UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of October, 2014
Commission File Number
Novogen
Limited
(Translation of registrant’s name into English)
16-20 Edgeworth David Ave, Hornsby, NSW 2077, Australia
(Address of principal executive office)
Indicate by check mark whether
the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F þ Form 40-F ¨
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ¨
Note: Regulation S-T Rule 101(b)(1) only permits the submission in paper of a Form 6-K if submitted
solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as
permitted by Regulation S-T Rule 101(b)(7): ¨
Note: Regulation S-T Rule 101(b)(7) only
permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated,
domiciled or legally organized (the registrant’s “home country”), or under the rules of the home country exchange on which the registrant’s securities are traded, as long as the report or other document is not a press release, is
not required to be and has not been distributed to the registrant’s security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
Indicate by check mark if the registrant by furnishing the information contained in this form is also thereby furnishing the information to the Commission
pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934. Yes ¨ No þ
If “yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b)
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned,
thereunto duly authorized.
Novogen Limited (Registrant)
Lionel Mateo
Lionel Mateo
Company Secretary
Date 22 October 2014
|
|
|
| NOVOGEN LIMITED
(ASX: NRT) |
|

|
ASX RELEASE
22 October
2014
STUDIES CONFIRM POTENTIAL OF TRILEXIUM FOR CHILDHOOD CANCERS
22 OCT 2014. SYDNEY: Australian biotechnology company, Novogen, today released the results of two recently-completed studies of one of its lead
candidate compounds, TRX-E-009-1, confirming its ability to kill paediatric brain cancer cells - diffuse intrinsic pontine glioma (DIPG) and paediatric neuroblastoma cells – and clearing the way for the compound to come into the clinic as a
potential treatment of childhood cancers.
TRX-E-009-1 is a super-benzopyran drug-candidate that shows broad anti-cancer activity in the laboratory
against a wide range cancer cell types. It has been designed specifically to cross the blood-brain barrier and has been selected for its particular ability to kill cancer cells of neural origin (relating to the nervous system).
TRX-E-009-1 is delivered in a construct in order to optimize the bioavailability of the drug candidate. The entire construct is known as Trilexium.
Novogen is developing Trilexium as a treatment for both primary and secondary brain cancer in adults. TRX-E-009-1 has already been confirmed in studies
conducted in the US as being highly cytotoxic to glioblastoma multiforme (GBM) stem-like cells derived from patients whose cancers have failed to respond to standard chemotherapy. TRX-E-009-1 is the first drug to show any significant activity
against a library of these highly chemo-resistant cancer cells. On this basis, Novogen is planning to bring Trilexium into the clinic in late 2015 for the treatment of GBM, with a Phase 1 study planned for Australian hospitals.
The question then was, whether Trilexium would be equally active against neural cancers such as brain cancer and neuroblastoma in children, with enough
differences in the behavior of adult and paediatric neural cancers to not allow that assumption to be made automatically. Brain cancers in children tend to involve different parts of the brain and different cell types to that of adults; also,
neuroblastoma occurs only in children.
To answer this question, studies have been underway in recent months looking at the effect of TRX-E-009-1 on DIPG,
neuroblastoma and medulloblastoma cells.
Australian researchers in the last week have now reported that TRX-E-009-1 is highly active in vitro against
3-dimensional spheroids of DIPG derived from patient biopsies.
NOVOGEN LTD – ACN
063 259 754
16-20 Edgeworth David Ave, Hornsby, NSW, 2077 | P: +61 (0) 2 9472 4100 - F: +61 (0) 2 9476 0388 | www.novogen.com
Dr. David Brown, Novogen Group CSO, said, “DIPG cells are highly resistant to standard chemotherapies, so
the ability of TRX-E-009-1 to impact the viability of these cells is particularly exciting. Importantly, the drug was relatively inactive against normal cells in the study, indicating that it has a good safety margin, an important factor in the
treatment of children.”
US researchers also have in the last week confirmed that TRX-E-009-1 is highly active against a panel of neuroblastoma cell
lines, irrespective of the type of mutations present, but more importantly including those mutations most commonly encountered in the clinic.
The
medulloblastoma study is ongoing.
Dr. Graham Kelly, Novogen Group CEO, said, “Children’s cancer is an area notoriously neglected by
pharmaceutical companies. Only one drug has been developed specifically with children’s cancers in mind, and children with brain cancer and neuroblastoma are being treated with drugs developed for adults, with the children then at risk of
bearing the results of toxic side-effects for the rest of their lives.”
