As filed with the Securities and Exchange Commission on __________, 2014.
Registration No 333-196243
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-3
AMENDMENT NO. 1
Registration Statement Under
THE SECURITIES ACT OF 1933
CEL-SCI CORPORATION
(Exact name of registrant as specified in charter)
Colorado
------------------------------------
(State or other jurisdiction of incorporation)
8229 Boone Blvd. #802
Vienna, Virginia 22182
84-09l6344 (703) 506-9460
---------------------------- ----------------------------------------
(IRS Employer I.D. Number) (Address, including zip code, and telephone
number including area of principal
executive offices)
Geert Kersten
8229 Boone Blvd. #802
Vienna, Virginia 22182
(703) 506-9460
------------------------------------
|
(Name and address, including zip code, and telephone
number, including area code, of agent for service)
Copies of all communications, including all communications sent
to the agent for service, should be sent to:
William T. Hart, Esq.
Hart & Trinen
1624 Washington Street
Denver, Colorado 80203
(303) 839-0061
APPROXIMATE DATE OF COMMENCEMENT OF PROPOSED SALE TO THE PUBLIC:
From time to time after this Registration Statement
becomes effective as determined by market conditions
If the only securities being registered on this Form are being offered pursuant
to dividend or interest reinvestment plans, please check the following box. [ ]
If any of the securities being registered on this Form are to be offered on a
delayed or continuous basis pursuant to Rule 415 under the Securities Act of
1933, other than securities offered only in connection with dividend or interest
reinvestment plans, check the following box. [X]
If this Form is filed to register additional securities for an offering pursuant
to Rule 462(b) under the Securities Act, please check the following box and list
the Securities Act registration statement number of the earlier effective
registration for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under
the Securities Act, check the following box and list the Securities Act
registration statement number of the earlier effective registration statement
for the same offering. [ ]
If this Form is a registration statement pursuant to General Instruction I.D. or
a post-effective amendment thereto that shall become effective upon filing with
the Commission pursuant to Rule 462(e) under the Securities Act, check the
following box. [ ]
If this Form is a post-effective amendment to a registration statement filed
pursuant to General Instruction I.D. filed to register additional securities or
additional classes of securities pursuant to Rule 413(b) under the Securities
Act, check the following box. [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting company. See
the definitions of "large accelerated filer", and "smaller reporting company" in
Rule 12b-2 of the Exchange Act.
Large accelerated filer [ ] Accelerated filer [X]
Non-accelerated filer [ ] Smaller reporting company [ ]
(Do not check if a smaller reporting company)
CALCULATION OF REGISTRATION FEE
Title of each Proposed
Class of Maximum Maximum Proposed
Securities Securities Offering Aggregate Amount of
to be to be Price Per Offering Registration
Registered Registered Share (1) Price Fee (1)
---------- ---------- ------------ --------- ----------
Common stock,
preferred stock,
convertible
preferred
stock, rights,
and warrants (2) (2) (2) (2)
Total $75,000,000 $75,000,000 $9,660
|
(1) The amount of registration fee, calculated in accordance with Rule 457(o),
is the maximum aggregate offering price at which the securities subject to
this registration statement are proposed to be offered.
(2) There are being registered hereunder an indeterminate amount and number of
securities as may be sold, from time to time, by the Company.
The Company hereby amends this Registration Statement on such date or dates
as may be necessary to delay its effective date until the registrant shall file
a further amendment which specifically states that this Registration Statement
shall thereafter become effective in accordance with Section 8(a) of the
Securities Act of l933 or until the Registration Statement shall become
effective on such date as the Commission, acting pursuant to said Section 8(a),
may determine.
2
PROSPECTUS
CEL-SCI CORPORATION
Common Stock
CEL-SCI Corporation may offer from time to time shares of common stock,
preferred stock, convertible preferred stock, rights, warrants, or securities
issuable upon the conversion or exercise of these securities, at an initial
offering price not to exceed $75,000,000, at prices and on terms to be
determined at or prior to the time of sale in light of market conditions at the
time of sale.
Specific terms pertaining to the securities offered by this prospectus will
be set forth in one or more accompanying prospectus supplements, together with
the terms of the offering and the initial price and the net proceeds to CEL-SCI
from the sale. The prospectus supplement will set forth, without limitation, the
terms of the offering and sale of such securities.
CEL-SCI may sell the securities offered by this prospectus directly,
through agents designated from time to time, or through underwriters or dealers.
If any agents of CEL-SCI or any underwriters or dealers are involved in the sale
of the securities, the names of the agents, underwriters or dealers, any
applicable commissions and discounts, and the net proceeds to CEL-SCI will be
set forth in the applicable prospectus supplement.
CEL-SCI may not use this prospectus to complete sales of its securities
unless this prospectus is accompanied by a prospectus supplement.
The securities offered by this prospectus are speculative and involve a
high degree of risk and should be purchased only by persons who can afford to
lose their entire investment. For a description of certain important factors
that should be considered by prospective investors, see "Risk Factors" beginning
on page 10 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities
commission has approved or disapproved of these securities or has passed upon
the accuracy or adequacy of this prospectus. Any representation to the contrary
is a criminal offense.
On June 25, 2013, CEL-SCI's shareholders approved a reverse split of
CEL-SCI's common stock. The reverse split became effective on the NYSE MKT on
September 25, 2013. On that date, every ten issued and outstanding shares of
CEL-SCI's common stock automatically converted into one outstanding share. All
references to shares of common stock and per share data for all periods
presented have been adjusted to reflect the reverse stock split on a retroactive
basis.
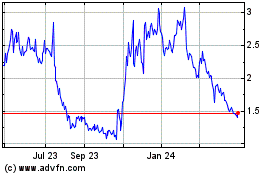
CEL-SCI's common stock is traded on the NYSE MKT under the symbol "CVM". On
June 16. 2014 the closing price of CEL-SCI's common stock on the NYSE MKT was
$1.09.
Date of this Prospectus is _______, 2014
3
PROSPECTUS SUMMARY
THIS SUMMARY IS QUALIFIED BY THE OTHER INFORMATION APPEARING ELSEWHERE IN THIS
PROSPECTUS.
CEL-SCI is dedicated to research and development directed at improving the
treatment of cancer and other diseases by utilizing the immune system, the
body's natural defense system. Its lead investigational immunotherapy is
Multikine(R) (Leukocyte Interleukin, Injection), currently being studied in a
pivotal global Phase III clinical trial as a potential first-line treatment for
advanced primary head and neck cancer. Multikine is also being used in a Phase I
study with the Naval Medical Center, San Diego under a Cooperative Research and
Development Agreement (CRADA) in HIV/HPV co-infected men and women with
peri-anal warts. The purpose of this study is to evaluate the safety and
clinical impact of Multikine as a treatment of peri-anal warts and assess its
effect on anal intraepithelial dysplasia (AIN) in HIV/HPV co-infected men and
women.
CEL-SCI's focus in HPV is not the development of an antiviral against HPV
in the general population. Instead it is the development of an immunotherapy to
be used in patients who are immune suppressed by diseases such as HIV and are
therefore less able or unable to control HPV and its resultant diseases. This
group of patients has no viable treatments available to them and there are, to
CEL-SCI's knowledge, no competitors at the current time. HPV is also relevant to
the head and neck cancer Phase III study since it is now known that HPV is a
cause of head and neck cancer. Multikine was shown to kill HPV in an earlier
study of HIV infected women with cervical dysplasia.
CEL-SCI is also investigating a different peptide-based immunotherapy
(LEAPS-H1N1-DC) as a possible treatment for H1N1 hospitalized patients and as a
vaccine (CEL-2000) for Rheumatoid Arthritis (currently in preclinical testing)
using its LEAPS technology platform. The investigational immunotherapy
LEAPS-H1N1-DC treatment involves non-changing regions of H1N1 Pandemic Flu
(www.jci.org/articles/view/67550), Avian Flu (H5N1), and the Spanish Flu, as
CEL-SCI scientists are very concerned about the possible emergence of a new more
virulent hybrid virus through the combination of H1N1 and Avian Flu, or possibly
Spanish Flu.
CEL-SCI Corporation was formed as a Colorado corporation in 1983. CEL-SCI's
principal office is located at 8229 Boone Boulevard, Suite 802, Vienna, VA
22182. CEL-SCI's telephone number is 703-506-9460 and its web site is
www.cel-sci.com. CEL-SCI does not incorporate the information on its website
into this prospectus supplement or accompanying prospectus, and you should not
consider it part of this prospectus supplement or accompanying prospectus.
CEL-SCI makes its electronic filings with the Securities and Exchange
Commission (SEC), including its annual reports on Form 10-K, quarterly reports
on Form 10-Q, current reports on Form 8-K and amendments to these reports
available on its website free of charge as soon as practicable after they are
filed or furnished to the SEC.
4
CEL-SCI'S PRODUCTS
CEL-SCI's business consists of the following:
1) Multikine(R) (Leukocyte Interleukin, Injection) investigational
immunotherapy against cancer and Human Papilloma Virus (HPV);
2) LEAPS technology, with two investigational therapies, LEAPS-H1N1-DC
pandemic flu treatment for hospitalized patients and CEL-2000, a
rheumatoid arthritis treatment vaccine.
MULTIKINE
CEL-SCI's lead investigational therapy, Multikine (Leukocyte Interleukin,
Injection), is currently being developed as a potential therapeutic agent
directed at using the immune system to produce an anti-tumor immune response.
Data from Phase I and Phase II clinical trials suggest that Multikine simulates
the activities of a healthy person's immune system, enabling it to use the
body's own anti-tumor immune response. Multikine (Leukocyte Interleukin,
Injection) is the full name of this investigational therapy, which, for
simplicity, is referred to in the remainder of this document as Multikine.
Multikine is the trademark that CEL-SCI has registered for this investigational
therapy, and this proprietary name is subject to FDA review in connection with
CEL-SCI's future anticipated regulatory submission for approval. Multikine has
not been licensed or approved for sale, barter or exchange by the FDA or any
other regulatory agency. Neither has its safety or efficacy been established for
any use.
Multikine has been cleared by the regulators in ten countries around the
world, including the U.S. FDA, for a global Phase III clinical trial in advanced
primary (not yet treated) head and neck cancer patients. The trial is currently
under the management of two new clinical research organizations (CROs) who are
adding 60-80 clinical centers in existing and new countries to increase the
speed of patient enrollment.
The trial will test the hypothesis that Multikine treatment administered
prior to the current standard therapy for head and neck cancer patients
(surgical resection of the tumor and involved lymph nodes followed by
radiotherapy or radiotherapy and concurrent chemotherapy) will extend the
overall survival, enhance the local/regional control of the disease and reduce
the rate of disease progression in patients with advanced oral squamous cell
carcinoma.
The primary clinical endpoint in CEL-SCI's ongoing Phase III clinical
trial is that a 10% improvement in overall survival in the Multikine treatment
arm, plus the current standard of care (SOC - consisting of surgery +
radiotherapy or surgery + radiochemotherapy), over that which can be achieved in
the SOC arm alone (in the well-controlled Phase III clinical trial currently
ongoing) must be achieved. Based on what is presently known about the current
survival statistics for this population, CEL-SCI believes that achievement of
this endpoint should enable CEL-SCI, subject to further consultations with FDA,
to move forward, prepare and submit a Biologic License Application to FDA for
Multikine.
5
In this clinical trial Multikine is given to cancer patients first, i.e.,
prior to their receiving any conventional treatment for cancer, including
surgery, radiation and/or chemotherapy. This could be shown to be important
because conventional therapy may weaken the immune system, and may compromise
the potential effect of immunotherapy. Because Multikine is given before
conventional cancer therapy, when the immune system may be more intact, CEL-SCI
believes the possibility exists for it to have a greater likelihood of
activating an anti-tumor immune response under these conditions. This likelihood
is one of the clinical aspects being evaluated in the ongoing global Phase III
clinical trial.
Multikine is a different kind of investigational therapy in the fight
against cancer; Multikine is a defined mixture of cytokines. It is a combination
immunotherapy, possessing both active and passive properties.
In October 2012, and again in November 2013, in an interim review of the
safety data from the Phase III study, an Independent Data Monitoring Committee
(IDMC) raised no safety concerns. The IDMC also indicated that no safety signals
were found that would call into question the benefit/risk of continuing the
study. CEL-SCI considers the results of the IDMC review to be important since
studies have shown that up to 30% of Phase III trials fail due to safety
considerations and the IDMC's safety findings from this interim review were
similar to those reported by investigators during CEL-SCI's Phase I-II trials.
