging subsidiary and the acquisition of our cellular-based technology from Cedars-Sinai.
Our principal executive offices are located at 23622 Calabasas Road, Suite 300, Calabasas, California 91302, and our telephone number at that address is (818) 264-2300.
|
|
|
|
|
|
SUMMARY OF THE OFFERING
|
|
Units Offered
|
10,000 Units, each consisting of (i) one share of our Series B Preferred Stock and (ii) one Series A Warrant to purchase 300 shares of our Common Stock.
|
|
Description of Preferred Stock
|
For additional information see “Description of the Securities We Are Offering — Preferred Stock.”
|
|
Preferred Stock Outstanding Immediately Before this Offering
|
None.
|
|
Preferred Stock Outstanding Immediately After this Offering
|
10,000 shares of Series B Preferred Stock.
|
|
Certificate of Designation for Preferred Stock
|
We intend to file a certificate of designation setting forth the preferences, rights and limitations, or “Certificate of Designation,” pertaining to the Preferred Stock with the Delaware Secretary of State. The Certificate of Designation will be controlling with regard to the preferences, rights and limitations of the Preferred Stock holders for all purposes.
|
|
Ranking of Preferred Stock
|
The Preferred Stock will rank senior to our Common Stock and other classes of capital stock with respect to dividend, redemption and distributions of assets upon liquidation, dissolution or winding up, unless the holders of a majority of the outstanding shares of Preferred Stock consent to the creation of parity stock or senior preferred stock.
|
|
Liquidation Preference of Preferred Stock
|
In the event of our liquidation, dissolution or winding up, holders of the Preferred Stock will receive a payment equal to the stated value of the Preferred Stock plus any accrued but unpaid dividends thereon and all liquidated damages and other amounts then due and owing, before any proceeds are distributed to the holders of our common stock.
|
|
|
|
|
Dividends on Preferred Stock
|
Holders of Preferred Stock are entitled to receive cumulative dividends at the rate of 6.0% per annum, payable quarterly on January 1, April 1, July 1 and October 1, beginning on the first such date after the original issue date and on each conversion date. We have the right to pay dividends in cash or in shares of common stock. If we do not have funds legally available to pay cash dividends, such dividends accrete to and increase the outstanding stated value of the Preferred Stock.
|
|
Conversion Price
|
The Preferred Stock is convertible into shares of Common Stock by dividing the stated value of the Preferred Stock by the conversion price. The conversion price is equal to the lesser of: (i) $ per share of Common Stock, referred to as the “Set Price;” and (ii) 87.5% of the lowest volume weighted average trading price of the Common Stock during the five trading days ending on, and including the date of delivery of a notice of conversion, subject to adjustment as provided for in the Certificate of Designation. The conversion price is subject to a floor of $1.00.
|
|
Mandatory Conversion
|
If after the effective date of the registration statement to which this prospectus forms a part, (i) the VWAP for each of any 10 consecutive Trading Day period, which 10 consecutive Trading Day period shall have commenced only after the effective date (“
Threshold Period
”), exceeds 300% of the then effective Conversion Price and (ii) the volume for each Trading Day during any Threshold Period exceeds $300,000 per Trading Day, within 1 Trading Day after the end of any such Threshold Period, the Preferred Stock is subject to mandatory conversion into shares of our Common Stock at a conversion price equal to the lesser of (i) the then-Set Price, or (ii) 87.5% of the lowest volume weighted average trading price of the Common Stock during the five trading days ending on, and including the date on which we provide notice of such mandatory conversion, upon written demand from us. The conversion price is subject to a floor of $1.00.
|
|
Series A Warrant Terms
|
Each Unit contains one Series A Warrant to purchase 300 shares of Common Stock at an initial exercise price equal to the last reported sale price of our Common Stock as of the close of the trading day immediately preceding the pricing of this offering, will be immediately exercisable and will expire on the fifth anniversary of the original issuance date. The exercise price of the Series A Warrants is subject to weighted-average anti-dilution adjustments if we issue or are deemed to issue additional shares of our common stock at a price per share less than the then effective exercise price, subject to certain exceptions. Such adjustment will not affect the number of shares of our common stock issuable upon the exercise of the Series A Warrants. The Series A Warrants will be issued in certificated form. See “Description of the Securities We Are Offering —Series A Warrants.”
|
|
|
|
|
|
|
|
|
|
Use of Proceeds
|
We currently intend to use the net proceeds of this offering, exclusive of deferred offering costs, to continue enrollment in our phase 3 clinical trial of ICT-107 (approximately $6.9 million), to continue our Stem-to-T-cell research program (approximately $0.7 million), to continue development of ICT-121 (approximately $0.1 million) and for working capital and general corporate purposes (approximately $1.1 million) as we work to evaluate strategic alternatives for our immuno-oncology research and development pipeline and technology platform, which may include a potential merger, consolidation, reorganization or other business combination, as well as the sale of the company or the company’s assets. We may use a portion of the net proceeds of this offering to acquire additional technologies.
|
|
|
|
|
Risk Factors
|
See “Risk Factors” beginning on page 8 of this prospectus, as well as other information included in this prospectus, for a discussion of factors you should read and consider carefully before investing in our securities.
|
|
|
|
|
Market Symbol and Trading
|
Our Common Stock is listed on the NYSE MKT under the symbol “IMUC.” We do not intend to list the Units, the Preferred Stock or the Series A Warrants on the NYSE MKT, any other national securities exchange or any other nationally recognized trading system.
|
|
Common Stock Outstanding Immediately After This Offering
|
10,773,863 shares, based on 7,329,004 shares of Common Stock are issuable upon the conversion of the Preferred Stock, assuming a conversion price of $2.31, which is equal to 87.5% of the lowest volume weighted average trading price of the Common Stock during the five trading days ending March 29, 2017, and the exercise of the Series A Warrants offered by this prospectus, based on their respective initial exercise prices.
|
The number of shares of our Common Stock to be outstanding after this offering as shown above is based on 3,444,859 shares outstanding as of December 31, 2016 and excludes as of that date:
|
|
|
|
•
|
162,665 shares of our Common Stock issuable upon exercise of outstanding options at a weighted average exercise price of $43.11 per share;
|
|
|
|
|
•
|
7,301 shares of our Common Stock issuable upon the settlement of outstanding restricted stock units;
|
|
|
|
|
•
|
1,708,557 shares of our Common Stock issuable upon exercise of outstanding warrants at a weighted average exercise price of $18.02 per share (without giving effect to any of the anti-dilution adjustment provisions thereof); and
|
|
|
|
|
•
|
199,197 shares of our Common Stock to be reserved for potential future issuance pursuant to our 2016 Equity Incentive Plan.
|
The number of shares of our Common Stock to be outstanding immediately after this offering as shown above does not include up to approximately $14.3 million of shares of our Common Stock that remained available for sale at December 31, 2016 under our Controlled Equity Offering
SM
Sales Agreement (Sales Agreement) with Cantor Fitzgerald & Co., as agent. Between December 31, 2016 and the date of this prospectus, no shares were sold under the Sales Agreement.
RISK FACTORS
Investing in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should carefully consider the risks described under the heading “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2016, as filed with the SEC on March 9, 2017, which description is incorporated in this prospectus by reference in its entirety and the risks described below, as well as in any prospectus supplement hereto, and other information in this prospectus before deciding to invest in or maintain your investment in our company. The risks described in these documents are not the only ones we face, but those that we consider to be material. There may be other unknown or unpredictable economic, business, competitive, regulatory or other factors that could have material adverse effects on our future results. Past financial performance may not be a reliable indicator of future performance, and historical trends should not be used to anticipate results or trends in future periods. If any of these risks actually occurs, our business, financial condition, results of operations or cash flow could be seriously harmed. This could cause the trading price of our Common Stock to decline, resulting in a loss of all or part of your investment. Please also read carefully the section below titled “Cautionary Statement Regarding Forward-Looking Statements.”
Risks Related To Our Business
The estimated cost of completing the development of any of our current immunotherapy product candidates and of obtaining all required regulatory approvals to market any of those product candidates is substantially greater than the amount of funds we currently have available. We will not have enough cash resources to fund the business for the next 12 months and successful completion of our research and development activities, and our transition to attaining profitable operations, is dependent upon obtaining financing.
As of December 31, 2016, we had working capital of $10,175,846, compared to working capital of $22,291,140 as of December 31, 2015. The estimated cost of completing the development of any of our current immunotherapy product candidates and of obtaining all required regulatory approvals to market any of those product candidates is substantially greater than the amount of funds we currently have available. We expect our costs will increase in 2017 primarily to fund the phase 3 trial of ICT-107, and that we will not have enough cash resources to fund the business for the next 12 months. Successful completion of our research and development activities, and our transition to attaining profitable operations, is dependent upon obtaining financing. Assuming we successfully close this offering, we expect that we will have sufficient cash to fund our operations for approximately 4 months assuming no revenue generation. Additional financing may not be available on acceptable terms or at all. If we issue additional equity securities to raise funds, the ownership percentage of existing stockholders would be reduced. New investors may demand rights, preferences or privileges senior to those of existing holders of common stock. If we cannot raise funds, we might be forced to make substantial reductions in the on-going clinical trials, thereby damaging our reputation in the biotech and medical communities which could adversely affect our ability to implement our business plan and our viability. These factors raise substantial doubt about our ability to continue as a going concern.
Risks Related to the Offering
Management will have broad discretion as to the use of the net proceeds from this offering, and we may not use these proceeds effectively.
We currently intend to use the net proceeds from this offering to continue enrollment in our phase 3 clinical trial of ICT-107, to complete phase 1 development of ICT-121, to continue our Stem-to-T-cell research program and for working capital and general corporate purposes as we work to evaluate strategic alternatives for our immuno-oncology research and development pipeline and technology platform, which may include a potential merger, consolidation, reorganization or other business combination, as well as the sale of the company or the company’s assets. We may also use a portion of the net proceeds of this offering to acquire additional technologies. Our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our Common Stock. Accordingly, you will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. Our failure to apply these funds effectively could have a material adverse effect on our business, delay the development of our product candidates and cause the price of our Common Stock to decline.
Investors in this offering will experience immediate and substantial dilution.
Since the public offering price per share of the Units offered pursuant to this prospectus is expected to be substantially higher than the net tangible book value per share of our Common Stock, you will suffer immediate and substantial dilution in the net tangible book value of the Common Stock underlying the Preferred Stock and Series A Warrants from the price per Unit that you pay in this offering. Our net tangible book value as of December 31, 2016 was approximately $6.0 million, or $1.75
per share. After giving effect to the assumed sale by us of 10,000 Units in this offering at a public offering price of $1,000 per Unit (and an assumed Conversion Price of $2.31 per share, which is equal to 87.5% of the lowest volume weighted average trading price of the Common Stock during the five trading days ending March 29, 2017), and after deducting underwriting discounts, commissions and other estimated offering expenses payable by us, and allocating approximately $6.5 million of the proceeds to the Series A Warrants and deducting approximately $1.6 million from our pro-forma net equity to account for the increase in the liability associated with the revaluation of our existing warrant derivatives, you will suffer immediate dilution of $1.44 per share in the net tangible book value of the Common Stock you acquire. In the event that you exercise Series A Warrants you will experience additional dilution to the extent that the exercise price of the Series A Warrants is higher than the tangible book value per share of our common stock. See the section titled “Dilution” below for a more detailed discussion of the dilution you would incur if you purchase Units in this offering.
In addition, we have a significant number of stock options and warrants outstanding. To the extent that outstanding stock options or warrants, including the Series A Warrants offered in this prospectus, have been or may be exercised or other shares issued, you may experience further dilution.
The offering price determined for this offering is not an indication of our value.
The per-Unit offering price and the initial conversion price of the Preferred Stock and the initial exercise price of the Series B Warrants may not necessarily bear any relationship to the book value of our assets, past operations, cash flows, losses, financial condition or any other established criteria for value. You should not consider the offering price for the Units as an indication of the value of the Common Stock underlying the Preferred Stock and the Series H Warrants. After the date of this prospectus, the Common Stock may trade at prices above or below the price per share of Common Stock imputed by the offering price.
Future sales of substantial amounts of our Common Stock could adversely affect the market price of our Common Stock.
We may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. If additional capital is raised through the sale of equity or convertible debt securities, or perceptions that those sales could occur, the issuance of these securities could result in further dilution to investors purchasing our Common Stock in this offering or result in downward pressure on the price of our Common Stock, and our ability to raise capital in the future.
The exercise of outstanding options and warrants to acquire shares of our Common Stock would cause additional dilution, which could cause the price of our Common Stock to decline.
In the past, we have issued options and warrants to acquire shares of our Common Stock. At December 31, 2016, there were 162,665 shares of Common Stock issuable upon exercise of outstanding options at a weighted average exercise price of $43.11 per share and 1,708,557 shares of Common Stock issuable upon exercise of outstanding warrants at a weighted average exercise price of $18.02 per share, and we may issue additional options, warrants and other types of equity in the future as part of stock-based compensation, capital raising transactions, technology licenses, financings, strategic licenses or other strategic transactions. In addition, after giving effect to the assumed sale by us of 10,000 Units in this offering with an assumed Conversion Price of $2.31 and Series A Warrant exercise price of $3.08 per share, the last reported sale price of our common stock on March 29, 2017, the weighted average exercise price would be adjusted to $12.71 per share as a result of exercise price protection provisions in certain warrants. To the extent these options and warrants are ultimately exercised, existing holders of our Common Stock would experience additional dilution which may cause the price of our Common Stock to decline.
Securities analysts may not continue to cover our Common Stock, and this may have a negative impact on our Common Stock’s market price.
The trading market for our Common Stock depends, in part, on the research and reports that securities analysts publish about us and our business. We do not have any control over these analysts. If our stock is downgraded by a securities analyst, our stock price would likely decline. If the analyst ceases to cover us or fails to publish regular reports on us, we could lose visibility in the financial markets, which could cause our stock price or trading volume to decline.
Anti-takeover provisions in our charter documents and under Delaware law may make an acquisition of us, which may be beneficial to our stockholders, more difficult.
Provisions of our amended and restated certificate of incorporation and amended and restated bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us, even if doing so would benefit our stockholders. These provisions:
|
|
|
|
•
|
establish that members of our board of directors may be removed upon an affirmative vote of stockholders owning a majority of our capital stock
|
|
|
|
|
•
|
authorize the issuance of “blank check” preferred stock that could be issued by our board of directors to increase the number of outstanding shares and thwart a takeover attempt;
|
|
|
|
|
•
|
limit who may call a special meeting of stockholders;
|
|
|
|
|
•
|
establish advance notice requirements for nominations for election to our board of directors; and
|
|
|
|
|
•
|
provide that the authorized number of directors may be changed by a resolution of our board of directors, provided that the number is within the limits established by the bylaws.
|
In addition, Section 203 of the Delaware General Corporation Law, which imposes certain restrictions relating to transactions with major stockholders, may discourage, delay or prevent a third party from acquiring us.
Risks Related to the Units, the Preferred Stock and the Series A Warrants.
There is no public market for the Units, the Preferred Stock and the Series A Warrants.
There is no established public trading market for the Units, the Preferred Stock and the Series A Warrants offered by this prospectus and we do not expect a market to develop. In addition, we do not intend to apply to list the Units, the Preferred Stock or the Series A Warrants on any national securities exchange or other nationally recognized trading system, including the NYSE MKT. Without an active market, the liquidity of the Units, the Preferred Stock and the Series A Warrants will be limited.
As a holder of the Preferred Stock or Series A Warrants you have no voting rights.
You will have no voting rights as a holder of the Preferred Stock or Series A Warrants. Our Common Stock is currently the only class of our securities that carries full voting rights.
Until you acquire shares of our common stock upon the conversion of your preferred stock or exercise of your warrants, you will have no rights with respect to shares of our common stock issuable upon conversion of your preferred stock or exercise of your warrants. Upon conversion of your preferred stock or exercise of your warrants, you will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
We may lose our current NYSE MKT listing of our common stock and may not be eligible to list our common stock on other exchanges. If we are unable to maintain compliance with NYSE MKT continued listing standards and policies, the NYSE MKT may commence proceedings to delist our common stock, and in some cases, determine to suspend trading in our common stock immediately without an opportunity to propose a plan that could enable us to regain compliance, which would likely cause the liquidity and market price of our common stock to decline and you could lose your investment.
Our common stock currently trades on the NYSE MKT under the symbol IMUC. If we fail to adhere to the NYSE MKT’s strict listing criteria, our stock may be delisted. For example, the NYSE MKT will consider suspending dealings in, or delisting, securities of an issuer that has stockholders’ equity of less than $6 million if that issuer has sustained losses from continuing operations and/or net losses in its five most recent fiscal years. We have had a loss from operations and net loss in each of our five most recent fiscal years and we expect to incur a loss from operations and net loss for 2017. At December 31, 2016, our stockholders’ equity was $6.1 million, and in March 2017, we received an early warning letter from the NYSE MKT indicating that if our stockholders’ equity falls below $6 million, the NYSE MKT may take formal action and determine that we are no longer suitable for listing and may commence delisting proceedings pursuant Section 1003(a)(iii) of the NYSE MKT Company Guide. This could potentially impair the liquidity of our securities not only in the number of shares that could be bought and sold at a given price, which might be depressed by the relative illiquidity, but also through delays in the timing of transactions and the potential reduction in media coverage. As a result, an investor might find it more difficult to dispose of our common stock. We believe that current and prospective investors would view an investment in our common stock more favorably if it continues to be listed on the NYSE MKT.
If our Common Stock is delisted, your ability to transfer or sell the Preferred Stock or Series A Warrants may be limited and the market value of the Preferred Stock or Series A Warrants will likely be materially adversely affected.
We expect that the value of the Preferred Stock and Series A Warrants to some extent is tied to the perceived value of their conversion and exercise features. If our Common Stock is delisted from the NYSE MKT, your ability to transfer or sell the Preferred Stock and Series A Warrants may be limited and the market value of the Preferred Stock and Series A Warrants will likely be materially adversely affected.
We expect that the market value of the Preferred Stock and Series A Warrants will be significantly affected by changes in the market price of our Common Stock, which could change substantially at any time.
We expect that the market value of the Preferred Stock and Series A Warrants will depend on a variety of factors, including, without limitation, the market price of our Common Stock. Each of these factors may be volatile, and may or may
not be within our control. For example, we expect the market value of the Preferred Stock and Series A Warrants will increase with increases in the market price of our Common Stock. The market price of the Common Stock has been volatile in the past and could fluctuate widely in response to various factors.
We may issue additional shares of our Common Stock or instruments convertible or exercisable into our Common Stock, including in connection with exercise of the Preferred Stock and Series A Warrants, and thereby materially and adversely affect the market price of our Common Stock, and, in turn, the market value of the Preferred Stock and Series A Warrants.
Subject to certain contractual limitations, under the Certificate of Designation and Underwriting Agreement, we are under, we may offer and sell additional shares of our Common Stock or other securities convertible into or exercisable for our Common Stock during the life of the Preferred Stock and Series A Warrants. We cannot predict the size of future issuances or the effect, if any, that they may have on the market price for our Common Stock. If we issue additional shares of our Common Stock or instruments convertible or exercisable into our Common Stock, it may materially and adversely affect the price of our Common Stock and, in turn, the market value of the Preferred Stock and Series A Warrants. Furthermore, the conversion or exercise of some or all of our outstanding derivative securities, including the Preferred Stock and Series A Warrants, will dilute the ownership interests of existing stockholders, and any sales in the public market of shares of our Common Stock issuable upon any such conversion or exercise could adversely affect prevailing market prices of our Common Stock or the market value of the Preferred Stock and Series A Warrants.
Holders of the Preferred Stock and Series A Warrants will be entitled to only limited rights with respect to our Common Stock, and will be subject to all changes made with respect to our Common Stock to the extent holders receive shares of Common Stock pursuant to the terms of the Preferred Stock and Series A Warrants.
Holders of the Preferred Stock and Series A Warrants will be entitled to only limited rights with respect to our Common Stock until the time at which they become holders of our Common Stock pursuant to the terms of the Preferred Stock and Series A Warrants, but will be subject to all changes affecting our Common Stock before that time. For example, if an amendment is proposed to our articles of incorporation requiring stockholder approval and the record date for determining the stockholders of record entitled to vote on the amendment occurs before the date you are deemed to be a record holder of our Common Stock, you generally will not be entitled to vote on the amendment, although you will nevertheless be subject to any changes affecting our Common Stock.
Provisions of the warrants offered by this prospectus could discourage an acquisition of us by a third party.
In addition to the discussion of the provisions of our Amended and Restated Certificate of Incorporation, as amended, certain provisions of the Series A Warrants offered by this prospectus could make it more difficult or expensive for a third party to acquire us. The Series A Warrants prohibit us from engaging in certain transactions constituting “fundamental transactions” unless, among other things, the surviving entity assumes our obligations under the warrants. Further, the Series A Warrants provide that, in the event of certain transactions constituting “fundamental transactions,” with some exception, holders of such warrants will have the right, at their option, to require us to repurchase such warrants at a price described in such warrants. These and other provisions of the Series A Warrants offered by this prospectus could prevent or deter a third party from acquiring us even where the acquisition could be beneficial to you.
A large number of shares issued in this offering may be sold in the market following this offering, which may depress the market price of our common stock.
A large number of shares that are issuable upon conversion of the Series B Preferred Stock or exercise of the Series A Warrants may be sold in the market following this offering, which may depress the market price of our common stock. Sales of a substantial number of shares of our common stock in the public market following this offering could cause the market price of our common stock to decline. If there are more shares of our common stock offered for sale than buyers are willing to purchase, then the market price of our common stock may decline to a market price at which buyers are willing to purchase the offered shares of our common stock and sellers remain willing to sell the shares. All of the securities issued in the offering will be freely tradable without restriction or further registration under the Securities Act of 1933, as amended (Securities Act).
The Series A Warrants may not have any value.
Each Series A Warrant sold in this offering will have an exercise price of $ per share, which is equal to the last reported sale price of our common stock as of the close of the trading day immediately preceding the pricing of this offering, and will expire on the fifth anniversary of the date they first become exercisable. In the event our common stock price does not exceed the exercise price of the base warrants during the period when the base warrants are exercisable, the base warrants may not have any value.
The exercise price of the Series A Warrants will not be adjusted for certain dilutive events.
The exercise price of the Series A Warrants is subject to adjustment for certain events, including, but not limited to, certain issuances of capital stock, options, convertible securities and other securities. However, the exercise prices will not be adjusted
for dilutive issuances of securities considered “excluded securities” and there may be transactions or occurrences that may adversely affect the market price of our Common Stock or the market value of such warrants without resulting in an adjustment of the exercise prices of such warrants.