“This situation is behind the creation of the Children’s Oncology
Drug Alliance (CODA). CODA is committed to changing this situation and Novogen is pleased to be a member of that alliance and to be making its two drug technology platforms available for the development of paediatric drugs.”
“The crucial first step was evidence that TRX-E-009-1 was active against paediatric neural cancer cells. These two studies reported here today have
provided that evidence. The fact that DIPG is a rare tumor of the brainstem with only 300-350 cases reported annually in the world is not the point. The importance lies in the fact that here is a paediatric neural cancer that is highly insensitive
to chemotherapy, and yet has proven to be sensitive to TRX-E-009-1. Combined with the confirmed sensitivity of neuroblastoma cells to the drug, the way is now cleared for us to begin the process of bringing Trilexium into the clinic for paediatric
neural cancers in parallel to adult brain cancers.”
About Neuroblastoma (NB)
Neuroblastoma is a childhood cancer that affects up to 100 children in Australia and around 650 in the United States each year. A neuroblastoma tumor develops
from immature nerve cells called neuroblasts and arises predominantly in the adrenal glands and abdomen. This cancer most commonly affects children aged 5 years or younger. The survival rate for patients that are diagnosed with “high risk”
disease is less than 40%. This poor survival rate is due to lack of response to current therapies.
About Diffuse Intrinsic Pontine Glioma (DIPG)
DIPG is a rare tumor of the brainstem that occurs almost exclusively in children usually under the age of ten. DIPG can affect critical functions such
as breathing and blood pressure and its location, as well as the way it infiltrates nearby brain tissue, makes treatment difficult.
NOVOGEN LTD – ACN
063 259 754
16-20 Edgeworth David Ave, Hornsby, NSW, 2077 | P: +61 (0) 2 9472 4100 - F: +61 (0) 2 9476 0388 | www.novogen.com
DIPG originates from the glial (connective/supporting) cells of the brain. Pontine gliomas grow quickly, so
symptoms can appear suddenly and progress rapidly. Treatment options include radiotherapy but are in the main palliative with the prognosis for DIPG patients remaining very poor.
About CODA
The Children’s Oncology Drug Alliance
(CODA) is an informal collection of organisations dedicated to achieving marketing approvals for new anti-cancer drugs specifically for childhood cancer indications. The Alliance comprises the Australia charity, The Kids Cancer Project (TKCP), The
University of New South Wales (Sydney, Australia), Nationwide Children’s Hospital (Columbus, Ohio) and Novogen.
About Novogen Limited
Novogen is a public, Australian drug-development company whose shares trade on both the Australian Securities Exchange (‘NRT’) and NASDAQ
(‘NVGN’). The Novogen Group includes a New Haven, Connecticut-based joint venture company, CanTx Inc., with Yale University.
Novogen has two
main drug technology platforms: super-benzopyrans (SBPs) and anti-tropomyosins (ATMs). SBP compounds have been created to kill the full range of cells within a tumor, but particularly the cancer stem cells. The ATM compounds target the microfilament
component of the cancer cell and when used in conjunction with standard anti-microtubular drugs, result in comprehensive and fatal destruction of the cancer cell’s cytoskeleton. Ovarian cancer, colorectal cancer, malignant ascites, prostate
cancer, neural cancers (glioblastoma, neuroblastoma in children) and melanoma are the key clinical indications being pursued, with the ultimate objective of employing both technologies as a unified approach to first-line therapy.
Further information is available on our website www.novogen.com
For more information please contact:
Novogen Enquiries
Dr Graham Kelly
CEO Novogen Group
Graham.Kelly@novogen.com
+(61 2) 9472 4100
NOVOGEN LTD – ACN
063 259 754
16-20 Edgeworth David Ave, Hornsby, NSW, 2077 | P: +61 (0) 2 9472 4100 - F: +61 (0) 2 9476 0388 | www.novogen.com
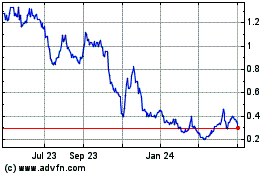
Kazia Therapeutics (NASDAQ:KZIA)
Historical Stock Chart
From Mar 2024 to Apr 2024
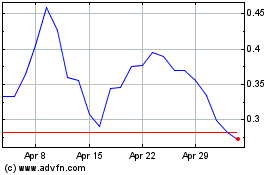
Kazia Therapeutics (NASDAQ:KZIA)
Historical Stock Chart
From Apr 2023 to Apr 2024