UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported):
September 28, 2015
Titan Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in
Charter)
|
Delaware |
0-27436 |
94-3171940 |
(State or Other Jurisdiction of
Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
400 Oyster
Point Blvd., Suite 505, South San Francisco, CA |
94080 |
| (Address of Principal Executive Offices) |
(Zip Code) |
Registrant’s telephone number, including
area code: 650-244-4990
(Former Name or Former Address, if Changed
Since Last Report)
Check the appropriate box below if the
Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions
(see General Instruction A.2. below):
| ¨ | Written communications
pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| | |
| ¨ | Soliciting material pursuant
to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| | |
| ¨ | Pre-commencement communications
pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| | |
| ¨ | Pre-commencement communications
pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
On September 28, 2015, Titan Pharmaceuticals, Inc. (the “Company”)
announced that the FDA has accepted for review the New Drug Application (“NDA”) for Probuphine® and has set February
27, 2016 as the target date for action on the NDA.
A copy of the press release issued by the
Company is attached hereto as Exhibit 99.1 and is incorporated herein by reference,
| Item 9.01. | Financial Statements and Exhibits. |
| Exhibit No. |
Description |
| 99.1 |
Press Release, dated September 28, 2015. |
SIGNATURE
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned
hereunto duly authorized.
| Dated: September 28, 2015 |
TITAN PHARMACEUTICALS, INC. |
|
| |
|
|
| |
By: |
/s/ Sunil Bhonsle |
|
| |
Name: |
Sunil Bhonsle |
|
| |
Title: |
President |
|
Exhibit Index
| Exhibit No. |
Description |
| 99.1 |
Press Release, dated September 28, 2015. |
Exhibit 99.1
 |
Titan Pharmaceuticals, Inc.
|
TITAN PHARMACEUTICALS ANNOUNCES FDA'S
ACCEPTANCE OF RESUBMISSION OF NEW DRUG APPLICATION FOR PROBUPHINE
Agency action expected by Feb. 27,
2016 on Probuphine for maintenance treatment of opioid dependence
SOUTH
SAN FRANCISCO, CA – Sept. 28, 2015 – Titan Pharmaceuticals, Inc. (OTCQB: TTNP) announced today that the
U.S. Food and Drug Administration has accepted for review the New Drug Application (NDA) for Probuphine®, a six-month subdermal
implant containing buprenorphine hydrochloride for the long-term maintenance treatment of opioid addiction. Probuphine was developed
utilizing Titan's long-term, continuous drug delivery technology, ProNeura™. The FDA has set Feb. 27, 2016 as a target date
for action on the application, which was submitted by Titan's development and commercialization partner Braeburn Pharmaceuticals.
"We are pleased that the FDA has accepted for review the
resubmission of the NDA for Probuphine, and will be working with the agency and Braeburn to achieve our goal of offering the first
product on the market to provide maintenance treatment of opioid addiction continuously for six months following a single treatment,"
said Kate Beebe, PhD, Titan's executive vice president and chief development officer.
U.S. Health and Human Services (HHS) Department Secretary Sylvia
Burwell recently announced that HHS will move to expand access to medication-assisted-treatment (MAT) by revising the regulations
related to the prescribing of buprenorphine to treat opioid dependence. The current cap on the number of patients that can be treated
with buprenorphine products by a physician limits the availability of this important medication-assisted-therapy. The HHS revision
to the regulation will be developed to provide a balance between expanding the supply of buprenorphine based treatment, encouraging
use of evidence-based MAT, and minimizing the risk of drug diversion.
"Opioid addiction has become a significant health epidemic,
and greater access to safe and effective treatments is essential," Titan President Sunil Bhonsle said. "As federal officials
seek to find a balance between increasing access and minimizing the risk of abuse and diversion, treatment options with an implant
such as Probuphine could play an expanding and crucial role in the maintenance treatment of patients."
The Probuphine NDA resubmission includes results from a Phase
3 double-blind, double-dummy clinical study. The study met the pre-specified primary endpoint of non-inferiority, as well as all
secondary efficacy endpoints.
