UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
AMENDMENT
NO. 1
FORM
S-1
REGISTRATION
STATEMENT UNDER THE SECURITIES ACT OF 1933

Immune
Therapeutics, Inc.
(Name
of small business issuer in our charter)
|
Florida
|
|
0001509957
|
|
59-3226705
|
|
(State
or other jurisdiction of
incorporation
or organization)
|
|
(Primary
Standard Industrial
Classification
Code Number)
|
|
IRS
Employer
Identification
Number
|
|
37
North Orange Ave, Suite 607,
Orlando,
Florida
|
|
32801
|
|
(Address
of principal executive offices)
|
|
(Zip
Code)
|
Telephone:
(888) 613-8802
Noreen
Griffin, Chief Executive Officer
37
North Orange Avenue, Suite 607
Orlando,
FL 32801
(888)
613-8802
(Name,
address and telephone number of agent for service)
Please
send copies of all communications to:
Gina
M. Austin, Esq. or
Arden
E. Anderson, Esq.
Austin
Legal Group, APC
3990
Old Town Ave., Suite A-112
San
Diego, A 92110
Approximate
date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under
the Securities Act of 1933, check the following box. [X]
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the
following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the
same offering. [ ]
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list
the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering. [ ]
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list
the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering. [ ]
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller
reporting company.
|
Large
accelerated filer
|
[ ]
|
Accelerated
Filer
|
[ ]
|
|
Non-accelerated
filer
|
[ ]
|
Smaller
reporting company
|
[X]
|
CALCULATION
OF REGISTRATION FEE
|
Title
of each class of securities to be registered
|
|
Amount
to be
registered [1]
|
|
|
Proposed
maximum
offering price
per unit [2]
|
|
|
Proposed
maximum
aggregate
offering price
|
|
|
Amount
of
registration
fee [3]
|
|
|
Common Stock offered by
the Selling Stockholders
|
|
|
4,015,621
|
|
|
$
|
0.14
|
|
|
$
|
562,187
|
|
|
$
|
56.1
2
|
|
|
(1)
|
Pursuant
to Rule 416 under the Securities Act, the securities being registered hereunder include such indeterminate number of additional
shares of common stock as may be issued after the date hereof as a result of stock splits, stock dividends or similar transactions.
|
|
|
|
|
(2)
|
Estimated
solely for purposes of calculating the registration fee pursuant to Rule 457(c) under the Securities Act of 1933, as amended
(“Securities Act”).
|
|
|
|
|
(3)
|
Calculated
by multiplying the proposed maximum aggregate offering price by .0001007.
|
|
|
|
|
(4)
|
$56.12
previously paid by the Company.
|
The
information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement
filed with the Securities and Exchange Commission, of which this prospectus is a part, shall have been declared effective. This
prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where
the offer or sale is not permitted.
SUBJECT
TO COMPLETION, DATED NOVEMBER 8 , 2016
Immune
Therapeutics, Inc.
4,015,621
Shares of Common Stock
Immune
Therapeutics, Inc. is a biotechnology company developing and seeking to commercialize patented therapies in emerging nations that
combat chronic, life-threatening diseases by stimulating or rebalancing the immune system. Our technology platform is based on
two interrelated cytokine drug therapies-Low-Dose Naltrexone (LDN) and Methionine Enkephalin (MENK)-which work by triggering opioid
receptors on immune cells to activate various cells of the immune system.
This
registration statement relates to the sale of 4,015,621 shares of the Company’s common stock, par value $0.0001, by selling
shareholder, JMJ Financial (“JMJ” or “Selling Shareholder”). Of the shares being offered, 500,000 are
issued and outstanding and the remaining shares are issuable upon the exercise of outstanding warrants issued to JMJ as part of
a securities purchase transaction with the Company in April, 2016. The Selling Shareholder will sell its shares in market transactions
at prevailing market prices or through privately negotiated prices
.
The Company will not receive any proceeds from the
sale of shares by the Selling Shareholder. The Company will pay for all fees relating to filing of this registration statement,
but otherwise will not incur any expense relating to the sale of shares by the Selling Shareholder. The Selling Shareholder will
pay all brokerage commissions and discounts attributable to the sale of the shares, plus brokerage fees, if applicable.
Our
common stock is not now listed on any national securities exchange or the NASDAQ stock market. However, our stock is quoted on
OTCQB under the symbol “IMUN.” While our common stock is on the OTCQB, there has been limited trading volume. There
is no guarantee that a more active trading market will develop in our securities.There is also no guarantee that our securities
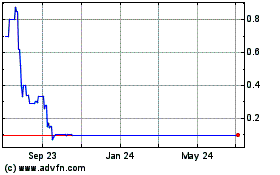
will ever trade on any listed exchange or even remain quoted on OTCQB. As of August 16, 2016, our securities were listed on the
OTCQB at a price of $0.111.
We
qualify as an “emerging growth company” as defined in the Jumpstart our Business Startups Act (“JOBS Act”).
This
offering is highly speculative and these securities involve a high degree of risk and should be considered only by persons who
can afford the loss of their entire investment. See “Risk Factors” on Page 9.
NEITHER
THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR
PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
This
Prospectus is dated ____________, 2016
TABLE
OF CONTENTS
SUMMARY
INFORMATION
This
summary highlights some of the information in this prospectus. It is not complete and may not contain all of the information that
you may want to consider. To understand this offering fully, you should carefully read the entire prospectus, including the section
entitled “Risk Factors,” before making a decision to invest in our securities. Unless otherwise noted or unless the
context otherwise requires, the terms “we,” “us,” “our,” the “Company,” “Immune
Therapeutics” and “IMUN” refers to Immune Therapeutics, Inc. together with its wholly owned subsidiaries. In
instances where we refer emphatically to “Immune Therapeutics, Inc.” or where we refer to a specific subsidiary of
ours by name, we are referring only to that specific legal entity.
The
Company
Immune
Therapeutics Inc. was incorporated in Florida on December 2, 1993 as Resort Clubs International, Inc. with a principal business
address at 37 North Orange Ave, Suite 607, Orlando, Florida 32801. As of March 21, 2016, the Company has 734 shareholders of record
and 220,989,542 shares of common stock outstanding as of August 12, 2016. Immune Therapeutics, Inc. is publicly traded under the
symbol “IMUN” and quoted on OTCQB.
Business
Overview
Immune
Therapeutics, Inc. was initially incorporated in Florida on December 2, 1993 as Resort Clubs International, Inc. (“Resort
Clubs”). It was formed to manage and market golf course properties in resort markets throughout the United States. Galliano
International Ltd. (“Galliano”) was incorporated in Delaware on May 27, 1998 and began trading in November 1999 through
the filing of a 15C-211. On November 10, 2004, Galliano merged with Resort Clubs. Resort Clubs was the surviving corporation.
On August 23, 2010, Resort Clubs changed its name to pH Environmental Inc. (“pH Environmental”).
On
April 23, 2012, pH Environmental completed a name change to TNI BioTech, Inc., and on April 24, 2012, executed a share exchange
agreement for the acquisition of all of the outstanding shares of TNI BioTech IP, Inc. On September 4, 2014, a majority of our
shareholders approved an amendment to our Amended and Restated Articles of Incorporation, as amended, to change our name to Immune
Therapeutics, Inc. We filed our name change amendment with the Secretary of State of Florida on October 27, 2014 changing our
name to Immune Therapeutics, Inc.
The
Company currently operates out of Orlando, Florida. In July 2012, the Company’s focus turned to acquiring patents that would
protect and advance the development of new uses of opioid-related immune- therapies, such as low dose naltrexone (“LDN”)
and Methionine [Met5]-enkephalin (“MENK”). The Company’s therapies are believed to stimulate and/or regulate
the immune system in such a way that they provide the potential to treat a variety of diseases. We believe our therapies may be
able to correct abnormalities or deficiencies in the immune system in diseases such as HIV infection, autoimmune disease, immune
disorders, or cancer; all of which can lead to disease progression and life-threatening situations when the immune system is not
functioning optimally.
In
October 2012, the Company formed TNI BioTech International, Ltd., a BVI company in Tortola, British Virgin Islands, which was
set up to allow the Company to market and sell LDN in those countries outside the U.S. in which we have been able to obtain approval
to sell the Company’s products.
In
August 2013, the Company formed its United Kingdom subsidiary, TNI BioTech, LTD (the “UK Subsidiary”). The UK Subsidiary
received approval to be considered a micro, small or medium-sized enterprise (“SME”) with the European Medicines Agency
(“EMA”) on August 21, 2013. The designation provides the UK Subsidiary with significant discounts when holding meetings
or submitting filings to the EMA. On September 19, 2013, the UK Subsidiary submitted a pre-submission package to the EMA regarding
Crohn’s Disease. The EMA granted the UK Subsidiary a meeting that took place on September 27, 2013. The UK Subsidiary is
eligible to benefit from the provisions for administrative and financial assistance for SMEs set out in Regulation (EC) No 2049/2005.
The Company will apply to obtain EMA benefits once funding becomes available.
In
December 2013, the Company formed a new subsidiary, Cytocom Inc., to focus on conducting LDN and MENK clinical trials in the United
States. In December 2014, the Company finalized the distribution of common stock of Cytocom Inc. to its shareholders. As part
of the transaction, the Company retained exclusive rights to all international patents, in-country approvals, formulations, trademarks,
manufacturing, marketing, sales, and distributions rights in emerging nations, including
Africa,
Central America, South America, Russia, India, China, Far East, and The Commonwealth of Independent States (former Soviet Union).
The Company will continue to have access to existing clinical data as well as any new data generated by Cytocom Inc. during drug
development. On December 8, 2014, the number of Cytocom Inc. shares of common stock that were issued to our shareholders totaled
113,242,522 shares. In connection with the transaction, Cytocom Inc. issued 140,100,000 shares of its common stock to the Company,
which gave the Company a 55.3% stake in Cytocom Inc. on that date. In April 2016, the Board of Directors and a majority of shareholders
of Cytocom approved a reverse stock split of Cytocom’s outstanding common stock with one new share of stock for each twenty
old shares of common stock. Cytocom effectuated and finalized the reverse split in June 2016. At June 30, 2016, the Company’s
equity interest had been further reduced to 41%, by subsequent issuances of Cytocom common stock to shareholders in settlement
of notes payable.
In
March 2014, the Company incorporated Airmed Biopharma Limited, an Irish corporation with an address in Dublin, Ireland, and Airmed
Holdings Limited, an Irish company domiciled in Bermuda. The Irish companies were set up to benefit from incentives granted by
the Irish government for the establishment of pharmaceutical companies (many of the world’s leading pharmaceutical companies
have located in Ireland), and so that the Company could take advantage of Ireland’s status as a member of the European Union
and the European Economic Area. An Irish limited liability company enjoys a low corporate income tax rate of 12.5%, one of the
lowest in the world. The Irish-domiciled company hopes to qualify for tax incentives for Irish holding/headquartered companies
and to benefit from the network of double tax treaties that reduce withholding taxes. TNI BioTech International, Ltd. will manage
our international distribution, using product that is manufactured in Ireland and elsewhere.
Immune
Therapeutics is focused on the commercialization of affordable non-toxic immunotherapies focused on the activation and rebalancing
of the body’s immune system. Stimulating the body’s immune system remains one of the most promising approaches in
the treatment of Cancers, HIV, Autoimmune Diseases, inflammatory conditions and other opportunistic infections for chronic often
life-threatening diseases through the mobilization of the body’s immune system in Emerging Nations using existing clinical
data.
Cytocom
Inc, is a clinical-stage pharmaceutical company focused on the development of the first affordable non-toxic immunodulator for
the treatment of inflammatory diseases, immune-related disorders, and cancer and is responsible for the development of our patented
therapies with the FDA and EMA.
As
of this date, neither we nor our collaboration partners are permitted to market our drug candidates in the United States until
we receive approval of a New Drug Application from the FDA. Neither we nor our collaboration partners have submitted an application
for or received marketing approval for any of our drug candidates. Obtaining approval of an NDA can be a lengthy, expensive and
uncertain process.
Some
of the Company’s more substantial risks include, but are not limited to, its lack of operating history, its high needs for
capital, strict government regulation, risk of law suits from trial participants and otherwise, requirement for drug approvals
which may never occur, changes in the industry, failure of the Company’s products to make it through trials, reliance on
third parties to conduct trials and manufacture and distribute the Company’s drugs, and fierce competition. All of these
factors and more could affect investors’ investments in the Company.
Emerging
Growth Company
We
qualify as an “emerging growth company” as defined in Section 101 of the Jumpstart our Business Startups Act (“JOBS
Act”), as we do not have more than $1,000,000,000 in annual gross revenue and did not have such amount as of December 31,
2015, the last day of our last fiscal year. We are electing to use the extended transition period for complying with new or revised
accounting standards under Section 102(b)(1) of the JOBS Act.
As
an emerging growth company, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements that are
otherwise applicable to public companies. These provisions include, but are not limited to:
|
|
●
|
being
permitted to present only two years of audited financial statements and only two years of related “Management’s
Discussion and Analysis of Financial Condition and Results of Operations” in this registration statement;
|
|
|
●
|
not
being requested to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended
(“Sarbanes-Oxley Act”);
|
|
|
|
|
|
|
●
|
reduced
disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements;
and
|
|
|
|
|
|
|
●
|
exemptions
from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden
parachute payments not previously approved.
|
We
will remain an emerging growth company until the earliest to occur of: (i) our reporting $1 billion or more in annual gross revenues;
(ii) the end of fiscal year 2019; (iii) our issuance, in a three-year period, of more than $1 billion in non-convertible debt;
and (iv) the end of the fiscal year in which the market value of our common stock held by non-affiliates exceeded $700 million
on the last business day of our second fiscal quarter.
Going
Concern
The
Company has incurred significant net losses since inception and has relied on its ability to fund its operations through private
equity financings. Management expects operating losses and negative cash flows to continue at more significant levels in the future.
As the Company continues to incur losses, transition to profitability is dependent upon the successful development, approval,
and commercialization of its product candidate and the achievement of a level of revenues adequate to support the Company’s
cost structure. The Company may never achieve profitability, and unless and until it does, the Company will continue to need to
raise additional cash. Management intends to fund future operations through additional private or public debt or equity offerings,
and may seek additional capital through arrangements with strategic partners or from other sources. Based on the Company’s
operating plan, existing working capital at December 31, 2015 was not sufficient to meet the cash requirements to fund planned
operations through December 31, 2016 without additional sources of cash. These conditions raise substantial doubt about the Company’s
ability to continue as a going concern. The accompanying financial statements have been prepared assuming that the Company will
continue as a going concern and do not include adjustments that might result from the outcome of this uncertainty. This basis
of accounting contemplates the recovery of the Company’s assets and the satisfaction of liabilities in the normal course
of business.
The
Company experienced a net loss from operations of $7,626,348 and used cash and cash equivalents from operations in the amount
of $1,399,729 during the six months ended June 30, 2016, resulting in stockholders deficit of $6,926,660 at June 30, 2016.
Securities
Purchase Agreement
On
April 12, 2016, we entered into Securities Purchase Agreement (“Purchase Agreement”) with JMJ. Pursuant to the Purchase
Agreement, JMJ loaned the Company $525,000 and was issued a promissory note in the amount of $656,250, 500,000 shares of common
stock, and a warrant exercisable for 3,515,621 additional common shares at a rate of $0.14 per share. The warrants have a cashless
exercise option if the Company were to fail to file this registration statement.
The
Offering
|
Securities
offered
|
|
Up
to 4,015,621 shares of our common stock.
|
|
|
|
|
|
Offering
price
|
|
The
Selling Shareholder will sell at prevailing market prices or through privately negotiated
transactions.
|
|
|
|
|
|
Offering
period
|
|
This
offering is being made on a continuous basis pursuant to Rule 415 under the Securities
Act and will expire two years from the date on which the registration statement related
to this prospectus becomes effective, unless earlier terminated or extended by our Company
by the filing of a post-effective amendment.
|
|
|
|
|
|
Common
stock outstanding before this offering
|
|
220,989,542
shares as of August 12, 2016.
|
|
Common
stock to be outstanding after this offering
|
|
Up
to 224,505,163 provided all outstanding warrants are exercised and no other shares issued.
|
|
|
|
|
|
Use
of
proceeds
|
|
The
Company will not receive any proceeds from this offering.
|
|
|
|
|
|
Risk
factors
|
|
See
“Risk Factors” beginning on page 10 and the other information set forth in this prospectus for a discussion of
factors you should consider before deciding to invest in our securities.
|
|
|
|
|
|
Market
for Common Stock
|
|
Our
common stock is not now listed on any national securities exchange or the NASDAQ stock market. However, our stock is quoted
on OTCQB under the symbol “IMUN.” While our common stock is on the OTCQB, there has been limited trading volume.
There is no guarantee that a more active trading market will develop in our securities.There is also no guarantee that our
securities will ever trade on any listed exchange or even remain quoted on OTCQB.
|
Financial
Summary
Because
this is only a financial summary, it does not contain all the financial information that may be important to you. Therefore, you
should carefully read all the information in this prospectus, including the financial statements and their explanatory notes before
making an investment decision.
|
|
|
For
the year ended
December
31, 2015
|
|
|
For
the year ended
December
31, 2014
|
|
|
Statements Of Operations
|
|
|
|
|
|
|
|
|
|
Revenues
|
|
$
|
16,197
|
|
|
$
|
0
|
|
|
Selling, general and administrative
|
|
$
|
2,734,414
|
|
|
$
|
4,072,277
|
|
|
Research and development
|
|
$
|
977,203
|
|
|
$
|
2,413,286
|
|
|
Depreciation and amortization
|
|
$
|
594,785
|
|
|
$
|
2,879,311
|
|
|
Other Expenses
|
|
$
|
11,512,684
|
|
|
$
|
35,899,378
|
|
|
Total Expenses
|
|
$
|
15,819,086
|
|
|
$
|
45,264,252
|
|
|
Loss from Operations
|
|
$
|
(15,802,889
|
)
|
|
$
|
(45,264,252
|
)
|
|
Net Loss
|
|
$
|
(16,949,451
|
)
|
|
$
|
(49,938,213
|
)
|
|
|
|
As
of
December 31, 2015
|
|
|
As
of
December 31, 2014
|
|
|
Balance Sheet
Data
|
|
|
|
|
|
|
|
|
|
Cash and Cash Equivalents
|
|
$
|
23,149
|
|
|
$
|
191,987
|
|
|
Other Assets
|
|
$
|
18,079
|
|
|
$
|
5,863,003
|
|
|
Total Assets
|
|
$
|
41,228
|
|
|
$
|
6,054,990
|
|
|
Total Liabilities
|
|
$
|
5,999,412
|
|
|
$
|
3,702,558
|
|
|
Stockholder’s Equity (Deficit)
|
|
$
|
(5,958,184
|
)
|
|
$
|
2,352,432
|
|
|
|
|
For
the three months
ended
June
30, 2016
(Q2 2016)
|
|
|
For
the three months ended
June
30, 2015
(Q1 2015)
|
|
|
Statements Of
Operations
|
|
|
|
|
|
|
|
|
|
Revenues
|
|
$
|
-
|
|
|
$
|
5,648
|
|
|
Selling, general and administrative
|
|
$
|
893,917
|
|
|
$
|
443,585
|
|
|
Research and development
|
|
$
|
67,286
|
|
|
$
|
414,492
|
|
|
Stock issues for services G&A
|
|
$
|
1,194,761
|
|
|
$
|
1,450,334
|
|
|
Depreciation and amortization
|
|
$
|
434
|
|
|
$
|
148,726
|
|
|
Warrant Valuation
|
|
$
|
490,355
|
|
|
|
-
|
|
|
Total Expenses
|
|
$
|
2,646,729
|
|
|
$
|
2,457,137
|
|
|
Loss from Operations
|
|
$
|
(2,646,729
|
)
|
|
$
|
(2,451,789
|
)
|
|
Net Loss
|
|
$
|
(4,038,648
|
)
|
|
$
|
(2,590,645
|
)
|
|
|
|
As
of June 30, 2016
(Q2 2016)
|
|
|
Balance Sheet
Data
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
64,289
|
|
|
Accounts receivable
|
|
$
|
2,661
|
|
|
Prepaids and other current assets
|
|
$
|
11,272
|
|
|
Total Current Assets
|
|
$
|
78,222
|
|
|
Computers and equipment, net of accumulated
depreciation
|
|
$
|
701
|
|
|
Deposits
|
|
$
|
200
|
|
|
Total Assets
|
|
$
|
79,123
|
|
|
Total Liabilities
|
|
$
|
(7,005,783
|
)
|
|
Stockholder’s Equity (Deficit)
|
|
$
|
(6,926,660
|
)
|
ABOUT
THIS PROSPECTUS
We
have prepared this prospectus as part of a registration statement that we filed with the SEC for our offering of securities. The
registration statement we filed with the SEC includes exhibits that provide more detailed descriptions of the matters discussed
in this prospectus. You should read this prospectus and the related exhibits filed with the SEC, together with additional information
described below under “Additional Information.”
You
should rely only on the information contained in this prospectus. Neither we nor any underwriters have authorized any other person
to provide you with any information different from that contained in this prospectus or information furnished by us upon request
as described herein. The information contained in this prospectus is complete and accurate only as of the date of this prospectus,
regardless of the time of delivery of this prospectus or sale of our shares. This prospectus contains summaries of certain other
documents, which summaries contain all material terms of the relevant documents and are believed to be accurate, but reference
is hereby made to the full text of the actual documents for complete information concerning the rights and obligations of the
parties thereto. Such information necessarily incorporates significant assumptions, as well as factual matters. All documents
relating to this offering and related documents and agreements, if readily available to us, will be made available to a prospective
investor or its representatives upon request.
No
information contained herein, nor in any prior, contemporaneous or subsequent communication should be construed by a prospective
investor as legal or tax advice. Each prospective investor should consult its, his or her own legal, tax and financial advisors
to ascertain the merits and risks of the transactions described herein prior to purchasing our shares. This written communication
is not intended to be “written advice,” as defined in Circular 230 published by the U.S. Treasury Department.
INDUSTRY
AND MARKET DATA
The
industry and market data used throughout this prospectus have been obtained from our own research, surveys or studies conducted
by third parties and industry or general publications. Industry publications and surveys generally state that they have obtained
information from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information. We believe
that each of these studies and publications is reliable.
TAX
CONSIDERATIONS
We
are not providing any tax advice as to the acquisition, holding or disposition of the securities offered herein. In making an
investment decision, investors are strongly encouraged to consult their own tax advisor to determine the U.S. Federal, state and
any applicable foreign tax consequences relating to their investment in our securities.
RISK
FACTORS
In
addition to the other information provided in this prospectus, you should carefully consider the following risk factors in evaluating
our business before purchasing any of our common stock. All material risks are discussed in this section.
Risks
Related to our Business
We
have a limited operating history and are expected to incur significant operating losses during the early stage of our corporate
development.
We
have a limited operating history. Our historical financial information consists only of an audit of our financial results at and
for the years ended December 31, 2015, 2014, 2013 and 2012. There is limited historical financial information upon which to base
an evaluation of our performance. We are an emerging company, and thus our prospects must be considered in light of the uncertainties,
risks, expenses, and difficulties frequently encountered by companies in their early stages of operation, particularly in the
pharmaceutical industry.
Since
inception, we have invested a substantial portion of our time and financial resources in the acquisition and development of our
most advanced drug candidate, LDN. We have generated cumulative losses of approximately $345 million and $6,926,660 stockholders’
deficit since inception, and we expect to continue to incur losses until IRT-103 (LDN) is approved by the FDA and foreign regulatory
authorities. Even if regulatory approval is obtained, there is a risk that we will not be able to generate material sales of IRT-103
(LDN), which would cause us to continue to incur losses.
We
may never generate revenue, are not profitable and may never become profitable.
We
expect to incur substantial losses and negative operating cash flow for the foreseeable future, and we may never achieve or maintain
profitability. Even if we are able to launch IRT-103 (LDN) we expect to incur substantial losses for the foreseeable future and
may never become profitable.
We
do not anticipate that we will generate revenue from the sale of our products for the foreseeable future. In addition, if approved,
we expect to incur significant costs to commercialize our drug candidates and our drugs may never gain market acceptance. If our
drug candidates fail to demonstrate safety and efficacy in clinical trials, do not gain regulatory approval, or do not achieve
market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain
profitability in subsequent periods. If we are unable to achieve and sustain profitability, the market value of our common stock
will likely decline. Because of the numerous risks and uncertainties associated with developing pharmaceutical products, we are
unable to predict the extent of any future losses or whether we will become profitable.
We
will see losses from our clinical trials conducted either directly or through our subsidiaries for the foreseeable future, and
if we fail at one or more of our clinical trials, it could affect the value of the Company’s stock.
We
rely on financings to fund and conduct clinical trials directly or through our subsidiaries needed for NDA submission with respect
to IRT-103 (LDN). Any of the following events or factors could have a material adverse effect on our ability to generate revenue
from the commercialization of IRT-103 (LDN):
|
|
●
|
The
Company may be unable to successfully complete the clinical development of IRT-103 (LDN);
|
|
|
|
|
|
|
●
|
The
Company must comply with any possible additional requests and recommendations from the FDA, including additional clinical
trials;
|
|
|
|
|
|
|
●
|
The
Company may not obtain all necessary approvals from the FDA and similar foreign regulatory agencies;
|
|
|
|
|
|
|
●
|
The
Company may not commit sufficient resources to the development, regulatory approval, marketing and distribution of IRT-103
(LDN);
|
|
|
|
|
|
|
●
|
IRT-103
(LDN) must be manufactured in compliance with requirements of the FDA and similar foreign regulatory agencies and in commercial
quantities sufficient to meet market demand;
|
|
|
●
|
IRT-103
(LDN) may not achieve market acceptance by physicians, patients and third party payers;
|
|
|
|
|
|
|
●
|
IRT-103
(LDN) may not successfully compete against alternative products and therapies; and
|
|
|
|
|
|
|
●
|
The
Company or any other pharmaceutical organization may independently develop products that compete with IRT-103 (LDN).
|
To
obtain approval from the FDA of an NDA, for IRT-103 (LDN), The Company will need to demonstrate through evidence of adequate and
well-controlled clinical trials that IRT-103 (LDN) is safe and effective for each proposed indication. However, IRT-103 (LDN)
may not be approved even though it achieved its specified endpoints in future Phase III clinical trials intended to support an
NDA, which may be conducted by the Company. The FDA may disagree with the trial design and the interpretation of data from clinical
trials, may ask the Company to conduct additional costly and time consuming clinical trials in order to obtain marketing approval
or approval to enter into an advanced phase of development, or may change the requirements for approval even after it has reviewed
and commented on the design for our future clinical trials. The FDA may also approve IRT-103 (LDN) for fewer or more limited indications
than the Company may request, or may grant approval contingent on the performance of costly post-approval clinical trials. In
addition, the FDA may not approve the labeling claims that we believe are necessary or desirable for the successful commercialization
of IRT-103 (LDN).
The
Company anticipates that if Cytocom initiates a clinical trial in the next 12 months, Cytocom would need approximately $7-$15
million to fully develop products and for Phase III clinical trials for Crohn’s disease. We expect that two-thirds of this
amount will be spent by Cytocom in the USA, the balance by Immune Therapeutics, Inc. and/or its subsidiaries for international
trials. Cytocom trials are expected to be split evenly between LDN and MENK. The international trials will focus the use of MENK
for treatment of cancer in Africa.
The
development of new drugs is a highly risky undertaking which involves a lengthy process, and therefore our drug discovery and
development activities may not result in products that are approved by the applicable regulatory authorities on the time schedule
we have planned, or at all.
Our
drug candidates are in early stages of drug discovery or clinical trials and are prone to the risks of failure inherent in drug
development. As of the date of this Form 10-K, both of our current drug candidates, IRT-101 (MENK) and IRT-103 (LDN) have been
tested on human beings. We will need to conduct additional clinical trials before we can demonstrate that our drug candidates
are safe and effective to the satisfaction of the FDA and other regulatory authorities. Clinical trials are expensive and uncertain
processes that can take multiple years to complete. We cannot assure you that our ongoing clinical trials or any future clinical
trial of any of our other drug candidates, will be completed on schedule, or at all, or whether our planned clinical trials will
start in a timely manner. The commencement of our planned clinical trials could be substantially delayed or prevented by a number
of factors, including:
|
|
●
|
delays or failures in obtaining sufficient
quantities of the API and/or drug product;
|
|
|
|
|
|
|
●
|
delays or failures in reaching an
agreement on acceptable clinical trial agreement terms or clinical trial protocols with prospective sites and with the FDA
or other foreign regulatory bodies;
|
|
|
|
|
|
|
●
|
delays or failures in obtaining Institutional
Review Board (“IRB”) or Ethics Committee (“EC”) approvals to conduct a clinical trial at a prospective
site;
|
|
|
|
|
|
|
●
|
the need to successfully complete,
on a timely basis, preclinical safety pharmacology studies (for IRT-101 (MENK));
|
|
|
|
|
|
|
●
|
the limited number of, and competition
for, suitable sites to conduct the clinical trials;
|
|
|
|
|
|
|
●
|
the limited number of, and competition
for, suitable patients for enrollment in the clinical trials; and
|
|
|
|
|
|
|
●
|
delays or failures in obtaining regulatory
approval to commence a clinical trial.
|
The
completion of our clinical trials could also be substantially delayed or prevented by a number of factors, including:
|
|
●
|
slower
than expected rates of patient recruitment and enrollment;
|
|
|
|
|
|
|
●
|
failure
of patients to complete the clinical trials;
|
|
|
|
|
|
|
●
|
failure
of our third party vendors to timely or adequately perform their contractual obligations relating to the clinical trials;
|
|
|
|
|
|
|
●
|
inability
or unwillingness of patients or medical investigators to follow our clinical trial protocols;
|
|
|
|
|
|
|
●
|
inability
to monitor patients adequately during or after treatment;
|
|
|
|
|
|
|
●
|
termination
of the clinical trials by one or more clinical trial sites;
|
|
|
|
|
|
|
●
|
unforeseen
safety issues;
|
|
|
|
|
|
|
●
|
lack
of efficacy demonstrated during clinical trial results;
|
|
|
|
|
|
|
●
|
lack
of adequate funding to continue the clinical trials;
|
|
|
|
|
|
|
●
|
the
need for unexpected discussions with the FDA or other foreign regulatory agencies regarding the scope or design of our clinical
trials or the need to conduct additional trials;
|
|
|
|
|
|
|
●
|
unforeseen
delays by the FDA or other foreign regulatory agencies after submission of our results;
|
|
|
|
|
|
|
●
|
an
unfavorable FDA inspection of our contract manufacturers of APIs or drug products; and/or
|
|
|
|
|
|
|
●
|
inspection
of the clinical trial operations or trial sites by the FDA or other regulatory authorities resulting in the imposition of
a clinical hold.
|
Any
failure or significant delay in completing clinical trials for our drug candidates will harm the commercial prospects for our
drug candidates and adversely affect our financial results.
Additionally,
changes in regulatory requirements and guidance may occur and we may need to amend clinical trial protocols to reflect these changes.
Amendments may require us to resubmit our clinical trial protocols to IRBs or ECs for reexamination, which may impact the costs,
timing or successful completion of a clinical trial. If we experience delays in completion of a clinical trial, or if we terminate
any of our clinical trials, the commercial prospects for our drug candidates may be harmed and our ability to generate product
revenues will be delayed. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of
clinical trials may also ultimately lead to the denial of regulatory approval of a drug candidate.
If
we are required to suspend or discontinue clinical trials due to side effects or other safety risks, or if we are required to
conduct studies on the long-term effects associated with the use of our drug candidates, our efforts to commercialize our products
could be delayed or halted.
Our
clinical trials may be suspended or terminated at any time for a number of safety-related reasons. For example, administering
any drug candidate to humans may produce undesirable side effects. We may voluntarily suspend or terminate our clinical trials
if at any time we believe that our drug candidates present an unacceptable safety risk to the clinical trial patients. In addition,
IRBs, ECs or regulatory agencies may order the temporary discontinuation or termination of our clinical trials at any time if
they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements, including
if they present an unacceptable safety risk to patients. The existence of undesirable side effects resulting from our drug candidates
could cause us or regulatory authorities, such as the FDA, to interrupt, delay or halt clinical trials of our drug candidates
and could result in the FDA or other regulatory agencies denying further development or approval of our drug candidates for any
or all targeted indications.
Further,
cytokine receptors and opiate growth factor receptors are a novel class of targets. As a result, we may experience unforeseen
adverse side effects with our existing and future drug candidates, including IRT-101 (MENK) and IRT-103
(LDN).
As of the date of this registration statement, although we have not observed harmful side effects in prior studies of LDN or MENK,
later trials could reveal such side effects. The pharmacokinetic profile and results of preclinical studies may not be indicative
of results in any clinical trial.
We
have not conducted studies on the long-term effects associated with the use of our drug candidates. Studies of long-term effects
and chronic dosing (approximately 1 year of dosing); will be required for regulatory approval and may delay introduction of our
therapies or our other drug candidates into the market. Additional studies could also be required at any time after regulatory
approval of any of our drug candidates. Some or all of our drug candidates may prove to be unsafe for human use.
Even
if our drug candidates do obtain regulatory approval they may never achieve market acceptance or commercial success.
Even
if we obtain FDA or other regulatory approval, our drug candidates may not achieve market acceptance among physicians, patients
and/or third party payers or they may be used only in applications more restricted than we anticipate, and ultimately, may not
be commercially successful. Our treatments, if successfully developed, will compete with a number of traditional products manufactured
and marketed by major pharmaceutical and biotechnology companies. Our treatments may also compete with new products currently
under development by such companies and others. Physicians will prescribe a product only if they determine, based on experience,
clinical data, side effect profiles and other factors, that it is beneficial as compared to other products currently available
and in use. Physicians also will prescribe a product based on their traditional preferences. Market acceptance of our drug candidates
for which we receive approval depend on a number of factors, including:
|
|
●
|
the
efficacy and safety of our drug candidates as demonstrated in clinical trials;
|
|
|
|
|
|
|
●
|
the
clinical indications for which the drug is approved;
|
|
|
|
|
|
|
●
|
acceptance
by physicians, major operators of clinics and patients of the drug as a safe and effective treatment;
|
|
|
|
|
|
|
●
|
the
potential and perceived advantages of our drug candidates over alternative treatments;
|
|
|
|
|
|
|
●
|
the
safety of drug candidates seen in a broader patient group, including its use outside the approved indications;
|
|
|
|
|
|
|
●
|
the
cost of treatment in relation to alternative treatments;
|
|
|
|
|
|
|
●
|
the
availability of adequate reimbursement and pricing by third parties and government authorities;
|
|
|
|
|
|
|
●
|
relative
convenience and ease of administration;
|
|
|
|
|
|
|
●
|
the
prevalence and severity of adverse side effects; and
|
|
|
|
|
|
|
●
|
the
effectiveness of our sales and marketing efforts.
|
If
our drug candidates that obtain regulatory approval fail to achieve market acceptance or commercial success, the Company’s
financial results will be adversely affected.
The
commercial success of IRT-103 depends, in part, on Cytocom’s ability to develop and market the drug in North America, and
if we fail in these initiatives, our ability to generate future revenue in the United States could be reduced.
If
Cytocom successfully completes the clinical development program in the U.S. for our lead independent drug candidate, IRT-103 (LDN),
we plan to retain commercial rights to IRT-103 as we have exclusive licensing rights. Any of the following events or factors could
have a material adverse effect on both the ability to generate revenue in the U.S. from the commercialization of IRT-103:
|
|
●
|
we
may be unable to successfully complete the clinical development of IRT-103;
|
|
|
●
|
our
lack of experience in commercializing and marketing drug products;
|
|
|
|
|
|
|
●
|
we
may not have or be able to obtain sufficient financial resources to develop and commercialize IRT-103;
|
|
|
|
|
|
|
●
|
we
may not be able to identify a suitable co-development partner;
|
|
|
|
|
|
|
●
|
we,
or any of our future partners, may fail to fulfill our responsibilities in a timely manner or fail to commit sufficient resources
to the development, regulatory approval, and commercialization efforts related to IRT-103;
|
|
|
|
|
|
|
●
|
we,
or any of our future partners, must comply with additional requests and recommendations from the FDA, including additional
clinical trials;
|
|
|
|
|
|
|
●
|
we,
or any of our future partners, may not obtain all necessary approvals from the FDA and similar foreign regulatory agencies;
|
|
|
|
|
|
|
●
|
IRT-103
must be manufactured in compliance with requirements of the FDA and similar foreign regulatory agencies and in commercial
quantities sufficient to meet market demand;
|
|
|
|
|
|
|
●
|
IRT-103
may not achieve market acceptance by physicians, patients and third party payers;
|
|
|
|
|
|
|
●
|
IRT-103
may not compete successfully against alternative products and therapies; and
|
|
|
|
|
|
|
●
|
we,
or any pharmaceutical company, may independently develop products that compete with IRT-103.
|
Changes
in pharmaceutical and biotechnology industry trends could adversely affect the Company’s operating results.
Industry
trends, economic and political factors that affect pharmaceutical, biotechnology, medical device companies and academic/government
entities sponsoring clinical research directly affect the Company’s business. For example, many companies in such industries
and government organizations have been hiring companies (like the Company) to conduct large development projects. The Company’s
operations, financial condition and growth rate could be materially and adversely affected if these industries reduce outsourcing
of such projects. In the past, mergers, product withdrawals, liability lawsuits and other factors in the pharmaceutical industry
have slowed decision making by pharmaceutical companies and correlating government bodies significantly delaying and/or halting
drug development projects. Continuation or increases in such trends could have an adverse effect on the Company’s business.
Additionally, numerous government agencies have undertaken efforts to control growing healthcare costs through legislation, regulation
and voluntary agreements with medical care providers and pharmaceutical companies. If future regulatory cost-containment efforts
limit potential profits derived from new drugs, the Company’s clients may reduce their drug discovery and development spending.
A reduction in drug discovery and development spending could have a material adverse effect on the Company’s results and
operations creating a significant reduction of the Company’s revenue.
We
currently rely on third parties to conduct all our clinical trials. If these third parties do not successfully carry out their
contractual duties or meet expected deadlines, we may be unable to obtain regulatory approval for or commercialize any of our
drug candidates.
We
currently do not have the ability to independently conduct clinical trials. We rely on medical institutions, clinical investigators,
contract laboratories, collaborative partners and other third parties, such as contract research organizations, to conduct clinical
trials on our drug candidates. The third parties with whom we contract for execution of our clinical trials play a significant
role in the conduct of these trials and the subsequent collection and analysis of data. These third parties are not our employees,
and except for contractual duties and obligations, we have limited ability to control the amount or timing of resources that they
devote to our programs. Our IND is being conducted per 21 Code of Federal Regulations Title 21, Part 312. In addition, we follow
ICH guidelines, including good clinical practices (ICH E6) and current good manufacturing practice (ICH Q7) throughout the development
process. After completion of Phase III clinical trials, the Company will file our NDA for LDN (IRT-103) as a 505(b)(2) application.
Although we rely on these third parties to conduct our clinical trials, we remain responsible for ensuring that each of our preclinical
studies and clinical trials is conducted in accordance with its investigational plan and protocol. Moreover, the FDA and foreign
regulatory authorities
require
us to comply with regulations and standards, commonly referred to as current Good Clinical Practices (“cGCPs”) for
conducting, monitoring, recording and reporting the results of clinical trials to ensure that the data and results are scientifically
credible and accurate and that the trial subjects are adequately informed of the potential risks of participating in clinical
trials.
In
addition, the execution of preclinical studies and clinical trials, and the subsequent compilation and analysis of the data produced,
requires coordination among various parties. In order for these functions to be carried out effectively and efficiently, it is
imperative that these parties communicate and coordinate with one another. Moreover, these third parties may also have relationships
with other commercial entities, some of which may compete with us. In most cases, these third parties may terminate their agreements
upon a material breach by us that is not cured within 30 days by providing us with 30 days’ prior written notice. Many of
these agreements may also be terminated by such third parties under certain other circumstances, including our insolvency or our
failure to comply with applicable laws. In general, these agreements require such third parties to reasonably cooperate with us
at our expense for an orderly winding down of services of such third parties under the agreements. If the third parties conducting
our clinical trials do not perform their contractual duties or obligations, experience work stoppages, do not meet expected deadlines,
terminate their agreements with us or need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised
due to their failure to adhere to our clinical trial protocols or cGCPs, or for any other reason, we may need to enter into new
arrangements with alternative third parties, which could be costly, and our clinical trials may be extended, delayed or terminated
or may need to be repeated, and we may not be able to obtain regulatory approval for or commercialize the drug candidate being
tested in such trials.
If
any of our drug candidates receive marketing approval, and the Company or others later identify undesirable side effects caused
by the drug candidate, our ability to market and derive revenue from the drugs could be compromised.
If
the Company or others identify undesirable side effects caused by one of our drug candidates, any of the following adverse events
could occur:
|
|
●
|
regulatory
authorities may withdraw approval of the drug or seize the drug;
|
|
|
|
|
|
|
●
|
we
may be required to recall the drug or change the way the drug is administered;
|
|
|
|
|
|
|
●
|
additional
restrictions may be imposed on the marketing or the manufacturing processes of the particular drug;
|
|
|
|
|
|
|
●
|
we
may be subject to fines, injunctions or the imposition of civil or criminal penalties;
|
|
|
|
|
|
|
●
|
regulatory
authorities may require the addition of labeling statements, such as a “black box” warning or a contraindication;
|
|
|
|
|
|
|
●
|
we
may be required to create a medication guide outlining the risks of such side effects for distribution to patients;
|
|
|
|
|
|
|
●
|
we
could be sued and held liable for harm caused to patients;
|
|
|
|
|
|
|
●
|
the
drug may become less competitive; and
|
|
|
|
|
|
|
●
|
our
reputation may suffer.
|
Any
of these could result in the loss of significant revenues, which would materially and adversely affect our results of operations
and business.
We
may need additional financing and may be unable to raise capital on acceptable terms, or at all, when needed, which could force
us to delay, reduce or eliminate our research and development programs and other operations or commercialization efforts.
We
are advancing multiple drug candidates through discovery and development and will require substantial funds to conduct development,
including preclinical studies and clinical trials, of our drug candidates. Commercialization of any drug candidate will also require
substantial expenditures. To further the development and commercialization efforts of our drug candidates, we may need additional
financing to hire additional employees to co-promote drug candidates or to commercialize drug candidates that may not be covered
by our current collaboration agreements.
At
December 31, 2015, we had $23,149 in cash and cash equivalents. We do not believe that our available cash and cash equivalents
will be sufficient to fund our anticipated level of operations for the next 12 months and we will likely need to seek outside
sources of funding. Assuming that anticipated investment and revenue does not materialize business operations would not be able
to continue more than 30 days. We believe we require at least $2,000,000 for our operations over the next 12 months. Our future
financing requirements will depend on many factors, some of which are beyond our control, including:
|
|
●
|
the
rate of progress and cost of our clinical trials, preclinical studies and other discovery and research and development activities;
|
|
|
|
|
|
|
●
|
the
timing of, and costs involved in, seeking and obtaining FDA and other regulatory approvals;
|
|
|
|
|
|
|
●
|
the
continuation and success of our strategic alliances and future collaboration partners;
|
|
|
|
|
|
|
●
|
the
exercise of remaining options under current collaborative agreements;
|
|
|
|
|
|
|
●
|
the
costs of preparing, filing, prosecuting, maintaining and enforcing any patent claims and other intellectual property rights,
including litigation costs and the results of such litigation;
|
|
|
|
|
|
|
●
|
our
ability to enter into additional collaboration, licensing, government or other arrangements and the terms and timing of such
arrangements;
|
|
|
|
|
|
|
●
|
potential
acquisition or in-licensing of other products or technologies; and
|
|
|
|
|
|
|
●
|
the
technologies or other adverse market developments.
|
Future
capital requirements will also depend on the extent to which we acquire or invest in additional complementary businesses, products
and technologies. We currently have no understandings, commitments or agreements relating to any of these types of transactions.
Until
we can generate a sufficient amount of product revenue to finance our cash requirements, which we may never do, we expect to finance
future cash needs primarily through public or private equity offerings, debt financings, government grants and contracts and/or
strategic collaborations. Additional financing may not be available to us when we need it or it may not be available on favorable
terms, if at all. If we are unable to obtain adequate financing when needed, we may have to delay, reduce the scope of or eliminate
one or more of our clinical trials or research and development programs or our commercialization efforts. We may be required to
enter into collaborative partnerships for one or more of our drug candidate programs at an earlier stage of development or on
less favorable terms, which may require us to relinquish rights to some of our drug candidates that we would otherwise have pursued
on our own. We may also be required to pursue strategic alternatives that may affect our business or corporate structure in order
to make ourselves more attractive to investors.
In
addition, If the Company or any of its future collaboration partners does not perform in the manner we expect or fulfill its responsibilities
in a timely manner, or at all, the clinical development, regulatory approval, and commercialization efforts related to IRT-103
(LDN) could be delayed or terminated. It may be necessary for us to assume the responsibility at our own expense for the development
of IRT-103 (LDN). In that event, we would likely be required to seek additional funding.
We
may form additional strategic alliances in the future with respect to our independent programs, and we may not realize any benefits
of such alliances.
We
may form strategic alliances, create joint ventures or collaborations or enter into licensing arrangements with third parties
with respect to our independent programs that we believe will complement or augment our existing business. For example, we plan
to find a partner to co-develop and commercialize IRT-101 (MENK) and IRT-103 (LDN) outside North America upon completion of clinical
development of IRT-103 (LDN) for the treatment of pediatric and adult patients with Crohn’s disease. We face significant
competition in seeking appropriate strategic partners. The negotiation process is time-consuming and complex. Moreover, we may
not be successful in our efforts to establish a strategic partnership or other alternative arrangements for any future product
candidates and programs because our research and development pipeline may be
insufficient,
our product candidates and programs may be deemed to be at too early a stage of development for collaborative effort and/or third
parties may not view our product candidates and programs as having the requisite potential to demonstrate safety and efficacy.
We cannot be certain that, following a strategic transaction or license, we will achieve the revenues or specific net income that
justifies such transactions. Any delays in entering into new strategic partnership agreements related to our product candidates
could also delay the development and commercialization of our product candidates and reduce their competitiveness even if they
reach the market.
We
do not currently manufacture IRT-103 Low Dose Naltrexone (LDN) and therefore must rely on third-party manufacturing to supply
the drug for clinical trials. If one of our suppliers or manufacturers fails to perform adequately or fulfill our needs, we may
be required to incur significant costs and devote significant efforts to find new suppliers or manufacturers, which would cause
delays in the development and commercialization of our drug candidates.
The
manufacture of pharmaceutical products in compliance with cGMPs requires significant expertise and capital investment, including
the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products often encounter
difficulties in production, including difficulties with production costs and yields, quality control, including stability of the
drug candidate and quality assurance testing, shortages of qualified personnel, as well as compliance with strictly enforced FDA
cGMP requirements, other federal and state regulatory requirements, and foreign regulations. If our manufacturers were to encounter
any of these difficulties or otherwise fail to comply with their obligations to us or under applicable regulations, our ability
to provide study drugs in our preclinical studies and clinical trials would be jeopardized. Any delay or interruption in the supply
of preclinical study or clinical trial materials could delay the completion of our preclinical studies and clinical trials, increase
the costs associated with maintaining our preclinical study and clinical trial programs and, depending upon the period of delay,
require us to commence new trials at significant additional expense or terminate the studies and trials completely.
All
manufacturers of our drug candidates must comply with cGMP requirements enforced by the FDA through its facilities inspection
program. These requirements include, among other things, quality control, quality assurance and the maintenance of records and
documentation. Manufacturers of our component materials may be unable to comply with these cGMP requirements and with other FDA,
state and foreign, regulatory requirements. The FDA or similar foreign regulatory agencies at any time may also implement new
standards, or change their interpretation and enforcement of existing standards for manufacture, packaging or testing of products.
We have little control over our manufacturers’ compliance with these regulations and standards. A failure to comply with
these requirements may result in fines and civil penalties, suspension of production, suspension or delay in product approval,
product seizure or recall, or withdrawal of product approval. If the safety of any product supplied is compromised due to our
manufacturers’ failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval
for or successfully commercialize our products, and we may be held liable for any injuries sustained as a result. Any of these
factors could cause a delay of clinical trials, regulatory submissions, approvals or commercialization of our drug candidates
or entail higher costs or impair our reputation.
We
source the API for IRT-103 (LDN) from a third-party manufacturing vendor. Another pharmaceutical company manufactures the API
for IRT-101. Our current agreements with our suppliers provide for the entire supply of the API necessary for additional clinical
trials or for full-scale commercialization. In the event that we and our suppliers cannot agree to the terms and conditions for
them to continue to provide some or all of our API clinical and commercial supply needs, or if any single source supplier terminates
the agreement in response to a breach by us, we would not be able to manufacture the API on a commercial scale until a qualified
alternative supplier is identified, which could also delay the development of, and impair our ability to commercialize, our drug
candidates.
Although
alternative sources of supplies exist, the number of third party suppliers with the necessary manufacturing and regulatory expertise
and facilities are limited, and it could be expensive and take a significant amount of time to arrange for alternative suppliers,
which could have a material adverse effect on our business. New suppliers of any API would be required to qualify under applicable
regulatory requirements and would need to have sufficient rights to the method of manufacturing such ingredients under applicable
intellectual property laws. Obtaining the necessary FDA approvals or other qualifications under applicable regulatory requirements
and ensuring non-infringement of third party intellectual property rights could result in a significant interruption of supply
and could require the new manufacturer to bear significant additional costs which may be passed on to us.
We
currently have only a limited distribution organization with no sales and marketing staff. If we are unable to develop sales and
marketing and expand distribution capability on our own or through collaborations with marketing partners, we will not be successful
in commercializing our future products.
We
currently have only a limited distribution organization with no sales or marketing staff. If our products are approved for sale
in the United States we will need to execute a number of sales and marketing agreements, but there can be no assurance that the
Company will be able to sign an agreement to market and distribute our products. To the extent we rely on third parties for marketing
and distributing our approved products, any revenue we receive will depend upon the efforts of third parties, which may not be
successful, and are only partially within our control. Our reliance on third parties makes it likely that our product revenue
is likely to be lower than if we directly marketed or sold our products. If we are unable to enter into arrangements with third
parties to commercialize the approved products on acceptable terms or at all, we may not be able to successfully commercialize
our future products or we will have to market these products ourselves, which will be expensive and require us to build our own
sales force, which we do not have experience doing. We cannot assure you we will be successful in any of these initiatives. If
we are not successful in commercializing our future products, either on our own or through collaborations with one or more third
parties, our future product revenue will be materially adversely affected.
We
are dependent on market acceptance of compounding pharmacies and compounded formulations, and physicians may be unwilling to prescribe,
and patients may be unwilling to use, our proprietary LDN compounded formulation.
We
are currently distributing our proprietary LDN formulation through Complete Pharmacy and Medical Solutions, LLC and expect to
distribute such formulation through other compounding pharmacies outside of the U.S. Formulations prepared and dispensed by compounding
pharmacies contain FDA-approved ingredients, but are not themselves approved by the FDA. As a result, our formulation has not
undergone the FDA approval process and only limited data, if any, may be available with respect to the safety and efficiency of
our formulation for any particular indication. Some physicians may be hesitant to prescribe, and some patients may be hesitant
to purchase and use, this non-FDA approved compounded formulation. In addition, certain compounding pharmacies have been the subject
of widespread negative media coverage in recent years, and the actions of these pharmacies have resulted in increased scrutiny
of compounding pharmacy activities from the FDA and state governmental agencies. As a result, physicians may be unwilling to prescribe
a compounded formulation when an FDA-approved alternative is available, even if they believe the compounded formulation to be
superior and less expensive. Other reasons physicians may be unwilling to prescribe or patients may be unwilling to use our proprietary
LDN compounded formulation could include the following, among others: our proprietary formulation is not required to be, and has
not been, approved for marketing and sale by the FDA; there may be limited or no data available with respect to the clinical efficacy
or safety of our compounded formulation the physician is prescribing; and to the extent there is such data available, we are limited
in our ability to discuss the efficacy or safety of our formulation with potential purchasers of our formulation.
Additionally,
some third-party payors, including the government Medicare and Medicaid programs, may not provide reimbursement for compounded
formulations. Physicians who may otherwise be interested in prescribing our formulation or utilizing our compounding pharmacy
services may be unwilling to do so if third party payor reimbursement, including Medicare and Medicaid reimbursement, is not available
for our compounded formulation. Any failure by physicians, patients and/or third-party payors to accept and embrace compounded
formulations could substantially limit our market and cause our operations to suffer.
We
aim to generate revenue from our proprietary LDN formulation through our licensing arrangement with Complete Pharmacy and Medical
Solutions, LLC and potentially other compounding pharmacies outside of the United States, but we may not be successful in our
efforts to generate revenue from such formulation.
One
aspect of our business strategy is to continue to develop our licensing arrangement with Complete Pharmacy and Medical Solutions,
LLC and potentially enter into other licensing arrangements with other compounding pharmacies outside of the U.S., through which
we can generate revenue from the sale of our proprietary LDN formulation. On December 8, 2014, we entered into a Contract for
the Compounding of Pharmaceutical Products with Complete Pharmacy and Medical Solutions, LLC pursuant to which Complete Pharmacy
and Medical Solutions, LLC will carry out the services of compounding, packaging and distributing tablets of our LDN formulation
in the U.S. We have limited experience commercializing our formulation through licensing arrangements with compounding pharmacies.
Even if we are successful, we may be unable to generate sufficient revenue to recover our costs.
We
have minimal experience licensing products to pharmacies and outsourcing facilities and we may not be successful in our efforts
to develop our licensing arrangements. If we elect to license our proprietary LDN formulation to one or more pharmacies or outsourcing
facilities outside of the U.S., we may not be able to enter into licensing agreements when desired,
on
acceptable terms, or at all. Establishing licensing or other relationships with pharmacies and outsourcing facilities could be
expensive and time consuming, disrupt our other operations, require significant capital expenditures and distract management and
our other employees from other aspects of our business.
Failure
to achieve and maintain effective internal controls could have a material adverse effect on our business.
Effective
internal controls are necessary for us to safeguard our assets and provide reliable financial reports. If we cannot provide reliable
financial reports, our operating results could be harmed. All internal control systems, no matter how well designed, have inherent
limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial
statement preparation and presentation.
While
we continue to evaluate and improve our internal controls, we are a small company with limited staff, and we cannot be certain
that the measures we implement will ensure that we design, undertake and maintain adequate controls over our financial processes
and reporting in the future. Any failure to implement required new or improved controls, or difficulties encountered in their
implementation, could harm our operating results or cause us to fail to meet our reporting obligations.
Failure
to achieve and maintain an effective internal control environment could cause investors to lose confidence in our reported financial
information, which could have a material adverse effect on our stock price. In addition, if our efforts to comply with new or
changed laws, regulations, and standards differ from the activities intended by regulatory or governing bodies due to ambiguities
related to practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
Our
independent registered public accounting firm has identified material weaknesses in our financial reporting process.
Our
independent registered public accounting firm has identified two material weaknesses in our financial reporting process. Specifically,
our independent registered public accounting firm identified material weaknesses with respect to:
|
|
●
|
currently
inadequate segregation of duties by management in the financial reporting area; and
|
|
|
|
|
|
|
●
|
the
lack of an audit committee to oversee the financial reporting process.
|
We
intend to remediate this weakness by increasing the size of our accounting staff in 2016 and by appointing an audit committee
with membership that is qualified to oversee the Company’s financial reporting. However, there can be no assurance that
we will be able to successfully implement our plans to remediate the material weaknesses in our financial reporting process. Our
failure to successfully implement our plans to remediate these material weaknesses could cause us to fail to meet our reporting
obligations, to produce timely and reliable financial information, and to effectively prevent fraud. Additionally, such failure,
or other weaknesses that we may experience in our financial reporting process or other internal controls, could cause investors
to lose confidence in our reported financial information, which could have a negative impact on our financial condition and stock
price.
We
will need to increase the size of our organization, but we may experience difficulties in managing growth.
We
will need to continue to expand our managerial, operational, financial and other resources in order to manage our operations and
clinical trials, continue our development activities and commercialize our drug candidates. Our current management, personnel
systems and facilities may not be adequate to support this future growth. Our need to effectively execute our growth strategy
requires that we:
|
|
●
|
manage
our clinical trials effectively, including our clinical trials for IRT-103 (LDN) which will be conducted at numerous trial
sites throughout the world;
|
|
|
|
|
|
|
●
|
manage
our internal development efforts effectively while carrying out our contractual obligations to licensors, contractors, collaborators,
government agencies and other third parties;
|
|
|
|
|
|
|
●
|
manage
operations in both regulated and unregulated businesses;
|
|
|
|
|
|
|
●
|
continue
to improve our operational, financial and management controls and reporting systems and procedures; and
|
|
|
●
|
identify,
recruit, maintain, motivate and integrate additional employees.
|
If
we are unable to expand our managerial, operational, financial and other resources to the extent required to manage our development
and commercialization activities, our business will be materially adversely affected.
We
face substantial competition. Our competitors may discover, develop or commercialize products faster or more successfully than
us.
The
biotechnology and pharmaceutical industries are highly competitive. We face significant competition from companies in the pharmaceutical,
biotechnology and other related markets that are researching and marketing products designed to address Crohn’s Disease,
multiple sclerosis, other autoimmune diseases or immune disorders, inflammatory disorders, HIV/AIDS and cancer. Established pharmaceutical
companies that currently sell or are developing drugs in our markets of interest include, for example; Abbott Laboratories, Amgen,
AstraZeneca, Biogen Idec, Bayer, Elan, Johnson & Johnson, Merck, Merck Serono, Takeda, Novartis, Pfizer, Reata, Sanofi-Aventis
and Teva. Many or all of these established competitors are also involved in research and drug development regarding various OGF
receptors. Pharmaceutical and biotechnology companies which are known to be involved in immunotherapy research and related drug
development include Pfizer, Bristol-Myers Squibb, Merck, Takeda, Sanofi-Aventis, Incyte, and UCB Pharma among others.
We
are developing small molecule therapeutics that will compete with other drugs and alternative therapies that are currently marketed
or are being developed to treat Crohn’s Disease, HIV/AIDS, other autoimmune diseases and inflammatory disorders, HIV/AIDS
and cancer. If approved for marketing by the FDA, IRT-103 (LDN), our lead Inflammatory Bowel Disease (“IBD”) drug
candidate, would compete against existing IBD treatments such as Sulfasalazine (
Azulfidine
); Mesalamine (
Asacol, Rowasa
)
Corticosteroids; Azathioprine (
Imuran
) and mercaptopurine (
Purinethol
); Infliximab (
Remicade
); Adalimumab
(
Humira
); Certolizumab pegol (
Cimzia
); Methotrexate (
Rheumatrex
); Cyclosporine (
Gengraf, Neoral, Sandimmune
)
and Natalizumab (
Tysabri
). Similarly, other future drug candidates we are pursuing would compete against numerous existing
and established drugs and potentially against other novel drugs and therapies that are currently in development. We also anticipate
that we will face increased competition in the future as new companies enter our target markets and scientific developments surrounding
the chemokine system continue to develop.
Many
of our competitors have greater name recognition and financial, manufacturing, marketing, research and drug development resources
than we do. Additional mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources
being concentrated in our competitors. Large pharmaceutical companies in particular have extensive expertise in preclinical and
clinical testing and in obtaining regulatory approvals for drugs. In addition, academic institutions, government agencies, and
other public and private organizations conducting research may seek patent protection with respect to potentially competitive
products or technologies. These organizations may also establish exclusive collaborative or licensing relationships with our competitors,
thus giving our competitors a significant advantage. We may be unable to respond to competitive forces presently in the marketplace,
which would severely impact our business.
In
addition, in terms of the licensing of our LDN formulation to Complete Pharmacy and Medical Solutions, LLC , we compete against
branded drug companies, generic drug companies, outsourcing facilities and other compounding pharmacies. We are currently and
expect to continue our efforts on making available our proprietary compounded formulation through Complete Pharmacy and Medical
Solutions, LLC and other compounding pharmacies outside of the U.S. The drug products available through branded and generic drug
companies with which our formulation competes have been approved for marketing and sale by the FDA and are required to be manufactured
in facilities compliant with cGMP standards. As a result, some physicians may be unwilling to prescribe them. Because our proprietary
LDN formulation is compounded in accordance with The U.S. Federal Food, Drug, and Cosmetic Act Section 503B and is not required
to be, and has not been, approved for marketing and sale by the FDA, our business may be subject to limitations our competitors
with FDA-approved drugs may not face.
Under
state and federal laws applicable to compounding pharmacies, Complete Pharmacy and Medical Solutions, LLC is not permitted to
prepare significant amounts of a specific formulation in advance of a prescription, compound quantities for office use or utilize
a wholesaler for distribution for our formulation; instead, our compounded formulation must be prepared and dispensed in connection
with a physician prescription for an individually identified patient. Pharmaceutical companies typically sell most of their FDA-approved
products to large pharmaceutical wholesalers, who in turn sell to and supply hospitals and retail pharmacies. As a result, the
sale of our formulation by Complete Pharmacy and Medical Solutions, LLC
is
not scalable on the scope available to our competitors with FDA-approved drugs, which may limit our potential for revenue.
We
may be subject to costly product liability claims related to our clinical trials and drug candidates and, if we are unable to
obtain adequate insurance or are required to pay for liabilities resulting from a claim excluded from, or beyond the limits of,
our insurance coverage, a material liability claim could adversely affect our financial condition.
Because
we conduct clinical trials with human patients, we face the risk that the use of our drug candidates may result in adverse side
effects to patients and to otherwise healthy volunteers in our clinical trials. We face even greater risks upon any commercialization
of our drug candidates. Although we will maintain product liability insurance for clinical trials, our insurance may be insufficient
to reimburse us for any expenses or losses we may suffer, and we will be required to increase our product liability insurance
coverage for the advanced clinical trials that we plan to initiate. We do not know whether we will be able to continue to obtain
product liability coverage and obtain expanded coverage on acceptable terms, or at all. We may not have sufficient resources to
pay for any liabilities resulting from a claim excluded from, or beyond the limits of, our insurance coverage. There is also a
risk that third parties that we have agreed to indemnify could incur liability. An individual may bring a product liability claim
against us if one of our drug candidates, products or compounded formulations cause, or is claimed to have caused, an injury or
is found to be unsuitable for consumer use. Any product liability claim brought against us, with or without merit, could result
in:
|
|
●
|
withdrawal
of clinical trial volunteers, investigators, patients or trial sites;
|
|
|
|
|
|
|
●
|
the
inability to commercialize our drug candidates;
|
|
|
|
|
|
|
●
|
decreased
demand for our drug candidates;
|
|
|
|
|
|
|
●
|
regulatory
investigations that could require costly recalls or product modifications;
|
|
|
|
|
|
|
●
|
loss
of revenues;
|
|
|
|
|
|
|
●
|
substantial
costs of litigation;
|
|
|
|
|
|
|
●
|
liabilities
that substantially exceed our product liability insurance, which we would then be required to pay ourselves;
|
|
|
|
|
|
|
●
|
an
increase in our product liability insurance rates or the inability to maintain insurance coverage in the future on acceptable
terms, if at all;
|
|
|
|
|
|
|
●
|
the
diversion of management’s attention from our business; and
|
|
|
|
|
|
|
●
|
damage
to our reputation and the reputation of our products.
|
Our
business involves the use of hazardous materials. As a result, we, including our third party manufacturers, must comply with environmental
laws and regulations, which may be expensive and restrict how we do business.
Our
third party manufacturers’ activities and our own activities involve the controlled storage, use and disposal of hazardous
materials, including the components of our pharmaceutical products, test samples and reagents, biological materials and other
hazardous compounds. We and our manufacturers are subject to federal, state and local, and foreign laws and regulations governing
the use, generation, manufacture, storage, handling and disposal of these hazardous materials. We currently carry no insurance
specifically covering environmental claims relating to the use of hazardous materials. Although we believe that our safety procedures
for handling and disposing of these materials and waste products comply with the standards prescribed by these laws and regulations,
we cannot eliminate the risk of accidental injury or contamination from the use, storage, handling or disposal of hazardous materials.
