UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant
to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 25, 2016
MEDIVATION, INC.
(Exact
name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-32836 |
|
13-3863260 |
| (State or other jurisdiction
of incorporation) |
|
(Commission
File No.) |
|
(IRS Employer
Identification No.) |
525 Market Street, 36th Floor
San Francisco, California 94105
(Address of principal executive offices and zip code)
Registrant’s telephone number, including area code: (415) 543-3470
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the
following provisions:
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Item 7.01. |
Regulation FD Disclosure. |
As previously disclosed, Medivation, Inc. has recently
concluded that the mechanism of action of MDV9300 (pidilizumab) is not as an inhibitor of PD-1, and work is underway to determine the mechanism of action. Under its investigational new drug application (IND) for MDV9300, Medivation has advised the
U.S. Food and Drug Administration (FDA) of this conclusion, and the FDA has placed the IND on partial clinical hold and requested Medivation to revise relevant statements in the related investigator brochure, protocols and informed consent
documents. Medivation may not enroll patients into its Phase 2 clinical trial evaluating the safety and efficacy of MDV9300 in patients with relapsed or refractory diffuse large B-cell lymphoma until such statements are revised and the partial
clinical hold is removed. Although the Phase 2 clinical trial was initiated in December 2015, no patients have been enrolled to date. Medivation believes the partial clinical hold does not relate to concerns regarding the safety of MDV9300.
Medivation plans to submit the requested documents in February 2016. The FDA has 30 days thereafter to notify Medivation if clinical studies with MDV9300 may be resumed. Under the partial clinical hold, patients who are currently receiving MDV9300
through investigator-sponsored trials of MDV9300 that cross-reference Medivation’s IND may continue on treatment, but Medivation must inform investigators to update protocols and informed consent documents to state that MDV9300 is not an
anti-PD-1 antibody.
In addition, Medivation intends to submit an amendment to the Chemistry, Manufacturing and Controls (CMC) section of
its IND for MDV9300 to address certain manufacturing changes. Medivation plans to submit the CMC amendment in the first half of 2016 and intends that patients in its Phase 2 clinical trial will be treated with MDV9300 manufactured in accordance with
the amended IND.
The Company has furnished an updated corporate presentation as Exhibit 99.1 to this Form 8-K.
The information in this Item 7.01, including Exhibit 99.1, is being furnished and shall not be deemed filed for purposes of
Section 18 of the Exchange Act, or otherwise subject to the liability of that section, nor shall such information be deemed to be incorporated by reference in any registration statement or other document filed under the Securities Act of 1933,
as amended, or the Exchange Act, except as otherwise stated in such filing.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits.
|
|
|
|
|
| 99.1 |
|
Updated corporate presentation |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
| |
|
|
|
MEDIVATION, INC. |
|
|
|
|
| Dated: January 25, 2016 |
|
|
|
By: |
|
/s/ Richard A. Bierly |
|
|
|
|
|
|
Richard A. Bierly Chief Financial
Officer |
EXHIBIT INDEX
|
|
|
|
|
| 99.1 |
|
Updated corporate presentation |

David Hung, M.D. Founder, President and
CEO January 25-29, 2016 NASDAQ: MDVN Exhibit 99.1

Safe Harbor This presentation includes
forward-looking statements concerning our business plans and prospects that are made pursuant to the safe harbor provisions of the federal securities laws. Forward-looking statements are any statements regarding future events or results, and may be
identified by the use of words such as “anticipate,” “expect,” “may,” “potentially,” “projected”, “target” and similar words. Forward-looking statements involve risks and
uncertainties that could cause Medivation's actual operations and results to differ significantly from those discussed herein, including, without limitation, risks related to: the inherent uncertainty associated with pharmaceutical product
development and clinical trials; the timing of our clinical trials, including difficulties or delays in initiating any of the trials planned for 2016, completing the enrollment of ongoing trials or manufacturing or supplying product for our clinical
trials; the timing and sufficiency of the data generated from completed, ongoing or planned clinical trials to support approval of our product candidates or expand the label for XTANDI® (enzalutamide); earlier pre-clinical and clinical results
may not be reproduced or confirmed in subsequent pivotal or controlled trials; the process of obtaining regulatory approval is uncertain, and may not be completed or may be unsuccessful; discovery of new safety information which may cause us to
re-evaluate our development programs; our ability to effectively and successfully commercialize our product candidates if or when approved by regulatory agencies, including our ability to obtain competitive pricing and reimbursement coverage; our
