UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant
to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): January 22, 2016
Delcath Systems, Inc.
(Exact Name of Registrant Specified in Charter)
|
|
|
|
|
| Delaware |
|
001-16133 |
|
06-1245881 |
| (State or Other Jurisdiction
of Incorporation) |
|
(Commission
File Number) |
|
(I.R.S. Employer
Identification No.) |
|
|
|
| 1301 Avenue of the Americas, 43rd Floor
New York, New York |
|
|
|
10019 |
| (Address of Principal Executive Offices) |
|
|
|
(Zip Code) |
Registrant’s telephone number, including area code: (212) 489-2100
Not Applicable
(Former
Name or Former Address, if Changed Since Last Report)
Check the appropriate box below
if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| |
¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Item 7.01. |
Regulation FD Disclosure. |
A copy of Delcath Systems, Inc.’s updated investor presentation slides
that the Company intends to use effective immediately is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated into this Item 7.01 by reference.
The information disclosed under this Item 7.01, including Exhibit 99.1 hereto, is being furnished and shall not be deemed “filed” for
purposes of Section 18 of the Securities Exchange Act of 1934, as amended, nor shall it be incorporated by reference into any registration statement or other document pursuant to the Securities Act of 1933, as amended, except as expressly set
forth in such filing.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
|
|
|
Exhibit
No. |
|
Description of Exhibit |
|
|
| 99.1 |
|
Delcath Systems, Inc. Investor Presentation Slides |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by
the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
DELCATH SYSTEMS, INC. |
|
|
|
|
| Date: January 22, 2016 |
|
|
|
By: |
|
/s/ Jennifer K. Simpson |
|
|
|
|
|
|
Jennifer K. Simpson |
|
|
|
|
|
|
Director, President and Chief Executive Officer |
EXHIBIT INDEX
|
|
|
Exhibit
No. |
|
Description of Exhibit |
|
|
| 99.1 |
|
Delcath Systems, Inc. Investor Presentation Slides |
Investor Presentation (NASDAQ: DCTH)
January 2016 Exhibit 99.1

Forward-looking Statements This
presentation contains forward-looking statements, within the meaning of the federal securities laws, related to future events and future financial performance which include statements about our expectations, beliefs, plans, objectives, intentions,
goals, strategies, assumptions and other statements that are not historical facts. Forward-looking statements are subject to known and unknown risks and uncertainties and are based on potentially inaccurate assumptions, which could cause
actual results to differ materially from expected results, performance or achievements expressed or implied by statements made herein. Our actual results could differ materially from those anticipated in forward-looking statements for many reasons,
including, but not limited to, uncertainties relating to: the timing and results of future clinical trials including without limitation the OM, HCC, ICC, and mCRC trials in the Company’s Clinical Development Program, clinical adoption, use and
resulting sales, if any, for the CHEMOSAT system in Europe, our ability to obtain reimbursement for the CHEMOSAT system in various markets, including without limitation Germany and the United Kingdom and the impact on sales, if any, of reimbursement
in these markets including ZE reimbursement in the German market, our ability to successfully commercialize the Melphalan/HDS system and the potential of the Melphalan/HDS system as a treatment for patients with primary and metastatic disease in the
liver, the Company's ability to satisfy the requirements of the FDA's Complete Response Letter relating to the ocular melanoma indication and the timing of the same, approval of the Melphalan/HDS system by the U.S. FDA, acceptance of the Phase 3
trial publication, the impact of presentations and abstracts at major medical meetings and congresses (SSO, ASCO, CIRSE, ESMO, EADO, RSNA) and future clinical results consistent with the data presented, approval of the current or future
Melphalan/HDS system for delivery and filtration of melphalan or other chemotherapeutic agents for various indications in the U.S. and/or in foreign markets, actions by the FDA or other foreign regulatory agencies, our ability to successfully enter
into strategic partnership and distribution arrangements in foreign markets and the timing and revenue, if any, of the same, uncertainties relating to the timing and results of research and development projects, and uncertainties regarding our
ability to obtain financial and other resources for any clinical trials, research, development, and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission
including the section entitled ‘‘Risk Factors’’ in our most recent Annual Report on Form 10-K and our Reports on Form 10-Q and Form 8-K.
