Filed Pursuant to
Rule 424(b)(5)
Registration No. 333-205168
PROSPECTUS SUPPLEMENT
(To Prospectus dated July 9, 2015)
3,750,000
SHARES

Common
Stock
We are offering shares of our common
stock pursuant to this prospectus supplement and the accompanying prospectus at a purchase price to investors of $4.00 per
share.
Our common
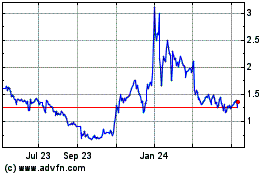
stock is currently quoted on the OTC Markets under the symbol “EKSO.” The last reported sale price of our common stock
on the OTC Markets (OTCQB) on August 5, 2016 was $6.24 per share. In conjunction with this offering, we applied to list our common stock on The NASDAQ Capital Market and
our common stock is expected to begin trading on The NASDAQ Capital Market under the ticker symbol “EKSO” on August
9, 2016.
We are an “emerging growth company” as defined
under the federal securities laws, and, as such, are eligible for reduced public company reporting requirements. See “Prospectus
Summary—Implications of Being an Emerging Growth Company.”
Investing in our securities involves
a high degree of risk. Please read “Risk Factors” beginning on page S-5 of this prospectus supplement and
in the documents incorporated by reference into this prospectus supplement and the accompanying prospectus.
Neither the Securities and Exchange Commission nor any state
securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying
prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
|
|
Per Share
|
|
|
Total
|
|
|
Public offering price
|
|
$
|
4.00
|
|
|
$
|
15,000,000
|
|
|
Underwriting discounts and commissions
(1)
|
|
$
|
0.26
|
|
|
$
|
975,000
|
|
|
Proceeds, before expenses, to us
|
|
$
|
3.74
|
|
|
$
|
14,025,000
|
|
(1) See “Underwriting” for a
description of the compensation payable to the underwriters.
We have granted the underwriters
an option for a period of 30 days from the date of this prospectus supplement to purchase up to an additional 562,500 shares
of common stock to cover overallotments at the public offering price, less underwriting discounts and commissions.
The underwriters expect to deliver the shares against payment
on or about August 12, 2016.
Sole Book-Running Manager
Cowen
and Company
Lead Manager
SunTrust
Robinson Humphrey
Co-Manager
B.
Riley & Co.
The date of this prospectus supplement
is August 9, 2016.
TABLE OF CONTENTS
Prospectus Supplement
Prospectus
Explanatory
note – stock split
Effective May 4, 2016, the Company effected
a one-for-seven reverse split of its common stock. As a result of the reverse stock split, every seven shares of issued and outstanding
common stock were converted into one share of issued and outstanding common stock. All references in this prospectus supplement
to shares and net tangible book value per share have been retroactively restated to reflect the reverse stock split.
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is in two parts. The first
part is this prospectus supplement, which describes the terms of this offering and also adds to and updates information contained
in the accompanying prospectus and the documents incorporated by reference into this prospectus supplement and the accompanying
prospectus. The second part, the accompanying prospectus dated July 9, 2015, including the documents incorporated by reference
therein, provides more general information, some of which may not apply to the securities offered by this prospectus supplement.
Generally, when we refer to this prospectus, we are referring to both parts of this document combined. To the extent there is
a conflict between the information contained in this prospectus supplement, on the one hand, and the information contained in
the accompanying prospectus or in any document incorporated by reference that was filed with the Securities and Exchange Commission
(the “SEC”) before the date of this prospectus supplement, on the other hand, you should rely on the information in
this prospectus supplement. If any statement in one of these documents is inconsistent with a statement in another document having
a later date—for example, a document incorporated by reference in the accompanying prospectus—the statement in the
document having the later date modifies or supersedes the earlier statement unless otherwise specified. You should assume that
the information contained in this prospectus supplement is accurate as of the date on the front cover of this prospectus supplement
only and that any information we have incorporated by reference or included in the accompanying prospectus is accurate only as
of the date given in the document incorporated by reference or as of the date of the prospectus, as applicable, regardless of
the time of delivery of this prospectus supplement or the accompanying prospectus or any sale of our securities. Our business,
financial condition, results of operations and prospects may have changed since that date.
We further note that the representations,
warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference
herein or in the accompanying prospectus were made solely for the benefit of the parties to such agreement, including, in some
cases, for the purpose of allocating risk among the parties to such agreement, and should not be deemed to be a representation,
warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made.
Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state
of our affairs.
You should rely
only on the information contained in this prospectus supplement or the accompanying prospectus, or incorporated by reference herein.
We have not authorized, and the underwriters have not authorized, anyone to provide you with information that is different. The
information contained in this prospectus supplement or the accompanying prospectus, or incorporated by reference herein is accurate
only as of the respective dates thereof, regardless of the time of delivery of this prospectus supplement and the accompanying
prospectus or of any sale of our securities. It is important for you to read and consider all information contained in this prospectus
supplement and the accompanying prospectus, including the documents incorporated by reference herein, in making your investment
decision. You should also read and consider the information in the documents to which we have referred you in the sections entitled
"Where You Can Find More Information" and "Incorporation of Documents by Reference" in this prospectus supplement
and in the accompanying prospectus.
We are offering
to sell, and seeking offers to buy, our securities only in jurisdictions where offers and sales are permitted. The distribution
of this prospectus supplement and the accompanying prospectus and the offering of the securities in certain jurisdictions may be
restricted by law. Persons outside the United States who come into possession of this prospectus supplement and the accompanying
prospectus must inform themselves about, and observe any restrictions relating to, the offering and the distribution of this prospectus
supplement and the accompanying prospectus outside the United States. This prospectus supplement and the accompanying prospectus
do not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities
offered by this prospectus supplement and the accompanying prospectus by any person in any jurisdiction in which it is unlawful
for such person to make such an offer or solicitation.
Unless the context requires otherwise,
all references in this prospectus supplement and the accompanying prospectus to “Ekso,” “the Company,”
“we,” “us,” “our,” or similar references refer to Ekso Bionics Holdings, Inc. and its
subsidiaries.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING
STATEMENTS
This
prospectus supplement, the accompanying prospectus and the documents incorporated by reference, include forward-looking statements
within the meaning of the Private Securities Litigation Reform Act of 1995.
These
forward-looking statements include all statements other than statements of historical facts contained or incorporated by reference
in this prospectus supplement and the accompanying prospectus, including statements regarding (i) the plans and objectives of
management for future operations, including plans or objectives relating to the design, development and commercialization of human
exoskeletons, (ii) a projection of income (including income/loss), earnings (including earnings/loss) per share, capital expenditures,
dividends, capital structure or other financial items, (iii) our future financial performance, including any such statement contained
in a discussion and analysis of financial condition by management or in the results of operations included pursuant to the rules
and regulations of the SEC, (iv) our beliefs regarding the potential for commercial opportunity for exoskeleton technology in
general and our exoskeleton products in particular, (v) our beliefs regarding potential clinical and other health benefits of
our medical devices, and (vi) the assumptions underlying or relating to any statement described in points (i), (ii), (iii), (iv)
or (v) above. The words “believe,” “may,” “will,” “estimate,” “continue,”
“anticipate,” “intend,” “expect” and similar expressions are intended to identify forward-looking
statements.
The
forward-looking statements are not meant to predict or guarantee actual results, performance, events or circumstances and may
not be realized because they are based upon our current projections, plans, objectives, beliefs, expectations, estimates and assumptions
and are subject to a number of risks and uncertainties and other influences, many of which we have no control over. Actual results
and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements
as a result of these risks and uncertainties. Factors that may influence or contribute to the inaccuracy of the forward-looking
statements or cause actual results to differ materially from expected or desired results may include, without limitation, our
inability to obtain adequate financing and necessary to develop or enhance our technology; the significant length of time and
resources associated with the development of our products; our failure to achieve broad market acceptance of our products; the
failure of our sales and marketing organization or partners to market our products effectively; adverse results in future clinical
studies of our medical device products; our failure to obtain or maintain patent protection for our technology; our failure to
obtain or maintain regulatory approval to market our medical devices; lack of product diversification; existing or increased competition;
our failure to implement our business plans or strategies; and other risks, uncertainties and assumptions included in our periodic
reports and in other documents that we file with the SEC, including those described in the section of this prospectus supplement
captioned “Risk Factors” beginning on page S-5.
Investors
are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this prospectus
supplement or the date of the document incorporated by reference in this prospectus supplement and the accompanying prospectus.
We are not under any obligation, and we expressly disclaim any obligation, to update any forward-looking statements, whether as
a result of new information, future events or otherwise.
Although
we undertake no obligation to revise or update any forward-looking statements, except as required by law, you are advised to consult
any additional disclosures we make in our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports
on Form 8-K filed with the SEC. See “Where You Can Find More Information.”
PROSPECTUS SUPPLEMENT SUMMARY
The following
is a summary of what we believe to be the most important aspects of our business and the offering of our securities under
this prospectus supplement. We urge you to read this entire prospectus supplement, the accompanying prospectus and any free
writing prospectus that we have authorized for use in connection with this offering, including the more detailed consolidated
financial statements, notes to the consolidated financial statements and other information incorporated by reference from our
other filings with the SEC or included in any applicable prospectus supplement. Investing in our common stock involves risks.
Therefore, you should carefully consider the risk factors set forth in this prospectus supplements and in our most recent
annual and quarterly filings with the SEC before purchasing our securities. Each of the risk factors could adversely affect
our business, operating results and financial condition, as well as adversely affect the value of an investment in our
securities.
Overview
We design, develop and
sell wearable bionic devices or “human exoskeletons” that have applications in healthcare, industrial, military, and
consumer markets. Our exoskeleton systems are worn over the user’s clothing to enhance human strength, endurance and mobility.
These systems serve multiple markets and can be used both by able-bodied users as well as by persons with physical disabilities.
We or our partners have sold, rented or leased devices that (a) enable individuals with neurological conditions affecting gait
(for example, spinal cord injury or stroke) to rehabilitate and to walk again; (b) allow industrial workers to perform heavy duty
work for extended periods; and (c) permit soldiers to carry heavy loads for long distances while mitigating lower back, knee, and
ankle injuries.
Our long-term goal is
to have one million persons stand and walk in Ekso exoskeletons by February 2022. The first step to achieving that goal is for
us to focus on selling our medical exoskeletons to rehabilitation centers and hospitals in the United States and Europe. We began
that effort with the February 2012 sale of Ekso, an exoskeleton for complete spinal cord injuries (“SCI”). We have
expanded that effort with the launch of our Variable Assist software and the announcement of our newest hardware platform, Ekso
GT. The Variable Assist software enables users with any amount of lower extremity strength to contribute their own power for either
leg to achieve self-initiated walking. The Ekso GT builds on the experience of the Ekso and incorporates Variable Assist, allowing
us to expand our sales and marketing efforts beyond SCI-focused centers to centers supporting stroke and related neurological patients.
In the U.S. there are about 5.9 million stroke and SCI rehabilitation sessions conducted on about 680,000 stroke and SCI patients
at 16,900 facilities. Globally, there are an estimated 50,000 rehabilitation facilities.
On April 4, 2016, we
received clearance from the U.S. Food and Drug Administration (“FDA”) to market its Ekso GT robotic exoskeleton for
use in the treatment of individuals with hemiplegia due to stroke, individuals with spinal cord injuries at levels T4 to L5, and
individuals with spinal cord injuries at levels of T3 to C7 (ASIA D), in accordance with the device’s labeling. On July 19,
2016, we received clearance from the FDA to expand/clarify the indications and labeling to expressly include individuals with hemiplegia
due to stroke who have upper extremity function of at least 4/5 in only one arm. Our prior cleared indications
for use statement required that individuals with hemiplegia due to stroke have upper extremity function of at least 4/5 in both
arms.
In parallel to the development
and early commercialization of medical exoskeletons, we have begun to work on the commercialization of exoskeletons for able-bodied
users, specifically for industrial and construction applications.
As we continue to develop,
commercialize and market our various exoskeleton technologies, we may seek to establish new strategic relationships with third
parties. Potential relationships may be in the form of technology or product development agreements, sales or distribution agreements,
or license agreements.
Corporate Information
We were incorporated in Nevada as PN Med
Group Inc. on January 30, 2012. Our original business was to distribute medical supplies and equipment to municipalities, hospitals,
pharmacies, care centers and clinics throughout the country of Chile. On January 15, 2014, a wholly-owned subsidiary of the Company,
Ekso Acquisition Corp., merged with and into Ekso Bionics, Inc. (the “Merger”). As a result of the Merger, the Company
discontinued its pre-merger operations and acquired and continues the business of Ekso Bionics, Inc. Ekso Bionics, Inc. was incorporated
on January 19, 2005, under the laws of the State of Delaware, to design, develop, and commercialize human exoskeletons to augment
human strength, endurance and mobility.
Effective May 4,
2016, we effected a one-for-seven reverse split of our common stock. As a result of the reverse stock split, every seven
shares of our issued and outstanding common stock were converted into one share of our common stock.
Our principal executive offices are located
at 1414 Harbour Way South, Suite 1201, Richmond, California 94804 and our telephone number is (203) 723-3576. Our website address
is
www.eksobionics.com
. The information contained on, or that can be accessed through, our website is not a part of this
prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
Our logo, trademarks and service marks are
the property of the Company. Other trademarks or service marks appearing in this prospectus are the property of their respective
holders.
Our Annual Reports on Form 10-K, Quarterly
Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to those reports filed or furnished pursuant to
Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, are available free
of charge through the investors page of our internet website as soon as reasonably practicable after we electronically file
such material with, or furnish it to, the SEC.
Implications of Being an Emerging Growth
Company
As a company with less than $1.0 billion
in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our
Business Startups, or JOBS, Act enacted in April 2012. An emerging growth company may take advantage of reduced reporting requirements
that are otherwise applicable to public companies. These provisions include, but are not limited to:
|
|
·
|
not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes Oxley Act of 2002, or Sarbanes Oxley Act;
|
|
|
·
|
reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and
|
|
|
·
|
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
|
We may take advantage of these provisions
for up to five years after the first sale of our common equity securities pursuant to an effective registration statement under
the Securities Act of 1933, as amended (the “Securities Act”). However, if certain events occur prior to the end of
such five year period, including if we become a “large accelerated filer,” our annual gross revenues exceed $1 billion
or we issue more than $1 billion of non-convertible debt in any three year period, we would cease to be an emerging growth company
prior to the end of such five year period.
We may choose to take advantage of some but
not all of these reduced burdens. We have taken advantage of certain of the reduced disclosure obligations regarding executive
compensation in this registration statement and may elect to take advantage of other reduced burdens in future filings. As a result,
the information that we provide to our stockholders may be different than you might receive from other public reporting companies
in which you hold equity interests.
Under the JOBS Act, emerging growth companies
can delay adopting new or revised accounting standards until such time as those standards apply to private companies. However,
we have irrevocably elected not to avail ourselves of this extended transition period for complying with new or revised accounting
standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are
not emerging growth companies.
THE OFFERING
|
Common Stock Offered by Us
|
3,750,000
shares
|
|
|
|
|
Offering Price per Share
|
$4.00
|
|
|
|
|
Common Stock to be Outstanding After This Offering
|
20,132,816
shares
|
|
|
|
|
Use of Proceeds
|
We
intend to use the net proceeds from this offering for our operations, including, but not limited to, increasing our
investments (i) in our clinical, sales and marketing initiatives to accelerate adoption of the Ekso robotic exoskeleton
in the rehabilitation market, (ii) in our research, development and commercialization activities with respect to an
Ekso robotic exoskeleton for home use, and/or (iii) in the development and commercialization of able-bodied
exoskeletons for industrial use; and for working capital and other general corporate purposes. See “Use of
Proceeds” on page S-22 of this prospectus supplement.
|
|
|
|
|
Risk Factors
|
Investing in our common stock involves a high degree of risk. You should carefully consider the “Risk Factors” included and incorporated by reference in this prospectus supplement and the accompanying prospectus, including the risk factors incorporated by reference from our filings with the SEC before deciding to invest in our common stock.
|
|
|
|
|
Trading Market
|
Our
common stock is currently quoted on the OTC Markets (OTCQB) under the symbol “EKSO.” In conjunction with this offering, we applied to list our common stock on The NASDAQ Capital Market and
our common stock is expected to begin trading on The NASDAQ Capital Market under the ticker symbol “EKSO” on August
9, 2016.
|
|
|
|
|
Lock-Up Agreements
|
Each
of our directors and executive officers have agreed that for a period of 90 days from the effective date of this offering,
they will be subject to a lockup prohibiting certain sales, transfers or hedging transactions in our securities held by them.
See “Underwriting - No Sales of Similar Securities.”
|
The number of shares of our common stock
to be outstanding after this offering is based on 16,382,816 shares outstanding as of June 30, 2016. Unless specifically stated
otherwise, the information in this prospectus supplement assumes no exercise by the underwriters of their overallotment option
and excludes:
|
|
·
|
546
,989 shares
of our common stock issuable upon conversion of our Series A Convertible Preferred Stock (the “Series A preferred
stock”) as of June 30, 2016, which number of shares will increase as a result of this offering
due to anti-dilution provisions contained in the Series A preferred stock;
|
|
|
·
|
4,085,175 shares of our common stock issuable upon the exercise of warrants outstanding as of June 30, 2016, at a weighted average exercise price of $9.81 per share;
|
|
|
·
|
2,303,307 shares of our common stock issuable upon the exercise of stock options outstanding as of June 30, 2016 under our 2014 Equity Incentive Plan, which we refer to as our 2014 Plan, at a weighted average exercise price of $6.87 per share;
|
|
|
·
|
1,148,548 shares of our common stock available for future issuance as of June 30, 2016 under our 2014 Plan; and
|
|
|
·
|
shares of our
common stock that may be issued to Equipois, LLC (“Equipois”) pursuant to the asset purchase agreement entered
into in connection with our acquisition of certain technologies of Equipois.
|
Anti-dilution Adjustment.
Because the sale price to the underwriters of the
common stock in this offering, determined on a per share basis (after the underwriting discount payable by us), is less than $7.07,
there will be an anti-dilution adjustment to the number of shares of common stock issuable upon conversion of the Series A preferred
stock. As a result of the anti-dilution provisions, the conversion price of the Series A preferred stock will be adjusted downwards
from $7.07 to $3.74 per share, which will result in the 3,443 shares of our Series A preferred stock currently outstanding becoming
convertible, for no additional consideration, into a total of 920,588 shares of our common stock. In addition,
the exercise price of our outstanding warrants issued in December 2015 to purchase a total of 2,121,642 shares of common stock
at a current exercise price of $8.75 per share (the “2015 Warrants”) will be subject to an anti-dilution adjustment
as a result of which the exercise price of the 2015 Warrants will be reduced to $3.74 per share. See “Risk Factors —
Risks related to this Offering — Holders of our Series A preferred stock will be entitled to an anti-dilution adjustment
as a result of this offering, which will result in dilution to the holders of our common stock, including the shares issued in
this offering “ and “— The exercise price of certain of our outstanding warrants may adjust as a result of this
offering, and the exercise of such warrants would result in dilution to our stockholders.”