In the event of an accident, state or federal or other applicable authorities may curtail our use of these materials and/or interrupt
our business operations. In addition, if an accident or environmental discharge occurs, or if we discover contamination caused
by prior operations, including by prior owners and operators of properties we acquire, we could be liable for cleanup obligations,
damages and fines. The substantial unexpected costs we may incur could significantly harm our financial condition and results
of operations.
Future
financings may adversely affect our stockholders or impose restrictions on our assets or operations, which may harm our business.
If
we raise additional capital by issuing equity securities or convertible debt securities, our existing stockholders’ ownership
will be diluted and the terms of any new equity securities may have preferences over our common stock. If we raise additional
capital through the issuance of debt securities, the debt will have rights senior to the holders of our common stock and may contain
covenants that restrict our operational flexibility or impose liens or other restrictions on our assets. In addition, the terms
of future financings may restrict our ability to raise additional capital, which would delay or prevent the further development
or commercialization of our drug candidates.
If
we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish potentially
valuable rights to our current drug candidates, potential products or proprietary technologies, or grant licenses on terms that
are not favorable to us. Additionally, we may consider pursuing strategic opportunity for our business and corporate structure
that may make us a more attractive investment candidate. If adequate funds are not available, our ability to achieve profitability
or to respond to competitive pressures would be significantly limited and we may be required to delay, significantly curtail or
eliminate the development of one or more of our drug candidates.
We
may be adversely affected by the current economic environment.
Our
ability to attract and retain collaboration partners or customers, invest in and grow our business and meet our financial obligations
depends on our operating and financial performance, which, in turn, is subject to numerous factors, including the prevailing economic
conditions and financial, business and other factors beyond our control, such as the rate of unemployment, the number of uninsured
persons in the United States and inflationary pressures. We cannot anticipate all the ways in which the current economic climate
and financial market conditions could adversely impact our business.
We
are exposed to risks associated with reduced profitability and potential financial instability of our collaboration partners or
customers, many of which may be adversely affected by volatile conditions in the financial markets. For example, unemployment
and underemployment, and the resultant loss of insurance, may decrease the demand for healthcare services and pharmaceuticals.
If fewer patients are seeking medical care because they do not have insurance coverage, our collaboration partners or customers
may experience reductions in revenues, profitability and/or cash flow that could lead them to reduce their support of our programs
or financing activities. If collaboration partners or customers are not successful in generating sufficient revenue or are precluded
from securing financing, they may not be able to pay, or may delay payment of, accounts receivable that are owed to us. This,
in turn, could adversely affect our financial condition and liquidity. To the extent economic challenges result in fewer individuals
pursuing or being able to afford our products once commercialized, our business, results of operations, financial condition and
cash flows could be adversely affected.
Our
internal computer systems, or the computer systems of our contractors or consultants, may fail or suffer security breaches, which
could result in a material disruption of our drug development programs.
Despite
the implementation of security measures, our internal computer systems and the computer systems of our contractors and consultants
are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and
electrical failures. While we have not experienced any such system failure, accident or security breach to date, if such an event
were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs.
For example, the loss of clinical trial data from completed or ongoing clinical trials for any of our drug candidates could result
in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent
that any disruption or security breach were to result in a loss of or damage to our data or applications, or inappropriate disclosure
of confidential or proprietary information, we could incur liability and the further development of our drug candidates could
be delayed.
Our
current and future operations substantially depend on our management team and our ability to have other key personnel, the loss
of any of whom could disrupt our business operations.
The
Company’s future success depends on the efforts and abilities of principal members of its senior management and scientific
staff to provide strategic direction, business development, operations management and maintenance of a cohesive and stable work
environment. The Company relies on the services of Dr. Nicholas P. Plotnikoff and Professor Fengping Shan.
If
we lost their services or the services of any other key member of management, it could be impossible to replace them.
Additionally,
the Company’s ability to maintain, expand and renew existing business with its clients and maximize potential business opportunities
from new clients (in both the drug development and the drug discovery areas) depends on its ability to hire and retain scientists
with necessary skills. The scientists working for the Company must have the ability to lead ahead of continuing changes and trends
in drug discovery and development technologies to create the most innovative products on the market in order to remain competitive
within the drug development industry. The Company faces risks, challenges and competition attracting and retaining experienced
scientists and healthcare providers.
The
Company’s inability to hire qualified personnel may increase the workload for both existing and new personnel. The Company
may not be successful in attracting new healthcare providers, scientists and management or in retaining/motivating existing personnel.
The shortage of experienced healthcare providers and scientists or other factors may lead to increased recruiting, relocation
and compensation costs for the Company. Such increased costs may reduce profit margins or make hiring necessary experts (i.e.
healthcare providers or scientists) impracticable. If the Company is unable to attract or retain any of its key personnel its
ability to execute a competitive and profitable business plan will be adversely affected. Services and products will be less competitive
if not obsolete. If competing companies introduce superior technologies that compete with the Company’s services and products,
the Company may not be able to make the necessary enhancements to its services and products that will maintain a competitive position
in the marketplace. The Company’s competitive position, business, revenues and financial condition will be materially and
adversely affected.
Any
failure by the Company to comply with existing health care and drug regulations could harm its reputation, operating results,
the quality of the Company’s business strategy and the quality of the Company’s products.
The
Company has not experienced any failure to comply and has not received any notice or violation of either good clinical practices,
laboratory practices or good manufacturing practices. Any future failure by the Company to comply with existing health care and
drug regulations could result in the termination of ongoing research and/or the disqualification of data for submission to regulatory
authorities. Failure to comply with existing regulations will harm the Company’s reputation, brand name, its prospects for
immediate and future work and its operating results. For example, if the Company fails to verify that informed consent is obtained
from patient participants in connection with a particular clinical trial or grant deviations from the inclusion/exclusion criteria
in a study protocol, the data collected from that trial could be disqualified at which point the Company may be required to conduct
the trial again at no further cost to its client. Furthermore, the issuance of a FDA notice based on a finding of a material violation
of good clinical practice, good laboratory practice or good manufacturing practice requirements could materially and adversely
affect the Company.
Proposed
and future legislation or regulation may increase the cost of the Company’s business or limit its service and product offerings.
Federal,
state, and/or international authorities might adopt healthcare legislation or regulations that are more burdensome than existing
regulations. For example, recent product safety concerns and the creation of the Drug Safety Oversight Board could change the
regulatory environment for drug products including the process for FDA product approval and post-approval safety surveillance.
Such changes and other possible changes in regulation could increase the Company’s expenses or limit its ability to offer
some of its services or products. For example, the confidentiality of patient-specific information and the circumstances under
which it may be released for inclusion in the Company’s databases or used in other aspects of business are subject to substantial
government regulation. Additional legislation or regulation governing the possession, use and dissemination of medical record
information or other personal health information may require the Company to implement new security measures requiring substantial
expenditures or limiting the ability to offer services and products. These regulations might also increase costs by creating new
privacy requirements for the Company’s business mandating additional privacy procedures for its clinical research business.
Requirements
associated with being a public company will increase our costs significantly, as well as divert significant company resources
and management attention.
Prior
to June 2013, we were not subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange
Act”). We are working with our legal, independent accounting and financial advisors to identify those areas in which changes
should be made to our financial and management control systems to manage our growth and our
obligations
as a public company. These areas include corporate governance, corporate control, internal audit, disclosure controls and procedures
and financial reporting and accounting systems. We have made, and will continue to make, changes in these and other areas. However,
the expenses that will be required in order to adequately prepare for being a public company could be material.
Compliance
with the various reporting and other requirements applicable to public companies will also require considerable time and attention
of management. As a public company, we incur significant legal, accounting and other expenses that we did not incur as a private
company. The Sarbanes-Oxley Act, as well as rules subsequently implemented by the SEC and NASDAQ, has imposed various requirements
on public companies. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives.
In addition, the changes we make may not be sufficient to allow us to satisfy our obligations as a public company on a timely
basis.
Moreover,
we anticipate that compliance with these rules and regulations will increase our legal, accounting and financial compliance costs
substantially. In addition, these rules and regulations may make our activities related to legal, accounting and financial compliance
more difficult, time-consuming and costly and may also place undue strain on our personnel, systems and resources. If these requirements
divert the attention of our management and personnel from other business concerns, they could have a material adverse effect on
our business, financial condition and results of operations.
In
addition, being a public company could make it more difficult or more costly for us to obtain certain types of insurance, including
directors’ and officers’ liability insurance, and we may be forced to accept reduced policy limits and coverage or
incur substantially higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult
for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers.
We
estimate the additional costs we may incur to respond to these requirements to range from $100 to $500 thousand annually, although
unforeseen circumstances could increase actual costs.
As
an “emerging growth company” under applicable law, we will be subject to lessened disclosure requirements, which could
leave our stockholders without information or rights available to stockholders of more mature companies.
For
as long as we remain an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (which
we refer to herein as the JOBS Act), we have elected to take advantage of certain exemptions from various reporting requirements
that are applicable to other public companies that are not “emerging growth companies” including, but not limited
to:
|
●
|
not
being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act;
|
|
|
|
|
●
|
taking
advantage of an extension of time to comply with new or revised financial accounting standards;
|
|
|
|
|
●
|
reduced
disclosure obligations regarding executive compensation in our periodic reports and proxy statements; and
|
|
|
|
|
●
|
exemptions
from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden
parachute payments not previously approved.
|
We
expect to take advantage of these reporting exemptions until we are no longer an “emerging growth company.” Because
of these lessened regulatory requirements, our stockholders would be left without information or rights available to stockholders
of more mature companies.
If
we are unable to attract suitable and willing investigators and volunteers for clinical trials and product development, business
may suffer.
Our
clinical research studies rely on the accessibility and participation of physician investigators and volunteer subjects. Investigators
are typically located at hospitals, clinics or other sites and supervise administration of the study drug to patients during the
course of a clinical trial. Volunteer subjects generally include individuals from the locale where the studies are conducted.
Our clinical research development business could be adversely affected if it is unable to attract suitable and willing investigators
or volunteers on a consistent basis.
We
may not obtain government approval for our products and/or uses.
The
development and commercialization of pharmaceutical products are subject to extensive governmental regulation in the United States
and foreign countries. Government approvals are required to develop, market and sell potential drug candidates. Obtaining government
approval to develop, market and sell drug candidates is time-consuming and expensive. The clinical trial results for a particular
drug candidate might not satisfy necessary requirements to obtain government approvals. Even if we are successful in obtaining
all required approvals to market and sell a drug candidate, post-approval requirements and the failure to comply with other regulations
could result in suspension or limitation of government approvals.
In
connection with drug discovery activities outside of the United States, we and our strategic partners will be subject to foreign
regulatory requirements governing testing, approval, manufacturing, labeling, marketing and sale of pharmaceutical products. These
requirements vary with location. Even if approval has been obtained for a product in the United States, approval in a foreign
country must be obtained prior to marketing the product. The approval process in foreign countries may be more or less rigorous
than the United States and the time required for approval may be longer or shorter. Clinical studies conducted outside of a specific
country may not be acceptable. The approval of a pharmaceutical product in one country does not guarantee approval in another.
Even
if approved, the products that we may develop and market may be later withdrawn from the market or subject to promotional limitations.
We
may not be able to obtain the labeling claims necessary or desirable for the promotion of our treatments if approved. We may also
be required to undertake post-marketing clinical trials. If the results of such post-marketing studies are not satisfactory or
if adverse events or other safety issues arise after approval, the FDA or a comparable regulatory agency in another country may
withdraw marketing authorization or may condition continued marketing on commitments from us that may be expensive and/or time
consuming to complete. In addition, if we or others identify adverse side effects after any of our products are on the market,
or if manufacturing problems occur, regulatory approval may be withdrawn and reformulation of our products, additional clinical
trials, changes in labeling of our products and additional marketing applications may be required. Any reformulation or labeling
changes may limit the marketability of our products if approved.
Florida
Law and our Articles of Incorporation may protect our Directors and Officers from certain types of lawsuits.
Florida
law provides that our officers and directors will not be liable to us or our stockholders for monetary damages for all but certain
types of conduct as officers and directors. Our Bylaws permit us broad indemnification powers to all persons against all damages
incurred in connection with our business to the fullest extent provided or allowed by law. The exculpation provisions may have
the effect of preventing stockholders from recovering damages against our officers and directors caused by their negligence, poor
judgment or other circumstances. The indemnification provisions may require us to use our limited assets to defend our officers
and directors against claims, including claims arising out of their negligence, poor judgment or other circumstances.
We
may seek to grow our business through acquisitions of or investments in new or complementary businesses, products or technologies,
and the failure to manage acquisitions or investments, or the failure to integrate them with our existing business, could have
a material adverse effect on us.
From
time to time we expect to consider opportunities to acquire or make investments in other technologies, products and businesses
that may enhance our capabilities, complement our current products or expand the breadth of our markets or customer base. Potential
and completed acquisitions and strategic investments involve numerous risks, including:
|
|
●
|
problems
assimilating the purchased technologies, products or business operations;
|
|
|
|
|
|
|
●
|
issues
maintaining uniform standards, procedures, controls and policies;
|
|
|
|
|
|
|
●
|
unanticipated
costs associated with acquisitions;
|
|
|
|
|
|
|
●
|
diversion
of management’s attention from our core business;
|
|
|
●
|
adverse
effects on existing business relationships with suppliers and customers;
|
|
|
|
|
|
|
●
|
risks
associated with entering new markets in which we have limited or no experience;
|
|
|
|
|
|
|
●
|
potential
loss of key employees of acquired businesses; and
|
|
|
|
|
|
|
●
|
increased
legal and accounting compliance costs.
|
We
have no current commitments with respect to any acquisition or investment. We do not know if we will be able to identify acquisitions
we deem suitable, whether we will be able to successfully complete any such acquisitions on favorable terms or at all, or whether
we will be able to successfully integrate any acquired business, product or technology into our business or retain any key personnel,
suppliers or distributors. Our ability to successfully grow through acquisitions depends upon our ability to identify, negotiate,
complete and integrate suitable target businesses and to obtain any necessary financing. These efforts could be expensive and
time-consuming, and may disrupt our ongoing business and prevent management from focusing on our operations. If we are unable
to integrate any acquired businesses, products or technologies effectively, our business, results of operations and financial
condition will be materially adversely affected.
We
may expend our limited resources to pursue a particular opportunity and fail to capitalize on current research and products that
may be more profitable or for which there is a greater likelihood of success.
Because
we have limited financial and managerial resources, we have focused on specific research programs, treatments, and products. As
a result, we may forego or delay pursuit of opportunities with other products or research that later may prove to have greater
commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial treatments or
profitable market opportunities. Our spending on current and future research and development programs may not yield any commercially
viable treatments.
We
are subject to risks associated with our non-U.S. operations.
The
Foreign Corrupt Practices Act (“FCPA”) and similar worldwide anti-bribery laws in non-U.S. jurisdictions generally
prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or
retaining business. The FCPA also imposes accounting standards and requirements on publicly traded U.S. corporations and their
foreign affiliates which are intended to prevent the diversion of corporate funds to the payment of bribes and other improper
payments, and to prevent the establishment of “off books” slush funds from which such improper payments can be made.
Because of the predominance of government-sponsored healthcare systems around the world, many of our customer relationships outside
of the United States are with governmental entities and are therefore subject to such anti-bribery laws. Our internal control
policies and procedures may not always protect us from reckless or criminal acts committed by our employees or agents. Violations
of these laws, or allegations of such violations, could disrupt our operations, involve significant management distraction and
result in a material adverse effect on our business, results of operations and financial condition. We also could suffer severe
penalties, including criminal and civil penalties, disgorgement and other remedial measures, including further changes or enhancements
to our procedures, policies and controls, as well as potential personnel changes and disciplinary actions.
Furthermore,
we are subject to the export controls and economic embargo rules and regulations of the United States, including, but not limited
to, the Export Administration Regulations and trade sanctions against embargoed countries, which are administered by the Office
of Foreign Assets Control within the Department of the Treasury, as well as the laws and regulations administered by the Department
of Commerce. These regulations limit our ability to market, sell, distribute or otherwise transfer our products or technology
to prohibited countries or persons. A determination that we have failed to comply, whether knowingly or inadvertently, may result
in substantial penalties, including fines and enforcement actions and civil and/or criminal sanctions, the disgorgement of profits,
the imposition of a court-appointed monitor, the denial of export privileges and/or an adverse effect on our reputation.
These
and other factors may have a material adverse effect on our international operations or on our business, results of operations
and financial condition generally.
Because
our some of our manufacturing activities occur in Nicaragua, which is subject to political, economic and other uncertainties,
situations may arise that could have a material adverse effect on our business.
The
status of Nicaragua as a developing country may make it difficult for us to obtain additional financing for our projects. Notwithstanding
the progress achieved in recent years in political institutions and revitalizing the Nicaraguan economy, the present administration,
or any successor government, may not be able to sustain the progress achieved. While the Nicaraguan economy has experienced growth
in recent years, such growth may not continue in the future at similar rates or at all. If the economy of Nicaragua fails to continue
its growth or suffers a recession, our manufacturing efforts may be affected.
Further,
Nicaragua has in the past experienced a difficult security environment as well as political instability. In particular, various
illegal groups that may be active in and around regions in which we are present may pose a credible threat of terrorism, extortion
and kidnapping, which could have an adverse effect on our operations in such regions. In the event that continued operations in
these regions compromise our security or business principles, we may withdraw from these regions on a temporary or permanent basis,
which in turn, could have an adverse impact on our results of operations and financial condition. Any changes in regulations or
shifts in political attitudes are beyond our control and may adversely affect our business.
Our
independent auditors have expressed substantial doubt about our ability to continue as a going concern.
Due
to our net losses, negative cash flow and negative working capital, in their report on our audited financial statements for the
years ended December 31, 2015 and 2014, our independent auditors included an explanatory paragraph regarding substantial doubt
about our ability to continue as a going concern.
We
have incurred substantial losses since inception. Because of these losses, we will require additional working capital to develop
our business operations. We intend to raise additional working capital through private placements, public offerings, bank financing
and/or advances from related parties or shareholder loans.
There
are no assurances that we will be able to achieve a level of revenues adequate to generate sufficient cash flow from operations.
To the extent that funds generated from operations and any private placements, public offerings and/or bank financing are insufficient,
we will have to raise additional working capital. No assurance can be given that additional financing will be available, or, if
available, will be on terms acceptable to us. If adequate working capital is not available we may not increase our operations.
These
conditions raise substantial doubt about our ability to continue as a going concern. The financial statements do not include any
adjustments relating to the recoverability and classification of asset carrying amounts or the amount and classification of liabilities
that might be necessary should we be unable to continue as a going concern.
Risks
Related to Intellectual Property
Our
inability to adequately protect our intellectual property rights could hurt business.
Our
commercial success will depend in part on obtaining and maintaining intellectual property protection for our products, formulations,
processes, methods and other technologies. We will only be able to protect these technologies and products from unauthorized use
by third parties to the extent that valid and enforceable intellectual property rights, including patents or other market exclusionary
rights apply.
The
patent positions of pharmaceutical companies, like ours, can be highly uncertain and involve complex legal and factual questions
for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies’
patents has emerged to date in the United States. The general environment for pharmaceutical patents outside the United States
also involves significant uncertainty. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced, or
that the scope of these patent rights could provide a sufficient degree of future protection that could permit us to gain or keep
our competitive advantage with respect to these products and technologies. For example, we cannot predict:
|
|
●
|
the
degree and range of protection any patents will afford us against competitors, including whether third parties will find ways
to make, use, sell, offer to sell or import competitive products without infringing our patents;
|
|
|
●
|
if
and when patents will issue;
|
|
|
|
|
|
|
●
|
whether
or not others will obtain patents claiming inventions similar to those covered by our patents and patent applications; or
|
|
|
|
|
|
|
●
|
whether
we will need to initiate litigation or administrative proceedings in connection with patent rights, which may be costly whether
we win or lose.
|
Some
of our patents we have licensed may be subject to challenge and possibly invalidated or rendered unenforceable by third parties.
Changes in either the patent laws or in the interpretations of patent laws in the United States or other countries may diminish
the value of our intellectual property.
In
addition, others may independently develop similar or alternative products and technologies that may be outside the scope of our
intellectual property. Furthermore, others may have invented technology claimed by our patents before our licensors or we did
so, and they may have filed patents claiming such technology before we did so, weakening our ability to obtain and maintain patent
protection for such technology. Should third parties obtain patent rights to similar products or technology, this may have an
adverse effect on our business.
We
may also rely on trade secrets to protect our technology, especially where we do not believe patent protection is appropriate
or obtainable. Trade secrets, however, are difficult to protect. While we believe that we will use reasonable efforts to protect
our trade secrets, our own or our strategic partners’ employees, consultants, contractors or advisors may unintentionally
or willfully disclose our information to competitors. We seek to protect this information, in part, through the use of non-disclosure
and confidentiality agreements with employees, consultants, advisors and others. These agreements may be breached, and we may
not have adequate remedies for a breach. In addition, we cannot ensure that those agreements will provide adequate protection
for our trade secrets, know-how or other proprietary information or prevent their unauthorized use or disclosure.
If
competitors that have greater experience and financial resources learn our trade secrets, the competitors may copy or use our
trade secrets and other proprietary information in the advancement of their products, methods or technologies. If we were to prosecute
a claim that a third party had illegally obtained and was using our trade secrets, it could be expensive and time consuming and
the outcome could be unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade
secrets than courts in the United States. Moreover, if our competitors independently develop equivalent knowledge, we would lack
any legal or contractual claim to prevent them from using such information, and our business could be harmed.
The
Company’s most important intellectual property includes:
|
|
●
|
For
IRT - 103 for Crohn’s disease, Patent Number 7879870, filed April 16, 2007, issued February 1, 2011, Methods for the
treatment of inflammatory and ulcerative diseases of the bowel (e.g., Crohn’s disease and ulcerative colitis) with low
dose opioid antagonists (e.g., naltrexone, nalmefene or naloxone), pharmaceutical compositions for use in such methods, and
methods for the manufacture of such pharmaceutical compositions.
|
|
|
|
|
|
|
●
|
We
depend extensively on our license agreement with Pennsylvania State University for the
development of IRT-101 for pancreatic cancer covered by patents US Patent Numbers 6,737,397,
CA 2,557,504, US 20010046968 , US 6737397 , US 6136780 , US 20080015211 , US 20070053838
, US 8003630 , US 20110123437 , US 7807368 , US 7576180 , US 7517649, US 20080146512
, US 7122651 , US 20060073565 , US 20050191241 , Patent No 8,003,630 issued between 2001
and 2011. Our license agreement with Pennsylvania State University may be terminated
if we materially breach the agreement and fail to cure our breach during an applicable
cure period. Our failure to use commercially reasonable efforts to develop and commercialize
OGF sometimes referred to as MENK (intravenous) and IRT-101 in the United States and
certain other specified countries or to perform our other diligence obligations under
the license agreement would constitute a material breach of the license agreement. In
the event our license agreement with Pennsylvania State University is terminated, we
will lose all of our rights to develop and commercialize the drug candidates covered
by such license, which would harm our business and future prospects. We own a number
of other patents having to do with the development of MENK which would allow us to continue
our development of those indications.
|
We
may become subject to intellectual property suits that could cause us to incur significant costs or pay significant damages or
that could prohibit us from selling its products.
The
Company’s competitors also seek to obtain patents or other protection of their proprietary rights. Third parties may claim
in the future that the Company’s products infringe upon their proprietary rights. To date, there have been no claims of
infringement. However, in the future, intellectual property claims could force the Company to alter its existing products or withdraw
them from the market or could delay the introduction of new products.
Various
patents have been issued to the Company’s competitors and these competitors may assert that the Company’s products
infringe their patent or other proprietary rights. If the Company’s products are found to infringe third-party intellectual
property rights, the Company may be unable to obtain a license to use such technology, and it could incur substantial costs to
redesign its products or to defend legal actions.
The
drug discovery and development industry has a history of patent and other intellectual property litigation; thus, we may be involved
in costly intellectual property lawsuits.
There
has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical
and biotechnology industries. We or one of our collaborators may be subject to third party claims in the future that would cause
us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages, including treble damages
and attorney’s fees if we are found to be willfully infringing a third party’s patents. Further, if a patent infringement
suit were brought against us or our collaborators, we or they could be forced to stop or delay research, development, manufacturing
or sales of the product or drug candidate that is the subject of the suit. As a result of patent infringement claims, or in order
to avoid potential claims, we or our collaborators may choose to seek, or be required to seek, a license from the third party
and would most likely be required to pay license fees or royalties or both. These licenses may not be available on acceptable
terms, or at all. Even if we or our collaborators were able to obtain a license, the rights may be nonexclusive, which would give
our competitors access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or
forced to redesign it, or to cease some aspect of our business operations if, as a result of actual or threatened patent infringement
claims, we or our collaborators are unable to enter into licenses on acceptable terms. This could harm our business significantly.
In
addition to infringement claims against us, if third parties prepare and file patent applications in the United States that also
claim technology to which we have rights, we may have to participate in interference proceedings with the United States Patent
and Trademark Office (“USPTO”) to determine the priority of invention. We may also become involved in similar opposition
proceedings in the European Patent Office regarding our intellectual property rights with respect to our products and technology.
The
failure to obtain or maintain patents, licensing agreements and other intellectual property could impact our ability to compete
effectively.
Our
success will depend, in part, on our ability to obtain and maintain patent protection for our drug candidates, preserve our trade
secrets, prevent third parties from infringing upon our proprietary rights and operate without infringing upon the proprietary
rights of others. While the patents we own have been issued, pending patent applications we have filed may not result in issued
patents or may take longer than we expect to result in issued patents. We cannot be certain that patents will be issued as a result
of any of our pending applications, and we cannot be certain that any of our issued patents, whether issued pursuant to our pending
applications or licensed from third parties, will give us adequate protection from competing products.
Composition
of Matter patents on APIs are generally considered to be the strongest form of intellectual property protection for pharmaceutical
products, as they apply without regard to any method of use. Entirely new individual chemical compounds, often referred to as
new chemical entities, are typically entitled to Composition of Matter coverage. However, we cannot be certain that the current
law will remain the same, or that our drug candidates will be considered novel and non-obvious by the USPTO and courts.
In
addition to Composition of Matter patents and patent applications, we also have filed Method of Use patent applications. This
type of patent protects the use of the product only for the specified method. However, this type of patent does not prevent a
competitor from making and marketing a product that is identical to our product for an indication that is outside the
scope
of the patented method. Moreover, even if these competitors do not actively promote their product for our targeted indication,
physicians may prescribe these products “off-label.” Although off-label prescriptions may infringe or contribute to
the infringement of Method of Use patents, the practice is common and such infringement is difficult to prevent or prosecute.
Patent
applications in the United States and most other countries are confidential for a period of time until they are published. The
publication of discoveries in scientific or patent literature typically lags actual discoveries by several months or more. As
a result, we cannot be certain whether the Company or another inventor were the inventors of the issued patents and applications
or that the Company or another inventor were the first to conceive of the inventions covered by such patents and pending patent
applications or that the Company or another inventor were the first to file patent applications covering such inventions.
Others
may obtain issued patents that could prevent us from commercializing our product candidates or require us to obtain licenses requiring
the payment of considerable fees or royalties in order to enable us to conduct our business. As to those patents that we have
licensed, our rights depend on maintaining our obligations to the licensor under the applicable license agreement, and we may
be unable to do so.
We
have numerous issued patents and some patent applications pending before the USPTO. The protection may lapse before we manage
to obtain commercial value from the patents, which might result in increased competition and materially affect our position in
the market.
We
may be subject to claims that we or our employees or consultants have wrongfully used or disclosed alleged trade secrets of our
employees’ or consultants’ former employers or their clients. These claims may be costly to defend and if we do not
successfully do so, we may be required to pay monetary damages and may lose valuable intellectual property rights or personnel.
Many
of our employees were previously employed at universities, biotechnology or pharmaceutical companies, including our competitors
or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees
or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers.
Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary
damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product
could hamper our ability to commercialize, or prevent us from commercializing our drug candidates, which could severely harm our
business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction
to management.
Some
of our intellectual property that was discovered through government funded programs may be subject to federal regulation such
as “march-in” rights, certain reporting requirements, and a preference for United States industry. Compliance with
such regulations may limit our exclusive rights, subject us to expenditure of resources with respect to reporting requirements,
and limit our ability to contract with foreign manufacturers.
Some
of our existing drug candidates, including LDN and MENK, and some of the research and development work conducted before we had
licensing rights may have been funded, at least in part, by the U.S. government and therefore would be subject to certain federal
regulations. Under the “march-in” provisions of the Bayh-Dole Act, the government may have the right under limited
circumstances to require the patent owners to grant exclusive, partially exclusive or non-exclusive rights to third parties for
intellectual property discovered through the government-funded program. The government can exercise its march-in rights if it
determines that action is necessary because the patent owner fails to achieve practical application of the new invention or because
action is necessary to alleviate health concerns or address the safety needs of the public. Intellectual property discovered under
the government-funded program is also subject to certain reporting requirements, compliance with which may require us to expend
substantial resources. Such intellectual property is also subject to a preference for U.S. industry, which may limit our ability
to contract with foreign product manufacturers for products covered by such intellectual property. We may apply for additional
U.S. government funding, and it is possible that we may discover compounds or drug candidates as a result of such funding. Intellectual
property under such discoveries would be subject to the applicable provisions of the Bayh-Dole Act.
Risks
Related to Government Regulation
The
regulatory approval process is expensive, time consuming and uncertain and may prevent us or our collaboration partners from obtaining
approvals for the commercialization of some or all of our drug candidates.
The
research, testing, manufacturing, labeling, approval, selling, import, export, marketing and distribution of drug products are
subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries which regulations
differ from country to country. Neither we nor our collaboration partners are permitted to market our drug candidates in the United
States until we receive approval of a NDA from the FDA. Neither we nor our collaboration partners have submitted an application
for or received marketing approval for any of our drug candidates. Obtaining approval of an NDA can be a lengthy, expensive and
uncertain process. In addition, failure to comply with FDA and other applicable U.S. and foreign regulatory requirements may subject
us to administrative or judicially imposed sanctions, including:
|
|
●
|
warning
letters;
|
|
|
|
|
|
|
●
|
civil
and criminal penalties;
|
|
|
|
|
|
|
●
|
injunctions;
|
|
|
|
|
|
|
●
|
withdrawal
of approved products;
|
|
|
|
|
|
|
●
|
product
seizure or detention;
|
|
|
|
|
|
|
●
|
product
recalls;
|
|
|
|
|
|
|
●
|
total
or partial suspension of production; and
|
|
|
|
|
|
|
●
|
refusal
to approve pending NDAs or supplements to approved NDAs.
|
Regulatory
approval of an NDA or NDA supplement is not guaranteed, and the approval process is expensive and may take several years. The
FDA also has substantial discretion in the approval process. Despite the time and expense exerted, failure can occur at any stage,
and we could encounter problems that cause us to abandon or repeat clinical trials, or perform additional preclinical studies
and clinical trials. The number of preclinical studies and clinical trials that will be required for FDA approval varies depending
on the drug candidate, the disease or condition that the drug candidate is designed to address, and the regulations applicable
to any particular drug candidate. The FDA can delay, limit or deny approval of a drug candidate for many reasons, including, but
not limited to, the following:
|
|
●
|
a
drug candidate may not be deemed safe or effective;
|
|
|
|
|
|
|
●
|
FDA
officials may not find the data from preclinical studies and clinical trials sufficient;
|
|
|
|
|
|
|
●
|
the
FDA might not approve our or our third party manufacturer’s processes or facilities; or
|
|
|
|
|
|
|
●
|
the
FDA may change its approval policies or adopt new regulations.
|
If
any of our drug candidates fail to demonstrate safety and efficacy in clinical trials or do not gain regulatory approval, our
business and results of operations will be materially and adversely harmed.
Even
if we receive regulatory approval for a drug candidate, we will be subject to ongoing regulatory obligations and continued regulatory
review which may result in consideerable additional expense and subject us to penalties if we fail to comply with applicable regulatory
requirements.
Once
regulatory approval has been granted, the approved product and its manufacturer are subject to continual review by the FDA and/or
non-U.S. regulatory authorities. Any regulatory approval that we or our collaboration partners receive for our drug candidates
may be subject to limitations on the indicated uses for which the product may be marketed or contain requirements for potentially
costly post-marketing follow-up studies to monitor the safety and efficacy of the product. In addition, if the FDA and/or non-U.S.
regulatory authorities approve any of our drug candidates, we will be subject to extensive and ongoing regulatory requirements
by the FDA and other regulatory authorities with regard to the labeling,
packaging,
adverse event reporting, storage, advertising, promotion and recordkeeping for our products. In addition, manufacturers of our
drug products are required to comply with current cGMP regulations which include requirements related to quality control and quality
assurance as well as the corresponding maintenance of records and documentation. Further, regulatory authorities must approve
these manufacturing facilities before they can be used to manufacture our drug products, and these facilities are subject to continual
review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMP regulations. If we or a regulatory
authority discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency,
or problems with the facility where the product is manufactured, a regulatory authority may impose restrictions on that product,
the manufacturer or us, including requiring withdrawal of the product from the market or suspension of manufacturing. If we, our
drug candidates or the manufacturing facilities for our drug candidates fail to comply with regulatory requirements of the FDA
and/or other non-U.S. regulatory authorities, we could be subject to administrative or judicially imposed sanctions, including:
|
|
●
|
warning
letters;
|
|
|
|
|
|
|
●
|
civil
or criminal penalties;
|
|
|
|
|
|
|
●
|
injunctions;
|
|
|
|
|
|
|
●
|
suspension
of or withdrawal of regulatory approval;
|
|
|
|
|
|
|
●
|
suspension
of any ongoing clinical trials;
|
|
|
|
|
|
|
●
|
voluntary
or mandatory product recalls and publicity requirements;
|
|
|
|
|
|
|
●
|
refusal
to approve pending applications for marketing approval of new drugs or supplements to approved applications filed by us;
|
|
|
|
|
|
|
●
|
restrictions
on operations, including costly new manufacturing requirements; or
|
|
|
|
|
|
|
●
|
seizure
or detention of our products or import bans.
|
The
regulatory requirements and policies may change and additional government regulations may be enacted for which we may also be
required to comply. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation
or administrative action, either in the United States or in other countries. If we are not able to maintain regulatory compliance,
we will not be permitted to market our future products and our business will suffer.
The
availability of adequate third-party coverage and reimbursement for newly approved drugs is uncertain, and failure to obtain adequate
coverage and reimbursement from third-party payers could impede our ability to market any future products we may develop and could
limit our ability to generate revenue.
There
is significant uncertainty related to the third-party payor coverage and reimbursement of newly approved drugs. The commercial
success of our future products in both domestic and international markets depends on whether such third-party coverage and reimbursement
is available for our future products. Governmental payers, including Medicare and Medicaid, health maintenance organizations and
other third-party payers are increasingly attempting to manage their healthcare expenditures by limiting both coverage and the
level of reimbursement of new drugs and, as a result, they may not cover or provide adequate reimbursement for our future products.
These payers may not view our future products as cost-effective, and coverage and reimbursement may not be available to our customers
or may not be sufficient to allow our future products to be marketed on a competitive basis. Third-party payers are exerting increasing
influence on decisions regarding the use of, and coverage and reimbursement levels for, particular treatments. Such third-party
payers, including Medicare, are challenging the prices charged for medical products and services, and many third-party payers
limit or delay coverage and reimbursement for newly approved healthcare products. In particular, third-party payers may limit
the covered indications. Cost-control initiatives could cause us to decrease the price we might establish for products, which
could result in lower than anticipated product revenues. If the prices for our drug candidates decrease or if governmental and
other third-party payers do not provide adequate coverage or reimbursement, our prospects for revenue and profitability will suffer.
Even
if we obtain FDA approval of any product candidate we may develop or acquire in the future, we may never obtain approval or commercialize
our products outside of the U.S., which would limit our ability to realize their full market potential. If foreign approval is
obtained, there are risks in conducting business in international markets.
We
have and continue to seek other distribution and marketing partners for IRT-101 and IRT-103 (LDN) outside North America that will
and may market future products in international markets. In order to market any of our products we may develop or acquire outside
of the U.S., we must establish and comply with numerous and varying regulatory requirements of other countries regarding safety
and efficacy. Clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory
approval in one country does not mean that regulatory approval will be obtained in any other country. Approval procedures vary
among countries and can involve additional product testing and validation and additional administrative review periods. Seeking
foreign regulatory approvals could result in significant delays, difficulties and costs for us and require additional preclinical
studies or clinical trials which would be costly and time consuming. Regulatory requirements can vary widely from country to country
and could delay or prevent the introduction of our products in those countries. Satisfying these and other regulatory requirements
is costly, time consuming, uncertain and subject to unanticipated delays. In addition, our failure to obtain regulatory approval
in the U.S. or any foreign country may delay or have negative effects on the process for regulatory approval in other countries.
If we fail to comply with regulatory requirements in a foreign country or to obtain and maintain required approvals, our potential
market for our products will be reduced and our ability to realize the full market potential of our products will be harmed.
The
Company, either directly or through its collaborating partners, is working with drug regulatory authorities in Nicaragua, China
and in those African Nations where an FDA equivalent exists. The Company is working with the agencies to obtain local approval
for the therapies for each modality that we intend to market for. We believe this will reduce our risk due to The Agreement on
Trade Related Aspects of Intellectual Property Rights (“TRIPS”) which is an international agreement administered by
the World Trade Organization (“WTO”). TRIPS allows emerging nations to manufacture drugs around existing patents.
If
approved for commercialization in a foreign country, we intend to enter into agreements with third parties to market our products
whenever they may be approved and wherever we have the right to market them. Consequently, we expect that we will be subject to
additional risks relating to entering into international business relationships, including:
|
|
●
|
lack
of adequate protection from intellectual property rights in foreign countries, which could occur if we do not have issued
patents in force in such foreign countries covering our products, their methods of use and methods of manufacture;
|
|
|
|
|
|
|
●
|
the
potential for so-called parallel importing, which is what happens when a local seller, faced with high or higher local prices
(for instance, because the goods have patent protection in such country), opts to import goods from a foreign market (with
low or lower prices) rather than buy them locally;
|
|
|
|
|
|
|
●
|
unexpected
changes in tariffs, trade barriers and regulatory requirements
|
|
|
|
|
|
|
●
|
economic
weakness, including inflation, or political instability in particular foreign economies and markets;
|
|
|
|
|
|
|
●
|
compliance
with laws for employees traveling abroad;
|
|
|
|
|
|
|
●
|
foreign
taxes, including withholding of payroll taxes;
|
|
|
|
|
|
|
●
|
foreign
currency fluctuations, which could result in increased operating expenses and reduced revenues;
|
|
|
|
|
|
|
●
|
workforce
uncertainty in countries where labor unrest is more common than in the U.S.;
|
|
|
|
|
|
|
●
|
production
shortages resulting from any events affecting the API and/or finished drug product supply or manufacturing capabilities abroad;
|
|
|
|
|
|
|
●
|
business
interruptions resulting from geo-political actions, including war and terrorism, or natural disasters including earthquakes,
typhoons, floods and fires; and
|
|
|
|
|
|
|
●
|
failure
to comply with Office of Foreign Asset Control rules and regulations and the Foreign Corrupt Practices Act
|
These
and other risks may materially adversely affect our ability to attain or sustain revenue from international markets.
Healthcare
policy changes may have a material adverse effect on us.
Our
business may be affected by the efforts of government and third-party payers to contain or reduce the cost of healthcare through
various means. For example, the Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation
Act of 2010 (collectively, the Affordable Care Act or ACA), enacted in March 2010, substantially changed the way healthcare is
financed by both governmental and private insurers, and significantly impacted the pharmaceutical industry. With regard to pharmaceutical
products, among other things, ACA is expected to expand and increase industry rebates for drugs covered under Medicaid programs
and make changes to the coverage requirements under the Medicare D program. ACA has been held constitutional. This adds to the
uncertainty of the legislative changes enacted as part of ACA, and we cannot predict the impact that ACA or any other legislative
or regulatory proposals will have on our business. We expect both government and private health plans to continue to require healthcare
providers, including healthcare providers that may one day purchase our products, to contain costs and demonstrate the value of
the therapies they provide.
If
we fail to comply with healthcare regulations, we could face substantial penalties and our business, operations and financial
condition could be adversely affected.
Even
though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party
payers, certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are
and will be applicable to our business. We could be subject to healthcare fraud and abuse and patient privacy regulation by both
the federal government and the states in which we conduct our business. The regulations that may affect our ability to operate
include, without limitation:
|
|
●
|
the
federal healthcare program Anti-Kickback Statute, which prohibits, among other things, any person from knowingly and willfully
offering, soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual
for an item or service or the purchasing or ordering of a good or service, for which payment may be made under federal healthcare
programs such as the Medicare and Medicaid programs;
|
|
|
|
|
|
|
●
|
the
federal False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing
to be presented, false claims, or knowingly using false statements to obtain payment from the federal government, and which
may apply to entities like us which may provide coding and billing advice to customers;
|
|
|
|
|
|
|
●
|
federal
criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating
to healthcare matters; and
|
|
|
|
|
|
|
●
|
the
federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic
and Clinical Health Act, which governs the conduct of certain electronic healthcare transactions and protects the security
and privacy of protected health information; and state law equivalents of each of the above federal laws, such as anti-kickback
and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers.
|
If
our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply
to us, we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring
of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability
to operate our business and our financial results. Any action against us for violation of these laws, even if we successfully
defend against it, could cause us to incur significant legal expenses and divert Management’s attention from the operation
of our business. Moreover, achieving and sustaining compliance with applicable federal and state privacy, security and fraud laws
may prove costly.
The
FDA may not accept the results of clinical trials conducted outside of the United States.
It
is possible that the FDA may not accept the results of our clinical trials; and this risk can increase when a clinical trial is
conducted
outside of the United States. All clinical trials and clinical trial sites that are outside of the United States but will be used
to support a FDA application will be run in accordance with all US guidelines and regulations; however, this does not guarantee
the FDA’s acceptance of the clinical trial results. Clinical studies to support US licensure will only be conducted in countries
that are typically used to support a US licensure such as Canada, Australia, and countries within the EU. We would need to obtain
approval from the FDA to conduct the trial outside of the United States and/or to allow clinical sites outside of the US, prior
to initiation of such study. We would also need to ensure that the study is conducted in accordance with local legal and regulatory
requirements and all applicable United States federal regulations, European Union regulations, International Conference on Harmonisation
of Good Clinical Practice guidelines and any other applicable regulatory requirements for the overall conduct of the clinical
investigation.
Risks
Related to this Offering our Common Stock
Because
of their significant stock ownership, our chief executive officer, our other executive officers, and our directors and principal
stockholders may be able to exert control over us and our significant corporate decisions. Our other stockholders will have limited
ability to influence corporate actions or decisions.
This
concentration of ownership may harm the value of our common stock by, among other things:
|
|
●
|
delaying,
deferring or preventing a change in control of our company;
|
|
|
|
|
|
|
●
|
impeding
a merger, consolidation, takeover or other business combination involving our company; or
|
|
|
|
|
|
|
●
|
causing
us to enter into transactions or agreements that are not in the best interests of all stockholders
|
As
a group, our officers and directors own 13.7% of the outstanding common stock of the Company. Our other stockholders will have
limited ability to influence corporate actions or decisions.
The
price of our common stock may be volatile, and you may not be able to resell your shares.
An
active and liquid trading market for our common stock may not develop or be sustainable. Shareholders may be unable to sell shares
of common stock at or above their purchase price due to fluctuations in the market price of our common stock. The market price
of our common stock may fluctuate significantly in response to factors, some of which are beyond our control. Factors that could
cause volatility in the market price of our common stock include, but are not limited to:
|
|
●
|
results
from, and delays in, clinical trial programs relating to our drug candidates, including the ongoing and planned clinical trials
for IRT-103 (LDN), IRT-101 (MENK) and other drug candidates;
|
|
|
|
|
|
|
●
|
announcements
of regulatory approvals or disapprovals of our drug candidates including IRT-103 (LDN) and IRT-101 (MENK) or delays in any
regulatory agency review or approval processes;
|
|
|
|
|
|
|
●
|
failure
or discontinuation of any of our research programs;
|
|
|
|
|
|
|
●
|
loss
of significant clients or customers;
|
|
|
|
|
|
|
●
|
loss
of significant strategic relationships;
|
|
|
|
|
|
|
●
|
announcements
relating to future collaborations or our existing collaborations;
|
|
|
|
|
|
|
●
|
our
failure to achieve and maintain profitability;
|
|
|
|
|
|
|
●
|
changes
in earnings estimates and recommendations by financial analysts;
|
|
|
|
|
|
|
●
|
changes
in market valuations of similar companies;
|
|
|
|
|
|
|
●
|
wholesalers’
buying patterns;
|
|
|
●
|
addition
or termination of clinical trials or funding support;
|
|
|
|
|
|
|
●
|
regulatory
developments affecting our drug candidates or those of our competitors;
|
|
|
|
|
|
|
●
|
the
Company’s sales decrease internationally;
|
|
|
|
|
|
|
●
|
variations
in the level of expenses related to our drug candidates or future development programs;
|
|
|
|
|
|
|
●
|
ability
to secure new government contracts and allocation of our resources to or away from performing work under government contracts;
|
|
|
|
|
|
|
●
|
general
economic conditions in the United States and abroad;
|
|
|
|
|
|
|
●
|
acquisitions
and sales of new products, technologies or business;
|
|
|
|
|
|
|
●
|
market
conditions in the pharmaceutical, biopharmaceutical and biotechnology sectors;
|
|
|
|
|
|
|
●
|
the
issuance of new or changed securities analysts’ reports or recommendations regarding us, our competitors or our industry
in general;
|
|
|
|
|
|
|
●
|
actual
and anticipated fluctuations in our quarterly operating results;
|
|
|
|
|
|
|
●
|
disputes
concerning our intellectual property or other proprietary rights;
|
|
|
|
|
|
|
●
|
introduction
of technological innovations or new products by us or our competitors;
|
|
|
|
|
|
|
●
|
manufacturing
issues related to our drug candidates for clinical trials or future products for commercialization;
|
|
|
|
|
|
|
●
|
market
acceptance of our future products;
|
|
|
|
|
|
|
●
|
deviations
in our operating results from the estimates of analysts;
|
|
|
|
|
|
|
●
|
third
party payor coverage and reimbursement policies;
|
|
|
|
|
|
|
●
|
new
legislation in the United States relating to the sale or pricing of pharmaceuticals;
|
|
|
|
|
|
|
●
|
FDA
or other U.S. or foreign regulatory actions affecting us or our industry;
|
|
|
|
|
|
|
●
|
product
liability claims or other litigation or public concern about the safety of our drug candidates or future drugs;
|
|
|
|
|
|
|
●
|
our
ability to obtain necessary intellectual property licenses including, if necessary, those relating to IRT-103 (LDN) and other
drug candidates;
|
|
|
|
|
|
|
●
|
the
outcome of any future legal actions to which we are a party;
|
|
|
|
|
|
|
●
|
sales
of our common stock by our officers, directors or significant stockholders;
|
|
|
|
|
|
|
●
|
frequent,
irregular, under market, or large sales of shares of our common stock by any shareholder;
|
|
|
|
|
|
|
●
|
additions
or departures of key personnel; and
|
|
|
|
|
|
|
●
|
external
factors, including natural disasters and other crises.
|
In
addition, the stock markets in general, and the markets for pharmaceutical, biopharmaceutical and biotechnology stocks in particular,
have experienced extreme volatility that has often been unrelated to the operating performance of the issuer. These broad market
fluctuations may adversely affect the trading price or liquidity of our common stock. In the past, when the
market
price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation suits against
the issuer. If any of our stockholders were to bring such a lawsuit against us, we could incur substantial costs defending the
lawsuit and the attention of our management would be diverted from the operation of our business.
Future
sales of our common stock or securities convertible or exchangeable for our common stock may depress our stock price.
If
our existing stockholders or holders of our convertible notes, options or warrants sell, or indicate an intention to sell substantial
amounts of our common stock in the public market, the trading price of our common stock could decline. The perception in the market
place that these sales may occur could also cause the trading price of our common stock to decline.
Certain
holders of shares of our common stock, warrants to purchase our common stock, and shares of common stock issuable upon exercise
of warrants will be entitled to rights with respect to the registration of their shares under the Securities Act. Registration
of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities
Act, except for shares purchased by affiliates. In addition, our directors may, and we expect that our executive officers will
establish programmed selling plans under Rule 10b5-1 of the Exchange Act, for the purpose of effecting sales of our common stock.
Any sales of securities by these stockholders, or the perception that those sales may occur, including the entry into such programmed
selling plans, could have a material adverse effect on the trading price of our common stock.
If
we sell shares of our common stock in future financings, common stockholders may experience immediate dilution and, as a result,
our stock price may decline.
We
may from time to time issue additional shares of common stock at a discount from the current trading price of our common stock.
As a result, our common stockholders would experience immediate dilution upon the purchase of any shares of our common stock sold
at such a discount. In addition, as opportunities present themselves, we may enter into financing or similar arrangements in the
future, including the issuance of debt securities, preferred stock or common stock.
Provisions
of our charter documents or Florida law could delay or prevent an acquisition of the Company, even if the acquisition would be
beneficial to our stockholders, and could make it more difficult for stockholders to change management.
Provisions
of our amended and restated articles of incorporation, as amended, and amended and restated bylaws may discourage, delay or prevent
a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which stockholders
might otherwise receive a premium for their shares. In addition, these provisions may frustrate or prevent any attempt by our
stockholders to replace or remove our current management by making it more difficult to replace or remove our board of directors.
We
do not anticipate paying any cash dividends on our capital stock in the foreseeable future, therefore capital appreciation, if
any, of our common stock will be our shareholders sole source of gain for the foreseeable future.
We
have never declared or paid cash dividends on our capital stock. We do not anticipate paying any cash dividends on our capital
stock in the foreseeable future. We currently intend to retain all available funds and any future earnings to fund the development
and growth of our business. As a result, capital appreciation, if any, of the common stock will be our shareholders sole source
of gain for the foreseeable future.
If
securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our
stock price and trading volume could decline.
The
trading market for our common stock will depend, in part, on the research and reports that securities or industry analysts publish
about us or our business. Securities and industry analysts do not currently, and may never, publish research on our Company. If
no securities or industry analysts commence coverage of our Company, the trading price for our stock would likely be negatively
impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade
our stock or publish inaccurate or unfavorable research about our business, our stock price would likely decline. In addition,
if our operating results fail to meet the forecast of analysts, our stock price would likely decline. If one or
more
of these analysts cease coverage of our Company or fail to publish reports on us regularly, demand for our stock could decrease,
which might cause our stock price and trading volume to decline.
Our
board of directors is authorized to issue and designate shares of our preferred stock in additional series without stockholder
approval.
Our
amended and restated articles of incorporation, as amended, authorize our board of directors, without the approval of our stockholders,
to issue shares of our preferred stock, subject to limitations prescribed by applicable law, rules and regulations and the provisions
of our amended and restated articles of incorporation, as amended, as shares of preferred stock in series, and to establish from
time to time the number of shares to be included in each such series, and to fix the designation, powers, preferences and rights
of the shares of each such series and the qualifications, limitations or restrictions thereof. The powers, preferences and rights
of these additional series of preferred stock may be senior to or on parity with our common stock, which may reduce its value.
We do not currently have any class of preferred stock authorized.
Our
shares may be subject to the “penny stock” rules, which may subject you to restrictions on marketability and limit
your ability to sell your shares.
Broker-dealer
practices in connection with transactions in “Penny Stocks” are regulated by certain penny stock rules adopted by
the SEC. Penny stocks generally are equity securities with a price of less than $5.00 per share (other than securities registered
on certain national securities exchanges or quoted on the NASDAQ system). The penny stock rules require a broker-dealer, prior
to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document that
provides information about penny stocks and the risk associated with the penny stock market. The broker-dealer must also provide
the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson
in the transaction, and monthly account statements showing the market value of each penny stock held in the customer’s account.
In addition, the penny stock rules generally require that prior to a transaction in a penny stock, the broker-dealer must make
a written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written
agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the
secondary market for a stock that becomes subject to the penny stock rules. The Company’s securities may be subject to the
penny stock rules, and investors may find it more difficult to sell their securities.
An
active and visible trading market for our common stock may not develop.
We
cannot predict whether an active market for our common stock will develop in the future. In the absence of an active trading market:
|
|
●
|
Investors
may have difficulty buying and selling or obtaining market quotations;
|
|
|
|
|
|
|
●
|
Market
visibility for our common stock may be limited; and
|
|
|
|
|
|
|
●
|
A
lack of visibility for our common stock may have a depressive effect on the market price for our common stock
|
Our
common stock is currently quoted on the OTC Market under the trading symbol “IMUN”. The OTC Market is unorganized,
inter-dealers, over-the-counter markets that provides significantly less liquidity than the New York Stock Exchange or NASDAQ.
No assurances can be given that we will ever obtain a listing for our securities on a senior exchange. The trading price of our
common stock is therefore expected to be subject to significant fluctuations in response to variations in quarterly operating
results, changes in analysts’ earnings estimates, announcements of innovations by us or our competitors, general conditions
in the industry in which we operate and other factors. These fluctuations, as well as general economic and market conditions,
may have a material or adverse effect on the market price of our common stock.
Because
we have elected to use the extended transition period for complying with new or revised accounting standards for an “emerging
growth company” our financial statements may not be comparable to companies that comply with public company effective dates.
We
have elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(1)
of the JOBS Act. This election allows us to delay the adoption of new or revised accounting standards that have
different
effective dates for public and private companies until those standards apply to private companies. As a result of this election,
our financial statements may not be comparable to companies that comply with public company effective dates, and thus investors
may have difficulty evaluating or comparing our business, performance or prospects in comparison to other public companies, which
may have a negative impact on the value and liquidity of our common stock.
We
may not register or qualify our securities with any state agency pursuant to blue sky regulations.
The
holders of our shares of common stock and persons who desire to purchase them in the future should be aware that there may be
significant state law restrictions upon the ability of investors to resell our shares. We currently do not intend to and may not
be able to qualify securities for resale in other states which require shares to be qualified before they can be resold by our
shareholders.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain
statements contained or incorporated by reference in this Form S-1 are considered forward-looking statements (within the meaning
of the Private Securities Litigation Reform Act of 1995) concerning our business, results of operations, economic performance
and/or financial condition, based on management’s current expectations, plans, estimates, assumptions and projections. Forward-looking
statements are included, for example, in the discussions about:
|
|
●
|
strategy;
|
|
|
|
|
|
|
●
|
new
product discovery and development;
|
|
|
|
|
|
|
●
|
current
or pending clinical trials;
|
|
|
|
|
|
|
●
|
our
products’ ability to demonstrate efficacy or an acceptable safety profile;
|
|
|
|
|
|
|
●
|
actions
by the FDA and other regulatory authorities;
|
|
|
|
|
|
|
●
|
product
manufacturing, including our arrangements with third-party suppliers;
|
|
|
|
|
|
|
●
|
product
introduction and sales;
|
|
|
|
|
|
|
●
|
royalties
and contract revenues;
|
|
|
|
|
|
|
●
|
expenses
and net income;
|
|
|
|
|
|
|
●
|
credit
and foreign exchange risk management;
|
|
|
|
|
|
|
●
|
liquidity;
|
|
|
|
|
|
|
●
|
asset
and liability risk management;
|
|
|
|
|
|
|
●
|
the
outcome of litigation and other proceedings;
|
|
|
|
|
|
|
●
|
intellectual
property rights and protection;
|
|
|
|
|
|
|
●
|
economic
factors;
|
|
|
|
|
|
|
●
|
competition;
and
|
|
|
|
|
|
|
●
|
legal
risks.
|
Any
statements contained in this report that are not statements of historical fact may be deemed forward-looking statements. Forward-looking
statements generally are identified by the words “expects,” “anticipates,” “believes,” “intends,”
“estimates,” “aims,” “plans,” “may,” “could,” “will,”
“will continue,” “seeks,” “should,” “predict,” “potential,” “outlook,”
“guidance,”
“target,”
“forecast,” “probable,” “possible” or the negative of such terms and similar expressions.
Forward-looking statements are subject to change and may be affected by risks and uncertainties, most of which are difficult to
predict and are generally beyond our control. Forward-looking statements speak only as of the date they are made, and we undertake
no obligation to update any forward-looking statement in light of new information or future events, except as required by law,
although we intend to continue to meet our ongoing disclosure obligations under the U.S. securities laws and other applicable
laws.
We
caution you that a number of important factors could cause actual results or outcomes to differ materially from those expressed
in, or implied by, the forward-looking statements, and therefore you should not place too much reliance on them. These factors
include, among others, those described herein, under “Risk Factors” and elsewhere in this Annual Report and in our
other public reports filed with the Securities and Exchange Commission. It is not possible to predict or identify all such factors,
and therefore the factors that are noted are not intended to be a complete discussion of all potential risks or uncertainties
that may affect forward-looking statements. If these or other risks and uncertainties materialize, or if the assumptions underlying
any of the forward-looking statements prove incorrect, our actual performance and future actions may be materially different from
those expressed in, or implied by, such forward-looking statements. We can offer no assurance that our estimates or expectations
will prove accurate or that we will be able to achieve our strategic and operational goals.
Forward-looking
statements are based on information we have when those statements are made or management’s good faith belief as of that
time with respect to future events, and are subject to significant risks and uncertainties that could cause actual performance
or results to differ materially from those expressed in or suggested by the forward-looking statements. Important factors that
could cause such differences include, but are not limited to:
|
|
●
|
our
lack of operating history;
|
|
|
|
|
|
|
●
|
our
current and future capital requirements and our ability to satisfy our capital needs;
|
|
|
|
|
|
|
●
|
our
inability to keep up with industry competition;
|
|
|
|
|
|
|
●
|
interpretations
of current laws and the passages of future laws;
|
|
|
|
|
|
|
●
|
acceptance
of our business model by investors and our ability to raise capital;
|
|
|
|
|
|
|
●
|
our
drug discovery and development activities may not result in products that are approved by the applicable regulatory authorities.
Even if our drug candidates do obtain regulatory approval they may never achieve market acceptance or commercial success;
|
|
|
|
|
|
|
●
|
our
reliance on key personnel, including our ability to attract and retain scientists;
|
|
|
|
|
|
|
●
|
our
reliance on third party manufacturing to supply drugs for clinical trials and sales;
|
|
|
|
|
|
|
●
|
our
limited distribution organization with no sales and marketing staff;
|
|
|
|
|
|
|
●
|
our
being subject to product liability claims;
|
|
|
|
|
|
|
●
|
our
reliance on key personnel, including our ability to attract and retain scientists;
|
|
|
|
|
|
|
●
|
legislation
or regulation that may increase the cost of our business or limit our service and product offerings;
|
|
|
|
|
|
|
●
|
risks
related to our intellectual property, including our ability to adequately protect intellectual property rights;
|
|
|
|
|
|
|
●
|
risks
related to government regulation, including our ability to obtain approvals for the commercialization of some or all of our
drug candidates, and ongoing regulatory obligations and continued regulatory review which may result in significant additional
expense and subject us to penalties if we fail to comply with applicable regulatory requirements; and
|
|
|
●
|
our
ability to obtain regulatory approvals in foreign jurisdictions to allow us to market our products internationally.
|
Moreover,
new risks regularly emerge and it is not possible for our management to predict or articulate all risks we face, nor can we assess
the impact of all risks on our business or the extent to which any risk, or combination of risks, may cause actual results to
differ from those contained in any forward-looking statements. All forward-looking statements included in this prospectus are
based on information available to us on the date of this registration statement. Except to the extent required by applicable laws
or rules, we undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information,
future events or otherwise. All subsequent written and oral forward-looking statements attributable to us or persons acting on
our behalf are expressly qualified in their entirety by the cautionary statements contained above and throughout this registration
statement.
USE
OF PROCEEDS
We
will not receive any proceeds from the resale of the common stock by the Selling Shareholder. However, we may receive up to $492,187
in gross proceeds from the exercise of outstanding warrants. Any proceeds received by us pursuant to the Selling Shareholder’s
exercise of the outstanding warrants may be used for general corporate purposes and working capital, acquisitions or assets, businesses
or operations or for other purposes that the board of directors, in its good faith, deems to be in the best interest of the Company.
DETERMINATION
OF OFFERING PRICE
In
determining the offering price of the common stock and the exercise price of the warrants, we have considered a number of factors
including, but not limited to, the current market price of our common stock, trading prices of our common stock over time, the
volatility of our common stock, our current financial condition and the prospects for our future cash flows and earnings, and
market and economic conditions at the time of the offering. The offering price for the common stock sold in this offering may
be less than the market price for our common stock.
DILUTION
No
dilution should be experienced due to the sale of shares by the Selling Shareholder. However, all shareholders in the Company
may be subject to dilution from the exercise of warrants underlying the shares offered hereby or other convertible securities
currently outstanding in the Company, or if the Company issues more of its authorized stock.
SELLING
SECURITY HOLDERS
Table
of Selling Shareholders
JMJ
Financial is the only Selling Shareholder. The below table lists its holdings as they relate to this offering. The table assumes
that all of the securities will be sold in this offering. However, any or all of the securities listed below may be retained by
any of the Selling Shareholder, and therefore, no accurate forecast can be made as to the number of securities that will be held
by the Selling Shareholder upon termination of this offering. The percentages in this table are based on a grand total of 220,989,542
common shares issued and outstanding, including those held by affiliates, as of August 12, 2016.
Except
as may be otherwise noted, we believe that the Selling Shareholder has sole voting and investment powers with respect to the securities
indicated. JMJ has never been an affiliate of the Company. The Company agreed in the Purchase Agreement with JMJ to register its
shares or give it a cashless exercise option for its warrants. We will not receive any proceeds from the sale of the securities
by the Selling Shareholder. JMJ is not, and is not affiliated with, a broker-dealer. The Selling Shareholder may be deemed an
underwriter or subject to Regulation M.
|
Name
of Shareholder
|
|
Total
Shares Owned
|
|
|
Shares
Registered
|
|
|
%
Before Offering
|
|
|
Remaining
Shares if All Registered Shares Sold (assuming sale of all shares registered hereunder)
|
|
|
%
After Offering (assuming sale of all shares registered hereunder)
|
|
|
Material
Transactions with Selling Shareholder in past 3 years (incl. nature of services provided and dates provided)
|
|
|
JMJ Financial [2]
|
|
|
4,015,621
|
[1]
|
|
|
4,015,621
|
|
|
|
1.82
|
%
|
|
|
0
|
|
|
|
0
|
%
|
|
|
[1]
|
|
(1)
Assumes exercise by JMJ of all 3,515,621 of its outstanding warrants.
(2)
Justin Keener is the natural person having voting and investment control over the shares beneficially owned by JMJ. JMJ acquired
its shares pursuant to the Purchase Agreement, previously disclosed herein in more detail.
PLAN
OF DISTRIBUTION
Of
the shares being offered, 500,000 are issued and outstanding and the remaining shares are issuable upon the exercise of outstanding
warrants issued to JMJ as part of a securities purchase transaction with the Company in April, 2016. Upon this registration statement
being declared effective, the Selling Shareholder may offer and sell shares from time to time until all of the shares registered
are sold; however, this offering will end two (2) years from the initial effective date of this registration statement, unless
extended or terminated by the Company pursuant to a post-effective amendment.
There
can be no assurances that the Selling Shareholder will sell any or all of the securities. In various states, the securities may
not be sold unless these securities have been registered or qualified for sale in such state or an exemption from registration
or qualification is available and is complied with.
We
will pay all the fees and expenses incident to the registration of the securities but will not pay any commissions or similar
fees on the sale of Selling Shareholder shares offered pursuant to this prospectus.
All
of the foregoing and following may affect the marketability of our securities. Should any substantial change occur regarding the
status or other matters concerning the Selling Shareholder or us, we will file a post-effective amendment to this registration
statement disclosing such matters.