Delcath Systems Seeking to make a
clinically meaningful difference for patients with cancers of the liver Focused on treatment of primary/metastatic liver cancers Proprietary system delivers high-dose chemotherapy (melphalan) directly to the liver with extra-corporeal filtration to
limit systemic toxicity Late-stage clinical development in the US Currently Commercial Stage in the EU Pursuing orphan indications in metastatic ocular melanoma (OM), hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma
(ICC)
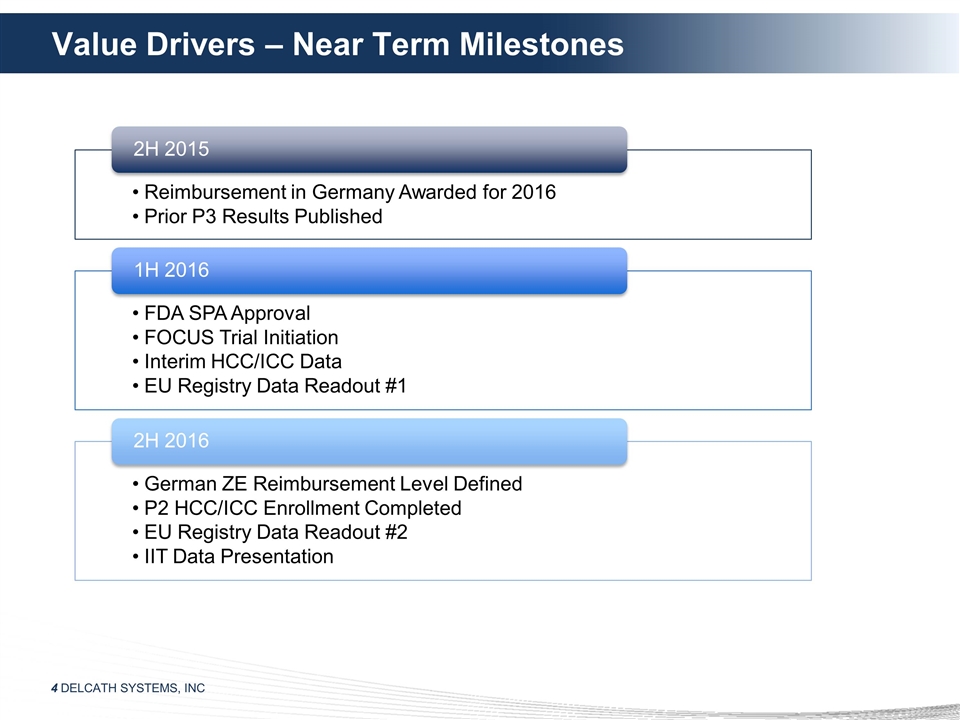
Value Drivers – Near Term
Milestones 2H 2015 Reimbursement in Germany Awarded for 2016 Prior P3 Results Published 1H 2016 FDA SPA Approval FOCUS Trial Initiation 2H 2016 EU Registry Data Readout #2 IIT Data Presentation Interim HCC/ICC Data EU Registry Data Readout #1 German
ZE Reimbursement Level Defined P2 HCC/ICC Enrollment Completed
Cancers of the Liver - A Major Unmet
Medical Need Large global patient population of ~1.2 million* patients diagnosed annually with primary or metastatic liver cancer Liver a common site of metastases and often the life-limiting organ for cancer patients Prognosis is poor, OS generally
<12 months Currently available/emerging therapies limited * SOURCE – 2008 GLOBOCAN
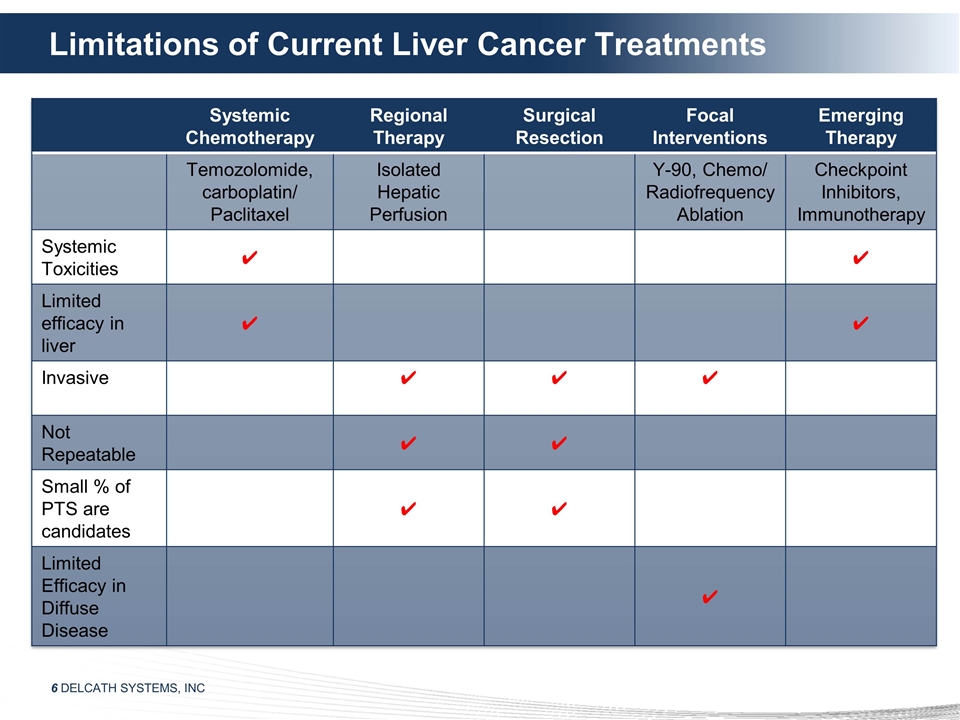
Limitations of Current Liver Cancer
Treatments Systemic Chemotherapy Regional Therapy Surgical Resection Focal Interventions Emerging Therapy Temozolomide, carboplatin/ Paclitaxel Isolated Hepatic Perfusion Y-90, Chemo/ Radiofrequency Ablation Checkpoint Inhibitors, Immunotherapy
Systemic Toxicities ✔ ✔ Limited efficacy in liver ✔ ✔ Invasive ✔ ✔ ✔ Not Repeatable ✔ ✔ Small % of PTS are candidates ✔ ✔ Limited Efficacy in Diffuse Disease ✔
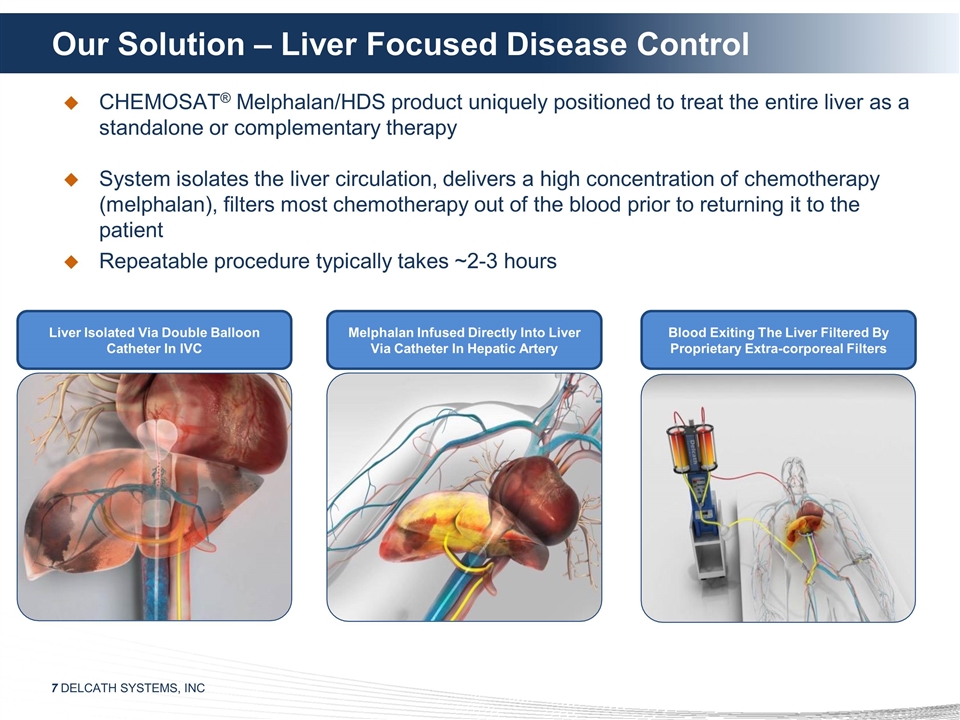
Our Solution – Liver Focused
Disease Control CHEMOSAT® Melphalan/HDS product uniquely positioned to treat the entire liver as a standalone or complementary therapy System isolates the liver circulation, delivers a high concentration of chemotherapy (melphalan), filters
most chemotherapy out of the blood prior to returning it to the patient Repeatable procedure typically takes ~2-3 hours Liver Isolated Via Double Balloon Catheter In IVC Melphalan Infused Directly Into Liver Via Catheter In Hepatic Artery Blood