To the extent we issue shares of our Common Stock to satisfy all or a portion of our conversion or exercise obligations, conversion of the Preferred Stock and exercise of the Series A Warrants will dilute the ownership interest of our existing stockholders, including holders who had previously converted or exercised their Preferred Stock or Series A Warrants.
Issuance of shares of Common Stock upon conversion of the Preferred Stock and exercise of the Series A Warrants will dilute the ownership interest of our existing stockholders. Further, any sales in the public market of our Common Stock issuable upon such exercise could adversely affect prevailing market prices of our Common Stock. In addition, the existence of the Preferred Stock and Series A Warrants may encourage short selling by market participants because the conversion of the Preferred Stock and exercise of the Series A Warrants could depress the price of our Common Stock.
You may receive less valuable consideration than expected because the value of our Common Stock may decline after you convert the Preferred Stock or exercise the Series A Warrants issued in this offering, but before we settle our obligation thereunder.
A converting or exercising holder will be exposed to fluctuations in the value of our Common Stock during the period from the date such holder converts the Preferred Stock or surrenders Series A Warrants for exercise until the date we settle our exercise obligation. Upon conversion of the Preferred Stock and exercise of the Series A Warrants, we will be required to deliver the shares of our Common Stock, on the third business day following the relevant conversion or exercise date. Accordingly, if the price of our Common Stock decreases during this period, the value of the shares that you receive will be adversely affected and would be less than the value on the exercise date.
If we explore or engage in future business combinations or other transactions, we may be subject to various uncertainties and risks.
From time-to-time, unrelated third-parties approach the Company about potential transactions, including business combinations. Recently, we have had preliminary discussions with one such third party to explore, among other things, a potential business combination. We have not, however, completed a review of matters that would be required prior to entering into any such transaction such as due diligence, negotiating and agreeing upon terms and obtaining approval from our board of directions. As such, to date, we have not entered into any agreements related to any business combination. While we may explore such opportunities when they arise, we could not pursue any proposed business combination or other transaction unless our board of directors first has determined that doing so would be in our and our stockholders’ interest. There can be no assurance that we will negotiate acceptable terms, enter into binding agreements or successfully consummate any business combination or other transaction with this private company or any other third parties.
We cannot currently predict the effects a future, potential business combination or other transaction would have on holders of the Preferred Stock, Series A Warrants or any of our other securities. There are various uncertainties and risks relating to our evaluation and negotiation of possible business combination or other transactions, our ability to consummate such transactions and the consummation of such transactions, including:
|
|
|
|
•
|
evaluation and negotiation of a proposed transaction may distract management from focusing our time and resources on execution of our operating plan, which could have a material adverse effect on our operating results and business;
|
|
|
|
|
•
|
the process of evaluating proposed transactions may be time consuming and expensive and may result in the loss of business opportunities;
|
|
|
|
|
•
|
perceived uncertainties as to our future direction may result in increased difficulties in retaining key employees and recruiting new employees, particularly senior management;
|
|
|
|
|
•
|
even if our board of directors negotiates a definitive agreement, successful integration or execution of a business combination or other transaction will be subject to additional risks;
|
|
|
|
|
•
|
the current market price of our Common Stock may reflect a market assumption that a transaction will occur, and during the period in which we are considering a transaction, the market price of our Common Stock could be highly volatile;
|
|
|
|
|
•
|
a failure to complete a transaction could result in a negative perception by our investors generally and could cause a decline in the market price of our Common Stock, as well as lead to greater volatility in the market price of our
|
Common Stock, all of which could adversely affect our ability to access the equity and financial markets, as well as our ability to explore and enter into different strategic alternatives;
|
|
|
|
•
|
expected benefits may not be successfully achieved;
|
|
|
|
|
•
|
such transactions may increase our operating expenses and cash requirements, cause us to assume or incur indebtedness or contingent liabilities, make it difficult to retain management and key personnel; and
|
|
|
|
|
•
|
dilution of our existing stockholders if such transaction involves our issuing dilutive securities.
|
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements, which reflect the views of our management with respect to future events and financial performance. These forward-looking statements are subject to a number of uncertainties and other factors that could cause actual results to differ materially from such statements. Forward-looking statements are identified by words such as “anticipates,” “believes,” “estimates,” “expects,” “intends,” “plans,” “projects,” “targets” and similar expressions. Readers are cautioned not to place undue reliance on these forward-looking statements, which are based on the information available to management at this time and which speak only as of this date. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. For a discussion of some of the factors that may cause actual results to differ materially from those suggested by the forward-looking statements, please read carefully the information under “Risk Factors.” Examples of our forward-looking statements include:
|
|
|
|
•
|
our current views with respect to our business strategy, business plan and research and development activities;
|
|
|
|
|
•
|
the progress of our product development programs, including clinical testing and the timing of commencement and results thereof;
|
|
|
|
|
•
|
our projected research and development expenses;
|
|
|
|
|
•
|
our ability to fund, enroll and successfully complete the phase 3 study of ICT-107 and any of our other product candidates;
|
|
|
|
|
•
|
the potential for and timing of development and commercial success of ICT-107;
|
|
|
|
|
•
|
our ability to continue development plans for ICT-140 and ICT-121;
|
|
|
|
|
•
|
our ability to pursue strategic alternatives, including for our immuno-oncology research and development pipeline and technology platform;
|
|
|
|
|
•
|
our ability to enter into a strategic collaboration with Memgen;
|
|
|
|
|
•
|
our anticipated use of proceeds from this offering; and
|
|
|
|
|
•
|
our ability to further develop our technologies into product candidates.
|
The identification in this document of factors that may affect future performance and the accuracy of forward-looking statements is meant to be illustrative and by no means exhaustive. All forward-looking statements should be evaluated with the understanding of their inherent uncertainty. You may rely only on the information contained in this prospectus.
We have not authorized anyone to provide information different from that contained in this prospectus. Neither the delivery of this prospectus nor the sale of securities means that information contained in this prospectus is correct after the date of this prospectus. This prospectus is not an offer to sell or solicitation of an offer to purchase these securities in any circumstances under which the offer or solicitation is unlawful.
USE OF PROCEEDS
Assuming that all 10,000 Units are sold in this offering, we estimate that our net proceeds from this offering will be approximately $8.8 million, excluding the proceeds, if any, from the exercise of the Series A Warrants, based on the assumed public offering price of $1,000 per Unit and after deducting underwriting discounts, commissions and other estimated offering expenses payable by us.
We currently intend to use the net proceeds of this offering to continue enrollment in our phase 3 clinical trial of ICT-107 (approximately $6.9 million), to continue our Stem-to-T-cell research program (approximately $0.7 million), to continue development of ICT-121 (approximately $0.1 million) and for working capital and general corporate purposes (approximately $1.1 million) as we work to evaluate strategic alternatives for our immuno-oncology research and development pipeline and technology platform, which may include a potential merger, consolidation, reorganization or other business combination, as well as the sale of the company or the company’s assets. We may also use a portion of the net proceeds to acquire or invest in businesses, products and technologies that are complementary to our own, although we are not currently planning or negotiating any such transactions. Pending these uses, we intend to invest our net proceeds from this offering primarily in investment grade, interest-bearing instruments. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds we will have upon completion of the offering. Accordingly, we will retain broad discretion over the use of these proceeds.
.
MARKET PRICE OF OUR COMMON STOCK
Market Information
Our Common Stock has been traded on the NYSE MKT since May 30, 2012 under the symbol “IMUC.” Our Common Stock previously traded on the OTC Bulletin Board over-the-counter market. The price information in the table below for periods prior to the listing of our Common Stock on the NYSE MKT reflects inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions:
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarter Ended
|
|
High
|
|
Low
|
|
December 31, 2014
|
|
$
|
41.20
|
|
|
$
|
21.38
|
|
|
March 31, 2015
|
|
$
|
32.81
|
|
|
$
|
19.20
|
|
|
June 30, 2015
|
|
$
|
21.60
|
|
|
$
|
17.06
|
|
|
September 30, 2015
|
|
$
|
25.40
|
|
|
$
|
14.00
|
|
|
December 31, 2015
|
|
$
|
21.20
|
|
|
$
|
13.61
|
|
|
March 31, 2016
|
|
$
|
15.03
|
|
|
$
|
8.07
|
|
|
June 30, 2016
|
|
$
|
13.60
|
|
|
$
|
8.07
|
|
|
September 30, 2016
|
|
$
|
10.60
|
|
|
$
|
4.44
|
|
|
December 31, 2016
|
|
$
|
4.80
|
|
|
$
|
1.83
|
|
|
March 31, 2017 (through March 29, 2017)
|
|
$
|
4.27
|
|
|
$
|
1.85
|
|
The reported last sale price of our Common Stock on the NYSE MKT on March 29, 2017 was $3.08 per share.
Stockholders
As of March 29, 2017, there were 32 holders of record of our Common Stock, not including any persons who hold their stock in “street name.”
DIVIDEND POLICY
We have not paid any dividends on our Common Stock to date and do not anticipate that we will pay dividends on our Common Stock in the foreseeable future. Any payment of cash dividends on our Common Stock in the future will be dependent upon the amount of funds legally available, our earnings, if any, our financial condition, our anticipated capital requirements and other factors that the board of directors may think are relevant.
DILUTION
If you purchase securities in this offering, you will experience dilution to the extent of the difference between the price per Unit you pay in this offering and the net tangible book value per share of our Common Stock underlying the Preferred Stock and Series A Warrants, assuming no value is attributed to the Series A Warrants, and such Series A Warrants are accounted for and classified as equity. The net tangible book value of our Common Stock on December 31, 2016 was $6.0 million, or $1.75 per share (based upon 3,444,859 shares of our Common Stock outstanding). Net tangible book value per share is equal to the amount of our total tangible assets, less total liabilities, divided by the aggregate number of shares of our Common Stock outstanding.
After giving effect to the assumed sale in this offering by us of 10,000 Units at a public offering price of $1,000 per Unit (assuming a Conversion Price of $2.31 per share, which is equal to 87.5% of the lowest volume weighted average trading price of the Common Stock during the five trading days ending March 29, 2017) and after deducting underwriting discounts, commissions and other estimated offering expenses payable by us, and allocating approximately $6.5 million of the proceeds to the Series A Warrants and deducting approximately $1.6 million to account for the estimated increase in the liability associated with the revaluation of our existing warrant derivatives, our as adjusted net tangible book value as of December 31, 2016 would have been $6.8 million, or $0.87 per share of Common Stock. This represents an immediate decrease in tangible net book value of $0.88 per share to existing stockholders and an immediate dilution of $1.44 per share to new investors purchasing Units in this offering, attributing none of the public offering price to the Series A Warrants offered hereby. The following table illustrates this per share dilution:
|
|
|
|
|
|
|
|
|
|
|
|
Assumed public offering price per share (assumed conversion of Preferred Stock as described above)
|
|
|
$
|
2.31
|
|
|
Net tangible book value per share as of December 31, 2016
|
$
|
1.75
|
|
|
|
|
Decrease per share attributable to new investors in this offering
|
(0.88
|
)
|
|
|
|
As adjusted net tangible book value per share as of December 31, 2016 after giving effect to this
offering
|
|
|
0.87
|
|
|
Dilution per share to investors participating in this offering
|
|
|
$
|
1.44
|
|
The discussion and table above assume (i) the sale by us of 10,000 Units, (ii) no exercise of the Series A Warrants, and (iii) no receipt of cash upon the exercise of the Series A Warrants. Upon the exercise of the Series A Warrants, if any, holders of the Series A Warrants will experience additional dilution. The calculations above exclude shares of Common Stock issuable upon the outstanding derivative securities described in this Prospectus. The discussion and table above do not take into account further dilution to new investors that could occur upon the exercise of outstanding options and warrants having a per share exercise price less than the public offering price per share in this offering.
The number of shares of our outstanding Common Stock reflected in the discussion and table above is based on 3,444,859 shares of Common Stock outstanding as of December 31, 2016 and excludes, as of that date:
|
|
|
|
•
|
162,665 shares of our Common Stock issuable upon exercise of outstanding options at a weighted average exercise price of $43.11 per share;
|
|
|
|
|
•
|
7,301 shares of our Common Stock issuable upon the settlement of outstanding restricted stock units;
|
|
|
|
|
•
|
1,708,557 shares of our Common Stock issuable upon exercise of outstanding warrants at a weighted average exercise price of $18.02 per share (without giving effect to any of the anti-dilution adjustment provisions thereof); and
|
|
|
|
|
•
|
199,197 shares of our Common Stock to be reserved for potential future issuance pursuant to our 2016 Equity Incentive Plan.
|
If we raise additional capital in the future, we may in the future sell substantial additional amounts of Common Stock or securities convertible into or exercisable for Common Stock. We may also choose to raise additional capital due to market conditions or other strategic considerations even if we believe we have sufficient funds for our current or future operating plans. The issuance of these securities could result in further dilution to our shareholders.
The number of shares of our Common Stock to be outstanding immediately after this offering as shown above does not include up to approximately $14.3 million of shares of our Common Stock that remained available for sale at December 31, 2016 under our Sales Agreement with Cantor Fitzgerald & Co., as agent. Between December 31, 2016 and the date of this prospectus, no shares were sold under the Sales Agreement.
MANAGEMENT
The following table sets forth information about our Board of Directors and Executive Officers as of March 22, 2017.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Name
|
|
Age
|
|
Position(s)
|
|
Anthony J. Gringeri, Ph.D.
|
|
63
|
|
President, Chief Executive Officer and Director
|
|
Steven J. Swanson, Ph.D.
|
|
60
|
|
Senior Vice President, Research
|
|
David Fractor
|
|
56
|
|
Vice President, Finance and Principal Accounting Officer
|
|
Gary S. Titus
(1)(3)(4)
|
|
56
|
|
Chairman of the Board and Secretary
|
|
Andrew Gengos
|
|
51
|
|
Director
|
|
Rahul Singhvi, Sc.D.
(2)(3)(4)(5)
|
|
51
|
|
Director
|
|
John S. Yu, M.D.
(4)
|
|
52
|
|
Director
|
|
Gregg A. Lapointe
(1)(5)
|
|
57
|
|
Director
|
|
Mark A. Schlossberg
(2)(3)(5)
|
|
55
|
|
Director
|
|
|
|
|
|
|
|
|
(1
|
)
|
Member of our Compensation Committee
|
|
(2
|
)
|
|
Member of our Nominating and Corporate Governance Committee
|
|
(3
|
)
|
|
Member of our Audit Committee
|
|
(4
|
)
|
|
Member of our Finance Committee
|
|
(5
|
)
|
|
Denotes independent Director
|
Anthony J. Gringeri, Ph.D.
Dr. Gringeri was appointed as Chief Executive Officer and a member of our board in December 2016. Previously he served as our Senior Vice President – Strategic Resources since August 2013. From 2011 to 2012, Dr. Gringeri served as Vice President and Chief Development Officer of ViaCyte, where he was responsible for all preclinical, clinical and regulatory activities. From 2006 to 2009, Dr. Gringeri served as Chief Operating Officer for Amsterdam Molecular Therapeutics, responsible for corporate strategy and operations, business development, building the company’s commercial capability, and playing a key role in its initial public offering. Prior to that, he worked with Amgen for 15 years in a series of executive leadership roles. He served as Vice President, Product Development, Executive Director, Scientific Operations, Vice President, Scientific Outreach and Licensing Operations, and Vice President, Project Management and Strategic Planning. Dr. Gringeri was the Product Development Team Leader responsible for the successful development and commercialization of ARANESP
®
(darbepoetin alfa), to treat anemia in patients with kidney failure as well as cancer patients. Dr. Gringeri holds a Ph.D. in pharmacology from the University of Rochester, and has authored multiple scientific publications. Dr. Gringeri was selected serve as a member of the Board of Directors in part due to his extensive leadership skills and operational expertise, including his operational experience and deep understanding of our business as our Chief Executive Officer.
David Fractor
Mr. Fractor has served as our Treasurer and Chief Financial Officer on a part-time basis since April 2011 and as our Vice President of Finance and Principal Accounting Officer on a part-time basis since March 2013. Since 2003, Mr. Fractor has been a consultant providing financial consulting and strategic planning services, including Sarbanes-Oxley compliance consulting services, to a variety of companies in a variety of industries. From 1999 through 2003, Mr. Fractor was the Chief Financial Officer of HemaCare Corporation, a publicly traded corporation which collects, manufactures, tests and distributes blood products to hospitals and provides blood services to patients in hospital settings on an outsourcing basis. Mr. Fractor received his B.S. in Accounting from the University of Southern California in 1982 and is a certified public accountant and a member of AICPA and the California Society of CPAs.
Steven J. Swanson, Ph.D.
Dr. Swanson has served as our Senior Vice President – Research since February 2015. Prior to joining ImmunoCellular, Dr. Swanson was an independent consultant advising biopharmaceutical companies on basic immunology research, bioanalytical procedures, immunogenicity assessment, regulatory affairs and product quality. Dr. Swanson spent 15 years at Amgen as Department Head for Clinical Immunology, a then-new department providing immunogenicity and cytometry support for all of Amgen’s therapeutic proteins. Prior to joining Amgen, he led the immunoassay laboratory in the Biotechnology department at Schering Plough Research Institute. Dr. Swanson has been actively involved in multiple industry professional associations, including the American Association of Pharmaceutical Scientists (AAPS), where he is a Fellow, and was a co-author of AAPS-sponsored Industry White Papers that were incorporated into FDA and EMA Guidance for Immunogenicity Assessment. He was also an industry representative for the EMA Committee that developed the first Immunogenicity Recommendations, and was engaged by the FDA to train reviewers on immunogenicity assessment. Dr. Swanson has authored more than 60 publications. He holds a BA in chemistry/biology from North Central College, a PhD in microbiology from the University of Iowa, and completed a post-doctoral fellowship at The Ohio State University.
Gary S. Titus
Mr. Titus was appointed as a director in December 2012 and was appointed as Chairman of the Board and Secretary in September 2015. Mr. Titus has more than 20 years of business experience in the healthcare and biopharmaceutical industries, primarily in senior management roles. Mr. Titus has served as Chief Financial Officer of UroGen Pharma LTD since July 2015. Prior to that from 2014 to 2015, Mr. Titus held the position of Chief Financial Officer of BioCardia, Inc. Prior to that, from 2008 to 2013, Mr. Titus was Senior Vice President and Chief Financial Officer at SciClone Pharmaceuticals, Inc. From 2006 to 2008, Mr. Titus was Senior Vice President of Finance and Chief Financial Officer at Kosan Biosciences, Inc. From 2003 to 2006, he was Chief Financial Officer and Vice President at Nuvelo, Inc. Earlier in his career, Mr. Titus held a variety of positions at other companies, including Metabolex, Inc., Intrabiotics Pharmaceuticals, Inc., Johnson & Johnson’s healthcare division and LifeScan, Inc. Mr. Titus holds a Bachelor of Science degree in Accounting from University of South Florida and a Bachelor of Science degree in Finance from University of Florida and is a Certified Public Accountant (Inactive). Mr. Titus also completed the Global BioExecutive Program at UC Berkeley’s Haas School of Business. Because of Mr. Titus’ extensive experience in the biomedical industry, we believe he is able to make valuable contributions to our Board of Directors.
Andrew Gengos
Mr. Gengos has served as a director since December 2012 and previously served as our President and Chief Executive Officer until December 2016. Mr. Gengos was the President and Chief Executive Officer of Neuraltus Pharmaceuticals from February 2010 until September 2012, where he led implementation of the company’s clinical, regulatory, fundraising and business development strategies while operating the company on a virtual business model. Previously, he served for more than seven years with Amgen where, as Vice President, Strategy and Corporate Development, he managed Amgen’s worldwide in-and-outbound business development activities, including a broad slate of acquisitions, licensing, spin-outs, divestitures, corporate venture capital investments, which included board of director positions, and alliance management. In addition, he led the execution of strategic projects and supported the long-range planning process for the company. Before joining Amgen, Mr. Gengos was Vice President, Chief Financial Officer, and Chief Business Officer of Dynavax Technologies, where he led the company’s business functions, including finance and accounting, fundraising, budgeting and planning, and business development. Earlier in his career, Mr. Gengos served as Vice President of Strategy at the Chiron Corporation and as Senior Engagement Manager at McKinsey & Company. Mr. Gengos holds an MBA degree from the UCLA Anderson School of Management and a BS degree in chemical engineering from the Massachusetts Institute of Technology. Because of Mr. Gengos’ more than 20 years in the life science industry, including experience in executive leadership positions in both large and emerging companies, with broad expertise in corporate strategy, business development and transactions, including mergers
and acquisitions, financing, operations, commercial planning and healthcare policy, we believe he is able to make valuable contributions to our Board of Directors.
Rahul Singhvi, Sc.D.
Dr. Singhvi has served as a director since June 2010 and served as our Lead Director from December 2010 until September 2015. He is the Chief Operating Officer of Takeda Vaccines, Inc. and is responsible for Takeda’s global vaccine operations. Before joining Takeda, Dr. Singhvi was with Novavax, Inc., a biopharmaceutical company focused on developing novel, highly potent recombinant vaccines beginning in 2004 and served as President, Chief Executive Officer and a director of Novavax from August 2005 to April 2011. Dr. Singhvi was the Senior Vice President and Chief Operating Officer of Novavax from April 2005 to August 2005 and Vice President – Pharmaceutical Development and Manufacturing Operations from April 2004 to April 2005. For ten years prior to joining Novavax, Dr. Singhvi served in various positions with Merck & Co., Inc., culminating as Director of the Merck Manufacturing Division, where he helped develop several vaccines, including Zostavax
®
, the only vaccine on the market to prevent shingles. Dr. Singhvi received his M.S. and Sc.D. degree in Chemical Engineering from the Massachusetts Institute of Technology. He also holds an M.B.A. from the Wharton School. Because of Dr. Singhvi’s experience and knowledge in the operation and leadership of early stage public healthcare companies and extensive expertise and experience in the development and manufacturing of vaccines which may assist the Board in its oversight of our cancer vaccine programs, we believe he is able to make valuable contributions to our Board of Directors.
John S. Yu, M.D.