Selected
CONSOLIDATED Financial Data
The following table
sets forth certain financial data with respect to our business. We have derived the following summary of our consolidated statement
of operations data for the six months ended June 30, 2016 and the consolidated balance sheet data as of June 30, 2016 from our
unaudited condensed consolidated financial statements incorporated by reference in this prospectus supplement from our Quarterly
Report on Form 10-Q for the quarterly period ended June 30, 2016. We derived the consolidated statement of operations data for
the years ended December 31, 2015, 2014 and 2013 from our audited consolidated financial statements. Our audited consolidated financial
statements for the years ended December 31, 2015 and 2014 are incorporated by reference in this prospectus supplement from our
Annual Report on Form 10-K for the year ended December 31, 2015. You should read this data together with our audited consolidated
financial statements and related notes and the information under the captions "Selected Consolidated Financial Data"
and "Management's Discussion and Analysis of Financial Condition and Results of Operations," which are included in our
Annual Report on Form 10-K for the year ended December 31, 2015 and our unaudited Quarterly Report on Form 10-Q for the six months
ended June 30, 2016 and incorporated by reference in this prospectus supplement. For more details on how you can obtain the documents
incorporated by reference in this prospectus supplement, “Where You Can Find More Information” and “Incorporation
of Documents by Reference” appearing elsewhere in this prospectus supplement. Our historical results are not necessarily
indicative of future results.
|
|
|
Six months ended
June 30,
|
|
|
Year ended December 31,
|
|
|
|
|
2016
|
|
|
2015
|
|
|
2014
|
|
|
2013
|
|
|
Statement of Operations Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues (1)
|
|
$
|
10,038
|
|
|
$
|
8,661
|
|
|
$
|
5,327
|
|
|
$
|
3,302
|
|
|
Loss from operations
|
|
|
(14,038
|
)
|
|
|
(21,561
|
)
|
|
|
(16,794
|
)
|
|
|
(10,294
|
)
|
|
Gain (loss) on warrant liability
|
|
|
4,650
|
|
|
|
2,505
|
|
|
|
(16,485
|
)
|
|
|
186
|
|
|
Net loss (2)(3)
|
|
|
(16,745
|
)
|
|
|
(19,590
|
)
|
|
|
(33,769
|
)
|
|
|
(11,887
|
)
|
|
Preferred deemed dividend
|
|
|
(7,329
|
)
|
|
|
4,655
|
|
|
|
–
|
|
|
|
–
|
|
|
Net loss per share, basic
|
|
|
(1.06
|
)
|
|
|
(0.24
|
)
|
|
|
(0.43
|
)
|
|
|
(0.57
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
|
4,661
|
|
|
|
19,552
|
|
|
|
25,190
|
|
|
|
805
|
|
|
Total assets
|
|
|
13,575
|
|
|
|
32,198
|
|
|
|
33,474
|
|
|
|
6,584
|
|
|
Warrant liability
|
|
|
4,545
|
|
|
|
9,195
|
|
|
|
–
|
|
|
|
378
|
|
|
|
(1)
|
The Company recognized medical device revenue previously
deferred at December 31, 2015 of $6.5 million (which represents the fair value of the already delivered devices) and associated
cost of revenue of $4.2 million, resulting in additional gross profit, reduction in net loss from operations, and reduction of
net loss applicable to common stockholders of $2.4 million in its results of operations for the six months ended June 30, 2016.
|
|
|
(2)
|
The net loss recorded in 2014 of $33.8 million includes a non-cash charge of $16.5 million associated with the issuance of
warrants in conjunction with our merger and subsequent private placement offering that included an anti-dilution
provision. The warrants were amended in November 2014 by a majority of common stock warrant holders to remove the
anti-dilution provision, among other things. In conjunction with the amendment, warrant holders exercised 22.9 million
warrant shares for which we received net proceeds of $21.4 million.
|
|
|
(3)
|
The net loss recorded in 2016 of $16.7 million includes a non-cash charge of $7.3 million associated with the conversion of Series A Convertible Preferred Stock into Common Stock, which at the time of conversion, triggers the amortization of a discount related to the warrants issued to the Series A Convertible Preferred Stockholders.
|
RISK FACTORS
An investment in our securities is highly
speculative and involves a high degree of risk. We face a variety of risks that may affect our operations or financial
results and many of those risks are driven by factors that we cannot control or predict. Before investing in our common
stock you should carefully consider the following risks, together with the financial and other information contained in this prospectus
supplement. If any of the following risks actually occurs, our business, prospects, financial condition and results
of operations could be materially adversely affected. In that case, the trading price of our common stock would likely
decline and you may lose all or a part of your investment. Only those investors who can bear the risk of loss of their
entire investment should invest in our common stock.
Please note that additional risks not currently known to us or
that we currently deem immaterial also may adversely affect our business, operations results of operations, financial condition
and prospects.
Risks Related to our Business and the
Industry in Which We Operate
We have a limited operating history
upon which investors can evaluate our future prospects.
Although we were incorporated in 2005,
we did not sell our first Ekso medical device until 2012. Therefore, we have limited operating history upon which an evaluation
of our business plan or performance and prospects can be made. Our business and prospects must be considered in the light of the
potential problems, delays, uncertainties and complications encountered in connection with a newly established business and creating
a new industry. The risks include, but are not limited to, the possibility that we will not be able to develop functional and scalable
products and services, or that although functional and scalable, our products and services will not be economical to market; that
our competitors hold proprietary rights that preclude us from marketing such products; that our competitors market a superior or
equivalent product; that we are not able to upgrade and enhance our technologies and products to accommodate new features and expanded
service offerings; or that we fail to receive necessary regulatory clearances for our products. To successfully introduce and market
our products at a profit, we must establish brand name recognition and competitive advantages for our products. There are no assurances
that we can successfully address these challenges. If we are unsuccessful, we and our business, financial condition and operating
results could be materially and adversely affected.
Given the limited operating history, management
has little basis on which to forecast future demand for our products from our existing customer base, much less new customers.
Our current and future expense levels are based largely on estimates of planned operations and future revenues rather than experience.
It is difficult to accurately forecast future revenues because our business is new and our market has not been developed. If our
forecasts prove incorrect, our business, operating results and financial condition will be materially and adversely affected. Moreover,
we may be unable to adjust our spending in a timely manner to compensate for any unanticipated reduction in revenue. As a result,
any significant reduction in revenues would immediately and adversely affect our business, financial condition and operating results.
The industries in which we operate
are highly competitive and subject to rapid technological change. If our competitors are better able to develop and market products
that are safer, more effective, less costly, easier to use, or are otherwise more attractive, we may be unable to compete effectively
with other companies.
The medical technology, industrial robotics
and military equipment industries are characterized by intense competition and rapid technological change, and we will face competition
on the basis of product features, clinical outcomes, price, services and other factors. Competitors may include large medical device
and other companies, some of which have significantly greater financial and marketing resources than we do, and firms that are
more specialized than we are with respect to particular markets. Our competition may respond more quickly to new or emerging technologies,
undertake more extensive marketing campaigns, have greater financial, marketing and other resources than we do or may be more successful
in attracting potential customers, employees and strategic partners. Our competitive position will depend on multiple, complex
factors, including our ability to achieve market acceptance for our products, develop new products, implement production and marketing
plans, secure regulatory approvals for products under development and protect our intellectual property. Competitors may offer,
or may attempt to develop, more efficacious, safer, cheaper, or more convenient alternatives to our products, including alternatives
that could make the need for robotic exoskeletons obsolete. The development of new or improved products, processes or technologies
by other companies may render our products or proposed products obsolete or less competitive. The entry into the market of manufacturers
located in low-cost manufacturing locations may also create pricing pressure, particularly in developing markets. Our future success
depends, among other things, upon our ability to compete effectively against current technology, as well as to respond effectively
to technological advances, and upon our ability to successfully implement our marketing strategies and execute our research and
development plan.
Our products or exoskeletons generally
may not be accepted in the market.
We cannot be certain that our current products
or any other products we may develop or market will achieve or maintain market acceptance. Market acceptance of our products depends
on many factors, including our ability to convince key opinion leaders to provide recommendations regarding our products, convince
distributors and customers that our technology is an attractive alternative to other technologies, convince health insurers and
other third party payers to cover and provide adequate payments for any products that are used for medical or therapy purposes,
demonstrate that our products are reliable and supported by us in the field, supply and service sufficient quantities of products
directly or through marketing alliances, and price products competitively in light of the current macroeconomic environment, which,
particularly in the case of the medical device industry, is becoming increasingly price sensitive.
In addition, the market for medical and
industrial exoskeletons is new and unproven. We cannot be certain that the market for robotic exoskeletons will continue to develop,
or that robotic exoskeletons for medical or industrial use will achieve market acceptance. If the exoskeleton market fails to develop,
or develops more slowly than we anticipate, we and our business, financial condition and operating results could be materially
and adversely affected.
Dependence on patent and other proprietary
rights and failing to attain, defend or maintain such rights or to be successful in litigation related to such rights may result
in our payment of significant monetary damages or adversely impact our product offerings.
Our long-term success largely depends on
our ability to market technologically competitive products. Failure to protect or to obtain, maintain or extend adequate patent
and other intellectual property rights could materially adversely impact our competitive advantage and impair our business. Our
issued patents may not be sufficient to protect our intellectual property and our patent applications may not result in issued
patents. Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful
protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors
may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner
or challenge the validity of our patents. Our attempts to prevent third parties from circumventing our intellectual property and
other rights ultimately may be unsuccessful. We may also fail to take the required actions or pay the necessary fees to maintain
any of our patents that issue.
Furthermore, we have not filed applications
for all of our patents internationally and may not be able to prevent third parties from using our proprietary technologies or
may lose access to technologies critical to our products in other countries. These include, in some cases, countries in which we
are currently selling products and countries in which we intend to sell products in the future.
Intellectual property litigation
and infringement claims could cause us to incur significant expenses or prevent us from selling certain of our products.
The industries in which we operate, including,
in particular, the medical device industry, are characterized by extensive intellectual property litigation and, from time to time,
we might be the subject of claims by third parties of potential infringement or misappropriation. Regardless of outcome, such claims
are expensive to defend and divert the time and effort of our management and operating personnel from other business issues. A
successful claim or claims of patent or other intellectual property infringement against us could result in our payment of significant
monetary damages and/or royalty payments or negatively impact our ability to sell current or future products in the affected category
and could have a material adverse effect on our business, cash flows, financial condition or results of operations.
Some of the patents in the intellectual
property portfolio are not within our complete control, which could reduce the value of such patents.
Some of our U.S. patent applications (which
have associated international applications) are co-owned by the Regents of the University of California Berkeley. The Regents of
the University of California Berkeley has licensed its rights under many of these patent applications to us, but we do not have
a license to their rights under three of these patent applications. With respect to two of these co-owned patent applications,
the Regents of the University of California Berkeley have licensed their rights in the U.S. to an unrelated third party. The third
patent application will need to be fully prosecuted before it can be determined which claims are exclusive to us (through a previous
license) and which claims the Regents of the University of California Berkeley may license to other entities. In addition, in connection
with our acquisition of certain assets from Equipois, we assumed the rights and obligations of Equipois with respect to certain
patents and patent applications under an in-license of intellectual property from a third party and a separate out-license of that
intellectual property to a third party for use in a particular field. We do not have complete control over the prosecution of these
patent applications. In addition, the license of patent rights under these patents to third parties could reduce the value of our
patent portfolio and limit any income or license fees that we might receive if we were to attempt to transfer or license our rights
under any of our co-owned or licensed patents.
Enforcing intellectual property rights
in foreign nations for military technology may be more problematic than enforcement in other industries.
In many countries, governments reserve
the right to allow local manufacturers to infringe patents in cases where it is beneficial to their national security to do so.
This could result in additional competition for us or our licensees from local manufacturers in foreign countries even though those
manufacturers are infringing patents we hold in those countries, which could adversely affect our ability to sell our products
in those countries for military use.
The regulatory approval and clearance
processes are expensive, time-consuming and uncertain and may prevent us from obtaining approvals or clearances, as the case may
be, for the continued commercialization of some or all of our products.
Our medical technology products and operations
are subject to regulation by the FDA, the European Union and other governmental authorities both inside and outside of the United
States. These agencies enforce laws and regulations that govern the development, testing, manufacturing, labeling, advertising,
marketing and distribution, and market surveillance of our medical products. It can be costly and time-consuming to obtain regulatory
approvals to market a medical device. Our failure to obtain and maintain clearances or approvals for medical device products could
have a material adverse effect on our business, results of operations, financial condition and cash flows. In general, unless an
exemption applies, we are not permitted to market our products in the United States until we receive a clearance letter under the
510(k) process or approval of a premarket approval application (PMA) from the FDA, depending on the nature of the device.
Regulatory clearance pursuant to a 510(k)
notification is not guaranteed, and the clearance process is expensive, uncertain and may take anywhere from several months to
over a year. The FDA also has substantial discretion in the medical device review process. Despite the time and expense exerted,
failure can occur at any stage, and we could encounter problems that cause us to repeat or perform additional development, standardized
testing, pre-clinical studies and clinical trials. The number of pre-clinical studies and clinical trials that will be required
for FDA clearance or approval varies depending on the medical device candidate, the disease or condition that the medical device
candidate is designed to address, and the regulations applicable to any particular medical device candidate. The FDA or other non-U.S.
regulatory authorities can delay, limit or deny clearance or approval of a medical device candidate for many reasons, including:
|
|
•
|
a medical device candidate may not be deemed to be substantially equivalent to a device lawfully marketed either as a grandfathered device or one that was cleared through the 510(k) premarket notification process;
|
|
|
•
|
a medical device candidate may not be deemed to be in conformance with applicable standards and regulations;
|
|
|
•
|
FDA or other regulatory officials may not find the data from pre-clinical studies and clinical trials or other product testing date to be sufficient;
|
|
|
•
|
other non-U.S. regulatory authorities may not approve our processes or facilities or those of any of our third-party manufacturers, thereby restricting export; or
|
|
|
•
|
the FDA or other non-U.S. regulatory authorities may change clearance or approval policies or adopt new regulations.
|
The Company has received 510(k) clearance
for its Ekso (Version 1.1) and Ekso GT (Version 1.2). The Ekso was originally marketed as a Class I 510(k)-exempt Powered Exercise
Equipment device since it was first sold in the United States in February 2012. The Company filed its first 510(k) Notice on December
25, 2014, following the creation of a new product classification for Powered Exoskeleton devices and receipt of an “Untitled
Letter” which informed us in writing of the agency’s belief that this new product classification applied to Ekso device.
On April 4, 2016, the Company received clearance from the FDA to market its Ekso GT robotic exoskeleton for use in the treatment
of individuals with hemiplegia due to stroke, individuals with spinal cord injuries at levels T4 to L5, and individuals with spinal
cord injuries at levels of T3 to C7 (ASIA D), in accordance with the device’s labeling. On July 19, 2016, the Company received
clearance from the FDA to expand/clarify the indications and labeling to expressly include individuals with hemiplegia due to
stroke who have upper extremity function of at least 4/5 in only one arm. Our prior cleared indications for use statement required
that individuals with hemiplegia due to stroke have upper extremity function of at least 4/5 in both arms.
Any failure or significant delay
in completing clinical trials for our products could materially harm our financial results and the commercial prospects for our
products.
Initiating and completing clinical trials
necessary to drive market adoption and support commercialization can be time consuming and expensive and the outcome uncertain.
Moreover, the results of early clinical trials are not necessarily predictive of future results, and any product we advance into
clinical trials may not have favorable results in later clinical trials. Conducting successful clinical studies requires the enrollment
of large numbers of patients, and suitable patients may be difficult to identify and recruit. Patient enrollment in clinical trials
and completion of patient participation and follow-up depends on many factors, including the size of the patient population, the
nature of the trial protocol, the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled
subjects, the availability of appropriate clinical trial investigators and support staff, proximity of patients to clinical sites,
patient ability to meet the eligibility and exclusion criteria for participation in the clinical trial and patient compliance.
For example, patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive
post-treatment procedures or follow-up to assess the safety and effectiveness of our products or if they determine that the treatments
received under the trial protocols are not attractive or involve unacceptable risks or discomforts. Patients may also not participate
in our clinical trials if they choose to participate in contemporaneous clinical trials of competitive products. In addition, patients
participating in clinical trials may die before completion of the trial or suffer adverse medical events unrelated to investigational
products.
To date, the Ekso device has been the subject
of several clinical trials, some of which have been partially sponsored by us, but most of which are non-Ekso-sponsored independent
studies conducted by rehabilitation institutions. Data from these studies was provided to the FDA as part of the 510(k) submissions.
In addition, there are several ongoing independent studies to investigate additional indications for use for the Ekso device, as
well as to evaluate clinical and non-clinical outcomes of using the Ekso device, and we are currently in the planning stage for
several Company-led studies. Sufficient and appropriate clinical protocols to demonstrate safety and efficacy may be required and
we may not adequately develop such protocols to support future clearances and approvals. Delays in patient enrollment or failure
of patients to continue to participate in a clinical trial may cause an increase in costs and delays in future clearances or approvals
of our products or result in the failure of the clinical trial. In addition, despite considerable time and expense invested in
our clinical trials, the FDA may not consider our data adequate to demonstrate safety and efficacy for future submissions. Such
increased costs and delays or failures could adversely affect our business, results of operations and prospects.
The results of clinical trials may
not support product submissions or claims or may result in the discovery of adverse side effects.
The Ekso device has been the subject of
several clinical trials, some sponsored by us, as well as non-Ekso-sponsored independent studies conducted by rehabilitation institutions.
We are currently conducting several studies to investigate additional indications for use for the Ekso device, as well as to evaluate
clinical and non-clinical outcomes of using the Ekso device. All clinical trial activities that we undertake are subject to extensive
regulation and review by numerous governmental authorities both in the United States and abroad. Clinical trials intended to support
a 510(k) or PMA must be conducted in compliance with the FDA’s Good Clinical Practice regulations and similar requirements
in foreign jurisdictions. Even if our clinical trials are completed as planned, we cannot be certain that their results will support
our intended claims or that the FDA or foreign authorities and Notified Bodies will agree with our conclusions regarding them.
Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we
cannot be sure that the later trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process
may fail to demonstrate that our product candidates are safe and effective for the proposed indicated uses, which could cause us
to abandon a product candidate and may delay development of others. Any delay or termination of our clinical trials or studies
could delay the filing of associated product submissions and, ultimately, our ability to commercialize products requiring submission
of clinical data or relying on clinical data for market acceptance. It is also possible that patients enrolled in a clinical trial
will experience adverse side effects that are not currently part of the product candidate’s safety profile, which could cause
us to delay or abandon development of such product.
Modifications to cleared or approved
products, including our Ekso GT, may require new 510(k) clearances, premarket approvals or new or amended CE Certificates of Conformity,
and may require us to cease marketing or recall the modified products until clearances, approvals or the relevant CE Certificates
of Conformity are obtained.
Any modification to a 510(k)-cleared device
that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, design,
or manufacture, requires a new 510(k) clearance or, possibly, a PMA. The FDA requires every manufacturer to make this determination
in the first instance, but the FDA may review such determinations. The FDA may not agree with our decisions regarding whether new
clearances or approvals are necessary. If the FDA disagrees with our determinations for any future changes, or prior changes to
previously marketed products, as the case may be, we may be required to cease marketing or to recall the modified products until
we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties. Furthermore, the FDA’s
ongoing review of its 510(k) program may make it more difficult for us to make modifications to our products, either by imposing
more strict requirements on when a new 510(k) for a modification to a previously cleared product must be submitted, or applying
more onerous review criteria to such submissions. In July and December 2011, respectively, the FDA issued draft guidance documents
addressing when to submit a new 510(k) due to modifications to 510(k) cleared products and the criteria for evaluating substantial
equivalence. The July 2011 draft guidance document was ultimately withdrawn, and as a result, the FDA’s original guidance
document regarding 510(k) modifications, which dates back to 1997, remains in place. It is uncertain when the FDA will seek to
issue new guidance on product modifications. Any efforts to do so could result in a more rigorous review process and make it more
difficult to obtain clearance for device modifications.