Exiting The Liver Filtered By Proprietary Extra-corporeal Filters
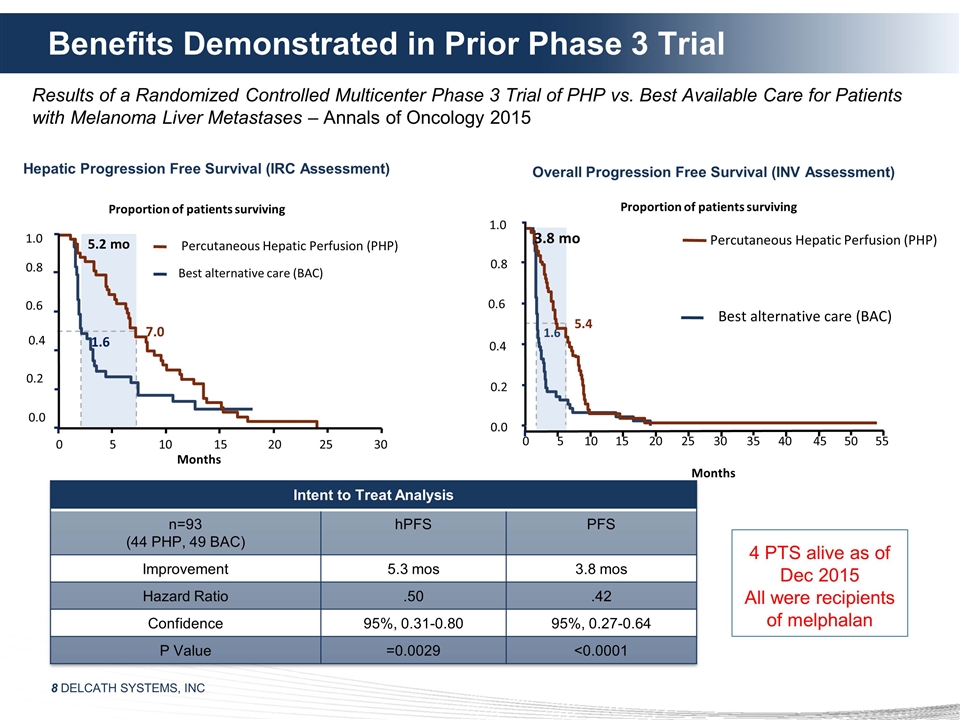
Months Benefits Demonstrated in Prior
Phase 3 Trial Percutaneous Hepatic Perfusion (PHP) Overall Progression Free Survival (INV Assessment) Months 1.0 0.8 0.6 0.4 0.2 0.0 Proportion of patients surviving 5.4 1.6 3.8 mo Best alternative care (BAC) 0 5 10 15 20 25 30 35 40 45 50 55 4 PTS
alive as of Dec 2015 All were recipients of melphalan Results of a Randomized Controlled Multicenter Phase 3 Trial of PHP vs. Best Available Care for Patients with Melanoma Liver Metastases – Annals of Oncology 2015 Proportion of patients
surviving 7.0 1.6 5.2 mo Percutaneous Hepatic Perfusion (PHP) 0 5 10 15 20 25 30 Best alternative care (BAC) Hepatic Progression Free Survival (IRC Assessment) 1.0 0.8 0.6 0.4 0.2 0.0 Intent to Treat Analysis n=93 (44 PHP, 49 BAC) hPFS PFS
Improvement 5.3 mos 3.8 mos Hazard Ratio .50 .42 Confidence 95%, 0.31-0.80 95%, 0.27-0.64 P Value =0.0029 <0.0001
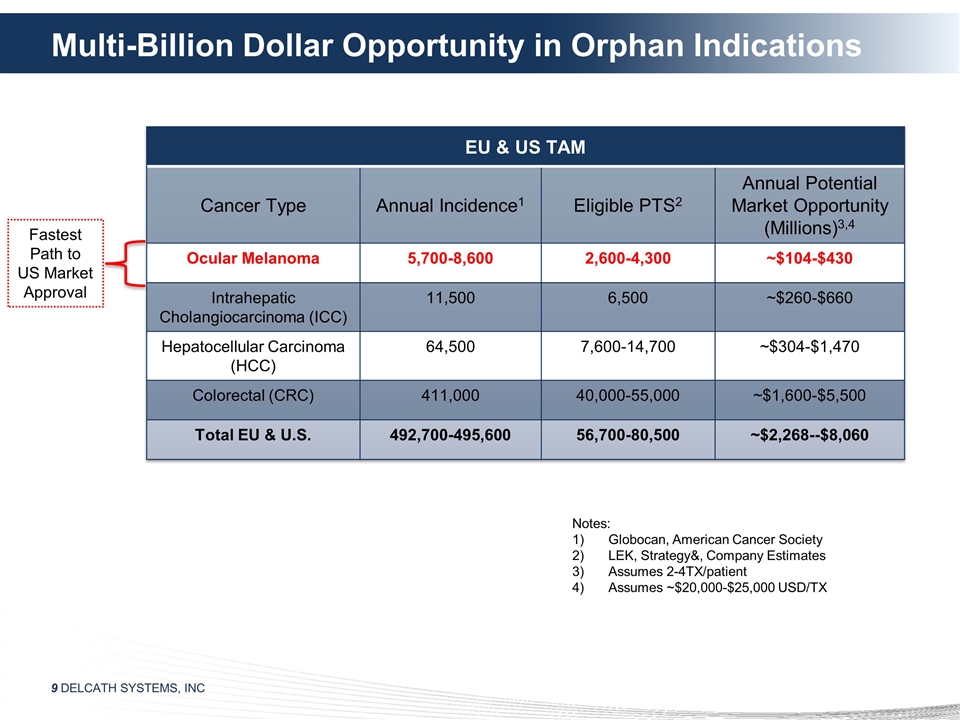
Multi-Billion Dollar Opportunity in
Orphan Indications EU & US TAM Cancer Type Annual Incidence1 Eligible PTS2 Annual Potential Market Opportunity (Millions)3,4 Ocular Melanoma 5,700-8,600 2,600-4,300 ~$104-$430 Intrahepatic Cholangiocarcinoma (ICC) 11,500 6,500 ~$260-$660
Hepatocellular Carcinoma (HCC) 64,500 7,600-14,700 ~$304-$1,470 Colorectal (CRC) 411,000 40,000-55,000 ~$1,600-$5,500 Total EU & U.S. 492,700-495,600 56,700-80,500 ~$2,268--$8,060 Notes: Globocan, American Cancer Society LEK, Strategy&,
Company Estimates Assumes 2-4TX/patient Assumes ~$20,000-$25,000 USD/TX Fastest Path to US Market Approval
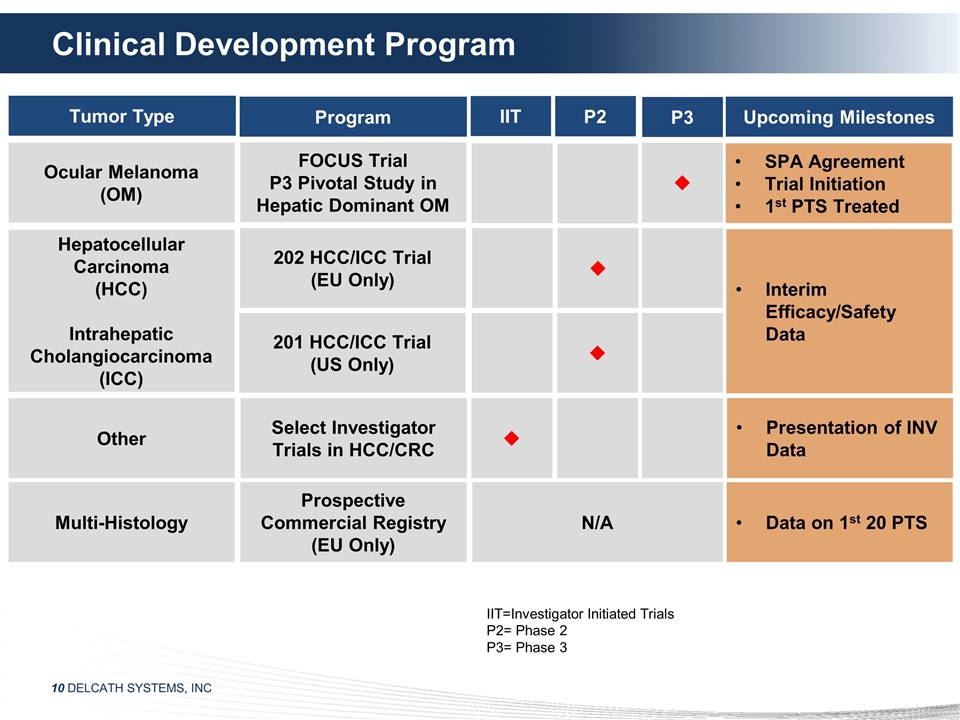
Clinical Development Program Program
Tumor Type IIT P2 P3 Upcoming Milestones FOCUS Trial P3 Pivotal Study in Hepatic Dominant OM u 202 HCC/ICC Trial (EU Only) u 201 HCC/ICC Trial (US Only) u SPA Agreement Trial Initiation 1st PTS Treated Interim Efficacy/Safety Data
Ocular Melanoma (OM) Hepatocellular Carcinoma (HCC) Intrahepatic Cholangiocarcinoma (ICC) Multi-Histology Prospective Commercial Registry (EU Only) Data on 1st 20 PTS Other Select Investigator Trials in HCC/CRC u Presentation of INV Data N/A