Dr. Yu has served as a director since November 2006. He served as Chairman of the Board and as our Chief Scientific Officer from January 2007 until September 2015. Dr. Yu also served as Interim Chief Executive Officer from August 2012 until November 2012. He is a member of the full-time faculty in the Department of Neurosurgery at Cedars-Sinai Medical Center where he has worked since 1997. An internationally renowned neurosurgeon, Dr. Yu’s clinical focus is on the treatment of malignant and benign brain and spinal tumors. He is also conducting extensive research in immune and gene therapy for brain tumors. He has also done extensive research in the use of neural stem cells as delivery vehicles for brain cancers and neurodegenerative diseases. He was inducted into Castle and Connelly’s America’s Top Doctors in 2005. Dr. Yu has published articles in a number of prestigious journals, including The Lancet, Cancer Research, Cancer Gene Therapy, Human Gene Therapy, Journal of Neuroimmunology, Journal of Neurological Science and Journal of Neurosurgery. Dr. Yu earned his bachelor’s degree in French literature and biological sciences from Stanford University and spent a year at the Sorbonne in Paris studying French literature. He also pursued a fellowship in immunology at the Institut Pasteur in Paris. He earned his medical degree from Harvard Medical School and master’s degree from the Harvard University’s Department of Genetics. He completed his neurosurgical residency at Massachusetts General Hospital in Boston. In addition, he was a Neuroscience Fellow at the National Institutes of Mental Health in the Neuroimmunology Unit at Massachusetts General Hospital from 1988 to 1989 and was a Culpepper Scholar at the Molecular Neurogenetics Unit at that hospital from 1993 to 1995. His other honors include the Preuss Award, Joint Section on Tumors, American Association of Neurological Surgeons and Congress of Neurologic Surgeons in 1995. He received the Academy Award from the American Academy of Neurological Surgery at its 1996 annual meeting. Other honors include the Young Investigator Award from the Congress of Neurological Surgeons in 2000, the National Brain Tumor Foundation Grant in 2001, and the Mahaley Clinical Research award from the American Association of Neurological Surgeons in 2005. Dr. Yu, as a recognized leader in the field of neurosurgery, has extensive knowledge of current therapies and therapies under development for the treatment of brain tumors and has participated in numerous clinical trials for potential therapies in this field. As our former Chief Scientific Officer and the co-inventor of our brain tumor vaccine technologies, Dr. Yu brings to the Board significant scientific expertise directly relevant to our product research and development activities.
Gregg A. Lapointe
Mr. Lapointe has served as a director since September 2015. Mr. Lapointe has served the Chief Executive Officer of Cerium Pharmaceuticals, Inc., a specialty pharmaceutical company, since April 2012. He previously served as Chief Operating Officer from November 2003 to March 2008 and then as Chief Executive Officer from April 2008 to March 2012 of Sigma-Tau Pharmaceuticals, Inc., a pharmaceutical company focused on rare disorders and the U.S. wholly-owned subsidiary of Sigma-Tau Finanziaria S.p.A. Lapointe is a Certified Public Accountant (Illinois). He holds a Bachelor of Commerce degree from Concordia University of Montreal, a Graduate Diploma in Public Accountancy from McGill University of Montreal and a Master of Business Administration degree from Duke University. Mr. Lapointe also serves on the Boards of Directors of SciClone Pharmaceuticals, Inc., Soligenix, Inc., and Raptor Pharmaceuticals Corp. Because of Mr. Lapointe’s deep and broad executive, financial, operational, commercial and general management experience in large and small biopharmaceutical companies, we believe he is able to make valuable contributions to our Board of Directors.
Mark A. Schlossberg
Mr. Schlossberg has served as a director since September 2015. Mr. Schlossberg has served as Senior Vice President, General Counsel and Corporate Secretary of Impax Laboratories, Inc. since May 2011, where he is part of the senior management team responsible for the strategic direction and financial condition of the company. He leads the legal team and previously had responsibility for the healthcare compliance team. From September 2004 to May 2011, he served as Vice President, Associate General Counsel of Amgen Inc., where he had legal responsibility for corporate governance, securities law, licensing, mergers and acquisitions, global operations, and sales and marketing compliance. Prior to joining Amgen, he held legal and business positions at Medtronic, Inc., and legal positions at Diageo plc, RJR Nabisco, Inc. and Mudge Rose Guthrie Alexander & Ferdon. Mr. Schlossberg holds a Bachelor of Sciences in business administration, finance from the University of Southern California and a Juris Doctor degree from Emory University. Because of Mr. Schlossberg’s more than 15 years of experience in the biotechnology, pharmaceutical and medical device industries, and broad expertise in general management, legal affairs, finance, business development, and mergers and acquisitions, we believe he is able to make valuable contributions to our Board of Directors.
Committees of the Board Of Directors
Our Board of Directors has established an Audit Committee, which currently consists of Mr. Titus, as Chair, Dr. Singhvi and Mr. Schlossberg. The Audit Committee held four meetings during the 2016 fiscal year.
The Audit Committee assists the Board of Directors in fulfilling its oversight responsibilities relating to:
|
|
|
|
•
|
the quality and integrity of our financial statements and reports;
|
|
|
|
|
•
|
the independent registered public accounting firm’s qualifications and independence; and
|
|
|
|
|
•
|
the performance of our internal audit function and independent registered public accounting firm.
|
The Audit Committee appoints the independent registered public accounting firm, reviews with that accounting firm the plans and results of the audit engagement, approves permitted non-audit services provided by our independent registered public accounting firm and reviews that firm’s independence. Mr. Titus has been designated as an “audit committee financial expert” as defined under Item 407(d)(5) of Regulation S-K of the Securities Exchange Act of 1934 (the “Exchange Act”).
Our Board of Directors has established a Compensation Committee, which currently consists of Mr. Lapointe, as Chair and Mr. Titus. The Compensation Committee reviews, and makes recommendations to the full Board of Directors relating to, the compensation of our officers and directors, including our officers’ annual salaries and bonuses and the terms and conditions of option grants to our officers and directors under our equity incentive plan. The Compensation Committee held three meetings during the 2016 fiscal year.
Our Board of Directors has established a Finance Committee currently consisting of Dr. Singhvi, as Chair, Mr. Titus and Dr. Yu. The Finance Committee has oversight responsibility for all material financial matters affecting the Company, including capital management, funding strategy and investing activities related to our financial position and financing activities. The Finance Committee held three meetings during the 2016 fiscal year.
Our Board of Directors has established a Nominating and Corporate Governance Committee, which currently consists of Dr. Singhvi, as Chair and Mr. Titus. The Nominating and Corporate Governance Committee develops and recommends corporate governance guidelines to the Board, selects or recommends for selection nominees to serve on the Board, and oversees the evaluation of the Board and its committees. The Nominating and Corporate Governance Committee held 1 meeting during the 2016 fiscal year.
The Charters of the Audit, Compensation and Nominating and Corporate Governance Committees are available on our website at
www.imuc.com
. Information found on, or accessible through, our website is not a part of, and is not incorporated into, this prospectus, and you should not consider it part of this prospectus. Our website address is included in this document as an inactive textual reference only.
Compensation Committee Interlocks and Insider Participation
No member of the Compensation Committee is currently, or ever has been, an officer or employee of the Company and no member will have any relationship with us requiring disclosure under Item 404 of SEC Regulation S-K. No executive officer of
the Company has served as a member of the Board of Directors or Compensation Committee of any entity that has one or more executive officers serving as a member of our Compensation Committee.
Transactions With Related Persons
Related-Person Transactions Procedures
It is our practice to comply with all applicable laws, rules and regulations regarding related-person transactions, including the Sarbanes-Oxley Act of 2002. A related-person is any executive officer, director, or more than 5% stockholder of the Company, including any of their immediate family members, and any entity owned or controlled by such persons. Under its charter and as required by the NYSE MKT rules, our Audit Committee is charged with reviewing and approving all related-party transactions, other than those previously reviewed and approved by an independent committee of the Company’s Board of Directors or an independent majority of the Company’s Board of Directors, after reviewing each such transaction for potential conflicts of interest and other improprieties.
Certain Transactions
We have entered into indemnification agreements with our directors.
Our Amended and Restated Certificate of Incorporation provides that, to the full extent permitted by the Delaware General Corporation Law, no director will be personally liable to us or our stockholders for or with respect to any acts or omissions in the performance of his or her duties as a director.
Our Amended and Restated Certificate of Incorporation also provides that each person who is or was or had agreed to become a director or officer, and each such person who is or was serving or who had agreed to serve at our request as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other entity, will be indemnified by us to the full extent permitted by the Delaware General Corporation Law and will be entitled to advancement of expenses in connection therewith. Our Bylaws have similar indemnification provisions.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
In addition, we have entered into indemnification agreements with our directors, whereby we have agreed to indemnify our directors to the fullest extent permitted by law, including advancement of expenses incurred in legal proceedings to which the director was, or is threatened to be made, a party by reason of the fact that such director is or was a director, officer, employee or agent of the Company, provided that such director acted in good faith and in a manner that the director reasonably believed to be in, or not opposed to, the best interest of the Company.
DIRECTOR COMPENSATION
On August 2, 2012, the Board of Directors adopted certain revisions to the compensation program for non-employee directors. The annual cash retainer was increased to $30,000 for serving as a director, the fee for telephonic board meetings lasting more than one hour was increased to $1,500, the fee for committee meetings will be paid to the chair and other members of the committee and invited board members and the fee for committee meetings lasting more than one hour was increased to $1,000. The annual retainers paid to the Chairman of the Board and the Lead Director were increased to $5,500 and the annual retainers paid to the Audit Committee Chairman and the Chairs of the other board committees were increased to $11,000 and $5,500, respectively. The members of the Executive Committee will receive an annual retainer of $5,250 and $750 for committee meetings lasting up to one hour and $1,500 for meetings lasting more than one hour. In addition, seven-year non-qualified stock options to purchase shares of our common stock are to be granted annually on the date of the annual stockholders’ meeting to each non-employee director at an exercise price equal to the last reported trading price of our common stock on that date, with such option to vest quarterly over the four-year period following the date of grant in the following amounts: Chairman of the Board – 1,250 shares, Lead Director – 1,250 shares, board members (other than Chair and Lead) 750 shares, Audit Committee Chair – 500 shares, Compensation Committee, Finance and Nominating and Corporate Governance Committee Chairs each to receive 250 shares, and members of Committees (other than Chairs) to receive 125 shares, with all vested options to be exercisable for 24 months after termination for any reason except termination for cause by us.
During the fiscal year ended December 31, 2016, we paid our non-employee directors cash compensation for serving on the Board of Directors and committees of the Board and granted a non-qualified option to purchase shares of common stock to each of our directors for their service as a director. Each of the options granted to the directors has a term of ten years, vests in a series of four equal quarterly installments following the date of grant, and may be exercised within their term during the period the grantee provides services to us and for 24 months after the grantee ceases providing services for any reason other than termination by us for cause. The amounts of the annual grants in June 2016 at an exercise price of $9.60 per share were: Gary Titus, 3,918 shares; Dr. Rahul Singhvi, 3,918 shares, Dr. John Yu, 3,918 shares, Gregg Lapointe, 3,918 shares and Mark Schlossberg, 3,918 shares.
The following table sets forth information concerning the compensation paid to each of our directors during 2016 for their services rendered as directors. The compensation of Mr. Gringeri, who serves as a director and as our President and Chief Executive Officer, is described in the Summary Compensation Table of Executive Officers. The compensation of Mr. Gengos, who serves as a director and previously served as our President and Chief Executive Officer, is described in the Summary Compensation Table of Executive Officers.
Director Compensation for Fiscal Year 2016
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Name
|
Fees
Earned
or Paid
in Cash
|
|
|
Stock
Awards
|
|
Option
Awards
(1)(7)
|
|
Non-Equity
Incentive
Plan
Compensation
|
|
Nonqualified
Deferred
Compensation
Earnings
|
|
All
Other
Compensation
|
|
Total
|
|
|
Gary S. Titus
|
$
|
72,250
|
|
|
—
|
|
$
|
25,000
|
(2
|
)
|
—
|
|
—
|
|
—
|
|
$
|
97,250
|
|
|
Rahul Singhvi, Sc.D
|
$
|
61,250
|
|
|
—
|
|
$
|
25,000
|
(3
|
)
|
—
|
|
—
|
|
—
|
|
$
|
86,250
|
|
|
John S. Yu, M.D
|
$
|
44,500
|
|
|
—
|
|
$
|
25,000
|
(4
|
)
|
—
|
|
—
|
|
—
|
|
$
|
69,500
|
|
|
Gregg A. Lapointe
|
$
|
48,500
|
|
|
—
|
|
$
|
25,000
|
(5
|
)
|
—
|
|
—
|
|
—
|
|
$
|
73,500
|
|
|
Mark A. Schlossberg
|
$
|
49,750
|
|
|
—
|
|
$
|
25,000
|
(6
|
)
|
—
|
|
—
|
|
—
|
|
$
|
74,750
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)
|
This column represents the aggregate grant date fair value of options awarded computed in accordance with FASB ASC topic 718, excluding the effect of estimated forfeitures related to service-based vesting conditions. For additional information on the valuation assumptions with respect to the option grants, refer to Note 2 of our financial statements. These amounts do not correspond to the actual value that will be recognized by the named directors from these awards.
|
|
(2)
|
|
On June 17, 2016, we granted to Mr. Titus a ten-year non-qualified option to purchase 3,918 shares of our common stock at an exercise price of $9.60 per share that vests quarterly over a period of four quarters.
|
|
(3)
|
|
On June 17, 2016, we granted to Dr. Singhvi a ten-year non-qualified option to purchase 3,918 shares of our common stock at an exercise price of $9.60 per share that vests quarterly over a period of four quarters.
|
|
(4)
|
|
On June 17, 2016, we granted to Dr. Yu a ten-year non-qualified option to purchase 3,918 shares of our common stock at an exercise price of $9.60 per share that vests quarterly over a period of four quarters.
|
|
(5)
|
On June 17, 2016, we granted to Mr. Lapointe a ten-year non-qualified option to purchase 3,918 shares of our common stock at an exercise price of $9.60 per share that vests quarterly over a period of four quarters.
|
|
(6)
|
On June 17, 2016, we granted to Mr. Schlossberg a ten-year non-qualified option to purchase 3,918 shares of our common stock at an exercise price of $9.60 per share that vests quarterly over a period of four quarters.
|
|
(7)
|
|
As of December 31, 2016, our non-employee directors held vested and unvested options, which they received as compensation for their services as directors to purchase the following number of shares of our common stock: Mr. Titus – 9,438 shares, Dr. Singhvi – 14,260 shares, Dr. Yu – 11,207 shares, Mr. Lapointe – 4,919 shares and Mr. Schlossberg – 4,919 shares.
|
EXECUTIVE COMPENSATION
Compensation of Named Executive Officers
The following table sets forth the compensation for services paid in all capacities for the two fiscal years ended December 31, 2016 to the following:
|
|
|
|
•
|
Andrew Gengos, who served as our President and Chief Executive Officer until December 13, 2016;
|
|
|
|
|
•
|
Anthony Gringeri, Ph.D., who served as our Senior Vice President - Strategic Resources from August 2013 to December 13, 2016 and who serves as our President and Chief Executive Officer effective December 13, 2016; and
|
|
|
|
|
•
|
Steven J. Swanson, M.D., our Senior Vice President – Research; and
|
|
|
|
|
•
|
David Fractor, our Vice President – Finance and Principal Accounting Officer.
|
In determining the compensation of executive officers, the Compensation Committee and the Board of Directors considered the performance of the executive officers, attainment of corporate goals, compensation of executive officers of similar biotechnology companies, and recommendations from a compensation consultant.
Summary Compensation Table
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year
|
|
Salary
($)
|
|
Bonus
($)
|
|
Stock
Awards
($)
(9)
|
|
Option
Awards
($)
(9)
|
|
Non-Equity
Incentive
Plan
Compensation
($)
|
|
Nonqualified
Deferred
Compensation
Earnings
($)
|
|
All Other Compensation($)
|
|
Total
Compensation
($)
|
|
AndrewGengos,
Former President and Chief Executive Officer
|
2016
|
|
$
|
481,057
|
|
(1)
|
—
|
|
|
$
|
49,500
|
|
(10)
|
$
|
81,034
|
|
(17)
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
447,762
|
|
(25)
|
$
|
1,059,353
|
|
|
|
2015
|
|
$
|
427,450
|
|
(2)
|
286,392
|
|
|
$
|
87,000
|
|
(11)
|
$
|
93,450
|
|
(18)
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
894,292
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anthony Gringeri
President and Chief Executive Officer
|
2016
|
|
$
|
341,443
|
|
(3)
|
27,548
|
|
|
$
|
24,750
|
|
(12)
|
$
|
52,093
|
|
(19)
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
$
|
445,834
|
|
|
|
2015
|
|
$
|
328,058
|
|
(4)
|
131,879
|
|
|
$
|
43,500
|
|
(13)
|
$
|
60,075
|
|
(20)
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
563,512
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Steven J. Swanson
Senior Vice President –Research
|
2016
|
|
$
|
294,500
|
|
(5)
|
19,071
|
|
|
$
|
8,250
|
|
(14)
|
$
|
34,729
|
|
(21)
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
356,550
|
|
|
|
2015
|
|
$
|
249,375
|
|
(6)
|
100,249
|
|
|
$
|
—
|
|
|
$
|
155,050
|
|
(22)
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
504,674
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
David Fractor
|
2016
|
|
$
|
191,910
|
|
(7)
|
10,595
|
|
|
$
|
11,550
|
|
(15)
|
$
|
23,153
|
|
(23)
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
237,208
|
|
|
VP of Finance
|
2015
|
|
$
|
182,911
|
|
(8)
|
49,020
|
|
|
$
|
20,300
|
|
(16)
|
$
|
26,700
|
|
(24)
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
278,931
|
|
|
|
|
|
|
|
(1)
|
Includes $35,878 per month for the period January 1, 2016 to February 29, 2016 and $37,314 per month for the period from March 1, 2016 to December 31, 2016.
|
|
|
|
|
(2)
|
Includes $34,333 per month for the period January 1, 2015 to February 28, 2015 and $35,878 per month for the period from March 1, 2015 to December 31, 2015.
|
|
|
|
|
|
|
(3)
|
Includes $27,536 per month for the period from January 1, 2016 through February 29, 2016 and $28,637 per month for the period from March 1, 2016 to December 31, 2016.
|
|
|
|
|
|
|
(4)
|
Includes $26,350 per month for the period from January 1, 2015 through February 28, 2015 and $27,536 per month for the period from March 1, 2015 to December 31, 2015.
|
|
|
|
|
|
|
(5)
|
Includes $23,750 per month for the period from January 1, 2016 through February 29, 2016 and $24,700 per month for the period from March 1, 2016 to December 31, 2016.
|
|
|
|
|
|
|
(6)
|
Includes $23,750 per month for the period February 16, 2015 through December 31, 2015.
|
|
|
|
|
|
|
(7)
|
Includes $15,353 per month for the period from January 1, 2016 through February 29, 2016 and $16,120 per month for the period from March 1, 2016 to December 31, 2016.
|
|
|
|
|
|
|
(8)
|
Includes $14,692 per month for the period from January 1, 2015 through February 28, 2015 and $15,353 per month for the period from March 1, 2015 to December 31, 2015.
|
|
|
|
|
|
|
(9)
|
These columns represent restricted stock unit and option awards computed in accordance with FASB ASC Topic 718, excluding the effect of estimated forfeitures related to service-based vesting conditions. For additional information on the valuation assumptions with respect to the restricted stock unit and option grants, refer to Note 2 of our financial statements. These amounts do not correspond to the actual value that will be recognized by the named executives from these awards.
|
|
|
|
|
|
|
(10)
|
Includes 3,750 restricted stock units granted on March 11, 2016, with each restricted stock unit representing a contingent right to receive one share of our common stock, vesting in full on the second anniversary of the grant.
|
|
|
|
|
|
|
(11)
|
Includes 3,750 restricted stock units granted on March 20, 2015, with each restricted stock unit representing a contingent right to receive one share of our common stock, vesting in full on the second anniversary of the grant.
|
|
|
|
|
|
|
(12)
|
Includes 1,875 restricted stock units granted on March 11, 2016, with each restricted stock unit representing a contingent right to receive one share of our common stock, vesting in full on the second anniversary of the grant.
|
|
|
|
|
|
|
(13)
|
Includes 1,875 restricted stock units granted on March 20, 2015, with each restricted stock unit representing a contingent right to receive one share of our common stock, vesting in full on the second anniversary of the grant.
|
|
|
|
|
|
|
(14)
|
Includes 625 restricted stock units granted on March 11, 2016, with each restricted stock unit representing a contingent right to receive one share of our common stock, vesting in full on the second anniversary of the grant.
|
|
|
|
|
|
|
(15)
|
Includes 875 restricted stock units granted on March 11, 2016, with each restricted stock unit representing a contingent right to receive one share of our common stock, vesting in full on the second anniversary of the grant.
|
|
|
|
|
|
|
(16)
|
Includes 875 restricted stock units granted on March 20, 2015, with each restricted stock unit representing a contingent right to receive one share of our common stock, vesting in full on the second anniversary of the grant.
|
|
|
|
|
|
|
(17)
|
Includes a ten-year option to purchase 8,750 shares of our common stock granted on March 11, 2016, at an exercise price of $13.20 per share, vesting in 48 equal monthly installment from the grant date.
|
|
|
|
|
|
|
(18)
|
Includes a ten-year option to purchase 4,375 shares of our common stock granted on March 20, 2015, at an exercise price of $22.80 per share, vesting in 48 equal monthly installment from the grant date and a ten-year option to purchase 4,375 shares of our common stock granted on March 20, 2015, at an exercise price of $22.80 per share, vesting immediately if the average daily closing price of our common stock on the NYSE MKT during any10 consecutive trading pay period exceeds a closing $80.00 weighted average bid and ask price per share (as adjusted for any stock splits and the like).
|
|
(19)
|
Includes a ten-year option to purchase 5,625 shares of our common stock granted on March 11, 2016, at an exercise price of $13.20 per share, vesting in 48 equal monthly installment from the grant date.
|
|
(20)
|
Includes a ten-year option to purchase 2,812 shares of our common stock granted on March 20, 2015, at an exercise price of $22.80 per share, vesting in 48 equal monthly installment from the grant date and a ten-year option to purchase 2,812 shares of our common stock granted on March 20, 2015, at an exercise price of $22.80 per share, vesting immediately if the average daily closing price of our common stock on the NYSE MKT during any10 consecutive trading pay period exceeds a closing $80.00 weighted average bid and ask price per share (as adjusted for any stock splits and the like).