dependence on our collaboration relationship with Astellas to fund the continued development, manufacturing and commercialization of XTANDI and the revenue generated from such collaboration to fund our other clinical development programs; the
introduction of generic competition or challenges to our intellectual property portfolio which may result in the loss of intellectual property protection and our competitive advantage; our past financial or operating performance may not be
indicative of future performance or results; unanticipated expenditures or liabilities; and other risks detailed in Medivation's filings with the Securities and Exchange Commission, or SEC, including our quarterly report on Form 10-Q for the quarter
ended September 30, 2015, which was filed on November 6, 2015. You are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this presentation. Medivation disclaims any obligation or undertaking
to update, supplement or revise any forward-looking statements contained in this presentation. This presentation includes non-GAAP financial measures, and a reconciliation between the non-GAAP and GAAP financial measures is available on
Medivation’s website at www.medivation.com within the investor relations section. Management believes this non-GAAP financial information is useful for management and investors, when considered in conjunction with Medivation’s GAAP
financial statements, because management uses such information internally for its operating, budgeting and financial planning purposes. Non-GAAP financial information is not prepared under a comprehensive set of accounting rules and should only be
used to supplement an understanding of Medivation’s operating results as reported under U.S. GAAP.
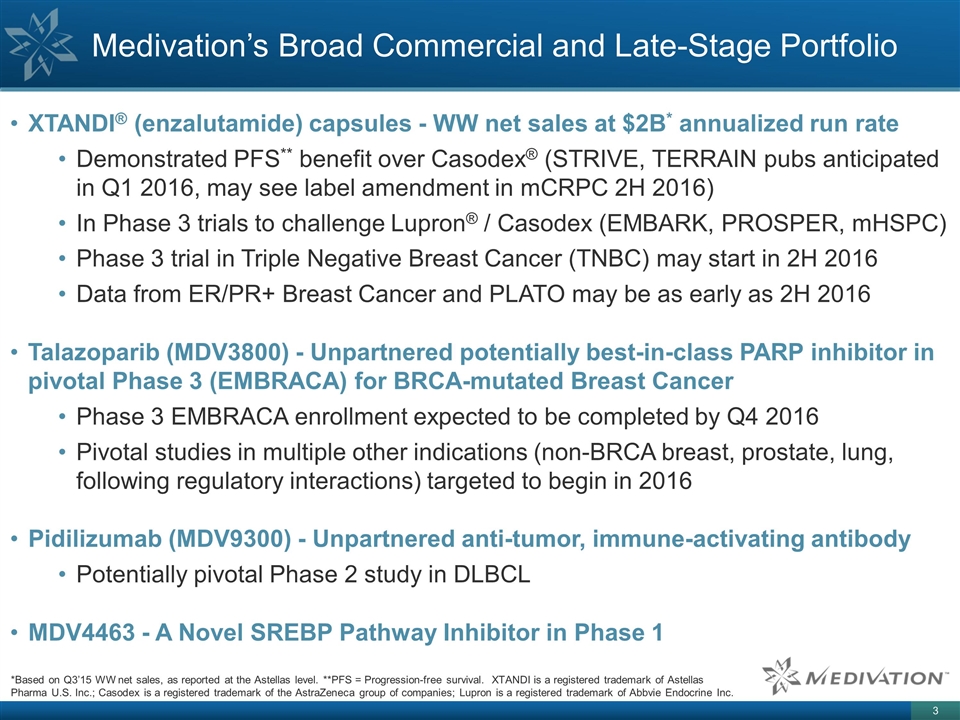
Medivation’s Broad Commercial and
Late-Stage Portfolio *Based on Q3’15 WW net sales, as reported at the Astellas level. **PFS = Progression-free survival. XTANDI is a registered trademark of Astellas Pharma U.S. Inc.; Casodex is a registered trademark of the AstraZeneca group
of companies; Lupron is a registered trademark of Abbvie Endocrine Inc. XTANDI® (enzalutamide) capsules - WW net sales at $2B* annualized run rate Demonstrated PFS** benefit over Casodex® (STRIVE, TERRAIN pubs anticipated in Q1 2016, may
see label amendment in mCRPC 2H 2016) In Phase 3 trials to challenge Lupron® / Casodex (EMBARK, PROSPER, mHSPC) Phase 3 trial in Triple Negative Breast Cancer (TNBC) may start in 2H 2016 Data from ER/PR+ Breast Cancer and PLATO may be as early
as 2H 2016 Talazoparib (MDV3800) - Unpartnered potentially best-in-class PARP inhibitor in pivotal Phase 3 (EMBRACA) for BRCA-mutated Breast Cancer Phase 3 EMBRACA enrollment expected to be completed by Q4 2016 Pivotal studies in multiple other
indications (non-BRCA breast, prostate, lung, following regulatory interactions) targeted to begin in 2016 Pidilizumab (MDV9300) - Unpartnered anti-tumor, immune-activating antibody Potentially pivotal Phase 2 study in DLBCL MDV4463 - A Novel SREBP
Pathway Inhibitor in Phase 1

Enzalutamide Prostate Cancer
Opportunity
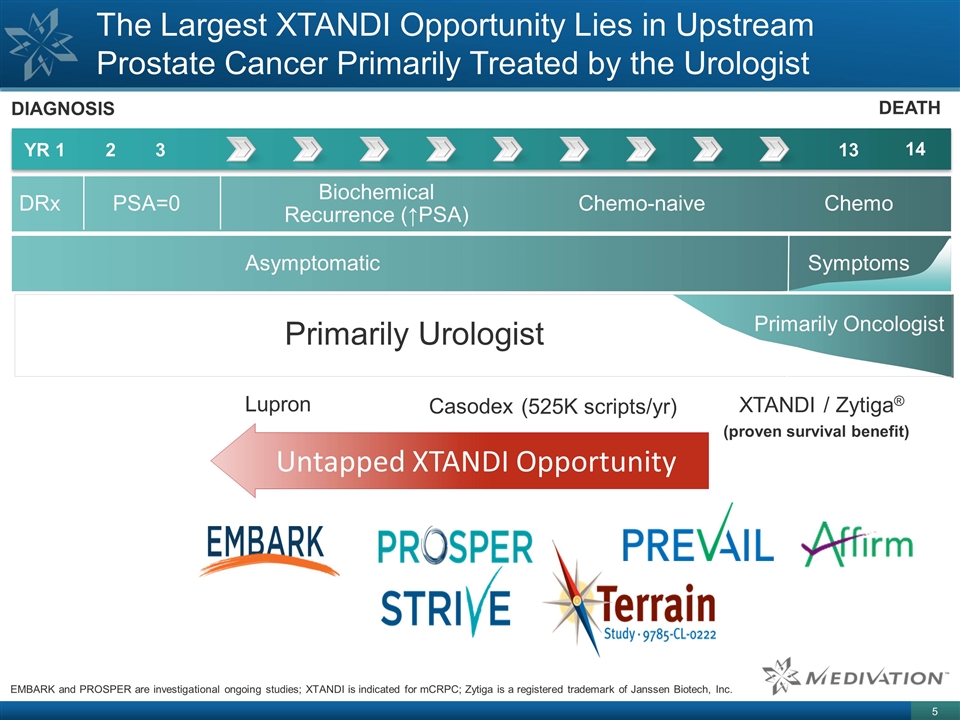
The Largest XTANDI Opportunity Lies in
Upstream Prostate Cancer Primarily Treated by the Urologist YR 1 2 14 DIAGNOSIS DEATH Primarily Oncologist Primarily Urologist DRx Chemo-naive Chemo PSA=0 Asymptomatic Symptoms 3 13 EMBARK and PROSPER are investigational ongoing studies; XTANDI is
indicated for mCRPC; Zytiga is a registered trademark of Janssen Biotech, Inc. Biochemical Recurrence (↑PSA) Lupron XTANDI / Zytiga® (proven survival benefit) Casodex Untapped XTANDI Opportunity (525K scripts/yr)
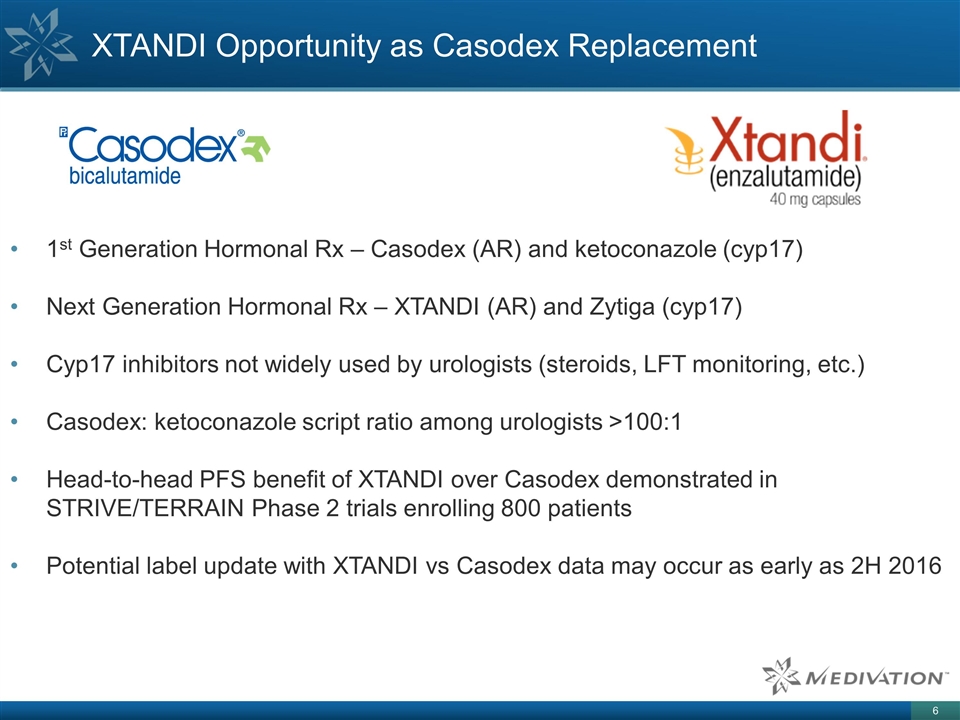
XTANDI Opportunity as Casodex
Replacement 1st Generation Hormonal Rx – Casodex (AR) and ketoconazole (cyp17) Next Generation Hormonal Rx – XTANDI (AR) and Zytiga (cyp17) Cyp17 inhibitors not widely used by urologists (steroids, LFT monitoring, etc.) Casodex:
ketoconazole script ratio among urologists >100:1 Head-to-head PFS benefit of XTANDI over Casodex demonstrated in STRIVE/TERRAIN Phase 2 trials enrolling 800 patients Potential label update with XTANDI vs Casodex data may occur as early as 2H
2016
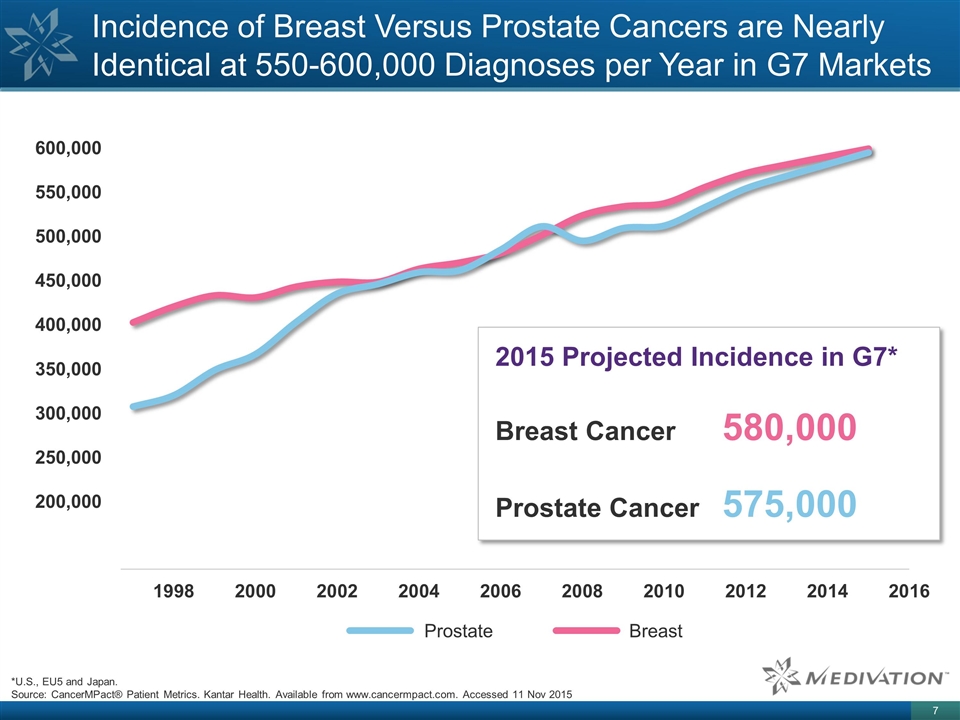
Incidence of Breast Versus Prostate
Cancers are Nearly Identical at 550-600,000 Diagnoses per Year in G7 Markets *U.S., EU5 and Japan. Source: CancerMPact® Patient Metrics. Kantar Health. Available from www.cancermpact.com. Accessed 11 Nov 2015 2015 Projected Incidence in G7*
Breast Cancer 580,000 Prostate Cancer 575,000 600,000 550,000 500,000 450,000 400,000 350,000 300,000 250,000 200,000 Prostate Breast
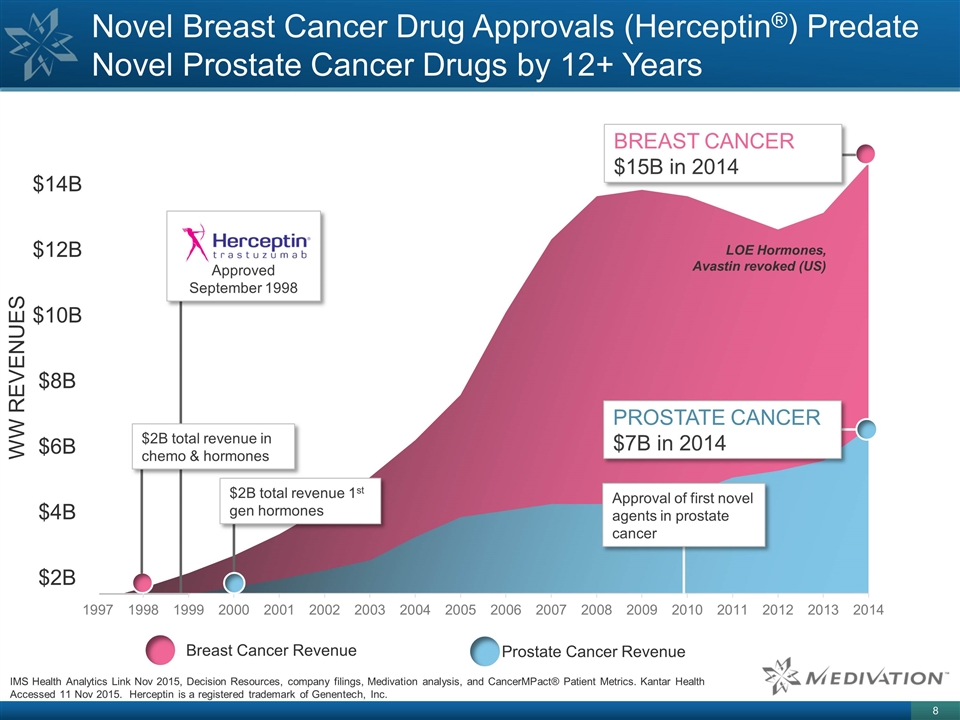
Novel Breast Cancer Drug Approvals
(Herceptin®) Predate Novel Prostate Cancer Drugs by 12+ Years $14B $12B $10B $8B $6B $4B $2B WW REVENUES Approved September 1998 $2B total revenue in chemo & hormones Approval of first novel agents in prostate cancer $2B total revenue 1st
gen hormones Prostate Cancer Revenue Breast Cancer Revenue PROSTATE CANCER $7B in 2014 BREAST CANCER $15B in 2014 LOE Hormones, Avastin revoked (US) IMS Health Analytics Link Nov 2015, Decision Resources, company filings, Medivation analysis, and
CancerMPact® Patient Metrics. Kantar Health Accessed 11 Nov 2015. Herceptin is a registered trademark of Genentech, Inc.