Ultimately, the decision as to whether a drug is safe is made by the FDA based
on an assessment of all of the data from a trial.
During the early investigational phase, in Phase I and Phase II clinical
trials in over 220 subjects who received the investigational therapy Multikine
in doses of 200 to 3200 IU (international units), no serious adverse events were
reported as being expressly due to administration of this investigational
therapy, and subjects in those clinical trials and the treating physicians
reported that this investigational therapy was well tolerated in those
early-stage clinical trials. Adverse events which were reported included pain at
the injection site, local minor bleeding and edema at the injection site,
diarrhea, headache, nausea, and constipation. No "abnormal" laboratory results
were reported following Multikine treatment - other than those commonly seen by
treating physicians in this patient population - regardless of Multikine
administration. Similarly, in these early-phase clinical studies in patients,
there was no reported increased toxicity of follow-on treatments as a result of
Multikine administration. No complications following surgery (such as increased
time for wound healing) were reported. No definitive conclusions can be drawn
from these data about the safety or efficacy profile of this investigational
therapy, further research is required and the global Phase III study is ongoing
in an effort to confirm these results.
The following is a summary of results from CEL-SCI's last Phase II study
conducted with Multikine. This study used the same treatment protocol as is
being used in CEL-SCI's Phase III study:
o In the final Phase II clinical study, using the same dosage and
treatment regimen as is being used in the Phase III study, head and
neck cancer patients with locally advanced primary disease who
received the investigational therapy Multikine as first-line
6
investigational therapy followed by surgery and radiotherapy were
reported by the clinical investigators to have had a 63.2% overall
survival (OS) rate at 3.5 years from surgery. This percentage OS was
arrived at as follows: of the 22 subjects enrolled in this final Phase
II study, the consent for the survival follow-up portion of the study
was received from 19 subjects. One subject did not consent to the
follow-up portion of the study. The other 2 subjects did not have
squamous cell carcinoma of the oral cavity and were thus not evaluable
per the protocol. The overall survival rate of subjects receiving the
investigational therapy in this study was compared to the overall
survival rate that was calculated based upon a review of 55 clinical
trials conducted in the same cancer population (with a total of 7,294
patients studied), and reported in the peer reviewed scientific
literature between 1987 and 2007. Review of this literature showed an
approximate survival rate of 47.5% at 3.5 year from treatment.
Therefore, the results of CEL-SCI's final Phase II study were
considered to be potentially favorable in terms of overall survival
recognizing the limitations of this early-phase study. It should be
noted that an earlier investigational therapy Multikine study appears
to lend support to the overall survival findings described above
-Feinmesser et al Arch Otolaryngol. Surg. 2003. However, no definitive
conclusions can be drawn from these data about the potential efficacy
or safety profile of this investigational therapy. Moreover, further
research is required, and these results must be confirmed in the
well-controlled Phase III clinical trial of this investigational
therapy that is currently in progress. Subject to completion of that
Phase III trial and FDA's review and acceptance of CEL-SCI's entire
data set on this investigational therapy, CEL-SCI believes that these
early-stage clinical trial results indicate the potential for this
investigational therapy to become a treatment for advanced primary
head and neck cancer.
o Reported average of 50% reduction in tumor cells in Phase II trials:
The clinical investigators who administered the three week Multikine
treatment regimen used in Phase II studies reported that, as was
determined in a controlled pathology study, Multikine administration
appeared to have caused, on average, the disappearance of about half
of the cancer cells present at surgery (as determined by
histopathology assessing the area of Stroma/Tumor (Mean+/- Standard
Error of the Mean of the number of cells counted per filed)) even
before the start of standard therapy such as radiation and
chemotherapy (Timar et al JCO 2005).
o Reported 12% complete response in the final Phase II trial: The
clinical investigators who administered the three week Multikine
investigational treatment regimen used in the final Phase II study
reported that, as was determined in a controlled pathology study, the
tumor apparently was no longer present (as determined by
histopathology) in approximately 12 % of patients (2 of 17 evaluable
by pathology). This determination was made by three pathologists
blinded to the study from the surgical specimen after a three week
treatment with Multikine (Timar et al JCO 2005).
o Adverse events reported in clinical trials: In clinical trials
conducted to date with the Multikine investigational therapy, adverse
events which have been reported by the clinical investigators as
possibly or probably related to Multikine administration included pain
at the injection site, local minor bleeding and edema at the injection
site, diarrhea, headache, nausea, and constipation.
7
The clinical significance of these and other data, to date, from the
multiple Multikine clinical trials is not yet known. These preliminary clinical
data do suggest the potential to demonstrate a possible improvement in the
clinical outcome for patients treated with Multikine.
Subject to completion of CEL-SCI's global Phase III clinical trial and
FDA's review of CEL-SCI's entire data set on this investigational therapy, if
the FDA were to conclude that the safety and efficacy of this investigational
therapy is established, the early-phase clinical data is encouraging in
suggesting the potential that approximately 60-66% (2/3) of head and neck cancer
patients with advanced primary disease could be candidates for this
investigational therapy if it were to be approved by FDA.
CEL-SCI has an agreement with Teva Pharmaceutical Industries, Ltd., which
provides Teva with the exclusive license to market and distribute Multikine in
Israel, Turkey, and, later on added Serbia and Croatia. Pursuant to the
agreement, Teva has signed up three hospitals and enrolled patients in Israel as
part of the Phase III trial. Revenues will be divided between CEL-SCI and Teva.
CEL-SCI has an agreement with Orient Europharma of Taiwan which provides
Orient Europharma with the exclusive marketing rights to Multikine for all
cancer indications in Taiwan, Singapore, Hong Kong, Malaysia, South Korea, the
Philippines, Australia and New Zealand. The agreement requires Orient Europharma
to fund the clinical trials needed to obtain marketing approvals in these
countries for head and neck cancer, naso-pharyngeal cancer and potentially
cervical cancer. Orient Europharma has signed up nine centers in Taiwan where it
has enrolled patients as part of the Phase III trial. Revenues will be divided
between CEL-SCI and Orient Europharma.
CEL-SCI has a licensing agreement with Byron Biopharma LLC ("Byron") under
which CEL-SCI granted Byron an exclusive license to market and distribute
Multikine in the Republic of South Africa. Pursuant to the agreement, Byron will
be responsible for registering the product in South Africa. Once Multikine has
been approved for sale, CEL-SCI will be responsible for manufacturing the
product, while Byron will be responsible for sales in South Africa. Revenues
will be divided between CEL-SCI and Byron.
In August 2011, CEL-SCI entered into an exclusive Sales, Marketing and
Distribution agreement with IDC-GP Pharm LLC ("IDC-GP Pharm") under which
CEL-SCI granted IDC-GP Pharm an exclusive license to market Multikine in the
countries of Argentina and Venezuela (the "Territory"). The agreement expired on
August 4, 2013 since IDC-GP Pharma did not receive regulatory approval of
Multikine in any country in the territory.
On April 23, 2013, the CEL-SCI announced that it has replaced the clinical
research organizations (CRO) running its Phase III clinical trial. This was
necessary since the patient enrollment in the study dropped off substantially
following a takeover of the CRO which caused most of the members of the CRO's
study team to leave the CRO. CEL-SCI has hired two CRO's who will manage the
global Phase III study; Aptiv Solutions and Ergomed who are both international
leaders in managing oncology trials. Both CRO's will help CEL-SCI expand the
trial by 60-80 clinical sites globally.
8
As of May 1, 2014, the last update given by CEL-SCI, the study had enrolled
183 patients. CEL-SCI expects to see a further increase in the number of
patients enrolled in the study at an accelerating pace as (i) the current
centers finalize all logistical issues and (ii) an additional 50-60 centers are
added throughout the world. Full enrollment of the planned 880 patients is
expected by the end of 2015.
In April 2013, CEL-SCI entered into a co-development agreement with
Ergomed. Under the co-development agreement, Ergomed will contribute up to $10
million towards the study in the form of offering discounted clinical services
in exchange for a single digit percentage of milestone and royalty payments, up
to a specified maximum amount, only from sales of Multikine. Ergomed, a
privately-held firm headquartered in Europe with global operations, has entered
into multiple similar co-development agreements, including one with Genzyme
(purchased by Sanofi in 2011 for over $20 billion). Ergomed will be responsible
for the majority of the new patient enrollment since it has a novel model for
clinical site management to accelerate patient recruitment and retention. For
example, Ergomed has almost 25 physicians who can directly call on clinical
sites to aid recruitment and retention. Some of the Ergomed physicians also have
the experience of being clinical investigators themselves. CEL-SCI believes that
this interaction on a physician to physician level is what is needed to help
increase enrollment in the Multikine study.
CEL-SCI estimates the total cash cost of the Phase III trial, with the
exception of the parts that will be paid by its partners, to be approximately
$31.3 million after June 16, 2014. This is in addition to approximately $13.3
million which has been spent as of June 16, 2014. This estimate is based on
information currently available in CEL-SCI's contracts with the Clinical
Research Organizations responsible for managing the Phase III trial. This number
can be affected by the speed of enrollment, foreign currency exchange rates and
many other factors, some of which cannot be foreseen today. It is therefore
possible that the cost of the Phase III trial will be higher than currently
estimated.
On October 7, 2013, CEL-SCI announced a Cooperative Research and
Development Agreement with the U.S. Naval Medical Center, San Diego. Pursuant to
this agreement, the Naval Medical Center will conduct Human Subjects
Institutional Review Board approved Phase I study of CEL-SCI's investigational
immunotherapy, Multikine, in HIV/HPV co-infected men and women with peri-anal
warts. Anal and genital warts are commonly associated with the Human Papilloma
Virus, the most common sexually transmitted disease. Men and women with a
history of anogenital warts have a 30 fold increased risk of anal cancer.
Persistent HPV infection in the anal region is thought to be responsible for up
to 80% of anal cancers. HPV is a significant health problem in the HIV infected
population as individuals are living longer as a result of greatly improved HIV
medications.
The purpose of this study is to evaluate the safety and clinical impact of
Multikine as a treatment of peri-anal warts and assess its effect on anal
intraepithelial dysplasia (AIN) in HIV/HPV co-infected men and women.
9
CEL-SCI will contribute the investigational study drug Multikine, will
retain all rights to any currently owned technology, and will have the right to
exclusively license any new technology developed from the collaboration.
Multikine will be given to the HIV/HPV co-infected patients with peri-anal
warts since promising early results were seen in another Institutional Review
Board approved Multikine Phase I study conducted at the University of Maryland.
In this study, investigational therapy Multikine was given to HIV/HPV
co-infected women with cervical dysplasia resulting in visual and histological
evidence of clearance of lesions. Furthermore, elimination of a number of HPV
strains was determined by in situ polymerase chain reaction (PCR) performed on
tissue biopsy collected before and after Multikine treatment. As reported by the
investigators in the earlier study, the study volunteers all appeared to
tolerate the treatment with no reported serious adverse events.
The treatment regimen for the study of up to 15 HIV/HPV co-infected patient
volunteers with peri-anal warts to be conducted by the Naval Medical Center will
be identical to the regimen that was used in the earlier Multikine cervical
study in HIV/HPV co-infected patients.
In October 2013, CEL-SCI entered into a co-development and profit sharing
agreement with Ergomed for Multikine in HIV/HPV co-infected men and women with
peri-anal warts. This agreement will initially be in support of the development
with the U.S. Navy. Ergomed will assume up to $3 million in clinical and
regulatory costs.
Also in October 2013, CEL-SCI entered into a co-development and profit
sharing agreement with Ergomed for Multikine in HIV/HPV co-infected women with
cervical dysplasia. Human Papilloma Virus (HPV) is the most common sexually
transmitted disease. HPV is a significant health problem in the HIV infected
population as individuals are living longer as a result of greatly improved HIV
medications. People living with HIV and others with compromised immunity are
more at risk for HPV-related complications. Persistent HPV infection can also be
a precursor to cervical cancer. Ergomed will assume up to $3 million in clinical
and regulatory costs.
CEL-SCI's focus in HPV is not the development of an antiviral against HPV
in the general population. Instead it is the development of an immunotherapy to
be used in patients who are immune suppressed by diseases such as HIV and are
therefore less able or unable to control HPV and its resultant diseases. This
group of patients has no viable treatments available to them and there are, to
CEL-SCI's knowledge, no competitors at the current time. HPV is also relevant to
the head and neck cancer Phase III study since it is now known that HPV is a
cause of head and neck cancer. Multikine was shown to kill HPV in an earlier
study of HIV infected women with cervical dysplasia.