IIT=Investigator Initiated Trials P2= Phase 2 P3= Phase 3
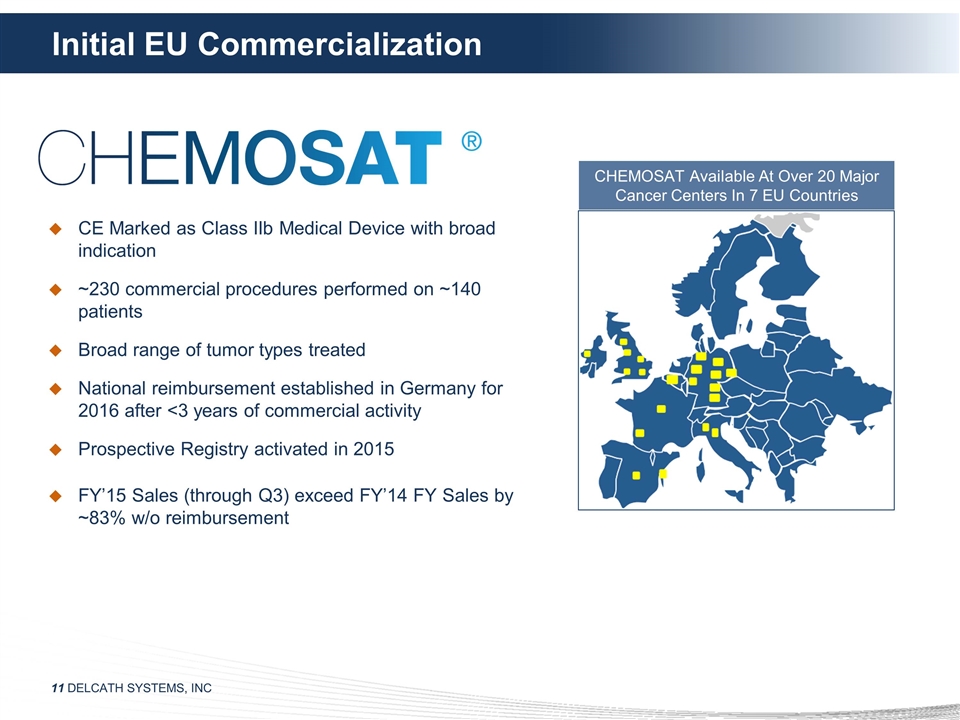
Initial EU Commercialization CE
Marked as Class IIb Medical Device with broad indication ~230 commercial procedures performed on ~140 patients Broad range of tumor types treated National reimbursement established in Germany for 2016 after <3 years of commercial activity
Prospective Registry activated in 2015 FY’15 Sales (through Q3) exceed FY’14 FY Sales by ~83% w/o reimbursement CHEMOSAT Available At Over 20 Major Cancer Centers In 7 EU Countries
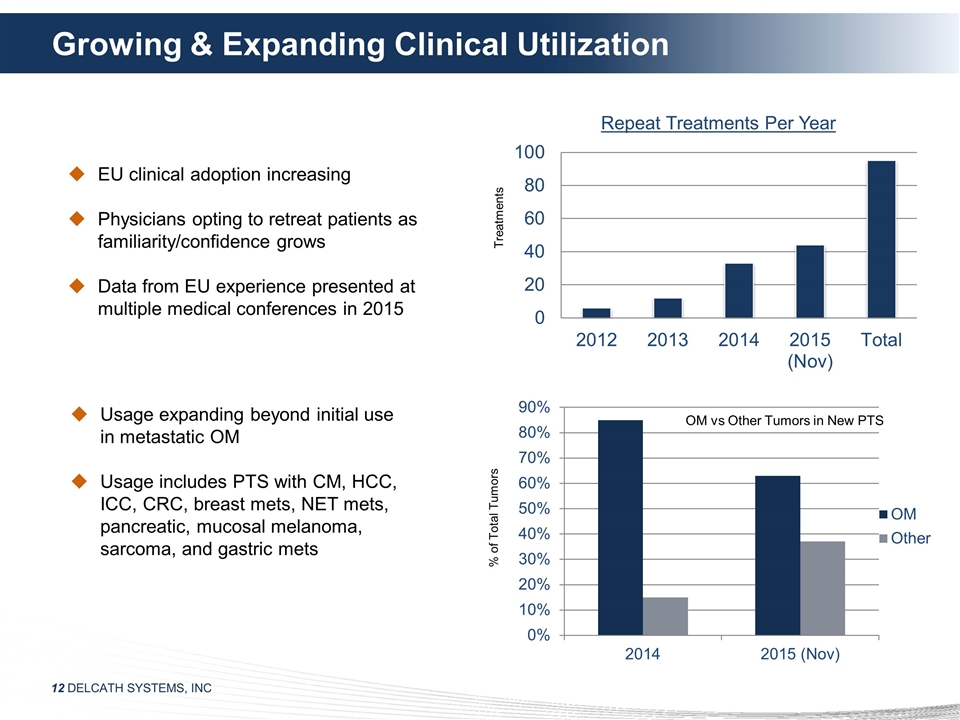
Growing & Expanding Clinical
Utilization OM vs Other Tumors in New PTS EU clinical adoption increasing Physicians opting to retreat patients as familiarity/confidence grows Data from EU experience presented at multiple medical conferences in 2015 Usage expanding beyond initial
use in metastatic OM Usage includes PTS with CM, HCC, ICC, CRC, breast mets, NET mets, pancreatic, mucosal melanoma, sarcoma, and gastric mets Treatments % of Total Tumors
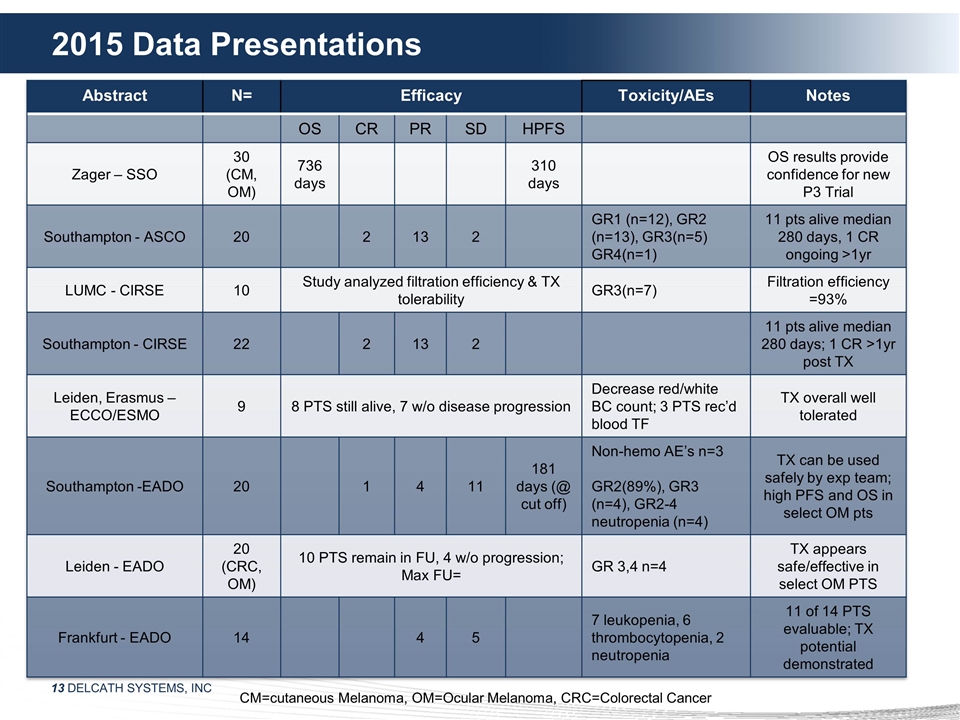
2015 Data Presentations Abstract N=
Efficacy Toxicity/AEs Notes OS CR PR SD HPFS Zager – SSO 30 (CM, OM) 736 days 310 days OS results provide confidence for new P3 Trial Southampton - ASCO 20 2 13 2 GR1 (n=12), GR2 (n=13), GR3(n=5) GR4(n=1) 11 pts alive median 280 days, 1 CR
ongoing >1yr LUMC - CIRSE 10 Study analyzed filtration efficiency & TX tolerability GR3(n=7) Filtration efficiency =93% Southampton - CIRSE 22 2 13 2 11 pts alive median 280 days; 1 CR >1yr post TX Leiden, Erasmus – ECCO/ESMO 9 8 PTS
still alive, 7 w/o disease progression Decrease red/white BC count; 3 PTS rec’d blood TF TX overall well tolerated Southampton -EADO 20 1 4 11 181 days (@ cut off) Non-hemo AE’s n=3 GR2(89%), GR3 (n=4), GR2-4 neutropenia (n=4) TX can be
used safely by exp team; high PFS and OS in select OM pts Leiden - EADO 20 (CRC, OM) 10 PTS remain in FU, 4 w/o progression; Max FU= GR 3,4 n=4 TX appears safe/effective in select OM PTS Frankfurt - EADO 14 4 5 7 leukopenia, 6 thrombocytopenia, 2