Selling
Shareholders
The
Selling Shareholder will offer its shares at prevailing market prices through broker dealer transactions or through privately
negotiated deals. The Selling Shareholder in this offering may be considered an underwriter, as that term is defined in Section
2(11) of the Securities Act. We are not aware of any underwriting arrangements that have been entered into by the Selling Shareholder.
The distribution of the securities by the Selling Shareholder may be effected in one or more transactions that may take place
in the OTC Markets, including broker’s transactions or privately negotiated transactions.
The
Selling Shareholder may pledge all or a portion of the securities owned as collateral for margin accounts or in loan transactions,
and the securities may be resold pursuant to the terms of such pledges, margin accounts or loan transactions. Upon default, the
pledgee in such loan transaction would have the same rights of sale as the Selling Shareholder under this prospectus. JMJ may
also enter into exchange traded listed option transactions, which require the delivery of the securities listed under this prospectus.
JMJ may also transfer securities owned in other ways not involving market makers or established trading markets, including directly
by gift, distribution, or other transfer without consideration, and upon any such transfer the transferee would have the same
rights of sale as such selling shareholders under this prospectus.
The
Selling Shareholder will be affected by the applicable provisions of the Securities Exchange Act of 1934, including, without limitation,
Regulation M, which may limit the timing of purchases and sales of any of the securities by the Selling Shareholder or any such
other person. We have instructed JMJ that they may not purchase any of our securities while they are selling shares under this
registration statement.
We
will not pay for any expenses relating to the sale of shares by JMJ except the fee for this registration statement, edgarizing
and other expenses related to preparation and filing this registration statement.
OTC
Markets Considerations
The
OTC Markets is separate and distinct from the NASDAQ stock market. NASDAQ has no business relationship with issuers of securities
quoted on the OTC Markets. The SEC’s order handling rules, which apply to NASDAQ-listed securities, do not apply to securities
quoted on the OTC Markets.
Although
the NASDAQ stock market has rigorous listing standards to ensure the high quality of its issuers, and can delist issuers for not
meeting those standards, the OTC Markets has no listing standards. Rather, it is the market maker who chooses to quote a security
on the system, files the application, and is obligated to comply with keeping information about the issuer in its files.
Although
we believe being listed on the OTC Markets increases liquidity for our stock, investors may have greater difficulty in getting
orders filled than if we were on NASDAQ or other exchange. Investors’ orders may be filled at a price much different than
expected when an order is placed. Trading activity in general is not conducted as efficiently and effectively on OTC Markets as
with exchange-listed securities. Also, because OTC Markets stocks are usually not followed by analysts, there may be lower trading
volume than for NASDAQ-listed securities.
Investors
must contact a broker-dealer to trade OTC Markets securities. Investors do not have direct access to the quotation service. For
OTC Markets securities, there only has to be one market maker.
DESCRIPTION
OF SECURITIES
The
following description is a summary of the material rights of shareholders. Shareholder rights are dictated via the Company’s
Articles of Incorporation and Bylaws. Each of the foregoing documents has been filed as an exhibit to the registration statement
of which this prospectus is a part.
Common
Stock
The
Company is authorized to issue 500,000,000 common shares at a par value of $0.0001 per share. As of August 12, 2016, the Company
had 220,989,542 shares of common stock outstanding.
Each
share of common stock entitles the holder to one vote, either in person or by proxy, at meetings of shareholders. The holders
are not permitted to vote their shares cumulatively. Accordingly, the shareholders of our common stock who hold, in the aggregate,
more than fifty percent of the total voting rights can elect all of our directors and, in such event, the holders of the remaining
minority shares will not be able to elect any of such directors. The vote of the holders of a majority of the issued and outstanding
shares of common stock entitled to vote thereon is sufficient to authorize, affirm, ratify or consent to such act or action, except
as otherwise provided by law. Shareholders may take action by written consent of over 50% of the issued and outstanding common
stock of the Company.
Holders
of common stock are entitled to receive ratably such dividends, if any, as may be declared by the Board of Directors out of funds
legally available. We have not paid any dividends since our inception, and we presently anticipate that all earnings, if any,
will be retained for development of our business. Any future disposition of dividends will be at the discretion of our Board of
Directors and will depend upon, among other things, our future earnings, operating and financial condition, capital requirements,
and other factors.
Holders
of our common stock have no pre-emptive rights or other subscription rights, conversion rights, redemption or sinking fund provisions.
Upon our liquidation, dissolution or winding up, the holders of our common stock will be entitled to share rateably in the net
assets legally available for distribution to shareholders after the payment of all of our debts and other liabilities. There are
not any provisions in our Articles of Incorporation or our Bylaws that would prevent or delay change in our control.
Stock
Warrants
In
the quarter ended June 30, 2016, the Company issued 23,654,908 warrants.
In
the quarter ended June 30, 2016, the Company extended to December 31, 2018 the maturity dates on 5,006,666 existing warrants that
were originally issued with expiration dates between July 25, 2016 to December 17, 2018. These warrants were originally issued
with 3 to 5 year terms, with exercise prices ranging between $0.75 and $1.00.
There
were no modifications of the terms of any warrants issued by the Company in the quarter ended June 30, 2015.
Following
is a summary of outstanding stock warrants at June 30, 2016 and activity during the six months then ended:
|
|
|
Number
of Shares
|
|
|
Exercise
Price
|
|
|
Weighted
Average Price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants as of December
31, 2015
|
|
|
9,131,500
|
|
|
$
|
0.07-15.00
|
|
|
$
|
1.47
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issued in 2016
|
|
|
23,654,908
|
|
|
$
|
0.14-2.00
|
|
|
$
|
0.32
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expired
|
|
|
(37,500
|
)
|
|
$
|
5.00
|
|
|
$
|
5.00
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
(0
|
)
|
|
$
|
0
|
|
|
$
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants as of June 30, 2016
|
|
|
32,748,908
|
|
|
$
|
0.07-15.00
|
|
|
$
|
0.54
|
|
Summary
of outstanding warrants as of June 30, 2016:
|
Expiration
Date
|
|
Number
of Shares
|
|
|
Exercise
Price
|
|
|
Remaining
Life
(years)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Third Quarter 2016
|
|
|
125,000
|
|
|
$
|
5.00
|
|
|
|
0.25
|
|
|
Fourth Quarter 2017
|
|
|
350,000
|
|
|
$
|
1.50-9.00
|
|
|
|
1.50
|
|
|
First Quarter 2018
|
|
|
127,500
|
|
|
$
|
15.00
|
|
|
|
1.75
|
|
|
Second Quarter 2018
|
|
|
33,334
|
|
|
$
|
15.00
|
|
|
|
2.00
|
|
|
Third Quarter 2018
|
|
|
250,000
|
|
|
$
|
1.50
|
|
|
|
2.25
|
|
|
Fourth Quarter 2018
|
|
|
6,089,166
|
|
|
$
|
1.00-1.50
|
|
|
|
2.50
|
|
|
First Quarter 2019
|
|
|
4,024,000
|
|
|
$
|
0.50-2.00
|
|
|
|
2.75
|
|
|
Second Quarter 2019
|
|
|
135,000
|
|
|
$
|
0.07–0.23
|
|
|
|
3.00
|
|
|
Third Quarter 2019
|
|
|
260,000
|
|
|
$
|
0.50-1.50
|
|
|
|
3.25
|
|
|
Fourth Quarter 2019
|
|
|
400,000
|
|
|
$
|
0.14
|
|
|
|
3.50
|
|
|
Second Quarter 2020
|
|
|
300,000
|
|
|
$
|
0.50
|
|
|
|
4.00
|
|
|
Third Quarter 2020
|
|
|
1,000,000
|
|
|
$
|
0.20
|
|
|
|
4.25
|
|
|
Fourth Quarter 2020
|
|
|
12,650,000
|
|
|
$
|
0.20
|
|
|
|
4.50
|
|
|
First Quarter 2021
|
|
|
7,004,908
|
|
|
$
|
0.10-0.20
|
|
|
|
4.75
|
|
A
total of up to 3,515,621 shares are issuable upon the exercise of the outstanding warrants issued to JMJ. The JMJ warrants have
a term of 5 years and the exercise price per share of Common Stock pursuant to the warrants issued to JMJ is $0.14 cents per share
unless this registration statement is not declared effective within six months at which time JMJ would be entitled to a cashless
exercise equal to the quotient obtained by dividing [(A-B) (X)] by (A), where:
|
|
(A)
=
|
the
VWAP on the trading day immediately preceding the date on which Holder elects to exercise this Warrant by means of a “cashless
exercise,” as set forth in the applicable Notice of Exercise;
|
|
|
|
|
|
|
(B)
=
|
the
Exercise Price of this Warrant, as adjusted hereunder; and
|
|
|
|
|
|
|
(X)
=
|
the
number of Warrant Shares that would be issuable upon exercise of this Warrant in accordance with the terms of this Warrant
if such exercise were by means of a cash exercise rather than a cashless exercise.
|
INTEREST
OF NAMED EXPERTS
The
financial statements of the Company for fiscal years ending December 31, 2014 and 2015 have been included herein in reliance upon
the reports of Turner, Stone & Company, L.L.P., certified public accountants upon the authority of said firm as experts in
accounting and auditing.
The
legality of the securities offered under this registration statement is being passed upon by Austin Legal Group, APC.
DESCRIPTION
OF BUSINESS
Organization
Immune
Therapeutics, Inc. was initially incorporated in Florida on December 2, 1993 as Resort Clubs International, Inc. It was formed
to manage and market golf course properties in resort markets throughout the United States. Galliano International Ltd. was incorporated
in Delaware on May 27, 1998 and began trading in November 1999 through the filing of a 15C-211. On November 10, 2004, Galliano
merged with Resort Clubs. Resort Clubs was the surviving corporation. On August 23, 2010, Resort Clubs changed its name to pH
Environmental Inc. (“pH Environmental”).
On
April 23, 2012, pH Environmental completed a name change to TNI BioTech, Inc., and on April 24, 2012, we executed a share exchange
agreement for the acquisition of all of the outstanding shares of TNI BioTech IP, Inc. On September 4, 2014, a majority of our
shareholders approved an amendment to our Amended and Restated Articles of Incorporation, as amended, to change our name to Immune
Therapeutics, Inc. We filed our name change amendment with the Secretary of State of Florida on October 27, 2014 changing our
name to Immune Therapeutics, Inc.
Business
The
Company currently operates out of Orlando, Florida. In July 2012, the Company’s focus turned to acquiring patents that would
protect and advance the development of new uses of opioid-related immune- therapies, such as low dose naltrexone and Methionine
[Met5]-enkephalin. The Company’s therapies are believed to stimulate and/or regulate the immune system in such a way that
they provide the potential to treat a variety of diseases. We believe our therapies may be able to correct abnormalities or deficiencies
in the immune system in diseases such as HIV infection, autoimmune disease, immune disorders, or cancer; all of which can lead
to disease progression and life-threatening situations when the immune system is not functioning optimally.
In
October 2012, the Company formed TNI BioTech International, Ltd., a BVI company in Tortola, British Virgin Islands, which was
set up to allow the Company to market and sell LDN in those countries outside the U.S. in which we have been able to obtain approval
to sell the Company’s products.
In
August 2013, the Company formed its United Kingdom subsidiary, TNI BioTech, LTD (the “UK Subsidiary”). The UK Subsidiary
received approval to be considered a micro, small or medium-sized enterprise (“SME”) with the European Medicines Agency
(“EMA”) on August 21, 2013. The designation provides the UK Subsidiary with significant discounts when holding meetings
or submitting filings to the EMA. On September 19, 2013, the UK Subsidiary submitted a pre-submission package to the EMA regarding
Crohn’s Disease. The EMA granted the UK Subsidiary a meeting that took place on September 27, 2013. The UK Subsidiary is
eligible to benefit from the provisions for administrative and financial assistance for SMEs set out in Regulation (EC) No 2049/2005.
The Company will apply to obtain EMA benefits once funding becomes available.
In
December 2013, the Company formed a new subsidiary, Cytocom Inc., to focus on conducting LDN and MENK clinical trials in the United
States. In December 2014, the Company finalized the distribution of common stock of Cytocom Inc. to its shareholders. As part
of the transaction, the Company retained exclusive rights to all international patents, in-country approvals, formulations, trademarks,
manufacturing, marketing, sales, and distributions rights in emerging nations, including Africa, Central America, South America,
Russia, India, China, Far East, and The Commonwealth of Independent States (former Soviet Union). The Company will continue to
have access to existing clinical data as well as any new data generated by Cytocom Inc. during drug development. On December 8,
2014, the number of Cytocom Inc. shares of common stock that were issued to our shareholders totaled 113,242,522 shares. In connection
with the transaction, Cytocom Inc. issued
140,100,000
shares of its common stock to the Company, which gave the Company a 55.3% stake in Cytocom Inc. on that date. In April 2016, the
Board of Directors and a majority of shareholders of Cytocom approved a reverse stock split of Cytocom’s outstanding common
stock with one new share of stock for each twenty old shares of common stock. Cytocom effectuated and finalized the reverse split
in June 2016. At June 30, 2016, the Company’s equity interest had been further reduced to 41%, by subsequent issuances of
Cytocom common stock to shareholders in settlement of notes payable.
In
March 2014, the Company incorporated Airmed Biopharma Limited, an Irish corporation with an address in Dublin, Ireland, and Airmed
Holdings Limited, an Irish company domiciled in Bermuda. The Irish companies were set up to benefit from incentives granted by
the Irish government for the establishment of pharmaceutical companies (many of the world’s leading pharmaceutical companies
have located in Ireland), and so that the Company could take advantage of Ireland’s status as a member of the European Union
and the European Economic Area. An Irish limited liability company enjoys a low corporate income tax rate of 12.5%, one of the
lowest in the world. The Irish-domiciled company hopes to qualify for tax incentives for Irish holding/headquartered companies
and to benefit from the network of double tax treaties that reduce withholding taxes. TNI BioTech International, Ltd. will manage
our international distribution, using product that is manufactured in Ireland and elsewhere.
One
aspect of our business strategy is to continue to develop our licensing arrangement with
Complete
Pharmacy and Medical Solutions, LLC (“Complete Pharmacy”)
and potentially enter into other licensing arrangements
with other compounding pharmacies outside of the U.S., through which we can generate revenue from the sale of our proprietary
LDN formulation. On May 20, 2016, we entered into a contract for the compounding of pharmaceutical products with
Complete
Pharmacy
pursuant to which
Complete Pharmacy
will carry out the services of
compounding, packaging and distributing tablets of our LDN formulation in the U.S. in exchange for 50% of the net revenue of its
sales. The Company has agreed to provide the raw materials to Complete Pharmacy pursuant to a 3-year contract. The contract may
be terminated by mutual agreement or upon 30 days written notice in if there is a material breach. As of October 1, 2016 no revenue
has been received from Complete Pharmacy.
The
Company does not market our product IRT-103 Low Dose Naltrexone as an off-label product. However, there are many doctors that
prescribe the drug off-label for uses that are not approved by the FDA. The Company believes that it is beneficial to provide
a cGMP manufactured product from an FDA approved 503(b) outsource facility rather than have a compounded product with no manufacturing
controls.
The
Compounding Quality Act allows an entity that compounds sterile drugs to register as an outsourcing facility. Once registered,
an outsourcing facility must meet certain conditions in order to be exempt from the FDCA’s approval requirements and the
requirement to label products with adequate directions for use. Under the new law, the drugs must be compounded in compliance
with CGMP by or under the direct supervision of a licensed pharmacist in a registered facility (section 503B(a)). The outsourcing
facility must also report specific information about the products that it compounds, including a list of all of the products it
compounded during the previous six months, and information about the compounded products, such as the source of the ingredients
used to compound (section 503B(3)). In addition, the outsourcing facility must meet other conditions described in the new law,
including reporting adverse events and labeling its compounded products with certain information (section 503B(b)(5) and section
503B(a)(10)). The Company has contracted with Complete Pharmacy because it is registered and in compliance with the Compounding
Quality Act.
Product
Development
The
Company’s technology platform is built surrounding two different immune therapies, IRT-103, low dose naltrexone herein sometimes
referred to as “LDN” or “Lodonal” and IRT-101, herein sometimes referred to as Methionine-Enkephalin or
“MENK.” Both therapies have been decades in the making, over forty clinical trials run by institutions such as the
Pennsylvania State University Medical School at Hershey, University of Chicago, State University of New York, and Multiple Sclerosis
Center at UCSF. When the Company acquired the assets from the either the patent holder or licensee we also acquired the rights
to the clinical data, orphan drug designations and IRB. The Company has completed one trial in Nigeria for Lodonal™ and
has a second trial underway for cervical cancer in Malawi. The Company has also submitted briefing packaged to the FDA for a phase
IIB/III trial for IRT-103 for Crohn’s Disease and is preparing a new briefing package for IRT-103 for pancreatic cancer.
In addition to the work in the United States we have been completing pre-clinical and clinical work for IRT-013 with Professor
Shan at the School of Immunology in China.
LDN
The
Company has branded our Immediate Release Low Dose Naltrexone as IRT-103
TM
when working with the FDA and EMA and trademarked
our product in Africa as Lodonal
TM
. Lodonal with an immediate release 4.5mg formulation of Naltrexone and is the brand
name for our HIV treatment. LDN can be any formulation between .05 and to 10mg and may or may not be immediate release and may
be an oral or liquid formulation.
The
FDA approved naltrexone HCl in 1984 for the treatment of opioid addiction. The typical daily dosage for opioid addiction is 50mg
to 100mg, and 50mg tablets are available commercial. There is no FDA-approved use for naltrexone at any lower dosage then 50mg
for the treatment of any other medical conditions or treatments.
Where
high dose Naltrexone at 50mg to 100mg and Slow release Naltrexone between .01mg and 10mg and Immediate Release Naltrexone between
.01mg and 10mg share commonality” in categories of genes and are considered the same drug the difference in dosing and delivery
method (immediate release) difference in the overall response to the immune system.
There
is a difference in the cell patterns of genes that are altered by the treatment of immediate release naltrexone verses high does
naltrexone and slow release low naltrexone between .01mg and 10mg. The differences are important because immediate release naltrexone
between .01 and 10mg acts as an immunomodulator.
Since
immediate release naltrexone blocks the opiate receptors only for a few hours before it is naturally excreted, what results is
a rebound effect; in which both the production
and
utilization of methionine-enkephalin or opiate growth factor are increased.
Once the immediate release naltrexone has been metabolized, the elevated endorphins produced as a result of the rebound effect
can now interact with the more sensitive and more-plentiful receptors and assist in regulating cell growth and immunity. There
is no rebound effect with either high dose naltrexone or slow release naltrexone and it is the rebound immunomodulatory effect
that effects the treatment of treating patients suffering from human immunodeficiency virus (HIV) acquired immune deficiency syndrome
(AIDS, autoimmune disease, opportunistic infections, cancer, inflammation, and neurodegenerative diseases
It
has been demonstrated in trials that in the presence of LDN, the numbers of T-cells, both CD4+ helper T cells and CD8+ cytotoxic
T cells, may increase by more than 300%.
The
Mechanism of Action of immediate release LDN is not fully understood at this time, but based on clinical work there are three
current theoretical models for how immediate release LDN works in autoimmune disease, inflammatory disease, cancer and HIV/AIDS.
Immediate
Release Low-Dose Naltrexone works by triggering a number of receptors including (1) the opioid and T Cell receptors on immune
cells which activate or balance various cells of the immune system, and (2) tolling receptors to shift Th1 (pro-inflammatory)
to Th2 (anti-inflammatory) which is critical when dealing with autoimmune and inflammatory disease. (1) Increases the production
of cytokines specifically an endorphin referred to as Methionine-enkephaline or OGF.
These
compounds then produce pain relief similar to opiates. The body responds to these compounds by inhibition of cell growth, promoting
healing, and reducing inflammation, all in an effort to restore homeostasis. IRLDN also causes increase in OGF receptor.
Regulatory
applications submitted, if any, to commence clinical trials and the current status of such applications.
Clinical
trials under the INDs are anticipated to be conducted in the United States and the EU.
|
IND
#
|
|
Indication
|
|
Product
Name
|
|
Sponsor
|
|
Status
|
|
34,442
|
|
HIV/AIDS
|
|
MENK (IRT-101)
|
|
Dr. Ronald Herberman and Dr.
Bernard Bihari
|
|
Inactive ; filed in 1997 (1)
|
|
|
|
|
|
|
|
|
|
|
|
67442
|
|
Crohn’s Disease
|
|
Naltrexone HCL (IRT-103)
|
|
Dr. Jill Smith
|
|
Active ; filed May 31, 2003 and filed with
the FDA in March, 2013
|
|
|
|
|
|
|
|
|
|
|
|
50987
|
|
Pancreatic Cancer
|
|
MENK (IRT-101)
|
|
Dr. Jill Smith
|
|
Active ; was filed with the FDA in March,
2013
|
(1) Currently
inactive but will soon be reactivated and transferred to TNIB.
Immune
Therapeutics Inc. Drug Development Plan
Nigeria
Immune
Therapeutics Inc., through its wholly owned subsidiary TNI BioTech Intl., completed a 90-day bridging trial for the treatment
of patients with HIV/AIDS in Nigeria. The National Agency for Food and Drug Administration and Control (NAFDAC) approval is based
on previous clinical data and the Nigeria Bridging Trial was a single center, open labeled, randomized, bridging study. The
trial consisted of a total of one hundred and fifty [150] patients of both genders between the ages of 18-60, each of whom was
infected with the human immunodeficiency virus (HIV).
The
90-Day Bridging Trial was undertaken at the State Specialist Hospital in Asubiaro, Osogbo, Osun State, Nigeria and the primary
objective of this Bridging Trial was to confirm that Lodonal
TM
has a beneficial effect on the immune system of immune
deficient patients and that it is safe. The trial separated the patients into a Control (placebo) Group and a Treatment Group
(which was administered Lodonal
TM
). The efficacy of increasing CD4 count [cell/mm
3
] between Day-1 and Day-90
by at least 25% was set as the criteria for demonstrating beneficial effect on the immune system. Safety was demonstrated
through quality of life assessment and vitals both of which were not adversely affected. Treatment Group patients were given a
daily dose of 4.5-mg/kg of Lodonal.
The
results yielded an average increase of 44% increase in CD4 count in the Lodonal Treatment Group compared to an 11% increase in
the Control Group. Additionally, there were no reported opportunistic infections and no toxicity levels uncovered. Liver function
remained normal and there was no negative impact on other systems based on blood results. No sleep disturbance or vivid dreams
were present enough to justify trial discontinuation. No appreciable adverse CNS, renal, cardiac, hepatic, musculoskeletal, hematopoietic
side effects were present.
NAFDAC
has issued approval of Lodonal as an immune system regulator in the management of HIV patients and the company is now in the process
of completing the registration to import the drug into Nigeria.
Malawi
The
Company, through its wholly owned subsidiary TNI BioTech International, received permission from the Pharmacy, Medicines and Poisons
Board (PMPB) and The College of Medicine; University of Malawi to initiate a clinical trial for a Single Visit Approach to Cervical
Cancer Prevention in the Republic of Malawi. The PMPB issued drug approval to import the drug in 2015.
The
Malawi Clinical Trial’s primary endpoint includes Safety, Acceptability, and Feasibility of a Single Visit Approach to Cervical
Cancer Prevention in patients. (Trial number: VIA-LDN-401 -0 I). The secondary objectives’ is to determine life extension;
to improve the immune system of HIV and Cancer positive patients by starting treatment with LDN (“Lodonal
TM”)
and to ensure marked improvement in Clinical benefit based upon parameters that reflect the overall well- being of the patient,
including Pain control, performance status, and body weight under the supervision of Dr. Frank Taulo, Dr. Gladys Gadama, Dr. Effie
Chipeta as Principal Investigators
.
The
Company intends to initiate a number of additional trials in Africa in the next 6 months, which will include trials as an adjunct
to chemotherapy in Kenya and Ghana and HIV/AIDS in Malawi.
FDA
and EMA Development Plan for Cytocom, Inc.
After
the completion of the spin-off of our subsidiary, Cytocom, Inc., to shareholders, all work with the FDA, EMA or any of the G7
countries will be under the supervision of Cytocom Inc. However, the Company plans to submit all trials and fees on Cytocom
Inc.’s behalf until such time as Cytocom, Inc. is sufficiently funded, as all funding is currently being provided through
the Company. Nonetheless, the trials will be supervised by Cytocom, Inc. following submission by the Company. At this time
the INDs have not been transferred to Cytocom; however they will be transferred before the trials begin. Studies and trials in
countries that are not part of the G7 are being conducted directly by the Company.
Our
lead product candidate is IRT-103 ™, a first-in-class, proprietary fixed-dose therapy entering Phase 2b clinical trials
for the treatment of adult and pediatric Crohn’s disease where we have orphan drug designation. The Company acquired all
of the clinical data from Dr. Jill Smith in conjunction with the acquisition of the license. The Company had no direct involvement
in the trials.
Crohn’s
disease affects over 1.6 million adults in the United States and an estimated 80,000 children. Data from the Phase 2a clinical
trial indicate that Lodonal™ was generally well-tolerated therapy that can provide promise for the treatment of Crohn’s
disease. The Department of Medicine at The Pennsylvania State University, (PSU) College of Medicine, GI Medicine were responsible
for three of the Crohn’s trials that were part of our briefing package to the FDA.
PSU
researchers have demonstrated in a mouse model of Crohn’s disease from with dextran sodium sulfate (a chemically induced
colitis) that opioid receptor blockade by a low dose of naltrexone resulted in less weight loss, lower disease activity index
scores and less histological evidence of inflammation when compared to controls (Matters, GL
et al
., 2008). Furthermore,
the researchers demonstrated that tissue inflammatory cytokine mRNA was reversed to baseline levels in the colons of mice treated
with naltrexone.
A
Phase 2 trial with low-dose naltrexone was completed on patients with Crohn’s Disease and the objective of the trial was
to determine the role of endogenous opioids and opioid antagonists in healing and repair of tissues. Eligible subjects with histologically
and endoscopically confirmed active Crohn’s disease activity index (CDAI) score of 220–450 were enrolled in a study
using 4.5 mg naltrexone per day. Infliximab was not allowed for a minimum of 8 weeks prior to study initiation. Other therapy
for Crohn’s disease that was at a stable dose for 4 weeks prior to enrollment was continued at the same doses. Patients
completed the inflammatory bowel disease questionnaire (IBDQ) and the short-form (SF-36) quality of life surveys and CDAI scores
were assessed pre-treatment, every 4 weeks while on therapy and 4 weeks after completion of the study. The drug was administered
by mouth each evening for a 12-week period. Seventeen patients with a mean CDAI score of 356 °æ 27 were enrolled. CDAI
scores decreased significantly (P = 0.01) with LDN, and remained lower than baseline 4 weeks after completing therapy. Eighty-nine
percent of patients exhibited a response to therapy and 67% achieved a remission (P < 0.001). Improvement was recorded in both
quality of life surveys with LDN compared with baseline. No laboratory abnormalities were noted. The most common side effect was
sleep disturbances, occurring in seven patients. The trial concluded that LDN therapy appears effective and safe in subjects with
active Crohn’s disease.
Another
Phase 2 trial therapy was conducted, entitled, Therapy with the Opioid Antagonist Naltrexone Promotes Mucosal Healing in Active
Crohn’s Disease: A Randomized Placebo-Controlled Phase 11 trial at the Department of Medicine, The Pennsylvania State University,
College of Medicine, GI Medicine [Smith, JP.
et al,
2011]. Aims – A randomized double-blind placebo-controlled study
was designed to test the efficacy and safety of an opioid antagonist for 12 weeks in adults with active Crohn’s disease.
The phase 2A trial had 3 major endpoints for the study including: 1) Clinical improvement based upon the Crohn’s Disease
Activity Index (CDAI) Score, 2) Mucosal healing by colonoscopy, and 3) Safety. The human studies were done with FDA approval under
IND 67442. Forty subjects with active Crohn’s disease were enrolled in the study. Randomized patients received daily oral
administration of 4.5-mg naltrexone or placebo. Providers and patients were masked to treatment assignment. The primary outcome
was the proportion of subjects in each arm with a 70-point decline in Crohn’s Disease Activity Index (CDAI) score. The secondary
outcome included mucosal healing based upon colonoscopy appearance and histology. Eighty-eight percent of those treated with naltrexone
had at least a 70-point decline in CDAI scores compared to 40% of placebo-treated patients (p = 0.009). After 12 weeks, 78% of
subjects treated with naltrexone exhibited an endoscopic response as indicated by a 5-point decline in the Crohn’s disease
endoscopy index severity score (CDEIS) from baseline compared to 28% response in placebo-treated controls (p = 0.008). 33% achieved
remission with a CDEIS score <6, whereas only 8% of those on placebo, showed the same change. Fatigue was the only side effect
reported that was significantly greater in subjects receiving placebo. The study concluded that Naltrexone improves clinical and
inflammatory activity of subjects with moderate to severe Crohn’s Disease compared to placebo-treated controls. Strategies
to alter the endogenous opioid system provide promise for the treatment of Crohn’s Disease.
A
pilot study was completed entitled Safety and Tolerability of Low-dose Naltrexone Therapy in Children With Moderate to Severe
Crohn’s Disease trial at the Department of Medicine, The Pennsylvania State University, College of Medicine, GI Medicine
Smith, ( JP.
et al,
2013). The aims of this study were to evaluate the safety and tolerability of an opioid antagonist,
naltrexone, in children with moderate to severe Crohn’s disease. The pilot clinical trial was conducted in children with
moderate to severe Crohn’s disease. Fourteen subjects with a mean age of 12.3 years (range, 8 to 17 y) were enrolled. Children
were randomized to placebo or naltrexone (0.1 mg/kg) orally for 8 weeks followed by open-labeled treatment with 8 additional weeks
of naltrexone. Safety and toxicity were monitored by physical examinations and blood chemistries. Clinical activity was assessed
by the Pediatric Crohn’s Disease Activity Index (PCDAI) and Quality of life was monitored by the Impact III survey. The
results indicated that oral naltrexone was well tolerated without any serious adverse events in children with moderate to severe
Crohn’s disease. PCDAI scores decreased from pre-treatment values (34.2Å 3.3) with an 8-week course of naltrexone
therapy (21.7Å 3.9) (P=0.005). Twenty-five percent of those treated with naltrexone were considered in remission (score
r10) and 67% had improved with mild disease activity (decrease in PCDAI score by at least 10 points) at the
end
of the study. Systemic and social quality of life improved with naltrexone treatment (P=0.035). The study concluded that naltrexone
therapy seems safe with limited toxicity when given to children with Crohn’s disease and may reduce disease activity.
Food
And Drug Administration
There
are three types of meetings with the FDA: Type A Meeting – is a meeting that is “immediately necessary for an otherwise
stalled drug development program to proceed.” This type of meeting refers to meetings to resolve disputes, talk about
clinical holds, special protocols. Type B Meetings are identified as (1) pre-IND meetings, (2) certain end of Phase I meetings,
(3) end of Phase 2/pre-Phase 3 meetings and (4) pre-NDA/BLA meetings. A type C Meeting is any other kind of meeting.
The
Company attended a Type C meeting with the FDA June 26, 2013 with the Division of Gastroenterology and Inborn Errors Products
regarding the clinical and regulatory aspects of the proposed Phase IIB/III development program and future 505(b)(2) application
for Low Dose Naltrexone (LDN) in the treatment of adults and pediatric patients with Crohn’s Disease. In principal the FDA
agreed that a 505(b)(2) application would be an acceptable approach at FDA recommends that sponsors considering the submission
of an application through the 505(b)(2) pathway consult FDA’s regulations at 21 CFR 314.54, and the draft guidance for industry
Applications Covered by Section 505(b)(2) (October 1999). The Company is planning to submit a request for “breakthrough
technology” designation. If this request is granted, what impact could it have on minimizing the Phase 3 study design(s)
and the package needed for filing the 505(b)(2) application.
The
Company retained the services of Cote Orphan to provide a new briefing package and work with the Division for Breakthrough Therapy
Designation is granted to determine the most efficient development program for your product and proposed indication. The Company
notes that a 505(b)(2) NDA will need to be submitted with all the same components that a regular NDA. The Company hopes to submit
our briefing package to the FDA because CDAI was previously been accepted by the FDA for phase 3 trials in CD, FDA has reconsidered
the use of the CDAI as a measure of clinical response to therapeutic intervention and we our new trial design endpoint will include
endoscopies to show true mescal healing. The Company expects to present our new briefing package to the FDA in third quarter 2016
with a meeting shortly thereafter.
Anticipated
developmental timelines for Cytocom, Inc.
Guidance
meeting held with FDA regarding pediatric and adult studies provided feedback and based on the new submission of a briefing package
we anticipate presenting final protocols for both adult and pediatric trial.
|
|
●
|
Current
development plan includes:
|
|
|
●
|
Two
adult studies planned:
|
|
|
●
|
Double-blind,
randomization, 24-week study of LDN vs. Placebo at 4.5mg
|
|
|
●
|
Double-blind,
randomization 12-week multi-dose study including 4.5mg
|
|
|
●
|
Both
studies will roll-over to an open label
|
|
|
●
|
Studies
to commence Q1 2017
|
|
|
●
|
Double-blind
randomization, 24 week study of LDN vs. Placebo at
|
|
|
●
|
Both
studies will roll-over to open label
|
|
|
●
|
Studies
to commence Q1 of 2017
|
Competitive
Advantage
The
Company believes many of the same advantages of our therapies apply to both the US market as well as the African market. Lodonal
TM
could provide the first affordable, non-toxic approach for treatment of immune dysfunction, cancer and d chronic inflammatory
state.
Some
of the Competitive Advantages and Benefits of Lodonal
TM
include the following:
Lower
production costs and sales price of treatments
Today
the majority of the drugs under development are both more expensive and more toxic. We do not believe this is the right way to
move forward. Biologic agents cost between $12,000 and $150,000 a year.
Lodonal
TM
/
IRT-103 can be manufactured and delivered in Emerging Nations for under $.90 cents a day and we estimate a price of $3,600 dollars
a year in developed country underwritten to $10 dollars per month and when not underwritten the company will provide to patients
for $30 dollars a month.
Lodonal
should be able to substantially reduce health care costs for a number of reasons:
|
|
●
|
IRT-103
and Lodonal can provides a new, non-toxic inexpensive method of medical treatment by mobilizing the natural defenses of one’s
own immune system. It can be used as a stand-alone therapy or and adjunct to existing immunosupressive therapies by reducing
the toxic side effects of immunosupressive drugs.
|
|
|
●
|
Patients
who are taking immunosuppressant drugs should see their doctor on a regular basis to monitor the patient for unwanted side
effects.
|
|
|
●
|
Lodonal
does not require the medical supervision of antiretroviral or immunosupressive therapies
|
|
|
●
|
Immunosupressive
drugs are very powerful and can cause such serious side effects as high blood pressure, kidney problems, malignancies and
liver disorder. Immunosuppressant drugs lower a person’s resistance to infection and can make infections harder to treat.
The drugs can also increase the chance of uncontrolled bleeding.
|
|
|
●
|
Lodonal
has no toxic side effects as it is an immunomodulator and activates and re-balances the immune system.
|
|
|
●
|
Avoiding
contact with people who have infections is also important. In addition, people who are taking or have been taking immunosuppressant
drugs should not have such immunizations as smallpox vaccinations without consulting their physician. Because their resistance
to infection has been lowered, people taking these drugs might get the disease that the vaccine is designed to prevent. People
taking immunosuppressant drugs should avoid contact with anyone who has had a recent dose of oral polio vaccine, as there
is a chance that the virus used to make the vaccine could be passed on to them.
|
|
|
●
|
Lodonal
has no toxic side effects
|
|
|
●
|
Indirect
Cost of Immunosupressive drugs
|
|
|
●
|
HIV/AIDS
still ranks 5th among the 14 diseases, with indirect per person costs ranging from $890 to $2663 in Zaire and from $2425 to
$5903 in Tanzania indirect costs are roughly 95% of the total costs which are not covered by donor dollars.
|
|
|
|
|
|
|
●
|
The
total economic burden of CD was $10.9-15.5 billion in the United States and euro 2.1-16.7 billion of which is approximately
30% to 50% are indirect cost.
|
|
|
●
|
Lodonal
has been shown to reduce the number of opportunistic infections with HIV/AIDS
|
Lodonal
can improve compliance
|
|
●
|
Lodonal
is a simple, once-daily regimen, which can be taken with or without a food,
|
|
|
●
|
Occasional
non-compliance will not affect the overall success therapy.
|
|
|
|
|
|
|
●
|
Does
not require the medical supervision of antiretroviral or immunosupressive therapies (people will not lose time from work)
|
|
|
|
|
|
|
●
|
Estimates
suggest that the average rates of non-adherence to antiretroviral therapy range from 50% to 70%.
|
|
|
●
|
The
principal factors associated with non-adherence appear to be patient-related, such as inconvenient dosing frequency, dietary
restrictions, pill burden, and the single biggest reason stated is the toxic side effects of the drugs.
|
|
|
●
|
Lodonal
No Toxic Side Effects
|
|
|
|
|
|
|
●
|
Improved
Quality of Life
|
Our
lead product candidate with the FDA is IRT-103 and is posed to initiate a pivotal phase IIB/III clinical trial for moderate-to-severe
adult Crohn’s disease as well for pediatric Crohn’s disease, an orphan indication. We will need to complete both a
Phase 2B and Phase 3 clinical trial for both Pediatric and Adult Crohn’s Disease for IRT-103 before we can obtain final
approval to market the drug in the U.S. from the FDA.
The
Company seeks to benefit patients with chronic and often life-threatening diseases through the stimulation and/or regulation of
the body’s immune system. Using our patented immunotherapy, management believes that the Company’s products, technologies
and patents will harness the power of the immune system to improve the treatment of cancer, HIV/AIDS, autoimmune diseases, opportunistic
infections and inflammatory disorders.
Recent
Accomplishments
The
Company obtained drug approval from NAFDAC (National Agency for Food and Drug Administration and Control) in April of 2016. In
May the Company began the regulatory process with NAFDAC for marketing authorization. There were a number of steps the Company
is required to complete before marketing approval will be granted despite the current approval of Lodonal™ as a treatment
of immune deficiency by NAFDAC.
The
Company is currently completing the regulatory process to begin marketing the drug in Nigeria. Beginning in July of 2016 NAFDAC
began requiring a manufacturing site inspection before authorizing the marketing of any new products, including Lodonal™.
On
October 3, 2016 the Company received a letter from NAFDAC requesting a site visit of our manufacturing facility as the final step
in regulatory marketing approval. Upon completion of the site visit the Company expects to receive marketing approval shortly
thereafter.
The
Company has been informed that the Nigerian approval allows the company to use the approval to be “fast tracked” for
approval in the Economic Community of West African States, a regional group of sixteen countries, of which Nigeria is a member.
The countries include Benin, Burkina, Faso, Cote d’Ivoire, Gambia, Ghana, Guinea-Bissau, Liberia, Mali, Nigeria, Senegal,
Sierra Leone, and Togo. These countries play a major role in our Africa development strategy, as they are part of the “Test
and Treat Program” for West and Central Africa. While the trend in international health funding for HIV/AIDS and ART (Antiretroviral
Therapy) and the policies that drive the funding, has been to focus on high-burden countries and HIV ‘hotspots’ in
Sub-Saharan Africa, most countries in the region classified by the UN as the West and Central Africa (WCA) region have been neglected.
In the WCA region, 76% of those who need antiretroviral therapy – a total of five million people – are still awaiting
treatment. The unmet needs in most of these countries are slipping further out of focus and we believe that Lodonal™ could
help the people of West and Central Arica.
In
September of 2016, the company retained the services of GLOBALMEDLINE SARL in Senegal to assist the drug registration of Lodonal™
in Senegal for HIV/AIDS and Cancer. The filing in Senegal is part of the company’s program to register Lodonal throughout
the francophone (French-speaking) countries in Africa using our approval in Senegal to fast track the process.
The
Company is currently in the process of filing in Kenya, Ghana, Liberia, Mali and Uganda. We will be adding additional countries
in 2017.
In
September of 2016 the Company signed a consulting agreement with the Honorable Joyce Banda as a member of the Champions for an
AIDS Free Generation. His Excellency Festus Mogae, the Former President of the Republic of Botswana, first launched The Champions
in 2008. The Champions program works to ensure that all children are born free from HIV in Africa and that all people have access
to quality HIV prevention and treatment services.
The
Company has been assigned two provision patent applications: No. 62/296,759, a method for Inducing a Sustained Immune Response
and No: 62/379, 272, a Method for Treating and Preventing Protozoan Infection. The Company expects to file additional patent applications
in the coming month. Our intellectual property portfolio includes biotech assets acquired either from acquisition or exclusive
licensing
The
Company has retained the services Coté Orphan. Cote Orphan is a boutique, full-service, lab-to-market regulatory group
with a laser focus on Orphan Drugs. They take your idea to the FDA and EMA for approval. Team Coté is led by Dr. Tim Coté,
the former Director of the FDA’s Office of Orphan Products Development (OOPD). He will work with us to file a briefing package
and final protocols for adult and pediatric Crohn’s Disease. We expect to finish this process in the next 60 days.
The
Company’s Board has authorized continued discussions with a number of potential partners in Far East as well as discussion
with drug development partners in both the US and EU.
MENK
MENK,
also herein referred to as IRT-101, opiate growth factor or OGF, and Methionine-enkephalin is a synthetic peptide that activates
natural killer (“NK”) cells of the immune system to seek and destroy cancer cells of the immune system to seek and
destroy cancer cells. IRT-101 is a small peptide normally made by nerve cells and immune cells.
Further
work in the laboratories of Plotnikoff, et al. has shown that daily injection of enkephalins into mice for 1 week resulted in
increases in the size and weight of the thymus gland and a concomitant decrease in size and weight of the spleen. These observations
led the same workers to study the antitumor action of the enkephalins. Mice carrying L 1210 tl.~mor cells were treated with methionine
and leucine enkephalins, and their survival was compared to placebo treated controls.
The
survival of the enkephalin-treated mice was longer than that of controls. Such observations in the animal model prompted these
and other researchers to evaluate the immune potentiating effects of enkephalins and endorphins in humans. Plotnikoff and his
group found that enkephalins and endorphins stimulated active rosettes in humans. Similar results were obtained by Gilman, et
al., and Wybran, et al. This work was expanded further to study the effect of enkephalins on an immunosuppressed population of
individuals. Lymphocytes from a group of lymphoma patients were treated with the enkephalins in vitro and then evaluated for rosetting
with sheep red blood cells (SRBC’s.) It was found that both methionine and leucine enkephalins enhanced the ability of these
cells to form T cell rosettes when compared to controls. Methionine enkephalin exhibited this immunopotentiation at a concentration
as low as 10-14 mg/ml.
Studies
have been undertaken in laboratories to investigate the effects of enkephalins on natural killer (NK) cell activity. The NK cells
are a population of cells that can selectively lyse certain tumor cells in vitro without prior sensitization. This is a heterogeneous
population of cells present in a variety of animals and humans. It is presently believed that these cells play a major role in
protective surveillance against cancer. Anticancer potential of many of the immune activators has been measured by their ability
to boost the (NK) defenses of the host. Results to date indicate enkephalins are capable of enhancing NK activity in vitro from
normal volunteers and more importantly from cancer patients, some of whom have been heavily pretreated with chemotherapy.
Three
separate groups have now reported in vivo clinical findings with the use of methionine enkephalin in patients with AIDS and symptomatic
HIV infection. A Low Dose Study (10 micrograms/kg l.V.3 times/wk. for 12 weeks) Zunich and Kirkpatrick administered 10 μg/kg
MEK intravenously three times weekly for up to 12 weeks to seven patients with various stages of HIV infection. This trial was
conducted prior to the advent of potent antiretroviral therapy. In evaluating cellular immunity, the authors stated that MEK appears
to temporarily enhance selected immune responses in patients infected with HIV. However, in this study, the results were neither
clinically, nor statistically significant. There were no adverse reactions or evidence of toxicity.
Moderate-High
Dose Studies (20, 25, 40, 50, 60, 80, and 100 micrograms/kg 1.V. 1 to 3 times/week for one to 24 months) have also been conducted.
The preliminary clinical studies of MEK in symptomatic HIV infection, AIDS and cancer patients (30, 32, 45, 46, 47, 48 and 49)
involved individualized treatment schedules with doses ranging up to 100 μg/kg 3x/week and durations extending beyond one year.
There were no serious adverse reactions attributed to treatment for any of the patients studied. All reactions were transitory
and appear to have been directly related to the infusion
Eight
Kaposi’s Sarcoma Patients were administered MEK (10-100 μg/kg 1-3 x week for 1-24 months). There were no serious adverse
reactions attributed to treatment for any of the patients studied.
Symptomatic
HIV Infection (Treatment one month up to 24 months, MEK administered at doses of 20-100 μg/kg 1-3x week). In a pilot study
in these patients, Dr. J. Wybran in Brussels, Belgium indicated that there may be immunological improvements with MEK treatment.
No adverse reactions attributable to MEK treatment were observed.
Asymptomatic
HIV+ patients Four asymptomatic HIV+ patients were treated with methionine enkephalin (60 μg/kg i.v.) once a week for 1-4 months.
The monoclonal marker Leu 19 (CD56) for natural killer and killer cells was found to be increased in all four patients (18/24
discrete measures). No adverse reactions to methionine enkephalin were reported. It appears, based on the above data, that a new
group of clinically useful immune modulators called enkephalins are emerging. Their in vitro magnitude of potency is at least
106 - 107 greater than that of interferon, interleukins and thymus hormones. Stimulatory effects were observed at concentrations
as low as 1012- 10-14 mg/ml (33-37). Such characteristics make endogenous peptides ideal for therapeutic trials. The following
additional facts are of interest in this regard: a) Methionine
enkephalin
stimulates humoral immunity (at low doses) and chemotaxis; b) Methionine enkephalin selectively stimulates production of cytotoxic
T cells. Cytotoxic T cells (CD3, CD8) have been reported to inhibit reverse transcriptase of HIV; c) Subacute safety studies in
rats and dogs at doses up to 25 mg/kg resulted in no toxicity; d) Human safety studies using doses up to 250 micrograms/kg resulted
in no toxicity.
e)
Enkephalins may in fact be the natural mediators for the endogenous release of both interleukin 2 (32, 44), gamma interferon (32,
44) and IL-12
Prior
clinical studies
8
Phase I and 4 Phase II clinical trials completed in cancer and HIV/AIDS Between the years
1997-2015
Noteably
promising data in HIV/AIDS and Cancer with strong efficacy signals
123
adults in Phase I/II trials in cancer Between the years 1997-2014
250
adults in Phase I/II for HIV/AIDS Between the years 2000 -2014
Our
first acquisition was the patents and intellectual property of Dr. Nicholas P. Plotnikoff and Professor Fengping Shan in 2012.
While Dr. Plotnikoff was with Oral Roberts University, he was a member of the team that developed and patented the specific application
of MENK as a treatment for cancer, HIV/AIDS, and infectious diseases. All of the clinical data generated by Dr. Plotnikoff has
been included in our briefing package to the FDA.
Dr.
Nicholas Plotnikoff initiated and completed Phase I and Phase II clinical studies of MENK under Investigational New Drug (“IND”)
protocols filed with the U.S. Food and Drug Administration (“FDA”). In these clinical trials, MENK has been shown
to reduce the symptoms of early AIDS and AIDS Related Complex (“ARC”), a condition also known as pre-AIDS which includes
symptoms such as fever, diarrhea, weight loss, swollen lymph nodes and herpes. In addition to the therapeutic effects of the treatments,
trial reports indicated an elevation in mood of the patients treated (Bihari, B., Plotnikoff, N., Freeman, K., Dowling, J., Duguid,
C., and Altmann, E., ’‘Methionine Enkephalin in the Treatment of ARC,’’ Seventh Int. Conf. on AIDS, Florence,
Italy, 1991).
A
double-blind, randomized controlled Phase II study of 46 patients was performed with ARC (Bihari B, Plotnikoff NP. Methionine
Enkephalin in the Treatment of AIDS-Related Complex.
CRC Press, LLC; Cytokines: Stress and Immunity
. 1999; 77-91) that
was designed to measure the effect of a regular weekly dosing schedule of MENK at two different dose levels. The study involved
randomized assignment to three arms: (i) patients on the first arm received weekly doses of 60µg/kg of MENK (low dose) for
12 weeks; (ii) patients on the second arm received a weekly infusion of 60µg/kg of MENK (low dose) for 2 weeks, followed
by 10 weekly doses of MENK at 125µg/kg (high dose); and (iii) the patients on the third arm received a placebo intravenously
for 12 weeks.
Product
Development Status
|
|
λ
|
Phase
I Trial Pancreatic was completed in 2007 – Department of Medicine, The Pennsylvania State University, College of Medicine,
GI Medicine
|
|
|
λ
|
Dose
is 250-300 μg/kg IV weekly.
|
|
|
λ
|
Showed
Minimal side effects
|
|
|
λ
|
Determine
the maximum tolerated dose standard 3+3 regimen starting at 25 μg/kg
|
|
|
λ
|
Safety
and toxicity of OGF (paresthesias, hypotension at 250μg/kg over 30 min)
|
|
|
λ
|
Compare
route of administration (IV vs. SC); solubility issues with sc in low volume
|
|
|
λ
|
Pharmacokinetic
assays; [Met
5
]-enkephalin by RIA
|
|
|
λ
|
Examine
safety of chronic administration
|
Phase
I combination trial OGF & Gemcitabine
Evaluate
the safety and toxicity of the combination of OGF biotherapy and gemcitabine chemotherapy in patients with advanced pancreatic
cancer / pharmacokinetics. N =20
Examine
the role of OGF given with gemcitabine on, patient survival.
Patients
were treatment naïve.

All
of the symptoms and possible side effects of MENK/OGF therapy such as nausea, constipation, dry mouth, flushing, diarrhea and
abdominal pain thought to be due to the advanced cancer rather than the treatment.
The
2 side effects thought to be due to the MENK/OGF therapy include the transient paresthesias or tingling at the beginning of the
infusion and the hypotension.
The
survival of patients with metastatic pancreatic cancer was increased to almost 9 months compared to standard historical medications
used.5 FU only lengthens the survival to 4.5 months and gemcitabine to 5.8 months.
Phase
II Trial Pancreatic Cancer
In
our larger Phase 2 study when we compared survival of OGF patients to 266 untreated control subjects, the survival was increased.
(Department of Medicine, The Pennsylvania State University, College of Medicine, GI Medicine)
|
|
λ
|
Funded
by FDA Orphan Drug Program
|
|
|
λ
|
Treatment
OGF 250mg/kg over 45 min weekly
|
|
|
λ
|
Open-labeled,
untreated controls
|
|
|
λ
|
Primary
endpoint: Survival
|
|
|
λ
|
Secondary
endpoints: efficacy, QOL
|
|
|
λ
|
25
OGF-treated subjects & 166 controls
|
|
|
λ
|
Eligible
patients:
|
|
|
λ
|
Unresectable
pancreatic cancer
|
|
|
λ
|
Failed
standard therapy
|
|
|
λ
|
Karnofsky
status 50%
|
Most
importantly, there were no changes in the blood laboratory tests with OGF. Compared to standard chemotherapy that reduces the
blood count from bone marrow toxicity, the blood count remained stable with OGF.
One
of the most important features of OGF therapy in cancer patients with advanced metastatic disease is the marked improvement in
Clinical Benefit. Clinical benefit is based upon parameters that reflect the overall well-being of the patient, including Pain
control, performance status, and body weight. 53% of those receiving OGF experienced a clinical benefit compared to patients treated
with standard chemotherapy.
The
Company is planning to rely on the following available information and historical data to support the initiation of the Phase
3 study and filing of the NDA:
1.
Clinical safety data from studies conducted under this IND (50,987 studies NIH# R03
CA80646,
NCT00109941, and IRB Protocol No. 26336;
2.
Clinical safety data from studies conducted under IND 34,442 (MENK for the treatment of AIDS/ARC and cancer patients and normal
healthy volunteers
Toxicology
Studies completed by Baxter and Travenol 1984 and 1985
Published
literature as summarized in this package (to provide nonclinical pharmacology and additional safety data). To support the NDA
filing, we are also planning to conduct the following additional study:
Phase
2b study will run in parallel with the Phase 1 PK and 3 month GLP toxicology studies.
|
|
●
|
Population
to be studied: Patients on first line therapy with local advanced or metastatic disease
|
|
|
●
|
The
Company is running the GLP toxicology at an FDA approved facility in China and those studies will be completed before the
end of Q4 2017.
|
|
|
●
|
The
company anticipates a database of 300 – 600 patients at the time of NDA filing.
|
|
|
●
|
The
FDA stated that a genotoxicity study will not need to be conducted.
|
|
|
●
|
The
MTD has been established previously under this IND in pancreatic cancer patients
|
|
|
●
|
Due
to safety data collected to date and established MTD, TNI BioTech believes that a Phase 1 PK study in healthy volunteers can
be run concurrently with a Phase 2 clinical trial in pancreatic cancer patients
|
|
|
●
|
Doses
will not exceed the previously established MTD (250 µg/kg)
|
Sponsor,
which in this case is the Company, will submit the Phase 2b clinical protocol for FDA review. The FDA confirmed that the Phase
2b study could be conducted as an open-label randomized study with two doses of MENK in combination with nap-paclitaxel + gemcitabine
versus nap-paclitaxel + gemcitabine. Exact study design will be determined by the Sponsor, and submitted to the FDA for review.
After
completion of the Phase 2b study, the Agency would grant the Sponsor an End-of-Phase 2 meeting.
Hubei
Qianjiang and Immune Therapeutics have a signed agreement for the development of MENK and have moved forward on that project since
2014-present.
PK
and Toxicology Studies were delayed in 2015 because the China Food and Drug Administration (CFDA) required Chemistry, Manufacturing
and Controls to be completed before starting the toxicology study. They have been started and will be completed by the end of
2017.
Hubei
Qianjiang signed Pharmaceutical Development Agreement for Formulation Development and CTM Manufacturing of Methionine–Enkephalin
for Quinjan and Immune Therapeutics with China Peptide Company (“CPC”) in Q3 2015
CPC
perform analytical, pre-formulation, formulation development, clinical trial manufacturing, release testing and ICH1 stability
for Methione- Enkephalin
CPC
is among only a handful of companies in the world that can claim both ISO Certification and cGMP licensing. In February 2012,
CPC became the first peptide company to successfully pass US FDA inspection outside of US and Europe regions.
CMC
and formulations are required for mass production of MENK, which is also required for pivotal trials with the FDA for this work
has been ongoing since our meeting with the FDA.
|
|
i.
|
Qianjiang
will provide Cote Orphan the CMC data which is required as part of filing MENK protocols.
|
In
addition Hubei has completed pre-clinical studies using MENK on various cancers in the lab using mouse models and has shown to
be successful in a number of cancers including colon, pancreatic and hepatic. The data will be translated and provided as part
of the briefing package for both pancreatic and liver cancer.
The
MENK treatment was generally well tolerated with no appreciable toxicity observed. The high dose of MENK increased adaptive cell
immunity resulting in increased activity of the body’s immune system (e.g. increased IL-2 receptors, CD56 NK and LAK cells,
CD3, CD4 and CD8 cells) and a reduction in the size of lymph nodes. One patient in the high dose group administered by rapid intravenous
infusion experienced dizziness, diaphoresis, elevated blood pressure and decreased pulse rate. These signs and symptoms were responded
to with supportive measures.
Recently,
Professor Fengping Shan and Dr. Plotnikoff have published, in a number of peer-reviewed international journals, that MENK inhibited
regulatory T-cells, increasing the functional activities of T cells and NK cells and, thus, is a key to improved cancer therapy.
They additionally published results showing that MENK alone or in combination with Interleukin-2 (“IL -2”) or Interferon-γ
(“IFN-γ”) can enhance the production of interferon- γ or IL-2 from CD4+T cells, respectively
(Shan
F, Yanjie Xia, Ning Wang, Jingjuan Meng, Changlong Lu, Yiming Meng, Nicolas P. Plotnikoff. Functional modulation of the pathway
between dendritic cells (DCs) and CD4+T cells by the neuropeptide: Methionine enkephalin (MENK).
Peptides
32. 2011; 929–937).
MENK also appeared to be more potent than IL -2 or IFN-γ, alone (Hua H, Changlong Lu, Weiwei Li, Jingjuan Meng, Danan Wang,
Nicolas Plotnikoff, Enhua Wang and Fengping Shan. Comparison of stimulating effect on subpopulations of lymphocytes in human peripheral
blood by methionine enkephalin with IL-2 and IFN-γ.
Human Vaccines & Immunotherapeutics
8:8, 2012; 1082-1089),
two widely known cytokines that have been approved by the FDA for marketing.
Plotnikoff
& Shan History
|
|
●
|
1983 Baxter takes license Conducts
sub-chronic multiple dose pathology and toxicology studies
|
|
|
|
|
|
|
●
|
Beginning 1984 Open Label clinical
studies started in cancer and AIDs patients
|
|
|
|
|
|
|
●
|
1989 NIH AID’s Committee recommends
Methionine-enkephalin for inclusion in AIDs clinical trials (low priority). Methionine-enkephalin discovered to activate LAK
cells which destroy AIDs virus
|
|
|
|
|
|
|
●
|
1990-1995 Double Blind placebo controlled
study in HIV patients begun at C.R.L in New York.
|
|
|
|
|
|
|
●
|
1995-2000 Open Label Tulsa, Belgium,
Denver and New York
|
|
|
|
|
|
|
●
|
2012-2014 Open Label 1Department
of Cord Blood Bank, Shengjing Hospital; China Medical University; Heping District, Shenyang, PR China; Department of dermatology;
No.1hospital; China Medical University; Heping District, Shenyang, PR China; Department of Immunology; School of Basic Medical
Science; China Medical University; Heping District, Shenyang, PR China and TNI Bio. Tech. Inc.; Orlando, FL USA
|
|
|
|
|
|
|
●
|
In 1984 Nicholas P Plotnikoff and
Gerald C miller and Joseph Wybran (etc) initiated a trial in 14 healthy volunteers and 8 cancer patients the Clinical pharmacology
of methionine-enkephalin was studied in normal volunteers at doses of 1, 10, 50, 100, 150, 200, and 250 pg/kg. Immunologically,
increases in total lymphocytes, B lymphocytes, active rosette-forming cells, T lymphocytes (OKTl 1), T-helper lymphocytes
(OKT4), and T-suppressor lymphocytes (OKT8) were seen after infusion with methionine-enkephalin. In addition, increased mitogen-stimulated
blastogenesis with PHA, Con A, and pokeweed were also seen with methionine-enkephalin treatment. No material changes were
seen in EKG, blood pressure, heart rate, respiratory rate, temperature, or neurologic reflexes of normal volunteers receiving
methionine enkephalin in doses of 1 to 200 pg/kg.
|
Our
human (in vivo) studies have demonstrated that methionine-enkephalin is an activator of T-cell subsets, NK cells, and potentiator
of blastogenesis in the presence of PHA, Con A, pokeweed, or Staph A. All of the clinical pharmacologic variables were normal,
including EKG, heart rate, blood pressure, respiration, temperature, and neurologic reflexes, as well as urinalysis and SMAC 26.
Transient side effects such as vasodilation and/or gastrointestinal cramps were seen only at high doses (100, 150, 200, and 250
pg/kg). Thus , in this study , methionine-enkephalin, a natural hormone, was administered without significant
adverse effect in a dose range of 1 to 250 pg/kg (by intravenous infusion).
In
our studies in cancer patients with Kaposi’s sarcoma (due to AIDS), melanoma, lung cancer, and hypernephroma increases in
T-cell subsets were also observed. Increased levels of blastogenesis with the mitogens PHA, Con A, and pokeweed were also observed.
An increased expression of interleukin-2 receptors was also observed, while Wybran reported increased blood levels of interleukin-2
in patients receiving methionine-enkephalin. In addition, Wybran et al. 28 have reported that methionine enkephalin elevates T-cell
subset numbers in pre-AIDS or ARC patients. Methionine enkephalin may well be useful as an immunomodulator in the treatment of
patient in the early stages of their illness and/or following surgery, radiation, or chemotherapy treatment.
Cancer
Patients
Methionine-enkephalin
was administrated to seven patients with lung cancer. These patients were newly diagnosed and had not yet received prior treatment
such as surgery, chemotherapy, radiotherapy, or immunotherapy. Immunologic tests were performed before Methionine-enkephalin injection,
and 2 hours, 24 hours, 6 days after Methionine-enkephalin perfusion. Th results can be summarized as follows: in four patients
active T-cells were increased in the blood; in five patients the
percentage
of OKTlO increased in the blood; and in seven the cells with the Leu 11 phenotype increased (to more than twice the initial value
in four of these patients). More interestingly, NK activity increased in five of seven patients (by more than 100 in three of
them, in whom NK activity increased from 30 to 60, 7 to 15%, and 18 to 36%).
Once
again, the absence of subjective or objective side effects should be stressed in this single-injection study.
ARC
Patients
Seven
patients with AIDS-related complex received Methionine-enkephalin three times a week intravenously for a minimum of 21 days; the
concentrations vaned from 20 to 100 pg/kg at each injection. Some patients have already been treated for 130 days. The immunologic
results can be summarized as follows after 21 days of treatment: increase in the numbers of blood OKT3 and OKT4 lymphocytes, no
increase in the absolute count of lymphocytes, borderline increase in NK activity (p < 0.10), and increase in IL-2 production
as well as in PHA response. The most striking result is the enhancement of the PHA response. Some patients were treated for a
longer period of time and one patient has shown a remarkable immunologic and clinical course. This 32-year-old Caucasian homosexual
male had a prior history of an 11-kg weight loss, night sweats, recurrent scrota infections, and lymphadenopathy for a period
of 2 years. Testing of his immunologic status showed a reduced OKT4 percentage (20%) and count 336/mm3), low NK activity (22%),
low ILL-2 production (0.5 units), and low PHA response (147,000 cpm). He was started on Methionine-enkephalin and followed immunologically
as well as clinically.
At
day 130, his OKT4 percentage is 38% and the OKT4 count 730/mm3. The NK activity has increased to 43% and the IL-2 production is
also normalized to 2.1 units. Finally, his PHA response has presently reached 414,000 cpm. Clinically, this patient has no more
scrota1 infections, he has gained 8 kg, the night sweats have disappeared, and the lymph nodes have completely regressed within
3 months. In summary, this patient shows an almost complete immunologic nonspecific functional reconstitution, except with respect
to the OKT4 subset. Clinically, he is in complete remission. All the data indicate that Methionine-enkephalin can enhance some
immunologic functions in ARC patients, and preliminary data suggest some therapeutic beneficial effect in some patients. These
results have to be confirmed in larger series in double-blind randomized trials.
AIDS
Patients
One
AIDS patient received a single injection of 20 pg/kg of Methionine-enkephalin and his PHA response increased from 4300 cpm to
14,000 cpm! Another AIDS patient with Kaposi’s Sarcoma is now receiving chronic treatment with Methionine-enkephalin. His
lesions have remained stable for 4 months. More interestingly, an AIDS patient (with Hodgkin’s lymphoma, Toxoplasma cerebral
abscess, atypical mycobacteria) was diagnosed as having Kaposi’s Sarcoma lesions (biopsy-proven). He was started on Methionine-enkephalin
treatment 1 month after the Kaposi’s Sarcoma lesions appeared, and after 4 weeks of treatment these lesions have flattened
and are in the process of disappearing (as determined by biopsy). In the case, Methionine-enkephalin administration was associated
with the regression of the Kaposi’s Sarcoma.
Clinical
Studies finished with 178 patients in Tulsa, Brussels the Belgium Medical School, New York SUNY, Denver Colorado Medical School
and Chicago to increase number of cytotoxic cells CD4, CD8 and NK Cells in AIDS and Cancer patients. The studies demonstrated
that Methionine-enkephalin is an effective and potent immunomodulator in HIV/AIDS patients. Methionine-enkephalin exhibited a
dose response between the dose of 10, 20, 50 and 100 micrograms/kg in terms of increasing number of CD3, CD4, CD8 and NK cells
in these patients (average increase of 50%). Higher doses of Methionine-enkephalin (150, 200, 250 and 300 micrograms/kg) exhibited
a plateau effect. No toxicity was seen in these patients. Methionine-enkephalin increases the number of cytotoxic cells (subsets
CD4, CD8 and NK) that are known to specifically destroy HIV. This “antiviral” effect was recorded by marked reduction
of p24 both in vitro and in vivo.