Manufacturing Facility
Before starting the Phase III trial, CEL-SCI needed to build a dedicated
manufacturing facility to produce Multikine. This facility has been completed
10
and validated, and has produced several clinical lots for the Phase III clinical
trial. The facility has also passed review by a European Qualified Person on two
different occasions.
CEL-SCI completed validation of its new manufacturing facility in January
2010. The state-of-the-art facility is being used to manufacture Multikine for
CEL-SCI's Phase III clinical trial. In addition to using this facility to
manufacture Multikine, CEL-SCI, only if the facility is not being used for
Multikine, may offer the use of the facility as a service to pharmaceutical
companies and others, particularly those that need to "fill and finish" their
drugs in a cold environment (4 degrees Celsius, or approximately 39 degrees
Fahrenheit). However, priority will always be given to Multikine as management
considers the Multikine supply to the clinical studies and preparation for a
final marketing approval to be more important than offering fill and finish
services. Fill and finish is the process of filling injectable drugs in a
sterile manner and is a key part of the manufacturing process for many
medicines.
LEAPS
CEL-SCI's patented T-cell Modulation Process, referred to as LEAPS (Ligand
Epitope Antigen Presentation System), uses "heteroconjugates" to direct the body
to choose a specific immune response. LEAPS is designed to stimulate the human
immune system to more effectively fight bacterial, viral and parasitic
infections as well as autoimmune, allergies, transplantation rejection and
cancer, when it cannot do so on its own. Administered like a vaccine, LEAPS
combines T-cell binding ligands with small, disease associated, peptide antigens
and may provide a new method to treat and prevent certain diseases.
The ability to generate a specific immune response is important because
many diseases are often not combated effectively due to the body's selection of
the "inappropriate" immune response. The capability to specifically reprogram an
immune response may offer a more effective approach than existing vaccines and
drugs in attacking an underlying disease.
Using the LEAPS technology, CEL-SCI has created a potential peptide
treatment for H1N1 (swine flu) hospitalized patients. This LEAPS flu treatment
is designed to focus on the conserved, non-changing epitopes of the different
strains of Type A Influenza viruses (H1N1, H5N1, H3N1, etc.), including "swine",
"avian or bird", and "Spanish Influenza", in order to minimize the chance of
viral "escape by mutations" from immune recognition. Therefore one should think
of this treatment not really as an H1N1 treatment, but as a pandemic flu
treatment. CEL-SCI's LEAPS flu treatment contains epitopes known to be
associated with immune protection against influenza in animal models.
In September 2009, the U.S. Food and Drug Administration advised CEL-SCI
that it could proceed with its first clinical trial to evaluate the effect of
LEAPS-H1N1 treatment on the white blood cells of hospitalized H1N1 patients.
This followed an expedited initial review of CEL-SCI's regulatory submission for
this study proposal.
In November 2009, CEL-SCI announced that The Johns Hopkins University
School of Medicine had given clearance for CEL-SCI's first clinical study to
11
proceed using LEAPS-H1N1. Soon after the start of the study, the number of
hospitalized H1N1 patients dramatically declined and the study has been unable
to complete the enrollment of patients.
Additional work on this treatment for the pandemic flu is being pursued in
collaboration with the National Institute of Allergy and Infectious Diseases
(NIAID), part of the National Institutes of Health, USA. In May 2011 NIAID
scientists presented data at the Keystone Conference on "Pathogenesis of
Influenza: Virus-Host Interactions" in Hong Kong, China, showing the positive
results of efficacy studies in mice of L.E.A.P.S. H1N1 activated dendritic cells
(DCs) to treat the H1N1 virus. Scientists at the NIAID found that H1N1-infected
mice treated with LEAPS-H1N1 DCs showed a survival advantage over mice treated
with control DCs. The work was performed in collaboration with scientists led by
Kanta Subbarao, M.D., Chief of the Emerging Respiratory Diseases Section in
NIAID's Division of Intramural Research, part of the National Institutes of
Health, USA.
In July 2013, CEL-SCI announced the publication of the results of
additional influenza studies by researchers from the NIAID in the Journal of
Clinical Investigation (www.jci.org/articles/view/67550). The studies described
in the publication show that when CEL-SCI's investigational J-LEAPS Influenza
Virus treatments were used "in vitro" to activate immune cells called dendritic
cells (DCs), these activated dendritic cells, when injected into influenza
infected mice, arrested the progression of lethal influenza virus infection in
these mice. The work was performed in the laboratory of Dr. Subbarao.
With its LEAPS technology, CEL-SCI also developed a second peptide named
CEL-2000, a potential rheumatoid arthritis vaccine. The data from animal studies
of rheumatoid arthritis using the CEL-2000 treatment vaccine demonstrated that
CEL-2000 is an effective treatment against arthritis with fewer administrations
than those required by other anti-rheumatoid arthritis treatments, including
Enbrel(R). CEL-2000 is also potentially a more disease type-specific therapy, is
calculated to be significantly less expensive and may be useful in patients
unable to tolerate or who may not be responsive to existing anti-arthritis
therapies.
In February 2010 CEL-SCI announced that its CEL-2000 vaccine demonstrated
that it was able to block the progression of rheumatoid arthritis in a mouse
model. The results were published in the scientific peer-reviewed Journal of
International Immunopharmacology (online edition) in an article titled
"CEL-2000: A Therapeutic Vaccine for Rheumatoid Arthritis Arrests Disease
Development and Alters Serum Cytokine/Chemokine Patterns in the Bovine Collagen
Type II Induced Arthritis in the DBA Mouse Model" with lead author Daniel
Zimmerman, Ph.D., Senior Vice President of Research, Cellular Immunology at
CEL-SCI. The study was co-authored by scientists from CEL-SCI, Washington
Biotech, Northeastern Ohio Universities Colleges of Medicine and Pharmacy and
Boulder BioPath.
In August 2012, Dr. Zimmerman gave a Keynote presentation at the OMICS 2nd
International Conference on Vaccines and Vaccinations in Chicago. This
presentation showed how the LEAPS peptides administered altered only select
cytokines specific for each disease model, thereby improving the status of the
test animals and even preventing death and morbidity. These results support the
12
growing body of evidence that provides for its mode of action by a common format
in these unrelated conditions by regulation of Th1 (e.g., IL12 and IFN-a) and
their action on reducing TNF-a and other inflammatory cytokines as well
regulation of antibodies to these disease associated antigens. This was also
illustrated by a schematic model showing how these pathways interact and result
in the overall effect of protection and regulation of cytokines in a beneficial
manner.
Even though the various LEAPS drug candidates have not yet been given to
humans, they have been tested in vitro with human cells. They have induced
similar cytokine responses that were seen in these animal models, which may
indicate that the LEAPS technology might translate to humans. The LEAPS
candidates have demonstrated protection against lethal herpes simplex virus
(HSV1) and H1N1 influenza infection, as a prophylactic or therapeutic agent in
animals. They have also shown efficacy in animals in two autoimmune conditions,
curtailing and sometimes preventing disease progression in arthritis and
myocarditis animal models. CEL-SCI's belief is that the LEAPS technology may be
a significant alternative to the vaccines currently available on the market
today for these diseases.
None of the LEAPS investigational products have been approved for sale,
barter or exchange by the FDA or any other regulatory agency for any use to
treat disease in animals or humans. The safety or efficacy of these products has
not been established for any use. Lastly, no definitive conclusions can be drawn
from the early-phase, preclinical-trials data involving these investigational
products. Before obtaining marketing approval from the FDA in the United States,
and by comparable agencies in most foreign countries, these product candidates
must undergo rigorous preclinical and clinical testing which is costly and time
consuming and subject to unanticipated delays. There can be no assurance that
these approvals will be granted.
THE OFFERING
Securities Offered:
CEL-SCI may offer from time to time shares of common stock, preferred
stock, rights and warrants, or securities issuable upon the conversion or
exercise of such securities at an initial offering price not to exceed
$75,000,000, at prices and on terms to be determined at or prior to the time of
sale in light of market conditions at the time of sale. CEL-SCI may not use this
prospectus to complete sales of its securities unless this prospectus is
accompanied by a prospectus supplement. See the "Plan of Distribution" section
of this prospectus for additional information concerning the manner in which
CEL-SCI's securities may be offered.
Common Stock Outstanding: As of June 16, 2014 CEL-SCI had 65,970,785
outstanding shares of common stock. The
number of outstanding shares does not give
effect to shares which may be issued upon
the exercise and/or conversion of options,
warrants or other convertible securities.
See "Comparative Share Data" for more
information.
13
Risk Factors:
The purchase of the securities offered by
this prospectus involves a high degree of
risk. Risk factors include the lack of
revenues and history of loss, need for
additional capital and need for FDA approval.
See the "Risk Factors" section of this
prospectus for additional Risk Factors.
Common Stock
NYSE MKT Symbol: CVM
Series S Warrants
NYSE MKT Symbol: CVM WS
|
FORWARD LOOKING STATEMENTS
This prospectus contains various forward-looking statements that are based
on CEL-SCI's beliefs as well as assumptions made by and information currently
available to CEL-SCI. When used in this prospectus, the words "believe",
"expect", "anticipate", "estimate" and similar expressions are intended to
identify forward-looking statements. Such statements may include statements
regarding seeking business opportunities, payment of operating expenses, and the
like, and are subject to certain risks, uncertainties and assumptions which
could cause actual results to differ materially from projections or estimates.
Factors which could cause actual results to differ materially are discussed at
length under the heading "Risk Factors". Should one or more of the enumerated
risks or uncertainties materialize, or should underlying assumptions prove
incorrect, actual results may vary materially from those anticipated, estimated
or projected. Investors should not place undue reliance on forward-looking
statements, all of which speak only as of the date made.
RISK FACTORS
Investors should be aware that this offering involves the risks described
below, which could adversely affect the price of CEL-SCI's common stock. In
addition to the other information contained in this prospectus, the following
factors should be considered carefully in evaluating an investment in the
securities offered by this prospectus.
Risks Related to CEL-SCI
Since CEL-SCI has earned only limited revenues and has a history of losses,
CEL-SCI will require additional capital to remain in operation, complete its
clinical trials and fund pre-marketing expenses.
CEL-SCI has had only limited revenues since it was formed in 1983. Since
the date of its formation and through March 31, 2014, CEL-SCI incurred net
losses of approximately $(230,000,000). CEL-SCI has relied principally upon the
proceeds of public and private sales of its securities to finance its activities
to date.
14
If CEL-SCI cannot obtain additional capital, CEL-SCI may have to postpone
development and research expenditures, which will delay CEL-SCI's ability to
produce a competitive product. Delays of this nature may depress the price of
CEL-SCI's common stock. In addition, although CEL-SCI is not aware of a direct
competitor for Multikine, it is possible that one exists. There are many
potential competitors of LEAPS. If competitors develop, any delay in the
development of CEL-SCI's products may provide opportunities to those
competitors.
The condition of the overall economy may continue to affect both the
availability of capital and CEL-SCI's stock price. In addition, future capital
raises, which will be necessary for CEL-SCI's survival, will be further dilutive
to current shareholders. There can be no assurance that CEL-SCI will be able to
raise the capital it will need.
All of CEL-SCI's potential products, with the exception of Multikine, are in the
early stages of development, and any commercial sale of these products will be
many years away.
Even potential product sales from Multikine are years away, since cancer
trials can be lengthy. Accordingly, CEL-SCI expects to incur substantial losses
for the foreseeable future.
Since CEL-SCI does not intend to pay dividends on its common stock, any
potential return to investors will result only from any increases in the price
of CEL-SCI's common stock.
At the present time, CEL-SCI intends to use available funds to finance its
operations. Accordingly, while payment of dividends rests within the discretion
of CEL-SCI's Directors, no common stock dividends have been declared or paid by
CEL-SCI and CEL-SCI has no intention of paying any common stock dividends in the
foreseeable future. Any gains for CEL-SCI's investors will most likely result
from increases in the price of CEL-SCI's common stock, which has been volatile
in the recent past. If CEL-SCI's stock price does not increase, which likely
will depend primarily upon the results of the Multikine clinical trials, an
investor is unlikely to receive any return on an investment in CEL-SCI's common
stock.
The costs of CEL-SCI's product development and clinical trials are difficult to
estimate and will be very high for many years, preventing CEL-SCI from making a
profit for the foreseeable future, if ever.