The terms of regulatory clearances
or approvals and ongoing regulation of our products may limit how we manufacture and market our products, which could materially
impair our ability to generate anticipated revenues or profit margins.
Once regulatory clearance or approval has
been granted, a cleared or approved product and its manufacturer are subject to continual review. Any cleared or approved product
may be promoted only for its indicated uses. In addition, if the FDA or other non-U.S. regulatory authorities clear or approve
any of our products, the labeling, packaging, adverse event reporting, storage, advertising and promotion for the product will
be subject to extensive regulatory requirements. If we fail to comply with the regulatory requirements of the FDA, either before
or after clearance or approval, or other non-U.S. regulatory authorities, or if previously unknown problems with our products or
manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions, including:
|
|
•
|
restrictions on the products, manufacturers or manufacturing process;
|
|
|
•
|
adverse inspectional observations (Form 483), warning letters, non-warning letters incorporating inspectional observations;
|
|
|
•
|
civil or criminal penalties or fines;
|
|
|
•
|
product seizures, detentions or import bans;
|
|
|
•
|
voluntary or mandatory product recalls and publicity requirements;
|
|
|
•
|
suspension or withdrawal of regulatory clearances or approvals;
|
|
|
•
|
total or partial suspension of production;
|
|
|
•
|
imposition of restrictions on operations, including costly new manufacturing requirements;
|
|
|
•
|
refusal to clear or approve pending applications or premarket notifications; and
|
|
|
•
|
import and export restrictions.
|
If imposed on us, any of these sanctions
could have a material adverse effect on our reputation, business, results of operations and financial condition.
We are subject to extensive post-market
regulation by the FDA. Our failure to meet strict regulatory requirements could require us to pay fines, incur other costs or even
close our facilities.
Once regulatory clearance or approval to
market a product is obtained, the FDA has the power to require us to conduct post-market studies. These studies can be very expensive
and time-consuming to conduct. Failure to complete such studies in a timely manner could result in the revocation of clearance
or approval and the recall or withdrawal of the product, which could prevent us from generating sales from that product in the
United States. The FDA has broad enforcement powers, and any regulatory enforcement actions or inquiries, or other increased scrutiny
on us, could dissuade some customers from using our products and adversely affect our reputation and the perceived safety and efficacy
of our products.
We are also required to comply with the
FDA’s Quality System Regulation, or QSR, which covers the methods used in, and the facilities and controls used for, the
design, manufacture, quality assurance, labeling, packaging, sterilization, storage, shipping, installation and servicing of our
marketed products. The FDA enforces the QSR through periodic announced and unannounced inspections of manufacturing facilities.
In addition, in the future, regulatory authorities and/or customers may require specific packaging of sterile products, which could
increase our costs and the price of our products. Later discovery of previously unknown problems with our products, including unanticipated
adverse events or adverse events of unanticipated severity or frequency, manufacturing problems, or failure to comply with regulatory
requirements such as QSR, may result in changes to labeling, restrictions on such products or manufacturing processes, withdrawal
of the products from the market, voluntary or mandatory recalls, a requirement to repair, replace or refund the cost of any medical
device we manufacture or distribute, fines, suspension of regulatory approvals, product seizures, injunctions or the imposition
of civil or criminal penalties which would adversely affect our business, operating results and prospects.
If our products, or malfunction of
our products, cause or contribute to a death or a serious injury, we will be subject to medical device reporting regulations, which
can result in voluntary corrective actions or agency enforcement actions. In addition, product defects could adversely affect the
results of our operations.
Under the FDA’s medical device reporting,
or MDR, regulations, we are required to report to the FDA any incident in which our product may have caused or contributed to a
death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute
to death or serious injury. For example, we have been informed of a limited number of events with respect to our Ekso GT devices
that have been determined to be reportable pursuant to the MDR regulations. There were no reported patient injuries related to
any of these events, and in each case we have filed the required MDR reports with the FDA.
Repeated product malfunctions may result
in a voluntary or involuntary product recall, which could divert managerial and financial resources, impair our ability to manufacture
our products in a cost-effective and timely manner, and have an adverse effect on our reputation, results of operations and financial
condition. We are also required to follow detailed recordkeeping requirements for all firm-initiated medical device corrections
and removals, and to report such corrective and removal actions to the FDA if they are carried out in response to a risk to health
and have not otherwise been reported under the MDR regulations.
When a medical human exoskeleton is used
by a paralyzed individual to walk, the individual relies completely on the exoskeleton to hold them upright. There are many exoskeleton
components that, if they were to fail catastrophically, could cause a fall resulting in severe injury or death of the patient.
Such occurrences could bring about costly litigation and could also bring about regulatory activity on the part of the FDA or its
foreign counterparts which could interfere with our ability to market our products.
When an industrial or military exoskeleton
is used by a healthy individual — for example to carry a heavy load — malfunction of the device at an inopportune moment
(such as when descending a stairway or navigating a precarious trail) could cause a fall resulting in severe injury or death of
the person using the device. Such occurrences could bring about costly litigation and could also bring about regulatory activity
on the part of OSHA or its foreign counterparts which could interfere with our ability to market our products.
All manufacturers bringing medical devices
to market in the European Economic Area are legally bound to report any incident that led or might have led to the death or serious
deterioration in the state of health of a patient, user or other person, and which the manufacturer’s device is suspected
to have caused, to the competent authority in whose jurisdiction the incident occurred. In such case, the manufacturer must file
an initial report with the relevant competent authority, which would be followed by further evaluation or investigation of the
incident and a final report indicating whether further action is required. The events described above that were reported to the
FDA were also reported to the relevant EU regulatory authorities.
Any adverse event involving our products
could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection
or enforcement action. We cannot guarantee that adverse events involving our products, such as the Ekso robotic exoskeleton, will
not occur in the future. Any corrective action, whether voluntary or involuntary, will require the dedication of our time and capital,
distract management from operating our business and may harm our reputation and financial results. Personal injuries relating to
the use of our products could also result in product liability claims being brought against us. In some circumstances, such adverse
events could also cause delays in new product approvals.
A recall of our products, either
voluntarily or at the direction of the FDA or another governmental authority, or the discovery of serious safety issues with our
products, could have a significant adverse impact on us.
The FDA and similar foreign governmental
authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects
in design or manufacture or in the event that a product poses an unacceptable risk to health. Manufacturers may, under their own
initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall by us could
occur as a result of an unacceptable risk to health, component failures, manufacturing errors, design or labeling defects or other
deficiencies and issues. To date, we have initiated only one field action in which we voluntarily accelerated our maintenance schedule
based on field usage.
Any future recalls of any of our products
would divert managerial and financial resources and could have an adverse effect on our reputation, results of operations and financial
condition, which could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’
demands. We may also be required to bear other costs or take other actions that may have a negative impact on our future sales
and our ability to generate profits.
We may be subject to fines, penalties
or injunctions if we are determined to be promoting the use of our products for unapproved or "off-label" uses.
Our promotional materials must comply with
FDA and other applicable laws and regulations. We believe that the specific use for which our products are marketed fall within
the scope of the indications for use that have been cleared by the FDA. However, if the FDA determines that our promotional materials
or training constitutes promotion of an unapproved use, it could request that we modify our promotional materials or subject us
to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil
fine and criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action
if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant
fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our
reputation could be damaged and adoption of the products would be impaired.
U.S. legislative or FDA regulatory
reforms may make it more difficult and costly for us to obtain regulatory approval of our product candidates and to manufacture
market and distribute our products after approval is obtained.
From time to time, legislation is drafted
and introduced in Congress that could significantly change the statutory provisions governing the regulatory approval, manufacture
and marketing of regulated devices. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in
ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing
regulations may impose additional costs or lengthen review times of future products. In addition, FDA regulations and guidance
are often revised or reinterpreted by the agency in ways that may significantly affect our business and our products. It is impossible
to predict whether legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and what the impact
of such changes, if any, may be.
For example, the FDA may change its clearance
and approval policies, adopt additional regulations or revise existing regulations, or take other actions which may prevent or
delay approval or clearance of our products under development or impact our ability to modify cleared products on a timely basis.
Any change in the laws or regulations that
govern the clearance and approval processes relating to our current and future products could make it more difficult and costly
to obtain clearance or approval for new products, or to produce, market and distribute existing products. Significant delays in
receiving clearance or approval, or the failure to receive clearance or approval for our new products would have an adverse effect
on our ability to expand our business.
Failure to comply with anti-kickback
and fraud regulations could result in substantial penalties and changes in our business operations.
Although we do not provide healthcare services,
submit claims for third-party reimbursement, or receive payments directly from Medicare, Medicaid or other third-party payers for
our products, we are subject to healthcare fraud and abuse regulation and enforcement by federal, state and foreign governments,
which could significantly impact our business. The principal U.S. federal laws implicated include those that prohibit (i) the filing
of false or improper claims for federal payment, known as the false claims laws, (ii) unlawful inducements for the referral of
business reimbursable under federally-funded health care programs, known as the anti-kickback laws, and (iii) health care service
providers from seeking reimbursement for providing certain services to a patient who was referred by a physician who has certain
types of direct or indirect financial relationships with the service provider, known as the Stark law. Many states have similar
laws that apply to reimbursement by state Medicaid and other funded programs as well as in some cases to all payers.
If our operations are found to be in violation
of any of the laws described above or any other governmental regulations that apply to us now or in the future, we may be subject
to penalties, including civil and criminal penalties, damages, fines, disgorgement, exclusion from governmental health care programs,
and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business
and our financial results.
We may be subject to penalties and
may be precluded from marketing our products if we fail to comply with extensive governmental regulations.
The FDA and various non-U.S. regulatory
authorities require that our products be manufactured according to rigorous standards. These regulatory requirements may significantly
increase our production costs and may even prevent us from making our products in amounts sufficient to meet market demand. If
we change our approved manufacturing process, the FDA may need to review the process before it may be used. Failure to comply with
applicable regulatory requirements discussed could subject us to enforcement actions, including warning letters, fines, injunctions
and civil penalties against us, recall or seizure of our products, operating restrictions, partial suspension or total shutdown
of our production, and criminal prosecution.
Federal, state and non-U.S. regulations
regarding the manufacture and sale of medical devices are subject to future changes. The complexity, timeframes and costs associated
with obtaining marketing clearances are unknown. Although we cannot predict the impact, if any, these changes might have on our
business, the impact could be material.
Certain of our competitors have reported
injuries caused by the malfunction of human exoskeleton devices (in at least one case to the FDA). Injuries caused by the malfunction
or misuse of human exoskeleton devices, even where such malfunction or misuse occurs with respect to one of our competitor’s
products, could cause regulatory agencies to implement more conservative regulations on the medical human exoskeleton industry,
which could significantly increase our operating costs.
We could be exposed to significant
liability claims if we are unable to obtain insurance at adequate levels or otherwise protect ourselves against potential product
liability claims.
The testing, manufacture, marketing and
sale of medical devices entail the inherent risk of liability claims or product recalls. Although we maintain product liability
insurance, the coverage is subject to deductibles and limitations, and may not be adequate to cover future claims. A successful
product liability claim or product recall could inhibit or prevent the successful commercialization of our products, cause a significant
financial burden on us, or both, which in either case could have a material adverse effect on our business and financial condition.
Warranty claims or any other service
and repairs provided by the Company at its expense could have a material adverse effect on our business.
Sales of our Ekso GT generally include
a one-year warranty for parts and services in the U.S. and a two-year warranty in Europe, the Middle East and Africa. We also generally
provide customers with an option to purchase an extended warranty for up to an additional three years. The costs associated with
such warranties, including any warranty-related legal proceedings, could have a material adverse effect on our results of operations,
cash flows and liquidity. As we enhance our product and in an effort to build our brand and drive adoption, the Company has elected
to incur increased service expenses related to an accelerated maintenance program, field corrections and the implementation of
technological improvements developed subsequent to many of our units being placed into service, sometimes outside of its warranty
and contractual obligations. Continuation of these activities could have a material adverse effect on our results of operations,
cash flows and liquidity.
If we are not able to both obtain
and maintain adequate levels of third-party reimbursement for our products, it would have a material adverse effect on our business.
Healthcare providers and related facilities
are generally reimbursed for their services through payment systems managed by various governmental agencies worldwide, private
insurance companies, and managed care organizations. The manner and level of reimbursement in any given case may depend on the
site of care, the procedure(s) performed, the final patient diagnosis, the device(s) utilized, available budget, or a combination
of these factors, and coverage and payment levels are determined at each payer’s discretion. The coverage policies and reimbursement
levels of these third-party payers may impact the decisions of healthcare providers and facilities regarding which medical products
they purchase and the prices they are willing to pay for those products. Thus, changes in reimbursement levels or methods may either
positively or negatively impact sales of our products.
We have no direct control over payer decision-making
with respect to coverage and payment levels for our medical device products. Additionally, we expect many payers to continue to
explore cost-containment strategies (e.g., comparative and cost-effectiveness analyses, so-called “pay-for-performance”
programs implemented by various public and private payers, and expansion of payment bundling schemes such as Accountable Care Organizations,
and other such methods that shift medical cost risk to providers) that may potentially impact coverage and/or payment levels for
our current products or products we develop.
Changes in reimbursement practices
of third-party payers could affect the demand for our products and the prices at which they are sold.
The sales of our products could depend,
in part, on the extent to which healthcare providers and facilities or individual users are reimbursed by government authorities,
private insurers and other third-party payers for the costs of our products or the services performed with our products. The coverage
policies and reimbursement levels of third-party payers, which can vary among public and private sources and by country, may affect
which products customers purchase and the prices they are willing to pay for those products in a particular jurisdiction. Reimbursement
rates can also affect the acceptance rate of new technologies. Legislative or administrative reforms to reimbursement systems in
the United States or abroad, or changes in reimbursement rates by private payers, could significantly reduce reimbursement for
procedures using our products or result in denial of reimbursement for those products, which would adversely affect customer demand
or the price customers may be willing to pay for such products.
Clinical outcome studies regarding
our products may not provide sufficient data to either cause third-party payers to approve reimbursement or to make human exoskeletons
a standard of care.
Our business plan relies on broad adoption
of human exoskeletons to provide neuro-rehabilitation in the form of gait training to individuals who have suffered a neurological
injury or disorder. Although use of human exoskeletons in neuro-rehabilitation is new, use of robotic devices to provide gait training
has been going on for over a decade and the clinical studies relating to such devices have had both positive and negative outcomes.
Much of the rehabilitation community has rejected the use of such devices based on the data from some of these studies. Although
we believe that human exoskeletons will outperform such robotic equipment, this has not been proven. Furthermore, it may prove
impossible to prove an advantage in a timely manner, or at all, which could prevent broad adoption of our products.
Part of our business plan relies on broad
adoption of the Ekso robotic exoskeleton to provide “early mobilization” of individuals who have been immobilized by
an injury, disease, or other condition. Although the health benefits of other methods of “early mobilization” have
been demonstrated in clinical studies in fields such as stroke, those studies did not test early mobilization with human exoskeletons
directly. It may be necessary to provide outcome studies on early mobilization with exoskeletons directly in order to convince
the medical community of their effectiveness. Such studies have not been designed at this time, and may be too large and too costly
for us and our partners to conduct. Failure to prove the health benefits of early mobilization with human exoskeletons could limit
our sales.
The technology of load carriage exoskeletons
(such as the HULC® human exoskeleton) is at a very early stage of development and the technology may not be broadly adopted
in military or other markets.
The most recent testing of our Human Universal
Load Carrier (“HULC®”) technology showed that the metabolic cost of load carriage while wearing the device varied
greatly from subject to subject. This implied that the device helped some subjects and hindered others. The source of this phenomenon
and whether it will go away with training of the subjects using the device remains unknown and requires further research and development.
This phenomenon and others like it could limit the adoption of such devices by militaries or other customers to a certain portion
of their personnel or in the worst case could make it impractical to deploy at all.
We may be unable to attract and retain
key employees.
Our success depends on our ability to identify,
hire, train and retain highly qualified managerial, technical and sales and marketing personnel. In addition, as we introduce new
products or services, we will need to hire additional personnel. Currently, competition for personnel with the required knowledge,
skill and experiences is intense, and we may not be able to attract, assimilate or retain such personnel. The inability to attract
and retain the necessary managerial, technical and sales and marketing personnel could have a material adverse effect on our business,
results of operations and financial condition.
We will experience long and variable
sales cycles, which could have a negative impact on our results of operations for any given quarter and may result in volatility
in our stock price.
The Ekso device has a lengthy sale and
purchase order cycle because it is a major capital item and generally requires the approval of senior management at purchasing
institutions, which may contribute to substantial fluctuations in our quarterly operating results. Other factors that may cause
our operating results to fluctuate include:
|
|
•
|
general economic uncertainties and political concerns;
|
|
|
•
|
the introduction of new products or product lines;
|
|
|
•
|
the level of market acceptance of new products;
|
|
|
•
|
the timing and amount of research and development and other expenditures;
|
|
|
•
|
timing of the receipt of orders from, and product shipments to, distributors and customers;
|
|
|
•
|
changes in the distribution arrangements for our products;
|
|
|
•
|
manufacturing or supply delays;
|
|
|
•
|
the time needed to educate and train additional sales and manufacturing personnel; and
|
|
|
•
|
costs associated with defending our intellectual property.
|
In addition to these factors, expenditures
are based, in part, on expected future sales. If sales levels in a particular quarter do not meet expectations, we may be unable
to adjust operating expenses quickly enough to compensate for the shortfall of sales, and our results of operations may be adversely
affected.
International sales of our products
account for a portion of our revenues, which will expose us to certain operating risks. If we are unable to successfully manage
our international activities, our net sales, results of operations and financial condition could be adversely impacted.
Our business currently depends in part
on our activities in Europe and other foreign markets, making it subject to a number of challenges that specifically relate to
international business activities. These include:
|
|
•
|
failure of local laws to provide the same degree of protection against infringement of our intellectual property rights;
|
|
|
•
|
protectionist laws and business practices that favor local competitors, which could slow our growth in international markets;
|
|
|
•
|
the expense of establishing facilities and operations in new foreign markets;
|
|
|
•
|
building an organization capable of supporting geographically dispersed operations;
|
|
|
•
|
challenges caused by distance, language and cultural differences;
|
|
|
•
|
challenges caused by differences in legal regulations, markets, and customer preferences, which may limit our ability to adapt our products or succeed in other regions;
|
|
|
•
|
multiple, conflicting, and changing laws and regulations, including complications due to unexpected changes in regulatory requirements, foreign laws, tax schemes, international import and export legislation, trading and investment policies, exchange controls and tariff and other trade barriers;
|
|
|
•
|
foreign tax consequences;
|
|
|
•
|
fluctuations in currency exchange rates and foreign currency translation adjustments;
|
|
|
•
|
foreign exchange controls that might prevent us from repatriating income earned outside the United States;
|
|
|
•
|
imposition of public sector controls;
|
|
|
•
|
political, economic and social instability; and
|
|
|
•
|
restrictions on the export or import of technology.
|
If we are unable to meet and overcome these
challenges, then our international operations may not be successful, which could adversely affect our net sales, results of operations
and financial condition and limit our growth.
We may be unable to manage our growth
and entry into new business areas.