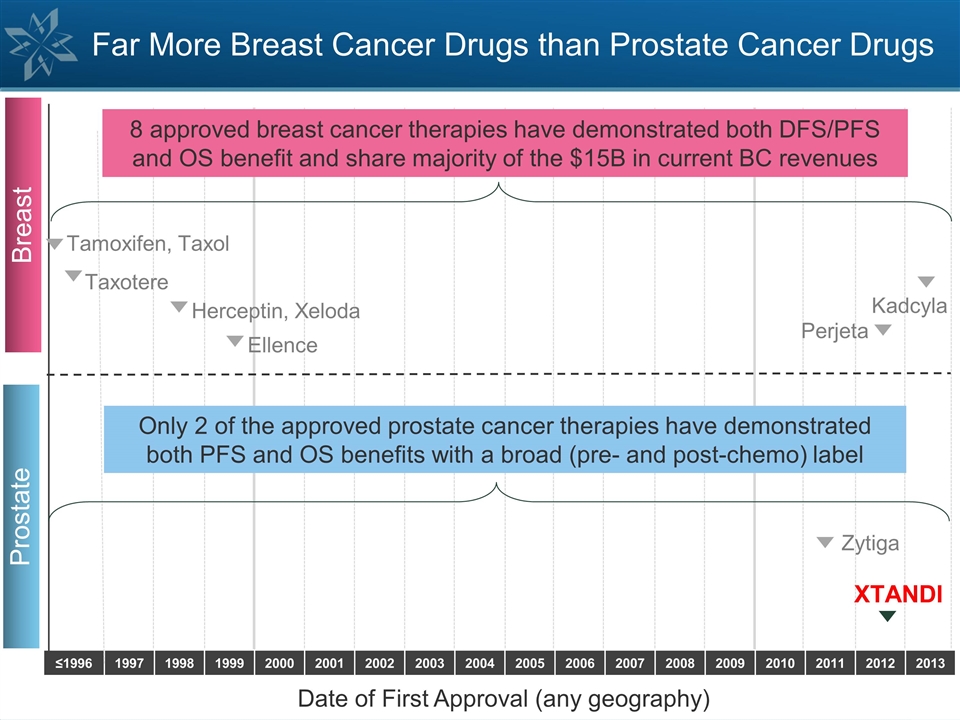
≤1996 1997 1998 1999 2000 2001
2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 Far More Breast Cancer Drugs than Prostate Cancer Drugs Prostate Zytiga XTANDI Only 2 of the approved prostate cancer therapies have demonstrated both PFS and OS benefits with a broad (pre-
and post-chemo) label Date of First Approval (any geography) Breast Perjeta Herceptin, Xeloda Ellence Kadcyla Tamoxifen, Taxol Taxotere 8 approved breast cancer therapies have demonstrated both DFS/PFS and OS benefit and share majority of the $15B
in current BC revenues

Enzalutamide Breast Cancer
Opportunity
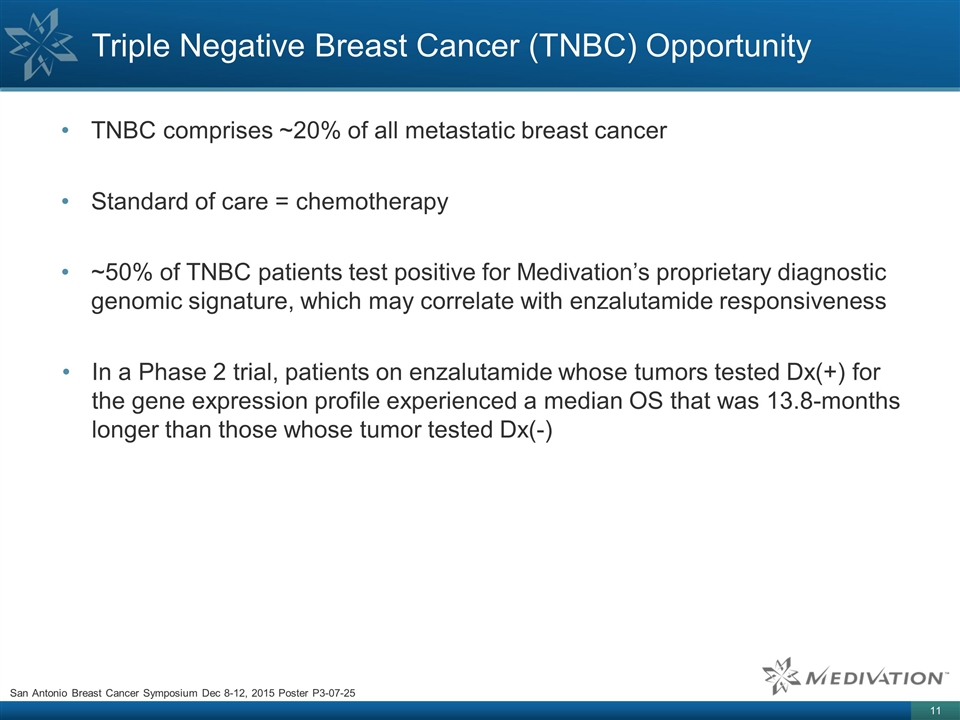
TNBC comprises ~20% of all
metastatic breast cancer Standard of care = chemotherapy ~50% of TNBC patients test positive for Medivation’s proprietary diagnostic genomic signature, which may correlate with enzalutamide responsiveness In a Phase 2 trial, patients on
enzalutamide whose tumors tested Dx(+) for the gene expression profile experienced a median OS that was 13.8-months longer than those whose tumor tested Dx(-) Triple Negative Breast Cancer (TNBC) Opportunity San Antonio Breast Cancer Symposium Dec
8-12, 2015 Poster P3-07-25
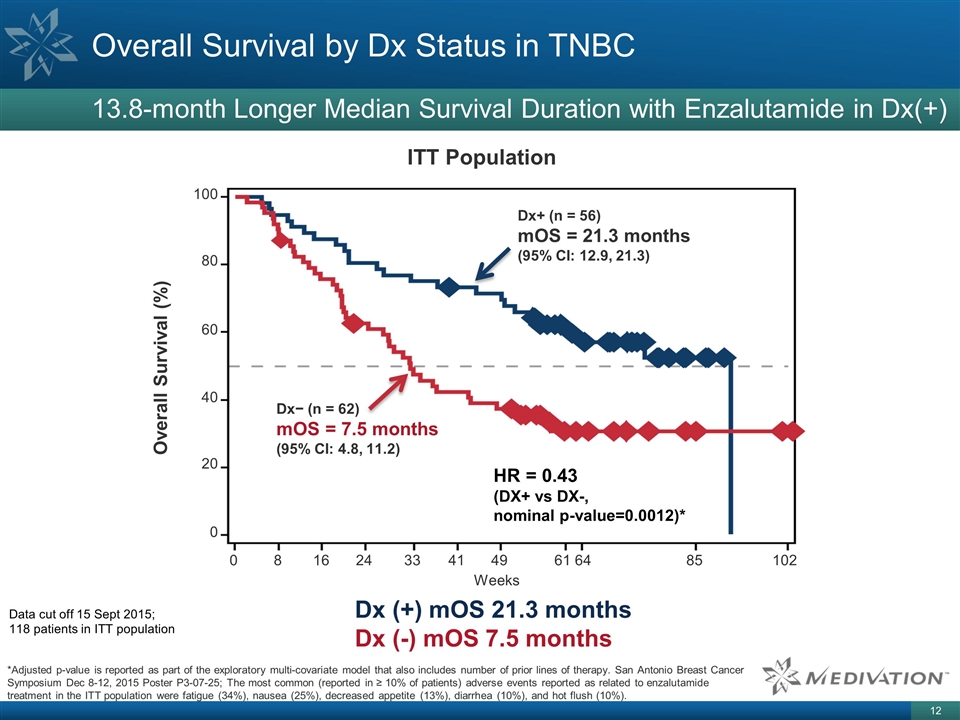
Overall Survival by Dx Status in
TNBC 13.8-month Longer Median Survival Duration with Enzalutamide in Dx(+) Data cut off 15 Sept 2015; 118 patients in ITT population Dx (+) mOS 21.3 months Dx (-) mOS 7.5 months *Adjusted p-value is reported as part of the exploratory
multi-covariate model that also includes number of prior lines of therapy. San Antonio Breast Cancer Symposium Dec 8-12, 2015 Poster P3-07-25; The most common (reported in ≥ 10% of patients) adverse events reported as related to enzalutamide
treatment in the ITT population were fatigue (34%), nausea (25%), decreased appetite (13%), diarrhea (10%), and hot flush (10%). ITT Population 100 80 60 40 20 0 Overall Survival (%) Weeks 0 8 16 24 33 41 49 61 64 102 85 Dx+ (n = 56) mOS = 21.3
months (95% CI: 12.9, 21.3) Dx− (n = 62) mOS = 7.5 months (95% CI: 4.8, 11.2) HR = 0.43 (DX+ vs DX-, nominal p-value=0.0012)*
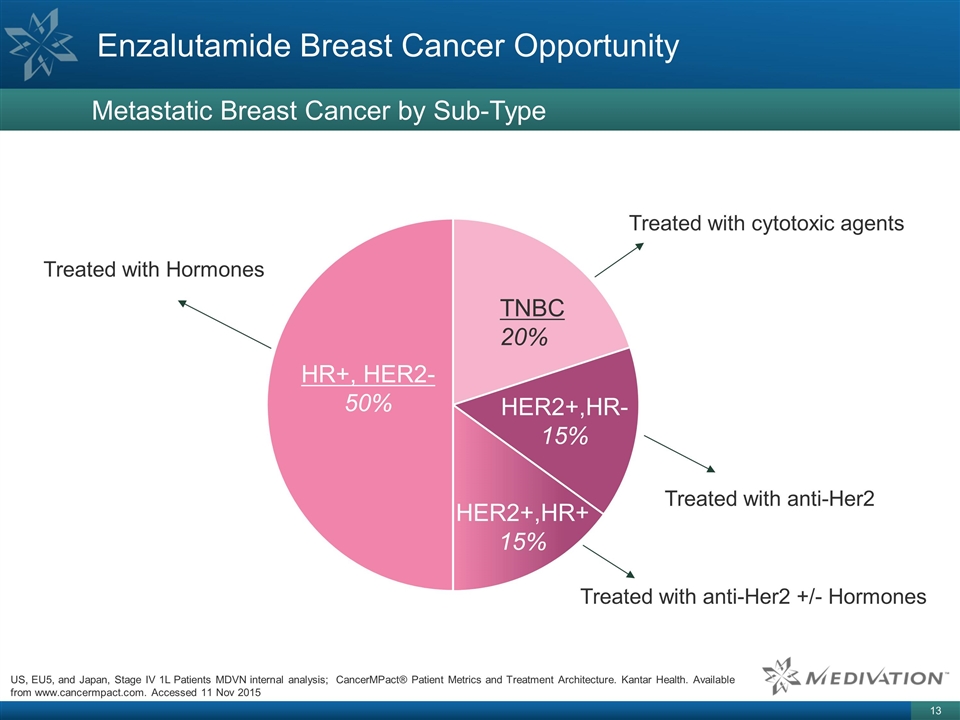
Enzalutamide Breast Cancer
Opportunity Metastatic Breast Cancer by Sub-Type HR+, HER2- 50% HER2+,HR- 15% TNBC 20% 90% urologists US, EU5, and Japan, Stage IV 1L Patients MDVN internal analysis; CancerMPact® Patient Metrics and Treatment Architecture. Kantar Health.
