Filed Pursuant to Rule 433
Issuer Free Writing Prospectus dated March 20, 2017
Relating to Preliminary Prospectus dated March 20, 2017
Registration Statement No. 333-216031

1 NASDAQ: ATOS WWW . ATOSSAGENETICS . COM ©2016, A TOSSA G ENETICS , I NC . A LL R IGHTS R ESERVED . C ORPORATE P RESENTATION M ARCH 20, 2017

2 Some of the information presented herein may contain projections or other forward - looking statements regarding future events or the future financial performance of the Company which the Company undertakes no obligation to update . These statements are based on management’s current expectations and are subject to risks and uncertainties that may cause actual results to differ materially from the anticipated or estimated future results, including the risks and uncertainties associated with clinical trials, actions by the FDA, regulatory clearances, responses to regulatory matters, the market demand for and acceptance of A tossa's products and services, performance of clinical research organizations and other risks detailed from time to time in A tossa's filings with the S ecurities and Exchange C ommission, including without limitation its most recent annual report on form 10 - K, subsequent quarterly reports on F orms 10 - Q and Forms 8 - K, each as amended and supplemented from time to time . Forward - Looking Statements

About Atossa Genetics 3 Atossa Genetics is a healthcare company focused on developing novel pharmaceuticals and drug delivery methods to treat breast cancer and other breast conditions.

Our Programs 4 Drug Development and Delivery Programs Intraductal Microcatheters – Phase 2 study for delivery fulvestrant for neoadjuvant treatment of ductal carcinoma in - situ (DCIS) and breast cancer Endoxifen – Phase 1 in Q2 2017 and Phase 2 in 2H 2017. Proprietary oral and topical. Initial program is for breast cancer patients who are refractory to tamoxifen

Mammo - graphy ; Suspicious Lump Biopsy Surgery* and Radiation/ Chemotherapy Diagnosis Breast Cancer Timeline Tamoxifen (5 years) Intraductal Fulvestrant Endoxifen Neoadjuvant Phase Adjuvant Phase *Mastectomy or lumpectomy

Program Pipeline 6 Pivota l Preclinical Phase 1 NDA* Market Drug/Device Program * Estimated FDA or Ex - US submission Phase 2 Phase 3 Fulvestrant : DCIS/ Breast Cancer Micro - Catheters 2018 Endoxifen Oral and Topical Phase 1 Q2 ‘17 Phase 2 Underway 2018 2019 2019 Phase 2 2H ‘17 Oral for Refractory Future: density; prevention Phase 2 2H ‘17

Endoxifen for Refractory Current therapies: up to 50% of patients taking tamoxifen are refractory – meaning they make inadequate e ndoxifen and have an increased rate of recurrence Our proprietary drug: potentially raises endoxifen levels Endoxifen for Density; Prevention No U.S. approved treatment for density; tamoxifen inadequate in prevention setting The Unmet Need 7 Intraductal Microcatheters Current therapies: use systemic delivery, which can have adverse effects and potentially limited tumor tissue concentration Our m icrocatheters : f ulvestrant and potentially other drugs targeted to site of the cancer/DCIS in the ducts

Endoxifen : Refractory Up to 1 million U.S. cancer survivors are eligible to take tamoxifen , but up to 50% are refractory $1B U.S market in treatment and prevention of breast cancer (Defined Health 1/17) Large Market Opportunities 8 Intraductal Microcatheters : Fulvestrant I ntramuscular Fulvestrant is $14,000 per dose; $700M expanding market $800M U.S. market in DCIS pre - surgery and replacement to surgery (Defined Health 1/17) Breast Cancer Statistics 250,000 breast cancers and 60,000 DCIS in U.S. in 2017 40,000 deaths in U.S. in 2017

Neoadjuvant Solution: Intraductal Microcatheters 9 • Phase 2 study for delivery of fulvestrant in patients with DCIS or breast cancer (initiated at Columbia; being transferred to Montefiore) • Advantages: potentially higher local drug exposure and lower systemic concentrations vs systemically delivered agents • 1 issued and 3 pending patent app’s (US and PCT) for intraductal drug delivery; potential “generic” use of other microcatheters • Fulvestrant is FDA approved for intramuscular admin (AstraZeneca); opportunities with other drugs and biotherapeutics

10 Microcatheter Fulvestrant - Clinical Trial Study Design Thirty women with ER + DCIS or Invasive Breast Cancer 6 24 Drug Administered 30 - 45 days B efore S urgery Assessments Efficacy Safety Pharmacokinetic Pathological Response, Ki - 67 Expression FACT - ES Tolerability Tissue and Blood Levels of Fulvestrant Intramuscular Administration Intraductal Administration

11 • IP: Composition of Matter and Methods of Treatment applications have been filed • E xisting data: Abundant pre - clinical and clinical data support approach • Speed to market: Potential for rapid approval • Potential competition: No known current commercial clinical development underway • Regulatory pathway: 505(b)(2) pathway potential • Developing oral and topical proprietary formulations • Other potential indications: breast density; prevention Endoxifen