If the initial response to our exoskeleton
products exceeds our capacity to provide services timely and efficiently, then we may need to expand our operations accordingly
and swiftly. Our management believes that establishing industry leadership will require us to:
|
|
•
|
test, introduce and develop new products and services including enhancements to our Ekso device;
|
|
|
•
|
develop and expand the breadth of products and services offered;
|
|
|
•
|
develop and expand our market presence through relationships with third parties; and
|
|
|
•
|
generate satisfactory revenues from such expanded products or services to fund the foregoing requirements while obtaining and maintaining satisfactory profit margins.
|
To be able to expand our operations in
a cost-effective or timely manner and increase the overall market acceptance of our products and services in this manner, we will
need additional capital and technical and managerial human resources. These additional resources may not be available to us. Our
failure to timely and efficiently expand our operations and successfully achieve the four requirements listed above could have
a material adverse effect on our business, results of operations and financial condition.
The disruption or loss of relationships
with vendors and suppliers for the components of our products could materially adversely affect our business.
Our ability to manufacture and market our
products successfully is dependent on relationships with both third party vendors and suppliers. Although most of the raw materials
that we use to manufacture our products are readily available from a number of suppliers, we generally procure raw materials and
components through purchase orders, with no guaranteed supply arrangements. Our inability to obtain sufficient quantities of various
components, if and as required in the future, may subject us to:
|
|
•
|
delays in delivery or shortages in components that could interrupt and delay manufacturing and result in cancellations of orders for our products;
|
|
|
•
|
increased component prices and supply delays as we establish alternative suppliers;
|
|
|
•
|
inability to develop alternative sources for product components;
|
|
|
•
|
required modifications of our products, which may cause delays in product shipments, increased manufacturing costs, and increased product prices; and
|
|
|
•
|
increased inventory costs as we hold more inventory than we otherwise might in order to avoid problems from shortages or discontinuance, which may result in write-offs if we are unable to use all such products in the future.
|
In addition, failure of any one supplier’s
components could result in a product recall, which could materially adversely affect our business, operations and cash flows.
New product introductions may adversely
impact our financial results.
We may introduce new products with enhanced
features and extended capabilities from time to time. The products may be subject to various regulatory processes, and we may need
to obtain and maintain regulatory approvals in order to sell our new products. If a potential purchaser believes that we plan to
introduce a new product in the near future or if a potential purchaser is located in a country where a new product that we have
introduced has not yet received regulatory approval, planned purchases may be deferred or delayed. As a result, new product introductions
may adversely impact our financial results.
The acquisition of other companies,
businesses, or technologies could result in operating difficulties, dilution, and other harmful consequences.
We may selectively pursue strategic acquisitions,
any of which could be material to our business, operating results, and financial condition. Future acquisitions could divert management’s
time and focus from operating our business. In addition, integrating an acquired company, business or technology is risky and may
result in unforeseen operating difficulties and expenditures associated with integrating employees from the acquired company into
our organization and integrating each company’s accounting, management information, human resources and other administrative
systems to permit effective management. The anticipated benefits of future acquisitions may not materialize. Future acquisitions
or dispositions could result in potentially dilutive issuances of our equity securities, the incurrence of debt, contingent liabilities
or amortization expenses, or write-offs of goodwill, any of which could harm our financial condition. Future acquisitions may also
require us to obtain additional financing, which may not be available on favorable terms or at all.
The impact of United States healthcare
reform legislation remains uncertain.
In 2010, the Patient Protection and Affordable
Care Act (“ACA”) was enacted into law. The legislation seeks to reform the United States healthcare system. It is far-reaching
and is intended to expand access to health insurance coverage, improve quality and reduce costs over time. We expect the new law
will have a significant impact upon various aspects of our business operations. The ACA reduces Medicare and Medicaid payments
to hospitals, clinical laboratories and pharmaceutical companies, and could otherwise reduce the volume of medical procedures.
These factors, in turn, could result in reduced demand for our products and increased downward pricing pressure. It is also possible
that the ACA will result in lower reimbursements. While the ACA is intended to expand health insurance coverage to uninsured persons
in the United States, the impact of any overall increase in access to healthcare on sales of our products remains uncertain. In
addition, the new law imposes a 2.3 percent excise tax on medical devices that will apply to United States sales of our medical
device product. Effective January 1, 2016, the excise tax on medical devices has been suspended until December 31, 2017. Many of
the details of the new law will be included in new and revised regulations, which have not yet been promulgated, and require additional
guidance and specificity to be provided by the Department of Health and Human Services, Department of Labor and Department of the
Treasury. Accordingly, while it is too early to understand and predict the ultimate impact of the new law on our business, the
legislation and resulting regulations could have a material adverse effect on our business, cash flows, financial condition and
results of operations.
Healthcare changes in the United
States and other countries resulting in pricing pressures could have a negative impact on our future operating results.
In addition to the ACA, initiatives sponsored
by government agencies, legislative bodies and the private sector to limit the growth of healthcare costs, including price regulation
and competitive pricing, are ongoing in markets where we will do business. Pricing pressure has also increased in these markets
due to continued consolidation among health care providers, trends toward managed care, the shift towards governments becoming
the primary payers of health care expenses and laws and regulations relating to reimbursement and pricing generally. Reductions
in reimbursement levels or coverage or other cost-containment measures could unfavorably affect our future operating results.
Continuing worldwide macroeconomic
instability, such as recent recessions in Europe and the debt crisis in certain countries in the European Union, could negatively
affect our ability to conduct business in those geographies.
Since 2008, the global economy has been
impacted by the sequential effects of an ongoing global financial crisis which has caused extreme disruption in the financial markets,
including severely diminished liquidity and credit availability. There can be no assurance that further deterioration will not
occur. Our customers and suppliers may experience financial difficulties or be unable to borrow money to fund their operations
which may adversely impact their ability to purchase our products or to pay for them on a timely basis, if at all. The continuing
debt crisis in certain European countries could cause the value of the euro to deteriorate, reducing the purchasing power of our
European customers. Failure to receive payment of all or a significant portion of our receivables could adversely affect our results
of operations. In addition, financial difficulties experienced by our suppliers could result in product delays and inventory issues.
Natural or other disasters could
disrupt our business and result in loss of revenue or in higher expenses.
Natural disasters, terrorist activities,
military conflict and other business disruptions could seriously harm our revenue and financial condition and increase our costs
and expenses. Our corporate headquarters are located in California, a seismically active region. A natural disaster in any of our
major markets in North America or Europe could have a material adverse impact on our operations, operating results and financial
condition. Further, any unanticipated business disruption caused by Internet security threats, damage to global communication networks
or otherwise could have a material adverse impact on our operating results.
Risks Related to our Financial Condition
We have a history of losses and we
may not achieve or sustain profitability in the future.
We have incurred losses in each fiscal
year since our incorporation in 2005. We anticipate that our operating expenses will increase in the foreseeable future as we continue
to invest to grow our business, acquire customers and develop our platform and new functionality. These efforts may prove more
expensive than we currently anticipate, and we may not succeed in increasing our revenues sufficiently to offset these higher expenses.
We may not be able to reduce the
cost to manufacture or service our products as planned.
Our business plan assumes that exoskeletons
can be manufactured more inexpensively than they are currently being manufactured. However, we have not yet found a way to significantly
reduce the manufacturing cost of our products and doing so may prove more difficult than expected or even impossible. For example,
if expectations for greater functionality of the products drive costs up as other factors drive costs down, the result may be that
the overall cost of manufacturing the product stays the same or even increases. Likewise, we currently provide service and support
of our products for our customers at a high standard (both in and out of warranty), and plan on continuing to do so. Our business
plan also assumes that as we continue to improve our product, we achieve improved levels of product reliability and decreased service
cost and frequency, which also may prove more difficult than expected.
We may not be able to leverage our
cost structure or achieve better margins.
Due to the early stage of our commercial
efforts, and particularly the early stage of customer adoption of our products, our current sales and marketing, research and development,
and general and administrative expenses are each a higher percentage of sales than they will need to be for us to reach profitability.
While we do expect these expenses to grow as our business grows, we also expect these expenses to decline as a percentage of revenues
over time. If we are unable to leverage these costs and grow revenues at a greater pace than these operating expenses as we expect,
we will not be able to achieve viable operating margins and profitability.
If we are unable to obtain additional
financing on acceptable terms, we may have to curtail our growth or cease our development plans and operations.
The operation of our business and
our growth efforts will require significant cash outlays and advance capital equipment expenditures and commitments. We have
been largely dependent on capital raised through our private placement offering that was completed in the first quarter of
2014, through the subsequent exercise of warrants that were issued in the same financing, and through our registered direct
offering completed in December 2015, and going forward will be largely dependent on capital raised through this offering and
in any future offerings, to implement our business plan and support our operations. This offering reflects our most immediate
arrangements to raise cash, and although we continue to explore additional alternative financing sources, we do not expect to
complete any other financing simultaneously, nor can assurances be given that we will do so in the immediate future. We
anticipate for the foreseeable future that cash on hand and cash generated from operations will not be sufficient to meet our
cash requirements, and that we will need to raise additional capital through investments to fund our operations and growth.
We cannot assure you that we will be able to raise additional working or growth capital as needed on terms acceptable to us,
if at all. If we are unable to raise capital as needed, we may be required to reduce the scope of our business development
activities, which could harm our business plans, financial condition and operating results, or cease our operations entirely.
Financings, if obtained, may be on terms that are dilutive to our stockholders, and the prices at which new investors would
be willing to purchase our securities may be lower than the price at which existing stockholders purchased their shares.
Our reported financial results may
be adversely affected by changes in our accounting policies or in accounting principles generally accepted in the United States.
Generally accepted accounting principles
in the United States are subject to interpretation by the Financial Accounting Standards Board, the American Institute of Certified
Public Accountants, the Securities and Exchange Commission and various bodies formed to promulgate and interpret appropriate accounting
principles. The accounting principles and accompanying accounting pronouncements, implementation guidelines and interpretations
for many aspects of our business, including revenue recognition, are highly complex and involve subjective judgments. Some of these
policies require the use of estimates and assumptions that may affect the value of our assets and liabilities, and financial results.
We may be required or determine that it is appropriate to change our accounting policies or the manner in which they are implemented
as circumstances change and additional information becomes known. A change in applicable rules, their interpretation, or their
application could have a significant effect on our reported financial results, and could affect the reporting of transactions completed
before the announcement of a change.
Changes in tax laws or exposure to
additional income tax liabilities could have a material adverse impact on our financial condition and results of operations.
We are subject to income taxes as well
as non-income based taxes, in both the U.S. and various jurisdictions outside the U.S. We are subject to ongoing tax audits in
various jurisdictions. Tax authorities may disagree with certain positions we have taken and assess additional taxes and penalties.
We regularly assess the likely outcomes of these audits in order to determine the appropriateness of our tax provision. However,
there can be no assurance that we will accurately predict the outcomes of these audits, and the actual outcomes of these audits
could have a material impact on our consolidated earnings and financial condition. Additionally, changes in tax laws or tax rulings
could materially impact our effective tax rate. Proposals for fundamental U.S. corporate tax reform, if enacted, could have a material
adverse impact on our future results of operations.
Risks Related to This Offering
You could lose all of your investment.
An investment in our common stock is speculative
and involves a high degree of risk. Potential investors should be aware that the value of an investment in us may go down as well
as up. In addition, there can be no certainty that the market value of an investment in us will fully reflect its underlying value.
You could lose your entire investment.
Management will have broad discretion
as to the use of the proceeds from this offering, and we may not use the proceeds effectively.
We currently intend to use the net
proceeds from this offering for our operations, including, but not limited to increasing our investments (i) in our clinical,
sales and marketing initiatives to accelerate adoption of the Ekso robotic exoskeleton in the rehabilitation market, (ii) in
our research, development and commercialization activities with respect to an Ekso robotic exoskeleton for home use, and/or
(iii) in the development and commercialization of able-bodied exoskeletons for industrial use; and for other general
corporate purposes. We have not allocated specific amounts of the net proceeds from this offering for any of the foregoing
purposes. Accordingly, our management will have broad discretion in the application of the net proceeds from this offering
and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock.
You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the
opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately. It is
possible that the net proceeds will be invested in a way that does not yield a favorable, or any, return for us. Our
failure to apply these funds effectively could have a material adverse effect on our business and cause the price of our
common stock to decline.
If you purchase our common stock in this offering, you
will incur immediate and substantial dilution in the book value of your shares.
The public offering price of our common stock is higher than
the net tangible book value per share of our common stock. Therefore, if you purchase shares of our common stock in this offering,
you will pay a price per share that substantially exceeds our net tangible book value per share after this offering. See “Dilution”
for a more detailed description of the dilution to new investors in the offering.
Holders of our Series A preferred stock will be entitled
to an anti-dilution adjustment as a result of this offering, which will result in dilution to the holders of our common stock,
including the shares issued in this offering.
Immediately prior to this offering, there were 3,443 shares
of our Series A preferred stock outstanding, which were convertible into a total of approximately 486,989 shares of our common
stock. The conversion price of the Series A preferred stock is subject to so-called full-ratchet anti-dilution provisions.
The sale price to the underwriters in
this offering, determined on a per share basis (after the underwriting discount payable by us), is $3.74. As a result of the anti-dilution
provisions, the conversion price of the Series A preferred stock will be adjusted downwards from $7.07 to $3.74 per share, which
will result in the 3,443 shares of our Series A preferred stock currently outstanding becoming convertible, for no additional
consideration, into a total of 920,588 shares of our common stock.
The exercise price of certain
of our outstanding warrants may adjust as a result of this offering, and the exercise of such warrants would result in dilution
to our stockholders.
Our 2015 Warrants to purchase a total of 2,121,642 shares of common stock at a current exercise price
of $8.75 per share contain so-called full-ratchet anti-dilution provisions. These anti-dilution provisions will be triggered by
the issuance of the securities in this offering. The
sale price to the underwriters in this offering, determined on a per share basis (after the underwriting discount payable by us),
is $3.74. As a result of the anti-dilution provisions in the 2015 Warrants, the exercise price of the 2015 Warrants will be adjusted
downwards to $3.74 per share.
Sales of a significant number of shares of our common
stock in the public markets, or the perception that such sales could occur, could depress the market price of our common stock.
Sales of a substantial number of shares of our common stock or
other equity-related securities in the public markets, or the perception that such sales could occur (including in connection with
sales of shares underlying outstanding options, warrants and Series A preferred stock), could depress the market price of our common
stock and impair our ability to raise capital through the sale of additional equity securities. Additionally, the Series A preferred
stock and 2015 Warrants are subject to a price-based anti-dilution adjustment in the event we sell common stock or common
stock equivalents (subject to exceptions for certain exempt issuances) at a price lower than the then-conversion price of the Series
A preferred stock or the then-exercise price of the 2015 Warrants, except that the price-based anti-dilution adjustment contained
in the 2015 Warrants expires at such time as we complete one or more future financings resulting in aggregate gross proceeds to
us of at least $10 million. We expect that following this offering, the 2015 Warrants will no longer be subject to price-based
anti-dilution. Future anti-dilution adjustments to the Series A preferred stock may result in substantial additional dilution to
existing stockholders and may depress the market price of our common stock. The issuance of the shares of common stock underlying
outstanding options, warrants and Series A preferred stock, or perception that issuance may occur, will have a dilutive impact
on other stockholders and could have a material negative effect on the market price of our common stock.
Risks Related to Our Securities
You may experience dilution of your
ownership interests because of the future issuance of additional shares of our common or preferred stock or other securities that
are convertible into or exercisable for our common or preferred stock.
In the future, we may issue our authorized
but previously unissued equity securities, resulting in the dilution of the ownership interests of our present stockholders. The
Company’s current Articles of Incorporation authorize the Company to issue an aggregate of 71,428,571 shares of common stock
and 10,000,000 shares of “blank check” preferred stock. We may issue additional shares of our common stock or other
securities that are convertible into or exercisable for our common stock in connection with hiring or retaining employees, future
acquisitions, future sales of our securities for capital raising purposes, or for other business purposes. The future issuance
of any such additional shares of our common stock will dilute the ownership interest of our current stockholders and may create
downward pressure on the trading price of the common stock. In addition, the terms of any new securities may include liquidation
or other preferences that may adversely affect the rights of our existing stockholders.
The ability of our Board of Directors
to issue additional stock may prevent or make more difficult certain transactions, including a sale or merger of the Company.
Our Board of Directors is authorized to
issue up to 10,000,000 shares of preferred stock with powers, rights and preferences designated by it. Shares of voting or convertible
preferred stock could be issued, or rights to purchase such shares could be issued, to create voting impediments or to frustrate
persons seeking to effect a takeover or otherwise gain control of us. The ability of the Board of Directors to issue such additional
shares of preferred stock, with rights and preferences it deems advisable, could discourage an attempt by a party to acquire control
of us by tender offer or other means. Such issuances could therefore deprive stockholders of benefits that could result from such
an attempt, such as the realization of a premium over the market price for their shares in a tender offer or the temporary increase
in market price that such an attempt could cause. Moreover, the issuance of such additional shares of preferred stock to persons
friendly to the Board of Directors could make it more difficult to remove incumbent managers and directors from office even if
such change were to be favorable to stockholders generally.
There currently is a limited trading
market for our common stock. Failure to maintain a trading market could negatively affect the value of our common stock and make
it difficult or impossible for existing stockholders to sell their shares.
Our common stock is currently quoted
on the OTC Markets under the symbol “EKSO.” In conjunction with this offering, we applied to list our common stock on The NASDAQ Capital Market and
our common stock is expected to begin trading on The NASDAQ Capital Market under the ticker symbol “EKSO” on August
9, 2016. Even after our common stock begins to trade on The NASDAQ Capital Market, we may
not be able to satisfy certain minimum financial and other requirements for continued listing. If we fail to meet all
applicable
Nasdaq
Capital Market requirements in the future and
Nasdaq
determines to delist our common stock, the delisting could adversely affect the market liquidity of our common stock and
adversely affect our ability to obtain financing for the continuation of our operations and could also impair the value of
your investment.
Our stock may be traded infrequently and in low volumes,
so our stock price may be volatile.
The market price of our common stock may fluctuate significantly in response to numerous factors, some
of which are beyond our control, such as:
|
|
•
|
our actual or anticipated operating and financial performance;
|
|
|
•
|
quarterly variations in the rate of growth of our financial indicators, such as net income per share, net income and cash flows, or those of companies that are perceived to be similar to us;
|
|
|
•
|
changes in revenue, cash flows or earnings estimates or publication of reports by equity research analysts;
|
|
|
•
|
speculation in the press or investment community;
|
|
|
•
|
public reaction to our press releases, announcements and filings with the SEC;
|
|
|
•
|
general financial market conditions;
|
|
|
•
|
the realization of any of the risk factors presented in this prospectus;
|
|
|
•
|
changes in market valuations of companies similar to ours; and
|
|
|
•
|
domestic and international economic, legal and regulatory factors unrelated to our performance.
|
In addition, each of our directors and
executive officers have agreed that for a period of 90 days from the effective date of this offering, they will be subject to a
lock-up prohibiting certain sales, transfers or hedging transactions in our securities held by them. Sales by our officers and directors
after the expiration of their lock-up agreements could impair the ability of a shareholder to sell our common stock in the amount
and at the price and time such holder desires.
Further, if we fail to meet the criteria
set forth in SEC regulations, various requirements would be imposed by law on broker-dealers who sell our securities to persons
other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending
or selling our common stock, which may further affect the liquidity of our common stock. This would also make it more difficult
for us to raise capital.
We have never paid and do not intend
to pay cash dividends.
Cash dividends have never been declared
or paid on our common stock, and we do not anticipate such a declaration or payment for the foreseeable future. We expect to use
future earnings, if any, to fund business growth. Therefore, stockholders will not receive any funds absent a sale of their shares
of common stock. If we do not pay dividends, our common stock may be less valuable because a return on investment will only occur
if our stock price appreciates.