A
phase II clinical study under IND was completed. The study, was a double-blind and placebo controlled, involved randomized assignment
to three arms receiving a weekly intravenous infusion of 60mpg/km (low Dose) or receiving a weekly infusion of 125 micrograms/kg
and the third receiving a placebo infusion of normal saline. Twenty subjects completed 12 weeks of the trial, twenty-six patients
completed 8 weeks, and 33 patients completed 4 weeks. Eligibility for this clinical study included a positive HIV serology, CD4
level between 200 and 500.
Substantial
differences from baseline were observed (at eight weeks in patients receiving 125 micrograms/km) for the following parameters:
CD4,DC8, DC35, CD38, CD56, NK, PHA, PWM and CMV. The most important findings of the study were the increase in CD4 (T helper cells)
and CD56 (cytotoxic cells NK-K-LAK). There were no serious adverse reactions.
Phase
I and Phase II studies have been conducted under the Company’s IND (previously held by Penn State University) that have
demonstrated that MENK can be delivered to patients suffering from advanced pancreatic cancer, hepatocellular carcinoma (PI: Eric
Kimchi, M.D. at Penn State University) and advanced head and neck cancer (PI: David Goldenberg, M.D., FACS at Penn State University).
The maximum tolerated dose has been found to be 250 µg/kg using the intravenous route of administration over a 30-min infusion
time in a Phase I trial in fourteen normal volunteers and eight cancer patients. This maximum tolerated dose was later confirmed
in Phase I and Phase II trials in patients with advanced unresectable pancreatic cancer.
Patients
with Kaposi’s Sarcoma (9 patients), lung cancer (12 patients), melanoma (3 patients), hypernephroma (1 patient), or pancreatic
cancer (1 patient) were treated with MENK for one week to 12 months at doses of 10 µg/kg three times per week up to 80 µg/kg
3 times per week. After 1-2 weeks increases in T cell subsets (CD3, CD4, CD8, and CD2 positive cells) were observed. An increase
also occurred in IL-2 receptor expression. NK cell activity was measured in 14 patients and an increased NK activity was present
in 12/14 patients. No toxicity attributable to treatment with MENK was observed in any patient.
Two
case studies have been reported in an infant and a 20-month old child who were treated with MENK. The infant was diagnosed with
hepatoblastoma and was treated with one course of neoadjuvant chemotherapy at approximately one week of age. Due to complications
from the chemotherapy (neutropenic fever, pneumonia and sepsis), the patient’s parents declined further chemotherapy, and
the infant was treated with surgical resection and MENK/LDN. She is currently close to ten years disease–free survival.
The 20-month-old child was diagnosed with hepatoblastoma. Due to existing comorbidities (including autosomal recessive polycystic
kidney disease and hypertension), and biopsy results that indicated the tumor might be insensitive to chemotherapy, the parents
elected not to proceed with neoadjuvant chemotherapy. The patient was treated with surgical resection and MENK/LDN, and is currently
at more than five years disease-free survival.
2013-
2014 Open Label China Medical School
2012-Present
We have been running trials both in-vitro and in-vino and have published on some of the work that was completed.
MENK,
a penta-peptide is considered as being involved in the regulatory feedback loop between the immune and neuroendocrine systems,
with marked modulation of various functions of human immune cells. The aim of the present work was to investigate change of lymphocyte
subpopulations in peripheral blood of 50 cancer patients before and after treatment with MENK. Peripheral blood mononuclear cells
(PBMCs) of peripheral blood from 50 cancer patients were isolated by density gradient centrifugation using Ficoll-Paque solution
and cultured with MENK. We measured proliferation of total nucleated cells, subpopulations of individual CD4+T cells, CD8+T cells,
CD4+CD25+ regulatory T cells (Treg), natural killer cells (NK) before and after treatment with 10-12M MENK in cell culture by
flow cytometry (FCM).
Our
results indicated that MENK showed a strong inhibiting effect on Treg cells while it stimulated marked proliferation of other
lymphocyte subpopulations. All data obtained were of significance statistically. It was therefore concluded that MENK could work
as a strong immune booster with great potential in restoring damaged human immune system and we could consider MENK as a drug
to treat cancer patients, whose immune systems are damaged by chemotherapy or radiotherapy. Furthermore we could consider MENK
as a chemotherapy additive, which would sustain immune system of cancer patients during the process of chemotherapy to get maximized
efficacy with minimized side effect.
Methionine
enkephalin (MENK) improves lymphocyte subpopulations in human peripheral blood of 50 cancer patients by inhibiting regulatory
T cells (Tregs). The patients recruited for this study were all with terminal cancers with broad metastasis, underwent chemotherapy
and their immune system were damaged severely. They failed to respond to any therapy available and were desperate. After they
signed informed consent we began to give treatment. The concrete cases distribution was as following: Rectal cancer: 4, Colon
cancer: 6, Stomach cancer: 3, Hepanocellular cancer: 2, Lung cancer: 7, Oval cancer: 3, Pancreatic cancer: 2, Breast cancer: 3,
Urinary bladder cancer: 1
This
approach helps us learn more knowledge about MENK’s action in rehabilitating human immune system and the conclusion of MENK
as an immune enhancer, drawn from present study is fully supported by the data obtained. We believe that this is the first time
that published data based on large samples show that MENK could stimulate proliferation of lymphocyte subpopulations by inhibiting
Tregs in peripheral blood of cancer patients.
Research
results indicate that MENK, at suitable doses, boosts the immune system through the following possible mechanisms:
|
|
●
|
increasing
proliferation and functional activities of CD4+T-cells and CD8+T-cells which will play a role in anti-virus and anti-tumor
activities;
|
|
|
|
|
|
|
●
|
increasing
maturation of dendritic cells which will initiate and intensify T-cell responses;
|
|
|
|
|
|
|
●
|
increasing
secretion of cytokines such as IL-2, TNF, IL-12 and IFN-γ which will amplify the T-cell response and mediate interaction
among immune cells, forming a modulated and balanced immunity;
|
|
|
|
|
|
|
●
|
increasing
functions of macrophages, resulting in enhanced cellular immunity through secreting a set of cytokines; and
|
|
|
|
|
|
|
●
|
increasing
activity of NK cells which have the ability to kill cancer cells and virus-infected cells.
|
Based
upon published literature, the Company believes that, in oncology in particular, MENK has two possible mechanisms of actions:
1.
Immune stimulation and regulation effects; and
2.
Direct anti-cancer inhibitory effects.
Based
upon data from multiple
in vivo
and
in vitro
studies conducted by Zagon
et al.
over the past 15 years, the
onset and/or progression of some cancers may be related to defects in MENK and/or OGFr, which would promote or exacerbate tumorigenesis.
These findings show there may be an advantage in up-regulating the peptide (e.g., MENK administration) to enhance anti-cancer
activity (Zagon IS, Donahue RN, McLaughlin PJ. Opioid growth factor-opioid growth factor receptor axis is a physiological determinant
of cell proliferation in diverse human cancers.
Am J Physiol Regul Integr Comp Physiol.
2009; 297: R1154–R1161).
Further exploration and clinical trials are needed to confirm MENK’s mechanism of action and its ability to stop the growth
of cancerous cells in human subjects with advanced cancer; however, supportive literature around the possible mechanism of actions
for MENK are provided below.
MENK
as an immune stimulator/regulator
While
Dr. Nicholas Plotnikoff was a faculty member at Oral Roberts University, he discovered that all three of the classical opioid
receptors are expressed on most subsets of immune cells, and that either
in vitro
incubation with MENK or parenteral administration
of and humans, especially those with immunodeficiencies associated with cancer or HIV/AIDS, MENK
in vivo
increased the
number and functional activities of T cells, including cytotoxic CD8+ T cells, and also natural killer (NK) cells (Plotnikoff
NP, Faith RE, Murgo AJ, Herberman RB, Good RA. Methionine Enkephalin: A New Cytokine – Human Studies.
Clinical Immunology
and Immunopathology
. February 1997; 82(2): 93-101). Following those pioneering studies, several other investigators observed
that administration of MENK to mice increased CD4+ and CD+ T cells, and increased various immune functions, including cytotoxic
activities of both T cells and NK cells (
Wybran J
,
Schandené L
,
Van Vooren JP
,
Vandermoten G
,
Latinne D
,
Sonnet J
,
De Bruyère M
,
Taelman H
,
Plotnikoff NP
. Immunologic properties
of methionine-enkephalin, and therapeutic implications in AIDS, ARC, and cancer.
Ann N Y Acad Sci.
1987; 496:108-14).
Recently, Drs. Fengping Shan and Nicholas Plotnikoff have reported that MENK treatment of mice stimulates the cytotoxic activities
of T cells and NK cells and reduces levels of T regulatory cells, and augments therapeutic effects in tumor-bearing immunocompetent
mice.
MENK
as an inhibitor of cancer cell growth
MENK
has been found to exert a profound inhibition on the initiation and progression of human pancreatic cancer
in vitro
and
in vivo
(Zagon IS, Smith JP, McLaughlin PJ. Opioid Growth Factor (OFG) Inhibits Human Pancreatic Cancer Transplanted into
Nude Mice.
Cancer Letters.
1997 Jan 30; 112(2):167-175. Zagon IS, Smith JP, Conter R, McLaughlin PJ. Identification and
Characterization of Opioid Growth Factor Receptor in Human Pancreatic Adenocarcinoma.
International J of Molecular Med.
2000 Jan; 5(1):77-84.). This led to the discovery that MENK interacted with a novel opioid receptor (OGFr) on human cancer cells
(ovarian, SCCHN, pancreatic, colorectal and others) creating a competitive inhibition profile
and
subcellular location that is different from other well-known “classic” opioid receptors [mu (µ), delta (δ)
and kappa (κ)]. The other “classic” opioid receptors have not been found to have any impact on cell growth;
thus there is specificity in the MENK-OGFr interaction which regulates cell proliferation. In an extensive number of experiments
that have been conducted on human pancreatic cancer cells in tissue culture exposed to a variety of opioid-related compounds,
MENK was the only compound that inhibited cell proliferation. Based upon data from multiple
in vivo
and
in vitro
studies conducted by Zagon
et al
., the onset and/or progression of some cancers may be related to defects in MENK and/or
OGFr, which would promote or exacerbate tumorigenesis. These findings show there may be an advantage in up-regulating the peptide
(e.g., MENK administration) to enhance anti-cancer activity.
Therefore,
as opioid receptors are not only found on cancer cells, but also on most subsets of immune cells, MENK has the ability to not
only inhibit cancer cell growth, but also have a direct impact on the patient’s immune system by increasing the number and
functional activities of T cells and NK cells.
Zagon
and McLaughlin have not recorded in any of their animal studies any side effects of MENK for non-oncological indications such
as experimental autoimmune encephalomyelitis (EAE, the mouse model of multiple sclerosis) or relapse-remitting EAE (RR-EAE) with
MENK being administered daily. Treatment with MENK or LDN did not exacerbate EAE and was able to halt progression of disease,
reverse neurological deficits, and prevent the onset of neurological dysfunction over time (Rahn KA,
McLaughlin PJ
,
Zagon
IS
. Prevention and diminished expression of experimental autoimmune encephalomyelitis by low dose naltrexone (LDN) or opioid
growth factor (OGF) for an extended period: Therapeutic implications for multiple sclerosis).
Management
believes clinical trials involving LDN hold great promise for the millions of people worldwide for the treatment of autoimmune
diseases or disorders, central nervous system disorders or those who face cancer. Management also believes it could be the first
low-cost, easy to administer therapy with minimal to no side-effects for the treatment of HIV/AIDS, autoimmune diseases and immune
disorders, in particular Crohn’s disease, multiple sclerosis, and/or fibromyalgia.
Naltrexone
is an orally effective opioid receptor antagonist, used as a treatment for opiate addiction. Naltrexone was originally synthesized
in 1963 and patented in 1967. In 1984, the FDA approved naltrexone in a 50 mg dose as a treatment for heroin addiction. Naltrexone
50 mg film-coated tablets have been approved in Europe since at least 1989 for the treatment of opiate addiction and more recently
alcohol dependency. At lower doses (approximately 4.5 mg/day), it has been gaining popularity as a treatment for signs and symptoms
of autoimmune diseases and immune disorders, HIV/AIDS and cancer. Research studies by others have indicated that the short-term
blockage of opioid receptors on circulating and tissue cells by LDN was followed by a substantial rebound in opioid receptor expression
and increased levels of β-endorphin and methionine-enkephalin (Zagon IS, McLaughlin PJ. Opioid antagonist modulation of murine
neuroblastoma: A profile of cell proliferation and opioid peptides and receptors.
Brain Res
. 1989; 480:16–28.)
Oral
administration of LDN has been shown to transiently (approximately 4 hours) inhibit opioid receptors which in turn provides a
remaining window of approximately 20 hours for the unregulated opioids and receptors to interact (
Donahue RN
,
McLaughlin
PJ
,
Zagon IS
. The opioid growth factor (OGF) and low dose naltrexone (LDN) suppress human ovarian cancer progression
in mice.
Gynecol Oncol.
2011 Aug; 122(2):382-8).
LDN
has been shown to increase the levels of endogenous opioid activity, thereby having the ability to play a direct role in enhancing
the human body’s stress resilience, improving psychiatric problems such as autism, in addition to being able to have a direct
impact on the immune system and regulation of how the immune system works when faced with disease. LDN is believed to facilitate
the body’s own resources to slow down or combat cancers, autoimmune diseases and HIV/AIDS; thus reducing the overall impact
and load on the body (Brown N, and Panksepp J. Low dose naltrexone for disease prevention and quality of life.
Med Hypotheses
.
2008 Mar: 72(3):293-6.).
Naltrexone
was originally patented in 1967 by the specialty pharmaceutical company Endo Health Solutions Inc. At the time, it seemed unlikely
that naltrexone would be developed because the experimental drug had relatively low market potential, and naltrexone’s patent
protection would likely expire before the completion of clinical trials. With the assistance of DuPont, a division of Merck &
Co. that acquired Endo in 1969, the US government’s National Institute on Drug Abuse (“NIDA”) advanced naltrexone
through the FDA approval process, leading to approval for marketing as a treatment for heroin addiction in a 50 mg dose in 1984.
Although its patent expired that same year, naltrexone gained seven additional years of marketing exclusivity for DuPont when
the FDA designated it (trademarked as Trexan) an Orphan Drug. Marketing exclusivity provides a pharmaceutical company the right
to sell its drug for a certain length of time free of competition from generic
versions
of the drug and is often granted to encourage companies to develop a use for a drug whose patent has expired or to encourage a
company to develop an already approved drug for a new use. With market exclusivity, the anticipated returns on investment are
higher, improving the profitability of a drug. With funding provided by the US National Institute on Alcohol Abuse and Alcoholism
(“NIAAA”) and the potential to gain three additional years of post-approval market exclusivity for naltrexone, DuPont
advanced naltrexone through additional clinical trials, and gained FDA approval for a 50 mg dose (trademarked as ReVia) as a treatment
for alcohol abuse in 1995. As naltrexone had already been on the market for 10 years as a treatment for heroin addiction, the
FDA’s confidence in its safety resulted in approval only six months after naltrexone’s regulatory application was
submitted.
Naltrexone
has a black box warning for liver toxicity, which was included based on liver enzyme elevations reported with daily dosing at
100 mg-300 mg. These doses were evaluated in clinical trials for obesity; however, they have not been approved for this use. Our
review of the literature and adverse effect reports in naltrexone clinical trials did not demonstrate a risk for liver damage
with daily dosing at 50 mg. Although the black box warning does remain, the FDA has stated that naltrexone does not appear to
be a hepatotoxin at the recommended doses for the currently approved indications. Recently, naltrexone at 4 mg and 8 mg, in combination
with the anti-depressant drug, bupropion at 90 mg, was evaluated as an anti-obesity drug by Orexigen Therapeutics Inc. and submitted
for FDA approval in 2010. Data from studies with the drug combination (trademarked Contrave) showed potential hepatotoxicity in
1.2% of subjects treated with Contrave (n = 3,239). [Note: After the FDA requested a long-term study to demonstrate the daily
recommended dose of the drug combination (two tablets each with 8 mg naltrexone plus 90 mg bupropion taken twice daily) does not
raise the risk of heart attacks, Orexigen initiated a Phase III trial to evaluate Contrave in a study expected to enroll more
than 9,000 subjects and is anticipated to be completed in 2017.] Other than its small potential association with liver toxicity
at high doses, the most common adverse effects reported with naltrexone are non-specific gastrointestinal complaints such as diarrhea
and abdominal cramping.
Noteable
published clinical trial evidence indicates that LDN, particularly daily dosing at 3mg - 4.5 mg, stimulates the immune system
and is effective in the treatment of some immunodeficiency diseases, such as HIV/AIDS diseases, and advanced cancer as shown in
the studies referenced herein. The first clinical trial results with LDN for immune disorders, however, were published only recently
in a peer-reviewed medical journal in 2007 which evaluated LDN treatment in a pilot phase II study of 17 patients with Crohn’s
disease, a form of inflammatory bowel disease that most commonly affects the ileum and the beginning of the colon. Two-Thirds
of patients in this study went into remission after 4.5 mg daily LDN treatment (p < 0.001), with 89% of patients overall showing
some degree of response.
An
open-label pilot study was conducted by Pennsylvania State University with LDN to evaluate response, safety and toxicity in adult
subjects with moderate to severe, active Crohn’s disease. Patients were treated with LDN orally each evening at a dose of
4.5 mg for 3 months. A total of 17 patients were enrolled, 16 of whom completed the study. No laboratory abnormalities were noted.
The most common side effect was sleep disturbances (occurred when dosing at night, at about bed-time), occurring in seven patients
(41%).
A
second clinical study was conducted by Pennsylvania State University as a randomized double-blind, placebo-controlled study to
test the efficacy and safety of LDN for 12 weeks in adults with moderate to severe active Crohn’s disease. Forty subjects
with moderate to severe active Crohn’s disease were enrolled in the study. Randomized patients received daily oral administration
LDN (4.5 mg/day) or placebo. Fatigue was the only side effect reported of statistical significance, and it was greater in subjects
receiving placebo.
A
pilot Phase II clinical trial was conducted by Pennsylvania State University in children with moderate to severe active Crohn’s
disease. Fourteen subjects were enrolled, 12 subjects were randomized and treated with a mean age of 12.3 years (range 8-17 years).
Children were randomized to placebo or LDN (0.1 mg/kg or a maximum dose of 4.5 mg) orally for 8 weeks followed by open-label treatment
for an additional 8 weeks of LDN at the same dose of 0.1 mg/kg or 4.5 mg. Oral LDN was well tolerated without any serious adverse
events.
Fourteen
(14) subjects were enrolled, 12 subjects were randomized and treated (Smith J.
et al
., 2007; Smith J.
et al
., 2011;
Smith J.
et al
., 2013).
Pennsylvania
State University researchers have also demonstrated in a mouse model of Crohn’s disease (chemically induced colitis with
dextran sodium sulfate) that opioid receptor blockade by LDN resulted in less weight loss, lower disease activity index scores
and less histological evidence of inflammation when compared to controls. Furthermore, the researchers
demonstrated
that tissue inflammatory cytokine mRNA was reversed to baseline levels in the colons of mice treated with LDN.
Management
believes in LDN’s potential treatment effects for Crohn’s disease, as the treatments currently available for Crohn’s
disease are expensive and carry black box warning due to the toxic side effects associated with virtually all of the currently
used drugs. Management believes that LDN provides an attractive alternative. Three published clinical trials in patients with
moderate to severe disease, two in adults and one in children, have shown notable disease and quality of life improvement by 12-weeks.
LDN was able to reverse the inflammatory activity, promote mucosal healing, and decrease histologic inflammation when compared
to placebo-treated controls (Smith J.
et al,
2011; Smith J.
et al,
2013). Based on these results, the Company has
placed a very high priority on implementing a pivotal Phase IIB/III study. By using the 505(b)(2) pathway, confirmation of efficacy
in our Phase III study is expected to result in approval by the FDA.
With
its increasing recognition in children and adolescents, Crohn’s disease has become one of the chronic diseases that affect
young people. Pediatric Crohn’s disease affects approximately 80,000 patients in the United States, and thus has led to
orphan drug designation with the FDA. In addition to the common GI symptoms due to inflammation in the small and/or large intestine,
children often experience growth failure, malnutrition, pubertal delay, bone demineralization, and psychological issues. Crohn’s
tends to be both severe and extensive in the pediatric population with a relatively high proportion of pediatric Crohn’s
patients having involvement of their small intestine, proximal to the ileum.
The
following table provides a summary of clinical trials for LDN that have recently been or are being conducted by Pennsylvania State
University:
|
Title
|
|
Indication(s)
|
|
|
Dose
|
|
|
ClinicalTrials.gov
Identifier / Status
|
|
Low Dose Naltrexone for Metastatic
Melanoma, Castrate Resistant Prostate Cancer and Renal Cancer
|
|
Metastatic Melanoma, Castrate
Resistant Prostate Cancer and Renal Cancer
|
|
|
5
mg/day
|
|
|
NCT01650350 /
Currently
Recruiting
(verified May 2013)
|
|
Effects of Low Dose Naltrexone
in Fibromyalgia
|
|
Fibromyalgia
|
|
|
3-4.5
mg/day
|
|
|
NCT00568555 / Completed June 2012
(verified June 2012)
|
|
Low Dose Naltrexone in Symptomatic
Inflammatory Bowel Disease
|
|
Inflammatory Bowel Disease
|
|
|
4.5
mg/day
|
|
|
NCT01810185 /
Not
yet recruiting
(verified June 2013)
|
|
Low dose Naltrexone for Glioma
Patients
|
|
Malignant Glioma
|
|
|
4.5
mg/day
|
|
|
NCT01303835 /
Active,
not recruiting
(verified January 2014)
|
|
Low dose Naltrexone for Depression
Relapse and Recurrence
|
|
Major Depressive Disorder
Depression,
Unipolar
Recurrence Relapse
|
|
|
1
mg/day
|
|
|
NCT01874951 /
Recruiting
(verified September 2013)
|
The
505(b)(2) Regulatory Pathway
Traditionally,
pharmaceutical drugs had to be approved by the FDA under the standard 505(b)(1) regulatory pathway, which could take as long as
15 years. Now, drugs approved under 505(b)(2) may rely in part on data from existing reference drugs meaning they can be developed
and achieve FDA approval in as little as 30 months with only a fraction of the number of required clinical trials and at a much
lower cost.
Developing
LDN using the 505(b)(2) regulatory pathway decreases the amount of development time and cost in order to obtain FDA approval.
As naltrexone is an FDA-approved product for alcohol or opiate dependence, prescriptions are currently being filled for naltrexone
in 50 mg doses by hundreds of local pharmacies and mail-order pharmacies around the United States.
The
FDA’s 505(b)(2) pathway for approving drugs opens the door for the Company to gain FDA approval of LDN (which is used at
doses of approximately 1/10th the approved dose) for new diseases. A 505(b)(2) drug application for LDN will contain full reports
of clinical investigations to support the safety and effectiveness in the new indication(s); however, at least some of the information
required for approval will come from studies not conducted by or for the applicant, and for which the applicant has not obtained
a right of reference or use, as many of these drugs are now off-patent. With the opportunity to use previous findings of safety,
the Company intends to use the 505(b)(2) pathway to study and gain approval for LDN in other diseases, with Crohn’s disease
slated as its first therapeutic indication.
As
there is a sufficient database of information around the safety of this product, and the reference listed drug (NDA 018932 REVIA)
is being used, the FDA agreed that a 505(b)(2) application would be an acceptable approach at this time.
The
Company, through its subsidiary, will need to complete both a Phase 2B and Phase 3 clinical trial for both Pediatric and Adult
Crohn’s Disease for IRT-103 before final approval to market the drug in the U.S. from the FDA. Similarly Phase 2 and Phase
3 clinical trials will need to be completed in the U.S. for IRT-101 before we can obtain final approval to market the drug in
the U.S. from the FDA. All clinical trials in the U.S. are being completed under the supervision of our subsidiary Cytocom, Inc.
Intellectual
Property
The
Company has been developing active forms of immunotherapies through the acquisition of patents, IND Applications, clinical data
and all proprietary technical information, know-how, procedures, protocols, methods, prototypes, designs, data and reports which
are not readily available to others through public means, and which were owned, generated or developed through experiments or
testing by Dr. Plotnikoff, Professor Shan, Dr. Bernard Bihari, Dr. Ian S. Zagon, Dr. Jill Smith, Dr. Patricia J. McLaughlin and
Moshe Rogosnitzky.
The
Company has been able to acquire many of the patents and intellectual property it was seeking, and has also been able to team
up with some of the leaders in the field of immunology, experts such as the late Dr. Ronald Herberman (1940-2013), Dr. Fenping
Shan, Dr. Jill Smith and Dr. Terry Grossman.
Dr.
Plotnikoff is the inventor behind a number of patents granted for cancer treatments and an adjunct to patents for autoimmune diseases
including: European Patent United Kingdom, Germany, France, Ireland EP 1401471 BI Methods for inducing sustained immune response;
Russian Patent Russian Federation patent number 2313364; The Patent Office of the People’s Republic of China, Application
No.: 200810165784.8 China Patent CN1015113407 A The Patent Office of the People’s Republic of China ISSN: 1006-2858 CN 21-1349/R;
Patent Agencies Government of India Patent, Application number 1627/KOLNP/2003 number 220265 an Enkephalin Peptide Composition;
and the US Patent Pending, US Patent Application 10/146.999 which was approved in January 2014 US patent number 20140024588 (the
“Plotnikoff Patents”). The Patent Cooperation Treaty (“PCT”) enables a U.S. applicant to file a single
application, known as “an international application,” in a standardized format in English in the U.S. Receiving Office
(the U.S. Patent and Trademark Office) that is acknowledged as a regular national or regional filing in any state or region that
is party to the PCT.
The
Company entered into a Sale of Technology Agreement with Dr. Nicholas P. Plotnikoff on March 4, 2012, wherein Dr. Plotnikoff agreed
to transfer and assign all of his rights, title and interest in the Plotnikoff Patents to the Company. The Company received all
the production formulations and technology designs from Dr. Plotnikoff necessary for the manufacturing, formulation, production
and protocols of the MENK treatment of cancer and HIV/AIDS. As consideration for entering into the Sale of Technology Agreement,
Dr. Plotnikoff received 6,000,000 shares of common stock, a royalty of a one percent on all sales of MENK in perpetuity and was
granted the position of Non-Executive Chairman of the Board of Directors. The Sale of Technology Agreement was filed with Amendment
No. 6 to the Form 10 Registration and incorporated herein by reference.
In
addition to the above patents, we also signed an exclusive licensing agreement for all of the intellectual property developed
at Pennsylvania State University by Dr. Ian S. Zagon, Dr. Patricia J. McLaughlin and Dr. Jill P. Smith for the treatment of cancer.
The patents cover methods and formulations related to the treatment and prevention of cancers. More specifically, the present
inventions describe the use of drugs that interact with opioid receptors (naltrexone, naloxone and the pentapeptide MENK) to inhibit
and arrest the growth of cancer. Such efficacy has been discovered to be partially due to the functional manipulation of the zeta
opioid receptor through exogenous and endogenous MENK. This receptor has been determined to be present in a variety of cancers,
including pancreatic, ovarian, liver, head and neck, and colon cancer. US Patent Numbers 6,737,397, CA 2,557,504,
US 20010046968
,
US 6737397
,
US 6136780
,
US 20080015211
,
US 20070053838
,
US 8003630
,
US 20110123437
,
US 7807368
,
US 7576180
,
US 7517649
,
US 20080146512
,
US 7122651
,
US 20060073565
,
US 20050191241
, Patent No 8,003,630. In addition to the approved patents we have four other patents pending: U.S. Patent
Application No. 11/061,932, U.S. Application No. 13/660,129; Israeli Patent Application No. 194734; Chinese Patent Application
No.: 200810165784.8 and US Application No. 62/296,759. The licensing agreement referenced in this paragraph was previously filed
with Amendment No. 7 to the Form 10 Registration Statement filed January 22, 2014 and is incorporated herewith.
We
also acquired the licensing rights to the patent portfolio and intellectual property developed by Dr. Bernard Bihari relating
to treatments with drugs that interact with opioid receptors such as LDN and MENK for a variety of diseases and conditions including
malignant lymphoma, chronic lymphocyctic leukemia, Hodgkin’s lymphoma, and non-Hodgkin’s lymphoma, chronic herpes
virus infections, and chronic infections due to the Epstein-Barr virus and a treatment method for humans infected with HTLV-III
(AIDS) virus including patients clinically diagnosed as suffering from HIV/AIDS and those suffering from ARC. The licensed rights
include all reissues or modifications, reexaminations, or other related U.S. patent filings directed to the same subject matter
and the use of
U.S. Patent Number 6,586,443
,
U.S. Patent Number 6,384,044
,
U.S. Patent Number 6,288,074
,
U.S. Patent Number 5,356,900
,
U.S. Patent Number 5,013,739
,
U.S. Patent Number 4,888,346
. The license agreement
with Dr. Bihari was previously filed with Amendment No. 1 to the Form 10 Registration Statement filed on June 7, 2013 and is incorporated
herewith.
Once
the Company acquired the above patents, it was then able to sign a licensing agreement to acquire the exclusive patent rights
for the intellectual property of the licensors, Dr. Jill Smith and LDN Research Group, LLC, whose members include Dr. Ian S. Zagon,
Dr. Patricia J. McLaughlin and Moshe Rogosnitzky. The patents cover methods and formulations for the treatment of the inflammatory
and ulcerative diseases of the bowel, using naltrexone in low doses as an opioid antagonist. Endogenous opioids and opioid antagonists
at low doses have been shown to play a role in stimulating and rebalancing the immune system and the healing and repair of tissues.
US Patent No. 6,136,780, Patent No. US 7879870. The Company then
negotiated
with Dr. Jill Smith to arrange the transfer of the Orphan Drug Designation for the use of naltrexone for the treatment of pediatric
Crohn’s disease with the FDA. Dr. Smith has since transferred the IND to the Company, and the FDA acknowledged that the
Company is now the sponsor for this IND. In September 2014, the Company and the licensors jointly agreed to terminate the license
agreement, and in place thereof, have the licensors grant a similar license in their patent rights to Cytocom Inc. pursuant to
a Patent License Agreement between the licensors, Cytocom Inc. and the Company with substantially similar terms as set forth in
the original license agreement. Pursuant to this agreement, the Company issued 1,000,000 shares of its common stock to the licensors
and the Company guaranteed the obligations of Cytocom Inc. to the licensors under the agreement.
The
Company originally acquired the patents and intellectual property from Dr. Smith and LDN Research Group, LLC because management
believed clinical trials involving LDN held great promise for the millions of people worldwide with autoimmune diseases or disorders,
central nervous system disorders or those who face cancer. Management also believed it could be the first low-cost, easy to administer
therapy with minimal to no side-effects for the treatment of HIV/AIDS, autoimmune diseases and immune disorders, in particular
Crohn’s disease, multiple sclerosis, and/or fibromyalgia.
Dr.
Nicholas Plotnikoff, Professor Fenping Shan and Noreen Griffin recently filed a Provisional Application for a Utility Patent US
Application No. 62/296,759 Method for Inducing a Sustained Immune Response, which was assigned to the Company in March 2016. U.S.
Patent and Trademark Office has issued the Official Filing Receipt in connection with this application, and accorded a filing
date of February 18, 2016 during its pendency in the USPTO.
Summaries
of the Company’s agreements are as follows:
Dr.
Nicholas Plotnikoff
In
connection with the share exchange, the following is the scope of the intellectual property transfer: we entered into a Sale of
Technology Agreement with Dr. Nicholas P. Plotnikoff on March 4, 2012, wherein Dr. Plotnikoff agreed to a 100% transfer and assign
all of his rights, title and interest in: European Patent United Kingdom, Germany, France, Ireland EP 1401471 BI Methods for inducing
sustained immune response; Russian Patent Russian Federation patent number 2313364; The Patent Office of the People’s Republic
of China, Application No.: 200810165784.8 China Patent CN1015113407 A The Patent Office of the People’s Republic of China
ISSN: 1006-2858 CN 21-1349/R; Patent Agencies Government of India Patent, Application number 1627/KOLNP/2003 number 220265 an
Enkephalin Peptide Composition; and the US Patent Pending, US Patent Application 10/146.999 e. The Company received all the production
formulations and technology designs from Dr. Plotnikoff necessary for the manufacturing, formulation, production and protocols
of the MENK treatment of cancer and HIV/AIDS. As consideration for entering into the Sale of Technology Agreement, Dr. Plotnikoff
received 8,000,000 shares of common stock, a royalty of a single-digit percentage on all sales of MENK and was granted the position
of Non-Executive Chairman of the Board of Directors. There were no other payment provisions included in the or termination provisions.
At
the time of the acquisition, the valuation of goodwill and other intangible assets were determined using the fair market price
for the Company’s common stock, which were exchanged for shares of TNI IP. In the fourth quarter of 2012, the Company performed
an annual valuation to determine whether any goodwill or intangible assets that had been acquired by the Company were impaired.
The result of this valuation was that material impairments were identified. The Company recognized an impairment of the goodwill
arising on the acquisition of TNI IP of $98,000,000.
Jacqueline
Young
On
August 13, 2012, the Company signed an exclusive License Agreement with Ms. Jacqueline Young (the “Young Agreement”)
for the intellectual property developed by Dr. Bernard Bihari relating to treatments with opioid antagonists such as naltrexone
and Met-enkephalin for a variety of diseases and conditions including malignant lymphoma, chronic lymphocytic leukemia, Hodgkin’s
lymphoma, and non-Hodgkin’s lymphoma, chronic herpes virus infections, chronic herpes viral infections such as chronic genital
herpes caused by the herpes simplex virus Type 2 and chronic infections due to the Epstein-Barr virus and a treatment method for
humans infected with HTLV-III (AIDS) virus, including patients clinically diagnosed as suffering from AIDS and those suffering
from AIDS-related complex (ARC).
The
Young Agreement is valid for the life of the patents and expires on a country by country basis in each country where patent rights
exist, upon the expiration of the last to expire patent in each country or in the event the patent in such country is held to
be invalid and/or unenforceable (by a court or government body of competent jurisdiction) or admitted to be invalid or unenforceable.
The
termination provision states that, additionally, we can cancel the Young Agreement upon 120 days’ written notice and shall
pay all royalties and fees that have accrued. We have the exclusive rights to the intellectual property; however, Ms. Young retains
a right to practice the patents licensed under the Young Agreement solely for noncommercial, academic research purposes.
The
Bihari patents were acquired in exchange for 540,000 shares of the Company’s common stock with a fair market value of $972,000
and assumed liabilities of $400,000, which is payable to Ms. Young over a twenty-four month period in equal installments to reimburse
her for the costs of a New York City office in accordance with the Young Agreement. The patent liability at December 31, 2013
totaled $118,333. The cost of the patent totaled $1,372,000. Additionally, the Company will pay the licensor a royalty payment
of 1% of gross MENK sales and provide the licensor a position as non-executive chairman of the Company.
Dr.
Jill Smith
On
December 24, 2012, the Company signed an agreement for the acquisition of patent rights (the “Smith Agreement”) for
the intellectual property of Dr. Jill Smith and LDN Research Group, LLC (collectively, the “Licensor Parties”), whose
members are Dr. Ian S. Zagon, Dr. Patricia J. McLaughlin and Moshe Rogosnitzky and orphan drug designation by the FDA to a novel
late-stage drug, trademarked “LDN,” for the treatment of Pediatric Crohn’s disease. The patent covers methods
and formulations for treatment of the inflammatory and ulcerative diseases of the bowel, using naltrexone in low doses as an opioid
antagonist. These patents were acquired in exchange for the purchase of 300,000 shares of our common stock with a fair market
value of $2,715,000 and an up-front payment of $165,384 (consisting of a $100,000 initial license fee and payment of $65,384 of
expenses), which totaled $2,880,384.
The
Smith Agreement requires the Company to (i) use commercially reasonable efforts to develop, commercialize, market and sell licensed
products in a manner consistent with a business plan, (ii) expend a minimum amount of funds per annum to develop and commercialize
licensed products as soon as practicable, (iii) obtain all requisite regulatory approvals needed to use or sell licensed products
in the field of use, and (iv) make the first commercial sale of a licensed product by March of 2017.
In
addition to the above stated 300,000 shares with a fair market value of $2,715,000 at the time of signing with the Company, it
was agreed that 1,000,000 shares of common stock at the time of signing of a licensing agreement with Cytocom Inc, with all fees
paid by the Compant, on behalf of the contract until such time as Cytocom is funded.
The
aggregate amounts paid to date under the agreement are: $100,000 in 2014, $100,000 in 2015, and $100,000 set to be paid in December
of 2016. The aggregate future potential milestone payments to be paid are: $250,000 upon the initiation of a phase III trial by
the FDA, $250,000 upon acceptance of a NDA by the FDA, and $750,000 upon marketing approval by the FDA. The royalty rates for
the transactions are .4% on Crohn’s Disease in the United States and .1% on sales in Emerging Markets.
Unless
terminated sooner pursuant to the Agreement, the Agreement will terminate upon the later of: (a) the expiration or abandonment
of the last patent to expire or become abandoned of the Patent Rights; or (b) Ten (10) years after the first Sale of the first
Licensed Product. The Company may terminate the Smith Agreement upon 90 days’ written notice, provided all sublicenses are
terminated and all amounts due and owing are paid to the Licensor Parties. The Licensor Parties may terminate the agreement ten
days’ after notice to the Company if the Company is ten days late in payment or there is a breach that remains uncured for
ten days after written notice of such breach.
The
Company is required to pay an annual license fee, an annual running royalty on net sales of each licensed product or a minimum
royalty, whichever is greater, and a sublicense fee on payments received by the Company from sublicensees. The Company has an
exclusive, worldwide license to make, have made, use, lease, import, offer for sale and sell licensed products and to use the
method under the patent rights.
The
Company is also required to pay milestone payments after substantial achievement of certain milestone events for each licensed
product including payment: upon initiation of each Phase III trial; upon positive completion of each Phase III clinical trial
of the therapeutic use of an LDN compound in the field of use; when a New Drug Application (“NDA”) is accepted for
review by the FDA; and when FDA approval to market the NDA is approved. The Company will issue shares upon reaching certain milestones
including upon the first dosing of the first patient in a Phase III clinical trial for each licensed product, upon the first sale
of each licensed product, and upon the achievement of a set dollar amount in cumulative sales for each licensed product covered
by NDAs.
As
part of the Smith Agreement, the Company has the right to apply to the FDA for the transfer of the orphan drug status for the
use of naltrexone for the treatment of pediatric Crohn’s disease and ulcerative colitis, the Investigation New Drug Application
(“IND”), and the right to acquire the relevant clinical data set from Dr. Jill Smith. Dr. Jill Smith made arrangements
to transfer the IND to the Company as well as the relevant clinical data set, and the FDA has acknowledged that the Company is
now the sponsor for this IND.
On
September 24, 2014, the Company and the Licensor Parties jointly agreed to terminate the Smith Agreement, and in place thereof,
have the Licensor Parties grant a similar license in their patent rights to Cytocom Inc. pursuant to a Patent License Agreement
between the Licensor Parties, Cytocom Inc. and the Company with substantially similar terms as set forth in the Smith Agreement.
Pursuant to this agreement, the Company issued 1,000,000 shares of its common stock valued at $270,000, upon execution to the
Licensor Parties and the Company guaranteed the obligations of Cytocom Inc. to the Licensor Parties under the agreement.
The
Penn State Research Foundation
On
January 18, 2013, the Company signed an exclusive licensing agreement with The Penn State Research Foundation to license all of
the intellectual property developed by Dr. Ian S. Zagon, Dr. Patricia J. McLaughlin and Dr. Jill P. Smith for the treatment of
cancer titled “Opioid Growth Factor and Cancer” and “Combination Therapy with Opioid Growth Factor and Taxanes
for the Treatment of Cancer” (the “Foundation Agreement”).
The
Foundation Agreement requires the Company to: (a) use commercially reasonable efforts to develop, commercialize, market and sell
licensed products in a manner consistent with a business plan; (b) expend a minimum amount of funds per annum to develop and commercialize
licensed products as soon as practicable; (c) obtain all requisite regulatory approvals needed to use or sell licensed products
in the field of use; and (d) make the first commercial sale of a licensed product by December 31, 2016.
The
Foundation Agreement provides that the Company must pay to the licensor an initial license fee, a license maintenance fee on each
anniversary of the effective date of the Foundation Agreement, and an annual running royalty on net sales for each licensed product
or a minimum royalty, whichever is greater. In addition, the Company must pay a sublicense fee on payments received by the Company
from sublicensees.
The
Foundation Agreement also requires the Company to make payments upon the achievement of certain milestone events including: initiation
of each Phase II trial; initiation of each Phase III trial; when the NDA is accepted for review by the FDA; and when FDA approval
to market is approved. The Company must also issue shares upon certain milestones including upon the first dosing of the first
patient in a Phase II clinical trial for each licensed product, upon the first dosing of the first patient in a Phase III clinical
trial for each licensed product, upon the first sale of each licensed product, and upon the achievement of a set dollar amount
of cumulative sales for each licensed product covered by NDAs.
The
Foundation Agreement terminates on the expiration or abandonment of the last patent to expire or become abandoned. The Company
may terminate the Foundation Agreement at any time upon 60 days’ prior written notice and ceasing to make and sell all licensed
products, the termination of all sublicenses and payment of all monies owed under the Foundation Agreement. The licensor may terminate
the agreement 30 days after notice to the Company if the Company is 30 days late in payment or a breach that remains uncured for
45 days after written notice of such breach.
Professor
Fengping Shan
In
May of 2013, the Company executed a Patent License Agreement with Professor Fengping Shan (the “Shan Agreement”) pursuant
to which it obtained exclusive rights to develop and commercialize the licensed technology. The licensed technology is the intellectual
property developed and owned by Professor Shan (i) relating to the treatment of a variety of diseases and conditions with MENK
including multiple forms of lymphoma and cancer and (ii) a treatment method for humans infected with the HLTV-III (AIDS) virus
including AIDS and AIDS related complex (ARC). The licensed technology includes the methods and formulations for these treatments
including all INDs, communications with regulatory agencies, patient data, and letters relating to these treatments. The licensed
technology also includes certain patents developed by Professor Shan.
Under
the Shan Agreement, the Company must issue 500,000 shares to Professor Shan upon final transfer of the licenses, and reimburse
Professor Shan for all out of pocket expenses in connection with the patents. The Company will pay Professor Shan a running royalty
on gross sales subject to decreases if third party intellectual property is needed to complete such sale or product. The Shan
Agreement lasts for the duration of each of the licensed patents however the Company may terminate the Shan Agreement on 120 days’
written notice to Professor Shan.
The
payment provisions under the Shan Agreement do not provide any up-front or executions payments and no aggregate amounts have been
paid or received under the agreement. No future potential milestone payments will be paid or received either. The royalty rate
under the agreement is .5% and there are no profit or revenue-sharing provisions to be followed.
On
August 6, 2014, Professor Fengping Shan executed an Assignment pursuant to which he transferred to the Company his entire right,
title and interest in and to the licensed patents under the Shan Agreement and CN 201210302259 Application of combination of low-dose
naltrexone and methionine-enkephalin to preparation of anti-cancer drug for the consideration of 500,000 shares of common stock
valued at $140,000.
Patents
Overview:
|
Patent:
|
|
Title:
|
|
Expiration:
|
|
License/Assigned:
|
|
Product
or Use:
|
|
|
|
|
|
|
|
|
|
|
|
U.S.
Patent Number 6,586,443
(Related
to US 5,356,900, 5,013,739 and 4,888,346 – all expired)
(No
related foreign patents)
|
|
Multiple
sclerosis in a human patient is treated by the administration preferably via a pharmacologically effective route of an essentially
pure opiate receptor antagonist.
|
|
January
3, 2019
|
|
Exclusive
License from Jacqueline Young.
|
|
IRT-103
(LDN)
|
|
|
|
|
|
|
|
|
|
|
|
U.S.
Patent Number 6,384,044
(No
related foreign patents)
|
|
Cancer
of the prostate in human male patients even at an advanced state with metastasis to other organs is preferably treated by
administration.
|
|
November
8, 2019
|
|
Exclusive
License from Jacqueline Young.
|
|
IRT-103
(LDN)
|
|
|
|
|
|
|
|
|
|
|
|
U.S.
Patent Number 6,288,074
(No
related foreign patents)
|
|
Lymphoproliferative
syndrome, including such diseases as malignant lymphoma, chronic lymphocytic leukemia, Hodgkin’s lymphoma, and non-Hodgkin’s
lymphoma, are treated in human patients via administration.
|
|
November
15, 2019
|
|
Exclusive
License from Jacqueline Young.
|
|
IRT-103
(LDN)
|
|
|
|
|
|
|
|
|
|
|
|
U.S.
Patent Number 6,136,780
(Related
to US 6,737,397)
(No
related foreign applications)
|
|
Control
of cancer growth through the interaction of [Met5] - Enkephalin and the zeta (s) receptor.
|
|
May
17, 2021
|
|
Exclusive
License: Penn State University.
|
|
IRT-101
(MENK)
and
IRT-103 (LDN)
|
|
U.S.
Patent No. 6,737,397
(Related
to US 6,136,780)
(No
related foreign applications)
|
|
Control
of cancer growth through the interaction of [Met5]-Enkephalin and the zeta receptor.
|
|
May
17, 2021
|
|
Exclusive
license: Penn State University.
|
|
IRT-101
(MENK)
and
IRT-103 (LDN)
|
|
|
|
|
|
|
|
|
|
|
|
U.S.
Patent No. 7,879,870
(US
PgPub 2008/0015211)
(No
related foreign patents)
|
|
Treatment
of inflammatory and ulcerative diseases of the bowel with opioid antagonists.
|
|
February
1, 2028
|
|
License
to Cytocom Inc.: Dr. Jill Smith and LDN Research Group, LLC.
|
|
IRT-103
(LDN)
|
|
|
|
|
|
|
|
|
|
|
|
Israeli
Patent mentioned in license
|
|
Treatment
of inflammatory and ulcerative diseases of the bowel with opioid antagonists.
|
|
Pending
|
|
License
to Cytocom Inc.: Dr. Jill Smith and LDN Research Group, LLC.
|
|
Treatment
of Crohn’s disease
|
|
U.S.
Application Number: 11/061,932
(Claims
Priority to US60/548,021)
Canadian
Application Number: 2,557,504 (Pending)
|
|
Combinatorial
therapies for the treatment of neoplasias using the opioid growth factor receptor.
|
|
Pending
application
|
|
Exclusive
license: Penn State University.
|
|
IRT-101 (MENK)
|
|
|
|
|
|
|
|
|
|
|
|
U.S.
Patent No. 8,003,630 (Application Number: 11/510,682)
(US
PgPub 2007/0053838)
(Claims
Priority to US60/548,021)
|
|
Combinatorial therapies
for the treatment of neoplasias using the opioid growth factor receptor.
|
|
May 22, 2028
|
|
Exclusive license:
Penn State University.
|
|
IRT-101 (MENK)
|
|
|
|
|
|
|
|
|
|
|
|
U.S.
PgPub 2013/0084242 A1
(Application
Number: 13/660,129)
(Claims
Priority to US60/548,021)
Patent
Cooperation Treaty (PCT) application:
PCT/US2010/030967
(Claims
priority to US61/173,351)
|
|
Combinatorial therapies
for the treatment of neoplasias using the opioid growth factor receptor.
|
|
Pending
|
|
Exclusive license:
Penn State University.
|
|
IRT-101 (MENK)
|
|
US
7,807,368
(US
PgPub 2008-0146512 A1)
(No
related foreign applications)
|
|
Cyclin-dependent
kinase inhibitors as targets for opioid growth factor treatment.
|
|
October
4, 2027
|
|
Exclusive
license: Penn State University.
|
|
IRT-101 (MENK)
|
|
|
|
|
|
|
|
|
|
|
|
US
7,576,180
(Claims
priority to US60/106,879)
(There
is a related PCT application PCT/US1999/025802, claiming priority to the US60/106,879, but no National Phase applications
were filed)
|
|
Opioid growth factor
receptors.
|
|
August 17, 2026
|
|
Exclusive license:
Penn State University.
|
|
IRT-101 (MENK)
|
|
|
|
|
|
|
|
|
|
|
|
US
7,122,651
(No
related foreign applications)
|
|
Novel nucleic acid
molecules encoding opioid growth factor receptors.
|
|
October 17, 2023
|
|
Exclusive license:
Penn State University.
|
|
Treatment of cancer
|
|
|
|
|
|
|
|
|
|
|
|
US
7,517,649
(US
PgPub 20060073565)
(No
related foreign applications)
|
|
Methods of detecting
opioid growth factor receptor (OGFr) in tissue.
|
|
April 13, 2026
|
|
Exclusive license:
Penn State University.
|
|
IRT-101 (MENK)
|
|
|
|
|
|
|
|
|
|
|
|
CN
200910011030
(No
related U.S. applications)
|
|
Shan Fengping, Nikola
Polonikov, Lu Changlong: Application of naloxone and composition thereof in preparing drug for treating cancer. Shan Fengping:
August 26, 2009.
|
|
August 23, 2026
|
|
Assigned by Fengping
Shan.
|
|
IRT-101
(MENK)
and
IRT-103 (LDN)
|
|
CN
200710051586
(No
related U.S. applications)
|
|
Huang
Jianyin, Zhang Ding, Shan Fengping, Luo Zhinong: Application of methionine enkephalin in preparing human or animal vaccination.
Huang Jianyin: August, 20 2008.
|
|
August
20, 2025
|
|
Assigned
by Fengping Shan.
|
|
IRT-101
(MENK)
|
|
|
|
|
|
|
|
|
|
|
|
CN
200710158742
(No
related U.S. applications)
|
|
Shan
Fengping, Lv Changlong, Nikola Polonikov, Huang Jianyin: Application of compounds Methionine Enkephalin for preparing medicine
for curing blood medulla hematopoietic system cancer. Dan Fengping: May 14, 2008.
|
|
May
13, 2025
|
|
Assigned
by Fengping Shan.
|
|
IRT-101
(MENK)
|
|
CN
200610046249
(No
related U.S. applications)
|
|
Shan
Fengping, Lv Changlong, Huang Jianyin, Zhang Ding, Luo Zhinong: Aerosol containing Met-Enkephalin. Shan Fengping: November
15, 2006.
|
|
November
14, 2023
|
|
Assigned
by Fengping Shan.
|
|
IRT-101
(MENK)
|
|
|
|
|
|
|
|
|
|
|
|
CN
200310120896
(No
related U.S. applications)
|
|
Shan
Fengping, Li Li: Integrated health food for regulating human body immune balance. Liaoning Academy of Microorganism Sciences:
July 6, 2005.
|
|
July
5, 2022
|
|
Exclusive
license: Nicholas Plotnikoff and Fengping Shan.
|
|
Oncology
treatments
and
cancer treatment
|
|
|
|
|
|
|
|
|
|
|
|
WO
2007/067753
(PCT/US2006/046925
|
|
Huang
John, Chang Ding, Lo Shi-Lung, Shan Fengping: Methods of reducing side effects in cancer therapy. Penta Biotech: June 14,
2007.
|
|
Pending
|
|
Exclusive
license: Fengping Shan.
|
|
IRT-101
(MENK)
|
|
|
|
|
|
|
|
|
|
|
|
CN
200510019964
|
|
Huang
Jianyin, Zhang Ding, Luo Zhinong, Shan Fengping: Use of Methionine Enkephalin in preparation of medicine for reducing toxic
side effects of chemical or radioactive therapy. Huang Jianyin: August 9, 2006.
|
|
August
8, 2023
|
|
Exclusive
license: Fengping Shan.
|
|
IRT-101
(MENK)
|
|
US
PgPub 2003/0148942 A1
(Application
Number: 10/146,999)
Our
Docket #6463-0101PUS1
(Claims
Priority to US60/291,237)
|
|
Methods
for inducing sustained immune response.
|
|
May
16, 2022
|
|
Assigned
and licensed: Nicholas Plotnikoff.
|
|
IRT-101
(MENK)
|
|
|
|
|
|
|
|
|
|
|
|
Russian
Application 2003136161/14
(Claims
Priority to US60/291,237)
|
|
Methods
for inducing sustained immune response.
|
|
May
16, 2022
|
|
Assigned
and licensed: Nicholas Plotnikoff.
|
|
IRT-101
(MENK)
|
|
PCT
application PCT/US2002/018529
(Claims
priority to the US60/291,237)
|
|
Methods
for inducing sustained immune response.
|
|
May
16, 2022
|
|
Assigned
and licensed: Nicholas Plotnikoff.
|
|
IRT-101
(MENK)
|
|
|
|
|
|
|
|
|
|
|
|
National
Phase entries filed off the PCT/US2002/018529
China
02814327.2
(Pending)
EP
App 2002746503 Granted: November 29, 2006
India
Patent No. 220265 (App 01627/KOLNP/2003)
Japan
App Withdrawn
|
|
Methods
for inducing sustained immune response.
|
|
May
16, 2022
|
|
Assigned
and licensed: Nicholas Plotnikoff.
|
|
IRT-101
(MENK)
|
|
|
|
|
|
|
|
|
|
|
|
China
Patent 200810229085
|
|
The
invention belongs to the technical field of treating tumors by immunization therapy. In particular, a method for treating
intestinal cancer and pancreatic cancer cells by Methionine Enkephalin under conditions of in-vivo injection and in-vitro
cell culture so as to achieve the treating aim.
|
|
March
21, 2026
|
|
Assigned
by Fengping Shan.
|
|
IRT-101
(MENK)
|
Employees
As
of August 18, 2016, the Company had 6 full time employees.
Reports
to Security Holders
Our
common stock is registered under the Securities Exchange Act of 1934 and we are required to file current, quarterly and annual
reports and other information with the SEC. You may read and copy any document that we file at the SEC's public reference
facilities at 100 F. Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-732-0330 for more information about its
public reference facilities. Our SEC filings are available to you free of charge at the SEC's web site at www.sec.gov.
We are an electronic filer with the SEC and, as such, our information is available through the Internet site maintained by the
SEC that contains reports, proxy and information statements and other information regarding issuers that file electronically with
the SEC. This information may be found at www.sec.gov and posted on our website at Immune Therapeutics.com.
Research
and Development
Our
research and development (“R&D”) organization focuses primarily on new uses for the opioid-related immuno-therapies,
such as LDN and MENK. These therapies stimulate the immune system in such a way that provides the potential to treat a variety
of diseases that have abnormalities in the immune system.
Our
R&D priorities include development of MENK IRT-101, a small synthetic pentapeptide that is naturally occurring in the body,
and LDN IRT-103, an opioid receptor antagonist. Our pipeline provides two therapies with an extremely wide range of indications
that can be pursued. Both molecules have the ability to stimulate and/or regulate the immune system in order to
treat
a variety of autoimmune diseases including multiple sclerosis, immune disorders such as Crohn’s disease, cancer, and viral
infections such as HIV/AIDS.
Our
R&D is overseen and managed internally, working with individuals, universities, and Contract Research Organizations (“CROs”)
in order to utilize patents that we have licensed or acquired since our inception. We continue to seek to expand our pipeline
by reviewing other compounds, technologies or capabilities. We also seek out promising compounds and innovative technologies developed
by third parties to incorporate into our discovery and development processes or projects.
Drug
discovery and development is time-consuming, expensive and unpredictable. According to the Pharmaceutical Research and Manufacturers
of America (PhRMA), out of 5,000-10,000 screened compounds, only 250 enter preclinical testing, five enter human clinical trials
and one is approved by the FDA. The process from early discovery or design to development to regulatory approval can take more
than 10 years. Drug candidates can fail at any stage of the process, and candidates may not receive regulatory approval even after
many years of research.
As
of December 31, 2015, we had two compounds (IRT-101 and IRT-103) in research and development. In 2015 our development programs
focused on both compounds, one in oncology and one in Crohn’s disease; which we are expecting to move into Phase II clinical
trials.
The
following table provides information about notable regulatory actions by, and filings pending with the FDA and regulatory authorities
in the EU, as well as additional indications and new drug candidates in late-stage development.
|
NEW
DRUG CANDIDATES IN LATE-STAGE DEVELOPMENT
|
|
CANDIDATE
|
|
INDICATION
|
|
REGULATORY
ACTIONS
|
|
IRT-101
|
|
Pancreatic
Cancer
|
|
End-of-Phase
1 Meeting with FDA Complete 3Q 2013
|
|
IRT-103
|
|
Crohn’s
Disease
|
|
Type
C Meeting with FDA Complete 2Q 2013
Scientific
Advice with EMA Complete 1Q 2014
|
The
Company expects it will incur future research and development expenditures in the next 12 months through Cytocom. Cytocom plans
to conduct Phase II and Phase IIB trials for the treatment of IRT-103 Crohn’s disease, at an estimated cost of $3,900,000
and $7,500,000 respectively for each phase. If the trials do not commence before 2017, the Company will be required to make a
payment of $100,000 in December 2016 under its license agreements. In prior years, the Company has been able to raise funds through
sales of notes payable, and it expects to do the same for the payment due in 2016. With funding Cytocom will be responsible for
the development of IRT-101 MENK for pancreatic cancer.
Government
Regulations
United
States
The
research, testing, manufacturing, labeling, approval, selling, import, export, marketing and distribution of drug products are
subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries with regulations
differing from country to country. Neither we nor our collaboration partners are permitted to market our drug candidates in the
United States until we receive approval of a New Drug Application (“NDA”) from the FDA. Neither we nor our collaboration
partners have submitted an application for or received marketing approval for any of our drug candidates. Obtaining approval of
an NDA can be a lengthy, expensive and uncertain process.
Prior
to receiving approval to commercialize any of our drug candidates in the United States or abroad, we and our collaboration partners
must demonstrate with substantial evidence from well controlled clinical trials, and to the satisfaction of the FDA and other
regulatory authorities abroad, that such drug candidates are safe and effective for their intended uses. Results from preclinical
studies and clinical trials can be interpreted in different ways. Regulatory approval of an NDA or NDA supplement is not guaranteed,
and the approval process is expensive and may take several years.
Before
a drug can be tested in people, the sponsor (in this case the Company) performs laboratory and animal tests to discover how the
drug works and whether it’s likely to be safe and effective in humans. As LDN and MENK have previously been used in clinical
trials, this phase of development was not required by the Company to initiate clinical trials under its applications.
Next,
a series of tests in people (i.e. clinical trials) is begun to determine whether the drug is safe when used to treat a disease
and whether it provides a real health benefit. The clinical phase typically starts at Phase 1 and progresses to Phase 3. The Company
will have an abbreviated list of clinical trials that need to be conducted due to published literature on previously conducted
studies, as well as utilizing the approval of naltrexone previously at 50 mg by the FDA.
Upon
completion of the clinical trials, the Company will send the FDA and/or the EMA the evidence from these tests to prove the drug
is safe and effective for its intended use (New Drug Application (NDA) in the US or Marketing Authorisation Application in the
EU). The regulatory bodies will review these data and determine if the sponsor has approval to market the product at the specified
dose(s) and formulation(s) for the specified indication(s) (
http://www.fda.gov/Drugs/DevelopmentApprovalProcess/default.htm)
.
Once
regulatory approval has been granted, the approved product and its manufacturer are subject to continual review by the FDA and/or
non-U.S. regulatory authorities. Any regulatory approval that we or our collaboration partners receive for our drug candidates
may be subject to limitations on the indicated uses for which the product may be marketed or contain requirements for potentially
costly post-marketing follow-up studies to monitor the safety and efficacy of the product. In addition, if the FDA and/or non-U.S.
regulatory authorities approve any of our drug candidates, we will be subject to extensive and ongoing regulatory requirements
by the FDA and other regulatory authorities with regard to the labeling, packaging, adverse event reporting, storage, advertising,
promotion and recordkeeping for our products. In addition, manufacturers of our drug products are required to comply with current
cGMP regulations which include requirements related to quality control and quality assurance, as well as the corresponding maintenance
of records and documentation. Further, regulatory authorities must approve these manufacturing facilities before they can be used
to manufacture our drug products, and these facilities are subject to continual review and periodic inspections by the FDA and
other regulatory authorities for compliance with cGMP regulations.
European
Union
We
intend to seek distribution and marketing partners for IRT-101 (MENK) and IRT-103 (LDN) in the European Union (“EU”).
To market our future products in the European Economic Area (“EEA”) (which is comprised of the 27 member states of
the EU plus Norway, Iceland and Liechtenstein) and many other foreign jurisdictions, we must obtain separate regulatory approvals.
More concretely, in the EEA, medicinal products can only be commercialized after obtaining a Marketing Authorization (“MA”).
|
|
●
|
The
Community MA is issued by the European Commission through the Centralized Procedure,
based on the opinion of the Committee for Medicinal Products for Human Use of the EMA,
and is valid throughout the entire territory of the EEA. The Centralized Procedure is
mandatory for certain types of products, such as biotechnology medicinal products, orphan
medicinal products, and medicinal products indicated for the treatment of AIDS, cancer,
neurodegenerative disorders, diabetes, auto-immune and viral diseases. The Centralized
Procedure is optional for products containing a new active substance not yet authorized
in the EEA, or for products that constitute a therapeutic, scientific or technical innovation
or which are in the interest of public health in the EU.
|
|
|
|
|
|
|
●
|
National
MAs, which are issued by the competent authorities of the member states of the EEA and only cover their respective territory,
are available for products not falling within the mandatory scope of the Centralized Procedure. Where a product has already
been authorized for marketing in a member state of the EEA, this National MA can be recognized in another member state through
the Mutual Recognition Procedure. If the product has not received a National MA in any member state at the time of application,
it can be approved simultaneously in various member states through the Decentralized Procedure.
|
Under
the procedures described above, before granting the MA, the EMA or the competent authorities of the member states of the EEA make
an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and
efficacy.
Our
IND is being conducted per 21 Code of Federal Regulations Title 21, Part 312. In addition, we follow ICH guidelines, including
good clinical practices (ICH E6) and current good manufacturing practice (ICH Q7) throughout the development process. After completion
of Phase III clinical trials, the Company will file our NDA for LDN (IRT-103) as a 505(b)(2) application. IRT-103 products will
follow the 505(b)(2) pathway relying on the Reference Listed Drug (RLD) REVIA to support the safety of the product. Efficacy will
be submitted by the Company directly to the LDN NDA. IRT-101 products will follow the traditional approval pathway as a RLD is
not available for MENK. However, published literature will support this program.
Nigeria
NAFDAC
is the equivalent in Nigeria of the FDA. It undertakes registration of food, drugs, medical devices, cosmetics, agrochemicals
and other similar products in Nigeria. At the end of the process, a registration number is given to the product and a registration
certificate is issued to the applicant.
Many
of the African countries do not have a local FDA equivalent organization or agency. We plan to use the NAFDAC Registration as
the guideline for submission in Africa for countries that do not have their own application and approval procedures.
The
Company has partnered with AHAR Pharma, a company duly incorporated and existing under the laws of the Republic of Nigeria to
distribute LDN in the Republic of Nigeria. The distribution agreement was originally signed in 2013 and extended in 2016 for 5
years with an optional 5 year extension. AHAR Pharma is responsible for securing all required governmental or regulatory approvals,
registrations, permits and licenses necessary to market, promote, offer for sale, sell, supply and distribute LDN in the Republic
of Nigeria. Pursuant to the distribution agreement, the Company shall pay AHAR Pharma twenty-five U.S. cents for each tablet sold.
Of the twenty-five cents paid, twelve and a half cents may be credited against the Company’s invoices and twelve and a half
U.S. cents shall be paid to AHAR Pharma’s affiliate GB Pharma.
In
addition, the Company has worked with GB Pharma Holdings since its formation in 2012. Over the last two years GB Pharma has worked
directly with the NAFDAC and Minister of Health’s in Africa and the African Union on the approval of Lodonal for HIV/AIDS
in Africa. GB Pharma has also worked to introduce LDN to the UNAIDS and World Health Organization. Dr. Gloria Herndon, the
President of GB Pharma Holdings has traveled extensively to Nigeria, Kenya, Angola and South Africa in the past two years meeting
with and holding discussion about the Company’s immunotherapies. Dr. Herndon has spoken on behalf of the Company at a number
of conferences and was responsible for the introduction of the therapy to the Africa Union and the Champions, a group of former
presidents of Africa to help promote the approval through the various in country agencies.