|
|
(21)
|
Includes a ten-year option to purchase 3,750 shares of our common stock granted on March 11, 2016, at an exercise price of $13.20 per share, vesting in 48 equal monthly installment from the grant date.
|
|
(22)
|
Includes a ten-year option to purchase 8,750 shares of our common stock granted on March 6, 2015, at an exercise price of $23.60 per share, with 2,187 shares vesting on March 6, 2016 and the remaining shares vesting in 36 equal monthly installments thereafter.
|
|
(23)
|
Includes a ten-year option to purchase 2,500 shares of our common stock granted on March 11, 2016, at an exercise price of $13.20 per share, vesting in 48 equal monthly installment from the grant date.
|
|
(24)
|
Includes a ten-year option to purchase 1,250 shares of our common stock granted on March 20, 2015, at an exercise price of $22.80 per share, vesting in 48 equal monthly installment from the grant date and a ten-year option to purchase 1,250 shares of our common stock granted on March 20, 2015, at an exercise price of $22.80 per share, vesting immediately if the average daily closing price of our common stock on the NYSE MKT during any10 consecutive trading pay period exceeds a closing $80.00 weighted average bid and ask price per share (as adjusted for any stock splits and the like).
|
|
(25)
|
Includes one year of severance payable in March 2017.
|
Outstanding Equity Awards
The following table sets forth information as of December 31, 2016 concerning unexercised options, unvested stock and equity incentive plan awards for the executive officers named in the Summary Compensation Table.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Option Awards
|
|
Stock Awards
|
|
Name
|
|
Grant
Date
|
|
Number of
Securities
Underlying
Unexercised
Options
(#)
Exercisable
|
|
Number of
Securities
Underlying
Unexercised
Options
(#)
Unexercisable
|
|
Option
Exercise
Price
($)
|
|
Option
Expiration
Date
|
|
Number of
Shares or
Units of Stock
That Have
Not Vested
|
|
Market Value of
Shares or
Units of Stock
That Have
Not Vested
($)
(11)
|
|
Andrew Gengos
|
|
12/3/2012
|
|
17,500
|
(1)
|
—
|
(1)
|
$
|
85.20
|
|
|
12/2/2019
|
|
—
|
|
—
|
|
|
|
|
3/7/2014
|
|
5,000
|
(1)
|
—
|
(1)
|
$
|
53.60
|
|
|
3/6/2024
|
|
—
|
|
—
|
|
|
|
|
3/20/2015
|
|
4,375
|
(1)
|
—
|
(1)
|
$
|
23.20
|
|
|
3/20/2025
|
|
—
|
|
—
|
|
|
|
|
3/20/2015
|
|
4,375
|
(1)
|
—
|
(1)
|
$
|
23.30
|
|
|
3/20/2025
|
|
—
|
|
—
|
|
|
|
|
3/11/2016
|
|
8,750
|
(1)
|
—
|
(1)
|
$
|
13.20
|
|
|
3/10/2026
|
|
—
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anthony Gringeri, Ph.D.
|
|
9/20/2013
|
|
6,719
|
(2)
|
781
|
(2)
|
$
|
108.00
|
|
|
9/19/2023
|
|
—
|
|
—
|
|
|
|
|
3/7/2014
|
|
1,445
|
(3)
|
430
|
(3)
|
$
|
53.60
|
|
|
3/6/2024
|
|
—
|
|
—
|
|
|
|
|
3/20/2015
|
|
1,465
|
(4)
|
1,348
|
(4)
|
$
|
23.20
|
|
|
3/20/2025
|
|
—
|
|
—
|
|
|
|
|
3/20/2015
|
|
—
|
(5)
|
2,813
|
(5)
|
$
|
23.20
|
|
|
3/20/2025
|
|
—
|
|
—
|
|
|
|
|
3/20/2015
|
|
—
|
|
—
|
|
—
|
|
|
—
|
|
1,875
|
(7)
|
$
|
3,844
|
|
|
|
|
3/11/2016
|
|
1,289
|
(4)
|
4,336
|
(4)
|
$
|
13.20
|
|
|
3/10/2026
|
|
—
|
|
—
|
|
|
|
|
3/11/2016
|
|
—
|
|
—
|
|
—
|
|
|
—
|
|
1,875
|
(7)
|
$
|
3,844
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Steven J. Swanson, Ph.D.
|
|
3/6/2015
|
|
4,557
|
(6)
|
4,193
|
(6)
|
$
|
23.20
|
|
|
3/5/2025
|
|
—
|
|
—
|
|
|
|
|
3/11/2016
|
|
859
|
(4)
|
2,891
|
(4)
|
$
|
13.20
|
|
|
3/10/2026
|
|
—
|
|
—
|
|
|
|
|
3/11/2016
|
|
—
|
|
—
|
|
—
|
|
|
—
|
|
625
|
(7)
|
$
|
1,281
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
David Fractor
|
|
4/4/2011
|
|
1,050
|
(4)
|
—
|
(4)
|
$
|
90.00
|
|
|
4/3/2021
|
|
—
|
|
—
|
|
|
|
|
10/24/2011
|
|
250
|
(8)
|
—
|
(8)
|
$
|
56.80
|
|
|
10/23/2021
|
|
—
|
|
—
|
|
|
|
|
2/24/2012
|
|
250
|
(9)
|
—
|
(9)
|
$
|
76.00
|
|
|
2/23/2022
|
|
—
|
|
—
|
|
|
|
|
3/1/2013
|
|
1,600
|
(10)
|
—
|
|
$
|
108.80
|
|
|
2/28/2013
|
|
—
|
|
—
|
|
|
|
|
3/7/2014
|
|
385
|
(4)
|
115
|
(4)
|
$
|
53.60
|
|
|
3/6/2024
|
|
—
|
|
—
|
|
|
|
|
3/19/2015
|
|
651
|
(4)
|
599
|
(4)
|
$
|
23.20
|
|
|
3/18/2025
|
|
—
|
|
—
|
|
|
|
|
3/19/2015
|
|
—
|
|
1,250
|
|
$
|
23.20
|
|
|
3/18/2025
|
|
—
|
|
—
|
|
|
|
|
3/20/2015
|
|
—
|
|
—
|
|
—
|
|
|
—
|
|
875
|
(7)
|
$
|
1,794
|
|
|
|
|
3/11/2016
|
|
469
|
(4)
|
2,031
|
(4)
|
$
|
13.20
|
|
|
3/10/2026
|
|
—
|
|
—
|
|
|
|
|
3/11/2016
|
|
—
|
|
—
|
|
—
|
|
|
—
|
|
875
|
(7)
|
$
|
1,794
|
|
|
|
|
|
|
|
(1)
|
Vested in full on December 31, 2016.
|
|
|
|
|
|
|
(2)
|
Vested 1,875 on the one year anniversary of the Option Grant Date and then in 36 equal monthly installments thereafter.
|
|
|
|
|
|
|
(3)
|
Vested 234 on the sixth month anniversary of the Option Grant Date and then in 42 equal monthly installments thereafter.
|
|
|
|
|
|
|
(4)
|
Vests in monthly installments over a four year period following the Option Grant Date.
|
|
|
|
|
|
|
(5)
|
Vests immediately if the average daily closing price of our common stock on the NYSE MKT during any10 consecutive trading pay period exceeds a closing $80.00 weighted average bid and ask price per share (as adjusted for any stock splits and the like).
|
|
|
|
|
|
|
(6)
|
Vests 2,188 on the one year anniversary of the Option Grant Date and then in 36 equal monthly installments thereafter.
|
|
|
|
|
|
|
(7)
|
Vests in full on the second anniversary of the grant date.
|
|
|
|
|
|
|
(8)
|
Vested in 36 equal monthly installments.
|
|
(9)
|
Vested in quarterly installments over a one year period following the Option Grant Date.
|
|
(10)
|
Vested 400 on the one year anniversary of the Option Grant Date and then in 36 equal monthly installments thereafter.
|
|
(11)
|
The market value is calculated using the closing price per share of our common stock of $2.05 per share on December 31, 2016.
|
|
|
|
Change of Control and Severance Agreements
Change of control and severance agreements
. We have entered into change of control and severance agreements with certain of our named executive officers, as set forth in their respective employment agreements.
The employment agreement with Mr. Gengos provides that in the event that we terminate his employment agreement without cause (as defined in the employment agreement) or Mr. Gengos terminates his employment agreement for good reason (as defined in the employment agreement), in either case prior to the closing of a change of control or more than 12 months following the closing of a change of control (as defined in the employment agreement and specifically excluding any sale of stock for capital raising purposes), then (i) we upon such termination will be required to make a lump sum payment to Mr. Gengos equal to twelve months of his base annual salary; (ii) provided that Mr. Gengos timely elects continued coverage under COBRA, we will pay Mr. Gengos’ COBRA premiums to continue his coverage through the period starting on the separation from service and ending on the earliest to occur of (a) 12 months following separation from service, (b) the date Mr. Gengos becomes eligible for group health insurance coverage through a new employer or (c) the date Mr. Gengos ceases to be eligible for COBRA continuation coverage for any reason; notwithstanding (a), (b) or (c), if we determine that we cannot pay the COBRA premiums without a substantial risk of violating applicable law, we will instead pay to Mr. Gengos, on the first day of each calendar month, a fully taxable cash payment equal to the applicable COBRA premiums for that month, subject to applicable tax withholdings, for the remainder of the COBRA premium period; and (iii) we will accelerate the vesting of Mr. Gengos’ option (as defined in the employment agreement) so that 100% of his option will vest as of his last day of employment and will be exercisable for 90 days after termination. The receipt of any payment described above is subject to Mr. Gengos signing and not revoking a separation agreement and release of claims in a form reasonably satisfactory to us.
On December 13, 2016, Mr. Gengos resigned from his position as President and Chief Executive Officer of the Company. On such date, the Company entered into a separation agreement with Mr. Gengos, pursuant to which Mr. Gengos remained an employee of the Company until December 31, 2016. Following the separation, Mr. Gengos remained a member of the Board of Directors.
The employment agreement with Dr. Gringeri provides that in the event that we terminate his employment agreement without cause (as defined in the employment agreement) or Dr. Gringeri terminates his employment agreement for good reason (as defined in the employment agreement), then (i) we will be required to make payments to Dr. Gringeri equal to six months of his base annual salary over the six month period following his separation, beginning 60 days after such termination; (ii) provided that Dr. Gringeri timely elects continued coverage under COBRA, we will pay Dr. Gringeri’s COBRA premiums to continue his coverage through the period starting on the separation from service and ending on the earliest to occur of (a) six months following separation from service, (b) the date Mr. Gringeri becomes eligible for group health insurance coverage through a new employer or (c) the date Dr. Gringeri ceases to be eligible for COBRA continuation coverage for any reason; notwithstanding (a), (b) or (c), if we determine that we cannot pay the COBRA premiums without a substantial risk of violating applicable law, we will instead pay to Dr. Gringeri, on the first day of each calendar month, a fully taxable cash payment equal to the applicable COBRA premiums for that month, subject to applicable tax withholdings, for the remainder of the COBRA premium period; and (iii) we will accelerate the vesting of Dr. Gringeri’s option (as defined in the employment agreement) such that the shares that would have vested in the six months following separation from service shall be deemed vested and exercisable as of his last day of employment and will be exercisable for 90 days after termination. In the event that we terminate Dr. Gringeri’s employment agreement without cause or Dr. Gringeri terminates his employment agreement for good
reason, in either case within 12 months following the closing of a change of control (as defined in the employment agreement and specifically excluding any sale of stock for capital raising purposes), then (i) we upon such termination will be required to make a lump sum payment to Dr. Gringeri equal to six months of his base annual salary; (ii) provided that Dr. Gringeri timely elects continued coverage under COBRA, we will pay Dr. Gringeri’s COBRA premiums to continue his coverage through the period starting on the separation from service and ending on the earliest to occur of (a) six months following separation from service, (b) the date Mr. Gringeri becomes eligible for group health insurance coverage through a new employer or (c) the date Dr. Gringeri ceases to be eligible for COBRA continuation coverage for any reason; notwithstanding (a), (b) or (c), if we determine that we cannot pay the COBRA premiums without a substantial risk of violating applicable law, we will instead pay to Dr. Gringeri, on the first day of each calendar month, a fully taxable cash payment equal to the applicable COBRA premiums for that month, subject to applicable tax withholdings, for the remainder of the COBRA premium period; and (iii) we will accelerate the vesting of Dr. Gringeri’s option such that 50% of the then-unvested shares subject to the option (or other equity interests) will vest as of his last day of employment and will be exercisable for 90 days after termination. The receipt of any payment described above is subject to Dr. Gringeri signing and not revoking a separation agreement and release of claims in a form reasonably satisfactory to us. In the event that Dr. Gringeri resigns without good reason, or if we terminate his employment for cause, then (i) he will no longer vest in any equity interests, (ii) all payments of compensation will terminate immediately (except as to amounts already earned), and (c) he will not be entitled to any severance benefits.
The employment agreement with Dr. Swanson provides that in the event that we terminate his employment agreement without cause (as defined in the employment agreement) or Dr. Swanson terminates his employment agreement for good reason (as defined in the employment agreement), then (i) we will be required to make payments to Dr. Swanson equal to six months of his base annual salary over the six month period following his separation, beginning 60 days after such termination; (ii) provided that Dr. Swanson timely elects continued coverage under COBRA, we will pay Dr. Swanson’s COBRA premiums to continue his coverage through the period starting on the separation from service and ending on the earliest to occur of (a) six months following separation from service, (b) the date Dr. Swanson becomes eligible for group health insurance coverage through a new employer or (c) the date Dr. Swanson ceases to be eligible for COBRA continuation coverage for any reason; notwithstanding (a), (b) or (c), if we determine that we cannot pay the COBRA premiums without a substantial risk of violating applicable law, we will instead pay to Dr. Swanson, on the first day of each calendar month, a fully taxable cash payment equal to the applicable COBRA premiums for that month, subject to applicable tax withholdings, for the remainder of the COBRA premium period; and (iii) we will accelerate the vesting of Dr. Swanson’s option (as defined in the employment agreement) such that the shares that would have vested in the six months following separation from service shall be deemed vested and exercisable as of his last day of employment and will be exercisable for 90 days after termination. In the event that we terminate Dr. Swanson’s employment agreement without cause or Dr. Swanson terminates his employment agreement for good reason, in either case within 12 months following the closing of a change of control (as defined in the employment agreement and specifically excluding any sale of stock for capital raising purposes), then (i) we upon such termination will be required to make a lump sum payment to Dr. Swanson equal to six months of his base annual salary; (ii) provided that Dr. Swanson timely elects continued coverage under COBRA, we will pay Dr. Swanson’s COBRA premiums to continue his coverage through the period starting on the separation from service and ending on the earliest to occur of (a) six months following separation from service, (b) the date Dr. Swanson becomes eligible for group health insurance coverage through a new employer or (c) the date Dr. Swanson ceases to be eligible for COBRA continuation coverage for any reason; notwithstanding (a), (b) or (c), if we determine that we cannot pay the COBRA premiums without a substantial risk of violating applicable law, we will instead pay to Dr. Swanson, on the first day of each calendar month, a fully taxable cash payment equal to the applicable COBRA premiums for that month, subject to applicable tax withholdings, for the remainder of the COBRA premium period; and (iii) we will accelerate the vesting of Dr. Swanson’s option such that 50% of the then-unvested shares subject to the option (or other equity interests) will vest as of his last day of employment and will be exercisable for 90 days after termination. The receipt of any payment described above is subject to Dr. Swanson signing and not revoking a separation agreement and release of claims in a form reasonably satisfactory to us. In the event that Dr. Swanson resigns without good reason, or if we terminate his employment for cause, then (i) he will no longer vest in any equity interests, (ii) all payments of compensation will terminate immediately (except as to amounts already earned), and (c) he will not be entitled to any severance benefits.
The employment agreement with Mr. Fractor provides that in the event that we terminate his employment agreement without cause (as defined in the employment agreement) or Mr. Fractor terminates his employment agreement for good reason (as defined in the employment agreement), then (i) we will be required to make payments to Mr. Fractor equal to six months of his base annual salary over the six month period following his separation, beginning 60 days after such termination; (ii) provided that Mr. Fractor timely elects continued coverage under COBRA, we will pay Mr. Fractor’s COBRA premiums to continue his coverage through the period starting on the separation from service and ending on the earliest to occur of (a) six months following separation from service, (b) the date Mr. Fractor becomes eligible for group health insurance coverage through a new employer or (c) the date Mr. Fractor ceases to be eligible for COBRA continuation coverage for any reason; notwithstanding (a), (b) or (c), if we determine that we cannot pay the COBRA premiums without a substantial risk of violating applicable law, we will instead pay to Mr. Fractor, on the first day of each calendar month, a fully taxable cash payment equal to the applicable COBRA premiums for that month, subject to applicable tax withholdings, for the remainder of the COBRA premium period; and (iii) we will accelerate the vesting of Mr. Fractor’s option (as defined in the employment agreement) such that the shares that would have vested in the six months following separation from service shall be deemed vested and exercisable as of his last day of employment. In the event that we terminate Mr. Fractor’s employment agreement without cause or Mr. Fractor terminates his employment agreement for good reason, in either case within 12 months following the closing of a change of control (as defined in the employment agreement and specifically excluding any sale of stock for capital raising purposes), then (i) we upon such termination will be required to make a lump sum payment to Mr. Fractor equal to six months of his base annual salary; (ii) provided that Mr. Fractor timely elects continued coverage under COBRA, we will pay Mr. Fractor’s COBRA premiums to continue his coverage through the period starting on the separation from service and ending on the earliest to occur of (a) six months following separation from service, (b) the date Mr. Fractor becomes eligible for group health insurance coverage through a new employer or (c) the date Mr. Fractor ceases to be eligible for COBRA continuation coverage for any reason; notwithstanding (a), (b) or (c), if we determine that we cannot pay the COBRA premiums without a substantial risk of violating applicable law, we will instead pay to Mr. Fractor, on the first day of each calendar month, a fully taxable cash payment equal to the applicable COBRA premiums for that month, subject to applicable tax withholdings, for the remainder of the COBRA premium period; and (iii) we will accelerate the vesting of Mr. Fractor’s options such that 25% of the then-unvested shares subject to the option (or other equity interests) will be deemed vested and exercisable as of his last day of employment. The receipt of any payment described above is subject to Mr. Fractor signing and not revoking a separation agreement and release of claims in a form reasonably satisfactory to us. In the event that Mr. Fractor resigns without good reason, or if we terminate his employment for cause, then (i) he will no longer vest in any equity interests, (ii) all payments of compensation will terminate immediately (except as to amounts already earned), and (c) he will not be entitled to any severance benefits.
Estimated Change of Control and Severance Benefits
The following charts present the approximate amount of the benefits that each of our named executive officers would have been entitled to have his employment terminated under the circumstances described in the preceding paragraphs on December 31, 2016.
Change of control benefits
. The following chart presents our estimates of the dollar values of the benefits to which each of the named executive officers would have been entitled had benefits for termination in connection with a change of control been triggered on December 31, 2016:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change of Control (
1)
|
|
Name
|
|
Salary
Continuation
($)
(2)
|
|
Continuation of
COBRA
($)
|
|
Equity
Acceleration
($)
(3)
|
|
Total
($)
|
|
Andrew Gengos
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Anthony Gringeri, Ph.D.
|
|
$
|
171,823
|
|
|
$
|
3,798
|
|
|
$
|
7,688
|
|
|
$
|
183,309
|
|
|
Steven J. Swanson, M.D.
|
|
$
|
142,500
|
|
|
$
|
16,645
|
|
|
$
|
1,281
|
|
|
$
|
160,426
|
|
|
David Fractor
|
|
$
|
92,117
|
|
|
$
|
13,624
|
|
|
$
|
3,588
|
|
|
$
|
109,329
|
|
|
|
|
|
|
|
(1)
|
As described above, these severance amounts are generally payable if the executive officer’s employment is terminated without cause or for good reason from 90 days prior to or 12 months following a change of control.
|
|
|
|
|
|
|
(2)
|
Amounts in this column are based upon salary in effect as of December 31, 2016.
|
|
|
|
|
|
|
(3)
|
The aggregate dollar value to be realized in connection with the acceleration of the equity awards represents the difference between the aggregate market value of our common stock underlying the accelerated stock options, which was based on our common stock’s closing price of $2.05 per share as of December 31, 2016, and the aggregate exercise price of the accelerated stock options.
|
Severance benefits
. The following chart presents our estimates of the dollar values of the benefits to which each of the named executive officers would have been entitled had his employment terminated by us under the circumstances described above without cause or by the executive for good reason (other than in connection with a change of control) on December 31, 2016:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termination by the Company
Without Cause or Involuntary
Termination for Good Reason
|
|
Name
|
|
Salary
Continuation
($)
(1)
|
|
Continuation of
COBRA
($)
|
|
Equity Acceleration
($)
|
|
Total
($)
|
|
Andrew Gengos
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Anthony Gringeri, Ph.D.
|
|
$
|
171,823
|
|
|
$
|
3,588
|
|
|
$
|
7,688
|
|
|
$
|
183,309
|
|
|
Steven J. Swanson, M.D.
|
|
$
|
142,500
|
|
|
$
|
13,656
|
|
|
$
|
1,281
|
|
|
$
|
160,426
|
|
|
David Fractor
|
|
$
|
92,117
|
|
|
$
|
13,624
|
|
|
$
|
3,588
|
|
|
$
|
109,329
|
|
|
|
|
|
|
|
(1)
|
Amounts in this column are based upon salary in effect as of December 31, 2016.
|
Employment Agreements and Offer Letter Agreement
We entered into employment agreements with certain of our named executive officers in connection with their commencement of employment with us. These employment agreements typically include the named executive officer’s initial base salary and stock option grant along with vesting provisions with respect to such initial stock option grant, as well as change of control and severance arrangements. See “Change of Control and Severance Agreements” above. We entered into an offer letter agreement with David Fractor in connection with his commencement of employment by us, including his initial base salary and stock option grant along with vesting provisions with respect to such initial stock option grant.
401(k) Plan
We have a 401(k) Plan to allow eligible employees to elect to reduce the amount of their compensation that is currently subject to income taxes. We currently do not make matching or other contributions to the 401(k) Plan on behalf of eligible employees, including our named executive officers.
Limitation on Liability and Indemnification Matters
Our Amended and Restated Certificate of Incorporation provides that, to the full extent permitted by the Delaware General Corporation Law, no director will be personally liable to us or our stockholders for or with respect to any acts or omissions in the performance of his or her duties as a director.