Major Tamoxifen Metabolites 12 Compound Plasma Level (nM) IC 50 Estrogen Receptor Effect (nM) PL/IC 50 Endoxifen 29.1 3 9.7 4 - OH - Tamoxifen 5.8 7 0.8 (8%) 3 - OH - Tamoxifen 0.7 94 <0.01 (0.1%) Murdter et al, Clin Pharmacol Therap. 2011 May

Low Plasma Endoxifen P redicts R ecurrence 13 CYP2D6 EM Mean (n=586) Dezentje ´ et. al. Breast Cancer Res Treat (2015) 153:583 – 590. 35 % increased Recurrence Rate Pre/Post - Menopausal (n=1370 ) Madlensky et. al. Clin Pharmacol Ther . (2011) 89:718 – 725 . 1.6 - 1.9 Increased Hazards Ratio for Recurrence Pre - Menopausal Only (n=587) Saladores et. al. The Pharmacogenomics Journal (2015) 15, 84 – 94

Endoxifen – Phase 1 Clinical Trial 14 Study to start in Q2 2017 in Australia Pharmacokinetics; safety and tolerability Placebo controlled, double - blinded 48 volunteers Oral and topical arms 28 day repeat dose

Endoxifen – Refractory Clinical Trial Design 15 Entry Criteria: Pre - menopausal ER + breast cancer patients on tamoxifen Measure Endoxifen Levels >TBD nM Endoxifen Continue on tamoxifen (20 mg/day) ≤TBD nM Endoxifen Add Oral Endoxifen (1 - 2 mg/day)

Endoxifen Regulatory Pathway 16 Program could qualify for designation under the 505(b)(2) status. Advantages: A single clinical study of safety and efficacy Limited additional clinical or pre - clinical studies Market exclusivity possible

Microcatheter / Fulvestrant Phase 2 trial (Columbia/Montefiore pending): August 2017 Steady progress: additional endoxifen program(s); Phase 1 updates Endoxifen : Phase 1 starts Q2 2017; Phase 2 2H 2017 2017 Planned Milestones 17

x I ntraductal fulvestrant in Phase 2 x Endoxifen Phase 1 planned for Q2 ’ 17, Phase 2 in 2H ‘17 x Favorable clinical and regulatory pathways x No debt, no preferred stock x $3M in cash as of Dec. 31, 2016 x Patent applications filed covering composition and methods x Experienced m anagement team 18 Investment/Financial Highlights

19 Seasoned Management Steven Quay, MD, PhD Chairman and CEO Kyle Guse, CPA, ESQ, MBA CFO and General Counsel Janet Rose Rea, MSPH VP Regulatory, Quality and Clinical Affairs

Atossa Genetics Inc. (IPO November 8, 2012) NASDAQ: ATOS Our Mission Develop novel pharmaceuticals and delivery systems to treat breast cancer and other breast conditions Debt (Dec. 31, 2016 ) NONE Cash (Dec. 31, 2016) $3M Shares Outstanding (Mar. 16, 2017) 3.8M Corporate Headquarters Seattle, Washington Corporate Summary 20

21 NASDAQ: ATOS WWW . ATOSSAGENETICS . COM ©2016, A TOSSA G ENETICS , I NC . A LL R IGHTS R ESERVED . F OR M ORE I NFORMATION : K YLE G USE , CFO AND G ENERAL C OUNSEL KYLE . GUSE @ ATOSSAGENETICS . COM

1 NASDAQ: ATOS WWW . ATOSSAGENETICS . COM ©2016, A TOSSA G ENETICS , I NC . A LL R IGHTS R ESERVED . Atossa Genetics Inc. has filed a registration statement (including a prospectus) on Form S - 1 with the SEC for the offering to which this communication relates. Before you invest, you should read the prospectus in such registration statement and other documents the issuer has filed with the SEC for more complete information about Atossa Genetics Inc. and this offering. You may get these documents for free by visiting EDGAR on the SEC Web site at www.sec.gov . Alternatively, we or any underwriter participating in the offering will arrange to send you the preliminary prospectus and, when available, the final prospectus and / or any supplements thereto if you contact Aegis Capital Corp., Prospectus Department, 810 Seventh Avenue, 18th Floor, New York, NY 10019, telephone: 212 - 813 - 1010, email: prospectus@aegiscap.com .
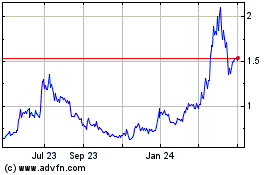
Atossa Therapeutics (NASDAQ:ATOS)
Historical Stock Chart
From Mar 2024 to Apr 2024
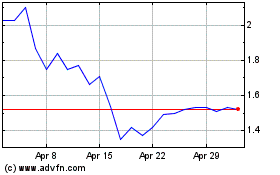
Atossa Therapeutics (NASDAQ:ATOS)
Historical Stock Chart
From Apr 2023 to Apr 2024