The
Company has a signed agreement with GB Holding LLC, and it is subsidiaries GB Pharma and GB Oncology & Imaging Group Ltd,
each led by Dr. Gloria B. Herndon. On September 25, 2012, GB Oncology & Imaging Group, in partnership with the
Company, signed an agreement with the Government of Malawi to open an outpatient clinic at Queen Elizabeth Central Hospital (in
Malawi) for the treatment of cancer and infectious disease. Due to a change in the Government the Company has abandoned
this contract.
The
Company’s wholly owned subsidiary, Airmed Biopharma Limited, entered into an Exclusive Agency Agreement with GB Parma Holdings,
Inc. on June 12, 2014 to distribute Lodonal™ in various emerging markets including Equatorial Guinea. Pursuant to the distribution
agreement, GB Pharma shall be entitled to a commission of 5% of the net product sales. The Company’s relationships
with the GB Pharma and its subsidiaries and affiliates are contractual, with the rights and obligations of the parties dictated
by their respective agreements. GB Pharma and certain of its affiliates may also now own, or in the past owned, minority share
positions in the Company via their investments or shares granted for services.
Malawi
No
formal governmental agency is in place in Malawi to govern the application of a new drug. Malawi is a member of the Southern Africa
Development Community (“SADC”). The SADC has been making efforts to synchronize the regulation of medication in the
SADC countries.
The
guidelines require filing an application prior to approval of registration. However, these guidelines are preliminary. The Regional
Indicative Strategic Development Plan (“RISDP”) is a comprehensive development and implementation framework guiding
the Regional Integration agenda of the SADC over a period of fifteen years (2005-2020). It is designed to provide clear strategic
direction with respect to SADC programs, projects and activities in line with the SADC Common Agenda and strategic priorities,
as enshrined in the SADC Treaty of 1992.
In
July 2014, the Republic of Malawi approved Lodonal™ as an adjunct for the treatment of cancer. Protocols for a Lodonal™
trial were approved in November 2015. The Brewer Group, Inc. has paid for production of the first shipment to Malawi of Lodonal™,
which was delivered to Malawi from Nicaragua in 2015.
The
Company with the Brewer Foundation arranged for the donation of the Wallach LL100 Cryosurgical system that were necessary to run
the trial and treat patients with cervical cancer. In July 2014, the Republic of Malawi approved Lodonal™ as an adjunct
for the treatment of cancer. Protocols for a Lodonal™ trial were approved in November 2015.
In
2012, the Company started its collaboration with the Brewer Foundation and the Brewer Group. Over the last two years The Brewer
Group and Foundation has worked directly with the Government of Malawi on the approval of the clinical trials and protocols. Once
the protocols were approved the Brewer Group help to arrange funding for the trials.
The
Brewer Group has entered into a distribution agreement with Airmed Bioparhma Limited, a wholly owned subsidiary of the Company,
to distribute Lodonal™ in emerging markets. The distribution agreement was entered into in 2014 and has a term of 5 years.
Pursuant to the distribution agreement the Company shall sell Lodonal™ to The Brewer Group at a 25% discount to the list
price. The Brewer Group has also arranged a number of meetings with various hedge funds in New York to assist with the funding
of the company.
The
Company’s relationships with the Brewer Group and Brewer Foundation are contractual, with the rights and obligations of
the parties dictated by their respective agreements. The Brewer Group, Brewer Foundation and certain of their affiliates may also
now own, or in the past owned, minority share positions in the Company via their investments or shares granted for services.
Equatorial
Guinea
Equatorial
Guinea does not have procedures for the official approval of traditional medical practices or remedies. Accordingly, the Company
was requested to make a presentation to the Health Sector and Minister on the use of naltrexone in the treatment of certain indications.
After due discussion, the Government approved the following:
|
|
1.
|
Drug
use naltrexone (4.5 mg) in the treatment of diseases requiring immune system stimulating cancer, HIV infection, multiple sclerosis,
etc., as demonstrated by the Company, at a cost of $1/day or 450 x F.CFA/day.
|
|
|
|
|
|
|
2.
|
Management
– laboratory for quality control and to analyze drugs imported in to Equatorial Guinea.
|
|
|
|
|
|
|
3.
|
The
implementation of local production of quality essential medicines.
|
The
Company’s wholly owned subsidiary Airmed Biopharma Limited, entered into an Exclusive Agency Agreement with GB Parma Holdings,
Inc. on June 12, 2014 to distribute Lodonal™ in various emerging markets including Equatorial Guinea. Pursuant to the distribution
agreement, GB Pharma shall be entitled to a commission of 5% of the net product sales.
China
On
October 18, 2012, the Company and Hubei Qianjiang Pharmaceutical Co., Ltd. (“Qianjiang Pharmaceutical”), signed a
Venture Cooperation Agreement on New Drug Methionine Enkephalin (the “Venture Agreement”) pursuant to which Qianjiang
Pharmaceutical acquired an exclusive license for the production of MENK in China. The Venture Agreement requires that Qianjiang
Pharmaceutical conduct drug research and pilot testing for MENK, organize pre-clinical studies, and apply for clinical trials
for MENK with the Chinese State Food and Drug Administration. Under the Venture Agreement, Qianjiang Pharmaceutical must open
a co-administration account for the development of MENK in China. Qianjiang Pharmaceutical must pay the Company, upon the marketing
of MENK products, a half-year amount equaling 6% of its gross sales from MENK of the preceding half year.
Qianjiang
Pharmaceutical is required to obtain all approvals and permits required for the importation and sale of the Company’s products
in China.
The
Company may cancel the Venture Agreement if Qianjiang Pharmaceutical does not pay expenses for a period exceeding nine months
or does not commence clinical trials within 12-months after receiving certain approvals. Qianjiang Pharmaceutical may cancel the
Venture Agreement if the Company fails to perform its obligations for a period of nine months or the failure to receive approval
of clinical trials is due to the Company’s MENK technologies.
On
August 6, 2014, the Company entered into a Supplementary Agreement on New Drug Methionine – Enkephalin Cooperation (the
“Amendment”) with Qianjiang Pharmaceutical, amending the Venture Agreement, as amended. The Company and Qianjiang
Pharmaceutical executed the Amendment to accelerate clinical trials in both the United States and China, and agreed to immediately
initiate three month Good Laboratory Practice (“GLP”) Toxicology Studies (rat and dog) within 30 days of signing the
Amendment. The Amendment requires that the GLP Toxicology Studies Trials are conducted in China in accordance with international
standards and standards acceptable to the FDA.
The
Company modified the agreement one more time due to the fact toxicology studies were not completed by the end of 2015 as others
steps were required before the studies could start.
Qianjiang
has completed the formulation and required Chemistry, Manufacturing, and Controls (“CMC”) for Methionine–Enkephalin.
All work was completed in China in accordance with international cGMP standards acceptable to the U.S. Food and Drug Administration
with Chinese Peptide Company (“CPC”). CPC is among only a handful of companies in the world that can claim both ISO
Certification and cGMP licensing. In February 2012, we became the first peptide company to successfully pass US FDA inspection
outside of US and Europe regions.
Nicaragua
Laboratorios
Ramos in Managua, Nicaragua has been issued approval from the Minster of Health to manufacture Naltrexone for the Company under
the trademark name Lodonal™. The certificate of free sale allows Naltrexone to be exported from the Managua facility to
other jurisdictions where the Company is approved to market and sell Naltrexone in satisfaction of the
import
requirements of such jurisdictions. The free sale certificate is not a license for export, which is issued separately for a specific
product in both the country of export as well as the country of import.
DESCRIPTION
OF PROPERTY
The
Company does not own any real property and leases all of its space. We maintain our headquarters at 37 North Orange Ave, Suite
607, Orlando, Florida 32801. The Company leases approximately 800 sq. feet at a monthly cost of approximately $1,300. The lease
expires on December 31, 2016. In August 2014, the Company closed its offices in Frederick, MD, where it had carried out research
and development. The Company will be required to obtain new research and development premises after it raises additional funding
to enable it to resume those activities.
LEGAL
PROCEEDINGS
N/A
MARKET
PRICE, DIVIDENDS, AND RELATED STOCKHOLDER MATTERS
Our
common stock is listed for quotation on the OTCQB marketplace under the symbol IMUN. Our common stock began trading in November
1999 on OTC under the name Galliano International Ltd. Trading under the name of TNI BioTech, Inc. commenced in March 2012 under
the symbol TNIB. The symbol was changed to IMUN on December 11, 2014. Trading in our common stock has historically lacked consistent
volume, and the market price has been volatile.
The
following table presents, for the periods indicated, the high and low bid prices of the Company’s common stock, and is based
upon information provided by the OTC Marketplace. These quotations below reflect inter-dealer prices, without retail mark-up,
mark-down, or commission, and may not necessarily represent actual transactions.
|
Period
|
|
Price
Range
|
|
|
|
|
High
|
|
|
Low
|
|
|
Quarter Ended June 30, 2016:
|
|
$
|
0.28
|
|
|
$
|
0.08
|
|
|
Quarter Ended:
|
|
|
|
|
|
|
|
|
|
March 31, 2016
|
|
$
|
0.44
|
|
|
$
|
0.16
|
|
|
December 31, 2015
|
|
$
|
0.08
|
|
|
$
|
0.28
|
|
|
September 30, 2015
|
|
$
|
0.05
|
|
|
$
|
0.29
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarter Ended June 30, 2015:
|
|
$
|
0.11
|
|
|
$
|
0.05
|
|
|
Quarter Ended
|
|
|
|
|
|
|
|
|
|
March 31, 2015
|
|
$
|
0.28
|
|
|
$
|
0.07
|
|
|
December 31, 2014
|
|
$
|
0.35
|
|
|
$
|
0.10
|
|
|
September 30, 2014
|
|
$
|
0.44
|
|
|
$
|
0.16
|
|
The
last reported sale price of the Company’s common stock as of August 22, 2016 was $0.112 per share.
As of
August 23, 2016, there were 734 shareholders of record.
While
an equity incentive plan was approved for 5,000,000 common shares in 2014, the Company has no current plans to implement the equity
incentive plan.
Equity
Compensation Plan Information
|
Plan
category
|
|
Number
of securities to be issued upon exercise of outstanding options, warrants and rights
|
|
Weighted-average
exercise price of outstanding options, warrants and rights
|
|
Number
of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column
(a))
|
|
Equity compensation plans
approved by security holders
|
|
|
0
|
|
|
|
0
|
|
|
|
5,000,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity compensation plans not approved
by security holders
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
0
|
|
|
|
0
|
|
|
|
5,000,000
|
|
The
Company paid no dividends in 2015 or 2014. We do not anticipate paying any cash dividends in the foreseeable future. The payment
of dividends is within the discretion of our Board of Directors and will depend on our earnings, capital requirements, financial
condition, and other relevant factors. There are no restrictions that currently limit our ability to pay dividends on its common
stock other than those generally imposed by applicable state law.
SELECTED
CONSOLIDATED FINANCIAL DATA
The
following tables summarize our selected consolidated financial data for the periods and as of the dates indicated. Our selected
statements of operations data for each of the years ended December 31, 2015 and 2014, and our selected balance
sheet
data as of December 31, 2015, have been derived from our audited consolidated financial statements and related notes included
elsewhere in this prospectus. Our selected statements of operations data for the three months ended June 30, 2016 and 2015, and
our selected balance sheet data as of June 30, 2016, have been derived from our unaudited interim condensed consolidated financial
statements and related notes included elsewhere in this prospectus. Our unaudited interim condensed consolidated financial statements
have been prepared in accordance with generally accepted accounting principles in the United States of America on the same basis
as the annual audited consolidated financial statements and, in the opinion of management, reflect all adjustments, consisting
only of normal recurring adjustments, necessary to state fairly our financial position as of June 30, 2016 and the results of
our operations for the three months ended June 30, 2016 and 2015. Our selected financial data should be read together with the
section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and
with our financial statements and their related notes, which are included elsewhere in this prospectus. Our historical results
are not indicative of the results that may be expected in the future.
(Unaudited)
|
|
|
Three
Months ended
|
|
|
Six
Months ended
|
|
|
|
|
June
30, 2016
|
|
|
June
30, 2015
|
|
|
June
30, 2016
|
|
|
June
30, 2015
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues, net
|
|
$
|
-
|
|
|
$
|
5,648
|
|
|
$
|
3,463
|
|
|
$
|
7,718
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative
|
|
|
893,917
|
|
|
|
443,585
|
|
|
|
1,833,258
|
|
|
|
845,572
|
|
|
Research and development expense
|
|
|
67,262
|
|
|
|
414,492
|
|
|
|
48,420
|
|
|
|
591,650
|
|
|
Stock issued for services G&A
|
|
|
1,194,761
|
|
|
|
1,450,334
|
|
|
|
3,178,598
|
|
|
|
3,611,893
|
|
|
Warrant valuation
|
|
|
490,355
|
|
|
|
-
|
|
|
|
2,568,554
|
|
|
|
-
|
|
|
Depreciation and amortization expense
|
|
|
434
|
|
|
|
148,726
|
|
|
|
981
|
|
|
|
297,452
|
|
|
Total operating
expenses
|
|
|
2,646,729
|
|
|
|
2,457,137
|
|
|
|
7,629,811
|
|
|
|
5,346,567
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(2,646,729
|
)
|
|
|
(2,451,489
|
)
|
|
|
(7,626,348
|
)
|
|
|
(5,338,849
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
(1,051,576
|
)
|
|
|
(50,711
|
)
|
|
|
(1,353,020
|
)
|
|
|
(68,757
|
)
|
|
Loss on settlement of debt
|
|
|
(340,343
|
)
|
|
|
(88,445
|
)
|
|
|
(1,704,683
|
)
|
|
|
(88,445
|
)
|
|
Total other
income (expense)
|
|
|
(1,391,919
|
)
|
|
|
(139,156
|
)
|
|
|
(3,057,703
|
)
|
|
|
(157,202
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss)
|
|
$
|
(4,038,648
|
)
|
|
$
|
(2,590,645
|
)
|
|
$
|
(10,684,051
|
)
|
|
$
|
(5,496,051
|
)
|
|
Net (loss) attributable to non-controlling interest
|
|
|
(109,929
|
)
|
|
|
(138,891
|
)
|
|
|
(154,049
|
)
|
|
|
(262,956
|
)
|
|
Net (loss) attributable to common shareholders
|
|
$
|
(3,928,719
|
)
|
|
$
|
(2,451,754
|
)
|
|
$
|
(10,530,002
|
)
|
|
$
|
(5,233,095
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share
attributable to common shareholders
|
|
$
|
(0.02
|
)
|
|
$
|
(0.02
|
)
|
|
$
|
(0.05
|
)
|
|
$
|
(0.04
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares
outstanding
|
|
|
207,229,469
|
|
|
|
147,387,763
|
|
|
|
199,076,428
|
|
|
|
142,563,007
|
|
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF
FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
The
following discussion and analysis of our financial condition and results of operations should be read in conjunction with “Selected
Consolidated Financial Data” and our consolidated financial statements and related notes appearing elsewhere in this prospectus.
Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus,
including
information with respect to our plans and strategy for our business, includes forward looking statements that involve risks and
uncertainties as described under the heading “Special Note Regarding Forward Looking Statements” elsewhere in this
prospectus. You should review the disclosure under the heading “Risk Factors” in this prospectus for a discussion
of important factors that could cause actual results to differ materially from the results described in or implied by the forward
looking statements contained in the following discussion and analysis.
Overview
General
Immune
Therapeutics, Inc. was initially incorporated in Florida on December 2, 1993 as Resort Clubs International, Inc. It was formed
to manage and market golf course properties in resort markets throughout the United States. Galliano International Ltd. (“Galliano”)
was incorporated in Delaware on May 27, 1998 and began trading in November 1999 through the filing of a 15C-211. On November 10,
2004, Galliano merged with Resort Clubs. Resort Clubs was the surviving corporation. On August 23, 2010, Resort Clubs changed
its name to pH Environmental Inc.
On
April 23, 2012, pH Environmental completed a name change to TNI BioTech, Inc., and on April 24, 2012, we executed a share exchange
agreement for the acquisition of all of the outstanding shares of TNI BioTech IP, Inc. On September 4, 2014, a majority of our
shareholders approved an amendment to our Amended and Restated Articles of Incorporation, as amended, to change our name to Immune
Therapeutics, Inc. We filed our name change amendment with the Secretary of State of Florida on October 27, 2014 changing our
name to Immune Therapeutics, Inc.
The
Company currently operates out of Orlando, Florida. In July 2012, the Company’s focus turned to acquiring patents that would
protect and advance the development of new uses of opioid-related immune- therapies, such as low dose naltrexone (“LDN”)
and Methionine [Met5]-enkephalin (“MENK”). The Company’s therapies are believed to stimulate and/or regulate
the immune system in such a way that they provide the potential to treat a variety of diseases. We believe our therapies may be
able to correct abnormalities or deficiencies in the immune system in diseases such as HIV infection, autoimmune disease, immune
disorders, or cancer; all of which can lead to disease progression and life-threatening situations when the immune system is not
functioning optimally.
Subsidiaries
In
October 2012, the Company formed TNI BioTech International, Ltd., a BVI company in Tortola, British Virgin Islands, which was
set up to allow the Company to market and sell LDN in those countries outside the U.S. in which we have been able to obtain approval
to sell the Company’s products.
In
August 2013, the Company formed its United Kingdom subsidiary, TNI BioTech, LTD. The UK Subsidiary received approval to be considered
a micro, small or medium-sized enterprise with the European Medicines Agency on August 21, 2013. The designation provides the
UK Subsidiary with significant discounts when holding meetings or submitting filings to the EMA. On September 19, 2013, the UK
Subsidiary submitted a pre-submission package to the EMA regarding Crohn’s Disease. The EMA granted the UK Subsidiary a
meeting that took place on September 27, 2013. The UK Subsidiary is eligible to benefit from the provisions for administrative
and financial assistance for SMEs set out in Regulation (EC) No 2049/2005. The Company will apply to obtain EMA benefits once
funding becomes available.
In
December 2013, the Company formed a new subsidiary, Cytocom Inc., to focus on conducting LDN and MENK clinical trials in the United
States. In December 2014, the Company finalized the distribution of common stock of Cytocom Inc. to its shareholders. As part
of the transaction, the Company retained exclusive rights to all international patents, in-country approvals, formulations, trademarks,
manufacturing, marketing, sales, and distributions rights in emerging nations, including Africa, Central America, South America,
Russia, India, China, Far East, and The Commonwealth of Independent States (former Soviet Union). The Company will continue to
have access to existing clinical data as well as any new data generated by Cytocom Inc. during drug development. On December 8,
2014, the number of Cytocom Inc. shares of common stock that were issued to our shareholders totaled 113,242,522 In connection
with the transaction, Cytocom Inc. issued 140,100,000 shares of its common stock to the Company, which gave the Company a 55.3%
stake in Cytocom Inc. on that date. . In April 2016, the Board of Directors and a majority of shareholders of Cytocom approved
a reverse stock split of Cytocom’s outstanding common stock with one new share of stock for each twenty old shares of common
stock. Cytocom effectuated and finalized the reverse split in June 2016. At June 30, 2016, the Company’s equity interest
had been further reduced to 41%, by subsequent issuances of Cytocom common stock to shareholders in settlement of notes payable.
In
March 2014, the Company incorporated Airmed Biopharma Limited, an Irish corporation with an address in Dublin, Ireland, and Airmed
Holdings Limited, an Irish company domiciled in Bermuda. The Irish companies were set up to benefit from incentives granted by
the Irish government for the establishment of pharmaceutical companies (many of the world’s leading pharmaceutical companies
have located in Ireland), and so that the Company could take advantage of Ireland’s status as a member of the European Union
and the European Economic Area. An Irish limited liability company enjoys a low corporate income tax rate of 12.5%, one of the
lowest in the world. The Irish-domiciled company hopes to qualify for tax incentives for Irish holding/headquartered companies
and to benefit from the network of double tax treaties that reduce withholding taxes. TNI BioTech International, Ltd. will manage
our international distribution, using product that is manufactured in Ireland and elsewhere.
We
are focused on the development and commercialization of therapeutic treatments for cancer, HIV/AIDS and autoimmune diseases and
immune disorders by combating these severe and fatal diseases through the stimulation and/or regulation of the body’s immune
system. TNI’s growth strategy includes the near-term commercialization of its existing immunotherapies targeting cancer,
Crohn’s disease and/or HIV/AIDS.
Results
of Operations
Three
Months Ended June 30, 2016 Compared to Three Months Ended June 30, 2015 (all dollar amounts and numbers in $000s, except percentages
or where otherwise indicated)
Revenues
We
had revenues from operations of $0 for the three months ended June 30, 2016, compared to revenues of $5,648 for three months ended
June 30, 2015.
Operating
Expenses
Selling,
general and administrative
Selling,
general and administrative expenses and related percentages for the three months ended June 30, 2016 and 2015 were as follows
(dollar amounts in thousands):
|
|
|
For
the three months ended June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Selling, general
and administrative
|
|
$
|
894
|
|
|
$
|
444
|
|
|
Increase/(decrease) from
prior year
|
|
$
|
450
|
|
|
$
|
(1,397
|
)
|
|
Percent increase/(decrease)
from prior year
|
|
|
101
|
%
|
|
|
(76
|
%)
|
For
the three months ended June 30, 2016 and 2015, selling, general and administrative expenses were made up as follows (dollar amounts
in thousands):
|
|
|
For
the three months ended June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Consulting
and contractors
|
|
$
|
160
|
|
|
$
|
334
|
|
|
Payroll
|
|
|
372
|
|
|
|
(43
|
)
|
|
Professional fees
|
|
|
50
|
|
|
|
31
|
|
|
Travel
|
|
|
26
|
|
|
|
17
|
|
|
Other expenses
|
|
|
286
|
|
|
|
105
|
|
In
the three months ended June 30, 2016, total cash and cash accruals for selling, general and administrative expense was $827 compared
to $444 for the corresponding period in 2015, an increase of $383 or 86%. Significant cash items included:
|
|
●
|
consulting
and contactor services obtained to assist the Company in raising capital, manage investor relations, and develop business
in new markets, in the amount of $160 in 2016, a decrease of $174 or 52% over the $334 spent in 2015. The decrease was the
result primarily of the conversion of amounts owed from prior periods to equity, which resulted in a large credit to this
expense category;
|
|
|
●
|
professional
fees for legal, tax and accounting services in the amount of $50 in 2016, an increase of $19 or 61% over the $31 spent in
2015. The increase was the result of billing for audit and tax services in 2016 ($12) and higher legal fees related to debt
settlements ($7);
|
|
|
|
|
|
|
●
|
payroll
in the amount of $372 in 2016, an increase of $ 415 or 965% over the $(43) reported in 2015. The increase reflects the
fact that, in 2015, there was a $282 conversion of deferred payroll owed to a Company officer into an agreement to pay the
officer a royalty on future product sales; and
|
|
|
|
|
|
|
●
|
travel
in the amount of $26 in 2016, an increase of $9 or 49% over the $17 spent in 2015. 2016 travel was related mostly to investor
relations.
|
Research
and development
Research
and development
R&D
expenses and related percentages for the three months ended June 30, 2016 and 2015 were as follows (dollar amounts in thousands):
|
|
|
For
the three months
ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Research and development
|
|
$
|
67
|
|
|
$
|
414
|
|
|
Increase decrease from prior year
|
|
$
|
(347
|
)
|
|
$
|
145
|
|
|
Percent decrease from prior year
|
|
|
(84
|
%)
|
|
|
(26
|
%)
|
Expenses
for research and development in the three months ended June 30, 2016 decreased by 84% compared to expenses in the same period
in 2015. The decrease occurred mainly as a result a reduction in the cost trials in Nigeria, and legal fees paid to maintain patents
and licenses.
In
the three months ended June 30, 2016, total cash spent on R&D was $67, a decrease of $347 or 84% over the $414 spent in the
same period in 2015. Significant cash items in 2016 included:
|
●
|
payments for contracted technical
services, $7 in2016, a decrease of $46 or 87 % over the $53 spent in 2015, reflecting the decreased use of contractors to
perform research activities;
|
|
|
|
|
●
|
payments for professional fees $6
in 2016, a decrease of $4 or 40% over the $10 spent in 2015;
|
|
|
|
|
●
|
patent expenses of $45 in 2016, an
increase of $42 or 1,400% over the $3 spent in 2015, reflecting payments for certain rights to use LDN.
|
All
of the R&D spending in 2016 was on LDN. In 2015, 75% of R&D spending was on the development of LDN; the balance was spent
on MENK.
Stock
issued for services
The
Company periodically issues stock to consultants who provide services to the Company under consulting contracts. The Company accrues
a liability for these services calculated by the number of shares issued for the services multiplied by the price of the Company’s
stock on the effective date of the consulting contract. The accrued liability is amortized over the period in which the services
are provided to the Company. The Company reports these costs separately from Selling, general and administrative costs, and Research
and development costs, to better demonstrate the true costs of Selling, general and administrative activities, and Research and
development.
Amortization
of accrued liabilities for stock issued for services G&A and related percentages for the three months ended June 30, 2016
and 2015 were as follows (dollar amounts in thousands):
|
|
|
For
the three months
ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Stock for services and Prepaid
consulting services expense G&A
|
|
$
|
1,195
|
|
|
$
|
1,450
|
|
|
Percentage decrease from prior year
|
|
|
(18
|
%)
|
|
|
(37
|
%)
|
The
decline in expense reflects the decrease in the price of the Company’s stock year over year and the fact that the cost of
shares issued for services prior to 2015 had been fully amortized prior to the third quarter of 2014.
The
number of shares issued for prepaid consulting services G&A in the three months ending June 30, 2016 was 4,600,000 (966,668
in the corresponding period in 2015).
Prepaid
consulting services G&A in the three months ended June 30, 2016 consisted of the following:
|
Amortization
of cost of stock issued prior to 2016
|
|
$
|
155
|
|
|
Amortization
of cost of stock issued in the first quarter of 2016
|
|
|
715
|
|
|
Amortization
of cost incurred for new stock issued second quarter of 2016 under consulting contracts entered into in 2016
|
|
|
325
|
|
Amortization
of stock issued for services R&D and related percentages for the three months ended June 30, 2016 and 2015 were as follows
(dollar amounts in thousands):
|
|
|
For
the three months
ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Prepaid consulting services
R&D
|
|
$
|
0
|
|
|
$
|
0
|
|
|
Percentage increase/(decrease) from prior year
|
|
|
0
|
%
|
|
|
(100
|
)%
|
The
cost of shares issued for R&D services have been fully amortized prior to the third quarter of 2014.
There
were no new shares issued for prepaid consulting services R&D in the three months ending June 30, 2016 or 2015.
Warrant
valuation expense
When
the Company sells its stock to stockholders for cash, it periodically issues warrants to those stockholders to acquire additional
stock at prices agreed at the date of the original sale. The Company incurs a cost for the rights attached to the warrants, which
is calculated using the Black-Scholes Model (see above 4. Capital Structure—Common Stock and Common Stock Purchase Warrants.)
This expense is reported in the Condensed Consolidated Statements of Operations above as the Warrant valuation expense.
In
the three months ended June 30, 2016, the Company issued 8,054,908 warrants. In the three months ended June 30, 2015, the Company
issued no warrants to stockholders.
Depreciation
and amortization
The
Company amortizes the costs incurred to acquire patents and licenses over the period of the related agreements. The decrease year
over year in depreciation and amortization expense reflects the fact that, at the end of 2015, the Company determined that the
unamortized carrying amount recorded for the acquisition of licenses and patents related to LDN were impaired, and recorded an
impairment loss of $5,226,352. In 2014, the Company determined that the carrying amount recoded for the acquisition of licenses
and patents related to MENK were impaired, and recorded an impairment loss of $9,908,477.
Depreciation
and amortization expenses for the three months ended June 30, 2016 and 2015 were as follows (dollar amounts in thousands):
|
|
|
For
the three months
ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Depreciation expense
|
|
$
|
0
|
|
|
$
|
1
|
|
|
Amortization expense
|
|
$
|
0
|
|
|
$
|
148
|
|
|
Decrease from prior year
|
|
$
|
(149
|
)
|
|
$
|
(570
|
)
|
|
Percentage decrease from prior year
|
|
|
(100
|
%)
|
|
|
(79
|
)%
|
The
decrease reflects the fact that all patents were fully amortized by the end of 2015.
Interest
Expense
Interest
expense for the three months ended June 30, 2016 and 2015 were as follows (dollar amounts in thousands):
|
|
|
For
the three months
ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Interest expense
|
|
$
|
1,052
|
|
|
$
|
51
|
|
|
Increase/(decrease) from prior year
|
|
$
|
1,001
|
|
|
$
|
(70
|
)
|
|
Percentage increase/(decrease) from prior year
|
|
|
1,963
|
%
|
|
|
(58
|
%)
|
The
increase reflects the higher levels of interest-bearing debt in the second quarter of 2016. Interest expense for the quarter ended
June 30, 2016 included $430 of penalties for late interest and principal payments ($0 for the quarter ended June 30, 2015).
Loss
on settlement of debt (dollar amounts in thousands)
In
three months ended June 30, 2016, certain lenders to the Company settled all or a portion of their notes or accounts payable by
converting them to equity. The Company recorded an expense of $340, reflecting the fair value of the shares of common stock issued
in exchange for the debt. In three months ended June 30, 2015, the Company recorded an expense of $88 to settle all or a portion
of notes or accounts payable.
Six
Months Ended June 30, 2016 Compared to Six Months Ended June 30, 2015 (all dollar amounts and numbers in $000s, except percentages
or where otherwise indicated)
Revenues
We
had revenues from operations of $3,463 for the six months ended June 30, 2016, compared to $7,718 for the six months ended June
30, 2015.
Operating
Expenses
Selling,
general and administrative
Selling,
general and administrative expenses and related percentages for the six months ended June 30, 2016 and 2015 were as follows (dollar
amounts in thousands):
|
|
|
For
the six months
ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Selling, general and administrative
|
|
$
|
1,833
|
|
|
$
|
846
|
|
|
Increase/(decrease) from prior year
|
|
$
|
987
|
|
|
$
|
(1,511
|
)
|
|
Percent increase/(decrease) from prior year
|
|
|
117
|
%
|
|
|
(64
|
%)
|
For
the six months ended June 30, 2016 and 2015, selling, general and administrative expenses were made up as follows (dollar amounts
in thousands):
|
|
|
For
the six months
ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
|
|
|
|
|
|
|
|
Stock listing and investor
relations expenses
|
|
$
|
155
|
|
|
$
|
38
|
|
|
Consulting and contractors
|
|
|
484
|
|
|
|
356
|
|
|
Payroll
|
|
|
767
|
|
|
|
179
|
|
|
Professional fees
|
|
|
136
|
|
|
|
104
|
|
|
Travel
|
|
|
45
|
|
|
|
121
|
|
|
Insurance
|
|
|
-
|
|
|
|
(126
|
)
|
|
Other expenses
|
|
|
246
|
|
|
|
174
|
|
In
the six months ended June 30, 2016, total cash and cash accruals for selling, general and administrative expense was $1,833 compared
to $846 for the corresponding period in 2015, an increase of $987 or 117%. Significant cash items included:
|
●
|
consulting and contractor
services obtained to assist the Company in raising capital, manage investor relations, and develop business in new markets,
in the amount of $484 in 2016, an increase of $ 128 or 36 % over the $356 spent in 2015. The increase was the result of the
conversion to equity of amounts owed to contractors from prior periods, and fees paid to advisors who are assisting the Company
with manufacturing and marketing activities in the United States and Africa;
|
|
|
|
|
●
|
professional fees
for legal, tax and accounting services in the amount of $136 in 2016, an increase of $32 or 31% over the $104 spent in 2015.
The increase reflects the higher spending on audit and tax fees and legal fees related to debt negotiations;
|
|
|
|
|
●
|
payroll in the amount
of $767 in 2016, an increase of $588 or 328% over the $179 spent in 2015. The increase is attributable to a $153 accrual for
future payment of payroll taxes, an increase in headcount, contractually-mandated payroll increases for officers, and the
conversion in 2015 of $282 of deferred payroll owed to a Company officer into an agreement to pay the officer a royalty on
future product sales; and
|
|
|
|
|
●
|
travel in the amount
of $45 in 2016, a decrease of $76 or 63% over the $121 spent in 2015, reflecting lower travel to Africa in 2016.
|
Research
and development
R&D
expenses and related percentages for the six months ended June 30, 2016 and 2015 were as follows (dollar amounts in thousands):
|
|
|
For
the six months
ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Research and development
|
|
$
|
48
|
|
|
$
|
592
|
|
|
Increase decrease from prior year
|
|
$
|
(544
|
)
|
|
$
|
(479
|
)
|
|
Percent decrease from prior year
|
|
|
(92
|
%)
|
|
|
(45
|
%)
|
Expenses
for research and development in the six months ended June 30, 2016 decreased by 92% compared to expenses in the same period in
2015. The decrease occurred mainly as a result a reduction in expenses recorded to settle payables related to amounts owing for
R&D contracted technical services in 2016, and the fact that in 2015 a charge totaling $339 had been recorded for Nigeria
trial expenses settled with Company stock.
In
the six months ended June 30, 2016, total spending on R&D was $48, a decrease of $544 or 92% over the $592 spent in the same
period in 2015. Significant cash items included:
|
●
|
expenses for contracted
technical services, $(48) in 2016, a decrease of $187 or 135% over the $139 spent in 2015, reflecting the settlement of payables
related to amounts owing for R&D contracted technical services;
|
|
|
|
|
●
|
payments for contracted
services of $37 in 2016, an increase of $37 or 100% over the $0 spent in 2015, related to meetings with the FDA for resumption
of trials by Cytocom;
|
|
|
|
|
●
|
expenses recorded
for future license payments totaling $101, an increase of $26 or 35% over the $75 recorded in 2015, reflecting timing of accruals
under license agreements.
|
|
|
|
|
●
|
rent expense of
$(84) in 2016, a decrease of 99 or 660% over the $15 spent in 2015, reflecting (i) the closure of all R&D offices and
(ii) the settlement of a rent dispute with a landlord in Maryland;
|
|
|
|
|
●
|
payments for professional
fees of $27 in 2016, an increase of $11 or 67% over the $16 spent in 2015, reflecting the higher fees incurred to protect
patent and intellectual property rights worldwide in 2016;
|
|
|
|
|
●
|
payroll of $(5)in
2016, a decrease of $10 or 200% over the $5 spent in 2015, reflecting the settlement in 2016 of claims for payroll taxes;
|
|
|
|
|
●
|
Spending on travel
of $10 in 2016, an increase of $10 or 100% over the $0 spent in 2015, to oversee trials in Africa in 2016; and
|
|
|
|
|
●
|
Spending on trials
of $10 in 2016, a decrease of $329 or 97% over the $339 spent in 2015, reflecting the fact that the Nigerian trial activity
had mostly concluded in 2015.
|
All
of the R&D spending in 2016 was for the development of LDN, compared to 75% of spending on LDN in 2015.
Stock
issued for services
The
Company periodically issues stock to consultants who provide services to the Company under consulting contracts. The Company accrues
a liability for these services calculated by the number of shares issued for the services multiplied by the price of the Company’s
stock on the effective date of the consulting contract. The accrued liability is amortized over the period in which the services
are provided to the Company. The Company reports these costs separately from Selling, general and administrative costs, and Research
and development costs, to better demonstrate the true costs of Selling, general and administrative activities, and Research and
development.
Amortization
of accrued liabilities for stock issued for services G&A and related percentages for the six months ended June 30, 2016 and
2015 were as follows (dollar amounts in thousands):
|
|
|
For
the six months
ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Stock for services and Prepaid
consulting services expense G&A
|
|
$
|
3,179
|
|
|
$
|
3,612
|
|
|
Percentage decrease from prior year
|
|
|
(12
|
%)
|
|
|
(70
|
%)
|
The
decline in expense reflects the decrease in the price of the Company’s stock year over year.
The
number of shares issued for prepaid consulting services G&A in the six months ending June 30, 2016 was 23,643,000 (10,239,170
in the corresponding period in 2015).
Prepaid
consulting services G&A in the six months ended June 30, 2016 consisted of the following:
|
|
|
Amortization of cost of stock
issued prior to 2016
|
|
$
|
539
|
|
|
|
|
|
|
|
|
Amortization of cost incurred for new stock
issued in the six months ended June 30, 2016 under consulting contracts entered into in 2016
|
|
|
2,640
|
|
Amortization
of stock issued for services R&D and related percentages for the six months ended June 30, 2016 and 2015 were as follows (dollar
amounts in thousands):
|
|
|
For
the six months
ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Prepaid consulting services
R&D
|
|
$
|
0
|
|
|
$
|
0
|
|
|
Percentage increase/(decrease) from prior year
|
|
|
0
|
%
|
|
|
(100
|
%)
|
The
cost of all shares issued for services had been fully amortized prior to the third quarter of 2014.
There
were no new shares issued for prepaid consulting services R&D in the six months ending June 30, 2016 or 2015.
Warrant
valuation expense (dollar amounts in thousands)
When
the Company sells its stock to stockholders for cash, it periodically issues warrants to those stockholders to acquire additional
stock at prices agreed at the date of the original sale. The Company incurs a cost for the rights attached to the warrants, which
is calculated using the Black-Scholes Model (see above 4. Capital Structure—Common Stock and Common Stock Purchase Warrants.)
This expense is reported in the Condensed Consolidated Statements of Operations above as the Warrant valuation expense.
In
the six months ended June 30, 2016, the Company issued 23,654,908 warrants to stockholders. In the six months ended June 30, 2015,
the Company issued no warrants to stockholders.
|
|
|
For
the six months
ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Warrant valuation expense
|
|
$
|
2,569
|
|
|
$
|
0
|
|
|
Percentage increase/(decrease) from prior year
|
|
|
100
|
%
|
|
|
0
|
%
|
Depreciation
and amortization
The
Company amortizes the costs incurred to acquire patents and licenses over the period of the related agreements.
Depreciation
and amortization expenses for the six months ended June 30, 2016 and 2015 were as follows (dollar amounts in thousands):
|
|
|
For
the six months
ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Depreciation expense
|
|
$
|
1
|
|
|
$
|
1
|
|
|
Amortization expense
|
|
$
|
0
|
|
|
$
|
296
|
|
|
Increase/ (decrease) from prior year
|
|
$
|
(296
|
)
|
|
$
|
(1,142
|
)
|
|
Percentage increase/(decrease) from prior year
|
|
|
(100
|
%)
|
|
|
(79
|
%)
|
The
decrease year over year in depreciation and amortization expense reflects the fact that all of the Company’s patents and
licenses had been fully amortized by December 31, 2015. At the end of 2015, the Company determined that the unamortized carrying
amount recorded for the acquisition of licenses and patents related to LDN were impaired, and recorded an impairment loss of $5,226,352.
In 2014, the Company determined that the carrying amount recoded for the acquisition of licenses and patents related to MENK were
impaired, and recorded an impairment loss of $9,908,477.
Interest
Expense
Interest
expense for the six months ended June 30, 2016 and 2015 was as follows (dollar amounts in thousands):
|
|
|
For
the six months
ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Interest
expense
|
|
$
|
1,353
|
|
|
$
|
69
|
|
|
Increase from prior
year
|
|
$
|
1,284
|
|
|
$
|
(213
|
)
|
|
Percentage decrease
from prior year
|
|
|
1,861
|
%
|
|
|
(76
|
%)
|
The
increase reflects the increased levels of interest-bearing debt in the first half of 2016. Interest expense for the six months
ended June 30, 2016 included $562 of penalties for late interest and principal payments ($0 for the six months ended June 30,
2015).
Loss
on settlement of debt (dollar amounts in thousands)
In
six months ended June 30, 2016, certain lenders to the Company settled all or a portion of their notes or accounts payable by
converting them to equity. The Company recorded an expense of $1,705, reflecting the fair value of the shares of common stock
issued in exchange for the debt. In six months ended June 30, 2015, the Company recorded an expense of $88 to settle all or a
portion of notes or accounts payable.
Year
Ended December 31, 2015 Compared to Year Ended December 31, 2014
The
table below and the discussion that follows summarizes our results of operations and certain selected operating statistics for
the last two fiscal years ended December 31, 2015, and 2014.
|
|
|
2015
|
|
|
2014
|
|
|
|
|
|
|
|
|
|
|
Revenues,
net
|
|
$
|
16,197
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
Selling,
general and administrative
|
|
|
2,734,414
|
|
|
|
4,072,277
|
|
|
Research
and development expense
|
|
|
977,203
|
|
|
|
2,413,286
|
|
|
Stock
issued for services general and administrative
|
|
|
6,240,143
|
|
|
|
19,116,075
|
|
|
Stock
issued for services research and development
|
|
|
-
|
|
|
|
5,126,250
|
|
|
Warrant
valuation
|
|
|
46,189
|
|
|
|
1,748,576
|
|
|
Depreciation
and amortization expense
|
|
|
594,785
|
|
|
|
2,879,311
|
|
|
Impairment
of intangible assets
|
|
|
5,226,352
|
|
|
|
9,908,477
|
|
|
Total
operating expenses
|
|
|
15,819,086
|
|
|
|
45,264,252
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss
from operations
|
|
|
(15,802,889
|
)
|
|
|
(45,264,252
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Other expense:
|
|
|
|
|
|
|
|
|
|
Interest
expense
|
|
|
(270,989
|
)
|
|
|
(388,221
|
)
|
|
Impairment of investment
|
|
|
(32,000
|
)
|
|
|
-
|
|
|
Foreign
exchange loss
|
|
|
-
|
|
|
|
(783
|
)
|
|
Loss
on settlement of debt
|
|
|
(843,573
|
)
|
|
|
(4,284,957
|
)
|
|
Total
other expense
|
|
|
(1,146,562
|
)
|
|
|
(4,673,961
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss
|
|
|
(16,949,451
|
)
|
|
|
(49,938,213
|
)
|
|
Net
loss attributable to non-controlling interest
|
|
|
(2,706,939
|
)
|
|
|
(1,684,996
|
)
|
|
Net loss attributable
to common shareholders
|
|
$
|
(14,242,512
|
)
|
|
$
|
(48,253,217
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Basic
and diluted loss per share attributed to common shareholders
|
|
$
|
(0.09
|
)
|
|
$
|
(0.49
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average
number of shares outstanding
|
|
|
153,247,023
|
|
|
|
98,508,458
|
|
Revenues
We
had revenues of $16,197 from operations for the year ended December 31, 2015. All revenues were earned under the agreement signed
on December 8, 2014 with KRS Global Biotechnology, Inc. We had no revenues from operations for year ended December 31, 2014.
Operating
Expenses
Selling,
general and administrative
Selling,
general and administrative expenses and related percentages for the years ended December 31, 2015 and 2014 were as follows (dollar
amounts in thousands):
|
|
|
2015
|
|
|
2014
|
|
|
Selling, general
and administrative
|
|
$
|
2,734
|
|
|
$
|
4,072
|
|
|
Increase / (decrease)
from prior year
|
|
$
|
(1,338
|
)
|
|
$
|
(1,265
|
)
|
|
Percent increase / (decrease)
from prior year
|
|
|
(33
|
)%
|
|
|
(23
|
)%
|
For
the years ended December 31, 2015 and 2014, selling, general and administrative expenses were made up as follows (dollar amounts
in thousands):
|
|
|
2015
|
|
|
2014
|
|
|
Stock listing
and investor relations expenses
|
|
$
|
44
|
|
|
$
|
472
|
|
|
Consulting and contractors
|
|
|
1,033
|
|
|
|
1,423
|
|
|
Payroll
|
|
|
833
|
|
|
|
1,103
|
|
|
Professional fees
|
|
|
265
|
|
|
|
818
|
|
|
Travel
|
|
|
174
|
|
|
|
244
|
|
|
Insurance
|
|
|
(107
|
)
|
|
|
239
|
|
|
Charitable donations
|
|
|
-
|
|
|
|
(750
|
)
|
|
Other
expenses
|
|
|
492
|
|
|
|
523
|
|
|
|
|
$
|
2,734
|
|
|
$
|
4,072
|
|
The
decrease year over year in selling, general and administrative expense was attributable primarily to decreased sales and marketing
activities to promote the manufacture and sale of the Company’s products in South America and Africa, the return of stock
previously issued for charitable donations, and the departure of in-house counsel, which reduced the Company’s payments
of professional fees.
In
2015, total cash spent on in selling, general and administrative expense was $2,734, compared to $4,822 for 2014, a decrease of
$2,088 or 43%. Significant cash items included:
|
|
●
|
consulting
services obtained to assist the Company in raising capital, manage investor relations, and develop business in new markets,
in the amount of $1,033 in 2015, a decrease of $390 or 27% of the $1,423 spent in 2014;
|
|
|
|
|
|
|
●
|
professional
fees for legal, tax and accounting services in the amount of $265 in 2015, a decrease of $553, or 68% over the $818 spent
in 2014;
|
|
|
|
|
|
|
●
|
payroll
in the amount of $833 in 2015, a decrease of $270 or 24% over the $1,103 spent in 2014; and
|
|
|
●
|
travel
in the amount of $174 in 2015, a decrease of $70 or 29% of the $244 spent in 2014.
|
Research
and development
R&D
expenses and related percentages for the years ended December 31, 2015 and 2014 were as follows (dollar amounts in thousands):
|
|
|
2015
|
|
|
2014
|
|
|
Research and development
|
|
$
|
977
|
|
|
$
|
2,413
|
|
|
Increase/ (decrease) from prior year
|
|
$
|
(1,436
|
)
|
|
$
|
(48
|
)
|
|
Percent increase/ (decrease) from prior
year
|
|
|
(60
|
)%
|
|
|
(2
|
)%
|
R&D
is overseen and managed internally, working with individuals, universities, and CROs in order to utilize patents that we have
licensed or acquired since our inception. We continue to seek to expand our pipeline of patents by reviewing other compounds,
technologies or capabilities. We also seek out promising compounds and innovative technologies developed by third parties to incorporate
into our discovery and development processes or projects.
Drug
discovery and development is time-consuming, expensive and unpredictable. According to PhRMA, out of 5,000-10,000 screened compounds,
only 250 enter preclinical testing, five enter human clinical trials and one is approved by the FDA. The process from early discovery
or design to development to regulatory approval can take more than 10 years. Drug candidates can fail at any stage of the process,
and candidates may not receive regulatory approval even after many years of research.
As
of December 31, 2015, we had two compounds (IRT-101 and IRT-103) in research and development in oncology and Crohn’s disease,
both of which are expected to move into Phase 3 clinical trials in 2016.
For
the years ended December 31, 2015 and 2014, research and development expenses were made up as follows (dollar amounts in thousands):
|
|
|
2015
|
|
|
2014
|
|
|
Contracted services
|
|
$
|
205
|
|
|
$
|
641
|
|
|
Patent expenses
|
|
|
156
|
|
|
|
466
|
|
|
Payroll
|
|
|
5
|
|
|
|
284
|
|
|
Trials
|
|
|
541
|
|
|
|
274
|
|
|
Professional fees
|
|
|
32
|
|
|
|
191
|
|
|
Supplies and materials
|
|
|
14
|
|
|
|
181
|
|
|
Rent
|
|
|
22
|
|
|
|
330
|
|
|
Travel
|
|
|
-
|
|
|
|
25
|
|
|
Conferences
|
|
|
2
|
|
|
|
21
|
|
|
Other
|
|
|
0
|
|
|
|
0
|
|
|
|
|
$
|
977
|
|
|
$
|
2,413
|
|
Most
of the R&D spending in both 2015 and 2014 was on the development of LDN.
Expenses
for research and development in 2015 decreased by 60% compared to expenses in the same period in 2014. The decrease occurred mainly
as a result of lower use of contractors for R&D services, lower payroll costs, reduced use of contractors and professional
services, the closure of our R&D office in Maryland, all as a result of lower funding available to conduct research and development.
These decreases were offset by increases in costs for trials to conduct R&D in Africa.
In
2015, total cash spent on R&D was $977, a decrease of $1,157 or 54% over the $2,134 spent in 2014. Significant cash items
included:
|
|
●
|
payments
for contracted services ($205 in 2015, a decrease of $436 or 68% over the $641 spent in 2014), reflecting the decreased use
of contractors to perform some of our research activities;
|
|
|
●
|
patent
expenses ($156 in 2015, compared to $466 for 2014, a decrease of $310 or 67% over the $466 spent in 2014), reflecting decreased
costs incurred in 2015 to maintain licenses and patents acquired in 2014 and 2015;
|
|
|
|
|
|
|
●
|
legal fees
($32 in 2015, compared to $191 in 2014), incurred for the support and acquisition of patents and licenses;
|
|
|
|
|
|
|
●
|
rent ($22 in
2015, compared to $51 in 2014), for premises in Maryland. (An additional non-cash expense of $279 was charged to rent for
a pending rent dispute in 2014. The corresponding amount in 2015 was $0);
|
|
|
|
|
|
|
●
|
payroll ($5
in 2015, a decrease $279, or 98% over the $284 spent in 2014), reflecting the reduction in the number of R&D employees
in 2015 due to lack of funding; and
|
|
|
|
|
|
|
●
|
travel ($0
in 2015, a decrease of $25 or 100% over the $25 in 2014), also reduced due to lack of funding.
|
Liquidity
Liquidity
is measured by the Company’s ability to secure enough cash to meet its contractual and operating needs as they arise. The
Company had cash of $64,289 at June 30, 2016, compared to $23,149 at December 31, 2015. For the six months ended June 30, 2016
and 2015, net cash used in operating activities was $1,399,729 and $1,265,077, respectively. $0 cash was used in investing activities
for the six months ended June 30, 2016 ($0 was used in 2015).
In
May 2016, the Company announced that it had received approval for sale of Lodonal
TM
in Nigeria. The Company expects
to commence sales to Nigeria by the end of 2016. The Company believes that those sales will generate sufficient cash flows for
the next 12 months to pay for operating expenses and to pay off current and past-due obligations. Until such sales commence, the
Company expects to fund operations through sales of equity and notes payable, and conversions of exiting obligations into equity.
The Company believes it will require between $300,000 and $350,000 monthly to meet its ongoing expenses and obligations.
If
the Company is unable to generate sufficient cash flows from sales, or if it does not raise additional working capital to meet
all of its operating obligations and expenditures, the Company may have to modify its business plan.
In
addition to the cost of its ongoing operations, the Company expects it will incur future research and development expenditures
in the next 12 months through Cytocom. Cytocom plans to conduct Phase II and Phase IIB trials for the treatment of Crohn’s
disease, at an estimated cost of $3,900,000 and $7,500,000 respectively for each phase. If the trials do not commence before 2017,
the Company will be required to make a payment of $100,000 in December 2016 under its license agreements. In prior years, the
Company has been able to raise funds through sales of notes payable, and it expects to do the same for the payment due in 2016.
During
the six months ended June 30, 2016 proceeds from the sale of stock and exercise of stock warrants totaled $50,000, compared to
$152,000 for the corresponding period in 2015. We also received $1,390,868 from the issuance of notes payable in six months ended
June 30, 2016, compared to $943,475 in 2015. There were $0 loan repayments made in cash in the six months ended June 30, 2016
($0 in 2015).
Our
ability to continue as a going concern is dependent entirely on raising funds through the sale of equity or debt. We anticipate
that we will continue our attempt to raise capital through private equity and debt transactions, develop a credit facility with
a lender, or the exercise of options and warrants; however, such additional capital may not be available to us at acceptable terms
or available at all. In the event that we are unable to obtain additional capital, we would be forced to cease operations altogether.
Off-Balance
Sheet Arrangements
During
the three months ended June 30, 2016 and 2015, we did not engage in any off balance sheet arrangements as defined in item 303(a)(4)
of the SEC’s Regulation S-K.
Critical
Accounting Policies
The
preparation of financial statements and related disclosures in conformity with U.S. generally accepted accounting principles (“GAAP”)
and the Company’s discussion and analysis of its financial condition and operating results require the Company’s management
to make judgments, assumptions, and estimates that affect the amounts reported in its consolidated financial statements and accompanying
notes. Note 1, “Summary of Significant Accounting Policies” of the Notes to Consolidated Financial Statements in Part
II, Item 8 of the Form 10-K/A describes the significant accounting policies and methods used in the preparation of the Company’s
consolidated financial statements. Management bases its estimates on historical experience and on various other assumptions it
believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying
values of assets and liabilities.
We
have identified the policies below as critical to our business operations and the understanding of its results of operations.
The Company’s senior management has reviewed these critical accounting policies and related disclosures with the Company’s
Board of Directors. The impact and any associated risks related to these policies on our business operations are discussed throughout
this section where such policies affect our reported and expected financial results. Our preparation of financial statements requires
us to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets
and liabilities at the date of our financial statements, and the reported amounts of revenues and expenses during the reporting
period. There can be no assurance that actual results will not differ from those estimates and such differences may be material.
Cash
and Cash Equivalents
The
Company considers all highly liquid investments with original maturities at the date of purchase of three months or less to be
cash equivalents. Cash and cash equivalents include bank demand deposits, marketable securities with maturities of three months
or less at purchase, and money market funds that invest primarily in certificates of deposits, commercial paper and U.S. government
and U.S. government agency obligations. Cash equivalents are reported at fair value.
Net
Loss Per Share of Common Stock
Basic
net loss per share is calculated by dividing the net loss attributable to common stockholders by the weighted average number of
common shares outstanding for the period, without consideration for common stock equivalents. Diluted net loss per share is calculated
by dividing the net loss by the weighted-average number of common share equivalents outstanding for the period determined using
the treasury-stock method and the if-converted method. Dilutive common stock equivalents are comprised of common stock purchase
warrants and options outstanding. For all periods presented, there is no difference in the number of shares used to calculate
basic and diluted shares outstanding due to the Company’s net loss position.
Income
Taxes
The
Company follows FASB ASC Topic 740,
“Income Taxes,”
which requires recognition of deferred tax assets and liabilities
for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this
method, deferred tax assets and liabilities are based on the differences between the financial statements and tax bases of assets
and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Deferred tax
assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the asset will
not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income
in the years in which those temporary differences are expected to be recovered or settled.
The
standard addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded
in the financial statements. Under ASC Topic 740, the Company may recognize the tax benefit from an uncertain tax position only
if it is more likely than not that the tax position will be sustained on examination by the tax authorities, based on the technical
merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based
on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate settlement. ASC Topic
740 also provides guidance on de-recognition, classification, interest and penalties on income taxes, accounting in interim periods
and requires increased disclosures. At the date of adoption, and as of December 31, 2015 and June 30, 2016, the Company does not
have a liability for unrecognized tax uncertainties.
The
Company’s policy is to record interest and penalties on uncertain tax positions as income tax expense. At the end of the
quarters ended June 30, 2016 and June 30, 2015, the Company had not accrued any interest or penalties related to uncertain tax
positions.
CHANGES
IN AND DISAGREEMENTS WITH ACCOUNTANTS
N/A
DIRECTORS,
EXECUTIVE OFFICERS, PROMOTERS, AND CONTROL PERSONS
Directors
As
of August 23, 2016, the number of voting members of our Board of Directors was five. Our Non-Executive Chairman of the Board is
a non-voting member of our Board. The members of our Board of Directors as of March 31, 2016 are as follows:
|
Name
|
|
Age
|
|
Director
Since
|
|
Position
|
|
Noreen
Griffin
|
|
63
|
|
March
2012
|
|
Chief
Executive Officer and Director
|
|
Dr.
Nicholas Plotnikoff
|
|
88
|
|
March
2012
|
|
Non-Executive
Chairman of the Board (non-voting director)
|
|
Christopher
Pearce
|
|
72
|
|
March
2012
|
|
Director,
Chief Operating Officer
|
|
Edward
Teraskiewicz
|
|
69
|
|
January
2015
|
|
Director
|
|
Dr.
Clifford Selsky
|
|
67
|
|
February
2016
|
|
Director
|
|
Paul
Akin
|
|
57
|
|
February
2016
|
|
Director
|
The
biographies of each director below contains information regarding the person’s service as a director, business experience,
director positions held currently or at any time during the last five years, and information regarding involvement in certain
legal or administrative proceedings, if applicable. Dr. Nicholas Plotnikoff, Edward Teraskiewicz, Dr. Clifford Selsky and Paul
Akin are all independent directors and do not hold any other positions with the Company.
Noreen
Griffin –
Ms. Griffin is one of our Founders and has served as our Chief Executive Officer and as a member of our Board
of Directors since March 2012. Ms. Griffin was a vital part of the acquisition of our patents and therapies involving MENK and
LDN. Ms Griffin knew Dr. Plotnikoff and had consulted with him in the past and knew the work of Dr. Bihari prior to forming the
Company. Ms. Griffin took over TNI Parmaceuticals, Inc. in November 2011 as the sole officer and director and filed for Chapter
11 bankruptcy on November 30, 2011 Case No. 11-13798 in the Delaware Bankruptcy Court. The case was converted to a Chapter 7 and
was subsequently discharged and closed on August 27, 2014.
Ms.
Griffin has handled the negotiations involving all of the intellectual property acquired by the Company. Ms. Griffin has been
working with Professor Shan and has been published a number of times over the last four years. In addition Ms. Griffin has been
involved with Professor Shan and Dr. Nicholas Plotnikoff in the filing of a number of new patents involving immediate release
naltrexone and menk. The patents have been assigned over to the Company. Prior to joining the Company, Ms. Griffin was the sole
officer and director of Supertrail Manufacturing Co., Inc., a private company in Aberdeen, Mississippi from 1998 to 2013. Ms.
Griffin was Chief Financial Officer of Environmental Remediation Holdings, Inc., a public reporting company in Lafayette, Louisiana
from 1997 to 2014. From 2004 to 2009, she was an advisor to Global Environmental Energy Corp, a public company, and assumed the
role of sole officer for the purpose of the company’s bankruptcy. From 2007 to 2013, Ms. Griffin was Chief Executive Officer
of pH Solutions, a private company in Boston, Massachusetts. Since 2008, Ms. Griffin has been a partner of Griffin Enterprises
Group. The firm provides chief financial officer services to its clients on a part- time basis. In that capacity, Ms. Griffin
acted as sole officer and director for the bankruptcy of James M. Jost and Company Inc. and Avalon from 2010 through 2013. Ms.
Griffin has over 25 years of industry experience, having founded and led a number of startup companies. She has played an integral
role in raising multiple rounds of private and venture capital funds on behalf of clients. Ms. Griffin has served as Chief Financial
Officer and Vice President of a number of small public companies over the last 10 years. In addition, Ms. Griffin has significant
experience in the administration of companies in bankruptcies.
Dr.
Nicholas Plotnikoff
– Dr. Plotnikoff has served as our non-voting, Non-Executive Chairman of the Board since March 2012.
Dr. Plotnikoff has a Ph.D. in Pharmacology and over 20 years’ experience in the pharmaceutical industry working in Pharmacology,
Toxicology, and Clinical Research. Prior to joining our Board, Dr. Plotnikoff was a Professor of Pharmacology at the University
of Illinois Medical Center in Chicago from December 1987 until his retirement in May 2008. Dr. Plotnikoff formed TNI Pharmaceutical,
Inc. in 1987, which became a public company in 1991. Dr. Plotnikoff served as its Chief Executive Officer and President until
November of 2011 when he and all of the remaining officers and directors
resigned.
During his 20 years with TNI Pharmaceutical, Inc. Mr. Plotnikoff helped develop the immunological effects of MENK. He successfully
managed the project teams that developed the new drug applications for Traxene (a Valium-like tranquilizer) and Cylert (a non-amphetamine
psychostimulant). Dr. Plotnikoff was responsible for the Phase I and Phase II trials for MENK in HIV/AIDS. Dr. In basic research,
he was the first to identify the central nervous system effects of hypothalamic releasing factors (brain hormones), resulting
in clinical development for treatment of depression and Parkinson’s disease. Dr. Plotnikoff has co-authored 25 publications
with Dr. Andrew Schally, covering basic research in the field of depression and Parkinson’s disease. Dr. Plotnikoff was
issued a number of international patents for the use of MENK in HIV/AIDS and cancer, which we acquired in 2012.
Dr.
Plotnikoff was the President and CEO of TNI Pharmaceuticals, Inc., until November of 2011 when he and all of the remaining officers
and directors resigned and Ms. Griffin took over as sole officer and director and filed for Chapter 11 bankruptcy on November
30, 2011. Case No. 11-13798 was filed in Delaware Bankruptcy Court and the case was converted to a Chapter 7, 2011 and terminated
on August 27, 2014.
Christopher
Pearce
– Mr. Pearce has served as a member of our Board of Directors since March 2012. Mr. Pearce served as our Chief
Financial Officer from March 2012 until January 2013 when Peter Aronstam, our current Chief Financial Officer, was appointed.
Mr. Pearce also served as our Chief Operating Officer from January 2013 until January 2015 when our current President and Chief
Operating Officer, Seth Elliott, was appointed. Mr. Pearce was born in the United Kingdom and educated at Lord Wandsworth College
and London University where he received his LLB (Bachelor of Law). He agreed to accept a position with us in late 2011 due to
his 10-year history and involvement with Dr. Plotnikoff. Mr. Pearce is one of the founding principals of pH Pharmaceutical, Inc.,
a pharmaceutical company focused on the commercial application and licensing of a patented technology, and its affiliated company,
pH Solutions, since its inception in December 2007. Mr. Pearce has had an association with independent filmmakers for over 30
years. He was head of Cannon Group production and served as Chairman and Chief Executive Officer from 1991 to 1994. Over the years
as director, chief operating officer and chief executive officer at Cannon Group, Mr. Pearce managed more than 5,000 employees
worldwide and $500,000,000 in revenue per annum. His responsibilities included running large operations that required sourcing
products and services, contract negotiation, marketing and advertising, scheduling, warehousing and inventory management and employee
oversight. When he left Cannon Group in 1994, he began working for Global Pictures, Inc. Mr. Pearce retired from filmmaking in
2000 and has since worked as an advisor on numerous projects.
Edward
Teraskiewicz
– Mr. Teraskiewicz has served on our Board since January 2015. He has over 35 years of financial services
experience. From 1992 to 2004, Mr. Teraskiewicz was 50% owner Co-Founder, Chief Executive Officer and Director of Prebon Yamane
International Limited, one of the world’s pre-eminent money brokerage firms, with over 1700 employees in 17 cities around
the world with revenue over $500,000,000 annually. Having retired from daily operations at the end of 1994, he continued his involvement
with Prebon Yamane as a member of the Board of Directors of Fulton Prebon Group U.K., the holding company which owns the money
brokerage business, until October 2004, at which time he resigned from the Board upon a merger with Collins, Steward, Tullett.
Since 2004, Mr. Teraskiewicz has been overseeing and managing various residential real estate development projects and has been
an investor in numerous other projects in the music and financial industry. Mr. Teraskiewicz is a graduate of the American Institute
of Banking, having commenced his career as a trainee at Citibank in 1964. In 1970, he joined Mabon, Nugent & Co., a New York
Stock Exchange member firm, where he advanced to the rank of Senior General Partner. Mr. Teraskiewicz is an experienced investor,
having developed several residential sub-divisions and luxury estate home projects in the United States, as well as pursuing other
transactions around the world.
Dr.
Clifford Selsky
— Dr. Selsky has served on our Board since February 2016. He has been a practicing pediatrician in Central
Florida for the past twenty years. He is the founder of the Children’s Center for Cancer and Blood Disease at Florida Hospital
cancer institute, which he established after training in pediatrics at Yale New Haven hospital and completing a pediatric hematology
and oncology fellowship at Yale University School of Medicine. Dr. Selsky is board certified in Pediatrics, Pediatric hematology
and oncology and Palliative medicine. Currently, he is a pediatrician at Family First Pediatrics which he established in 2013.