Our Amended and Restated Certificate of Incorporation also provides that each person who is or was or had agreed to become a director or officer, and each such person who is or was serving or who had agreed to serve at our request as a director, officer, employee, or agent of another corporation, partnership, joint venture, trust, or other entity, will be indemnified by us to the full extent permitted by the Delaware General Corporation Law and will be entitled to advancement of expenses in connection therewith. Our Bylaws have similar indemnification provisions.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
In addition, we have entered into indemnification agreements with our directors, whereby we have agreed to indemnify our directors to the fullest extent permitted by law, including advancement of expenses incurred in legal proceedings to which the director was, or is threatened to be made, a party by reason of the fact that such director is or was a director, officer, employee or agent of the Company, provided that such director acted in good faith and in a manner that the director reasonably believed to be in, or not opposed to, the best interest of the Company.
Rule 10b5-1 Sales Plans
Our directors and executive officers may adopt written plans, known as Rule 10b5-1 plans, in which they will contract with a broker to buy or sell shares of our common stock on a periodic basis. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the director or officer when entering into the plan, without further direction from them. A director or officer can only enter into a 10b5-1 plan when he or she is not in possession of material non-public information regarding the Company. Under certain circumstances, a broker may subsequently execute trades pursuant to such plan, even if the director or officer has subsequently come into possession of material non-public information. The director or officer may amend or terminate the plan in some circumstances. Our directors and executive officers may also buy or sell additional shares outside of a Rule 10b5-1 plan when they are not in possession of material, nonpublic information.
Employment Agreements
Andrew Gengos
On December 3, 2012, the Company entered into an agreement with Mr. Andrew Gengos pursuant to which Mr. Gengos serves as the Company’s President and Chief Executive Officer. The Agreement was terminated effective December 31, 2016.
Anthony Gringeri, Ph.D.
On August 19, 2013, the Company entered into an agreement with Dr. Anthony Gringeri pursuant to which Dr. Gringeri served as the Company’s Senior Vice President of Strategic Resources and now serves as the Company’s President and Chief Executive Officer. The agreement may be terminated be either party with or without cause.
Steven J. Swanson, M.D.
On January 30, 2015, the Company entered into an agreement with Dr. Steven J. Swanson pursuant to which Dr. Swanson serves as the Company’s Senior Vice President of Research. The agreement may be terminated be either party with or without cause.
David Fractor
On April 4, 2011, we entered into an offer letter agreement with David Fractor in connection with his commencement of employment by us. This agreement was last modified on September 17, 2015.
Equity Compensation Plan Information
The following table summarizes, as of December 31, 2016, (i) the number of shares of our common stock that are issuable under our equity compensation plans upon the exercise of outstanding options, warrants and other rights, (ii) the weighted-average exercise price of such options, warrants and rights, and (iii) the number of securities remaining available for future issuance under our equity compensation plans.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Plan Category
|
|
Number of
securities to be
issued upon
exercise of
outstanding options,
warrants and rights
|
|
Weighted-average
exercise price of
outstanding options,
warrants and rights
|
|
Number of securities
remaining available for
future issuance under
equity compensation
plans (excluding
securities reflected in
column (a))
|
|
|
|
(a)
|
|
(b)
|
|
(c)
|
|
Equity compensation plans approved by stockholders
|
|
158,915
|
|
|
$
|
43.09
|
|
|
199,197
|
|
|
Equity compensation plans not approved by stockholders
|
|
3,750
|
|
|
$
|
44.00
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
162,665
|
|
|
$
|
43.11
|
|
|
199,197
|
|
In February 2005, the Company adopted an Equity Incentive Plan (the Plan) that was approved by our stockholders. Initially, the Company reserved 150,000 shares of common stock for issuance under the Plan, which was subsequently increased by the Company's stockholders to 300,000 shares. Options to purchase 110,846 common shares were granted under the Plan and are outstanding as of December 31, 2016. Additionally, 6,500 shares of restricted common stock were granted to management and 1,000 shares of restricted common stock were granted to members of the Company’s Board of Directors under the Plan and 3,250 shares remain unvested. This plan expired in January 2016.
On March 11, 2016, the Company's Board of Directors adopted the 2016 Equity Incentive Plan (the 2016 Plan) and reserved 250,000 shares of common stock for issuance under the 2016 Plan. The 2016 Plan was approved by the Company's stockholders at its 2016 Annual Meeting of Stockholders. During the year ended December 31, 2016, the Company’s Board of Directors granted 48,444 stock options and 7,862 restricted stock units to certain directors, officers and employees. The options have an exercise price equal to the closing stock price on the date of grant. The stock options vest over a period of four years and the restricted stock units vest over a period of two years.
Equity compensation plans not approved by stockholders include a ten year option to purchase 37,500 shares at an exercise price of $44.00 per share (of which 33,750 have been exercised) issued to Dr. Keith Black, on January 8, 2007 in connection with his appointment to our Scientific Advisory Board.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding beneficial ownership of our common stock as of February 28, 2017 (a) by each person known by us to own beneficially 5% or more of our common stock, (b) by each of our named executive officers as set forth in our proxy statement for our 2016 Annual Meeting of Stockholders and our directors and (c) by all our executive officers and directors as a group. Our calculation of the percentage of beneficial ownership prior to this offering is based on 3,459,859 shares of our common stock outstanding as of December 31, 2016. We have based our calculation of the percentage of beneficial ownership after this offering on 7,788,863 shares of our common stock outstanding immediately after the closing of this offering, assuming conversion of all shares of Series B Preferred Stock at an assumed Conversion Price of $2.31, which is equal to 87.5% of the lowest volume weighted average trading price of the Common Stock during the five trading days ending March 29, 2017, and no exercise of the Series A Warrants. Unless otherwise noted, we believe that all persons named in the table have sole voting and investment power with respect to all the shares beneficially owned by them.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Percentage of Shares Beneficially Owned
|
|
Name and Address of Beneficial Owner
(1)
|
|
Shares
Beneficially
Owned
(2)
|
|
Before Offering
|
After Offering
|
|
John S. Yu, M.D.
|
|
15,221
(3)
|
|
*
|
*
|
|
|
|
|
|
|
|
|
Andrew Gengos
|
|
47,147
(4)
|
|
1.36%
|
*
|
|
|
|
|
|
|
|
|
Anthony Gringeri, Ph.D.
|
|
17,077
(5)
|
|
*
|
*
|
|
|
|
|
|
|
|
|
David Fractor
|
|
7.945
(6)
|
|
*
|
*
|
|
|
|
|
|
|
|
|
Rahul Singhvi, Sc.D.
|
|
11,072
(7)
|
|
*
|
*
|
|
|
|
|
|
|
|
|
Steven J. Swanson, M.D.
|
|
6,197
(8)
|
|
*
|
*
|
|
|
|
|
|
|
|
|
Gary S. Titus
|
|
6,550
(9)
|
|
*
|
*
|
|
|
|
|
|
|
|
|
Gregg A. Lapointe
|
|
2,334
(10)
|
|
*
|
*
|
|
|
|
|
|
|
|
|
Mark A. Schlossberg
|
|
2,334
(11)
|
|
*
|
*
|
|
|
|
|
|
|
|
|
All executive officers and directors as a group (9 persons)
|
|
115,877
(12)
|
|
3.24%
|
1.47%
|
_________
|
|
|
|
(1)
|
The address of each of the persons shown is c/o ImmunoCellular Therapeutics, Ltd., 23622 Calabasas Road, Suite 300, Calabasas, California 91302.
|
|
|
|
|
(2)
|
Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. Shares of common stock subject to options, warrants and convertible securities currently exercisable or convertible, or exercisable or convertible within 60 days of February 28, 2017, are deemed outstanding, including for purposes of computing the percentage ownership of the person holding such option, warrant or convertible security, but not for purposes of computing the percentage of any other holder.
|
|
|
|
|
(3)
|
Includes 14,713 shares of common stock underlying stock options that are exercisable within 60 days of February 28, 2017.
|
|
|
|
|
(4)
|
Includes 40,000 shares of common stock underlying stock options that are exercisable within 60 days of February 28, 2017 and warrants to purchase 723 shares of common stock.
|
|
|
|
|
(5)
|
Includes 11,152 shares of common stock underlying stock options that are exercisable within 60 days of February 28, 2017 and warrants to purchase 525 shares of common stock.
|
|
|
|
|
(6)
|
Includes 4,863 shares of common stock underlying stock options that are exercisable within 60 days of February 28, 2017 and warrants to purchase 291 shares of common stock.
|
|
|
|
|
(7)
|
Includes 10,697 shares of common stock underlying stock options that are exercisable within 60 days of February 28, 2017.
|
|
|
|
|
(8)
|
Includes 5,572 shares of common stock underlying stock options that are exercisable within 60 days of February 28, 2017.
|
|
|
|
|
(9)
|
Includes 5,850 shares of common stock underlying stock options that are exercisable within 60 days of February 28, 2017.
|
|
|
|
|
(10)
|
Includes 2,334 shares of common stock underlying stock options that are exercisable within 60 days of February 28, 2017.
|
|
|
|
|
(11)
|
Includes 2,334 shares of common stock underlying stock options that are exercisable within 60 days of February 28, 2017.
|
|
|
|
|
(12)
|
Includes 97,515 shares of common stock underlying stock options that are exercisable within 60 days of February 28, 2017 and warrants to purchase 1,539 shares of common stock.
|
DESCRIPTION OF CAPITAL STOCK
As of the date of this prospectus, our authorized capital stock consists of 25,000,000 shares of common stock, $0.0001 par value, and 1,000,000 shares of preferred stock, $0.0001 par value. A description of material terms and provisions of our amended and restated certificate of incorporation and amended and restated bylaws affecting the rights of holders of our capital stock is set forth below. The description is intended as a summary, and is qualified in its entirety by reference to our amended and restated certificate of incorporation and our amended and restated bylaws.
Common Stock
The holders of our common stock are entitled to equal dividends and distributions per share with respect to the common stock when, as and if declared by our board of directors from funds legally available therefor. No holder of any shares of our common stock has a preemptive right to subscribe for any of our securities, nor are any common shares subject to redemption or convertible into other securities. Upon liquidation, dissolution or winding-up of our company, and after payment of creditors and preferred stockholders, if any, the assets will be divided pro rata on a share-for-share basis among the holders of the shares of our common stock. All shares of our common stock now outstanding are fully paid, validly issued and non-assessable. Each share of our common stock is entitled to one vote with respect to the election of any director or any other matter upon which stockholders are required or permitted to vote.
On November 18, 2016, we effected a one-for-forty reverse stock split or our common stock. Upon the effectiveness of the reverse stock split (i) every forty shares of our outstanding common stock was combined into one share of common stock, (ii) the number of shares of common stock for each outstanding option or warrant to purchase common stock is exercisable was proportionately adjusted, and (iii) the exercise price of each outstanding option or warrant to purchase common stock was proportionately increased.
Preferred Stock
Our board of directors is authorized, subject to limitations prescribed by Delaware law, to issue up to 1,000,000 shares preferred stock in one or more series, to establish from time to time the number of shares to be included in each series and to fix the designation, powers, preferences and rights of the shares of each series and any of its qualifications, limitations or restrictions. Our board of directors can also increase or decrease the number of shares of any series, but not below the number of shares of that series then outstanding, without any further vote or action by our stockholders. Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the common stock. The issuance of preferred stock, while providing flexibility in connection with financings, possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring, discouraging or preventing a change in control of our company, may adversely affect the market price of our common stock and the voting and other rights of the holders of common stock, and may reduce the likelihood that common stockholders will receive dividend payments and payments upon liquidation.
Warrants
January 2012 Warrants
The following summary description of the material features of the outstanding warrants that we issued in January 2012 is general and is qualified in its entirety by reference to the form of warrant, a copy of which has been filed with the SEC as an exhibit to the registration statement of which this prospectus is a part. See “Where You Can Find More Information.”
Each warrant represents the right to purchase shares of our common stock at an exercise price of $56.40 per share. Each warrant may be exercised after the date of issuance through and including the date that is five years after the date the warrant is first exercisable.
Exercise.
The warrants may be exercised on or prior to the expiration date at the offices of the warrant agent, with the delivery of a written notice in the form attached to the warrant completed and executed as indicated, accompanied by full payment of the exercise price for the number of warrants being exercised in the form discussed below. Within three trading days, certificates representing the shares of our common stock purchased will be delivered to the warrant holder, or at the warrant holder’s request, the warrant shares will be credited to the warrant holder’s account with the Depository Trust Company. The warrants may be exercised in whole or in part.
Payment.
The holder shall pay the exercise price in immediately available funds; provided, however, if at any time there is (i) no effective registration statement registering the relevant common stock and (ii) no effective registration statement registering the resale of or no current prospectus available for the resale of the relevant common stock by the holder, the holder may elect to satisfy its obligation to pay the exercise price through a “cashless exercise.”
Fractional Shares.
No fractional shares will be issued upon exercise of the warrants. If, upon exercise of the warrants, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Limitations on Exercise.
The number of shares of our common stock that may be acquired by a holder upon any exercise of a warrant shall be limited so that the total number of shares of our common stock then beneficially owned by such holder does not exceed 4.99% (subject to increase, not to exceed 9.99%, in certain circumstances) of the total number of issued and outstanding shares of our common stock (including for such purpose the shares of common stock issuable upon such exercise). Our obligation to issue shares of our common stock upon the exercise of a warrant shall be suspended until such time, if any, as shares of our common stock may be issued in compliance with such limitation.
Adjustment.
The exercise price and the number of shares underlying the warrants are subject to appropriate adjustment in the event of stock splits, stock dividends on our common stock, stock combinations or similar events affecting our common stock. In addition, in the event we consummate any merger, consolidation, sale or other reorganization event in which our common stock is converted into or exchanged for securities, cash or other property, then following such event, the holders of the warrants will be entitled to receive upon exercise of such warrants the kind and amount of securities, cash or other property which the holders would have received had they exercised such warrants immediately prior to such reorganization event.
Rights as Stockholders.
The warrant holders do not have the rights or privileges of holders of our common stock and any voting rights until they exercise their warrants and receive shares of our common stock. After the issuance of shares of our common stock upon exercise of the warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
October 2012 Warrants
The following summary description of the material features of the outstanding warrants that we issued in October 2012 is general and is qualified in its entirety by reference to the form of warrant, a copy of which has been filed with the SEC as an exhibit to the registration statement of which this prospectus is a part. See “Where You Can Find More Information.”
Each warrant represents the right to purchase shares of our common stock at an exercise price of $106.00 per share. Each warrant may be exercised after the date of issuance through and including the date that is five years after the date the warrant is first exercisable.
Exercise.
The warrants may be exercised on or prior to the expiration date at the offices of the warrant agent, with the delivery of a written notice in the form attached to the warrant completed and executed as indicated, accompanied by full payment of the exercise price for the number of warrants being exercised in the form discussed below. Within three trading days, certificates representing the shares of our common stock purchased will be delivered to the warrant holder, or at the warrant holder’s request, the warrant shares will be credited to the warrant holder’s account with the Depository Trust Company. The warrants may be exercised in whole or in part.
Payment.
The holder shall pay the exercise price in immediately available funds; provided, however, if at any time there is (i) no effective registration statement registering the relevant common stock and (ii) no effective registration statement registering the resale of or no current prospectus available for the resale of the relevant common stock by the holder, the holder may elect to satisfy its obligation to pay the exercise price through a “cashless exercise.”
Fractional Shares.
No fractional shares will be issued upon exercise of the warrants. If, upon exercise of the warrants, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Limitations on Exercise.
The number of shares of our common stock that may be acquired by a holder upon any exercise of a warrant shall be limited so that the total number of shares of our common stock then beneficially owned by such holder does not exceed 4.99% (subject to increase, not to exceed 9.99%, in certain circumstances) of the total number of issued and outstanding shares of our common stock (including for such purpose the shares of our common stock issuable upon such exercise). Our obligation to issue shares of our common stock upon the exercise of a warrant shall be suspended until such time, if any, as shares of common stock may be issued in compliance with such limitation.
Adjustment.
The exercise price and the number of shares underlying the warrants are subject to appropriate adjustment in the event of stock splits, stock dividends on our common stock, stock combinations or similar events affecting our common stock. In addition, in the event we consummate any merger, consolidation, sale or other reorganization event in which our common stock is converted into or exchanged for securities, cash or other property, then following such event, the holders of the warrants will be entitled to receive upon exercise of such warrants the kind and amount of securities, cash or other property which the holders would have received had they exercised such warrants immediately prior to such reorganization event.
Rights as Stockholders.
The warrant holders do not have the rights or privileges of holders of our common stock and any voting rights until they exercise their warrants and receive shares of our common stock. After the issuance of shares of our common stock upon exercise of the warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
February 2015 Warrants
The following summary description of the material features of the outstanding warrants that we issued in February 2015 is general and is qualified in its entirety by reference to the form of warrant, a copy of which has been filed with the SEC as an exhibit to the registration statement of which this prospectus is a part. See “Where You Can Find More Information.”
Each warrant represents the right to purchase one share of our common stock at an exercise price of $20.00 per share. Each warrant may be exercised after the date of issuance through and including the date that is five years after the date the warrant is first exercisable.
Exercise.
The warrants may be exercised on or prior to the expiration date at the offices of the warrant agent, with the delivery of a written notice in the form attached to the warrant completed and executed as indicated, accompanied by full payment of the exercise price for the number of warrants being exercised in the form discussed below. Within three trading days, certificates representing the shares of our common stock purchased will be delivered to the warrant holder, or at the warrant holder’s request, the warrant shares will be credited to the warrant holder’s account with the Depository Trust Company. The warrants may be exercised in whole or in part.
Payment.
The holder shall pay the exercise price in immediately available funds; provided, however, if at any time there is (i) no effective registration statement registering the relevant common stock and (ii) no effective registration statement registering the resale of or no current prospectus available for the resale of the relevant common stock by the holder, the holder may elect to satisfy its obligation to pay the exercise price through a “cashless exercise.”
Fractional Shares.
No fractional shares will be issued upon exercise of the warrants. If, upon exercise of the warrants, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Limitations on Exercise.
The number of shares of our common stock that may be acquired by a holder upon any exercise of a warrant shall be limited so that the total number of shares of our common stock then beneficially owned by such holder does not exceed 4.99% (subject to increase, not to exceed 9.99%, in certain circumstances) of the total number of issued and outstanding shares of our common stock (including for such purpose the shares of our common stock issuable upon such exercise). Our obligation to issue shares of our common stock upon the exercise of a warrant shall be suspended until such time, if any, as shares of common stock may be issued in compliance with such limitation.
Adjustment.
The exercise price and the number of shares underlying the warrants are subject to appropriate adjustment in the event of stock splits, stock dividends on our common stock, stock combinations or similar events affecting our common stock. The warrants provide for a weighted-average adjustment to the exercise price if we issue or are deemed to issue additional shares of our common stock at a price per share less than the then effective exercise price of the warrants, subject to certain exceptions. In addition, in the event of any fundamental transaction, as described in the warrants and generally including any merger with or into another entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our common stock, then upon any subsequent exercise of a warrant, the holder will have the right to receive as alternative consideration, for each share of our common stock that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of our common stock of the successor or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares of our common stock for which the warrant is exercisable immediately prior to such event. In addition, in the event of a fundamental transaction, we or any successor entity will be required to purchase at a holder’s option, exercisable at any time concurrently with or within thirty (30) days after the consummation of the fundamental transaction, such holder’s warrants for cash in an amount equal to the value of the unexercised portion of such holder’s warrants, determined in accordance with the Black Scholes option pricing model as specified in the warrants.
Rights as Stockholders.
The warrant holders do not have the rights or privileges of holders of our common stock and any voting rights until they exercise their warrants and receive shares of our common stock. After the issuance of shares of our common stock upon exercise of the warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
August 2016 Warrants
The following summary description of the material features of the outstanding base warrants and pre-funded that we issued in August 2016 is general and is qualified in its entirety by reference to each form of warrant, copies of which has been filed with the SEC as an exhibit to the registration statement of which this prospectus is a part. See “Where You Can Find More Information.”
Each base warrant represents the right to purchase one share of our common stock at an exercise price of $7.68 per share. Each pre-funded warrant represents the right to purchase one share of our common stock at an exercise price of $0.40 per share. Each warrant may be exercised after the date of issuance through and including the date that is five years after the date the warrant is first exercisable.
Exercise.
The warrants may be exercised on or prior to the expiration date at the offices of the warrant agent, with the delivery of a written notice in the form attached to the warrant completed and executed as indicated, accompanied by full payment of the exercise price for the number of warrants being exercised in the form discussed below. Within three trading days, certificates representing the shares of our common stock purchased will be delivered to the warrant holder, or at the warrant holder’s request, the warrant shares will be credited to the warrant holder’s account with the Depository Trust Company. The warrants may be exercised in whole or in part.
Payment.
The holder shall pay the exercise price in immediately available funds; provided, however, if at any time there is (i) no effective registration statement registering the relevant common stock and (ii) no effective registration statement registering the resale of or no current prospectus available for the resale of the relevant common stock by the holder, the holder may elect to satisfy its obligation to pay the exercise price through a “cashless exercise.”
Fractional Shares.
No fractional shares will be issued upon exercise of the warrants. If, upon exercise of the warrants, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Limitations on Exercise.
A holder (together with its affiliates) may not exercise any portion of a base warrant or pre-funded warrant to the extent that the holder would own more than 4.99% of the outstanding common stock after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase or decrease the amount of ownership of outstanding stock after exercising the holder’s warrants, as applicable, up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants. No fractional shares of common stock will be issued in connection with the exercise of a warrant. In lieu of fractional shares, we will round down to the next whole share.
Adjustment.
The exercise price of the base warrants is subject to weighted-average adjustment to the exercise price if we issue or are deemed to issue additional shares of our common stock at a price per share less than the then effective exercise price. For purposes of these adjustments, dilutive issuances do not include (i) up to $15,000,000, in the aggregate, of shares of common stock issued or issuable at an effective price per share less than the exercise price then in effect pursuant to (A) our Sales Agreement with Cantor Fitzgerald & Co. or (B) any similar agreement that may be entered into while this base warrant is outstanding, (ii) securities issued pursuant to board-approved equity incentive plans;
provided, however
, issuances to consultants shall not exceed 25,000 shares (to be appropriately adjusted for any stock dividend, stock split, stock combination or other similar transaction), in the aggregate, of common stock or common stock equivalents in any twelve month period, (iii) securities issued in certain strategic transactions, and (iv) securities issued upon the exercise of warrants outstanding prior to the issuance of the base warrants and pre-funded warrants.