Available from www.cancermpact.com. Accessed 11 Nov 2015 Treated with Hormones Treated with cytotoxic agents Treated with anti-Her2 HER2+,HR+ 15% Treated with anti-Her2 +/- Hormones
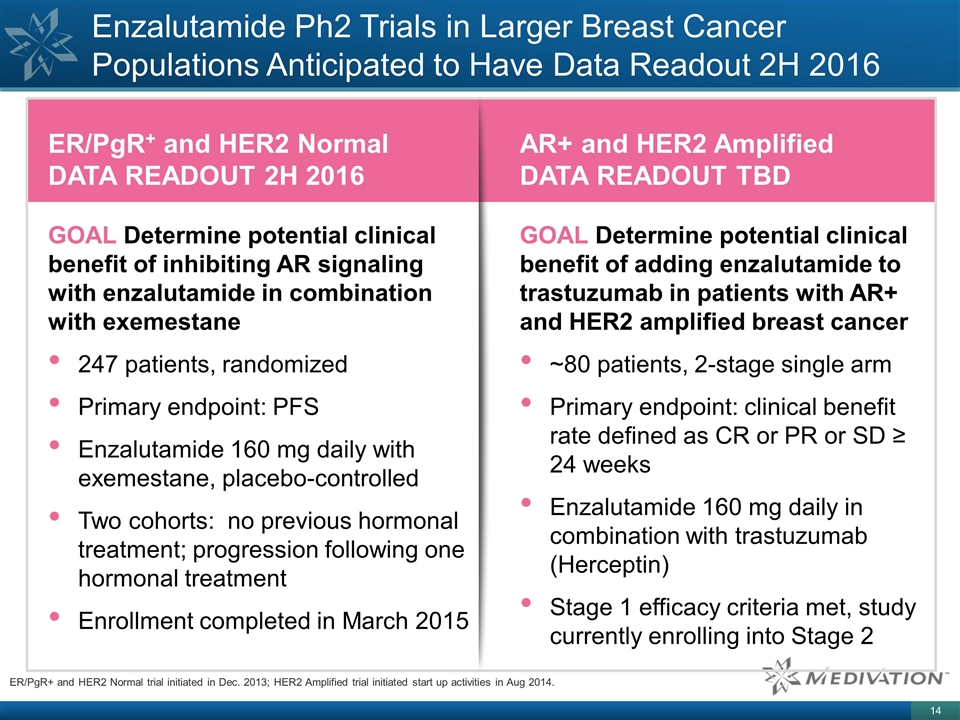
Enzalutamide Ph2 Trials in Larger
Breast Cancer Populations Anticipated to Have Data Readout 2H 2016 ER/PgR+ and HER2 Normal trial initiated in Dec. 2013; HER2 Amplified trial initiated start up activities in Aug 2014. ER/PgR+ and HER2 Normal DATA READOUT 2H 2016 GOAL Determine
potential clinical benefit of inhibiting AR signaling with enzalutamide in combination with exemestane 247 patients, randomized Primary endpoint: PFS Enzalutamide 160 mg daily with exemestane, placebo-controlled Two cohorts: no previous hormonal
treatment; progression following one hormonal treatment Enrollment completed in March 2015 AR+ and HER2 Amplified DATA READOUT TBD GOAL Determine potential clinical benefit of adding enzalutamide to trastuzumab in patients with AR+ and HER2
amplified breast cancer ~80 patients, 2-stage single arm Primary endpoint: clinical benefit rate defined as CR or PR or SD ≥ 24 weeks Enzalutamide 160 mg daily in combination with trastuzumab (Herceptin) Stage 1 efficacy criteria met, study
currently enrolling into Stage 2

Enzalutamide Opportunity in Other
Areas
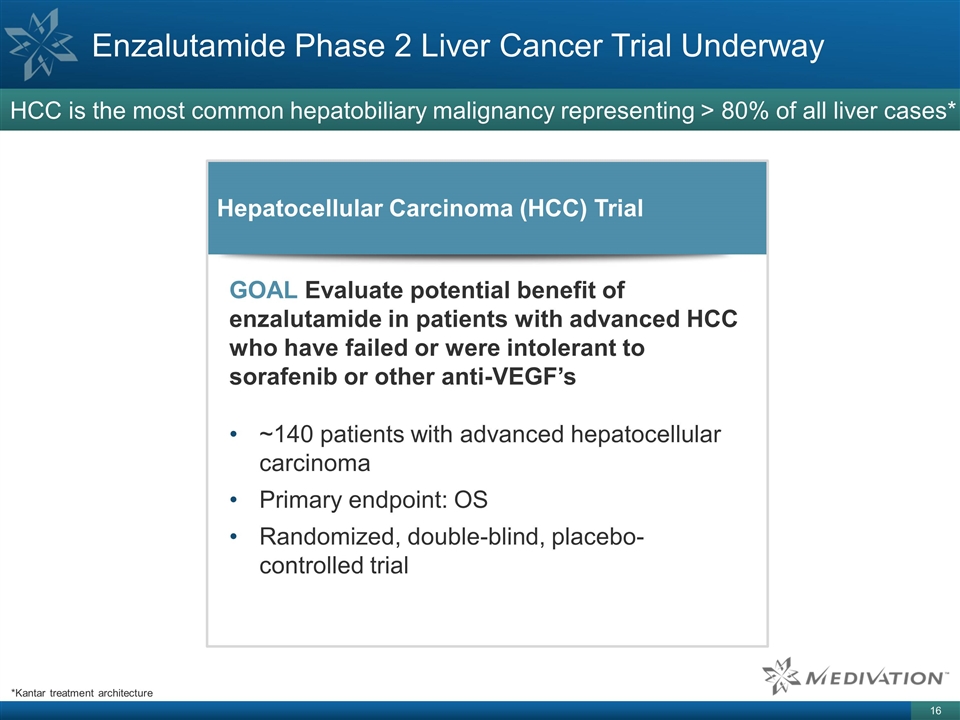
Enzalutamide Phase 2 Liver Cancer
Trial Underway HCC is the most common hepatobiliary malignancy representing > 80% of all liver cases* *Kantar treatment architecture GOAL Evaluate potential benefit of enzalutamide in patients with advanced HCC who have failed or were intolerant
to sorafenib or other anti-VEGF’s ~140 patients with advanced hepatocellular carcinoma Primary endpoint: OS Randomized, double-blind, placebo-controlled trial Hepatocellular Carcinoma (HCC) Trial
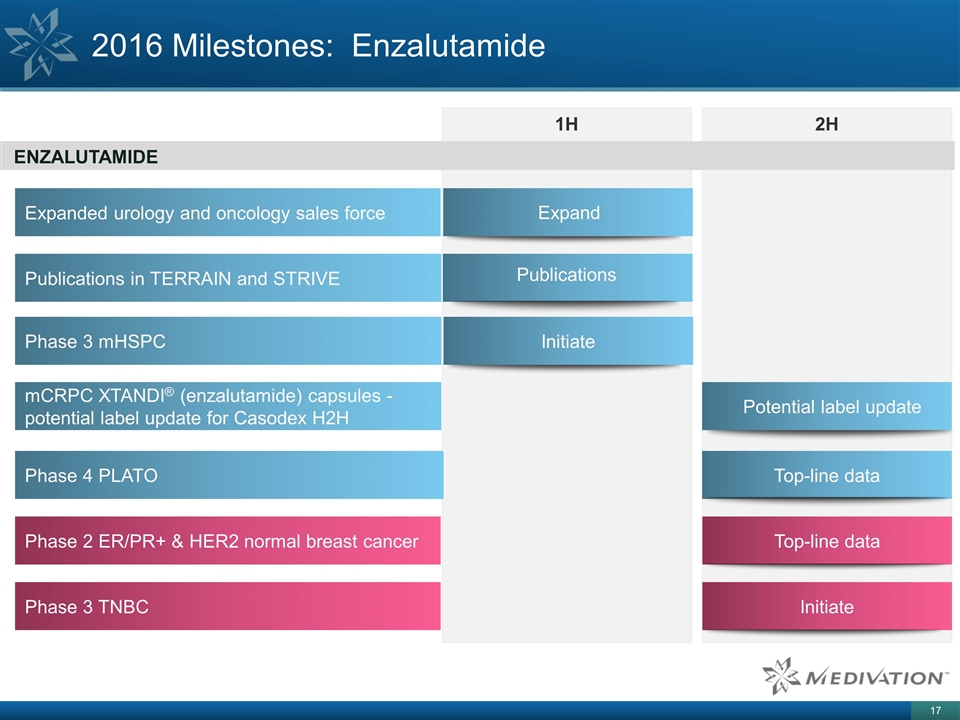
2016 Milestones: Enzalutamide 1H 2H
ENZALUTAMIDE Phase 3 mHSPC Initiate Phase 4 PLATO Top-line data Phase 3 TNBC Initiate Phase 2 ER/PR+ & HER2 normal breast cancer Top-line data Publications in TERRAIN and STRIVE Publications Expanded urology and oncology sales force Expand mCRPC
XTANDI® (enzalutamide) capsules - potential label update for Casodex H2H Potential label update

Talazoparib (MDV3800) Opportunity
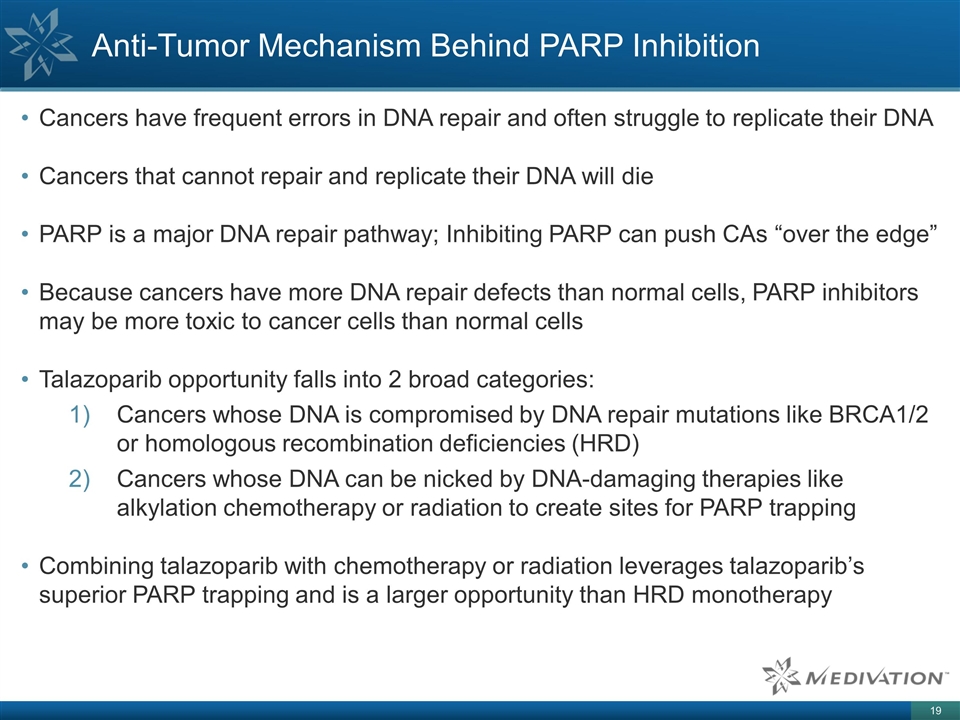
Cancers have frequent errors in DNA
repair and often struggle to replicate their DNA Cancers that cannot repair and replicate their DNA will die PARP is a major DNA repair pathway; Inhibiting PARP can push CAs “over the edge” Because cancers have more DNA repair defects
than normal cells, PARP inhibitors may be more toxic to cancer cells than normal cells Talazoparib opportunity falls into 2 broad categories: Cancers whose DNA is compromised by DNA repair mutations like BRCA1/2 or homologous recombination
deficiencies (HRD) Cancers whose DNA can be nicked by DNA-damaging therapies like alkylation chemotherapy or radiation to create sites for PARP trapping Combining talazoparib with chemotherapy or radiation leverages talazoparib’s superior PARP
trapping and is a larger opportunity than HRD monotherapy Anti-Tumor Mechanism Behind PARP Inhibition
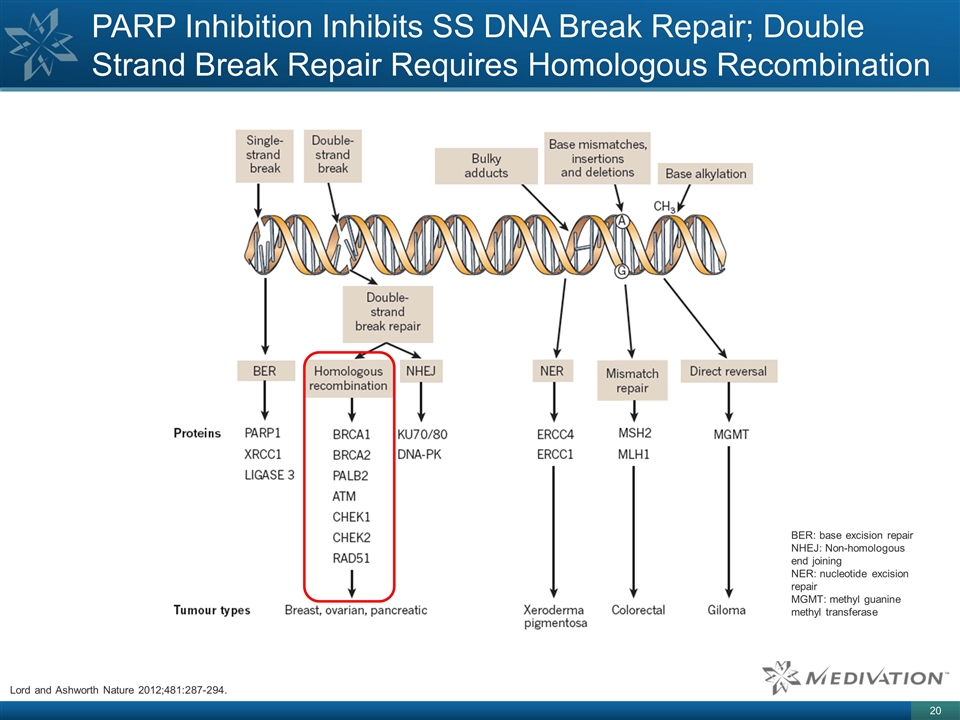
PARP Inhibition Inhibits SS DNA
Break Repair; Double Strand Break Repair Requires Homologous Recombination BER: base excision repair NHEJ: Non-homologous end joining NER: nucleotide excision repair MGMT: methyl guanine methyl transferase Lord and Ashworth Nature 2012;481:287-294.