Clinical and other studies necessary to obtain approval of a new drug can
be time consuming and costly, especially in the United States, but also in
foreign countries. CEL-SCI's estimates of the costs associated with future
clinical trials and research may be substantially lower than what CEL-SCI
actually experiences. It is impossible to predict what CEL-SCI will face in the
development of a product, such as LEAPS. The purpose of clinical trials is to
provide both CEL-SCI and regulatory authorities with safety and efficacy data in
humans. It is relatively common to revise a trial or add subjects to a trial in
progress. These examples of common vagaries in product development and clinical
investigations demonstrate how predicted costs may exceed reasonable
expectations. The different and often complex steps necessary to obtain
regulatory approval, especially that of the United States Food and Drug
Administration ("FDA") and the European Union's European Medicine's Agency
15
("EMA"), involve significant costs and may require several years to complete.
CEL-SCI expects that it will need substantial additional financing over an
extended period of time in order to fund the costs of future clinical trials,
related research, and general and administrative expenses.
The extent of CEL-SCI's clinical trials and research programs are primarily
based upon the amount of capital available to CEL-SCI and the extent to which it
receives regulatory approvals for clinical trials. CEL-SCI has established
estimates of the future costs of the Phase III clinical trial for Multikine,
but, as explained above, that estimate may not prove correct.
Compliance with changing regulations concerning corporate governance and public
disclosure may result in additional expenses.
Changing laws, regulations and standards relating to corporate governance
and public disclosure may create uncertainty regarding compliance matters. New
or changed laws, regulations and standards are subject to varying
interpretations in many cases. As a result, their application in practice may
evolve over time. CEL-SCI is committed to maintaining high standards of
corporate governance and public disclosure. Complying with evolving
interpretations of new or changing legal requirements may cause CEL-SCI to incur
higher costs as it revises current practices, policies and procedures, and may
divert management time and attention from potential revenue-generating
activities to compliance matters. If CEL-SCI's efforts to comply with new or
changed laws, regulations and standards differ from the activities intended by
regulatory or governing bodies due to ambiguities related to practice, CEL-SCI's
reputation may also be harmed. Further, CEL-SCI's board members, chief executive
officer and president could face an increased risk of personal liability in
connection with the performance of their duties. As a result, CEL-SCI may have
difficulty attracting and retaining qualified board members and executive
officers, which could harm its business.
CEL-SCI has not established a definite plan for the marketing of Multikine.
CEL-SCI has not established a definitive plan for marketing nor has it
established a price structure for any of its products. However, CEL-SCI intends,
if it is in a position to do so, to sell Multikine itself in certain markets and
to enter into written marketing agreements with various major pharmaceutical
firms with established sales forces. The sales forces in turn would, CEL-SCI
believes, target CEL-SCI's products to cancer centers, physicians and clinics
involved in head and neck cancer. CEL-SCI has already licensed Multikine to
three companies, Teva Pharmaceuticals in Israel, Turkey, Serbia and Croatia,
Orient Europharma in Taiwan, Singapore, Hong Kong, Malaysia, South Korea, the
Philippines, Australia and New Zealand, and Byron BioPharma, LLC in South
Africa. CEL-SCI believes that these companies have the resources to market
Multikine appropriately in their respective territories, but there is no
guarantee that they will. There is no assurance that CEL-SCI will find qualified
parties willing to market CEL-SCI's product in other areas.
CEL-SCI may encounter problems, delays and additional expenses in
developing marketing plans with outside firms. In addition, even if Multikine is
cost effective and proven to increase overall survival, CEL-SCI may experience
16
other limitations involving the proposed sale of Multikine, such as uncertainty
of third-party reimbursement. There is no assurance that CEL-SCI can
successfully market any products which it may develop.
CEL-SCI hopes to expand its clinical development capabilities in the future, and
any difficulties hiring or retaining key personnel or managing this growth could
disrupt CEL-SCI's operations.
CEL-SCI is highly dependent on the principal members of CEL-SCI's
management and development staff. If the Multikine clinical trial is successful,
CEL-SCI expects to expand its clinical development and manufacturing
capabilities, which will involve hiring additional employees. Future growth will
require CEL-SCI to continue to implement and improve CEL-SCI's managerial,
operational and financial systems and to continue to retain, recruit and train
additional qualified personnel, which may impose a strain on CEL-SCI's
administrative and operational infrastructure. The competition for qualified
personnel in the biopharmaceutical field is intense. CEL-SCI is highly dependent
on its ability to attract, retain and motivate highly qualified management and
specialized personnel required for clinical development. Due to CEL-SCI's
limited resources, CEL-SCI may not be able to manage effectively the expansion
of its operations or recruit and train additional qualified personnel. If
CEL-SCI is unable to retain key personnel or manage its growth effectively,
CEL-SCI may not be able to implement its business plan.
Multikine is made from components of human blood, which involves inherent risks
that may lead to product destruction or patient injury.
Multikine is made, in part, from components of human blood. There are
inherent risks associated with products that involve human blood such as
possible contamination with viruses, including Hepatitis or HIV. Any possible
contamination could require CEL-SCI to destroy batches of Multikine or cause
injuries to patients who receive the product, thereby subjecting CEL-SCI to
possible financial losses, lawsuits, and harm to its business.
Although CEL-SCI has product liability insurance for Multikine, the
successful prosecution of a product liability case against CEL-SCI could have a
materially adverse effect upon its business if the amount of any judgment
exceeds CEL-SCI's insurance coverage. Such a suit also could damage the
reputation of Multikine and make successful marketing of the product less
likely. CEL-SCI commenced the Phase III clinical trial for Multikine in December
2010. Although no claims have been brought to date, participants in CEL-SCI's
clinical trials could bring civil actions against CEL-SCI for any unanticipated
harmful effects arising from the use of Multikine or any drug or product that
CEL-SCI may attempt to develop.
Risks Related to Government Approvals
CEL-SCI's product candidates must undergo rigorous preclinical and clinical
testing and regulatory approvals, which could be costly and time-consuming and
subject CEL-SCI to unanticipated delays or prevent CEL-SCI from marketing any
products.
17
Therapeutic agents, drugs and diagnostic products are subject to approval,
prior to general marketing, from the FDA in the United States, the EMA in the
European Union, and by comparable agencies in most foreign countries. Before
obtaining marketing approval, these product candidates must undergo costly and
time consuming preclinical and clinical testing which could subject CEL-SCI to
unanticipated delays and may prevent CEL-SCI from marketing its product
candidates. There can be no assurance that such approvals will be granted.
CEL-SCI cannot be certain when or under what conditions it will undertake
future clinical trials. A variety of issues may delay CEL-SCI's Phase III
clinical trial for Multikine or preclinical and early clinical trials for other
products. For example, early trials, or the plans for later trials, may not
satisfy the requirements of regulatory authorities, such as the FDA. CEL-SCI may
fail to find subjects willing to enroll in CEL-SCI's trials. CEL-SCI
manufactures Multikine, but relies on third party vendors for managing the trial
process and other activities, and these vendors may fail to meet appropriate
standards. Accordingly, the clinical trials relating to CEL-SCI's product
candidates may not be completed on schedule, the FDA or foreign regulatory
agencies may order CEL-SCI to stop or modify its research, or these agencies may
not ultimately approve any of CEL-SCI's product candidates for commercial sale.
Varying interpretations of the data obtained from pre-clinical and clinical
testing could delay, limit or prevent regulatory approval of CEL-SCI's product
candidates. The data collected from CEL-SCI's clinical trials may not be
sufficient to support regulatory approval of its various product candidates,
including Multikine. CEL-SCI's failure to adequately demonstrate the safety and
efficacy of any of its product candidates would delay or prevent regulatory
approval of its product candidates in the United States, which could prevent
CEL-SCI from achieving profitability. Although CEL-SCI had positive results in
its Phase II trials for Multikine, those results were for a very small sample
set, and CEL-SCI will not know definitively how Multikine will perform until
CEL-SCI is well into, or completes, its Phase III clinical trial.
The requirements governing the conduct of clinical trials, manufacturing,
and marketing of CEL-SCI's product candidates, including Multikine, outside the
United States vary from country to country. Foreign approvals may take longer to
obtain than FDA approvals and can require, among other things, additional
testing and different trial designs. Foreign regulatory approval processes
include all of the risks associated with the FDA approval process. Some of those
agencies also must approve prices for products approved for marketing. Approval
of a product by the FDA or the EMA does not ensure approval of the same product
by the health authorities of other countries. In addition, changes in regulatory
requirements for product approval in any country during the clinical trial
process and regulatory agency review of each submitted new application may cause
delays or rejections.
CEL-SCI has only limited experience in filing and pursuing applications
necessary to gain regulatory approvals. CEL-SCI's lack of experience may impede
its ability to obtain timely approvals from regulatory agencies, if at all.
CEL-SCI will not be able to commercialize Multikine and other product candidates
until it has obtained regulatory approval. In addition, regulatory authorities
may also limit the types of patients to which CEL-SCI or others may market
Multikine or CEL-SCI's other products. Any failure to obtain or any delay in
obtaining required regulatory approvals may adversely affect the ability of
CEL-SCI or potential licensees to successfully market CEL-SCI's products. Even
if CEL-SCI obtains regulatory approval for its product candidates, CEL-SCI will
be subject to stringent, ongoing government regulation.
18
If CEL-SCI's products receive regulatory approval, either in the United
States or internationally, CEL-SCI will continue to be subject to extensive
regulatory requirements. These regulations are wide-ranging and govern, among
other things:
o product design, development and manufacture;
o product application and use
o adverse drug experience;
o product advertising and promotion;
o product manufacturing, including good manufacturing practices
o record keeping requirements;
o registration and listing of CEL-SCI's establishments and products with
the FDA, EMA and other state and national agencies;
o product storage and shipping;
o drug sampling and distribution requirements;
o electronic record and signature requirements; and
o labeling changes or modifications.
CEL-SCI and any third-party manufacturers or suppliers must continually
adhere to federal regulations setting forth requirements, known as current Good
Manufacturing Practices, or cGMPs, and their foreign equivalents, which are
enforced by the FDA, the EMA and other national regulatory bodies through their
facilities inspection programs. If CEL-SCI's facilities, or the facilities of
CEL-SCI's contract manufacturers or suppliers, cannot pass a pre-approval plant
inspection, the FDA, EMA, or other national regulators will not approve the
marketing applications of CEL-SCI's product candidates. In complying with cGMP
and foreign regulatory requirements, CEL-SCI and any of its potential
third-party manufacturers or suppliers will be obligated to expend time, money
and effort in production, record-keeping and quality control to ensure that
CEL-SCI's products meet applicable specifications and other requirements.
If CEL-SCI does not comply with regulatory requirements at any stage,
whether before or after marketing approval is obtained, CEL-SCI may be subject
to license suspension or revocation, criminal prosecution, seizure, injunction,
fines, be forced to remove a product from the market or experience other adverse
consequences, including restrictions or delays in obtaining regulatory marketing
approval for such products or for other products for which it seeks approval.
This could materially harm CEL-SCI's financial results, reputation and stock
price. Additionally, CEL-SCI may not be able to obtain the labeling claims
necessary or desirable for product promotion. CEL-SCI may also be required to
undertake post-marketing trials, which will be evaluated by applicable
authorities to determine if CEL-SCI's products may remain on the market. If
CEL-SCI or other parties identify adverse effects after any of CEL-SCI's
products are on the market, or if manufacturing problems occur, regulatory
approval may be suspended or withdrawn. CEL-SCI may be required to reformulate
19
its products, conduct additional clinical trials, make changes in product
labeling or indications of use, or submit additional marketing applications to
support any changes. If CEL-SCI encounters any of the foregoing problems, its
business and results of operations will be harmed and the market price of its
common stock may decline.
CEL-SCI cannot predict the extent of adverse government regulations which
might arise from future legislative or administrative action. Without government
approval, CEL-SCI will be unable to sell any of its products.
Foreign governments often impose strict price controls, which may adversely
affect CEL-SCI's future profitability.
CEL-SCI intends to seek approval to market Multikine in both the United
States and foreign jurisdictions. If CEL-SCI obtains approval in one or more
foreign jurisdictions, CEL-SCI will be subject to rules and regulations in those
jurisdictions relating to Multikine. In some foreign countries, particularly in
the European Union, prescription drug pricing is subject to governmental
control. In these countries, pricing negotiations with governmental authorities
can take considerable time after the receipt of marketing approval for a drug
candidate. To obtain reimbursement or pricing approval in some countries,
CEL-SCI may be required to conduct a clinical trial that compares the
cost-effectiveness of Multikine to other available therapies. If reimbursement
of Multikine is unavailable or limited in scope or amount, or if pricing is set
at unsatisfactory levels, CEL-SCI may be unable to achieve or sustain
profitability.