neutropenia 11 of 14 PTS evaluable; TX potential demonstrated CM=cutaneous Melanoma, OM=Ocular Melanoma, CRC=Colorectal Cancer

Cash & Capital Resources Cash
& Cash Equivalents $16.7 million at Sept 30, 2015 Debt None ATM Program1 $40 million available at Sept 30, 2015 Shares Outstanding 21.8 million (41.3 million fully diluted2) at Sept 30, 2015 Subject to market conditions and certain limitations
Fully diluted includes approximately 0.8 million options, 0.6 million in unvested restricted shares and 18.1 million warrants Focused Spending and Resources to Support Execution of Near-term Plan
Investment Highlights Attractive
multi-billion dollar orphan drug business model Unique, highly differentiated solution Late-stage asset in US with active clinical development program Early commercial activity in EU with increasing sales/procedure volumes Imminent valuation drivers
FDA SPA Agreement P3 Trial Initiation 2016 German Reimbursement


Appendix
SSO 2015 Hepatic Progression Free
and Overall Survival after Regional Therapy to the Liver for Metastatic Melanoma - Moffitt Cancer Center (Tampa, FL) Methods – Retrospective analysis of 30 patients with ocular or cutaneous melanoma treated with Melphalan/HDS (n=10),
chemoembolization (CE, n=12), and yttrium-90 (Y90, n=6) Results - Study showed significant difference in hepatic progression free survival (HPFS) for Melphalan/HDS (310 days), CE (80 days), Y90 (54 days) Median overall survival (OS) longest for
Melphalan/HDS (736 days) vs Y90 (285 days) CE (265 days), but did not reach statistical significance Conclusion - Authors concluded that HPFS and progression free survival (PFS) were significantly prolonged with Melphalan/HDS vs CE and
Y90
ASCO 2015 Single Centre Experience
of Chemosaturation Percutaneous Hepatic Perfusion in the Treatment of Metastatic Uveal Melanoma - Southampton University Hospital (UK) Methods - Analysis of 20 ocular melanoma patients who received 34 TX Results - Eleven patients remain alive after
a median of 280 days with one complete response ongoing at >1 year From the diagnosis of liver metastases, 11 patients (55%) survived to one year and 3 (15%) for >2 years; no procedure related deaths were seen ORR 85%: 2 patients (10%)