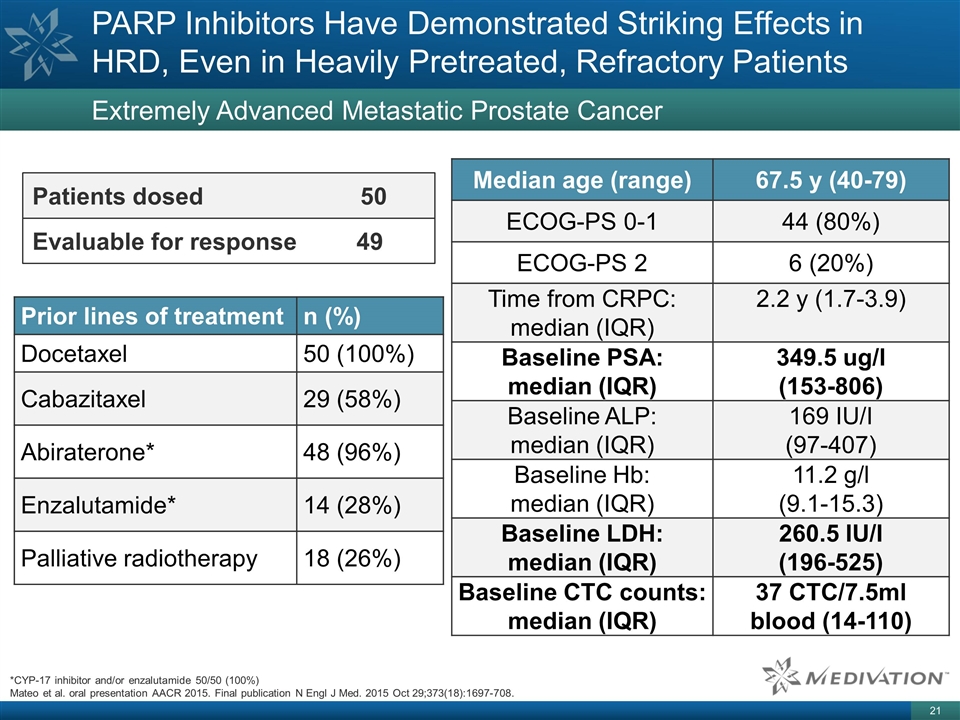
PARP Inhibitors Have Demonstrated
Striking Effects in HRD, Even in Heavily Pretreated, Refractory Patients Extremely Advanced Metastatic Prostate Cancer Patients dosed 50 Evaluable for response 49 Prior lines of treatment n (%) Docetaxel 50 (100%) Cabazitaxel 29 (58%) Abiraterone*
48 (96%) Enzalutamide* 14 (28%) Palliative radiotherapy 18 (26%) Median age (range) 67.5 y (40-79) ECOG-PS 0-1 44 (80%) ECOG-PS 2 6 (20%) Time from CRPC: median (IQR) 2.2 y (1.7-3.9) Baseline PSA: median (IQR) 349.5 ug/l (153-806) Baseline ALP:
median (IQR) 169 IU/I (97-407) Baseline Hb: median (IQR) 11.2 g/l (9.1-15.3) Baseline LDH: median (IQR) 260.5 IU/I (196-525) Baseline CTC counts: median (IQR) 37 CTC/7.5ml blood (14-110) *CYP-17 inhibitor and/or enzalutamide 50/50 (100%) Mateo et
al. oral presentation AACR 2015. Final publication N Engl J Med. 2015 Oct 29;373(18):1697-708.
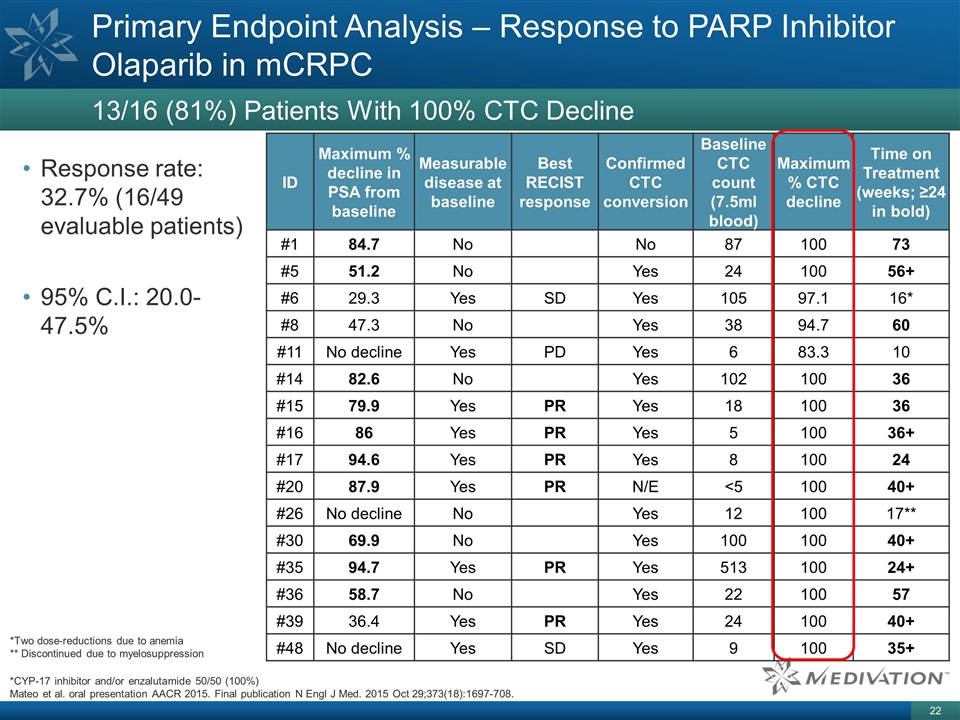
ID Maximum % decline in PSA from
baseline Measurable disease at baseline Best RECIST response Confirmed CTC conversion Baseline CTC count (7.5ml blood) Maximum % CTC decline Time on Treatment (weeks; ≥24 in bold) #1 84.7 No No 87 100 73 #5 51.2 No Yes 24 100 56+ #6 29.3 Yes
SD Yes 105 97.1 16* #8 47.3 No Yes 38 94.7 60 #11 No decline Yes PD Yes 6 83.3 10 #14 82.6 No Yes 102 100 36 #15 79.9 Yes PR Yes 18 100 36 #16 86 Yes PR Yes 5 100 36+ #17 94.6 Yes PR Yes 8 100 24 #20 87.9 Yes PR N/E <5 100 40+ #26 No decline No
Yes 12 100 17** #30 69.9 No Yes 100 100 40+ #35 94.7 Yes PR Yes 513 100 24+ #36 58.7 No Yes 22 100 57 #39 36.4 Yes PR Yes 24 100 40+ #48 No decline Yes SD Yes 9 100 35+ Response rate: 32.7% (16/49 evaluable patients) 95% C.I.: 20.0-47.5% Primary
Endpoint Analysis – Response to PARP Inhibitor Olaparib in mCRPC 13/16 (81%) Patients With 100% CTC Decline *Two dose-reductions due to anemia ** Discontinued due to myelosuppression *CYP-17 inhibitor and/or enzalutamide 50/50 (100%) Mateo et
al. oral presentation AACR 2015. Final publication N Engl J Med. 2015 Oct 29;373(18):1697-708.
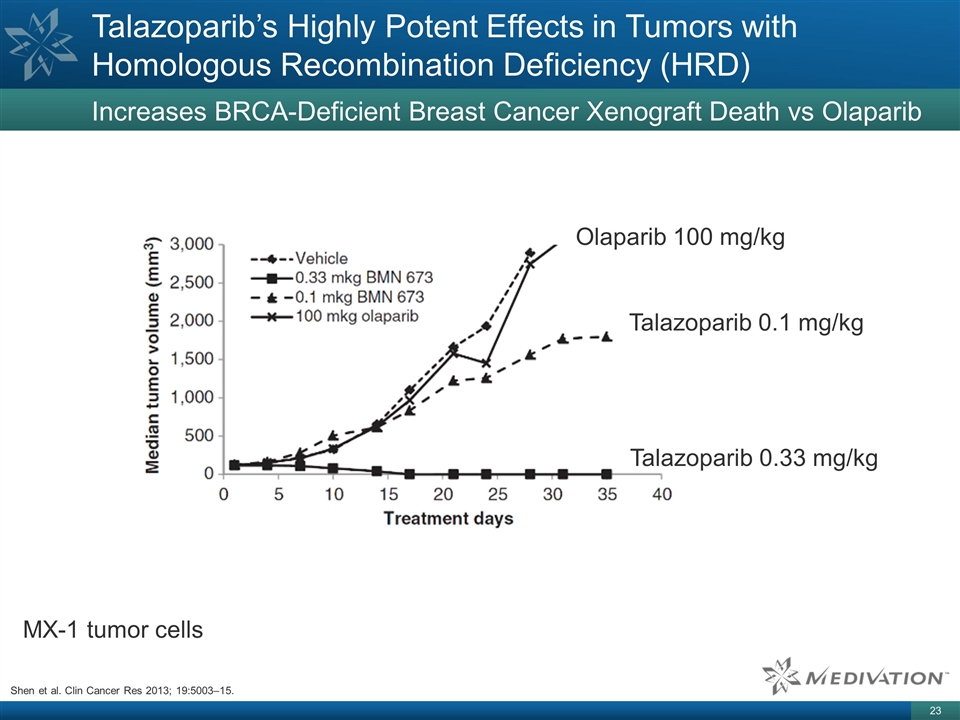
Talazoparib’s Highly Potent
Effects in Tumors with Homologous Recombination Deficiency (HRD) Increases BRCA-Deficient Breast Cancer Xenograft Death vs Olaparib MX-1 tumor cells Talazoparib 0.1 mg/kg Talazoparib 0.33 mg/kg Olaparib 100 mg/kg Shen et al. Clin Cancer Res 2013;
19:5003–15.