Risks Related to Intellectual Property
CEL-SCI may not be able to achieve or maintain a competitive position, and other
technological developments may result in CEL-SCI's proprietary technologies
becoming uneconomical or obsolete.
CEL-SCI is involved in a biomedical field that is undergoing rapid and
significant technological change. The pace of change continues to accelerate.
The successful development of products from CEL-SCI's compounds, compositions
and processes through CEL-SCI-financed research, or as a result of possible
licensing arrangements with pharmaceutical or other companies, is not assured.
Many companies are working on drugs designed to cure or treat cancer or
cure and treat viruses, such as HPV or H1N1. Many of these companies have
financial, research and development, and marketing resources, which are much
greater than CEL-SCI's, and are capable of providing significant long-term
competition either by establishing in-house research groups or by forming
collaborative ventures with other entities. In addition, smaller companies and
non-profit institutions are active in research relating to cancer and infectious
diseases. CEL-SCI's market share will be reduced or eliminated if CEL-SCI's
competitors develop and obtain approval for products that are safer or more
effective than CEL-SCI's products.
20
CEL-SCI's patents might not protect CEL-SCI's technology from competitors, in
which case CEL-SCI may not have any advantage over competitors in selling any
products which it may develop.
Certain aspects of CEL-SCI's technologies are covered by U.S. and foreign
patents. In addition, CEL-SCI has a number of new patent applications pending.
There is no assurance that the applications still pending or which may be filed
in the future will result in the issuance of any patents. Furthermore, there is
no assurance as to the breadth and degree of protection any issued patents might
afford CEL-SCI. Disputes may arise between CEL-SCI and others as to the scope
and validity of these or other patents. Any defense of the patents could prove
costly and time consuming and there can be no assurance that CEL-SCI will be in
a position, or will deem it advisable, to carry on such a defense. A suit for
patent infringement could result in increasing costs, delaying or halting
development, or even forcing CEL-SCI to abandon a product. Other private and
public concerns, including universities, may have filed applications for, may
have been issued, or may obtain additional patents and other proprietary rights
to technology potentially useful or necessary to CEL-SCI. CEL-SCI currently is
not aware of any such patents, but the scope and validity of such patents, if
any, and the cost and availability of such rights are impossible to predict.
Also, as far as CEL-SCI relies upon unpatented proprietary technology, there is
no assurance that others may not acquire or independently develop the same or
similar technology.
Much of CEL-SCI's intellectual property is protected as a trade secret, not as a
patent.
Much of CEL-SCI's intellectual property pertains to its manufacturing
system, certain aspects of which may not be suitable for patent filing and must
be protected as trade secrets. Those trade secrets must be protected diligently
by CEL-SCI to protect their disclosure to competitors, since legal protections
after disclosure may be minimal or non-existent. Accordingly, much of CEL-SCI's
value is dependent upon its ability to keep its trade secrets confidential.
Although CEL-SCI takes measures to ensure confidentiality, CEL-SCI may fail in
that attempt. In addition, in some cases a regulator considering CEL-SCI's
application for product approval may require the disclosure of some or all of
CEL-SCI's proprietary information. In such a case, CEL-SCI must decide whether
to disclose the information or forego approval in a particular country. If
CEL-SCI is unable to market its products in key countries, CEL-SCI's
opportunities and value may suffer.
Risks Related to CEL-SCI's Common Stock
Since the market price for CEL-SCI's common stock is volatile, investors may not
be able to sell any of CEL-SCI's shares at a profit.
The market price of CEL-SCI's common stock, as well as the securities of
other biopharmaceutical and biotechnology companies, have historically been
highly volatile, and the market has from time to time experienced significant
price and volume fluctuations that are unrelated to the operating performance of
particular companies. During the twelve months ended May 31, 2014, CEL-SCI's
post-split stock price has ranged from a low of $0.53 per share to a high of
21
$2.70 per share. Factors such as fluctuations in CEL-SCI's operating results,
announcements of technological innovations or new therapeutic products by
CEL-SCI or its competitors, governmental regulation, developments in patent or
other proprietary rights, public concern as to the safety of products developed
by CEL-SCI or other biotechnology and pharmaceutical companies, publications by
market analysts, law suits, and general market conditions may have a significant
effect on the future market price of CEL-SCI's common stock.
Future sales of CEL-SCI's securities may dilute the value of current investors'
holdings.
The provisions in CEL-SCI's Articles of Incorporation relating to CEL-SCI's
preferred stock allow CEL-SCI's directors to issue preferred stock with rights
to multiple votes per share and dividend rights which would have priority over
any dividends paid with respect to CEL-SCI's common stock. The issuance of
preferred stock with such rights may make more difficult the removal of
management even if such removal would be considered beneficial to shareholders
generally, and will have the effect of limiting shareholder participation in
certain transactions such as mergers or tender offers if such transactions are
not favored by incumbent management. In addition, CEL-SCI has issued warrants in
the past and may do so in the future. These warrants, providing a future right
to purchase shares of CEL-SCI's common stock at an established price, may
further dilute the ownership of current shareholders.
In order to raise additional capital, CEL-SCI may need to sell shares of
its common stock, or securities convertible into common stock, at prices that
may be below the prevailing market price of CEL-SCI's common stock at the time
of sale. Since CEL-SCI's stock price has been volatile, even a sale at market
price one week may represent a substantial "discount" over the prior week's
price. Future sales of CEL-SCI's securities will dilute CEL-SCI's current
stockholders and investors and may have a negative effect on the market price of
its common stock.
Shares issuable upon the conversion of a note or upon the exercise of
outstanding warrants and options may substantially increase the number of shares
available for sale in the public market and may depress the price of CEL-SCI's
common stock.
As of June 16, 2014, there were outstanding options which allows the
holders to purchase approximately 5,996,000 shares of CEL-SCI's common stock, at
prices ranging between $0.85 and $20.00 per share, outstanding warrants which
allow the holders to purchase approximately 36,767,000 shares of CEL-SCI's
common stock, at prices ranging between $0.53 and $17.50 per share, and a
convertible note which allows the holder to acquire approximately 276,000 shares
of CEL-SCI's common stock at a conversion price of $4.00. The outstanding
options and warrants could adversely affect CEL-SCI'S ability to obtain future
financing or engage in certain mergers or other transactions, since the holders
of options and warrants can be expected to exercise them at a time when CEL-SCI
may be able to obtain additional capital through a new offering of securities on
terms more favorable to CEL-SCI than the terms of the outstanding options and
warrants. For the life of the options, warrants and the convertible note, the
holders have the opportunity to profit from a rise in the market price of
CEL-SCI's common stock without assuming the risk of ownership. The issuance of
shares upon the exercise of outstanding options and warrants, or the conversion
of the note, will also dilute the ownership interests of CEL-SCI's existing
stockholders.
22
Substantially all of the shares of common stock that are issuable upon the
conversions of the note, of the exercise of outstanding options and warrants,
may be sold in the public market. The sale of common stock described above, or
the perception that such sales could occur, may adversely affect the market
price of CEL-SCI's common stock.
Any decline in the price of CEL-SCI's common stock may encourage short
sales, which could place further downward pressure on the price of CEL-SCI's
common stock. Short selling is a practice of selling shares which are not owned
by a seller at that time, with the expectation that the market price of the
shares will decline in value after the sale, providing the short seller a
profit.
We may have exposure for certain securities we sold in October 2013.
In September 2012, we filed a shelf registration statement covering the
sale of $50,000,000 of securities (the "2012 Registration Statement"), and in
January 2013 we filed another shelf registration statement covering the sale of
an additional $50,000,000 of securities (the "2013 Registration Statement"). In
October 2013, we filed a prospectus supplement to the 2012 Registration
Statement for the sale in an underwritten public offering of 17,826,087 shares
of our common stock, 20,475,000 Series S Warrants, as well as up to 20,475,000
shares of common stock issuable upon the exercise of the Series S warrants (the
"October Prospectus"). Collectively, we offered approximately $43.4 million of
securities pursuant to the October Prospectus. This amount includes
approximately $17.8 million for the sale of the common stock and Series S
warrants and $25.6 million upon the exercise of the Series S Warrants. We
subsequently realized that at the time of the October 2013 offering we had
approximately $28.9 million available for issuance under the 2012 Registration
Statement. As a result, we issued securities that were not registered with the
SEC, and that may not have been eligible for an exemption from registration
under the federal or state securities laws. We had securities available under
the 2013 Registration Statement to register all of the securities not covered by
the 2012 Registration Statement. In December 2013, we filed a prospectus
supplement to the 2013 Registration Statement registering the offer and sale of
all of the shares of common stock issuable upon exercise of the Series S
Warrants included in the October 2013 offering to assure that the offering and
sale of all of the shares issuable upon exercise of the Series S Warrants were
registered (the "December Prospectus"). Prior to the filing of the December
Prospectus, no Series S Warrants issued in the October offering had been
exercised. Notwithstanding the above, the actions we have taken to mitigate our
possible non-compliance with securities laws will not prevent regulators from
asserting that we violated the law, from imposing penalties and fines against us
with respect to any potential violations of securities laws, and may subject us
to possible claims for damages from certain investors.
COMPARATIVE SHARE DATA
Number of Shares
Shares outstanding as of June 16, 2014 65,970,785
23
The number of shares outstanding as of June 16, 2014 excludes shares which
may be issued upon the exercise of the options or warrants, or the conversion of
the note, described below.
Other Shares Which May Be Issued:
Number of Note
Shares Reference
------ ---------
Shares issuable upon exercise of Series L warrants 70,000 A
Shares issuable upon the exercise of
Series N warrants 2,844,627 B
Shares issuable upon the exercise of warrants
held by private investors 54,250 C
Shares issuable upon exercise of options granted
to CEL-SCI's officers, directors, employees,
consultants, and third parties 5,995,681 D
Shares issuable upon exercise of Series A warrants 130,347 E
Shares issuable upon conversion of note payable to
officer and director 276,014 F
Shares issuable upon exercise of warrants held by
officer and director 349,754 F
Shares issuable upon exercise of Series B warrants 50,000 G
Shares issuable upon exercise of Series C warrants 463,487 H
Shares issuable upon exercise of Series E warrants 71,428 I
Shares issuable upon exercise of Series F warrants 1,200,000 J
Shares issuable upon exercise of Series G warrants 66,667 J
Shares issuable upon exercise of Series H warrants 1,200,000 K
Shares issuable upon exercise of Series P warrants 590,001 L
Shares issuable upon exercise of Series Q warrants 1,200,000 M
Shares issuable upon exercise of Series R warrants 2,625,000 N
|
24
Shares issuable upon exercise of Series S warrants 23,624,326 O
Shares issuable upon exercise of Series T warrants 1,782,057 P
Shares issuable upon exercise of Series U warrants 445,514 P
A. The Series L warrants allow the holders to purchase up to:
o 70,000 shares of CEL-SCI's common stock at a price of $2.50 per share
at any time on or before April 2, 2015.
B. On August 18, 2008, CEL-SCI sold 138,339 shares of common stock and 207,508
Series N warrants in a private financing for $1,037,500. In June 2009, an
additional 116,667 shares and 181,570 Series N warrants were issued to the
investors. In October 2011, an additional 83,333 shares and 129,693 Series N
warrants were issued to the investors. In October 2013, an additional 764,602
shares and 1,189,961 Series N warrants were issued to the investors. In December
2013, an additional 798,481 shares and 1,242,688 Series N warrants were issued
to the investors. In January 2014, CEL-SCI offered to the investors to extend
the Series N warrants by one year and allow for cashless exercise in exchange
for cancelling the reset provision in the warrant agreement. One of the
investors accepted this offer. As of June 16, 2014, 106,793 Series N Warrants
had been exercised. The remaining 2,844,627 Series N warrants entitle the
holders to purchase one share of CEL-SCI's common stock at a price of $0.52731
per share at any time prior to August 18, 2015.