demonstrated stable disease for >3 months, 13 patients (65%) had a partial response, 2 patients (10%) demonstrated complete response Nine deaths from disease progression occurred after a median of 264 days from the first procedure Adverse Events
- Early AEs often expected with percutaneous hepatic perfusion (PHP) were observed including coagulopathy, electrolyte disturbances and transient transaminases (elevated liver enzymes). Rare late AEs (1 patient each) included hair loss, skin
rash, myelosuppression and persistent transaminases (elevated liver enzymes) AEs seen were grade 1 (n=12), 2 (n=13), 3 (n=5) and 4 (n=1) Grade 4 complication was pulmonary edema due to fluid overload Conclusion - results show that PHP (CHEMOSAT) can
be used safely to control hepatic metastases in selected UM patients with a high rate of hepatic progression free and excellent overall survival

CIRSE 2015 Safety and efficacy of
Delcath 2nd Generation filter in PHP with melphalan for unresectable hepatic metastases of colorectal cancer and uveal melanoma - Leiden University Medical Center (the Netherlands) 15 PHP procedures performed with CHEMOSAT on 10 pts; PK blood
samples @ baseline, set intervals PHP performed with melphalan dose of 3mg/kg; 1st blood sample filtration efficiency = 93% Grade 3 complications (mostly leukocytopenia, thrombocytopenia) in 7 pts; febrile neutropenia (w/bacterial pharyngitis) in 1
pts (not seen following introduction of growth factors) Conclusion - 2nd Generation filter efficiency very high; PHP associated with no mortality and acceptable morbidity Lessons and Early Results from the Largest Single Center Experience in Europe
of Treating Ocular Melanoma Liver Metastases with Chemosaturation via Percutaneous Hepatic Perfusion - Southampton University (United Kingdom) Retrospective analysis of 22 pts; 20 received TX w/PHP 11 pts alive after median 280 days; one complete
response > 1yr post TX 9 deaths from disease progress after median 264 days post TX Complete response in liver in 2 pts (10%), 13 pts (65%) had partial liver response, 2 pts (10%) had stable disease > 3mos Conclusion - PHP effective palliative
TX in bleak disease with an acceptable side-effect profile