Fundamental Transactions
. In the event of any fundamental transaction, as described in the warrants and generally including any merger with or into another entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our common stock, then upon any subsequent exercise of a warrant, the holder will have the right to receive as alternative consideration, for each share of our common stock that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of common stock of the successor or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares of our common stock for which the warrant is exercisable immediately prior to such event. In addition, in the event of a fundamental transaction, we or any successor entity will be required to purchase, at a holder’s option, exercisable at any time concurrently with or within thirty (30) days after the consummation of the fundamental transaction, such holder’s warrants for cash in an amount equal to the value of the unexercised portion of such holder’s warrants, determined in accordance with the Black Scholes option pricing model as specified in the warrants.
Rights as Stockholders.
The warrant holders do not have the rights or privileges of holders of our common stock and any voting rights until they exercise their warrants and receive shares of our common stock. After the issuance of shares of our common stock upon exercise of the warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
Anti-Takeover Provisions
Section 203 of the Delaware General Corporation Law
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
|
|
|
|
•
|
before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder;
|
|
|
|
|
•
|
upon closing of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned by (1) persons who are directors and also officers and (2) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
|
|
|
|
|
•
|
on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder.
|
In general, Section 203 defines business combination to include the following:
|
|
|
|
•
|
any merger or consolidation involving the corporation and the interested stockholder;
|
|
|
|
|
•
|
any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder;
|
|
|
|
|
•
|
subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder;
|
|
|
|
|
•
|
any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or
|
|
|
|
|
•
|
the receipt by the interested stockholder of the benefit of any loss, advances, guarantees, pledges or other financial benefits by or through the corporation.
|
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the person’s affiliates and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A. The transfer agent and registrar’s address is 350 Indiana Street, Suite 750, Golden, Colorado 80401. Its phone number is (303) 262-0600.
Listing on the NYSE MKT
Our common stock is listed on the NYSE MKT under the symbol “IMUC." We do not intend to list the Units, the Preferred Stock or the Series A Warrants on the NYSE MKT, any other national securities exchange or any other nationally recognized trading system. Without an active trading market, the liquidity of the Units, the Preferred Stock and the Series A Warrants will be limited.
DESCRIPTION OF THE SECURITIES WE ARE OFFERING
We are offering an aggregate of up to 10,000 Units, each consisting of (i) one share of our Preferred Stock and (ii) one Series A Warrant to purchase 300 shares of our Common Stock. The Units will not be issued or certificated. The Preferred Stock and Series A Warrants are immediately separable and will be issued separately, but will be purchased together as a unit in this offering.
Common Stock
The material terms and provisions of our common stock and each other class of our securities which qualifies or limits our common stock are described under the caption “Description of Capital Stock” in this prospectus.
Preferred Stock
Our authorized preferred stock consists of 1,000,000 shares. As of December 31, 2016, there were no shares of preferred stock outstanding. The following is a summary of the material terms of the Preferred Stock. This summary is not complete and is qualified in its entirety by reference to the Certificate of Designations and our Certificate of Incorporation. You should review a copy of the Certificate of Designation filed as an exhibit to the registration statement of which this prospectus forms a part for a complete description of the terms and conditions applicable to the Preferred Stock offered by this prospectus.
Form
. The Preferred Stock will be issued in electronic book entry form at closing. Investors will be permitted to request the issuance of individual stock certificates through their brokers after closing. Delivery of paper certificates is expected to take between 4 and 10 business days following request.
Amount of Preferred Stock Shares
. Each purchaser of Units will receive one share of Preferred Stock for each Unit purchased. Each share of Preferred Stock has a par value of $0.0001 and an initial stated value of $1,000 (subject to adjustment).
Ranking
. The Preferred Stock will rank senior to our Common Stock and other classes of capital stock with respect to dividend, redemption and distributions of assets upon liquidation, dissolution or winding up, unless the holders of a majority of the outstanding shares of Preferred Stock consent to the creation of parity stock or senior preferred stock.
Voting Rights
. With certain exceptions, as described in the Certificate of Designation, the Preferred Shares have no voting rights. However, as long as any shares of Preferred Shares remain outstanding, we may not, without the affirmative vote of holders of a majority of the then outstanding shares of Preferred Stock: (a) alter or change adversely the powers, preferences or rights given to the Preferred Stock or alter or amend the Certificate of Designation; (b) authorize or create any class of stock ranking as to dividends, redemption or distribution of assets upon liquidation, dissolution or winding up senior to, or otherwise pari passu with, the Preferred Stock; (c) amend our articles of incorporation or other charter documents in any manner that adversely affects any rights of the holders of Preferred Stock; (d) increase the number of authorized shares of Preferred Stock; or (e) enter into any agreement with respect to any of the foregoing.
Liquidation Preference
. Upon our voluntary or involuntary liquidation, dissolution or winding up, before the payment of any amount to the holder of shares of junior stock, but pari passu with any parity stock, the holders of Preferred Stock are entitled to receive an amount equal to the stated value of the Preferred Stock plus any accrued but unpaid dividends thereon and all liquidated damages and other amounts then due and owing. If there are insufficient assets to pay in full such amounts, then the available assets shall be ratably distributed to the holders of Preferred Stock in accordance with the respective amounts that would be payable on such shares if all amounts payable thereon were paid in full. The Certificate of Designation does not provide for any restriction on the repurchase of Preferred Shares by us while there is an arrearage in the payment of dividends of the Preferred Shares.
Dividends
. Holders of Preferred Stock are entitled to receive cumulative dividends at the rate of 6.0% per annum, payable quarterly on January 1, April 1, July 1 and October 1, beginning on the first such date after the original issue date and on each
conversion date to the converting holder. We have the right to pay dividends in cash or in shares of Common Stock on the Maturity Date. If we do not have funds legally available to pay cash dividends, such dividends accrete to and increase the outstanding stated value of the Preferred Stock and will be considered fully paid and no longer accrued and unpaid dividends. Any dividends that are not paid within three trading days following the applicable payment date other than dividends that accrete to and increase the stated value shall continue to accrue and shall incur a late fee at the rate of 18% per annum or the lesser maximum rate permitted by applicable law, which shall accrue daily from the applicable payment date through and including the date of actual payment in full. There are no sinking fund provisions applicable to the Preferred Stock.
Voluntary Conversion
. Each share of Preferred Stock is convertible at any time from and after the original issue date following the date of issuance, at the holder’s option into a number of shares of Common Stock equal to its then stated value divided by the conversion price.
Mandatory Conversion
. If after the effective date of the registration statement to which this prospectus forms a part (the “Effective Date”) (i) the VWAP for each of any 10 consecutive trading day period, which 10 consecutive trading day period shall have commenced only after the Effective Date (“Threshold Period”), exceeds 300% of the then effective conversion price and (ii) the volume for each trading day during any Threshold Period exceeds $300,000 per trading day, we will have the right to cause each holder of Preferred Stock to convert all or part of such holder’s Preferred Stock, plus all accrued but unpaid dividends thereon and all liquidated damages and other amounts then due and owing, to shares the Preferred Stock, subject to the satisfaction of certain equity and other conditions set forth in the Certificate of Designation.
Limitations on Conversion
. A holder of Preferred Stock may not convert shares of Preferred Stock and we may not issue shares of Common Stock under the Preferred Stock if, after giving effect to the conversion or issuance, the holder together with its affiliates would beneficially own in excess of 4.99% of the outstanding shares of our Common Stock.
Conversion Price
. The conversion price for the Preferred Stock shall be equal to the lesser of: (a) the Set Price of $ , subject to certain adjustments and a floor; and (b) 87.5% of the lowest volume weighted average price for our Common Stock as reported at the close of trading on the market reporting trade prices for the Common Stock during the five trading days ending on, and including, the date that a notice of conversion is tendered to us. In connection with a mandatory conversion by us, the conversion price shall be the lesser of: (y) the then Set Price; and (z) 87.5% of the lowest volume weighted average price for our Common Stock as reported at the close of trading on the market reporting trade prices for the Common Stock during the five trading days ending on the date that we provide notice of the conversion. In no event shall the conversion price be less than $1.00. The Set Price is also subject to adjustment for stock splits, stock dividends, distributions of Common Stock or securities convertible, exercisable or exchangeable for Common Stock, subdivisions, combinations and reclassifications. Further, The Set Price is subject to adjustment if we issue or are deemed to issue additional shares of our Common Stock at a price per share less than the then effective Set Price. For purposes of these adjustments, dilutive issuances do not include (i) up to $15,000,000, in the aggregate, of shares of Common Stock issued or issuable at an effective price per share less than the exercise price then in effect pursuant to (A) our Sales Agreement with Cantor Fitzgerald & Co. or (B) any similar agreement that may be entered into while the Series A Warrants are outstanding, (ii) securities issued pursuant to board-approved equity incentive plans;
provided, however
, issuances to consultants shall not exceed 1,000,000 shares (to be appropriately adjusted for any stock dividend, stock split, stock combination or other similar transaction), in the aggregate, of Common Stock or Common Stock equivalents in any twelve month period, (iii) securities issued in certain strategic transactions, and (iv) securities issued upon the exercise of warrants outstanding prior to the issuance of the Preferred Stock.
Purchase Rights
. Holders of Preferred Stock are entitled to acquire our securities or rights to purchase our securities or property granted, issued or sold pro rata to the holders of our Common Stock on an “as if exercised for Common Stock” basis.
Rights Upon Distribution
. Holders of Preferred Stock are entitled to receive any dividend or other distribution of our assets (or rights to acquire our assets) declared or made to holders of our Common Stock on an “as if exercised for Common Stock” basis.
Fundamental Transactions
. In the event of any fundamental transaction, as described in the Certificate of Designations and generally including any merger with or into another entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our common stock, then upon any subsequent conversion of the Preferred Stock, the holder will have the right to receive as alternative consideration, for each share of our common stock that would have been issuable upon such conversion immediately prior to the occurrence of such fundamental transaction, the number of shares of common stock of the successor or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares of our common stock for which the Preferred Stock is convertible immediately prior to such event.
Market and Exchange Listing
. The Preferred Stock is a new issue of securities and currently there is no market for the securities. We do not intend to list or qualify for quotation the Preferred Stock on any securities exchange or market.
Negative Covenants
. So long as any Preferred Stock is outstanding, without the consent of holders of at least 51% of the stated value of the then outstanding Preferred Stock, we may not (a) other than Permitted Indebtedness as described in the Certificate of Designations, enter into, create, incur, assume, guarantee or suffer to exist any indebtedness, (b) other than Permitted Liens as described in the Certificate of Designations, enter into, create, incur, assume or suffer to exist any Liens of any kind, on or with respect to any of its property or assets, (c) amend our charter documents in any manner that materially and adversely affects any rights of the holders of Preferred Stock, (d) repay, repurchase or offer to repay, repurchase or otherwise acquire more than a de minimis number of shares of our Common Stock and other junior securities, subject to certain exceptions, (e) pay cash dividends or make distribution on junior securities, (f) enter into certain affiliate transactions, and (g) enter into any agreement with respect to any of the foregoing.
Series A Warrants
The following is a brief summary of certain terms and conditions of the Series A Warrants offered by this prospectus and is subject in all respects to the provisions contained in the Series A Warrant, a form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. As of the date of this prospectus, there are no Series A Warrants outstanding. You should review a copy of the attached form of Series A Warrant for a complete description of the terms and conditions applicable to the warrant offered by this prospectus.
Amount of Series A Warrant Shares
. A Series A Warrant to purchase 300 shares of Common Stock will be issued for every one share of Preferred Stock sold in this offering.
Duration and Exercise Price
.
Each Series A Warrant offered hereby will have an exercise price of $ per share, which is equal to the last reported sale price of our common stock as of the close of the trading day immediately preceding the pricing of this offering. The Series A Warrants will be immediately exercisable and will expire on the fifth anniversary of the original issuance date. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price.
Exercisability
. The Series A Warrants will be exercisable immediately after closing of this offering, with the delivery of a written notice in the form attached to the warrant completed and executed as indicated, accompanied by full payment of the exercise price for the number of Series A Warrants being exercised (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of a Series A Warrant to the extent that the holder would own more than 4.99% of the outstanding common stock after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase or decrease the amount of ownership of outstanding stock after exercising the holder’s warrants, as applicable, up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants. No fractional shares of common stock will be issued in connection with the exercise of a warrant. In lieu of fractional shares, we will round down to the next whole share. The Series A Warrants may be exercised in whole or in part.
Cashless Exercise
. If, at the time a holder exercises its warrant, there is no effective registration statement registering, or the prospectus contained therein is not available for an issuance of the shares underlying the warrant to the holder, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise
price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the warrant.
Payment.
The holder shall pay the exercise price in immediately available funds; provided, however, if at any time there is (i) no effective registration statement registering the relevant common stock and (ii) no effective registration statement registering the resale of or no current prospectus available for the resale of the relevant common stock by the holder, the holder may elect to satisfy its obligation to pay the exercise price through a “cashless exercise.”
Fractional Shares.
No fractional shares will be issued upon exercise of the Series A Warrants. If, upon exercise of the Series A Warrants, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Limitations on Exercise.
A holder (together with its affiliates) may not exercise any portion of a Series A Warrant to the extent that the holder would own more than 9.99% of the outstanding common stock after exercise.
Adjustment.
The exercise price of the Series A Warrants is subject to weighted-average adjustment to the exercise price if we issue or are deemed to issue additional shares of our Common Stock at a price per share less than the then effective exercise price. For purposes of these adjustments, dilutive issuances do not include (i) up to $15,000,000, in the aggregate, of shares of Common Stock issued or issuable at an effective price per share less than the exercise price then in effect pursuant to (A) our Sales Agreement with Cantor Fitzgerald & Co. or (B) any similar agreement that may be entered into while this base warrant is outstanding, (ii) securities issued pursuant to board-approved equity incentive plans;
provided, however
, issuances to consultants shall not exceed 1,000,000 shares (to be appropriately adjusted for any stock dividend, stock split, stock combination or other similar transaction), in the aggregate, of Common Stock or Common Stock equivalents in any twelve month period, (iii) securities issued in certain strategic transactions, and (iv) securities issued upon the exercise of warrants outstanding prior to the issuance of the Series A Warrants.
Fundamental Transactions
. In the event of any fundamental transaction, as described in the Series A Warrants and generally including any merger with or into another entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our Common Stock, then upon any subsequent exercise of a Series A Warrant, the holder will have the right to receive as alternative consideration, for each share of our common stock that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of common stock of the successor or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares of our common stock for which the warrant is exercisable immediately prior to such event. In addition, in the event of a fundamental transaction, we or any successor entity will be required to purchase, at a holder’s option, exercisable at any time concurrently with or within thirty (30) days after the consummation of the fundamental transaction, such holder’s warrants for cash in an amount equal to the value of the unexercised portion of such holder’s warrants, determined in accordance with the Black Scholes option pricing model as specified in the warrants.
Rights as Stockholders.
The warrant holders do not have the rights or privileges of holders of our Common Stock and any voting rights until they exercise their warrants and receive shares of our common stock. After the issuance of shares of our common stock upon exercise of the warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
Transferability
. Subject to applicable laws and the restriction on transfer set forth in the warrant, the warrant may be transferred at the option of the holder upon surrender of the warrant to us together with the appropriate instruments of transfer.
Waivers and Amendments
. Subject to certain exceptions, any term of the warrants may be amended or waived with our written consent and the written consent of the holders of at least a majority of the then-outstanding warrants.
Market and Exchange Listing
. The Series A Warrants are a new issue of securities and currently there is no market for the securities. We do not intend to list or qualify for quotation the Series A Warrants on any securities exchange or market.
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following is a general discussion of the material U.S. federal income considerations relating to the purchase, ownership and disposition of our common stock, Series B Preferred Stock or warrants. This discussion is based on current provisions of the Internal Revenue Code of 1986 (the “Code”), existing and proposed U.S. Treasury Regulations promulgated or proposed thereunder and current administrative and judicial interpretations thereof, all as in effect as of the date of this prospectus and all of which are subject to change or to differing interpretation, possibly with retroactive effect. We have not sought and will not seek any rulings from the Internal Revenue Service (the “IRS”) regarding the matters discussed below. There can be no assurance that the IRS or a court will not take a contrary position.
This discussion is limited to U.S. holders and non-U.S. holders who hold our common stock, Series B Preferred Stock or warrants as capital assets within the meaning of Section 1221 of the Code (generally, as property held for investment). This discussion does not address all aspects of U.S. federal income taxation, such as the U.S. alternative minimum income tax and the Medicare contribution tax on net investment income, nor does it address any aspect of state, local or non-U.S. taxes, or U.S. federal taxes other than income taxes, such as federal estate taxes. This discussion does not consider any specific facts or circumstances that may apply to a holder and does not address the special tax considerations that may be applicable to particular holders, such as:
|
|
|
|
•
|
tax-exempt organizations;
|
|
|
|
|
•
|
financial institutions;
|
|
|
|
|
•
|
brokers or dealers in securities;
|
|
|
|
|
•
|
traders in securities that elect to use a mark-to-market method of accounting;
|
|
|
|
|
•
|
regulated investment companies;
|
|
|
|
|
•
|
real estate investment trusts;
|
|
|
|
|
•
|
controlled foreign corporations;
|
|
|
|
|
•
|
passive foreign investment companies;
|
|
|
|
|
•
|
corporations that accumulate earnings to avoid U.S. federal income tax;
|
|
|
|
|
•
|
certain U.S. expatriates;
|
|
|
|
|
•
|
persons that have a “functional currency” other than the U.S. dollar;
|
|
|
|
|
•
|
persons that acquire our common stock as compensation for services;
|
|
|
|
|
•
|
persons deemed to sell the stock or the warrants under the constructive sale provisions of the Code;
|
|
|
|
|
•
|
owners that hold our common stock, Series B Preferred Stock or warrants as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment; and
|
|
|
|
|
•
|
partnerships or other entities treated as partnerships for U.S. federal income tax purposes.
|
If any entity taxable as a partnership for U.S. federal income tax purposes holds our common stock, Series B Preferred Stock or warrants, the U.S. federal income tax treatment of a partner in the partnership generally will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. A partner in a partnership or other pass-through entity that holds our common stock, Series B Preferred Stock or warrants should consult his, her or its own tax advisor regarding the applicable tax consequences.
For purposes of this discussion, the term “U.S. holder” means a beneficial owner of our common stock, Series A Preferred Stock or warrants that is, for U.S. federal income tax purposes:
|
|
|
|
•
|
an individual who is a citizen or resident of the United States;
|
|
|
|
|
•
|
a corporation created or organized in or under the laws of the United States or of any political subdivision of the United States;
|
|
|
|
|
•
|
a corporation created or organized in or under the laws of the United States or of any political subdivision of the United States;
|
|
|
|
|
•
|
a corporation created or organized in or under the laws of the United States or of any political subdivision of the United States;
|
|
|
|
|
•
|
a trust, if (1) a U.S. court is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust or (2) the trust has a valid election to be treated as a U.S. person under applicable U.S. Treasury Regulations.
|
A “non-U.S. holder” is a beneficial owner of our common stock, Series B Preferred Stock or warrants that is not a U.S. holder or an entity taxable as a partnership for U.S. federal income tax purposes.
Prospective investors should consult their own tax advisors regarding the U.S. federal, state, local and non-U.S. income and other tax considerations of purchasing, holding and disposing of our common stock, Series B Preferred Stock or warrants.
U.S. Holders
Purchase of Units
For U.S. federal income tax purposes, the purchase of a Unit will be treated as the purchase of two components: a component consisting of one share of our Series B Preferred Stock and a component consisting of one warrant to purchase 300 shares of our common stock. The purchase price for each Unit will be allocated between its components in proportion to the relative fair market value of each at the time the Unit is purchased by the holder. This allocation of the purchase price for each Unit will establish a holder’s initial tax basis for U.S. federal income tax purposes in the shares and warrants that compose each Unit.
Exercise of Warrants
A U.S. holder generally will not recognize gain or loss on the exercise of a warrant and related receipt of shares of our common stock (except to the extend that cash is received in lieu of the issuance of a fractional share of our common stock). A U.S. holder’s initial tax basis in the shares of our common stock received (including the fractional share deemed received) upon the exercise of a warrant will be equal to the sum of (a) such U.S. holder’s tax basis in such warrant plus (b) the exercise price paid by such U.S. holder on the exercise of such warrant. A U.S. holder’s holding period for the shares of our common stock received upon the exercise of a warrant will begin on the day after the date that the warrant is exercised.
In certain limited circumstances, a U.S. holder may be permitted to undertake a cashless exercise of warrants into shares of our common stock. The U.S. federal income tax treatment of a cashless exercise of warrants into shares of common stock is unclear, and the tax consequences of a cashless exercise could differ from the consequences upon the exercise of a warrant described in the preceding paragraph. U.S. holders should consult their own tax advisors regarding the U.S. federal income tax consequences of a cashless exercise of warrants.
Certain Adjustments to the Warrants or Series B Preferred Stock
An adjustment to the number of shares of our common stock that will be issued upon the exercise of a warrant or conversion of a share of Series B Preferred Stock, or an adjustment to the exercise price of a warrant, may be treated as a constructive distribution to a U.S. holder of the warrant or share depending on the circumstances of such adjustment. In addition, the failure to provide for such an adjustment (or to adequately adjust) may also result in a deemed distribution to U.S. holders of our Series B Preferred Stock or the warrants. Any such constructive distribution would be taxable whether or not there is an actual distribution of cash or other property. However, adjustments to the exercise price of warrants or conversion price of Series B Preferred Stock made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the holders thereof generally should not be considered to result in a constructive distribution. Generally, such deemed distributions will be taxable in the same manner as an actual distribution as described below under "—Distributions on Common Stock or Series B Preferred Stock," below, except that it is unclear whether such deemed distributions would be eligible for the reduced tax rate applicable to certain dividends paid to non-corporate holders or the dividend-received deduction applicable to certain dividends paid to corporate holders. You should consult your tax advisor as to whether such
deemed distributions are eligible for dividends-received deduction or the preferential rate's applicable to certain dividends. Generally, a U.S. holder's tax basis in the underlying stock will be increased to the extent any such constructive distribution is treated as a dividend.
Expiration of the Warrants without Exercise
Upon the lapse or expiration of a warrant, a U.S. holder will recognize a loss in an amount equal to such U.S. holder’s tax basis in the warrant. Any such loss generally will be a capital loss and will be long-term capital loss if the warrant is held for more than one year. Deductions for capital losses are subject to significant limitations.