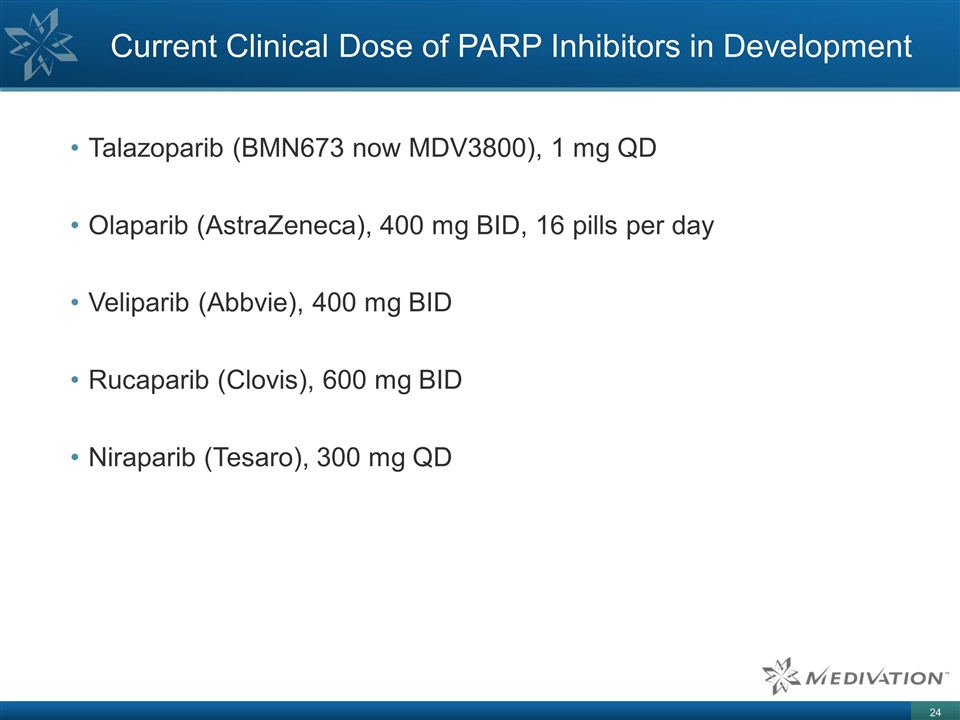
Current Clinical Dose of PARP
Inhibitors in Development Talazoparib (BMN673 now MDV3800), 1 mg QD Olaparib (AstraZeneca), 400 mg BID, 16 pills per day Veliparib (Abbvie), 400 mg BID Rucaparib (Clovis), 600 mg BID Niraparib (Tesaro), 300 mg QD 24
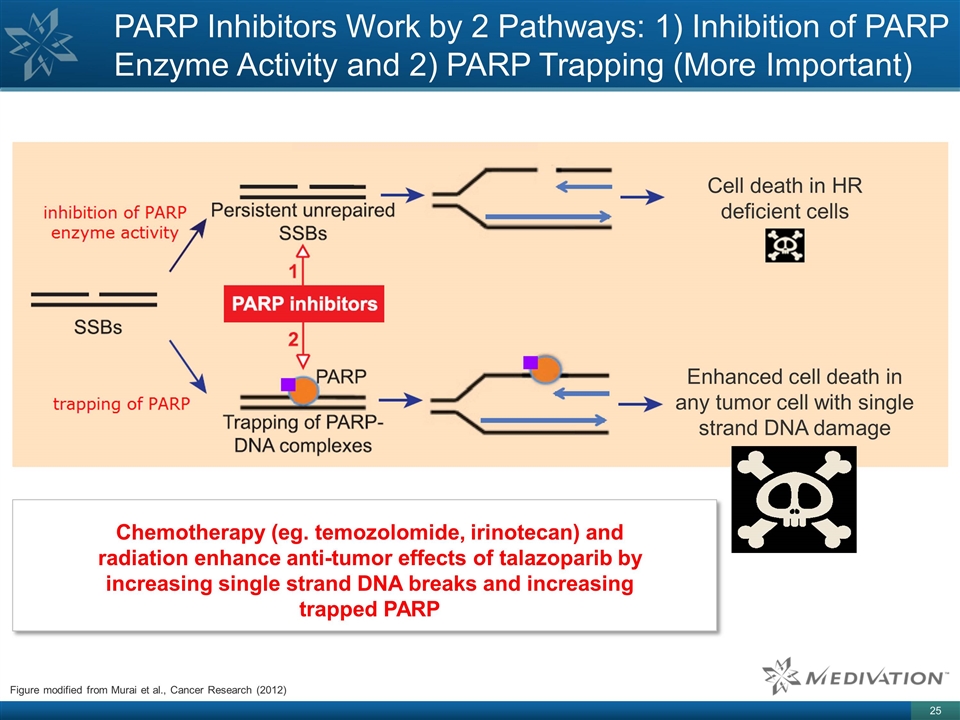
Figure modified from Murai et al.,
Cancer Research (2012) PARP Inhibitors Work by 2 Pathways: 1) Inhibition of PARP Enzyme Activity and 2) PARP Trapping (More Important) Cell death in HR deficient cells Enhanced cell death in any tumor cell with single strand DNA damage Chemotherapy
(eg. temozolomide, irinotecan) and radiation enhance anti-tumor effects of talazoparib by increasing single strand DNA breaks and increasing trapped PARP
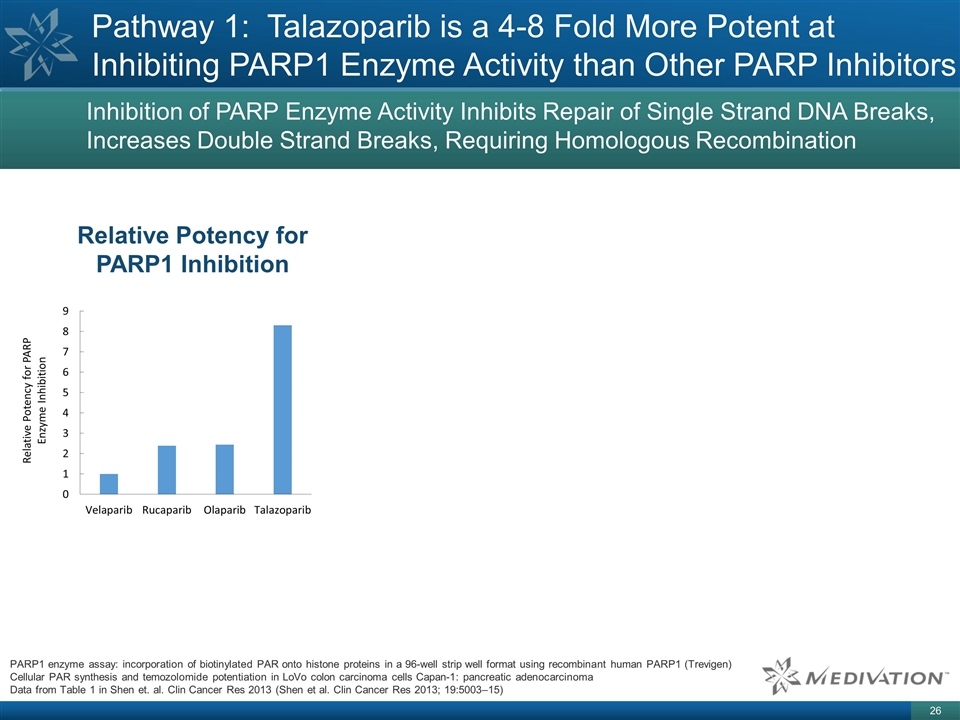
Pathway 1: Talazoparib is a 4-8 Fold
More Potent at Inhibiting PARP1 Enzyme Activity than Other PARP Inhibitors Relative Potency for PARP1 Inhibition PARP1 enzyme assay: incorporation of biotinylated PAR onto histone proteins in a 96-well strip well format using recombinant human PARP1
(Trevigen) Cellular PAR synthesis and temozolomide potentiation in LoVo colon carcinoma cells Capan-1: pancreatic adenocarcinoma Data from Table 1 in Shen et. al. Clin Cancer Res 2013 (Shen et al. Clin Cancer Res 2013; 19:5003–15) Inhibition
of PARP Enzyme Activity Inhibits Repair of Single Strand DNA Breaks, Increases Double Strand Breaks, Requiring Homologous Recombination
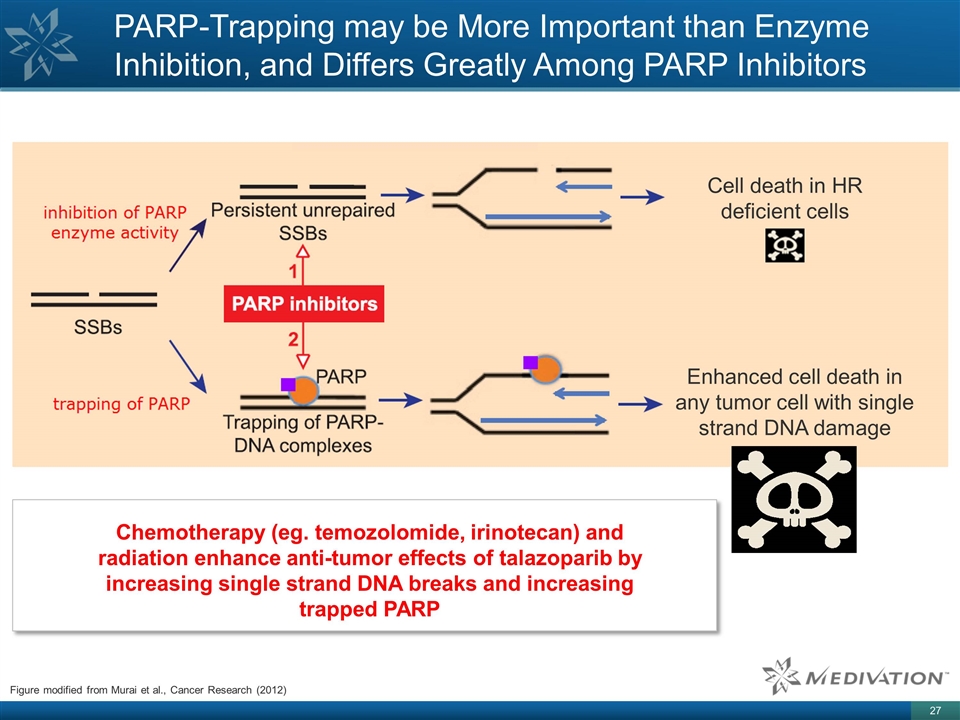
Figure modified from Murai et al.,
Cancer Research (2012) PARP-Trapping may be More Important than Enzyme Inhibition, and Differs Greatly Among PARP Inhibitors Cell death in HR deficient cells Enhanced cell death in any tumor cell with single strand DNA damage Chemotherapy (eg.
temozolomide, irinotecan) and radiation enhance anti-tumor effects of talazoparib by increasing single strand DNA breaks and increasing trapped PARP
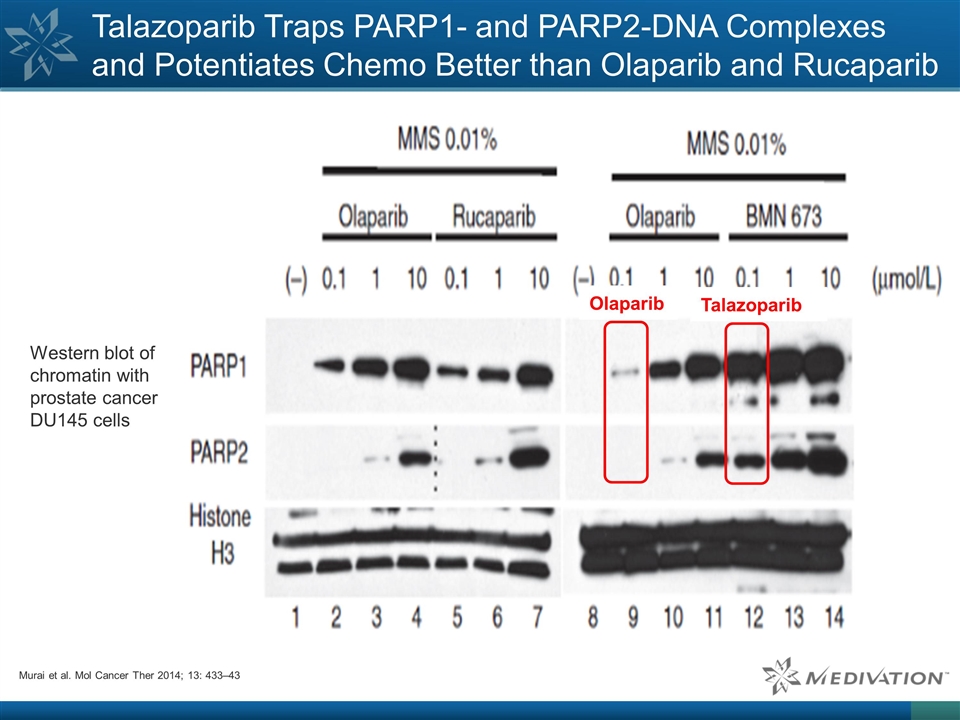
Talazoparib Traps PARP1- and
PARP2-DNA Complexes and Potentiates Chemo Better than Olaparib and Rucaparib Western blot of chromatin with prostate cancer DU145 cells Murai et al. Mol Cancer Ther 2014; 13: 433–43 Talazoparib Olaparib
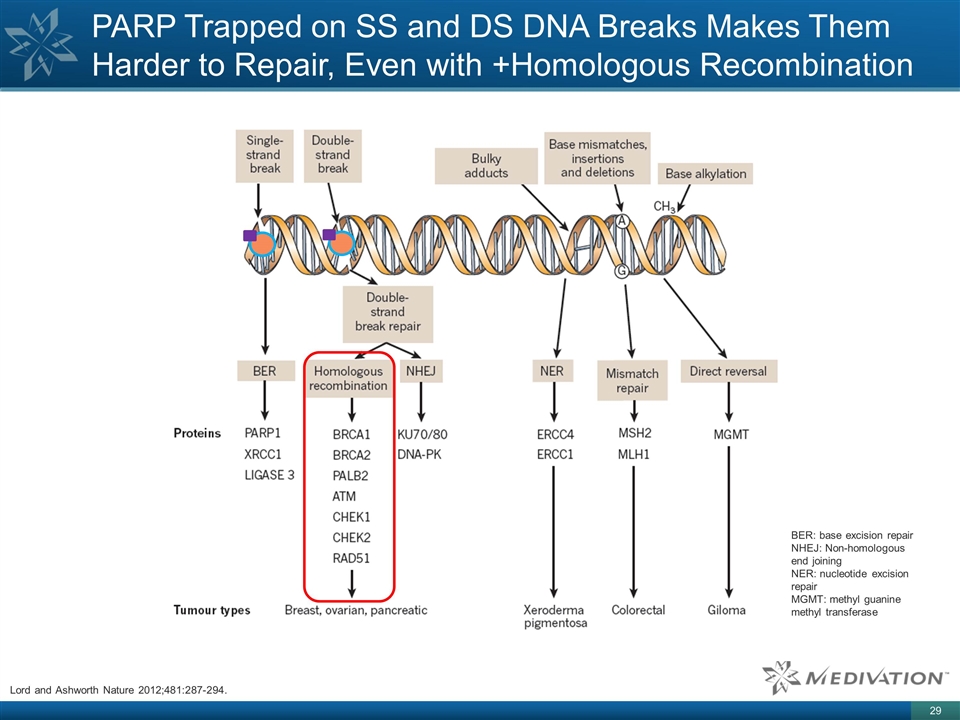
PARP Trapped on SS and DS DNA Breaks
Makes Them Harder to Repair, Even with +Homologous Recombination BER: base excision repair NHEJ: Non-homologous end joining NER: nucleotide excision repair MGMT: methyl guanine methyl transferase Lord and Ashworth Nature 2012;481:287-294.