ECCO 2015 Treating Unresectable
Liver Metastases of Uveal Melanoma with (Percutaneous) Isolated Hepatic Perfusion with Melphalan: Results from Two Experienced Centers - Leiden University Medical Center, Erasmus Medical Center (the Netherlands) Methods - Investigators compared TX
with IHP and PHP for ocular melanoma liver metastases Results - IHP cohort (n=30) treated between 1999 and 2009 PFS was 6 mos Disease recurrence mainly in the liver OS was 10 mos (vs 6 mos historic avg for IHP) PHP cohort (n=9); PTS received 15 PHP
TX since Feb 2014 Max follow up period was 14 mos 8 PTS still alive, 7 without disease progression Decrease in red/white blood cell count observed following TX; 3 PTS received blood transfusion TX overall was well tolerated Conclusion - PHP appears
to be effective/safe procedure in select patients with unresectable liver metastases from CRC or OM and can be repeated
EADO 2015 Liver Directed Treatment
Of Metastatic Uveal Melanoma By Chemosaturation Via Percutaneous Hepatic Perfusion – A Single Centre Experience Southampton University (United Kingdom) Methods – A retrospective evaluation of 20 patients treated with CHEMOSAT over 3
years; analysis of survival, tumor response, time to progression and treatment related adverse events; 18 patients able to receive treatment, 17 were evaluable Results - 10 patients remained alive after median 256 days 1 complete response (6%), 4
partial responses (24%), 11 (65%) stable disease >90 days Progression free survival for patients who had progressed was 181 days at the time of data cut off 6 patients alive >1 year following their first treatment 8 deaths from disease
progression occurred median 241 days following first treatment; no treatment related deaths Adverse Events (AEs) - treatment overall was well tolerated Non-hematological AEs were rare (3) Most common adverse events was transient, mild grade 2
transaminitis and thrombocytopenia (89%); grade 3 anemia seen in 4 patients, grade 2-4 neutropenia was seen in 4 patients Conclusion - CHEMOSAT can be used safely by experience team to deliver liver directed therapy in selected uveal melanoma
patients with high progression free and excellent overall survival