C. Between May 30, 2003 and July 8, 2009, CEL-SCI sold shares of its common
stock in private transactions. In some cases warrants were issued as part of the
financings. The names of the warrant holders and the terms of the warrants are
shown below:
Shares Issuable
Issue Upon Exercise Exercise Expiration
Warrant Holder Date of Warrants Price Date
-------------- ----- ------------ ----- ----
Cher Ami Holdings 7/18/05 37,500 $ 6.50 7/18/14
Christian Schleuning 7/8/09 16,750 $ 5.00 1/8/15
---------
|
54,250
The shares of common stock issuable upon the exercise of these warrants
were registered by means of a separate registration statement.
D. The options are exercisable at prices ranging from $0.85 to $20.00 per share.
CEL-SCI may also grant options to purchase additional shares under its Incentive
Stock Option and Non-Qualified Stock Option Plans.
25
E. Between June 23 and July 1, 2009, CEL-SCI sold 1,509,935 shares of its common
stock at a price of $4.00 per share. The investors in this offering also
received 1,011,656 Series A warrants. Each Series A warrant entitles the holder
to purchase one share of CEL-SCI's common stock. The Series A warrants may be
exercised at any time prior to December 24, 2014 at a price of $5.00 per share.
As of June 16, 2014, 881,309 Series A warrants had been exercised. The remaining
130,347 Series A warrants entitle the holders to purchase one share of CEL-SCI's
common stock at a price of $5.00 per share.
F. Between December 2008 and June 2009, Maximilian de Clara, CEL-SCI's President
and a director, loaned CEL-SCI $1,104,057. The loan was initially payable at the
end of March, 2009, but was extended to the end of June, 2009. At the time the
loan was due, and in accordance with the loan agreement, CEL-SCI issued Mr. de
Clara a warrant which entitles Mr. de Clara to purchase 164,824 shares of
CEL-SCI's common stock at a price of $4.00 per share. The warrant is exercisable
at any time prior to December 24, 2014. Although the loan was to be repaid from
the proceeds of a financing, CEL-SCI's Directors deemed it beneficial not to
repay the loan and negotiated a second extension of the loan with Mr. de Clara
on terms similar to the June 2009 financing. Pursuant to the terms of the second
extension the note is now due on July 6, 2014, but, at Mr. de Clara's option,
the loan can be converted into shares of CEL-SCI's common stock. The number of
shares which will be issued upon any conversion will be determined by dividing
the amount to be converted by $4.00. As further consideration for the second
extension, Mr. de Clara received warrants which allow Mr. de Clara to purchase
184,930 shares of CEL-SCI's common stock at a price of $5.00 per share at any
time prior to January 6, 2015. On May 13, 2011, to recognize Mr. de Clara's
willingness to agree to subordinate his note to convertible preferred shares and
convertible debt, CEL-SCI extended the maturity date of the note to July 6,
2015. The loan from Mr. de Clara bears interest at 15% per year and is secured
by a lien on substantially all of CEL-SCI's assets. CEL-SCI does not have the
right to prepay the loan without Mr. de Clara's consent. As of June 16, 2014,
none of the warrants issued to Mr. De Clara had been exercised.
G. On August 31, 2009, CEL-SCI borrowed $2,000,000 from two institutional
investors. The loans are evidenced by CEL-SCI's Series B promissory notes which
were repaid in September 2009. The Series B note holders also received Series B
warrants which allow the holders to purchase up to 50,000 shares of CEL-SCI's
common stock at a price of $6.80 per share. The Series B warrants may be
exercised at any time prior to September 4, 2014. As of June 16, 2014, none of
the Series B Warrants had been exercised.
H. On August 20, 2009, CEL-SCI sold 1,078,444 shares of its common stock to a
group of private investors for $4,852,995 or $4.50 per share. The investors also
received Series C warrants which entitle the investors to purchase 539,220
shares of CEL-SCI's common stock. The Series C warrants may be exercised at any
time prior to February 20, 2015 at a price of $5.50 per share. As of June 16,
2014, 75,733 Series C warrants had been exercised. The remaining 463,487 Series
C warrants entitle the holders to purchase one share of CEL-SCI's common stock
at a price of $5.50 per share.
26
I. On September 21, 2009, CEL-SCI sold 1,428,572 shares of its common stock to a
group of private investors for $20,000,000 or $14.00 per share. The investors
also received Series D warrants which entitle the investors to purchase up to
471,428 shares of CEL-SCI's common stock. The Series D warrants could be
exercised at any time prior to September 21, 2011 at a price of $15.00 per
share. On September 21, 2011, all Series D warrants expired.
CEL-SCI paid Rodman & Renshaw, LLC, the placement agent for the offering, a
cash commission of $1,000,000, as well as an expense reimbursement of $37,500.
CEL-SCI also issued Rodman & Renshaw 71,428 Series E warrants. Each Series E
warrant entitles the holder to purchase one share of CEL-SCI's common stock. The
Series E warrants may be exercised at any time prior to August 12, 2014 at a
price of $17.50 per share. As of June 16, 2014, none of the Series E warrants
had been exercised.
J. On October 3, 2011 CEL-SCI sold 1,333,333 shares of its common stock to a
group of private investors for $4,000,000 or $3.00 per share. The investors also
received Series F warrants which entitle the investors to purchase up to
1,200,000 shares of CEL-SCI's common stock. The Series F warrants may be
exercised at any time prior to October 6, 2014 at a price of $4.00 per share.
CEL-SCI paid Chardan Capital Markets, LLC, the placement agent for this
offering, a cash commission of $140,000, and issued 66,667 Series G warrants to
Chardan. Each Series G warrant entitles the holder to purchase one share of
CEL-SCI's common stock. The Series G warrants may be exercised at any time prior
to August 12, 2014 at a price of $4.00 per share. As of June 16, 2014, none of
the Series F or G warrants had been exercised.
K. On January 25, 2012, CEL-SCI sold 1,600,000 shares of its common stock to
institutional investors for $5,760,000 or $3.60 per share. The investors also
received Series H warrants which entitle the investors to purchase up to
1,200,000 shares of CEL-SCI's common stock. The Series H warrants may be
exercised at any time prior to August 1, 2015 at a price of $5.00 per share. As
of June 16, 2014, none of the Series H Warrants had been exercised.
L. On February 10, 2012, CEL-SCI issued 590,001 Series P warrants to the former
holder of the Series O warrants as an inducement for the early exercise of the
Series O warrants. The Series P warrants allow the holder to purchase up to
590,001 shares of CEL-SCI's common stock at a price of $4.50 per share. The
Series P warrants are exercisable at any time prior to March 7, 2017. As of June
16, 2014, none of the Series P Warrants had been exercised.
M. On June 21, 2012, CEL-SCI sold 1,600,000 shares of its common stock to
institutional investors for $5,600,000 or $3.50 per share. The investors also
received Series Q warrants which allow the investors to purchase up to 1,200,000
shares of CEL-SCI's common stock. The Series Q warrants may be exercised at any
time after prior to December 22, 2015 at a price of $5.00 per share. As of June
16, 2014, none of the Series Q Warrants had been exercised.
N. On December 4, 2012, CEL-SCI sold 3,500,000 shares of its common stock to
institutional investors for $10,500,000 or $3.00 per share. The investors also
received Series R warrants which entitle the investors to purchase up to
2,625,000 shares of CEL-SCI's common stock. The Series R warrants may be
27
exercised at any time prior to December 7, 2016 at a price of $4.00 per share.
As of June 16, 2014, none of the Series R Warrants had been exercised.
O. On October 11, 2013, CEL-SCI, in an underwritten public offering, sold
17,826,087 shares of its common stock, as well as 20,475,000 Series S warrants,
for net proceeds of approximately $16,424,000, after deduction for underwriting
discounts and commissions. The Series S warrants may be exercised at any time
prior to October 11, 2018 at a price of $1.25 per share.
On December 24, 2013, CEL-SCI closed a public offering of 4,761,905 units
of common stock and warrants at a price of $0.63 per unit for net proceeds of
$2,790,000, net of underwriting discounts and commissions and offering expenses
of the Company. Each unit consisted of one share of common stock and one Series
S warrant to purchase one share of common stock. The Series S warrants are
immediately exercisable, expire on October 11, 2018, and have an exercise price
of $1.25. The underwriters purchased an additional 476,190 units of common stock
and warrants pursuant to the overallotment option, for which the Company
received net proceeds of approximately $279,000.
On February 7, 2014, the warrants issued in connection with the public
offerings in October and December 2013 began trading on the NYSE MKT under the
ticker symbol "CVM WS". As of June 16, 2014, 2,088,769 Series S Warrants had
been exercised. The remaining 23,624,326 Series S warrants entitle the holders
to purchase one share of CEL-SCI's common stock at a price of $1.25 per share.
P. On April 17, 2014, CEL-SCI closed a public offering of 7,128,229 shares of
common stock and warrants to purchase an aggregate of 1,782,057 shares of common
stock. The units were sold at a price of $1.40 per unit and resulted in net
proceeds of approximately $9.23 million. The warrants are immediately
exercisable, expire on October 17, 2014, and have an exercise price of $1.58 per
share. As of June 16, 2014, none of the Series T Warrants had been exercised.
CEL-SCI issued Dawson James Securities, Inc. and Laidlaw & Company (UK)
Ltd. 445,514 Series U warrants for being joint book-running managers and
underwriters for this offering. Each Series U warrant entitles the holder to
purchase one share of CEL-SCI's common stock. The Series U warrants may be
exercised beginning October 17, 2014 at a price of $1.75 per share and expire on
October 17, 2017. As of June 16, 2014, none of the Series U warrants had been
exercised.
MARKET FOR CEL-SCI'S COMMON STOCK
As of June 16, 2014 there were approximately 1,370 record holders of
CEL-SCI's common stock. CEL-SCI's common stock is traded on the NYSE MKT under
the symbol "CVM".
On June 25, 2013, CEL-SCI's shareholders approved a reverse split of
CEL-SCI's common stock. The reverse split became effective on the NYSE MKT on
September 25, 2013. On that date, every ten issued and outstanding shares of
28
CEL-SCI's common stock automatically converted into one outstanding share.
As a result of the reverse stock split, the number of CEL-SCI's outstanding
shares of common stock decreased from 310,005,272 (pre-split) shares to
31,001,686 (post-split) shares. In addition, by reducing the number of CEL-SCI's
outstanding shares, CEL-SCI's loss per share in all prior periods will increase
by a factor of ten.
Shown below are the post-split range of high and low quotations for
CEL-SCI's common stock for the periods indicated as reported on the NYSE MKT.
The market quotations reflect inter-dealer prices, without retail mark-up,
mark-down or commissions and may not necessarily represent actual transactions.
Quarter Ending High Low
12/31/11 $4.20 $2.70
3/31/12 $6.50 $2.80
6/30/12 $5.80 $3.30
9/30/12 $4.70 $3.10
12/31/12 $3.90 $2.60
3/31/13 $2.90 $2.10
6/30/13 $3.10 $2.00
9/30/13 $2.70 $1.60
12/31/13 $1.80 $0.53
3/31/14 $1.90 $0.59
|
Holders of common stock are entitled to receive dividends as may be
declared by the Board of Directors out of legally available funds and, in the
event of liquidation, to share pro rata in any distribution of CEL-SCI's assets
after payment of liabilities. The Board of Directors is not obligated to declare
a dividend. CEL-SCI has not paid any dividends on its common stock and CEL-SCI
does not have any current plans to pay any common stock dividends.
The provisions in CEL-SCI's Articles of Incorporation relating to CEL-SCI's
preferred stock would allow CEL-SCI's directors to issue preferred stock with
rights to multiple votes per share and dividend rights which would have priority
over any dividends paid with respect to CEL-SCI's common stock. The issuance of
preferred stock with such rights may make more difficult the removal of
management, even if such removal would be considered beneficial to shareholders
generally, and will have the effect of limiting shareholder participation in
certain transactions such as mergers or tender offers if such transactions are
not favored by incumbent management.
The market price of CEL-SCI's common stock, as well as the securities of
other biopharmaceutical and biotechnology companies, have historically been
highly volatile, and the market has from time to time experienced significant
price and volume fluctuations that are unrelated to the operating performance of
29
particular companies. Factors such as fluctuations in CEL-SCI's operating
results, announcements of technological innovations or new therapeutic products
by CEL-SCI or its competitors, governmental regulation, developments in patent
or other proprietary rights, public concern as to the safety of products
developed by CEL-SCI or other biotechnology and pharmaceutical companies, and
general market conditions may have a significant effect on the market price of
CEL-SCI's common stock.