Also
an accomplished scientist, Dr. Selsky obtained his PH.D. in Microbiology and Molecular Genetics at the University of Miami School
of Medicine. He then did DNA repair research studies at the radiobiology laboratory at Harvard School of Public Health and the
biophysics laboratory at Stanford University. Dr. Selsky has numerous publications in peer reviewed journals relating to DNA repair
and clinical conditions such as angiocentric lymphoma and chemotherapy related neurological disorders. As a toxicologist for Stauffer
Chemical Company, he designed and implemented research on molecular dosimetry and genetic risk estimation, including DNA adduct
separation and quantitation.
Over
the course of his career, Dr. Selsky has served as principal investigator for both the Pediatric Oncology Group at Florida Hospital
Cancer Institute and the Children’s Oncology group at Florida Hospital overseeing more than 140 cooperative group protocols.
He was department chair for Pediatrics at Florida Hospital for Children for seven years. Additionally, he has
served
on numerous committees including Florida Hospital Cancer Center Medical Advisory committee, Florida Hospital Ethics committee,
Florida Hospital Quality Assurance committee and Florida Hospital Pharmacy and Therapeutics committee. Dr. Selsky was elected
president of the Orange County Medical Society in 2016 and has received numerous awards including the Florida Hospital Medical
Staff recognition Award for Excellence 2008 and being named Top Doctor, Orlando Magazine in 2001, 2005, 2006, 2007, 2008, 2009,
2010, 2011 and 2015.
Paul
Akin
— Mr. Akin has been a member of our Board since February 2016. Mr. Akin is a significant investor and active strategist
for Immune Therapeutics. As an early investor, he was instrumental in the strategic planning and coordination of market penetration,
expansion and capitalization. In 2015, he drove the research, development and subsequent creation of LDN Information Management,
Inc. (“LDNIM”), whose goal is twofold: to educate the general population on the benefits of LDN, and to distribute
LDN domestically through partnership with the Company. LDNIM also serves as an adjunct marketing team with the goal of accelerating
consumer and physician education and distributing LDN in certain states in the USA over the next 12 months.
Mr.
Akin currently serves as Chief Executive Officer and Executive Chairman of Collier Warehouse Inc., which sells residential and
commercial products and services for architects, contractors, homeowners, and developers. Outside of the day-to-day operations
of Collier and LDNIM, Mr. Akin is an active investor in a variety of emerging growth companies, having served in a number of business
roles including Executive Chairman, Independent Board Director, Strategic Advisor, Venture Capital Limited Partner, market pundit,
guest speaker/moderator, private investor and trustee. An avid sportsman, Mr. Akin is an active member at the San Francisco Olympic
Club and competes on the basketball and triathlon teams, as well as a distance runner and golfer. He currently resides in San
Francisco.
Executive
Officers
Biographical
information concerning Noreen Griffin, our Chief Executive Officer, and Christopher Pearce, our Chief Operating Officer, are set
forth above. Biographical information concerning our other executive officers is set forth below.
Peter
Aronstam
– Mr. Aronstam, age 64, has been our Chief Financial Officer since January 2013. Mr. Aronstam brings more than
30 years of experience in accounting, finance, banking, international trade and law to his clients. His career is marked by a
progression of senior finance roles with growth and performance-driven enterprises, from start-up technology and internet companies
to chief financial officer roles with small service providers to very large international manufacturers to global banks. Mr. Aronstam
has advised publicly-held and privately-owned businesses since 1978, providing both full-time and part-time CFO services. His
background includes start-up VC-backed entrepreneurial companies, manufacturing technology and service companies, serving as a
public-company CFO, corporate and international banking in major multinational banks, managing HR and IT functions, and raising
more than $500,000,000 in debt and equity for his companies and their customers. From 2001 to 2006, Mr. Aronstam was the Chief
Financial Officer of Airspan Networks, Inc., a public reporting company in Boca Raton, Florida. He also was the CFO of private
company Mainstream Holdings, LLC in West Palm Beach, Florida from 2007 to 2008 and private company The Neptune Society in Plantation,
Florida from 2008 to 2009. Since 2010, Mr. Aronstam has been a partner of B2B CFO Partners, LLC, doing business as B2B CFO©.
The firm provides CFO services to its clients on a part time basis. Born and educated in South Africa, Mr. Aronstam earned his
Bachelor of Commerce, Bachelor of Law and PhD from the University of the Witwatersrand in South Africa. Mr. Aronstam has worked
in South Africa, Canada, and Florida.
Dr.
Fengping Shan
– Dr. Shan, age 57, has been our Chief Science Officer since March 2012 and was a member of our Board
from March 2012 until September 2014. Dr. Shan has a Ph.D. in Microbiology and Tumor Immunology. Dr. Shan is Professor of Immunology
and Vice Director of the Institute of Immunology, China Medical University, in Shenyang, China. He has been with the University
from 2006 to present. Dr. Shan was the Senior Scientist for Penta Biotech from 2000 to 2006, Chief Scientist for the China Liaoning
Institute of Microbiological Science from 1995 to 2000 and studied at the National Cancer Research in Paris, France from 1990
to 1994. Dr. Shan has authored 90 publications, been issued 11 patents, and is the unique inventor of a thrombolytic enzyme from
the earthworm. From 2000 to present, Dr. Shan has worked both in the United States and China on the clinical trials with Dr. Nicholas
Plotnikoff involving new immunotherapies for the treatment of cancer. Based on the trials, a number of patents were filed in China
beginning in 2009 and 2010, and approved in 2011.
Board
Committees
At
August 23, 2016, we had not yet established an audit committee, compensation committee, or nominating committee. During 2015,
the functions ordinarily handled by these committees were handled by our entire Board. In February of 2015, the Board authorized
formation of and adopted charters for an audit committee, compensation committee and nominating committee. As of the date of filing
this annual report, no members were appointed to the audit committee, compensation committee or nominating committee. The audit
committee, compensation committee and nominating committee have not yet held any meetings.
EXECUTIVE
COMPENSATION
The
following table summarizes the annual compensation of our Named Executive Officers (defined below), as of December 31, 2015. “Named
Executive Officers,” consistent with Item 402(m) of Regulation S-K promulgated under the Exchange Act, include: (i) the
Company’s Principal Executive Officer and individuals acting in a similar capacity during fiscal year 2015, regardless of
compensation level; (ii) the Company’s two most highly compensated executive officers other than the Principal Executive
Officer who were serving as executive officers at the end of fiscal year 2015; and (iii) up to two additional individuals who
would have been included under (ii) above but for the fact that the applicable individual was not serving as an executive officer
of the Company at the end of fiscal year 2015.
The
Officers receive an annual salary as described in the table below for the services rendered on behalf of the Company.
|
Name
and Principal Position
|
|
Year
|
|
|
Salary
|
|
|
Bonus
|
|
|
Stock
Awards
|
|
|
Option
Awards
|
|
|
All
Other Compen-sation
|
|
|
Total($)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Noreen
Griffin
|
|
|
2015
|
|
|
$
|
381,281
|
(1)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
18,000
|
(2)
|
|
$
|
399,281
|
|
|
Chief
Executive Officer
|
|
|
2014
|
|
|
$
|
364,583
|
(1)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
18,000
|
(2)
|
|
$
|
382,583
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Christopher
Pearce
|
|
|
2015
|
|
|
$
|
285,961
|
(1)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
18,000
|
(3)
|
|
$
|
303,961
|
|
|
Chief
Operating Officer, Director(*)
|
|
|
2014
|
|
|
$
|
272,344
|
(1)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
18,000
|
(3)
|
|
$
|
290,344
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Peter
Aronstam(*)
|
|
|
2015
|
|
|
$
|
90,000
|
|
|
$
|
—
|
|
|
$
|
—
|
(4)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Chief
Financial Officer
|
|
|
2014
|
|
|
$
|
60,000
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
32,007
|
(4)
|
|
$
|
92,007
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr. Fengping
Shan
|
|
|
2015
|
|
|
$
|
20,000
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
20,000
|
|
|
Chief
Science Officer
|
|
|
2014
|
|
|
$
|
30,000
|
|
|
$
|
—
|
|
|
$
|
140,000
|
(5)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
170,000
|
|
(1)
In 2014 and 2015, Ms. Griffin and Mr. Pearce agreed to defer a portion of their salaries until the Company had sufficient income
to pay them in cash in full. In April 2015, Mr. Pearce agreed to waive payment of $282,252 of his deferred compensation in return
for a royalty payable in perpetuity in an amount equal to (a) $0.01 per tablet or capsule of LDN sold by the Company outside of
the United States, and (b) $0.005 per tablet or capsule of LDN sold by the Company in the United States. At December 31, 2015,
the amounts of salaries deferred for Ms. Griffin and Mr. Pearce were $613,109 and $126,562 respectively, ($407,593 and $256,343,
respectively, at December 31, 2014).
(2)
The Company pays Ms. Griffin $1,500 per month for unaccountable expenses during each month of the term of her employment agreement.
Unaccountable expenses refer to costs incurred by Ms. Griffin in the course of business that are not required to be reported on
a monthly expense report. These costs include expenses incurred related to international travel.
(3)
The Company pays Mr. Pearce $1,500 per month for unaccountable expenses during each month of the term. Unaccountable expenses
refer to costs incurred by Mr. Pearce in the course of business that are not required to be reported on a monthly expense report.
These costs include expenses incurred related to international travel.
(4)
In 2015, there were no awards to Mr. Aronstam of Company common stock. In 2015, Mr. Aronstam was awarded 50,000 shares of common
stock of Cytocom Inc. In December 2014, Mr. Aronstam received warrants to purchase 250,000 shares of the Company’s common
stock for $0.14 per share, together with warrants to purchase 250,000 shares of Cytocom Inc. for
$0.14
per share. The warrants expire in December 2019. The warrants were recorded at $31,909 and $98 respectively, which was determined
to be the fair value as of the date of the warrant grants.
(5)
No shares were issued and no cash payments were made to Dr. Shan in 2015. In 2015, the Company recorded a liability of $20,000
owed to Dr. Shan for services provided. In 2014, Dr. Shan received 500,000 shares of the Company’s common stock. The stock
was recorded at $140,000, which was determined to be the fair value as of the date of the stock award measured based on the closing
fair market value of the Company’s common stock on the date of award. 250,000 of the shares were issued in lieu of payment
of cash compensation to Dr. Shan.
(*)
Mr. Pearce was the Chief Financial Officer of the Company during the 2012 term. He was appointed Chief Operating Officer on January
1, 2013 when Mr. Aronstam was appointed Chief Financial Officer. Mr. Pearce resigned his position as Chief Operating Officer on
January 13, 2015, when he was replaced in that capacity by Mr. Seth Elliott. He was reappointed as Chief Operating Officer in
2015, when Mr. Elliott left the Company.
Employment
and Related Agreements
Noreen
Griffin, the Company’s Chief Executive Officer, entered into an employment agreement with the Company in March 2012. The
agreement has an initial two-year term and automatically renews for additional one-year periods at the election of our Board,
unless sooner terminated in accordance with the agreement. The agreement provides for payment of an annual salary of $275,000,
increasing by 5% per year during the term, payment of a monthly auto allowance of $1,500, payment for medical and insurance benefits,
and payment of annual bonuses as determined by the Company. Ms. Griffin is also entitled to receive 2 million shares of the Company’s
common stock when the Company commences shipments of its LDN product, in addition to the 3 million founder shares to which Ms.
Griffin was already entitled. The agreement entitles Ms. Griffin to certain payments if more than 50.1% of the Company’s
issued stock is acquired in a merger. The agreement was amended in March 2013, increasing Ms. Griffin’s annual salary to
$350,000. The agreement was further amended as of March 14, 2016 extending the term to March 25, 2017. The amendment to the agreement
also entitles Ms. Griffin warrants to purchase 1,000,000 shares of the Company’s common stock at $0.20 cents.
Christopher
Pearce, previously the Company’s Chief Operating Officer, entered into an employment agreement with the Company in January
2012. The agreement has an initial two-year term and automatically renews for additional one-year periods at the election of our
Board, unless sooner terminated in accordance with the agreement. The agreement provides for payment of an annual salary of $250,000,
increasing by 5% per year during the term, payment of a monthly auto allowance of $1,500, payment for medical and insurance benefits,
and payment of annual bonuses as determined by the Company. At signing, Mr. Pearce was also entitled to receive 2 million shares
of the Company’s common stock, in addition to the 2 million founder shares to which Mr. Pearce was already entitled. The
agreement entitles Mr. Pearce to certain payments if more than 50.1% of the Company’s issued stock is acquired in a merger.
The agreement was further amended as of March 14, 2016 extending the term to March 25, 2017. The amendment to the agreement also
entitles Mr. Pearce warrants to purchase 1,000,000 shares of the Company’s common stock at $0.20 cents.
Peter
Aronstam, our Chief Financial Officer, entered into a services agreement with the Company on December 15, 2014. The agreement
expired on December 14, 2015 and was subsequently extended to June 30, 2017. Pursuant to the agreement, Mr. Aronstam is required
to devote twenty-five hours per month to carry out the chief financial officer services identified in the agreement. The agreement
provides for a monthly payment of $7,500 and requires the Company to issue Mr. Aronstam a warrant, which is exercisable for five
years, to purchase 100,000 shares of our common stock at an exercise price of $0.17 per share.
Outstanding
Equity Awards at Fiscal Year-End
Warrants
were granted to Mr. Aronstam in December 2014 to acquire 250,000 shares of common stock of the Company and 250,000 shares of common
stock of Cytocom Inc. at a price of $0.14 each. The warrants expire on December 14, 2019.
Summary
Director Compensation Table
The
following table shows information regarding the compensation earned or paid during 2015 to Non-Employee Directors who served on
the Board during the year. The compensation paid to Ms. Griffin, Mr. Pearce, and Dr. Shan is shown under
“Executive
Compensation” and the related explanatory tables. Ms. Griffin, Mr. Pearce, and Dr. Shan did not receive any compensation
for their service as members of the Board.
|
Name
and Principal Position
|
|
Year
|
|
|
Fees
Paid or Earned in Cash ($)
|
|
|
Stock
Awards ($)
|
|
|
Option
Awards ($)
|
|
|
Non-equity
incentive plan compensation
|
|
|
Non-qualified
incentive plan compensation
|
|
|
All
Other Compensation ($)
|
|
|
Total
($)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr. Nicholas
Plotnikoff
|
|
|
2015
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Non-Executive Chairman
of the Board
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Edward Teraskiewicz,
|
|
|
2015
|
|
|
|
50,000
|
(1)
|
|
|
53,990
|
(1)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
103,990
|
|
|
Director*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)
For services as a director in 2015, Mr. Teraskiewicz was entitled to receive payment of $50,000. Mr. Teraskiewicz agreed to defer
payment until 2016. In 2015, Mr. Teraskiewic was awarded 350,000 shares of common stock the Company for services as director.
The stock was recorded at $53,990.
For
2015, all members of the Board of Directors who are not our employees, or Non-Employee Directors, currently receive an annual
retainer of $60,000 per year, payable monthly in arrears. In addition, Non-Employee Directors are eligible to receive stock upon
their appointment to the Board. Certain members of the Board of Directors are also entitled to receive an annual payment of Company
shares for their services. We do not currently have minimum stock ownership guidelines for Non-Employee Directors.
We
reimburse Non-Employee Directors for actual out-of-pocket costs incurred to attend board meetings. No additional compensation
is paid for attendance in person or by telephone at board meetings.
CERTAIN
RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
On
January 3, 2013, the Company formalized the terms under which Kelly O’Brien Wilson, the daughter-in-law of Noreen Griffin,
the Company’s Chief Executive Officer, is employed. Ms. Wilson had been working with the Company since 2012 and her three-year
employment agreement is effective as of December 1, 2012. The terms of the agreement define her base salary, a grant of a common
stock, and health insurance coverage. Ms. Wilson was issued 500,000 shares of common stock of the Company in January 2014. In
October 2015, the expiration date of the agreement was extended to October 1, 2018. In the year ended December 31, 2015, the Company
paid compensation to Ms. Wilson totaling $163,954 ($157,582 in the year ended December 31, 2014).
In
May 2013, the Company executed a Patent License Agreement with Professor Fengping Shan. The Company obtained exclusive rights
to develop and commercialize the licensed technology. The licensed technology is the intellectual property developed and owned
by Professor Shan (i) relating to the treatment of a variety of diseases and conditions with MENK including multiple forms of
lymphoma and cancer and (ii) a treatment method for humans infected with the HLTV-III (AIDS) virus including AIDS and AIDS related
complex (ARC). The licensed technology includes the methods and formulations for these treatments including but not limited to
all INDs, communications with regulatory agencies, patient data, and letters relating to these treatments. The licensed technology
also includes the following patents: 200710158742.7 MENK, its application is in treating leukemia and other blood cancers; No.
200710051586.4 MENK, its application is in preparation of human and animal vaccines; No. 200610046249.1, a nasal spray formulation
containing MENK; No. 201210290150.1 LDN, combined with MENK, its application is in preparation of an anticancer drug (Pending);
No. 201210302259.2 LDN, combined with MENK, its application is in preparation of leukophoresis for anticancer (Pending); No. 200810229085.5
Compound MENK as a drug for colon cancer and pancreatic cancer; No. 200910011030.1, Naltrexone as well as analogues being anticancer
drug. In August 2014, Professor Shan executed an Assignment under which he transferred to the Company his entire right, title
and interest in and to the licensed patents under the Patent License Agreement and to CN 201210302259 Application of combination
of LDN and MENK to preparation of anti-cancer drug for the consideration of 500,000 shares of our common stock.
The
Company entered into a Sale of Technology Agreement with Dr. Nicholas P. Plotnikoff on March 4, 2012, wherein Dr. Plotnikoff agreed
to transfer and assign all of his rights, title and interest in: European Patent United Kingdom, Germany, France, Ireland EP 1401471
BI Methods for inducing sustained immune response; Russian Patent Russian Federation patent number 2313364; The Patent Office
of the People’s Republic of China, Application No.: 200810165784.8 China Patent CN1015113407 A The Patent Office of the
People’s Republic of China ISSN: 1006-2858 CN 21-1349/R; Patent Agencies Government of India Patent, Application number
1627/KOLNP/2003 number 220265 an Enkephalin Peptide Composition; and the US Patent Pending, US Patent Application 10/146.999 e.
The Company received all the production formulations and technology designs from Dr. Plotnikoff necessary for the manufacturing,
formulation, production and protocols of the MENK treatment of cancer and HIV/AIDS. As consideration for entering into the Sale
of Technology Agreement, Dr. Plotnikoff received 8,000,000 shares of common stock, a royalty of a single-digit percentage on all
sales of MENK and was granted the position of Non-Executive Chairman of the Board of Directors.
On
April 23, 2012, the Company acquired TNI BioTech IP, Inc. (“TNI IP”), its wholly-owned subsidiary, in exchange for
20,250,000 shares of the Company’s common stock, of which 8,000,000 shares were issued to Dr. Plotnikoff for TNI IP’s
acquisition of the patents and the remaining 12,250,000 shares were issued to the founders of TNI IP in exchange for all of their
right, title and interest in their TNI IP shares.
In
April 2015, the Company entered into an agreement with LDN Information Management, LLC, (“LDNIM”), a California limited
liability company. Paul Akin, a member of the Company’s board of directors, is the managing member of LDNIM. Under the contract,
LDNIM is required to provide outreach and educational services to physicians relating to the Company’s LDN formulation in
Arizona, California, Hawaii, Nevada, Oregon and Washington (the Territory”). This includes attending seminars and conferences
and otherwise providing educational information about the Company’s licensing of its LDN formulation to Complete Pharmacy
and Medical Solutions, LLC.
LDNIM
has agreed that its outreach efforts should result in a threshold set of patients filling prescriptions at Complete Pharmacy and
Medical Solutions, LLC , reaching 60,000 patients by December 31, 2017 (excluding patients who purchase from Complete Pharmacy
and Medical Solutions, LLC via prescriptions from the specified physicians). As compensation, the Company has agreed to pay LDNIM
monthly an amount equal to ten percent (10%) of the licensing revenue paid by Complete Pharmacy and Medical Solutions, LLC for
revenues generated in the Territory. Revenues generated from patients that have placed an order with Complete Pharmacy and Medical
Solutions, LLC via prescriptions written by specified physicians are not included. In a related agreement, as compensation for
the services to be provided via LDN in May 2015, Mr. Akin also received 190,000 shares of common stock of the Company, for which
the Company recorded an expense of $12,350.
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED
STOCKHOLDER MATTERS
The
following table sets forth certain information regarding the ownership of our common stock as of March 16, 2016 (the “Determination
Date”) by: (i) each current director of our company; (ii) each of our named executive officers; (iii) all current executive
officers and directors of our company as a group; and (iv) all those known by us to be beneficial owners of more than five percent
(5%) of our common stock.
Beneficial
ownership and percentage ownership are determined in accordance with the rules of the SEC. Under these rules, beneficial ownership
generally includes any shares as to which the individual or entity has sole or shared voting power or investment power and includes
any shares that an individual or entity has the right to acquire beneficial ownership of within 60 days of the Determination Date,
through the exercise of any option, warrant or similar right (such instruments being deemed to be “presently exercisable”).
In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of our common
stock that could be issued upon the exercise of presently exercisable options and warrants are considered to be outstanding. These
shares, however, are not considered outstanding as of the Determination Date when computing the percentage ownership of each other
person.
To
our knowledge, except as indicated in the footnotes to the following table, and subject to state community property laws where
applicable, all beneficial owners named in the following table have sole voting and investment power with respect to all shares
shown as beneficially owned by them. Percentage of ownership is based on 206,913,301 shares of common stock outstanding as of
the Determination Date. Unless otherwise indicated, the business address of each person in the table below is c/o Immune Therapeutics,
Inc., 37 North Orange Avenue, Suite 607, Orlando, Florida 32801.
|
Name
and Address
|
|
Amount
of
Beneficial
Ownership
|
|
|
Percentage
of
Class %
|
|
|
|
|
|
|
|
|
|
|
Dr. Nicholas
Plotnikoff, Non-Executive Chairman of the Board
|
|
|
6,650,000
|
|
|
|
3.2
|
|
|
|
|
|
|
|
|
|
|
|
|
Noreen Griffin, Chief
Executive Officer and Director
|
|
|
4,317,710
|
(2)
|
|
|
2.1
|
|
|
Christopher Pearce, Chief
Operating Officer and Director
|
|
|
3,776,125
|
(3)
|
|
|
1.8
|
|
|
Edward Teraskiewicz, Director
|
|
|
707,000
|
(4)
|
|
|
*
|
|
|
Paul Akin, Director (6)
|
|
|
4,836,449
|
|
|
|
2.3
|
|
|
Dr. Clifford Selsky, Director
(6)
|
|
|
0
|
|
|
|
*
|
|
|
Dr. Fengping Shan, Chief
Science Officer
|
|
|
2,264,999
|
|
|
|
1.1
|
|
|
Peter Aronstam, Chief
Financial Officer
|
|
|
325,000
|
(5)
|
|
|
*
|
|
|
Robert J. Dailey (7)
|
|
|
9,320,895
|
|
|
|
4.5
|
|
|
|
|
|
|
|
|
|
|
|
|
All directors and officers
as a group (8 persons)
|
|
|
32,198,178
|
|
|
|
15.09
|
|
|
(1)
|
These
shares are held by the Plotnikoff Family Trust.
|
|
|
|
|
(2)
|
Represents
shares held in the name of Griffin Enterprises Group, Inc., which is 50% owned and managed by Robert Wilson, Ms. Griffin’s
son; shares held by the Griffin Family Trust, an irrevocable trust that is not managed by Ms. Griffin; and shares held by
Noreen Griffin, individually.
|
|
|
|
|
(3)
|
1,876,125
shares are held by Mr. Pearce individually; and 1,900,000 shares are held by the Pearce Family Trust over which Mr. Pearce
has no voting or dispositive control.
|
|
|
|
|
(4)
|
These
shares are held by the Raster Investments, Inc., an irrevocable trust that is not managed by Mr. Teraskiewicz.
|
|
|
|
|
(5)
|
Represents
warrants to purchase 325,000 shares of common stock for prices of $1.00 (75,000 shares) and $0.14 (250,000 shares) per share
until December 2018 (75,000 shares) and December 2019 (250,000 shares). In addition, Mr. Aronstam holds a warrant to purchase
250,000 shares of Cytocom Inc. at $0.14 per share until December 2019.
|
|
|
|
|
(6)
|
As
compensation for their appointment as directors, the Company has agreed to issue Dr. Selsky and Mr. Akin each a total of 500,000
shares of restricted common as follows: 250,000 shares of stock will be issued and fully earned upon their agreement to serve
as directors, and 250,000 shares will be issued and fully earned after each serves as director for 12 consecutive months.
|
|
|
|
|
(7)
|
Mr.
Dailey’s address is 401 First Street, Los Altos, California 94002.
|
(*)
Beneficial ownership of less than 1.0 percent is omitted.
WHERE
YOU CAN FIND ADDITIONAL INFORMATION
We
have filed with the Securities and Exchange Commission a registration statement on Form S1 under the Securities Act with respect
to the shares of common stock being offered by this prospectus. This prospectus does not contain all of the information in the
registration statement and its exhibits. For further information with respect to us and the common stock offered by this prospectus,
we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any
contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the
contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects
by this reference.
You
can read our SEC filings, including the registration statement, over the Internet at the SEC's website at www.sec.gov.
You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street NE, Washington,
D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the
SEC at 100 F Street N.E., Washington, D.C. 20549. Please call the SEC at 1800SEC0330 for further information on the operation
of the public reference facilities. You may also request a copy of these filings, at no cost, by writing us at 37 North Orange
Ave., Ste 607, Orlando, FL 32801 or telephoning us at (888) 613-8802. We are subject to the information and periodic reporting
requirements of the Exchange Act, and we file periodic reports, proxy statements and other information with the SEC. These periodic
reports, proxy statements and other information are available for inspection and copying at the public reference room and website
of the SEC referred to above. We maintain a website at
http://www.immunetherapeutics.com.
You may access our annual reports
on Form 10K, quarterly reports on Form 10Q, current reports on Form 8K and amendments to those reports filed or furnished pursuant
to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after
such material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through,
our website is not incorporated by reference in, and is not part of, this prospectus.
FINANCIAL
STATEMENTS
INDEX
TO FINANCIAL STATEMENTS
CONDENSED
CONSOLIDATED BALANCE SHEET
|
|
|
June
30, 2016
|
|
|
December
31, 2015
|
|
|
|
|
(Unaudited)
|
|
|
(Audited)
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
Current Assets:
|
|
|
|
|
|
|
|
|
|
Cash
and cash equivalents
|
|
$
|
64,289
|
|
|
$
|
23,149
|
|
|
Accounts receivable
|
|
|
2,661
|
|
|
|
16,197
|
|
|
Prepaids
and other current assets
|
|
|
11,272
|
|
|
|
-
|
|
|
Total
current assets
|
|
|
78,222
|
|
|
|
39,346
|
|
|
|
|
|
|
|
|
|
|
|
|
Fixed Assets:
|
|
|
|
|
|
|
|
|
|
Computer equipment,
net of accumulated depreciation of $7,312 and $6,331 respectively
|
|
|
701
|
|
|
|
1,682
|
|
|
|
|
|
|
|
|
|
|
|
|
Deposits
|
|
|
200
|
|
|
|
200
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
79,123
|
|
|
$
|
41,228
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND
STOCKHOLDERS’ DEFICIT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
1,517,788
|
|
|
$
|
1,924,672
|
|
|
Accrued liabilities
|
|
|
2,030,559
|
|
|
|
1,281,039
|
|
|
Current
portion of notes payable
|
|
|
3,457,436
|
|
|
|
2,793,701
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current
liabilities
|
|
|
7,005,783
|
|
|
|
5,999,412
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current liabilities:
|
|
|
|
|
|
|
|
|
|
Notes
payable, less current portion
|
|
|
-
|
|
|
|
-
|
|
|
Total
non-current liabilities
|
|
|
-
|
|
|
|
-
|
|
|
Total liabilities
|
|
|
7,005,783
|
|
|
|
5,999,412
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and Contingencies (Note
10)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Deficit:
|
|
|
|
|
|
|
|
|
|
Common stock - par
value $0.0001; 500,000,000 shares authorized; 214,447,611 and 174,850,047 shares issued and outstanding respectively
|
|
|
21,445
|
|
|
|
17,485
|
|
|
Additional paid
in capital
|
|
|
354,333,473
|
|
|
|
343,434,786
|
|
|
Stock issuances
due
|
|
|
1,275,838
|
|
|
|
1,140,303
|
|
|
Prepaid services
|
|
|
(1,983,024
|
)
|
|
|
(660,417
|
)
|
|
Accumulated
deficit
|
|
|
(358,319,891
|
)
|
|
|
(347,789,889
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Deficit attributable
to common shareholders
|
|
|
(4,672,159
|
)
|
|
|
(3,857,732
|
)
|
|
Non-controlling
interest
|
|
|
(2,254,501
|
)
|
|
|
(2,100,452
|
)
|
|
Total
stockholders’ deficit
|
|
|
(6,926,660
|
)
|
|
|
(5,958,184
|
)
|
|
Total
liabilities and stockholders’ deficit
|
|
$
|
79,123
|
|
|
$
|
41,228
|
|
The
accompanying notes are an integral part of these condensed consolidated financial statements.
CONDENSED
CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
|
|
|
Three
Months ended
|
|
|
Six
Months ended
|
|
|
|
|
June
30, 2016
|
|
|
June
30, 2015
|
|
|
June
30, 2016
|
|
|
June
30, 2015
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues, net
|
|
$
|
-
|
|
|
$
|
5,648
|
|
|
$
|
3,463
|
|
|
$
|
7,718
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative
|
|
|
893,917
|
|
|
|
443,585
|
|
|
|
1,833,258
|
|
|
|
845,572
|
|
|
Research and development expense
|
|
|
67,262
|
|
|
|
414,492
|
|
|
|
48,420
|
|
|
|
591,650
|
|
|
Stock issued for services G&A
|
|
|
1,194,761
|
|
|
|
1,450,334
|
|
|
|
3,178,598
|
|
|
|
3,611,893
|
|
|
Warrant valuation
|
|
|
490,355
|
|
|
|
-
|
|
|
|
2,568,554
|
|
|
|
-
|
|
|
Depreciation
and amortization expense
|
|
|
434
|
|
|
|
148,726
|
|
|
|
981
|
|
|
|
297,452
|
|
|
Total
operating expenses
|
|
|
2,646,729
|
|
|
|
2,457,137
|
|
|
|
7,629,811
|
|
|
|
5,346,567
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(2,646,729
|
)
|
|
|
(2,451,489
|
)
|
|
|
(7,626,348
|
)
|
|
|
(5,338,849
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
(1,051,576
|
)
|
|
|
(50,711
|
)
|
|
|
(1,353,020
|
)
|
|
|
(68,757
|
)
|
|
Loss on settlement
of debt
|
|
|
(340,343
|
)
|
|
|
(88,445
|
)
|
|
|
(1,704,683
|
)
|
|
|
(88,445
|
)
|
|
Total
other income (expense)
|
|
|
(1,391,919
|
)
|
|
|
(139,156
|
)
|
|
|
(3,057,703
|
)
|
|
|
(157,202
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss)
|
|
$
|
(4,038,648
|
)
|
|
$
|
(2,590,645
|
)
|
|
$
|
(10,684,051
|
)
|
|
$
|
(5,496,051
|
)
|
|
Net (loss) attributable to non-controlling
interest
|
|
|
(109,929
|
)
|
|
|
(138,891
|
)
|
|
|
(154,049
|
)
|
|
|
(262,956
|
)
|
|
Net (loss) attributable to common shareholders
|
|
$
|
(3,928,719
|
)
|
|
$
|
(2,451,754
|
)
|
|
$
|
(10,530,002
|
)
|
|
$
|
(5,233,095
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted
loss per share attributable to common shareholders
|
|
$
|
(0.02
|
)
|
|
$
|
(0.02
|
)
|
|
$
|
(0.05
|
)
|
|
$
|
(0.04
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares
outstanding
|
|
|
207,229,469
|
|
|
|
147,387,763
|
|
|
|
199,076,428
|
|
|
|
142,563,007
|
|
The
accompanying notes are an integral part of these condensed consolidated financial statements.
CONDENSED
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
|
|
|
Six
Months Ended
|
|
|
|
|
June
30, 2016
|
|
|
June
30, 2015
|
|
|
CASH FLOWS FROM OPERATING
ACTIVITIES
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(10,684,051
|
)
|
|
$
|
(5,496,051
|
)
|
|
Adjustments to reconcile net loss to
net cash flows used in operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation
|
|
|
981
|
|
|
|
1,335
|
|
|
Amortization
|
|
|
-
|
|
|
|
296,117
|
|
|
Stock issued, and
amortization of stock issued, for prepaid services
|
|
|
1,539,928
|
|
|
|
3,530,894
|
|
|
Loss on settlement
of debt
|
|
|
1,704,683
|
|
|
|
600,562
|
|
|
Stock issued for
services
|
|
|
1,661,169
|
|
|
|
-
|
|
|
Stock issued for
legal settlement
|
|
|
-
|
|
|
|
81,000
|
|
|
Amortization of
debt discount
|
|
|
385,288
|
|
|
|
-
|
|
|
Stock warrant expense
|
|
|
2,568,554
|
|
|
|
-
|
|
|
Stock (returned)
issued for donation
|
|
|
|
|
|
|
-
|
|
|
Stock issued for
interest
|
|
|
149,000
|
|
|
|
-
|
|
|
Changes in operating
assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
|
104,131
|
|
|
|
(158,931
|
)
|
|
Accounts receivable
|
|
|
13,536
|
|
|
|
(7,718
|
)
|
|
Accrued liabilities
|
|
|
1,168,324
|
|
|
|
(112,285
|
)
|
|
Prepaid
expenses and deposits
|
|
|
(11,272
|
)
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used
in operating activities
|
|
|
(1,399,729
|
)
|
|
|
(1,265,077
|
)
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING
ACTIVITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used
in investing activities
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING
ACTIVITIES
|
|
|
|
|
|
|
|
|
|
Proceeds from sale
of stock and exercise of warrants
|
|
|
50,000
|
|
|
|
152,000
|
|
|
Proceeds
from issuance of notes payable
|
|
|
1,390,869
|
|
|
|
943,475
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided
by financing activities
|
|
|
1,440,869
|
|
|
|
1,095,475
|
|
|
Net increase (decrease) in cash
|
|
|
41,140
|
|
|
|
(169,602
|
)
|
|
Cash and cash
equivalents at beginning of period
|
|
|
23,149
|
|
|
|
191,987
|
|
|
Cash and cash
equivalents at end of period
|
|
$
|
64,289
|
|
|
$
|
22,385
|
|
|
|
|
Six
Months Ended
|
|
|
|
|
June
30, 2016
|
|
|
June
30, 2015
|
|
|
SUPPLEMENTAL DISCLOSURES
OF CASH FLOW INFORMATION:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for interest
|
|
$
|
10,317
|
|
|
$
|
7,500
|
|
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL DISCLOSURES
OF NON-CASH INVESTING AND FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of debt and accrued interest
to common stock
|
|
$
|
3,064,259
|
|
|
$
|
600,562
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-controlling interest
|
|
$
|
154,049
|
|
|
$
|
262,956
|
|
The
accompanying notes are an integral part of these condensed consolidated financial statements.
Notes
to the Condensed Consolidated Financial Statements
June
30, 2016
(Unaudited)
1.
Organization and Description of Business
Immune
Therapeutics, Inc. (the “Company”) was initially incorporated in Florida on December 2, 1993 as Resort Clubs International,
Inc. (“Resort Clubs”). It was formed to manage and market golf course properties in resort markets throughout the
United States. Galliano International Ltd. (“Galliano”) was incorporated in Delaware on May 27, 1998 and began trading
in November 1999 through the filing of a 15C-211. On November 10, 2004, Galliano merged with Resort Clubs. Resort Clubs was the
surviving corporation. On August 23, 2010, Resort Clubs changed its name to pH Environmental Inc. (“pH Environmental”).
On
April 23, 2012, pH Environmental completed a name change to TNI BioTech, Inc., and on April 24, 2012, we executed a share exchange
agreement for the acquisition of all of the outstanding shares of TNI BioTech IP, Inc. On September 4, 2014, a majority of our
shareholders approved an amendment to our Amended and Restated Articles of Incorporation, as amended, to change our name to Immune
Therapeutics, Inc. We filed our name change amendment with the Secretary of State of Florida on October 27, 2014 changing our
name to Immune Therapeutics, Inc.
The
Company currently operates out of Orlando, Florida. In July 2012, the Company’s focus turned to acquiring patents that would
protect and advance the development of new uses of opioid-related immune- therapies, such as low dose naltrexone (“LDN”)
and Methionine [Met5]-enkephalin (“MENK”). Today, the Company is focused on the development and commercialization
of therapeutic treatments for cancer, HIV/AIDS, malaria, and opportunistic infections arising from their treatment, and commercializing
affordable, non-toxic therapies in Africa, to be followed by Asia and South America, and autoimmune diseases and immune disorders
by combating these severe and fatal diseases through the stimulation and/or regulation of the body’s immune system. The
Company’s therapies are believed to stimulate and/or regulate the immune system in such a way that they provide the potential
to treat a variety of diseases. We believe our therapies may be able to correct abnormalities or deficiencies in the immune system
in diseases such as HIV infection, autoimmune disease, immune disorders, or cancer; all of which can lead to disease progression
and life-threatening situations when the immune system is not functioning optimally.
In
October 2012, the Company formed TNI BioTech International, Ltd., a BVI company in Tortola, British Virgin Islands, which was
set up to allow the Company to market and sell LDN in those countries outside the U.S. in which we have been able to obtain approval
to sell the Company’s products.
In
August 2013, the Company formed its United Kingdom subsidiary, TNI BioTech, LTD (the “UK Subsidiary”). The UK Subsidiary
received approval to be considered a micro, small or medium-sized enterprise (“SME”) with the European Medicines Agency
(“EMA”) on August 21, 2013. The designation provides the UK Subsidiary with significant discounts when holding meetings
or submitting filings to the EMA. On September 19, 2013, the UK Subsidiary submitted a pre-submission package to the EMA regarding
Crohn’s Disease. The EMA granted the UK Subsidiary a meeting that took place on September 27, 2013. The UK Subsidiary is
eligible to benefit from the provisions for administrative and financial assistance for SMEs set out in Regulation (EC) No 2049/2005.
The Company will apply to obtain EMA benefits once funding becomes available.
In
December 2013, the Company formed a new subsidiary, Cytocom Inc. (“Cytocom”). Cytocom is a clinical-stage pharmaceutical
company focused on the development of the first affordable non-toxic immunodulator for the treatment of inflammatory diseases,
immune-related disorders, and cancer, and is responsible for the development of the Company’s patented therapies under the
auspices of the FDA and EMA. In December 2014, the Company finalized the distribution of common stock of Cytocom to its shareholders.
As part of the transaction, the Company retained exclusive rights to all international patents, in-country approvals, formulations,
trademarks, manufacturing, marketing, sales, and distributions rights in emerging nations, including Africa, Central America,
South America, Russia, India, China, Far East, and The Commonwealth of Independent States (former Soviet Union). The Company will
continue to have access to existing clinical data as well as any new data generated by Cytocom during drug development. On December
8, 2014, the number of Cytocom shares of common stock that were issued to our shareholders totaled 113,242,522 shares. In connection
with the transaction, Cytocom issued 140,100,000 shares of its common stock to the Company, which gave the Company a 55.3% equity
interest in Cytocom on that date. In April 2016, the Board of Directors and a majority of shareholders of Cytocom approved a reverse
stock
split of Cytocom’s outstanding common stock with one new share of stock for each twenty old shares of common stock. Cytocom
effectuated and finalized the reverse split in June 2016. At June 30, 2016, the Company’s equity interest had been further
reduced to 41%, by subsequent issuances of Cytocom common stock to shareholders in settlement of notes payable.
In
March 2014, the Company incorporated Airmed Biopharma Limited, an Irish corporation with an address in Dublin, Ireland, and Airmed
Holdings Limited, an Irish company domiciled in Bermuda. The Irish companies were set up to benefit from incentives granted by
the Irish government for the establishment of pharmaceutical companies (many of the world’s leading pharmaceutical companies
have located in Ireland), and so that the Company could take advantage of Ireland’s status as a member of the European Union
and the European Economic Area. An Irish limited liability company enjoys a low corporate income tax rate of 12.5%, one of the
lowest in the world. The Irish-domiciled company hopes to qualify for tax incentives for Irish holding/headquartered companies
and to benefit from the network of double tax treaties that reduce withholding taxes. TNI BioTech International, Ltd. will manage
our international distribution, using product that is manufactured in Ireland and elsewhere.
Going
Concern
The
Company has incurred significant net losses since inception and has relied on its ability to fund its operations through private
equity financings. Management expects operating losses and negative cash flows to continue at more significant levels in the future.
As the Company continues to incur losses, transition to profitability is dependent upon the successful development, approval,
and commercialization of its product candidate and the achievement of a level of revenues adequate to support the Company’s
cost structure. The Company may never achieve profitability, and unless and until it does, the Company will continue to need to
raise additional cash. Management intends to fund future operations through additional private or public debt or equity offerings,
and may seek additional capital through arrangements with strategic partners or from other sources. Based on the Company’s
operating plan, existing working capital at December 31, 2015 was not sufficient to meet the cash requirements to fund planned
operations through December 31, 2016 without additional sources of cash. These conditions raise substantial doubt about the Company’s
ability to continue as a going concern. The accompanying financial statements have been prepared assuming that the Company will
continue as a going concern and do not include adjustments that might result from the outcome of this uncertainty. This basis
of accounting contemplates the recovery of the Company’s assets and the satisfaction of liabilities in the normal course
of business.
The
Company experienced a net loss from operations of $(7,626,348) and used cash and cash equivalents from operations in the amount
of $1,399,729 ) during the six months ended June 30, 2016, resulting in stockholders equity of $(6,926,660) at June 30,
2016.
2.
Summary of Significant Accounting Policies
Basis
of Presentation
The
consolidated financial statements included herein have been prepared by the Company, without audit, pursuant to the rules and
regulations of the Securities and Exchange Commission. Certain information and footnote disclosures normally included in financial
statements prepared in accordance with U.S. generally accepted accounting principles have been omitted. However, in the opinion
of management, all adjustments (which include only normal recurring adjustments, unless otherwise indicated) necessary to present
fairly the financial position and results of operations for the periods presented have been made. The results for interim periods
are not necessarily indicative of trends or of results to be expected for the full year. These financial statements should be
read in conjunction with the financial statements of the Company for the year ended December 31, 2015 (including the notes thereto)
set forth in Form 10-K/A.
The
Company qualifies as an “emerging growth company” as defined in Section 101 of the Jumpstart our Business Startups
Act (“JOBS Act”) as we do not have more than $1,000,000,000 in annual gross revenue for the year ended December 31,
2015. We are electing to use the extended transition period for complying with new or revised accounting standards under Section
102(b)(1) of the JOBS Act.
Use
of Estimates
The
preparation of the Company’s financial statements in conformity with U.S. generally accepted accounting principles requires
management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes.
Actual results could differ from such estimates.
Cash,
Cash Equivalents, and Short-Term Investments
The
Company considers all highly liquid investments with original maturities at the date of purchase of three months or less to be
cash equivalents. Cash and cash equivalents include bank demand deposits, marketable securities with maturities of three months
or less at purchase, and money market funds that invest primarily in certificates of deposits, commercial paper and U.S. government
and U.S. government agency obligations. Cash equivalents are reported at fair value.
Concentration
of Credit Risk
Financial
instruments that potentially subject the Company to concentrations of credit risk are primarily cash and cash equivalents. The
Company is exposed to credit risk, subject to federal deposit insurance, in the event of a default by the financial institutions
holding its cash and cash equivalents to the extent of amounts recorded on the balance sheets. The cash accounts are insured by
the Federal Deposit Insurance Corporation up to $250,000. At June 30, 2016, the Company has no uninsured cash balances.
Segment
and Geographic Information
Operating
segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the
chief operating decision maker, or decision making group, in deciding how to allocate resources and in assessing performance.
The Company views its operations and manages its business in one operating segment and does not segment the business for internal
reporting or decision making.
Fair
Value of Financial Instruments
In
accordance with the reporting requirements of Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC)
Topic 825, “
Financial Instruments”
, the Company calculates the fair value of its assets and liabilities which
qualify as financial instruments under this standard and includes this additional information in the notes to the financial statements
when the fair value is different than the carrying value of those financial instruments. Cash and cash equivalents, accounts receivable
and accounts payable are accounted for at cost which approximates fair value due to the relatively short maturity of these instruments.
The carrying value of notes payable also approximates fair value since they bear market rates of interest and other terms. None
of these instruments are held for trading purposes.
Fair
Value Measurements
The
ASC Topic 820,
Fair Value Measurements,
defines fair value, establishes a framework for measuring fair value in accordance
with U.S. generally accepted accounting principles, and requires certain disclosures about fair value measurements. In general,
fair values of financial instruments are based upon quoted market prices, where available. If such quoted market prices are not
available, fair value is based upon internally developed models that primarily use, as inputs, observable market-based parameters.
Valuation adjustments may be made to ensure that financial instruments are recorded at fair value. These adjustments may include
amounts to reflect counterparty credit quality and the customer’s creditworthiness, among other things, as well as unobservable
parameters. Any such valuation adjustments are applied consistently over time.
Property
and Equipment
Property
and equipment are stated at cost, less accumulated depreciation. Depreciation is determined on a straight-line basis over the
estimated useful lives of the assets, which generally range from three to five years. Maintenance and repairs are charged against
expense as incurred. Depreciation expense for the quarters ended June 30, 2016 and June 30, 2015 was $434 and $667, respectively.
Intangible
Assets
Costs
incurred to acquire and/or develop the Company’s product licenses and patents are capitalized and amortized by straight-line
methods over estimated useful lives of seven to sixteen years. Intangible assets are stated at the lower of cost or estimated
fair market value. At the end of 2015, the Company determined that the unamortized carrying amount recorded for the acquisition
of licenses and patents related to LDN were impaired, and recorded an impairment loss of $5,226,352. In 2014, the Company determined
that the carrying amount recoded for the acquisition of licenses and patents related to MENK were impaired, and recorded an impairment
loss of $9,908,477.
During
the quarters ended June 30, 2016 and June 30, 2015, the Company did not capitalize any costs to acquire and/or develop the Company’s
product licenses and patents. (See Note 10). Amortization expense for the quarters ended June 30, 2016 and June 30, 2015 was $0
and $148,059, respectively.
Impairment
of Long-Lived Assets
The
Company evaluates long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount
of an asset may not be recoverable as prescribed by ASC Topic 360-10-05, “
Property, Plant and Equipment
.” If
the carrying amount of the asset, including any intangible assets associated with that asset, exceeds its estimated undiscounted
net cash flow, before interest, the Company will recognize an impairment loss equal to the difference between its carrying amount
and its estimated fair value. No impairment losses were recognized for the quarters ended June 30, 2016 and June 30, 2015.
Research
and Development Costs
Research
and development costs are charged to expense as incurred and are typically comprised of salaries and benefits, pre-clinical studies,
clinical trial activities, drug development and manufacturing, fees paid to consultants and other entities that conduct certain
research and development activities on the Company’s behalf and third-party service fees, including clinical research organizations
and investigative sites. Costs for certain development activities, such as clinical trials are recognized based on an evaluation
of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations, or information
provided by vendors on their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements,
which may differ from the pattern of costs incurred, and are reflected in the financial statements as operating expenses.
Income
Taxes
The
Company follows FASB ASC Topic 740,
“Income Taxes,”
which requires recognition of deferred tax assets and liabilities
for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this
method, deferred tax assets and liabilities are based on the differences between the financial statements and tax bases of assets
and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Deferred tax
assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the asset will
not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income
in the years in which those temporary differences are expected to be recovered or settled.
The
standard addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded
in the financial statements. Under ASC Topic 740, the Company may recognize the tax benefit from an uncertain tax position only
if it is more likely than not that the tax position will be sustained on examination by the tax authorities, based on the technical
merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based
on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate settlement. ASC Topic
740 also provides guidance on de-recognition, classification, interest and penalties on income taxes, accounting in interim periods
and requires increased disclosures. At the date of adoption, and as of December 31, 2015 and June 30, 2016, the Company does not
have a liability for unrecognized tax uncertainties.
The
Company’s policy is to record interest and penalties on uncertain tax positions as income tax expense. At the end of the
quarters ended June 30, 2016 and June 30, 2015, the Company had not accrued any interest or penalties related to uncertain tax
positions.
Stock-Based
Compensation and Issuance of Stock for Non-Cash Consideration
The
Company measures and recognizes compensation expense for all share-based payment awards made to employees and directors, including
employee stock options, based on estimated fair values equaling either the market value of the shares issued or the value of consideration
received, whichever is more readily determinable. The majority of the non-cash consideration pertains to services rendered by
consultants and others and has been valued at the fair market value of the Company’s common stock at the date of the agreement.
The
Company’s accounting policy for equity instruments issued to consultants and vendors in exchange for goods and services
follows the provisions of ASC Topic 505-50, “
Equity-Based Payments to Non-Employees
.” The measurement date
for the fair value of the equity instruments issued is determined at the earlier of (i) the date at which a commitment for performance
by the consultant or vendor is reached or (ii) the date at which the consultant or vendor’s performance is complete.
Non-controlling
Interest
In
accordance with ASC 810,
Consolidation
, the Company consolidates Cytocom, Inc. The non-controlling interests in Cytocom
represent the interests of outside shareholders in the equity and results of operations of Cytocom.
Net
Loss per Share
Basic
net loss per share is calculated by dividing the net loss attributable to common stockholders by the weighted average number of
common shares outstanding for the period, without consideration for common stock equivalents. Diluted net loss per share is calculated
by dividing the net loss by the weighted-average number of common share equivalents outstanding for the period determined using
the treasury-stock method and the if-converted method. Dilutive common stock equivalents are comprised of common stock purchase
warrants and options outstanding. For all periods presented, there is no difference in the number of shares used to calculate
basic and diluted shares outstanding due to the Company’s net loss position.
A
calculation of basic and diluted net loss per share follows:
|
|
|
For
the three months ended
June 30,
|
|
|
For
the six months ended
June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
2016
|
|
|
2015
|
|
|
Net loss per share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Numerator
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(4,038,648
|
)
|
|
$
|
(2,590,645
|
)
|
|
$
|
(10,684,051
|
)
|
|
$
|
(5,496,051
|
)
|
|
Net loss attributed to Common stockholders
|
|
$
|
(3,928,719
|
)
|
|
$
|
(2,451,754
|
)
|
|
$
|
(10,530,002
|
)
|
|
$
|
(5,233,095
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Denominator
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average common shares outstanding—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Denominator for basic and diluted net
loss per share
|
|
|
207,229,469
|
|
|
|
147,387,763
|
|
|
|
199,076,428
|
|
|
|
142,563,007
|
|
|
Basic and diluted
net loss per share attributed to common stockholders
|
|
$
|
(0.02
|
)
|
|
$
|
(0.02
|
)
|
|
$
|
(0.05
|
)
|
|
$
|
(0.04
|
)
|
The
Company’s potential dilutive securities which include stock, stock warrants and convertible debt have been excluded from
the computation of diluted net loss per share as the effect would be to reduce the net loss per share. Therefore, the weighted-average
Common stock outstanding used to calculate both basic and diluted net loss per share is the same.
The
following shares of potentially dilutive securities have been excluded from the computations of diluted weighted average shares
outstanding as the effect of including such securities would be antidilutive:
|
|
|
As
of June 30,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
Warrants
to purchase Common stock
|
|
|
32,748,908
|
|
|
|
9,372,750
|
|
|
|
|
|
32,748,908
|
|
|
|
9,372,750
|
|
Recent
Accounting Standards
During
the quarter ended June 30, 2016, there were several new accounting pronouncements issued by the Financial Accounting Standards
Board. Each of these pronouncements, as applicable, has been or will be adopted by the Company. Management does not believe the
adoption of any of these accounting pronouncements has had or will have a material impact on the Company’s consolidated
financial statements.
The
Company qualifies as an “emerging growth company” as defined in Section 101 of the Jumpstart our Business Startups
Act (“JOBS Act”) as we do not have more than $1,000,000,000 in annual gross revenue and did not have such amount as
of December 31, 2015, our last fiscal year. We are electing to use the extended transition period for complying with new or revised
accounting standards under Section 102(b)(1) of the JOBS Act.
3.
Property and Equipment
|
|
|
June
30, 2016
|
|
|
December
31, 2015
|
|
|
Property and equipment:
|
|
|
|
|
|
|
|
|
|
Computer equipment
|
|
$
|
8,013
|
|
|
$
|
8,013
|
|
|
|
|
|
|
|
|
|
|
|
|
Less accumulated
depreciation
|
|
|
(7,312
|
)
|
|
|
(6,331
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Property and
equipment, net
|
|
$
|
701
|
|
|
$
|
1,682
|
|
The
Company utilizes the straight-line method for depreciation, using three to five-year depreciable asset lives. Depreciation expense
was not material for all periods presented.
4.
Accrued Liabilities
Accrued
expenses and other liabilities consist of the following:
|
|
|
June
30, 2016
|
|
|
December
31, 2015
|
|
|
|
|
(in thousands)
|
|
|
Accrued payroll to officers
and others
|
|
$
|
1,013
|
|
|
$
|
758
|
|
|
Accrued interest and penalties - notes
payable
|
|
|
843
|
|
|
|
237
|
|
|
Accrued legal settlements
|
|
|
174
|
|
|
|
282
|
|
|
Other accrued
liabilities
|
|
|
1
|
|
|
|
4
|
|
|
Total accrued expenses and other liabilities
|
|
$
|
2,031
|
|
|
$
|
1,281
|
|
5.
Notes Payable
Notes
payable consist of the following:
|
|
|
June
30, 2016
|
|
|
December
31, 2015
|
|
|
Promissory note issued
July 29, 2014 to Ira Gaines. The note matures on January 27, 2015 and earns interest at a rate of 18% per annum. The Company
was unable to repay the note at maturity and the note is in default, although no demand for repayment has been made by the
lender.
|
|
$
|
100,000
|
|
|
$
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Promissory notes
issued between November 26, 2014 and September 30, 2015, to raise up to $2,000,000 in debt. Lenders earn interest at a rate
of 10% per annum, plus a pro-rata share of two percent of the Company’s gross receipts for sales of IRT-103-LDN in perpetuity.
Notes will be repaid in 36 monthly installments of principal and interest commencing no later than October 15, 2015. Notes
aggregating $346,000 were in default at June 30, 2015, as the Company was unable to pay installments on those notes on their
due dates. No demands for repayment have been made by the lenders. One of these notes ($60,000), together with interest accrued
on the note to March 31, 2016 ($8,183), was converted to equity on August 4, 2016 for 852,292 shares of the Company’s
common stock. In addition, the note holder agreed to waive future payments of his pro-rata share of two percent of the Company’s
gross receipts for sales of Lodonal
TM
in perpetuity in return for 187,500 shares of the common stock of Cytocom.
|
|
|
346,000
|
|
|
|
711,500
|
|
|
Promissory note issued
October 17, 2014 to Roger Bozarth. The note matures on October 17, 2015 and earns interest at a rate of 2% per annum. The
lender converted this note to common stock in January 2016.
|
|
|
-
|
|
|
|
7,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Promissory notes issued between May
1, 2015 and June 30, 2016, and maturing between June 14, 2015 and June 30, 2017. Lenders on loans aggregating $834,927 earn
interest at rates between 10% and 18% per annum. On loans aggregating $198,500, interest is payable in a fixed amount not
tied to a specific interest rate. One of these notes ($278,933), together with interest accrued to April 1, 2016 ($18,828),
was converted on August 4, 2016 to 3,722,013 shares of the Company’s common stock. Notes aggregating $198,500 were in
default at June 30, 2016, as the Company was unable to repay those notes on their due dates. No demands for repayment have
been made by the lenders.
|
|
|
1,033,427
|
|
|
|
669,933
|
|
|
|
|
|
|
|
|
|
|
|
|
Promissory note issued January 26,
2015 to Robert J. Dailey. The note is senior to, and has priority in right of payment over, all indebtedness of the Company.
The note earns interest at a rate of 2% per annum and was due on July 30, 2015. The Company was unable to repay the note at
maturity and the note is in default, although no demand for repayment has been made by the lender. This note, together with
interest accrued to April 1, 2016 ($4,778), was converted on August 4, 2016 to 2,559,725 shares of the Company’s common
stock.
|
|
|
200,000
|
|
|
|
200,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Promissory notes issued by Cytocom
Inc. between April 29, 2015 and December 31, 2015. Lenders earn interest at rates between 5% and 10% per annum. These notes
mature on September 30, 2016. One of these notes ($350,000), together with interest accrued on the note to March 31, 2016
($11,304), was converted to equity on August 4, 2016 for 4,516,302 shares of the Company’s common stock.
|
|
|
775,000
|
|
|
|
800,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Promissory notes issued in December
2015. Lenders earn interest at a rate of 10% per month. Notes are repayable on March 9, 2016. The Company was unable to repay
the note at maturity and the note is in default. The Company is obligated to pay late-payment penalties totaling $5,000 per
day.
|
|
|
100,000
|
|
|
|
130,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Promissory note issued November 24,
2015 as settlement of amounts owing to a law firm. The Lender earns interest at the rate of 10% per annum. The note is repayable
in full on December 1, 2016. This note, together with interest accrued on the note to July 19, 2016 ($10,536), was converted
to equity on July 19, 2016 for 1,235,356 shares of the Company’s common stock.
|
|
|
175,268
|
|
|
|
175,268
|
|
|
|
|
|
|
|
|
|
|
|
|
Promissory notes issued between April
6, 2016 and June 2, 2016 that mature between October 1, 2016 and January 31, 2017, and include stock conversion features,
warrants and original issue debt discounts.
|
|
|
1,161,250
|
|
|
|
-
|
|
|
Promissory notes issued
to an officer of the Company effective November 3, 2015 and maturing November 3, 2016 for settlement of accrued payroll, bearing
interest at 10% per annum and including a stock conversion feature. One of these notes ($50,000), together with interest accrued
on the note to July 19, 2016 ($3,479), was converted to equity on July 19, 2016 for 1,069,589 shares of the Company’s
common stock.
|
|
|
162,737
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Less: Original
issue discounts on notes payable and warrants issued with notes.
|
|
|
(596,246
|
)
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
3,457,436
|
|
|
|
2,793,701
|
|
|
|
|
|
|
|
|
|
|
|
|
Less: Current
Portion
|
|
$
|
(3,457,436
|
)
|
|
$
|
(2,793,701
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Long-Term debt, less current portion
|
|
$
|
-
|
|
|
$
|
-
|
|
As
of June 30, 2016, the Company had accrued $843,275 in unpaid interest, compared to $236,671 as of December 31, 2015. These amounts
included default of penalties of $581,324 at June 30, 2016, compared to $18,954 at December 31, 2015. During the six months ended
June 30, 2016, 896,296 shares with a fair value of $149,000 were issued by the Company for interest expense under promissory notes.
6.
Capital Structure—Common Stock and Common Stock Purchase Warrants
Each
holder of common stock is entitled to vote on all matters and is entitled to one vote for each share held. No holder of shares
of stock of any class shall be entitled as a matter of right to subscribe for or purchase or receive any part of any new or additional
issue of shares of stock of any class, or of securities convertible into shares of stock or any class, whether now hereafter authorized
or whether issued for money, for consideration other than money, or by way of dividend.
As
of June 30, 2016 and 2015, the Company was authorized to issue 500,000,000 common shares at a par value of $0.0001 per share.
As
of June 30, 2016, the Company had 214,447,611 shares of common stock outstanding, and 174,850,047 outstanding as of December 31,
2015.
Stock
Warrants
In
the quarter ended June 30, 2016, the Company issued 23,654,908 warrants.
In
the quarter ended June 30, 2016, the Company extended to December 31, 2018 the maturity dates on 5,006,666 existing warrants that
were originally issued with expiration dates between July 25, 2016 to December 17, 2018. These warrants were originally issued
with 3 to 5 year terms, with exercise prices ranging between $0.75 and $1.00.
There
were no modifications of the terms of any warrants issued by the Company in the quarter ended June 30, 2015.
Following
is a summary of outstanding stock warrants at June 30, 2016 and activity during the six months then ended:
|
|
|
Number of Shares
|
|
|
Exercise
Price
|
|
|
Weighted
Average
Price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants as of December 31, 2015
|
|
|
9,131,500
|
|
|
$
|
0.07-15.00
|
|
|
$
|
1.47
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issued in 2016
|
|
|
23,654,908
|
|
|
$
|
0.14-2.00
|
|
|
$
|
0.32
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expired
|
|
|
(37,500
|
)
|
|
$
|
5.00
|
|
|
$
|
5.00
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
(0
|
)
|
|
$
|
0
|
|
|
$
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants as of June 30, 2016
|
|
|
32,748,908
|
|
|
$
|
0.07-15.00
|
|
|
$
|
0.54
|
|
Summary
of outstanding warrants as of June 30, 2016:
|
Expiration
Date
|
|
Number of Shares
|
|
|
Exercise
Price
|
|
|
Remaining
Life
(years)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Third Quarter 2016
|
|
|
125,000
|
|
|
$
|
5.00
|
|
|
|
.25
|
|
|
Fourth Quarter 2017
|
|
|
350,000
|
|
|
|
1.50-9.00
|
|
|
|
1.50
|
|
|
First Quarter 2018
|
|
|
127,500
|
|
|
$
|
15.00
|
|
|
|
1.75
|
|
|
Second Quarter 2018
|
|
|
33,334
|
|
|
$
|
15.00
|
|
|
|
2.00
|
|
|
Third Quarter 2018
|
|
|
250,000
|
|
|
$
|
1.50
|
|
|
|
2.25
|
|
|
Fourth Quarter 2018
|
|
|
6,089,166
|
|
|
$
|
1.00-1.50
|
|
|
|
2.50
|
|
|
First Quarter 2019
|
|
|
4,024,000
|
|
|
$
|
0.50-2.00
|
|
|
|
2.75
|
|
|
Second Quarter 2019
|
|
|
135,000
|
|
|
$
|
0.07–0.23
|
|
|
|
3.00
|
|
|
Third Quarter 2019
|
|
|
260,000
|
|
|
$
|
0.50-1.50
|
|
|
|
3.25
|
|
|
Fourth Quarter 2019
|
|
|
400,000
|
|
|
$
|
0.14
|
|
|
|
3.50
|
|
|
Second Quarter 2020
|
|
|
300,000
|
|
|
$
|
0.50
|
|
|
|
4.00
|
|
|
Third Quarter 2020
|
|
|
1,000,000
|
|
|
$
|
0.20
|
|
|
|
4.25
|
|
|
Fourth Quarter 2020
|
|
|
12,650,000
|
|
|
$
|
0.20
|
|
|
|
4.50
|
|
|
First Quarter 2021
|
|
|
7,004,908
|
|
|
$
|
0.10-0.20
|
|
|
|
4.75
|
|
7.
Stock Compensation
Shares
Issued for Services
During
the six months ended June 30, 2016 and 2015, the Company issued 23,643,000 and 10,239,170 shares of common stock respectively
for consulting fees. The Company valued these shares at $4,543,170 and $1,810,876 respectively, based upon the fair value of the
common stock at the dates of the agreements. The consulting fees are amortized over the contract periods which are typically between
12 and 24 months. The amortization of prepaid services totaled $1,539,928 and $3,530,894 for the six months ended June 30, 2016
and 2015.
8.
Income Taxes
-
Results of Operations
There
was no income tax expense reflected in the results of operations for the quarters ended June 30, 2016 and 2015 because the Company
incurred a net loss in both quarters.
The
Company has recognized no tax benefit for the losses generated for the periods through December 31, 2015. ASC Topic 740 requires
that a valuation allowance be provided if it is more likely than not that some portion or all of a deferred tax asset will not
be realized. The Company’s ability to realize the benefit of its deferred tax asset will depend on the generation of future
taxable income. Because the Company has yet to recognize revenue, we believe that the full valuation allowance should be provided.
The
Company’s effective tax rate for fiscal years 2015 and 2013 was 0%. The Company’s tax rate can be affected by recurring
items, such as tax rates in foreign jurisdictions and the relative amount of income we earn in jurisdictions. It may also be affected
by discrete items that may occur in any given year, but are not consistent from year to year.