We are an “emerging growth
company,” and may elect to comply with reduced public company reporting requirements applicable to emerging growth companies,
which could make our common stock less attractive to investors.
We are an “emerging growth company,”
as defined in the JOBS Act enacted in April 2012. For as long as we continue to be an emerging growth company, we may choose to
take advantage of exemptions from various public company reporting requirements. These exemptions include, but are not limited
to, (a) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, (b) reduced
disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements,
and (c) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval
of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years after the
first sale of our common equity securities pursuant to an effective registration statement under the Securities Act of 1933. However,
if certain events occur prior to the end of such five year period, including if we become a “large accelerated filer,”
our annual gross revenues exceed $1 billion or we issue more than $1 billion of non-convertible debt in any three year period,
we would cease to be an emerging growth company prior to the end of such five year period. We have taken advantage of certain of
the reduced disclosure obligations regarding executive compensation in the filings we have made with the SEC and may elect to take
advantage of other reduced burdens in future filings. As a result, the information that we provide to our stockholders may be different
than information received from other public reporting companies. We cannot predict if investors will find our common stock less
attractive as a result of our reliance on these exemptions. If some investors find our common stock less attractive as a result
of any choice we make to reduce disclosure, there may be a less active trading market for our common stock and our stock price
may be more volatile.
Under the JOBS Act, emerging growth companies
can delay adopting new or revised accounting standards until such time as those standards apply to private companies. However,
we have irrevocably elected not to avail ourselves of this extended transition period for complying with new or revised accounting
standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are
not emerging growth companies.
Being a public company is expensive
and administratively burdensome.
As a public reporting company, we are subject
to the information and reporting requirements of the Securities Act of 1933, the Exchange Act of 1934 and other federal securities
laws, rules and regulations related thereto, including compliance with the Sarbanes-Oxley Act. Complying with these laws and regulations
requires the time and attention of our Board of Directors and management, and increases our expenses. Among other things, we are
required to:
|
|
•
|
maintain and evaluate a system of internal controls over financial reporting in compliance with the requirements of Section 404 of the Sarbanes-Oxley Act and the related rules and regulations of the SEC and the Public Company Accounting Oversight Board;
|
|
|
•
|
maintain policies relating to disclosure controls and procedures;
|
|
|
•
|
prepare and distribute periodic reports in compliance with our obligations under federal securities laws;
|
|
|
•
|
institute a more comprehensive compliance function, including with respect to corporate governance; and
|
|
|
•
|
involve, to a greater degree, our outside legal counsel and accountants in the above activities.
|
The costs of preparing and filing annual
and quarterly reports, proxy statements and other information with the SEC and furnishing audited reports to stockholders is expensive
and much greater than that of a privately-held company, and compliance with these rules and regulations may require us to hire
additional financial reporting, internal controls and other finance personnel, and will involve a material increase in regulatory,
legal and accounting expenses and the attention of management. There can be no assurance that we will be able to comply with the
applicable regulations in a timely manner, if at all. In addition, being a public company makes it more expensive for us to obtain
director and officer liability insurance. In the future, we may be required to accept reduced coverage or incur substantially higher
costs to obtain this coverage. These factors could also make it more difficult for us to attract and retain qualified executives
and members of our Board of Directors, particularly directors willing to serve on our audit committee.
Any failure to maintain effective
internal control over our financial reporting could materially adversely affect us.
Section 404 of the Sarbanes-Oxley Act of
2002 requires us to include in our annual reports on Form 10-K and quarterly reports on Form 10-Q an assessment by management of
the effectiveness of our internal control over financial reporting. We previously reported a material weakness in internal control
over financial reporting related to the timing of the implementation of certain policies, processes and procedures that we have
put in place since the Merger. Throughout 2014 and 2015, we continued to strengthen our internal control environment by implementing
new policies, processes and procedures. Our remediation efforts, including the testing of these controls, continued into 2015.
This material weakness was considered remediated in the fourth quarter of 2015, once these controls were shown to be operational
for a sufficient period of time to allow management to conclude that these controls were operating effectively. In addition, our
independent registered public accounting firm has reported on management’s assessment of the effectiveness of such internal
control over financial reporting as of December 31, 2015. While we believe that the policies, processes and procedures we put in
place are sufficient to render our internal controls over financial reporting effective, our initiatives may not prove successful
and in the future management may not be able to conclude that our internal control over financial reporting is effective. Furthermore,
even if management were to reach such a conclusion, if our independent registered public accounting firm is not satisfied with
the adequacy of our internal control over financial reporting, or if the independent auditors interpret the requirements, rules
or regulations differently than we do, then (if required in the future) they may decline to attest to management’s assessment
or may issue a report that is qualified. Any of these events could result in a loss of investor confidence in the reliability of
our financial statements, which in turn could negatively affect the price of our common stock.
In particular, we must perform system and
process evaluation and testing of our internal control over financial reporting to allow management and our independent registered
public accounting firm to report on the effectiveness of our internal control over financial reporting, as required by Section
404. Our compliance with Section 404 may require that we incur substantial accounting expense and expend significant management
efforts.
***
The risks above do
not necessarily comprise all of those associated with an investment in us. This prospectus contains forward looking statements
that involve unknown risks, uncertainties and other factors that may cause our actual results, financial condition, performance
or achievements to be materially different from any future results, performance or achievements expressed or implied by such forward
looking statements. Factors that might cause such a difference include, but are not limited to, those set out above.
USE OF PROCEEDS
We estimate
that the net proceeds we will receive from this offering will be approximately $13.7 million, or $15.8 million if the
underwriters exercise their full overallotment option to purchase up to 562,500 additional shares of common stock, after
deducting the underwriting discount and estimated offering expenses payable by us.
We intend to
use the net proceeds from this offering for our operations, including, but not limited to, increasing our investments (i) in
our clinical, sales and marketing initiatives to accelerate adoption of the Ekso robotic exoskeleton in the rehabilitation
market, (ii) in our research, development and commercialization activities with respect to an Ekso robotic exoskeleton for
home use, and/or (iii) in the development and commercialization of able-bodied exoskeletons for industrial use; and for
working capital and other general corporate purposes. The occurrence of unforeseen events or changed business conditions,
however, could result in the application of the net proceeds from this offering in a manner other than as described in this
prospectus supplement. As a result, our management will retain broad discretion over the allocation of the net proceeds from
this offering.
Pending our use of the
net proceeds from this offering, we plan to invest the net proceeds in short-term, investment-grade, interest-bearing instruments
and U.S. government securities.
Capitalization
The following table sets forth
our capitalization as of June 30, 2016 on an actual basis and as adjusted to reflect the (i) sale of the 3,750,000 shares of
common stock offered by us in this offering at a public offering price of $4.00 per share, after deducting underwriting
discounts and commissions, but before deducting estimated offering expenses payable by us, and assuming no exercise of
the underwriters’ overallotment option,
|
|
|
As of June 30, 2016
|
|
|
(In
thousands, except share and per share information)
|
|
Actual
|
|
|
As adjusted
for this
offering
|
|
|
Cash and cash equivalents
|
|
$
|
4,661
|
|
|
$
|
18,686
|
|
|
Stockholders’ Equity
|
|
|
|
|
|
|
|
|
Common stock, $0.001 par
value; 71,428,571 shares authorized, actual and as adjusted; 16,382,816
shares issued and outstanding, actual;
20,132,816 shares issued and outstanding as adjusted
|
|
|
16
|
|
|
|
20
|
|
|
Additional paid-in capital
|
|
|
102,341
|
|
|
|
116,362
|
|
|
Accumulated other comprehensive income
|
|
|
10
|
|
|
|
10
|
|
|
Accumulated deficit
|
|
|
(100,806
|
)
|
|
|
(100,806
|
)
|
|
Total stockholders’ equity
|
|
|
1,561
|
|
|
|
15,586
|
|
|
Total capitalization
|
|
$
|
1,561
|
|
|
|
15,586
|
|
|
|
(1)
|
Excludes the following as of June 30, 2016:
|
|
|
·
|
546,989
shares of
our common stock issuable upon conversion of our Series A Convertible Preferred Stock, which number of shares will increase
as a result of this offering due to anti-dilution provisions contained in the Series A preferred stock;
|
|
|
·
|
4,085,175 shares of our common stock issuable upon the exercise of warrants outstanding, at a weighted average exercise price of $9.81 per share;
|
|
|
·
|
2,303,307 shares of our common stock issuable upon the exercise of stock options outstanding under our 2014 Plan at a weighted average exercise price of $6.87 per share;
|
|
|
·
|
1,148,548 shares of our common stock available for future issuance under our 2014 Plan;
|
|
|
·
|
shares of our
common stock that may be issued to Equipois pursuant to the asset purchase agreement entered into in connection with our
acquisition of certain technologies of Equipois;
|
|
|
·
|
the effects of the
anti-dilution provisions of the Series A preferred stock and the 2015 Warrants, which will be triggered in connection with
this offering because the sale price to the underwriters is less than $7.07 per share and $8.75 per share, respectively (see
“Risk Factors — Risks Related to This Offering” and “Dilution”); and
|
|
|
·
|
any shares that we may issue in the future pursuant to the registration statement of which this prospectus supplement forms a part.
|
For a discussion of our stock option and employee benefit plans,
the Series A preferred stock and the 2015 Warrants, and the common stock issuance to Equipois, see the Notes to our consolidated
financial statements for the year ended December 31, 2015, which are incorporated by reference in this prospectus supplement. See
“Risk Factors — Risks Related to This Offering,” for a discussion of the anti-dilution provisions of the Series
A preferred stock and the 2015 Warrants.
Please read the capitalization table together with the sections
of this prospectus supplement entitled “Selected Financial Information” and “Management’s Discussion and
Analysis of Financial Condition and Results of Operations,” and our financial statements and related notes thereto which
are incorporated by reference in this prospectus supplement.
DILUTION
Our net
tangible book value at June 30, 2016 was $75,000, or $0.00 per share. We calculate net tangible book value per share by
subtracting our total liabilities from our total tangible assets and dividing the difference by the number of outstanding
shares of our common stock. If the Company's warrant liability were excluded from this calculation, our net tangible book
value at June 30, 2016 would be $4.6 million, or $0.28 per share. Dilution with respect to net tangible book value per share
represents the difference between the amount per share paid by purchasers of shares of common stock in this offering and the
net tangible book value per share of our common stock immediately after this offering.
After giving
effect to the sale of shares of common stock in this offering at a public offering price of $4.00 per share, and after
deducting the underwriting discount and estimated offering expenses payable by us, our as adjusted net tangible book value at
June 30, 2016 would have been $13.8 million or $0.68 per share of common stock. This represents an
immediate increase in net tangible book value of $0.68 per share to existing stockholders and an immediate dilution of $3.32 per
share to investors in this offering. The following table illustrates this per share dilution:
|
Public offering price per share
|
|
|
|
|
|
$
|
4.00
|
|
|
Net tangible book value per share as of June 30, 2016
|
|
$
|
0.00
|
|
|
$
|
|
|
|
Increase per share attributable to net proceeds of this offering
|
|
$
|
0.68
|
|
|
$
|
|
|
|
Net tangible book value per share as of June 30, 2016 after giving effect to this offering
|
|
|
|
|
|
$
|
0.68
|
|
|
Dilution per share to new investors participating in this offering
|
|
|
|
|
|
$
|
3.32
|
|
If the
underwriters exercise in full their option to purchase up to 562,500 additional shares of common stock, the as adjusted net
tangible book value after this offering would be $0.77 per share, representing an increase in net tangible book value of
$0.77 per share to existing stockholders and immediate dilution of $3.23 per share to investors in this offering.
The above discussion
is based on 16,382,816 shares of our common stock outstanding as of June 30, 2016 and excludes:
|
|
·
|
546,989 shares of our common stock issuable
upon conversion of our Series A Convertible Preferred Stock, which number of shares will increase as a result of this
offering due to anti-dilution provisions contained in the Series A preferred stock;
|
|
|
·
|
4,085,175 shares of our common stock issuable upon the exercise of warrants outstanding, at a weighted average exercise price of $9.81 per share;
|
|
|
·
|
2,303,307 shares of our common stock issuable upon the exercise of stock options outstanding as of under our 2014 Plan at a weighted average exercise price of $6.87 per share;
|
|
|
·
|
1,148,548 shares of our common stock available for future issuance as of under our 2014 Plan;
|
|
|
·
|
shares of our common stock that may be issued to Equipois pursuant to the asset purchase agreement entered
into in connection with our acquisition of certain technologies of Equipois;
|
|
|
·
|
the effects of the
anti-dilution provisions of the Series A preferred stock and the 2015 Warrants, which will be triggered in connection with
this offering because the sale price to the underwriters is less than $7.07 per share and $8.75 per share, respectively (see
“Risk Factors — Risks Related to This Offering” and “Dilution”); and
|
|
|
·
|
any shares that we may issue in the future pursuant to the registration statement of which this prospectus supplement forms a part.
|
To the extent that outstanding
options or warrants outstanding as of June 30, 2016 have been or may be exercised, preferred stock is converted into common stock
or other shares are issued, investors purchasing our common stock in this offering may experience further dilution. In addition,
we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient
funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or
convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
Anti-dilution Adjustment.
Because
the sale price to the underwriters of the common stock in this offering, determined on a per share basis (after the underwriting
discount payable by us), is less than $7.07, there will be an anti-dilution adjustment to the number of shares of common stock
issuable upon conversion of the Series A preferred stock. As a result of the anti-dilution provisions, the conversion price of
the Series A preferred stock will be adjusted downwards from $7.07 to $3.74 per share, which will result in the 3,443 shares of
our Series A preferred stock currently outstanding becoming convertible, for no additional consideration, into a total of 920,588
shares of our common stock. In addition, the exercise price of our 2015 Warrants will be
reduced to $3.74 per share as a result of anti-dilution provisions contained in the 2015 Warrants.
UNDERWRITING
Subject to the terms and conditions set
forth in the Underwriting Agreement, dated August 9, 2016, between us and Cowen and Company, LLC (Cowen), as the representative
of the underwriters named below, we have agreed to sell to the underwriters, and each of the underwriters has agreed, severally
and not jointly, to purchase from us, the respective number of shares of common stock shown opposite its name below:
|
Underwriters
|
|
Number
of Shares
|
|
|
Cowen and Company, LLC
|
|
|
2,718,750
|
|
|
SunTrust
Robinson Humphrey, Inc.
|
|
|
750,000
|
|
|
B. Riley &
Co., LLC
|
|
|
281,250
|
|
|
Total
|
|
|
3,750,000
|
|
The Underwriting Agreement provides that
the obligations of the several underwriters are subject to certain conditions precedent such as the receipt by the underwriters
of officers’ certificates and legal opinions and approval of certain legal matters by their counsel. The Underwriting Agreement
provides that the underwriters will purchase all of the shares of common stock if any of them are purchased. If an underwriter
defaults, the Underwriting Agreement provides that the purchase commitments of the nondefaulting underwriters may be increased
or the Underwriting Agreement may be terminated. We have agreed to indemnify the underwriters and certain of their controlling
persons against certain liabilities, including liabilities under the Securities Act of 1933, as amended, and to contribute to payments
that the underwriters may be required to make in respect of those liabilities.
The underwriters are offering the shares
of common stock subject to their acceptance of the shares of common stock from us and subject to prior sale. The underwriters reserve
the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Discounts and Commissions.
The underwriters
have advised us that they propose to offer the shares of common stock to the public at the public offering price set forth on the
cover page of this prospectus supplement and to certain dealers, which may include the underwriters, at that price less a concession
not in excess of $0.156 per share of common stock. After the offering, the public offering price and concession
may be reduced by the representatives. No such reduction will change the amount of proceeds to be received by us as set forth on
the cover page of this prospectus supplement.
The underwriting fee is equal to the public
offering price per share of common stock less the amount paid by the underwriters to us per share of common stock. The underwriting
fee is $0.26 per share. The following table shows the public offering price, the underwriting discounts and commissions
that we are to pay the underwriters and the proceeds, after expenses, to us in connection with this offering. Such amounts are
shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
|
|
|
|
|
|
Total
|
|
|
|
|
Per Share
|
|
|
Without Option
|
|
|
With Option
|
|
|
Public offering price
|
|
$
|
4.00
|
|
|
$
|
15,000,000
|
|
|
$
|
17,250,000
|
|
|
Underwriting discount
|
|
|
0.26
|
|
|
|
975,000
|
|
|
|
1,121,250
|
|
|
Proceeds, before expenses, to us
|
|
|
3.74
|
|
|
|
14,025,000
|
|
|
|
16,128,750
|
|
We estimate that the total expenses of this
offering, excluding the underwriting discounts and commissions, will be approximately $350,000, which includes
$75,000 that we have agreed to reimburse the underwriters for the fees incurred by them in connection with the offering.
Listing.
Our common stock is currently
quoted on the OTC Markets under the symbol “EKSO.” In conjunction with this offering, we applied to list our common stock on The NASDAQ Capital Market and
our common stock is expected to begin trading on The NASDAQ Capital Market under the ticker symbol “EKSO” on August
9, 2016.
Option to Purchase Additional Shares.
We have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus supplement, to purchase,
from time to time, in whole or in part, up to an aggregate of 562,500 additional shares of common
stock from us at the public offering price set forth on the cover page of this prospectus supplement, less underwriting discounts
and commissions. If the underwriters exercise this option, each underwriter will be obligated, subject to specified conditions,
to purchase a number of additional shares of common stock proportionate to that underwriter’s initial purchase commitment
as indicated in the table above.
No Sales of Similar Securities.
We,
our executive officers and directors have agreed, subject to specified exceptions, not to directly or
indirectly:
|
|
·
|
offer, sell, assign, transfer, pledge, contract to sell, or otherwise dispose of, or announce the intention to otherwise dispose of, any shares of common stock (including, without limitation, common stock that may be deemed to be beneficially owned by the undersigned in accordance with the rules and regulations promulgated under the Securities Exchange Act of 1934, as amended (Exchange Act), or securities convertible into or exercisable or exchangeable for common stock,
|
|
|
·
|
enter into any swap, hedge or similar agreement or arrangement that transfers in whole or in part, the economic risk of ownership of beneficially owned shares common stock or securities convertible into or exercisable or exchangeable for common stock, or
|
|
|
·
|
engage in any short selling of common stock or securities convertible into or exercisable or exchangeable for common stock.
|
These
restrictions terminate after the close of trading of the common stock on and including the 90th day after the date of this
prospectus supplement.
Cowen may, in its sole discretion and at
any time or from time to time before the termination of the 90-day period, release all or any portion of the securities subject
to lock-up agreements. There are no existing agreements between the underwriters and any of our stockholders who will execute a
lock-up agreement, providing consent to the sale of shares prior to the expiration of the lock-up period.
Stabilization.
The underwriters
have advised us that, pursuant to Regulation M under the Exchange Act, certain persons participating in the offering may engage
in short sale transactions, stabilizing transactions, syndicate covering transactions or the imposition of penalty bids in connection
with this offering. These activities may have the effect of stabilizing or maintaining the market price of the common stock at
a level above that which might otherwise prevail in the open market. Establishing short sales positions may involve either ‘‘covered’’
short sales or ‘‘naked’’ short sales.
‘‘Covered’’ short
sales are sales made in an amount not greater than the underwriters’ option to purchase additional shares of our common stock
in this offering. The underwriters may close out any covered short position by either exercising their option to purchase additional
shares of our common stock or purchasing shares of our common stock in the open market. In determining the source of shares to
close out the covered short position, the underwriters will consider, among other things, the price of shares of common stock available
for purchase in the open market as compared to the price at which they may purchase shares through the option to purchase additional
shares.