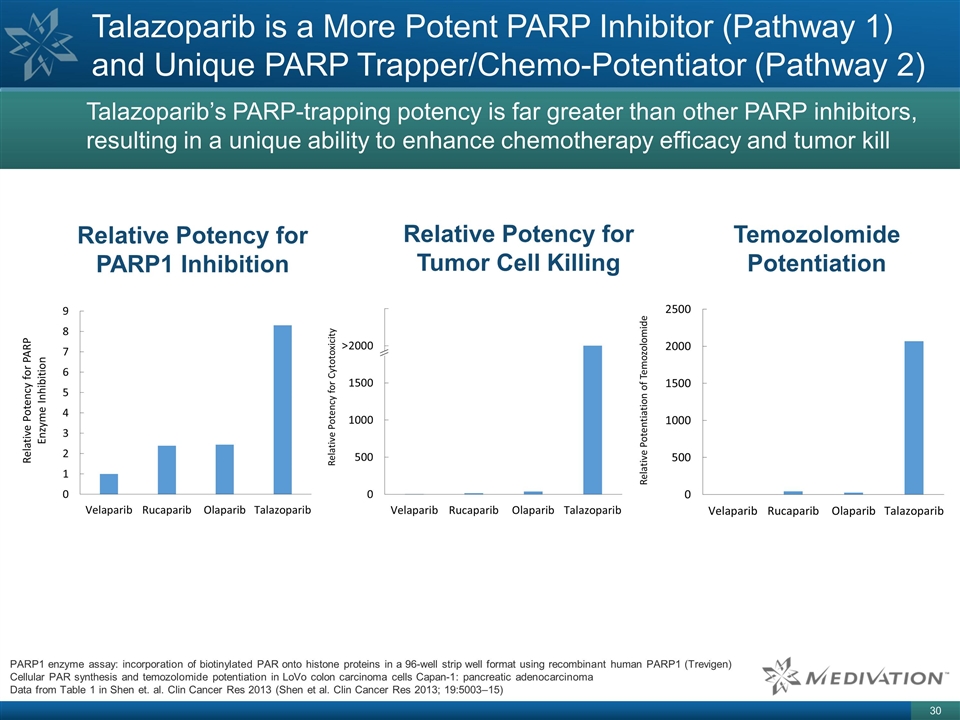
Talazoparib is a More Potent PARP
Inhibitor (Pathway 1) and Unique PARP Trapper/Chemo-Potentiator (Pathway 2) Relative Potency for PARP1 Inhibition Relative Potency for Tumor Cell Killing Temozolomide Potentiation PARP1 enzyme assay: incorporation of biotinylated PAR onto histone
proteins in a 96-well strip well format using recombinant human PARP1 (Trevigen) Cellular PAR synthesis and temozolomide potentiation in LoVo colon carcinoma cells Capan-1: pancreatic adenocarcinoma Data from Table 1 in Shen et. al. Clin Cancer Res
2013 (Shen et al. Clin Cancer Res 2013; 19:5003–15) Talazoparib’s PARP-trapping potency is far greater than other PARP inhibitors, resulting in a unique ability to enhance chemotherapy efficacy and tumor kill
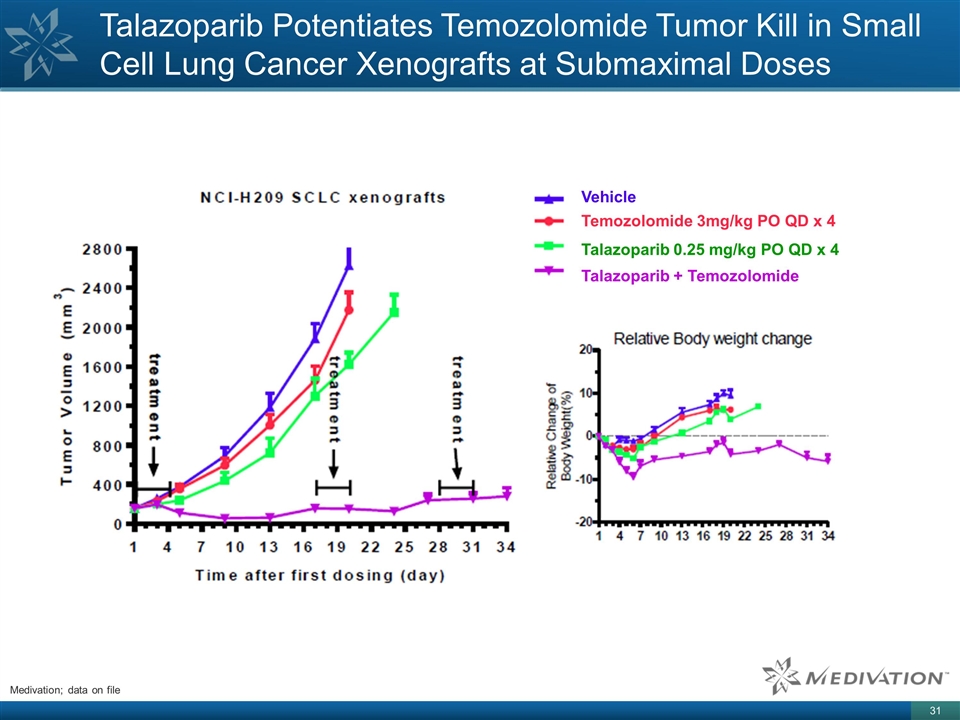
Talazoparib Potentiates Temozolomide
Tumor Kill in Small Cell Lung Cancer Xenografts at Submaximal Doses Vehicle Temozolomide 3mg/kg PO QD x 4 Talazoparib 0.25 mg/kg PO QD x 4 Talazoparib + Temozolomide Medivation; data on file
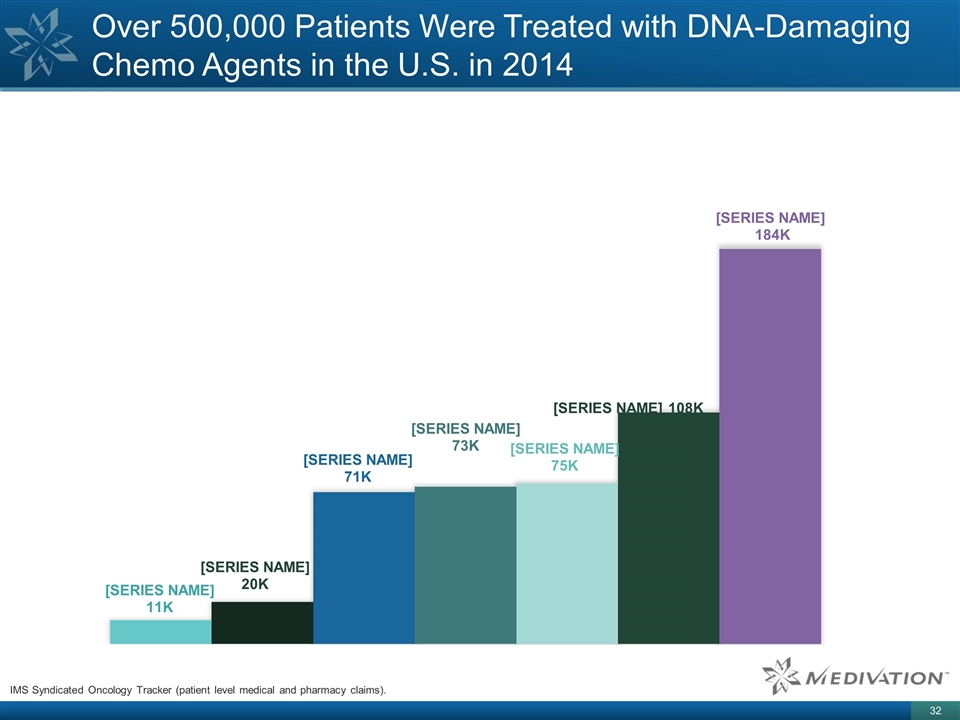
Over 500,000 Patients Were Treated
with DNA-Damaging Chemo Agents in the U.S. in 2014 IMS Syndicated Oncology Tracker (patient level medical and pharmacy claims).
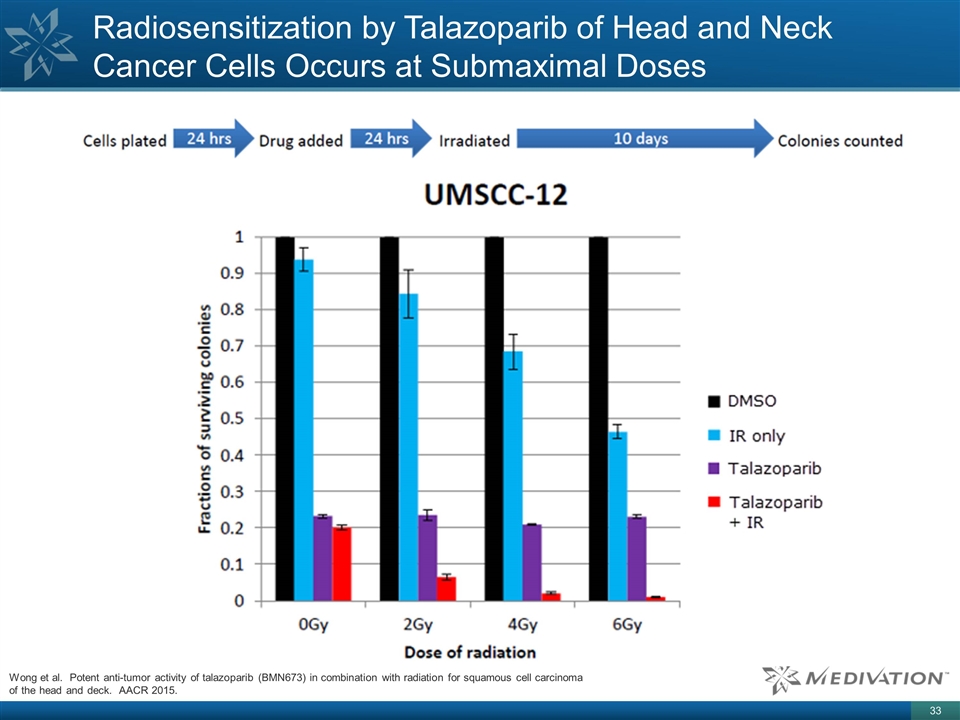
Radiosensitization by Talazoparib of
Head and Neck Cancer Cells Occurs at Submaximal Doses Wong et al. Potent anti-tumor activity of talazoparib (BMN673) in combination with radiation for squamous cell carcinoma of the head and deck. AACR 2015.