EADO 2015 Treating Unresectable
Liver Metastases Of Uveal Melanoma With Percutaneous Hepatic Perfusion With Melphalan, Leiden University Medical Center, Erasmus Cancer Institute (the Netherlands) presented by Dr. Mark Burgmans (Leiden) Methods - two-center Phase 2 study aims to
evaluate 20 patients with uveal, or ocular melanoma treated with percutaneous hepatic perfusion (PHP) performed with CHEMOSAT; data from the first 11 patients with a maximum follow up period of 16 months were presented Primary endpoints are response
rate (RECIST criteria) following two treatments at 6 week interviews, percentage of patients with stable disease Secondary endpoints are safety, overall survival, hepatic progression free survival, quality of life Results -18 treatments performed on
11 patients, maximum follow up currently 16 months 10 patients remain in follow up, 4 without progression of disease Adverse Events - grade 3 or 4 toxicity observed in 4 patients, managed with blood transfusion or platelet infusion Conclusion -
CHEMOSAT appears to be effective and safe procedure in select patients with unresectable liver metastases of uveal melanoma and can be repeated
EADO 2015 Chemosaturation with
Percutaneous Hepatic Perfusion of Melphalan for Hepatic Metastases from Uveal Melanoma: Multi-institutional Evaluation, poster presented by lead author Prof. Thomas Vogl, Frankfurt University Hospital Methods - retrospective evaluation of
non-resectable hepatic metastases from uveal melanoma treated with CHEMOSAT 14 patients treated 2012-2014; patients received 1-3 treatments 11 PTS evaluated by RECIST criteria; survival time analysis performed, complications registered Results - 4
(36%) partial response, 5 (46%) stable disease, 2 (18%) progressive disease Survival time ranged from 1. 5 months to 23 months (median OS 6.5 months) Time to progression for two patients who progressed was 6.2 months in one patient; the other died
1.6 months after evaluation Adverse Events – treatment well tolerated by all 14 patients 7 leukopenia, 6 thrombocytopenia, 2 neutropenia Conclusion - CHEMOSAT has been manifested as a potential treatment for patient with non-resectable hepatic
metastases of uveal melanoma