‘‘Naked’’ short
sales are sales in excess of the option to purchase additional shares of our common stock.
The underwriters must
close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created
if the underwriters are concerned that there may be downward pressure on the price of the shares of our common stock in the open
market after pricing that could adversely affect investors who purchase in this offering.
A stabilizing bid is a bid for the purchase
of shares of common stock on behalf of the underwriters for the purpose of fixing or maintaining the price of the common stock.
A syndicate covering transaction is the bid for or the purchase of shares of common stock on behalf of the underwriters to reduce
a short position incurred by the underwriters in connection with the offering. Similar to other purchase transactions, the underwriters’
purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock
or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be
higher than the price that might otherwise exist in the open market. A penalty bid is an arrangement permitting the underwriters
to reclaim the selling concession otherwise accruing to a syndicate member in connection with the offering if the common stock
originally sold by such syndicate member are purchased in a syndicate covering transaction and therefore have not been effectively
placed by such syndicate member.
Neither we, nor any
of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions
described above may have on the price of our common stock. The underwriters are not obligated to engage in these activities and,
if commenced, any of the activities may be discontinued at any time.
The underwriters may also engage in passive
market-making transactions in our common stock on The NASDAQ Capital Market in accordance with Rule 103 of Regulation M during
a period before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion
of distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security.
However, if all independent bids are lowered below the passive market maker’s bid, that bid must then be lowered when specified
purchase limits are exceeded. However, if all independent bids are lowered below the passive market maker’s bid, that bid
must then be lowered when specified purchase limits are exceeded.
United Kingdom.
Each of the underwriters
has represented and agreed that:
|
|
·
|
it has not made or will not make an offer of the securities to the public in the United Kingdom within the meaning of section 102B of the Financial Services and Markets Act 2000,as amended (FSMA), except to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities or otherwise in circumstances which do not require the publication by us of a prospectus pursuant to the Prospectus Rules of the Financial Services Authority (FSA);
|
|
|
·
|
it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) to persons who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 or in circumstances in which section 21 of FSMA does not apply to us; and
|
|
|
·
|
it has complied with and will comply with all applicable provisions of FSMA with respect to anything done by it in relation to the securities in, from or otherwise involving the United Kingdom.
|
European Economic Area.
In relation
to each Member State of the European Economic Area (Iceland, Norway and Lichtenstein in addition to the member states of the European
Union) that has implemented the Prospectus Directive (each, a Relevant Member State), each underwriter has represented and agreed
that with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State, or
the Relevant Implementation Date, it has not made and will not make an offer of the securities to the public in that Relevant Member
State prior to the publication of a prospectus in relation to the securities that has been approved by the competent authority
in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority
in that Relevant Member State, all in accordance with the Prospectus Directive, except that it may, with effect from and including
the Relevant Implementation Date, make an offer of the securities to the public in that Relevant Member State at any time:
|
|
·
|
to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
|
|
|
·
|
to any legal entity which has two or more of (i) an average of at least 250 employees during the last financial year; (ii) a total balance sheet of more than €43,000,000 and (iii) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts; and
|
|
|
·
|
in any other circumstances which do not require the publication by the issuer of a prospectus pursuant to Article 3 of the Prospectus Directive.
|
Each person in a Relevant Member State
who receives any communication in respect of, or who acquires any securities under, the offer contemplated in this prospectus will
be deemed to have represented, warranted and agreed to and with us and each underwriter that:
|
|
·
|
it is a qualified investor within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive; and
|
|
|
·
|
in the case of any securities acquired by it as a financial intermediary, as that term is used in Article 3(2) of the Prospectus Directive, (i) the securities acquired by it in the offer have not been acquired on behalf of, nor have they been acquired with a view to their offer or resale to, persons in any Relevant Member State other than qualified investors, as that term is defined in the Prospectus Directive, or in circumstances in which the prior consent of the representative of the underwriters has been given to the offer or resale; or (ii) where securities have been acquired by it on behalf of persons in any Relevant Member State other than qualified investors, the offer of those securities to it is not treated under the Prospectus Directive as having been made to such persons.
|
For the purposes of the provisions in the
two immediately preceding paragraphs, the expression an “offer of the securities to the public” in relation to the
securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms
of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities,
as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member
State, and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing
measure in each Relevant Member State.
Electronic Offer, Sale and Distribution
of Shares.
A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters
or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering
may distribute prospectuses electronically. The representatives may agree to allocate a number of shares to underwriters and selling
group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters
and selling group members that will make internet distributions on the same basis as other allocations. Other than the prospectus
in electronic format, the information on these websites is not part of this prospectus supplement, the accompanying prospectus
or the registration statement of which the accompanying prospectus forms a part, has not been approved or endorsed by us or any
underwriter in its capacity as underwriter, and should not be relied upon by investors.
Other Activities and Relationships.
The underwriters and certain of their affiliates are full service financial institutions engaged in various activities, which may
include securities trading, commercial and investment banking, financial advisory, investment management, investment research,
principal investment, hedging, financing and brokerage activities. The underwriters and certain of their affiliates have, from
time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services
for us and our affiliates, for which they received or will receive customary fees and expenses.
In the ordinary
course of their various business activities, the underwriters and certain of their affiliates may make or hold a broad array
of investments and actively traded debt and equity securities (or related derivative securities) and financial instruments
(including bank loans) for their own account and for the accounts of their customers, and such investment and securities
activities may involve securities and/or instruments issued by us and our affiliates. The underwriters and certain of their
respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or
publish or express independent research views in respect of such securities or instruments and may at any time hold, or
recommend to clients that they acquire, long and/or short positions in such securities and instruments.
LEGAL MATTERS
The validity of the
securities offered by this prospectus supplement and the accompanying prospectus will be passed upon by Nutter, McClennen &
Fish, LLP, Boston, Massachusetts. Certain legal matters will be passed upon for the underwriters by Goodwin Procter LLP, New York,
New York.
EXPERTS
The consolidated financial
statements of Ekso Bionics Holdings, Inc. as of, and for the fiscal years ended December 31, 2015, 2014 and 2013, incorporated
by reference into this prospectus, have been audited by OUM & Co. LLP, independent registered public accounting firm, as stated
in their report which is incorporated by reference. Such financial statements have been incorporated by reference in reliance on
the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We file reports, proxy
statements and other information with the SEC as required by the Exchange Act. You can find, copy and inspect information we file
at the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. You can call the SEC at 1-800-SEC-0330
for further information about the public reference room. You can review our electronically filed reports, proxy and information
statements on the SEC’s web site at http://www.sec.gov or on our web site at http://www.eksobionics.com. Information included
on our web site is not incorporated into or a part of this prospectus supplement or any prospectus supplement.
This prospectus supplement
is part of a registration statement we filed with the SEC. This prospectus supplement and the accompanying prospectus omit some
information contained in the registration statement in accordance with SEC rules and regulations. You should review the information
and exhibits in the registration statement for further information about us and the securities we are offering. Statements in this
prospectus supplement and the accompanying prospectus concerning any document we filed as an exhibit to the registration statement
or that we otherwise filed with the SEC are not intended to be comprehensive and are qualified by reference to these filings. You
should review the complete document to evaluate these statements. You can obtain a copy of the registration statement from the
SEC at the address listed above or from the SEC’s website.
INCORPORATION BY REFERENCE
The SEC allows us to
incorporate by reference much of the information we file with the SEC, which means that we can disclose important information to
you by referring you to those publicly available documents. The information that we incorporate by reference in this prospectus
supplement is considered to be part of this prospectus supplement. Because we are incorporating by reference future filings with
the SEC, this prospectus supplement is continually updated and those future filings may modify or supersede some of the information
included or incorporated in this prospectus supplement. This means that you must look at all of the SEC filings that we incorporate
by reference to determine if any of the statements in this prospectus supplement or in any document previously incorporated by
reference have been modified or superseded. This prospectus supplement incorporates by reference the documents listed below and
any future filings we make with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as
amended, or the Exchange Act (in each case, other than those documents or the portions of those documents not deemed to be filed)
between the date of the initial registration statement and the effectiveness of the registration statement and following the effectiveness
of the registration statement until the offering of the securities under the registration statement is terminated or completed:
|
|
·
|
our Annual Report on Form 10-K for the fiscal year ended December 31, 2015 filed on March 14, 2016;
|
|
|
·
|
our Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2016, filed on May 10, 2016 and our Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2015, filed on August 3, 2016;
|
|
|
·
|
our Current
Reports on Form 8-K filed on February 26, 2016, April 7, 2016, April 21, 2016, May 5, 2016, May 31, 2016 and August
9, 2016;
|
|
|
·
|
our Definitive Proxy Statement on Schedule 14A filed on April 25, 2016, but only to the extent that such information was incorporated by reference into our Annual Report on Form 10-K for the year ended December 31, 2015;
|
|
|
·
|
the description of our common stock contained in our Registration Statement on Form 8-A, filed on May 6, 2015 pursuant to Section 12(b) of the Exchange Act, which incorporates by reference the description of the shares of our common stock contained in our Registration Statement on Form S-1 (File No. 333-195783) filed on May 7, 2014 and declared effective by the SEC on June 20, 2014, and any amendment or report filed with the SEC for purposes of updating such description; and
|
|
|
·
|
all reports and other documents subsequently filed by us pursuant to Sections 13(a), 13(c), 14 and 15(d) of the Exchange Act after the date of this prospectus supplement and prior to the termination or completion of the offering of securities under this prospectus supplement shall be deemed to be incorporated by reference in this prospectus supplement and to be a part hereof from the date of filing such reports and other documents.
|
A statement contained
in a document incorporated by reference into this prospectus supplement and the accompanying prospectus shall be deemed to be modified
or superseded for purposes of this prospectus supplement and the accompanying prospectus to the extent that a statement contained
in this prospectus supplement, the accompanying prospectus or in any other subsequently filed document which is also incorporated
in this prospectus supplement modifies or replaces such statement. Any statements so modified or superseded shall not be deemed,
except as so modified or superseded, to constitute a part of this prospectus supplement and the accompanying prospectus.
You may request a copy
of these documents, which will be provided to you at no cost, by writing or telephoning us using the following contact information:
Ekso Bionics Holdings, Inc.
Attention: Investor Relations
1414 Harbour Way South, Suite 1201
Richmond, CA 94804
Phone: (203) 723-3576
PROSPECTUS
Ekso Bionics Holdings, Inc.
$75,000,000
COMMON STOCK
PREFERRED STOCK
WARRANTS
RIGHTS
UNITS
Ekso Bionics Holdings, Inc., a Nevada corporation
(“Ekso Bionics”), may offer and sell from time to time, pursuant to this prospectus, in one or more series or issuances
and on terms that Ekso Bionics will determine at the time of the offering, any of the securities described in this prospectus,
and any combination of those securities, up on an aggregate amount of $75,000,000.
This prospectus describes the general terms
of these securities and the general manner in which these securities will be offered. We will provide you with the specific
terms of any offering in one or more supplements to this prospectus. The prospectus supplements will also describe the specific
manner in which these securities will be offered and may also supplement, update or amend information contained in this document.
You should read this prospectus and any prospectus supplement, as well as any documents incorporated by reference into this prospectus
or any prospectus supplement, carefully before you invest.
We may offer and sell these securities
directly to you, through agents designed by time to time, or to or through underwriters or dealers. For additional information
on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus and in the
applicable prospectus supplement. If any underwriters or agents are involved in the sale of our securities with respect to
which this prospectus is being delivered, the names of such underwriters or agents and any applicable fees, commissions or discounts
and over-allotment options will be set forth in the applicable prospectus supplement. The price to the public of such securities
and the net proceeds that we expect to receive from such sale will also be set forth in a prospectus supplement.
Our common stock is listed on the OTC Markets
under the symbol “EKSO.” On June 22, 2015, the last reported sale price of our common stock was $1.25 per share.
The applicable prospectus supplement will contain information, where applicable, as to any other listing, if any, on the OTC Markets
or any securities market or other securities exchange of the securities covered by the prospectus supplement. Prospective purchasers
of our securities are urged to obtain current information as to the market prices of our securities, where applicable.
Investing
in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully
the risks that we have described on page 4 of this prospectus under the caption “Risk Factors.” We may include
specific risk factors in supplements to this prospectus under the caption “Risk Factors.” This prospectus may not be
used to sell our securities unless accompanied by a prospectus supplement.
Neither the Securities and Exchange
Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus
is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is July 9,
2015.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration
statement on Form S-3 that we filed with the Securities and Exchange Commission, or SEC, using a “shelf” registration
process. Under this shelf registration process, we may offer and sell, from time to time, shares of our common stock and
preferred stock, various series of warrants or rights to purchase any of such securities, either individually or in units, in one
or more offerings, with a total value of up to $75,000,000. This prospectus provides you with a general description of the
securities we may offer. Each time we offer a type or series of securities under this prospectus, we will provide a prospectus
supplement that will contain specific information about the terms of that offering.
This prospectus does not contain all of
the information included in the registration statement. For a more complete understanding of the offering of the securities,
you should refer to the registration statement, including its exhibits. The prospectus supplement may also add, update or
change information contained or incorporated by reference in this prospectus. However, no prospectus supplement will offer
a security that is not registered and described in this prospectus at the time of its effectiveness. This prospectus, together
with the applicable prospectus supplements and the documents incorporated by reference into this prospectus, includes all material
information relating to the offering of securities under this prospectus. You should carefully read this prospectus, the
applicable prospectus supplement, the information and documents incorporated herein by reference and the additional information
under the heading “Where You Can Find More Information” before making an investment decision.
You should rely only on the information
we have provided or incorporated by reference in this prospectus or any prospectus supplement. We have not authorized anyone
to provide you with information different from that contained or incorporated by reference in this prospectus. No dealer,
salesperson or other person is authorized to give any information or to represent anything not contained or incorporated by reference
in this prospectus. You must not rely on any unauthorized information or representation. This prospectus is an offer
to sell only the securities offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so.
You should assume that the information in this prospectus or any prospectus supplement is accurate only as of the date on the front
of the document and that any information we have incorporated herein by reference is accurate only as of the date of the document
incorporated by reference, regardless of the time of delivery of this prospectus or any sale of a security.
We further note that the representations,
warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference
in the accompanying prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for
the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty
or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made.
Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state
of our affairs.
This prospectus may not be used to consummate
sales of our securities, unless it is accompanied by a prospectus supplement. To the extent there are inconsistencies between
any prospectus supplement, this prospectus and any documents incorporated by reference, the document with the most recent date
will control.
Unless the context otherwise requires,
“Ekso Bionics,” “the Company,” “we,” “us,” “our” and similar terms
refer to Ekso Bionics Holdings, Inc. and our subsidiaries.
PROSPECTUS SUMMARY
The following is a summary of
what we believe to be the most important aspects of our business and the offering of our securities under this prospectus.
We urge you to read this entire prospectus, including the more detailed consolidated financial statements, notes to the consolidated
financial statements and other information incorporated by reference from our other filings with the SEC or included in any applicable
prospectus supplement. Investing in our securities involves risks. Therefore, carefully consider the risk factors set forth
in any prospectus supplements and in our most recent annual and quarterly filings with the SEC, as well as other information in
this prospectus and any prospectus supplements and the documents incorporated by reference herein or therein, before purchasing
our securities. Each of the risk factors could adversely affect our business, operating results and financial condition,
as well as adversely affect the value of an investment in our securities.
Overview
Ekso Bionics designs, develops and sells
wearable bionic or robotic exoskeletons that have applications in healthcare, industrial, military, and consumer markets. Our exoskeletons
systems are strapped over the user’s clothing and augment human strength, endurance and mobility. These systems serve multiple
markets and can be used both by able-bodied users as well as by persons with physical disabilities. We or our partners have sold,
rented or leased devices that (a) enable individuals with neurological conditions affecting gait (e.g., spinal cord injury or stroke)
to rehabilitate and to walk again; (b) allow industrial workers to perform heavy duty work for extended periods; and (c) permit
soldiers to carry heavy loads for long distances while mitigating lower back, knee, and ankle injuries.
Additional Information
For additional information related to our
business and operations, please refer to the reports incorporated herein by reference, including our Annual Report on Form 10-K
for the year ended December 31, 2014, as described under the caption “Incorporation of Documents by Reference” on page 14
of this prospectus.
Corporate Information
We were incorporated under the laws of
the State of Nevada in January 2012 under the name PN Med Group, Inc. and changed our name to Ekso Bionics Holdings, Inc. in December
2013. Ekso Bionics, Inc. was incorporated under the laws of the State of Delaware in January 19, 2005 and in January 2014 completed
a reverse merger transaction with and became a wholly-owned subsidiary of Ekso Bionics Holdings, Inc.
Our principal executive offices are located
at 1414 Harbour Way South, Suite 1201, Richmond, California 94804 and our telephone number is (510) 984-1761. Our website address
is
www.eksobionics.com
. The information contained on, or that can be accessed through, our website is not a part of this
prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
Our logo, trademarks and service marks
are the property of Ekso Bionics. Other trademarks or service marks appearing in this prospectus are the property of their respective
holders.
Our Annual Reports on Form 10-K, Quarterly
Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to those reports filed or furnished pursuant to
Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, are available free
of charge through the investors page of our internet website as soon as reasonably practicable after we electronically file
such material with, or furnish it to, the SEC.
Implications of Being an Emerging Growth Company
As a company with less than $1.0 billion
in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our
Business Startups, or JOBS, Act enacted in April 2012. An emerging growth company may take advantage of reduced reporting requirements
that are otherwise applicable to public companies. These provisions include, but are not limited to:
|
|
·
|
not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes Oxley Act of 2002, or
Sarbanes Oxley Act;
|
|
|
·
|
reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration
statements; and
|
|
|
·
|
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of
any golden parachute payments not previously approved.
|
We may take advantage of these provisions
for up to five years after the first sale of our common equity securities pursuant to an effective registration statement under
the Securities Act of 1933, as amended (the “Securities Act”). However, if certain events occur prior to the end of
such five year period, including if we become a “large accelerated filer,” our annual gross revenues exceed $1 billion
or we issue more than $1 billion of non-convertible debt in any three year period, we would cease to be an emerging growth company
prior to the end of such five year period.
We may choose to take advantage of some
but not all of these reduced burdens. We have taken advantage of certain of the reduced disclosure obligations regarding executive
compensation in this registration statement and may elect to take advantage of other reduced burdens in future filings. As a result,
the information that we provide to our stockholders may be different than you might receive from other public reporting companies
in which you hold equity interests.
Under the JOBS Act, emerging growth companies
can delay adopting new or revised accounting standards until such time as those standards apply to private companies. However,
we have irrevocably elected not to avail ourselves of this extended transition period for complying with new or revised accounting
standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are
not emerging growth companies.
Offerings Under This Prospectus
Under this prospectus, we may offer shares
of our common stock and preferred stock, various series of warrants or rights to purchase any of such securities, either individually
or in units, with a total value of up to $75,000,000, from time to time at prices and on terms to be determined by market conditions
at the time of the offering. This prospectus provides you with a general description of the securities we may offer.
Each time we offer a type or series of securities under this prospectus, we will provide a prospectus supplement that will describe
the specific amounts, prices and other important terms of the securities.
The prospectus supplement also may
add, update or change information contained in this prospectus or in documents we have incorporated by reference into this prospectus.
However, no prospectus supplement will fundamentally change the terms that are set forth in this prospectus or offer a security
that is not registered and described in this prospectus at the time of its effectiveness.