As
of December 31, 2015, the Company has estimated federal and state income tax net operating loss (“NOL”) carry-forwards
of approximately $66,500,000, which will expire in 2032-2035.
9.
Licenses and Supply Agreements
Patent
and Subsidiary Acquisition
The
Company has not entered into any new licenses or supply agreements during quarter ending June 30, 2016. All prior licenses, supply
agreements, and patents remain unchanged from the prior quarter.
10.
Commitments and Contingencies
Except
as described below,
t
he Company has not created any new commitments or contingency obligations during quarter ending June
30, 2016. All other prior commitments and contingent obligations remain unchanged from the prior quarter.
Distribution
of Lodonal
TM
in Nigeria
Through
its wholly-owned subsidiary, TNI BioTech International, Ltd., in December 2015 the Company completed a 90-day bridging trial for
the treatment of patients with HIV/AIDS. The trial consisted of a total of 150 patients of both genders between the ages of 18-60,
each of whom was infected HIV/AIDS. The primary objective of this bridging trial was to confirm that Lodonal
TM
has
a beneficial effect on the immune system of immune deficient patients and that it is safe. The trial separated patients into a
Control (placebo) Group and a Treatment Group (which was administered Lodonal
TM
). The efficacy of increasing CD4 count
[cell/mm3] between Day-1 and Day-90 by at least 25% was set as the criteria for demonstrating beneficial effect on the immune
system. Safety was demonstrated through quality of life assessment and vitals both of which were not adversely affected. Treatment
Group patients were given a daily dose of 4.5-mg/kg of Lodonal.
In
April 2016, the Company announced that Nigeria’s National Agency for Food and Drug Administration and Control (“NAFDAC”)
had approved its Lodonal
TM
as an over the counter, non-toxic adjunct therapy in the treatment of HIV/AIDS and immune
system regulator. NAFDAC is the Nigerian agency under the Federal Ministry of Health that is responsible for regulating and controlling
the manufacture, import/export, distribution, sale and use of food and drugs. The NAFDAC approval clears the way for the Company
and its distribution partners to complete the registration of Lodonal
TM
for sale in Nigeria. The Company is now in
the process of completing the registration to import the drug into Nigeria
Contract
Manufacturing Agreements
On
May 16, 2016, the Company entered into an agreement with Complete Pharmacy and Medical Solutions, LLC (“CPMS”) to
compound, package and distribute the LDN tablets, capsules and/or creams in the United States . The initial term of the agreement
is three years, with the option to renew for an additional year. The agreement may be terminated by (i) mutual agreement, (ii)
in the event of a breach, provided however that if the Company terminates the contract to reimburse CPMS for all unused packaging
materials for the LDN, which unused packaging materials CPMS will provide to IMUN. If CPMS does not receive and ship at least
1,000 orders (prescriptions), the Company will be required to reimburse CPMS for 100% of the “ramp up costs” (defined
as all costs and expenses of labor and materials related to the testing, and required FDA and other governmental documentation/approvals
of test data) of providing and producing the LDN, even where the Company cancels/terminates the Agreement, which provision shall
survive the cancellation/termination of this Agreement.
Operating
Leases
At
June 30, 2016, the Company was a party to an agreement to lease office space in Orlando, Florida. Rent expense for the quarters
ended June 30, 2016 and 2015 was $4,011 and $18,319, respectively.
Legal
Proceedings
None.
11.
Subsequent Events
In
July 2016, the Company amended the terms of a $100,000 note payable that was due of March 29, 2016. In accordance with the amendment,
the amount of the note was increased to $130,000, the maturity date was extended to July 31, 2016, and the number of Company shares
due on the original note was increased from 100,000 to 225,000. The note is in default.
Between
July 1, 2016 and August 12, 2016, the Company repaid or amended the following notes payable through conversions of obligations
into shares of the Company’s common stock as follows:
|
|
●
|
A
note payable to an officer ($50,000), together with interest accrued on the note to July 14, 2016 ($3,479), was converted
to equity on July 19, 2016 for 1,069,589 shares of the Company’s common stock.
|
|
|
|
|
|
|
●
|
A
note payable ($175,267), together with interest accrued on the note to June 30, 2016 ($10,536), was converted to equity on
July 19, 2016 for 1,235,356 shares of the Company’s common stock.
|
|
|
●
|
A
note payable by Cytocom ($350,000), together with interest accrued on the note to April 1, 2016 ($11,304), was converted to
equity on August 4, 2016 for 4,516,302 shares of the Company’s common stock .
|
|
|
|
|
|
|
●
|
A
note payable ($60,000), together with interest accrued on the note to April 1, 2016 ($8,183), was converted to equity on August
4, 2016 for 852,292 shares of the Company’s common stock. In addition, the note holder agreed to waive future payments
of his pro-rata share of two percent of the Company’s gross receipts for sales of Lodonal
TM
in perpetuity
in return for 187,500 shares of the common stock of Cytocom.
|
|
|
|
|
|
|
●
|
Two
notes payable ($200,000 and $278,933), together with interest accrued on the note to April 1, 2016 ($23,606), were converted
to equity on August 4, 2016 for 6,281,738 shares of the Company’s common stock.
|
In
July 2016, the Company issued promissory notes as follows:
|
|
●
|
A
note payable, dated July 5, 2016, for $50,000, paying interest at 6% per month, and maturing on October 5, 2016.
|
|
|
|
|
|
|
●
|
A
note payable, issued on July 7, 2016, for $180,000, maturing on March 07, 2017. The Company received $150,000 upon issuance
of the note. The Company must apply the consideration towards obtaining governmental approvals for the use and sale of Lodonal™
in Malawi, Africa. If the Company fails to repay the note at maturity, the holder has the right, at any time after the maturity
date, at its election, to convert all or part of the outstanding and unpaid principal and accrued into shares common stock
of the Company, in number equal to the dollar conversion amount divided by the lesser of $0.15 or 80% of the lowest trade
price in the 25 trading days prior to the conversion.
|
In
July 2016, the Company issued shares of the Company’s common stock for services as follows:
|
|
●
|
1,000,000
shares issued on July 14, 2016 as a bonus under an agreement to provide investor relations services.
|
|
|
|
|
|
|
●
|
200,000
shares issued on July 19, 2016 for services related to the trial of Lodonal
TM
in Nigeria.
|
Between
July 1, 2016 and August 12, 2016, the Company issued warrants as follows:
|
|
●
|
A
warrant issued to Paul Akin, director, on July 1, 2016, for services as director, to purchase 1,000,000 shares of the Company’s
common stock at $0.15 per share. The warrant expires on June 30, 2021.
|
|
|
|
|
|
|
●
|
A
warrant issued to Nicholas Plotnikoff, director, on August 5, 2016, as payment for services previously rendered as director,
to purchase 150,000 shares of the Company’s common stock at $0.20 per share. The warrant expires on July 28, 2021.
|
REPORT
OF INDEPENDENT REGISTERED Public Accounting Firm
Board
of Directors and Stockholders
Immune
Therapeutics, Inc.
Orlando,
Florida
We
have audited the accompanying consolidated balance sheets of Immune Therapeutics, Inc. and its subsidiaries (the “Company”)
as of December 31, 2015 and 2014 and the related consolidated statements of operations, stockholders’ equity/(deficit) and
cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our
responsibility is to express an opinion on these financial statements based on our audits.
We
conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are
free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in
the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management,
as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for
our opinion.
In
our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position
of Immune Therapeutics, Inc. and its subsidiaries as of December 31, 2015 and 2014, and the results of their consolidated operations
and their cash flows for the years then ended in conformity with accounting principles generally accepted in the United States
of America.
The
accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern.
As discussed in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations since
inception and has a working capital deficiency both of which raise substantial doubt about its ability to continue as a going
concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements
do not include any adjustments that might result from the outcome of this uncertainty.
|
/s/
Turner, Stone & Company, L.L.P.
|
|
|
|
|
|
Dallas,
Texas
|
|
|
March
30, 2016
|
|
CONSOLIDATED
BALANCE SHEETS
DECEMBER
31, 2015 AND 2014
|
|
|
2015
|
|
|
2014
|
|
|
|
|
|
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current Assets:
|
|
|
|
|
|
|
|
|
|
Cash
and cash equivalents
|
|
$
|
23,149
|
|
|
$
|
191,987
|
|
|
Accounts receivable
|
|
|
16,197
|
|
|
|
-
|
|
|
Prepaids
and other current assets
|
|
|
-
|
|
|
|
30,000
|
|
|
Total
current assets
|
|
|
39,346
|
|
|
|
221,987
|
|
|
|
|
|
|
|
|
|
|
|
|
Fixed Assets:
|
|
|
|
|
|
|
|
|
|
Computer equipment,
net of accumulated depreciation of $6,331 and $3,778 respectively
|
|
|
1,682
|
|
|
|
4,235
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible Assets:
|
|
|
|
|
|
|
|
|
|
Patents and licenses,
net of amortization of $1,793,911 and $1,201,678, respectively
|
|
|
-
|
|
|
|
5,818,585
|
|
|
|
|
|
|
|
|
|
|
|
|
Deposits
|
|
|
200
|
|
|
|
10,183
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
41,228
|
|
|
$
|
6,054,990
|
|
|
LIABILITIES AND
STOCKHOLDERS’ EQUITY/(DEFICIT)
|
|
|
|
|
|
|
|
|
|
Current Liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
1,924,672
|
|
|
$
|
2,077,194
|
|
|
Accrued liabilities
|
|
|
1,281,039
|
|
|
|
1,111,839
|
|
|
Current
portion of notes payable
|
|
|
2,793,701
|
|
|
|
186,067
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
current liabilities
|
|
|
5,999,412
|
|
|
|
3,375,100
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current liabilities:
|
|
|
|
|
|
|
|
|
|
Notes
payable, less current portion
|
|
|
-
|
|
|
|
327,458
|
|
|
Total
non-current liabilities
|
|
|
-
|
|
|
|
327,458
|
|
|
Total liabilities
|
|
|
5,999,412
|
|
|
|
3,702,558
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and Contingencies (Note
10)
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity/(Deficit):
|
|
|
|
|
|
|
|
|
|
Common stock - par
value $0.0001; 500,000,000 shares authorized; 174,850,047 and 134,417,210 shares issued and outstanding respectively
|
|
|
17,485
|
|
|
|
13,442
|
|
|
Additional paid
in capital
|
|
|
343,434,786
|
|
|
|
337,985,787
|
|
|
Stock issuances
due
|
|
|
1,140,303
|
|
|
|
847,279
|
|
|
Prepaid services
|
|
|
(660,417
|
)
|
|
|
(3,553,186
|
)
|
|
Accumulated
deficit
|
|
|
(349,861,173
|
)
|
|
|
(333,547,377
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Equity/(Deficit)
attributable to common shareholders
|
|
|
(3,857,732
|
)
|
|
|
1,745,945
|
|
|
Non-controlling
interest
|
|
|
(2,100,452
|
)
|
|
|
606,487
|
|
|
Total
stockholders’ equity/ (deficit)
|
|
|
(5,958,184
|
)
|
|
|
2,352,432
|
|
|
Total
liabilities and stockholders’ equity/(deficit)
|
|
$
|
41,228
|
|
|
$
|
6,054,990
|
|
The
accompanying footnotes are an integral part of these consolidated financial statements
CONSOLIDATED
STATEMENTS OF OPERATIONS
FOR
THE YEARS ENDED DECEMBER 31, 2015 AND 2014
|
|
|
2015
|
|
|
2014
|
|
|
|
|
|
|
|
|
|
|
Revenues,
net
|
|
$
|
16,197
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative
|
|
|
2,734,414
|
|
|
|
4,072,277
|
|
|
Research and development expense
|
|
|
977,203
|
|
|
|
2,413,286
|
|
|
Stock issued for services general and
administrative
|
|
|
6,240,143
|
|
|
|
19,116,075
|
|
|
Stock issued for services research and
development
|
|
|
-
|
|
|
|
5,126,250
|
|
|
Warrant valuation
|
|
|
46,189
|
|
|
|
1,748,576
|
|
|
Depreciation and amortization expense
|
|
|
594,785
|
|
|
|
2,879,311
|
|
|
Impairment of
intangible assets
|
|
|
5,226,352
|
|
|
|
9,908,477
|
|
|
Total operating expenses
|
|
|
15,819,086
|
|
|
|
45,264,252
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(15,802,889
|
)
|
|
|
(45,264,252
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Other expense:
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
(270,989
|
)
|
|
|
(388,221
|
)
|
|
Impairment of investment
|
|
|
(32,000
|
)
|
|
|
-
|
|
|
Foreign exchange loss
|
|
|
-
|
|
|
|
(783
|
)
|
|
Loss on settlement
of debt
|
|
|
(843,573
|
)
|
|
|
(4,284,957
|
)
|
|
Total other expense
|
|
|
(1,146,562
|
)
|
|
|
(4,673,961
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
(16,949,451
|
)
|
|
|
(49,938,213
|
)
|
|
Net loss attributable
to non-controlling interest
|
|
|
(2,706,939
|
)
|
|
|
(1,684,996
|
)
|
|
Net loss attributable
to common shareholders
|
|
$
|
(14,242,512
|
)
|
|
$
|
(48,253,217
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted
loss per share attributed to common shareholders
|
|
$
|
(0.09
|
)
|
|
$
|
(0.49
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares
outstanding
|
|
|
153,247,023
|
|
|
|
98,508,458
|
|
The
accompanying footnotes are an integral part of these consolidated financial statements.
CONSOLIDATED
STATEMENTS OF STOCKHOLDERS’ EQUITY/ (DEFICIT)
FOR
THE YEARS ENDED DECEMBER 31, 2015 AND 2014
|
|
|
Common
Stock
|
|
|
Additional
Paid-in
|
|
|
Stock
to
|
|
|
Prepaid
|
|
|
Accumulated
|
|
|
Non-Controlling
|
|
|
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Be
Issued
|
|
|
Services
|
|
|
Deficit
|
|
|
Interest
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance December 31, 2013
|
|
|
74,161,639
|
|
|
$
|
7,416
|
|
|
$
|
308,180,119
|
|
|
$
|
4,893,499
|
|
|
$
|
(13,447,109
|
)
|
|
$
|
(283,002,678
|
)
|
|
$
|
-
|
|
|
$
|
16,631,247
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for prepaid
services
|
|
|
17,178,294
|
|
|
|
1,718
|
|
|
|
11,275,282
|
|
|
|
787,501
|
|
|
|
(12,962,001
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(897,500
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Return of common stock for charitable
donation
|
|
|
(100,000
|
)
|
|
|
(10
|
)
|
|
|
(749,990
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(750,000
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of prepaid services
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
22,855,925
|
|
|
|
-
|
|
|
|
-
|
|
|
|
22,855,925
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for patents
and license fees
|
|
|
1,600,000
|
|
|
|
160
|
|
|
|
467,840
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
468,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for legal settlement
|
|
|
650,000
|
|
|
|
65
|
|
|
|
448,435
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
448,500
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock to employees
and consultants
|
|
|
6,329,000
|
|
|
|
633
|
|
|
|
3,176,424
|
|
|
|
(1,028,157
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
2,148,900
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock in exchange
for debt
|
|
|
24,037,450
|
|
|
|
2,404
|
|
|
|
8,250,396
|
|
|
|
(1,150,000
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
7,102,800
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for loan expenses
and interest
|
|
|
670,000
|
|
|
|
67
|
|
|
|
527,183
|
|
|
|
(224,800
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
302,450
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for cash and
exercise of warrants
|
|
|
9,890,827
|
|
|
|
989
|
|
|
|
4,661,522
|
|
|
|
(2,430,764
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
2,231,747
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance and modification of common
stock warrants
|
|
|
-
|
|
|
|
-
|
|
|
|
1,748,576
|
|
|
|
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,748,576
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
-
|
|
|
|
(48,253,217
|
)
|
|
|
(1,684,996
|
)
|
|
|
(49,938,213
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-controlling
interest in Cytocom, Inc. (Note 2)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
-
|
|
|
|
(2,291,483
|
)
|
|
|
2,291,483
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance December 31, 2014
|
|
|
134,417,210
|
|
|
$
|
13,442
|
|
|
$
|
337,985,787
|
|
|
$
|
847,279
|
|
|
$
|
(3,553,185
|
)
|
|
$
|
(333,547,378
|
)
|
|
$
|
606,487
|
|
|
$
|
2,352,432
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for prepaid
services
|
|
|
16,672,504
|
|
|
|
1,667
|
|
|
|
2,730,708
|
|
|
|
535,000
|
|
|
|
(1,436,000
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
1,831,376
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of prepaid services
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
4,328,768
|
|
|
|
-
|
|
|
|
-
|
|
|
|
4,328,768
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for investment
|
|
|
400,000
|
|
|
|
40
|
|
|
|
31,960
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
32,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for legal settlement
|
|
|
1,000,000
|
|
|
|
100
|
|
|
|
79,900
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
80,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for interest
expense
|
|
|
62,500
|
|
|
|
6
|
|
|
|
15,619
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
15,625
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock in exchange
for debt
|
|
|
14,058,833
|
|
|
|
1,406
|
|
|
|
1,954,971
|
|
|
|
(257,000
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
1,699,377
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for cash and
exercise of warrants
|
|
|
8,239,000
|
|
|
|
824
|
|
|
|
589,651
|
|
|
|
15,025
|
|
|
|
-
|
|
|
|
|
|
|
|
-
|
|
|
|
605,500
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance and modification of common
stock warrants
|
|
|
-
|
|
|
|
|
|
|
|
46,189
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
46,189
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(14,242,512
|
)
|
|
|
(2,706,939
|
)
|
|
|
(16,949,451
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance December 31, 2015
|
|
|
174,850,047
|
|
|
$
|
17,485
|
|
|
$
|
343,434,786
|
|
|
$
|
1,140,303
|
|
|
$
|
(660,417
|
)
|
|
$
|
(347,789,890
|
)
|
|
$
|
(2,100,452
|
)
|
|
$
|
(5,958,184
|
)
|
The
accompanying footnotes are an integral part of these consolidated financial statements.
CONSOLIDATED
STATEMENTS OF CASH FLOWS
FOR
THE YEARS ENDED DECEMBER 31, 2015 AND 2014
|
|
|
2015
|
|
|
2014
|
|
|
CASH FLOWS FROM OPERATING
ACTIVITIES
|
|
|
|
|
|
|
|
|
|
Net
loss including non-controlling interest
|
|
$
|
(16,949,451
|
)
|
|
$
|
(49,938,213
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments to reconcile
net loss to net cash flows used in operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation
|
|
|
2,552
|
|
|
|
2,485
|
|
|
Amortization
|
|
|
592,233
|
|
|
|
2,876,826
|
|
|
Stock issued for
services
|
|
|
1,911,375
|
|
|
|
1,251,401
|
|
|
Stock issued for
legal settlement
|
|
|
|
|
|
|
448,500
|
|
|
Stock issued for
patents and license fees
|
|
|
-
|
|
|
|
468,000
|
|
|
Amortization of
stock issued for prepaid services
|
|
|
4,328,768
|
|
|
|
22,891,924
|
|
|
Impairment of intangible
assets
|
|
|
5,226,352
|
|
|
|
9,908,477
|
|
|
Impairment of investment
|
|
|
32,000
|
|
|
|
0
|
|
|
Debt discount
|
|
|
|
|
|
|
25,000
|
|
|
Loss on settlement
of debt and accounts payable
|
|
|
843,573
|
|
|
|
4,248,957
|
|
|
Stock warrant expense
|
|
|
46,189
|
|
|
|
1,748,576
|
|
|
Stock issued for
donation
|
|
|
-
|
|
|
|
(750,000
|
)
|
|
Stock issued for
loan expenses and interest
|
|
|
15,625
|
|
|
|
302,450
|
|
|
Changes in operating
assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(16,197
|
)
|
|
|
-
|
|
|
Prepaid expenses
and other current assets
|
|
|
39,983
|
|
|
|
246,342
|
|
|
Accrued liabilities
|
|
|
194,201
|
|
|
|
630,662
|
|
|
Accounts payable
|
|
|
900,484
|
|
|
|
1,795,518
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(2,832,313
|
)
|
|
|
(3,843,095
|
)
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING
ACTIVITIES
|
|
|
|
|
|
|
|
|
|
Purchase of licenses
|
|
|
-
|
|
|
|
(57,340
|
)
|
|
Purchase of computer
equipment
|
|
|
-
|
|
|
|
(1,113
|
)
|
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided
by/ (used in) investing activities
|
|
|
-
|
|
|
|
(58,453
|
)
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING
ACTIVITIES
|
|
|
|
|
|
|
|
|
|
Proceeds from sale
of common stock and exercise of warrants
|
|
|
605,500
|
|
|
|
2,231,747
|
|
|
Proceeds from notes
payable
|
|
|
2,057,975
|
|
|
|
1,623,525
|
|
|
Payments made on
patent liability
|
|
|
-
|
|
|
|
(118,333
|
)
|
|
Repayment of notes
payable
|
|
|
-
|
|
|
|
(50,000
|
)
|
|
Net cash provided
by financing activities
|
|
|
2,663,475
|
|
|
|
3,686,939
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Decrease in cash
|
|
|
(168,838
|
)
|
|
|
(214,609
|
)
|
|
Cash and cash equivalents,
beginning of year
|
|
|
191,987
|
|
|
|
406,596
|
|
|
Cash and cash equivalents,
end of year
|
|
$
|
23,149
|
|
|
$
|
191,987
|
|
The
accompanying footnotes are an integral part of these consolidated financial statements.
CONSOLIDATED
STATEMENTS OF CASH FLOWS
FOR
THE YEARS ENDED DECEMBER 31, 2015 and 2014
|
|
|
2015
|
|
|
2014
|
|
|
SUPPLEMENTAL DISCLOSURES
OF CASH FLOW INFORMATION:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for interest
|
|
$
|
20,500
|
|
|
$
|
4,538
|
|
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL DISCLOSURES
OF NON-CASH INVESTING AND FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of debt and accrued interest
to common stock
|
|
$
|
0
|
|
|
$
|
2,159,248
|
|
|
Shares issued for accounts payable and
accrued liabilities
|
|
$
|
855,804
|
|
|
$
|
580,404
|
|
|
Non-controlling interest
|
|
$
|
635,655
|
|
|
$
|
2,105,062
|
|
|
Settlement of note payable by issuance
of new note payable
|
|
$
|
50,000
|
|
|
$
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes payable for expenses paid by lender
|
|
$
|
46,933
|
|
|
$
|
0
|
|
The
accompanying footnotes are an integral part of these consolidated financial statements.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
1.
Organization and Description of Business
Immune
Therapeutics, Inc. (the “Company,” “we,” or “our”) was initially incorporated in Florida on
December 2, 1993 as Resort Clubs International, Inc. (“Resort Clubs”). It was formed to manage and market golf course
properties in resort markets throughout the United States. Galliano International Ltd. (“Galliano”) was incorporated
in Delaware on May 27, 1998 and began trading in November 1999 through the filing of a 15C-211. On November 10, 2004, Galliano
merged with Resort Clubs. Resort Clubs was the surviving corporation. On August 23, 2010, Resort Clubs changed its name to pH
Environmental Inc. (“pH Environmental”).
On
April 23, 2012, pH Environmental completed a name change to TNI BioTech, Inc., and on April 24, 2012, we executed a share exchange
agreement for the acquisition of all of the outstanding shares of TNI BioTech IP, Inc. On September 4, 2014, a majority of our
shareholders approved an amendment to our Amended and Restated Articles of Incorporation, as amended, to change our name to Immune
Therapeutics, Inc. We filed our name change amendment with the Secretary of State of Florida on October 27, 2014 changing our
name to Immune Therapeutics, Inc.
The
Company currently has an office in Orlando, Florida. In July 2012, the Company’s focus turned to acquiring patents that
would protect and advance the development of new uses of opioid-related immune- therapies, such as low dose naltrexone (“LDN”)
and Methionine [Met5]-enkephalin (“MENK”).
In
October 2012, the Company formed TNI BioTech International, Ltd., a BVI company in Tortola, British Virgin Islands, which was
set up to allow the Company to market and sell LDN in those countries outside the U.S. in which we have been able to obtain approval
to sell the Company’s products.
In
August 2013, the Company formed its United Kingdom subsidiary, TNI BioTech, LTD (the “UK Subsidiary”). The UK Subsidiary
received approval to be considered a micro, small or medium-sized enterprise (“SME”) with the European Medicines Agency
(“EMA”) on August 21, 2013. The designation provides the UK Subsidiary with significant discounts when holding meetings
or submitting filings to the EMA. On September 19, 2013, the UK Subsidiary submitted a pre-submission package to the EMA regarding
Crohn’s Disease. The EMA granted the UK Subsidiary a meeting that took place on September 27, 2013. The UK Subsidiary is
eligible to benefit from the provisions for administrative and financial assistance for SMEs set out in Regulation (EC) No 2049/2005.
The Company will apply to obtain EMA benefits once funding becomes available.
In
December 2013, the Company formed a new subsidiary, Cytocom Inc., to focus on conducting LDN and MENK clinical trials in the United
States. In December 2014, the Company completed the distribution of common stock of Cytocom Inc. to its shareholders. As part
of the transaction, the Company retained exclusive rights to all international patents, in-country approvals, formulations, trademarks,
manufacturing, marketing, sales, and distributions rights in emerging nations, including Africa, Central America, South America,
Russia, India, China, Far East, and The Commonwealth of Independent States (former Soviet Union). The Company will continue to
have access to existing clinical data as well as any new data generated by Cytocom Inc. during drug development. On December 8,
2014, the number of Cytocom Inc. shares of common stock that were issued to our shareholders totaled 113,242,522 shares. In connection
with the transaction, Cytocom Inc. issued 140,100,000 shares of its common stock to the Company, which gave the Company a 55.3%
stake in Cytocom Inc. on that date. The stake has since been reduced to 50.2% at December 31, 2015, by subsequent issuances of
Cytocom common stock to shareholders.
In
March 2014, the Company incorporated Airmed Biopharma Limited, an Irish corporation with an address in Dublin, Ireland, and Airmed
Holdings Limited, an Irish company domiciled in Bermuda. The Irish companies were set up to benefit from incentives granted by
the Irish government for the establishment of pharmaceutical companies (many of the world’s leading pharmaceutical companies
have located in Ireland), and so that the Company could take advantage of Ireland’s status as a member of the European Union
and the European Economic Area. An Irish limited liability company enjoys a low corporate income tax rate of 12.5%, one of the
lowest in the world. The Irish-domiciled company hopes to qualify for tax incentives for Irish holding/headquartered companies
and to benefit from the network of double tax treaties that reduce withholding taxes. TNI BioTech International, Ltd. will manage
our international distribution, using product that is manufactured in Ireland and elsewhere.
We
are focused on the development and commercialization of therapeutic treatments for cancer, HIV/AIDS and autoimmune diseases and
immune disorders by combating these severe and fatal diseases through the stimulation and/or regulation of the body’s immune
system. Our growth strategy includes the near-term commercialization of our existing immunotherapies targeting cancer, Crohn’s
disease and/or HIV/AIDS.
Going
Concern
The
Company has incurred significant net losses since inception and has relied on its ability to fund its operations through private
equity financings. Management expects operating losses and negative cash flows to continue at more significant levels in the future.
As the Company continues to incur losses, transition to profitability is dependent upon the successful development, approval,
and commercialization of its product candidate and the achievement of a level of revenues adequate to support the Company’s
cost structure. The Company may never achieve profitability, and unless and until it does, the Company will continue to need to
raise additional cash. Management intends to fund future operations through additional private or public debt or equity offerings,
and may seek additional capital through arrangements with strategic partners or from other sources. Based on the Company’s
operating plan, existing working capital at December 31, 2015 was not sufficient to meet the cash requirements to fund planned
operations through December 31, 2016 without additional sources of cash. These conditions raise substantial doubt about the Company’s
ability to continue as a going concern. The accompanying financial statements have been prepared assuming that the Company will
continue as a going concern and do not include adjustments that might result from the outcome of this uncertainty. This basis
of accounting contemplates the recovery of the Company’s assets and the satisfaction of liabilities in the normal course
of business.
The
Company has experienced a net loss from operations of $16,949,451 and has used cash and cash equivalents for operations in the
amount of $2,832,213 during the year ended December 31, 2015, resulting in a stockholder’s deficit of $5,958,184.
2.
Summary of Significant Accounting Policies
Basis
of Presentation
The
accompanying consolidated financial statements have been prepared in accordance with United States (U.S.) generally accepted accounting
principles (U.S. GAAP) and include all adjustments necessary for the fair presentation of the Company’s financial position
for the periods presented.
The
Company qualifies as an “emerging growth company” as defined in Section 101 of the Jumpstart our Business Startups
Act (“JOBS Act”) as we do not have more than $1,000,000,000 in annual gross revenue for the year ended December 31,
2014. We are electing to use the extended transition period for complying with new or revised accounting standards under Section
102(b)(1) of the JOBS Act.
Revenue
Recognition
Revenue
from product sales is recognized upon passage of title and risk of loss to customers. Provisions for discounts, rebates and sales
incentives to customers, and returns and other adjustments are provided for in the period the related sales are recorded. Historical
data are not yet readily available and reliable for use in estimating the amount of the reduction in gross sales. Revenue from
the launch of a new product, from an improved version of an existing product, or for shipments in excess of a customer’s
normal requirements will be recorded when the conditions noted above are met. In those situations, management will record a returns
reserve for such revenue, if necessary. If in future the Company participates in selling arrangements that include multiple deliverables
(e.g., instruments, reagents, procedures, and service agreements), under these arrangements, the Company will recognize revenue
upon delivery of the product or performance of the service and will allocate the revenue based on the relative selling price of
each deliverable, which will be based primarily on vendor specific objective evidence. Revenue from license of product rights
is recorded over the periods earned.
In
May 2014, the Financial Accounting Standards Board issued Accounting Standards Update No. 2014-09, Revenue from Contracts with
Customers, which provides a single comprehensive model for accounting for revenue from contracts with customers and will supersede
most existing revenue recognition guidance. Early adoption is not permitted. The standard becomes effective for the Company in
the first quarter of 2017. The Company is currently evaluating the effect, if any, that the standard will have on its consolidated
financial statements and related disclosures.
Use
of Estimates
The
preparation of the Company’s financial statements in conformity with U.S. generally accepted accounting principles requires
management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes.
Actual results could differ from such estimates.
Cash,
Cash Equivalents, and Short-Term Investments
The
Company considers all highly liquid investments with original maturities at the date of purchase of three months or less to be
cash equivalents. Cash and cash equivalents include bank demand deposits, marketable securities with maturities of three months
or less at purchase, and money market funds that invest primarily in certificates of deposits, commercial paper and U.S. government
and U.S. government agency obligations. Cash equivalents are reported at fair value.
Concentration
of Credit Risk
Financial
instruments that potentially subject the Company to concentrations of credit risk are primarily cash and cash equivalents. The
Company is exposed to credit risk, subject to federal deposit insurance, in the event of a default by the financial institutions
holding its cash and cash equivalents to the extent of amounts recorded on the balance sheets. The cash accounts are insured by
the Federal Deposit Insurance Corporation up to $250,000. At December 31, 2015, the Company has no cash balances in excess of
insured limits.
Segment
and Geographic Information
Operating
segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the
chief operating decision maker, or decision making group, in deciding how to allocate resources and in assessing performance.
The Company views its operations and manages its business in one operating segment and does not segment the business for internal
reporting or decision making.
Fair
Value of Financial Instruments
In
accordance with the reporting requirements of Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC)
Topic 825, “
Financial Instruments”
, the Company calculates the fair value of its assets and liabilities which
qualify as financial instruments under this standard and includes this additional information in the notes to the financial statements
when the fair value is different than the carrying value of those financial instruments. Cash and accounts payable are accounted
for at cost which approximates fair value due to the relatively short maturity of these instruments. The carrying value of notes
payable also approximate fair value since they bear market rates of interest and other terms. None of these instruments are held
for trading purposes.
Fair
Value Measurements
The
ASC Topic 820,
Fair Value Measurement,
defines fair value, establishes a framework for measuring fair value in accordance
with U.S. generally accepted accounting principles, and requires certain disclosures about fair value measurements. In general,
fair values of financial instruments are based upon quoted market prices, where available. If such quoted market prices are not
available, fair value is based upon internally developed models that primarily use, as inputs, observable market-based parameters.
Valuation adjustments may be made to ensure that financial instruments are recorded at fair value. These adjustments may include
amounts to reflect counterparty credit quality and the customer’s creditworthiness, among other things, as well as unobservable
parameters. Any such valuation adjustments are applied consistently over time.
Property
and Equipment
Property
and equipment are stated at cost, less accumulated depreciation. Depreciation is determined on a straight-line basis over the
estimated useful lives of the assets, which generally range from three to five years. Maintenance and repairs are charged against
expense as incurred. Depreciation expense from continuing operations for the years ended December 31, 2015 and December 31, 2014
was $2,552 and $2,485, respectively.
Intangible
Assets
Costs
incurred to acquire and/or develop the Company’s product licenses and patents are capitalized and amortized by straight-line
methods over estimated useful lives of ten to sixteen years. Intangible assets are stated at the lower of cost or estimated fair
value. During the years ended December 31, 2015 and December 31, 2014, the Company capitalized $nil and $57,340 respectively,
of such costs incurred for the Company’s acquisition of licenses for the patents. (See Note 10). Amortization expense for
the years ended December 31, 2015 and December 31, 2014 was $592,233 and $2,876,826, respectively.
Impairment
of Long-Lived Assets
The
Company evaluates long-lived assets for impairment whenever events or change in circumstances indicate that the carrying amount
of an asset may not be recoverable as prescribed by ASC Topic 360-10-05, “
Property, Plant and Equipment
.”
If the carrying amount of the asset, including any intangible assets associated with that asset, exceeds its estimated undiscounted
net cash flow, before interest, the Company will recognize an impairment loss equal to the difference between its carrying amount
and its estimated fair value. In 2015, the Company determined that the carrying amount recorded for the acquisition of licenses
and patents related to LDN were impaired, and recorded an impairment loss of $5,226,352. In 2014, the Company determined that
the carrying amount recorded for the acquisition of licenses and patents related to MENK were impaired, and recorded an impairment
loss of $9,908,477.
Research
and Development Costs
Research
and development costs are charged to expense as incurred and are typically comprised of salaries and benefits, pre-clinical studies,
clinical trial activities, drug development and manufacturing, fees paid to consultants and other entities that conduct certain
research and development activities on the Company’s behalf and third-party service fees, including clinical research organizations
and investigative sites. Costs for certain development activities, such as clinical trials are recognized based on an evaluation
of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations, or information
provided by vendors on their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements,
which may differ from the pattern of costs incurred, and are reflected in the financial statements as operating expenses.
Income
Taxes
The
Company follows ASC Topic 740,
Income Taxes,
which requires recognition of deferred tax assets and liabilities for the
expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method,
deferred tax assets and liabilities are based on the differences between the financial statements and tax bases of assets and
liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Deferred tax assets
are reduced by a valuation allowance to the extent management concludes it is more likely than not that the asset will not be
realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the
years in which those temporary differences are expected to be recovered or settled.
The
standard addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded
in the financial statements. Under ASC Topic 740, the Company may recognize the tax benefit from an uncertain tax position only
if it is more likely than not that the tax position will be sustained on examination by the tax authorities, based on the technical
merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based
on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate settlement. ASC Topic
740 also provides guidance on de-recognition, classification, interest and penalties on income taxes, accounting in interim periods
and requires increased disclosures. At the date of adoption, and as of December 31, 2015, and 2014, the Company does not have
a liability for unrecognized tax uncertainties.
The
Company’s policy is to record interest and penalties on uncertain tax positions as income tax expense. As of December 31,
2015, and 2014, the Company has not accrued any interest or penalties related to uncertain tax positions.
Stock-Based
Compensation and Issuance of Stock for Non-Cash Consideration
The
Company measures and recognizes compensation expense for all share-based payment awards made to employees and directors, including
employee stock options, based on estimated fair values equaling either the market value of the shares issued or the value of consideration
received, whichever is more readily determinable. The majority of the non-cash consideration pertains to services rendered by
consultants and others and has been valued at the fair value of the Company’s common stock at the date of the agreement.
The
Company’s accounting policy for equity instruments issued to consultants and vendors in exchange for goods and services
follows the provisions of ASC Topic 505-50, “
Equity-Based Payments to Non-Employees
.” The measurement date
for the fair value of the equity instruments issued is determined at the earlier of (i) the date at which a commitment for performance
by the consultant or vendor is reached or (ii) the date at which the consultant or vendor’s performance is complete.
Non-controlling
Interest
In
accordance with ASC Topic 810,
Consolidation
, the Company consolidates Cytocom, Inc. The non-controlling interests in
Cytocom represent the interests of outside shareholders in the equity and results of operations of Cytocom.
Net
Loss per Share
Basic
net loss per share is calculated by dividing the net loss attributable to common stockholders by the weighted average number of
common shares outstanding for the period, without consideration for common stock equivalents. Diluted net loss per share is calculated
by dividing the net loss by the weighted-average number of common share equivalents outstanding for the period determined using
the treasury-stock method and the if-converted method. Dilutive common stock equivalents are comprised of common stock purchase
warrants outstanding. For all periods presented, there is no difference in the number of shares used to calculate basic and diluted
shares outstanding due to the Company’s net loss position.
A
calculation of basic and diluted net loss per share follows:
|
|
|
For
the year ended December 31,
|
|
|
|
|
2015
|
|
|
2014
|
|
|
Historical net loss per share:
|
|
|
|
|
|
|
|
Numerator
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(16,949,451
|
)
|
|
$
|
(49,938,213
|
)
|
|
Non-controlling
interest
|
|
|
(2,706,939
|
)
|
|
|
(1,684,996
|
)
|
|
Net loss attributed
to Common stockholders
|
|
$
|
(14,242,512
|
)
|
|
$
|
(48,253,217
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Denominator
|
|
|
|
|
|
|
|
|
|
Weighted-average common shares outstanding—Denominator
for basic and diluted net loss per share
|
|
|
153,247,023
|
|
|
|
98,508,458
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted
net loss per share attributed to common stockholders
|
|
$
|
(0.09
|
)
|
|
$
|
(0.49
|
)
|
The
Company’s potential dilutive securities which include stock and warrants have been excluded from the computation of diluted
net loss per share as the effect would be to reduce the net loss per share. Therefore, the weighted-average Common stock outstanding
used to calculate both basic and diluted net loss per share is the same.
The
following shares of potentially dilutive securities have been excluded from the computations of diluted weighted average shares
outstanding as the effect of including such securities would be antidilutive:
|
|
|
Year
Ended December 31,
|
|
|
|
|
2015
|
|
|
2014
|
|
|
Common Stock Purchase Warrants
|
|
|
9,131,500
|
|
|
|
9,296,750
|
|
Recent
Accounting Standards
During
the year ended December 31, 2015 and through March 30, 2016, there were several new accounting pronouncements issued by the Financial
Accounting Standards Board. Each of these pronouncements, as applicable, has been or will be adopted by the Company. Management
does not believe the adoption of any of these accounting pronouncements has had or will have a material impact on the Company’s
consolidated financial statements.
The
Company qualifies as an “emerging growth company” as defined in Section 101 of the Jumpstart our Business Startups
Act (“JOBS Act”) as we do not have more than $1,000,000,000 in annual gross revenue and did not have such amount as
of December 31, 2015, our last fiscal year. We are electing to use the extended transition period for complying with new or revised
accounting standards under Section 102(b)(1) of the JOBS Act.
3.
Property and Equipment
|
|
|
December
31,
|
|
|
|
|
2015
|
|
|
2014
|
|
|
Property and equipment:
|
|
|
|
|
|
|
|
|
|
Computer equipment
|
|
$
|
8,013
|
|
|
$
|
8,013
|
|
|
|
|
|
|
|
|
|
|
|
|
Less accumulated
depreciation
|
|
|
(6,331
|
)
|
|
|
(3,778
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Property and
equipment, net
|
|
$
|
1,682
|
|
|
$
|
4,235
|
|
The
Company utilizes the straight-line method for depreciation, using three to five-year depreciable asset lives. Depreciation expense
was not material for all periods presented.
4.
Accrued Liabilities
Accrued
expenses and other liabilities consist of the following:
|
|
|
December
31,
|
|
|
|
|
2015
|
|
|
2014
|
|
|
Retainer for legal services
|
|
$
|
-
|
|
|
$
|
60,000
|
|
|
Accrued payroll to officers and others
|
|
|
758,342
|
|
|
|
762,633
|
|
|
Accrued interest - notes payable
|
|
|
236,671
|
|
|
|
1,808
|
|
|
Estimated legal settlement
|
|
|
282,136
|
|
|
|
278,681
|
|
|
Other accrued liabilities
|
|
|
323
|
|
|
|
6,933
|
|
|
State payroll
taxes
|
|
|
3,567
|
|
|
|
1,784
|
|
|
|
|
|
|
|
|
|
|
|
|
Total accrued
expenses and other liabilities
|
|
$
|
1,281,039
|
|
|
$
|
1,111,839
|
|
5.
Notes Payable
Notes
payable consist of the following:
|
|
|
December
31, 2015
|
|
|
December
31, 2014
|
|
|
Promissory note issued July
29, 2014 to Ira Gaines. The note matures on January 27, 2015 and earns interest at a rate of 18% per annum. The Company was
unable to repay the note at maturity and the note is in default, although no demand for repayment has been made by the lender.
|
|
$
|
100,000
|
|
|
$
|
100,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Promissory notes issued between November
26, 2014 and September 30, 2015, to raise up to $2,000,000 in debt. Lenders earn interest at a rate of 10% per annum, plus
a pro-rata share of two percent of the Company’s gross receipts for sales of IRT-103-LDN in perpetuity. Notes will be
repaid in 36 monthly installments of principal and interest commencing no later than October 15, 2015. Notes aggregating $711,500
were in default at December 31, 2015, as the Company was unable to pay installments on those notes on their due dates. No
demands for repayment have been made by the lenders. Subsequent to December 31, 2015, $380,500 of these notes and their accompanying
interest were converted into 5,389,409 shares of Immune Therapeutics common stock.
|
|
|
711,500
|
|
|
|
406,525
|
|
|
|
|
|
|
|
|
|
|
|
|
Promissory note issued October 17, 2014
to Roger Bozarth. The note matures on October 17, 2015 and earns interest at a rate of 2% per annum. The Company was unable
to repay the note at maturity and the note is in default, although no demand for repayment has been made by the lender. Subsequent
to December 31, 2015, this note and the accompanying interest was converted into 89,639 shares of Immune Therapeutics common
stock.
|
|
|
7,000
|
|
|
|
7,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Promissory notes issued between May
1, 2015 and September 30, 2015, and maturing between June 14, 2015 and December 31, 2015. Lenders on loans aggregating $506,433
earn interest at rates between 10% and 18% per annum. On loans aggregating $98,500, interest is payable in a fixed amount
not tied to a specific interest rate. Notes aggregating $669,933 were in default at December 31, 2015, as the Company was
unable to repay those notes on their due dates. No demands for repayment have been made by the lenders. Subsequent to December
31, 2015, $135,000 of these notes and their accompanying interest were converted into 2,870,377 shares of Immune Therapeutics
common stock.
|
|
|
669,933
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Promissory note issued January 26, 2015
to Robert J. Dailey. The note is senior to, and has priority in right of payment over, all indebtedness of the Company. The
note earns interest at a rate of 2% per annum and was due on July 30, 2015. The Company was unable to repay the note at maturity
and the note is in default, although no demand for repayment has been made by the lender.
|
|
|
200,000
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Promissory notes issued by Cytocom Inc.
between April 29, 2015 and December 31, 2015. Lenders earn interest at rates between 5% and 10% per annum. These notes mature
on September 30, 2016.
|
|
|
800,000
|
|
|
|
-
|
|
|
Promissory notes issued
in December 2015. Lenders earn interest at a rate of 10% per month. Notes are repayable on March 9, 2016. The Company was
unable to repay the note at maturity and the note is in default. The Company is obligated to pay late-payment penalties totaling
$6,667 per day.
|
|
|
130,000
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Promissory note
issued November 24, 2015 as settlement of amounts owing to a law firm. The Lender earns interest at the rate of 10% per annum.
The note is repayable in full on December 1, 2016.
|
|
|
175,268
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
2,793,701
|
|
|
|
513,525
|
|
|
|
|
|
|
|
|
|
|
|
|
Less: Current
Portion
|
|
|
(2,793,701
|
)
|
|
|
(186,067
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Long-Term debt,
less current portion
|
|
$
|
-
|
|
|
$
|
327,458
|
|
As
of December 31, 2015, the Company had accrued $236,671 in unpaid interest, compared to $1,808 as of December 31, 2014. During
the year ended December 31, 2015, 62,500 shares with a fair value of $15,625 were issued by the Company for interest expense under
promissory notes (compared to 670,000 shares issued for interest and loan expenses with a fair value $302,450 in the year ended
December 31, 2014).
6.
Capital Structure—Common Stock and Common Stock Purchase Warrants
Each
holder of common stock is entitled to vote on all matters and is entitled to one vote for each share held. No holder of shares
of stock of any class shall be entitled as a matter of right to subscribe for or purchase or receive any part of any new or additional
issue of shares of stock of any class, or of securities convertible into shares of stock or any class, whether now hereafter authorized
or whether issued for money, for consideration other than money, or by way of dividend.
As
of December 31, 2015 and 2014, the Company was authorized to issue 500,000,000 common shares at a par value of $0.0001 per share.
As
of December 31, 2015, the Company had 174,850,047 shares of common stock outstanding and 134,417,210 outstanding as of December
31, 2014.
Stock
Warrants
In
2015, the Company issued 435,000 warrants, exercisable into one share of common stock of the Company for each warrant at prices
between $0.07 and $0.50 per share. The warrants expire between Jan 2016 and Dec 2021.
When
the Company sells its stock to stockholders for cash, it periodically issues warrants to those stockholders to acquire additional
stock at prices agreed at the date of the original sale. The Company incurs a cost for the rights attached to the warrants, which
is calculated using the Black-Scholes Model. This expense is reported in the Condensed Consolidated Statements of Operations above
as the Warrant valuation expense.
During
2015, there were no modifications of the terms of any warrants issued by the Company.
Following
is a summary of outstanding stock warrants at December 31, 2015 and 2014 and activity during the years then ended:
|
|
|
Number
of
Shares
|
|
|
Exercise
Price
|
|
|
Weighted
Average Price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants
as of December 31, 2014
|
|
|
9,396,750
|
|
|
$
|
0.14-15
|
|
|
$
|
1.72
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issued
|
|
|
435,000
|
|
|
$
|
0.07-0.50
|
|
|
$
|
0.40
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expired
|
|
|
(676,250
|
)
|
|
$
|
1.50
|
|
|
$
|
1.50
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
(24,000
|
)
|
|
$
|
0.50
|
|
|
$
|
0.50
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants
as of December 31, 2015
|
|
|
9,131,500
|
|
|
$
|
0.07-15.00
|
|
|
$
|
1.47
|
|
Summary
of outstanding warrants as of December 31, 2015:
|
Expiration
Date
|
|
Number
of Shares
|
|
|
Exercise
Price
|
|
|
Remaining
Life (years)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Second Quarter 2016
|
|
|
37,500
|
|
|
$
|
5.00
|
|
|
|
.50
|
|
|
Third Quarter 2016
|
|
|
525,000
|
|
|
$
|
1.00-5.00
|
|
|
|
.75
|
|
|
Third Quarter 2017
|
|
|
1,500,000
|
|
|
$
|
1.00
|
|
|
|
1.75
|
|
|
Fourth Quarter 2017
|
|
|
2,941,666
|
|
|
$
|
1.00-9.00
|
|
|
|
2.00
|
|
|
First Quarter 2018
|
|
|
127,500
|
|
|
$
|
15.00
|
|
|
|
2.25
|
|
|
Second Quarter 2018
|
|
|
33,334
|
|
|
$
|
15.00
|
|
|
|
2.50
|
|
|
Third Quarter 2018
|
|
|
650,000
|
|
|
$
|
1.00-1.50
|
|
|
|
2.75
|
|
|
Fourth Quarter 2018
|
|
|
1,197,500
|
|
|
$
|
1.00-1.50
|
|
|
|
3.00
|
|
|
First Quarter 2019
|
|
|
1,024,000
|
|
|
$
|
1.50
|
|
|
|
3.25
|
|
|
Second Quarter 2019
|
|
|
135,000
|
|
|
$
|
0.07–0.23
|
|
|
|
3.50
|
|
|
Third Quarter 2019
|
|
|
260,000
|
|
|
$
|
0.50
|
|
|
|
3.75
|
|
|
Fourth Quarter 2019
|
|
|
400,000
|
|
|
$
|
0.14
|
|
|
|
4.00
|
|
|
Second Quarter 2020
|
|
|
300,000
|
|
|
$
|
0.50
|
|
|
|
4.50
|
|
7.
Stock Compensation
Shares
Issued for Services
During
the years ended December 31, 2015 and 2014, the Company issued 16,672,504 and 17,178,294 shares of common stock respectively for
consulting fees. The Company valued these shares based upon the fair value of the common stock at the dates of the agreements.
The consulting fees are amortized over the contract periods which are typically between 12 and 24 months. The amortization of
prepaid services totaled $4,328,768 and $22,855,925 for the years ended December 31, 2015 and 2014.
8.
Income Taxes
-
Results of Operations
There
was no income tax expense reflected in the results of operations for the years ended December 31, 2015 and 2014 because the Company
incurred a net loss in both years.
Deferred
tax assets:
|
|
|
2015
|
|
|
2014
|
|
|
|
|
|
|
|
|
|
|
Net operating losses
|
|
$
|
22,611,000
|
|
|
$
|
20,710,000
|
|
|
Stock based compensation
|
|
|
60,914,000
|
|
|
|
59,032,000
|
|
|
Amortization, depreciation , and impairment
|
|
|
6,765,000
|
|
|
|
4,786,000
|
|
|
Capitalization of start-up costs for
tax purposes
|
|
|
1,854,000
|
|
|
|
1,854,000
|
|
|
Loss on debt
conversion of debt
|
|
|
502,000
|
|
|
|
502,000
|
|
|
Total deferred tax assets
|
|
|
92,646,000
|
|
|
|
86,884,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Valuation allowance
|
|
|
(92,646,000
|
)
|
|
|
(86,884,000
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred
tax assets, net
|
|
$
|
-
|
|
|
$
|
-
|
|
The
Company has recognized no tax benefit for the losses generated for the periods through December 31, 2015. ASC Topic 740 requires
that a valuation allowance be provided if it is more likely than not that some portion or all of a deferred tax asset will not
be realized. The Company’s ability to realize the benefit of its deferred tax asset will depend on the generation of future
taxable income. Because the Company has yet to recognize revenue, we believe that the full valuation allowance should be provided.
Our
effective tax rate for fiscal years 2015 and 2014 was 0%. Our tax rate can be affected by recurring items, such as tax rates in
foreign jurisdictions and the relative amount of income we earn in jurisdictions. It may also be affected by discrete items that
may occur in any given year, but are not consistent from year to year.
As
of December 31, 2015, we have estimated federal and state income tax net operating loss (“NOL”) carry-forwards of
approximately $66,500,000, which will expire in 2032-2035.
|
|
|
2015
|
|
|
2014
|
|
|
|
|
Amount
|
|
|
Percent
|
|
|
Amount
|
|
|
Percent
|
|
|
Benefits for income tax
at federal statutory rate
|
|
$
|
5,763,000
|
|
|
|
34
|
%
|
|
$
|
16,978,000
|
|
|
|
34
|
%
|
|
Change in valuation allowance
|
|
|
(5,762,000
|
)
|
|
|
(34
|
)%
|
|
$
|
(16,973,000
|
)
|
|
|
(34
|
)
|
|
Permanent differences
|
|
|
(1,000
|
)
|
|
|
-
|
|
|
|
(5,000
|
)
|
|
|
-
|
|
|
Non-deductible
impairment of goodwill
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
$
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
-
|
%
|
9.
Licenses and Supply Agreements
Patent
and Subsidiary Acquisition
The
Company entered into a share exchange agreement on April 24, 2012 to acquire all of the outstanding shares of TNI BioTech IP,
Inc. (“TNI IP”), a biotechnology firm incorporated in Florida and formed to acquire patents related to the treatment
of cancer and HIV/AIDS and autoimmune diseases, using Met-enkephalin (“MENK”) and Naltrexone (“LDN”).
The goal of TNI IP’s management was to enable mankind and civilization to combat fatal diseases by activating and mobilizing
the body’s own immune system using TNI IP’s patented use of MENK. The first patents acquired by TNI IP were acquired
from Dr. Nicholas P. Plotnikoff and Professor Fengping Shan in 2012. TNI IP was acquired in exchange for 20,250,000 shares of
the Company’s common stock, of which 8,000,000 shares were issued to Dr. Plotnikoff for the acquisition of patents and the
remaining 12,250,000 shares were issued to the founders of TNI IP in exchange for all of their right, title and interest in their
TNI IP shares. The goodwill arising on the acquisition of TNI BioTech IP, Inc. was valued at $98,000,000 and license agreements
arising from the acquisition of TNI IP were valued at $16,006,000.
In
connection with the share exchange, we entered into a Sale of Technology Agreement with Dr. Nicholas P. Plotnikoff on March 4,
2012, wherein Dr. Plotnikoff agreed to transfer and assign all of his rights, title and interest in: European Patent United Kingdom,
Germany, France, Ireland EP 1401471 BI Methods for inducing sustained immune response; Russian Patent Russian Federation patent
number 2313364; The Patent Office of the People’s Republic of China, Application No.: 200810165784.8 China Patent CN1015113407
A The Patent Office of the People’s Republic of China ISSN: 1006-2858 CN 21-1349/R; Patent Agencies Government of India
Patent, Application number 1627/KOLNP/2003 number 220265 an Enkephalin Peptide Composition; and the US Patent Pending, US Patent
Application 10/146.999 e. The Company received all the production formulations and technology designs from Dr. Plotnikoff necessary
for the manufacturing, formulation, production and protocols of the MENK treatment of cancer and HIV/AIDS. As consideration for
entering into the Sale of
Technology
Agreement, Dr. Plotnikoff received 8,000,000 shares of common stock, a royalty of a single-digit percentage on all sales of MENK
and was granted the position of Non-Executive Chairman of the Board of Directors.
At
the time of the acquisition, the valuation of goodwill and other intangible assets were determined using the fair market price
for the Company’s common stock, which were exchanged for shares of TNI IP. In the fourth quarter of 2012, the Company performed
an annual valuation to determine whether any goodwill or intangible assets that had been acquired by the Company were impaired.
The result of this valuation was that material impairments were identified. The Company recognized an impairment of the goodwill
arising on the acquisition of TNI IP of $98,000,000.
Patent
License Agreements
On
August 13, 2012, the Company signed an exclusive License Agreement with Ms. Jacqueline Young (the “Young Agreement”)
for the intellectual property developed by Dr. Bernard Bihari relating to treatments with opioid antagonists such as naltrexone
and Met-enkephalin for a variety of diseases and conditions including malignant lymphoma, chronic lymphocytic leukemia, Hodgkin’s
lymphoma, and non-Hodgkin’s lymphoma, chronic herpes virus infections, chronic herpes viral infections such as chronic genital
herpes caused by the herpes simplex virus Type 2 and chronic infections due to the Epstein-Barr virus and a treatment method for
humans infected with HTLV-III (AIDS) virus, including patients clinically diagnosed as suffering from AIDS and those suffering
from AIDS-related complex (ARC). The Young Agreement is valid for the life of the patents and expires on a country by country
basis in each country where patent rights exist, upon the expiration of the last to expire patent in each country or in the event
the patent in such country is held to be invalid and/or unenforceable (by a court or government body of competent jurisdiction)
or admitted to be invalid or unenforceable. The Bihari patents were acquired in exchange for 540,000 shares of the Company’s
common stock with a fair market value of $972,000 and assumed liabilities of $400,000, which is payable to Ms. Young over a twenty-four
month period in equal installments to reimburse her for the costs of a New York City office in accordance with the Young Agreement.
The patent liability at December 31, 2013 totaled $118,333. The cost of the patent totaled $1,372,000. Additionally, the Company
will pay the licensor a royalty payment of 1% of gross MENK sales and provide the licensor a position as non-executive chairman
of the Company.
On
December 24, 2012, the Company signed an agreement for the acquisition of patent rights (the “Smith Agreement”) for
the intellectual property of Dr. Jill Smith and LDN Research Group, LLC (collectively, the “Licensor Parties”),
whose members are Dr. Ian S. Zagon, Dr. Patricia J. McLaughlin and Moshe Rogosnitzky and orphan drug designation
by the FDA to a novel late-stage drug, trademarked “LDN,” for the treatment of Pediatric Crohn’s disease. The
patent covers methods and formulations for treatment of the inflammatory and ulcerative diseases of the bowel, using naltrexone
in low doses as an opioid antagonist. These patents were acquired in exchange for 300,000 shares of our common stock with a fair
market value of $2,715,000 and payment of $165,384 (consisting of a $100,000 initial license fee and payment of $65,384 of expenses),
which totaled $2,880,384.
The
Smith Agreement requires the Company to (i) use commercially reasonable efforts to develop, commercialize, market and sell licensed
products in a manner consistent with a business plan, (ii) expend a minimum amount of funds per annum to develop and commercialize
licensed products as soon as practicable, (iii) obtain all requisite regulatory approvals needed to use or sell licensed products
in the field of use, and (iv) make the first commercial sale of a licensed product by March of 2017.
The
Company is required to pay an annual license fee, an annual running royalty on net sales of each licensed product or a minimum
royalty, whichever is greater, and a sublicense fee on payments received by the Company from sublicensees. The Company has an
exclusive, worldwide license to make, have made, use, lease, import, offer for sale and sell licensed products and to use the
method under the patent rights. The Smith Agreement will terminate on the expiration or abandonment of the last patent to expire
or ten years after the sale of the first licensed product. The Company may terminate the Smith Agreement upon 90 days’ written
notice, provided all sublicenses are terminated and all amounts due and owing are paid to the Licensor Parties. The Licensor Parties
may terminate the agreement ten days’ after notice to the Company if the Company is ten days late in payment or there is
a breach that remains uncured for ten days after written notice of such breach.
The
Company is also required to pay milestone payments after substantial achievement of certain milestone events for each licensed
product including payment: upon initiation of each Phase III trial; upon positive completion of each Phase III clinical trial
of the therapeutic use of an LDN compound in the field of use; when a New Drug Application (“NDA”) is accepted for
review by the FDA; and when FDA approval to market the NDA is approved. The Company will issue shares upon reaching certain milestones
including upon the first dosing of the first patient in a Phase III clinical trial for each licensed product, upon the first sale
of each licensed product, and upon the achievement of a set dollar amount in cumulative sales for each licensed product covered
by NDAs.
As
part of the Smith Agreement, the Company has the right to apply to the FDA for the transfer of the orphan drug status for the
use of naltrexone for the treatment of pediatric Crohn’s disease and ulcerative colitis, the Investigation New Drug Application
(“IND”), and the right to acquire the relevant clinical data set from Dr. Jill Smith. Dr. Jill Smith made arrangements
to transfer the IND to the Company as well as the relevant clinical data set, and the FDA has acknowledged that the Company is
now the sponsor for this IND.
On
September 24, 2014, the Company and the Licensor Parties jointly agreed to terminate the Smith Agreement, and in place thereof,
have the Licensor Parties grant a similar license in their patent rights to Cytocom Inc. pursuant to a Patent License Agreement
between the Licensor Parties, Cytocom Inc. and the Company with substantially similar terms as set forth in the Smith Agreement.
Pursuant to this agreement, the Company issued 1,000,000 shares of its common stock valued at $270,000, upon execution to the
Licensor Parties and the Company guaranteed the obligations of Cytocom Inc. to the Licensor Parties under the agreement.
On
January 18, 2013, the Company signed an exclusive licensing agreement with The Penn State Research Foundation to license all of
the intellectual property developed by Dr. Ian S. Zagon, Dr. Patricia J. McLaughlin and Dr. Jill P. Smith for the treatment of
cancer titled “Opioid Growth Factor and Cancer” and “Combination Therapy with Opioid Growth Factor and Taxanes
for the Treatment of Cancer” (the “Foundation Agreement”).
The
Foundation Agreement requires the Company to: (a) use commercially reasonable efforts to develop, commercialize, market and sell
licensed products in a manner consistent with a business plan; (b) expend a minimum amount of funds per annum to develop and commercialize
licensed products as soon as practicable; (c) obtain all requisite regulatory approvals needed to use or sell licensed products
in the field of use; and (d) make the first commercial sale of a licensed product by December 31, 2016.
The
Foundation Agreement provides that the Company must pay to the licensor an initial license fee, a license maintenance fee on each
anniversary of the effective date of the Foundation Agreement, and an annual running royalty on net sales for each licensed product
or a minimum royalty, whichever is greater. In addition, the Company must pay a sublicense fee on payments received by the Company
from sublicensees.
The
Foundation Agreement also requires the Company to make payments upon the achievement of certain milestone events including: initiation
of each Phase II trial; initiation of each Phase III trial; when the NDA is accepted for review by the FDA; and when FDA approval
to market is approved. The Company must also issue shares upon certain milestones including upon the first dosing of the first
patient in a Phase II clinical trial for each licensed product, upon the first dosing of the first patient in a Phase III clinical
trial for each licensed product, upon the first sale of each licensed product, and upon the achievement of a set dollar amount
of cumulative sales for each licensed product covered by NDAs.
The
Foundation Agreement terminates on the expiration or abandonment of the last patent to expire or become abandoned. The Company
may terminate the Foundation Agreement at any time upon 60 days’ prior written notice and ceasing to make and sell all licensed
products, the termination of all sublicenses and payment of all monies owed under the Foundation Agreement. The licensor may terminate
the agreement 30 days after notice to the Company if the Company is 30 days late in payment or a breach that remains uncured for
45 days after written notice of such breach.
In
May of 2013, the Company executed a Patent License Agreement with Professor Fengping Shan (the “Shan Agreement”) pursuant
to which it obtained exclusive rights to develop and commercialize the licensed technology. The licensed technology is the intellectual
property developed and owned by Professor Shan (i) relating to the treatment of a variety of diseases and conditions with MENK
including multiple forms of lymphoma and cancer and (ii) a treatment method for humans infected with the HLTV-III (AIDS) virus
including AIDS and AIDS related complex (ARC). The licensed technology includes the methods and formulations for these treatments
including all INDs, communications with regulatory agencies, patient data, and letters relating to these treatments. The licensed
technology also includes certain patents developed by Professor Shan. Under the Shan Agreement, the Company must issue 500,000
shares to Professor Shan upon final transfer of the licenses, and reimburse Professor Shan for all out of pocket expenses in connection
with the patents. The Company will pay Professor Shan a running royalty on gross sales subject to decreases if third party intellectual
property is needed to complete such sale or product. The Shan Agreement lasts for the duration of each of the licensed patents
however the Company may terminate the Shan Agreement on 120 days’ written notice to Professor Shan.
On
August 6, 2014, Professor Fengping Shan executed an Assignment pursuant to which he transferred to the Company his entire right,
title and interest in and to the licensed patents under the Shan Agreement and CN 201210302259 Application of combination of low-dose
naltrexone and methionine-enkephalin to preparation of anti-cancer drug for the consideration of 500,000 shares of common stock
valued at $140,000.
10.
Commitments and Contingencies
Malawi
Treatment Facilities
On
July 14, 2012, GB Oncology and Imaging Group LTD (“GBOIG”) in partnership with the Company signed a letter of intent
agreement to collaborate with the Government of Malawi to assist in expanding the treatment of cancer, HIV/AIDS and other infectious
diseases.
In
December of 2014, the Government of Malawi completed an oncology clinic at the Queen Elizabeth Central Hospital in Blantyre, Malawi
for the treatment of cancer and infectious diseases. In 2015, the Company submitted protocols seeking permission from the Pharmacy,
Medicines and Poisons Board of Malawi (“PMPB”) to conduct two trials involving Lodonal™ in Malawi:
|
a.
|
The
first protocol, submitted jointly with The Jack Brewer Foundation (“JBF Worldwide”), received PMPB approval on
November 11, 2015. The protocol covers a 12-month trial for a “Single Visit Approach to Cervical Cancer Prevention.”