Conversion of Series B Preferred Stock
A U.S. holder generally will not recognize gain or loss upon the conversion of a share of Series B Preferred Stock into common stock (except to the extent that cash is received in lieu of the issuance of a fractional share of our common stock). A U.S. holder’s initial tax basis in the shares of our common stock received upon the conversion of a share of Series B Preferred Stock (including fractional share deemed received) will be equal to such U.S. holder’s tax basis in the share of Series B Preferred Stock (reduced by the basis of any fractional share paid out in cash). A U.S. holder’s holding period for the shares of our common stock received upon the conversion of a share of Series B Preferred Stock will include the U.S. holder’s holding period in such share of Series B Preferred Stock.
Treatment of Fractional Shares
A U.S. holder who receives cash in lieu of a fractional share of our common stock upon the exercise of the warrants or the conversion of the Series B Preferred Stock will allocate the tax basis in the common stock received in the exercise or the conversion, as applicable, as described above over all of the shares of common received including the fractional share deemed received and will recognize capital gain or loss in the amount of the difference between the cash received and the tax basis allocated to the fractional share. Such gain or loss will be long-term capital gain or loss if at the time of the exercise or the conversion, as applicable, the Series B Preferred Stock or warrants have been held by the U.S. holder for more than one year. Preferential tax rates may apply to long-term capital gain of a U.S. holder that is an individual, estate or trust. Deductions for capital losses are subject to significant limitations.
Distributions on Common Stock or Series B Preferred Stock
If we pay distributions of cash or property (including our common stock) with respect to our common stock or Series B Preferred Stock (including constructive distributions as described above under the heading “Certain Adjustments to the Warrants or Series B Preferred Stock”), those distributions generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a tax-free return of the U.S. holder’s investment, up to such holder’s tax basis in its shares of our common stock or Series B Preferred Stock. Any remaining excess will be treated as capital gain, subject to the tax treatment described below under the heading “—Gain on Sale, Exchange or Other Taxable Disposition.” Dividends received by a corporate U.S. holder may be eligible for the dividends received deduction, and dividends received by non-corporate U.S. holders generally will be subject to tax at the current lower applicable capital gains rates, provided, in each case, that certain holding period and other applicable requirements are satisfied.
Gain on Sale, Exchange or Other Taxable Disposition
Upon the sale or other taxable disposition of common shares, Series B Preferred Stock or warrants, a U.S. holder generally will recognize capital gain or loss in an amount equal to the difference between (a) the amount of cash plus the fair market value of any property received and (b) such U.S. holder’s tax basis in such common shares, Series B Preferred Stock or warrants sold or otherwise disposed of. Such gain or loss generally will be long-term capital gain or loss if, at the time of the sale or other disposition, the common shares, Series B Preferred Stock or warrants have been held by the U.S. holder for more than one year. Preferential tax rates may apply to long-term capital gain of a U.S. holder that is an individual, estate or trust. Deductions for capital losses are subject to significant limitations.
Non-U.S. Holders
Distributions on Common Stock or Series B Preferred Stock
If we pay distributions of cash or property (including our common stock) with respect to our common stock or Series B Preferred Stock (including constructive distributions as described above under the heading “Certain Adjustments to the Warrants or Series B Preferred Stock”), those distributions generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a tax-free return of the non-U.S. holder’s investment, up to such holder’s tax basis in its shares of our common stock or Series B Preferred Stock. Any remaining excess will be treated as capital gain, subject to the tax treatment described below under the heading “—Gain on Sale, Exchange or Other Taxable Disposition.” Dividends paid to a non-U.S. holder generally will be subject to withholding of U.S. federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence. In the case of any constructive distribution, it is possible that this tax would be withheld from any amount owed to the non-U.S. holder, including, but not limited to, distributions of cash, common stock or sales proceeds subsequently paid or credited to that holder. If we are unable to determine, at the time of payment of a distribution, whether the distribution will constitute a dividend, we may nonetheless choose to withhold any U.S. federal income tax on the distribution as permitted by U.S. Treasury Regulations.
Distributions that are treated as effectively connected with a trade or business conducted by a non-U.S. holder within the United States (and if an income tax treaty applies and so requires, such distribution is attributable to a permanent establishment or fixed base maintained by such non-U.S. holder in the United States) are generally not subject to the 30% withholding tax if the non-U.S. holder provides a properly executed IRS Form W-8ECI (which should be reviewed periodically) stating that the distributions are not subject to withholding because they are effectively connected with the non-U.S. holder’s conduct of a trade or business in the United States. If a non-U.S. holder is engaged in a trade or business in the United States and the distribution is effectively connected with the conduct of that trade or business, the distribution will generally have the consequences described above for a U.S. holder (subject to any modification provided under an applicable income tax treaty). Any U.S. effectively connected income received by a non-U.S. holder that is treated as a corporation for U.S. federal income tax purposes may also, under certain circumstances, be subject to an additional “branch profits tax” at a 30% rate (or such lower rate as may be specified by an applicable income tax treaty).
A non-U.S. holder who claims the benefit of an applicable income tax treaty between the United States and such holder’s country of residence generally will be required to provide a properly executed IRS Form W-8BEN or W-8BEN-E, as applicable (which should be reviewed periodically), and satisfy applicable certification and other requirements. A non-U.S. holder that is eligible for a reduced rate of U.S. withholding tax under an income tax treaty generally may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim with the IRS. Non-U.S. holders should consult their own tax advisors regarding their entitlement to benefits under a relevant income tax treaty.
Distributions on our common stock or Series B Preferred Stock paid to a non-U.S. holder may also be subject to the withholding described under "—The Foreign Account Tax Compliance Act" below.
Gain on Sale, Exchange or Other Taxable Disposition
Subject to the discussion below in “—Information Reporting and Backup Withholding” and “—Foreign Account Tax Compliance Act,” a non-U.S. holder generally will not be subject to U.S. federal income tax on gain recognized on a sale, exchange or other taxable disposition of our common stock, Series B Preferred Stock or warrants unless:
|
|
|
|
•
|
the gain is effectively connected with the non-U.S. holder’s conduct of a trade or business in the United States and, if an applicable income tax treaty so provides, the gain is attributable to a permanent establishment or fixed base maintained by the non-U.S. holder in the United States; in these cases, the non-U.S. holder will be taxed on a net income basis at the regular graduated rates and in the manner applicable to a U.S. holder, and, if the non-U.S. holder is a corporation, an additional branch profits tax at a rate of 30%, or a lower rate as may be specified by an applicable income tax treaty, may also apply;
|
|
|
|
|
•
|
the non-U.S. holder is an individual present in the United States for 183 days or more in the taxable year of the disposition and certain other conditions are met, in which case the non-U.S. holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty) on the amount by which such non-U.S. holder’s capital gains allocable to U.S. sources exceed capital losses allocable to U.S. sources during the taxable year of the disposition; or
|
|
|
|
|
•
|
we are or were a “U.S. real property holding corporation” during the shorter of the five-year period ending on the date of the disposition or the period that the non-U.S. holder held our common stock or Series B Preferred Stock. Generally, a corporation is a “U.S. real property holding corporation” if the fair market value of its “U.S. real property interests” (within the meaning of the Code) equals or exceeds 50% of the sum of the fair market value of its
|
worldwide real property interests plus its other assets used or held for use in a trade or business. We believe that we are not currently, and we do not anticipate becoming, a “U.S. real property holding corporation” for U.S. federal income tax purposes. Even if we are treated as a U.S. real property holding corporation, gain realized by a non-U.S. holder on a disposition of our common stock will not be subject to U.S. federal income tax so long as (1) the non-U.S. holder owned, directly, indirectly and constructively, no more than five percent of our common stock at all times within the shorter of (i) the five-year period preceding the disposition or (ii) the holder's holding period and (2) our common stock is regularly traded on an established securities market. There can be no assurance that our common stock will continue to qualify as regularly traded on an established securities market. Disposition by a non-U.S. holder of our Series B Preferred Stock and the warrants (that are not expected to be regularly traded on an established securities market) may also be eligible for an exemption from withholding even if we are treated as a U.S. real property holding corporation, if on the date such Series B Preferred Stock and/or warrants were acquired by such non-U.S. holder such holdings had a fair market value no greater than the fair market value on that date of five percent of our regularly-traded common stock, provided that, if a non-U.S. holder holding our not regularly-traded Series B Preferred Stock and warrants subsequently acquires additional such securities, then such interests would be aggregated and valued as of the date of the subsequent acquisition in order to apply this five percent limitation. Non-U.S. holder should consult their tax advisors.
Dividend Equivalents
Section 871(m) of the Code requires withholding (up to 30%, depending on whether a treaty applies) on certain financial instruments to the extent that the payments or deemed payments on the financial instruments are contingent upon or determined by reference to U.S.-source dividends. Under U.S. Treasury regulations promulgated under Section 871(m), certain payments or deemed payments to non-U.S. holders with respect to certain equity-linked instruments ("specified ELIs") that reference U.S. stocks may be treated as dividend equivalents ("dividend equivalents") that are subject to U.S. withholding tax at a rate of 30% (or lower treaty rate). Under these regulations, withholding may be required even in the absence of any actual dividend related payment or adjustment made pursuant to the terms of the instrument. The regulations as originally adopted were to be effective for all covered contracts and instruments issued on or after January 1, 2017. In December 2016, the IRS and Treasury issued guidance which granted temporary transitional relief from the requirements of the regulations in certain circumstances and announced prospective changes to the regulations. Under such guidance Section 871(m) will apply to "delta-one" transactions only for 2017. The application of Section 871(m) to non-delta-one transactions is delayed and Section 871(m) will apply only to non-delta one transactions issued on or after January 1, 2018. A "delta one" instrument is one in which the ratio of the change in the fair market value of the instrument to the change in the fair market value of the property referenced by the contract is equal to 1.00. We do not believe that the Series B Preferred Stock or the warrants are delta one instruments. Accordingly, non-U.S. holders of the Series B Preferred Stock or the warrants should not be subject to tax under Section 871(m). However, it is possible that Section 871(m) withholding tax could apply to the Series B Preferred Stock or the warrants under these rules in the future if either the Series B Preferred Stock or the warrants were amended and thereby deemed reissued after January 1, 2018 or the Section 871(m) regulations when further amended by the IRS are broader in scope than is anticipated under the current IRS and U.S. Treasury guidance. If withholding is required, we (or the applicable paying agent) would be entitled to withhold such taxes without being required to pay any additional amounts with respect to amounts so withheld. Non-U.S. Holders should consult with their tax advisors regarding the application of Section 871(m) and the regulations thereunder in respect of their acquisition and ownership of the Series B Preferred Stock and the warrants.
Information Reporting and Backup Withholding
Distributions on, and the payment of the proceeds of a disposition of, our common stock, Series B Preferred Stock or warrants generally will be subject to information reporting if made within the United States or through certain U.S.-related financial intermediaries. Information returns are required to be filed with the IRS and copies of information returns may be made available to the tax authorities of the country in which a holder resides or is incorporated under the provisions of a specific treaty or agreement.
Backup withholding may also apply if the holder fails to provide certification of exempt status or a correct U.S. taxpayer identification number and otherwise comply with the applicable backup withholding requirements. Generally, a holder will not be subject to backup withholding if it provides a properly completed and executed IRS Form W-9 or appropriate IRS Form W-8, as applicable. Backup withholding is not an additional tax. Amounts withheld under the backup withholding rules may be refunded or credited against the holder’s U.S. federal income tax liability, if any, provided certain information is timely filed with the IRS.
Foreign Account Tax Compliance Act
Legislation commonly known as the Foreign Account Tax Compliance Act, or FATCA, and guidance issued thereunder may impose withholding taxes on certain types of payments made to "foreign financial institutions" (as specifically defined) and certain other non-U.S. entities (including financial intermediaries). Under FATCA, failure to comply with certification, information reporting and other specified requirements could result in withholding tax being imposed on payments of dividends and sales proceeds of any property of a type which can produce U.S. source dividends to foreign intermediaries and certain non-U.S. holders. FATCA imposes a 30%withholding tax on dividends or gross proceeds from the sale or other disposition of the Series B Preferred Stock and the shares of our common stock issuable on conversion of such Series B Preferred Stock or exercise of such warrants paid to a foreign financial institution or to anon-financial foreign entity, unless (i) the foreign financial institution undertakes certain diligence and reporting obligations, (ii) the non- financial foreign entity either certifies it does not have any "substantial United States owners" as specifically defined in FATCA or furnishes identifying information regarding each substantial United States owner or (iii) the foreign financial institution or the non-financial foreign entity qualifies for an exemption from the withholding tax. We intend to treat the warrants as also subject to FATCA. If the payee is a foreign financial institution, in the absence of any applicable exemption, it must enter into an agreement with the United States Treasury requiring, among other things, that it undertake to identify accounts held by certain United States persons or United States-owned foreign entities, annually report certain information about such accounts, and withhold 30% on "withholdable payments" (as specifically defined) made to certain account holders. An intergovernmental agreement between the United States and the foreign entity's jurisdiction may modify these requirements. Under certain transition rules, any obligation to withhold under FATCA with respect to payments of dividends on the Series B Preferred Stock and our dividends on common stock is currently in effect, but with respect to the gross proceeds of a sale or other disposition of the Series B Preferred Stock, the warrants or our common stock the obligation to withhold under FATCA will not begin until January 1, 2019. Prospective investors should consult their tax advisors regarding the implications of FATCA with respect to an investment in the Units.
UNDERWRITING
We have entered into an underwriting agreement with Maxim Group LLC with respect to the Units subject to this offering. Subject to certain conditions, we have agreed to sell to the underwriter, and the underwriter has agreed to purchase, the number of Units provided below opposite its name.
|
|
|
|
|
|
|
|
|
|
|
Underwriter
|
|
Number of Units
|
|
Maxim Group LLC
|
|
|
|
Total
|
|
|
The underwriter is offering the Units subject to its acceptance of the Units from us and subject to prior sale. The underwriting agreement provides that the obligations of the underwriter to pay for and accept delivery of the Units offered by this prospectus are subject to the approval of certain legal matters by its counsel and to certain other conditions. The underwriter is obligated to take and pay for all of the Units if any such Units are taken.
The underwriter has advised us that it proposes to offer the Units to the public at the respective public offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per Unit. After this offering, the public offering price and concession to dealers may be changed by the representative. No such change shall change the amount of proceeds to be received by us as set forth on the cover page of this prospectus. The Units are offered by the underwriter as stated herein, subject to receipt and acceptance by it and subject to its right to reject any order in whole or in part. The underwriter has informed us that it does not intend to confirm sales to any accounts over which it exercises discretionary authority.
Discounts and Expenses
The following table summarizes the public offering price, underwriting discount and proceeds before expenses to us:
|
|
|
|
|
|
|
|
|
Per Unit
|
|
Total
|
|
Public offering price
|
$
|
|
$
|
|
Underwriting discount
|
$
|
|
$
|
|
Proceeds to us (before expenses)
|
$
|
|
$
|
In addition, we have agreed to reimburse the underwriters for reasonable out-of-pocket expenses not to exceed $60,000 in the aggregate. We estimate that total expenses payable by us in connection with this offering, other than the underwriting discount referred to above, will be approximately $ .
We have also agreed to give the underwriter a right of first refusal to act as our agent in any subsequent financing for six months following the commencement of sales in this offering.
Our Common Stock is currently traded on the NYSE MKT under the symbol “IMUC.” On March 29, 2017 the closing price of our Common Stock was $3.08 per share.
The public offering price of the securities offered by this prospectus will be determined by negotiation between us and the underwriter. Among the factors considered in determining the public offering price of the shares were:
|
|
|
|
•
|
our history and our prospects;
|
|
|
|
|
•
|
the industry in which we operate;
|
|
|
|
|
•
|
our past and present operating results;
|
|
|
|
|
•
|
the previous experience of our executive officers; and
|
|
|
|
|
•
|
the general condition of the securities markets at the time of this offering.
|
The offering price stated on the cover page of this prospectus should not be considered an indication of the actual value of the securities sold in this offering. That price is subject to change as a result of market conditions and other factors and we cannot assure you that the securities sold in this offering can be resold at or above the public offering price.
Indemnification
We have agreed to indemnify the underwriter against certain liabilities, including liabilities under the Securities Act of 1933, as amended or to contribute to payments that the underwriter may be required to make for these liabilities.
Lock-up Agreements
We, our officers, directors and certain of our stockholders have agreed, subject to limited exceptions, for a period of 90 days after the date of the underwriting agreement, not to offer, sell, contract to sell, pledge, grant any option to purchase, make any short sale or otherwise dispose of, directly or indirectly any shares of Common Stock or any securities convertible into or exchangeable for our Common Stock either owned as of the date of the underwriting agreement or thereafter acquired without the prior written consent of the underwriter. After 30 days following the date of this prospectus, the foregoing restrictions will not apply to shares that may be sold by us pursuant to our Sales Agreement with Cantor Fitzgerald & Co., as agent, or shares that may be sold by our officers and directors pursuant to trading plans established pursuant to Rule 10b5-1 under the Securities Exchange Act of 1934, as amended, that are in place as of the date of this prospectus. The underwriter may, in its sole discretion and at any time or from time to time before the termination of the lock-up period, without notice, release all or any portion of the securities subject to lock-up agreements.
Price Stabilization, Short Positions and Penalty Bids
The underwriter has advised us that it does not intend to conduct any stabilization or over-allotment activities in connection with this Offering.
Electronic Distribution
This prospectus in electronic format may be made available on websites or through other online services maintained by the underwriter, or by its affiliates. Other than this prospectus in electronic format, the information on the underwriter’s website and any information contained in any other website maintained by the underwriter is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the underwriter in its capacity as underwriter, and should not be relied upon by investors.
Other
From time to time, the underwriter and/or its affiliates have provided, and may in the future provide, various investment banking and other financial services for us for which services it has received and, may in the future receive, customary fees.
Except for the services provided in connection with this offering, the underwriter has not provided any investment banking or other financial services during the 180-day period preceding the date of this prospectus and we do not expect to retain the underwriter to perform any investment banking or other financial services for at least 90 days after the date of this prospectus.
Offers Outside the United States
Other than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons
into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
NOTICE TO INVESTORS
Notice to Investors in the United Kingdom
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”) an offer to the public of any securities which are the subject of the offering contemplated by this prospectus may not be made in that Relevant Member State except that an offer to the public in that Relevant Member State of any such securities may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
|
|
|
|
a.
|
to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
|
|
|
|
|
b.
|
to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts;
|
|
|
|
|
c.
|
by an underwriter to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive); or
|
|
|
|
|
d.
|
in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of these securities shall result in a requirement for the publication by the issuer or the underwriter of a prospectus pursuant to Article 3 of the Prospectus Directive.
|
For the purposes of this provision, the expression an “offer to the public” in relation to any of the securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any such securities to be offered so as to enable an investor to decide to purchase any such securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
The underwriter has represented, warranted and agreed that:
|
|
|
|
a.
|
it has only communicated or caused to be communicated and will only communicate or cause to be communicated any invitation or inducement to engage in investment activity (within the meaning of section 21 of the Financial Services and Markets Act 2000 (the FSMA)) received by it in connection with the issue or sale of any of the securities in circumstances in which section 21(1) of the FSMA does not apply to the issuer; and
|
|
|
|
|
b.
|
it has complied with and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the securities in, from or otherwise involving the United Kingdom.
|
European Economic Area
In particular, this document does not constitute an approved prospectus in accordance with European Commission’s Regulation on Prospectuses no. 809/2004 and no such prospectus is to be prepared and approved in connection with this offering. Accordingly, in relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (being the Directive of the European Parliament and of the Council 2003/71/EC and including any relevant implementing measure in each Relevant Member State) (each, a Relevant Member State), with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State (the Relevant Implementation Date) an offer of securities to the public may not be made in that Relevant Member State prior to the publication of a prospectus in relation to such securities which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member
State, all in accordance with the Prospectus Directive, except that it may, with effect from and including the Relevant Implementation Date, make an offer of securities to the public in that Relevant Member State at any time:
|
|
|
|
•
|
to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
|
|
|
|
|
•
|
to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000; and (3) an annual net turnover of more than €50,000,000, as shown in the last annual or consolidated accounts; or
|
|
|
|
|
•
|
in any other circumstances which do not require the publication by the Issuer of a prospectus pursuant to Article 3 of the Prospectus Directive.
|
For the purposes of this provision, the expression an “offer of securities to the public” in relation to any of the securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State. For these purposes the securities offered hereby are “securities.”
LEGAL MATTERS
Cooley LLP, Palo Alto, California, will pass upon the validity of the Preferred Stock, the Series A Warrants and the Common Stock issuable upon conversion of the Preferred Stock and exercise of the Series A Warrants offered hereby. The underwriter is being represented by Ellenoff Grossman & Schole LLP of New York, New York in connection with the offering.
EXPERTS
Marcum LLP, an independent registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2016, as set forth in their report dated March 9, 2017, which is incorporated by reference in this prospectus and elsewhere in the registration statement. Our consolidated financial statements are incorporated by reference in reliance on Marcum LLP’s report, given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION AND INCORPORATION BY REFERENCE
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the Units, Preferred Stock and Series A Warrants offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits filed with the registration statement. For further information about us and the Units, Preferred Stock and the Series A Warrants offered hereby, we refer you to the registration statement and the exhibits filed with the registration statement. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. A copy of the registration statement and the filed exhibits may be inspected without charge at the public reference room maintained by the SEC, located at 100 F Street, NE, Washington, DC 20549, and copies of all or any part of the registration statement may be obtained from that office at prescribed rates. Please call the SEC at 1-800-SEC-0330 for further information about the public reference room. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of the website is www.sec.gov.
We are subject to the information and reporting requirements of the Exchange Act and, in accordance with this law, are required to file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other information are available for inspection and copying at the SEC’s public reference facilities and the website of the
SEC referenced above. We make available free of charge, on or through the investor relations section of our website, annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. The information found on our website, other than as specifically incorporated by reference in this prospectus, is not part of this prospectus.
The SEC allows us to incorporate by reference the information we file with it, which means that we can disclose important information to you by referring you to another document that we have filed separately with the SEC. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus. We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC (Commission File No. 001-35560), excluding any portions of any Current Report on Form 8-K that are not deemed “filed” pursuant to the General Instructions of Form 8-K:
|
|
|
|
•
|
our Annual Report on Form 10-K for the year ended December 31, 2016 filed with the SEC on March 9, 2017.
|
10,000 Units
Each Consisting of
One Share of Series B 6.0% Mandatorily Convertible Preferred Stock
Series A Warrants to Purchase 300 Shares of Common Stock
Prospectus
Maxim Group LLC
, 2017
PART II - INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13.
OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
We estimate that expenses in connection with the distribution described in this registration statement (other than brokerage commissions, discounts or other expenses relating to the sale of the shares by the selling stockholders) will be as set forth below. We will pay all of the expenses with respect to the distribution, and such amounts, with the exception of the Securities and Exchange Commission registration fee and FINRA fee, are estimates.
|
|
|
|
|
|
|
|
|
|
SEC registration fee
|
$
|
2,241
|
|
|
|
|
FINRA filing fee
|
$
|
3,400
|
|
|
|
|
NYSE MKT listing fee
|
$
|
*
|
|
|
Accounting fees and expenses
|
$
|
*
|
|
|
Legal fees and expenses
|
$
|
*
|
|
|
Printing and related expenses
|
$
|
*
|
|
|
Miscellaneous
|
$
|
*
|
|
|
Total
|
$
|
*
|
|
* To be completed by amendment.
ITEM 14.
INDEMNIFICATION OF DIRECTORS AND OFFICERS
Our amended and restated certificate of incorporation provides that no officer or director shall be personally liable to this corporation or our stockholders for monetary damages except as provided pursuant to Delaware law. Our amended and restated certificate of incorporation and our amended and restated bylaws also provide that we shall indemnify and hold harmless each person who serves at any time as a director, officer, employee or agent of our from and against any and all claims, judgments and liabilities to which such person shall become subject by reason of the fact that he is or was a director, officer, employee or agent of our and shall reimburse such person for all legal and other expenses reasonably incurred by him or her in connection with any such claim or liability. We also have the power to defend such person from all suits or claims in accord with the Delaware law. The rights accruing to any person under our amended and restated certificate of incorporation and our amended and restated bylaws do not exclude any other right to which any such person may lawfully be entitled, and we may indemnify or reimburse such person in any proper case, even though not specifically provided for by our amended and restated certificate of incorporation and our amended and restated bylaws.
We have entered into an indemnity agreement with each of our directors. In our employment agreements with Andrew Gengos and Anthony Gringeri, we agreed to indemnify each of these officers for all claims arising out of performance of his duties, other than those arising out of his breach of the agreement or his gross negligence or willful misconduct.
Insofar as indemnification for liabilities for damages arising under the Securities Act of 1933 may be permitted to our directors, officers, and controlling persons pursuant to the foregoing provision, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
The underwriting agreement to be filed as Exhibit 1.1 to this Registration Statement provides for indemnification by the underwriter of us and our directors and officers for certain liabilities under the Securities Act, or otherwise.
ITEM 15.
RECENT SALES OF UNREGISTERED SECURITIES
None.
ITEM 16.
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) Exhibits.
The exhibits to the registration statement are listed in the Exhibit Index attached hereto and incorporated by reference herein.
(b) Financial Statement Schedules.
Financial statement schedules have been omitted, as the information required to be set forth therein is included in the Consolidated Financial Statements or Notes thereto appearing in the prospectus made part of this registration statement.
ITEM 17.
UNDERTAKINGS
The undersigned Registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement to:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act.
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
provided, however
, that paragraphs (1)(i), (ii) and (iii) do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to securities offered therein, and the offering of the securities at that time shall be deemed to be the initial bona fide offering thereof;
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering;
(4) That, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this registration statement to be signed on its behalf by the undersigned, in Calabasas, California, on April 3, 2017.
|
|
|
|
|
|
|
|
IMMUNOCELLULAR THERAPEUTICS, LTD.
|
|
|
|
|
|
|
By:
|
/s/ Anthony Gringeri, Ph.D.
|
|
|
|
Anthony Gringeri, Ph.D.
President and Chief Executive Officer
|
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed below by the following persons in the capacities and on the dates indicated:
|
|
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
/s/ Anthony Gringeri, Ph.D.
|
|
President, Chief Executive Officer and Director
(Principal Executive Officer)
|
|
April 3, 2017
|
|
Anthony Gringeri, Ph.D.
|
|
|
|
|
|
|
|
/s/ David Fractor
|
|
Vice President, Finance and Principal Accounting Officer
(Principal Financial and Accounting Officer)
|
|
April 3, 2017
|
|
David Fractor
|
|
|
|
*
|
|
|
|
April 3, 2017
|
|
Andrew W. Gengos
|
|
Director
|
|
April 3, 2017
|
|
|
|
|
|
*
|
|
Director
|
|
April 3, 2017
|
|
Gregg A. Lapointe
|
|
|
|
|
|
|
|
*
|
|
Director
|
|
April 3, 2017
|
|
Rahul Singhvi, Sc.D.
|
|
|
|
|
|
|
|
*
|
|
Director
|
|
April 3, 2017
|
|
Mark A. Schlossberg
|
|
|
|
|
|
|
|
*
|
|
Director
|
|
April 3, 2017
|
|
Gary S. Titus
|
|
|
|
|
|
|
|
*
|
|
Director
|
|
April 3, 2017
|
|
John S. Yu, M.D.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Pursuant to Power of Attorney
|
|
|
|
|
By:
|
|
/s/ Anthony Gringeri, Ph.D.
|
|
|
|
Anthony Gringeri, Ph.D.
|
|
|
|
Attorney-in-Fact
|
EXHIBIT INDEX
|
|
|
|
|
|
|
|
|
|
|
Exhibit
|
Description
|
Incorporation by Reference
|
Filed Herewith
|
|
Form
|
SEC File No.
|
Exhibit
|
Filing Date
|
|
1.1*
|
Form of Underwriting Agreement
|
|
|
|
|
|
|
2.1
|
Agreement and Plan of Reorganization dated as of May 5, 2005, as amended, among Patco Industries Subsidiary, Inc., William C. Patridge, and Spectral Molecular Imaging, Inc., as amended on June 30, 2005, September 26, 2005 and January 20, 2006
|
8-K
|
033-17624-NY
|
2.1
|
1/26/2006
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation
|
8-K
|
001-35560
|
3.1
|
11/3/2006
|
|
|
3.2
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation
|
8-K
|
001-35560
|
3.2
|
11/3/2006
|
|
|
3.3
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation
|
8-K
|
001-35560
|
3.1
|
05/09/2007
|
|
|
3.4
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation
|
10-Q
|
001-35560
|
3.1
|
11/14/2011
|
|
|
3.5
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation
|
8-K
|
001-35560
|
3.1
|
09/24/2013
|
|
|
3.6
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation
|
8-K
|
001-35560
|
3.1
|
11/19/2015
|
|
|
3.7
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation
|
8-K
|
001-35560
|
3.1
|
11/18/2016
|
|
|
3.8
|
Amended and Restated Bylaws
|
S-8
|
333-171652
|
3.1
|
1/11/2011
|
|
|
3.9
|
Amendment to the Amended and Restated Bylaws
|
8-K
|
001-35560
|
3.1
|
5/25/2012
|
|
|
3.10*
|
Certificate of Designation of Preferences, Rights and Limitations of Series B 6.0% Mandatorily Convertible Preferred Stock
|
|
|
|
|
|
|
4.1
|
Form of Common Stock Certificate of the Registrant
|
SB-2
|
333-140598
|
4.1
|
2/12/2007
|
|
|
4.2
|
Form of Warrant to Purchase Common Stock, originally issued in February 2011
|
8-K
|
033-17264-NY
|
4.1
|
2/25/2011
|
|
|
4.3
|
Form of Warrant to Purchase Common Stock, originally issued in January 2012
|
8-K
|
033-17264-NY
|
4.1
|
1/10/2012
|
|
|
4.4
|
Form of Warrant to Purchase Common Stock, originally issued in October 2012
|
8-K
|
001-35560
|
10.1
|
10/19/2012
|
|
|
4.5
|
Form of Warrant to Purchase Common Stock, originally issued in February 2015
|
10-Q
|
001-35560
|
4.1
|
5/11/2015
|
|
|
4.6
|
Form of Base Warrant to Purchase Common Stock, originally issued in August 2016
|
S-1/A
|
333-211763
|
4.8
|
8/4/2016
|
|
|
4.7
|
Form of Pre-Funded Warrant to Purchase Common Stock, originally issued in August 2016
|
S-1/A
|
333-211763
|
4.9
|
8/4/2016
|
|
|
4.8*
|
Form of Series A Warrant to Purchase Common Stock
|
|
|
|
|
|
|
5.1*
|
Opinion of Cooley LLP
|
|
|
|
|
|
|
10.1
|
Amended and Restated 2006 Equity Incentive Plan of ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.1
|
11/14/2011
|
|
|
10.2
|
Form of Non-Qualified Stock Option Agreement for the 2006 Equity Incentive Plan of ImmunoCellular Therapeutics, Ltd.
|
S-8
|
333-147278
|
4.5
|
11/9/2007
|
|
|
10.3
|
Form of Incentive Stock Option Agreement for the 2006 Equity Incentive Plan of ImmunoCellular Therapeutics, Ltd.
|
S-8
|
333-147278
|
4.6
|
11/9/2007
|
|
|
|
|
|
|
|
|
|
|
|
|
10.4†
|
Exclusive License Agreement dated as of November 17, 2006 between Cedars-Sinai Medical Center and ImmunoCellular Therapeutics, Ltd.
|
8-K
|
033-17264-NY
|
10.1
|
11/22/2006
|
|
|
10.5†
|
First Amendment to Exclusive License Agreement dated as of June 16, 2008, between Cedars-Sinai Medical Center and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
033-17264-NY
|
10.2
|
08/14/2008
|
|
|
10.6
|
Stock Purchase Agreement dated as of November 17, 2006 between Cedars-Sinai Medical Center and ImmunoCellular Therapeutics, Ltd.
|
8-K
|
033-17264-NY
|
10.3
|
11/22/2006
|
|
|
10.7
|
Registration Rights Agreement dated as of November 17, 2006 between Cedars-Sinai Medical Center and ImmunoCellular Therapeutics, Ltd.
|
8-K
|
033-17264-NY
|
10.4
|
11/22/2006
|
|
|
10.8
|
Securities Purchase Agreement dated as of November 17, 2006 between Dr. John Yu and ImmunoCellular Therapeutics, Ltd.
|
8-K
|
033-17264-NY
|
10.5
|
11/22/2006
|
|
|
10.9
|
Agreement dated as of November 17, 2006 between Dr. John Yu and ImmunoCellular Therapeutics, Ltd.
|
8-K
|
033-17264-NY
|
10.2
|
11/22/2006
|
|
|
10.10
|
Nonqualified Stock Option Agreement dated as of November 17, 2006 between Dr. John Yu and ImmunoCellular Therapeutics, Ltd.
|
8-K
|
033-17264-NY
|
10.6
|
11/22/2006
|
|
|
10.11
|
Registration Rights Agreement dated as of November 17, 2006 between Dr. John Yu and ImmunoCellular Therapeutics, Ltd.
|
8-K
|
033-17264-NY
|
10.7
|
11/22/2006
|
|
|
10.12
|
Agreement dated as of February 14, 2008 between Molecular Discoveries, LLC and ImmunoCellular Therapeutics, Ltd.
|
10KSB
|
033-17264-NY
|
10.20
|
03/25/2008
|
|
|
10.13
|
Registration Rights Agreement dated as of April 14, 2008, between Molecular Discoveries, LLC and ImmunoCellular Therapeutics, Ltd.
|
S-1
|
333-150277
|
10.24
|
04/16/2008
|
|
|
10.14
|
Agreement dated as of August 1, 2008 between Dr. Cohava Gelber and ImmunoCellular Therapeutics, Ltd.
|
10-K
|
001-35560
|
10.1
|
03/30/2009
|
|
|
10.15
|
Second Amendment dated August 1, 2009 to Exclusive License Agreement dated as of November 17, 2006 between Cedars-Sinai Medical Center and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
033-17264-NY
|
10.1
|
11/13/2009
|
|
|
10.16
|
Preferred Stock Purchase Agreement dated as of December 3, 2009 between ImmunoCellular Therapeutics, Ltd. and Socius Capital Group, LLC d/b/a Socius Life Sciences Capital Group, LLC
|
8-K
|
033-17264-NY
|
10.1
|
12/03/2009
|
|
|
10.17
|
Agreement dated March 1, 2010 between Dr. John Yu and ImmunoCellular Therapeutics, Ltd.
|
10-K
|
033-17264-NY
|
10.36
|
03/31/2010
|
|
|
10.18
|
Securities Purchase Agreement dated March 11, 2010 between participants in the March 2010 private placement and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
033-17264-NY
|
10.6
|
05/181/2010
|
|
|
10.19
|
Form of Registration Rights Agreement dated as of March 29, 2010 between participants in the March 2010 private placement and ImmunoCellular Therapeutics, Ltd.
|
S-1/A
|
333-150277
|
10.27
|
05/12/2010
|
|
|
10.20
|
Modification Agreement dated May 2, 2010 among Socius CG II, Ltd., Socius Life Sciences Capital Group, LLC and ImmunoCellular Therapeutics, Ltd.
|
S-1/A
|
333-150277
|
10.33
|
05/12/2010
|
|
|
|
|
|
|
|
|
|
|
|
|
10.21
|
Third Amendment dated March 26, 2010 to Exclusive License Agreement dated as of November 17, 2006 between Cedars-Sinai Medical Center and ImmunoCellular Therapeutics, Ltd.
|
S-1/A
|
333-150277
|
10.35
|
05/12/2010
|
|
|
10.22
|
Securities Purchase Agreement dated May 12, 2010 between participants in the May 2010 private placement and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
033-17264-NY
|
10.11
|
05/18/2010
|
|
|
10.23
|
Form of Registration Rights Agreement between participants in the May 2010 private placement and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
033-17264-NY
|
10.12
|
05/18/2010
|
|
|
10.24
|
Purchase Agreement, dated as of February 22, 2011, by and between the ImmunoCellular Therapeutics, Ltd. and each investor named therein
|
10-Q
|
001-35560
|
10.1
|
5/11/2015
|
|
|
10.25
|
Registration Rights Agreement, dated as of February 22, 2011, by and among ImmunoCellular Therapeutics, Ltd. and the investors named therein
|
8-K
|
033-17264-NY
|
10.2
|
02/25/2011
|
|
|
10.26†
|
Exclusive Sublicense Agreement dated May 28, 2010 between Targepeutics, Inc. and ImmunoCellular Therapeutics, Ltd.
|
10-K
|
033-17264-NY
|
10.48
|
03/31/2011
|
|
|
10.27†
|
Sponsored Research and Vaccine Production Agreement dated January 1, 2011 between The Trustees of the University of Pennsylvania and ImmunoCellular Therapeutics, Ltd.
|
10-K
|
033-17264-NY
|
10.49
|
03/31/2011
|
|
|
10.28
|
Placement agent agreement dated March 30, 2010 between Gilford Securities Incorporated and ImmunoCellular Therapeutics, Ltd.
|
10-K
|
033-17264-NY
|
10.50
|
03/31/2011
|
|
|
10.29
|
Placement agent agreement dated April 7, 2010 between Scarsdale Equities LLC and ImmunoCellular Therapeutics, Ltd.
|
10-K
|
033-17264-NY
|
10.51
|
03/31/2011
|
|
|
10.30
|
Consulting Agreement dated October 1, 2010 between JFS Investments and ImmunoCellular Therapeutics, Ltd.
|
10-K
|
033-17264-NY
|
10.52
|
03/31/2011
|
|
|
10.31
|
Advisory services agreement dated October 1, 2010 between Garden State Securities Inc. and ImmunoCellular Therapeutics, Ltd.
|
10-K
|
033-17264-NY
|
10.53
|
03/31/2011
|
|
|
10.32
|
Co-placement Agents Agreement dated January 31, 2011 among Summer Street Research Partners, Dawson James Securities, Inc. and ImmunoCellular Therapeutics, Ltd.
|
10-K
|
033-17264-NY
|
10.54
|
03/31/2011
|
|
|
10.33
|
Agreement dated as of March 13, 2011 between Dr. John Yu and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
033-17264-NY
|
10.4
|
08/18/2011
|
|
|
10.34†
|
Patent License Agreement, effective February 10, 2012, among The Trustees of the University of Pennsylvania and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
033-17264-NY
|
10.50
|
03/21/2012
|
|
|
10.35†
|
Exclusive License Agreement, effective February 16, 2012, between the Johns Hopkins University and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
033-17264-NY
|
10.51
|
03/21/2012
|
|
|
10.36
|
Office Lease dated July 1, 2012 between Regent Business Centers and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.1
|
08/14/2012
|
|
|
10.37
|
Employment Agreement dated December 3, 2012 between Andrew Gengos and ImmunoCellular Therapeutics, Ltd.
|
10-K
|
001-35560
|
10.54
|
03/11/2013
|
|
|
10.38
|
Form of Stock Option Grant Notice for the 2006 Equity Incentive Plan of ImmunoCellular Therapeutics, Ltd.
|
10-K
|
001-35560
|
10.55
|
03/11/2013
|
|
|
|
|
|
|
|
|
|
|
|
|
10.39
|
Controlled Equity Offering
SM
Sales Agreement dated April 18, 2013 between ImmunoCellular Therapeutics, Ltd. and Cantor Fitzgerald & Co.
|
8-K
|
001-35560
|
10.1
|
04/18/2013
|
|
|
10.40
|
Form of Indemnity Agreement between ImmunoCellular Therapeutics, Ltd. and each of its directors and executive officers
|
10-Q
|
001-35560
|
10.1
|
05/10/2013
|
|
|
10.41
|
Office Lease dated May 13, 2013 between Calabasas/Sorrento Square, LLC and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.1
|
08/08/2013
|
|
|
10.42
|
Master Services Agreement dated September 1, 2010 between Averion International Corp. and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.2
|
08/08/2013
|
|
|
10.43
|
Employment Agreement dated August 19, 2013 between Anthony Gringeri and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.1
|
11/07/2013
|
|
|
10.44†
|
Amendment No. 1 to the Exclusive License Agreement between the Johns Hopkins University and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.2
|
11/07/2013
|
|
|
10.45
|
Amended and Restated 2006 Equity Incentive Plan of ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.3
|
11/07/2013
|
|
|
10.46
|
Amendment No. 1 to Amended and Restated 2006 Equity Incentive Plan of ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.4
|
11/07/2013
|
|
|
10.47
|
Form of Stock Option Grant Notice for the 2006 Equity Incentive Plan of ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.5
|
11/07/2013
|
|
|
10.48
|
Master Services Agreement dated February 19, 2014 between Aptiv Solutions, Inc. and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10
|
03/14/2014
|
|
|
10.49
|
Employment Agreement dated January 30, 2015 between Steven J. Swanson and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.1
|
5/11/2015
|
|
|
10.50†
|
Agreement for GMP Manufacturing of ICT-107 dated March 13, 2015 between PharmaCell B.V. and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.2
|
5/11/2015
|
|
|
10.51†
|
Amended & Restated Exclusive License Agreement dated May 13, 2015 between Cedars-Sinai Medical Center and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.2
|
8/7/2015
|
|
|
10.52
|
Form of Restricted Stock Unit Agreement for the 2006 Equity Incentive Plan of ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.1
|
8/7/2015
|
|
|
10.53†
|
Services Agreement dated June 11, 20015 between ImmunoCellular Therapeutics, Ltd. and PCT, LLC, a Caladrius Company
|
10-Q
|
001-35560
|
10.3
|
8/7/2015
|
|
|
10.54†
|
Second Amendment to Exclusive License Agreement dated August 7, 2015 between ImmunoCellular Therapeutics, Ltd. and Johns Hopkins University
|
10-Q
|
001-35560
|
10.1
|
11/9/2015
|
|
|
10.55
|
Employment Agreement dated September 17, 2015 between David Fractor and ImmunoCellular Therapeutics. Ltd., as amended on September 14, 2016
|
10-Q
|
001-35560
|
10.2
|
11/9/2015
|
|
|
10.56
|
Independent Contractor Services Agreement effective as of October 1, 2015 between John Yu and ImmunoCellular Therapeutics, Ltd.
|
10-K
|
001-35560
|
10.57
|
3/30/2016
|
|
|
10.57
|
Amended and Restated Independent Contractor Services Agreement dated February 1, 2016 between John Yu and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.1
|
5/13/2016
|
|
|
10.58
|
2016 Equity Incentive Plan
|
S-1/A
|
333-211763
|
10.59
|
7/11/2016
|
|
|
|
|
|
|
|
|
|
|
|
|
10.59
|
Forms of Stock Option Agreement, Notice of Grant of Stock Option, Restricted Stock Unit Grant Notice and Restricted Stock Award Grant Notice under the 2016 Equity Incentive Plan
|
S-1/A
|
333-211763
|
10.60
|
7/11/2016
|
|
|
10.60
|
Non-Employee Director Compensation Plan
|
S-1/A
|
333-211763
|
10.61
|
7/11/2016
|
|
|
10.61
|
First Amendment to Lease Extending Term executed on May 18, 2016 between Calabasas/Sorrento Square, LLC and ImmunoCellular Therapeutics, Ltd.
|
10-Q
|
001-35560
|
10.1
|
8/22/2016
|
|
|
10.62
|
Separation Agreement dated December 13, 2016 between Andrew Gengos and ImmunoCellular Therapeutics, Ltd.
|
10-K
|
001-35560
|
10.62
|
3/9/2017
|
|
|
23.1
|
Consent of Marcum LLP, independent registered public accounting firm
|
|
|
|
|
X
|
|
23.2*
|
Consent of Cooley LLP (See Exhibit 5.1)
|
|
|
|
|
|
|
24.1
|
Power of Attorney
|
|
|
|
|
|
_______________________________________________________________________________________________
|
|
|
|
*
|
To be filed by amendment.
|
|
|
|
|
†
|
Confidential treatment has been granted with respect to certain portions of this exhibit by the Securities and Exchange Commission. The omitted portions of the exhibit have been separately filed with the Securities and Exchange Commission.
|
EOM Pharmaceutical (PK) (USOTC:IMUC)
Historical Stock Chart
From Mar 2024 to Apr 2024
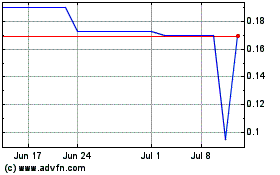
EOM Pharmaceutical (PK) (USOTC:IMUC)
Historical Stock Chart
From Apr 2023 to Apr 2024