We may sell the securities directly to
investors or to or through agents, underwriters or dealers. We, and our agents, underwriters or dealers, reserve the right
to accept or reject all or part of any proposed purchase of securities. If we offer securities through agents, underwriters
or dealers, we will include in the applicable prospectus supplement:
|
|
·
|
the names of those agents, underwriters or dealers;
|
|
|
·
|
applicable fees, discounts and commissions to be paid to them;
|
|
|
·
|
details regarding over-allotment options, if any; and
|
|
|
·
|
the net proceeds to us.
|
This prospectus may not be used
to consummate a sale of any securities unless it is accompanied by a prospectus supplement.
RISK FACTORS
Investing in our securities involves
significant risk. The prospectus supplement applicable to each offering of our securities will contain a discussion of the
risks applicable to an investment in Ekso Bionics. Prior to making a decision about investing in our securities, you should
carefully consider the specific factors discussed under the heading “Risk Factors” in the applicable prospectus supplement,
together with all of the other information contained or incorporated by reference in the prospectus supplement or appearing or
incorporated by reference in this prospectus. You should also consider the risks, uncertainties and assumptions discussed
under the heading “Risk Factors” included in our most recent annual report on Form 10-K, as revised or supplemented
by our subsequent quarterly reports on Form 10-Q or our current reports on Form 8-K on file with the SEC, all of which
are incorporated herein by reference, and which may be amended, supplemented or superseded from time to time by other reports we
file with the SEC in the future. The risks and uncertainties we have described are not the only ones we face. Additional
risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our operations. The
occurrence of any of these risks might cause you to lose all or part of your investment in the offered securities.
SPECIAL NOTE REGARDING FORWARD-LOOKING
STATEMENTS
The SEC encourages companies to disclose
forward-looking information so that investors can better understand a company’s future prospects and make informed investment
decisions. This prospectus and the documents we have filed with the SEC that are incorporated herein by reference contain
such “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995.
Such statements in connection with any
discussion of future operations or financial performance are identified by the use of words such as “may,” “anticipate,”
“estimate,” “expect,” “project,” “intend,” “plan,” “believe,”
and other words and terms of similar meaning. Forward-looking statements include, but are not limited to, statements regarding:
(i) the plans and objectives of management for future operations, including plans or objectives relating to the design, development
and commercialization of human exoskeletons, (ii) a projection of income (including income/loss), earnings (including earnings/loss)
per share, capital expenditures, dividends, capital structure or other financial items, (iii) our future financial performance,
including any such statement contained in a discussion and analysis of financial condition by management or in the results of operations
included pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”), (iv) our beliefs
regarding the potential for commercial opportunity for exoskeleton technology in general and our exoskeleton products in particular,
(v) our beliefs regarding potential clinical and other health benefits of our medical devices, and (vi) the assumptions underlying
or relating to any statement described in points (i), (ii), (iii), (iv) or (v) above.
We may not actually achieve the plans,
intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking
statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking
statements we make. We have included important cautionary statements in this Registration Statement, particularly in the “Risk
Factors” section, that we believe could cause actual results or events to differ materially from the forward-looking statements
that we make. For a summary of such factors, please refer to the section entitled “Risk Factors” in this prospectus,
as updated and supplemented by the discussion of risks and uncertainties under “Risk Factors” contained in any supplements
to this prospectus and in our most recent annual report on Form 10-K, as revised or supplemented by our subsequent quarterly
reports on Form 10-Q or our current reports on Form 8-K, as well as any amendments thereto, as filed with the SEC and
which are incorporated herein by reference. The information contained in this document is believed to be current as of the date
of this document. We do not intend to update any of the forward-looking statements after the date of this document to conform
these statements to actual results or to changes in our expectations, except as required by law.
In light of these assumptions, risks and
uncertainties, the results and events discussed in the forward-looking statements contained in this prospectus or in any document
incorporated herein by reference might not occur. Investors are cautioned not to place undue reliance on the forward-looking
statements, which speak only as of the date of this prospectus or the date of the document incorporated by reference in this prospectus.
We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether
as a result of new information, future events or otherwise. All subsequent forward-looking statements attributable to us
or to any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred
to in this section.
About
Ekso Bionics
Ekso Bionics designs,
develops and sells wearable bionic or robotic exoskeletons that have applications in healthcare, industrial, military, and consumer
markets. Our exoskeletons systems are strapped over the user’s clothing and augment human strength, endurance and mobility.
These systems serve multiple markets and can be used both by able-bodied users as well as by persons with physical disabilities.
We or our partners have sold, rented or leased devices that (a) enable individuals with neurological conditions affecting gait
(e.g., spinal cord injury or stroke) to rehabilitate and to walk again; (b) allow industrial workers to perform heavy duty work
for extended periods; and (c) permit soldiers to carry heavy loads for long distances while mitigating lower back, knee, and ankle
injuries.
Our long-term goal is to have one million
people stand and walk in an Ekso exoskeleton by February 2022. Our first step to achieving that goal was for us to focus on selling
our medical exoskeletons to rehabilitation centers and hospitals in the United States and Europe. We began that journey in February
2012 with our first sale of the Ekso, an exoskeleton for complete spinal cord injuries (“SCI”). We have since expanded
that effort with the July 2013 launch of our Variable Assist software and the December 2013 release of our next generation Ekso
hardware platform, Ekso GT. The Variable Assist software enables users with any amount of lower extremity strength to contribute
their own power for either leg to achieve self-initiated walking. The Ekso GT builds on the experience of the Ekso and incorporates
the Variable Assist software, allowing us to expand our sales and marketing efforts beyond SCI-focused centers to centers supporting
stroke and related neurological patients.
Ekso Labs, our engineering services division,
furthers exoskeleton technology for current medical applications and conducts research and development work which may have potential
use in future, able-bodied models of the Ekso human exoskeleton. To date, the majority of our Ekso Labs revenue has been in the
form of research grants from government organizations. Many of the research projects funded by grants are focused on researching
future medical applications and capabilities not yet ready for commercial development. Other projects, often funded by commercial
partners or the U.S. military, focus on able-bodied human exoskeleton applications, such as industrial models that are intended
to increase an individual’s workload, endurance and efficiency, allowing workers to carry heavy objects for much longer while
reducing employee injuries. Our military and industrial able-bodied exoskeleton products are all in the developmental stage.
USE OF PROCEEDS
We cannot assure you that we will receive
any proceeds in connection with securities which may be offered pursuant to this prospectus. Unless otherwise indicated in
the applicable prospectus supplement, we intend to use any net proceeds from the sale of securities under this prospectus for our
operations and to increase our investments (i) in our clinical, sales and marketing initiatives to accelerate adoption of the Ekso
in the rehabilitation market, (ii) in our research, development and commercialization activities with respect to an Ekso robotic
exoskeleton for home use, and/or (iii) in the development and commercialization of able-bodied exoskeletons for industrial use,
and for other general corporate purposes, including, but not limited to, working capital, intellectual property protection and
enforcement, capital expenditures, investments, acquisitions and collaborations. We have not determined the amounts we plan
to spend on any of the areas listed above or the timing of these expenditures. As a result, our management will have broad
discretion to allocate the net proceeds, if any, we receive in connection with securities offered pursuant to this prospectus for
any purpose. Pending application of the net proceeds as described above, we may initially invest the net proceeds in short-term,
investment-grade, or interest-bearing securities.
PLAN OF DISTRIBUTION
General Plan of Distribution
We may offer securities under this prospectus
from time to time pursuant to underwritten public offerings, negotiated transactions, “at the market” sales, block
trades or a combination of these methods. We may sell the securities:
|
|
·
|
to or through underwriters or dealers;
|
|
|
·
|
directly to one or more purchasers;
|
|
|
·
|
through a combination of such methods; or
|
|
|
·
|
through any other method described in a prospectus supplement.
|
The distribution of
securities may be effected, from time to time, in one or more transactions, including:
|
|
·
|
block transactions (which may involve crosses) and transactions on the OTCBB or any other organized market where the securities
may be traded;
|
|
|
·
|
purchases by a dealer as principal and resale by the dealer for its own account pursuant to a prospectus supplement;
|
|
|
·
|
ordinary brokerage transactions and transactions in which a dealer solicits purchasers;
|
|
|
·
|
sales “at the market” to or through a market maker or into an existing trading market, on an exchange or otherwise;
and
|
|
|
·
|
sales in other ways not involving market makers or established trading markets, including direct sales to purchasers.
|
We may distribute the securities from time
to time in one or more transactions at:
|
|
·
|
a fixed price or prices, which may be changed from time to time;
|
|
|
·
|
market prices prevailing at the time of sale;
|
|
|
·
|
prices related to the prevailing market prices; or
|
We may directly
solicit offers to purchase the securities being offered by this prospectus. We may also designate agents to solicit offers to purchase
the securities from time to time. The prospectus supplement relating to a series of the offered securities will name any underwriters,
dealers or agents involved in the offer or sale of the securities.
If
we utilize a dealer in the sale of the securities being offered by this prospectus, we will sell the securities to the dealer,
as principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time
of resale.
If we utilize
an underwriter in the sale of the securities being offered by this prospectus, we will execute an underwriting agreement with the
underwriter at the time of sale, and we will provide the name of any underwriter in the prospectus supplement which the underwriter
will use to make resales of the securities to the public. In connection with the sale of the securities, we, or the purchasers
of the securities for whom the underwriter may act as agent, may compensate the underwriter in the form of underwriting discounts
or commissions. The underwriter may sell the securities to or through dealers, and the underwriter may compensate those dealers
in the form of discounts, concessions or commissions.
With respect to
underwritten public offerings, negotiated transactions and block trades, we will provide in the applicable prospectus supplement
information regarding any compensation we pay to underwriters, dealers or agents in connection with the offering of the securities,
and any discounts, concessions or commissions allowed by underwriters to participating dealers. Underwriters, dealers and agents
participating in the distribution of the securities may be deemed to be underwriters within the meaning of the Securities Act,
and any discounts and commissions received by them and any profit realized by them on resale of the securities may be deemed to
be underwriting discounts and commissions. We may enter into agreements to indemnify underwriters, dealers and agents against civil
liabilities, including liabilities under the Securities Act, or to contribute to payments they may be required to make in respect
thereof.
If so indicated in the applicable prospectus
supplement, we will authorize underwriters, dealers or other persons acting as our agents to solicit offers by certain institutions
to purchase securities from us pursuant to delayed delivery contracts providing for payment and delivery on the date stated in
each applicable prospectus supplement. Each contract will be for an amount not less than, and the aggregate amount of securities
sold pursuant to such contracts shall not be less nor more than, the respective amounts stated in each applicable prospectus supplement.
Institutions with whom the contracts, when authorized, may be made include commercial and savings banks, insurance companies, pension
funds, investment companies, educational and charitable institutions and other institutions, but shall in all cases be subject
to our approval. Delayed delivery contracts will not be subject to any conditions except that:
|
|
·
|
the purchase by an institution of the securities covered under that contract shall not at the time of delivery be prohibited
under the laws of the jurisdiction to which that institution is subject; and
|
|
|
·
|
if the securities are also being sold to underwriters acting as principals for their own account, the underwriters shall have
purchased such securities not sold for delayed delivery. The underwriters and other persons acting as our agents will not
have any responsibility in respect of the validity or performance of delayed delivery contracts.
|
One or more firms, referred to as “remarketing
firms,” may also offer or sell the securities, if a prospectus supplement so indicates, in connection with a remarketing
arrangement upon their purchase. Remarketing firms will act as principals for their own accounts or as our agents. These remarketing
firms will offer or sell the securities in accordance with the terms of the securities. Each prospectus supplement will identify
and describe any remarketing firm and the terms of its agreement, if any, with us and will describe the remarketing firm’s
compensation. Remarketing firms may be deemed to be underwriters in connection with the securities they remarket. Remarketing firms
may be entitled under agreements that may be entered into with us to indemnification by us against certain civil liabilities, including
liabilities under the Securities Act, and may be customers of, engage in transactions with or perform services for us in the ordinary
course of business.
Certain underwriters may use this prospectus
and any accompanying prospectus supplement for offers and sales related to market-making transactions in the securities. These
underwriters may act as principal or agent in these transactions, and the sales will be made at prices related to prevailing market
prices at the time of sale. Any underwriters involved in the sale of the securities may qualify as “underwriters” within
the meaning of Section 2(a)(11) of the Securities Act. In addition, the underwriters’ commissions, discounts or concessions
may qualify as underwriters’ compensation under the Securities Act and the rules of the Financial Industry Regulatory
Authority, Inc., or FINRA.
Shares of our common stock sold pursuant
to the registration statement of which this prospectus is a part will be authorized for quotation and trading on the OTC Markets.
The applicable prospectus supplement will contain information, where applicable, as to any other listing, if any, on the OTC Markets
or any securities market or other securities exchange of the securities covered by the prospectus supplement. Underwriters
may make a market in our common stock, but will not be obligated to do so and may discontinue any market making at any time without
notice. We can make no assurance as to the liquidity of or the existence, development or maintenance of trading markets for any
of the securities.
In order to facilitate the offering of
the securities, certain persons participating in the offering may engage in transactions that stabilize, maintain or otherwise
affect the price of the securities. This may include over-allotments or short sales of the securities, which involve the
sale by persons participating in the offering of more securities than we sold to them. In these circumstances, these persons
would cover such over-allotments or short positions by making purchases in the open market or by exercising their over-allotment
option. In addition, these persons may stabilize or maintain the price of the securities by bidding for or purchasing the
applicable security in the open market or by imposing penalty bids, whereby selling concessions allowed to dealers participating
in the offering may be reclaimed if the securities sold by them are repurchased in connection with stabilization transactions.
The effect of these transactions may be to stabilize or maintain the market price of the securities at a level above that which
might otherwise prevail in the open market. These transactions may be discontinued at any time.
The underwriters, dealers and agents may
engage in other transactions with us, or perform other services for us, in the ordinary course of their business.
DESCRIPTION OF COMMON STOCK
We are authorized to issue 500,000,000
shares of common stock, par value $0.001 per share. On June 18, 2015, we had 102,123,767 shares of common stock outstanding
and approximately 313 stockholders of record.
The following summary of certain provisions
of our common stock does not purport to be complete. You should refer to our articles of incorporation and our bylaws, both
of which are included as exhibits to the registration statement of which this prospectus is a part. The summary below is
also qualified by provisions of applicable law.
General
The holders of outstanding shares of common
stock are entitled to receive dividends out of assets or funds legally available for the payment of dividends of such times and
in such amounts as the board from time to time may determine. Holders of common stock are entitled to one vote for each share held
on all matters submitted to a vote of stockholders. There is no cumulative voting of the election of directors then standing for
election. The common stock is not entitled to pre-emptive rights and is not subject to conversion or redemption. Upon liquidation,
dissolution or winding up of our company, the assets legally available for distribution to stockholders are distributable ratably
among the holders of the common stock after payment of liquidation preferences, if any, on any outstanding payment of other claims
of creditors. Each outstanding share of common stock is duly and validly issued, fully paid and non-assessable.
Transfer Agent and Registrar
The transfer agent and registrar for our
common stock is VStock Transfer, LLC. The transfer agent’s address is 18 Lafayette Place, Woodmere, New York 11598 and its
telephone number is (212) 828-8436.
OTC Markets
Our common stock is listed for quotation
on the OTC Markets under the symbol “EKSO.”
DESCRIPTION OF PREFERRED STOCK
We are authorized to issue 10,000,000 shares
of preferred stock, par value $0.001 per share. As of June 18, 2015, no shares of our preferred stock were outstanding or
designated. The following summary of certain provisions of our preferred stock does not purport to be complete. You
should refer to our articles of incorporation and our bylaws, both of which are included as exhibits to the registration statement
of which this prospectus is a part. The summary below is also qualified by provisions of applicable law.
General
Our board of directors may, without further
action by our stockholders, from time to time, direct the issuance of shares of preferred stock in one or more series and may,
at the time of issuance, determine the rights, preferences and limitations of each series, including voting rights, dividend rights
and redemption and liquidation preferences. Satisfaction of any dividend preferences of outstanding shares of preferred stock
would reduce the amount of funds available for the payment of dividends on shares of our common stock. Holders of shares
of preferred stock may be entitled to receive a preference payment in the event of any liquidation, dissolution or winding-up of
our company before any payment is made to the holders of shares of our common stock. In some circumstances, the issuance
of shares of preferred stock may render more difficult or tend to discourage a merger, tender offer or proxy contest, the assumption
of control by a holder of a large block of our securities or the removal of incumbent management. Upon the affirmative vote
of our board of directors, without stockholder approval, we may issue shares of preferred stock with voting and conversion rights
which could adversely affect the holders of shares of our common stock.
If we offer a specific series of preferred
stock under this prospectus, we will describe the terms of the preferred stock in the prospectus supplement for such offering and
will file a copy of the certificate establishing the terms of the preferred stock with the SEC. To the extent required, this
description will include:
|
|
·
|
the title and stated value;
|
|
|
·
|
the number of shares offered, the liquidation preference, if any, per share and the purchase price;
|
|
|
·
|
the dividend rate(s), period(s) and/or payment date(s), or method(s) of calculation for such dividends;
|
|
|
·
|
whether dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate;
|
|
|
·
|
the procedures for any auction and remarketing, if any;
|
|
|
·
|
the provisions for a sinking fund, if any;
|
|
|
·
|
the provisions for redemption, if applicable;
|
|
|
·
|
any listing of the preferred stock on any securities exchange or market;
|
|
|
·
|
whether the preferred stock will be convertible into our common stock, and, if applicable, the conversion price (or how it
will be calculated) and conversion period;
|
|
|
·
|
whether the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange price (or how it will
be calculated) and exchange period;
|
|
|
·
|
voting rights, if any, of the preferred stock;
|
|
|
·
|
a discussion of any material and/or special U.S. federal income tax considerations applicable to the preferred stock;
|
|
|
·
|
the relative ranking and preferences of the preferred stock as to dividend rights and rights upon liquidation, dissolution
or winding up of the affairs of the Company; and
|
|
|
·
|
any material limitations on issuance of any class or series of preferred stock ranking pari passu with or senior to the series
of preferred stock as to dividend rights and rights upon liquidation, dissolution or winding up of the Company.
|
Transfer Agent and Registrar
The transfer agent and registrar for our
preferred stock will be set forth in the applicable prospectus supplement.
DESCRIPTION OF WARRANTS
General
As of June 18, 2015, warrants to purchase
an aggregate of 13,747,161 shares of our common stock are issued and outstanding, of which warrants to purchase 621,361 shares
of common stock have an exercise price of $1.38 per share and expire on May 20, 2020 (the “Pre-Merger Warrants”), warrants
to purchase 2,600,000 shares of common stock have an exercise price of $1.00 per share and expire on January 15, 2017, warrants
to purchase 2,981,300 shares of common stock have an exercise price of $1.00 per share and expire on January 15, 2019, and warrants
to purchase 7,544,500 shares of common stock have an exercise price of $2.00 per share and expire on January 15, 2019. The Pre-Merger
Warrants may, at the option of the holders, be exercised on a cashless exercise basis, which means that in lieu of paying the aggregate
exercise price for the shares being purchased upon exercise of the warrants for cash, the holder will forfeit a number of shares
underlying the warrants with a fair market value (as defined in the warrant) equal to such aggregate exercise price. We will not
receive additional proceeds to the extent these warrants are exercised on a cashless exercise basis.
We may issue warrants to purchase shares
of our common stock and/or preferred stock in one or more series together with other securities or separately, as described in
the applicable prospectus supplement. Below is a description of certain general terms and provisions of the warrants that
we may offer. Particular terms of the warrants will be described in the warrant agreements and the prospectus supplement
relating to the warrants.