PLAN OF DISTRIBUTION
CEL-SCI may sell shares of its common stock, preferred stock, convertible
preferred stock, rights, or warrants, as well as any issuable shares upon the
conversion or exercise of such securities, in and/or outside the United States:
(i) through underwriters or dealers; (ii) directly to a limited number of
purchasers or to a single purchaser; or (iii) through agents. The applicable
prospectus supplement with respect to the offered securities will set forth the
name or names of any underwriters or agents, if any, the purchase price of the
offered securities and the proceeds to CEL-SCI from such sale, any delayed
delivery arrangements, any underwriting discounts and other items constituting
underwriters' compensation, the public offering price and any discounts or
concessions allowed or reallowed or paid to dealers and any compensation paid to
an underwriter or a placement agent. The public offering price and any discounts
or concessions allowed or reallowed or paid to dealers may be changed from time
to time.
Notwithstanding the above, the maximum commission or discount to be
received by any NASD member or independent broker-dealer will not be greater
than 10% in connection with the sale of any securities offered by means of this
prospectus or any related prospectus supplement, exclusive of any
non-accountable expense allowance. Any securities issued by CEL-SCI to any FINRA
member or independent broker-dealer in connection with an offering of CEL-SCI's
securities will be considered underwriting compensation and may be restricted
from sale, transfer, assignment, or hypothecation for a number of months
following the effective date of the offering, except to officers or partners
(not directors) of any underwriter or member of a selling group and/or their
officers or partners.
CEL-SCI's securities may be sold:
o At a fixed price.
o As the result of the exercise of warrants or rights, or the conversion
of preferred shares, at fixed or varying prices, as determined by the
terms of the warrants, rights or convertible securities.
o At varying prices in at the market offerings.
o In privately negotiated transactions, at fixed prices which may be
changed, at market prices prevailing at the time of sale, at prices
related to such prevailing market prices or at negotiated prices.
If underwriters are used in the sale, the offered securities will be
acquired by the underwriters for their own account and may be resold from time
to time in one or more transactions, including negotiated transactions, at a
30
fixed public offering price or at varying prices determined at the time of sale.
The securities may be offered to the public either through underwriting
syndicates represented by one or more managing underwriters or directly by one
or more firms acting as underwriters. The underwriter or underwriters with
respect to a particular underwritten offering of securities will be named in the
prospectus supplement relating to such offering and, if an underwriting
syndicate is used, the managing underwriter or underwriters will be set forth on
the cover of such prospectus supplement. Unless otherwise set forth in the
prospectus supplement, the obligations of the underwriters to purchase the
offered securities will be subject to conditions precedent and the underwriters
may be obligated to purchase all the offered securities if any are purchased.
If dealers are utilized in the sale of offered securities in respect of
which this prospectus is delivered, CEL-SCI will sell the offered securities to
the dealers as principals. The dealers may then resell the offered securities to
the public at varying prices to be determined by the dealers at the time of
resale. The names of the dealers and the terms of the transaction will be set
forth in the prospectus supplement relating to the securities sold to the
dealers.
If an agent is used in an offering, the agent will be named, and the terms
of the agency will be set forth, in the prospectus supplement. Unless otherwise
indicated in the prospectus supplement, an agent will act on a best efforts
basis for the period of its appointment.
The securities may be sold directly by CEL-SCI to institutional investors
or others, who may be deemed to be underwriters within the meaning of the
Securities Act of 1933 with respect to any resale of the securities purchased by
the institutional investors. The terms of any of the sales, including the terms
of any bidding or auction process, will be described in the applicable
prospectus supplement.
CEL-SCI may permit agents or underwriters to solicit offers to purchase its
securities at the public offering price set forth in a prospectus supplement
pursuant to a delayed delivery arrangement providing for payment and delivery on
the date stated in the prospectus supplement. Any delayed delivery contract will
contain definite fixed price and quantity terms. The obligations of any
purchaser pursuant to a delayed delivery contract will not be subject to any
market outs or other conditions other than the condition that the delayed
delivery contract will not violate applicable law. In the event the securities
underlying the delayed delivery contract are sold to underwriters at the time of
performance of the delayed delivery contract, those securities will be sold to
those underwriters. Each delayed delivery contract shall be subject to CEL-SCI's
approval. CEL-SCI will pay the commission indicated in the prospectus supplement
to underwriters or agents soliciting purchases of securities pursuant to delayed
delivery arrangements accepted by CEL-SCI.
Notwithstanding the above, while prospectus supplements may provide
specific offering terms, or add to or update information contained in this
prospectus, any fundamental changes to the offering terms will be made by means
of a post-effective amendment.
Agents, dealers and underwriters may be entitled under agreements entered
into with CEL-SCI to indemnification from CEL-SCI against certain civil
31
liabilities, including liabilities under the Securities Act, or to contribution
with respect to payments made by such agents, dealers or underwriters.
DESCRIPTION OF SECURITIES
Common Stock
CEL-SCI is authorized to issue 600,000,000 shares of common stock, (the
"common stock"). Holders of common stock are each entitled to cast one vote for
each share held of record on all matters presented to shareholders. Cumulative
voting is not allowed; hence, the holders of a majority of the outstanding
common stock can elect all directors.
Holders of common stock are entitled to receive such dividends as may be
declared by the Board of Directors out of funds legally available therefor and,
in the event of liquidation, to share pro rata in any distribution of CEL-SCI's
assets after payment of liabilities. The board is not obligated to declare a
dividend. It is not anticipated that dividends will be paid in the foreseeable
future.
Holders of common stock do not have preemptive rights to subscribe to
additional shares if issued by CEL-SCI. There is no conversion, redemption,
sinking fund or similar provision regarding the common stock. All of the
outstanding shares of common stock are fully paid and non-assessable.
Preferred Stock
CEL-SCI is authorized to issue up to 200,000 shares of preferred stock.
CEL-SCI's Articles of Incorporation provide that the Board of Directors has the
authority to divide the preferred stock into series and, within the limitations
provided by Colorado statute, to fix by resolution the voting power,
designations, preferences, and relative participation, special rights, and the
qualifications, limitations or restrictions of the shares of any series so
established. As the Board of Directors has authority to establish the terms of,
and to issue, the preferred stock without shareholder approval, the preferred
stock could be issued to defend against any attempted takeover of CEL-SCI. As of
June 16, 2014 no shares of preferred stock were outstanding.
Warrants Held by Private Investors
See "Comparative Share Data" for information concerning CEL-SCI's
outstanding options, warrants and convertible securities.
Transfer Agent
Computershare Trust Company, Inc., of Denver, Colorado, is the transfer
agent for CEL-SCI's common stock.
32
EXPERTS
The financial statements as of September 30, 2013 and 2012 and for each of
the three years in the period ended September 30, 2013 and management's
assessment of the effectiveness of internal control over financial reporting as
of September 30, 2013 incorporated by reference in this Prospectus, have been so
incorporated in reliance on the reports of BDO USA, LLP, an independent
registered public accounting firm, incorporated herein by reference, given on
the authority of said firm as experts in auditing and accounting.
INDEMNIFICATION
CEL-SCI's bylaws authorize indemnification of a director, officer, employee
or agent of CEL-SCI against expenses incurred by him in connection with any
action, suit, or proceeding to which he is named a party by reason of his having
acted or served in such capacity, except for liabilities arising from his own
misconduct or negligence in performance of his duty. In addition, even a
director, officer, employee, or agent of CEL-SCI who was found liable for
misconduct or negligence in the performance of his duty may obtain such
indemnification if, in view of all the circumstances in the case, a court of
competent jurisdiction determines such person is fairly and reasonably entitled
to indemnification. Insofar as indemnification for liabilities arising under the
Securities Act of 1933 may be permitted to directors, officers, or persons
controlling CEL-SCI pursuant to the foregoing provisions, CEL-SCI has been
informed that in the opinion of the Securities and Exchange Commission, such
indemnification is against public policy as expressed in the Act and is
therefore unenforceable.
ADDITIONAL INFORMATION
CEL-SCI is subject to the requirements of the Securities Exchange Act of
l934 and is required to file reports, proxy statements and other information
with the Securities and Exchange Commission. Copies of any such reports, proxy
statements and other information filed by CEL-SCI can be read and copied at the
Commission's Public Reference Room at 100 F Street, N.E., Washington, D.C.,
20549. The public may obtain information on the operation of the Public
Reference Room by calling the Commission at 1-800-SEC-0330. The Commission
maintains an Internet site that contains reports, proxy and information
statements, and other information regarding CEL-SCI. The address of that site is
http://www.sec.gov.
CEL-SCI will provide, without charge, to each person to whom a copy of this
prospectus is delivered, including any beneficial owner, upon the written or
oral request of such person, a copy of any or all of the documents incorporated
by reference below (other than exhibits to these documents, unless the exhibits
are specifically incorporated by reference into this prospectus). Requests
should be directed to:
CEL-SCI Corporation
8229 Boone Blvd., #802
Vienna, Virginia 22182
(703) 506-9460
33
The following documents filed with the Commission by CEL-SCI (Commission
File No. 001-11889) are incorporated by reference into this prospectus:
(1) Annual Report on Form 10-K for the fiscal year ended September 30,
2013.
(2) Report on Form 8-K filed on October 10, 2013.
(3) Report on Form 8-K filed on October 11, 2013.
(4) Report on Form 8-K filed on November 1, 2013.
(5) Report on Form 8-K filed on December 19, 2013.
(6) Report on Form 8-K filed on December 24, 2013.
(7) Report on Form 8-K filed on April 14, 2014.
(8) Report on Form 8-K filed on April 15, 2014.
(9) Report on Form 8-K filed on April 18, 2014.
(10) Quarterly report on Form 10-Q for the three months ended December 31,
2013.
(11) Quarterly report on Form 10-Q for the six months ended March 31, 2014.
(12) Proxy statement relating to the annual meeting of shareholders to
be held on July 22, 2014.
All documents filed with the Commission by CEL-SCI pursuant to Sections
13(a), 13(c), 14 or 15(d) of the Exchange Act subsequent to the date of this
prospectus and prior to the termination of this offering shall be deemed to be
incorporated by reference into this prospectus and to be a part of this
prospectus from the date of the filing of such documents. Any statement
contained in a document incorporated or deemed to be incorporated by reference
shall be deemed to be modified or superseded for the purposes of this prospectus
to the extent that a statement contained in this prospectus or in any
subsequently filed document which also is or is deemed to be incorporated by
reference in this prospectus modifies or supersedes such statement. Such
statement so modified or superseded shall not be deemed, except as so modified
or superseded, to constitute a part of this prospectus.
Investors are entitled to rely upon information in this prospectus or
incorporated by reference at the time it is used by CEL-SCI to offer and sell
securities, even though that information may be superseded or modified by
information subsequently incorporated by reference into this prospectus.
CEL-SCI has filed with the Securities and Exchange Commission a
Registration Statement under the Securities Act of l933, as amended, with
respect to the securities offered by this prospectus. This prospectus does not
contain all of the information set forth in the Registration Statement. For
further information with respect to CEL-SCI and such securities, reference is
made to the Registration Statement and to the exhibits filed with the
Registration Statement. Statements contained in this prospectus as to the
contents of any contract or other documents are summaries which are not
necessarily complete, and in each instance reference is made to the copy of such
contract or other document filed as an exhibit to the Registration Statement,
each such statement being qualified in all respects by such reference. The
Registration Statement and related exhibits may also be examined at the
Commission's internet site.
34
No dealer salesman or other person has been authorized to give any
information or to make any representations, other than those contained in this
prospectus. Any information or representation not contained in this prospectus
must not be relied upon as having been authorized by CEL-SCI. This prospectus
does not constitute an offer to sell, or a solicitation of an offer to buy, the
securities offered hereby in any state or other jurisdiction to any person to
whom it is unlawful to make such offer or solicitation. Neither the delivery of
this prospectus nor any sale made hereunder shall, under any circumstances,
create an implication that there has been no change in the affairs of CEL-SCI
since the date of this prospectus.
35
TABLE OF CONTENTS
Page
Prospectus Summary....................................................
Forward Looking Statements ...........................................
Risk Factors .........................................................
Comparative Share Data ...............................................
Market for CEL-SCI's Common Stock ....................................
Plan of Distribution .................................................
Description of Securities ............................................
Experts ..............................................................
Indemnification ......................................................
Additional Information ...............................................