About Opioid Addiction
According to recent estimates, there are 2.2 million people
with opioid addiction in the U.S. Approximately 20 percent of this population is addicted to illicit opioids, such as heroin, and
the other 80 percent to prescription opioids, such as oxycodone, hydrocodone, methadone, hydromorphone and codeine. Before the
year 2000, medication-assisted therapies for opioid dependence had been sanctioned to a limited number of facilities in the U.S.
The Drug Addiction Treatment Act of 2000 (DATA 2000) allowed medical office-based treatment of opioid dependence and greatly expanded
patient access to medication-assisted treatments. Sales of buprenorphine drug products for treatment of opioid addiction in 2014
were approximately $1.75 billion in the United States.
About Probuphine®
Probuphine is an investigational subdermal implant designed
to deliver buprenorphine continuously for six months following a single treatment, and to promote patient compliance and retention.
Buprenorphine, which is the active ingredient in multiple FDA-approved drug products for the treatment of opioid dependence, is
currently available in tablet and film formulations that require self-administration by patients on a daily basis.
Probuphine was developed using ProNeura™, Titan's continuous
drug delivery system that consists of a small, solid implant made from a mixture of ethylene-vinyl acetate (EVA) and a drug substance.
The resulting construct is a solid matrix that is placed subdermally, normally in the upper arm in an outpatient office procedure,
and removed in a similar manner at the end of the treatment period.
The efficacy and safety of Probuphine has previously been studied
in several clinical trials, including a 163-patient, placebo-controlled study over a 24-week period (published in the Journal
of the American Medical Association (JAMA)), and a follow on study of 287 patients (published in the journal Addiction).
About Titan Pharmaceuticals
Titan Pharmaceuticals Inc. (OTCQB: TTNP), based in South San
Francisco, CA, is a specialty pharmaceutical company developing proprietary therapeutics primarily for the treatment of serious
medical disorders. The company’s lead product candidate is Probuphine®, a novel and long-acting formulation of buprenorphine
for the long-term maintenance treatment of opioid dependence. Probuphine employs Titan’s proprietary drug delivery system
ProNeura™, which is capable of delivering sustained, consistent levels of medication for three months or longer. Titan has
granted U.S. and Canadian commercial rights for Probuphine to Braeburn Pharmaceuticals. If approved, Probuphine would be the first
and only commercialized treatment of opioid dependence to provide continuous, around-the-clock blood levels of buprenorphine for
six months following a single procedure. The ProNeura technology has the potential to be used in developing products for treating
other chronic conditions, such as Parkinson’s disease, where maintaining consistent blood levels of a therapeutic agent
may benefit the patient and improve medical outcomes. For more information about Titan, please visit www.titanpharm.com.
This press release may contain "forward-looking statements"
within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such statements
include, but are not limited to, any statements relating to our product development programs and any other statements that are
not historical facts. Such statements involve risks and uncertainties that could negatively affect our business, operating results,
financial condition and stock price. Factors that could cause actual results to differ materially from management's current expectations
include those risks and uncertainties relating to the regulatory approval process, the development, testing, production and marketing
of our drug candidates, patent and intellectual property matters and strategic agreements and relationships. We expressly disclaim
any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to
reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based,
except as required by law.
###
CONTACT:
Titan Pharmaceuticals, Inc.:
Sunil Bhonsle, President
(650) 244-4990
Investors:
Stephen Kilmer
(647) 872-4849
skilmer@titanpharm.com
Media:
Susan Thomas
(619) 540-9195
sthomas@titanpharm.com
Titan Pharmaceuticals (NASDAQ:TTNP)
Historical Stock Chart
From Mar 2024 to Apr 2024
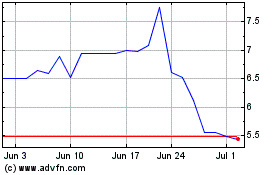
Titan Pharmaceuticals (NASDAQ:TTNP)
Historical Stock Chart
From Apr 2023 to Apr 2024