The applicable prospectus supplement will
contain, where applicable, the following terms of and other information relating to the warrants:
|
|
·
|
the specific designation and aggregate number of, and the price at which we will issue, the warrants;
|
|
|
·
|
the currency or currency units in which the offering price, if any, and the exercise price are payable;
|
|
|
·
|
the designation, amount and terms of the securities purchasable upon exercise of the warrants;
|
|
|
·
|
if applicable, the exercise price for shares of our common stock and the number of shares of common stock to be received upon
exercise of the warrants;
|
|
|
·
|
if applicable, the exercise price for shares of our preferred stock, the number of shares of preferred stock to be received
upon exercise, and a description of that series of our preferred stock;
|
|
|
·
|
the date on which the right to exercise the warrants will begin and the date on which that right will expire or, if you may
not continuously exercise the warrants throughout that period, the specific date or dates on which you may exercise the warrants;
|
|
|
·
|
whether the warrants will be issued in fully registered form or bearer form, in definitive or global form or in any combination
of these forms, although, in any case, the form of a warrant included in a unit will correspond to the form of the unit and of
any security included in that unit;
|
|
|
·
|
any applicable material U.S. federal income tax consequences;
|
|
|
·
|
the identity of the warrant agent for the warrants and of any other depositaries, execution or paying agents, transfer agents,
registrars or other agents;
|
|
|
·
|
the proposed listing, if any, of the warrants or any securities purchasable upon exercise of the warrants on any securities
exchange;
|
|
|
·
|
if applicable, the date from and after which the warrants and the common stock and/or preferred stock will be separately transferable;
|
|
|
·
|
if applicable, the minimum or maximum amount of the warrants that may be exercised at any one time;
|
|
|
·
|
information with respect to book-entry procedures, if any;
|
|
|
·
|
the anti-dilution provisions of the warrants, if any;
|
|
|
·
|
any redemption or call provisions;
|
|
|
·
|
whether the warrants may be sold separately or with other securities as parts of units; and
|
|
|
·
|
any additional terms of the warrants, including terms, procedures and limitations relating to the exchange and exercise of
the warrants.
|
Transfer Agent and Registrar
The transfer agent and registrar for any
warrants will be set forth in the applicable prospectus supplement.
DESCRIPTION OF RIGHTS
General
We may issue rights to our stockholders
to purchase shares of our common stock, preferred stock or the other securities described in this prospectus. We may offer
rights separately or together with one or more additional rights, preferred stock, common stock, or warrants, or any combination
of those securities in the form of units, as described in the applicable prospectus supplement. Each series of rights will
be issued under a separate rights agreement to be entered into between us and a bank or trust company, as rights agent. The
rights agent will act solely as our agent in connection with the certificates relating to the rights of the series of certificates
and will not assume any obligation or relationship of agency or trust for or with any holders of rights certificates or beneficial
owners of rights. The following description sets forth certain general terms and provisions of the rights to which any prospectus
supplement may relate. The particular terms of the rights to which any prospectus supplement may relate and the extent, if
any, to which the general provisions may apply to the rights so offered will be described in the applicable prospectus supplement.
To the extent that any particular terms of the rights, rights agreement or rights certificates described in a prospectus supplement
differ from any of the terms described below, then the terms described below will be deemed to have been superseded by that prospectus
supplement. We encourage you to read the applicable rights agreement and rights certificate for additional information before
you decide whether to purchase any of our rights.
We will provide in a prospectus supplement
the following terms of the rights being issued:
|
|
·
|
the date of determining the stockholders entitled to the rights distribution;
|
|
|
·
|
the aggregate number of shares of common stock, preferred stock or other securities purchasable upon exercise of the rights;
|
|
|
·
|
the aggregate number of rights issued;
|
|
|
·
|
whether the rights are transferrable and the date, if any, on and after which the rights may be separately transferred;
|
|
|
·
|
the date on which the right to exercise the rights will commence, and the date on which the right to exercise the rights will
expire;
|
|
|
·
|
the method by which holders of rights will be entitled to exercise;
|
|
|
·
|
the conditions to the completion of the offering, if any;
|
|
|
·
|
the withdrawal, termination and cancellation rights, if any;
|
|
|
·
|
whether there are any backstop or standby purchaser or purchasers and the terms of their commitment, if any;
|
|
|
·
|
whether stockholders are entitled to oversubscription rights, if any;
|
|
|
·
|
any applicable material U.S. federal income tax considerations; and
|
|
|
·
|
any other terms of the rights, including terms, procedures and limitations relating to the distribution, exchange and exercise
of the rights, as applicable.
|
Each right will entitle the holder of rights
to purchase for cash the principal amount of shares of common stock, preferred stock or other securities at the exercise price
provided in the applicable prospectus supplement. Rights may be exercised at any time up to the close of business on the
expiration date for the rights provided in the applicable prospectus supplement.
Holders may exercise rights as described
in the applicable prospectus supplement. Upon receipt of payment and the rights certificate properly completed and duly executed
at the corporate trust office of the rights agent or any other office indicated in the prospectus supplement, we will, as soon
as practicable, forward the shares of common stock, preferred stock or other securities, as applicable, purchasable upon exercise
of the rights. If less than all of the rights issued in any rights offering are exercised, we may offer any unsubscribed
securities directly to persons other than stockholders, to or through agents, underwriters or dealers or through a combination
of such methods, including pursuant to standby arrangements, as described in the applicable prospectus supplement.
Rights Agent
The rights agent for any rights we offer
will be set forth in the applicable prospectus supplement.
DESCRIPTION OF UNITS
The following description, together with
the additional information that we include in any applicable prospectus supplements summarizes the material terms and provisions
of the units that we may offer under this prospectus. While the terms we have summarized below will apply generally to any
units that we may offer under this prospectus, we will describe the particular terms of any series of units in more detail in the
applicable prospectus supplement. The terms of any units offered under a prospectus supplement may differ from the terms
described below.
We will incorporate by reference from reports
that we file with the SEC, the form of unit agreement that describes the terms of the series of units we are offering, and any
supplemental agreements, before the issuance of the related series of units. The following summaries of material terms and
provisions of the units are subject to, and qualified in their entirety by reference to, all the provisions of the unit agreement
and any supplemental agreements applicable to a particular series of units. We urge you to read the applicable prospectus
supplements related to the particular series of units that we may offer under this prospectus, as well as any related free writing
prospectuses and the complete unit agreement and any supplemental agreements that contain the terms of the units.
General
We may issue units consisting of common
stock, preferred stock, one or more warrants or rights for the purchase of common stock or preferred stock in one or more series,
in any combination. Each unit will be issued so that the holder of the unit is also the holder of each security included
in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each security included in the unit.
The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred
separately, at any time or at any time before a specified date.
We will describe in the applicable prospectus
supplement the terms of the series of units being offered, including:
|
|
·
|
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances
those securities may be held or transferred separately;
|
|
|
·
|
any provisions of the governing unit agreement that differ from those described below; and
|
|
|
·
|
any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the
units.
|
The provisions described in this section,
as well as those set forth in any prospectus supplement or as described under “Description of Common Stock,” “Description
of Preferred Stock,” “Description of Warrants,” and “Description of Rights” will apply to each unit,
as applicable, and to any common stock, preferred stock, warrant, or right included in each unit, as applicable.
Unit Agent
The name and address of the unit agent
for any units we offer will be set forth in the applicable prospectus supplement.
Issuance in Series
We may issue units in such amounts and
in such numerous distinct series as we determine.
Enforceability of Rights by Holders of Units
Each unit agent will act solely as our
agent under the applicable unit agreement and will not assume any obligation or relationship of agency or trust with any holder
of any unit. A single bank or trust company may act as unit agent for more than one series of units. A unit agent will
have no duty or responsibility in case of any default by us under the applicable unit agreement or unit, including any duty or
responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a unit may,
without the consent of the related unit agent or the holder of any other unit, enforce by appropriate legal action its rights as
holder under any security included in the unit.
CERTAIN PROVISIONS OF NEVADA LAW AND
OF THE COMPANY’S ARTICLES OF INCORPORATION AND BYLAWS
Anti-Takeover Provisions
Nevada Law
We may in the future become subject to
Nevada’s control share laws. A corporation is subject to Nevada’s control share law if it has more than 200 stockholders
of record, at least 100 of whom are residents of Nevada, and if the corporation does business in Nevada, including through an affiliated
corporation. This control share law may have the effect of discouraging corporate takeovers. The Company currently has fewer than
100 stockholders of record who are residents of Nevada.
The control share law focuses on the acquisition
of a “controlling interest,” which means the ownership of outstanding voting shares that would be sufficient, but for
the operation of the control share law, to enable the acquiring person to exercise the following proportions of the voting power
of the corporation in the election of directors: (1) one-fifth or more but less than one-third; (2) one-third or more but less
than a majority; or (3) a majority or more. The ability to exercise this voting power may be direct or indirect, as well as individual
or in association with others.
The effect of the control share law is
that an acquiring person, and those acting in association with that person, will obtain only such voting rights in the control
shares as are conferred by a resolution of the stockholders of the corporation, approved at a special or annual meeting of stockholders.
The control share law contemplates that voting rights will be considered only once by the other stockholders. Thus, there is no
authority to take away voting rights from the control shares of an acquiring person once those rights have been approved. If the
stockholders do not grant voting rights to the control shares acquired by an acquiring person, those shares do not become permanent
non-voting shares. The acquiring person is free to sell the shares to others. If the buyer or buyers of those shares themselves
do not acquire a controlling interest, the shares are not governed by the control share law any longer.
If control shares are accorded full voting
rights and the acquiring person has acquired control shares with a majority or more of the voting power, a stockholder of record,
other than the acquiring person, who did not vote in favor of approval of voting rights for the control shares, is entitled to
demand fair value for such stockholder’s shares.
In addition to the control share law, Nevada
has a business combination law, which prohibits certain business combinations between Nevada corporations and “interested
stockholders” for two years after the interested stockholder first becomes an interested stockholder, unless (a) the corporation’s
board of directors approves the combination in advance or (b) the corporation’s board of directors and at least 60% of the
corporation’s disinterested stockholders approve the combination at an annual or special meeting. For purposes of Nevada
law, an interested stockholder is any person who is: (a) the beneficial owner, directly or indirectly, of 10% or more of the voting
power of the outstanding voting shares of the corporation, or (b) an affiliate or associate of the corporation and at any time
within the previous two years was the beneficial owner, directly or indirectly, of 10% or more of the voting power of the then-outstanding
shares of the corporation. The definition of “business combination” contained in the statute is sufficiently broad
to cover virtually any kind of transaction that would allow a potential acquirer to use the corporation’s assets to finance
the acquisition or otherwise to benefit its own interests rather than the interests of the corporation and its other stockholders.
The effect of Nevada’s business combination
law is to potentially discourage a party interested in taking control of the Company from doing so if it cannot obtain the approval
of our board of directors.
Authorized but Unissued Shares
The
authorized but unissued shares of common stock and preferred stock are available for future issuance without stockholder approval,
subject to any limitations imposed by the listing standards of any exchange on which our shares are listed.
These
additional shares may be used for a variety of corporate finance transactions, acquisitions and employee benefit plans. The existence
of authorized but unissued and unreserved common stock and preferred stock could make more difficult or discourage an attempt to
obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Special Meeting of Stockholders;
Advance Notice Requirements; Stockholder Action
Our
by-laws provide that, except as otherwise required by law or the articles of incorporation, special meetings of the stockholders
can only be called by our board of directors, by the chairman of our board of directors or by certain of our officers. In addition,
our by-laws establish an advance notice procedure for stockholder proposals to be brought before an annual meeting of stockholders,
including proposed nominations of candidates for election to our board of directors. Stockholders at an annual meeting may only
consider proposals or nominations specified in the notice of the meeting or brought before the meeting by or at the direction of
our board of directors, or by a stockholder of record on the record date for the meeting who is entitled to vote at the meeting
and who has delivered timely written notice in proper form to our secretary of the stockholder's intention to bring such business
before the meeting. These provisions could have the effect of delaying until the next stockholder meeting stockholder actions that
are favored by the holders of a majority of our outstanding voting securities. In addition, our by-laws require that stockholder
actions must be effected at a duly called stockholders meeting and prohibit actions by our stockholders by written consent.
Limitation of Liability and Indemnification
Nevada Revised Statutes (NRS) Sections
78.7502 and 78.751 provide us with the power to indemnify any of our directors, officers, employees and agents. The
person entitled to indemnification must have conducted himself in good faith, and must reasonably believe that his conduct was
in, or not opposed to, our best interests. In a criminal action, the director, officer, employee or agent must not have
had reasonable cause to believe that his conduct was unlawful.
Under NRS Section 78.751, advances for
expenses may be made by agreement if the director or officer affirms in writing that he has met the standards for indemnification
and will personally repay the expenses if it is determined that such officer or director did not meet those standards.
Our by-laws state that we shall indemnify
every (i) present or former director, officer, employee or agent of us and (ii) any person who served at our request as a director,
officer, member, manager, partner, trustee, fiduciary, employee or agent of another corporation, limited liability company, partnership,
joint venture, trust, employee benefit plan or other enterprise (each an “Indemnitee”).
Our by-laws provide that we shall indemnify
an Indemnitee against expenses, including attorneys’ fees and disbursements, and costs (and in connection with a proceeding
other than a proceeding by or in the right of the Company, judgments, fines and amounts paid in settlement) actually and reasonably
incurred by such person in connection with any proceeding in which such Indemnitee was, is or is threatened to be named as defendant
or respondent, or in which he was or is a witness without being named a defendant or respondent, by reason, in whole or in part,
of his serving or having served, or having been nominated or designated to serve, if it is determined that the Indemnitee (a) conducted
himself in good faith and in a manner which such Indemnitee reasonably believed to be in or not opposed to our best interests,
or with respect to any criminal proceeding, had no reasonable cause to believe that his conduct was unlawful or (b) is not liable
pursuant to NRS Section 78.138; provided, however, that in the event that an Indemnitee is found liable to us, we will have no
obligation to indemnify such Indemnitee unless, and only to the extent that the court in which such action or suit was brought
or other court of competent jurisdiction determines that, in view of all the circumstances of the case, such person is fairly and
reasonably entitled to indemnity for such expenses and costs as a court of competent jurisdiction or such other court shall deem
proper.
The termination of any proceeding by judgment,
order, settlement or conviction, or on a plea of nolo contendere or its equivalent, is not of itself determinative that the Indemnitee
did not meet the requirements set forth in clauses (a) or (b) above. An Indemnitee shall be deemed to have been found liable in
respect of any claim, issue or matter only after the Indemnitee shall have been so adjudged by a court of competent jurisdiction
after exhaustion of all appeals therefrom.
In addition to our by-laws, have entered
into an Indemnification Agreement with each of our directors and executive officers pursuant to which we are required to indemnify,
and to advance expenses on behalf of, such persons to the fullest extent permitted by applicable law and our governing documents.
We believe that entering into these agreements helps us to attract and retain highly competent and qualified persons to serve the
Company.
Insofar as indemnification for liabilities
arising under the Securities Act may be permitted to our directors, officers and controlling persons, we have been advised that
in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is, therefore,
unenforceable.
LEGAL MATTERS
Nutter, McClennen and Fish, LLP, Boston,
Massachusetts, will pass upon the validity of the issuance of the securities to be offered by this prospectus.
EXPERTS
OUM & Co., LLP, independent registered
public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K for the
year ended December 31, 2014, as set forth in their report, which is incorporated by reference in this prospectus and elsewhere
in the registration statement. Our financial statements are incorporated by reference in reliance on OUM & Co., LLP’s
report, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We are subject to the reporting requirements
of the Securities Exchange Act of 1934, as amended, and file annual, quarterly and current reports, proxy statements and other
information with the SEC. You may read and copy these reports, proxy statements and other information at the SEC’s
public reference facilities at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You can request copies of these
documents by writing to the SEC and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for more information
about the operation of the public reference facilities. SEC filings are also available at the SEC’s web site at http://www.sec.gov.
We
have filed with the SEC a registration statement under the Securities Act relating to the offering of these securities. The registration
statement, including the attached exhibits, contains additional relevant information about us and the securities. This prospectus
does not contain all of the information set forth in the registration statement. You can obtain a copy of the registration statement,
at prescribed rates, from the SEC at the address listed above.
The
registration statement and the documents referred to below under "Incorporation by Reference" are also available on our
Internet website www.eksobionics.com. We have not incorporated by reference into this prospectus the information on our website,
and you should not consider it to be a part of this prospectus
.
INCORPORATION OF DOCUMENTS BY REFERENCE
The
SEC allows us to incorporate by reference into this prospectus certain information we file with it, which means that we can disclose
important information by referring you to those documents. The information incorporated by reference is considered to be a part
of this prospectus, and information that we file later with the SEC will automatically update and supersede information contained
in this prospectus and any accompanying prospectus supplement. We incorporate by reference the documents listed below that we have
previously filed with the SEC (excluding any portions of any Form 8-K that are not deemed "filed" pursuant to the
General Instructions of Form 8-K):
|
|
·
|
our Annual Report on Form 10-K for the fiscal year ended December 31, 2014 filed on March 19, 2015;
|
|
|
·
|
our Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2015, filed on May 11, 2015;
|
|
|
·
|
our Current Reports on Form 8-K filed on April 10, 2015 and June 16, 2015 ; and
|
|
|
·
|
the description of our common stock contained in our Registration Statement on Form 8-A filed on May 6, 2015, including
any amendment or report filed for the purpose of updating such description.
|
We
also incorporate by reference into this prospectus additional documents that we may file with the SEC under Sections 13(a),
13(c), 14 or 15(d) of the Exchange Act prior to the completion or termination of the offering, including all such documents we
may file with the SEC after the date of the initial registration statement and prior to the effectiveness of the registration statement,
but excluding any information deemed furnished and not filed with the SEC.
Any statement contained in this prospectus
or in a document incorporated or deemed to be incorporated by reference into this prospectus will be deemed to be modified or superseded
for purposes of this prospectus to the extent that a statement contained in this prospectus or any other subsequently filed document
that is deemed to be incorporated by reference into this prospectus modifies or supersedes the statement. Any statement so modified
or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
We
will provide to each person, including any beneficial owner, to whom this prospectus is delivered, upon written or oral request,
at no cost to the requester, a copy of any or all of the information that is incorporated by reference in this prospectus. Requests
for such documents should be directed to:
Investor Relations, Ekso Bionics Holdings, Inc., 1414 Harbour Way South, Suite
1201 Richmond, California 94804, (510) 984-1761.
You
may also access the documents incorporated by reference in this prospectus through our website at www.eksobionics.com. Except for
the specific incorporated documents listed above, no information available on or through our website shall be deemed to be incorporated
in this prospectus or the registration statement of which it forms a part.
This
prospectus may contain information that updates, modifies or is contrary to information in one or more of the documents incorporated
by reference in this prospectus. You should rely only on the information incorporated by reference or provided in this prospectus.
We have not authorized anyone else to provide you with different information. You should not assume that the information in this
prospectus is accurate as of any date other than the date of this prospectus or the date of the documents incorporated by reference
in this prospectus.
3,750,000
Shares of Common Stock

PROSPECTUS SUPPLEMENT
Cowen
and Company
SunTrust
Robinson Humphrey
B.
Riley & Co.
August 9, 2016
Ekso Bionics (NASDAQ:EKSO)
Historical Stock Chart
From Mar 2024 to Apr 2024
Ekso Bionics (NASDAQ:EKSO)
Historical Stock Chart
From Apr 2023 to Apr 2024