Common Stock
CEL-SCI CORPORATION
PROSPECTUS
PART II
Information Not Required in Prospectus
Item 14. Other Expenses of Issuance and Distribution
SEC Filing Fee $9,660
Legal Fees and Expenses 30,000
Accounting Fees and Expenses 10,000
Miscellaneous Expenses 5,340
------
TOTAL $55,000
=======
|
All expenses other than the S.E.C. filing fees are estimated.
Item 25. Indemnification of Officers and Directors.
It is provided by Section 7-109-102 of the Colorado Revised Statutes and
CEL-SCI's Bylaws that CEL-SCI may indemnify any and all of its officers,
directors, employees or agents or former officers, directors, employees or
agents, against expenses actually and necessarily incurred by them, in
connection with the defense of any legal proceeding or threatened legal
proceeding, except as to matters in which such persons shall be determined to
not have acted in good faith and in the best interest of CEL-SCI.
Item 16. Exhibits
3(a) Articles of Incorporation Incorporated by reference to
Exhibit 3(a) of CEL-SCI's combined
Registration Statement on Form S-1
and Post-Effective Amendment
("Registration Statement"),
Registration Nos. 2-85547-D and
33-7531.
3(b) Amended Articles Incorporated by reference to
Exhibit 3(a) of CEL-SCI's
|
Registration Statement on Form S-1,
Registration Nos. 2-85547-D and
33-7531.
3(c) Amended Articles (Name change only) Filed as Exhibit 3(c) to CEL-SCI's
Registration Statement on Form S-1
Registration Statement (No. 33-34878).
3(d) Bylaws Incorporated by reference to
Exhibit 3(b) of CEL-SCI's
Registration Statement on Form S-1,
Registration Nos. 2-85547-D and
33-7531.
|
4 Shareholders Rights Agreement
Incorporated by reference to Exhibit 4
of CEL-SCI'S report on Form 8-K dated
November 7, 2007.
5 Opinion of Counsel _______________
10(d) Employment Agreement with Incorporated by reference to
Maximilian de Clara Exhibit 10(d) of CEL-SCI's report
on Form 8-K (dated April 21, 2005) and
Exhibit 10(d) to CEL-SCI's report on
Form 8-K dated September 8, 2006.
10(f) Distribution and Royalty Incorporated by reference to Agreement
with Eastern Biotech Exhibit 10(x) to Amendment No. 2 to
CEL-SCI's Registration Statement on
Form S-3 (Commission File No.
333-106879).
10(g) Securities Purchase Agreement Incorporated by reference to
(together with schedule required Exhibit 10 to CEL-SCI's report on
by Instruction 2 to Item 601 of Form 8-K dated August 4, 2006.
Regulation S-K) pertaining to
Series K notes and warrants,
together with the exhibits to
the Securities Purchase
Agreement.
10(h) Subscription Agreement (together Incorporated by reference to
with Schedule required by Exhibit 10 of CEL-SCI's report on
Instruction 2 to Item 601 of Form 8-K dated April 18, 2007.
Regulation S-K) pertaining to
April 2007 sale of 20,000,000
shares of CEL-SCI's common stock,
10,000,000 Series L warrants and
10,000,000 Series M Warrants.
10(i) Warrant Adjustment Agreement with Incorporated by reference to Exhibit
Laksya Ventures 10(i) of CEL-SCI's report on Form 8-K
dated August 3, 2010.
10(j) Employment Agreement with Incorporated by reference to Exhibit
Patricia Prichep 10(j) of CEL-SCI's report on Form 8-K
dated August 30, 2010.
10(k) Employment Agreement with Incorporated by reference to Exhibit
Eyal Talor 10(k) of CEL-SCI's report on Form 8-K
dated August 30, 2010.
|
10(l) Amendment to Employment Agreement Incorporated by reference to Exhibit
with Maximilian de Clara 10(l) of CEL-SCI's report on Form 8-K
dated August 30, 2010.
10(m) Amendment to Development Supply Incorporated by reference to Exhibit
and Distribution Agreement with 10(m) filed with CEL-SCI's 10-K report
Orient for the Europharma. (part for year ended September 30, 2010.
of Exhibit 10(m) has been omitted
pursuant to a request for
confidential treatment).
10(n) Licensing Agreement with Teva Incorporated by reference to Exhibit
Pharmaceutical Industries Ltd. 10(n) filed with CEL-SCI's 10-K report
(parts of Exhibit 10(n) for the year ended September 30, 2010.
have been omitted pursuantto a
request for confidential
treatment.)
10(o) Lease Agreement (parts of Exhibit Incorporated by reference to
10(o) have been omitted pursuant Exhibit 10(o) filed with CEL-SCI's
to a request for confidential 10-K report for the year ended
treatment). September 30, 2010.
10(p) Loan Agreements with Incorporated by reference to Exhibit
Maximilian de Clara 10(p) filed with CEL-SCI's 10-K report
for the year ended September 30, 2010.
10(q) Licensing Agreement with Incorporated by reference to Exhibit
Byron Biopharma 10(i) of CEL-SCI's report on Form 8-K
dated March 27, 2009.
10(r) At Market Issuance Sales Incorporated by reference to Exhibit
Agreement with McNicoll, 10(r) filed with CEL-SCI's 10-K report
Lewis & Vlak LLC for the year ended September 30, 2010.
10(z) Development, Supply and Incorporated by reference to Exhibit
Distribution Agreement 10(z)filed with CEL-SCI's report on
with Orient Europharma Form 10-K for the year ended
September 30, 2003.
10(za) Employment Agreement with Incorporated by reference to Exhibit
Geert Kersten 10(za) to CEL-SCI's report on Form 8-K
dated September 1, 2011.
10(aa) Securities Purchase Agreement Incorporated by reference to Exhibit
and form of the Series F 10(aa) of CEL-SCI's report on Form
warrants, which is and exhibit 8-K dated October 3, 2011.
to the Securities Purchase
Agreement.
10(bb) Placement Agent Agreement Incorporated by reference to Exhibit
10(bb) of CEL-SCI's report on Form 8-K
dated October 3, 2011.
10(cc) Securities Purchase Agreement Incorporated by reference to
together with the form of the Exhibit 10(cc) of CEL-SCI's
Series H warrants, which is an report on Form 8-K dated January
exhibit to the securities 25, 2012.
Purchase Agreement.
|
10(dd) Placement Agent Agreement Incorporated by reference to Exhibit
10(dd) of CEL-SCI's report on Form 8-K
dated January 25, 2012.
10(ee) Warrant Amendment Agreement, Incorporated by reference to Exhibit
together with the form of the 10(ee) of CEL-SCI's report on Form 8-K
Series P warrant, which is an dated February 10, 2012. exhibit to
the Warrant Amendment Agreement.
10(ff) Placement Agent Agreement Incorporated by reference to Exhibit
10(ff) of CEL-SCI's report on Form 8-K
dated February 10, 2012.
10(gg) Securities Purchase Agreement Incorporated by reference to Exhibit
and the form of the Series Q 10(gg) of CEL-SCI's report on Form 8-K
warrant which is an exhibit dated June 18, 2012.
to the Securities Purchase
Agreement.
10(hh) Placement Agent Agreement Incorporated by reference to Exhibit
10(hh) of CEL-SCI's report on Form 8-K
dated June 18, 2012.
10(ii) Amendment to Employment Incorporated by reference to Exhibit
Agreement with Maximilian de 10(L) of CEL-SCI's report on Form 8-K
Clara dated August 30, 2013.
10(jj) Amendment to Employment Incorporated by reference to Exhibit
Agreement with Geert Kersten 10(ZA) of CEL-SCI's report on Form
8-K dated August 30, 2013.
10(kk) Employment Agreement with Incorporated by reference to Exhibit
Patricia Prichep 10(J) of CEL-SCI's report on Form
8-K dated August 30, 2013.
10(ll) Employment Agreement with Incorporated by reference to Exhibit
Eyal Talor 10(K) of CEL-SCI's report on Form
8-K dated August 30, 2013.
10(mm) Securities Purchase Agreement, Incorporated by reference to Exhibit
together with the form of the 10(ii) of CEL-SCI's report on Form
Series R warrant, which is an 8-K dated December 5, 2012.
exhibit to the securities
Purchase Agreement.
10(nn) Underwriting agreement, Incorporated by reference to Exhibit
together with the form of 1.1 of CEL-SCI's report on Form 8-K
Series S warrant which is an dated October 8, 2013.
exhibit to the underwriting
agreement.
|
10(oo) Underwriting agreement, Incorporated by reference to Exhibit
together with the form of 1.1 of CEL-SCI's report on Form 8-K
Series S warrant which is an dated December 19, 2013.
exhibit to the underwriting
agreement.
10(pp) Underwriting agreement, Incorporated by reference to Exhibit
together with the form of 1.1 of CEL-SCI's report on Form 8-K
Series T warrant which is an dated April 14, 2014.
exhibit to the underwriting
agreement.
23.1 Consent of Hart & Hart _________________
23.2 Consent of BDO USA, LLP _________________
|
Item 17. Undertakings.
The undersigned Registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made,
a post-effective amendment to this Registration Statement.
(i) To include any prospectus required by Section l0(a)(3) of the
Securities Act of l933;
(ii) To reflect in the prospectus any facts or events arising
after the effective date of the Registration Statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate,
represent a fundamental change in the information set forth in the Registration
Statement;
(iii)To include any material information with respect to the plan
of distribution not previously disclosed in the Registration Statement or any
material change to such information in the Registration Statement, including
(but not limited to) any addition or deletion of a managing underwriter.
(2) That, for the purpose of determining any liability under the
Securities Act of l933, each such post-effective amendment shall be deemed to be
a new registration statement relating to the securities offered therein, and the
offering of such securities at that time shall be deemed to be the initial bona
fide offering thereof.
(3) To remove from registration by means of a post-effective amendment
any of the securities being registered which remain unsold at the termination of
the offering.
Insofar as indemnification for liabilities arising under the Securities Act
of l933 may be permitted to directors, officers and controlling persons of the
Registrant, the Registrant has been advised that in the opinion of the
Securities and Exchange Commission such indemnification is against public policy
as expressed in the Act and is, therefore, unenforceable. In the event that a
claim for indemnification against such liabilities (other than the payment by
the Registrant of expenses incurred or paid by a director, officer or
controlling person of the Registrant in the successful defense of any action,
suit or proceeding) is asserted by such director, officer or controlling person
in connection with the securities being registered, the Registrant will, unless
in the opinion of its counsel the matter has been settled by controlling
precedent, submit to a court of appropriate jurisdiction the question of whether
such indemnification by it is against public policy as expressed in the Act and
will be governed by the final adjudication of such issue.
POWER OF ATTORNEY
The registrant and each person whose signature appears below hereby
authorizes the agent for service named in this Registration Statement, with full
power to act alone, to file one or more amendments (including post-effective
amendments) to this Registration Statement, which amendments may make such
changes in this Registration Statement as such agent for service deems
appropriate, and the Registrant and each such person hereby appoints such agent
for service as attorney-in-fact, with full power to act alone, to exe- cute in
the name and in behalf of the Registrant and any such person, individually and
in each capacity stated below, any such amendments to this Registration
Statement.
SIGNATURES
Pursuant to the requirements of the Securities Act of l933, the Registrant
certifies that it has reasonable grounds to believe that it meets all the
requirements for filing on Form S-3 and has duly caused this Registration
Statement to be signed on its behalf by the undersigned, thereunto duly
authorized, in the City of Vienna, State of Virginia, on the 20th day of June
2014.
CEL-SCI CORPORATION
By:/s/ Maximilian de Clara
---------------------------
Maximilian de Clara, President
|
Pursuant to the requirements of the Securities Act of l933, this
Registration Statement has been signed by the following persons in the
capacities and on the dates indicated.
Signature Title Date
/s/ Maximilian de Clara Director and Principal June 20, 2014
-------------------------- Executive Officer
Maximilian de Clara
/s/ Geert R. Kersten Director, Principal Financial June 20, 2014
-------------------------- Officer, Principal Accounting
Geert R. Kersten Officer and Chief Executive
Officer
Director
--------------------------
Alexander G. Esterhazy
Director
------------------------
C. Richard Kinsolving, Ph.D.
/s/ Peter R. Young Director June 20, 2014
------------------------
Peter R. Young, Ph.D.
|
CEL-SCI CORPORATION
FORM S-3
EXHIBITS
Cel Sci (AMEX:CVM)
Historical Stock Chart
From Mar 2024 to Apr 2024
Cel Sci (AMEX:CVM)
Historical Stock Chart
From Apr 2023 to Apr 2024