The approach is designed to deliver a preventive and simple procedure that can be performed in a clinical setting without
the use of a laboratory and to allow for immediate treatment of any precancerous lesions utilizing Wallach LL100 Cryosurgical
systems. The protocol provides for 50% of the patient group to be put on Lodonal™ to determine if the drug lowers the
number of opportunistic infections during the year, and if it can be shown that LDN increases CD4, CE8, NK and T cell count,
which would show that the incidence rates of opportunistic infection could decrease with Lodonal™ and that Lodonal™
could be used as a prophylaxis to prevent substantial HIV-related morbidity in Malawi. The Company expects the final trial
agreement with PMPB to be signed by the end of 2015. Lodonal™ pills have been produced in Nicaragua in anticipation
of the trial. Shipments will commence once the trial is approved.
|
|
|
|
|
b.
|
The
second protocol, which has not yet been approved, covers a trial using Lodonal™ for the treatment of cancer. The protocol
is still under discussion with the PMPB, and the Company expects a final protocol to be submitted for approval by year end.
|
The
Company and GB will work with the government of Malawi to open and operate other clinics that provide treatments for HIV/AIDS,
cancer and other infectious diseases. Under the letter of intent, the Company and GBOIG intend to provide HIV/AIDS treatment to
25,000 patients and hopefully expanding to 500,000 within 24 months. The Company shall contribute $1,000 in initial capital to
the venture. The Company shall be allocated 50% of the net income from the venture. Either party may terminate the venture with
180 days’ notice to the other party prior to the one-year anniversary of the Agreement. After the one-year anniversary,
the agreement may only be terminated with 180 days’ notice to the other party if the other party has breached the Agreement.
GBOIG,
a subsidiary of GB Energie LLC, is a Washington D.C. based minority woman-owned business managed by Dr. Gloria B. Herndon. Dr.
Herndon is a former director of the Company. Dr. Herndon’s directorship with the Company ended September 4, 2014.
Open
an Oncology and Infectious Disease Center in Malawi at Queen Elizabeth Central Hospital
On
September 25, 2012, GBOIG, in partnership with the Company, signed an agreement with the Government of Malawi to open an outpatient
clinic at Queen Elizabeth Central Hospital (in Malawi) for the treatment of cancer and infectious disease.
The
duration of the Agreement shall be for 25 years with an optional 10-year renewal to be indicated by the Government of Malawi at
least three years prior to the expiration of the term. The Government of Malawi shall bear the upfront costs for the agreement
of $2,500,000.
Distribution
Agreements in Nigeria
Effective
November 9, 2012, we signed an exclusive Distribution Agreement with G-Ex Technologies/St. Maris Pharma and GB Pharma Holdings,
LLC for the Federal Republic of Nigeria. Under the terms of the Distribution Agreement, G-Ex Technologies/St. Maris Pharma and
GB Pharma Holdings LLC will have exclusive marketing and distribution rights to IRT-103 LDN and IRT-104 LDN cream in Nigeria until
the end of the term of the agreement on December 31, 2017. We will be responsible for the manufacture and supply of IRT-103 LDN
and IRT-104 LDN cream. As part of the Distribution Agreement, G-Ex Technologies/St. Maris Pharma will provide us with a revolving
letter of credit for the minimum purchase of 750,000 doses monthly of IRT-103 LDN or IRT-104 LDN cream priced at $1.00 per dose.
There are no upfront fees.
The
Distribution Agreement calls for G-Ex Technologies/St. Maris Pharma and GB Pharma Holdings, LLC to purchase a minimum of 15,000,000
doses monthly within 24 months to maintain the exclusivity of the agreement. Once G-Ex Technologies/St. Maris Pharma and GB Pharma
Holdings, LLC reach sales of 1,000,000 doses per day, we have agreed to joint venture a factory in the Federal Republic of Nigeria
to meet local demands.
G-Ex
Technologies/St. Maris Pharma is a consortium of companies organized under the laws of the Republic of Nigeria operated by management,
a consultant, general pharmaceutical, clinical pharmacy and marketing executives, each with over 25 years of industry experience
and well versed in the changing dynamics of the prescription and over-the-counter drug international marketplace. G-Ex Technologies/St.
Maris Pharma has been actively supported by medical practice professionals in business and academia who have been involved in
the management of related drug therapies for many years.
The
parties have been unable to perform under the agreement because a certificate of free sale was not obtained by the Company until
November of 2013, and no extension has been granted.
In
October 2013, the Company announced the signing of a Distribution Agreement with AHAR Pharma, a Nigerian company, to market Lodonal™,
in Nigeria for the treatment of autoimmune diseases and cancer. AHAR intends to distribute Lodonal™ through a local distributor
network, an Internet client base and directly to hospitals, pharmacists and doctors in Nigeria. The Company expects to implement
the agreement in 2016. Under the agreement, the Company is obligated to provide delivery of an initial supply of between 1 million
and 1.5 million doses of Lodonal™ product to cover AHAR Pharma’s first-year purchase commitment.
In
August 2015, the Company announced the signing of a letter of intent with GB Pharma/AHAR and Fidson Healthcare Plc., in terms
of which Fidson will promote Lodonal
TM
upon execution of a definitive agreement between the companies and receipt
of NAFDAC and other approvals to distribute Lodonal
TM
in Nigeria.
Agreements
with Hubei Qianjiang Pharmaceutical Company
On
October 18, 2012, the Company and Hubei Qianjiang Pharmaceutical Co., Ltd. (“Qianjiang Pharmaceutical”), signed a
Venture Cooperation Agreement on New Drug Methionine Enkephalin (the “Venture Agreement”) pursuant to which Qianjiang
Pharmaceutical acquired an exclusive license for the production of MENK in China. The Venture Agreement requires that Qianjiang
Pharmaceutical conduct drug research and pilot testing for MENK, organize pre-clinical studies, and apply for clinical trials
for MENK with the Chinese State Food and Drug Administration. Under the Venture Agreement, Qianjiang Pharmaceutical must open
a co-administration account for the development of MENK in China. Qianjiang Pharmaceutical must pay the Company, upon the marketing
of MENK products, a half-year amount equaling 6% of its gross sales from MENK of the preceding half year. The Company may cancel
the Venture Agreement if Qianjiang Pharmaceutical does not pay expenses for a period exceeding six months or does not commence
clinical trials within 12-months after receiving certain approvals. Qianjiang Pharmaceutical may cancel the Venture Agreement
if the Company fails to perform its obligations for a period of six months or the failure to receive approval of clinical trials
is due to the Company’s MENK technologies. The Venture Agreement was amended on February 24, 2013 to expand the clinical
trials from pancreatic to both pancreatic and liver cancer and amended on March 6, 2014 to require Qianjiang Pharmaceutical to
commence studies and clinical trials in China and place funds in the co-administration account.
On
August 6, 2014, the Company entered into a Supplementary Agreement on New Drug Methionine – Enkephalin Cooperation (the
“Amendment”) with Qianjiang Pharmaceutical, amending the Venture Agreement, as amended. The Company and Qianjiang
Pharmaceutical executed the Amendment to accelerate clinical trials in both the United States and China, and agreed to immediately
initiate three month Good Laboratory Practice (“GLP”) Toxicology Studies (rat and dog) within 30 days of signing the
Amendment. The Amendment requires that the GLP Toxicology Studies Trials are conducted in China in accordance with international
standards and standards acceptable to the FDA and that the studies include the following:
Exploratory
Toxicology (nGLP)
|
|
●
|
Dose
range finding studies
|
|
|
●
|
Different
species and methods of administration
|
|
|
●
|
Multiple
dosing regimens
|
|
|
●
|
Estimate
the response vs. dose given
|
Definitive
Toxicology (GLP)
|
|
●
|
Performed
in collaboration with Calvert Laboratories (USA) and MPI/Medicillon (China)
|
|
|
●
|
General
toxicology studies
|
|
|
●
|
Different
species and methods of administration
|
|
|
●
|
Immunogenicity
study with NHPs
|
Special
Toxicology Studies (planned)
Pursuant
to the Amendment, Qianjiang Pharmaceutical has made certain funds available from the co-administrative account opened by Qianjiang
Pharmaceutical under the Venture Agreement, in accordance with an approved budget and timeline set forth in the Amendment. A portion
of these funds are expected to be used by Cytocom to run PK and Dosing trials for MENK in the United States in 2016. The Amendment
requires Cytocom and Qianjiang Pharmaceutical to meet with the China State Food and Drug Administration to determine that PK and
Dosing Trials completed in the United States will be acceptable. All developments and trials run by Cytocom in the U.S. or the
European Union will be used for requesting registration approval in China.
In
February 2013, the Company signed a Strategic Framework Agreement for Cooperation with Qianjiang Pharmaceutical. Under the agreement,
the parties will work together to further the development of new products and conduct research and development on the Company’s
licensed patented technology. Specifically, the parties aim to co-invest to develop and market products focusing on HIV, cancer
and related autoimmune system therapies, develop co-ventured manufacturing facilities in China, and develop co-ventured distribution
of the developed products in China and Africa. The agreement does not have a definitive term, as each new agreement resulting
from the cooperation will set forth the material terms, including, but not limited to, fees, duration and termination therein.
Supervision
and Inspection of Manufacturing in Nicaragua
On
April 23, 2013, the Company signed a Contract with ViPharma for the Supervision and Inspection of Manufacturing Processes as part
of its negotiations for a contract for the manufacturing of LDN in a tablet, capsule and/or cream. The contract sets out the terms
and conditions under which ViPharma will carry out the services of inspecting and supervising the manufacturing and packaging
processes of LDN and ensure compliance with the FDA’s Current Good Manufacturing Practice regulations (“cGMP”)
and the Company’s specifications. ViPharma will carry out its obligations in whatever Latin American country the Company
ultimately decides to manufacture LDN. Under the contract, ViPharma has the exclusive rights to supervise and inspect all manufacturing
processes of LDN in Latin America. The initial term of the agreement is ten years commencing in September 2013, with automatic
five-year renewal terms provided neither party is in breach. The agreement may be terminated by (i) mutual agreement, (ii) in
the event of a breach after a 45 day cure period or (iii) by either party upon provision of written notice at least 90 days before
the end of the agreement, provided however that if the Company terminates the contract without cause it will be required to pay
ViPharma a $10 million penalty.
Operating
Leases
At
December 31, 2015, the Company was a party to an agreement to lease office space in Orlando, Florida. Cash rental expense for
the years ended December 31, 2015 and 2014 was $53,205 and $147,236 respectively.
Legal
Proceedings
N/A.
11.
Related Party Transactions
In
2012, Webfoot, Inc. provided a loan to the Company in the amount of $121,128. Webfoot, Inc. is owned by the son of Noreen Griffin,
the Company’s Chief Executive Officer. On February 21, 2013, the Company entered into a formal loan agreement to evidence
the amount owed by the Company. In the nine months ended September 30, 2014, the Company repaid $40,000 of the loan. On September
30, 2014, the remaining balance of $71,128 owed under the agreement, together with accrued and unpaid interest totaling $10,212,
was converted into 813,404 shares of the Company’s common stock in full and final settlement of the loan. The loss on conversion
was $162,681.
In
2012, Noreen Griffin made payments totaling $30,000 on the Company’s behalf covering the costs of incorporation and merger-related
expenses. On February 13, 2013, the Company entered into a formal loan agreement to evidence repayment of the amount owed on December
31, 2012. On September 30, 2014, the full balance of the loan, together with accrued and unpaid interest totaling $2,870, was
converted into 328,701 shares of the Company’s common stock in full and final settlement of the loan. The loss on conversion
was $65,740.
In
2012, Griffin Enterprises, LLC made payments totaling $46,000 on the Company’s behalf covering the cost of incorporation
and merger-related expenses. Griffin Enterprises, LLC is owned 50% by Noreen Griffin and 50% by Robert Wilson. On February 13,
2013, the Company entered into a formal loan agreement to evidence repayment of the amount owed on December 31, 2012. On September
30, 2014, the full balance of the loan, together with accrued and unpaid interest totaling $4,401, was converted into 504,009
shares of the Company’s common stock in full and final settlement of the loan. The loss on conversion was $100,802.
On
January 3, 2013, the Company formalized the terms under which Kelly O’Brien Wilson, the daughter-in-law of the Company’s
Chief Executive Officer is employed. Ms. Wilson had been working with the Company in 2012 and her three-year employment agreement
is effective as of December 1, 2012. The terms of the agreement define her base salary, a grant of a common stock, and health
insurance coverage. Ms. Wilson was issued 500,000 shares of common stock of the Company in January 2014. In 2014, the Company
paid compensation to Ms. Wilson totaling $157,582.
In
May 2013, the Company entered into the Shan Agreement and obtained exclusive rights to develop and commercialize the intellectual
property developed and owned by Professor Shan as described in detail above under “Patent License Agreements.” In
August 2014, Professor Shan executed an Assignment under which he transferred to the Company his entire right, title and interest
in and to the licensed patents under the Shan Agreement and to CN 201210302259 Application of combination of LDN and MENK to preparation
of anti-cancer drug for the consideration of 500,000 shares of our common stock.
In
April 2014, the Company entered into an agreement with Pixelheads, Inc. for the provision of public-company web services, maintenance
and updates of its server infrastructure and technology, and website content management systems. Pixelheads, Inc. is owned by
the son of Noreen Griffin, the Company’s Chief Executive Officer. The Company currently pays a monthly fee of $7,500 under
the agreement.
12.
Subsequent Events
Between
January 1, 2016 and March 30, 2016, the Company issued shares as follows:
|
|
|
Number
of Shares
|
|
|
Issuance of common stock
to employees and consultants, including employee settlement
|
|
|
16,493,000
|
|
|
Issuance of common stock for cash
|
|
|
312,500
|
|
|
Issuance of common stock in exchange
for debt
|
|
|
8,349,425
|
|
As
of March 31, 2016, the Company had outstanding 206,913,301 shares of common stock.
Between
January 1, 2016 and March 21, 2016, the Company issued 13,050,000 warrants for services and employment, exercisable into one share
of common stock of the Company for each warrant at a price of $0.20 per share. The warrants expire between January 2021 and March
2021. The Company also issued 3,000,000 warrants to a contractor, exercisable into one share of common stock of the Company for
each warrant, at prices between $0.50 and $2.00 per share. These warrants fully vest between October 1, 2016 and October 1, 2017,
and expire between October 2019 and October 2020.
PART
II- INFORMATION NOT REQUIRED IN PROSPECTUS
OTHER
EXPENSES OF ISSUANCE AND DISTRIBUTION
The
following table is an itemization of all
estimated
expenses, without consideration to future contingencies, incurred or
expected to be incurred by us in connection with the issuance and distribution of the securities being offered by this prospectus.
|
ITEM
|
|
AMOUNT
|
|
|
|
|
|
|
|
SEC Registration Fee
|
|
$
|
56.12
|
|
|
Legal Fees and Expenses
|
|
$
|
25,000
|
|
|
Accounting Fees and Expenses
|
|
$
|
5,000
|
|
|
Transfer Agent
Fees
|
|
$
|
0
|
|
|
Total
|
|
$
|
30,056.12
|
|
INDEMNIFICATION
OF OFFICERS AND DIRECTORS
Florida
law provides that our officers and directors will not be liable to us or our stockholders for monetary damages for all but certain
types of conduct as officers and directors. Our Bylaws permit us broad indemnification powers to all persons against all damages
incurred in connection with our business to the fullest extent provided or allowed by law. The exculpation provisions may have
the effect of preventing stockholders from recovering damages against our officers and directors caused by their negligence, poor
judgment or other circumstances.
RECENT
SALES OF UNREGISTERED SECURITIES
2013
For
fiscal year 2013, beginning quarter two on April 1, 2013, the Company issued the following securities:
In
April 2013 the Company issued two short-term promissory notes to third party investors totaling $200,000. Under the terms of the
notes, the Company was required to issue a total of 20,000 shares of restricted common stock to the note holders as loan origination
fees. The notes matured 14 days from the date of issuance. Under the terms of the notes, if the loans were not repaid, the note
holders would collectively receive 20,000 shares of restricted common stock on the maturity date and every 30 days thereafter
that the notes remain unpaid. As of the date of this filing, the notes have not been repaid.
On
March 11, 2013 the Company issued four short-term promissory notes to third party investors totaling $249,000. Under the terms
of the notes, the Company was required to issue a total of 25,000 shares of restricted common stock to the note holders as loan
origination fees. The notes matured on March 25, 2013. Under the terms of the notes, if the loans were not repaid, the note holders
would collectively receive 25,000 shares of restricted common stock on the maturity date and every 30 days thereafter that the
notes remain unpaid. As of the date of this filing, the notes have not been repaid.
During
the second quarter of 2013, the Company issued 107,900 shares of its restricted common stock through common stock purchase warrant
exercises. The warrants were exercised at a price of $0.75 per share and the Company receive proceeds of $80,925 from the exercise
of the warrants.
Between July
1, 2013 and September 30, 2013, various warrant holders purchased an aggregate total of 1,652,500 shares of TNI BioTech,
Inc. (the Company) at an exercise price of $0.50 per share. In addition, the Company sold an aggregate sum of
465,000
shares of its common stock at $1.25 and $1.00 per share pursuant to a private placement . In total, the Company received $826,250
as consideration for the exercise of the previously-issued warrants and $531,250 for the purchase of common stock, for an aggregate
sum of $1,357,500.
The
following warrants were issued in 2013:
|
Warrant
Issued
|
|
Warrant
Shares
|
|
|
Issued
Price
|
|
|
Years
|
|
|
Warrant
Expiry
|
|
1/9/2013
|
|
|
125,000
|
|
|
$
|
15.00
|
|
|
|
5
|
|
|
1/8/2018
|
|
2/21/2013
|
|
|
1,750
|
|
|
$
|
15.00
|
|
|
|
5
|
|
|
2/20/2018
|
|
3/8/2013
|
|
|
750
|
|
|
$
|
15.00
|
|
|
|
5
|
|
|
3/7/2018
|
|
5/29/2013
|
|
|
33,334
|
|
|
$
|
15.00
|
|
|
|
5
|
|
|
5/28/2018
|
|
5/31/2013
|
|
|
12,500
|
|
|
$
|
5.00
|
|
|
|
3
|
|
|
5/30/2016
|
|
5/10/2013
|
|
|
25,000
|
|
|
$
|
5.00
|
|
|
|
3
|
|
|
5/9/2016
|
|
7/17/2013
|
|
|
12,500
|
|
|
$
|
5.00
|
|
|
|
3
|
|
|
7/16/2016
|
|
7/2/2013
|
|
|
12,500
|
|
|
$
|
5.00
|
|
|
|
3
|
|
|
7/1/2016
|
|
7/11/2013
|
|
|
12,500
|
|
|
$
|
5.00
|
|
|
|
3
|
|
|
7/10/2016
|
|
7/11/2013
|
|
|
12,500
|
|
|
$
|
5.00
|
|
|
|
3
|
|
|
7/10/2016
|
|
7/26/2013
|
|
|
400,000
|
|
|
$
|
1.00
|
|
|
|
3
|
|
|
12/31/2018
|
|
12/5/2013
|
|
|
24,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
12/4/2018
|
|
12/5/2013
|
|
|
36,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
12/4/2018
|
|
12/5/2013
|
|
|
24,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
12/4/2018
|
|
12/10/2013
|
|
|
48,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
12/9/2018
|
|
12/11/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
12/10/2018
|
|
12/12/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
12/11/2018
|
|
12/13/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
12/12/2018
|
|
12/18/2013
|
|
|
60,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
12/17/2018
|
|
12/18/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
12/17/2018
|
|
12/20/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
12/19/2018
|
|
12/23/2013
|
|
|
18,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
12/22/2018
|
|
12/11/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
12/10/2018
|
|
12/18/2013
|
|
|
75,000
|
|
|
$
|
1.00
|
|
|
|
5
|
|
|
12/31/2018
|
|
12/18/2013
|
|
|
40,000
|
|
|
$
|
1.00
|
|
|
|
5
|
|
|
12/31/2018
|
|
11/1/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
10/31/2018
|
|
11/1/2013
|
|
|
96,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
10/31/2018
|
|
11/6/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
11/5/2018
|
|
11/13/2013
|
|
|
48,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
11/12/2018
|
|
10/9/2013
|
|
|
512,500
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
10/8/2018
|
|
10/10/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
10/9/2018
|
|
10/18/2013
|
|
|
24,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
10/17/2018
|
|
10/22/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
10/21/2018
|
|
10/22/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
10/21/2018
|
|
10/23/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
10/22/2018
|
|
10/28/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
10/27/2018
|
|
10/30/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
10/29/2018
|
|
10/30/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
10/29/2018
|
|
10/31/2013
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
10/30/2018
|
|
7/2/2013
|
|
|
75,000
|
|
|
$
|
5.00
|
|
|
|
3
|
|
|
7/1/2016
|
|
8/1/2013
|
|
|
400,000
|
|
|
$
|
1.00
|
|
|
|
5
|
|
|
12/31/2018
|
2014
For
fiscal year 2014, the Company issued the following securities:
Between January
1, 2014 and March 31, 2014, various warrant holders exercised their warrants for an aggregate of 627,250 shares
of the Company at an exercise price of $0.50 per share. In addition, the Company sold an aggregate 627,250 shares of its common
stock at $0.58 per share and 48,000 warrants in a private placement. The warrants are exercisable for a period of five years at
an exercise price of $1.50 per share. In total, the Company received $290,625 as consideration for the exercise of the previously-issued
warrants and $366,400 for the purchase of common stock under the private placement, for an aggregate sum of $657,025.
In
the three months ended March 31, 2014, the Company issued a total of 5,490,000 shares, at an average price of $1.45 per share,
and 1,400,000 five-year warrants, with an average exercise price of $1.36, in accordance with consulting agreements entered
into in 2013 and 2014. The consulting agreements cover the provision of product and market development services, business development
and strategy services, and investor and media relations services to the Company.
Between January
1, 2014 and June 30, 2014, various warrant holders exercised their warrants for an aggregate of 918,750 shares
of the Company at an exercise price of $0.50 per share. In addition, the Company sold an aggregate 3,557,250 shares of its common
stock at $0.87 per share. In total, the Company received $459,375 as consideration for the exercise of the previously-issued warrants
and $2,838,532 for the purchase of common stock under the private placement, for an aggregate sum of $3,297,907.
In
the six months ended June 30, 2014, the Company issued a total of 7,140,000 shares, at an average price of $1.25 per share, and
1,400,000 five-year warrants, with an average exercise price of $1.36, in accordance with consulting agreements entered into in
2013 and 2014. The consulting agreements cover the provision of product and market development services, business development
and strategy services, and investor and media relations services to the Company.
Between January
1, 2014 and September 30, 2014, various warrant holders exercised their warrants for an aggregate of 918,750 shares
of the Company at an exercise price of $0.50 per share. In addition, the Company sold an aggregate 6,373,410 shares of its common
stock at $0.28 per share. In total, the Company received $2,474 as consideration for the exercise of the previously-issued warrants
and $1,758 for the purchase of common stock under the Private Placement, for an aggregate sum of $4,232.
In
the nine months ended September 30, 2014, the Company issued a total of 22,719,000 shares, at an average price of $0 .65
per share, and five-year warrants, with an average exercise price of $1.00, in accordance with consulting agreements entered into
in 2013 and 2014. The consulting agreements cover the provision of product and market development services, business development
and strategy services, and investor and media relations services to the Company.
In
the nine months ended September 30, 2014, the Company issued a total of $20,848,767 shares, at a price range of $0.10 to $0.25
per share settle amounts owed under notes payable, including accrued and unpaid interest as applicable, to common stock as repayment
of the notes.
In
the nine months ended September 30, 2014, the Company issued a total of 1,838,683 shares, at prices between $0.08 and $0.25 per
share settle amounts owed to certain of the Company’s vendors.
The
Company issued the following warrants in 2014:
|
Warrant
Issued
|
|
Warrant
Shares
|
|
|
Issued
Price
|
|
|
Years
|
|
|
Warrant
Expiry
|
|
2/18/2014
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
2/17/2019
|
|
1/29/2014
|
|
|
12,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
1/28/2019
|
|
7/29/2014
|
|
|
250,000
|
|
|
$
|
0.50
|
|
|
|
5
|
|
|
7/28/2019
|
|
9/29/2014
|
|
|
250,000
|
|
|
$
|
1.50
|
|
|
|
4
|
|
|
9/28/2018
|
|
9/30/2014
|
|
|
10,000
|
|
|
$
|
1.50
|
|
|
|
5
|
|
|
9/29/2019
|
|
12/15/2014
|
|
|
150,000
|
|
|
$
|
0.14
|
|
|
|
5
|
|
|
12/14/2019
|
|
12/15/2014
|
|
|
250,000
|
|
|
$
|
0.14
|
|
|
|
5
|
|
|
12/14/2019
|
The
Company issued the following promissory notes in 2014:
|
Loan
date
|
|
Loan
amount (in $)
|
|
|
Royalty
|
|
|
Interest
rate p.a.
|
|
|
3/7/2014
|
|
|
100,000
|
|
|
|
|
|
|
|
1500 p/m cash
|
|
|
3/7/2014
|
|
|
100,000
|
|
|
|
|
|
|
|
1500 p/m cash
|
|
|
11/30/14
|
|
|
50,000
|
|
|
|
2
|
%
|
|
|
10
|
%
|
|
12/31/14
|
|
|
50,000
|
|
|
|
2
|
%
|
|
|
10
|
%
|
|
11/26/14
|
|
|
60,000
|
|
|
|
2
|
%
|
|
|
10
|
%
|
|
12/2/14
|
|
|
36,500
|
|
|
|
2
|
%
|
|
|
10
|
%
|
2015
The
Company issued the following securities in 2015:
Between January
1, 2015 and March 31, 2015, warrant holders exercised warrants to purchase 24,000 shares. The Company received $12,000
from the warrant holders.
The
Company sold an aggregate 240,000 shares of its common stock at $0.10 per share. In total, the Company received $5,975 as consideration
for the exercise of the previously-issued warrants and $24,000 for the purchase of common stock under the Private Placement, for
an aggregate sum of $29,975.
Between January
1, 2015 and June 30, 2015, the Company sold an aggregate 1,640,000 shares of its common stock at an average price of $0.085
per share, and received $140,000 therefor.
2,803,240
shares were issued by the Company in the quarter ended June 30, 2015 to settle amounts aggregating $600,562 owed to certain of
the Company’s vendors.
Between
April 29, 2015 and June 22, 2015 promissory notes totaling $378,500 were issued by the Company’s subsidiary Cytocom, Inc.
Lenders earn interest at rates between 5% and 10% per annum. Notes will be repaid between June 30, 2015 and September 30, 2015
During
the six months ended June 30, 2015 and 2014, the Company issued 10,239,170 and 7,140,000 shares of common stock respectively for
consulting fees. The Company valued these shares at $1,810,876 and $8,922,500 respectively, based upon the fair value of the common
stock at the dates of the agreements. The consulting fees are amortized over the contract periods which are typically between
12 and 24 months. The amortization of prepaid services totaled $3,073,518 and $16,577,236 for the six months ended June 30, 2015
and 2014.
The
following table describes all unregistered equity securities we issued during 2015
|
Date
|
|
|
Description
|
|
Number
|
|
|
Purchaser
|
|
|
Proceeds
|
|
|
Consideration
|
|
|
Exemption
|
|
|
7/9/2015
|
|
|
Common Stock Purchase
|
|
|
500,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Advisory
services
|
|
|
Sec. 4(a)(2)
|
|
|
7/15/2015
|
|
|
Common Stock Purchase
|
|
|
400,000
|
|
|
|
Subsidiary company
|
|
|
$
|
Nil
|
|
|
|
Incorporation new
subsidiary
|
|
|
Sec. 4(a)(2)
|
|
|
8/31/2015
|
|
|
Common Stock Purchase
|
|
|
4,000,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
8/31/2015
|
|
|
Common Stock Purchase
|
|
|
1,200,000
|
|
|
|
Shareholder
|
|
|
$
|
60,000
|
|
|
|
Purchase
|
|
|
Sec. 4(a)(2)
|
|
|
8/31/2015
|
|
|
Common Stock Purchase
|
|
|
280,000
|
|
|
|
Shareholder
|
|
|
$
|
14,000
|
|
|
|
Purchase
|
|
|
Sec. 4(a)(2)
|
|
|
8/31/2015
|
|
|
Common Stock Purchase
|
|
|
200,000
|
|
|
|
Shareholder
|
|
|
$
|
10,000
|
|
|
|
Purchase
|
|
|
Sec. 4(a)(2)
|
|
|
8/31/2015
|
|
|
Common Stock Purchase
|
|
|
200,000
|
|
|
|
Shareholder
|
|
|
$
|
10,000
|
|
|
|
Purchase
|
|
|
Sec. 4(a)(2)
|
|
|
8/31/2015
|
|
|
Common Stock Purchase
|
|
|
200,000
|
|
|
|
Shareholder
|
|
|
$
|
10,000
|
|
|
|
Purchase
|
|
|
Sec. 4(a)(2)
|
|
|
8/31/2015
|
|
|
Common Stock Purchase
|
|
|
40,000
|
|
|
|
Shareholder
|
|
|
$
|
2,000
|
|
|
|
Purchase
|
|
|
Sec. 4(a)(2)
|
|
|
8/31/2015
|
|
|
Common Stock Purchase
|
|
|
500,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
9/1/2015
|
|
|
Common Stock Purchase
|
|
|
2,500,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
9/1/2015
|
|
|
Common Stock Purchase
|
|
|
462,500
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Debt settlement
|
|
|
Sec. 4(a)(2)
|
|
|
9/1/2015
|
|
|
Common Stock Purchase
|
|
|
100,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Debt settlement
|
|
|
Sec. 4(a)(2)
|
|
|
9/1/2015
|
|
|
Common Stock Purchase
|
|
|
1,000,000
|
|
|
|
Officer
|
|
|
$
|
Nil
|
|
|
|
Employment services
|
|
|
Sec. 4(a)(2)
|
|
|
9/1/2015
|
|
|
Common Stock Purchase
|
|
|
2,000,000
|
|
|
|
Officer
|
|
|
$
|
Nil
|
|
|
|
Employment services
|
|
|
Sec. 4(a)(2)
|
|
|
9/2/2015
|
|
|
Common Stock Purchase
|
|
|
1,200,000
|
|
|
|
Officer
|
|
|
$
|
Nil
|
|
|
|
Employment services
|
|
|
Sec. 4(a)(2)
|
|
|
9/2/2015
|
|
|
Common Stock Purchase
|
|
|
1,000,000
|
|
|
|
Employee
|
|
|
$
|
Nil
|
|
|
|
Settlement agreement
|
|
|
Sec. 4(a)(2)
|
|
|
9/3/2015
|
|
|
Common Stock Purchase
|
|
|
4,501,880
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
9/4/2015
|
|
|
Common Stock Purchase
|
|
|
16,664
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
9/4/2015
|
|
|
Common Stock Purchase
|
|
|
80,000
|
|
|
|
Shareholder
|
|
|
$
|
4,000
|
|
|
|
Purchase
|
|
|
Sec. 4(a)(2)
|
|
|
9/4/2015
|
|
|
Common Stock Purchase
|
|
|
33,335
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
9/4/2015
|
|
|
Common Stock Purchase
|
|
|
33,335
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
9/4/2015
|
|
|
Common Stock Purchase
|
|
|
773,600
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
9/17/2015
|
|
|
Common Stock Purchase
|
|
|
1,300,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Employment services
|
|
|
Sec. 4(a)(2)
|
|
|
9/17/2015
|
|
|
Common Stock Purchase
|
|
|
1,300,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Employment services
|
|
|
Sec. 4(a)(2)
|
|
|
9/22/2015
|
|
|
Common Stock Purchase
|
|
|
1,000,000
|
|
|
|
Shareholder
|
|
|
$
|
10,000
|
|
|
|
Purchase
|
|
|
Sec. 4(a)(2)
|
|
|
9/22/2015
|
|
|
Common Stock Purchase
|
|
|
1,000,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
9/23/2015
|
|
|
Common Stock Purchase
|
|
|
500,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
The
Company issued the following warrants in 2015:
|
Warrant
Issued
|
|
Warrant
Shares
|
|
|
Issued
Price
|
|
|
Years
|
|
|
Warrant
Expiry
|
|
4/30/15
|
|
|
15,000
|
|
|
$
|
0.07
|
|
|
|
4
|
|
|
4/1/2019
|
|
5/31/15
|
|
|
15,000
|
|
|
$
|
0.10
|
|
|
|
4
|
|
|
4/1/2019
|
|
6/30/15
|
|
|
15,000
|
|
|
$
|
0.08
|
|
|
|
4
|
|
|
4/1/2019
|
|
4/8/15
|
|
|
300,000
|
|
|
$
|
0.50
|
|
|
|
5
|
|
|
4/8/2020
|
|
7/31/15
|
|
|
15,000
|
|
|
$
|
0.20
|
|
|
|
4
|
|
|
4/1/2019
|
|
8/31/15
|
|
|
15,000
|
|
|
$
|
0.23
|
|
|
|
4
|
|
|
4/1/2019
|
|
9/30/15
|
|
|
15,000
|
|
|
$
|
0.22
|
|
|
|
4
|
|
|
4/1/2019
|
|
10/31/15
|
|
|
15,000
|
|
|
$
|
0.12
|
|
|
|
3
|
|
|
4/1/2019
|
|
11/30/15
|
|
|
15,000
|
|
|
$
|
0.22
|
|
|
|
3
|
|
|
4/1/2019
|
|
12/31/15
|
|
|
15,000
|
|
|
$
|
0.22
|
|
|
|
3
|
|
|
4/1/2019
|
The
Company issued the following promissory notes in 2015:
|
Loan
date
|
|
Loan
amount (in $)
|
|
|
Royalty
|
|
|
Interest
rate p.a.
|
|
|
1/5/15
|
|
|
33,500
|
|
|
|
2
|
%
|
|
|
10
|
%
|
|
3/10/15
|
|
|
50,000
|
|
|
|
2
|
%
|
|
|
10
|
%
|
|
3/31/15
|
|
|
13,000
|
|
|
|
2
|
%
|
|
|
10
|
%
|
|
7/1/2015
|
|
|
77,500
|
|
|
|
|
|
|
|
5
|
%
|
|
1/26/2015
|
|
|
200,000
|
|
|
|
|
|
|
|
2
|
%
|
|
8/1/2015
|
|
|
278,933
|
|
|
|
|
|
|
|
10
|
%
|
|
5/15/2015
|
|
|
98,500
|
|
|
|
|
|
|
$
|
10,000
|
/mo
|
|
3/3/2015
|
|
|
50,000
|
|
|
|
2
|
%
|
|
|
10
|
%
|
|
4/9/2015
|
|
|
3,000
|
|
|
|
2
|
%
|
|
|
10
|
%
|
|
12/10/2015
|
|
|
100,000
|
|
|
|
|
|
|
|
10%
per month
|
|
|
11/24/2015
|
|
|
175,267
|
|
|
|
|
|
|
|
10
|
%
|
|
7/10/2015
|
|
|
100,000
|
|
|
|
|
|
|
|
|
|
|
11/3/2015
|
|
|
162,737
|
|
|
|
|
|
|
|
10
|
%
|
|
12/1/2015
|
|
|
53,494
|
|
|
|
|
|
|
|
10
|
%
|
|
7/1/15
|
|
|
77,500
|
|
|
|
|
|
|
|
5
|
%
|
|
8/1/15
|
|
|
278,933
|
|
|
|
|
|
|
|
10
|
%
|
|
5/15/2015
|
|
|
98,500
|
|
|
|
|
|
|
$
|
10,000
|
/mo
|
|
7/10/2015
|
|
|
100,000
|
|
|
|
|
|
|
|
|
|
|
1/26/15
|
|
|
200,000
|
|
|
|
|
|
|
|
2
|
%
|
|
12/10/2015
|
|
|
100,000
|
|
|
|
|
|
|
|
10%
per month
|
|
|
11/24/2015
|
|
|
175,267
|
|
|
|
|
|
|
|
10
|
%
|
|
11/3/2015
|
|
|
162,737
|
|
|
|
|
|
|
|
10
|
%
|
|
12/1/2015
|
|
|
53,494
|
|
|
|
|
|
|
|
10
|
%
|
2016
The
Company has issued the following securities in 2016, as of June 30, 2016.
In
the quarter ended March 31, 2016, the Company issued a total of 30,277,483 shares of common stock (net of stock cancellations).
8,503,041 of those shares were issued to settle amounts owed under notes payable, including accrued and unpaid interest as applicable,
to common stock as repayment of the notes (0 in 2015). 2,418,942 of the shares were issued to settle amounts owed to certain of
the Company’s vendors and employees (0 in 2015). Between January 1, 2016 and March 31, 2016, warrant holders exercised no
warrants to purchase shares. The Company sold an aggregate 312,500 shares of its common stock at $0.08 per share. In total, the
Company received $0 as consideration for the exercise of the previously-issued warrants and $25,000 for the purchase of common
stock, for an aggregate sum of $25,000.
In
the quarter ended June 30, 2016, the Company issued a total of 9,320,081 shares of common stock (net of stock cancellations),
compared to 14,706,410 in the corresponding period in 2015. 3,511,285 of those shares were issued to settle amounts owed under
notes payable, including accrued and unpaid interest as applicable, to common stock as repayment of the notes (2,803,240 in 2015).
4,450,000 of the shares were issued to as payment for prepaid services to Company’s vendors
(10,239,170
in 2015). Between April 1, 2016 and June 30, 2016, warrant holders exercised no warrants to purchase shares (0 in 2015). The Company
sold an aggregate 312,500 shares of its common stock at an average of $0.08 per share. In total, the Company received $0.00 as
consideration for the exercise of the previously-issued warrants and $25,000 for the purchase of common stock, for an aggregate
sum of $25,000.
The
following table lists all equity securities issued without registering the securities under the Securities Act, up to June 30,
2016:
|
Date
|
|
|
Description
|
|
Number
|
|
|
Purchaser
|
|
|
Proceeds
|
|
|
Consideration
|
|
Exemption
|
|
|
|
1/8/2016
|
|
|
Common
Stock Purchase
|
|
|
5,000,000
|
|
|
|
Ex-officer
|
|
|
$
|
Nil
|
|
|
Debt
Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
1/11/2016
|
|
|
Common Stock
Purchase
|
|
|
967,900
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Settlement of
Accounts Payable
|
|
|
Sec. 4(a)(2)
|
|
|
|
1/21/2016
|
|
|
Common Stock
Purchase
|
|
|
1,453,313
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
1/26/2016
|
|
|
Common Stock
Purchase
|
|
|
172,656
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
1/26/2016
|
|
|
Common Stock
Purchase
|
|
|
999,392
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
1/26/2016
|
|
|
Common Stock
Purchase
|
|
|
208,114
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
1/26/2016
|
|
|
Common Stock
Purchase
|
|
|
269,097
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
1/26/2016
|
|
|
Common Stock
Purchase
|
|
|
160,829
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
1/26/2016
|
|
|
Common Stock
Purchase
|
|
|
160,829
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
1/28/2016
|
|
|
Common Stock
Purchase
|
|
|
312,500
|
|
|
|
Shareholder
|
|
|
$
|
25,000
|
|
|
Purchase
|
|
|
Sec. 4(a)(2)
|
|
|
|
1/29/2016
|
|
|
Common Stock
Purchase
|
|
|
497,387
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
1/29/2016
|
|
|
Common Stock
Purchase
|
|
|
706,250
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
2/11/2016
|
|
|
Common Stock
Purchase
|
|
|
7,000,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
2/23/2016
|
|
|
Common Stock
Purchase
|
|
|
300,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Settlement of
Accounts Payable
|
|
|
Sec. 4(a)(2)
|
|
|
|
2/23/2016
|
|
|
Common Stock
Purchase
|
|
|
1,000,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
2/23/2016
|
|
|
Common Stock
Purchase
|
|
|
1,000,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
2/23/2016
|
|
|
Common
Stock Purchase
|
|
|
500,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory
services
|
|
|
Sec. 4(a)(2)
|
|
|
|
2/23/2016
|
|
|
Common Stock
Purchase
|
|
|
100,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
2/23/2016
|
|
|
Common Stock
Purchase
|
|
|
100,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
2/23/2016
|
|
|
Common Stock
Purchase
|
|
|
143,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
2/23/2016
|
|
|
Common Stock
Purchase
|
|
|
600,000
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/1/2016
|
|
|
Common Stock
Purchase
|
|
|
1,000,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Settlement of
Accounts Payable
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/1/2016
|
|
|
Common Stock
Purchase
|
|
|
1,000,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/1/2016
|
|
|
Common Stock
Purchase
|
|
|
197,500
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/1/2016
|
|
|
Common
Stock Purchase
|
|
|
500,000
|
|
|
|
Officer
|
|
|
$
|
Nil
|
|
|
Employment
services
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/3/2016
|
|
|
Common Stock
Purchase
|
|
|
651,042
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Settlement of
Accounts Payable
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/3/2016
|
|
|
Common Stock
Purchase
|
|
|
1,250,000
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/10/2016
|
|
|
Common Stock
Purchase
|
|
|
100,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/10/2016
|
|
|
Common Stock
Purchase
|
|
|
250,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/10/2016
|
|
|
Common Stock
Cancellation
|
|
|
(1,000,000
|
)
|
|
|
Officer
|
|
|
$
|
Nil
|
|
|
Employment services,
forfeited
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/16/2016
|
|
|
Common Stock
Purchase
|
|
|
1,337,431
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/18/2016
|
|
|
Common Stock
Purchase
|
|
|
500,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/21/2016
|
|
|
Common Stock
Purchase
|
|
|
100,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/23/2016
|
|
|
Common Stock
Purchase
|
|
|
2,000,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/29/2016
|
|
|
Common Stock
Purchase
|
|
|
490,243
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt Settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
3/31/2016
|
|
|
Common Stock
Purchase
|
|
|
250,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
4/7/2016
|
|
|
Common Stock Purchase
|
|
|
250,000
|
|
|
|
Director
|
|
|
$
|
Nil
|
|
|
Board services
|
|
|
Sec. 4(a)(2)
|
|
|
|
4/8/2016
|
|
|
Common Stock Purchase
|
|
|
680,035
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
5/4/2016
|
|
|
Common Stock Purchase
|
|
|
40,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
5/4/2016
|
|
|
Common Stock Purchase
|
|
|
40,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
5/4/2016
|
|
|
Common Stock Purchase
|
|
|
40,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
5/4/2016
|
|
|
Common Stock Purchase
|
|
|
40,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
5/4/2016
|
|
|
Common Stock Purchase
|
|
|
40,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
6/7/2016
|
|
|
Common Stock Purchase
|
|
|
296,296
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Origination Fees
|
|
|
Sec. 4(a)(2)
|
|
|
|
6/23/2016
|
|
|
Common Stock Purchase
|
|
|
1,000,000
|
|
|
|
Officer
|
|
|
$
|
Nil
|
|
|
Employment services
|
|
|
Sec. 4(a)(2)
|
|
|
|
6/23/2016
|
|
|
Common Stock Purchase
|
|
|
437,500
|
|
|
|
Officer
|
|
|
$
|
Nil
|
|
|
Employment services
|
|
|
Sec. 4(a)(2)
|
|
|
|
6/29/2016
|
|
|
Common Stock Purchase
|
|
|
100,000
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
6/30/2016
|
|
|
Common Stock Purchase
|
|
|
2,000,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Consulting
|
|
|
Sec. 4(a)(2)
|
|
|
|
6/30/2016
|
|
|
Common Stock Purchase
|
|
|
1,000,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
6/30/2016
|
|
|
Common Stock Purchase
|
|
|
312,500
|
|
|
|
Shareholder
|
|
|
$
|
25,000
|
|
|
Share purchase agreement
|
|
|
Sec. 4(a)(2)
|
|
|
|
4/20/2016
|
|
|
Common Stock Purchase
|
|
|
500,000
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Origination fees/
|
|
|
Sec. 4(a)(2)
|
|
|
|
5/10/2016
|
|
|
Common Stock Purchase
|
|
|
156,250
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Settlement of accounts
payable
|
|
|
Sec. 4(a)(2)
|
|
|
|
6/1/2016
|
|
|
Common Stock Purchase
|
|
|
987,500
|
|
|
|
Lender
|
|
|
$
|
Nil
|
|
|
Debt settlement
|
|
|
Sec. 4(a)(2)
|
|
|
|
6/16/2016
|
|
|
Common Stock Purchase
|
|
|
50,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
6/16/2016
|
|
|
Common Stock Purchase
|
|
|
50,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
6/16/2016
|
|
|
Common Stock Purchase
|
|
|
50,000
|
|
|
|
Consultant
|
|
|
$
|
Nil
|
|
|
Advisory services
|
|
|
Sec. 4(a)(2)
|
|
|
|
6/21/2016
|
|
|
Common Stock Purchase
|
|
|
625,000
|
|
|
|
Ex-officer
|
|
|
$
|
Nil
|
|
|
Board and employment services
|
|
|
Sec. 4(a)(2)
|
|
|
|
6/21/2016
|
|
|
Common Stock Purchase
|
|
|
625,000
|
|
|
|
Ex-officer
|
|
|
$
|
Nil
|
|
|
Board and consulting services
|
|
|
Sec. 4(a)(2)
|
|
The
Company has issued the following warrants, up to June 30, 2016
|
Warrant
Issued
|
|
Warrant
Shares
|
|
|
Issued
Price
|
|
|
Years
|
|
|
Warrant
Expiry
|
|
3/1/2016
|
|
|
1,000,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
2/28/2021
|
|
2/26/2016
|
|
|
2,000,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
2/25/2021
|
|
2/19/2016
|
|
|
2,000,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
2/18/2021
|
|
2.18/2016
|
|
|
2,000,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
2/17/2021
|
|
2/17/2016
|
|
|
2,000,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
2/16/2021
|
|
3/2/2016
|
|
|
1,000,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
3/1/2021
|
|
3/17/2016
|
|
|
1,000,000
|
|
|
$
|
0.50
|
|
|
|
3
|
|
|
3/16/2019
|
|
3/17/2016
|
|
|
1,000,000
|
|
|
$
|
1.00
|
|
|
|
3
|
|
|
3/16/2019
|
|
3/17/2016
|
|
|
1,000,000
|
|
|
$
|
2.00
|
|
|
|
3
|
|
|
3/16/2019
|
|
3/18/2016
|
|
|
1,000,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
3/17/2021
|
|
3/18/2016
|
|
|
1,000,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
3/17/2021
|
|
3/30/2016
|
|
|
500,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
3/29/2021
|
|
3/30/2016
|
|
|
50,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
3/29/2021
|
|
3/7/2016
|
|
|
50,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
3/2/2021
|
|
6/2/2016
|
|
|
1,339,287
|
|
|
$
|
0.14
|
|
|
|
5
|
|
|
6/1/2021
|
|
4/6/2016
|
|
|
3,515,621
|
|
|
$
|
0.14
|
|
|
|
5
|
|
|
4/5/2021
|
|
1/1/2016
|
|
|
1,000,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
12/30/2020
|
|
5/19/2016
|
|
|
1,000,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
5/18/2021
|
|
3/3/2016
|
|
|
50,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
3/2/2021
|
|
6/30/2016
|
|
|
100,000
|
|
|
$
|
0.17
|
|
|
|
5
|
|
|
6/29/2021
|
|
6/30/2016
|
|
|
50,000
|
|
|
$
|
0.17
|
|
|
|
5
|
|
|
6/29/2021
|
|
5/5/2016
|
|
|
1,000,000
|
|
|
$
|
0.20
|
|
|
|
5
|
|
|
5/4/2021
|
The
following are all promissory notes issued in 2016:
|
Loan
date
|
|
Loan
amount (in $)
|
|
Royalty
|
|
Interest
rate p.a.
|
|
2/23/2016
|
|
|
|
37,000
|
|
|
|
|
|
|
|
10
|
%
|
|
3/9/2016
|
|
|
|
25,000
|
|
|
|
|
|
|
|
10
|
%
|
|
1/8/2016
|
|
|
|
80,000
|
|
|
|
|
|
|
|
10
|
%
|
|
3/9/2016
|
|
|
|
20,000
|
|
|
|
|
|
|
|
10
|
%
|
|
2/8/2016
|
|
|
|
50,000
|
|
|
|
|
|
|
|
10
|
%
|
|
3/31/2016
|
|
|
|
100,000
|
|
|
|
|
|
|
|
10
|
%
|
|
2/3/2016
|
|
|
|
|
|
|
|
|
|
|
|
prepaid
|
|
|
4/11/2016
|
|
|
|
21,000
|
|
|
|
|
|
|
|
10
|
%
|
|
4/11/2016
|
|
|
|
21,000
|
|
|
|
|
|
|
|
10
|
%
|
|
4/11/2016
|
|
|
|
21,000
|
|
|
|
|
|
|
|
10
|
%
|
|
7/13/2016
|
|
|
|
656,250
|
|
|
|
|
|
|
|
prepaid
|
|
|
5/10/2016
|
|
|
|
305,000
|
|
|
|
|
|
|
|
prepaid
|
|
|
06/2016
|
|
|
|
32,000
|
|
|
|
|
|
|
|
10
|
%
|
|
06/2016
|
|
|
|
18,000
|
|
|
|
|
|
|
|
10
|
%
|
|
06/2016
|
|
|
|
200,000
|
|
|
|
|
|
|
|
prepaid
|
|
|
2/23/2016
|
|
|
|
37,000
|
|
|
|
|
|
|
|
10
|
%
|
|
3/9/2016
|
|
|
|
25,000
|
|
|
|
|
|
|
|
10
|
%
|
|
1/8/2016
|
|
|
|
80,000
|
|
|
|
|
|
|
|
10
|
%
|
|
3/9/2016
|
|
|
|
20,000
|
|
|
|
|
|
|
|
10
|
%
|
|
2/8/2016
|
|
|
|
50,000
|
|
|
|
|
|
|
|
10
|
%
|
|
4/11/2016
|
|
|
|
21,000
|
|
|
|
|
|
|
|
10
|
%
|
|
4/11/2016
|
|
|
|
21,000
|
|
|
|
|
|
|
|
10
|
%
|
|
4/11/2016
|
|
|
|
21,000
|
|
|
|
|
|
|
|
10
|
%
|
|
3/31/2016
|
|
|
|
100,000
|
|
|
|
|
|
|
|
10
|
%
|
|
06/2016
|
|
|
|
32,000
|
|
|
|
|
|
|
|
10
|
%
|
|
06/2016
|
|
|
|
18,000
|
|
|
|
|
|
|
|
10
|
%
|
|
7/13/2016
|
|
|
|
656,250
|
|
|
|
|
|
|
|
prepaid
|
|
|
5/10/2016
|
|
|
|
305,000
|
|
|
|
|
|
|
|
prepaid
|
|
|
06/2016
|
|
|
|
200,000
|
|
|
|
|
|
|
|
prepaid
|
|
All
of the above issuances of the Company’s securities were completed in private transactions by the Company not involving any
public offering pursuant to Section 4(a)(2) and/or Rule 506 of Regulation D of the Securities Act. The shares were issued bearing
a restrictive legend and may not be resold by the holders unless such securities are registered or an exemption from
registration
is available. The Company determined, based on representations of the investors, that the investors were “accredited investors”
as defined under Rule 501(a) of the Securities Act.
EXHIBITS
The
following exhibits are filed with this registration statement:
|
Exhibit
|
|
Description
|
|
|
|
|
|
3.1
|
|
Restated
Articles of Incorporation*
|
|
3.1.1
|
|
Articles
of Amendment for Name Change
|
|
3.2
|
|
Bylaws*
|
|
4.1
|
|
Form
of Common Stock Certificate
|
|
5.1
|
|
Opinion
of Austin Legal Group, APC regarding the legality of the securities being registered
|
|
10.1
|
|
Sale
and Assignment of Patent and Transfer of Technology Agreement with Nicholas Plotnikoff †>
|
|
10.2
|
|
Agreement
with Professor Shan †>
|
|
10.3
|
|
Patent
License Agreement with Penn State Research Foundation r†
|
|
10.4
|
|
Patent
License Agreement Between Immune Therapeutics, Inc. and Jacqueline Young for the intellectual property of Dr. Bernard Bihari#
|
|
10.5
|
|
Strategic
Framework Agreement for Cooperation with Hubei Qianjiang Pharmaceutical Company, and Commissioned Processing Contract, and
Addendum to Venture Cooperation †>
|
|
10.6
|
|
Malawi
Memorandum of Agreement with GB Oncology & Imaging Group Ltd.#
|
|
10.7
|
|
Letter
of Intent between GB Oncology & Imaging Group Ltd. and G-Ex Technologies St. Maris Pharma Limited #
|
|
10.8
|
|
Distribution
Agreement in Nigeria with GB Pharma Holdings Inc. †>
|
|
10.9
|
|
ViPharma
Agreement †>
|
|
10.10
|
|
Strategic
Framework Agreement, Addendum to Venture Cooperation and Supplementary Agreement with Hubei Qianjiang Pharmaceutical Company
(MENK) (filed as Exhibit 10.11 to our Annual Report on Form 10-K for the fiscal year ended December 31, 2013, and incorporated
herein by reference).
|
|
10.11
|
|
Manufacturing
Agreement with Laboratorios Ramos (and English translation)>
|
|
10.12
|
|
Engagement
Agreement for Corporate Advisory Services by the Brewer Group?
|
|
10.13
|
|
Employment
Agreement with Noreen Griffin (filed as Exhibit 10.14 to our Annual Report on Form 10-K for the fiscal year ended December
31, 2013, and incorporated herein by reference).
|
|
10.14
|
|
Master
Service Agreement with American Peptide Company (filed as Exhibit 10.17 to our Annual Report on Form 10-K for the fiscal year
ended December 31, 2013, and incorporated herein by reference).
|
|
10.15
|
|
Agreement
with AHAR Pharma (filed as Exhibit 10.18 to our Annual Report on Form 10-K for the fiscal year ended December 31, 2013, and
incorporated herein by reference).
|
|
10.16
|
|
Consulting
agreement with Dr. Graham Burton (filed as Exhibit 10.19 to our Annual Report on Form 10-K for the fiscal year ended December
31, 2013, and incorporated herein by reference).
|
|
10.17
|
|
Promissory
note to Robert J. Dailey, issued February 6, 2014, for $200,000 (filed as Exhibit 10.1 to our Quarterly Report on Form 10-Q
for the quarter ended March 31, 2014, and incorporated herein by reference).
|
|
10.18
|
|
Promissory
note to Robert J. Dailey, issued March 7, 2014, for $200,000 (filed as Exhibit 10.2 to our Quarterly Report on Form 10-Q for
the quarter ended March 31, 2014, and incorporated herein by reference).
|
|
10.19
|
|
Promissory
note to Robert J. Dailey, issued March 28, 2014, for $200,000 (filed as Exhibit 10.3 to our Quarterly Report on Form 10-Q
for the quarter ended March 31, 2014, and incorporated herein by reference).
|
|
10.20
|
|
Promissory
Note Settlement Agreement, dated September 26, 2014, between Immune Therapeutics, Inc. and Robert Dailey for notes equaling
$399,000 µ
|
|
10.21
|
|
Promissory
Note Settlement Agreement, dated September 26, 2014, between Immune Therapeutics, Inc. and Robert Dailey for notes equaling
$400,000 µ
|
|
10.22
|
|
Distribution
Agreement, effective June 11, 2014, between Airmed Biopharma Limited and PanAm Global Logistics, Inc. (filed as Exhibit 1.1
to our Current Report on Form 8-K dated June 25, 2014, and incorporated herein by reference).
|
|
10.23
|
|
Immune
Therapeutics, Inc. 2014 Stock Incentive Plan (filed as Appendix A to our Definitive Proxy Statement on Schedule 14A filed
on July 16, 2014, and incorporated herein by reference).
|
|
10.24
|
|
Supplementary
Agreement on New Drug Methionine – Enkephalin Cooperation, dated August 6, 2014, between Immune Therapeutics, Inc. and
Hubei Qianjiang Pharmaceutical Co., Ltd. (filed as Exhibit 1.1 to our Current Report on Form 8-K dated August 13, 2014, and
incorporated herein by reference).
|
|
10.25
|
|
Assignment
by Professor Fengping Shan Ph.D. to Immune Therapeutics, Inc., executed August 6, 2014 (filed as Exhibit 1.2 to our Current
Report on Form 8-K dated August 13, 2014, and incorporated herein by reference).
|
|
10.26
|
|
Clinical
Trial Agreement, dated October 20, 2014, between TNI BioTech International Ltd. and American Hospital and Resorts (filed as
Exhibit 10.1 to our Current Report on Form 8-K dated October 30, 2014, and incorporated herein by reference).
|
|
10.27
|
|
Contract
for the Compounding of Pharmaceutical Products, dated December 8, 2014, between Immune Therapeutics, Inc. and KRS Global Biotechnology,
Inc. (filed as Exhibit 10.1 to our Current Report on Form 8-K dated December 15, 2014, and incorporated herein by reference).
|
|
10.28
|
|
Services
Agreement, dated December 15, 2014, between Immune Therapeutics, Inc. and Aronstam Management Services, Inc. µ
|
|
10.29
|
|
Royalty
Agreement (Filed as Exhibit 10.1 to our Current Report on Form 8-K dated May 21, 2015, and incorporated herein by reference.)
|
|
10.30
|
|
Change
of Transfer Agent Agreement (Filed as Exhibit 99.1 to our Current Report on Form 8-K dated January 26, 2015, and incorporated
herein by reference.)
|
|
10.31
|
|
Real
Property Lease for Florida Office and Extension
|
|
10.32
|
|
Compounding
Contract with Complete Pharmacy and Medical Solutions, LLC (filed as Exhibit 10.1 to
our Current Report 8-K dated May 20, 2016, and incorporated herein by reference.)
|
|
10.33
|
|
Employment Agreement with Robert
Wilson (filed as Exhibit 10.1 to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2016, and incorporated herein
by reference).
|
|
10.34
|
|
Letter of Intent with Super T-Cell
Cancer Co., (filed as Exhibit 10.1 to our Current Report on Form 8-K dated April 21, 2016, and incorporated herein by reference.)
|
|
10.35
|
|
Extension of Employment Agreement
with Noreen Griffin
|
|
10.36
|
|
Extension of AHAR Agreement
|
|
10.37
|
|
Extension of Employment Agreement
with Peter Aronstam
|
|
10.38
|
|
Agreement with Joyce Banda
|
|
10.39
|
|
Patent License Agreement with Cytocom
|
|
10.40
|
|
Agreement with Cote Orphan
|
|
10.41
|
|
Securities Purchase Agreement
with JMJ Financial
|
|
21.1
|
|
List of Subsidiaries
|
|
23.1
|
|
Consent
of Turner Stone & Company, L.L.P.
|
|
23.2
|
|
Consent
of Austin Legal Group, APC (included in Exhibit 5.1)
|
|
*
|
filed
with the Form 10 Registration Statement filed with the SEC on April 22, 2013 and the Amendment No. 1 to the Form 10 Registration
Statement filed with the SEC on June 7, 2013 and incorporated herein by reference.
|
|
#
|
filed
with the Amendment No. 1 to the Form 10 Registration Statement filed with the SEC on June 7, 2013 and incorporated herein
by reference.
|
|
+
|
filed
with the Amendment No. 2 to the Form 10 Registration Statement filed with the SEC on July 18, 2013 and incorporated herein
by reference.
|
|
^
|
filed
with the Amendment No. 3 to the Form 10 Registration Statement filed with the SEC on August 23, 2013 and incorporated herein
by reference.
|
|
?
|
filed
with the Amendment No. 4 to the Form 10 Registration Statement filed with the SEC on September 25, 2013 and incorporated herein
by reference.
|
|
Î
|
filed
with the Amendment No. 5 to the Form 10 Registration Statement filed with the SEC on October 11, 2013 and incorporated herein
by reference.
|
|
†
|
Portions
of this exhibit have been redacted pursuant to a confidential treatment order granted by the Securities and Exchange Commission.
|
|
>
|
Filed
with the Amendment No. 6 to the Form 10 Registration Statement filed with the SEC on November 21, 2013 and incorporated hereby
by reference.
|
|
R
|
Filed
with the Amendment No. 7 to the Form 10 Registration Statement filed with the SEC on January 22, 2014 and incorporated hereby
by reference.
|
|
µ
|
Filed
with the Form 10k filed with the SEC on March 30, 2016 and incorporated hereby by reference.
B
Filed herewith.
|
UNDERTAKINGS
|
(a)
|
The
undersigned registrant hereby undertakes:
|
|
(1)
|
To
file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
|
|
i.
|
To
include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
|
|
|
|
|
ii.
|
To
reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most
recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information
set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered
(if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low
or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and
Exchange Commission (the “Commission”) pursuant to Rule 424(b) if, in the aggregate, the changes in volume and
price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation
of Registration Fee” table in the effective registration statement;
|
|
|
|
|
iii.
|
To
include any material information with respect to the plan of distribution not previously disclosed in the registration statement
or any material change to such information in the registration statement.
|
|
(2)
|
That,
for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be
deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities
at that time shall be deemed to be the initial bona fide offering thereof.
|
|
|
|
|
(3)
|
To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold
at the termination of the offering.
|
|
|
|
|
(4)
|
That,
for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant
to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on
Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration
statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration
statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated
by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser
with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration
statement or prospectus that was part of the registration statement or made in any such document immediately prior to such
date of first use.
|
|
(5)
|
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling
persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the
opinion of the Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is,
therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by
the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful
defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with
the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by
controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against
public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue.
|
|
|
|
|
(6)
|
The
undersigned Registrant hereby undertakes that it will:
|
For
the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution
of the securities: the undersigned registrant undertakes that in a primary offering of the securities of the undersigned registrant
pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if
the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant
will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i)
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant
to Rule 424;
(ii)
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred
to by the undersigned registrant;
(iii)
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned
registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv)
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
DEALER
PROSPECTUS DELIVERY OBLIGATION
Until
____________all dealers that effect transactions in these securities, whether or not participating in this offering, may be required
to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters
and with respect to their unsold allotments or subscriptions.
SIGNATURES
Pursuant
to the requirements of the Securities Act, the Registrant has duly caused this Registration Statement to be signed on our behalf
by the undersigned, thereunto duly authorized, on November 9 , 2016.
|
|
Immune
Therapeutics, Inc.
|
|
|
|
|
|
Date:
November 9, 2016
|
By:
|
/s/
Noreen Griffin
|
|
|
Name:
|
Noreen Griffin
|
|
|
Title:
|
Chief Executive
Officer
|
|
|
|
(Principal Executive
Officer)
|
|
|
|
|
|
|
By:
|
/s/
Peter Aronstam
|
|
|
Name:
|
Peter Aronstam
|
|
|
Title:
|
Chief Financial
Officer
|
|
|
|
(Principal Accounting
Officer)
|
In
accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in
the capacities and on the dates indicated.
|
Person
|
|
Capacity
|
|
Date
|
|
|
|
|
|
|
|
/s/
Noreen Griffin
|
|
Director, Principal
Executive Officer
|
|
November 9, 2016
|
|
Noreen Griffin
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Christopher Pearce
|
|
Director
|
|
November
9, 2016
|
|
Christopher Pearce
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Paul Akin
|
|
Director
|
|
November
9, 2016
|
|
Paul Akin
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Clifford Selsky
|
|
Director
|
|
November
9, 2016
|
|
Clifford
Selsky
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Edward Teraskiewicz
|
|
Director
|
|
November
9, 2016
|
|
Edward Teraskiewicz
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Peter Aronstam
|
|
Principal Accounting Officer
|
|
November 9, 2016
|
|
Peter Aronstam
|
|
|
|
|
Immune Therapeutics (PK) (USOTC:IMUN)
Historical Stock Chart
From Mar 2024 to Apr 2024
Immune Therapeutics (PK) (USOTC:IMUN)
Historical Stock Chart
From Apr 2023 to Apr 2024