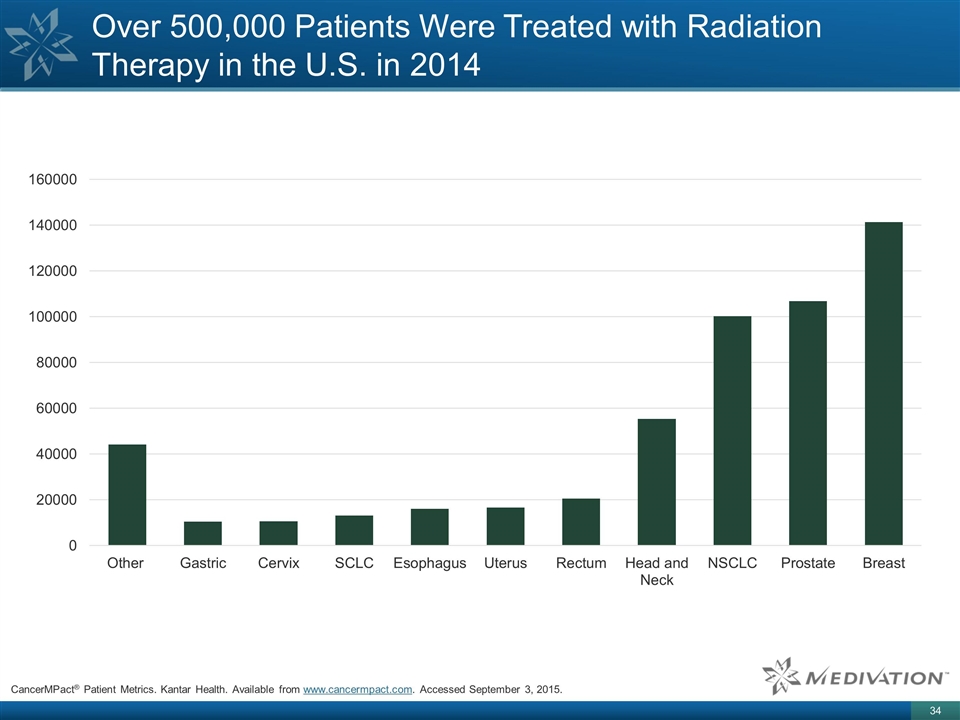
Over 500,000 Patients Were Treated
with Radiation Therapy in the U.S. in 2014 CancerMPact® Patient Metrics. Kantar Health. Available from www.cancermpact.com. Accessed September 3, 2015.

Non-Specific Off-Target Cell
Toxicity is Lower with Talazoparib than with Olaparib and Rucaparib Murai et al. Mol Cancer Ther 2014; 13: 433–43 DT40: PARP1-/- avian B lymphoblast cells are equivalent to PARP1 and PARP2 double knockout cells and do not have detectable
levels of PARP Talazoparib
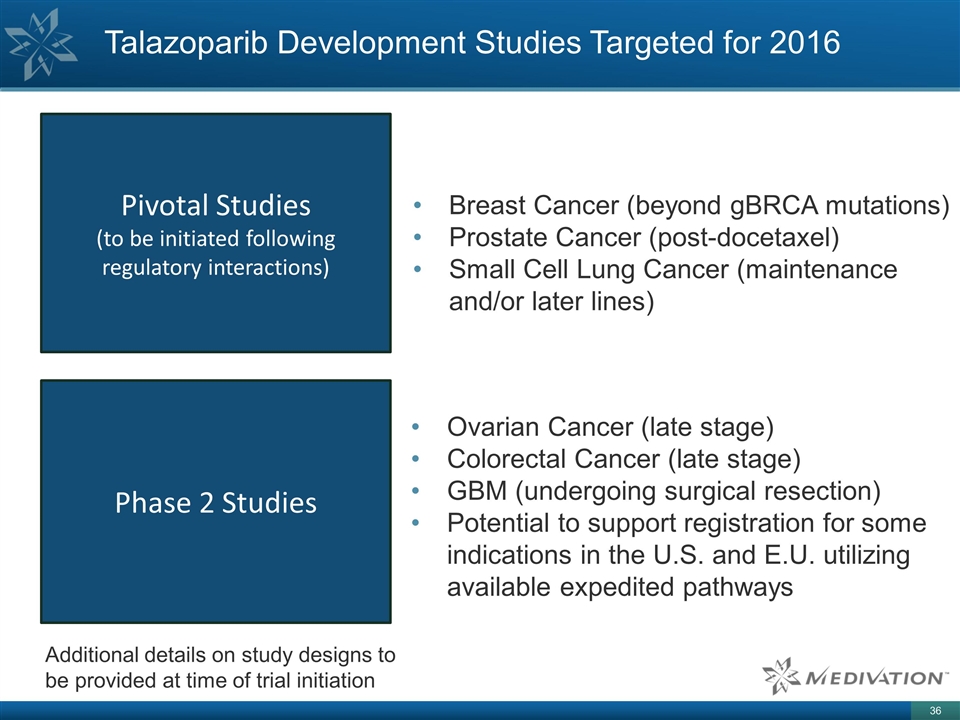
Talazoparib Development Studies
Targeted for 2016 Breast Cancer (beyond gBRCA mutations) Prostate Cancer (post-docetaxel) Small Cell Lung Cancer (maintenance and/or later lines) Additional details on study designs to be provided at time of trial initiation Ovarian Cancer (late
stage) Colorectal Cancer (late stage) GBM (undergoing surgical resection) Potential to support registration for some indications in the U.S. and E.U. utilizing available expedited pathways Pivotal Studies (to be initiated following regulatory
interactions) Phase 2 Studies
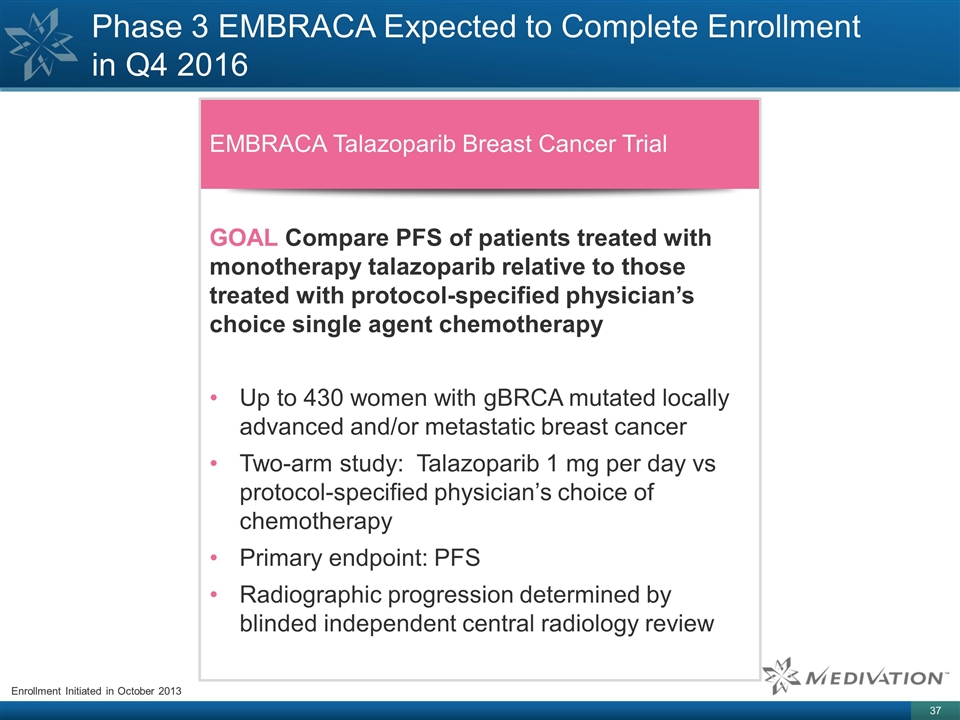
Phase 3 EMBRACA Expected to Complete
Enrollment in Q4 2016 Enrollment Initiated in October 2013 EMBRACA Talazoparib Breast Cancer Trial GOAL Compare PFS of patients treated with monotherapy talazoparib relative to those treated with protocol-specified physician’s choice single
agent chemotherapy Up to 430 women with gBRCA mutated locally advanced and/or metastatic breast cancer Two-arm study: Talazoparib 1 mg per day vs protocol-specified physician’s choice of chemotherapy Primary endpoint: PFS Radiographic
progression determined by blinded independent central radiology review

Pidilizumab (MDV9300)
Opportunity
Exclusive WW rights from CureTech,
Ltd., in Q4 2014, for all potential indications Administered to several hundred patients and activity demonstrated in multiple Phase 2 trials (eg. 52% complete response rate in B-Cell Lymphoma) Pidilizumab effects on DLBCL published in Journal of
Clinical Oncology (Vol. 31, 4199-4206, 2013) was one of the most frequently cited publications of 2013 Generated vs lymphoblast cell line, not vs single purified recombinant PD1 protein Mechanism of action NOT via PD1 binding; activation of natural
killer (NK) cells described Potentially pivotal study in Diffuse Large B-Cell Lymphoma (DLBCL) Other hematological malignancies such as multiple myeloma being considered Pidilizumab (MDV9300) – Anti-Tumor, Immune-Activating Antibody Benson et
al. Blood 2010; 116:2286-2294
Pivotal Phase 2 Trial in Diffuse
Large B-Cell Lymphoma Pidilizumab DLBCL Trial GOAL Determine best overall response rate in patients with an incomplete response following salvage therapy or autologous stem cell transplantation ~180 patients Two parallel cohorts First cohort will
enroll patients who have received an autologous stem cell transplant Second cohort will enroll patients who have received salvage chemotherapy, but are transplant-ineligible Pidilizumab 200 mg by IV infusion Primary endpoint: Best overall response
rate
MDV4463 is a novel small molecule
inhibitor of the sterol regulatory element binding proteins (SREBP) pathway SREBPs are master regulators of cholesterol and lipid biosynthesis Modulates the expression of multiple metabolic proteins including, HMG-CoA reductase, the target of all
statins Preclinical studies demonstrated that MDV4463 lowered triglycerides, cholesterol, glucose, insulin and weight in animals. MDV4463 also reduced fat in the liver in animal models of Non-Alcoholic Hepatic Steatosis (NASH) Potential indications
being considered include NASH, hyperlipidemia, diabetes, obesity, metabolic syndrome Currently in a Phase 1 trial in healthy volunteers Medivation’s First Internal NCE – MDV4463

Why We Are Here—Graeme’s
Story
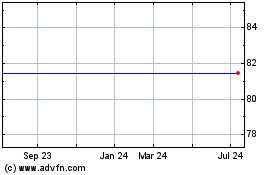
Medivation, Inc. (MM) (NASDAQ:MDVN)
Historical Stock Chart
From Mar 2024 to Apr 2024
Medivation, Inc. (MM) (NASDAQ:MDVN)
Historical Stock Chart
From Apr 2023 to Apr 2024